Abstract
The recurrence rate after surgery in patients with hepatocellular carcinoma (HCC) is very high, while prognosis is quite poor. However, there is no standard treatment to prevent recurrence of HCC after a curative operation. In this study, we investigated the clinical utilization of an autologous tumor lysate-pulsed dendritic cell vaccine plus ex vivo activated T cell transfer (ATVAC) in an adjuvant setting for postoperative HCC as a non-randomized controlled trial. Ninety-four patients with invasive HCC received informed consent information regarding the study, and 42 opted to have the ATVAC after surgery. Their recurrence-free survival (RFS) and overall survival (OS) were measured after 5 years and compared with those of 52 patients who selected to have the curative operation alone. The median RFS and OS were 24.5 months and 97.7 months in the patients receiving adjuvant ATVAC and 12.6 months and 41.0 months in the group receiving surgery alone (P = 0.011 and 0.029). In the treated group, patients with positive delayed-type hypersensitivity (DTH) had a better prognosis (RFS P = 0.019, OS P = 0.025). No adverse events of grade 3 or more were observed.
A postoperative dendritic cell vaccine plus activated T cell transfer would be a feasible and effective treatment for preventing recurrence in HCC patients and achieving long-term survival especially in DTH positive patients.
Keywords: dendritic cell, cancer vaccine, hepatocellular carcinoma, adjuvant therapy, adoptive transfer, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is highly likely to reoccur after surgery, and there is no standard therapy to prevent such recurrences.1 Therefore, development of new treatment modalities is needed, and a cancer vaccine is one such possible treatment. The clinical efficacy of postoperative adoptive immunotherapy with CD3-activated T cells (CAT) has been reported to improve the recurrence-free survival (RFS) of patients who received surgery plus adoptive transfer of CAT; however, overall survival (OS) was not improved.2 Recent research has shown that dendritic cells are potent antigen-presenting cells, capable of inducing antigen-specific immune responses both in vivo and in vitro.3 It has been reported that autologous tumor lysate-pulsed dendritic cells plus activated T cell transfer (ATVAC) improved both postoperative RFS and OS in patients with intrahepatic cholangiocarcinoma.4 HCCs are often associated with cirrhosis of the liver caused by Hepatitis B or C infection.5 This leads to decreased of WBC (including monocytes and lymphocytes) in most patient with HCC. A cancer vaccine could induce antigen-specific cytotoxic T lymphocytes (CTLs) in vivo, which could in turn attack tumors. It may thus be that the number of lymphocytes in vivo is important to obtaining clinical effects. For this reason, it seems that a dendritic cell vaccine plus activated T cell transfer would be a better combination for inducing tumor-specific immunity in such immune-compromised individuals.
In this study, autologous tumor lysate-pulsed dendritic cells were injected and CD3-activated T cells were transferred in patients with invasive HCC after surgery in an adjuvant setting to prevent recurrence and improve OS. This Phase II study was performed as a non-randomized trial with self-selected patients at a single center. We presented the informed consent form to 94 patients scheduled to undergo a curative operation in our hospital. Forty-two patients chose to have the adjuvant immunotherapy, while 52 patients opted for surgery alone. We analyzed and compared postoperative RFS and OS for these 2 groups.
Results
Patient characteristics
The 94 patients who were given the informed consent form were divided into two groups. The 42 who elected to have the adjuvant immunotherapy were enrolled in the ” ATVAC” group, and the 52 patients who chose to have only surgery were enrolled in the “Operation alone” group. The “Operation alone” group did not receive any adjuvant therapies. There was no critical surgical morbidity or mortality in the enrolled patients, and no significant differences in patient characteristics between the two groups (Table 1).
Table 1. Patient characteristics.
| Factor | “ATVAC” group(n = 42) | Operation alone group (n = 52) | P value |
|---|---|---|---|
| Sex (Men) | 35 | 40 | 0.606 |
| Age (y) | 62.9 ± 8.5 | 65.7 ± 11.2 | 0.192 |
| HBs Ag-positive | 12 | 10 | 0.333 |
| HCV-positive | 20 | 32 | 0.213 |
| ICG R15(%) | 19.9 ± 15.4 | 17.5 ± 10.9 | 0.356 |
| AST (U/L) | 62.1 ± 30.3 | 71.2 ± 41.5 | 0.223 |
| ALT (U/L) | 58.1 ± 36.2 | 66.5 ± 44.4 | 0.328 |
| Child (A) | 34 | 44 | 0.772 |
| Portal invasion (+) | 18 | 14 | 0.131 |
| im (+) | 11 | 16 | 0.65 |
| Liver (LC) | 15 | 22 | 0.523 |
| AFP (μg/ml) | 5.4 ± 15.5 | 35.3 ± 225.3 | 0.399 |
| Tumor size (cm) | 6.7 ± 4.3 | 6.0 ± 4.8 | 0.442 |
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATVAC, adoptive transfer of T cells plus dendritic cell vaccine; ICG R15, indocyanine green retention rate at 15 min; im, intrahepatic metastasis; LC, liver cirrhosis.
Quality assessment of DCs and CATs
The results of the bacterial and fungal testing were all negative. The sampling inspection showed >90% CD11b, CD86, and HLA-DR positive on DCs, and >90% CD3 positive on CATs.
Assessment of toxicity
The ATVAC was well-tolerated without any treatment-associated adverse events of grade 3 or higher. Twenty-two of the 42 treatment patients developed grade 1 local skin reaction, with redness at the injection site; no ulceration was seen, however. No high-grade fever, fatigue, diarrhea, rash, or itching was observed. No hematologic, cardiovascular, hepatic, or renal toxicity was observed during or after treatment.
Post operative recurrence-free survival
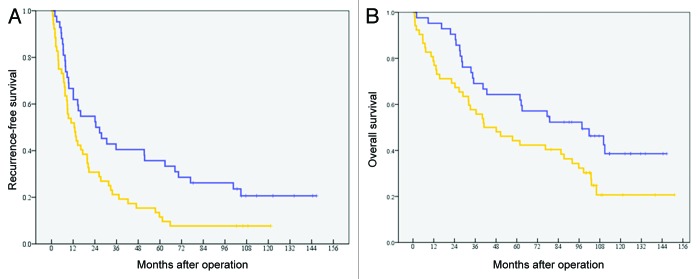
The postoperative recurrence-free survival (RFS) analysis was conducted when all patients who received surgery plus ATVAC were 5 y out from surgery. The 5-y RFS of patients in the “ATVAC” group (n = 42) was 35.7%, with a median RFS of 24.5 mo (95%CI: 7.8–41.2). The 5-y RFS of patients in the “Operation alone” group (n = 52) was 11.5%, with a median RFS of 12.6 mo (95% CI: 6.9–18.3) (Fig. 1A). The difference between the 2 groups was statistically significant (P = 0.011).
Figure 1. (A) Postoperative recurrence-free survival. The 5-y RFS of patients in the “ATVAC” group (n = 42) was 35.7%, with a median RFS of 24.5 mo. The 5-y RFS of patients in the “Operation alone” group (n = 52) was 11.5%, with a median RFS of 12.6 mo (P = 0.011). (B) Postoperative overall survival. The 5-y OS of patients in the “ATVAC” group was 64.3%, with a median OS of 97.7 mo. The 5-y OS of patients in the “Operation alone” group was 44.2%, with a median OS was 41.0 mo (P = 0.029).
Post operative overall survival
The postoperative overall survival (OS) analysis was conducted when all patients who received surgery plus ATVAC were 5 y out from surgery. The 5-y OS of patients in the “ATVAC” group was 64.3%, with a median OS of 97.7 mo (95% CI: 48.6–146.7). In contrast, the 5-y OS of patients in the “Operation alone” group was 44.2%, with a median OS was 41.0 mo (95% CI: 16.3–65.8). The difference between the 2 groups was statistically significant (P = 0.029) (Fig. 1B).
Univariate analysis of recurrence-free survival and overall survival
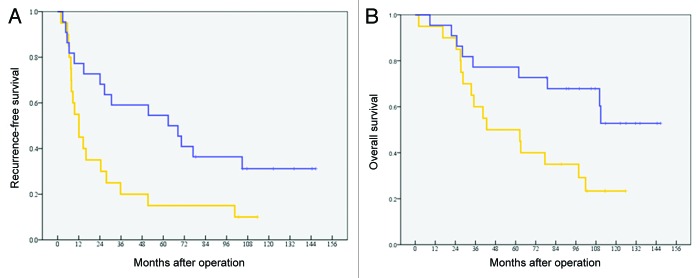
Among the 42 patients in the ATVAC group, 9 patients had RFS and 18 were still alive. A univariate analysis was performed to compare the characteristics of these patients with those of 33 patients with tumor recurrence or those of 24 patients who died. Within the factors, the serum level of alanine aminotransferase (ALT) seemed related to recurrence, and the serum level of aspartate aminotransferase (AST) and DTH response seemed related to overall survival (Table 2). In the analysis of the prognostic factors, the postoperative RFS and OS of patients who with positive DTH were better than that of patients with negative DTH (Table 3). The 5-y RFS of the patients with positive DTH (n = 22) was 54.5%, with a median RFS of 62.8 mo (95% CI: 17.2–108.5). The 5-y RFS of the patients with negative DTH (n = 20) was 15.0%, with a median RFS of 12.1 mo (95% CI: 6.6–17.6). There was a statistically significant difference (P = 0.019) between these 2 groups (Fig. 2A). The 5-y OS of the patients with positive DTH was 77.3% (a median OS was not reached). The 5-y OS of the patients with negative DTH was 50.0%, with a median OS of 42.7 mo (95% CI: 0.0–91.0). There was a statistically significant difference (P = 0.025) between these 2 groups (Fig. 2B). The RFS and OS of patients who with negative DTH were almost same as that of patients in the “Operation alone” group.
Table 2. Characteristics of patients receiving ATVAC.
| Factor | Characteristics on RFS | Characteristics on OS | ||||
|---|---|---|---|---|---|---|
| Recur(-) (n = 9) | Recur(+) (n = 33) | P value | Alive(n = 18) | Dead(n = 24) | P value | |
| Sex (Men) | 8 | 27 | 1 | 16 | 19 | 0.679 |
| Age (y) | 58.7 ± 10.5 | 64.1 ± 7.6 | 0.091 | 62.9 ± 9.3 | 62.9 ± 8.0 | 0.979 |
| HBs Ag-positive | 4 | 8 | 0.406 | 6 | 6 | 0.732 |
| HCV-positive | 3 | 17 | 0.46 | 8 | 12 | 0.764 |
| ICG R15(%) | 22.9 ± 27.3 | 19.6 ± 10.8 | 0.575 | 22.4 ± 21.3 | 18.7 ± 9.2 | 0.452 |
| AST (U/L) | 48.3 ± 16.9 | 65.8 ± 32.2 | 0.125 | 51.9 ± 20.8 | 69.8 ± 34.2 | 0.043 |
| ALT (U/L) | 37.8 ± 8.9 | 63.7 ± 38.9 | 0.001 | 46.9 ± 27.0 | 66.5 ± 40.3 | 0.083 |
| AFP (μg/ml) | 3.1 ± 5.6 | 6.0 ± 17.3 | 0.623 | 7.0 ± 21.0 | 4.1 ± 9.5 | 0.555 |
| Child (A) | 6 | 28 | 0.268 | 12 | 22 | 0.354 |
| Portal invasion (+) | 5 | 13 | 0.462 | 8 | 10 | 1.000 |
| im (+) | 1 | 10 | 0.403 | 3 | 8 | 0.299 |
| Liver (LC) | 1 | 14 | 0.123 | 5 | 10 | 0.517 |
| Tumor size (cm) | 8.3 ± 5.6 | 6.3 ± 3.8 | 0.226 | 6.9 ± 4.8 | 6.6 ± 3.9 | 0.811 |
| DC (times) | 4.0 ± 0.7 | 3.5 ± 0.8 | 0.86 | 3.8 ± 0.6 | 3.4 ± 0.9 | 0.094 |
| DC (× 106 cells) | 13.5 ± 8.7 | 16.2 ± 13.7 | 0.572 | 13.9 ± 13.7 | 16.9 ± 12.1 | 0.455 |
| CAT (× 108 cells) | 11.8 ± 5.5 | 10.2 ± 5.7 | 0.457 | 11.6 ± 5.0 | 9.8 ± 6.1 | 0.32 |
| DTH (+) | 7 | 15 | 0.135 | 13 | 9 | 0.033 |
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATVAC, adoptive transfer of T cells plus dendritic cell vaccine; DC, dendritic cell; DC(times), the number of times of DC injection; CAT, CD3-activated T cell; DTH, delayed-type hypersensitivity; ICG R15, indocyanine green retention rate at 15 min; im, intrahepatic metastasis; LC, liver cirrhosis; RFS, recurrence-free survival; OS, overall survival.
Table 3. Prognostic factors of RFS or OS.
| Factors | RFS | OS |
|---|---|---|
| Sex (male/female) | 0.54 | 0.481 |
| Age (≥63/ <63) | 0.468 | 0.848 |
| HBs Ag(+/−) | 0.292 | 0.628 |
| HCV Ab(+/−) | 0.711 | 0.996 |
| AST(≥60/ <60 U/L) | 0.06 | 0.246 |
| ALT(≥60/ <60 U/L) | 0.13 | 0.359 |
| ICG R15(≥20/ <20%) | 0.674 | 0.977 |
| AFP(≥5/ < 5μg/ml) | 0.303 | 0.404 |
| DC(times) (≥4/ <4 times) | 0.2 | 0.132 |
| DC (≥15/ <15 × 106 cells) | 0.755 | 0.419 |
| CAT(≥10/ <10 × 108 cells) | 0.383 | 0.27 |
| Tumor size(≥6/ <6 cm) | 0.351 | 0.952 |
| Portal invasion(+/−) | 0.748 | 0.743 |
| im(+/−) | 0.125 | 0.145 |
| LC(+/−) | 0.06 | 0.131 |
| Child (A/B) | 0.283 | 0.265 |
| DTH (positive/negative) | 0.019 | 0.025 |
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DC, dendritic cell; DC(times), the number of times of DC injection; CAT, CD3-activated T cell; LC, liver cirrhosis; DTH, delayed-type hypersensitivity; ICG R15, indocyanine green retention rate at 15 min; im, intrahepatic metastasis; RFS, recurrence-free survival; OS, overall survival.
Figure 2. (A) Postoperative recurrence-free survival in patients with a positive DTH and negative DTH. The 5-y RFS of the patients with positive DTH (n = 22) was 54.5%, with a median RFS of 62.8 mo. The 5-y RFS of the patients with negative DTH (n = 20) was 15.0%, with a median RFS of 12.1 mo (P = 0.019). (B) Postoperative overall survival in patients with a positive DTH or negative DTH. The 5-y OS of the patients with positive DTH was 77.3% (a median OS was not reached). The 5-y OS of the patients with negative DTH was 50.0%, with a median OS of 42.7 mo (P = 0.025).
Discussion
HCC is highly likely to recur or metastasize after surgery.6 Although there is no standard therapy to prevent recurrence at this time, several clinical trials used Sorafenib,7 Interferon,8 or Vitamins9 have been conducted. CATs have also been reported as a useful tool for improving postoperative recurrence-free survival, but not overall survival, because of an increase in recurrence 1 y after operation. DC vaccines were shown to prevent recurrence 1 y after operation in our study, however. Therefore a combination CAT and DC therapy could succeed in improving both RFS and OS. In the meantime, cancer vaccines like the DC vaccine often show a delayed clinical effect.10Our data indicated that DC vaccines failed to prevent recurrence within the first year after operation. To ensure success of treatment, the combination of CAT and DC might be required as an adjuvant therapy for HCC.
Currently, many clinical trials using DCs have been conducted, but very few have been approved.11Provenge has been approved in the US and Europe based on the data of randomized Phase III trials.12 Our results encourage advancing to the next step in randomized trials to assess the true clinical effect of ATVAC.
Recently, a synthetic peptide was investigated as a potential peptide vaccine for in vivo injection.13-15 Glypican-3 (GPC3), which is overexpressed on HCC cells, is one candidate for a peptide vaccine.16 The phase-I study, already completed for advanced HCC, has confirmed its safety and immunologic response.17 If the patients meet the criteria of HLA typing, the combination therapy with a peptide vaccine and CATs might be an effective tool in the future treatment.
While immunotherapy such as cancer vaccines or T cell transfer may be useful tools, their clinical efficacy remains limited because of the immune suppression in the patients with advanced cancer. Some cytokines as TGFβ, IL-4, IL-10, or regulatory T cells are the critical factors in the suppression of immune response. Nonmyeloablative chemotherapy to deplete regulatory T cells is a promising technique for overcoming those problems.18 Another method using denileukindiftitox has also been examined in both animal and human models.19,20 Recent research has indicated that blockading immune checkpoints seems to be very important in obtaining the clinical effect of immunotherapy in patients with advanced cancer. The anti-PD-1 antibody,21 anti-PD-L1 antibody,22 and anti-CTLA-4 antibody23 are all likely candidates for improving the immune response of T cells to cancer cells. These studies have demonstrated that regulation of the host immune setup is crucial for obtaining a good immune response in a clinical study.24 The infusion of CATs might improve the host immune condition in order to allow it to acquire the DC vaccine-induced immune response in advanced cancer patients who show a decreased number of lymphocytes.25 Additionally, the efficacy of the CATs might be boosted by DC vaccines.26,27 Thus, CATs and DCs could both be enhanced if used together. In the meantime, neither RFS nor OS improved in patients who showed negative DTH. To obtain a positive DTH response, it is first necessary to remove immune suppressive factors in order to achieve a better prognosis.
In this study, we have shown the feasibility and potential efficacy of the combination therapy of DC vaccines and CD3-activated T cell transfer. Postoperative ATVAC treatment resulted in improved RFS and OS in patients with HCC, in particular in those with a positive DTH response. In order to assess the effect of ATVAC treatment, randomized control trials on an appropriate number of candidates are needed. Furthermore, the selection of patients based on immune condition is an important issue for discussion to obtain good results in further clinical trials,28 if it is difficult to remove the immune suppressive factors from the patients.
Patients and Methods
Patient eligibility
All patients with an invasive HCC 2.5 cm in diameter or larger who had surgery in our hospital between October 2000 and March 2005 were eligible for this study. Invasive HCCs were diagnosed by their capsular invasions. Additional inclusion criteria were age (between 20 and 80 y), white blood cell count (>2500/mL), hemoglobin (>9 mg/dL), and platelet count (>100 000/mL). An informed consent was obtained from all patients.
Study design and endpoints
This study was performed as a non-randomized controlled trial. Subjects were drawn from 94 patients who received surgery for invasive HCC who met the inclusion criteria. The 42 patients who chose to have adjuvant DC vaccine plus T cell transfer were enrolled to the ATVAC (adoptive transfer of T cells plus dendritic cell vaccine) group; the 52 patients who did not choose to adjuvant immunotherapy consented to serve as the control group. None of the patients had received other therapies between their curative operation and diagnosis of recurrence at the follow-up examination. The ATVAC group received 4 cycles of leukapheresis and the cells obtained were used to induce the dendritic cell (DC) vaccine and CD3-activated T (CAT) cells for each treatment. Each patient was vaccinated with the DC i.d. near an inguinal nodal region. A total of 3 DC vaccinations and CAT transfer were performed within 2 mo of surgery. The patients were followed by radiological and chemical examination to assess tumor recurrence. If a patient applied for a continuation of adjuvant therapy, an additional ATVAC was performed once a month until their tumor lysate was finished. The primary endpoint of this study was the assessment of RFS after surgery; the secondary endpoint was the assessment of OS. This study was approved by the Institutional Review Board of Tokyo Women's Medical University (TWMU) and the procedures were in accord with the Helsinki Declaration. Trial registration was UMIN000005820.
Preparation of autologous tumor lysates
Autologous tumor tissues were obtained by surgical operation. Fresh tumor tissues were cut mechanically and a single cell suspension made by enzyme as soon as possible. The cells were filtered on mesh and washed 3 times in AIM-V (therapeutic grade; Invitrogen Corporation) medium. The tumor cell suspensions were centrifuged (1200 rpm, 5 min) and the tumor cell pellets were subjected to 5 freeze-thaw cycles to lyse the tumor cells. Tumor lysates were stored in a freezer at –80 °C for further use.
Preparation of autologous DC vaccines
Autologous peripheral-blood mononuclear cells (PBMCs) were obtained by leukapheresis performed on a Heamonetics CCS (Heamonetics) at the TWMU Hospital. Leukapheresis consisted of 4 cycles, each approximately 1 h long, in which 1.5–2 L of blood were collected, containing approximately 1 × 109 cells per patient. PBMCs were plated in AIM-V medium at 1 × 107 cells/mL for a total volume of 50 mL in each T225-cm2 flask (Corning) for 2 h at 37 °C in 5% CO2, and the non-adherent cells were removed for further use. The adherent cells were cultured in AIM-V medium containing GM-CSF (Leukine; 50 ng/mL) and IL-4 (50 ng/mL; Primmune), for a total volume of 50 mL per flask. On day 6, the DCs were harvested from the flasks and resuspended at 1 × 106 cells/ml in AIM-V medium containing GM-CSF (50 ng/ml), IL-4 (50 ng/ml), and recombinant TNFα (10 ng/ml; PeproTech). Thirty-five ml of the cell suspension was placed in 75-cm2 flasks (1 × 106 cells/ml) and pulsed with tumor-lysates which were made from the same numbers of DCs to adjust at a 1:1 cell equivalent ratio and 1ml of keyhole limpet hemocyanin (KLH; Calbiochem) diluted in PBS (50 ng/ml). DCs were then incubated for 24 h at 37 °C in an atmosphere of 5% CO2. After incubation, the DCs were harvested and adjusted to a total volume of 0.5 ml of saline for intradermal administration. To allow release, samples had to meet the following release criteria were >80% alive, endotoxin test negative, and bacterial and fungal test negative. Where possible, samples were taken for phenotypical characterization via flowcytometric analysis using a FACSCanflowcytometer (Beckton Dickinson) with anti-human CD11b, CD86, and HLA-DR monoclonal antibodies (Beckman Coulter).
Preparation of CAT cells
A T225 cm2 flask was coated with anti-CD3 monoclonal antibody for 1 h at 37 °C in 5% CO2. The non-adherent cells obtained by the procedure described above were resuspended in AIM-V medium at 1 × 107 cells/ml. The resuspended cells were placed in the coated flasks for a total volume of 50 ml per flask, yielding 3 × 108 cells/flask. On day 2, the cells were collected and resuspended in AIM-V medium at 1 × 106 cells/ml for a total volume of 2000 ml in a culture bag (Nipro). Recombinant hIL-2 (Proluekin; Chiron) was added to the cell suspension at 60 IU/ml. On day 7, the cells were harvested and washed 3 times in buffered saline. To allow release, samples had to meet the following release criteria were >80% alive, endotoxin test negative, and bacterial and fungal test negative. Where possible, samples were taken for phenotypical characterization via flowcytometric analysis using a FACSCanflowcytometer (Beckton Dickinson) with anti-human CD3 monoclonal antibodies (Beckman Coulter). The obtained cells were resuspended for a total volume of 100 ml in a saline bag and infused intravenously into the patients immediately after DC injection. The cell cultures of both DC and CAT were performed in the Cell Processing Center (CPC) of the TWMU Hospital, which operates under good manufacturing procedures (GMP).
Clinical assessment
All patients were followed by clinical examination, computed tomography (CT), ultrasonography (US), and measurement of tumor markers at the outpatients clinic in order to evaluate tumor recurrence. Delayed-type hypersensitivity (DTH) was tested after at least three vaccinations. For this, 0.2 ml of tumor lysates or 0.2 ml of saline were injected intradermally. After 48 h, redness and induration were assessed and judged as positive if the measurement of the reaction exceeded 10 mm.
Statistical analysis
Statistical analysis between the treatment and control group was done using chi-square test and non-parametrical t test or the Fisher exact test (2sided P value). Postoperative RFS and OS were calculated using the Kaplan-Meier method, and evaluated by log-rank test. A P value of less than 0.05 was considered statistically significant. Values were expressed as the means ± standard deviation. Subgroup analysis was done with several factors as secondary analyses.
Glossary
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ATVAC
adoptive transfer of T cells plus dendritic cell vaccine
- CAT
CD3-activated T cells
- DC
dendritic cell
- DC(times)
the number of times of DC injection
- DTH
delayed-type hypersensitivity
- HCC
hepatocellular carcinoma
- ICG R15
indocyanine green retention rate at 15 minutes
- im
intrahepatic metastasis
- LC
liver cirrhosis
- OS
overall survival
- RFS
recurrence-free survival
Disclosure of Potential Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Acknowledgments
This study was supported in part by funds from the Nakayama Cancer Research Institute, Tokyo, Japan.
References
- 1.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, et al. . Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003; 38:200 - 7; http://dx.doi.org/ 10.1016/S0168-8278(02)00360-4; PMID: 12547409 [DOI] [PubMed] [Google Scholar]
- 2.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, et al. . Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802 - 7; http://dx.doi.org/ 10.1016/S0140-6736(00)02654-4; PMID: 11022927 [DOI] [PubMed] [Google Scholar]
- 3.Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. . Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer ImmunolImmunother 2009; 58:1 - 14; http://dx.doi.org/ 10.1007/s00262-008-0568-4; PMID: 18719915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. . Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J HepatobiliaryPancreatSci 2012; 19:171 - 8; http://dx.doi.org/ 10.1007/s00534-011-0437-y; PMID: 21874278 [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, Cao G. . Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst 2009; 101:1066 - 82; http://dx.doi.org/ 10.1093/jnci/djp180; PMID: 19574418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altekruse SF, McGlynn KA, Reichman ME. . Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J ClinOncol 2009; 27:1485 - 91; http://dx.doi.org/ 10.1200/JCO.2008.20.7753; PMID: 19224838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. , SHARP Investigators Study Group. . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378 - 90; http://dx.doi.org/ 10.1056/NEJMoa0708857; PMID: 18650514 [DOI] [PubMed] [Google Scholar]
- 8.Zhang CH, Xu GL, Jia WD, Ge YS. . Effects of interferon alpha treatment on recurrence and survival after complete resection or ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Int J Cancer 2009; 124:2982 - 8; http://dx.doi.org/ 10.1002/ijc.24311; PMID: 19296539 [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, et al. . Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011; 54:532 - 40; http://dx.doi.org/ 10.1002/hep.24430; PMID: 21574174 [DOI] [PubMed] [Google Scholar]
- 10.Chen T-T. . Statistical issues and challenges in immuno-oncology. J Immunother Cancer 2013; 1:18; http://dx.doi.org/ 10.1186/2051-1426-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogi C, Aruga A. . Clinical evaluation of therapeutic cancer vaccines. Hum VaccinImmunother 2013; 9:1049 - 57; http://dx.doi.org/ 10.4161/hv.23917; PMID: 23454867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheever MA, Higano CS. . PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011; 17:3520 - 6; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-3126; PMID: 21471425 [DOI] [PubMed] [Google Scholar]
- 13.Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T, Takeda K, Yamamoto M. . Long-term vaccination with multiple peptides derived from cancer-testis antigens can maintain a specific T-cell response and achieve disease stability in advanced biliary tract cancer. Clin Cancer Res 2013; 19:2224 - 31; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-3592; PMID: 23479678 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita N, Aruga A, Inoue Y, Kotera Y, Takeda K, Yamamoto M. . Phase I clinical trial of a peptide vaccine combined with tegafur-uracil plus leucovorin for treatment of advanced or recurrent colorectal cancer. Oncol Rep 2013; 29:951 - 9; PMID: 23314271 [DOI] [PubMed] [Google Scholar]
- 15.Kono K, Iinuma H, Akutsu Y, Tanaka H, Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R, et al. . Multicenter, phase II clinical trial of cancer vaccination for advanced esophageal cancer with three peptides derived from novel cancer-testis antigens. J Transl Med 2012; 10:141; http://dx.doi.org/ 10.1186/1479-5876-10-141; PMID: 22776426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen IP, Ariizumi S, Nakano M, Yamamoto M. . Positive glypican-3 expression inearly hepatocellular carcinoma predicts recurrence after hepatectomy. J Gastroenterol 2013; (Forthcoming); http://dx.doi.org/ 10.1007/s00535-013-0793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, et al. . Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012; 18:3686 - 96; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-3044; PMID: 22577059 [DOI] [PubMed] [Google Scholar]
- 18.Koike N, Pilon-Thomas S, Mulé JJ. . Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother 2008; 31:402 - 12; http://dx.doi.org/ 10.1097/CJI.0b013e31816cabbb; PMID: 18391755 [DOI] [PubMed] [Google Scholar]
- 19.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. . Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods 2008; 333:167 - 79; http://dx.doi.org/ 10.1016/j.jim.2008.01.012; PMID: 18295790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. . Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood 2008; 112:610 - 8; http://dx.doi.org/ 10.1182/blood-2008-01-135319; PMID: 18519811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. . Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134 - 44; http://dx.doi.org/ 10.1056/NEJMoa1305133; PMID: 23724846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. . Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455 - 65; http://dx.doi.org/ 10.1056/NEJMoa1200694; PMID: 22658128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122 - 33; http://dx.doi.org/ 10.1056/NEJMoa1302369; PMID: 23724867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogi C, Aruga A. . Immunological monitoring of anticancer vaccines in clinical trials. Oncoimmunology 2013; 2:e26012; http://dx.doi.org/ 10.4161/onci.26012; PMID: 24083085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott RL, Head JF. . Adjuvant breast cancer vaccine improves disease specific survival of breast cancer patients with depressed lymphocyte immunity. SurgOncol 2013; 22:172 - 7; http://dx.doi.org/ 10.1016/j.suronc.2013.05.003; PMID: 23791552 [DOI] [PubMed] [Google Scholar]
- 26.Chang AE, Aruga A, Cameron MJ, Sondak VK, Normolle DP, Fox BA, Shu S. . Adoptive immunotherapy with vaccine-primed lymph node cells secondarily activated with anti-CD3 and interleukin-2. J ClinOncol 1997; 15:796 - 807; PMID: 9053507 [DOI] [PubMed] [Google Scholar]
- 27.Kandalaft LE, Powell DJ Jr., Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Trigian DA, et al. . Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccin-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. OncoImmunlogy 2013; 2:1 - 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. . The immune score as a new possible approach for the classification of cancer. J Transl Med 2012; 10:1; http://dx.doi.org/ 10.1186/1479-5876-10-1; PMID: 22214470 [DOI] [PMC free article] [PubMed] [Google Scholar]