Abstract
We evaluated the cell-mediated immune (CMI) response to RSV acute infection including the magnitude, kinetics and correlates with morbidity and age. Twenty-nine RSV-infected patients with mean ± SD age of 15 ± 14 months were enrolled during their first week of disease. Th1, Th2, Th9, Th17 and Th22 responses were measured at entry and 2 and 6 weeks later. All subjects were hospitalized for a median (range) of 5 (3–11) days. RSV-specific effector and memory Th1 CMI measured by lymphocyte proliferation and IFNγ ELISPOT significantly increased over time (P ≤ 0.03). In contrast, Th22 responses decreased over time (P ≤ 0.03). Other changes did not reach statistical significance. The severity of RSV disease measured by the length of hospitalization positively correlated with the magnitude of Th9, Th22 and TNFα inflammatory responses (rho ≥ 0.4; P ≤ 0.04) and negatively with memory CMI (rho = –0.45; P = 0.04). The corollary of this observation is that robust Th1 and/or low Th9, Th22, and TNFα inflammatory responses may be associated with efficient clearance of RSV infection and therefore desirable characteristics of an RSV vaccine. Young age was associated with low memory and effector Th1 responses (rho ≥ 0.4; P ≤ 0.04) and high Th2, Th9, Th17, Th22 and TNFα inflammatory responses (rho ≤ –0.4; P ≤ 0.04), indicating that age at vaccination may be a major determinant of the CMI response pattern.
Keywords: Th1, Th17, Th2, Th22, Th9, cell-mediated immunity, morbidity, respiratory syncytial virus
Introduction
RSV is the leading cause of respiratory disease in infants and young children worldwide, with almost all children being infected within their first 2 y of life.1 A recent meta-analysis estimated that in 2005, at least 33.8 million episodes of RSV-associated acute lower respiratory infections occurred worldwide in children younger than 5 y.2 RSV can also cause life-threatening disease in elderly patients, immunocompromised patients and adults with cardiopulmonary disease.3
Despite over 4 decades of research, there are currently no licensed vaccines for the prevention of RSV disease. Ideally, an RSV vaccine should generate both antibody responses, which are required for protection against RSV infection, and T-cell-mediated cellular responses, which have a critical role in viral clearance. This goal may be met by live-attenuated RSV vaccines, which are currently in clinical trials.4,5 A complete understanding of the T cell-mediated response to natural RSV infection may help in the optimization of RSV candidate vaccines.
The memory CD4+ T cell response to RSV includes T helper 1 (Th1), Th2 and more recently described Th9 and Th17 cells. Th cells are defined by the cytokines that they secrete: Th1 cytokines typically include interleukin-2 (IL-2), TNFα, IFNγ, and IL-12; Th2 cytokines are represented by IL-4, IL-5 and IL-13,6,7 Th9 by IL-9,8,9 Th17 by IL6, IL-17A and F10,11 and Th22 by IL-22.12,13 There is some overlap in cytokine production between different types of cells such that Th17 cells were shown to sometimes produce TNFα and/or IFNγ14,15
Th1 lymphocytes have important roles in immune modulation and function and, to some extent, are self-stimulatory and inhibit other responses.16 Th1 responses are generally associated with reduced disease during RSV infection.17 CD8+ Th1 cells that secrete perforin and TNFα play a key role in viral clearance,18 and in infected lungs of mice were noted to suppress the development of Th2 responses, thus limiting the development of pulmonary eosinophilia.19
By contrast, Th2 and Th9 responses, particularly those represented by IL-9 and IL-13, were shown to contribute to the severity of the RSV disease in mice by generating eosinophilia, goblet cell hyperplasia and mucus overproduction,16,20 and to contribute to the pathogenesis of RSV bronchiolitis in humans.21 Recent studies in mice and humans have also postulated that Th9 may play a critical function in the regulation of allergic asthma and inflammation.22-25
The recently described T cell subsets Th17 and Th2211,13 have an important role in the pathogenesis of inflammatory disorders of the gastrointestinal tract and other autoimmune diseases,13,26 but also a protective role against candida infections. Little is known about the role of these two subsets in viral infections, including RSV. Th17 might inhibit virus-induced cell apoptosis, resulting in enhanced viral replication and persistence in mice.27 However, earlier studies in mice showed that IL-17 promoted clearance of vaccinia virus infection.28 Further studies are required, especially in humans, to understand if the Th17 and Th22 subsets play a role in the pathogenesis of RSV infections.
The goal of our study was to inform RSV vaccine development by characterizing the kinetics of Th1, Th2, Th9, Th17, and Th22 CD4+ and CD8+ T cells responses to RSV during the first 6 wk of infection and by evaluating correlations of cell-mediated immunity (CMI) measures with the severity of the disease.
Results
Demographic characteristics
We enrolled 29 RSV-infected children, of whom 20 completed all 3 visits (69%). The average age ± SD at enrollment was 15 ± 14 mo (Table 1). The study included 59% males, 59% white, and 34% black subjects. All participants were hospitalized for an average of 5 d (range of 3 to 11 d). Five also required ICU admission. Three participants (10%) had viral co-infections with either parainfluenza virus 3 or entero/rhinovirus. In 4 cases (14%), participants had been on inhaled corticosteroids for asthma prior to admission. Only 2 participants had received Synagis® (MedImmune) prior to admission. All 29 participants required oxygen on admission and 12 (41%) were discharged on oxygen.
Table 1. Demographic and clinical characteristics.
| Characteristic | Measure |
|---|---|
| N subjects enrolled (%) | 29 (100) |
| N subjects who completed all 3 visits (%) | 20 (69) |
| N males (%) | 17 (59) |
| Months of age: Mean ± SD | 15 ± 14 |
| N (%) <6 months | 12 (41) |
| 6-12 months | 4 (14) |
| 13-24 months | 9 (31) |
| 25-60 months | 4 (14) |
| Race/Ethnicity: N (%) | |
| White | 17 (59) |
| Black | 10 (34) |
| Hispanic | 2 (7) |
| Days of hospitalization: mean (range) | 5 (3; 11) |
| N (%) ICU admission | 5 (17) |
| N (%) Co- Infections | 3 (10) |
| N (%) Oxygen requirement admission | 29 (100) |
| N (%) Oxygen requirement discharge | 12 (41) |
Kinetics of memory responses measured by Lymphocyte Proliferation Assay (LPA)
RSV-specific LPA increased over time from median simulation index (SI) (interquartile range [IQR]) of 3 (2; 10) at week 1 to 30 (11; 63) at week 2 and to 26 (8; 56) at week 6 (P < 0.001 for weeks 1 vs. 2 or 6; Figure 1). There were no significant changes over time in the SI’s of pokeweed mitogen (PWM)-stimulated cultures.

Figure 1. LPA responses to RSV during acute infection and convalescence. Data were derived from 29 subjects with acute RSV disease. Panel (A) shows SI calculated by dividing median cpm of RSV-stimulated by control wells; and panel (B) shows adjusted cpm calculated by subtracting median cpm of control from RSV-stimulated wells. N indicates the number of subjects who contributed data at each time point. Bars represent median and interquartile ranges. P values were calculated using Wilcoxon matched signed-rank test and are shown only for significant differences (P < 0.05).
Kinetics of effector Th1 responses measured by IFNγ and IL-2 ELISPOT
IFNγ ELISPOT, measured on 26 samples for week 1 and 2 and 20 samples for week 6, increased from a median (interquartile range [IQR]) of 0 (0; 2) to 5 (1; 8) SFC/106 PBMC from week 1 to week 2 (Fig. 2: Panel A; P = 0.03) followed by a plateau up to week 6 (4 [0; 8] SFC/106 PBMC). Median (IQR) IL-2 ELISPOT responses were 0 (0; 4) at entry, 3 (0; 10) at week 2, and 6 (0.5; 17) SFC/106 PBMC at week 6, but the differences were not statistically significant (Fig. 2; Panel B). There were no significant changes in IFNγ or IL-2 responses to phytohemagglutinin (PHA) mitogen or in unstimulated wells over time.

Figure 2. IFNγ (A) and IL-2 (B) ELISPOT responses during acute RSV infection and convalescence. Data were derived from 29 subjects with acute RSV disease. Results are reported as spot forming cells (SFC) per 10^6 PBMC. Bars represent median and interquartile ranges. N indicates the number of subjects who contributed data at each time point. P values were calculated using Wilcoxon matched signed-rank test and are shown only for significant differences (P < 0.05).
Th1, Th2, Th9, Th17 and Th22 responses measured by flow cytometry
Th1 responses, represented by TNFα and perforin production were already present at week 1 (Table 2). RSV-specific CD4+ and CD8+TNFα+% tended to decrease from week 1 to week 6. Although none of the changes reached statistical significance, there was a strong decreasing trend for CD8+TNFα+% from week 1 to week 6 (P = 0.08). CD4+ and CD8+perforin+% tended to increase from week 1 to week 6. However, the only difference that approached statistical significance was for CD8+perforin+% between weeks 2 and 6 (P = 0.07).
Table 2. RSV-specific T cell responses during the first 6 weeks of disease.
| Parameter (%) & Study visit | N | Median | IQR |
P value wk 1 vs. wk 2 |
P value wk 1 vs. wk 6 |
P value wk 2 vs. wk 6 |
|---|---|---|---|---|---|---|
| CD4+Perforin+ | ||||||
| Week 1 | 25 | 3.5 | 2.5; 5.2 | 0.72 | 0.2 | 0.43 |
| Week 2 | 24 | 4 | 2.9; 5.4 | |||
| Week 6 | 20 | 4.5 | 5.5; 5.7 | |||
| CD8+Perforin+ | ||||||
| Week 1 | 25 | 5.1 | 3.2; 8.9 | 0.6 | 0.7 | 0.07 |
| Week 2 | 24 | 4.8 | 3.2; 7.0 | |||
| Week 6 | 20 | 5.4 | 4.3; 7.0 | |||
| CD4+TNFα+ | ||||||
| Week 1 | 25 | 1.5 | 0.4; 2.0 | 0.38 | 0.86 | 0.23 |
| Week 2 | 24 | 1.6 | 0.9; 2.6 | |||
| Week 6 | 20 | 1 | 0.8; 2.2 | |||
| CD8+TNFα+ | ||||||
| Week 1 | 25 | 1.6 | 1.1; 3.0 | 0.52 | 0.08 | 0.28 |
| Week 2 | 24 | 1.3 | 0.9; 2.2 | |||
| Week 6 | 20 | 1.2 | 0.8; 1.7 | |||
| CD4+IL9+ | ||||||
| Week 1 | 25 | 2.2 | 1.3; 2.7 | 0.4 | 0.1 | 0.5 |
| Week 2 | 24 | 2.6 | 1.9; 3.0 | |||
| Week 6 | 20 | 2.9 | 1.6; 4.0 | |||
| CD8+IL9+ | ||||||
| Week 1 | 25 | 2.4 | 1.9; 4.3 | 0.6 | 0.8 | 0.6 |
| Week 2 | 24 | 3.1 | 1.8; 4.4 | |||
| Week 6 | 20 | 3.6 | 2.1; 5.0 | |||
| CD4+IL13+ | ||||||
| Week 1 | 25 | 1.7 | 1.1; 2.0 | 0.7 | 0.8 | 0.8 |
| Week 2 | 24 | 1.5 | 1.0; 2.3 | |||
| Week 6 | 20 | 1.5 | 1.1; 2.7 | |||
| CD8+IL13+ | ||||||
| Week 1 | 25 | 2.1 | 1.3; 3.6 | 0.6 | 0.7 | 0.7 |
| Week 2 | 24 | 2.2 | 1.3; 3.1 | |||
| Week 6 | 20 | 2 | 1.2; 3.0 | |||
| CD4+IL17+ | ||||||
| Week 1 | 25 | 2 | 1.2; 2.8 | 0.3 | 0.8 | 0.4 |
| Week 2 | 24 | 2 | 1.5; 3.0 | |||
| Week 6 | 20 | 1.7 | 1.3; 2.7 | |||
| CD8+IL17+ | ||||||
| Week 1 | 25 | 2.7 | 0.6; 3.6 | 0.8 | 0.3 | 0.1 |
| Week 2 | 24 | 2.3 | 1.5; 4.0 | |||
| Week 6 | 20 | 2.2 | 1.7; 2.7 | |||
| CD4+IL22+ | ||||||
| Week 1 | 25 | 1.54 | 0.9; 2.0 | 0.3 | 0.6 | 0.03 |
| Week 2 | 24 | 1.37 | 1.0; 2.4 | |||
| Week 6 | 20 | 1.19 | 0.8; 1.7 | |||
| CD8+ IL22+ | ||||||
| Week 1 | 25 | 2.2 | 1.2; 3.1 | 0.27 | 0.01 | 0.01 |
| Week 2 | 24 | 1.975 | 1.3; 2.5 | |||
| Week 6 | 20 | 1.625 | 1.0; 2.2 | |||
Bold and italic underscore P values < 0.1 calculated by the Wilcoxon paired signed rank test.
RSV-specific Th9 and Th2 were detected at week 1 in most participants (Table 2). IL-9 expression tended to increase over time while IL-13 tended to decrease, but none of the differences reached statistical significance (P ≥ 0.1).
RSV-specific Th17 and Th22 responses were maximal at week 1 (Table 2). RSV-specific CD4+ and CD8+IL-17+% tended to decrease over time without reaching statistical significance (P ≥ 0.1). RSV-specific CD4+ and CD8+IL-22+% also decreased over time. Differences were significant for CD4+IL-22+% between weeks 2 and 3 (P = 0.03) and for CD8+IL-22+% between weeks 1 and 6 and weeks 2 and 6 (P = 0.01 for both).
There were no significant changes over time in unstimulated T cell subsets.
Correlation of T cell responses to RSV infection with the severity of the disease
We investigated the association of early RSV-specific Th1, Th2, Th9, Th17, or Th22 responses with the severity of the disease. Because all children were hospitalized and required O2 therapy on admission, we used the length of the hospitalization as our primary measure of the severity of the disease.
Hospitalization was longer in children with higher RSV-specific CD8+IL-9+% (rho = 0.40, P = 0.03, Fig. 3A) and CD4+IL-22+% (rho = 0.40, P = 0.04, Fig. 3B) during the first week of disease. There were no other significant correlations between early T-cell responses to RSV and duration of hospitalization (data not shown).
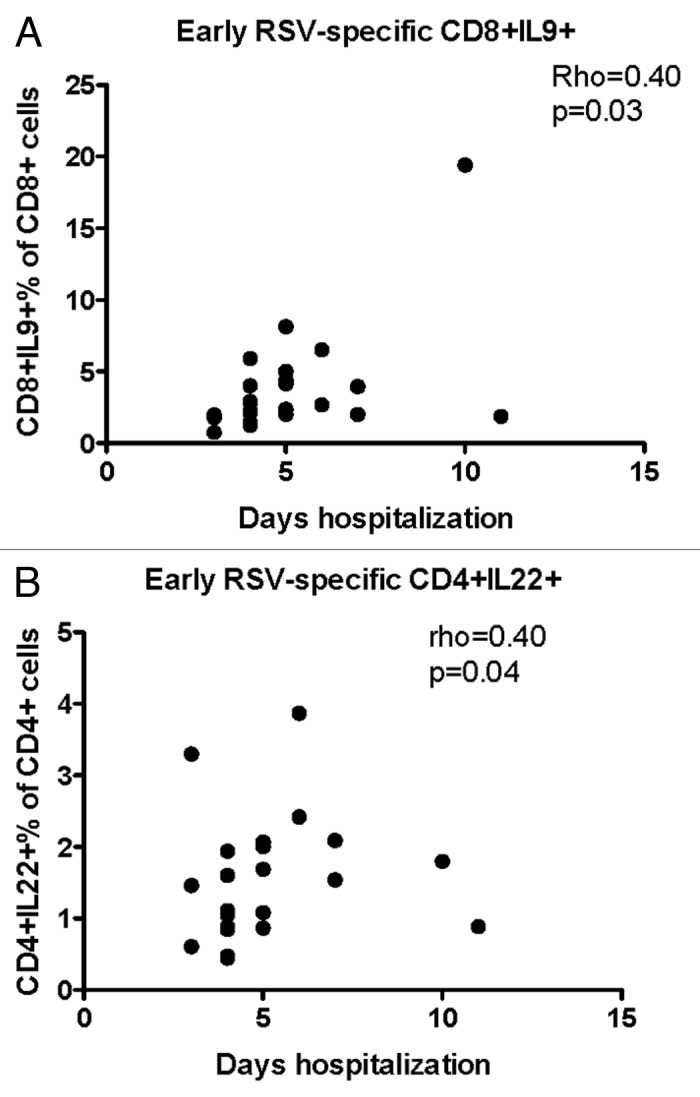
Figure 3. Significant correlations of the severity of RSV infection with early T cell responses (first week of disease). Data were derived from 25 subjects with acute RSV disease. Severity of RSV disease was measured by the duration of hospitalization. RSV-specific CD8+IL-9+ (A) and CD4+IL-22+ (B) were enumerated by flow cytometry and expressed as percentages of the parent CD4+ population. Coefficients of correlations and P values were generated using the Spearman correlation analysis.
We also sought to determine if the progression of the T cell responses over time was associated with the severity of RSV disease and performed correlation analysis of duration of hospitalization with RSV-specific Th1, Th2, Th9, Th17, and Th22 responses at week 6. We found that hospitalization significantly increased in children with low RSV-specific LPA results (rho = -0.45; P = 0.04 Fig. 4A) and high CD4+TNFα+% (rho = 0.47, P = 0.04, Fig. 4B) during convalescence.
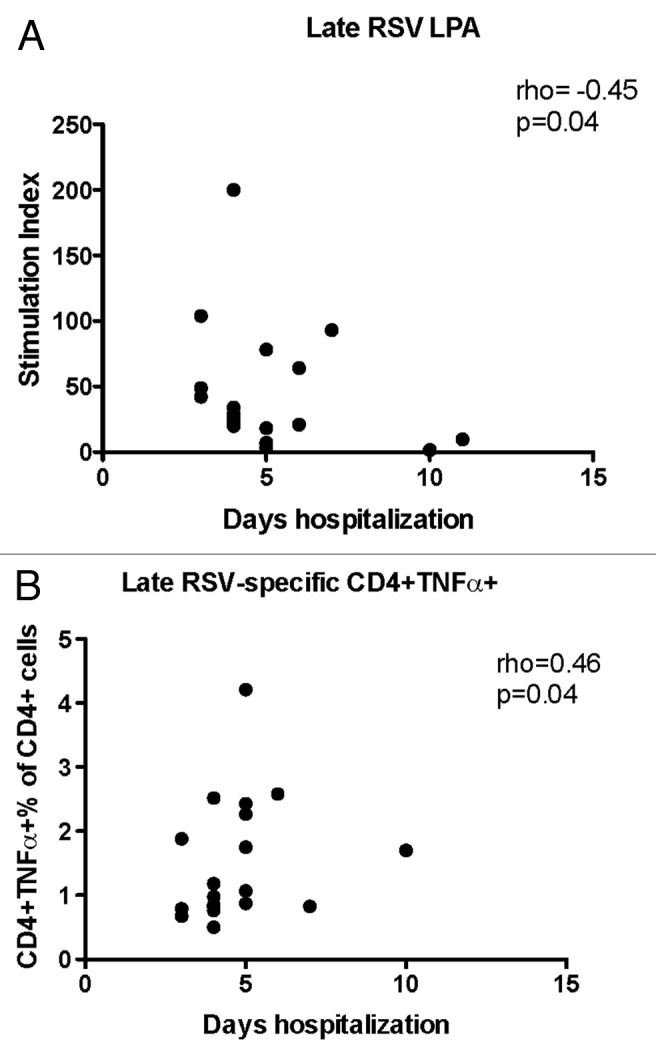
Figure 4. Significant correlations of the severity of RSV infection with convalescent CMI responses (week 6). Data were derived from subjects with acute RSV disease. Severity of RSV disease was measured by the duration of hospitalization. Panel (A) shows stimulation indices measured by LPA (21 subjects). Panel (B) shows RSV-specific CD4+TNFα+% measured by flow cytometry (20 subjects). Coefficients of correlations and p values were generated using Spearman correlation analysis.
The Effect of Age on Early and Convalescent T Cell Responses to RSV Infection
Early in the infection (week 1; Table 3) young age was associated with low RSV-specific IFNγ ELISPOT responses (rho = 0.4, P = 0.04) and with high CD4+ and CD8+TNFα+% (rho = –0.5, P = 0.02; and rho = –0.6, P = 0.0007 respectively), CD4+IL-9+% (rho –0.4, P = 0.04); CD4+IL-13+% (rho = –0.5, P = 0.007); and CD4+IL-17+% (rho = –0.4, P = 0.04),
Table 3. Correlations of RSV-specific T cell response with age at start of the disease.
| Parameter | Study visit | N | Spearman rho | P value |
|---|---|---|---|---|
| LPA | Week 1 | 27 | 0.3 | 0.1 |
| Week 2 | 26 | 0.4 | 0.02 | |
| Week 6 | 21 | 0.6 | 0.006 | |
| IFNγ ELISPOT | Week 1 | 26 | 0.4 | 0.04 |
| Week 2 | 26 | 0.3 | 0.1 | |
| Week 6 | 20 | 0.2 | 0.4 | |
| IL2 ELISPOT | Week 1 | 26 | 0.3 | 0.2 |
| Week 2 | 26 | 0.2 | 0.2 | |
| Week 6 | 20 | 0.1 | 0.6 | |
| CD4+Perforin+% | Week 1 | 25 | 0.07 | 0.7 |
| Week 2 | 24 | –0.2 | 0.3 | |
| Week 6 | 20 | 0.2 | 0.4 | |
| CD8+Perforin+% | Week 1 | 25 | 0.05 | 0.8 |
| Week 2 | 24 | –0.06 | 0.8 | |
| Week 6 | 20 | –0.07 | 0.8 | |
| CD4+TNFα+% | Week 1 | 25 | –0.5 | 0.02 |
| Week 2 | 24 | –0.3 | 0.1 | |
| Week 6 | 20 | –0.6 | 0.005 | |
| CD8+TNFα+% | Week 1 | 25 | –0.6 | P <0.001 |
| Week 2 | 24 | –0.3 | 0.1 | |
| Week 6 | 20 | –0.3 | 0.1 | |
| CD4+IL9+% | Week 1 | 25 | –0.4 | 0.04 |
| Week 2 | 24 | –0.5 | 0.02 | |
| Week 6 | 20 | –0.3 | 0.1 | |
| CD8+IL9+% | Week 1 | 25 | –0.3 | 0.2 |
| Week 2 | 24 | –0.4 | 0.06 | |
| Week 6 | 20 | –0.4 | 0.1 | |
| CD4+IL13+% | Week 1 | 25 | –0.5 | 0.007 |
| Week 2 | 24 | –0.4 | 0.07 | |
| Week 6 | 20 | –0.7 | p<0.001 | |
| CD8+IL13+% | Week 1 | 25 | –0.3 | 0.2 |
| Week 2 | 24 | –0.3 | 0.1 | |
| Week 6 | 20 | –0.6 | 0.009 | |
| CD4+ IL17+% | Week 1 | 25 | –0.4 | 0.04 |
| Week 2 | 24 | –0.2 | 0.3 | |
| Week 6 | 20 | –0.3 | 0.2 | |
| CD8+IL17+% | Week 1 | 25 | –0.05 | 0.8 |
| Week 2 | 24 | –0.1 | 0.7 | |
| Week 6 | 20 | –0.07 | 0.8 | |
| CD4+IL22+% | Week 1 | 25 | –0.3 | 0.07 |
| Week 2 | 24 | –0.2 | 0.3 | |
| Week 6 | 20 | –0.6 | 0.008 | |
| CD8+IL22+% | Week 1 | 25 | –0.2 | 0.4 |
| Week 2 | 24 | –0.1 | 0.5 | |
| Week 6 | 20 | –0.4 | 0.05 |
Bold font underscores correlations with P < 0.1.
During convalescence (week 6; Table 3) young age was associated with low RSV-specific memory responses measured by LPA (rho = 0.6, P = 0.006) and with high CD4+ and CD8+IL-13+% (rho = –0.7, P < 0.001 and rho = –0.6, P = 0.009, respectively) and CD4+IL-22+% (rho = –0.6, P = 0.008).
Discussion
Our data show that effector and memory RSV-specific Th1 responses measured by IFNγ, IL-2 and LPA were low during the first week of the disease, but had a rapid and robust increase between the first and second weeks. In contrast, the RSV-specific cytotoxic T lymphocytes measured by CD8+perforin+% were already close to peak during the first week of the disease and modestly increased up to week 6. The increase in Th1 responses paralleled the decrease in symptoms. Furthermore, high LPA responses during convalescence were significantly associated with low severity of the disease as measured by duration of hospitalization. Both findings are in agreement with the protective role ascribed to Th1 responses against RSV disease. There are several mechanisms by which Th1 responses may modulate the severity of RSV disease, but the best studied is the IFNγ secretion. IFNγ has only modest direct antiviral activity, but is a potent stimulator of NK and CD8+ T cell cytotoxicity.29 In previous studies, the magnitude of CD8+ cytotoxic and of IFNγ+CD4+ T cell responses to RSV correlated with the clearance of virally infected cells both in mice and humans.30-32 IFNγ was shown to play a critical role in limiting allergic and pro-inflammatory Th responses as demonstrated by the excess respiratory tract eosinophilia and mucus production in RSV-infected IFNγ knockout mice.33 It is also important to note that there has not been a consensus regarding the importance of Th1 responses in RSV infection. A few studies in infants and children failed to show a significant increase in RSV-specific Th1 compared with Th2 CMI in parallel with the clearance of the infection.29,34-39 Our results support the notion that robust RSV-specific Th1 responses play an important role in controlling the severity of disease.
In contrast to the kinetics of Th1 CMI, RSV-specific Th17 and Th22 inflammatory responses peaked during the first week of the disease and gradually decreased into convalescence. The decrease in Th17 and Th22 responses may have resulted from Th1-mediated downregulation. An alternative hypothesis is that Th17 and Th22 represent early effector responses to RSV that rapidly subside as the amount of antigen decreases. IL-22 has been shown to play both protective and pathogenic roles in chronic inflammatory diseases.13 Very few studies explored the role of IL-17 or IL-22 in viral infections. Studies in the influenza mouse models yielded contradictory results showing that IL-22 deficiency did not affect the outcome of infection,40 but also that IL-22 secretion limited airway inflammation and tissue damage and provided significant amelioration from airway constriction.41 We found that high RSV-specific Th22 responses during the first week of the disease were associated with increased length of hospitalization, arguing in favor of a harmful role of IL-22 in the pathogenesis of RSV disease.
Interestingly, the RSV-specific T cell expression of TNFα, generally considered a Th1 mediator, showed kinetics similar to those of IL-17 and IL-22 and opposite to those of IFNγ and IL-2. Specifically, the frequency of TNFα-producing T cells tended to decrease from the first week of disease to convalescence. Recent studies showed that Th17 T cell subsets sometimes produce TNFα,15,42 which might also be the case in the context of RSV infection and may explain the kinetics of TNFα observed in this study. We could not determine the frequency of cells with dual secretion of TNFα and IL-17 and/or IL-22, because these analytes were used in separate flow cytometry panels.
We did not see any significant or trend changes in RSV-specific Th2 responses over the 6-wk period of observation. RSV-specific Th9 responses were maximal during the first week of disease, but the subsequent decrease did not reach statistical significance. High RSV-specific CD4+IL-9+% cells during the first week of the disease was associated with increased length of hospitalization. This is in contrast with data recently generated in RSV-infected mice showing that early IL-9 production was critical for the antiviral activity of T cells recruited to the lung.20 However, our findings are in agreement with previously described deleterious effects of enhanced Th2/Th9 responses on RSV pathogenesis both in humans and in mice.21,43-45 Furthermore, studies in humans showed increased IL-9 production in asthma and other allergic disorders.22,46,47 Overall, these data indicate that increased IL-9 production may be harmful in the context of RSV infection.
The outcome of childhood RSV infection is dependent on age such that serious consequences are most commonly found in the neonate.48 This has been attributed to the well described predominance of Th2 over Th1 responses in the first year of life and was confirmed in the neonatal mouse RSV infection model.49 In our study, older age at RSV infection was associated with higher RSV-specific IFNγ responses during the first week of disease, whereas younger age was associated with higher RSV-specific IL-9, IL-13, IL-17 and TNFα expression. To our knowledge, this is the first demonstration that younger children mount higher Th17 and/or Th22 RSV-specific responses than older children. Furthermore, the persistence of increased RSV-specific pro-inflammatory CMI after clearing the initial infection may also affect the morbidity of subsequent episodes of infection. This phenomenon may constitute a challenge in the use of live attenuated RSV vaccines in young children and needs to be carefully considered in the design of new RSV vaccines.
Our study has limitations, including the relatively small number of subjects and the fact that they were all hospitalized. Nevertheless, some infants with mild to moderate disease may have been admitted because of perceived increased risk for dehydration and apnea and discharged early in the course of the disease. Additionally, as this study required multiple blood draws, many parents were hesitant to enroll children with mild disease, who were followed as outpatients. As there was a range in age from 2 mo to 5 y, this study may have enrolled children with primary RSV and secondary infection. It is noteworthy, however, that all early measures of RSV-specific CMI, with the exception of IFNγ secretion, were negatively or insignificantly associated with age at infection, which suggests that older children were naïve to RSV before entry in this study.
Our study offers a comprehensive analysis of RSV CMI responses during acute infection and identify those that may play a role in limiting the viral replication and the severity of the disease. The findings are highly relevant to the development of an effective RSV vaccine, because such a vaccine may rely not only on neutralizing antibodies, but also on CMI, which may be needed to clear the virus that escapes neutralization. Since a large portion of the RSV morbidity is due to inflammation, it is important that vaccine candidates target CMI responses that clear the virus without extensive tissue damage. Our findings indicate that vaccine candidates with the ability to promote robust Th1 and weak Th2, Th17, and Th22 responses may achieve this goal. Young children, who tend to mount strong TNFα, Th9, Th17, and Th22 and weak Th1 RSV-specific responses, will need particular attention during the evaluation of RSV vaccine candidates.
Subjects and Methods
Subjects
From January 1, 2009 to April 1, 2012, infants and children from 31 d to 5 y of age diagnosed at Children’s Hospital Colorado with an acute episode of RSV respiratory tract infection by PCR, culture or direct immunofluorescence were offered this study, which was approved by the Colorado Multiple Institutions IRB. Parents/guardians provided signed informed consent. Children with underlying chronic medical conditions, receiving immunosuppressive agents, as well as children on prolonged systemic steroids were excluded from the study. Children were enrolled during the first 7 d after onset of symptoms (week 1) with follow up visits 7–14 d (week 2) and 30–40 d after enrollment (week 6). Each study visit included collection of blood for immunologic assays, updated medical history and targeted physical exam. Additional clinical, laboratory, and demographic information were obtained by review of medical records.
Virus and cells
An RSV clinical isolate was expanded in Hep-2 cells grown in RPMI 1640 containing 2% fetal calf serum (Gibco). At 80% cytopathic effect, the culture supernatant was collected, aliquoted and stored at –80 °C until use. The titer of the virus pool, measured in the presence of 10% human AB serum (Nabi), was 104 TCID50/mL. An aliquot of the virus was inactivated by exposure to UV light for 30 min.
PBMCs were separated from heparinized blood and cryopreserved as previously described.50 The following priority assay order was observed for samples with insufficient cells for all assays: lymphocyte proliferation assay (LPA) followed by IFNγ/IL-2 dual-color ELISPOT and/or flow cytometry depending on the number of cells.
Lymphocyte proliferation assay (LPA)
This assay was performed as previously described.50 Stimulation medium consisted of RPMI 1640 with glutamine (Gibco), 10% human AB serum (Nabi) and 1% antibiotics (Gibco). PBMC at 105 cells/well were added to triplicate wells containing RSV at a net multiplicity of infection (MOI) = 1000 TCID50 /106 PBMC, medium control or PWM (Sigma) control at 10 µg/ml in 200 µL of total volume/well. After 6 d at 37 °C in a 5% CO2 humidified atmosphere, cells were pulsed with 20 µCi/mL 3H-thymidine and their DNA was harvested 6 h later onto unifilter plates (Perkin Elmer). Radioactivity on the filters was counted in a microplate scintillation counter (Packard). Results were expressed as SI = median counts-per-minute (CPM) in RSV (or PWM)-stimulated wells / median CPM in control wells and as adjusted CPM = median RSV CPM – median control CPM. Assays were considered valid if the PWM SI were ≥ 10.
ELISPOT assays
Dual color IFNγ and IL-2 ELISPOT assays were performed using MabTech Fluorospot kits as per manufacturer’s instructions with study-specific modifications.
Ninety-six-well low fluorescence PVDF membrane plates (IPFL) were coated with a mixture of monoclonal antibodies (mAbs): 1-D1K for IFNγ and IL-2-I/249 for IL-2. PBMC were added at 250 000 cells/well in 100 µL of stimulation medium as above and infected with RSV at a MOI = 1000 TCID50/ 106 PBMC or stimulated with medium or 10 µg of phytohemagglutinin A (PHA), each in duplicate wells. After 48 h at 37 °C in a 5% CO2 humidified atmosphere, plates were washed; bound IFNγ was detected with 7-B6-1-FS FITC and bound IL-2 with 11-Biotin. Spots were revealed using a mixture of anti-FITC-Green fluorochrome (IFNγ) and SA-Red fluorochrome (IL-2) and analyzed with an Immunospot plate reader (CTL). Results were reported as mean SFC/106 PBMC in RSV-infected wells after subtraction of the mean SFC in medium control wells. Assays were considered valid if PHA-stimulated wells had ≥ 100 IFNγ and IL-2 SFC/106 PBMC.
Flow Cytometry
T-cell phenotype was assessed by flow cytometry using thawed PBMC. PBMC at 106 cells in 1 mL of stimulation medium described above were infected with RSV at a MOI = 1000 TCID50/ 106 PBMC or mock-stimulated for 48 h at 37 °C in a 5% CO2 humidified atmosphere. Brefeldin A (Sigma-Aldrich) was added to a final concentration of 10µg/mL for the last 12–15 h of the incubation. Optimization experiments determined that CD8+ T-cell responses could be elicited after 48 h of in vitro viral infection, but not after 24 h of in vitro infection or 24 or 48 h of stimulation with UV-inactivated RSV preparations. This suggested that 48 h in vitro infection was necessary for MHC class I presentation.
Cells were stained using the following fluorochrome-conjugated mAbs: anti-CD3 APC Cy7 (BD BioSciences, cat# 557851), anti-CD8 PerCp (BD BioSciences, cat# 347314), anti-IL-9 PE (BD BioSciences, cat# 560807), anti-IL-13 APC (BD BioSciences, cat# 561162), anti-IL-17 AF 467 (BD BioSciences, cat# 560437), anti-IL-22 PE (Biolegend, cat# 515303), anti-Perforin FITC (BD BioSciences, cat# 556577), and anti-TNFα PeCy7 (eBioscience, cat# 25-7349-82). Cells were treated with Cytofix/Cytoperm solution (BD BioSciences, cat# 554722) prior to intracellular staining. Following surface and intracellular staining, cells were washed and fixed with 2% paraformaldehyde in PBS and analyzed with Guava easyCyte 8HT (Millipore) and FlowJo (Treestar) software. Subsets were expressed as a percentage of the parent CD4+ or CD8+ T cell population. Assays were considered valid if there were ≥100 events in the CD4+ and CD8+ lymphocyte anchor gates. The gating strategy is shown in Figure 5.
Figure 5. Gating strategy. The plots present the strategy used for identifying perforin-expressing cells as a typical example of the overall gating strategy. The left panel shows the selection of cells most likely to represent lymphocytes based on their size (x-axis) and granularity (y-axis); the middle panel shows the selection of CD4+ and CD8+ T cell populations based on the expression of the CD3 receptor (y-axis) and on the absence or presence, respectively, of the CD8 receptor (x-axis); the right panel shows the selection of the CD4+(CD8-)perforin+ cells out of the total CD4+(CD8-) cells based on the expression of perforin (x-axis) plotted against side scatter (y-axis) for improved visualization.
Statistical Analysis
Statistical Analysis was performed using PRISM 5.04 (GraphPad). Wilcoxon signed rank tests were used to compare CMI at different visits. Spearman correlation test was used to evaluate associations. P value < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by a grant from Medimmune. We thank Ms Sharolene Goodman for assistance with patient enrollment; and Ms Kelly Richardson, MS, and Adriana Tovar-Salazar, MS, for technical advice and assistance.
References
- 1.Hall CB. . Respiratory syncytial virus and parainfluenza virus. N Engl J Med 2001; 344:1917 - 28; http://dx.doi.org/ 10.1056/NEJM200106213442507; PMID: 11419430 [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et al. . Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545 - 55; http://dx.doi.org/ 10.1016/S0140-6736(10)60206-1; PMID: 20399493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. . Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 2012; 206:56 - 62; http://dx.doi.org/ 10.1093/infdis/jis309; PMID: 22529314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, et al. . Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 2005; 191:1093 - 104; http://dx.doi.org/ 10.1086/427813; PMID: 15747245 [DOI] [PubMed] [Google Scholar]
- 5.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE Jr., Boyce TG, Halburnt LL, Reed GW, Whitehead SS, Anderson EL, et al. . Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 2000; 182:1331 - 42; http://dx.doi.org/ 10.1086/315859; PMID: 11010838 [DOI] [PubMed] [Google Scholar]
- 6.Johnson TR, Graham BS. . Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol 1999; 73:8485 - 95; PMID: 10482601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TR, Teng MN, Collins PL, Graham BS. . Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J Virol 2004; 78:6024 - 32; http://dx.doi.org/ 10.1128/JVI.78.11.6024-6032.2004; PMID: 15141000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabeen R, Kaplan MH. . The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol 2012; 24:303 - 7; http://dx.doi.org/ 10.1016/j.coi.2012.02.001; PMID: 22365614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stassen M, Schmitt E, Bopp T. . From interleukin-9 to T helper 9 cells. Ann N Y Acad Sci 2012; 1247:56 - 68; http://dx.doi.org/ 10.1111/j.1749-6632.2011.06351.x; PMID: 22235761 [DOI] [PubMed] [Google Scholar]
- 10.Stockinger B, Veldhoen M. . Differentiation and function of Th17 T cells. Curr Opin Immunol 2007; 19:281 - 6; http://dx.doi.org/ 10.1016/j.coi.2007.04.005; PMID: 17433650 [DOI] [PubMed] [Google Scholar]
- 11.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. . A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010; 207:2479 - 91; http://dx.doi.org/ 10.1084/jem.20101376; PMID: 20921287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, et al. . Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009; 119:3573 - 85; PMID: 19920355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Pan HF, Ye DQ. . Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem 2011; 353:41 - 6; http://dx.doi.org/ 10.1007/s11010-011-0772-y; PMID: 21384158 [DOI] [PubMed] [Google Scholar]
- 14.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. . Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 2012; 484:514 - 8; http://dx.doi.org/ 10.1038/nature10957; PMID: 22466287 [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Korn T, Oukka M, Kuchroo VK. . Induction and effector functions of T(H)17 cells. Nature 2008; 453:1051 - 7; http://dx.doi.org/ 10.1038/nature07036; PMID: 18563156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins PL, Graham BS. . Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol 2008; 82:2040 - 55; http://dx.doi.org/ 10.1128/JVI.01625-07; PMID: 17928346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J. . Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol 2007; 17:5 - 34; http://dx.doi.org/ 10.1002/rmv.518; PMID: 17004293 [DOI] [PubMed] [Google Scholar]
- 18.Oshansky CM, Zhang W, Moore E, Tripp RA. . The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol 2009; 4:279 - 97; http://dx.doi.org/ 10.2217/fmb.09.1; PMID: 19327115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens WW, Sun J, Castillo JP, Braciale TJ. . Pulmonary eosinophilia is attenuated by early responding CD8(+) memory T cells in a murine model of RSV vaccine-enhanced disease. Viral Immunol 2009; 22:243 - 51; http://dx.doi.org/ 10.1089/vim.2009.0016; PMID: 19594395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodd JS, Lum E, Goulding J, Muir R, Van Snick J, Openshaw PJ. . IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. J Immunol 2009; 183:7006 - 13; http://dx.doi.org/ 10.4049/jimmunol.0900085; PMID: 19915054 [DOI] [PubMed] [Google Scholar]
- 21.McNamara PS, Flanagan BF, Baldwin LM, Newland P, Hart CA, Smyth RL. . Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 2004; 363:1031 - 7; http://dx.doi.org/ 10.1016/S0140-6736(04)15838-8; PMID: 15051283 [DOI] [PubMed] [Google Scholar]
- 22.Xing J, Wu Y, Ni B. . Th9: a new player in asthma pathogenesis?. J Asthma 2011; 48:115 - 25; http://dx.doi.org/ 10.3109/02770903.2011.554944; PMID: 21294663 [DOI] [PubMed] [Google Scholar]
- 23.Blanco JC, Boukhvalova MS, Shirey KA, Prince GA, Vogel SN. . New insights for development of a safe and protective RSV vaccine. Hum Vaccin 2010; 6:482 - 92; http://dx.doi.org/ 10.4161/hv.6.6.11562; PMID: 20671419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, et al. . IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol 2000; 105:108 - 15; http://dx.doi.org/ 10.1016/S0091-6749(00)90185-4; PMID: 10629460 [DOI] [PubMed] [Google Scholar]
- 25.Levitt RC, McLane MP, MacDonald D, Ferrante V, Weiss C, Zhou T, Holroyd KJ, Nicolaides NC. . IL-9 pathway in asthma: new therapeutic targets for allergic inflammatory disorders. J Allergy Clin Immunol 1999; 103:S485 - 91; http://dx.doi.org/ 10.1016/S0091-6749(99)70165-X; PMID: 10329852 [DOI] [PubMed] [Google Scholar]
- 26.Mesquita D Jr., Cruvinel WM, Câmara NO, Kállas EG, Andrade LE. . Autoimmune diseases in the TH17 era. Braz J Med Biol Res 2009; 42:476 - 86; http://dx.doi.org/ 10.1590/S0100-879X2009000600002; PMID: 19448894 [DOI] [PubMed] [Google Scholar]
- 27.Hou W, Kang HS, Kim BS. . Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 2009; 206:313 - 28; http://dx.doi.org/ 10.1084/jem.20082030; PMID: 19204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. . IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol 2007; 179:3917 - 25; PMID: 17785829 [DOI] [PubMed] [Google Scholar]
- 29.Román M, Calhoun WJ, Hinton KL, Avendaño LF, Simon V, Escobar AM, Gaggero A, Díaz PV. . Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med 1997; 156:190 - 5; http://dx.doi.org/ 10.1164/ajrccm.156.1.9611050; PMID: 9230746 [DOI] [PubMed] [Google Scholar]
- 30.Bueno SM, González PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE, Fennelly GJ, Eugenin EA, Jacobs WR Jr., Riedel CA, et al. . Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A 2008; 105:20822 - 7; http://dx.doi.org/ 10.1073/pnas.0806244105; PMID: 19075247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson MR, Hartwig SM, Varga SM. . The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol 2008; 181:7958 - 68; PMID: 19017987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castilow EM, Olson MR, Meyerholz DK, Varga SM. . Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J Virol 2008; 82:2196 - 207; http://dx.doi.org/ 10.1128/JVI.01949-07; PMID: 18094193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, Joetham A, Gelfand EW, Dakhama A. . IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med 2008; 177:208 - 18; http://dx.doi.org/ 10.1164/rccm.200612-1890OC; PMID: 17962634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker Y. . Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review. Virus Genes 2006; 33:235 - 52; PMID: 16972040 [DOI] [PubMed] [Google Scholar]
- 35.Anderson LJ, Tsou C, Potter C, Keyserling HL, Smith TF, Ananaba G, Bangham CR. . Cytokine response to respiratory syncytial virus stimulation of human peripheral blood mononuclear cells. J Infect Dis 1994; 170:1201 - 8; http://dx.doi.org/ 10.1093/infdis/170.5.1201; PMID: 7963714 [DOI] [PubMed] [Google Scholar]
- 36.Bont L, Heijnen CJ, Kavelaars A, van Aalderen WM, Brus F, Draaisma JM, Pekelharing-Berghuis M, van Diemen-Steenvoorde RA, Kimpen JL. . Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 2001; 184:355 - 8; http://dx.doi.org/ 10.1086/322035; PMID: 11443563 [DOI] [PubMed] [Google Scholar]
- 37.Bendelja K, Gagro A, Bace A, Lokar-Kolbas R, Krsulovic-Hresic V, Drazenovic V, Mlinaric-Galinovic G, Rabatic S. . Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol 2000; 121:332 - 8; http://dx.doi.org/ 10.1046/j.1365-2249.2000.01297.x; PMID: 10931150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen ZM, Mao JH, Du LZ, Tang YM. . Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr 2002; 91:914 - 22; http://dx.doi.org/ 10.1111/j.1651-2227.2002.tb02877.x; PMID: 12412865 [DOI] [PubMed] [Google Scholar]
- 39.Tripp RA, Moore D, Barskey A 4th, Jones L, Moscatiello C, Keyserling H, Anderson LJ. . Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis 2002; 185:1388 - 94; http://dx.doi.org/ 10.1086/340505; PMID: 11992272 [DOI] [PubMed] [Google Scholar]
- 40.Guo H, Topham DJ. . Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol 2010; 84:7750 - 9; http://dx.doi.org/ 10.1128/JVI.00187-10; PMID: 20504940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taube C, Tertilt C, Gyülveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, et al. . IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One 2011; 6:e21799; http://dx.doi.org/ 10.1371/journal.pone.0021799; PMID: 21789181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, et al. . IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol 2011; 41:1894 - 901; http://dx.doi.org/ 10.1002/eji.201041197; PMID: 21469124 [DOI] [PubMed] [Google Scholar]
- 43.Castilow EM, Meyerholz DK, Varga SM. . IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol 2008; 180:2376 - 84; PMID: 18250447 [DOI] [PubMed] [Google Scholar]
- 44.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. . Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2003; 168:633 - 9; http://dx.doi.org/ 10.1164/rccm.200210-1148OC; PMID: 12773328 [DOI] [PubMed] [Google Scholar]
- 45.Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, Lincoln P, Maassab H. . Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol 2001; 167:1060 - 5; PMID: 11441116 [DOI] [PubMed] [Google Scholar]
- 46.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, et al. . Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A 1997; 94:13175 - 80; http://dx.doi.org/ 10.1073/pnas.94.24.13175; PMID: 9371819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soussi-Gounni A, Kontolemos M, Hamid Q. . Role of IL-9 in the pathophysiology of allergic diseases. J Allergy Clin Immunol 2001; 107:575 - 82; http://dx.doi.org/ 10.1067/mai.2001.114238; PMID: 11295641 [DOI] [PubMed] [Google Scholar]
- 48.Semple MG, Dankert HM, Ebrahimi B, Correia JB, Booth JA, Stewart JP, Smyth RL, Hart CA. . Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One 2007; 2:e1038; http://dx.doi.org/ 10.1371/journal.pone.0001038; PMID: 17940602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culley FJ, Pollott J, Openshaw PJ. . Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med 2002; 196:1381 - 6; http://dx.doi.org/ 10.1084/jem.20020943; PMID: 12438429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberg A, Song LY, Wilkening C, Sevin A, Blais B, Louzao R, Stein D, Defechereux P, Durand D, Riedel E, et al. , Pediatric ACTG Cryopreservation Working Group. . Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin Vaccine Immunol 2009; 16:1176 - 86; http://dx.doi.org/ 10.1128/CVI.00342-08; PMID: 19515870 [DOI] [PMC free article] [PubMed] [Google Scholar]



