Abstract
Background
Clear cell ependymoma is one of the 4 main histological subtypes of ependymomas defined by the World Health Organization (WHO) classification of tumors of the CNS. DNA methylation profiling can distinguish 4 subgroups of intracranial ependymomas, including supratentorial (ST) ependymomas with Yes-associated protein 1 fusion (YAP1), ST ependymomas with fusion of v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA), posterior fossa ependymomas with balanced genome, and posterior fossa ependymomas with chromosomal instability. In addition, trisomy 19 is a genomic hallmark of ependymomas with rich branching capillaries. However, the relation of histological and molecular subtypes is unclear.
Methods
Here, we report a series of 20 ependymomas histologically defined by clear cells and branching capillaries.
Results
We observed a strong male predominance. Median age at surgery was 10.4 years (range, 0.8–68.4). All cases were ST, cortical, contrast enhancing, and most often frontal, cystic, and calcified. All tumors qualified as WHO grade III. Some of them exhibited neuronal differentiation. Trisomy 19 was recorded in 13 cases. All samples strongly accumulated p65RelA protein within nuclei, indicating pathological activation of the nuclear factor-kappaB pathway. We identified causative C11ORF95-RELA fusion in almost all cases. Median progression-free survival and overall survival were 11.4 years (95% CI: 5.1–17.8) and not reached, respectively.
Conclusion
ST clear cell ependymomas with branching capillaries display characteristic clinicopathological features and are associated with pathological activation of nuclear factor-kappaB signaling, which may indicate a potential novel target for therapy in these patients.
Keywords: clear cell ependymomas, intracranial, NF kappa B, RelA, trisomy 19
Ependymomas are rare tumors of the brain and spinal cord. Statistical reports by the Central Brain Tumor Registry of the United States show that they account for 6.7% of all glial neoplasms. They are more frequent in children and young patients (<20 y) than in adults.1
Although ependymomas develop in all age groups, the incidence depends on histological variant, molecular group, and location.2 In children, 90% of ependymomas occur intracranially, with two thirds being located in the posterior fossa (PF) and one third in the supratentorial (ST) compartment.3 In contrast, intracranial ependymomas are rare in adults.4 Two factors are consistently prognostic across different series in the literature: age and extent of surgical excision. The 10-year overall survival (OS) is 64% in pediatric patients but ranges from 70% to 89% in adult patients.1 Total surgical removal is associated with a better OS independently of age.4–6
In contrast, the prognostic value of histological grade varies from one study to another, likely because of poor interobserver reproducibility, but also because of high intratumoral heterogeneity, especially in the infratentorial location.7
The current World Health Organization (WHO) classification since 2007 has distinguished 4 main histological subtypes of ependymomas: classic, tanycytic, clear cell, and papillary. In their recent study (which includes also myxopapillary ependymomas and subependymomas, both WHO grade I), Pajtler and colleagues2 classified 500 ependymal tumors using DNA methylation profiling into 9 molecular subgroups, 4 of them occurring in the intracranial compartment: ST ependymomas with Yes-associated protein 1 (YAP1) fusion, ST ependymomas with v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA) fusion, PF ependymomas with balanced genome (group A), and PF ependymomas with chromosomal instability (group B). Except for grade, the histological subtype of the ependymomas included in this series was unknown. Besides, Rousseau et al8 pointed out recurrent trisomy 19 often associated with deletion of 13q21.31–31.2 and 3 copies of 11q13.3–13.4 in ST ependymomas showing a rich branched capillary network, some of them being clear cell ependymomas. Here we focus on a series of 20 clear cell ependymomas that were selected because of their common histopathological features: clear cells and branching vessels mimicking oligodendroglioma. Neuroradiological and molecular analyses showed that all the clear cell ependymomas selected on histological criteria shared common neuroradiological features and molecular alterations. They were all ST and cortical, strongly contrast enhancing, most often cystic, and calcified. They all accumulated p65RelA protein within nuclei, indicating pathological activation of the nuclear factor-kappaB (NFκB) pathway, and all except one carried the most frequent C11ORF95-RELA fusion.
Materials and Methods
Patient's Samples
Formalin fixed, paraffin-embedded (FFPE) samples from 20 ependymomas showing clear cell features and rich networks of branching vessels mimicking oligodendroglioma were included retrospectively in the study. Cases were either from 9 patients operated on at the APHM or sent by French pathologists in Marseille for pathological review (11 patients). One case out of 11 was previously reported.9 Patients included retrospectively in this study have provided their written consent for clinical data collection and genetic analysis according to national policies and all studies have been reviewed by the appropriate ethics committee. In all cases, the following pathological features were assessed as present or absent: clear cell cytology, branching vessels, calcifications, perivascular pseudorosettes, ependymal rosettes, microvascular proliferation, necrosis, and mitotic count (cutoff = 5/10 high-power fields). In addition, when possible, we evaluated the delimitation of the tumor from the surrounding brain. Immunohistochemical staining for glial fibrillary acidic protein (GFAP), epithelial membrane antigen, Mib1, Olig2, NeuN, neurofilament, and isocitrate dehydrogenase 1 (IDH1)R132H was performed in all cases with an automated Ventana-Roche Benchmark XT and each item was scored as present or absent, except for Mib1 expression, which was evaluated as a percentage. Analysis of chromosome 19 status was done by fluorescence in situ hybridization (FISH) in all samples.
Patients and Imaging
A file was sent to clinicians who took care of the patients to assess the following clinical items: date of birth, date of surgery, date of relapse if appropriate, date of last follow-up, clinical status at last follow-up (alive or dead), extent of surgical removal (evaluated by postoperative imaging in 14 cases and by neurosurgeon's impression in the others), and postoperative treatment. In addition, we asked for MRI and CT data to assess tumor location, contrast enhancement, calcification, and cystic changes.
Status of RELA and L1 Cell Adhesion Molecule
Immunohistochemistry
FFPE tissue slides from the 20 samples included in the study were immunostained on the Ventana-Roche Benchmark XT system using the rabbit anti-NFκB p65 antibody D14E12 (1:400; Cell Signaling) and the mouse monoclonal anti–L1 cell adhesion molecule (L1CAM) antibody (clone UJ127.11, 1:1500; Sigma-Aldrich) after antigen retrieval (30 min, buffer CC1; Ventana-Roche). Significant nuclear staining was rated positive for the anti-NFκB p65 antibody, whereas L1CAM staining was rated as positive or negative. All positive cases displayed strong diffuse membranous and interstitial immunostaining.
In addition, immunohistochemistry was performed in a further 69 ependymoma samples included in a tissue microarray (TMA) that contained cases from a previously reported series of adult intracranial ependymomas.4 Fifty-two cases were located in the infratentorial compartment and 17 in the ST (including 10 intraparenchymatous cases). Fifty-three were classified as grade II and 16 as grade III. None of these cases had clear cells and branching capillaries.
Reverse-transcription PCR for C11ORF95-RELA fusion mRNA
Total RNA of 20 ependymoma cases was extracted from FFPE tissue using the AllPrep DNA/RNA FFPE Kit from Qiagen according to the manufacturer's instructions. Five hundred nanograms, 250 ng, or 150 ng of total RNA (as measured by 260 nm extinction) were then reversed-transcribed using the Prime Script RT reagent kit (Perfect Real Time, Takara Bio) and random primers.
PCR of cDNA was performed with primers located in exon 2 of C11ORF95 (5′ aggaagtcatcagcaacagc 3′) and in exon 3 of RELA (5′ tcttggtggtatctgtgctc 3′) as described previously.10 PCR was performed under the following conditions: annealing temperature, 65°C; elongation, 72°C for 30 s, 50 cycles. The generated 321 bp PCR fragments were analyzed on a 2% agarose gel. PCR products were visualized and documented on a Gel Doc 1000 system (Bio-Rad). PCR products were purified using the Qiagen PCR purification kit. Direct Sanger sequencing reactions were performed in duplicate (forward and reverse) by Eurofins MWG Operon.
Statistical Analyses
Categorical variables were presented as frequencies and percentages, continuous variables as median and range. OS was defined to be time from the date of surgery to death from any cause, censored at the date of last contact. Progression-free survival (PFS) was defined to be time from the date of surgery to documented progression or death, censored at the date of the last documented disease evaluation. The Kaplan–Meier method was used to estimate survival distributions. Log-rank tests were used for univariate comparisons. All the tests were 2-sided and P < .05 was considered significant for each statistical analysis. Statistical analyses were conducted using the statistical package SPSS software v17.
Results
Pathological Features
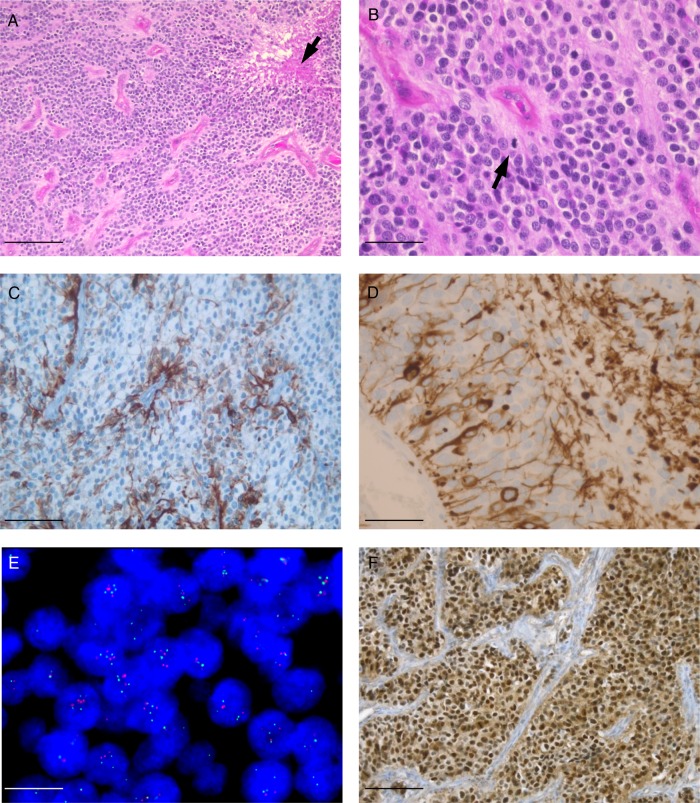
Clear cells and a rich network of branching vessels were observed in all cases (inclusion criteria) (Fig. 1A and B). Perivascular pseudorosettes were also recorded in all cases but were difficult to see because of lack of long tapering processes in most cases. True ependymal rosettes were observed in only one case. Calcifications were a common finding, recorded in 12/20 cases. Sharp delimitation from the surrounding brain was recorded in all assessable cases (12/12). All cases were scored as anaplastic WHO grade III, showing more than 5 mitoses per 10 high-power fields, with microvascular proliferation associated with necrosis in 13 cases (Fig. 1A and B).
Fig. 1.
(A, B, hematoxylin/eosin staining) Clear cell ependymomas appeared as densely cellular lesions, composed of uniform closely packed cells, with discrete perivascular pseudorosettes and presenting a branched capillary network reminiscent of oligodendroglioma. Note microvascular proliferation and necrosis (arrow in A) and mitotic figure (arrow in B). (C) GFAP immunostaining shows a perivascular pattern. (D) Some tumors express neurofilament and exhibit bipolar morphology. (E) Interphase FISH analysis using 19p13.3 (green) and 19q13.2 (orange) dual-color probes showing numerous nuclei with 3 or 4 copies of 19q. (F) Nuclear p65RelA accumulation was detected in a clear cell ependymoma. Scale bars: 100 µm (A), 50 µm (B, C, F), 25 µm (D), and 10 µm (E).
Immunohistochemistry showed perivascular accumulation of GFAP (Fig. 1C) and dot-like inclusion with epithelial membrane antigen and lack of IDH1R132H expression in all cases. Olig2 expression was recorded in 7/20 cases, but the number of immunoreactive cells remained low except in one case showing strong Olig2 accumulation in up to 20% of tumor cells. NeuN expression was observed in 4 cases, but the percentage of immunoreactive nuclei remained low (≤10). Neurofilament expression was observed in 6 cases and reached in some cases 15% of tumor cells (Fig. 1D). Interestingly, immunopositive cells were often characterized with long bipolar processes, one of them reaching blood vessels.
Chromosome 19 copy number status was successfully assessed by FISH. It demonstrated trisomy 19 in 13 cases (associated with tetrasomy in 2) and polysomy in 2 (Fig. 1E) and was normal in 5.
Clinical Features and Imaging
Patient characteristics are summarized in Table 1. We observed a strong male predominance (14 males, 6 females). Most of the patients were <18 years at surgery (15/20) and the median age at surgery was 10.4 years (range, 0.8–68.4).
Table 1.
Patient characteristics and follow-up
| ID | Sex | Age, y | Topography | Cystic | Symptoms | Type of Surgery | RT/CT | Mib1 | NF | Chromosome 19 | Relapse | Death | PFS, y | OS, y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 9.6 | Frontal | 0 | Seizure | Complete | RT | 15 | 0 | Normal | Remission | Alive | 7.6 | 7.6 |
| 2 | M | 0.8 | Fronto-parietal | 0 | Seizure | Complete | CT | 40 | 5 | Normal | Remission | Alive | 7.8 | 7.8 |
| 3 | M | 11.7 | Frontal | 1 | Seizure | Complete | RT + CT | 20 | 0 | Polysomy | Remission | Alive | 15.9 | 15.9 |
| 4 | M | 21.2 | Occipital | 1 | Headache | Complete | RT | 15 | 0 | Trisomy | Remission | Alive | 5.1 | 5.1 |
| 5 | M | 7.0 | Frontal | ICH | Complete | None | 10 | 0 | Trisomy | Progressive | Dead | 0.3 | 1.3 | |
| 6 | M | 11.5 | Occipital | 1 | Headache | Complete | None | 30 | 0 | Trisomy | Progressive | Dead | 1.2 | 2.4 |
| 7 | F | 31.8 | Temporal | 1 | Seizure and ICH | Complete | RT | 25 | 0 | Trisomy | Progressive | Dead | 0.5 | 1.1 |
| 8 | F | 10.7 | Frontal | 1 | Headache | 40 | 0 | Trisomy | Progressive | Alive | 8.5 | 17.5 | ||
| 9 | M | 0.9 | Parietal | 1 | Deficit | Complete | CT | 25 | 15 | Trisomy | Remission | Alive | 9.0 | 9.0 |
| 10 | M | 6.5 | Parieto-occipital | 0 | ICH | Complete | RT | 10 | 0 | Normal | Remission | Alive | 10.6 | 10.6 |
| 11 | M | 19.1 | Frontal | 1 | Deficit | Complete | RT | 40 | 5 | Trisomy | Progressive | Dead | 1.7 | 3.8 |
| 12 | F | 10.1 | Parietal | 1 | ICH | Complete | RT | 30 | 5 | Trisomy | Remission | Alive | 7.0 | 7.0 |
| 13 | M | 36.8 | Frontal | 1 | Headache | Subtotal | RT + CT | 40 | 0 | Normal | Progressive | Dead | 11.5 | 11.5 |
| 14 | M | 12.7 | Parieto-occipital | 1 | ICH | Complete | RT | 25 | 0 | Normal | Progressive | Dead | 4.9 | 4.9 |
| 15 | M | 2.5 | Fronto-Parieto-occipital | 0 | ICH | Complete | RT + CT | 10 | 10 | Trisomy | Remission | Alive | 7.4 | 7.4 |
| 16 | M | 10.6 | Parietal | 0 | Seizure | Complete | None | 30 | 0 | Polysomy | Progressive | Alive | 11.8 | 16.2 |
| 17 | F | 68.4 | Frontal | 0 | Headache | Subtotal | RT | 20 | 0 | Trisomy | Progressive | Alive | 4.7 | 9.7 |
| 18 | F | 4.4 | Frontal | 1 | Headache | Complete | None | 15 | 0 | Trisomy | Progressive | Alive | 15.8 | 22.2 |
| 19 | M | 9.8 | Frontal | 0 | Headache | Complete | RT | 60 | 15 | Trisomy and tetrasomy | Remission | Alive | 0.5 | 0.5 |
| 20 | M | 9.4 | Temporal | 1 | Seizure | Complete | RT | 25 | 0 | Trisomy and tetrasomy | Remission | Alive | 0.8 | 0.8 |
Abreviations: ICH, intracranial hypertension; RT, radiotherapy; CT, chemotherapy; NF, neurofilament.
PFS and OS are in years. 1 = present; 0 = absent. Mib1 and NF expression rates are given in percentage of immunoreactive cells.
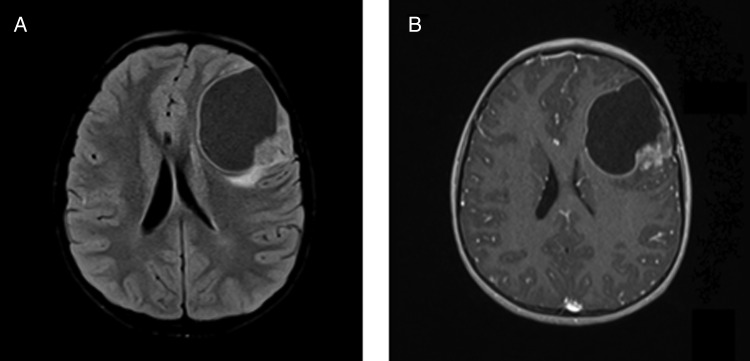
Initial symptoms were headache (7 patients), increased intracranial pressure (5 patients), epilepsy (6 patients), and neurological deficit (2 patients). Neuroimaging data (19/20) showed that all tumors were located in the ST compartment. Except for 4 patients who demonstrated huge lesions, all cases were cortically located (Fig. 2). Tumor location was frontal in 11 cases (including 1 fronto-parieto-occipital and 1 fronto-parietal), parietal in 5 (including 2 parieto-occipital), temporal in 2, and occipital in 2. Contrast enhancement was recorded in 19/19 cases, cysts in 12 cases, and calcifications in 7.
Fig. 2.
Typical neuroradiological features of clear cell ependymoma: cortical and cystic lesion on (A) T1-weighted sequence with (B) a mural nodule strongly enhanced after gadolinium injection. Case #19.
Extent of surgical removal was recorded in 19 patients—complete in 17 and subtotal in 2. Fifteen of 19 patients underwent adjuvant/postoperative treatment: radiotherapy alone in 10, chemotherapy alone in 2, and radiotherapy + chemotherapy in 3. It was noteworthy that the 2 patients who recurred almost 20 years later were treated by surgery only. For both patients, first relapse was treated by surgery and radiotherapy. Interestingly, in these 2 patients, the pathological and immunohistochemical features (including p65RelA and L1CAM staining) were similar between the first and the second surgery.
Median follow-up was 8.4 years (range, 0.5–22.2). At the time of last follow-up, 10 patients had recurred, of whom 6 died. Five- and 10-year PFS rates were 67.5% (SE = 11.0) and 57.9% (SE = 13.0), respectively, and 5- and 10-year OS rates were both 72.2% (SE = 10.6).
RELA and L1CAM Status
Nuclear immunostaining with anti-p65RelA antibody was recorded in all cases. It was strong and diffuse in 15 (Fig. 1E) and weak in 3; and in 2 cases (<10%), only a few nuclei were immunoreactive. In 9 cases, additional cytoplasmic staining was recorded. Except for one case, all cases demonstrated the most common C11ORF95-RELA fusion mRNA by reverse transcription (RT)-PCR.
Interestingly, the case that was negative for C11ORF95-RELA fusion showed strong nuclear immunostaining with anti-p65RelA antibody. Regarding the ependymoma series included in the TMA, immunohistochemical detection of p65RelA antibody was negative in 65/69 cases.
Among the positive cases, 1 demonstrated nuclei accumulation only and 3 demonstrated cytoplasmic as well as nuclear expression. All positive cases were located within ventricles. Two were in the fourth ventricle (occurring in a 48- and a 56-y-old man), one in the third ventricle (occurring in a 33-y-old woman), and the fourth in the lateral ventricle (in a 34-y-old woman). They were all classic at histology and grade II. Except for one patient, surgical removal was total. All patients were alive with no relapse at last follow-up (6 and 10 y for the patients with fourth ventricle tumor and 15 and 21 mo for patients operated on for third and lateral ventricle ependymomas). Because FFPE blocks of these cases were no longer available, it was not possible to search for C11ORF95-RELA fusions by RT-PCR in these cases. Regarding the other 65 cases that were included in the TMA but remained p65RelA negative, we recorded 49 grade II and 16 grade III tumors. Fifty were infratentorial and 15 ST. Among the 49 grade II tumors, histological subtypes were cellular in 11 cases, classic in 32 cases, papillary in 1, and tanycytic in 5.
Eighteen out of 20 clear cell ependymoma cases demonstrated strong and diffuse L1CAM immunostaining (only cases #7 and #18 in Table 1 were negative). In addition, we found that 3 out of 69 ependymoma cases included in the TMA were positive. These 3 cases were ST, and 2 out of 3 were the cases also found to express p65RelA by immunohistochemistry. The remaining one occurred in a 22-year-old man and was grade III.
Survival Analyses
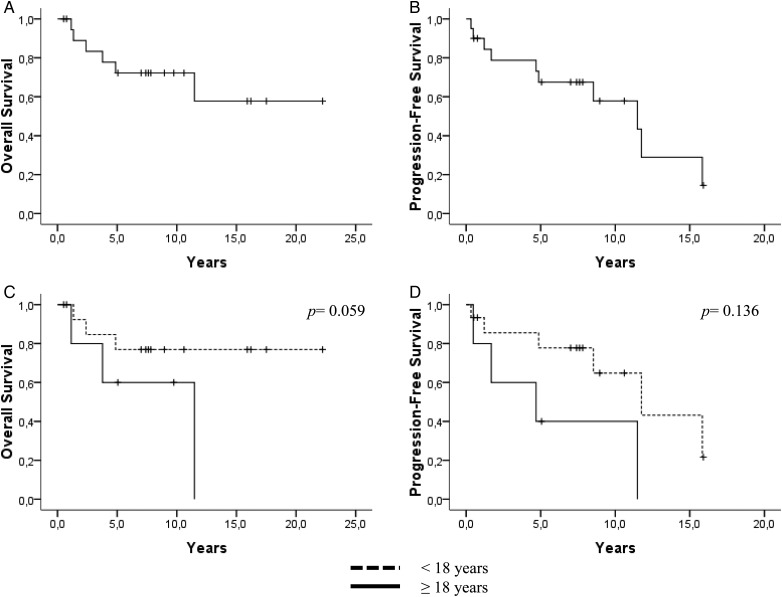
Median PFS and OS were 11.4 years (95% CI: 5.1–17.8) and not reached, respectively (Fig. 3). In univariate analyses, only the patient age (cutoff = 18 y) tended to be associated with both PFS (P = .136) and OS (P = .059) (Fig. 3). Other factors—such as the proliferative index, chromosome 19 copy number status, neurofilament expression, the type of adjuvant treatment, and tumor localization—were not associated with patient outcome.
Fig. 3.
(A) OS and (B) PFS for the clear cell ependymoma cohort. (C) OS and (D) PFS for patients with clear cell ependymoma stratified according to age (< or ≥ 18 y).
Discussion
We report here a series of 20 clear cell ependymomas with branching capillaries and show that these tumors have a characteristic clinico-radiological presentation, pathological features, and pathological activation of the NFκB signaling pathway, with nuclear p65RelA accumulation in almost all cases as a result of C11ORF95-RELA fusions as described by Pietsch et al10 and Parker et al.11 Although the frequency of clear cell ependymomas is unknown in the literature, it seems very rare according to the few series reported.12,13 The 20 cases included in our work were collected over a period of 20 years. Clear cell ependymomas occur mainly in the ST compartment12,13 (present series) and develop within the parenchyma, often in a cortical location. If we take into account the 20 cases of the present series and the 12 intraparenchymatous cases of the 2 major previous series,12,13 we observe a strong predominance for the frontal location. When available, neuroradiological features showed in all cases a contrast-enhancing mass with cystic compartments and/or calcifications. These features are in accordance with a previous series of 10 clear cell ependymomas: among them, 6 were ST and cystic and most of them displayed punctate calcifications on CT scans.12 The triad of contrast enhancement, cyst, and calcifications was also recorded in most of the cases investigated by Min and Scheithauer.13
ST clear cell ependymomas share light microscopic similarities: they are composed of uniform closely packed cells with round, centrally located nuclei and clear or focally eosinophilic cytoplasm associated with a prominent branched capillary network. In accordance with neuroradiological features, calcifications are common (12/20 cases in the present series). Because true rosettes are almost always absent and perivascular pseudorosettes might be difficult to recognize because in most cases long fibrillary processes are lacking, the differential diagnosis with oligodendroglioma in case of intraparenchymatous location might be tricky without further assessment of immunophenotype.
However, in contrast to oligodendrogliomas, ST clear cell ependymomas are sharply demarcated from the surrounding parenchyma. Most importantly, they lack IDH1R132H expression, which is common in most 1p19q codeleted oligodendrogliomas.14 Moreover, in contrast to 1p19q codeleted oligodendrogliomas, which show strong and intense Olig2 expression, Olig2 expression in clear cell ependymomas remains rare and never exceeds 20% of nuclei when recorded.
Central neurocytoma or extraventricular neurocytoma is another differential diagnosis that should be considered, because ependymomas may have a similar location and histology. Furthermore, neuronal differentiation can occur in ST clear cell ependymoma with NeuN expression, as in some of the cases we described here. However, the number of immunoreactive nuclei remains low in contrast to what is observed in central neurocytoma.15 Neurofilament expression was also recorded in 6/20 cases of the present series. Neurofilament expression as well as NeuN expression was found in a high number of ST ependymomas, and strong expression of neurofilament (>5% positive cells) was associated with a better PFS.16 Both the glial and neuronal differentiation recorded in ST clear cell ependymoma is in accordance with the presumptive origin of ependymoma from radial glia, which indeed represent neural stem cells.17,18
The ST clear cell ependymomas reported here were all WHO grade III, which was also the case for the 7 ST clear cell ependymomas included in the series of the 12 “trisomy 19 ependymoma” cases previously reported.8 These cases were histologically defined as “compact lesions characterized by a rich branched capillary network amongst which tumoral cells are regularly distributed; when containing clear cells they are called clear cell ependymomas.” Therefore, clear cell ependymomas with branching capillaries are a subset of trisomy 19 ependymomas.8 However, by using FISH analysis, we recorded trisomy 19 in only 13 cases, and 2 cases demonstrated polysomy (more than 3 spots). It is likely that FISH analysis with a locus-specific probe was not fully appropriate to detect trisomy 19 ependymomas, because trisomy 19 appeared not complete in some cases.8 Importantly, the so-called trisomy 19 ependymomas as previously defined by Rousseau et al were frequently associated with deletion of 13q21.31–31.2, three copies of 11q13.3–13.4, and/or deletions on chromosome 9, this latter finding being also previously reported in clear cell ependymomas.19 Frequent loss of the entire chromosome 9 or chromosomal arm 9p was also a common feature of the ST RELA fused ependymomas previously reported.2 In contrast, gain of chromosome 19 was a rare finding in this series, observed in only 10% of the cases.2 This can rely on the age of the patients included in this cohort and on the technique used, which can miss partial trisomy 19. In fact, 75% of the patients in the series reported by Pajtler et al2 were under 18 years, and the age of the patients harboring gain of chromosome 19 was unknown. Among the 12 patients reported by Rousseau et al8 with a trisomy 19, seven of 12 were 18 years or older (up to 30 y). In our series we recorded 13/20 patients with trisomy 19 and 4/5 patients older than 18 years who had trisomy 19. Therefore, it appears that among ST clear cell ependymomas with branching capillaries, the likelihood to demonstrate trisomy 19 is higher in older patients than in patients younger than 18 years.
Fusions between RELA, which encodes the NFκB component p65 and C11ORF95, a poorly characterized gene on chromosome 11, were recently identified by large-scale genomic and epigenomic studies as well as RNA sequencing.10,11 Although 7 distinct C11ORF95-RELA fusion transcripts have been reported,11 the most frequent included exons 1–2 of C11ORF95 and, except for the first codons, the entire open reading frame of RELA (this fusion was called RELAFUS1 by the authors).
Ependymomas carrying the C11ORF95-RELA fusion are characterized by a nuclear accumulation of p65RelA indicating a pathological activation of the NFκB signaling pathway.10 C11ORF95-RELA fusion was recorded in up to 70% of ST ependymomas, and this subset of tumors were reported as ST EPN-RELA.10,11 Here we showed that pathological NFκB activation by this mechanism characterized human ST clear cell ependymomas with branching capillaries, which therefore represent a subset of ST EPN-RELA. All cases included in the present series strongly expressed NFκB p65 in nuclei, and all but one carried the RELAFUS1 fusion transcript. It is likely that the peculiar case showing activation but lacking this usual fusion may exhibit a different fusion partner not covered by our RT-PCR analysis. Our study also emphasizes the usefulness of the immunohistochemical technique (which is an easy method) to pinpoint cases with C11ORF95-RELA fusions. Importantly, neural stem cells isolated from Ink4a/Arfnull Blbp-eGFP, transduced with RELAFUS1-RFP retroviruses, and implanted into the cerebrum of CD1-nude mice generated tumors.11 These tumors recapitulate the clear cell morphology and finely branched vasculature characteristic of clear cell ependymomas.11 Indeed, we recorded C11ORF95-RELA fusions in almost all clear cell ependymomas. However, the oncogene fusion event is not exclusively found in clear cell ependymomas and accounts for a large percentage of supratentorial ependymomas in young patients.2,10 Therefore, clear cell ependymoma with branching vessels might represent a possible morphological appearance of RELA-fused ependymoma. In contrast, this genetic alteration is very rare in adult ependymomas. By using a TMA from a cohort of adult ependymoma cases that do not exhibit clear cell features, we found very few cases of ependymomas with p65RelA nuclear accumulation (2/52 infratentorial ependymomas and 2/17 ST ependymomas). Unfortunately, it was not possible to test those cases for C11ORF95-RELA fusions because of lack of material available, and we cannot be sure that in these cases p65RelA nuclear accumulation relies on C11ORF95-RELA fusion or another mechanism, especially for the 2 PF cases. However, although C11ORF95-RELA fusion characterized a subset of supratentorial ependymomas, it was also reported by Pajtler et al in 4 PF ependymomas.2 Besides, according to the results by Parker et al,11 we observed a very strong correlation between p65RelA nuclear accumulation and diffuse L1CAM expression.
The prognostic value of RELA fusions in ependymomas showed discrepant results. In the large series reported by Pajtler et al, the 5- and 10-year PFS rates were 29% and 19%, respectively,2 whereas it was 67.5% and 57.9% in our series. Along the same lines, the 5- and 10-year OS rates were 75% and 49% in the Pajtler et al series, whereas it was 72.2% (at 5 and 10 y) in our series. Importantly, the percentage of complete removal, which is a major factor of prognosis, was higher in our series (84%) in comparison with the Pajtler et al series (65%). Interestingly, we also observed in our series a trend toward a shorter OS for patients above age 18. Although age has been reported as a prognostic factor in some series, the cutoff was usually different depending on the cohort analyzed.4–6 Taken together, clear cell ependymomas with branching vessels might represent a subset of ST EPN-RELA ependymomas with a more favorable prognosis, but this has to be confirmed by independent series. Moreover, it would be of high importance to compare the clinical course of RELA-positive clear cell ependymomas with branching capillaries to RELA-positive non–clear cell ependymomas.
In conclusion, ST clear cell ependymomas with branching capillaries display characteristic clinico-pathological features: they occur mainly in young patients; are cortical, cystic, and often calcified; and exhibit the potency of a divergent neural and glial differentiation in keeping with their neural stem cell origin. In addition, these ependymomas are associated with a more favorable prognosis than other ST EPN-RELAs reported so far. Nevertheless, pathological NFκB pathway activation may indicate a potential novel target for therapy in NFκB-activated ependymomas independently of their histological appearance.
Funding
This study was supported by a grant from the German Childhood Cancer Foundation. This work was completed in the SIRIC (Site de Recherche Intégrée en Cancérologie) of Marseille, grant INCa-DGOS-Inserm 6038.
Acknowledgments
We acknowledge the GENOP (Groupe d'Étude de Neuropathologie Oncologique Pediatrique) and the TUCERA (Tumeurs cérébrales rares) networks. We are grateful to all physicians for providing information regarding MRI features and follow-up of the patients (Pr C. Nuti, Dr C. Berger, Dr T. Dufour, Pr E. Emery, Dr A. Busson, Dr O. Lejars, Dr M. Lourmiere). We warmly thank Karen Silva for performing L1CAM immunohistochemistry. Specimens from Marseille's patients were retrieved from the APHM tumor bank AC 2013-1786.
Conflict of interest statement. None declared.
References
- 1.Ostrom QT, Gittleman H, Liao P et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pajtler KW, Witt H, Sill M et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilday JP, Rahman R, Dyer S et al. Pediatric ependymoma: biological perspectives. Mol Cancer Res. 2009;7(6):765–786. [DOI] [PubMed] [Google Scholar]
- 4.Metellus P, Barrie M, Figarella-Branger D et al. Multicentric French study on adult intracranial ependymomas: prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130(Pt 5):1338–1349. [DOI] [PubMed] [Google Scholar]
- 5.Figarella-Branger D, Civatte M, Bouvier-Labit C et al. Prognostic factors in intracranial ependymomas in children. J Neurosurg. 2000;93(4):605–613. [DOI] [PubMed] [Google Scholar]
- 6.Merchant TE, Li C, Xiong X et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison DW, Kocak M, Figarella-Branger D et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau E, Palm T, Scaravilli F et al. Trisomy 19 ependymoma, a newly recognized genetico-histological association, including clear cell ependymoma. Mol Cancer. 2007;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payet M, Conter C, Labrousse F et al. Clear cell ependymoma with trisomy 19 developing bone metastases. Childs Nerv Syst. 2012;28(5):739–742. [DOI] [PubMed] [Google Scholar]
- 10.Pietsch T, Wohlers I, Goschzik T et al. Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-kappaB signaling pathway. Acta Neuropathol. 2014;127(4):609–611. [DOI] [PubMed] [Google Scholar]
- 11.Parker M, Mohankumar KM, Punchihewa C et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouladi M, Helton K, Dalton J et al. Clear cell ependymoma: a clinicopathologic and radiographic analysis of 10 patients. Cancer. 2003;98(10):2232–2244. [DOI] [PubMed] [Google Scholar]
- 13.Min KW, Scheithauer BW. Clear cell ependymoma: a mimic of oligodendroglioma: clinicopathologic and ultrastructural considerations. Am J Surg Pathol. 1997;21(7):820–826. [DOI] [PubMed] [Google Scholar]
- 14.Figarella-Branger D, Mokhtari K, Dehais C et al. Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro Oncol. 2014;16(9):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasiljevic A, Francois P, Loundou A et al. Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J Surg Pathol. 2012;36(2):220–227. [DOI] [PubMed] [Google Scholar]
- 16.Andreiuolo F, Puget S, Peyre M et al. Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol. 2010;12(11):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkle FT, Tramontin AD, Garcia-Verdugo JM et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101(50):17528–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor MD, Poppleton H, Fuller C et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. [DOI] [PubMed] [Google Scholar]
- 19.Rickert CH, Korshunov A, Paulus W. Chromosomal imbalances in clear cell ependymomas. Mod Pathol. 2006;19(7):958–962. [DOI] [PubMed] [Google Scholar]