Abstract
This randomized, partially-blind study (ClinicalTrials.gov registration number NCT00541970) evaluated the immunogenicity and safety of 2-dose (2D) schedules of the HPV-16/18 AS04-adjuvanted vaccine. Results to month (M) 24 have been reported previously and we now report data to M48 focusing on the licensed vaccine formulation (20 μg each of HPV-16 and -18 antigens) administered at M0,6 compared with the standard 3-dose (3D) schedule (M0,1,6). Healthy females (age stratified: 9–14, 15–19, 20–25 years) were randomized to receive 2D at M0,6 (n = 240) or 3D at M0,1,6 (n = 239). In the according-to-protocol immunogenicity cohort, all initially seronegative subjects seroconverted for HPV-16 and -18 antibodies and remained seropositive up to M48. For both HPV-16 and -18, geometric mean antibody titer (GMT) ratios (3D schedule in women aged 15–25 years divided by 2D schedule in girls aged 9–14 years) at M36 and M48 were close to 1, as they were at M7 when non-inferiority was demonstrated. The kinetics of HPV-16, -18, -31, and -45 antibody responses were similar for both groups and HPV-16 and -18 GMTs were substantially higher than natural infection titers. The vaccine had a clinically acceptable safety profile in both groups. In summary, antibody responses to a 2D M0,6 schedule of the licensed vaccine formulation in girls aged 9–14 years appeared comparable to the standard 3D schedule in women aged 15–25 years up to 4 years after first vaccination. A 2D schedule could facilitate implementation of HPV vaccination programs and improve vaccine coverage and series completion rates.
Keywords: human papillomavirus vaccine, randomized controlled trial, administration schedule, female adolescents, women, immunogenicity, safety
Introduction
The prophylactic human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Vaccines) has been shown to be immunogenic, efficacious, and to have a clinically acceptable safety profile in clinical studies.1-6 The vaccine is formulated with the AS04 Adjuvant System, containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL; 50 µg) adsorbed on aluminum salt (500 µg Al3+), which has been shown to enhance the vaccine’s immunogenicity.7 The licensed vaccine schedule is 3 doses given at months (M) 0,1,6, with each dose formulated to contain 20 µg of HPV-16 L1 protein virus-like particles (VLPs) and 20 µg of HPV-18 L1 VLPs.
High coverage and compliance rates can be difficult to achieve with 3 dose (3D) regimens of HPV vaccines, particularly in low resource settings where the need for cervical cancer prevention is greatest.8 Proof-of-principle that a two-dose (2D) regimen may be sufficient to protect against cervical cancer was demonstrated in a large trial of the HPV-16/18 AS04-adjuvanted vaccine in women aged 18–25 y conducted in Costa Rica.9 In a post-hoc analysis of that study, 2 doses of the vaccine were still highly efficacious in protecting against incident HPV-16 or -18 infections that persisted for one year.9
An immunobridging study demonstrated that 3 doses of the HPV-16/18 AS04-adjuvanted vaccine induced geometric mean antibody titers (GMTs) in 10–14 y-old adolescent girls that were approximately 2-fold higher than those elicited in women aged 15–25 y.10 The study reported here (ClinicalTrials.gov registration number NCT00541970) was designed to evaluate the immunogenicity and safety of 2D schedules of the licensed formulation of the HPV-16/18 AS04-adjuvanted vaccine (20 μg each of HPV-16 and -18 L1 VLPs; 20/20) or an alternative formulation (40 μg of each of HPV-16 and -18 L1 VLPs; 40/40) in different age groups, compared with the standard 3D schedule of the licensed formulation. Study data up to month 24 (including primary and secondary endpoints) have been published previously.11 We now report results up to month 48, including antibody titers against vaccine types (HPV-16 and -18), and longer-term safety. We also report exploratory results for cross-reactive antibody titers against non-vaccine types (HPV-31 and -45).
Based on results of the current trial up to month 24, it was suggested that further development of the 40/40 formulation was not justified; the 2D 20/20 M0,6 formulation elicited an immune response in girls that was non-inferior to the 3D 20/20 schedule in young women, and the 2D 40/40 formulation gave no added benefit.11 Therefore, in this analysis up to month 48 we focus on data for the 2D 20/20 formulation in girls aged 9–14 y and the standard 3D 20/20 schedule in women aged 15–25 y.
Results
Study population
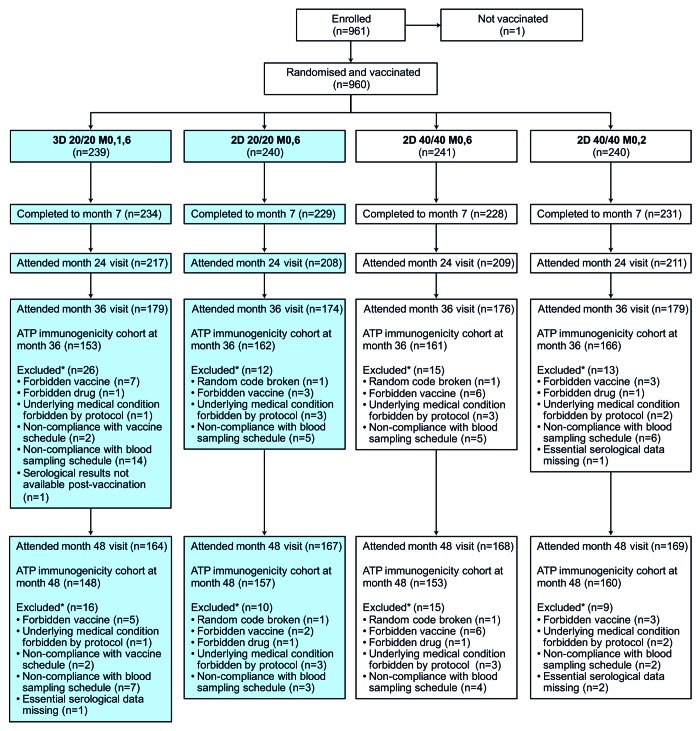
Of the 960 girls and young women in the total vaccinated cohort, 239 received the standard 3D M0,1,6 schedule and 240 received the 2D 20/20 M0,6 schedule; the remaining subjects received 2D 40/40 schedules (M0,2 or M0,6) (Fig. 1). More than 72% of vaccinated participants in each group attended the clinic at month 36 and more than 68% of participants in each group attended at month 48. Reasons for exclusion from the according-to-protocol (ATP) immunogenicity cohort at months 36 and 48 are shown in Figure 1.
Figure 1. Flow of participants through the trial. 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 40/40, 40 μg each of HPV-16 and -18 L1 virus-like particles; ATP, according-to-protocol; M, month. This article focuses on subjects randomized to the 3D 20/20 M0,1,6 group and the 2D 20/20 M0,6 group (shaded boxes). Disposition data are also shown for subjects enrolled in other study groups (2D 40/40 M0,6 and 2D 40/40 M0,2) for completeness.
The standard 3D and 2D 20/20 groups were comparable in terms of baseline demographics, as reported previously.11 Here, we focus on results from the licensed formulation given as a 2D schedule in girls aged 9–14 y and as a 3D schedule in women aged 15–25 y. In these sub-groups, the mean (standard deviation) age of vaccinated participants was 19.7 (3.07) years in the 3D group and 12.6 (1.56) years in the 2D 20/20 group and more than 96% of participants in each group were of Caucasian/European ethnicity. In the total vaccinated cohort at month 48 with pre-vaccination results available, 85.7% (90/105) of women aged 15–25 y in the standard 3D group and 96.7% (58/60) of girls aged 9–14 y in the 2D 20/20 group were seronegative for HPV-16 at baseline, and 85.6% (89/104) in the 3D group and 93.3% (56/60) in the 2D 20/20 group were seronegative for HPV-18 at baseline.
Immunogenicity
In the ATP month 48 immunogenicity cohort, all initially seronegative subjects aged 9–25 y seroconverted for HPV-16 and -18 antibodies and remained seropositive to month 48, as measured by enzyme-linked immunosorbent assay (ELISA) (Table 1).
Table 1. HPV-16 and HPV-18 antibody responses up to month 48 in subjects aged 9–25 y (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline).
| Antigen | Age (y) | Timing (months) | 3D 20/20 M0,1,6 |
2D 20/20 M0,6 |
||||
|---|---|---|---|---|---|---|---|---|
| N | Seropositive, n (%) | GMT* (95% CI), EU/mL | N | Seropositive, n (%) | GMT* (95% CI), EU/mL | |||
| HPV-16 | 9–25 | 0 | 129 | 0 (0.0) | 4.0 (4.0, 4.0) | 139 | 0 (0.0) | 4.0 (4.0, 4.0) |
| 7 | 129 | 129 (100) | 15177.0 (12640.2, 18223.0) | 139 | 139 (100) | 8675.3 (7505.6, 10027.3) | ||
| 12 | 128 | 128 (100) | 5131.1 (4207.5, 6257.4) | 139 | 139 (100) | 2600.1 (2256.5, 2996.0) | ||
| 18 | 128 | 128 (100) | 3380.3 (2791.2, 4093.7) | 138 | 138 (100) | 1757.3 (1518.0, 2034.2) | ||
| 24 | 127 | 127 (100) | 2617.3 (2156.2, 3176.8) | 136 | 136 (100) | 1354.7 (1175.3, 1561.4) | ||
| 36 | 129 | 129 (100) | 2228.8 (1847.5, 2688.7) | 136 | 136 (100) | 1116.6 (968.4, 1287.5) | ||
| 48 | 129 | 129 (100) | 1825.7 (1518.3, 2195.5) | 139 | 139 (100) | 968.5 (840.6, 1115.9) | ||
| HPV-18 | 9–25 | 0 | 129 | 0 (0.0) | 3.5 (3.5, 3.5) | 135 | 0 (0.0) | 3.5 (3.5, 3.5) |
| 7 | 129 | 129 (100) | 5640.7 (4801.1, 6627.2) | 135 | 135 (100) | 5295.2 (4633.1, 6051.9) | ||
| 12 | 128 | 128 (100) | 1885.0 (1559.3, 2278.8) | 135 | 135 (100) | 1493.7 (1271.6, 1754.7) | ||
| 18 | 128 | 128 (100) | 1223.5 (1015.4, 1474.2) | 135 | 135 (100) | 911.2 (773.4, 1073.5) | ||
| 24 | 127 | 127 (100) | 984.8 (814.8, 1190.3) | 132 | 132 (100) | 717.5 (609.3, 845.0) | ||
| 36 | 129 | 129 (100) | 879.2 (727.9, 1062.0) | 132 | 132 (100) | 628.9 (530.1, 746.3) | ||
| 48 | 129 | 129 (100) | 721.7 (599.4, 868.8) | 135 | 135 (100) | 529.6 (448.6, 625.1) | ||
2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month; N, number of evaluable seronegative subjects in the according-to-protocol month 48 immunogenicity cohort; n (%), number (percentage) of seropositive subjects with antibody titer greater than or equal to the assay cut-off (≥8 EU/mL for HPV-16 and ≥7 EU/mL for HPV-18). *Antibody titers below the assay cut-off were given an arbitrary value of half the cut-off for the purpose of GMT calculation.
The GMT ratios for the 3D schedule in women aged 15–25 y divided by the 2D schedule in girls aged 9–14 y at months 36 and 48 were, respectively, 1.00 (95% confidence interval [CI]: 0.73, 1.37) and 1.08 (0.78, 1.48) for HPV-16 antibodies and 1.03 (0.72, 1.49) and 1.11 (0.78, 1.58) for HPV-18 antibodies (Table 2).
Table 2. HPV-16 and HPV-18 GMT ratios for 3D schedule in women aged 15–25 y over 2D schedule in girls aged 9–14 y at months 36 and 48 (according-to-protocol month 36 and 48 immunogenicity cohorts, subjects seronegative at baseline).
| Antigen | Group | Age (y) | N | GMT (95% CI), EU/mL | GMT ratio (3D/2D) (95% CI) |
|---|---|---|---|---|---|
| Month 36 | |||||
| HPV-16 | 3D 20/20 M0,1,6 | 15–25 | 85 | 1592.0 (1282.6, 1975.9) | 1.00 (0.73, 1.37) |
| 2D 20/20 M0,6 | 9–14 | 53 | 1595.1 (1298.2, 1960.0) | - | |
| HPV-18 | 3D 20/20 M0,1,6 | 15–25 | 81 | 712.3 (560.3, 905.6) | 1.03 (0.72, 1.49) |
| 2D 20/20 M0,6 | 9–14 | 52 | 689.3 (530.4, 895.9) | - | |
| Month 48 | |||||
| HPV-16 | 3D 20/20 M0,1,6 | 15–25 | 80 | 1419.6 (1133.9, 1777.2) | 1.08 (0.78, 1.48) |
| 2D 20/20 M0,6 | 9–14 | 53 | 1319.8 (1084.1, 1606.7) | - | |
| HPV-18 | 3D 20/20 M0,1,6 | 15–25 | 79 | 604.5 (475.9, 768.0) | 1.11 (0.78, 1.58) |
| 2D 20/20 M0,6 | 9–14 | 52 | 542.9 (426.5, 691.0) | - | |
2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month; N, number of evaluable seronegative subjects in the according-to-protocol immunogenicity cohort.
The kinetics of the antibody response against both HPV-16 and HPV-18 in the 2D 20/20 group in girls aged 9–14 y followed a similar pattern to that observed in women aged 15–25 y in the 3D group, i.e., after a peak response at month 7, GMTs for both antibodies gradually declined until approximately month 24 when they reached a plateau phase between month 24 and month 48 (Fig. 2). At month 48, for initially seronegative girls aged 9–14 y in the 2D 20/20 group, HPV-16 and HPV-18 GMTs were 44-fold higher and 24-fold higher, respectively, than titers after natural infection observed in women aged 15–25 y participating in a previous clinical trial12 (1319.8 vs 29.8 ELISA units [EU]/mL for HPV-16 antibodies; 542.9 vs 22.6 EU/mL for HPV-18 antibodies) (Fig. 2). HPV-16 and HPV-18 GMTs at month 48 also remained above the plateau titers of 397.8 EU/mL and 297.3 EU/mL, respectively, observed at month 45–50 for women aged 15–25 y participating in a previous trial, in whom sustained protection has been shown (Fig. 2).4,13
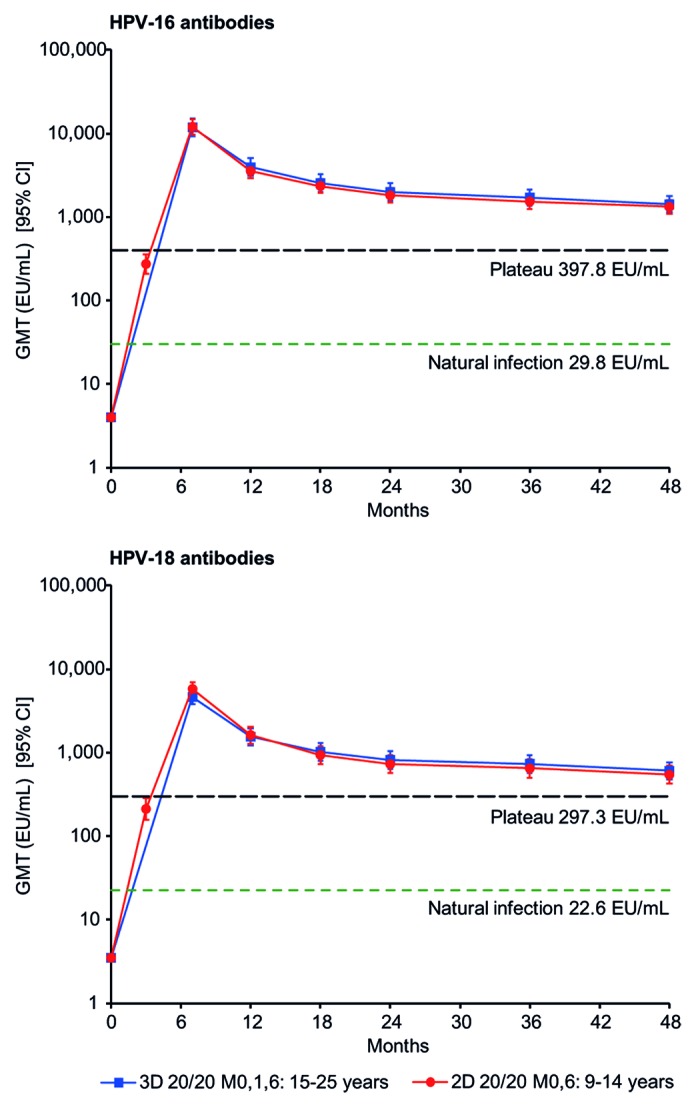
Figure 2. Kinetics of HPV-16 and HPV-18 antibody responses for girls aged 9–14 y in the 2D 20/20 M0,6 group and women aged 15–25 y in the 3D 20/20 M0,1,6 group (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline). 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month. Natural infection, GMT in subjects who had cleared a natural infection.12 Plateau, GMT at the plateau level (month 45–50) in women aged 15–25 y (total vaccinated cohort) in a study in which sustained protection with the HPV-16/18 AS04-adjuvanted vaccine has been shown (i.e., 397.8 EU/mL for HPV-16 and 297.3 EU/mL for HPV-18).13
Antibody responses to non-vaccine types HPV-31 and HPV-45 appeared similar in girls aged 9–14 y in the 2D 20/20 group and in women aged 15–25 y in the standard 3D group in terms of seroconversion rates and GMTs up to month 48, as measured by ELISA (Table 3). At month 48, GMTs for HPV-31 antibodies for girls aged 9–14 y in the 2D 20/20 group and women aged 15–25 y in the 3D group were 195.5 and 241.7 EU/mL, respectively, and GMTs for HPV-45 antibodies were 156.6 and 147.2 EU/mL, respectively. The kinetics of the antibody response against HPV-31 and HPV-45 in the 2D 20/20 group in girls aged 9–14 y followed a similar pattern to that observed in women aged 15–25 y in the standard 3D group, i.e., after a peak response at month 7, GMTs for both antibodies gradually declined until approximately month 24 and reached a plateau between month 24 and month 48 (Fig. 3).
Table 3. HPV-31 and HPV-45 antibody responses for the 3D schedule in women aged 15–25 y and the 2D schedule in girls aged 9–14 y up to month 48 (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline).
| Antigen | Timing (months) | 3D 20/20 M0,1,6 (women 15–25 y) |
2D 20/20 M0,6 (girls 9–14 y) |
||||
|---|---|---|---|---|---|---|---|
| N | Seropositive, n (%) | GMT* (95% CI), EU/mL | N | Seropositive, n (%) | GMT* (95% CI), EU/mL | ||
| HPV-31 | 0 | 36 | 0 (0.0) | 29.5 (29.5, 29.5) | 40 | 0 (0.0) | 29.5 (29.5, 29.5) |
| 7 | 36 | 36 (100) | 1201.8 (819.2, 1763.0) | 40 | 40 (100) | 1321.7 (1011.7, 1726.7) | |
| 12 | 36 | 35 (97.2) | 370.0 (246.4, 555.6) | 40 | 39 (97.5) | 262.4 (197.0, 349.6) | |
| 18 | 36 | 35 (97.2) | 270.7 (190.9, 383.8) | 40 | 34 (85.0) | 184.7 (134.8, 253.0) | |
| 24 | 36 | 31 (86.1) | 215.2 (145.5, 318.3) | 40 | 35 (87.5) | 166.7 (122.8, 226.3) | |
| 36 | 36 | 33 (91.7) | 237.5 (166.7, 338.4) | 39 | 35 (89.7) | 173.7 (126.7, 238.1) | |
| 48 | 36 | 32 (88.9) | 241.7 (166.7, 350.3) | 40 | 37 (92.5) | 195.5 (144.8, 263.9) | |
| HPV-45 | 0 | 38 | 0 (0.0) | 29.5 (29.5, 29.5) | 37 | 0 (0.0) | 29.5 (29.5, 29.5) |
| 7 | 38 | 38 (100) | 928.4 (652.0, 1322.0) | 37 | 37 (100) | 1574.5 (1156.8, 2142.8) | |
| 12 | 38 | 37 (97.4) | 306.1 (207.9, 450.7) | 37 | 35 (94.6) | 305.7 (209.3, 446.4) | |
| 18 | 38 | 33 (86.8) | 204.3 (140.0, 298.2) | 37 | 32 (86.5) | 191.6 (133.0, 275.8) | |
| 24 | 38 | 33 (86.8) | 178.4 (124.8, 254.9) | 37 | 32 (86.5) | 176.7 (121.9, 256.3) | |
| 36 | 38 | 32 (84.2) | 169.3 (117.8, 243.4) | 36 | 31 (86.1) | 177.4 (124.4, 252.8) | |
| 48 | 38 | 30 (78.9) | 147.2 (102.0, 212.5) | 37 | 34 (91.9) | 156.6 (114.2, 214.7) | |
2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month; N, number of evaluable seronegative subjects in the according-to-protocol month 48 immunogenicity cohort; n (%), number (percentage) of seropositive subjects with antibody titer greater than or equal to the cut-off value (≥59 EU/mL for HPV-31 and HPV-45). *Antibody titers below the cut-off of the assay were given an arbitrary value of half the cut-off for the purpose of GMT calculation.
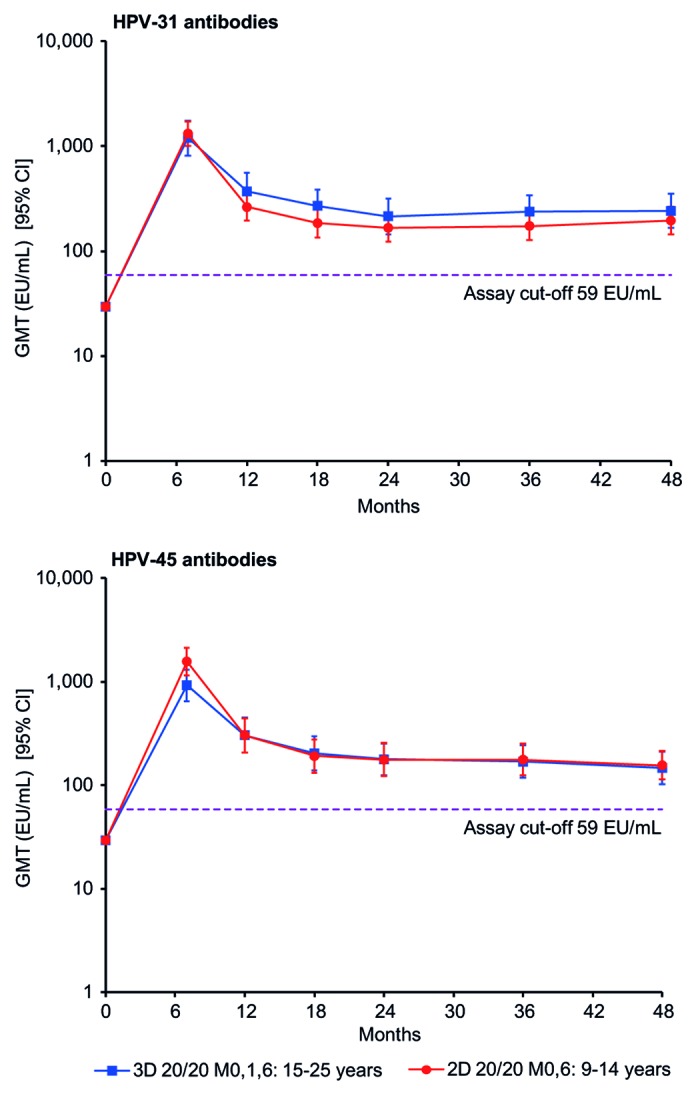
Figure 3. Kinetics of cross-reactive HPV-31 and HPV-45 antibody responses for girls aged 9–14 y in the 2D 20/20 M0,6 group and women aged 15–25 y in the 3D 20/20 M0,1,6 group (according-to-protocol month 48 immunogenicity cohort, subjects seronegative at baseline). 2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; EU/mL, ELISA unit per milliliter; GMT, geometric mean antibody titer; M, month.
Safety
All vaccine formulations and schedules evaluated in this study have been shown previously to have a clinically acceptable reactogenicity and safety profile up to month 24.11 In this longer-term evaluation up to month 48, the safety profile of the licensed 20/20 formulation of the HPV-16/18 AS04-adjuvanted vaccine appeared similar whether administered as a 2D or 3D schedule (Table 4).
Table 4. Summary of safety and pregnancy outcomes from month 0 to month 48 (total vaccinated cohort).
| 3D 20/20 M0,1,6 N = 239 |
2D 20/20 M0,6 N = 240 |
|
|---|---|---|
| Safety outcomes | ||
| Adverse event leading to withdrawal | ||
| Subjects with at least one event, n (%) | 0 (0.0) | 0 (0.0) |
| Serious adverse event | ||
| Subjects with at least one event, n (%) [95% CI] | 13 (5.4) [2.9, 9.1] | 19 (7.9) [4.8, 12.1] |
| Number of events | 19 | 23 |
| Medically significant condition | ||
| Subjects with at least one event, n (%) [95% CI] | 82 (34.3) [28.3, 40.7] | 88 (36.7) [30.6, 43.1] |
| Number of events | 135 | 155 |
| New onset chronic disease | ||
| Subjects with at least one event, n (%) [95% CI] | 6 (2.5) [0.9, 5.4] | 13 (5.4) [2.9, 9.1] |
| Number of events | 7 | 15 |
| New onset autoimmune disease | ||
| Subjects with at least one event, n (%) [95% CI] | 4 (1.7) [0.5, 4.2] | 5 (2.1) [0.7, 4.8] |
| Number of events | 4 | 6 |
| Pregnancy outcomes | ||
|---|---|---|
| Number of subjects with pregnancies | 20 | 24 |
| Ectopic pregnancy, n (%) | 0 | 0 |
| Elective termination no apparent congenital anomaly, n (%) | 5 (25.0) | 3 (12.5) |
| Elective termination congenital anomaly, n (%) | 0 | 1 (4.2) |
| Live infant no apparent congenital anomaly, n (%) | 12 (60.0) | 15 (62.5) |
| Lost to follow up, n (%) | 0 | 0 |
| Pregnancy ongoing at time of reporting, n (%) | 2 (10.0) | 2 (8.3) |
| Spontaneous abortion no apparent congenital anomaly, n (%) | 1 (5.0) | 3 (12.5) |
2D, 2-dose schedule; 3D, 3-dose schedule; 20/20, 20 μg each of HPV-16 and -18 L1 virus-like particles; 95% CI, exact 95% confidence interval; N, number of subjects with at least one administered dose; n (%), number (percentage) of subjects with at least one event within the given category. Medically significant conditions were adverse events prompting an emergency room or physician visit that were not related to common diseases. As described previously,18 all adverse events reported during the trial were compared with a pre-defined list of potential chronic diseases derived from the Medical Dictionary for Regulatory Activities. Determination of whether a chronic disease was of new onset was based on blinded review of the reported symptoms and the subject’s pre-vaccination medical history by a physician from GlaxoSmithKline. A separate list, restricted to potential autoimmune events which excluded allergy-related events or isolated signs and symptoms and events not considered to be autoimmune in origin, was used to identify new onset autoimmune diseases among events identified as new onset chronic diseases.
Discussion
We demonstrated that the licensed formulation of the HPV-16/18 AS04-adjuvanted vaccine (20 µg each of HPV-16 and HPV-18 L1 VLPs) given to girls aged 9–14 y as a 2D schedule at months 0 and 6 elicited HPV-16 and -18 antibody titers that were similar to the licensed 3D schedule in young women aged 15–25 y. GMT ratios for these 2 groups of subjects were close to 1 at months 36 and 48, as they were at month 7 when non-inferiority was statistically demonstrated. Both schedules had clinically acceptable reactogenicity11 and safety profiles up to month 48.
Protection against HPV infection is thought to be mediated by neutralizing antibodies that transude across the cervical epithelium,14 with serum antibody titers measured by ELISA serving as a good surrogate for neutralizing antibody titers.15,16 As it is not ethical, nor feasible, to conduct HPV vaccine efficacy trials in adolescent girls, we measured antibodies against HPV and used the principle of immunobridging to infer efficacy in younger subjects. As the 2D schedule in adolescent girls induced a similar immune response to a 3D schedule in young women through 4 y of observation, a similarly high level of protection against HPV infection and subsequent disease is expected.1,2 This principle of immunobridging was previously used to infer efficacy of the 3D schedule of the HPV-16/18 AS04-adjuvanted vaccine in younger subjects, by showing that immunogenicity in girls aged 10–14 y was similar to that in women aged 15–25 y, with GMTs in adolescents being approximately 2-fold higher than those seen in young women.10
The AS04 Adjuvant System utilized in the HPV-16/18 vaccine may contribute to the longevity of the immune response observed in the current trial.7,17 AS04 contains the toll-like receptor 4 agonist MPL, adsorbed on aluminum salt, which enhances humoral and cell-mediated responses.7 The added value of this Adjuvant System is exemplified in a previous clinical trial in which the HPV-16/18 AS04-adjuvanted vaccine elicited superior HPV-16 and -18 humoral and cell-mediated immune responses up to 24 mo after vaccination, compared with the HPV-6/11/16/18 vaccine (Gardasil®, Merck and Co., Inc.), which contains a proprietary aluminum-containing adjuvant.18,19 Other explanations for differences in immunogenicity between the 2 HPV vaccines could be related to structural distinctions in the constituent L1 VLPs arising from differing manufacturing processes. The HPV-16/18 AS04-adjuvanted vaccine contains C-terminally truncated L1 VLPs which are produced using the Baculovirus expression vector system, with assembly of the VLPs achieved in vitro following purification of the L1 proteins,20 whereas the HPV-6/11/16/18 vaccine contains full-length L1 proteins produced in yeast.21
Proof-of-principle that fewer than 3 doses of the HPV-16/18 AS04-adjuvanted vaccine may be adequate to protect against HPV-16 and -18 infection was shown in a post-hoc analysis from a clinical efficacy trial conducted in Costa Rica in women aged 18–25 y at the time of first vaccination. In this trial, women were scheduled to receive 3 vaccine doses, but in those women who received 2 doses only, the vaccine was still highly efficacious in the prevention of HPV-16 and -18 infections that persisted for at least one year.9 Preliminary long-term immunogenicity data from the Costa Rican study showed that HPV-16 and HPV-18 antibody titers following 2 vaccine doses remained persistently higher than titers after natural infection (at least 24- and 14-fold higher, respectively, at 4 y).22
Limited data from the Costa Rican efficacy trial suggested that 2 doses of the HPV-16/18 AS04-adjuvanted vaccine offered no cross-protection against HPV-31, HPV-33, or HPV-45 infections that persisted for at least one year.9 This may be explained by the fact that the Costa Rican trial included an older population (18–25 y), the 2D schedule was an incomplete 3D schedule (majority of administrations at months 0 and 1) and by the low number of cases with non-16/18 HPV types. In the current trial, the magnitude and kinetics of cross-reactive antibody responses to HPV-31 and -45 appeared similar following administration of a 2D schedule to girls aged 9–14 y compared with a 3D schedule administered to young women aged 15–25 y. Thus, data from the current trial suggest that girls vaccinated with a 2D schedule may have a similar level of cross-protection against non-vaccine HPV types compared with women vaccinated with the standard 3D schedule.23
A previous Phase III trial showed that for HPV-naïve subjects, the estimated efficacy of the HPV-16/18 AS04-adjuvanted vaccine was 93% against grade 3 or greater cervical intraepithelial neoplasia (CIN3+), irrespective of HPV type in the lesion.2 This high overall efficacy is partly due to cross-protective efficacy of the vaccine against non-vaccine types, including HPV-31, -33, -45, and -51,23 which together with HPV-16 and -18 become increasingly more prevalent in high-grade cervical lesions.24 Extrapolating the results from our immunogenicity study, it is hypothesized that high overall efficacy, including cross-protection against non-vaccine HPV types, might also be observed following vaccination of adolescent girls with a 2D regimen.
The longevity of immune responses to vaccine and non-vaccine HPV types observed in this trial with a 2D schedule is important, since vaccinated girls will require many years of protection against HPV infection. Previous studies with the 3D schedule of the HPV-16/18 AS04-adjuvanted vaccine in adult women have shown that HPV-16 and -18 antibody titers were more than 10-fold above natural infection titers for at least 9.4 y after first vaccination,25 and cross-reacting antibody titers to HPV-31 and -45 persist at detectable levels for at least 7 y after first vaccination.26 Sustained efficacy has also been demonstrated up to 6.4 y after first vaccination for CIN2+ lesions4 and up to 9.4 y for virological endpoints.25 Therefore, it is expected that girls administered a 2D schedule will continue to remain protected over a number of years.
The principle finding of this 4-y follow-up was the apparent non-inferiority of HPV-16 and -18 antibody responses over the longer term for the 2D schedule in girls compared with the 3D schedule in women. However, a limitation is that the trial was not primarily designed to demonstrate non-inferiority at months 36 and 48, since this was an exploratory endpoint after month 7, and the study was not powered on this basis. An appropriately powered registration trial (NCT01381575) with a larger sample size is currently ongoing for the primary purpose of demonstrating non-inferiority in this context. This registration trial will also characterize immune responses in more depth, including an evaluation of neutralizing antibodies, avidity, and cell-mediated immunity.
Our trial was conducted in healthy participants enrolled at centers in Canada and Germany, therefore, relevance of results to less developed areas of the world may be perceived as a limitation. A previous trial with the standard 3D schedule of the HPV-16/18 AS04-adjuvanted vaccine in sub-Saharan Africa showed that antibody responses in an African setting were similar compared with a European setting and were not impacted by helminth or malaria infections.27 A 3D course of the vaccine has also been shown to be immunogenic in HIV-positive women aged 18–25 y, with HPV-16 and -18 antibody titers at month 12 substantially above levels associated with natural infection, and above the plateau level associated with sustained protection against HPV-16/18 infection and associated CIN2+ lesions in healthy young women aged 15–25 y in a previous efficacy trial.28 Therefore, we believe that our findings are generalizable to other populations, including those in low resource settings who may have co-morbidities or poor nutritional status, although further evaluation of reduced-dose schedules in preteen/adolescent girls in such settings would be desirable.
A study of the quadrivalent HPV-6/11/16/18 vaccine (Gardasil®) also demonstrated non-inferior antibody responses to vaccine components for a 2D M0,6 schedule in girls aged 9–13 y vs. a 3D M0,2,6 schedule of the same vaccine in women aged 16–26 y up to 3 y after first vaccination.29 However, a nested substudy found that age of the recipient and the number of doses of quadrivalent vaccine administered impacted the development of B-cell and T-cell memory, respectively, and that HPV-18 specific B-cells following administration of the 2D schedule to girls aged 9–13 y failed to reach statistical significance when compared with pre-vaccination levels.30 A post-hoc analysis of another trial of the quadrivalent vaccine in adolescent girls aged 11–13 y showed that antibody titers to vaccine components appeared similar 12 mo after administration of 2 doses at M0,12 and 29 mo after administration of 3 doses at M0,2,6, although these findings should be interpreted with caution as antibody titers were not compared at the same time point and girls were not randomized to the 2D schedule.31 Despite the finding of non-inferiority immunogenicity of 2D vs. 3D schedules for both the quadrivalent HPV-6/11/16/18 vaccine and the HPV-16/18 AS04-adjuvanted vaccine, it should not be inferred that 2D schedules of these 2 HPV vaccines will elicit similar immune responses in adolescent girls, since each trial used the 3D schedule of the matching vaccine in women as the reference. Similar immunogenicity can only be demonstrated in a head-to-head trial.
In summary our data suggest that a 2D schedule of the HPV-16/18 AS04-adjuvanted vaccine in adolescent girls elicits a similar immune response to the standard 3D schedule in young women, and would be expected to provide a similar level of protection against HPV infection and subsequent development of high-grade cervical lesions and cancer. A 2D schedule may be more convenient for physicians and vaccinees, improve compliance, and be less costly both in terms of vaccine doses and administration costs. These benefits could facilitate implementation of HPV vaccination programs in low resource settings, and improve the low vaccine coverage and series completion rates that are observed in some higher resource settings.32-34
Participants and Methods
Study design and participants
The design of this study has been reported previously.11 Briefly, this was a phase I/II, partially-blind, controlled, randomized, age-stratified, parallel group trial conducted at 21 centers in Canada and Germany. It was initiated in October 2007 and data for the month 48 analysis were collected up to February 2012. The trial is ongoing to month 60. The trial was approved by the appropriate Independent Ethics Committee for each center and was conducted according to the Declaration of Helsinki and Good Clinical Practice. The trial is registered with ClinicalTrials.gov (registration number NCT00541970).
Healthy girls and young women aged 9 to 25 y at the time of first vaccination were eligible for the trial. Exclusion criteria have been described previously.11 All participants provided written informed consent, or informed assent with written consent from a parent or legal representative (if below the legal age of consent).
Eligible subjects were randomized (1:1:1:1) and stratified by age (9–14, 15–19, 20–25 y), using an internet-based centralised randomization system, to 1 of 4 groups to receive 3 doses of the HPV-16/18 (20 μg/20 μg) AS04-adjuvanted vaccine at months 0, 1, and 6 (Group 20/20 M0,1,6), 2 doses of vaccine (20 μg/20 μg) at months 0 and 6 (Group 20/20 M0,6), 2 doses of vaccine (40 μg/40 μg) at months 0 and 6 (Group 40/40 M0,6), or 2 doses of vaccine (40 μg/40 μg) at months 0 and 2 (Group 40/40 M0,2).11 The randomization schedule was generated by GlaxoSmithKline Vaccines using validated software. The trial was partially blinded within the 2D groups (observers were blinded to group assignment), with blinding maintained to month 7, and open in the 3D group.
Endpoints
Primary and secondary objectives to month 24 have been reported previously.11 Secondary objectives reported herein were to evaluate the safety of the 2D schedule and to evaluate HPV-16 and -18 antibody responses up to month 48.
Exploratory objectives were to descriptively compare HPV-16 and -18 GMTs induced by the 2D (20/20 M0,6) formulation in girls aged 9–14 y and the licensed 3D schedule in women aged 15–25 y, and to describe the kinetics of HPV-16, -18, -31, and -45 antibody responses up to month 48.
Serologic evaluation
Blood samples for serologic evaluation were drawn prior to first vaccination (month 0), at month 3 (2D groups only), and at months 7, 12, 18, 24, 36, and 48. Antibodies to HPV-16 and HPV-18 and cross-reactive antibodies to HPV-31 and HPV-45 were measured by ELISA, as described previously.15,35 Seropositivity was defined as an antibody titer ≥8 EU/mL for HPV-16, ≥7 EU/mL for HPV-18, and ≥59 EU/mL for HPV-31 and HPV-45.
Safety evaluation
Serious adverse events, adverse events leading to withdrawal, other medically significant conditions (i.e., adverse events prompting emergency room or physician visits that were not related to common diseases), new onset chronic diseases including new onset autoimmune diseases and pregnancies occurring through month 48 were documented. Pregnancies were followed until delivery. New onset chronic diseases and new onset autoimmune diseases (potential autoimmune events, which excluded allergy-related events or isolated signs and symptoms) were identified as described previously.18
Statistical methods
The total vaccinated cohort, for analysis of safety, included all subjects who received at least one dose of vaccine. The ATP cohort for analysis of immunogenicity at months 36 and 48 included all evaluable subjects (i.e., those meeting all eligibility criteria, complying with the procedures defined in the protocol, with no elimination criteria during the trial) for whom data concerning immunogenicity endpoints were available. This included subjects who returned for blood sampling at months 36 or 48 and for whom assay results were available for antibodies against at least one study vaccine antigen component after vaccination. Analyses of immune responses were stratified by serostatus for the corresponding antigen at baseline.
The exploratory objective of this follow-up analysis was to compare the serum antibody responses to HPV-16 and -18 induced by the 2D and 3D schedules by calculating the GMT ratios with exact 95% CI (3D schedule in women aged 15–25 y over the 2D schedule in girls aged 9–14 y) in initially seronegative subjects in the ATP immunogenicity cohort at months 36 and 48. Seroconversion or seropositivity rates and GMTs for HPV-16 and HPV-18 antibodies were calculated with exact 95% CI.
The percentages of subjects with serious adverse events, adverse events leading to withdrawal, medically significant conditions, new onset chronic diseases, and new onset autoimmune diseases over the 48-mo monitoring period were calculated with exact 95% CI.
Glossary
Abbreviations:
- 20/20
formulation containing 20 µg of HPV-16 and 20 µg of HPV-18 L1 protein virus-like particles
- 40/40
formulation containing 40 µg of HPV-16 and 40 µg of HPV-18 L1 protein virus-like particles
- 2D
2-dose
- 3D
3-dose
- AS04
Adjuvant System containing 3-O-desacyl-4’-monophosphoryl lipid A (MPL, 50 µg) adsorbed on aluminum salt (500 µg Al3+)
- ATP
according-to-protocol
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- ELISA
enzyme-linked immunosorbent assay
- EU
ELISA unit
- GMT
geometric mean antibody titer
- HPV
human papillomavirus
- M
month
- VLP
virus-like particle
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies.
Gardasil is a registered trade mark of Merck and Co., Inc.
Disclosure of Potential Conflicts of Interest
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf. Institutions of B Romanowski received grants from the GlaxoSmithKline group of companies for the present study and for other vaccine clinical trials sponsored by the GlaxoSmithKline group of companies. Through her institution, K.S. received financial support from the GlaxoSmithKline group of companies as clinical investigator for the present study. The Colchester Research Group and its medical staff (M.F. and L.F.) received funding from the GlaxoSmithKline group of companies for the present study and for conducting other clinical trials sponsored by Merck, Sanofi, Pfizer, Novartis, and the GlaxoSmithKline group of companies. L.F., M.F., P.H., and T.S. received support either directly or through their institution from the GlaxoSmithKline group of companies for attending meetings and conferences related to the study. Through her corporation, B Romanowski received reimbursement for travel expenses incurred during the study, consultancy fees, and payment for lectures and expert testimony. P.H. received financial support from the GlaxoSmithKline group of companies for consulting activities and expert testimony. L.F. and T.S. received payment from the GlaxoSmithKline group of companies for consultancy, lectures, and development of educational presentations. T.S. received honoraria from the GlaxoSmithKline group of companies for participating in advisory board meetings. F.S., F.T., and P.S. are employees of the GlaxoSmithKline group of companies. F.S. and F.T. own stock in the GlaxoSmithKline group of companies. K.P., U.B., and B Ramjattan declare no conflicts of interest.
Acknowledgments
The co-first authors (B Romanowski and T.S.) and the sponsor clinical team (P.S., F.T., and F.S.) wrote the first draft of the manuscript with the support of a professional medical writer (Julie Taylor, Peak Biomedical, UK) and 2 publication managers (Dirk Saerens and Jérôme Leemans, Keyrus Biopharma, Belgium) working on behalf of GlaxoSmithKline Vaccines. All authors contributed to the development of the subsequent drafts, with the writing and editorial assistance of the sponsor. All authors had full access to the data and gave final approval before submission. The authors received no financial support or other form of compensation for the development of the manuscript. GlaxoSmithKline Biologicals SA took in charge all the costs associated with the development and publishing of the present publication.
The authors thank the study participants and their families, as well as the study investigators and their staff members who are not named as authors but who substantially contributed to the HPV-048 study at months 36 and 48, i.e., Rolf Ebert, Wolf-D. Höpker, Klaus Kindler, Ulrich Kohoutek (private practices, Germany), and Rizwan Somani and Barbara Trainor (Glover Medical Centre, Canada). The authors are also indebted to the local teams of GlaxoSmithKline Vaccines for the coordination of the study, in particular the Canadian team, i.e., Robyn Widenmaier (Study Manager), Christine Wolfe, Ruth Ackerman, and Belinda Grouchy (Clinical Research Associates)—and the German team, i.e., Jens Vollmar (Head of Vaccine Department) and Alexandra Fritsch (Clinical Research Associate). The authors gratefully acknowledge at GlaxoSmithKline Vaccines (Belgium) Garry Edwards (Global Study Manager) who contributed to the central coordination of the study; at GlaxoSmithKline Vaccines (Belgium) Global Vaccine Clinical laboratories, Sylviane Poncelet and the Clinical Immunology and Applied Microbiology team for the enzyme-linked immunosorbent assay analysis of sera. The authors would like also to acknowledge Gary Dubin (GlaxoSmithKline Biologicals, USA), Fabian Santiago Tibaldi, Fatoumata Gassama, Olivier Godeaux, Philippe Marius, and Toufik Zahaf (GlaxoSmithKline Vaccines, Belgium) for their contributions to the study design and study protocol writing; Edwin Kolp (GlaxoSmithKline Vaccines, Belgium) for the coordination of laboratory operations; Marie-Pierre David (GlaxoSmithKline Vaccines, Belgium) and Amulya Jayadev (GlaxoSmithKline, India) for their input on statistical analyses; Stéphanie Genevrois, Nele Martens (GlaxoSmithKline Vaccines, Belgium) and Sanchaita Ukil (GlaxoSmithKline, India) for scientific writing of the clinical study reports; Nicolas Folschweiller (GlaxoSmithKline Vaccines, Belgium) for regulatory affairs support.
Sponsor and Author Contributions
The study reported here (HPV-048; NCT00541970) was funded by GlaxoSmithKline Biologicals SA, which was involved in all stages of the study, from design to final report. M.F. (Canada) and P.H. (Germany) were coordinating investigators and together with B Ramjattan, B Romanowski, K.P., K.S., L.F., M.D., U.B., and T.S. participated in the recruitment and/or follow-up of subjects. T.S. designed the study in collaboration with GlaxoSmithKline Vaccines. At GlaxoSmithKline (India), P.S. contributed toward data analyses and interpretation, and prepared the statistical analysis report. F.S. and F.T. supervised the conduct of the study at GlaxoSmithKline Vaccines (Belgium), and together with M.F. and P.H. critically reviewed the study report.
References
- 1.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. , HPV PATRICIA Study Group. . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/ 10.1016/S0140-6736(09)61248-4; PMID: 19586656 [DOI] [PubMed] [Google Scholar]
- 2.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al. , HPV PATRICIA Study Group. . Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:89 - 99; http://dx.doi.org/ 10.1016/S1470-2045(11)70286-8; PMID: 22075171 [DOI] [PubMed] [Google Scholar]
- 3.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. , GlaxoSmithKline HPV Vaccine Study Group. . Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 65; http://dx.doi.org/ 10.1016/S0140-6736(04)17398-4; PMID: 15541448 [DOI] [PubMed] [Google Scholar]
- 4.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, et al. , GlaxoSmithKline Vaccine HPV-007 Study Group. . Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 85; http://dx.doi.org/ 10.1016/S0140-6736(09)61567-1; PMID: 19962185 [DOI] [PubMed] [Google Scholar]
- 5.Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, Izurieta P, Dubin G, Breuer T. . Analysis of adverse events of potential autoimmune aetiology in a large integrated safety database of AS04 adjuvanted vaccines. Vaccine 2008; 26:6630 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.049; PMID: 18845199 [DOI] [PubMed] [Google Scholar]
- 6.Angelo M-G, David M-P, Zima J, Baril L, Dubin G, Arellano F, Struyf F. . Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf 2014; Forthcoming http://dx.doi.org/ 10.1002/pds.3554; PMID: 24644063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garçon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. . Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs 2011; 25:217 - 26; http://dx.doi.org/ 10.2165/11591760-000000000-00000; PMID: 21815697 [DOI] [PubMed] [Google Scholar]
- 8.Goldie SJ, O’Shea M, Diaz M, Kim SY. . Benefits, cost requirements and cost-effectiveness of the HPV16,18 vaccine for cervical cancer prevention in developing countries: policy implications. Reprod Health Matters 2008; 16:86 - 96; http://dx.doi.org/ 10.1016/S0968-8080(08)32409-4; PMID: 19027626 [DOI] [PubMed] [Google Scholar]
- 9.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al. , CVT Vaccine Group. . Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103:1444 - 51; http://dx.doi.org/ 10.1093/jnci/djr319; PMID: 21908768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. , HPV Vaccine Adolescent Study Investigators Network. . Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564 - 71; http://dx.doi.org/ 10.1016/j.jadohealth.2007.02.015; PMID: 17531764 [DOI] [PubMed] [Google Scholar]
- 11.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al. . Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin 2011; 7:1374 - 86; http://dx.doi.org/ 10.4161/hv.7.12.18322; PMID: 22048171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsagué X, et al. , HPV PATRICIA study group. . Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 70; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5; PMID: 17602732 [DOI] [PubMed] [Google Scholar]
- 13.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Schulze K, Poncelet SM, Catteau G, Thomas F, Descamps D. . Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years. Hum Vaccin 2011; 7:958 - 65; http://dx.doi.org/ 10.4161/hv.7.9.15999; PMID: 21892005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley M, Lowy DR, Frazer I. . Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine 2006; 24:Suppl 3 S106 - 13; http://dx.doi.org/ 10.1016/j.vaccine.2006.05.110; PMID: 16949996 [DOI] [PubMed] [Google Scholar]
- 15.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. . Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425 - 34; http://dx.doi.org/ 10.4161/hv.4.6.6912; PMID: 18948732 [DOI] [PubMed] [Google Scholar]
- 16.Schwarz TF, Kocken M, Petäjä T, Einstein MH, Spaczynski M, Louwers JA, Pedersen C, Levin M, Zahaf T, Poncelet S, et al. . Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine. Hum Vaccin 2010; 6:1054 - 61; http://dx.doi.org/ 10.4161/hv.6.12.13399; PMID: 21157180 [DOI] [PubMed] [Google Scholar]
- 17.Roteli-Martins CM, Naud P, De Borba P, Teixeira JC, De Carvalho NS, Zahaf T, Sanchez N, Geeraerts B, Descamps D. . Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother 2012; 8:390 - 7; http://dx.doi.org/ 10.4161/hv.18865; PMID: 22327492 [DOI] [PubMed] [Google Scholar]
- 18.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. , HPV-010 Study Group. . Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705 - 19; http://dx.doi.org/ 10.4161/hv.5.10.9518; PMID: 19684472 [DOI] [PubMed] [Google Scholar]
- 19.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. , HPV-010 Study Group. . Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7:1343 - 58; http://dx.doi.org/ 10.4161/hv.7.12.18281; PMID: 22048173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschuyteneer M, Elouahabi A, Plainchamp D, Plisnier M, Soete D, Corazza Y, Lockman L, Giannini S, Deschamps M. . Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix™, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum Vaccin 2010; 6:407 - 19; http://dx.doi.org/ 10.4161/hv.6.5.11023; PMID: 20953154 [DOI] [PubMed] [Google Scholar]
- 21.Barr E, Sings HL. . Prophylactic HPV vaccines: new interventions for cancer control. Vaccine 2008; 26:6244 - 57; http://dx.doi.org/ 10.1016/j.vaccine.2008.07.056; PMID: 18694795 [DOI] [PubMed] [Google Scholar]
- 22.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, et al. , CVT Group. . Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013; 6:1242 - 50; http://dx.doi.org/ 10.1158/1940-6207.CAPR-13-0203; PMID: 24189371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, et al. , HPV PATRICIA Study Group. . Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100 - 10; http://dx.doi.org/ 10.1016/S1470-2045(11)70287-X; PMID: 22075170 [DOI] [PubMed] [Google Scholar]
- 24.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, Clifford GM. . Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349 - 59; http://dx.doi.org/ 10.1002/ijc.27485; PMID: 22323075 [DOI] [PubMed] [Google Scholar]
- 25.Naud P, Roteli-Martins CM, De Carvalho N, Teixeira J, Borba P, Sanchez N, Zahaf T, Geeraerts B, Descamps D. HPV-16/18 vaccine: sustained immunogenicity and efficacy up to 9.4 years. Abstract presented at 27th International Papillomavirus Conference and Clinical Workshop, Berlin, Germany; September 17-22, 2011 [Internet]. Applied/Clinical Science Abstract Book; p.153 [cited 2013 Nov 4]. Available from: http://www.hpv2011.org/pics/1/4/Abstract%20Book%202%20APSC%20WEBB%20110922.pdf
- 26.Moscicki AB, Wheeler CM, Romanowski B, Hedrick J, Gall S, Ferris D, Poncelet S, Zahaf T, Moris P, Geeraerts B, et al. . Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012; 31:234 - 41; http://dx.doi.org/ 10.1016/j.vaccine.2012.09.037; PMID: 23063422 [DOI] [PubMed] [Google Scholar]
- 27.Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, Bousso K, Kavishe B, Andreasen A, Toure M, et al. . Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10-25-year-old HIV-Seronegative African girls and young women. J Infect Dis 2013; 207:1753 - 63; http://dx.doi.org/ 10.1093/infdis/jis619; PMID: 23242542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere K, Durand C, Hervé C, Descamps D. . Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine 2013; 31:5745 - 53; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.032; PMID: 24091311 [DOI] [PubMed] [Google Scholar]
- 29.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. . Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793 - 802; http://dx.doi.org/ 10.1001/jama.2013.1625; PMID: 23632723 [DOI] [PubMed] [Google Scholar]
- 30.Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES 3rd, Kollmann TR. . Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30:3572 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.051; PMID: 22469863 [DOI] [PubMed] [Google Scholar]
- 31.Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. . Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 Years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. J Infect Dis 2013; 208:1325 - 34; http://dx.doi.org/ 10.1093/infdis/jit363; PMID: 23901077 [DOI] [PubMed] [Google Scholar]
- 32.Taylor LD, Hariri S, Sternberg M, Dunne EF, Markowitz LE. . Human papillomavirus vaccine coverage in the United States, National Health and Nutrition Examination Survey, 2007-2008. Prev Med 2011; 52:398 - 400; http://dx.doi.org/ 10.1016/j.ypmed.2010.11.006; PMID: 21108962 [DOI] [PubMed] [Google Scholar]
- 33.European Centre for Disease Prevention and Control. Introduction of HPV vaccines in EU countries - an update. Stockholm: ECDC; 2012 [Internet]; p.6 [cited 2013 Nov 4]. Available from: http://ecdc.europa.eu/en/publications/Publications/20120905_GUI_HPV_vaccine_update.pdf.
- 34.Centers for Disease Control and Prevention (CDC). . Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013 - United States. MMWR Morb Mortal Wkly Rep 2013; 62:591 - 5; PMID: 23884346 [PMC free article] [PubMed] [Google Scholar]
- 35.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Lebacq M, van der Most R, et al. , HPV-010 Study Group. . Comparison of the immunogenicity of the human papillomavirus (HPV)-16/18 vaccine and the HPV-6/11/16/18 vaccine for oncogenic non-vaccine types HPV-31 and HPV-45 in healthy women aged 18-45 years. Hum Vaccin 2011; 7:1359 - 73; http://dx.doi.org/ 10.4161/hv.7.12.18282; PMID: 22048172 [DOI] [PMC free article] [PubMed] [Google Scholar]