Abstract
Multi-antigen immunotherapy approaches against Staphylococcus aureus are expected to have the best chance of clinical success when used in combinatorial therapy, potentially incorporating opsonic killing of bacteria and toxin neutralization. We recently reported the development of a murine monoclonal antibody specific for the immunodominant staphylococcal antigen A (IsaA), which showed highly efficient staphylococcal killing in experimental infection models of S. aureus. If IsaA-specific antibodies are to be used as a component of combination therapy in humans, the binding specificity and biological activity of the humanized variant must be preserved. Here, we describe the functional characterization of a humanized monoclonal IgG1 variant designated, hUK-66. The humanized antibody showed comparable binding kinetics to those of its murine parent, and recognized the target antigen IsaA on the surface of clinically relevant S. aureus lineages. Furthermore, hUK-66 enhances the killing of S. aureus in whole blood (a physiological environment) samples from healthy subjects and patients prone to staphylococcal infections such as diabetes and dialysis patients, and patients with generalized artery occlusive disease indicating no interference with already present natural antibodies. Taken together, these data indicate that hUK-66 mediates bacterial killing even in high risk patients and thus, could play a role for immunotherapy strategies to combat severe S. aureus infections.
Keywords: Staphylococcus aureus, immunotherapy, immunotherapeutics, monoclonal antibodies, humanization, opsonophagocytosis, bacterial killing, fc-receptors, vaccinology, MRSA
Introduction
Staphylococcus aureus causes severe hospital- and community-associated infections and its resistance against multiple classes of antibiotics leads primarily to high therapeutic failure rates.1 Several resistant strains are now endemic in hospitals around the world causing, for example, an estimated 1.5 million cases of pneumonia per year.2,3 Therefore, various immunotherapeutic approaches have been mooted as potential solutions to inadequate treatment of invasive infections using antibiotics.4 During the last decade, various active and passive immunization strategies have been examined in clinical trials and in preclinical animal models. Despite promising experimental data, clinical trials in humans have not yielded positive results.5 This has prompted a general discussion about whether immunotherapeutic strategies have limited efficacy for treating or preventing S. aureus infections. The main defense against microbes is our dermal and mucosal barriers. In case that microbes cross these natural border, the importance of immune clearance is underscored by the fact that humans with intact immune systems either do not suffer from staphylococcal infections or combat them successfully. Furthermore, recent studies provide evidence supporting the role of protective antibodies; these studies show that patients with higher levels of antibodies against different S. aureus antigens including extracellular toxins have a lower risk of developing invasive infections.6,7 However, antibodies generated from carriage and not during disease, are often not functional or protective. There are 3 major issues regarding the successful development of immunotherapeutics against S. aureus that need to be consistently addressed. First, the selected target must be expressed by all relevant S. aureus strains that infect humans. Second, antibodies must have proven anti-staphylococcal activity and block important cellular functions or essential virulence mechanisms relevant to the pathogen (a combination of different functional antibodies).8 Third, the targeted patient population, including immunocompromised patients, must have the ability to benefit from immunotherapy.
Most recently, we described the monoclonal IgG1 mouse antibody UK-66P which is specific for the immunodominant antigen A (IsaA) of S. aureus and demonstrated its therapeutic efficacy (opsonization and killing of bacteria) in 2 mouse models.9 We selected IsaA, a supposed lytic transglycosylase, as the target for antibody-based therapy, because all analyzed patients surviving staphylococcal sepsis produced significant levels of IsaA-specific antibodies.10 Moreover, IsaA is expressed on the surface of S. aureus, and is evolutionary highly conserved.11 We, and others, believe that humans do not generate sufficient baseline levels of functional opsonophagocytic antibodies against S. aureus; however, infected individuals mount a rapid functional immune response if required.5 This concept is strongly supported by recent studies of the humoral immune response to IsaA in patients with bacteremia.12 Based on these observations, we choose the antibody UK-66P as the basis for a humanized variant, hUK-66, as a prerequisite to further clinical development for therapeutic application in humans.
Here, we report the successful humanization and subsequent functional characterization of hUK-66, which showed binding specificity and biological activity similar to that of the parent antibody. The antibody hUK-66 induced significant killing activity when tested alongside functional immune cells derived from healthy subjects and from potential at risk patients, such as those with diabetes, end-stage renal disease, or artery occlusive disease (AOD). The results clearly show that this humanized variant of a murine anti-IsaA antibody represents a promising component for an immunotherapeutic approach to the treatment of S. aureus infections.
Results
Humanization of the mouse UK-66 IgG1 antibody
Our previous study used an intravenous catheter-associated mouse model and a mouse bacteremia model to demonstrate the therapeutic efficacy of the monoclonal IgG1 mouse antibody UK-66P.9 These encouraging results prompted us to select UK-66P for humanization as a prerequisite for further clinical development as a component for passive immunotherapeutic approach to treating S. aureus infections. Based on the mouse VH and VL sequences, the UK-66 antigen binding site was humanized by grafting the CDRs onto human frameworks obtained from the closest human germline V segments.
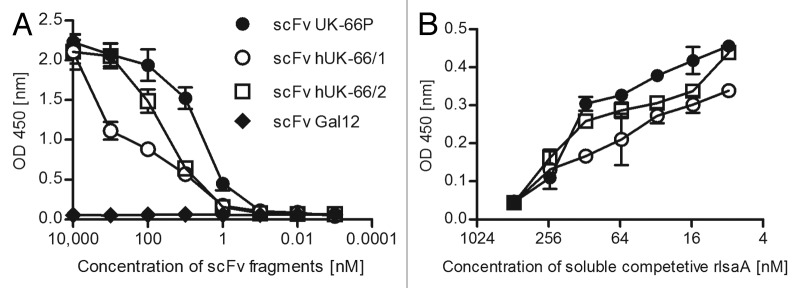
To determine the binding characteristics of the humanized variant, the VH and VL domains were assembled into a scFv, which was produced in E. coli TG1 cells. Binding assays revealed that both the recombinant murine and both humanized scFv UK-66 fragments recognized recombinant IsaA (rIsaA) in a specific and concentration-dependent manner (Fig. 1). hUK-66 variant 2 showed a higher binding affinity for rIsaA (KD = 8 nm) than variant 1 (KD = 80 nm). This binding affinitiy was similar to that of the parent mouse scFv (Fig. 1B), confirming the conservation of binding activity after humanization. Based on these results, we chose hUK-66 variant 2 as starting point for generating full-length IgG (κ) antibodies.
Figure 1. Binding affinities of scFv hUK-66 fragments after humanization analyzed by comparative ELISA studies. (A) Affinity of scFv hUK-66 fragments against rIsaA were analyzed by ELISA studies. The scFv fragment against Gal12 served as control. Binding of scFv fragments were detected by HRP-conjugated anti-Myc antibody. (B) Specificity of scFv UK-66 fragments against rIsaA was determined by competitive ELISA studies using HRP-conjugated anti-Myc antibody. Presented data illustrate one representative experiment done in triplicate.
hUK-66 IgG1 shows superior biological activity
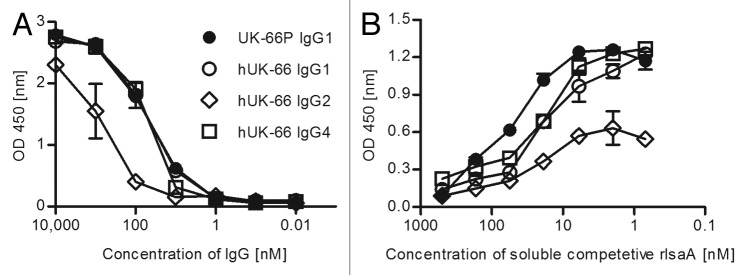
To assess the effector functions of the humanized antibody, including antibody-dependent killing of S. aureus, we constructed different hUK-66 isotypes: IgG1, IgG2, and IgG4. First, the binding properties of purified hUK-66 IgGs to rIsaA were studied in a competitive ELISA (Fig. 2A and B). As result, hUK-66 isotypes IgG1 and IgG4 showed stronger binding to rIsaA than hUK-66 IgG2 isotype (IgG1 = IgG4 > IgG2). Next, we examined the bactericidal activity of the full-length hUK-66 IgG isotypes. In our preliminary studies, human polymorphonuclear neutrophils (PMNs) of donors were purified and incubated with bacteria and antibody. Data of these studies clearly demonstrate that the presence of hUK-66 increases killing (data not shown). Based on this result, we assumed the same effect in whole blood assays. Furthermore, binding studies with FcγRIa, FcγRIIa (high responder), and FcγRIIa (low responder) revealed that hUK-66 IgG1 is recognized by these receptors (see below). Human whole blood from healthy donors was incubated with S. aureus Newman in the presence of hUK-66 IgG1, IgG2, or IgG4 for 60 min at 37°. After lysing eukaryotic cells, the amount of viable S. aureus was determined and the results presented as the mean percentages with standard deviation (SD) in parenthesis relative to that in the untreated bacterial samples (which were set at 100%). Figure 3 shows that addition of hUK-66 IgG1 led to a significant reduction in the amount of viable of S. aureus when compared with that in the untreated controls (44% [10.8%]; P < 0.05, t test). The addition of hUK-66 IgG4 resulted in a moderate decrease of viable bacteria (15% [6.2%]) compared with that in the control samples, whereas addition of the IgG2 variant actually led to a slight increase in the proportion of viable bacteria (12.3% [4%]) compared with that in controls. Taken together, these results indicate that hUK-66 IgG1 showed the highest bactericidal activity. This isotype was, therefore, used in all subsequent experiments.
Figure 2. Comparative binding studies of complete humanized UK-66 IgG isotypes against rIsaA. (A) ELISA plates were coated with 10 µg/ ml rIsaA and incubated with the indicated full humanized UK-66 IgG isotypes. Binding of isotypes were determined by isotype specific, HRP-conjugated anti human IgG antibodies. (B) Specificity of hUK-66 IgGs was analyzed by competitive ELISA. Binding of UK-66 IgGs to coated rIsaA was analyzed by isotype specific, HRP-conjugated anti-human IgG antibodies. Presented data illustrate one representative experiment done in triplicate.
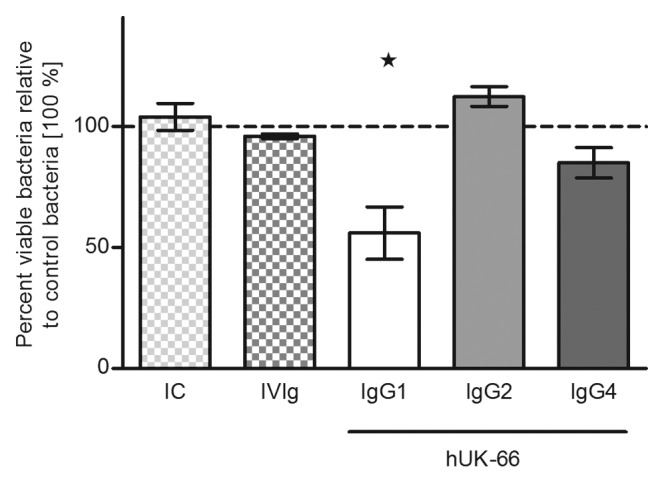
Figure 3. Impact of immunoglobulins isotype structure on hUK-66-dependent staphylococcal killing. Heparinized blood samples from healthy donors were infected with S. aureus Newman and either humanized UK-66 IgG1, IgG2, or IgG4 for 60 min at 37°. The monoclonal antibody Trastuzumab served as matched IgG1 isotype control (IC) and the intravenous immunoglobulin mixture Gammunex® (IVIg) as a second positive control. Viable bacteria were recovered after whole eukaryotic cell lysis and enumerated by plate counting. The values were expressed as mean percentage with standard deviation (SD) in parenthesis relative to the level of untreated blood/bacterial samples (set at 100%). Data represent three independent experiments from three different donors. The dashed line in the graphs demonstrates the 100% reference value of control bacteria samples. *P > 0.05, t test.
Humanization of UK-66 IgG1 does not affect its binding characteristics
SPR was used to compare the affinities of mouse UK-66P (the parental antibody) and hUK-66 IgG1. Affinity constants were obtained by calculating the ratio of the respective association and dissociation constants (Fig. 4). The equilibrium dissociation constant (KD) for hUK-66 was 4.8 nM and that for murine UK-66P was 1.7 nM.9 The association (ka) and dissociation (kd) rate constants for the interaction between hUK-66 and rIsaA were 3.7 × 105 M−1s−1 and 1.8 × 10−3 s−1, respectively, whereas those for murine UK-66P were 1.8 × 105 M−1s−1 and 3.1 × 10−4 s−1, respectively. These results show that hUK-66 IgG1 binds specifically to rIsaA with high affinity (rapid association and relatively slow dissociation), and confirming, that the hUK-66 antibody retains its high affinity for IsaA after humanization.
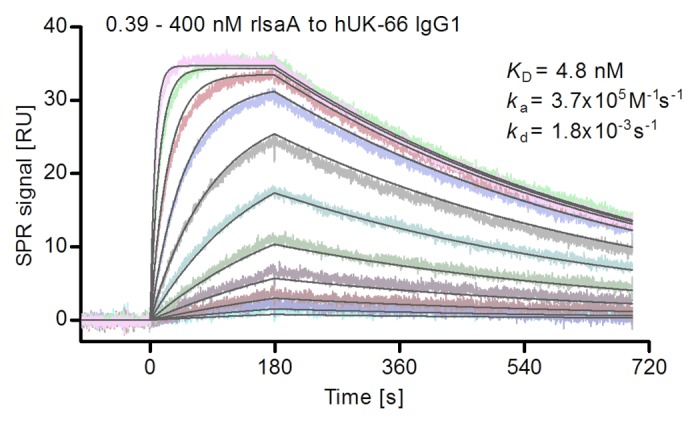
Figure 4. Quantitative analysis of the interaction of hUK-66 IgG1 with rIsaA performed using surface plasmon resonance (SPR). Various concentrations of rIsaA (0.39 to 400 nM) were flushed over the antibody hUK-66 IgG1, immobilized on the sensor chip surface. Sensorgrams were recorded at a flow rate of 30 µl/ min at 25 °C. From these Sensorgrams, an equilibrium dissociation constant (KD) of 4.8 nM was determined. Rate constants for association (ka) and dissociation (kd) were determined to be 3.7 × 105 M−1s−1 and 1.8x10−3 s−1, respectively.
hUK-66 IgG1 recognizes IsaA expressed by clinically relevant strains of S. aureus
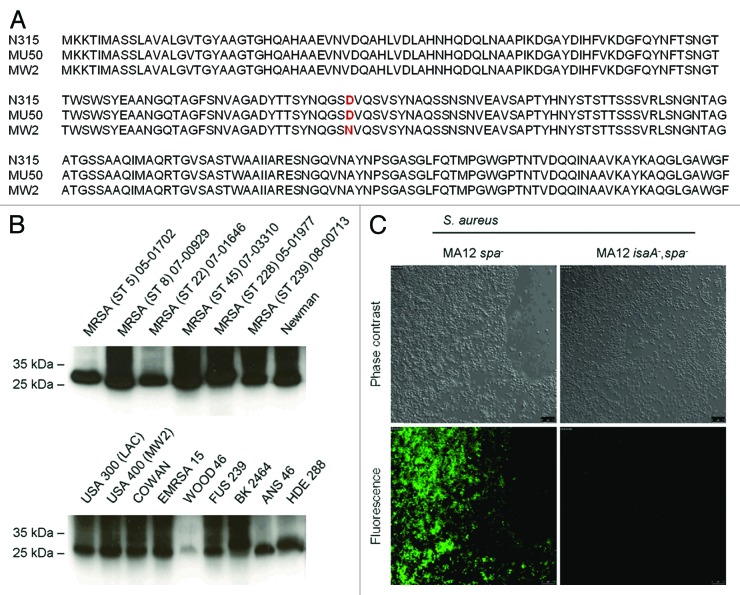
Target expression in vivo is critical for effective anti-staphylococcal immunotherapy. IsaA was originally identified as immunogenic component by screening patient sera suffering from sepsis for seroconversion confirming its in vivo expression.10 The anti-IsaA antibody response of patients was significantly increased during sepsis compared with anti-IsaA IgG titer of the same patients before onset of S. aureus disease.10 Comparison of genomic data of IsaA (SA2356) by using KEGG and UniProt databases demonstrate high homology between IsaA sequences of different S. aureus strains. There are 230 S. aureus strains in the database which have 100% identical IsaA sequences (www.uniprot.org/uniprot/P99160). The most abundant point mutation is an amino acid exchange of D111 to N111 (Fig. 5A). However, immunoblot analysis demonstrates that hUK-66 recognizes this variation of IsaA (Fig. 5B). Even five point mutations in IsaA sequence compared with the reference IsaA sequence of strain N315 (SA2356) have no effect on antibody binding by hUK-66 IgG1. Moreover, we confirmed binding of hUK-66 IgG1 to native IsaA expressed by S. aureus by immunoblot analysis using clinically relevant S. aureus strains, including both MRSA and MSSA.13 hUK-66 recognized the 29 kDa IsaA antigen from all strains tested independently from multi-locus sequence type (ST) and resistance background (Fig. 5B). Immunofluorescence analysis was performed to examine binding of hUK-66 IgG1 to IsaA expressed on the cell surface of viable bacteria. For these experiments we used the strains MA12∆spa and MA12∆spaΔisaA to exclude non-specific interaction between the monoclonal antibody with staphylococcal protein A. Viable MA12∆spa cells showed an IsaA-dependent fluorescence signal, but MA12∆spaΔisaA cells did not (Fig. 5C). Thus, hUK-66 IgG1 is specific for IsaA expressed on the bacterial cell surface without protein A cross-reaction. Finally, in vitro expression of IsaA was analyzed by ELISA studies confirming the binding of hUK-66 to IsaA (Fig. S1).
Figure 5. IsaA conservation and expression by different clinically relevant S. aureus strains. (A) Alignment of IsaA sequence of S. aureus strain N315, MW2, and MU 50 (B) Immunoblot analysis of IsaA in cell pellets of different clinically relevant strains. (C) Specific binding of IsaA by hUK-66 IgG1 was performed with S. aureus MA12 spa- and its isogenic isa- mutant by indirect immunofluorescence studies.
hUK-66 IgG1 induces a respiratory burst by activated neutrophils
We previously showed that anti-IsaA antibodies induce the antibody-dependent killing of S. aureus by triggering an increase in the respiratory burst produced by activated neutrophils.9 Therefore, we next tested the ability of hUK-66 IgG1 to induce a respiratory burst by activated neutrophils, the key immunological effector cells involved in staphylococcal phagocytosis. We examined neutrophils in blood samples from patients with end-stage renal disease (ESRD), diabetes, or AOD, as such patients show increased susceptibility to staphylococcal infections due to an impairment of innate immunity.14-17 Blood samples were infected with S. aureus Newman for 20 min and the percentage of neutrophils that generated an oxidative burst in the presence or absence of hUK-66 IgG1 was measured by flow cytometry.
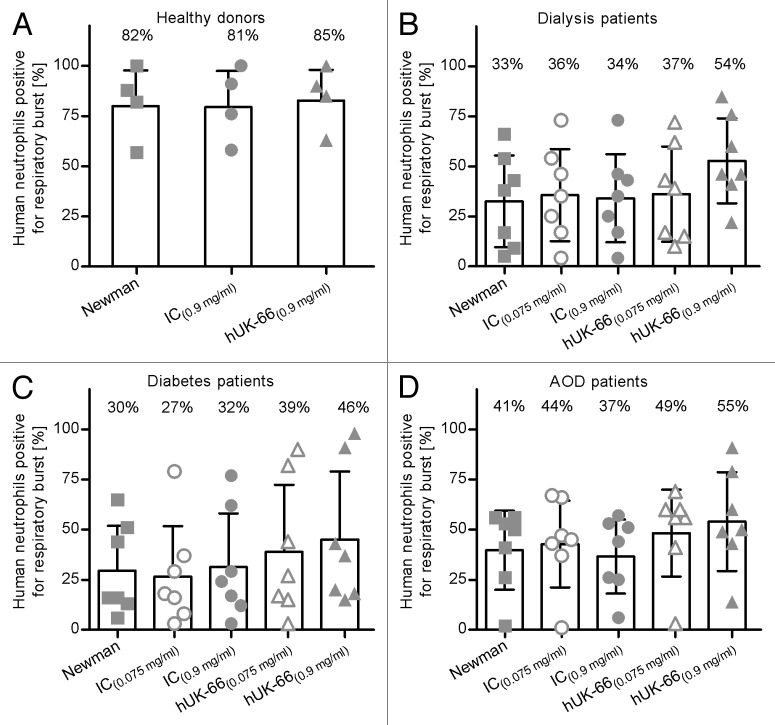
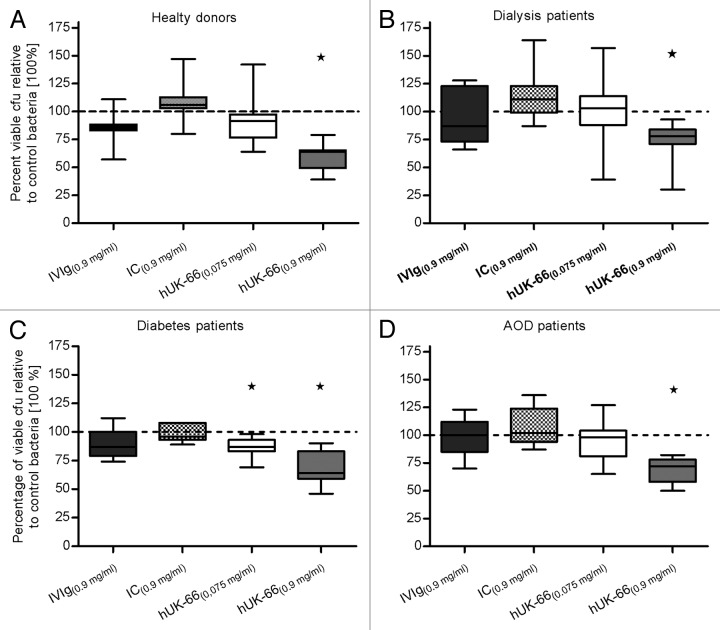
In healthy donors, the majority of neutrophils generated an oxidative burst upon contact with bacteria regardless of the presence of hUK-66 (mean percentage [SD] 81.8% [18.1%]), whereas 81.2% (18.4%) generated a respiratory burst in the presence of the isotype-matched control antibody, and 84.5% (15.6%) generated a burst in the presence of the hUK-66 IgG1 antibody (Fig. 6). hUK-66 IgG1 (5.81 µM; 0.9 mg/ ml) induced a greater oxidative burst by neutrophils in the blood of dialysis patients, diabetes patients, and AOD patients (53.7% [21.6%], 46% [34.7%], and 55.1% [25.1%], respectively) compared with those in the respective control blood/bacteria samples (33.1% [23.4%], 30.1% [22.8%], and 40.6% [20.1%]). Although the isotype-matched control antibody induced a respiratory burst at the same molar concentration, the percentages were much lower than those achieved by hUK-66 (34.7%% [22.4%], 32% [27.3%], and 37.4% [18.8%] for dialysis patients, diabetes patients, and AOD patients, respectively). The addition of hUK-66 IgG1 generated a burst in the presence of the hUK-66 IgG1 antibody at 0.483 µM (0.075 mg/ ml) had no measurable effect in any of the samples. These results illustrate that neutrophils from patients with diabetes, ESRD, and AOD show a reduced response to S. aureus compared with those from healthy donors; however, hUK-66 IgG1 increases the ability of these neutrophils to generate a respiratory burst.
Figure 6. hUK-66 IgG1 augments the respiratory burst in neutrophils from clinical relevant patient groups. Heparinized human blood samples (n = 4–7) from healthy donors, patients undergoing dialysis, diabetes, and artery occlusive disease (AOD) were infected with S. aureus Newman in the presence of two different concentrations of hUK-66 as indicated for 20 min at 37°. The monoclonal antibody Trastuzumab served as matched IgG1 isotype control (IC). The generation of reactive oxygen species (ROS) by neutrophils was analyzed by flow cytometry and the values were plotted relative to the burst of unstimulated samples (not shown). (A) ROS-positive neutrophils in blood from healthy donors (B–D) ROS-positive neutrophils in blood from patients with dialysis (B), diabetes, (C) and artery occlusive disease (D). Individual data points of one blood sample (triplicate) were superimposed with bar. Mean values of each group are shown as percentage.
hUK-66 IgG1 binds specifically to Fcγ receptors
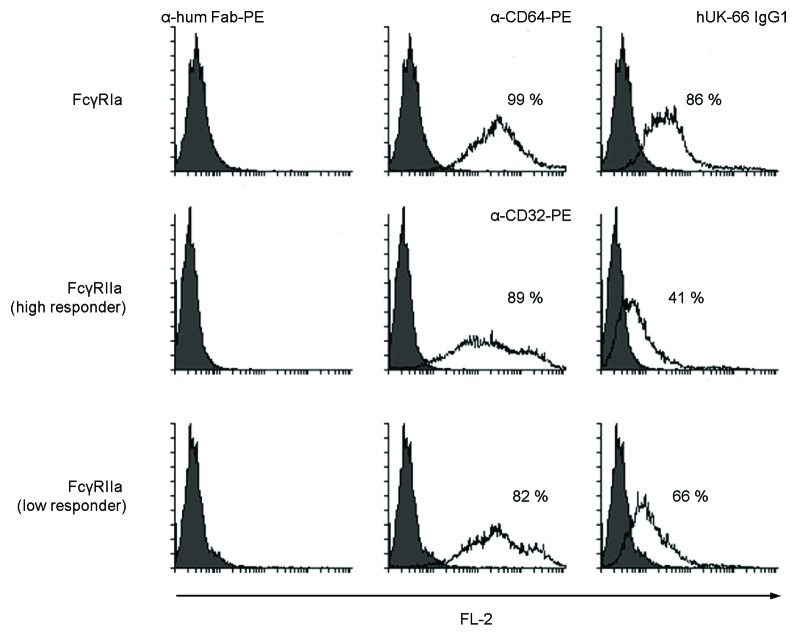
The interaction of hUK-66 IgG1 to IgG-specific Fcγ−receptors (FcγR) is as a prerequisite for phagocytosis and the production of reactive oxygen metabolites by immune effector cells. The FcγRI and FcγRIIa expressed by neutrophils, the primary cells of phagocytosis, trigger the phagocytosis of opsonized staphylococci.18-20 The reason for investigating primarily the interaction to these receptors is further justified due to the fact that the receptor FcγRIIIa is not expressed on human neutrophils,21 and the receptor FcγRIIIb has no detectable role in staphylococcal phagocytosis.22 To determine the interaction of hUK-66 IgG1 to FcγRs, HEK293 cells were transiently transfected with plasmids expressing recombinant FcγRIa, FcγRIIa (high responder), and FcγRIIa (low responder) on their surface and binding of different concentrations of hUK-66 IgG between 6.61 pM (1.024 ng/ ml) and 20.65 µM (3.2 mg/ ml) were analyzed by flow cytrometry. Here, hUK-66 IgG1 interacted concentration dependent with FcγRIa, FcγRIIa (high responder), and FcγRIIa (low responder) with a maximum of 86%, 41%, and 66% positive cells, respectively at a concentration of 0.516 µM (0.08 mg/ ml) hUK-66 IgG1 (Fig. 7).
Figure 7. hUK-66 IgG1 binds to FcγRIa and FcγRIIa expressing HEK293 transiently transfected by CaPO4. The expression of the FcγRs was analyzed by α-CD64 and α-CD32-PE antibodies, respectively. Binding of different concentrations of hUK-66 IgG between 6.61 pM (1.024 ng/ ml) and 20.65 µM (3.2 mg/ ml) on FcγRs was detected via a α-human Fab IgG1-PE.
hUK-66 IgG1 promotes the killing of S. aureus under physiological conditions
Next, we examined the functionality of hUK-66 IgG1 under the physiological conditions likely to be encountered during therapy. Antibody-mediated killing of S. aureus Newman was investigated using whole blood samples from patients with either ESRD, diabetes, or AOD in the presence or absence of hUK-66 IgG1. The addition of hUK-66 (5.81 µM; 0.9 mg/ ml) resulted in a significant increase in bactericidal activity with a mean percentage (SD) reduction in viable bacteria of 41.3% (11.5%) relative to that in untreated control blood/bacteria samples (P < 0.05, t test; Fig. 8A). Adding the immunoglobulin preparation, Gammunex® (0.9 mg/ ml), to the samples resulted in a much smaller reduction in the percentage of viable bacteria (15.9% [12.1%]), whereas the isotype-matched control IgG1 antibody, Trastuzumab (0.9 mg/ ml), resulted in a reduction of only 8.9% (15.7%). A lower molar concentration of hUK-66 IgG1 (0.483 µM; 0.075 mg/ ml) had no significant effect (10.1% [19.2%]). hUK-66 (5.81 µM; 0.9 mg/ ml) also showed bactericidal activity when added to blood samples from dialysis patients, diabetes patients, and patients suffering from AOD, with reductions in viable bacteria of 27% (20.3%), 32.7% (15%), and 31.3% (11.7%), respectively, relative to those in untreated control blood/bacteria samples (P < 0.05, t test; Fig. 8B–D). Furthermore, a lower concentration of hUK-66 IgG1 (0.483 µM; 0.075 mg/ ml) was effective when added to samples from diabetes patients (a mean percentage reduction of 13.6% [9.1%]). Neither Trastuzumab nor Gammunex® had any significant effect when added to samples from the three patient groups. Gammunex® was analyzed for anti-staphylococcal and anti-IsaA IgG antibodies by ELISA studies. The concentration of anti-IsaA IgG antibodies (<0.075 mg/ml) was not sufficient to promote anti-IsaA dependent killing. Furthermore, the presence of anti-staphylococcal antibodies did not reveal antibody dependent killing in this experimental setting (Fig. S2).
Figure 8. Killing capacity of S. aureus by whole blood from patients at risk for staphylococcal infections is enhanced by the humanized UK-66 IgG1. Heparinized human blood samples (n = 7) were infected with S. aureus Newman in the presence of two different concentrations of hUK-66 as indicated for 60 min at 37°. The monoclonal antibody Trastuzumab served as matched IgG1 isotype control (IC) and the intravenous human immunoglobulin preparation Gammunex® (IVIg) as a second positive control. Total viable bacteria were recovered after eukaryotic cell lysis and enumerated by plate counting. The values were plotted relative to the level of control bacteria samples (set at 100%). Results were expressed in mean percentage (SD) of viable S. aureus recovered in the blood from (A) healthy donors, (B) dialysis patients, (C) diabetes patients, and (D) patients with artery occlusive disease (AOD). Data are illustrated as box-and-whisker plot. The dashed line in the graphs demonstrates the 100% reference value of control bacteria samples. *P < 0.05, t test.
Discussion
Here, we generated a humanized anti-staphylococcal antibody and characterized its antigen binding properties and biological activity against the immunodominant staphylococcal antigen IsaA. The results showed that hUK-66 bound specifically to its target antigen IsaA and effectively induced the killing of bacteria in blood samples taken from patients at high risk of S. aureus infections (patients with diabetes, patients on dialysis, and patients with AOD). Because microbes must be viable to cause a serious infection, reducing the percentage of viable bacteria in the blood is of paramount importance if therapeutic approaches are to be effective. Thus, the results presented herein suggest that hUK-66 is a strong candidate for inclusion in future immunotherapeutic strategies.
A critical step in the development of any immunotherapeutic strategy is the selection of a suitable target molecule. Generally, molecules expressed on the surface of bacteria, as well as factors that are essential for growth or virulence are the most promising targets for antibodies. Immunotherapeutic strategies based on virulence-associated determinants such as adhesins or toxins show varying efficacy, which is dependent on disease type and the causative strain. This is because the expression of S. aureus virulence-associated factors is controlled by complex regulatory pathways, resulting in different expression levels during infection in a particular strain.13 Therefore, we selected the lytic transglycosylase IsaA as target antigen for immunotherapy due to its outstanding immunodominance during infection.7,10,23 Here, we confirmed binding of the humanized hUK-66 IgG1 to IsaA expressed by major clinical and community-associated MRSA lineages including CC5, CC8, CC22, CC45, CC239, USA300, and USA400.24,25 Moreover, the target IsaA is present on the surface of S. aureus during different growth phases, and most importantly, during human infection indicated by the specific antibody response in patients.10,23 Notable, antibody responses to IsaA are associated with protection against invasive S. aureus infections.7
In addition to selecting the appropriate target, a therapeutic antibody must be functional in humans. It is thought that the baseline level of critical functional anti-staphylococcal antibodies are not always sufficient to protect from invasive infections.5 Consequently, when designing anti-staphylococcal antibodies, it is important to select candidates that not only bind to the staphylococcal antigen on the bacterial surface but also have the capability to trigger the elimination of the bacteria, e.g., by inducing opsonophagocytosis or antibody dependent killing. Here, we showed that hUK-66 IgG1 was more effective at inducing the killing of S. aureus in whole blood samples than pooled human immunoglobulin formulations or other hUK-66 isotype variants (IgG2 and IgG4). However, although hUK-66 bound to FcγRIa and FcγRIIa receptors in vitro, it did not significantly increase the oxidative burst generated by phagocytic neutrophils in the blood of healthy donors compared with that in control antibodies. It is worth noting that a previous study examining the functional role of anti-IsdB monoclonal antibodies (a functional component of Merck`s V710 vaccine) introduced a mutation into the Fc fragment of the mAb (at aa 297), which completely abolished its Fc-mediated effector functions.26 Although the mAb, called mutein, showed markedly reduced opsonophagocytic activity in vitro compared with the wild-type mAb, it did confer some level of protection in a murine sepsis model. This was due to mechanisms involving complement, phagocytes, and lymphocytes, rather than FcγR-mediated functions. Antibodies modulate several secondary immune mechanisms, including the induction of proinflammatory cytokines, T and B cell activation, FcγR expression, NK and dendritic cell activation, and neutrophil adhesion. However, effective staphylococcal killing triggered by antigen-specific antibodies is always critical to combat S. aureus infections.27 For this reason, we performed whole blood assays in an attempt to mimic the physiological milieu in which the hUK-66 antibody is to function.
One of the unresolved issues for clinicians wishing to use immunotherapy to treat S. aureus infections is the selection of patients who would most likely benefit. Clinical trials in patients with end-stage renal disease, infants, or patients undergoing cardiothoracic surgery have shown only limited efficacy.28-30 In general, patients with immunocompromised conditions are most vulnerable to S. aureus infections. However, underlying serious comorbidities or adjunctive treatments such as surgery limit the detection of the overall efficacy of an immunotherapeutic approach once chosen only these patient groups for clinical trials. The best way to differentiate between disease-associated morbidity and confounding variables assessing clinical efficacy should be possible in patients with pneumonia caused by S. aureus. In line, the consensus position paper of the Infectious Diseases Society of America, American Thoracic Society, American College of Chest Physicians, and Society of Critical Care Medicine makes clear that validated methods and/or tools are available to design, conduct, and analyze hospital-acquired or ventilator-associated bacterial pneumonia trials.31,32 Nevertheless, the target populations for clinical trials and those for clinical treatment are somewhat different. Next, the in vivo effect of hUK-66 IgG1 during S. aureus infection will be investigated in mouse infection models. The effective dose for these experiments has to be defined by dose titration experiments. A probably effective dose range can now be calculated based on the in vitro killing results reported here.
We examined the killing capacity of S. aureus in blood samples taken from patients with diabetes, end-stage renal disease, and AOD; all conditions that are related to immune cell dysfunction. It has been described that patients with end stage renal ESRD show reduced killing of S. epidermidis but this could be corrected by continuous ambulatory peritoneal dialysis (CAPD).33 In contrast to CAPD patients and healthy controls, conservatively treated dialysis patients produced less intracellular hydrogen peroxide.34 These reports suggest that reduced intracellular hydrogen peroxide concentrations, which indicate oxidative burst activity, are the reason for a reduced killing capacity of PMNs in dialysis patients. In our study, we detected no significant difference in oxidative burst activity between dialysis patients and healthy donors but clearly observed a reduced killing of S. aureus in the patient group. Obviously, other mechanisms of killing e.g., due to antimicrobial peptides may be activated by hUK-66. In addition, the exact therapy status of the dialysis patients whose blood was used for phagotest experiments was unknown. It would be an interesting issue for future research to compare hUK-66-related killing of S. aureus in different dialysis patient groups.
In conclusion, the results of this study show that the hUK-66 antibody was effective in antibody-dependent killing of S. aureus using whole blood samples of patients vulnerable to S. aureus infections. Most importantly, hUK-66 induced the killing of S. aureus in blood from both healthy controls and patients, suggesting that the antibody does not interfere with naturally occurring antibodies against S. aureus. This is the first example of an anti-S. aureus antibody that effectively induces the killing of S. aureus in the blood of vulnerable patient groups. One shortcoming of the study is related to the activity of therapeutic antibodies in tissues of infected patients. Phagocytosis may be influenced by extracellular products of S. aureus such as proteases and immunological active toxins or by inflammatory processes which interfere with antibacterial killing activity measured under in vitro conditions. Future studies are warranted to dissect the action of hUK-66 in vivo and in combination with other antibodies directed against S. aureus proteins. These results may aid the future development of immunotherapies for S. aureus infections.
Materials and Methods
Ethics statement
Blood samples of the subjects were drawn according to the guidelines of the University Hospital of Wuerzburg.
Bacterial strains
Bacterial strains were propagated in lysogenic broth (LB) at 37 °C with shaking at 175 rpm. When required, antibiotics were added to the medium at the following concentrations: kanamycin, 50 μg/ml; spectinomycin, 100 μg/ml; erythromycin, 5 μg/ml; and ampicillin, 100 μg/ml. The successful generation of isaA and spa mutants was achieved by allelic replacement and confirmed by PCR and immunoblot.
Humanization of the mouse UK-66 antibody
Total RNA was isolated from the hybridoma clone, UK-66, and double stranded cDNA was synthesized using reverse transcriptase (Invitrogen, Cat. No. 18080-093). PCR reactions were set up using the cDNA as a template. The PCR fragments encoding the VL and VH regions were then cloned into the pGEMT vector (Invitrogen, Cat. No. A1360) to determine the sequence of the variable region of mouse UK-66 IgG. Humanization of the UK-66 antibody was performed using the complementarity-determining region (CDR) grafting method.35 Briefly, the framework region (FR) residues of potential importance for antigen binding were identified by computer-assisted homology modeling. Two humanized versions of mouse UK-66, denoted hUK66–1 and hUK66–2, were generated by transferring the key murine CDR residues onto a human antibody framework (selected based on its homology to the mouse antibody framework). The light and heavy chain variable regions were then directly grafted into the human antibody light and heavy chains. The CDR-grafted antibody light and heavy chain genes were commercially synthesized and were cloned into the expression vector, pEE12.4 (Lonza, Gene expression system Lot. No. L05048/48).
Expression of antibodies and antibody fragments
The Lonza® glutamine synthetase (GS) gene expression system was used to express the proteins in Chinese Hamster Ovary (CHO) cells (Lonza, Lot. No. 028-W4). GS activity is selectively inhibited by methionine sulphoximine (MSX); the selection pressure in CHO.K1 cells is provided by culture in glutamine-free medium containing 25 μM MSX (Sigma-Aldrich Alderich, Cat. No. M5379K). A double gene vector, pEE12.4, containing expression cassettes for the light and heavy chains of humanized UK-66 IgG1 was stably transfected into CHO.K1 cells using Nanofectamine (Invitrogen, Cat. No. Q0002-006). Plasmid DNA (used for subsequent transfections) was isolated and purified according to the manufacturer’s protocol (Qiagen, QIAquick PCR Purification Kit, Cat. No. 28104). Cells were sub-cultured every 3–4 d. On the day before transfection, cells were trypsinized and seeded into six-well plates at a density of 1 × 106 cells/ well. On the day of transfection, 200 μl of transfection solution was prepared, which comprised 5 μg of GS vector DNA and 7 μl of Nanofectamine in OptiMEM medium (Invitrogen, Cat. No. 31985062). The transfection solution was added to the cells, which were then incubated overnight at 37 °C in a humidified CO2 incubator. The OptiMEM medium was replaced with 3 ml of glutamine-free selective RPMI containing 10% dFCS and 25 μM MSX on the following day. MSX-resistant colonies appeared about two weeks after transfection. CHO cell transfectants that stably produced antibodies were obtained using a positive selection procedure. A binding ELISA was used to test cell culture supernatants for hUK-66 IgG1 production.
Antibody fragments comprising the antigen binding domain (mouse or humanized UK-66) and a human IgG1 Fc fragment (single-chain Fv (sc)Fv-Fc) were produced by transient transfection of HEK293 cells. The scFv-Fc fragments were constructed using the vector pSEC system (Life Technologies, Cat. No. 17839), which incorporates a Zeocin resistance gene (Invitrogen, Cat. No. ant-zn-1). HEK293 cells were then transfected as described above. The OptiMEM medium was replaced with 3 ml of glutamine-free selective RPMI containing 10% FCS and Zeocin (300 μg/ ml) at the following day. After 10 d, the cell culture supernatant was purified and tested for the presence of murine and humanized scFv-Fc UK-66 IgG1.
Overexpression and purification of IsaA
The isaA open reading frame was amplified and cloned into the overexpression vector pQE30 (Qiagen, Cat. No. 33203), to incorporate a C-terminal His6 fusion tag. The resulting plasmid was then transformed into E. coli strain TG1 and the recombinant protein expressed as previously described.9 For purification of rIsaA, the supernatant of lysed bacteria was passed through a nickel-charged Hi-Trap column (GE Healthcare Cat. No. 17040801) and the bound proteins eluted using 350 mM imidazole. The resulting protein was dialyzed into phosphate-buffered saline (PBS) containing 350 mM NaCl and the correct product was confirmed by analysis in SDS-PAGE gels.
Determination of binding affinity of antibodies using SPR
The affinity of hUK-66 IgG1 for rIsaA was measured by surface plasmon resonance (SPR) using a Biacore 2000 system. Briefly, hUK-66 was reversibly immobilized by binding to an anti-human Fab antibody covalently coupled at high density to a CM5 sensor chip according to the manufacturer’s instructions (GE Healthcare, antibody Fab capture kit, Cat. No. 28-9583-25). The average concentration of hUK-66 on the anti-human Fab surface was between 0.39 and 400 nM. Sensor chips coated with the capture antibody alone served as controls for monitoring non-specific binding. Affinity measurements were performed in buffer containing 10 mM HEPES (pH 7.4, 150 mM NaCl, 3.4 mM EDTA, 0.005% Tween 20). Sensorgrams were recorded at a flow rate of 30 μl/ min at 25 °C and the capture surface was regenerated after each cycle using 10 mM glycine (pH 2.1). The dissociation constants were calculated using BIAevaluation software 4.0.1 after fitting the sensorgrams to a 1: 1 Langmuir binding model.
Immunofluorescence analysis
Immunofluorescence analysis was performed to confirm the expression of IsaA on the surface of viable S. aureus. Bacteria were grown overnight and harvested by centrifugation at 11 000 × g. The bacterial pellet was washed twice with PBS and the cell density was adjusted to 5 × 108 cfu/ ml. After blocking with 5% FCS in PBS for 1 h, the bacteria were incubated with humanized UK-66 IgG1 (diluted 1: 5000) for 30 min at 37 °C. The bacterial pellet was washed three times with PBS/ 0.05% Tween 20 and then stained with fluorescein isothiocyanate (FITC)-conjugated anti-human IgG1 (diluted 1: 10 000) for 30 min at 37 °C (Sigma-Aldrich, Cat. No. F4512). The fluorescently-labeled bacteria were re-suspended in 100 μl PBS, dried, and fixed on slides. Surface expression of IsaA was determined by examining the slides under a fluorescence microscope with the same exposure settings for each comparison group. Images were processed using the GIMP program 2.8.6.
Determination of binding affinity of antibodies using ELISA
An ELISA was performed to measure the affinity of murine and humanized UK-66 for rIsaA according to standard protocols (all stages were performed in a volume of 50 μl/well, apart from the blocking steps [200 μl/ well]). Briefly, 96-well plates (Nunc, MaxiSorp, Cat. No. 44240421) were coated with rIsaA diluted in PBS (pH 7) to a final concentration of 10 μg/ ml. The plates were then incubated overnight at 4 °C. After blocking non-specific sites with 5% bovine serum albumin (BSA) in PBS, the mouse and humanized UK-66 IgG1 antibodies were added at 1:10 dilutions. The optimal concentration of hUK-66 IgG1 was 10 μM (1: 5 dilution). The plates were incubated for 1 h at 37 °C, washed with PBS-0.05% Tween 20 (PBS-T), and then incubated with horseradish peroxidase-conjugated mouse anti-human IgG1 antibodies (diluted 1: 5000) for a further 1 h at 37 °C (ab-online, Cat. No. ABIN567704). After a final wash with PBS-T, 50 µl of 3,3′,5,5′-tetramethylbenzidine (TMB) was added to each well for 10 min at 37 °C (Invitrogen Cat.No. 002023) and titers were measured at 450 nm on a Multiskan ELISA reader. All titers were measured in duplicate. The binding specificity of the mouse and humanized UK-66 antibodies was assessed in a competitive ELISA. Briefly, a 96-well plate (Nunc, MaxiSorp, Cat. No. 44240421) was coated with 10 μg/ ml rIsaA and incubated overnight at 4 °C. The plate was then washed with PBS-T and blocked with 5% BSA for 2 h at room temperature (RT). In parallel tubes, the mouse and humanized UK-66 antibodies were pre-incubated with an equal volume of rIsaA (serially 1: 5 diluted from a starting concentration of 100 μg/ml). The antibody/antigen mixtures were then transferred to rIsaA pre-coated plates and incubated for 15 min at RT. The plates were washed six times and then incubated with HRP-conjugated anti-human IgG1 (1: 5000). After incubation for 1 h at 37 °C, 50 µl of TMB substrate (Invitrogen, Cat.No. 002023) was added to each well and incubated 15 min at RT. The plates were then read in an ELISA reader at OD 450 nm.
Fcγ receptor binding assay
The Fcγ receptor (FcγR) binding assay were performed as previously described.9 Briefly, HEK293 cells were transiently transfected with the expression plasmid, pCMVXL-6, harboring full-length human FcγRIa or FcγRIIa (low responder), or with pEE12.4 harboring FcγRIIa (high responder) using calcium phosphate. After trypsinization, the transfected HEK293 cells were treated with concentration of hUK-66 IgG1 between 6.61 pM (1.024 ng/ ml) and 20.65 µM (3.2 mg/ ml) for 1 h at 4 °C. After washing, the cells were incubated with a PE-conjugated α-human Fab antibody for 1 h (Biozol Cat. No. BS0364R). Receptor expression by viable cells was detected using a CD32/CD64-specific PE-conjugated mouse monoclonal antibody (Biozol Cat. No. LSC21937100). Binding of hUK-66 IgG1 to cell surface FcγRs expressed by HEK293 cells was analyzed by flow cytometry and compared with that of secondary antibody-treated cells.
Phagocytosis assay
Phagocytosis by neutrophils was analyzed using carboxyfluorescein succinimidyl ester (CFSE, BioLegend Cat. No. 422701)-stained strain S. aureus Newman. The intensity of CFSE fluorescence by human neutrophils is dependent on the number of ingested bacteria. Briefly, bacteria were stained at 37 °C for 20 min with 20 μg CFSE in 500 μl PBS containing 5% FCS. After washing, the bacteria were added to neutrophils (MOI 50) for 20 min at 37 °C. Same samples incubated at 4 °C serve as negative controls. All samples were prepared for flow cytometry analysis using the phagotest kit according to the manufacturers’ protocol (Glycotope, Cat. No. 10-0100).
Whole blood bacterial killing assays
Human blood samples were incubated with S. aureus Newman strain to measure antibody-dependent killing and the loss of viable bacteria as determined by colony counting. In brief, bacteria were washed twice with PBS and the cell density was adjusted to 5 × 107 cfu in 30 μl PBS. Bacteria were then mixed with 100 μl human whole blood and incubated at 37 °C on a rotating shaker for 60 min. hUK-66 (0.075 and 0.9 mg/ ml) was then added. The isotype-matched control antibody, Trastuzumab, and the intravenous immunoglobulin, Gammunex®, were used as controls. Subsequently, all eukaryotic cells were lysed by exposure to 1% saponin solution for 20 min at 4 °C. The samples were then serially diluted 1: 10 in duplicates to 10−5. Duplicates of each serial dilution were then plated onto LB agar plates and incubated overnight at 37 °C. Colonies were counted and the percentage of surviving bacteria was calculated as follows: (blood + bacteria + antibody)/ (blood + bacteria)*100% = survival [%].
Respiratory burst assay
The killing of ingested bacteria by human phagocytes occurs via both oxygen-dependent and oxygen-independent mechanisms. The production of reactive oxygen species by neutrophils was determined using the fluorogenic substrate dihydrorhodamine (DHR) 123 as previously described.9 The percentage of neutrophils showing a positive oxidative burst was determined by flow cytometry. To this end, the manufacturers’ protocol was adapted to evaluate the antibody-dependent oxidative burst by human neutrophils using the phagoburst kit (Glycototope, Cat. No. 10–0200). Briefly, 100 μl of heparinized human whole blood was infected with 5 × 107 cfu bacteria and the antibody was added at different concentrations. Human whole blood with bacteria serves as negative control. After incubation for 10 min at 37 °C, DHR was added to the samples and the mixture incubated for a further 10 min. All samples were prepared for analysis by flow cytometry according to the manufacturers protocol.
Immunoblot analysis
Proteins were separated by one-dimensional sodium dodecyl sulfate-PAGE (SDS-PAGE) and transferred to a nitrocellulose membrane using a semi-dry transfer system. After blocking in 5% BSA in PBS, hUK-66 was added at a dilution of 1: 5000. HRP-conjugated mouse anti-human IgG1 (Biozol, Cat. No BSS-BS-0297M-HRP) at a dilution of 1: 10 000 was used for detecting the immune complexes. The signal was developed using the ECL detection system (Amersham Biosciences, Cat. No. RPN3004).
Purification of IgG
IgG was purified from CHO.K1 culture supernatants by affinity chromatography on immobilized Protein G according to manufacturer`s instructions (UltraLink immobilized protein G Cat. No. 20397; Thermo Fisher). Briefly, samples of cell culture supernatant were incubated with the protein G resin and then loaded onto columns equilibrated with binding buffer (TBS, pH 5.0). The columns were washed with 10 volumes of binding buffer and the IgG was eluted in elution buffer (100 mM glycine HCl, pH 2.7). The collected fractions were immediately pH-neutralized with 1 M TRIS-HCl, pH 9.0, and the antibody concentration was measured at an OD of 280 nm. The antibodies were also examined by SDS-PAGE.
Statistical analysis
Statistical analysis was performed using the Student t test. Differences were considered significant at P < 0.05. All data were analyzed using GraphPad Prism software.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Go-Bio program of the Federal Ministry of Education and Research in Germany (BMBF).
References
- 1.Sánchez García M. . Early antibiotic treatment failure. Int J Antimicrob Agents 2009; 34:Suppl 3 S14 - 9; http://dx.doi.org/ 10.1016/S0924-8579(09)70552-7; PMID: 19596109 [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein E, Kollef MH, Nathwani D. . Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008; 46:Suppl 5 S378 - 85; http://dx.doi.org/ 10.1086/533594; PMID: 18462093 [DOI] [PubMed] [Google Scholar]
- 3.Kollef MH, Morrow LE, Niederman MS, Leeper KV, Anzueto A, Benz-Scott L, Rodino FJ. . Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 2006; 129:1210 - 8; http://dx.doi.org/ 10.1378/chest.129.5.1210; PMID: 16685011 [DOI] [PubMed] [Google Scholar]
- 4.Verkaik NJ, van Wamel WJ, van Belkum A. . Immunotherapeutic approaches against Staphylococcus aureus.. Immunotherapy 2011; 3:1063 - 73; http://dx.doi.org/ 10.2217/imt.11.84; PMID: 21913829 [DOI] [PubMed] [Google Scholar]
- 5.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. . Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine 2013; 31:2723 - 30; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.002; PMID: 23624095 [DOI] [PubMed] [Google Scholar]
- 6.Adhikari RP, Ajao AO, Aman MJ, Karauzum H, Sarwar J, Lydecker AD, Johnson JK, Nguyen C, Chen WH, Roghmann MC. . Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206:915 - 23; http://dx.doi.org/ 10.1093/infdis/jis462; PMID: 22807524 [DOI] [PubMed] [Google Scholar]
- 7.van der Kooi-Pol MM, de Vogel CP, Westerhout-Pluister GN, Veenstra-Kyuchukova YK, Duipmans JC, Glasner C, Buist G, Elsinga GS, Westra H, Bonarius HP, et al. . High anti-staphylococcal antibody titers in patients with epidermolysis bullosa relate to long-term colonization with alternating types of Staphylococcus aureus.. J Invest Dermatol 2013; 133:847 - 50; http://dx.doi.org/ 10.1038/jid.2012.347; PMID: 23014336 [DOI] [PubMed] [Google Scholar]
- 8.Bagnoli F, Bertholet S, Grandi G. . Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16; http://dx.doi.org/ 10.3389/fcimb.2012.00016; PMID: 22919608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz U, Lorenz B, Schmitter T, Streker K, Erck C, Wehland J, Nickel J, Zimmermann B, Ohlsen K. . Functional antibodies targeting IsaA of Staphylococcus aureus augment host immune response and open new perspectives for antibacterial therapy. Antimicrob Agents Chemother 2011; 55:165 - 73; http://dx.doi.org/ 10.1128/AAC.01144-10; PMID: 20956605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenz U, Ohlsen K, Karch H, Hecker M, Thiede A, Hacker J. . Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus.. FEMS Immunol Med Microbiol 2000; 29:145 - 53; http://dx.doi.org/ 10.1111/j.1574-695X.2000.tb01517.x; PMID: 11024354 [DOI] [PubMed] [Google Scholar]
- 11.Sakata N, Mukai T. . Production profile of the soluble lytic transglycosylase homologue in Staphylococcus aureus during bacterial proliferation. FEMS Immunol Med Microbiol 2007; 49:288 - 95; http://dx.doi.org/ 10.1111/j.1574-695X.2006.00200.x; PMID: 17328763 [DOI] [PubMed] [Google Scholar]
- 12.den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, Verkaik NJ, van Belkum A, Verbrugh HA, van Wamel WJ. . Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One 2013; 8:e53391; http://dx.doi.org/ 10.1371/journal.pone.0053391; PMID: 23308212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy AJ, Lindsay JA. . Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol 2010; 10:173; http://dx.doi.org/ 10.1186/1471-2180-10-173; PMID: 20550675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. . Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol 2009; 6:36 - 41; http://dx.doi.org/ 10.1080/15476910802604564; PMID: 19519161 [DOI] [PubMed] [Google Scholar]
- 15.Yano H, Kinoshita M, Fujino K, Nakashima M, Yamamoto Y, Miyazaki H, Hamada K, Ono S, Iwaya K, Saitoh D, et al. . Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect Immun 2012; 80:4409 - 16; http://dx.doi.org/ 10.1128/IAI.00787-12; PMID: 23027538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mylotte JM, Tayara A. . Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 2000; 31:1170 - 4; http://dx.doi.org/ 10.1086/317421; PMID: 11073748 [DOI] [PubMed] [Google Scholar]
- 17.Jaber BL. . Bacterial infections in hemodialysis patients: pathogenesis and prevention. Kidney Int 2005; 67:2508 - 19; http://dx.doi.org/ 10.1111/j.1523-1755.2005.00364.x; PMID: 15882306 [DOI] [PubMed] [Google Scholar]
- 18.Nibbering PH, Broug-Holub E, Bezemer AC, Jansen R, van de Winkel JG, Geertsma MF. . Phagocytosis and intracellular killing of serum-opsonized Staphylococcus aureus by mouse fibroblasts expressing human Fcgamma receptor type IIa (CD32). Front Biosci 1996; 1:a25 - 33; PMID: 9159191 [DOI] [PubMed] [Google Scholar]
- 19.Schiff DE, Rae J, Martin TR, Davis BH, Curnutte JT. . Increased phagocyte Fc gammaRI expression and improved Fc gamma-receptor-mediated phagocytosis after in vivo recombinant human interferon-gamma treatment of normal human subjects. Blood 1997; 90:3187 - 94; PMID: 9376602 [PubMed] [Google Scholar]
- 20.Rigby KM, DeLeo FR. . Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 2012; 34:237 - 59; http://dx.doi.org/ 10.1007/s00281-011-0295-3; PMID: 22080185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruhns P. . Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640 - 9; http://dx.doi.org/ 10.1182/blood-2012-01-380121; PMID: 22535666 [DOI] [PubMed] [Google Scholar]
- 22.Fossati G, Moots RJ, Bucknall RC, Edwards SW. . Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum 2002; 46:1351 - 61; http://dx.doi.org/ 10.1002/art.10230; PMID: 12115243 [DOI] [PubMed] [Google Scholar]
- 23.Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, Boyd AP, Söllner J, Schmidt W, von Ahsen U, et al. . Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus.. Proc Natl Acad Sci U S A 2002; 99:6573 - 8; http://dx.doi.org/ 10.1073/pnas.092569199; PMID: 11997460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, et al. . Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 2010; 15:19688; PMID: 20961515 [DOI] [PubMed] [Google Scholar]
- 25.David MZ, Daum RS. . Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23:616 - 87; http://dx.doi.org/ 10.1128/CMR.00081-09; PMID: 20610826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pancari G, Fan H, Smith S, Joshi A, Haimbach R, Clark D, Li Y, Hua J, McKelvey T, Ou Y, et al. . Characterization of the mechanism of protection mediated by CS-D7, a monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB). Front Cell Infect Microbiol 2012; 2:36; http://dx.doi.org/ 10.3389/fcimb.2012.00036; PMID: 22919628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. . Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol 2013; 67:629 - 50; http://dx.doi.org/ 10.1146/annurev-micro-092412-155746; PMID: 23834243 [DOI] [PubMed] [Google Scholar]
- 28.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. . Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309:1368 - 78; http://dx.doi.org/ 10.1001/jama.2013.3010; PMID: 23549582 [DOI] [PubMed] [Google Scholar]
- 29.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. . Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491 - 6; http://dx.doi.org/ 10.1056/NEJMoa011297; PMID: 11844850 [DOI] [PubMed] [Google Scholar]
- 30.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, et al. . Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr 2007; 151:260 - 5, e1; http://dx.doi.org/ 10.1016/j.jpeds.2007.04.060; PMID: 17719934 [DOI] [PubMed] [Google Scholar]
- 31.Talbot GH. . Considerations in undertaking a clinical development program for hospital-acquired bacterial pneumonia and/or ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51:Suppl 1 S144 - 9; http://dx.doi.org/ 10.1086/653064; PMID: 20597665 [DOI] [PubMed] [Google Scholar]
- 32.Spellberg B, Talbot G, Infectious Diseases Society of America (IDSA), American College of Chest Physicians (ACCP), American Thoracic Society (ATS), Society of Critical Care Medicine (SCCM). . Recommended design features of future clinical trials of antibacterial agents for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 2010; 51:Suppl 1 S150 - 70; http://dx.doi.org/ 10.1086/653065; PMID: 20597666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter CJ, Burden RP, Morgan AG, Daniels I, Fletcher J. . Impaired polymorphonuclear neutrophil function in end-stage renal failure and its correction by continuous ambulatory peritoneal dialysis. Nephron 1995; 71:133 - 7; http://dx.doi.org/ 10.1159/000188700; PMID: 8569942 [DOI] [PubMed] [Google Scholar]
- 34.Porter CJ, Burden RP, Morgan AG, Daniels I, Fletcher J. . Impaired bacterial killing and hydrogen peroxide production by polymorphonuclear neutrophils in end-stage renal failure. Nephron 1997; 77:479 - 81; http://dx.doi.org/ 10.1159/000190328; PMID: 9434073 [DOI] [PubMed] [Google Scholar]
- 35.Kettleborough CA, Saldanha J, Heath VJ, Morrison CJ, Bendig MM. . Humanization of a mouse monoclonal antibody by CDR-grafting: the importance of framework residues on loop conformation. Protein Eng 1991; 4:773 - 83; http://dx.doi.org/ 10.1093/protein/4.7.773; PMID: 1798701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.