Significance
Leukotriene E4 (LTE4), a lipid mediator detected in asthma exacerbations triggered by allergen, viruses, and aspirin, elicits airflow obstruction and lung inflammation in asthmatics. GPR99 is the recently identified high-affinity receptor for LTE4 and is resistant to blockade by commercially available cysteinyl leukotriene (cysLT) receptor antagonists. Here we find that GPR99 is expressed in respiratory epithelial cells and mediates mucin release and submucosal swelling in response to LTE4 or to cysLTs elicited by Alternaria, a common airborne fungus. Furthermore, among cysLT receptors, only GPR99 regulates baseline numbers of mucin-containing goblet cells. These studies demonstrate a unique role for GPR99 in epithelial cell homeostasis and activation and indicate that epithelial cells may be a dominant site of LTE4 action in the lung.
Keywords: cysteinyl leukotrienes, mast cells, mucosal immunology, lung, epithelial cell
Abstract
Cysteinyl leukotrienes (cysLTs), leukotriene C4 (LTC4), LTD4, and LTE4 are proinflammatory lipid mediators with pathobiologic function in asthma. LTE4, the stable cysLT, is a weak agonist for the type 1 and type 2 cysLT receptors (CysLTRs), which constrict airway smooth muscle, but elicits airflow obstruction and pulmonary inflammation in patients with asthma. We recently identified GPR99 as a high-affinity receptor for LTE4 that mediates cutaneous vascular permeability. Here we demonstrate that a single intranasal exposure to extract from the respiratory pathogen Alternaria alternata elicits profound epithelial cell (EpC) mucin release and submucosal swelling in the nasal mucosa of mice that depends on cysLTs, as it is absent in mice deficient in the terminal enzyme for cysLT biosynthesis, LTC4 synthase (LTC4S). These mucosal changes are associated with mast cell (MC) activation and absent in MC-deficient mice, suggesting a role for MCs in control of EpC function. Of the three CysLTRs, only GPR99-deficient mice are fully protected from EpC mucin release and swelling elicited by Alternaria or by intranasal LTE4. GPR99 expression is detected on lung and nasal EpCs, which release mucin to doses of LTE4 one log lower than that required to elicit submucosal swelling. Finally, mice deficient in MCs, LTC4S, or GPR99 have reduced baseline numbers of goblet cells, indicating an additional function in regulating EpC homeostasis. These results demonstrate a novel role for GPR99 among CysLTRs in control of respiratory EpC function and suggest that inhibition of LTE4 and of GPR99 may have therapeutic benefits in asthma.
Cysteinyl leukotrienes (cysLTs), leukotriene C4 (LTC4), LTD4, and LTE4 are lipid mediators detected during asthma exacerbations triggered by allergen (1), aspirin (2, 3), and respiratory viruses (4). The cysLTs elicit vascular permeability, inflammation, and bronchoconstriction through three G-protein–coupled receptors. The type 1 cysLT receptor (CysLTR), CysLT1R, is the high-affinity receptor for LTD4 and the dominant CysLTR mediating airway smooth muscle constriction (5–8). The type 2 CysLTR, CysLT2R, has prominent effects on the vascular endothelium (9–12) and also elicits bronchial constriction (13, 14). LTE4, the stable cysLT (15–18), is a weak agonist for CysLT1R and CysLT2R in transfected cells (5, 19), but elicits airflow obstruction in patients with asthma (20–22). Moreover, LTE4 has comparable activity to LTC4 and LTD4 in eliciting a wheal and flare response in human skin (23), and LTE4 elicits cutaneous vascular permeability in mice lacking both CysLT1R and CysLT2R, suggesting the existence of a high-affinity receptor for LTE4, which was recently identified as GPR99 (24, 25). However, the mechanism by which LTE4 induces lung pathobiology and the role of GPR99 remain poorly understood.
The cysLTs are derived from arachidonate through the serial enzymatic actions of 5-lipoxygenase and leukotriene C4 synthase (LTC4S). LTC4, the terminal product of intracellular biosynthesis, is generated in activated leukocytes, exported extracellularly, and rapidly metabolized to LTD4 and then to LTE4. cysLT generation is elicited in the effector phase of allergic inflammation by the IgE-dependent cross-linking of FcεR1 on mast cells (MCs). However, cysLT generation can also be elicited by some allergen extracts in an IgE-independent fashion (26, 27), and recent reports demonstrate a role for cysLTs in shaping primary type 2 immune responses in the lung (28, 29). Thus, we sought to better understand cysLT function in the innate response to Alternaria alternata, a ubiquitous airborne fungus that contains potent aeroallergens. Here we demonstrate that a single intranasal (i.n.) exposure to Alternaria extract elicits profound epithelial cell (EpC) mucin release and submucosal swelling in the nasal mucosa of mice, which depends on MC activation, cysLT generation, and the CysLTR GPR99. Furthermore, GPR99, expressed on respiratory EpCs, mediates mucin release in response to low doses of i.n. LTE4 injection and regulates baseline numbers of goblet cells, demonstrating a novel role for GPR99 among CysLTRs in control of respiratory EpCs.
Results
cysLT-Dependent Submucosal Swelling and Mucin Release.
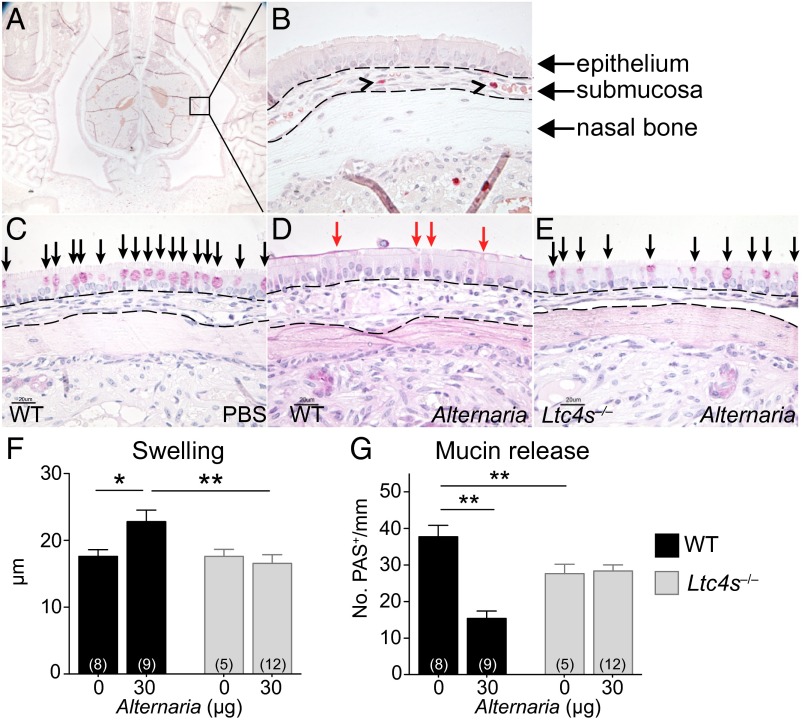
To determine cysLT function in innate immune responses in the airway, we evaluated mucosal changes in the nose of WT and LTC4S-deficient (Ltc4s‒/‒) mice 1 h after a single i.n. application of Alternaria. In the nasal septum (Fig. 1A), the highly vascular submucosa (Fig. 1B, submucosal area marked with punctated lines) responded to 30 μg Alternaria with marked swelling in WT mice, compared with PBS-treated controls (Fig. 1 C and D). Submucosal swelling was due to both vasodilatation and interstitial edema. There was no detectable cellular infiltration. The nasal epithelium of naive or PBS-challenged mice contains a large number of mucin granule-containing Periodic acid Schiff (PAS)-reactive goblet cells (GCs) (Fig. 1C, black arrows). Alternaria challenge led to extrusion of mucin from EpC granules with loss of PAS reactivity (Fig. 1D). Both submucosal edema and EpC mucin release were absent in Alternaria-treated Ltc4s‒/‒ mice (Fig. 1 E–G), indicating a critical role for cysLTs in the response of respiratory tissue. Baseline numbers of PAS-reactive GCs were reduced in Ltc4s‒/‒ mice compared with WT mice (Fig. 1G).
Fig. 1.
cysLT-dependent submucosal swelling and mucin release elicited by Alternaria. (A) Representative histological coronal section through the incisors of the mouse skull of a PBS-challenged mouse with CAE stain (50× magnification). (B) A 630× magnification of Inset in A, rotated 90°. Black arrowheads point to MCs. (C–E) PAS staining of nasal septum 1 h after i.n. administration of (C) PBS or (D and E) 30 µg of Alternaria (magnification: 630×). (Scale bar, 20 µm.) Black arrows indicate mucin-containing GCs. Red arrows indicate GCs with partial extrusion of mucin. (F and G) WT (black bars) and Ltc4s−/− (gray bars) mice. (F) Submucosal swelling, indicated by the difference in the submucosal area between PBS (0 µg) and Alternaria-treated (30 µg) mice. (G) Mucin release, indicated by the difference in the total number of PAS+ cells detected between PBS and Alternaria-treated mice. Results are means ± SEM pooled from four independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01.
Innate Activation of MCs Is Required for Alternaria-Elicited Mucosal Responses.
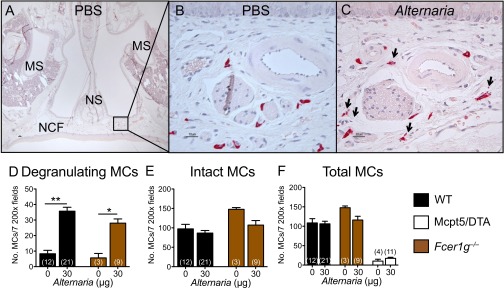
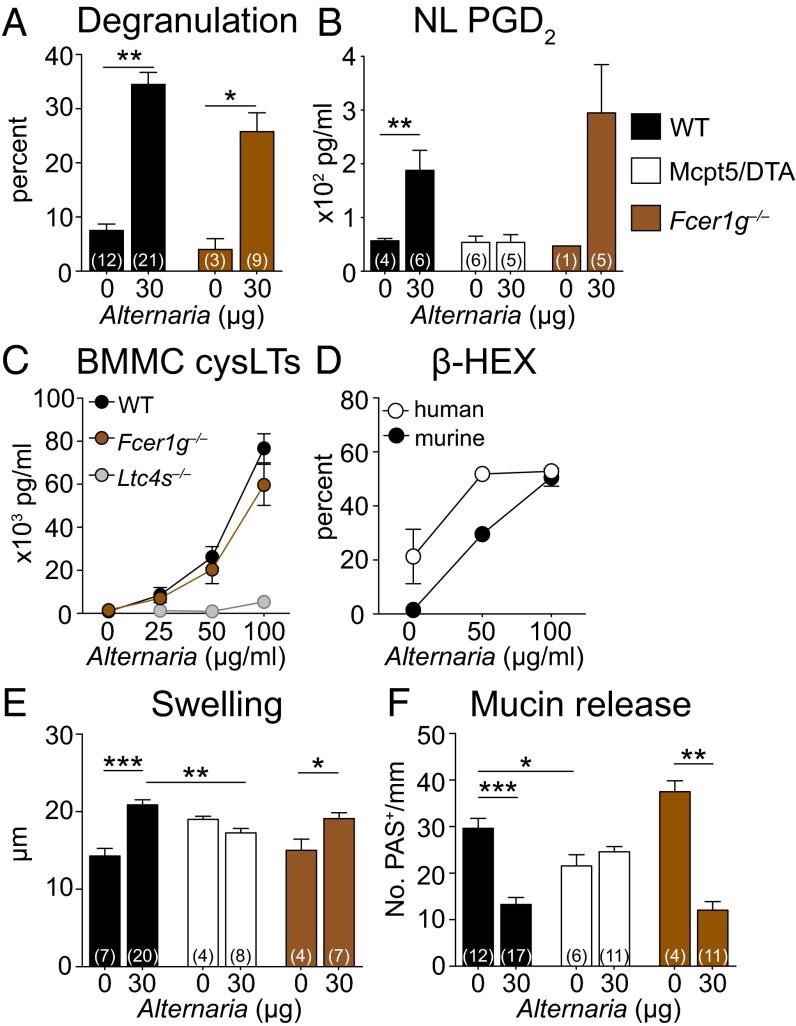
The submucosa of the nasal septum and nasal cavity floor contained MCs, and Alternaria challenge elicited release of their chloroacetate esterase (CAE)-reactive granules (Fig. S1 A–C). Degranulation, defined by the presence of more than five extracellular granules per cell, was elicited in 35 ± 2% of MCs in Alternaria-challenged WT mice, compared with 8 ± 1% in PBS-challenged WT mice (Fig. 2A). MC activation was confirmed by the detection of prostaglandin D2 (PGD2) in the nasal lavage (NL) of Alternaria-treated WT mice, which was absent in Alternaria-treated MC-deficient (Mcpt5/DTA) controls (Fig. 2B). To establish that Alternaria activated MCs in an innate fashion, we assessed Fcer1g‒/‒ mice. The percentage of degranulating MCs (26 ± 4%) and the levels of PGD2 in the NL of Alternaria-treated Fcer1g‒/‒ mice were comparable to those of WT mice (Fig. 2 A and B and Fig. S1 D–F), demonstrating that Alternaria activates MCs independently of signaling through FcεR1 or activating FcγRs.
Fig. S1.
MCs in the nasal cavity degranulate in response to Alternaria. (A) Representative histological coronal section through the first molar teeth of the mouse skull of a PBS-challenged mouse with CAE stain. MS, maxillary sinuses; NCF, nasal cavity floor; NS, nasal septum (50× magnification). (B) Magnification of Inset from A; CAE-reactive MCs stain red. (C) Degranulating MCs in the nasal cavity floor 1 h after Alternaria administration. Arrows point to degranulating MCs (magnification: 630×). (D) Number of degranulating MCs. (E) Number of intact nondegranulating MCs. (F) Total number of MCs (degranulated + intact MCs) enumerated in seven 200× fields in WT (black bars), Fcer1g‒/‒ (brown bars), and Mcpt5/DTA (open bars) mice. Data are means ± SEM pooled from three to four independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01.
Fig. 2.
The innate activation of MCs is required for Alternaria-elicited mucosal responses. (A) Percentage of degranulating MCs in the nasal submucosa 1 h after i.n. administration of PBS (0 µg) or Alternaria (30 µg) to WT (black bars) and Fcer1g‒/‒ (brown bars) mice. (B) ELISA quantification of PGD2 in the NL 1 h after i.n. administration of PBS or Alternaria to WT, Fcer1g‒/‒, and Mcpt5/DTA (white bars) mice. (C) BMMCs from WT, Fcer1g‒/‒, or Ltc4s‒/‒ mice were harvested after 5–8 wk of culture and stimulated with Alternaria. The concentrations of cysLTs in the supernatants were measured by enzyme immunoassay at 30 min. Results are means ± SEM pooled from three independent experiments with cultures obtained from 10 WT mice, 3 Ltc4s‒/‒ mice, and 3 Fcer1g‒/‒ mice. (D) β-Hexosaminidase release into the supernatant at 30 min as a percentage of total. Results are means ± SEM pooled from three independent BMMC cultures and three independent human CD34+ cell-derived MC cultures. (E) Submucosal swelling and (F) mucin release in WT, Mcpt5/DTA, and Fcer1g‒/‒ mice 1 h after a single dose of PBS or Alternaria. Results are means ± SEM pooled from three (A) or five (B, E, and F) independent experiments with the number of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001.
Alternaria also elicited robust cysLT generation from WT bone marrow-derived mast cells (BMMCs) in a dose-dependent fashion with a peak response at 100 µg/mL Alternaria (Fig. 2C). There was no cytotoxicity at these doses. As expected, no cysLTs were detected from Alternaria-treated Ltc4s‒/‒ BMMCs, whereas Fcer1g‒/‒ BMMCs had no attenuation in Alternaria-elicited cysLT production (Fig. 2C). Finally, we found a robust dose-dependent release of β-hexosaminidase from Alternaria-stimulated BMMCs and from human CD34+ cell-derived MCs (Fig. 2D), indicating that Alternaria induces MC activation across species. Innate MC activation was essential for the epithelial and vascular changes in the Alternaria-challenged mucosa as both submucosal edema (Fig. 2E) and mucin release (Fig. 2F) were absent in Alternaria-treated Mcpt5/DTA mice and present in Alternaria-treated Fcer1g‒/‒ mice. Mcpt5/DTA mice had reduced baseline numbers of mucin-containing goblet cells, similar to Ltc4s‒/‒ mice.
GPR99 Controls Alternaria-Elicited EpC Mucin Release and Baseline EpC Composition.
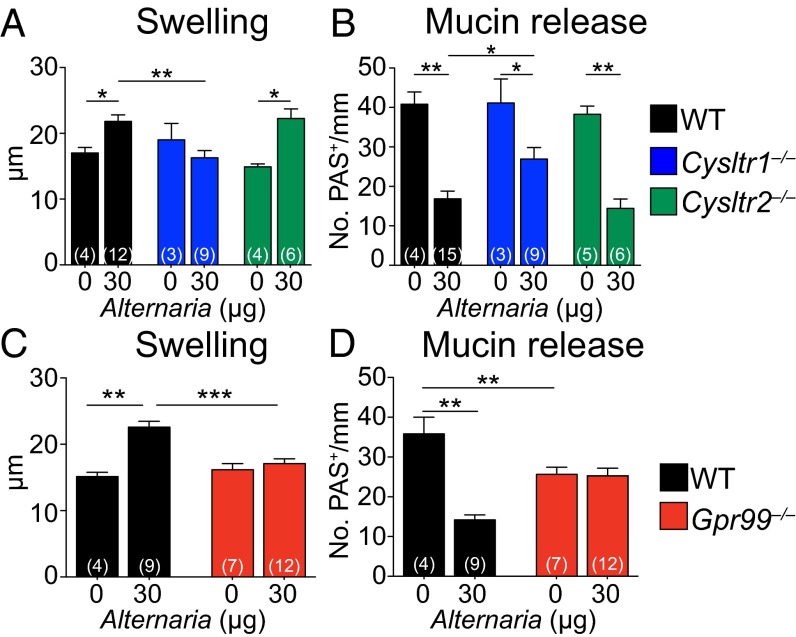
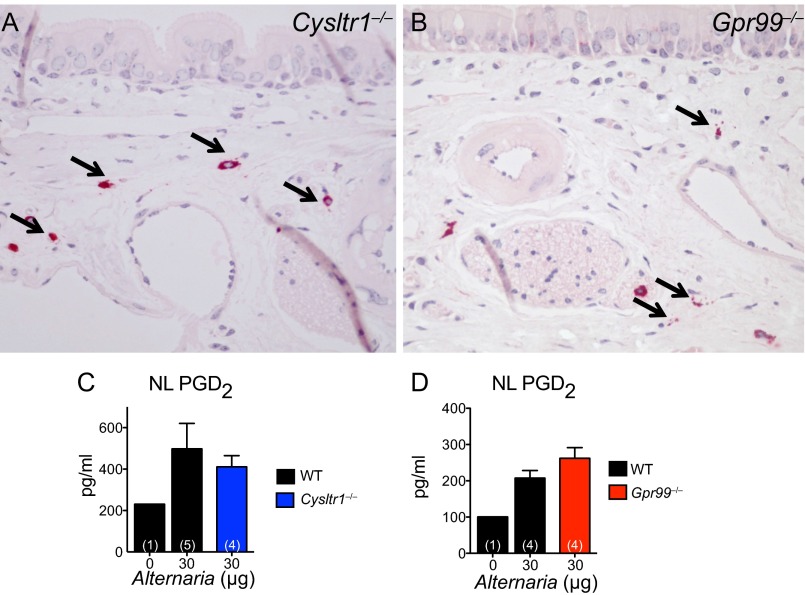
We next addressed which CysLTR might regulate these epithelial and submucosal changes. Alternaria-elicited submucosal swelling was absent in mice deficient in the type 1 CysLTR, CysLT1R (Cysltr1‒/‒), but intact in CysLT2R-deficient (Cysltr2‒/‒) mice, compared with WT controls (Fig. 3A). EpC mucin release was partially attenuated in Cysltr1‒/‒ mice and intact in Cysltr2‒/‒ mice (Fig. 3B). Notably, both nasal submucosal swelling and mucin release were completely abrogated in Alternaria-treated Gpr99‒/‒ mice (Fig. 3 C and D). MC degranulation in the submucosa and PGD2 levels in the NL of Alternaria-treated Cysltr1‒/‒ and Gpr99‒/‒ mice were comparable to Alternaria-treated WT mice (Fig. S2), indicating no defects in MC activation.
Fig. 3.
GPR99 controls Alternaria-elicited EpC mucin release and baseline EpC composition. (A–D) Alternaria (30 µg) or PBS (0 µg) was administered to WT (black bars), Cysltr1‒/‒ (blue bars), Cysltr2‒/‒ (green bars), and Gpr99‒/‒ (red bars) mice. (A and C) Swelling and (B and D) mucin release were measured in histological samples from the nasal mucosa 1 h after i.n. challenge, as in Fig. 1. Results are means ± SEM pooled from three independent experiments with the numbers of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S2.
MCs in the nasal cavity of Cysltr1‒/‒ and Gpr99‒/‒ mice are activated after i.n. Alternaria administration. (A and B) Degranulating MCs in the nasal submucosa of (A) Cysltr1‒/‒ and (B) Gpr99‒/‒ mice. Magnification: 400×. Arrows indicate degranulating MCs. (C and D) PGD2 in the NL of WT (black bars), Cysltr1‒/‒ (blue bars), and Gpr99‒/‒ (red bars) mice. Data are means ± SEM from one experiment with the number of mice per group indicated on each bar in parentheses.
Baseline numbers of PAS-reactive GCs in PBS-treated Gpr99‒/‒ mice were reduced by 29% compared with PBS-treated WT mice (Fig. 3D). Although there was no reduction in baseline GC numbers in Fcer1g‒/‒ or Cysltr1‒/‒ and Cysltr2‒/‒ mice (Figs. 2F and 3B), baseline GC numbers were decreased by 34% in Ltc4s‒/‒ mice (Fig. 1G) and by 27% in Mcpt5/DTA mice (Fig. 2F), compared with WT controls. These results demonstrate a constitutive effect of MCs, cysLTs, and GPR99 on EpC composition.
GPR99 Is Expressed in Respiratory EpCs and Mediates LTE4-Elicited Submucosal Swelling and Mucin Release.
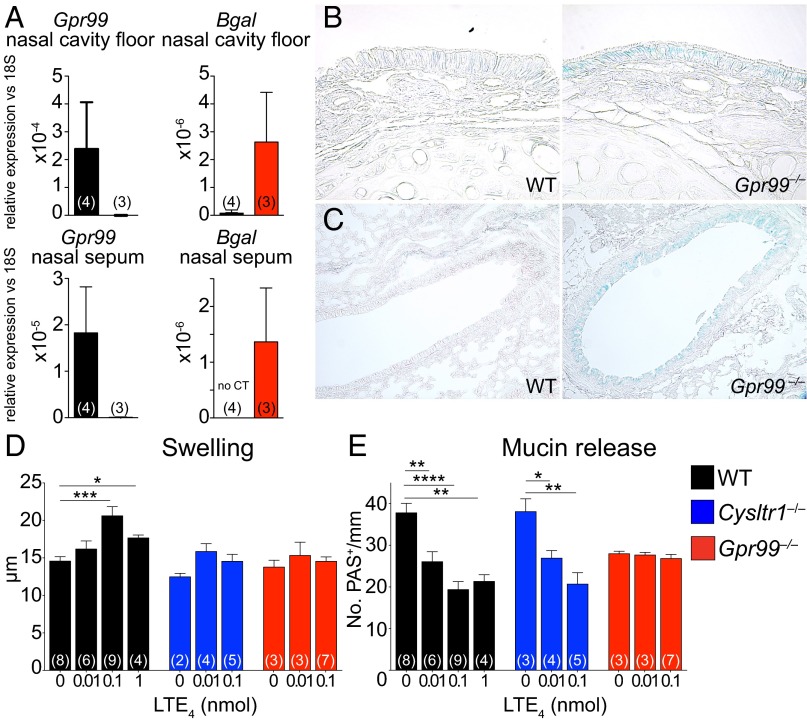
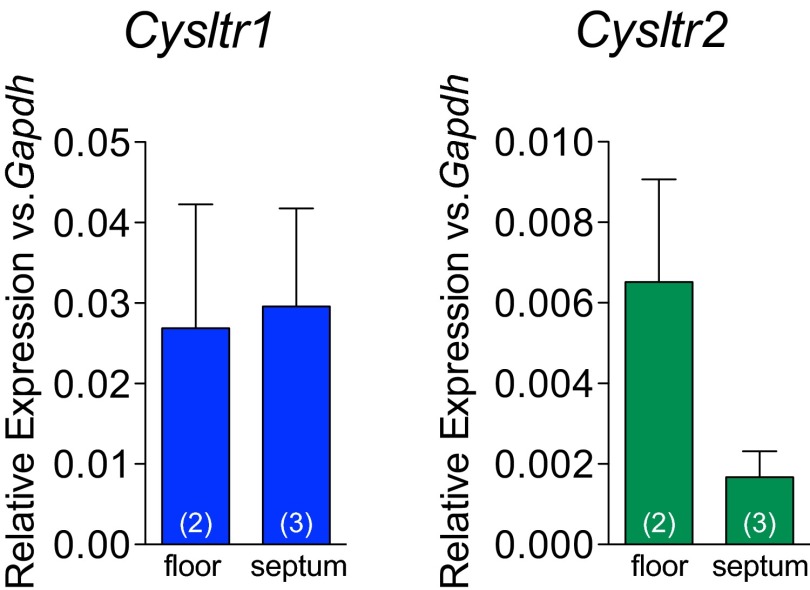
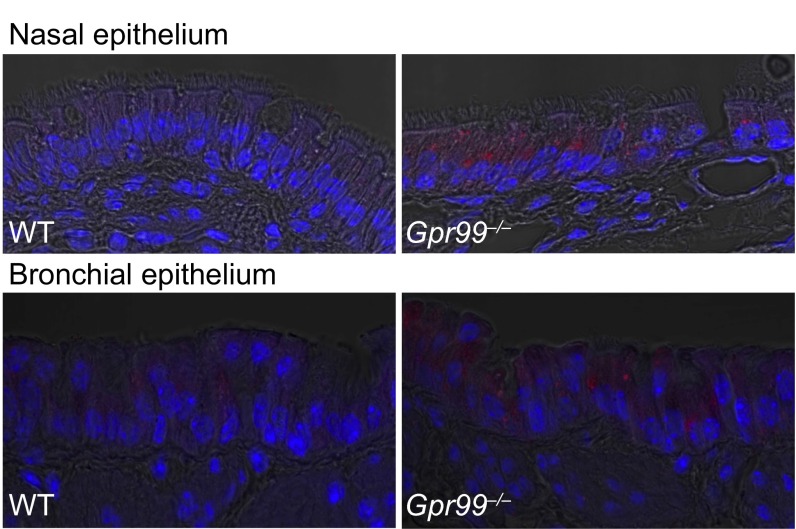
Quantitative RT-PCR showed expression of all three CysLTRs in the nasal mucosa of naive WT mice (Fig. 4A and Fig. S3). Additionally, Gpr99‒/‒ mice expressed Escherichia coli β-galactosidase (Fig. 4A), inserted in the targeted deletion of the Gpr99 gene, confirming GPR99 expression in the nasal mucosa. Strong X-gal staining in naive Gpr99‒/‒ nasal mucosa localized to respiratory EpCs and was not detected in WT mice (Fig. 4B and Fig. S4). X-gal staining also confirmed GPR99 expression in bronchial EpCs (Fig. 4C and Fig. S4). We did not detect GPR99 expression in the vascular endothelium, but note the limited sensitivity of this technique.
Fig. 4.
GPR99 is expressed in respiratory EpCs and mediates LTE4-elicited submucosal swelling and mucin release. (A) Quantitative RT-PCR analysis of expression of Gpr99 and β-galactosidase (Bgal) genes in the nasal cavity floor (Upper panels) and nasal mucosa of the septum and side wall (Lower panels) compared with 18S rRNA. Results are means ± SEM pooled from two independent experiments with the numbers of mice per group indicated in parentheses in each column. (B and C) X-gal staining in the nasal mucosa of Gpr99‒/‒ mice (Right panels) and WT mice (Left panels) in the (B) nasal cavity (magnification: 630×) and (C) large bronchi (magnification: 200×). Positive X-gal reactivity produces a blue precipitate. (D and E) LTE4 (0.01, 0.1, or 1 nmol) or PBS (0 nmol) was administered i.n. to WT (black bars), Cysltr1‒/‒ (blue bars), and Gpr99‒/‒ (red bars) mice. (D) Submucosal swelling and (E) mucin release were measured 1 h after LTE4 administration in histological samples from the nasal mucosa. Data are means ± SEM pooled from three independent experiments with the numbers of mice per group indicated on each bar in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fig. S3.
Quantitative RT-PCR analysis of CysLT1R (blue bars) and CysLT2R (green bars) expression in the nasal cavity floor (“floor”) and nasal septum (“septum”) of WT mice. Data are means ± SEM from one experiment with number of mice per group indicated on each bar in parentheses.
Fig. S4.
Confocal microscopy of nasal (Upper panels) and bronchial (Lower panels) epithelia stained with X-gal. The X-gal precipitate fluoresces in the far red spectrum. Nuclei stained blue with Hoechst 33342 solution. Magnification: 630×.
Intranasal injection of LTE4 induced a dose-dependent increase in submucosal swelling in WT mice (Fig. 4D), reaching a plateau at 0.1 nmol. Similar to Alternaria, LTE4 caused expansion of the extracellular space and dilatation of the submucosal vessels with no detectable cellular infiltration at 1 h. Both Cysltr1‒/‒ and Gpr99‒/‒ mice were protected from LTE4-elicited submucosal edema. LTE4 also induced EpC mucin release in a dose-dependent fashion with a plateau at 0.1 nmol (Fig. 4E). Strikingly, only Gpr99‒/‒ mice were protected from mucin release, demonstrating that GPR99 is the dominant CysLTR for LTE4-elicited EpC secretory function.
Discussion
The role(s) of LTE4 in airway biology has received little attention, in part because of its poor activity at the classical cysLT receptors, CysLT1R and CysLT2R (5, 19), in transfected cells. Here we find that the high-affinity receptor for LTE4, GPR99, is expressed on airway EpCs and controls EpC mucin release and submucosal swelling in response either to exogenous LTE4 or to endogenously generated cysLTs elicited by Alternaria. Moreover, we find reduced nasal GC numbers in both naive Ltc4s‒/‒ and Gpr99‒/‒ mice, but not in Cysltr1‒/‒ or Cysltr2‒/‒ mice. These results demonstrate a selective role for GPR99 in EpC homeostasis and secretory function. Furthermore, they suggest that targeting GPR99, which is resistant to commercially available CysLT1R antagonists, may have therapeutic benefit in airway diseases characterized by abnormalities in mucin secretion and clearance.
LTE4 elicited mucin release at 0.01 nmol in WT mice and reached a plateau at 0.1 nmol. The dose of LTE4 required for mucin release was one log lower than that required to elicit submucosal swelling, demonstrating a dominant effect for LTE4 on airway EpCs that was solely mediated by GPR99 in the presence of the classical receptors. By contrast, LTE4-induced submucosal swelling, elicited at higher doses, was reduced in both Cysltr1‒/‒ and Gpr99‒/‒ mice, indicating nonredundant functions for these receptors in control of the nasal microvasculature. In response to a fixed dose of Alternaria, we again saw the nonredundant effects of these receptors for swelling and did find that mucin release was partially reduced in Cysltr1‒/‒ mice. A role for CysLT1R may reflect LTE4 action at CysLT1R at higher doses.
The decreased numbers of PAS-reactive EpCs in both PBS-treated Ltc4s‒/‒ and Gpr99‒/‒ mice, and the intact numbers in Cysltr1‒/‒ or Cysltr2‒/‒ mice, seem a credible functional coupling of ligand production and a receptor-selective response. Remarkably, a reduced number of GCs was also seen in unmanipulated MC-deficient Mcpt5/DTA mice, but not in Fcer1g‒/‒, suggesting an innate function of MCs in controlling EpC composition in the absence of specific activation. Moreover, we found that IgE-independent MC activation controlled EpC mucin release in response to airway Alternaria challenge. These findings are in keeping with an innate immune cell that is present in sea squirts, preceding adaptive immunity, and which expresses a conserved transcriptional signature between mouse and human (30). Our findings surprisingly suggest that some allergen-elicited EpC pathways may be regulated indirectly by tissue-resident constitutive mast cells.
GPR99 was initially reported as an oxoglutarate receptor (31). As GPR99 belongs to the same nucleotide P2Y receptor family as CysLT1R and CysLT2R and recognizes dicarboxylic acids, it was identified as a candidate LTE4 receptor and subsequently demonstrated to mediate cutaneous swelling in response to low doses of LTE4 (25). Our findings for GPR99 control of mucin secretion and EpC homeostasis indicate a central role for this CysLTR at low doses of LTE4. Although we do find GPR99 expressed in both nasal and lung EpCs, a function for GPR99 in other cell types cannot be excluded. There are no reports of GPR99 function in human airways; however, LTE4 elicits mucin release from both human conjunctival GCs (32) and human airway EpCs (33). Notably, asthmatic patients are more sensitive to LTE4-elicited airflow obstruction than healthy controls, a hyperresponsiveness not seen with either LTC4 or LTD4 (20–22, 34). This suggests a mechanism for LTE4-mediated airflow obstruction that is distinct from the bronchoconstricting effects of the other CysLTRs and that depends on precedent lung disease. As mucus secretion can contribute to airway obstruction (35, 36), it is possible that the sensitivity to LTE4-induced obstruction reflects a contribution of GPR99 in the setting of the GC expansion seen in asthmatic lung. Furthermore, targeting GPR99, which is resistant to commercially available CysLTR antagonists, may have therapeutic benefit in airway diseases characterized by abnormalities of mucin secretion and clearance. In sum, our findings define GPR99 as the airway receptor for LTE4 and identify a function for this third CysLTR in airway epithelial integrity and secretion that is distinct from the classical receptors.
Materials and Methods
Mice.
Ltc4s‒/‒ mice were generated on a 129Sv background (37) and backcrossed for 15 generations onto the C57BL/6 background. Cysltr1‒/‒ and Cysltr2‒/‒ mice were generated on a C57BL/6 background as reported previously (38, 39). C57BL/6 Gpr99+/‒ mice were obtained from the National Institutes of Health Knock-Out Mouse Project and intercrossed to obtain Gpr99‒/‒ mice (25). Fcer1g‒/‒ mice (B6;129P2-Fcer1gtm1Rav/J) were purchased from Jackson Laboratories. Mcpt5/DTA mice were generated by crossing mice with MC-specific expression of Cre recombinase (40) (kindly provided by Axel Roers, Technische Universitaet Dresden) on a C57BL/6 background with ROSA-diphtheria toxin-α mice (B6.129P2-Gt(ROSA)26Sortm1(DTA)Lky/J from Jackson Laboratories). WT littermates from the intercrossing of Cysltr1−/−, Cysltr2−/−, and Mcpt5/DTA mice (Cre+DTA‒, Cre‒DTA+, and Cre‒DTA‒) or age- and sex-matched C57BL/6 mice (Charles River Laboratories) were used as controls. In 18% of the Mcpt5/DTA mice, significant numbers of MCs were detectable in the nasal mucosa (41), and these mice were excluded. Male and female mice 3–8 mo of age were used. All mice were housed in a specific pathogen-free facility in groups of four to five mice per cage with a standard light/dark cycle of 12 h. Mice were provided food and water ad libitum. All mice except C57BL/6 mice purchased from Charles River Laboratories were bred in the Dana Farber Cancer Institute mouse facility, and pups were weaned between 19 and 28 d old. All experiments were performed during the day. The use of mice for these studies was in accordance with review and approval by the Animal Care and Use Committee of the Dana Farber Cancer Institute.
Alternaria and LTE4 Administration.
Mice received a single application of A. alternata culture filtrate (Greer Laboratories) intranasally after anesthesia with an i.p. injection of ketamine (10 mg/kg) and xylazine (20 mg/kg) for full sedation. Alternaria culture filtrate was delivered in a total volume of 20 μL of sterile PBS. In a separate set of experiments, 0.01, 0.1, or 1 nmol of LTE4 (Cayman Chemical) was dissolved and diluted in 20 μL PBS after ethanol was evaporated and immediately administered intranasally. Mice of a given genotype were randomized to treatment dose, and challenges were performed in groups organized by genotype and treatment dose. Mice were euthanized exactly 1 h after Alternaria or LTE4 administration with isoflurane overdose, and tissues were harvested for histology and fixed with 4% (wt/vol) paraformaldehyde (PFA). NL was performed with 500 μL of PBS, and after acetone precipitation, PGD2 was measured in the lipid fraction using a PGD2–MOX ELISA kit (Cayman Chemical) with the following reported reactivity: Prostaglandin D2-MOX (100%), Prostaglandin D2 (0.2%), Prostaglandin E2-MOX (<0.01%), 6-keto Prostaglandin F1α-MOX (<0.01%), Prostaglandin F2α (<0.01%), Tetranor-PGEM (<0.01%), Tetranor-PGFM (<0.01%), and Thromboxane B2-MOX (<0.01%) (42, 43).
In Vitro Culture and Stimulation.
BMMCs were generated as previously described (37). In brief, bone marrow was collected from femurs and tibiae of mice and cultured for 4–6 wk in RPMI medium 1640 containing 10% (vol/vol) FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, penicillin (100 units/mL), streptomycin (100 μg/mL), and 1% culture supernatant from Chinese hamster ovary cells expressing mouse interleukin-3. The culture medium for the BMMC was changed every week, and the cell density was adjusted to 3 × 105/mL at every passage. After 4 wk, more than 97% of the cells were BMMCs as assessed by staining with Wright–Giemsa and toluidine blue. Human MCs were derived from fresh heparinized peripheral blood using a CD34+ isolation kit (Miltenyi Biotec) and cultured in Iscove’s modified Dulbecco’s media with 4 mM l-glutamine, penicillin, streptomycin, 30% (vol/vol) charcoal-treated characterized FCS, and holo-Transferrin (50 μg/mL) in the presence of stem-cell factor (100 ng/mL) and GM-CSF (10 pg/mL).
BMMCs and human MCs were harvested and stimulated with Alternaria at the concentration of 0–100 μg/mL. After 15 min, the reaction was stopped by centrifugation at 120 × g for 5 min at 4 °C, and the supernatants were retained for assays of β-hexosaminidase (β-HEX) and cysLTs. For β-HEX assay, a marker of MC degranulation, the cell pellets were suspended in Hanks’ balanced salt solution and disrupted by repeated freeze–thawing. β-HEX was quantitated by spectrophotometric analysis of the hydrolysis of p-nitrophenyl-β-d-2-acetamido-2-deoxyglucopyranoside. The percentage release of β-HEX was calculated by the formula [S/(S+P)] × 100, where S and P are the β-HEX contents of equal portions of supernatant and cell pellet, respectively.
cysLTs in the supernatants of BMMCs and human MCs were measured 30 min after stimulation with Alternaria culture filtrate by enzyme immunoassay according to the manufacturer’s protocol (Amersham Biosciences) with a lower limit of detection at 60 pg/mL and the following reported reactivity: Leukotriene C4 (100%), Leukotriene D4 (100%), Leukotriene E4 (91%), Leukotriene B4 (<1.35%), Prostaglandin D2 (<0.006%), Prostaglandin F2α (<0.006%), Prostaglandin E2 (<0.006%), 6-Keto-prostaglandin F1α (<0.006%), Thromboxane B2 (<0.006%), and Glutathione (<0.006%) (44).
RT-PCR.
The nasal cavity floor was harvested by separating the palate with nasal cavity floor from the base of the skull. The remaining mucosa covering the nasal septum and side wall was scraped with a scalpel. Total RNA was isolated with TRIzol Reagent (Invitrogen) according to the manufacturer’s protocol and further purified with Qiagen microcolumn (SABiosciences). Total RNA (1 μg) was reverse-transcribed with SuperScript III (Invitrogen), and quantitative RT-PCR was performed with primers specific for mouse GPR99, the E. coli β-galactosidase gene, mouse CysLT1R, mouse 18S rRNA (SABiosciences), and for GAPDH and CysLT2R (Sigma-Aldrich) using the Mx3005P Real-Time PCR System (Agilent Technologies) under the following condition: 98 °C for 15 s, 58 °C for 30 s, 72 °C for 1 min, 40 cycles. The ratio of each mRNA relative to the 18S rRNA was calculated with the ddCt method.
Histochemistry and Quantitative Assessment of MC Degranulation, Mucin Release, and Submucosal Swelling.
The nasal cavity was harvested from mouse snouts obtained from euthanized mice 1 h after i.n. administration of Alternaria. After separation from the spine, the skull skin was stripped, the lower jaw and brain were removed, and the tissue was fixed in 4% (wt/vol) PFA in PBS for 18 h, changed to PBS, and decalcified using 14% (wt/vol) EDTA in NH4OH solution (pH 7.2–7.4) for 7–14 d. When the snouts were deemed to be sufficiently decalcified, they were rinsed in PBS and cross-sectioned behind the incisors and in between the first three palatal ridges, yielding four coronal sections through the nasal cavity. For histochemical evaluation, the tissues were embedded in glycolmethacrylate. Tissue sections, 2.5 μm thick, were assessed by PAS for quantitation of mucin-containing goblet cells and by CAE reactivity for quantitation of MC number and degranulation. Slides were counterstained with hematoxylin for general morphologic examination. All histologic assessments were done in a blinded fashion by a single investigator.
The number of PAS-reactive cells for each animal was enumerated from 6 to 8,200× digital photographs spanning a 4- to 8-mm basement membrane over the four coronal sections. Submucosal swelling for each animal was determined using ImageJ software (National Institutes of Health). The area of submucosal tissue between the epithelial basement membrane and nasal septum bone in two to four photographs spanning the 2- to 4-mm basement membrane over two coronal sections containing nasal septum was measured. This area was divided by the length of basement membrane to define the average thickness of the submucosal space. Degranulated and intact MCs were counted, as previously described (45), in 1.89 mm2 over four coronal sections of the nasal cavity floor and septum.
X-gal staining was performed on mouse snouts from WT and Gpr99‒/‒ mice. Briefly, tissues were fixed in 4% (wt/vol) PFA for 2 h, washed with PBS, and then incubated in X-gal staining solution for 48 h (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 2 mM MgCl2, 5 mM EGTA, 0.02% IGEPAL, 0.01% sodium deoxycholate + 10 mM K3FeCN6, 10 mM K4FeCN6, 0.5 mg/mL X-gal). Subsequently, the mouse snouts were decalcified with 14% (wt/vol) EDTA solution and embedded in paraffin. Visulization was performed in 8-μm deparaffinized sections with a Leica DM LB2 microscope equipped with a Nikon DXM 1200 camera. For confocal microscopy, direct visualization of X-gal–stained tissue was achieved after excitation at 633 nm and recording fluorescence emission in the 650- to 770-nm range. Nuclei were stained with Hoechst 33342 nuclear stain (1:10,000; Sigma). Images were acquired with a Zeiss LSM 700 Laser Scanning Confocal microscope with a 63× Zeiss plan-APOCHROMAT oil, 1.4 N.A. objective. Transmitted and fluorescence images were overlayed using ImageJ (NIH).
Statistics.
Analysis was performed with GraphPad Prism software (version 5.01, GraphPad). Nonparametric two-sided Mann–Whitney tests were used to determine significance. A value of P < 0.05 was considered significant. Sample sizes were not predetermined by statistical methods.
Acknowledgments
We thank Anthony Hill (Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Cellular Imaging Core) and Li Li, Thomas Parsons, Julia Charles, and Joerg Ermann for their technical assistance. This work was supported by National Institutes of Health Grants R01 HL120952 (to N.A.B.) and U19 AI095219 (to Y.K., L.G.B, and J.A.B.), by the Steven and Judy Kaye Young Innovators Award (N.A.B.), and by the Joycelyn C. Austen Fund for Career Development of Women Physician Scientists (L.G.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605957113/-/DCSupplemental.
References
- 1.Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis. 1990;142(1):112–119. doi: 10.1164/ajrccm/142.1.112. [DOI] [PubMed] [Google Scholar]
- 2.Christie PE, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 3.Laidlaw TM, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drazen JM, et al. Recovery of leukotriene E4 from the urine of patients with airway obstruction. Am Rev Respir Dis. 1992;146(1):104–108. doi: 10.1164/ajrccm/146.1.104. [DOI] [PubMed] [Google Scholar]
- 5.Lynch KR, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 6.Figueroa DJ, et al. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Respir Crit Care Med. 2001;163(1):226–233. doi: 10.1164/ajrccm.163.1.2003101. [DOI] [PubMed] [Google Scholar]
- 7.Mechiche H, et al. Effects of cysteinyl leukotrienes in small human bronchus and antagonist activity of montelukast and its metabolites. Clin Exp Allergy. 2003;33(7):887–894. doi: 10.1046/j.1365-2222.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- 8.Leff JA, et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339(3):147–152. doi: 10.1056/NEJM199807163390302. [DOI] [PubMed] [Google Scholar]
- 9.Uzonyi B, et al. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci USA. 2006;103(16):6326–6331. doi: 10.1073/pnas.0601223103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, et al. Endothelial cysteinyl leukotriene 2 receptor expression mediates myocardial ischemia-reperfusion injury. Am J Pathol. 2008;172(3):592–602. doi: 10.2353/ajpath.2008.070834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirasaki H, et al. Localization and up-regulation of cysteinyl leukotriene-2 receptor in human allergic nasal mucosa. Allergol Int. 2013;62(2):223–228. doi: 10.2332/allergolint.12-OA-0490. [DOI] [PubMed] [Google Scholar]
- 12.Duah E, et al. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT2 and CysLT1 receptors. Sci Rep. 2013;3:3274. doi: 10.1038/srep03274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonetomi Y, et al. Leukotriene C4 induces bronchoconstriction and airway vascular hyperpermeability via the cysteinyl leukotriene receptor 2 in S-hexyl glutathione-treated guinea pigs. Eur J Pharmacol. 2015;754:98–104. doi: 10.1016/j.ejphar.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Sekioka T, et al. Expression of CysLT2 receptors in asthma lung, and their possible role in bronchoconstriction. Allergol Int. 2015;64(4):351–358. doi: 10.1016/j.alit.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Orning L, Kaijser L, Hammarström S. In vivo metabolism of leukotriene C4 in man: Urinary excretion of leukotriene E4. Biochem Biophys Res Commun. 1985;130(1):214–220. doi: 10.1016/0006-291x(85)90404-8. [DOI] [PubMed] [Google Scholar]
- 16.Verhagen J, et al. The excretion of leukotriene E4 into urine following inhalation of leukotriene D4 by human individuals. Biochem Biophys Res Commun. 1987;148(2):864–868. doi: 10.1016/0006-291x(87)90955-7. [DOI] [PubMed] [Google Scholar]
- 17.Lam S, Chan H, LeRiche JC, Chan-Yeung M, Salari H. Release of leukotrienes in patients with bronchial asthma. J Allergy Clin Immunol. 1988;81(4):711–717. doi: 10.1016/0091-6749(88)91043-3. [DOI] [PubMed] [Google Scholar]
- 18.Sala A, Voelkel N, Maclouf J, Murphy RC. Leukotriene E4 elimination and metabolism in normal human subjects. J Biol Chem. 1990;265(35):21771–21778. [PubMed] [Google Scholar]
- 19.Heise CE, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275(39):30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 20.Davidson AB, et al. Bronchoconstrictor effects of leukotriene E4 in normal and asthmatic subjects. Am Rev Respir Dis. 1987;135(2):333–337. doi: 10.1164/arrd.1987.135.2.333. [DOI] [PubMed] [Google Scholar]
- 21.O’Hickey SP, Arm JP, Rees PJ, Spur BW, Lee TH. The relative responsiveness to inhaled leukotriene E4, methacholine and histamine in normal and asthmatic subjects. Eur Respir J. 1988;1(10):913–917. [PubMed] [Google Scholar]
- 22.Arm JP, Spur BW, Lee TH. The effects of inhaled leukotriene E4 on the airway responsiveness to histamine in subjects with asthma and normal subjects. J Allergy Clin Immunol. 1988;82(4):654–660. doi: 10.1016/0091-6749(88)90979-7. [DOI] [PubMed] [Google Scholar]
- 23.Soter NA, Lewis RA, Corey EJ, Austen KF. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. J Invest Dermatol. 1983;80(2):115–119. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci USA. 2008;105(43):16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanaoka Y, Maekawa A, Austen KF. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem. 2013;288(16):10967–10972. doi: 10.1074/jbc.C113.453704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182(2):1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suram S, et al. Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the beta-glucan receptor. J Biol Chem. 2006;281(9):5506–5514. doi: 10.1074/jbc.M509824200. [DOI] [PubMed] [Google Scholar]
- 28.Barrett NA, et al. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011;208(3):593–604. doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty TA, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer DF, Barrett NA, Austen KF. Transcriptional profiling of tissue resident mast cells reveals a unique identity within the immune system. Nat Immunol. 2016 doi: 10.1038/ni.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He W, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429(6988):188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 32.Dartt DA, et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186(7):4455–4466. doi: 10.4049/jimmunol.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirasaki H, Kanaizumi E, Seki N, Himi T. Leukotriene E4 induces MUC5AC release from human airway epithelial NCI-H292 cells. Allergol Int. 2015;64(2):169–174. doi: 10.1016/j.alit.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Arm JP, et al. Asthmatic airways have a disproportionate hyperresponsiveness to LTE4, as compared with normal airways, but not to LTC4, LTD4, methacholine, and histamine. Am Rev Respir Dis. 1990;142(5):1112–1118. doi: 10.1164/ajrccm/142.5.1112. [DOI] [PubMed] [Google Scholar]
- 35.Evans CM, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuyper LM, et al. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115(1):6–11. doi: 10.1016/s0002-9343(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 37.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276(25):22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 38.Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004;279(44):46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- 39.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277(23):20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 40.Scholten J, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17(2):307–315. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudeck A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34(6):973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Cayman Chemical Company 2015 Prostaglandin D2-MOX ELISA Kit. Available from: https://www.caymanchem.com/pdfs/512011.pdf.
- 43.Kelly RW, Deam S, Cameron MJ, Seamark RF. Measurement by radioimmunoassay of prostaglandins as their methyl oximes. Prostaglandins Leukot Med. 1986;24(1):1–14. doi: 10.1016/0262-1746(86)90201-5. [DOI] [PubMed] [Google Scholar]
- 44.GE Healthcare 2015 Amersham Leukotriene C4/D4/E4 Biotrak Enzymeimmunoassay (EIA) System. Available at https://www.gelifesciences.com/gehcls_images/GELS/Related%20Content/Files/1314716762536/litdoc28953094AF_20110830172927.pdf. Accessed April 29, 2016.
- 45.Bankova LG, et al. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol. 2014;192(6):2812–2820. doi: 10.4049/jimmunol.1301794. [DOI] [PMC free article] [PubMed] [Google Scholar]