Abstract
Prophylactic vaccines against hepatitis B Virus (HBV) infection were produced in different expression systems under different processing conditions. Since the recombinant HBV surface antigen (HBsAg) in these vaccines is a cysteine-rich protein with 14 cysteines among a total of 226 amino acids, the epitopes are dependent on the formation of intra- and intermolecular disulfide bonds. A panel of 22 monoclonal antibodies (mAbs) were developed and evaluated with respect to their sensitivity to disulfide reduction treatment of recombinant HBsAg. Not surprisingly, different mAbs showed different degree of sensitivity to controlled HBsAg disulfide reduction. With a view to exploring the functionality of anti-HBsAg mAbs to be used in HBsAg quality analysis, in vitro neutralization activity for the mAbs was assessed. One of the mAbs tested, 5F11, which showed high sensitivity to the disulfide integrity in HBsAg, was shown also to be highly effective in neutralizing HBV in vitro. Conversely, 42B6, while exhibiting similar neutralization activity, showed comparable binding HBsAg with or without reduction treatment. Based on these mAb characteristics, a sandwich ELISA with 42B6 being the capture Ab and detection Ab was developed to quantify HBsAg (like a “mass” assay) during antigen bioprocessing or in vaccine products. In parallel, when 5F11 was used as the detection Ab (with the same capture Ab), the assay can be used to probe disulfide-dependent and virion-like epitopes in intermediates or final products of hepatitis B vaccine, serving as a surrogate marker for vaccine efficacy to elicit neutralizing antibodies. This approach enables the comparative epitope specific antigenicity analysis of HBsAg antigen preparations from different sources.
Keywords: epitope, HBsAg, disulfide, monoclonal antibody, neutralization activity, hepatitis B virus
Introduction
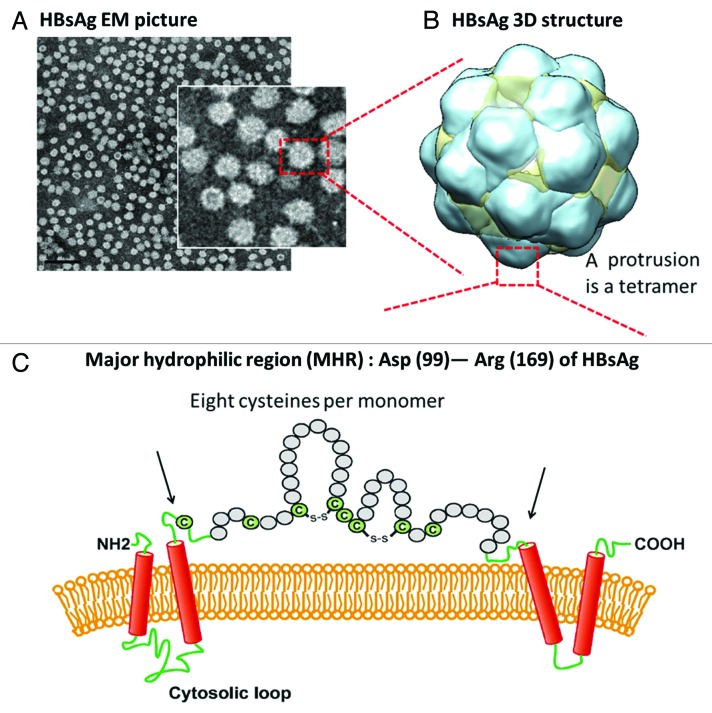
Hepatitis B virus (HBV) is the most important member of the taxonomic family Hepadnaviridiae that causes hepatitis B, liver cancer, and liver cirrhosis. Despite the progress made over the past 3 decades through vaccination, HBV remains to be a major challenge and a constant threat in the field of public health; current estimates suggest that there are more than 350 million hepatitis B carriers worldwide.1,2 Hepatitis B virus surface antigen (HBsAg) based vaccine Heptavax-B (Merck) was introduced initially in 1981 with the plasma derived non-infectious HBsAg subviral lipid-protein particle as antigen. Subsequently, plasma-derived antigen was replaced with a recombinant HBsAg based vaccine with the commercial name RECOMBIVAX HB® (licensed by Merck) in 1986 as the first vaccine produced using modern recombinant DNA technology. RECOMBIVAX HB® is also the first human vaccine developed with virus-like particles (VLP) approach, followed by other globally successful vaccines including Engerix-B (by GSK) and other products in various countries.3,4 The structure of the hepatitis B subviral vaccine particle consists of lipids (~1/3 of the total mass) and HBsAg protein. HBV HBsAg produced in vivo or recombinantly self-assembles upon expression in cells into 22 nm spherical VLP, smaller than the infectious 42 nm Dane particles.4 The self-assembled 22 nm spherical HBsAg particlescomprise of HBsAg oligomers embedded in the lipid layers. The spherical lipid-protein HBsAg particles were decorated with distinct surface protrusions, harboring key epitopes. These protrusions (24 protrusions per particle) in the octahedral structure were recently determined to be the HBsAg tetramers with their trans-membrane helical segments inserted in the lipid layers in the spherical particles.5,6 These protrusions, harboring the “a” determinant (aa 124–147) epitopes near the tip of these protrusions, comprised of the ~70 aa major hydrophilic region (MHR) of each HBsAg monomer. This monomer-monomer interface area is the area with immuno dominant epitopes for neutralizing antibodies found in the sera from HBV infected individuals or in the sera from vaccinees.7 HBsAg is a cysteine-rich protein with a total of 14 cysteines (with a total of 226 aa) in each monomer. Most strikingly, in the MHR with ~70 aa, there are 8 cysteines in each monomeric HBsAg, making a total of 32 cysteines in each protrusion (Fig. 1). Therefore, disulfide-bond pairing patterns are conceivably complicated with potential disulfide reshuffling during antigen preparation and/or during vaccine storage. Due to the level of complexity and heterogeneity of multiple disulfide bonds, there was no definitive assignment on the disulfide bonds, which are critical for forming the neutralizing epitopes that resemble those of native virus.8-10 The heterogeneity in disulfide pairing and the presence of the lipids in the particles could be the reasons impeding the disulfide assignment and the determination of the full HBsAg or subdomain structure with high resolution even after over 3 decades successful application as a human vaccine.
Figure 1. Recombinant cysteine-rich HBsAg, the active component of HBV vaccines, is a protein self-assembled into spherical particles upon expression with proper lipids. (A) Transmission electron-microscopy (TEM) obtained with CHO-derived HBsAg. Scale bar = 100 nm. (B) Three-dimensional octahedral HBsAg particle structure (yeast-derived HBsAg) with reconstructed cryo TEM data (adopted from Mulder et al.)5 (C) Major hydrophilic region (MHR), Asp99 to Arg169, in each monomer is illustrated with 8 cysteines per monomer. Therefore, a total of 32 cysteines are present in each protrusion which comprises of a tetrameric HBsAg MHR. It is conceivable that the disulfide pairing in MHR is complex. The presence of cysteines and formation of disulfides were shown to be crucial in virions for budding and viral post-replication transport.9,10 These disulfides are also important in the HBsAg VLPs in maintaining the virion-like epitopes,8 Therefore, making stable and cross-linked HBsAg antigens is the prime goal during vaccine bioprocess.19
Without high resolution structure, the important epitopes of HBsAg have been mapped using various functional assays with monoclonal antibodies (mAbs), notably with neutralizing mAb RF-1 and A1.2, as molecular probes to assess the integrity and function of key epitopes.5,11 Binding activity of HBsAg in vaccine preparations to neutralizing mAbs has been used as a surrogate marker for vaccine efficacy to elicit functional antibodies.12,13 In this report, the characteristics of a panel of anti-HBsAg mAbs, probing different HBsAg surface epitopes, are described. These mAbs showed different degree of dependence on the presence of disulfides.5 The neutralization activities of these murine mAbs were assessed using a recombinant HBV system in HepaRG cells.14 Two representative mAbs, 5F11 and 42B6, one being highly sensitive to disulfide bond reduction and another being in-sensitive to such a reduction, were used in different assays to probe disulfide bond status on the HBsAg particles, a critical attribute for an efficacious vaccine. Probing the disulfide bond status, thus the epitope integrity of HBsAg, could potentially be useful in process monitoring and product characterization or even release and stability testing during HBV vaccine production and analysis.
Results
Characterization of murine anti-HBsAg mAbs
The cysteine-rich HBsAg protein, self-assembling into 22 nm spherical particles with images from transmission electron microscopy shown in Figure 1, was expressed in Chinese hamster ovary cancer cells. The HBsAg preparation was used the immunogen and screening antigen during preparation of murine anti-HBsAg hybridoma. A panel of anti-HBsAg 22 mAbs were tested for their binding activity to surface immobilized recombinant HBsAg in a direct binding ELISA. The effective concentrations yielding the half maximal binding signals or EC50 values, were derived for these anti-HBsAg mAbs. They were ranked based on their affinity, reflected by EC50 values, being grouped into 4 groups, i.e., very strong, strong, medium, and weak (Table 1). To probe the sensitivity of each mAb to disulfide bond reduction, HBsAg was treated with different concentration of DTT during plate coating, yielding different levels of disulfide reduction (Fig. 2). A quantitative analysis on the binding activity to HBsAg (native particle antigen) and DTT-treated HBsAg (disulfides being reduced to free thiols) with a serially diluted mAb in each assay was performed in parallel to probe the mAb sensitivity to HBsAg reduction. EC50 values for these mAbs with untreated HBsAg as the coating antigen are tabulated in last column of Table 1.
Table 1. Characteristics of a panel of anti-HBsAg monoclonal antibodies.
| Antibody | Subclass | Degree of disulfide sensitivitya | Epitope typeb | Dimer HBsAgc | Monomer HBsAgc | Binding strengthd | EC50 (ng/mL)e |
|---|---|---|---|---|---|---|---|
| Highly sensitive to DTT treatment (>10) | |||||||
| 22F10 | IgG1 | >440 | C | - | - | very strong | 0.18 |
| A2C1 | IgG1 | >240 | C | - | - | very strong | 0.34 |
| 15D1 | IgG1 | >220 | C | - | - | very strong | 0.36 |
| 5F11 | IgG2a | >220 | C | + | +/− | very strong | 0.37 |
| 127D7 | IgG1 | >33.5 | L | + | + | strong | 2.4 |
| 20A2 | IgG2b | 10.1 | C | - | +/− | strong | 2.9 |
| Sensitive (1.3~10) | |||||||
|---|---|---|---|---|---|---|---|
| SF | IgM | >9.02 | C | +/− | - | medium | 8.9 |
| A10C2 | IgG1 | 6.74 | L | + | + | very strong | 0.59 |
| SA1 | IgG2a | >3.63 | C | - | - | weak | 22.0 |
| E2A9 | IgG2a | 3.22 | L | + | + | strong | 1.1 |
| 129G1 | IgG1 | 2.01 | L | + | + | very strong | 0.91 |
| 6C10 | IgM | 1.43 | L | + | + | strong | 1.4 |
| Not sensitive (0.7~1.3) | |||||||
|---|---|---|---|---|---|---|---|
| 13H10 | IgM | 1.21 | L | + | + | medium | 7.5 |
| A13A2 | IgG2b | 0.97 | L | + | + | strong | 2.2 |
| 42B6 | IgG1 | 0.89 | L | + | + | very strong | 0.94 |
| G12F5 | IgG1 | 0.77 | L | + | + | very strong | 0.75 |
| 75C12 | IgG1 | 0.72 | L | + | + | weak | 29.8 |
| Preferring reduced HBsAg (<0.7) | |||||||
|---|---|---|---|---|---|---|---|
| E7D4 | IgG2a | 0.49 | L | + | + | medium | 4.2 |
| E11E4 | IgG2a | 0.39 | L | + | + | strong | 1.2 |
| 83H12 | IgG1 | 0.23 | L | + | + | medium | 4.6 |
| 45E9E | IgG3 | 0.22 | L | + | + | weak | 56.8 |
| E9B3 | IgG3 | 0.12 | L | + | + | weak | 34.4 |
The degree of sensitivity to DTT treatment for a given mAb was indicated by fold change in EC50 value in direct binding ELISA. aDegree of disulfide sensitivity was assessed in a direct binding ELISA on HBsAg-coated plates. Relative binding data were derived from (DDT-treated HBsAg EC50)/ (HBsAg in PBS EC50) based on curve fitting results. bEpitope type identified via Western Blotting,15 “C” means conformational and “L” means linear. cMonomer HBsAg and dimer HBsAg15 were used by SIA (strip immunoblot assay), SIA strips contained 2 individual bands: a SDS-treated HBsAg dimer (5 μg, obtained from SDS-treated HBsAg by electro-elution), a SDS-treated HBsAg monomer (5 μg, obtained from SDS-treated HBsAg by electro-elution). dBinding strength for a given mAb was assigned based on the EC50 value of direct binding ELISA data. These mAbs were classified into 4 different groups: very strong (EC50 < 1 ng/mL), strong (1 ng/mL < EC50 < 3 ng/mL), medium (3 ng/mL < EC50 < 10 ng/mL), weak (10 ng/mL < EC50). eEC50 values were derived from 4-parameter logistics fits of the direct binding ELISA data. Binding affinity as reflected EC50 values may not correlate to the neutralization activity for different mAbs. The neutralization activity is more dependent on the epitope recognized by a given mAb.
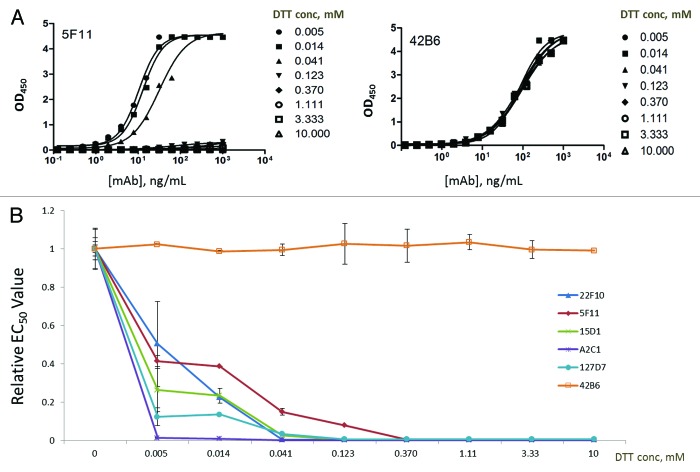
Figure 2. The changing affinities certain monoclonal antibodies (mAbs) to immobilized HBsAg as a function of DTT concentration (unit: mM) during plate coating. (A) The binding profiles for 2 representative mAbs - 5F11 (highly sensitive for DTT treatment), 42B6 (insensitive to DTT treatment), with different DTT concentrations during plate coating. (B) Relative EC50 values of 22F10, 5F11, 15D1, A2C1, 127D7, and 42B6 as a function of DTT concentration. All 5 disulfide-sensitive mAbs lost most of their binding capacities to HBsAg with as low as 0.005 mM DTT during plate coating.
Subtle perturbation to the HBsAg by varying levels of DTT to yield different level of reduction was probed with different mAbs in a direct binding ELISA. While DTT was present during plate coating, the plate was washed thoroughly to remove any residual DTT, not impacting subsequent Ag–Ab interaction during the ensuing ELISA steps. The level of sensitivity for each mAb to DTT treatment was quantitatively assessed by deriving the EC50 values in the HBsAg binding activity for control HBsAg and HBsAg treated with different concentrations of DTT (0.005, 0.014, 0.123, 0.37, 1.11, 3.33, and 10.0 mM) (Fig. 2). The mAbs can be grouped into 2 groups, those sensitive to DTT treatment (e.g., 5F11) and those that showed comparable binding to HBsAg regardless of the DTT treatment (e.g., 42B6). Five of the mAbs, i.e., 22F10, 5F11, 15D1, A2C1, 127D7, were found to be sensitive to disulfide bond reduction even with very low level (i.e., 0.005 mM) of DTT as indicated by markedly increased EC50 values (Fig. 2B). Conversely, other mAbs such as 42B6 showed little or no sensitivity to DTT treatment (Fig. 2B). The plot of relative EC50 against DTT concentration for HBsAg treatment is shown in Figure 2B for 6 representative mAbs, showing progressively lower binding activities to reduced HBsAg for 5 mAbs except 42B6, whose epitope is known to be linear (Cys137 – Gly145).15
Quantitative analysis of mAb sensitivity to disulfide reduction
As shown in Figure 2, 5 highly disulfide-bond sensitive mAbs—22F10, A2C1, 15D1, 5F11, and 127D7—were identified. The concentration of DTT could be as low as 0.005 mM while still yielding a significant decreasing effect on the binding activity of these disulfide sensitive mAbs to HBsAg. Other mAbs had also shown altered affinities for the DTT-treated HBsAg, however, they were either not as significant (e.g., 42B6 in Figure 2) or having slight increase in affinity (data not shown). With 10 mM DTT treatment, all 5 highly disulfide-sensitive mAbs showed no activity to bind HBsAg after reduction treatment, suggesting that 10 mM DTT would be a suitable reference point to categorize the mAbs according to their degrees of disulfide-bond sensitivity. Based on the level of sensitivity to disulfide reduction as reflected by relative EC50 (rEC50) values, the mAbs were ranked and categorized into 4 different groups: (Fig. 3B; Table 1)
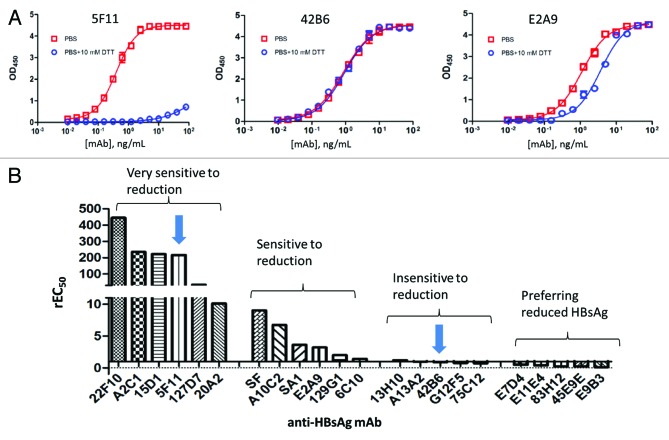
Figure 3. The binding profiles of 3 representative monoclonal antibodies to HBsAg and the different levels of sensitivity to DTT treatment for different mAbs. (A) The primary ELISA binding profiles are shown for 3 mAbs (5F11, 42B6 and E2A9) with distinctively different binding properties to fully reduced HBsAg as compared to the control. (B) These mAbs were divided 4 different groups based on their relative EC50 values (derived from four parameter logistic fits) reflecting the degree of change in HBsAg binding activity to due to disulfide bond reduction in HBsAg. rEC50 ≈1.0 would indicate a mAb being insensitive for DTT treatment. Any significant change in Relative EC50 from 1.0 would indicate a significant change in binding affinities, reflecting conformational changes on HBsAg, probed by different mAbs. Two arrows indicate the 2 different mAbs (i.e., mAb 5F11 from Group I and mAb 42B6 from Group III) chosen for developing assays for different HBsAg antigenicity analyses. The mAbs preferring reduced HBsAg (in the last group) showed slightly higher binding activity to DTT-treated HBsAg as compared with the untreated HBsAg, likely recognizing linear epitopes.
•Type I, Highly sensitive for DTT-treated HBsAg (rEC50, >10)
•Type II, Sensitive for DTT-treated HBsAg (rEC50, 1.3–10)
•Type III, Insensitive for DTT-treated HBsAg (rEC50, 0.7–1.3)
•Type IV, Preferring reduced HBsAg (rEC50, <0.7)
Among the 22 mAbs, there are 6 mAbs classified as Type I with 22F10 being the most sensitive to DTT treatment of HBsAg, 6 mAbs as Type II, 5 mAbs as Type III, and 5 mAbs as Type IV. (Table 1 and Fig. 3B) Based on the direct binding ELISA results, the degree of sensitivity to reduction treatment and the grouping based on their affinities, along with other characteristics were tabulated these mAbs (Table 1). Not surprisingly, the Type I mAbs, exhibiting highest level of sensitivity to disulfide reduction, were also shown to be highly sensitive to HBsAg conformation as most of mAbs in Type I did not show much bind activity to denatured dimers or monomers in Western Blot or strip immuno assay (Table 1). Representative mAbs from Type I and Type III were chosen to be analyzed further with respect to their antigenicity toward HBsAg using sandwich ELISA (see below). Type IV mAbs, preferring reduced HBsAg, likely recognize buried linear epitopes where reduction could have resulted in better epitope access.
Anti-HBsAg mAb neutralization activity
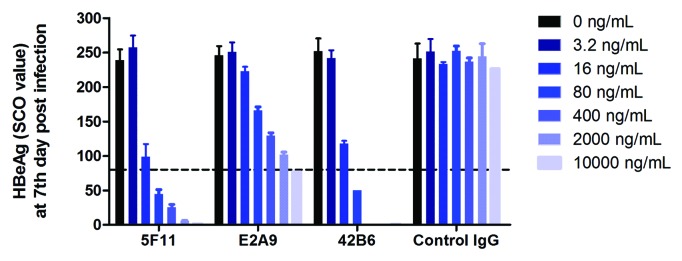
Recombinant HBV system in HepaRG cells was used to evaluate the mAb neutralization activity on HBV replication in cell culture. HBeAg was quantified in culture medium as the marker for in vitro HBV viral replication, as shown in Figure 4. The virus concentrations in cell culture, as reflected by HBV HBeAg levels, in mAb-treated groups were significantly lower as compared with the control group. The neutralizing effect is clearly antibody concentration dependent. Similar neutralizing activities was observed for 5F11 and 42B6 (IC50 ~16 ng/mL), where the E2A9 exhibited slightly lower neutralization activity (IC50 ~100 ng/mL). (Fig. 4) All of 3 epitopes, as probed by these mAbs, seem to be essential for HBV infection/replication and for maintaining the structure of HBsAg. Therefore, the presence of these epitopes on the HBsAg VLP surface is a good surrogate marker for the ability of an immunogen to elicit neutralizing antibodies.
Figure 4. HBV neutralizing activities of mouse anti-HBsAg mAbs evaluated using HBV replication system in HepaRG cells. Decreased virus concentrations, as indicated with HBeAg levels, were observed with increasing level of mAbs. Control mouse IgG showed no impact on the virus replication. The neutralizing activities of 5F11 and 42B6 were comparable (IC50 ~16 ng/mL), both having higher neutralization activity than E2A9. All of 3 epitopes, as recognized by these mAbs, are essential for HBV infection, and likely for maintaining the structure of HBsAg.
HBsAg antigenicity development as probed with mAb-based sandwich ELISAs
From Table 1, 4 Type I mAbs (22F10, A2C1, 15D1, 5F11) and 4 Type III mAbs (13H10, A13A2, 42B6, G12F5), all with high binding activity, were chosen to be analyzed during sandwich ELISA development. Not surprisingly, due to the multiplicity of epitopes (i.e., 48 dimers or 24 tetramers) on the 22 nm HBsAg VLP surface, the same mAb can be used as a detection Ab with the same capture Ab. It was the case for 42B6, when a nice binding curve was obtained for HBsAg antigenicity assay with the same mAb functioning as capture and detection Ab in the sandwich ELISA. Due to the nature of 42B6 epitope being insensitive to disulfide reduction, this assay, i.e., 42B6: HBsAg: 42B6-HRP, can be used for quantitation of HBsAg antigen, similar to a “mass ELISA.” In a different assay, still with 42B6 as capture Ab, the antigenicity was revealed by a disulfide-sensitive mAb 5F11. This assay is highly disulfide-dependent, making it a useful tool for tracking VLP epitope status in recombinant HBsAg VLP preparations.
Since the maturation phenomenon has been observed for freshly purified HBsAg,4 it would be of interests to ascertain how the epitopes develop on DTT-treated HBsAg during oxidative maturation as indicated by these 2 distinctively different ELISAs. Hence, with aliquots drawn at different time intervals, standard curves of 2 assays were used to determine the antigenicity development of reduced HBsAg during heat-induced oxidative maturation. As illustrated in Figure 5, disulfide-sensitive 42B6: HBsAg: 5F11-HRP assay showed an anticipated steady increase in antigenicity during heat treatment over time, whereas with the same sample set, the disulfide-insensitive 42B6: HBsAg: 42B6-HRP assay showed no change in antigenicity over time, as one might expect from a “mass-ELISA.” Moreover, cupric ion was shown to promote the oxidative maturation process, an expected phenomenon where disulfide bond formation or shuffling is occurring during the maturation process.
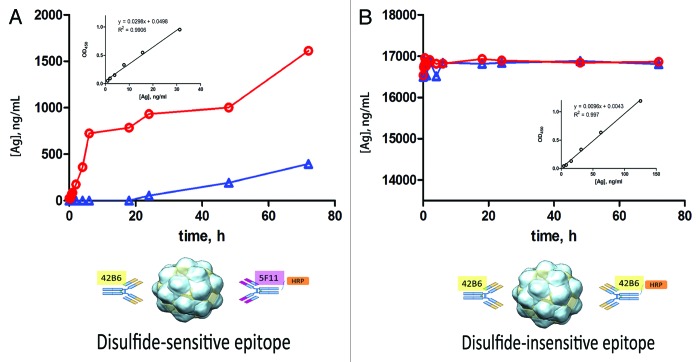
Figure 5. Maturation process of HBsAg (post DTT removal) at 37 °C as monitored with an “antigenicity ELISA” and a “mass-ELISA.” In the antigenicity assays, the HBsAg was captured using the disulfide insensitive 42B6 regardless of the disulfide status. Two different mAbs, same mAb 42B6 or disulfide sensitive 5F11, were employed as detection Ab in 2 different sandwich ELISAs to reveal the binding activity yielding unique information on different epiopes. (A) This assay (42B6: HBsAg: 5F11-HRP) is disulfide-dependent, detecting the HBsAg epitope development as a function oxidative maturation time. The red circles/line are the data obtained with facilitated maturation with cupric ion and the blue triangles or line are the data for the control (PBS only). Please note the expected promoting effect of cupric ion on the oxidative maturation of DTT-reduced HBsAg as indicated with the conformation-sensitive assays. (B) The other assay, with 42B6 on both sides (42B6: HBsAg: 42B6-HRP) is disulfide-independent. For this “mass-like” ELISA, it showed constant level during the whole oxidative maturation process. The standard curves used for antigenicity analysis were shown in the inset Figures in (A) and (B). The minimal detection level of HBsAg was for 2 assays shown in Figure (A) and (B) insets, ~0.98 ng/mL and ~3.9 ng/mL, respectively.
Discussion
Globally available HBV vaccines comprised of HBsAg antigens expressed with different host cells (Saccharomyces cerevisiae, Hansenula polymorpha, Chinese hamster ovary cell), in some cases showing different morphology (symmetrical octahedron, irregular octahedron).16 For marketed vaccines for routine immunization, monitoring vaccine safety and efficiency is essential during vaccine manufacturing. It is also of interests to perform comparative analysis on antigenicity and immunogenicity of vaccines from difference sources for comparison or possible replacement for vaccination. HBV vaccines from different sources are dosed on protein content as measured in a colorimetric assay (such as a Lowry or BCA method) at the aqueous bulk stage rather than on antigen content from an activity based immunoassay.
As a lipid protein particle, the HBsAg VLP stability depends on intermolecular cross-linking of HBsAg and trans-membrane helix-helix interactions and lipid-protein associations.17-19 The top of each S-protein tetramer forms a protrusion, where it displays the critical clinically relevant epitopes.5 The MHR projected out of the lipid layer surface is a tetramer of S-protein monomers, thus containing a total of 32 cysteine residues per protrusion.5 The epitopes on these surface protrusions, depending upon the proper disulfide bond pairings at the dimeric and tetrameric interface, are critical for HBsAg VLP as an immunogen to elicit functional and neutralizing titers against HBV infection. Therefore, the correct disulfide bond pairing could be critical for the structure and formation of virion-like epitopes on the surface of HBsAg VLPs. As reported recently, comparative immunization studies indicated that changes in the disulfide bonding pattern modulate the HBsAg VLP immunogenicity most likely due to structural changes.20
To probe changes in immune reactivity due to structural perturbation, this study took advantage of the disulfide–dependent nature of MHR conformation in HBsAg. By fully reducing all the disulfides with DTT during plate coating, the changes in mAb binding activity to surface immobilized HBsAg were assessed. Different levels of spiked DTT in the coating buffer resulted in different levels of activity loss probed by various mAbs in the subsequent binding assays (Figs. 2 and 3). Post-DTT removal assay time for Ag and Ab binding is just a couple of hours at ambient temperature, thus there is no discernible reoxidation of the reduced HBsAg which could take days to complete.12 Based on the binding data from DTT treatment study for various mAbs, a pair of sandwich ELISAs were proposed using 2 different anti-HBsAg mAbs (5F11 and 42B6) as detection Abs in sandwich ELISAs. These 2 different assays probe distinctly different binding properties of HBsAg VLPs (Fig. 5). A disulfide-sensitive mAb 5F11 was used to probe HBsAg VLP conformational changes, whereas the other mAb 42B6 being disulfide-insensitive was employed to monitor HBsAg antigen concentration or “antigen mass.” The antigenicity developed during heat treatment of HBsAg, as reported with 5F11, was similar to the antigenicity enhancement of HBsAg as probed by neutralizing mAb A1.2 or RF-1.5,12,13 The virus neutralization results showed both epitopes of 5F11 (disulfide-sensitive) and of 42B6 (disulfide-insensitive) were essential for HBV infection/replication. Therefore, this conformation-sensitive and neutralizing mAb, 5F11, could be used to monitor the epitope formation, vaccine potency, and particle stability during antigen bioprocessing and vaccine formulation and storage.
When HBsAg was initially developed as a vaccine in 1980s, mouse potency assay was used as release assay for vaccine potency. In vitro relative potency (IVRP) assays for hepatitis B vaccine, replacing animal based potency assays, were later developed for product characterization and lot release by various institutes or vaccine manufacturers (Table 2).21,22 The development of these binding based IVRPs is mainly driven by initiatives in reduction of animal use and the need for an assay with shorter turn-around time and better assay precision.13,22-25 Bead-based sandwich ELISA Auszyme kit made by Abbott Laboratories, once widely used, was discontinued around 2005. Both inhibition ELISA and sandwich ELISA were used by different manufacturers to develop replacement assays for HBV vaccine antigenicity analysis. Sandwich ELISA could be highly specific and reproducible,26 while inhibition ELISA maybe more sensitive to subtle conformational changes as it probes antigenicity in solution.21,27 Polyclonal antibodies may have renewability and sustainability issues, if they are used for release assays in the long run (Table 2). This problem is apparent as the quality analysis of vaccine potency is needed during the whole product life cycle.
Table 2. In vitro relative potency assays of Hepatitis B vaccine reported in the literature and proposed assays based on the results in this report3.
| Method | Key reagent | Information on epitopea |
Information on disulfide- dependent epitope | Comment | Country | Reference |
|---|---|---|---|---|---|---|
| Sandwich ELISAb (Bead-based) | mAbs | + | No | Discontinued in 2005 | USA | Auszyme kit. Schofield et al. 200222 |
| Inhibition ELISAc | pAb | - | No | One site assayd | Brazil | Cardoso et al. 200135 |
| Cuba | Cuervo et al. 200421 | |||||
| Cuba | Cuervo et al. 200827 | |||||
| Inhibition ELISAe | pAb and mAb | + | No | One site assayd | Belgium | Giffroy et al. 200636 |
| Sandwich ELISAf | pAb as capture Ab mAb as detection Ab | + | No | mAb affinity was determination | India | Shanmugham et al. 201037 |
| Sandwich ELISAg | mAbs as capture Ab pAb as detection Ab | - | No | Iran | Karimzadeh et al. 201038 | |
| Sandwich ELISAh | mAbs | + | No | Brazil | Costa, et al. 201126 | |
| Sandwich ELISAi | mAbs | Multifaceted, highly specific, and sensitive | China | This work | ||
| 5F11 as detection Ab | ++ | Yes | ||||
| 42B6 as detection Ab | + | No |
a The “+” represents that method aim at specific epitope on HBsAg, “-” otherwise. The “++” means against specific epitope, furthermore, monitoring the HBsAg conformational changes due to changes in disulfide bonds. bMethod was based on AuszymeTM kit, a bead based assay with anti-HBsAg and mAbs. (http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/ucm077519.pdf). cPlates were coated HBsAg polyclonal antibodies.The quantity of non-neutralized antibodies was then measured via HBsAg-HRP conjugated. dThe assays were potentially useful in monitoring the vaccine antigenicity on adjuvants. ePlated were coated HBsAg polyclonal antibodies, non-neutralized mAb was used compete Ab for the method, then the quantity of antibodies were measured via goat anti-human IgG antibodies labeled with peroxidase. fPlates were coated polyclonal guinea pig anti-HBsAg antibody and mouse anti-HBsAg antibody was for measuring HBsAg. gPlates coated 2 monoclonal antibodies and a polyclonal antibody conjugated with biotin was used for detect Ab. hTwo human monoclonal antibodies were used in this method, one was coated Ab and the other for detecting. iIn this paper, we proposed a pair of assays (sandwich conformational / sandwich linear) for monitoring our HBsAg. The assay has advantage of detecting conformational changes by an “antigenicity ELISA” with mAb which is highly sensitive to the disulfide bond status and quantifying HBsAg by a “mass ELISA” with mAb recognizing a linear epitope.
In this report, based on a comprehensive characterization data set on a panel of mAbs, 2 unique mAbs were chosen to be used in 2 different assays for vaccine potency/antigenicity determination, yielding non-overlapping and orthogonal information on 2 distinctly different epitopes (one linear and another conformational). Long-term supplies can be guaranteed for these mAbs (available upon request) produced from mouse hybridoma. The assay with 42B6 as a detection Ab yields information similar to a “mass ELISA” for vaccine antigen content. The assay with a conformation-sensitive and neutralizing mAb 5F11 as the revelation Ab functions like the IVRP assays (Table 2) reported in the field. Antigenicity analysis with 5F11 would be highly sensitive in detecting subtle conformational changes in HBsAg during manufacturing and storage. Multifaceted analysis on HBsAg VLP antigenicity with mAbs directed toward different epitopes could provide more informative data on routine or investigational analysis of vaccine products.
The maturation of HBsAg by disulfide bond formation and disulfide bond exchange was observed during the downstream purification process and under storage conditions via air oxidation.5,12,13 Cross-linking through intermolecular disulfide bonds is likely the molecular basis for causing certain conformational changes, making the epitopes more similar to those in native virions under the conditions where conformational optimization was allowed. As previously reported, cross-linking, likely coupled with conformational consolidation which can be probed with conformation-sensitive mAbs, is important for both antigenicity and immunogenicity of theses in vitro assembled VLPs.12,28-30 In early years of vaccine development, HBsAg VLP characterization was performed mainly with SDS-PAGE gel to show the degree of cross-linking, lacking information on 3-dimensional features of key epitopes.19 Now with different anti-HBsAg mAbs against various epitopes available, the proposed assays with mAbs of choice can be performed on process intermediates or final products, yielding quantitative data and orthogonal information on antigen content (“mass ELISA”) or integrity of clinically relevant epitopes (e.g., RF-1, or A1.2-like mAb binding activity, a surrogate marker for vaccine clinical efficacy).
When these 2 different sandwich ELISAs were run in parallel, different HBsAg VLP epitopes would be assessed in a quantitative manner. This novel approach with mAb-based assays for HBV vaccine provides quantitative analytical tools forantigen mass as well as epitope-specific antigenicity assays. Addition of these new analytical tools for process monitoring and product characterization upgrades the HBV vaccine analytical toolbox, better ensuring the process reproducibility and product consistency during the whole vaccine life cycle. Application of the proposed assays to reduced HBsAg confirmed the oxidative maturation phenomenon (Fig. 5), demonstrating the disulfide bond dependent nature of key epitopes, much similar to the results obtained with neutralizing mAbs RF-1 and A1.2.12,13,29 Distinctly different kinetic profiles of HBsAg maturation were obtained with 2 different assays, one (42B6: HBsAg: 5F11-HRP) being highly disulfide-sensitive and another (42B6: HBsAg: 42B6-HRP) being disulfide-insensitive. Epitope mapping of 42B6 and 5F11 were previously done with HBsAg variants with mutations in the MHR.15 The epitope for 42B6 is linear, which located at Cys137-Gly145. For the conformation-sensitive 5F11, its epitope is clearly discontinuous. Changes in amino acids in both loops (A loop, Ile110 to Thr123 and B loop, Cys138 to Cys147) in MHR were showed to impact the binding activity of 5F11. The following “naturally occurring” HBsAg variants, G119R, C124R, S136P, K141E, D144A, and G145R, showed more than 50% reduction in binding to 5F11 as compared with wild type HBsAg.15
These 2 assays with 2 different revealing mAbs, when performed in parallel, can serve as quantitative tools to establish the antigenicity profiles of multiple antigen lots. Such a database from these assays would help to ensure manufacturing consistency, to monitor product stability and to aid in the comparability exercise for a process upgrade or scaling up.4 Multifaceted analysis using a panel of mAbs for recombinant human papillomavirus vaccines has been performed, yielding most critical data sets for antigen characterization in the comparability package.4 More importantly, this approach paves the way for comparing the widely used HBV vaccine antigens made using different expression host and/or with different bioprocessing conditions.
In addition to analyzing HBsAg in solution, the same assay set could be used on recovered antigen from final vaccine products, as most HBV vaccines are adjuvanted with aluminum containing adjuvants. The proposed assays in this paper could be performed post-dissolution of adjuvants, releasing the HBsAg back into solution without any damage to antigen structure or antigenicity based on our own experience as well as published work.31 There were no discernible changes in HBsAg size and morphology for recovered HBsAg antigen after adjuvant dissolution, as observed with electron microscopy. Moreover, antigenicity analysis showed no difference between native and desorbed HBsAg for HBsAg-specific mAb RF-1, a conformation-sensitive and neutralizing mAb.31 Therefore, with proper dissolution treatments, vaccines from different manufacturers could conceivably be compared with respect to their epitope integrity by performing of the proposed assays after normalization of the protein concentration with the “mass-ELISA” as an additional control. While the proposed assays could provide new insights into the conformation status of the vaccine antigen by extending the analysis on process samples and on the final vaccine products, the assignment of the native-like disulfides in HBsAg was not addressed in this study. Such an assignment along with high resolution structure of HBsAg particle or even the MHR domain structure could help to better understand the structure activity relationship of this highly successful prophylactic vaccine introduced about 3 decades ago. Another caveat for this work is, while the neutralization activity of 5F11 has been confirmed, that correlation between antigenicity reported by 5F11 and immunogenicity has not been established. Extending the work to heat-stressed samples and vaccine products from various manufacturers and establishing the correlation between 5F11 binding activities to in vitro mouse potency are the subjects for future studies.
In summary, an in-depth characterization of a panel of 22 anti-HBsAg mAbs was performed particularly with respect to their sensitivity to the presence of disulfides. Two different in vitro antigenicity analysis methods were proposed to monitor 2 different HBsAg epitopes in parallel. This epitope-specific approach with unique information on epitope integrity provides an improved toolbox over earlier methods for more informative HBsAg vaccine characterization. It offers multifaceted assessment on epitopes in a quantitative way,32 enabling across comparison of recombinant HBsAg or final vaccine products undergone different bioprocessing conditions from different sources, with respect to their disulfide-dependent antigenicity of HBsAg.
Materials and Methods
Recombinant protein and monoclonal antibodies
Recombinant HBsAg proteins
The recombinant HBsAg protein was expressed in Chinese Hamster Ovary (CHO) cells, and purified as described previously (Wantai Biopharm).15,33 The samples (1 mg/mL) were applied to a carbon-coated copper grid. After 2 min, the excess liquid on the grid was removed by blotting with filter paper. The grid was then washed with water prior to staining with 2% phosphotungstic acid (pH 5.0) for 30 s and was dried for 2 min. The samples were then viewed using a JEM-2100HC (JEOL Ltd.) microscope with a side-mounted Morada camera at 120 KV. Electron micrographs of the samples were captured and visualized using iTEM software.
Monoclonal antibodies (mAbs)
A total of 22 anti-HBsAg mAb hybridomas were generated in house using standard mouse hybridoma technology. Certain characterization and specific binding analysis to HBsAg on these mAbs has been previously reported.15 These mAbs were produced using mouse ascites fluids and then affinity purified using Protein A chromatography (GE Healthcare). The concentration of the purified IgG was determined with optical density at 280 nm.
HBV neutralizing activity for mAbs
HepaRG cell line was used to assess the in vitro HBV neutralizing capacity of anti-HBsAg mAbs. The HepaRG cells were cultured as previously described in William’s E medium supplemented with 10% fetal calf serum, 100 unit/mL penicillin, 100μg/mL streptomycin, 5μg/mL insulin, and 5 × 10−7 M hydrocortisone hemisuccinate for 2 wk.14 For differentiation and infection, HepaRG cells were cultured for 2 wk in medium supplemented with 2% DMSO, prior to the HBV infection procedure. To test the mAb neutralization activity, HepAD38, which expresses HBV under the control of the inducible tetracycline Promoter 2 was used. About 1.4 × 108 copies of HBV from cultured supernatant were pre-incubated with a serial dilutions (10 000, 2000, 400, 80, 16, 3.2 ng/mL, and buffer without mAb) of each anti-HBsAg mAb or a control IgG for 1 h at 37 °C. Subsequently, the mixtures of mAbs and HepAD38 were added to HepaRG cells in medium with 4% (w/v) PEG 8000. After overnight incubation, the HepaRG cells were gently washed 3 times with medium and then cultured with fresh medium. At the seventh day post infection, HBeAg, a marker for HBV concentration was quantified with an HBeAg quantification kit (Wantai Biopharm).34 Signal-to-cut-off ratio from the ELISA assay was calculated to indicate the viral concentration in each well.
mAb binding activity to HBsAg with or without disulfide reduction
Direct binding ELISA on antigen-coated plates was employed to assess the median effective concentration (EC50) of mAbs in solution with half-maximal binding against the surface-immobilized recombinant HBsAg, with or without a strong reductant, dithiothreitol (DTT), during plate coating. After plate blocking, plates were incubated at 37 °C for 60 min with 100 μL 2-fold serial dilutions (starting at 80 ng/mL mAb) of 22 different mAbs using assay diluent. Following 5 wash cycles using the standardized wash-buffer PBS–0.5% (w/v) Tween 20 (PBS-T), secondary antibody goat anti-mouse IgG horseradish peroxidase (HRP) conjugate (DAKO, Glostrup, Denmark) (diluted 1:5000 in assay diluent) was added in plates. After incubated at 37 °C for 45 min, the plates were washed 5 times with PBS-T. Subsequently, 100 μL per well tetramethylbenzidine substrate solution (Wantai Biopharm) was added and incubated for 10 min. The reaction was stopped by adding 50 μL per well 2.0 M sulfuric acid. Absorbance in each well was determined at 450 nm with that at 620 nm as a background.
Sandwich ELISAs for HBsAg antigenicity analysis
Sandwich ELISA, with mAbs as capture and detection Abs, was used to determine the immuno-reactivity of HBsAg preparations. The define value is the median effective concentration (EC50) of antigen (HBsAg or DTT-treated HBsAg) in solution with half-maximal binding. Four different mAbs (13H10, A13A2, 42B6, G12F5) were screened as coating Ab in the development of sandwich ELISAs for HBsAg antigenicity analysis. After plate coating and plate blocking, the plates were incubated with 100 μL per well (starting at 1000 ng/mL) antigen HBsAg or DTT-treated HBsAg (after DTT removal via dialysis) at 37 °C for 2 h. Following 5 washes using the wash buffer PBS-T, HRP-conjugated detection mAbs (diluted 1:1000 in assay diluent) were added and the plates were incubated for 30 min at 37 °C. The plates were washed 5 times with PBS-T, 100 μL per well of tetramethylbenzidine substrate solution was added and incubated for 15 min prior to quenching with 50 μL per well of 2.0 M sulfuric acid. The intensity of the signals in each well was read at 450 nm using a 96-well micro-plate reader (Autobio Labtec).
HBsAg maturation as monitored with sandwich ELISAs
HBsAg solution was treated by 10 mM DTT at room temperature for 30 min to reduce all the disulfide bonds in HBsAg. The mixture was then extensively dialyzed in PBS or PBS containing 50μM CuCl2 at 4 °C. Spontaneous HBsAg maturation induced by heat treatment was followed for 72 h at 37 °C, in a similar way with previously reported experiments.12,13 Samples were taken at different time points: 0, 1/6, 1/3, 1/2, 3/4, 1, 2, 4, 6, 18, 24, 48, 72 (unit: h) for antigenicity analysis. Aliquots of 100 μL were drawn at each time interval and then rapidly frozen in liquid nitrogen. These samples were stored at –20 °C until analysis. The samples were analyzed with 2 sandwich ELISAs in parallel in one batch for better consistency for antigenicity of HBsAg samples from different time intervals. The antigen concentration of HBsAg, as indicated in each assay, was derived from a corresponding standard curve. To assess the antigenicity in an epitope specific way, different detection Abs were used to reveal different epitope status. For consistency in the capture step, 42B6 was used as a coating Ab in both assays with either, HRP-labeled 42B6 or HRP-labeled 5F11 were used as detection Abs.
Curve-fitting and statistical analysis
Curve-fitting using 4-parameter logistic fit of the ELISA data from direct binding ELISA or sandwich ELISA assays to derive EC50 values, or for antigen concentration determination using standard curve, was performed with GraphPad Prism (GraphPad Software). The standard deviation in replicate EC50 values was calculated using Excel (Microsoft).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Professor Zhiqiang An of University of Texas at Houston and Professor Patricia Wouters of Xiamen University/University of Dundee for critical reading and helpful comments on the manuscript. Support from China Ministry of Science and Technology via Major Project (2012AA02A408, 2013ZX10002002 and 2012ZX10002005) and National Science Foundation of China (30925030, 81273327, and 81371819) was acknowledged.
References
- 1.Hilleman MR. . Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine 2001; 19:1837 - 48; http://dx.doi.org/ 10.1016/S0264-410X(00)00364-9; PMID: 11228353 [DOI] [PubMed] [Google Scholar]
- 2.Hilleman MR. . Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine 2003; 21:4626 - 49; http://dx.doi.org/ 10.1016/S0264-410X(03)00529-2; PMID: 14585670 [DOI] [PubMed] [Google Scholar]
- 3.Kushnir N, Streatfield SJ, Yusibov V. . Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 2012; 31:58 - 83; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.083; PMID: 23142589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Q, Li S, Yu H, Xia N, Modis Y. . Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol 2013; 31:654 - 63; http://dx.doi.org/ 10.1016/j.tibtech.2013.09.002; PMID: 24125746 [DOI] [PubMed] [Google Scholar]
- 5.Mulder AM, Carragher B, Towne V, Meng Y, Wang Y, Dieter L, Potter CS, Washabaugh MW, Sitrin RD, Zhao Q. . Toolbox for non-intrusive structural and functional analysis of recombinant VLP based vaccines: a case study with hepatitis B vaccine. PLoS One 2012; 7:e33235; http://dx.doi.org/ 10.1371/journal.pone.0033235; PMID: 22493667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert RJ, Beales L, Blond D, Simon MN, Lin BY, Chisari FV, Stuart DI, Rowlands DJ. . Hepatitis B small surface antigen particles are octahedral. Proc Natl Acad Sci U S A 2005; 102:14783 - 8; http://dx.doi.org/ 10.1073/pnas.0505062102; PMID: 16203986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. . Vaccine-induced escape mutant of hepatitis B virus. Lancet 1990; 336:325 - 9; http://dx.doi.org/ 10.1016/0140-6736(90)91874-A; PMID: 1697396 [DOI] [PubMed] [Google Scholar]
- 8.Mangold CMT, Streeck RE. . Mutational analysis of the cysteine residues in the hepatitis B virus small envelope protein. J Virol 1993; 67:4588 - 97; PMID: 8392600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abou-Jaoudé G, Sureau C. . Entry of hepatitis delta virus requires the conserved cysteine residues of the hepatitis B virus envelope protein antigenic loop and is blocked by inhibitors of thiol-disulfide exchange. J Virol 2007; 81:13057 - 66; http://dx.doi.org/ 10.1128/JVI.01495-07; PMID: 17898062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisse J, Sureau C. . A function essential to viral entry underlies the hepatitis B virus “a” determinant. J Virol 2009; 83:9321 - 8; http://dx.doi.org/ 10.1128/JVI.00678-09; PMID: 19570861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YC, Delbrook K, Dealwis C, Mimms L, Mushahwar IK, Mandecki W. . Discontinuous epitopes of hepatitis B surface antigen derived from a filamentous phage peptide library. Proc Natl Acad Sci U S A 1996; 93:1997 - 2001; http://dx.doi.org/ 10.1073/pnas.93.5.1997; PMID: 8700874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Wang Y, Abraham D, Towne V, Kennedy R, Sitrin RD. . Real time monitoring of antigenicity development of HBsAg virus-like particles (VLPs) during heat- and redox-treatment. Biochem Biophys Res Commun 2011; 408:447 - 53; http://dx.doi.org/ 10.1016/j.bbrc.2011.04.048; PMID: 21527246 [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q, Towne V, Brown M, Wang Y, Abraham D, Oswald CB, Gimenez JA, Washabaugh MW, Kennedy R, Sitrin RD. . In-depth process understanding of RECOMBIVAX HB® maturation and potential epitope improvements with redox treatment: multifaceted biochemical and immunochemical characterization. Vaccine 2011; 29:7936 - 41; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.070; PMID: 21871939 [DOI] [PubMed] [Google Scholar]
- 14.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. . Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 2002; 99:15655 - 60; http://dx.doi.org/ 10.1073/pnas.232137699; PMID: 12432097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CH, Yuan Q, Chen PJ, Zhang YL, Chen CR, Zheng QB, Yeh SH, Yu H, Xue Y, Chen YX, et al. . Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J Hepatol 2012; 57:720 - 9; http://dx.doi.org/ 10.1016/j.jhep.2012.05.009; PMID: 22634131 [DOI] [PubMed] [Google Scholar]
- 16.Chuang L, Tingyu Z, Jin L, Zhao W, Rui M, Xi W, Changcheng Y. . Structure analysis of HBsAg produced by recombinant yeast cells Hansenula polymorpha. Chinese Medicinal Biotechnology 2009; 4:441 - 4 [Google Scholar]
- 17.Milhiet PE, Dosset P, Godefroy C, Le Grimellec C, Guigner JM, Larquet E, Ronzon F, Manin C. . Nanoscale topography of hepatitis B antigen particles by atomic force microscopy. Biochimie 2011; 93:254 - 9; http://dx.doi.org/ 10.1016/j.biochi.2010.09.018; PMID: 20887766 [DOI] [PubMed] [Google Scholar]
- 18.Greiner VJ, Egelé C, Oncul S, Ronzon F, Manin C, Klymchenko A, Mély Y. . Characterization of the lipid and protein organization in HBsAg viral particles by steady-state and time-resolved fluorescence spectroscopy. Biochimie 2010; 92:994 - 1002; http://dx.doi.org/ 10.1016/j.biochi.2010.04.014; PMID: 20420879 [DOI] [PubMed] [Google Scholar]
- 19.Wampler DE, Lehman ED, Boger J, McAleer WJ, Scolnick EM. . Multiple chemical forms of hepatitis B surface antigen produced in yeast. Proc Natl Acad Sci U S A 1985; 82:6830 - 4; http://dx.doi.org/ 10.1073/pnas.82.20.6830; PMID: 2931722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong WS, Hyakumura M, Yuen L, Warner N, Locarnini S, Netter HJ. . Modulation of the immunogenicity of virus-like particles composed of mutant hepatitis B virus envelope subunits. Antiviral Res 2012; 93:209 - 18; http://dx.doi.org/ 10.1016/j.antiviral.2011.11.011; PMID: 22138713 [DOI] [PubMed] [Google Scholar]
- 21.Cuervo MLC, de Castro Yanes AF. . Comparison between in vitro potency tests for Cuban hepatitis B vaccine: contribution to the standardization process. Biologicals 2004; 32:171 - 6; http://dx.doi.org/ 10.1016/j.biologicals.2004.03.003; PMID: 15572098 [DOI] [PubMed] [Google Scholar]
- 22.Schofield T. In vitro versus in vivo concordance: A case study of the replacement of an animal potency test with an immunochemical assay. Dev Biol (Basel, Karger) 2002; 111:299-304. [PubMed] [Google Scholar]
- 23.Shank-Retzlaff M, Wang F, Morley T, Anderson C, Hamm M, Brown M, Rowland K, Pancari G, Zorman J, Lowe R, et al. . Correlation between mouse potency and in vitro relative potency for human papillomavirus Type 16 virus-like particles and Gardasil vaccine samples. Hum Vaccin 2005; 1:191 - 7; http://dx.doi.org/ 10.4161/hv.1.5.2126; PMID: 17012876 [DOI] [PubMed] [Google Scholar]
- 24.Metz B, Hendriksen CF, Jiskoot W, Kersten GF. . Reduction of animal use in human vaccine quality control: opportunities and problems. Vaccine 2002; 20:2411 - 30; http://dx.doi.org/ 10.1016/S0264-410X(02)00192-5; PMID: 12057596 [DOI] [PubMed] [Google Scholar]
- 25.Metz B, Brunel F, Chamberlin C, van der Gun J, Halder M, Jiskoot W, Kersten G, van Opstal O, Petersen JW, Ravetkar SD, et al. . The potential of physicochemical and immunochemical assays to replace animal tests in the quality control of toxoid vaccines. The report and recommendations of ECVAM workshop 61. Altern Lab Anim 2007; 35:323 - 31; PMID: 17650951 [DOI] [PubMed] [Google Scholar]
- 26.Costa CI, Delgado IF, da Costa JAC, de Carvalho RF, Mouta SdaS Jr., Vianna CO, de Moraes MT. . Establishment and validation of an ELISA for the quantitation of HBsAg in recombinant hepatitis B vaccines. J Virol Methods 2011; 172:32 - 7; http://dx.doi.org/ 10.1016/j.jviromet.2010.12.010; PMID: 21185330 [DOI] [PubMed] [Google Scholar]
- 27.Cuervo ML, Sterling AL, Nicot IA, Rodríguez MG, García OR. . Validation of a new alternative for determining in vitro potency in vaccines containing Hepatitis B from two different manufacturers. Biologicals 2008; 36:375 - 82; http://dx.doi.org/ 10.1016/j.biologicals.2008.06.005; PMID: 18693036 [DOI] [PubMed] [Google Scholar]
- 28.Mangold CM, Unckell F, Werr M, Streeck RE. . Analysis of intermolecular disulfide bonds and free sulfhydryl groups in hepatitis B surface antigen particles. Arch Virol 1997; 142:2257 - 67; http://dx.doi.org/ 10.1007/s007050050240; PMID: 9672591 [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q, Wang Y, Freed D, Fu TM, Gimenez JA, Sitrin RD, Washabaugh MW. . Maturation of recombinant hepatitis B virus surface antigen particles. Hum Vaccin 2006; 2:174 - 80; http://dx.doi.org/ 10.4161/hv.2.4.3015; PMID: 17012892 [DOI] [PubMed] [Google Scholar]
- 30.Sitrin R, Wample D. D, E. & Ellis, R, D. Survey of licensed hepatitis B vaccines and their production processes. In: Ellis RW, ed. Hepatitis B Vaccines in Clinical Practice, 1993:83-102. [Google Scholar]
- 31.Greiner VJ, Ronzon F, Larquet E, Desbat B, Estèves C, Bonvin J, Gréco F, Manin C, Klymchenko AS, Mély Y. . The structure of HBsAg particles is not modified upon their adsorption on aluminium hydroxide gel. Vaccine 2012; 30:5240 - 5; http://dx.doi.org/ 10.1016/j.vaccine.2012.05.082; PMID: 22705175 [DOI] [PubMed] [Google Scholar]
- 32.Towne V, Zhao Q, Brown M, Finnefrock AC. . Pairwise antibody footprinting using surface plasmon resonance technology to characterize human papillomavirus type 16 virus-like particles with direct anti-HPV antibody immobilization. J Immunol Methods 2013; 388:1 - 7; http://dx.doi.org/ 10.1016/j.jim.2012.11.005; PMID: 23159495 [DOI] [PubMed] [Google Scholar]
- 33.Yuan Q, Ge S, Xiong J, Yan Q, Li Z, Hao X, Tian D, Niu J, Su Z, Chen C, et al. . A novel immunoassay for PreS1 and/or core-related antigens for detection of HBsAg variants. J Virol Methods 2010; 168:108 - 13; http://dx.doi.org/ 10.1016/j.jviromet.2010.04.029; PMID: 20451558 [DOI] [PubMed] [Google Scholar]
- 34.Tajiri K, Ozawa T, Jin A, Tokimitsu Y, Minemura M, Kishi H, Sugiyama T, Muraguchi A. . Analysis of the epitope and neutralizing capacity of human monoclonal antibodies induced by hepatitis B vaccine. Antiviral Res 2010; 87:40 - 9; http://dx.doi.org/ 10.1016/j.antiviral.2010.04.006; PMID: 20412816 [DOI] [PubMed] [Google Scholar]
- 35.Cardoso OR, Llera GG, López MI, Ezquivel MQ, Marcelo JL. . Ensayo de potencia in vitro para la vacuna cubana recombinante contra la hepatitis B. Biotecnol Apl 2001; 18:154 - 8 [Google Scholar]
- 36.Giffroy D, Mazy C, Duchêne M. . Validation of a new ELISA method for in vitro potency assay of hepatitis B-containing vaccines. Pharmeuropa Bio 2006; 2006:7 - 14; PMID: 17270127 [PubMed] [Google Scholar]
- 37.Nimmagadda SV, Aavula SM, Biradhar N, Rao VS, Shanmugham R, Chandran D, Thirumeni N, Singanallur NB, Villuppanoor SA. . Recombinant diabody-based immunocapture enzyme-linked immunosorbent assay for quantification of rabies virus glycoprotein. Clin Vaccine Immunol 2010; 17:1261 - 8; http://dx.doi.org/ 10.1128/CVI.00204-10; PMID: 20573881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karimzadeh H, Ajdary S, Jazayeri SM, Pakzad SR. . Validation of an in-vitro method for Hepatitis B vaccine potency assay: specification setting. Panminerva Med 2010; 52:177 - 82; PMID: 21045773 [PubMed] [Google Scholar]