Abstract
Alzheimer disease (AD) process involves the accumulation of amyloid plaques and tau tangles in the brain, nevertheless the attempts at targeting the main culprits, neurotoxic β-amyloid (Aβ) peptides, have thus far proven unsuccessful for improving cognitive function. Important lessons about anti-Aβ immunotherapeutic strategies were learned from the first active vaccination clinical trials. AD progression could be safely prevented or delayed if the vaccine (1) induces high titers of antibodies specific to toxic forms of Aβ; (2) does not activate the harmful autoreactive T cells that may induce inflammation; (3) is initiated before or at least at the early stages of the accumulation of toxic forms of Aβ. Data from the recent passive vaccination trials with bapineuzumab and solanezumab also indicated that anti-Aβ immunotherapy might be effective in reduction of the AD pathology and even improvement of cognitive and/or functional performance in patients when administered early in the course of the disease. For the prevention of AD the active immunization strategy may be more desirable than passive immunotherapy protocol and it can offer the potential for sustainable clinical and commercial advantages. Here we discuss the active vaccine approaches, which are still in preclinical development and vaccines that are already in clinical trials.
Keywords: Alzheimer disease, DNA vaccine, protein vaccine, liposome-based vaccination, adjuvants, electroporation, immune responses
Introduction
According to World Health Organization, there were an estimated 35.6 million people with dementia worldwide, and this number is projected to nearly double every 20 y. AD is the most common cause of dementia and may contribute to 60–70% of all dementia cases.1 It is characterized clinically by an insidious onset and progressive cognitive decline that impacts memory, language, judgment, and orientation to time and space, eventually resulting in death, usually within 10 y of diagnosis. The neuropathological features of the disease include extracellular plaques composed primarily of Aβ, and intracellular neurofibrillary tangles composed mainly of a cytoskeletal protein, tau.2-5 These pathological changes result in a profound loss of neuronal synapses over the course of the disease, thereby contributing to a progressive reduction in the functional capacity of the patients. Aβ abnormalities precede and accelerate tau pathology, therefore, the first immunotherapy strategy was aimed at eliminating Aβ peptide from the brain of AD patients. However, it is well known that the clinical trials with the first-in-human anti-Aβ vaccine (e.g., AN1792 which is a fibrillar Aβ formulated in QS21 adjuvant with or without polysorbate B) has been halted due to induction of meningoencephalitis in the small subset of vaccinated AD patients.6 To eliminate the harmful effect of autoreactive Th cells and have therapeutically relevant concentrations of anti-Aβ antibodies several groups and companies decided to use passive vaccination strategy.7-10 Recently announced results of phase III clinical trials that evaluated frequent administration of high concentrations of 2 humanized monoclonal antibodies (bapineuzumab and solanezumab) into patients with mild to moderate AD indicated that the cognitive and functional primary endpoints were not met.11-13 However, reduction of the amyloid burden and a significant decrease of CSF p-tau were detected in some AD patients in Pfizer/Janssen AIP studies.12,13 Administration of high dose of solanezumab (400 mg per wk) was well tolerated by vaccinated subjects,14 but unfortunately it was not effective in 2 separate studies conducted by Eli-Lilly. At the same time analysis of pooled data from the mild AD, but not in the moderate AD subgroup of these 2 studies showed a small, statistically significant advantage over placebo on a cognitive measure in the patients given solanezumab,11 suggesting that immunotherapy should be initiated at the earliest stages of AD or even in asymptomatic people at AD risk to minimize synaptic and neuronal loss. Based on these results 3 passive vaccinations studies have been initiated: (1) 770-patient phase II/III gantenerumab (Roche) study in prodromal and mild AD15; (2) 210-patient prevention study in individuals with inherited autosomal-dominant mutations (the Dominantly Inherited Alzheimer Network [DIAN] study)16; and ADCS/A4 trials in normal individuals with positive “Aβ/PetScan” test.17 Our team believes that frequent and long-term administration of high dose of expensive humanized Aβ-specific antibodies in patients at very early stages of sporadic AD and, a fortiori, in asymptomatic pre-AD patients is not feasible for conventional treatment. Instead, active immunization is a more practical approach if vaccine is safe, fairly immunogenic in elderly people, and will not activate potentially harmful autoreactive Th cells in vaccinated subjects.
Immunogenicity of Protein-Based AD Epitope Vaccines Formulated in Various Adjuvants
Almost 8 y ago we proposed a vaccine strategy18 and based on that strategy generated various peptide/recombinant protein epitope vaccines composed of a small immunodominant self-B cell epitope of Aβ42 and one or two universal foreign Th epitopes. These epitope vaccines induced high levels of therapeutically potent anti-Aβ42 antibodies without activation of potentially harmful autoreactive Th cells.18-21 Immunizations of Tg2576 mouse model of AD with peptide and recombinant protein based epitope vaccines did not activate autoreactive Th cells as well. Importantly, vaccinations induced production of therapeutically relevant titers of anti-Aβ antibodies (≥50 µg/mL), which in turn inhibited accumulation of Aβ42 pathology in the brains of older mice, reduced glial activation and prevented the development of behavioral deficits in aged animals without increasing the incidence of microhemorrhages.21,22 Five other protein vaccines based on the same strategy are already being tested in various phase I–III clinical trials23-25 and one vaccine, ACI-24 has been added to this list recently,26,27 (Table 1).
Table 1. Ongoing clinical trials for Alzheimer disease.
| Company | Vaccine | Composition | Adjuvant | Trial phase | References |
|---|---|---|---|---|---|
| Novartis | CAD106 | Aβ1–6 on Qβ VLP | VLP; VLP/Alum; VLP/MF59 | Phase III | 32 |
| AFFiRiS AG | AD02/AD03 | Aβ1–6 Mimatope + carrier |
Alum | Phase II | 33 |
| Pfizer/J&J | ACC-001 | Aβ1–6 + carrier CRM197 | QS-21 | Phase II | 10, 23, 24 |
| Merck | V950 | N-terminal region from Aβ1–42 | ISCOMATRIX; ISCOMATRIX+Alum |
Phase II | 10, 23, 24 |
| United Biomedical | UB-311 | Aβ1–14 + T cell epitope from MVF, HBVsa, PT, TT. | CpG+Alum | Phase II | 10, 23, 24 |
| AC Immune | ACI-24 | Aβ1–15 on a liposome membrane | Liposome and MPLA inside the liposome | Phase I/II | 10, 26, 27 |
Although, all protein-based AD vaccines share some common characteristics they are vary from one another and each presents distinct challenges which must be addressed on a case-by-case basis. However, the major challenge for all type of protein/peptide-based AD vaccines based on self-Aβ and tau antigens is how to induce a sufficiently high and long lasting immune response to immunizations. In order to achieve this objective it is necessary to formulate protein antigens in an adjuvant, the compound that can boost the immune response against a vaccine antigen. There are several adjuvants that can enhance immune responses to protein antigens without causing significant harmful side effects: (1) Mineral salts/gels (e.g., alum); (2) Oil-in-water emulsions (e.g., MF59, AS03, Montanides/ISA); (3) Water-in-oil emulsions (Mas-1/MER5); (4) Saponin-based (e.g., QS21); (5) Delta-Inulin-based (e.g., Advax™); (6) Microbial derivatives (e.g., TLR agonists such as MPLA, imiquimod, CpG, LT, etc); (7) Endogenous human immunomodulators (cytokines); (8) Virosomal/particle (e.g., VLP); (9) Cationic liposomes (10) Combinations of these adjuvant systems (e.g., Iscomatrix [structural complex of saponin with phospholipids/cholesterol] or AS04 [combination of alum and MPLA]).28 However, from all these adjuvants only Alum is generally used to enhance immune responses to many human vaccines,29 while, 2 other adjuvants recently have been licensed in Europe for use as the components for few viral vaccines (MF59 for flu vaccine in elderly people and AS04 for HBV and HPV vaccines30). Although, Alum is a relatively weak adjuvant it has been used either alone (for AD01/92) or in combinations with VLP (CAD106), ISCOMATRIX (V950), and CpG (UB-311) to enhance immune responses to AD vaccines (Table 1) and.31 In addition, AC Immune is using liposomes in combination with MPLA in ACI-24 vaccine for enhancing the antibody responses to Aβ1–15 B cell epitope, and Novartis is using VLP particles (CAD106) formulated in MF59 adjuvant. Unfortunately, there are only few published results on the immunological efficacy of 2 vaccines, CAD10632 and AD01/02.33
More specifically, CAD106, which is composed of Aβ1–6 B cell epitope coupled to the coat protein of bacteriophage Qβ on the surface of virus-like particles, was shown to be safe in subjects with mild-to-moderate AD. Antibody responses directed to Aβ were detected in 62% of low dose and 82% of high dose subjects; however, quantification of the antibody titers was done relative to serum from rhesus macaques immunized with CAD106, making it difficult to interpret the actual magnitude of the humoral responses and possible therapeutic value of these concentrations of antibodies.32 The one of advantages of this vaccine has been the assumption that the repetitive and ordered exposure of Aβ1–6 peptides on the surface of viral particles should lead to the induction of high titers of anti-Aβ antibodies without adjuvant. However, apparently, the antibody response was still low, since the adjuvant (Alum or MF59) was incorporated into the vaccine formulation.34
According to AFFiRiS33 they have completed clinical phase I studies with AD01/02 composed of 6 aa peptide mimicking N-terminus of Aβ42 formulated with Alum adjuvant and demonstrated safety for both vaccine candidates in 48 mild-moderate AD patients. The company stated that: (1) AD02 formulation demonstrated stabilization of cognitive parameters over the 18 mo observation period in 9 from 12 patients; (2) lmmunological data supported a potential correlation between post-vaccination antibody levels and cognitive function.35 Although, phase I trials by AFFiRiS were completed and they decided to move to phase II trials with 420 patients with early AD, unfortunately, the data analyses are not published yet. Of note, AFFiRiS are also testing an AD03 vaccine targeting N-terminal-truncated and pyroglutamated Aβ in phase I trials.33
United Biomedical uses UB-311 vaccine based on N-terminal Aβ1–14 and formulated with CpG/Alum adjuvants. Phase I open-label study to evaluate the safety, tolerability, and immunogenicity of this vaccine was initiated 4 y ago in Taiwan and completed in 2010, but the results of the study are not yet disclosed.36
V-950 is a multivalent Aβ vaccine conjugated with Alum/Iscomatrix adjuvant that triggers production of anti-Aβ antibodies in serum and CSF of animal models that are targeting various N-terminal truncated fragments of Aβ.24,37 At present there is no information about exact B cell epitope(s) of amyloid and possible carrier molecule that can provide Th cell immune responses to these B cells.
ACC-001 vaccine, a short N-terminal Aβ1–7 fragment attached to a carrier protein, CRM197 (non-toxic variant of diphtheria toxin) and formulated in the QS-21 adjuvant is currently tested in 6 different phase II studies.23,24
Thus, at least 6 peptide/protein based AD vaccines are being tested in clinical trials in patients with mild-moderate AD and at least one more vaccine, Lu AF20513 vaccine recently tested in our laboratory38 is moving to phase I trial in Europe in 2014.39 If the safety and at least immunogenic efficacy of these vaccines will be proved in the above mentioned trials, we believe that the most immunogenic AD vaccine(s) should be used as a preventive measure in subjects with very early AD pathology (prodromal AD40) or even in asymptomatic subjects at AD risk.
DNA Based AD Vaccines and Electroporation System for Enhancing of Immune Responses
Unlike to protein based AD vaccines there is no reports on DNA vaccine that are in clinical trials.
However, DNA vaccines exhibit several significant advantages when compared with a recombinant protein or peptide-based vaccines including less complicated technologies of production, high stability, the capability to modify genes encoding desired antigen/s, the ability to make changes in the cellular localization of an antigen by means of adding or removing signal sequences or transmembrane domains, and the ability to target the desired type of immune response. However, the application of DNA immunization methods used in mice did not provide encouraging results in humans or large animals.41 More specifically, although DNA vaccines can be immunogenic without any adjuvant, the efficacy of in vivo transfection of DNA vaccines in humans and large animal species is low and delivery devices such as gene gun or electroporation (EP) are required to make these vaccines immunogenic in humans.42,43 The gene gun delivers gold particles coated with plasmid into the epidermal and dermal layers of the skin.44 It is believed that gene gun directly delivers DNA into the cell and even the nucleus and that is why the immune responses can be induced with significantly lower doses of naked DNA than in other delivery systems.41 Based on this we tested Aβ42-based DNA vaccine strategy and demonstrated the immunogenicity of this vaccine in wild type mice.45 Dr Rozenberg’s group also showed that gene-gun–administration of Aβ42 dimer gene can effectively elicit humoral immune responses not only in wild type, but also in APP/Tg mice.46 While this and other groups continue to test DNA vaccines-based on full-length Aβ4247-50 in preclinical models of AD we decided to move to another direction. More specifically, to avoid potentially harmful autoreactive Th cell responses generated by full-length Aβ42 (AN1792), we designed a DNA epitope vaccine composed of 3Aβ11 and a non-self, universal Th cell epitope, PADRE.20,51,52 Other groups supported this strategy for DNA vaccines against AD using short peptides spanning Aβ42 and various viral53,54 and non viral carriers.55
More recently, we hypothesized that to make this vaccine more immunogenic in humans with highly polymorphic MHC genes additional universal Th epitopes may be needed. Accordingly we developed a novel MultiTEP platform based DNA epitope vaccines, AV-1955D and AV-1959D and tested the efficacy of these vaccines in mice, rabbits, and rhesus macaques.38,56,57 In these studies we decided to enhance immune responses to DNA vaccinations with electroporation device from Ichor Medical Systems acceptable for humans instead of using gen gun system from Bio Rad that can be used only for animals. It was shown that EP destabilizes the cell membrane for a short time period to allow DNA to enter the cells more efficiently.58 In fact, EP could increase gene expression in vivo by 100- to 1000-fold compared with needle injection of naked plasmid DNA59,60 inducing a strong immune response to DNA vaccines. Importantly, EP-mediated delivery of DNA vaccines is now being tested for safety and immunogenicity in several phase I clinical trials (http://www.clinicaltrials.gov). Although, EP delivery of DNA vaccines, AV-1955D and AV-1959D activated both humoral and cellular immune responses in all tested species the most interesting data have been developed in monkeys.38,56,57 More specifically, data showed that both vaccines activated a broad and individualized repertoire of Th cells specific to peptides from different pathogens incorporated into the MultiTEP platform design and induced high titers of potentially protective anti-Aβ antibodies. We further hypothesized that MultiTep platform based vaccine may (1) provide broad coverage of human population with highly polymorphic MHC class molecules and (2) activate in vaccinated subjects pre-existing memory T cells, formed after conventional vaccinations and infections received during the lifespan. Finally, recruitment of memory T cells may overcome nonresponsiveness of elderly people to new vaccines due to immunosenescence.
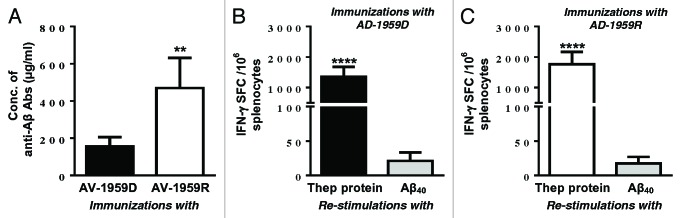
Recently, we decided to compare the immunogenic efficacy of DNA-based vaccine, AV-1959D to homologous protein-based vaccine, AV-1959R in wild type mice. Delivered by EP device AV-1959D vaccine induces cellular immune responses comparable with that generated after immunizations of mice with AV-1959R formulated in a strong adjuvant, Quil-A (analog of QS21 for animals) (Fig. 1 A and B). As shown in Figure 1C, both vaccines also induced strong humoral immune responses after 3 immunizations, however AV-1959R generated significantly higher levels of anti-Aβ antibodies than DNA vaccine, AV-1959D. We believe that this superior antibody response might be associated with Quil-A, which is a strong, Th1-type adjuvant. In fact, our recent data generated with the same, AV-1959D vaccine delivered by AgilePulse in vivo EP system form Cellectis (SA/BTX-Harvard Apparatus) supported this hypothesis, since vaccinated mice of the same haplotype induces significantly higher humoral immune responses (data not shown, paper in preparation). Based on these data we concluded that both DNA- and protein-based AD epitope vaccines described above can be immunogenic in humans if appropriate adjuvant or delivery system will be used in clinical trials.
Figure 1. Humoral and cellular immune responses generated in mice by DNA-based epitope vaccine using TDS-IM EP system and protein-based AD epitope vaccine formulated with Quil-A adjuvant. (A and B) Cellular responses are specific to Thep protein, but not Aβ40 peptide. Splenocytes were re-stimulated with 10 µg/mL protein or Aβ40 peptide. (C) Concentrations of anti-Aβ antibodies were detected after 3rd immunizations in sera from individual mice. Bars indicate average ± SD (n = 5 per group, **P ≤ 0.01, ****P ≤ 0.0001).
DNA- and Protein-Based AD Epitope Vaccines and Liposomes for Enhancing of Immune Responses
Cationic mannosylated liposomes are very promising adjuvants and delivery systems for DNA, proteins and other biological molecules and drugs. They are discrete particulate structures based on lipid bilayers with characteristics that depend on the exact components and the protocol of manufacturing. Entrapment of protein/peptide antigen or DNA encoding the antigen into the liposomes can protect them from interaction with plasma, alter pharmacokinetic characteristics and the distribution compared with free compounds. These changes may lead to more effective uptake of antigen by APC and longer half-life of antigen therefore increasing immune responses. Previously it was shown that mice injected with liposome containing plasmid encoding HBsAg induced much greater (up to 100-fold) antibody responses against the encoded antigen than animals immunized with the naked DNA.61 In addition, we studied the effects of various combinations of DNA and protein vaccines entrapped in the liposomes on the antibody response using hemagglutinin and hepatitis-B surface antigen. We observed a strong synergistic effect on the immune response when protein antigen and DNA encoding the same antigen were entrapped in the same liposomal compartment. This synergistic response was not seen when the protein and DNA materials were contained in separate liposomal vehicles and administered as a combination immunisation dose suspension.62 Here we decided to test this strategy for enhancing of immune responses against self-antigens such as Aβ peptide.
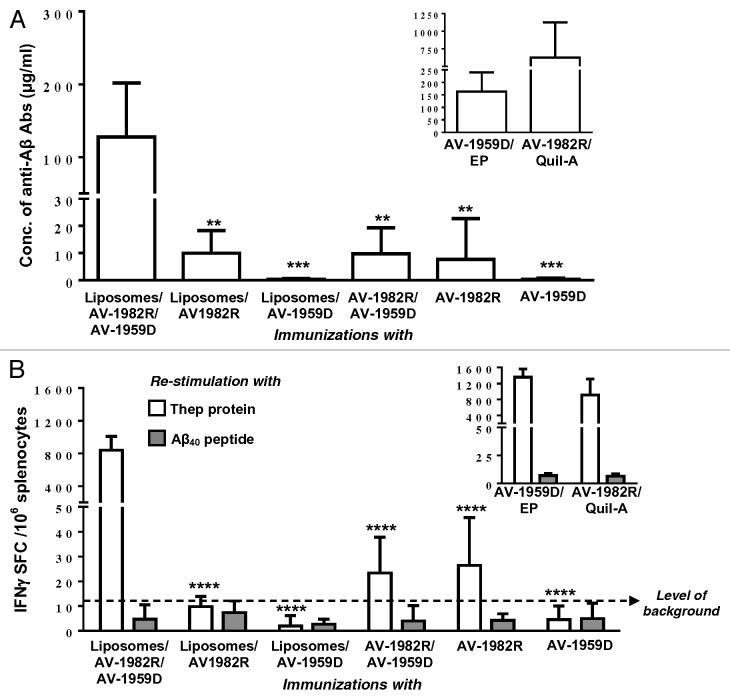
More specifically, wild-type mice were immunized with liposomes containing DNA vaccine, protein vaccine, and DNA/protein together. Briefly, Liposomes were prepared from >95% purity lipid from Lipoid GmbH as described previously.62 The liposomes were characterized in terms of particle size / zeta potential, and DNA/ protein dose content (Table 2). As DNA vaccine we chose AV-1959R, while, to avoid the interference with endotoxins, in this study we used our GMP grade protein vaccine composed of 3 copies of Aβ1–11 fused to promiscuous synthetic Th epitope PADRE and universal epitope from tetanus toxin, P30 (designated as AV-1982R). As control groups, mice were immunized with naked AV-1959D, AV-1982R, and mix of both. In addition, as a positive control, mice were immunized with AV-1982R formulated in Quil-A adjuvant and AV-1959D delivered by EP device. As shown in Figure 2A, AV-1959D and AV-1982R entrapped into the liposomes together induced significantly higher anti-Aβ antibody response compared with either liposomes/AV1959D or liposomes/AV1982R. We observed also significantly higher immune response in mice immunized with liposome containing DNA and protein vaccines regardless are they used alone or in combination. Animals immunized with AV-1959D/EP generated comparable level of humoral immune responses to vaccinations with liposome containing DNA and protein based vaccines, in contrary to immunizations with AV-1982R/Quil-A (Fig. 2A, inscribed Figure).
Table 2. Liposomal dose formulations composition and characterization.
| Compositiona, mg | Dose characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Liposomal Formulation | SUV (lipid) |
DNA (AV-1959D) |
Protein (AV-1982R) |
Sucrose | Size nm, PDI |
Zeta mV |
DNA dose µg |
Protein dose µg |
| A (DNA and Protein) | 6.250 | 0.025 | 0.050 | 18.750 | 149 ± 2, 0.46 ± 0.05 |
18 ± 4 | 9.7 ± 1.0 | 18.6 ± 0.8 |
| B (DNA) | 6.250 | 0.025 | Nil | 18.750 | 134 ± 9, 0.35 ± 0.09 |
18 ± 5 | 10.0 ± 0.3 | NA |
| C (Protein) | 6.250 | Nil | 0.050 | 18.750 | 112 ± 3, 0.25 ± 0.01 |
17 ± 8 | NA | 22.3 ± 2.5 |
2.5 dose vial fill, ave ± stdev with n > 3.
Figure 2. Humoral and cellular immune responses generated by different formulations of protein- and DNA-based AD epitope vaccines, AV-1982R and AV-1959D, respectively. (A) Concentrations of anti-Aβ antibodies were detected after 3rd immunizations in sera from individual mice. (B) Cellular responses are specific to Thep protein, but not Aβ40 peptide. Splenocytes were re-stimulated with 10 µg/mL Thep protein or Aβ40 peptide. Bars indicate average ± SD. Statistical differences in all groups were calculated relative to Liposomes/AV-1982R/AV-1959D immunized group using two-tailed t test (n = 6 per group, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
Cellular immune responses specific to Th epitopes or to Aβ40 peptide were analyzed in splenocytes of immunized mice re-stimulated in vitro with recombinant protein composed of Th epitopes as well as with Aβ40 peptide. Again, in splenocytes of mice immunized with AV1959D and AV-1982R entraped into the liposomes together we detected significantly higher number of splenocytes producing IFNγ than in splenocytes of mice from other groups. Importantly, no Aβ-specific response was seen in all mice (Fig. 2B). Positive control groups of mice generated similar level of cellular responses after immunization with AV-1959D/EP or AV-1982R/Quil-A (Fig. 2B, inscribed Figure). This new vaccination approach has been termed “co-delivery” and may derive from the simultaneous presentation of antigen via MHC class-I (DNA) and MHC class-II (protein) pathways to CD8+ and CD4+ cells at the same antigen presenting cell -a mode of presentation that would commonly occur with live viral pathogens. Additionally the liposome composition employed a surface presented mannose moiety, ManDOG lipid, to specifically target liposomal uptake in APCs (dendriric cells) via the mannose receptor.63 Although it is recognized an extensive formulation control immunological response study would be required to prove these mechanisms in this specific vaccine scenario.
Conclusions and Perspectives
These studies highlight the importance to consider the advantages and disadvantages of a particular approach to the development of vaccines in order to avoid undesirable side effects while achieving the desired result. It is obvious that protein based vaccine may be a better choice in case of availability of safe and effective adjuvant. Although, DNA based vaccines are less immunogenic but they are considered to be safe and if good EP device is available, they can be quite effective. Finally, liposomes containing DNA and Protein based vaccines together are interesting approach for development of AD vaccine, however are premature for translation into the clinic yet.
Disclosure of Potential Conflicts of Interest
A.B. is an employee and shareholder of Xenetic Biosciences.
Acknowledgments
We would like to thank Armine Hovakimyan and Arpine Davtyan for technical help. This work was supported by funding from NIH (R01-NS050895 and R01-AG020241) and Alzheimer Association (IIRG-12–239626 and NIRG-13–281227). The β-amyloid peptide used in this project was provided by the University of California Alzheimer's Disease Research Center (UCI-ADRC) that is funded by NIH/NIA Grant P50 AG16573.
References
- 1.Dementia WHO. key facts. http://www.who.int/mediacentre/factsheets/fs362/en/, 2012.
- 2.Price DL, Sisodia SS. . Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med 1994; 45:435 - 46; http://dx.doi.org/ 10.1146/annurev.med.45.1.435; PMID: 8198393 [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. . The molecular pathology of Alzheimer’s disease. Neuron 1991; 6:487 - 98; http://dx.doi.org/ 10.1016/0896-6273(91)90052-2; PMID: 1673054 [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. . Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol 1994; 53:438 - 47; http://dx.doi.org/ 10.1097/00005072-199409000-00003; PMID: 8083687 [DOI] [PubMed] [Google Scholar]
- 5.Esler WP, Wolfe MS. . A portrait of Alzheimer secretases--new features and familiar faces. Science 2001; 293:1449 - 54; http://dx.doi.org/ 10.1126/science.1064638; PMID: 11520976 [DOI] [PubMed] [Google Scholar]
- 6.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, et al. . Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003; 61:46 - 54; http://dx.doi.org/ 10.1212/01.WNL.0000073623.84147.A8; PMID: 12847155 [DOI] [PubMed] [Google Scholar]
- 7.Imbimbo BP, Ottonello S, Frisardi V, Solfrizzi V, Greco A, Seripa D, Pilotto A, Panza F. . Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease. Expert Rev Clin Immunol 2012; 8:135 - 49; http://dx.doi.org/ 10.1586/eci.11.93; PMID: 22288451 [DOI] [PubMed] [Google Scholar]
- 8.Kerchner GA, Boxer AL. . Bapineuzumab. Expert Opin Biol Ther 2010; 10:1121 - 30; http://dx.doi.org/ 10.1517/14712598.2010.493872; PMID: 20497044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delrieu J, Ousset PJ, Vellas B. . Gantenerumab for the treatment of Alzheimer’s disease. Expert Opin Biol Ther 2012; 12:1077 - 86; http://dx.doi.org/ 10.1517/14712598.2012.688022; PMID: 22583155 [DOI] [PubMed] [Google Scholar]
- 10.Lemere CA. . Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener 2013; 8:36; http://dx.doi.org/ 10.1186/1750-1326-8-36; PMID: 24148220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eli Lilly and Company Announcment. Eli Lilly and Company Announces Top-Line Results on Solanezumab Phase 3 Clinical Trials in Patients with Alzheimer's Disease. Available at http://newsroom.lilly.com/releasedetail.cfm?releaseid=702211, 2012.
- 12.Sperling R, Salloway S, Raskind MA, Ferris S, Liu E, Yuen E, et al. A randomized double-blind, placebocontrolled clinical trila of intravenous bapineuzumab in patients with Alzheimer's disease who are apolipoprotein E ϵ4 carriers. 16th Congress of the European Federation of Neurological Societies. Stokholm, Sweden, 2012.
- 13.Salloway S, Sperling R, Honig L, Porsteinsson A, Sabbagh M, Liu E, et al. A randomized double-blind, placebocontrolled clinical trila of intravenous bapineuzumab in patients with Alzheimer's disease who are apolipoprotein E ϵ4 non-carriers. 16th Congress of the European Federation of Neurological Societies. Stokholm, Sweden, 2012.
- 14.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, Friedrich S, Dean RA, Gonzales C, Sethuraman G, et al. . Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement 2012; 8:261 - 71; http://dx.doi.org/ 10.1016/j.jalz.2011.09.224; PMID: 22672770 [DOI] [PubMed] [Google Scholar]
- 15.NIH. Gantenerumab for Prodromal Alzheimer's Disease. http://www.nia.nih.gov/alzheimers/clinical-trials/gantenerumab-prodromal-alzheimers-disease.
- 16.NIH. Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or With a Type of Early Onset Alzheimer's Disease Caused by a Genetic Mutation. (DIAN TU). http://clinicaltrials.gov/ct2/show/NCT01760005.
- 17.NIH. NIH-supported Alzheimer’s studies to focus on innovative treatments. http://www.nih.gov/news/health/jan2013/nia-14.htm. 2013.
- 18.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. . Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol 2005; 174:1580 - 6; PMID: 15661919 [DOI] [PubMed] [Google Scholar]
- 19.Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. . Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine 2006; 24:2275 - 82; http://dx.doi.org/ 10.1016/j.vaccine.2005.11.039; PMID: 16368167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davtyan H, Mkrtichyan M, Movsesyan N, Petrushina I, Mamikonyan G, Cribbs DH, Agadjanyan MG, Ghochikyan A. . DNA prime-protein boost increased the titer, avidity and persistence of anti-Abeta antibodies in wild-type mice. Gene Ther 2010; 17:261 - 71; http://dx.doi.org/ 10.1038/gt.2009.140; PMID: 19865176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, Marleau AM, Movsesyan N, Kiyatkin A, Rasool S, et al. . Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer’s disease: prelude to a clinical trial. J Neurosci 2013; 33:4923 - 34; http://dx.doi.org/ 10.1523/JNEUROSCI.4672-12.2013; PMID: 23486963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG. . Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J Neurosci 2007; 27:12721 - 31; http://dx.doi.org/ 10.1523/JNEUROSCI.3201-07.2007; PMID: 18003852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delrieu J, Ousset PJ, Caillaud C, Vellas B. . ‘Clinical trials in Alzheimer’s disease’: immunotherapy approaches. J Neurochem 2012; 120:Suppl 1 186 - 93; http://dx.doi.org/ 10.1111/j.1471-4159.2011.07458.x; PMID: 21883222 [DOI] [PubMed] [Google Scholar]
- 24.Lobello K, Ryan JM, Liu E, Rippon G, Black R. . Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int J Alzheimers Dis 2012; 2012:628070; http://dx.doi.org/ 10.1155/2012/628070; PMID: 22292124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemere CA, Masliah E. . Can Alzheimer disease be prevented by amyloid-beta immunotherapy?. Nat Rev Neurol 2010; 6:108 - 19; http://dx.doi.org/ 10.1038/nrneurol.2009.219; PMID: 20140000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman DT, López-Deber MP, Ndao DM, Silva AB, Nand D, Pihlgren M, Giriens V, Madani R, St-Pierre A, Karastaneva H, et al. . Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem 2011; 286:13966 - 76; http://dx.doi.org/ 10.1074/jbc.M110.186338; PMID: 21343310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhs A, Hickman DT, Pihlgren M, Chuard N, Giriens V, Meerschman C, van der Auwera I, van Leuven F, Sugawara M, Weingertner MC, et al. . Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc Natl Acad Sci U S A 2007; 104:9810 - 5; http://dx.doi.org/ 10.1073/pnas.0703137104; PMID: 17517595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman JLUS. Food and Drug Administration Workshop: Adjuvants and Adjuvanted Preventive and Therapeutic Vaccines for Infectious Disease Indications 2008. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/UCM095732.pdf
- 29.Baylor NW, Egan W, Richman P. . Aluminum salts in vaccines--US perspective. Vaccine 2002; 20:Suppl 3 S18 - 23; http://dx.doi.org/ 10.1016/S0264-410X(02)00166-4; PMID: 12184360 [DOI] [PubMed] [Google Scholar]
- 30.Rappuoli R, Mandl CW, Black S, De Gregorio E. . Vaccines for the twenty-first century society. Nat Rev Immunol 2011; 11:865 - 72; PMID: 22051890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galimberti D, Ghezzi L, Scarpini E. . Immunotherapy against amyloid pathology in Alzheimer’s disease. J Neurol Sci 2013; 333:50 - 4; http://dx.doi.org/ 10.1016/j.jns.2012.12.013; PMID: 23299047 [DOI] [PubMed] [Google Scholar]
- 32.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, et al. . Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol 2012; 11:597 - 604; http://dx.doi.org/ 10.1016/S1474-4422(12)70140-0; PMID: 22677258 [DOI] [PubMed] [Google Scholar]
- 33.Schneeberger A, Mandler M, Mattner F, Schmidt W. . AFFITOME® technology in neurodegenerative diseases: the doubling advantage. Hum Vaccin 2010; 6:948 - 52; http://dx.doi.org/ 10.4161/hv.6.11.13217; PMID: 20980801 [DOI] [PubMed] [Google Scholar]
- 34.International WHO. Clinical Trials. In: GmbH NP, ed., http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2009-012394-35-DE, 2010.
- 35.Genetic Engineering and Biotechnology News. AFFiRiS Invests in Public-Funded Lab to Develop Vaccine Against Diabetes and Cardiometabolic Diseases. http://www.genengnews.com/keywordsandtools/print/4/23255/.
- 36.NIH. Study to Evaluate Safety, Tolerability and Immunogenicity of Vaccine (UB 311) in Subjects With Alzheimer's Disease. http://clinicaltrials.gov/ct2/show/NCT00965588.
- 37.Savage MJ, Wu GY, McCampbell A, Wessner KR, Citron M, Liang X, et al. . A novel multivalent Abeta peptide vaccine with preclinical evidence of a central immune response that generates antisera recognizing a wide range of abeta peptide species. Alzheimers Dement 2010; 6:S142; http://dx.doi.org/ 10.1016/j.jalz.2010.05.437 [DOI] [Google Scholar]
- 38.Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Cribbs DH, Agadjanyan MG. . The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement 2014; Forthcoming http://dx.doi.org/ 10.1016/j.jalz.2013.12.003; PMID: 24560029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.H. Lundbeck A/S. Lundbeck and Otsuka to co-develop a vaccine, Lu AF20513, their third collaborative development project to tackle Alzheimer's disease. http://investor.lundbeck.com/releasedetail.cfm?ReleaseID=813105. 2013.
- 40.Mura T, Proust-Lima C, Jacqmin-Gadda H, Akbaraly TN, Touchon J, Dubois B, Berr C. . Measuring cognitive change in subjects with prodromal Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2013; Forthcoming http://dx.doi.org/ 10.1136/jnnp-2013-305078; PMID: 23840054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babiuk LA, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. . Induction of immune responses by DNA vaccines in large animals. Vaccine 2003; 21:649 - 58; http://dx.doi.org/ 10.1016/S0264-410X(02)00574-1; PMID: 12531334 [DOI] [PubMed] [Google Scholar]
- 42.Li L, Saade F, Petrovsky N. . The future of human DNA vaccines. J Biotechnol 2012; 162:171 - 82; http://dx.doi.org/ 10.1016/j.jbiotec.2012.08.012; PMID: 22981627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. . Clinical applications of DNA vaccines: current progress. Clin Infect Dis 2011; 53:296 - 302; http://dx.doi.org/ 10.1093/cid/cir334; PMID: 21765081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregersen JP. . DNA vaccines. Naturwissenschaften 2001; 88:504 - 13; http://dx.doi.org/ 10.1007/s00114-001-0270-2; PMID: 11824223 [DOI] [PubMed] [Google Scholar]
- 45.Ghochikyan A, Vasilevko V, Petrushina I, Movsesyan N, Babikyan D, Tian W, Sadzikava N, Ross TM, Head E, Cribbs DH, et al. . Generation and characterization of the humoral immune response to DNA immunization with a chimeric beta-amyloid-interleukin-4 minigene. Eur J Immunol 2003; 33:3232 - 41; http://dx.doi.org/ 10.1002/eji.200324000; PMID: 14635031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu B, Rosenberg RN, Li L, Boyer PJ, Johnston SA. . Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease. Arch Neurol 2004; 61:1859 - 64; http://dx.doi.org/ 10.1001/archneur.61.12.1859; PMID: 15596606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto Y, Niimi N, Kohyama K. . Development of a new DNA vaccine for Alzheimer disease targeting a wide range of aβ species and amyloidogenic peptides. PLoS One 2013; 8:e75203; http://dx.doi.org/ 10.1371/journal.pone.0075203; PMID: 24086465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lambracht-Washington D, Qu BX, Fu M, Anderson LD Jr., Eagar TN, Stüve O, Rosenberg RN. . A peptide prime-DNA boost immunization protocol provides significant benefits as a new generation Aβ42 DNA vaccine for Alzheimer disease. J Neuroimmunol 2013; 254:63 - 8; http://dx.doi.org/ 10.1016/j.jneuroim.2012.09.008; PMID: 23036592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu BX, Xiang Q, Li L, Johnston SA, Hynan LS, Rosenberg RN. . Abeta42 gene vaccine prevents Abeta42 deposition in brain of double transgenic mice. J Neurol Sci 2007; 260:204 - 13; http://dx.doi.org/ 10.1016/j.jns.2007.05.012; PMID: 17574274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian S, Divya Shree AN. . Enhanced Th2 immunity after DNA prime-protein boost immunization with amyloid beta (1-42) plus CpG oligodeoxynucleotides in aged rats. Neurosci Lett 2008; 436:219 - 22; http://dx.doi.org/ 10.1016/j.neulet.2008.03.024; PMID: 18394801 [DOI] [PubMed] [Google Scholar]
- 51.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. . Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS One 2008; 3:e2124; http://dx.doi.org/ 10.1371/journal.pone.0002124; PMID: 18461171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davtyan H, Ghochikyan A, Movsesyan N, Ellefsen B, Petrushina I, Cribbs DH, Hannaman D, Evans CF, Agadjanyan MG. . Delivery of a DNA vaccine for Alzheimer’s disease by electroporation versus gene gun generates potent and similar immune responses. Neurodegener Dis 2012; 10:261 - 4; http://dx.doi.org/ 10.1159/000333359; PMID: 22301697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olkhanud PB, Mughal M, Ayukawa K, Malchinkhuu E, Bodogai M, Feldman N, Rothman S, Lee JH, Chigurupati S, Okun E, et al. . DNA immunization with HBsAg-based particles expressing a B cell epitope of amyloid β-peptide attenuates disease progression and prolongs survival in a mouse model of Alzheimer’s disease. Vaccine 2012; 30:1650 - 8; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.136; PMID: 22248819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HD, Jin JJ, Maxwell JA, Fukuchi K. . Enhancing Th2 immune responses against amyloid protein by a DNA prime-adenovirus boost regimen for Alzheimer’s disease. Immunol Lett 2007; 112:30 - 8; http://dx.doi.org/ 10.1016/j.imlet.2007.06.006; PMID: 17686533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu YZ, Wang S, Bai JY, Zhao M, Chen A, Wang WB, Chang Q, Liu S, Qiu WY, Pang XB, et al. . Effective DNA epitope chimeric vaccines for Alzheimer’s disease using a toxin-derived carrier protein as a molecular adjuvant. Clin Immunol 2013; 149:11 - 24; http://dx.doi.org/ 10.1016/j.clim.2013.05.016; PMID: 23886550 [DOI] [PubMed] [Google Scholar]
- 56.Ghochikyan A, Davtyan H, Petrushina I, Hovakimyan A, Movsesyan N, Davtyan A, Kiyatkin A, Cribbs DH, Agadjanyan MG. . Refinement of a DNA based Alzheimer’s disease epitope vaccine in rabbits. Hum Vaccin Immunother 2013; 9:1002 - 10; http://dx.doi.org/ 10.4161/hv.23875; PMID: 23399748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG, Ghochikyan A. . Epitope-based DNA vaccine for Alzheimer’s disease: Translational study in macaques. Alzheimers Dement 2013; Forthcoming http://dx.doi.org/ 10.1016/j.jalz.2013.04.505; PMID: 23916838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banga AK, Prausnitz MR. . Assessing the potential of skin electroporation for the delivery of protein- and gene-based drugs. Trends Biotechnol 1998; 16:408 - 12; http://dx.doi.org/ 10.1016/S0167-7799(98)01238-4; PMID: 9807837 [DOI] [PubMed] [Google Scholar]
- 59.Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, Leung L, Otten GR, Thudium K, Selby MJ, et al. . Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000; 164:4635 - 40; PMID: 10779767 [DOI] [PubMed] [Google Scholar]
- 60.Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, et al. . Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A 1999; 96:6417 - 22; http://dx.doi.org/ 10.1073/pnas.96.11.6417; PMID: 10339602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregoriadis G, Bacon A, Caparros-Wanderley W, McCormack B. . A role for liposomes in genetic vaccination. Vaccine 2002; 20:Suppl 5 B1 - 9; http://dx.doi.org/ 10.1016/S0264-410X(02)00514-5; PMID: 12477411 [DOI] [PubMed] [Google Scholar]
- 62.Laing P, Bacon A, McCormack B, Gregoriadis G, Frisch B, Schuber F. . The ‘co-delivery’ approach to liposomal vaccines: application to the development of influenza-A and hepatitis-B vaccine candidates. J Liposome Res 2006; 16:229 - 35; http://dx.doi.org/ 10.1080/08982100600880432; PMID: 16952877 [DOI] [PubMed] [Google Scholar]
- 63.Espuelas S, Thumann C, Heurtault B, Schuber F, Frisch B. . Influence of ligand valency on the targeting of immature human dendritic cells by mannosylated liposomes. Bioconjug Chem 2008; 19:2385 - 93; http://dx.doi.org/ 10.1021/bc8002524; PMID: 19053315 [DOI] [PubMed] [Google Scholar]