Abstract
Background
Both smoking and diabetes can increase the risk and influence the manifestations and outcomes of tuberculosis (TB). It is not clear whether the influence of smoking on pulmonary TB differs between non-diabetic and diabetic patients. Herein, we assessed the manifestations and outcomes of TB in relation to smoking in both diabetic and non-diabetic TB patients.
Methodology/Principal Findings
All diabetic culture-positive pulmonary TB patients notified from 2005–2010 at three teaching hospitals in Taiwan were enrolled. A culture-positive pulmonary TB patient without DM who was notified to the health authority immediately prior to each diabetic TB patient was selected for comparison. The 972 patients in this study cohort included 365 (37.6%) non-diabetic non-smokers, 149 (15.3%) non-diabetic smokers, 284 (29.2%) diabetic non-smokers, and 174 (17.9%) diabetic smokers. The adjusted relative risk of a pretreatment positive smear for a smoker compared with a non-smoker was 2.19 (95% CI 1.38–3.47) in non-diabetic patients and 2.23 (95% CI 1.29–3.87) in diabetic culture-positive pulmonary TB patients. The adjusted relative risk for a positive smear among diabetic smokers was 5.61 (95% CI 3.35–9.41) compared with non-diabetic non-smokers. Smoking was significantly associated with an increased frequency of bilateral lung parenchyma involvement (AdjOR 1.84, 95% CI 1.16–2.93), far-advanced pulmonary TB (AdjOR 1.91, 95% CI 1.04–3.50), cavitary lesions (AdjOR 2.03, 95% CI 1.29–3.20), and unfavorable outcomes of TB (AdjOR 2.35, 95% CI 1.02–5.41) in non-diabetic patients. However, smoking was not associated with cavitary lung parenchyma lesions regarding the location, number or size of the cavity in diabetic TB patients.
Conclusions/Significance
Smoking and diabetes have joint effects on a pretreatment positive smear. Diabetic smokers had more than a 5-fold increased risk of a pretreatment positive smear than did non-diabetic non-smokers, indicating remarkable joint effects of diabetes and smoking on the risk of TB transmission.
Introduction
Smoking is significantly associated with increased risks of tuberculous infection, tuberculosis (TB) disease, TB mortality and recurrent TB [1–5]. TB patients who have smoked are more likely to transmit TB to their child contacts [6]. A recent systematic review and meta-analysis reported that exposure to environmental tobacco smoke increases the risks of developing childhood TB disease and tuberculous infection [7]. Smoking influences the clinical manifestations and outcomes of TB. ‘Ever smokers’ were more likely to have experienced cough, dyspnea, cavity, miliary lung involvement, positive sputum culture and poor outcomes of TB [5, 8–11]. Diabetes mellitus (DM) is also associated with an increased risk of TB disease [12]. Diabetic TB patients have an increased risk of treatment failure, death and recurrent TB compared with non-diabetic TB patients [13, 14]. DM also influences the manifestations of TB. It has been reported that diabetic TB patients have more symptoms [15], are more likely to be smear-positive [16, 17], and have an increased frequency of cavitary lesions [18] compared with non-diabetic TB patients. Therefore, the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (The Union) have encouraged National TB Programme to address the combined challenges of smoking, diabetes and TB [19, 20].
Although smoking and diabetes are both important risk factors for TB, it is unclear whether there is a differential influence of smoking on pulmonary TB between non-diabetic and diabetic patients. Previously, we have investigated the influence of diabetes on TB using representative samples of diabetic and non-diabetic culture positive pulmonary TB patients treated at three teaching hospitals in Taiwan [18, 21]. The smoking status of TB patients with or without diabetes was documented, which provided an opportunity to investigate joint effects and interactions between the influences of smoking and diabetes on TB. Herein, we report the manifestations and outcomes of pulmonary TB in association with smoking in both diabetic and non-diabetic culture positive pulmonary TB patients.
Materials and Methods
Study population
This present study was nested in a previous study of glycemic control and pulmonary TB, which was conducted at three teaching hospitals with respective locations in Northern, Eastern and Southern Taiwan [18, 21]. We obtained a list of all TB patients who were notified to health authorities from 2005–2010 and were managed by these three hospitals from the national TB registry at Taiwan CDC. Patients with DM were defined as those who 1) were treated with insulin or diabetes-specific hypoglycemic agents, 2) had been assigned an ICD-9 code related to DM during admission, 3) had been assigned an ICD-9 code related to DM two or more times on outpatient visits, or 4) had a history of DM. All diabetic culture-positive pulmonary TB patients who were notified from 2005–2010 were enrolled. For each diabetic culture-positive pulmonary TB patient, a culture-positive pulmonary TB patient without DM who was notified to the health authority immediately prior to the diabetic patient was selected for inclusion in a comparison group. As the non-diabetic culture-positive pulmonary TB patients were systematically selected, this was a representative sample of all non-diabetic culture-positive pulmonary TB patients who were treated at the three teaching hospitals from 2005–2010, which enabled us to assess the influence of smoking on pulmonary TB in relation to diabetes.
Among the 1209 culture-positive pulmonary TB patients (581 with DM and 628 without DM) who were included in the study of glycemic control and radiographic manifestations of pulmonary TB [18], 972 (80.4%; 649 non-smokers and 323 smokers) were included in this present study; 211 ex-smokers (a previous smoker who had stopped smoking prior to the diagnosis of TB), 21 patients whose smoking status was unknown, and 5 patients whose outcome was not available were excluded.
Clinical records were reviewed to determine patient age, sex, pretreatment smear (negative, positive, and positivity grade, scanty, 1+, 2+, 3+ 4+), type of TB case (new versus retreatment), smoking status, and treatment outcome. Patients were classified as a current smoker (those individuals who smoked at the time of TB diagnosis) or a non-smoker (those individuals who never smoked). Pretreatment drug susceptibility tests for isoniazid (H), rifampicin (R), ethambutol and streptomycin were collected and patients were classified as 1) susceptible, 2) showing resistance to H but not R (HrRs), 3) resistance to at least both H and R (HrRr), or 4) other resistance patterns. Data were collected from medical charts using a structured questionnaire.
The approach used to read chest x-rays has been reported previously [18]. Briefly, a pre-treatment postero-anterior chest radiograph was read by two qualified pulmonologists (readers) at each hospital. Readings were independent without discussion between the readers who were blinded to patients’ diabetic and smoking status. Films with any discordant reading were read by a third reader, who was a senior pulmonologist at each of the participating hospitals. Readings of the chest radiographs focused on lung parenchymal opacity and cavitation. Recordings of abnormal opacity of the lung parenchyma included location (right-upper, right-lower, left-upper, or left-lower) and the extent of disease (minimal, moderately-advanced, or far advanced). The extent of disease was estimated based on the sum of all areas of abnormality in which a boundary of abnormal opacity could be drawn. Minimal lesions were defined as an area less than that above a horizontal line across the 2nd chondrosternal conjunction of one lung. Moderately advanced lesions were defined as an area greater in size than the minimal lesions but smaller than that of one entire lung. Far advanced lesions were defined as an area equivalent to or greater than one lung. The size of the largest cavity was dichotomized into small and large by the median diameter.
Data entry and statistical analysis
To ensure the accuracy of data entry, the data set was “double entered” and validated using EpiData Entry 3.1 (EpiData Association, Odense, Denmark). Any discrepant records were checked and corrected against the original data on the questionnaires. STATA Version 12 (StataCorp LP, College Station, Texas, USA) was used for statistical analyses. A P-value <0.05 was considered to indicate a statistically significant difference.
We assessed the association between smoking and radiographic manifestations, symptoms, pre-treatment smears and smear positivity grades, as well as the outcome of TB for both diabetic and non-diabetic TB patients. Categorical data were analyzed using Pearson’s χ2 test. Logistic regression models were constructed for outcome variables with 2 categories, including the location of abnormal opacities and cavities, and the number and size of cavities; multinomial logistic regression models were constructed for outcome variables with 3 categories or more, including pretreatment smear findings (negative, positive, not done) and smear positivity grades (negative, low positivity grade [scanty, 1+, and 2+], high positivity grade [3+ and 4+]), and the extent of abnormal opacities (negative, minimal, moderately advanced, far advanced). In a treatment outcome analysis, outcomes were dichotomized into successful (treatment success) and unfavorable (died, failed, loss to follow-up). The association between smoking and patient outcomes was computed using multivariate logistic regression analysis, adjusted for age, sex, type of case, and anti-TB drug resistance. To assess whether diabetes is an effect modifier of smoking on TB, we performed analysis stratified by diabetes and regression models were constructed separately for diabetes and non-diabetic patients. Furthermore, regression models including a product term of smoking and diabetes were constructed for the total study population to assess interaction of diabetes and smoking.
Ethics
This study was approved by the Joint Institute Review Board of Taipei Medical University. Written informed consent from each participant for his or her clinical records to be used in this study was waived. Patient information was anonymized and de-identified prior to analysis.
Results
The cohort of 972 patients enrolled in this study included 365 (37.6%) non-diabetic non-smokers, 149 (15.3%) non-diabetic smokers, 284 (29.2%) diabetic non-smokers, and 174 (17.9%) diabetic smokers. Table 1 shows the characteristics of the 972 patients who were included in this study.
Table 1. Characteristics of the 972 culture-positive pulmonary tuberculosis patients included in this study, by diabetes and smoking.
| Non-Diabetes | Diabetes | |||
|---|---|---|---|---|
| Non smoker | Smoker | Non smoker | Smoker | |
| Total | 365 | 149 | 284 | 174 |
| Sex | ||||
| Male | 202 (55.3%) | 135 (90.6%) | 170 (59.9%) | 166 (95.4%) |
| Female | 163 (44.7%) | 14 (9.4%) | 114 (40.1%) | 8 (4.6%) |
| Age (years) | ||||
| <45 | 124 (34.0%) | 48 (32.2%) | 22 (7.8%) | 33 (19.0%) |
| 45–64 | 91 (24.9%) | 62 (41.6%) | 127 (44.7%) | 101 (58.1%) |
| ≥65 | 150 (41.1%) | 39 (26.2%) | 135 (47.5%) | 40 (23.0%) |
| Case type | ||||
| New | 312 (85.5%) | 128 (85.9%) | 243 (85.6%) | 157 (90.2%) |
| Retreatment | 53 (14.5%) | 21 (14.1%) | 41 (14.4%) | 17 (9.8%) |
| Smear | ||||
| Negative | 171 (46.9%) | 46 (30.9%) | 93 (32.8%) | 25 (14.4%) |
| Positive | 157 (43.0%) | 96 (64.4%) | 174 (61.3%) | 134 (77.0%) |
| Scanty | 1 (0.3%) | 1 (0.7%) | 3 (1.1%) | 4 (2.3%) |
| 1+ | 47 (12.9%) | 17 (11.4%) | 38 (13.4%) | 24 (13.8%) |
| 2+ | 28 (7.7%) | 24 (16.1%) | 35 (12.3%) | 22 (12.6%) |
| 3+ | 26 (7.1%) | 26 (17.5%) | 47 (16.6%) | 27 (15.5%) |
| 4+ | 55 (15.1%) | 28 (18.8%) | 51 (18.0%) | 57 (32.8%) |
| Not done | 37 (10.1%) | 7 (4.7%) | 17 (6.0%) | 15 (8.6%) |
| DST pattern* | ||||
| Susceptible | 265 (72.6%) | 110 (73.8%) | 210 (73.9%) | 133 (76.4%) |
| HrRs | 23 (6.3%) | 10 (6.7%) | 19 (6.7%) | 15 (8.2%) |
| HrRr | 12 (3.3%) | 6 (4.0%) | 14 (4.9%) | 5 (2.9%) |
| Other | 7 (1.9%) | 8 (5.4%) | 8 (2.8%) | 7 (4.0%) |
| Not done | 58 (15.9%) | 15 (10.1%) | 33 (11.6%) | 14 (8.1%) |
* DST, drug susceptibility testing; Susceptible, susceptible to isoniazid, rifampicin, ethambutol, and streptomycin; HrRs, any resistance to isoniazid but not resistance to rifampicin; HrRr, resistance to at least both isoniazid and rifampicin; Other, other resistant patterns.
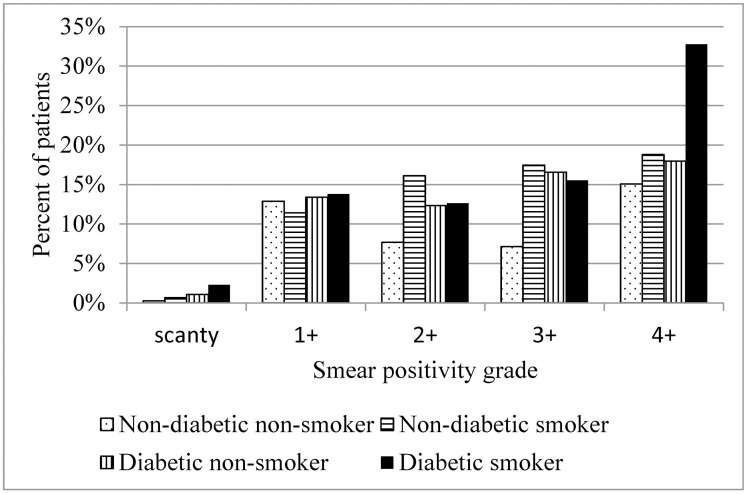
The proportion of patients with a pretreatment positive smear was 43.0% in non-diabetic non-smokers, 64.4% in non-diabetic smokers, 61.3% in diabetic non-smokers, and 77.0% in diabetic smokers (P<0.001). The proportion of patients with high smear positivity grades (3+ or 4+) was 22.2% in non-diabetic non-smokers, 36.2% in non-diabetic smokers, 34.5% in diabetic non-smokers, and 48.3% in diabetic smokers (P<0.001; Fig 1). In a multinomial logistic regression analysis stratified by diabetes and adjusted for age and sex, the adjusted relative risk of pretreatment positive smears for smokers compared with non-smokers was 2.19 (95% CI 1.38–3.47) in non-diabetic and 2.23 (95% CI 1.29–3.87) in diabetic culture-positive pulmonary TB patients. In a multinomial logistic regression analysis of the whole study population without stratification and including a product term of diabetes and smoking, there was no significant interaction of smoking and diabetes for pretreatment positive smear (p = 0.517). The relative risk for a pretreatment positive smear among diabetic smokers was more than 5-fold higher than for non-diabetic non-smokers (AdjRRR 5.61, 95% CI 3.35–9.41). Smoking was also significantly associated with a high (3+ or 4+) smear positivity grade in both non-diabetic (AdjRRR 2.32, 95% CI 1.36–3.98) and diabetic (AdjRRR 2.29, 95% CI 1.26–4.15) culture-positive pulmonary TB patients in a multinomial logistic regression analysis stratified by diabetes and adjusted for age and sex (Table 2). In a multinomial logistic regression analysis of the whole study population without stratification and including a product term of diabetes and smoking, there was no significant interaction of smoking and diabetes for a pretreatment high smear positivity grade (p = 0.546). The relative risk for a pretreatment high smear positivity grade among diabetic smokers was more than 6-fold higher than for non-diabetic non-smokers (AdjRRR 6.60, 95% CI 3.74–11.66). (Table 2)
Fig 1. Pre-treatment smear positivity grades by smoking and diabetes.
Table 2. Pretreatment-positive sputum smears and high smear positivity grades (3+ or 4+) in relation to smoking in culture-positive pulmonary tuberculosis patients at three tertiary referral hospitals in Taiwan, 2005–2010, stratified by diabetes.
| Multivariate analysis* | ||||||
|---|---|---|---|---|---|---|
| Overall | Stratified by diabetes | |||||
| N | % | AdjRRR | 95% CI | AdjRRR | 95% CI | |
| Smear positive | ||||||
| Non-diabetes | ||||||
| Non-smoker | 157 | 43.0 | 1 | 1 | ||
| Smoker | 96 | 64.4 | 2.08 | 1.33–3.25 | 2.19 | 1.38–3.47 |
| Diabetes | ||||||
| Non-smoker | 174 | 61.3 | 2.19 | 1.53–3.13 | 1 | |
| Smoker | 134 | 77.0 | 5.61 | 3.35–9.41 | 2.23 | 1.29–3.87 |
| High positivity grade | ||||||
| Non-diabetes | ||||||
| Non-smoker | 81 | 22.2 | 1 | 1 | ||
| Smoker | 54 | 36.2 | 2.17 | 1.29–3.64 | 2.32 | 1.36–3.98 |
| Diabetes | ||||||
| Non-smoker | 98 | 34.5 | 2.43 | 1.60–3.70 | 1 | |
| Smoker | 84 | 48.3 | 6.60 | 3.74–11.66 | 2.29 | 1.26–4.15 |
*Adjusted relative risk ratio, adjusted for age group and sex; 95% CI, 95% confidence interval.
Table 3 shows the location and extent of abnormal opacities in the lung parenchyma on chest radiographs in relation to smoking in culture-positive non-diabetic and diabetic pulmonary TB patients. Smoking was associated with an increased frequency of bilateral lung parenchyma involvement (AdjOR 1.84, 95% CI 1.16–2.93) and far-advanced pulmonary TB (AdjOR 1.91, 95% CI 1.04–3.50) in non-diabetic TB patients. However, smoking was not found to be associated with lung parenchyma lesions in terms of the location or extent of disease in diabetic TB patients.
Table 3. Location and extent of abnormal opacities of the lung parenchyma on chest radiographs in relation to smoking in culture-positive pulmonary tuberculosis patients at three tertiary-referral hospitals in Taiwan, 2005–2010, stratified by diabetes.
| Non-smoker | Smoker | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | AdjOR | 95% CI | ||
| Non-diabetic | 365 | 100 | 149 | 100 | |||
| Any opacity | 357 | 97.8 | 146 | 98.0 | 1.09 | 0.29 | 4.17 |
| Location | |||||||
| Upper field | 321 | 88.0 | 143 | 96.0 | 2.01 | 0.77 | 5.30 |
| Lower field | 228 | 62.5 | 106 | 71.1 | 1.57 | 0.98 | 2.50 |
| Right field | 299 | 81.9 | 133 | 89.3 | 1.76 | 0.93 | 3.36 |
| Left field | 262 | 71.8 | 121 | 81.2 | 1.51 | 0.89 | 2.54 |
| Bilateral field | 204 | 55.9 | 108 | 72.5 | 1.84 | 1.16 | 2.93 |
| Extent | |||||||
| Minimal | 157 | 43.0 | 42 | 28.2 | Base | ||
| Moderately-advanced | 149 | 40.8 | 69 | 46.3 | 1.48 | 0.90 | 2.42 |
| Far-advanced | 51 | 14.0 | 35 | 23.5 | 1.91 | 1.04 | 3.50 |
| Diabetic | 284 | 100 | 174 | 100 | |||
| Any opacity | 282 | 99.3 | 171 | 98.3 | 0.40 | 0.07 | 2.44 |
| Location | |||||||
| Upper field | 267 | 94.0 | 164 | 94.3 | 0.87 | 0.33 | 2.30 |
| Lower field | 209 | 73.6 | 133 | 76.4 | 0.98 | 0.59 | 1.61 |
| Right field | 235 | 82.8 | 142 | 81.6 | 0.88 | 0.50 | 1.56 |
| Left field | 222 | 78.2 | 137 | 78.7 | 0.77 | 0.45 | 1.32 |
| Bilateral field | 175 | 61.6 | 108 | 62.1 | 0.86 | 0.55 | 1.35 |
| Extent | |||||||
| Minimal | 84 | 29.6 | 38 | 21.8 | Base | ||
| Moderately-advanced | 149 | 52.5 | 90 | 51.7 | 1.02 | 0.60 | 1.73 |
| Far-advanced | 49 | 17.3 | 43 | 24.7 | 1.64 | 0.86 | 3.13 |
Note: No, number; CI, confidence interval; AdjOR, adjusted odds ratio, adjusted for sex and age group.
Table 4 shows the location and size of cavitary lesions in the lung parenchyma on a chest radiograph in relation to smoking in culture-positive non-diabetic and diabetic pulmonary TB patients. Smoking was found to be significantly associated with an increased frequency of cavitary lesions (AdjOR 2.03, 95% CI 1.29–3.20) in non-diabetic pulmonary TB patients; it mainly affected the upper-lung field (AdjOR 2.25, 95% CI 1.42–3.57), but not the lower lung field (AdjOR 0.67, 95% CI 0.23–1.93). Smoking was also significantly associated with an increased frequency of multiple cavities (AdjOR 1.88, 95% CI 1.13–3.14) and large (≥3 cm) cavities (AdjOR 2.03, 95% CI 1.29–3.20) in non-diabetic TB patients. However, smoking was not associated with cavitary lung parenchyma lesions in terms of the location, number, or size of cavitary lesions in diabetic TB patients.
Table 4. Location and size of cavitary lesions in the lung parenchyma on chest radiographs in relation to smoking in culture-positive pulmonary tuberculosis patients at three tertiary-referral hospitals in Taiwan, 2005–2010, stratified by diabetes.
| Non-smoker | Smoker | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | AdjOR | 95% CI | ||
| Non-diabetes | 365 | 100 | 149 | 100 | |||
| Any cavity | 86 | 23.6 | 68 | 45.6 | 2.03 | 1.29 | 3.20 |
| Location | |||||||
| Upper field | 79 | 21.6 | 67 | 45.0 | 2.25 | 1.42 | 3.57 |
| Lower field | 15 | 4.1 | 6 | 4.0 | 0.67 | 0.23 | 1.93 |
| Right field | 48 | 13.2 | 48 | 32.2 | 2.35 | 1.40 | 3.93 |
| Left field | 58 | 15.9 | 45 | 30.2 | 1.69 | 1.02 | 2.81 |
| Bilateral field | 20 | 5.5 | 25 | 16.8 | 2.43 | 1.21 | 4.89 |
| Number and size | |||||||
| Multiple | 51 | 14.0 | 45 | 30.2 | 1.88 | 1.13 | 3.14 |
| Large (≥3 cm) | 35 | 9.6 | 35 | 23.5 | 2.03 | 1.29 | 3.20 |
| Diabetes | 284 | 100 | 174 | 100 | |||
| Any cavity | 126 | 44.4 | 105 | 60.3 | 1.20 | 0.76 | 1.88 |
| Location | |||||||
| Upper field | 113 | 39.8 | 98 | 56.3 | 1.28 | 0.82 | 2.00 |
| Lower field | 36 | 12.7 | 29 | 16.7 | 0.91 | 0.49 | 1.67 |
| Right field | 74 | 26.1 | 61 | 35.1 | 0.91 | 0.57 | 1.46 |
| Left field | 74 | 26.1 | 64 | 36.8 | 1.23 | 0.76 | 1.98 |
| Bilateral field | 22 | 7.8 | 20 | 11.5 | 0.82 | 0.40 | 1.68 |
| Number and size | |||||||
| Multiple | 70 | 24.7 | 69 | 39.7 | 1.21 | 0.75 | 1.95 |
| Large (≥3 cm) | 55 | 19.4 | 57 | 32.8 | 1.20 | 0.76 | 1.88 |
Note: No, number; CI, confidence interval; AdjOR, adjusted odds ratio, adjusted for sex and age group.
Among the 972 TB patients, 885 (91.1%) were successfully treated, 63 (6.5%) died, 9 (0.9%) were lost to follow-up, and 15 (1.5%) failed. Table 5 shows that smoking was significantly associated with an unfavorable treatment outcome in non-diabetic pulmonary TB patients (P = 0.032), and was associated with a higher frequency of death (6.7% vs. 3.8%) and failure (2.7% vs. 0.6%) among smokers compared with non-smokers. However, smoking was not significantly associated with an unfavorable outcome for TB among diabetic pulmonary TB patients (P = 0.407). In a multivariate logistic regression analysis of the whole study population without stratification that adjusted for age, sex, type of case, anti-TB drug resistance, there was significant negative interaction of smoking and diabetes(p = 0.013) and the effect of smoking on outcome of TB was attenuated by diabetes. However, we found that non-diabetic smokers (AdjOR 3.12, 95% CI 1.40–6.98), diabetic non-smokers (AdjOR 3.32, 95% CI 1.75–6.31), and diabetic smokers (AdjOR 2.95, 95% CI 1.31–6.66) were significantly associated with increased risks of unfavorable outcomes for TB treatment compared with non-diabetic non-smokers (Table 6). When stratified by diabetes, smoking was found to be significantly associated with an unfavorable outcome for TB treatment in non-diabetic TB patients (AdjOR 2.35, 95% CI 1.02–5.41), but did not increase the risk of an unfavorable outcome among diabetic TB patients (AdjOR 1.14, 95% CI 0.55–2.37).
Table 5. Outcomes of treatment in relation to smoking in culture-positive pulmonary tuberculosis patients at three tertiary-referral hospitals in Taiwan, 2005–2010, stratified by diabetes.
| Success | Died | Lost | Failed | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| Non-diabetes | 0.032 | ||||||||
| Non-smoker | 349 | 95.6 | 14 | 3.8 | 0 | 0 | 2 | 0.6 | |
| Smoker | 134 | 89.9 | 10 | 6.7 | 1 | 0.7 | 4 | 2.7 | |
| Diabetes | 0.407 | ||||||||
| Non-smoker | 244 | 85.9 | 29 | 10.2 | 5 | 1.76 | 6 | 2.1 | |
| Smoker | 158 | 90.8 | 10 | 5.8 | 3 | 1.7 | 3 | 1.7 | |
Note: treatment success (documented sputum culture conversion and remained culture negative till completion of a treatment course); failed (sputum culture positive at 5 months of treatment or later); lost-to-follow-up (interruption of treatment for 2 consecutive months or lack of outcome assessment); died (died of any cause during TB treatment)
Table 6. Smoking and unfavorable tuberculosis outcomes (died, loss-to-follow-up, or failed) in the study population, stratified by diabetes.
| Total | Unfavorable | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Overall | Stratified by diabetes | ||||||
| N | % | AdjOR* | 95% CI | AdjOR* | 95% CI | ||
| Non-diabetes | |||||||
| Non-smoker | 365 | 16 | 4.4 | 1 | 1 | ||
| Smoker | 149 | 15 | 10.1 | 3.12 | 1.40–6.98 | 2.35 | 1.02–5.41 |
| Diabetes | |||||||
| Non-smoker | 284 | 40 | 14.1 | 3.32 | 1.75–6.31 | 1 | |
| Smoker | 174 | 16 | 9.2 | 2.95 | 1.31–6.66 | 1.14 | 0.55–2.37 |
*Adjusted for age group, sex, type of case (new vs. previously treated) and drug resistance.
Discussion
Our present study shows that the influence of smoking on pulmonary TB is different between non-diabetic and diabetic patients. Smoking was found to be significantly associated with an increased frequency of bilateral lung parenchyma involvement, far-advanced pulmonary TB, cavitary lesions, multiple cavities, large cavities, and an unfavorable outcome of TB treatment in non-diabetic, but not in diabetic culture-positive pulmonary TB patients because the effect of smoking was attenuated by diabetes. However, smoking was found to be associated with an increased frequency of pretreatment-positive smears in both non-diabetic and diabetic culture-positive pulmonary TB patients and the effect was not attenuated by diabetes.
An association between smoking and the manifestations of TB has been previously reported in several publications, although these findings have not been consistent. Leung et al assessed 851 TB patients and reported that ‘ever smokers’ were more likely to have upper zone involvement (OR 1.67), cavities (OR 1.76), miliary lung involvement (OR 2.77), and positive sputum cultures (OR 1.43) [8]. Altet-Gómez et al assessed 8903 pulmonary TB patients in whom 35.8% had cavitary lesions on CXR and 66.2% were smear positive; smokers were more likely to have cavitary lesions (OR 2.2, 95% CI 2.0–2.4%) and to be smear positive (OR 2.0, 95% CI 1.8–2.3%) than non-smokers [9]. Wang investigated 523 patients and reported that ‘ever smokers’ were more likely to have upper-lung predominance and cavitation compared with ‘never smokers’, but there was no significant difference in the pretreatment positive smears between ‘ever smokers’ and ‘never smokers’ [10]. Leung et al investigated 16,345 TB patients in Hong Kong and reported that smoking was associated with more extensive lung disease, lung cavitation, and positive sputum smears (29.2% among non-smokers, 39.6% among ex-smokers, and 36.7% among smokers) and culture results at the baseline [5].
In our present study, the proportion of patients with upper-lung field involvement was higher among smokers than non-smokers in non-diabetic patients, although the difference did not meet our threshold for statistical significance. Our findings confirm the finding that smoking is associated with extensive lung disease and cavitary lesions, but these were only observed in non-diabetic and not in diabetic TB patients. Several studies have reported that diabetes is associated with cavitary lesions on CXR [14, 16–18, 22, 23]. Our study reveals that the effect of smoking on cavitary lesions or far advanced lesions on CXR was attenuated by diabetes.
The most striking finding of our study was that smoking and diabetes showed joint effects on pretreatment positive smears: smokers had more than a 2-fold increased risk of pretreatment positive smears in both non-diabetic and diabetic TB patients and diabetic smokers had more than a 5-fold increased risk of pretreatment positive smears than did non-diabetic non-smokers. Furthermore, the increased frequency in positive smears was mainly of a high positivity grade, indicating remarkable joint effects of diabetes and smoking that increased the risk of transmission of TB. This finding implies that radiographic manifestations may not be sufficiently sensitive to capture the joint effects of diabetes and smoking on the bacillary load of TB.
Regarding outcomes of TB, Wang et al reported that smoking was not associated with a poor prognosis for TB in a multi-variate survival analysis [10]. Leung et al reported that the proportion of patients with treatment success was 76.7% among ex-smokers and 81.5% among smokers, which were both lower than the 84.7% among non-smokers as a consequence of the higher proportion of death among ex-smokers and current smokers [5]. Reed et al reported that the risk of death in the first 12 months of enrollment was 2.2-fold higher than in non-diabetic patients and that the risk of death among diabetic smokers who smoked one or more pack of cigarettes daily prior to enrollment was 4.3-fold higher than for non-diabetic non-smokers [24]. Our study confirmed that smoking was significantly associated with an unfavorable outcome of TB, but the increased risk of unfavorable outcome occurred only in non-diabetic patients. In our present study, diabetes was associated with an increased risk of an unfavorable outcome of TB. The effect of smoking was attenuated by diabetes and smoking was not associated with an unfavorable outcome of TB in diabetic patients.
Our study has several limitations. First, we found that smoking was significantly associated with extensive disease, cavitary lesions, and unfavorable outcomes of TB in non-diabetic, but not in diabetic culture-positive pulmonary TB patients. The sample size of our study may not have been sufficient to detect the influence of smoking on the radiographic manifestations and outcomes of pulmonary TB in diabetic patients. Second, we did not have data regarding the daily use of cigarettes, so we could not assess the dose response relationship between smoking and TB. Third, several studies have reported the underlying mechanisms of the impact of smoking on TB [25, 26]. Our present study had an observational design, which by nature did not allow us to investigate how smoking and diabetes jointly modify the innate and adaptive immune responses to TB. Fourth, the study is a retrospective study based on charts review. There might be mis-classification of smoking status of TB patients. However, mis-classification of exposure tends to bias results toward the null. Thus, our analysis may under-estimate the influence of smoking on TB.
Nevertheless, our study clearly established that smoking is significantly associated with an increased frequency of bilateral lung parenchyma involvement, far-advanced pulmonary TB, cavitary lesions, multiple cavities, large cavities, and unfavorable outcomes of TB treatment in non-diabetic TB patients. Furthermore, smoking and diabetes have joint effects on pretreatment-positive smears and a high proportion of smokers and diabetic patients have high smear positivity grades. Therefore, the benefit of tobacco control on TB responses should be noted [27]. The ‘MPOWER’ package developed by the WHO to address the global tobacco epidemic includes the following suggestions: Monitor tobacco use and prevention policies, Protect people from tobacco smoke, Offer help to quit tobacco use, Warn about the dangers of tobacco, Enforce bans on tobacco advertising, promotion and sponsorship, and Raise taxes on tobacco. Levy et al reported that in 41 countries that adopted at least one of the highest-level MPOWER policies between 2007 and 2010, the number of smokers was estimated to have dropped by 14.8 million [28]. Moreover, several studies have reported that even briefly providing advice regarding smoking cessation among TB patients could result in a high quit rate among TB patients who smoked [29–32]. The findings of our present study provide additional evidence to highlight the potential contributions of tobacco control on TB control.
Supporting Information
(DTA)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by a research grant DOH101-DC-1103 of Taiwan Centers for Disease Control. http://www.cdc.gov.tw. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Slama K, Chiang C-Y, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11:1049–61. [PubMed] [Google Scholar]
- 2.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.d'Arc Lyra Batista J, de Fátima Militão de Albuquerque M, Arraes de Alencar Ximenes R, Rodrigues LC. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol. 2008;37:841–51. 10.1093/ije/dyn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 5.Leung CC, Yew WW, Chan CK, Chang KC, law WS, Lee SN, et al. Smoking adversely affects treatment response, outcome and relapse in tuberculosis. Eur Respir J. 2015;45:583–5. [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Tchetgen Tchetgen E, Becerra MC, Cohen T, Galea J, Calderon R, et al. Cigarette smoking among tuberculosis patients increases risk of transmission to child contacts. Int J Tuberc Lung Dis. 2014;14:1285–91. [DOI] [PubMed] [Google Scholar]
- 7.Jafta N, Jeena PM, Barregard L, Naidoo RN. Childhood tuberculosis and exposure to indoor air pollution: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19:596–602. 10.5588/ijtld.14.0686 [DOI] [PubMed] [Google Scholar]
- 8.Leung CC, Yew WW, Chan CK, Tam CM, Lam CW, Chang KC, et al. Smoking and tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2003;7:980–6. [PubMed] [Google Scholar]
- 9.Altet-Gómez MN, Alcaide J, Godoy P, Romero MA, Hernández del Rey I. Clinical and epidemiological aspects of smoking and tuberculosis: a study of 13 038 cases. Int J Tuberc Lung Dis. 2005;9:430–6. [PubMed] [Google Scholar]
- 10.Wang JY, Hsueh PR, Jan IS, Lee LN, Liaw YS, Yang PC, et al. The effect of smoking on tuberculosis: different patterns and poorer outcomes. Int J Tuberc Lung Dis. 2007;11:143–9. [PubMed] [Google Scholar]
- 11.Wampande EM, Mupere E, Debanne SM, Asiimwe BB, Nsereko M, Mayanja H, et al. Long-term dominance of Mycobacterium tuberculosis Uganda family in peri-urban Kampala-Uganda is not associated with cavitary disease. BMC Infect Dis. 2013;1471-2334/13/484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7): e152: 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Medicine. 2011;1741-7015/9/81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiménez-Corona ME, Cruz-Hervert LP, García-García L, Ferreyra-Reyes L, Delgado-Sánchez G, Bobadilla-del-Valle M, et al. Association of diabetes and tuberculosis: impact on treamtent and post-treatment outcomes. Thorax. 2013;68:214–20. 10.1136/thoraxjnl-2012-201756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alisjahbana B, Sahiratmadja E. The Effect of Type 2 Diabetes Mellitus on the Presentation and Treatment Response of Pulmonary Tuberculosis. Clin Infect Dis. 2007;45:428–35. [DOI] [PubMed] [Google Scholar]
- 16.Wang CS, Yang CJ, Chen HC, Chuang SH, Chong IW, Hwang JJ, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–10. 10.1017/S0950268808000782 [DOI] [PubMed] [Google Scholar]
- 17.Chang JY, Dou HY, Yen CL, Wu YH, Huang RM, Lin HJ, et al. Effect of type 2 Diabetes Mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug-resistance. J Formos Med Assoc. 2011;110(6):372–8. 10.1016/S0929-6646(11)60055-7 [DOI] [PubMed] [Google Scholar]
- 18.Chiang C-Y, Lee J-J, Chien S-T, Enarson DA, Chang Y-C, Chen Y-T, et al. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PLoS ONE. 2014;9(4): e93397: 10.1371/journal.pone.0093397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissell K, Fraser T, Chiang C-Y, Enarson DA. Smoking cessation and smokefree environments for tuberculosis patients. Second edition In: Disease IUATaL, editor. 2 ed. Paris: International Union Against Tuberculosis and Lung Disease; 2010. p. 1–50. [Google Scholar]
- 20.International Union Against Tuberculosis and Lung Disease, World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes. World Health Organization Document. 2011;WHO/HTM/TB/2011.15:1–40.
- 21.Chiang C-Y, Bai K-J, Lin H-H, Chien S-T, Lee J-J, Enarson DA, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS ONE. 2015;10:e0121698 10.1371/journal.pone.0121698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee MJ, kempker RR, Kipiani M, Gandhi NR, Darchia L, Tukvadze N, et al. Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis. 2015;19:685–92. 10.5588/ijtld.14.0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SW, Shin JW, Kim JY, Park IW, Choi BW, Choi JC, et al. The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2012;31:1305–10. 10.1007/s10096-011-1443-3 [DOI] [PubMed] [Google Scholar]
- 24.Reed GW, Choi H, Lee SY, Lee M, Kim Y, Park H, et al. Impact of diabetes and smoking on mortality in tuberculosis. PLoS ONE. 2013;8(2): e58044: 10.1371/journal.pone.0058044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zyl-Smit RN, Binder A, Meldau R, Semple PL, Evans A, Smith P, et al. Cigarette smoke impairs cytokine responses and BCG containment in alveolar macrophages. Thorax. 2014;69:363–70. 10.1136/thoraxjnl-2013-204229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Leary SM, Coleman MM, Chew WM, Morrow C, McLaughlin AM, Gleeson LE, et al. Cigarette smoking impairs human pulmonary immunity to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2014;190:1430–6. 10.1164/rccm.201407-1385OC [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Stuckler D, Bitton A, Glantz SA. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343: 10.1136/bmj.d5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy D T, Ellis J A, Mays D, Huang A T. Smoking-related deaths averted due to three years of policy progress. Bull World Health Organ 2013; 91: 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Wang LX, Qiu LX, Huang Q, Shu Q, Lin HX, et al. A smoking cessation intervention among tuberculosis patients in rural China. Public Health Action. 2015;5:183–7. 10.5588/pha.15.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bam TS, Aditama TY, Chiang C-Y, Rubaeah R, Suhaemi A. Smoking cessation and smokefree environments for tuberculosis patients in Indonesia—a cohort study. BMC Public Health. 2015;15:604 10.1186/s12889-015-1972-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiquea BN, Islam MA, Bam TS, Satyanarayana S, Enarson DA, Reid AJ, et al. High quit rate among smokers with tuberculosis in a modified smoking cessation programme in Dhaka, Bangladesh. Public Health Action. 2013;3:243–6. 10.5588/pha.13.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur J, Sachdeva KS, Modi B, Jain DC, Chauhan LS, Dave P, et al. Promoting tobacco cessation by integrating "brief advice" in tuberculosis control programme. WHO South-East Asia J Public Health. 2013;2:28–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DTA)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.