Abstract
Ischemic stroke represents a leading cause of death worldwide and the leading cause of disability in the United States. Greater than 8% of all deaths are attributed to ischemic stroke. This rate is consistent with the heightened burden of cardiovascular disease deaths. Treatments for acute ischemic stroke remain limited to tissue plasminogen activator and mechanical thrombolysis, both of which require significant medical expertise and can only be applied to a select number of patients based on time of presentation, imaging, and absence of contraindications. Over 1,000 compounds that were successful in treating ischemic stroke in animal models have failed to correlate to success in clinical trials. The search for alternative treatments is ongoing, drawing greater attention to the importance of preclinical models that more accurately represent the clinical population through incorporation of common risk factors. This work reviews the contribution of these commonly observed risk factors in the clinical population highlighting both the pathophysiology as well as current clinical diagnosis and treatment standards. We also highlight future potential therapeutic targets, areas requiring further investigation, and recent changes in best-practice clinical care.
Keywords: Ischemic Stroke, Metabolic Syndrome
Introduction
Ischemic stroke has been recognized as a leading cause of morbidity and mortality in the United States.1 Disruption of cerebral blood flow causes activation of neuroinflammatory cascades, which can ultimately disrupt brain metabolism and lead to decreased neuronal survival. These chronic changes account for why ischemic stroke is the leading cause of disability in the United States.2 Several risk factors are associated with the occurrence of ischemic stroke. Risk factors fall into two categories: modifiable factors such as hypertension and diabetes and non-modifiable factors such as age and gender. While age remains the greatest risk factor for stroke, evident by the exponential increase in risk with each decade,3 modifiable risk factors such as smoking, hypertension, diabetes, dyslipidemia, and obesity are contributing to a significantly heightened risk for ischemic stroke.4 In fact, population-based analysis suggests that the metabolic syndrome may account for approximately 19% of all strokes.4 By understanding the factors that increase the risk of ischemic stroke and are associated with poor outcomes, alternative therapeutic targets may be identified.
Hypertension and Stroke
Classification
Hypertension can be classified as either primary or secondary hypertension. Secondary hypertension can be caused by medical conditions or as the result of various medications.5 Unlike secondary hypertension the cause of primary hypertension is idiopathic. Primary hypertension is responsible for 95% of all hypertension cases and negatively affects multiple organ systems.6 Several factors may contribute to the 20% increase in hypertension cases from 1976–20047. One factor is the increased screening for hypertension following the United States Preventive Services Task Force (USPSTF) 1996 recommendation for sphygmomanometry readings for all adult patients.8 Other factors that account for the increase in hypertension may include increased alcohol consumption, high salt intake, obesity, high cholesterol, stress, and advanced age. The VIPER-Bp clinical study reported that successful management of hypertension in the clinic involves early diagnosis, a multi-drug treatment approach, and eliminating environmental and social factors that lead to an increase in hypertension.9
Hypertension Outcomes
Age is one of the most predominant risk factors for hypertension according to a recent National Health and Nutrition Examination Study report. This is of particular relevance as the median age across the nation increases.10 By the year 2040, the population over the age of 65 is predicted to double.11 The rate of hypertension for individuals over 60 was 65.4% which dwarfs the 28.7% overall rate for the general population.12 One of the reasons for this large difference may be due to the body’s response to aging including but not limited to an increase in inflammatory reactivity. Aging affects blood pressure through age-related changes in blood vessel structure. Elevated blood pressure increases the shear force placed on artery walls as blood is pumped throughout the body.5 This pressure change can cause damage to the vascular wall as well as smooth muscle thickening, decreased elasticity, and a narrowed lumen. Patients with diagnosed hypertension during mid-life have increased susceptibility for ischemic injury as they age.10
Pathophysiology
In addition to thrombosis, cardiovascular disease (CVD) is a suggested secondary effect of primary hypertension. The major cells of the vessel wall that play a role in CVD are the endothelial cells (EC). These cells line the vessels in every organ system and have the ability to control secretory, synthetic, metabolic, and immunologic functions.13 The regulation of blood flow in the body is partly orchestrated through a wide variety of molecules acting on membrane bound receptors. These molecules include coagulant and anticoagulant proteins, metabolites, cytokines, lipid transporting proteins, and hormones.13 In correct proportions these molecules help maintain homeostasis, but in excess or scarcity may lead to damage. Damage initiated through dysregulated signaling pathways can have detrimental consequences on any organ in the body.
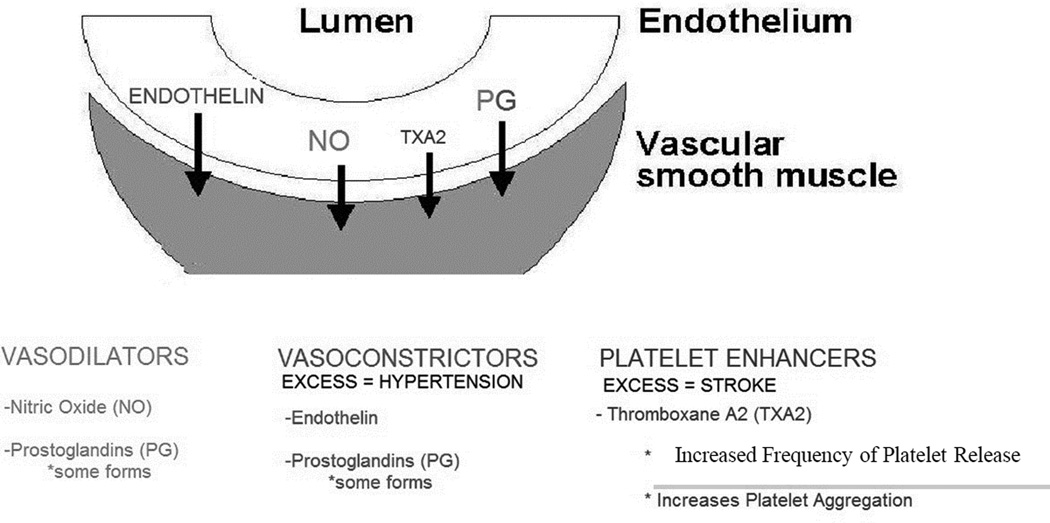
For example, ECs respond to the surrounding environment and release vasoconstrictors and vasodilators in order to maintain the appropriate blood pressure and proper blood flow. When ECs are healthy they prevent platelet adhesion to the vessel wall by favoring release of vasodilators such as nitric oxide (NO) and prostacyclin (PGI2).13 On the other hand, during inflammatory driven responses due to shear and other physiologic processes, ECs may become damaged due to oxidative stress and cytokine mediated responses. Damaged ECs have a lower availability of NO and PGI2 and an increased level of vasoconstrictors and platelet enhancers such as platelet activation factor (PAF) and Thromboxane A2 (TXA2) (Figure 1). This change in balance has a series of effects ultimately leading to the development of hypertension and increased risk of thrombosis. Inflamed ECs produce cytokines and adhesion factors such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule (VCAM) leading to leukocyte adhesion to the underlying damaged tissue.14 During this process circulating platelets create a hemostatic plug by interacting with adherent platelets and other adhesion factors already on the EC surface. This interaction induces the generation of thrombin which leads to the formation of a fibrin clot aiding vessel wall repair.13 Unfortunately, this process is also known to be the earliest stages of atherothrombosis. Atherothrombosis is the cause of 50% of ischemic strokes, which comprise approximately 80% of all strokes. Furthermore, stroke is the second most common cause of death worldwide.15 Therefore, continuous high blood pressure is associated with chronic vessel wall damage and indicates an increased likelihood for future stroke, necessitating close management of hypertension as a preventative measure.
Figure 1.
Balance of vasodilator-associated and vasoconstrictor-associated signaling molecules is altered in hypertension. Damage to endothelial cells can lead to the development of hypertension and vice-versa. Further damage can result in release of pro-thrombotic factors leading to platelet adhesion and thrombosis, producing ischemic stroke.
Diagnosis and Treatment
Unfortunately, no clear signs of primary hypertension exist and hence it is sometimes referred to as a silent killer. At the time of discovery, treatment involves basic lifestyle modifications such as diet alterations, increasing physical activity, lowering sodium and alcohol intake, lowering LDL cholesterol, smoking cessation, and reducing stress. If lifestyle modifications are not sufficient, antihypertensive medications such as beta blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, and diuretics may be prescribed.16 Based on previous studies, only 34% of patients with hypertension are controlled, indicating a clear shortcoming in current management and/or screening/prevention efforts. Since hypertension is a silent killer, few people understand its severity as a risk factor for ischemic vascular events. With increased focus on improving patient understanding of the disease, the percentage of controlled cases will increase and more people will get screened. The USPSTF suggests that adults be screened a minimum of every 2 years and more frequently in the elderly. Therefore, increased emphasis on screening and prevention programs, particularly those that target elderly populations, may reduce the burden of ischemic stroke.17
Diabetes Mellitus and Stroke
Diabetes Mellitus Classification
Type II diabetes (T2D) is one of the fastest growing diseases in terms of new diagnoses around the world. It is characterized by insulin resistance and insulin deficiency. The CDC has claimed that 7% of the American population has the disease.18 T2D has been linked to lifestyle habits as well as certain genetic patterns. The growing number of individuals affected by the disease is due to limited health service access, socioeconomic factors, and the restricted amount of nutritional resource availability in commercially packaged foods. The growing levels of obesity are directly associated with the increasing prevalence of T2D.19 T2D has been linked as a leading risk factor for ischemic stroke.20
T2D Stroke Outcomes
Both stroke and T2D are typically diagnosed in the aging population. Aging contributes to a heightened state of inflammatory response and microglia activation.21 Inflammatory cytokines, such as interleukin-6 (IL-6), have been shown to increase concomitantly with increasing levels of blood glucose.22 It has become well known that inflammatory cytokines play a major role in neural injury. The increasing release of inflammatory cytokines associated with ischemic stroke in T2D patients has been shown to exacerbate the damage caused by the stroke,23 and lead to worsened outcome.24 While T2D is not the sole cause of stroke, it does negatively affect the outcome of ischemic injury.
T2D and an increased stroke risk have also been linked with hypertension. T2D can potentially exacerbate hypertension, due to added stress placed on the arterial walls, thereby also increasing the risk of thromboembolic stroke.25, 26 Unfortunately, most patients at risk for stroke present with a metabolic syndrome consisting of T2D, hypertension, and obesity.27 The metabolic syndrome has been linked to poor cardiovascular outcomes in adults as well.28 According to a recent study, a person with hypertension is 2.4 times more likely to have cerebrovascular disease.29 The synchrony of clinical data reveals a stark comorbidity between stroke and diabetes; however, the underlying mechanism behind this relationship has yet to be fully unveiled.
Pathophysiology
T2D causes acute microvasculature changes such as retinopathy, and contributes to macrovascular changes related to atherosclerosis.30 After ischemic stroke, hyperglycemia is an acute indicator of endocrine stress and neuroinflammation.31 Sustained hyperglycemia leads to the formation of advanced glycosylated end products, which trigger the release of reactive oxygen species.32 Advanced glycosylated end products also lead to increased vascular permeability and decreased vascular dilation, thus worsening ischemic stroke outcome. Controlling hyperglycemia has been shown to have a 42% relative risk reduction for ischemic stroke.33 The appropriate management of T2D is therefore an urgent necessity.
Diagnosis and Treatment
Diagnosis of T2D consists of either fasting plasma glucose above 126 mg/dl or an HbA1c above 6.5%. Appropriate management of T2D is lowering the HbA1c below 6.5%. The most successful treatment regimens include a combination of healthy diet, regular exercise, and anti-hyperglycemic therapy.34 50% of T2D patients however, are not adequately managed. Long terms results of unmanaged T2D include optic retinopathy, diabetic neuropathy, and increased risk for ischemic stroke. A clear need exists for an increase in prevention and monitoring programs based on the high prevalence of diabetes across the nation.35 When considering ischemic stroke specifically, management and control of diabetes may lessen the impact of stroke. Rapid and sufficient correction of hyperglycemia has been shown to reduce infarct development and expansion in ischemic stroke.36 Furthermore, diabetes increase rates of intracerebral hemorrhage following tPA administration, emphasizing the need for blood-glucose control upon patient arrival.37, 38
Obesity and Stroke
Obesity Classification
The obesity epidemic continues to plague the United States, where nearly 70% of Americans are overweight (Body Mass Index (BMI) ≥ 25) and 35% are obese (BMI ≥ 30).39 Moreover, health problems including diabetes, coronary artery disease, ischemic stroke, respiratory failure, and cancer are strongly associated with excess weight gain.40
Obesity is a multifactorial disease influenced by many variables including environment, social structures, genetics, behavior, and diet. Furthermore, twin, adoption, and family lineage studies suggest that heritable factors contribute to 40–70% of inter-individual variation seen in the obesity population.41, 42 At the simplest level, obesity can be defined as a state of positive energy balance. This positive energy balance is in part fueled by our current environment, which encourages overeating and discourages physical activity. Recent data suggests the adult population is consuming an excess of 100kcal/day.43
A substantial body of evidence has documented that increased adiposity is associated with an increased risk of stroke.44–47 A meta-analysis found that between the BMI ranges of 25 to 50, each increase of 5 on the BMI scale was associated with a 40% increased risk of stroke mortality. There was no relationship in the lower BMI ranges.48 Recently, the American Heart Association and American Stroke Association recommended that the treatment of obesity is critical for both primary5 and secondary49 stroke prevention.
Obesity Stroke Outcomes
Interestingly, inconsistent results about the association between obesity and stroke risk have been reported. A recent analysis showed that BMI and abdominal obesity do not increase cardiovascular disease risk when data about systolic blood pressure, history of diabetes, and lipid dysfunction are accounted by controlling for confounding.50 On the other hand, while one study reported that BMI was associated with an increased risk of stroke in both sexes and abdominal obesity only in men51; another study reported women aged 35 to 54 years are more likely to be obese and morbidly obese than in the previous decade and their abdominal obesity however was an independent risk factor for stroke.52 Abdominal obesity may be a stronger risk factor of stroke than BMI in future studies considering the current trends.53 Apart from stroke morbidity, a recent study has proposed a paradoxical “protective” effect of obesity in acute stroke survivors.54 This inverse relationship between obesity and outcome was first documented in those recovering from an intracerebral hemorrhage55 as well as those suffering from chronic diseases.56 Clearly, further research is needed to sufficiently assess the best measure of obesity on stroke risk and to find better tests to predict likelihood of stroke mortality.
Pathophysiology
A possible mechanism linking obesity and stroke involves the pleiotropic effects that cytokines secreted by adipose tissue may exert on insulin resistance, inflammation, and changes in the vascular wall. An accumulation of lipids in adipocytes and the expansion of adipose tissue initiate a host of inflammatory processes. The hypertrophic adipocytes produce proinflammatory cytokines such as tissue necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1) as outlined in Table 1. Local endothelial cells respond with an up-regulation of adhesion molecular synthesis and increased vascular permeability, which along with the chemokines serve to recruit circulating monocytes. Together, the endothelial cells, adipocytes, and immune cells create an inflammatory milieu that induces a state of local and systemic insulin resistance and increase the risk for atherosclerosis.57 In addition, the dysfunction of adipose tissue may lead to the dysregulation of cytokines acting on the sympathetic nervous system, renin-angiotensin axis, and endothelial cells. These changes increase the risk for hypertension,58 which is the number one modifiable risk factor for stroke.
Table 1.
Cytokine secretion from adipose tissue and the role of these cytokines in the development of insulin resistance.39
| Cytokine | Function | Role in insulin resistance |
|---|---|---|
| TNF-α | Inflammatory cytokine | Plays role in insulin resistance by stimulating intracellular signaling through NF-κB activation; knockout mice have shown improved insulin sensitivity and insulin signaling |
| IL-6 | Inflammatory cytokine | Implicated as pathogenic mediator of insulin resistance; human polymorphism in IL6 gene, with resulting decrease in circulating IL-6 levels, is associated with increased insulin sensitivity |
| resistin | Inflammatory cytokine | Plays role in insulin resistance by stimulating intracellular signaling through NF-κB activation; knockout mice have shown improved glucose tolerance on high-fat diet |
| MCP-1 | Chemoattractive protein that recruits immune cells to sites of inflammation |
Expression in adipocytes have resulted in hepatic steatosis and insulin resistance in liver, muscle and fat |
| PAI-1 | Inhibitor of plasminogen activators, which converts plasminogen into plasmin during fibrinolysis |
Knockout mice have shown improved insulin sensitivity |
Diagnosis and Treatment
The need for appropriate diagnosis of obesity is necessary to prevent serious long-term consequences. The relative risk for ischemic stroke after 10 years of obesity is 1.64.59 If obesity is diagnosed early, effective treatment options can be implemented to prevent serious long-term outcomes such as stroke.60 Diagnosis of obesity is more likely when patients are referred to a cardiology specialist.61 Furthermore, preventing obesity has gained widespread support, and the NIH is sponsoring consortiums to find effective interventions for children and adolescents.62 Current treatment includes lifestyle modifications, followed by phentermine-topiramate or lorcaserin, and as a last resort bariatric surgery.63 Unfortunately, only 50% of obese patients attempt to lose weight.59 Patients diagnosed as obese instead of simply being classified as overweight have a higher likelihood of participating in weight loss programs.61 Several novel approaches for treating obesity such as encouraging patients to watch less television and to walk after eating are currently being investigated with randomized control trials.64
Future Directions
As highlighted in the previous sections, comorbidities contribute substantially to ischemic stroke risk. Due to the limited success of translating compounds from preclinical trials to FDA approved ischemic stroke treatments, it will be necessary to promote preventative care. Preventive approaches must incorporate healthcare professionals, research scientists, and members of the local communities. A three tier approach should be implemented. Tier one incorporates focused research to improve our understanding of stroke pathophysiology. The goal is to ascertain how subsets of patients presenting with different comorbidities may respond to individualized therapies and focused treatment plans that will lower the risk for ischemic stroke. Inherent to this goal is early diagnosis and treatment. Tier two is an educational approach that engages the community to encourage healthy food availability, to teach about comorbidities, and to improve recreational and park resources. Regular physician check-ups should be emphasized in these educational meetings. In order to create an environment more conducive to healthy lifestyles, tier three requires physician led advocacy for changes in social infrastructure. One such change is encouraging regulations on nutrient-poor food and drinks. Other changes include improving transport protocols from tertiary care centers following stroke, and increasing the number of care facilities in areas with high ischemic stroke prevalence.
Conclusion
In summary, ischemic stroke is the leading cause of disability and a major cause of mortality worldwide. It is predominantly seen in the elderly and in patients with the metabolic syndrome.65, 66 We looked specifically at the pathophysiology of hypertension, T2D, and obesity. Continued research is necessary to improve our understanding of exactly how the aforementioned comorbidities increase ischemic stroke risk. A stroke is a devastating event for the individual, family, and local community. Currently, those at risk for stroke have limited understanding of why they are at risk. To adequately address the overall lack of awareness, it will be important for an interdisciplinary healthcare team to implement the three tiers mentioned in the future direction section. Tier 1 requires early diagnosis, adequate treatment, and focused preclinical and clinical research. Tier 2 provides educational resources in easy accessible formats such as through school assemblies or town-hall meetings. Simple targets should be highlighted: improving nutritional food choices, increasing physical activity, and scheduling annual physical exams. Tier 3 involves physician led advocacy for nutritional regulations. Furthermore, when a stroke does occur, it is critical that patients know how to activate the emergency response system and where to go to receive help.67 It is also important for limited care facilities to initiate transport protocols quickly and efficiently in order for patients to receive the best care available.68 Although the need for improved care for stroke patients is dire, the potential for better preventive measures orchestrated through the three tier approach offers to be promising.
Acknowledgments
We would like to acknowledge West Virginia University Health Sciences Center for use of its facility. Research reported in this publication was supported by the NIGMS of the National Institutes of Health under award number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
No conflicts of interest to report.
References
- 1.Floel A, Warnecke T, Duning T, et al. Granulocyte-colony stimulating factor (G-CSF) in stroke patients with concomitant vascular disease--a randomized controlled trial. PloS one. 2011;6(5):e19767. doi: 10.1371/journal.pone.0019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen F, Qi Z, Luo Y, et al. Non-pharmaceutical therapies for stroke: Mechanisms and clinical implications. Prog Neurobiol. 2014 doi: 10.1016/j.pneurobio.2013.12.007. ePub(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacco RL. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology. 1997 Nov;49(5 Suppl 4):S39–S44. doi: 10.1212/wnl.49.5_suppl_4.s39. [DOI] [PubMed] [Google Scholar]
- 4.Boden-Albala B, Sacco RL, Lee HS, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke; a journal of cerebral circulation. 2008 Jan;39(1):30–35. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2011 Feb;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 6.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000 Jan 25;101(3):329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 7.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012 Oct 23;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 8.Sheridan S, Pignone M, Donahue K. Screening for high blood pressure: A review of the evidence for the U.S. preventive services task force. Am J Prev Med. 2003;25(2):151–158. doi: 10.1016/s0749-3797(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Carrington MJ, Swemmer C, Kurstjens N, Jennings GL. Optimising management of hypertension in primary care: The valsartan intensified primary care reduction of blood pressure (viper-bp) study. Int J Cardiol. 2011;153(3):317–322. doi: 10.1016/j.ijcard.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Liu H, Yang J, Yang S, Lin J, Lee T. Longitudinal MR imaging study in the prediction of ischemic susceptibility after cerebral hypoperfusion in rats: Influence of aging and hypertension. Neuroscience. 2014;257(0):31–40. doi: 10.1016/j.neuroscience.2013.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the united states. Am J Med. 2011;124(9):827–833. e5. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasan ZN, Hussein MQ, Haji GF. Hypertension as a risk factor: is it different in ischemic stroke and acute myocardial infarction comparative cross-sectional study? International journal of hypertension. 2011;2011:701029. doi: 10.4061/2011/701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998 May 15;91(10):3527–3561. [PubMed] [Google Scholar]
- 14.Viles-Gonzalez JF, Fuster V, Badimon JJ. Atherothrombosis: a widespread disease with unpredictable and life-threatening consequences. European heart journal. 2004 Jul;25(14):1197–1207. doi: 10.1016/j.ehj.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Krafft PR, Bailey EL, Lekic T, et al. Etiology of stroke and choice of models. International journal of stroke : official journal of the International Stroke Society. 2012 Jul;7(5):398–406. doi: 10.1111/j.1747-4949.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Kim M, Han S, et al. Characteristics of hypertension subtypes and treatment outcome among elderly korean hypertensives. Journal of the American Society of Hypertension. (0) doi: 10.1016/j.jash.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke; a journal of cerebral circulation. 2006 Jun;37(6):1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 18.Billings LK, Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS? Annals of the New York Academy of Sciences. 2010 Nov;1212:59–77. doi: 10.1111/j.1749-6632.2010.05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tekola-Ayele F, Adeyemo AA, Rotimi CN. Genetic epidemiology of type 2 diabetes and cardiovascular diseases in africa. Prog Cardiovasc Dis. 2013;56(3):251–260. doi: 10.1016/j.pcad.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka R, Ueno Y, Miyamoto N, et al. Impact of diabetes and prediabetes on the short-term prognosis in patients with acute ischemic stroke. J Neurol Sci. 2013;332(1–2):45–50. doi: 10.1016/j.jns.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Suridjan I, Rusjan PM, Voineskos AN, et al. Neuroinflammation in healthy aging: A PET study using a novel translocator protein 18 kDa (TSPO) radioligand, [18F]-FEPPA. Neuroimage. 2014;84(0):868–875. doi: 10.1016/j.neuroimage.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen OE. Cardiovascular disease and type 2 diabetes mellitus: a multifaceted symbiosis. Scandinavian journal of clinical and laboratory investigation. 2007;67(8):786–800. doi: 10.1080/00365510701408558. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan K, Sharma SS. Sodium phenylbutyrate ameliorates focal cerebral ischemic/reperfusion injury associated with comorbid type 2 diabetes by reducing endoplasmic reticulum stress and DNA fragmentation. Behavioural brain research. 2011 Nov 20;225(1):110–116. doi: 10.1016/j.bbr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Bellolio MF, Gilmore RM, Stead LG. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev. 2011;(9):CD005346. doi: 10.1002/14651858.CD005346.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008 Nov;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA : the journal of the American Medical Association. 2010 Apr 28;303(16):1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanchaiphiboolkul S, Suwanwela NC, Poungvarin N, et al. Risk of metabolic syndrome for stroke is not greater than the sum of its components: Thai epidemiologic stroke (TES) study. Journal of Stroke and Cerebrovascular Diseases. 2013;22(8):e264–e270. doi: 10.1016/j.jstrokecerebrovasdis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Shin AM, Kim MK, Kim YN. Comorbidity study on type 2 diabetes mellitus using data mining. The Korean journal of internal medicine. 2012 Jun;27(2):197–202. doi: 10.3904/kjim.2012.27.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung JY, Kang HT, Lee DC, Lee HR, Lee YJ. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Archives of gerontology and geriatrics. 2013 Jan-Feb;56(1):270–278. doi: 10.1016/j.archger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. The Lancet Neurology. 2012;11(3):261–271. doi: 10.1016/S1474-4422(12)70005-4. [DOI] [PubMed] [Google Scholar]
- 31.Radermecker RP, Scheen AJ. Management of blood glucose in patients with stroke. Diabetes Metab. 2010;36(Supplement 3(0)):S94–S99. doi: 10.1016/S1262-3636(10)70474-2. [DOI] [PubMed] [Google Scholar]
- 32.Biessels GJ. Sweet memories: 20 years of progress in research on cognitive functioning in diabetes. Eur J Pharmacol. 2013;719(1–3):153–160. doi: 10.1016/j.ejphar.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Liu D, Sun L, Lu Y, Zhang Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J Neurol Sci. 2012;317(1–2):1–5. doi: 10.1016/j.jns.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Bleich SN, Pickett-Blakely O, Cooper LA. Physician practice patterns of obesity diagnosis and weight-related counseling. Patient Educ Couns. 2011;82(1):123–129. doi: 10.1016/j.pec.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth JL. In: The Burden of Diabetes in West Virginia 2009. Resources WVDoHH, editor. Charleston, WV: Bureau for Public Health; 2009. pp. 1–52. [Google Scholar]
- 36.Bruno A, Liebeskind D, Hao Q, Raychev R. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol. 2010 Nov;12(6):492–503. doi: 10.1007/s11940-010-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan X, Ning M, Lo EH, Wang X. Early Insulin Glycemic Control Combined With tPA Thrombolysis Reduces Acute Brain Tissue Damages in a Focal Embolic Stroke Model of Diabetic Rats. Stroke; a journal of cerebral circulation. 2013 Jan;44(1):255–259. doi: 10.1161/STROKEAHA.112.663476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan X, Qiu J, Yu Z, et al. A rat model of studying tissue-type plasminogen activator thrombolysis in ischemic stroke with diabetes. Stroke; a journal of cerebral circulation. 2012 Feb;43(2):567–570. doi: 10.1161/STROKEAHA.111.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS data brief. 2012 Jan;(82):1–8. [PubMed] [Google Scholar]
- 40.Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? The American journal of medicine. 2009 Apr;122(4 Suppl 1):S4–S11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Hjelmborg J, Fagnani C, Silventoinen K, et al. Genetic influences on growth traits of BMI: a longitudinal study of adult twins. Obesity (Silver Spring Md.) 2008 Apr;16(4):847–852. doi: 10.1038/oby.2007.135. [DOI] [PubMed] [Google Scholar]
- 42.Loos RJ. Recent progress in the genetics of common obesity. British journal of clinical pharmacology. 2009 Dec;68(6):811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science (New York, N.Y.) 2003 Feb 7;299(5608):853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 44.Kurth T, Gaziano JM, Berger K, et al. Body mass index and the risk of stroke in men. Archives of internal medicine. 2002 Dec 9–23;162(22):2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 45.Kurth T, Gaziano JM, Rexrode KM, et al. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005 Apr 19;111(15):1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 46.Suk SH, Sacco RL, Boden-Albala B, et al. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke; a journal of cerebral circulation. 2003 Jul;34(7):1586–1592. doi: 10.1161/01.STR.0000075294.98582.2F. [DOI] [PubMed] [Google Scholar]
- 47.Strazzullo P, D'Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke; a journal of cerebral circulation. 2010 May;41(5):e418–e426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 48.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar 28;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olson DM, Cox M, Pan W, et al. Death and Rehospitalization after Transient Ischemic Attack or Acute Ischemic Stroke: One-year Outcomes from the Adherence Evaluation of Acute Ischemic Stroke-Longitudinal Registry. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2012 Dec 25; doi: 10.1016/j.jstrokecerebrovasdis.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011 Mar 26;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Archives of internal medicine. 2007 Jul 9;167(13):1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 52.Towfighi A, Zheng L, Ovbiagele B. Weight of the obesity epidemic: rising stroke rates among middle-aged women in the United States. Stroke; a journal of cerebral circulation. 2010 Jul;41(7):1371–1375. doi: 10.1161/STROKEAHA.109.577510. [DOI] [PubMed] [Google Scholar]
- 53.Winter Y, Rohrmann S, Linseisen J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke; a journal of cerebral circulation. 2008 Dec;39(12):3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 54.Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke; a journal of cerebral circulation. 2011 Jan;42(1):30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- 55.Kim BJ, Lee SH, Ryu WS, Kim CK, Lee J, Yoon BW. Paradoxical longevity in obese patients with intracerebral hemorrhage. Neurology. 2011 Feb 8;76(6):567–573. doi: 10.1212/WNL.0b013e31820b7667. [DOI] [PubMed] [Google Scholar]
- 56.Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: facts and numbers. Journal of cachexia, sarcopenia and muscle. 2012 Mar;3(1):1–4. doi: 10.1007/s13539-012-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007 May;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 58.Dorresteijn JA, Visseren FL, Spiering W. Mechanisms linking obesity to hypertension. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012 Jan;13(1):17–26. doi: 10.1111/j.1467-789X.2011.00914.x. [DOI] [PubMed] [Google Scholar]
- 59.Lebrun LA, Chowdhury J, Sripipatana A, Nair S, Tomoyasu N, Ngo-Metzger Q. Overweight/obesity and weight-related treatment among patients in U.S. federally supported health centers. Obesity Research & Clinical Practice. 2013;7(5):e377–e390. doi: 10.1016/j.orcp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Taveras EM, Marshall R, Horan CM, et al. Rationale and design of the STAR randomized controlled trial to accelerate adoption of childhood obesity comparative effectiveness research. Contemporary Clinical Trials. 2013;34(1):101–108. doi: 10.1016/j.cct.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Singh S, Somers VK, Clark MM, et al. Physician diagnosis of overweight status predicts attempted and successful weight loss in patients with cardiovascular disease and central obesity. Am Heart J. 2010;160(5):934–942. doi: 10.1016/j.ahj.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pratt CA, Boyington J, Esposito L, et al. Childhood obesity prevention and treatment research (COPTR): Interventions addressing multiple influences in childhood and adolescent obesity. Contemporary Clinical Trials. 2013;36(2):406–413. doi: 10.1016/j.cct.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56(4):465–472. doi: 10.1016/j.pcad.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Raynor HA, Steeves EA, Bassett DR, Jr, Thompson DL, Gorin AA, Bond DS. Reducing TV watching during adult obesity treatment: Two pilot randomized controlled trials. Behavior Therapy. 2013;44(4):674–685. doi: 10.1016/j.beth.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Hendryx M, Zullig KJ. Higher coronary heart disease and heart attack morbidity in Appalachian coal mining regions. Prev Med. 2009 Nov;49(5):355–359. doi: 10.1016/j.ypmed.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Reyes B, Trotter C, Richards M, et al. Mildly reduced preoperative ejection fraction increases the risk of stroke in older adults undergoing coronary artery bypass grafting. W V Med J. 2012 Sep-Oct;108(5):28. 30-24. [PubMed] [Google Scholar]
- 67.Alkadry MG, WIlson C, Nicholas D. Stroke Awareness Among Rural Residents. Social work in health care. 2006;42(2):73–92. doi: 10.1300/J010v42n02_05. [DOI] [PubMed] [Google Scholar]
- 68.Riggs JE, Libell DP, Brooks CE, Hobbs GR. Impact of institution of a stroke program upon referral bias at a rural academic medical center. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association. 2005 Summer;21(3):269–271. doi: 10.1111/j.1748-0361.2005.tb00094.x. [DOI] [PubMed] [Google Scholar]