Abstract
Background
Recent transcriptomic approaches have demonstrated that there are at least 4 distinct subgroups in medulloblastoma (MB); however, survival studies of molecular subgroups in adult MB have been inconclusive because of small sample sizes. The aim of this study is to investigate the molecular subgroups in adult MB and identify their clinical and prognostic implications in a large, single-institution cohort.
Methods
We determined gene expression profiles for 13 primary adult MBs. Bioinformatics tools were used to establish distinct molecular subgroups based on the most informative genes in the dataset. Immunohistochemistry with subgroup-specific antibodies was then used for validation within an independent cohort of 201 formalin-fixed MB tumors, in conjunction with a systematic analysis of clinical and histological characteristics.
Results
Three distinct molecular variants of adult MB were identified: the SHH, WNT, and group 4 subgroups. Validation of these subgroups in the 201-tumor cohort by immunohistochemistry identified significant differences in subgroup-specific demographics, histology, and metastatic status. The SHH subgroup accounted for the majority of the tumors (62%), followed by the group 4 subgroup (28%) and the WNT subgroup (10%). Group 4 tumors had significantly worse progression-free and overall survival compared with tumors of the other molecular subtypes.
Conclusions
We have identified 3 subgroups of adult MB, characterized by distinct expression profiles, clinical features, pathological features, and prognosis. Clinical variables incorporated with molecular subgroup are more significantly informative for predicting adult patient outcome.
Keywords: adult medulloblastoma, histology, molecular subgroup, prognosis, survival
Medulloblastoma (MB) is the most common malignant pediatric brain tumor but accounts for <1% of adult intracranial tumors.1 Over the past 2 decades, MB survival has been improved by considerable advancements in therapeutic strategies on the basis of clinical risk stratification.2 However, MB is still associated with a poor outcome overall, and most survivors are left with long-term disabilities secondary to treatment.3–5 Studies are urgently needed to understand the biology of the disease, develop novel targets, and identify biomarkers that predict prognosis and response to treatment.
Recently, several transcriptional profiling studies have shown that MB is not a single disease, but a collection of clinically and molecularly diverse tumor subgroups. The current consensus is that there are 4 major subgroups: wingless (WNT), sonic hedgehog (SHH), group 3, and group 4, with disparate demographics, clinical characteristics, genetics, and transcriptomes.6–10 Due to the low incidence of MB in adults, studies of the biological and clinical features in this age group are limited. It has been shown that adult MB is distinct from pediatric MB in genomic aberrations, histopathology, and prognostic outcomes11,14,15; however, because of the lack of knowledge in adult MB, the clinical treatment strategies for adult MB are mainly adapted from pediatric protocols.11–13 Studies with combination of the clinical variables and subgroup affiliation are urgently needed to improve patient prognostication and therapy strategy.
In this study, we performed gene expression analyses to identify molecular subgroups of adult MB. The findings were validated in an independent cohort of 201 adult MBs using an immunohistochemistry (IHC)-based classification system, to evaluate the characteristics of molecular subgroups and their prognostic factors and to provide a basis for future therapeutic applications.
Patients and Methods
Ethics Statement
The study was approved by the institutional review board (CWO [commissie wetenschappelijk onderzoek, in Dutch]) of Altrecht Psychosomatic Medicine, Zeist, the Netherlands. All patients provided written informed consent.
Tumor Material and Patient Characteristics
All tumor samples were obtained from adult patients (aged ≥18 years at diagnosis) during initial surgery before any adjuvant therapy, at the Neurosurgical Centre of Beijing Tiantan Hospital, Beijing Neurosurgical Institute, Capital Medical University. Histological diagnoses according to the criteria of the 2007 World Health Organization (WHO) classification were confirmed by 2 neuropathologists.16 The MB tumors were classified by histology as classic (CMB), desmoplastic/nodular (DNMB), anaplastic (AMB), or large cell (LCMB) (see Supplementary Fig. S1). Gene expression analysis was performed on 13 frozen tumor tissue samples containing all 4 histological subtypes. And an independent cohort of paraffin-embedded samples collected from 1997 to 2014 from 201 adult patients was analyzed using IHC. In this cohort, the mean age of all patients was 28.7 years (range: 18–61 y) and the sex ratio was 1.96:1 (male:female). A more detailed description of the tumor material and patient characteristics of the 2 cohorts is provided in Supplementary Table S1.
In the 201-case validation cohort, the patients were staged using the classic Chang system definitions for tumor (T) and metastasis (M) parameters.17 All patients were treated with postoperative craniospinal irradiation (30–36 Gy and 54–60 Gy to a primary tumor site), except for 15 patients who received whole-brain irradiation alone because of toxicity reactions or problems with local facilities. Chemotherapy (4–6 cycles of carboplatin, vincristine, lomustine, or cisplatin) was administered to 147 patients: 72 high-risk patients and 75 average-risk patients. The 201 patients in the cohort were observed for a median of 60 months (range: 8–187 mo) from date of diagnosis to last contact or death. There had been 58 deaths by the end of the study, and the median follow-up for surviving patients was 74 months (range: 15–187 mo).
Biostatistics and Bioinformatics
The Agilent Whole Human Genome Oligo Microarray Kit, 4×44K (Gene Expression Omnibus accession no. GPL6480) was used for gene expression profiling of the samples (n = 13). Data were extracted with Feature Extraction Software v10.7 (Agilent Technologies). Raw data were normalized by Quantile algorithm, Gene Spring Software v11.0 (Agilent Technologies). Genes with a fold change ≥2 and P < .05 were selected for further analysis. Pathway annotation was performed by Ingenuity Systems.
Immunohistochemistry
Molecular subgroups of MB were identified by IHC using a combination of 2 groups of markers7,8: (1) CTNNB1 (WNT/wingless marker, 1:100; ab610154, BD Transduction Laboratories), SFRP1 (SHH marker, 1:2000; ab4193, Abcam), NPR3 (subgroup 3 marker, 1:200; ab37617, Abcam), and KCNA1 (subgroup 4 marker, 1:2000; ab32433, Abcam); and (2) β-catenin (1:800; BD #610154), GAB1 (1:50; Abcam #ab27439;), filamin A (1:100; Fitzgerald #10R-F113A), and YAP1 (1:50; Santa Cruz #sc-101199). Immunostaining was done on formalin-fixed paraffin-embedded tumors after dewaxing and rehydrating slides. Antigen retrieval was conducted by pretreatment at 95°C. Endogenous peroxidase was blocked with 0.5% hydrogen peroxide in water for 30 min.
For staining evaluation, 5 sections of each case were evaluated independently. The percentage of positive cells was evaluated for each protein. The pattern of expression (cytoplasmic, membranous, and nuclear) was recorded. All scores were evaluated separately by 2 neuropathologists; the mean score calculated was used to represent the whole immunoreactivity in tumors. Tumors were considered negative for a marker if no stained cells were detected in the 5 sections. Positive control tissues for these antibodies were: CTNNB1, colonic carcinoma; SFRP1, breast carcinoma; NPR3, breast carcinoma; KCNA1, mouse brain; β-catenin, colonic carcinoma; GAB1, tonsil; filaminA, appendix; YAP1, placenta. Substitution of the primary antibody with phosphate-buffered saline was used as a negative control.
Statistical Analysis
The distribution of survival times was determined using Kaplan–Meier estimates. The log-rank test was used to compare survival curves between groups. Overall survival (OS) for all analyses was defined as the time from diagnosis until death. Progression-free survival (PFS) was defined as the time from date of surgical resection until date of tumor progression confirmed by imaging (or by death if no progression had been confirmed previously).
Univariate and multivariate analyses were performed for multiple variables with regard to impact on PFS and OS. Multivariate Cox regression models with the backward selection method were performed to further assess molecular subgroups and histological subtypes for PFS and OS, adjusted with clinical factors. P < .05 in univariate analysis and covariates were deemed to be important prognostic factors. Factors with P-values of .05 and .10 in the multivariate analysis were included and excluded, respectively. All statistical analyses were performed using SPSS software v19.0 (IBM). P < .01 was considered statistically significant.
Results
Transcriptomic Profiling Reveals 3 Subgroups of Adult Medulloblastoma
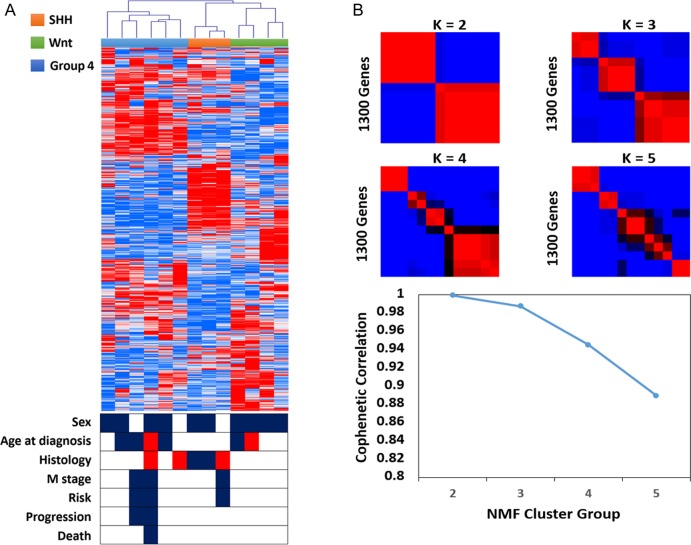
Unsupervised hierarchical cluster analysis delineated 3 distinct sample clusters: WNT, SHH, and non-WNT/non-SHH (Fig. 1A). Support tree analysis of the clustering data indicated high confidence for these 3 subgroups (90%; Fig. 1B). Pathway annotation was performed to reveal characteristic gene expression patterns for each of the clusters (see Supplementary Table S2). WNT signaling was enriched in the WNT group tumors (P = .0004, n = 4); SHH signaling was enriched in the SHH subgroup (P = .0013, n = 3); and other tumors (non-WNT/non-SHH) were characterized by overexpression of genes involved in neuroactive ligand–receptor interaction and the calcium signaling pathway (P = .00017, n = 6).
Fig. 1.
Gene expression profiles for 13 primary adult MBs display 3 subgroups with distinct demographics and histology. (A) Unsupervised hierarchical clustering of 13 adult medulloblastomas based on 1300 transcripts with high standard deviation. Hierarchical clustering is performed using average linkage and dissimilarity based on the Pearson correlation metric. Clinical features of the study population are indicated below the dendrogram: sex (male, black; female, white), age at diagnosis (18–20 y, red; 20–40 y, white; >40 y, black), histology (C, white; DN, black; LC/A, red), M stage (M0, white; M+, black), risk (low, white; high, black), and progression and death (no event, white; event, black). (B) Application of consensus non-negative matrix factorization (NMF), an unsupervised bioinformatics tool for determining the number of independent classes within an expression dataset, strongly supports the existence of 3 adult medulloblastoma subgroups.
Known marker genes of the WNT pathway (ie, WIF1, DKK1, and DKK2) and the SHH pathway (ie, HHIP, SFRP1, and MYCN) showed differential expression in their respective subgroups, and the non-WNT/non-SHH group showed high levels of expression of known oncogenes (ie, OTX2, FOXG1B, and KCNA1) and lack of MYC family expression (see Supplementary Fig. S2).6,7 An 80-gene classifier of MB subgroup signature genes was also applied to recapitulate subgroups (see Supplementary Fig. S3). The non-WNT/non-SHH tumors in this study were identified as belonging to subgroup 4.
The distinction of 3 stable clusters was further supported by semi-nonnegative matrix factorization using the same 1300-gene list. Direct comparison of clustering results obtained by hierarchical clustering and nonnegative matrix factorization confirmed strong concordance between the independent analyses (Rand index: 0.987). Subgroup-specific signature genes were identified using a multivariate permutation test restricted on the proportion of false discoveries and one-way analysis of variance.
Clinicopathological comparison reveals different characteristics of the adult MB subgroups in this cohort (Fig. 1). The patient age distribution for SHH tumors is concentrated in the range of 20–40 years, whereas group 4 and WNT tumors have a more widely distributed age of onset (18–55 y). Metastasis was found in 3 cases of SHH/group 4 tumors. DNMBs were mainly found to be of the SHH subgroup, and LCMB/AMBs were only found in the SHH and group 4 subgroups. Classic histology was found in all molecular variants.
Validation of Molecular Subgroups in Adult Medulloblastoma by IHC
Molecular subtype-specific markers were stained in 201 formalin-fixed paraffin-embedded samples of adult MB (see Supplementary Figs. S4 and S5). In this cohort, most adult MBs were classified as SHH tumors (124/201, 62%), followed by group 4 tumors (28%) and WNT tumors (10%). All 201 samples were immune-negative for NPR3. There were significant clinical differences among the 3 molecular subgroups (Table 1). Age distributions of the 3 subgroups are shown in Supplementary Fig. S6. Male patients predominantly had SHH and group 4 tumors, whereas female patients predominantly had tumors in the WNT group (P= .007). SHH tumors showed an overrepresentation of hemispheric location (P < .001), and WNT tumors had a lower incidence of fourth ventricular floor involvement (P< .001). Tumors with classic histology accounted for the largest proportion of all molecular variants, especially in the WNT subtype, in which 95% of the tumors had classic histology. Most DNMB tumors belonged to the SHH subtype (P < .001). Anaplastic MBs and LCMBs were found to be only SHH or group 4 tumors.
Table 1.
Clinical characteristics of the 3 molecular subtypes of adult medulloblastoma
| Characteristic | SHH (n = 124) | WNT (n = 20) | Group 4 (n = 57) | P (χ2) |
|---|---|---|---|---|
| No. of Patients (%) | No. of Patients (%) | No. of Patients (%) | ||
| Sex | ||||
| Male | 88 (70.97) | 7 (35.00) | 38 (66.67) | .007 |
| Female | 36 (29.03) | 13 (65.00) | 19 (33.33) | |
| Tumor location | ||||
| Midline | 55 (44.35) | 14 (70.00) | 43 (75.44) | 1.9–e4 |
| Hemisphere | 69 (55.65) | 6 (30.00) | 14 (24.56) | |
| Tumor size | ||||
| ≤4 cm | 59 (47.58) | 13 (65.00) | 29 (50.88) | .349 |
| >4 cm | 65 (52.42) | 7 (35.00) | 28 (49.12) | |
| V4 floor involvement | ||||
| Yes | 53 (42.74) | 9 (45.00) | 40 (70.18) | .002 |
| No | 71 (57.26) | 11 (55.00) | 17 (29.82) | |
| Metastasis classification | ||||
| M0 | 104 (83.87) | 18 (90.00) | 44 (77.19) | .282 |
| M+ | 20 (16.13) | 2 (10.00) | 13 (22.81) | |
| Surgical resection | ||||
| GTR | 77 (62.10) | 11 (55.00) | 26 (45.61) | .114 |
| STR | 47 (37.90) | 9 (45.00) | 31 (54.39) | |
| Histology | ||||
| CMB | 58 (46.77) | 19 (95.00) | 37 (64.91) | 1.5–e6 |
| DNMB | 43 (34.68) | 1 (5.00) | 3 (5.26) | |
| LC/A MB | 23 (18.55) | 0 (0) | 17 (29.83) | |
| Risk | ||||
| Average | 70 (56.45) | 17 (85.00) | 26 (45.61) | .020 |
| High | 54 (43.55) | 3 (15.00) | 31 (54.39) | |
Abbreviations: GTR, gross total resection; STR, subtotal resection; V4, fourth ventricle.
Clinicopathological and Molecular Prognosticators in Adult Medulloblastoma by IHC
In the IHC-based cohort of 201 adult patients, the estimated 5-year PFS and OS rates were 60% (95% CI, 52%–67%) and 73% (95% CI, 66%–80%), respectively, and the estimated 10-year PFS and OS rates were 52% (95% CI, 45%–62%) and 65% (95% CI, 57%–73%), respectively.
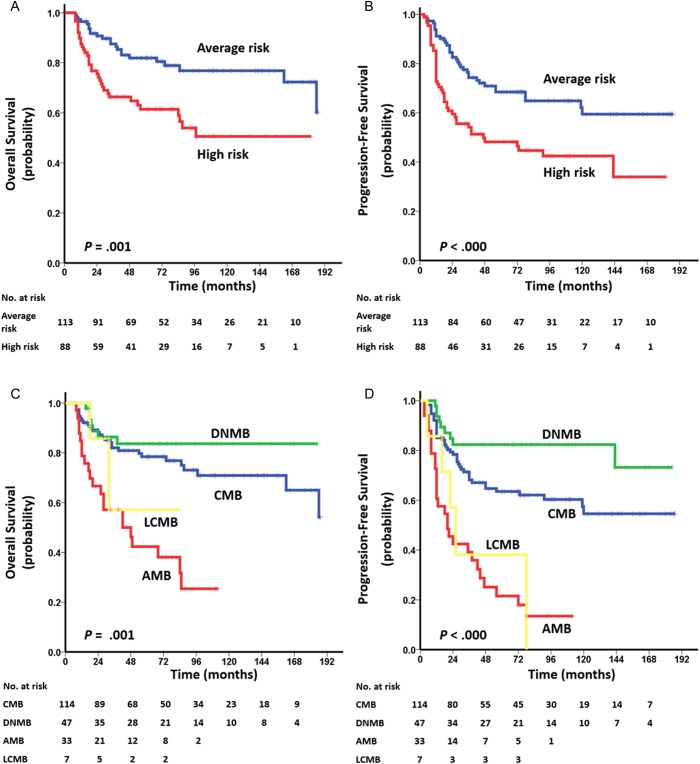
Among the clinical variables, metastatic dissemination (M+ stage) at diagnosis and whole-brain irradiation alone were associated with significant unfavorable prognosis (PFS and OS, respectively: P< .001; Table 2). Poorer PFS and OS were also noted for adults with tumors located in the midline than for those with tumors located in the hemispheres (OS: P= .039, PFS: P = .045). There was no statistically significant survival difference between the groups with and without chemotherapy (OS: P= .548; PFS: P= .787; Supplementary Fig. S7). Stratification of risk classification showed that average-risk patients had better PFS and OS than high-risk patients (PFS/OS: P < .001; Fig. 2A and B).
Table 2.
Univariate analysis for clinical, histopathological, and molecular variables with regard to impact on survival for adult MBs
| Variables | OS |
PFS |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Sex, male vs female | 1.450 | 0.805–2.611 | .216 | 1.145 | 0.720–1.822 | .567 |
| Tumor location, midline vs hemisphere | 0.562 | 0.325–0.973 | .039 | 0.633 | 0.404–0.990 | .045 |
| Tumor size (cm), >4 vs ≤4 | 1.067 | 0.636–1.790 | .806 | 1.088 | 0.707–1.673 | .703 |
| V4 floor involvement, yes vs no | 1.630 | 0.962–2.762 | .070 | 1.386 | 0.898–2.140 | .141 |
| Metastasis, M+ vs M0 | 3.669 | 2.123–6.342 | 3.2e-6 | 3.311 | 2.061–5.318 | 7.3e-7 |
| VP shunt, yes vs no | 0.633 | 0.299–1.337 | .230 | 0.677 | 0.367–1.248 | .211 |
| Resection, GTR vs STR | 0.647 | 0.385–1.085 | .099 | 0.670 | 0.435–1.031 | .068 |
| Radiotherapy, WBI vs CSI | 4.284 | 2.207–8.316 | 1.7e-6 | 3.712 | 2.005–6.874 | 3.0e-5 |
| Chemotherapy, yes vs no | 0.845 | 0.488–1.465 | .548 | 1.069 | 0.660–1.731 | .787 |
| Histology | ||||||
| DNMB vs classic | 0.607 | 0.264–1.394 | .239 | 0.480 | 0.234–0.986 | .046 |
| Anaplastic vs classic | 1.910 | 1.428–2.553 | 1.3e-5 | 1.828 | 1.429–2.339 | 1.5e-6 |
| Large cell vs classic | 1.190 | 0.734–1.930 | .480 | 1.406 | 1.029–1.920 | .032 |
| Molecular subgroup | ||||||
| WNT vs SHH | 0.377 | 0.089–1.594 | .185 | 0.376 | 0.116–1.217 | .103 |
| Group 4 vs SHH | 1.887 | 1.443–2.467 | 3.5e-6 | 1.845 | 1.476–2.307 | 7.8e-8 |
| WNT vs group 4 | 0.056 | 0.008–0.416 | .005 | 0.091 | 0.027–0.308 | 1.2e-4 |
Abbreviations: CSI, craniospinal irradiation; GTR, gross total resection; STR, subtotal resection; V4, fourth ventricle; VP, ventriculoperitoneal; WBI, whole-brain irradiation.
Fig. 2.
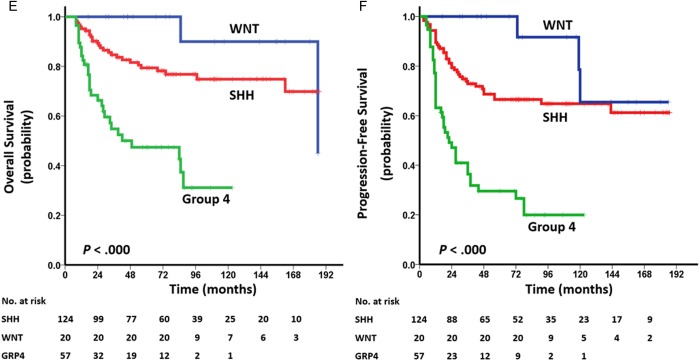
Kaplan–Meier plots of estimated OS (left side) and PFS (right side) time distributions for the 201-tumor cohort of adult MB according to: (A, B) risk staging: average risk vs high risk, (C, D) histology: classic vs desmoplastic/nodular vs anaplastic vs large cell, and (E, F) molecular subgroup: WNT vs SHH vs group 4. Survival differences are calculated using continuous log-rank tests.
Stratification of histological subtypes and molecular subgroups in survival rates was also observed. Compared with the CMB and DNMB subtypes, AMBs had poorer OS and PFS (OS/PFS: P < .001; Fig. 2C and D) and LCMBs had poorer PFS (P = .024 and P= .001, respectively; Fig. 2C and D). Among the 3 molecular subgroups, MBs classified as group 4 had the most unfavorable prognosis (OS/PFS: P < .001; Fig. 2E and F). In SHH-subgroup MB, adult patients with LCMB/AMB had poorer prognosis than those with DNMB or CMB (OS: P <.001; PFS: P= .002; Supplementary Fig. S8A and B). In both SHH and group 4 tumors, the survival rates of adult patients with M+ stage were lower than those of adult patients with M0 stage (OS/PFS: P= .001; OS: P < .001, PFS: P= .002, respectively; Supplementary Fig. S8C–F).
The multivariate Cox proportional hazards model was applied to selected independent variables useful for OS and PFS prediction. Radiation, metastatic disease, histological subtype (ie, CMB, DNMB, LCMB, or AMB) and molecular subgroup (ie, SHH, WNT, or group 4) were identified as independent significant predictors for PFS and OS (Table 3).
Table 3.
Multivariate Cox proportional hazards model for selected clinical, histopathological, and molecular variables with regard to impact on survival for adult medulloblastomas
| Variables | OS |
PFS |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Metastasis, M+ vs M0 | 0.370 | 0.208–0.657 | 0.001 | 0.333 | 0.202–0.551 | 1.8e-5 |
| Radiotherapy, WBI vs CSI | 5.277 | 2.574–10.815 | 5.6e-6 | 5.590 | 2.873–10.877 | 4.0e-7 |
| Histology | ||||||
| DNMB vs classic | 1.024 | 0.415–2.528 | 0.958 | 0.713 | 0.330–1.539 | 0.389 |
| Anaplastic vs classic | 2.645 | 1.422–4.920 | 0.002 | 2.537 | 1.506–4.274 | 4.7e-4 |
| Large cell vs classic | 1.335 | 0.309–5.772 | 0.699 | 1.807 | 0.698–4.678 | 0.223 |
| Molecular subgroup | ||||||
| WNT vs SHH | 0.367 | 0.085–1.592 | 0.181 | 0.303 | 0.091–1.008 | 0.051 |
| Group 4 vs SHH | 2.881 | 1.617–5.134 | 3.3e-4 | 2.720 | 1.686–4.389 | 4.1e-5 |
Abbreviations: CSI, craniospinal irradiation; WBI, whole-brain irradiation.
Discussion
Recent expression profiling studies have indicated the existence of 4 major molecular subgroups associated with activation of specific signaling pathways in MB.6,7,18,19 The identification of such molecular subgroups provides important opportunities to improve our understanding of the disease and its clinical treatment. Several studies have shown that adult and pediatric MBs are genetically distinct and may require different algorithms for molecular risk stratification.6,11,14,15,20 However, due to the low incidence of adult MB, the lack of a common treatment strategy, and the frequent occurrence of late relapses after more than 5 years, systematic studies of the biologic and clinical characteristics in adult MB have been hindered. Remke et al21 identified 3 molecular subgroups in adult MB, and tumors classified as subgroup D had worse prognosis. Additionally, this study also showed that most clinicopathological factors were of no statistical significance when taking molecular variables into account.
In our study, we selected 13 adult MBs with 4 histological subtypes and identified 3 subgroups of adult MB, characterized by distinct expression profiles, clinical features, pathological features, and prognosis. To determine whether the molecular subgroups could support clinical variables for prognostication in adult MB, these distinct molecular subsets were also confirmed by IHC in a large independent cohort of 201 adult MBs treated at one institution over a 10-year period and with a median follow-up of over 60 months. Multiple clinicopathological variables, especially including treatment strategy, were analyzed for prognostication by combining molecular subgroups. We have demonstrated that subgroup status incorporated with clinicopathological parameters (metastasis and histology) and treatment strategy is more informative for predicting patient outcome.
In this cohort, our data showed a preponderance of SHH-activated tumors (62%), followed by group 4 tumors (28%) and WNT-activated tumors (10%). This is consistent with previous studies which reported 50%–80% SHH tumors of all cases,7,18,21 whereas group 4 MB is always the most common subgroup at all ages.6,9 In several studies, group 3 tumors accounted for <2% of adult MBs.9,19,21 We found that the adult tumors were devoid of group 3 cases, suggesting that this subgroup may be restricted to pediatric MBs. The combination of molecular and clinicopathological data has provided valuable insights into the clinical features of the molecular subgroups. Most DNMBs were found in the SHH group, to the extent that DN histology may be considered a surrogate marker for SHH activation in adult MBs. The preponderance of the SHH subgroup in adult MB may explain its clinical differences from pediatric MB, such as its higher incidence of hemispheric localization and DN histology. The presence of severe anaplasia or the LC subtype of MB is considered to be predictive of survival in pediatric MB.22 In this study, the LC/A variant of MB accounted for only a minority of SHH subgroup cases, and these tumors were associated with a worse outcome than other tumors (P < .0001). The WNT subgroup in adult MB predominantly comprised the classic variant (accounting for 95% of cases), and LC/A histology was rare. The sex ratio of the WNT subgroup was obviously skewed, with a male-to-female ratio of 1:2. In WNT tumors, there was rarely metastasis present at diagnosis; however, the OS and PFS of this group were similar to those of the SHH group. Because of the excellent prognosis of childhood WNT MB with survival rates in excess of 90%,6,7,18 it seems that adult WNT MB is quite different from pediatric WNT MB.6,7,18 Notably, group 4 MB in adults is associated with a high rate of high-risk disease and LC/A histology, which may be the reason that this subgroup has the worst outcome among all 3 subgroups.
In our study, we also found that M-stage significantly correlated with OS and PFS in both univariate and multivariate analyses and was an important prognostic predictor in adult patients. This is consistent with previous studies that reported localized disease (M0) at diagnosis as an independent prognostic factor,11,14 and M+ patients as a high-risk group should receive more intensive treatment. Other conventional clinical parameters for risk stratification (such as tumor size, location, extent of tumor resection, and fourth ventricle floor involvement) were not prognostic predictors. Previous studies were controversial concerning whether the extent of tumor resection is of prognostic value.5,11–13 In our opinion, complete resection would result in severely reduced postoperative performance status, especially for patients with tumor invaded in the fourth ventricle or brainstem.
It has been demonstrated that the histopathological subtypes of adult MB have substantially different prognoses. We found that the presence of moderate or severe anaplasia in adult MB was associated with markedly poorer prognosis. This is consistent with previous studies of pediatric MB.22,23 As an independent subgroup, LCMB is a rare variant and has a poor prognosis in pediatric studies.25,26 In our study, LCMBs were present in 3.5% of adult MBs and had worse PFS than CMBs and DNMBs. In early childhood cases, DNMBs have been linked to better survival than CMBs,22–24 but there is no apparent difference in adult MBs.
Adjuvant chemotherapy did not correlate with prognosis in either the overall patient population or the patients designated as having average or high risk in this study, indicating that the current chemotherapy regimen needs to be optimized for adult MB patients. The efficacy of postoperative chemotherapy, with or without postoperative radiotherapy, has been well established in the pediatric population.3–5,22–24,27 However, its use is still controversial in adult MB, and major questions remain as to which subgroups could benefit from adjuvant chemotherapy. Several studies show that postoperative chemotherapy reduced the risk of recurrence and death among high-risk adult patients.12,14,28,29 In our study, chemotherapy also showed no significant difference on PFS and OS in high-risk patients or average-risk patients. It may be that high-dose craniospinal irradiation reduced the prognostic significance of widespread disease. Therefore, a larger randomized controlled clinical trial is needed to study the outcome of postoperative chemotherapy.
The demographic and clinical differences among the subgroups emphasize the need for subgroup-specific trials of novel therapies. In adult MB, a distinct risk stratification for molecular subgroups should be further developed and validated. Given the more favorable outcome, WNT and SHH tumors may be controlled by less intensive treatment, whereas the terrible prognosis for group 4 patients indicates that more intensive treatment is needed for this subgroup, especially when metastasis is present at diagnosis. The urgent need for appropriate therapies for SHH tumors has already led to investigation of specifically targeted therapies.9 In conclusion, our study shows that histopathological and molecular subtypes are useful prognostic factors for adult MB. Patients with AMB variants or group 4 tumors have a significantly worse outcome and require more intensive treatment, although it should still be acknowledged that the issues with the use of IHC include differences in fixation and embedding procedures. We showed that IHC markers are helpful in identification and validation of molecular subgroups, thereby providing the level of confidence necessary for subgroup assignment in the setting of a clinical trial. Prognostication algorithms incorporating factors such as clinical variables, histopathology, and molecular subtype should be developed to guide individualized treatment for adult MB patients.
Supplementary Material
Funding
This work was supported by the National Science and Technology Support Program of the 12th Five-Year Plan of China (grant number: 2012BAI12B03), the National Science Foundation of China (grant number: 7112049), and the Capital Health Development and Research Special Projects of Beijing (grant number: 2011-1015-01). Additional support came from an American Cancer Society Research Scholar award, an NIH/National Cancer Institute Outstanding Investigator grant (R35CA197743), a Department of Defense New Investigator award, a Children's Tumor Foundation Clinical Research award, and the Drug Discovery Initiative (L.X.).
Conflict of interest statement. The authors indicate no potential conflicts of interest.
Supplementary Material
References
- 1.Giordana MT, Schiffer P, Lanotte M et al. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80(5):689–692. [DOI] [PubMed] [Google Scholar]
- 2.Polkinghorn WR, Tarbell NJ. Medulloblastoma: tumorigenesis, current clinical paradigm, and efforts to improve risk stratification. Nat Clin Pract Oncol. 2007;4(5):295–304. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RE, Bailey CC, Robinson K et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21(8):1581–1591. [DOI] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 6.Kool M, Korshunov A, Remke M et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northcott PA, Korshunov A, Witt H et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison DW, Dalton J, Kocak M et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121(3):381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudin CM, Hann CL, Laterra J et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remke M, Hielscher T, Korshunov A et al. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J Clin Oncol. 2011;29(29):3852–3861. [DOI] [PubMed] [Google Scholar]
- 11.Padovani L, Sunyach MP, Perol D et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68(2):433–440. [DOI] [PubMed] [Google Scholar]
- 12.Kunschner LJ, Kuttesch J, Hess K et al. Survival and recurrence factors in adult medulloblastoma: the M.D. Anderson Cancer Center experience from 1978 to 1998. Neuro Oncol. 2001;3(3):167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai R. Survival of patients with adult medulloblastoma: a population-based study. Cancer. 2008;112(7):1568–1574. [DOI] [PubMed] [Google Scholar]
- 14.Korshunov A, Remke M, Werft W et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010;28(18):3054–3060. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez FJ, Eberhart C, O'Neill BP et al. Histopathologic grading of adult medulloblastomas. Cancer. 2007;109(12):2557–2565. [DOI] [PubMed] [Google Scholar]
- 16.Giangaspero F, Eberhart CG, Haapasalo H et al. Medulloblastoma. In: Louis DN, Ohgaki H, Wiestler OD, et al., eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007:132–140. [Google Scholar]
- 17.Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic [sic] for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 18.Shih DJ, Northcott PA, Remke M et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kool M, Koster J, Bunt J et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang C, Hauerstock D, Guiot MC et al. Characteristics and outcomes of medulloblastoma in adults. Pediatr Blood Cancer. 2008;51(5):603–607. [DOI] [PubMed] [Google Scholar]
- 21.Remke M, Hielscher T, Northcott PA et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–2723. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski S, von Hoff K, Emser A et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. [DOI] [PubMed] [Google Scholar]
- 23.Eberhart CG, Kepner JL, Goldthwaite PT et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94(2):552–560. [DOI] [PubMed] [Google Scholar]
- 24.Rutkowski S, Bode U, Deinlein F et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 25.Giangaspero F, Rigobello L, Badiali M et al. Large-cell medulloblastomas. A distinct variant with highly aggressive behavior. Am J Surg Pathol. 1992;16:687–693. [PubMed] [Google Scholar]
- 26.Brown HG, Kepner JL, Perlman EJ et al. “Large cell/anaplastic” medulloblastomas: a Pediatric Oncology Group study. J Neuropathol Exp Neurol. 2000;59:857–865. [DOI] [PubMed] [Google Scholar]
- 27.Dunkel IJ, Gardner SL, Garvin JH Jr et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrie C, Lasset C, Alapetite C et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74(8):2352–2360. [DOI] [PubMed] [Google Scholar]
- 29.Brandes AA, Franceschi E, Tosoni A et al. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110(9):2035–2041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.