Significance
Glycosyltransferase enzymes synthesize complex sugar-containing macromolecules that play pivotal roles in the biology of all cells. Bacteria produce a remarkable range of these glycoconjugate structures, often containing unusual sugars. For example, Gram-negative bacteria exploit an unusual eight-carbon sugar (Kdo) as a linkage point between diverse glycan structures and conserved lipid termini in LPS and (some) capsules. Here, we describe the distribution and phylogenetic relationships of a new family of β-Kdo glycosyltransferases. Although these enzymes resemble some other glycosyltransferases, including those forming α-Kdo linkages, they are not readily identified as glycosyltransferases by bioinformatics approaches. The structure of a prototypical enzyme reveals extensive insertions, deletions, and rearrangements in the normally highly conserved GT-B–fold, highlighting the unusual structure of this glycosyltransferase family.
Keywords: microbial glycobiology, 3-deoxy-D-manno-oct-2-ulosonic acid, Kdo, glycosyltransferase, polysaccharide
Abstract
Kdo (3-deoxy-d-manno-oct-2-ulosonic acid) is an eight-carbon sugar mostly confined to Gram-negative bacteria. It is often involved in attaching surface polysaccharides to their lipid anchors. α-Kdo provides a bridge between lipid A and the core oligosaccharide in all bacterial LPSs, whereas an oligosaccharide of β-Kdo residues links “group 2” capsular polysaccharides to (lyso)phosphatidylglycerol. β-Kdo is also found in a small number of other bacterial polysaccharides. The structure and function of the prototypical cytidine monophosphate-Kdo–dependent α-Kdo glycosyltransferase from LPS assembly is well characterized. In contrast, the β-Kdo counterparts were not identified as glycosyltransferase enzymes by bioinformatics tools and were not represented among the 98 currently recognized glycosyltransferase families in the Carbohydrate-Active Enzymes database. We report the crystallographic structure and function of a prototype β-Kdo GT from WbbB, a modular protein participating in LPS O-antigen synthesis in Raoultella terrigena. The β-Kdo GT has dual Rossmann-fold motifs typical of GT-B enzymes, but extensive deletions, insertions, and rearrangements result in a unique architecture that makes it a prototype for a new GT family (GT99). The cytidine monophosphate-binding site in the C-terminal α/β domain closely resembles the corresponding site in bacterial sialyltransferases, suggesting an evolutionary connection that is not immediately evident from the overall fold or sequence similarities.
Carbohydrates coat the surface of cells in the form of complex macromolecules, collectively known as glycoconjugates. These molecules are implicated in many crucial biological processes and the diversity of carbohydrate structures is a result of the activities of a large group of enzymes, called glycosyltransferases (GTs). GTs catalyze formation of glycosidic linkages by transferring a sugar moiety from a glycosyl donor to an acceptor substrate. The glycosyl donors are various sugar-1-phosphates and their derivatives, with sugar mono- or diphospho-nucleotides being most common. GTs are classified as either retaining or inverting, depending on whether the anomeric configuration of the glycosyl donor is retained or inverted in the final product (1). Based on amino acid sequence similarities, GTs are currently classified to 98 families in the Carbohydrate-Active enZYmes (CAZy) database (2), reflecting the breadth of biological systems and product chemistry. Each family typically has members with different donor or acceptor specificities. However, the 3D structures of GTs are more conserved; for nucleotide sugar-dependent enzymes, only two structural folds, GT-A and GT-B, are currently known. The fold does not dictate activity (i.e., inverting or retaining). Currently less than 1% of the overall ∼225,000 GT sequences in the CAZy database have been biochemically characterized, and less than 0.1% have solved 3D structures. Understanding the relationship between sequence, 3D structure, and substrate specificity of GTs represents a fundamental challenge in glycobiology. Moreover, increasing interest in GTs is emerging from the perspective of developing new therapeutics, as well as application to enzymatic and chemo-enzymatic synthesis of medically and industrially important glycoconjugates, including glycoconjugate vaccines (3, 4).
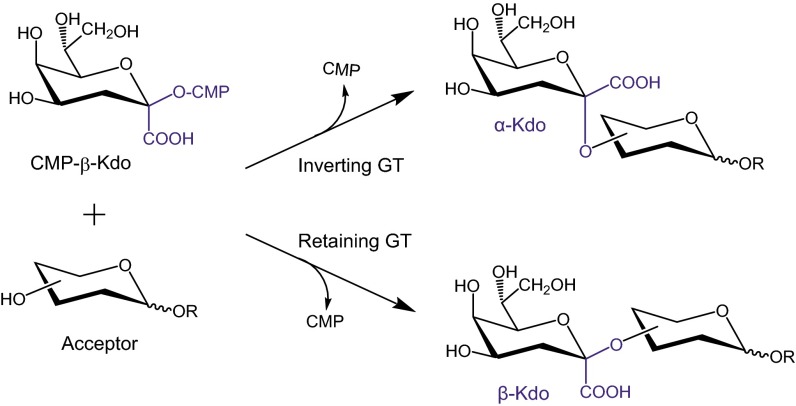
Bacteria possess a remarkable range of GTs, which transfer sugars found nowhere else in nature: they generate glycoconjugates involved in the interface between pathogens and the host immune system. We recently discovered a novel family of 3-deoxy-β-d-manno-oct-2-ulosonic acid (β-Kdo) transferases (5). Kdo is eight-carbon keto-sugar structurally related to sialic acids. Kdo is a ubiquitous component of bacterial LPSs. In eukaryotes, Kdo has been identified as a component of rhamnogalacturonan II in the primary cell walls of higher plants (6, 7), and in cell wall polysaccharides of some green algae (8, 9), but it has never been found in the animal kingdom. LPS is a glycolipid that forms the outer leaflet of the characteristic outer membrane of the Gram-negative bacterial cell; it is essential for the viability of almost all of these bacteria. LPS molecules typically consist of three domains: a hydrophobic moiety (lipid A) forming the outer leaflet of the membrane, a polysaccharide chain (O-antigen, OPS), and a bridging oligosaccharide called the core. Kdo is the most conserved sugar residue in all studied LPS, where it possesses the α-configuration and provides the linkage between lipid A and carbohydrate moieties. In contrast, β-Kdo is found in a small number of bacterial glycoconjugates. Its most conserved application lies in a recently discovered (10) β-Kdo oligosaccharide that bridges a lipid anchor (lyso-phosphatidylglycerol) to a long-chain capsular polysaccharide (CPS) in organisms, including Escherichia coli, Neisseria meningitidis, Haemophilus influenzae, and others (11). These capsules are collectively known as “group 2” based on their shared biosynthetic pathway. Identification of the β-Kdo oligosaccharide led to definition of the β-Kdo GTs activities of a group of enzymes (pfam05159), previously described only as “capsule polysaccharide biosynthesis proteins” with no assigned function (5). These retaining β-Kdo transferases show no significant sequence similarity to their well-studied inverting α-Kdo counterparts of the LPS core biosynthetic pathway (Fig. 1), or to any other known GT family.
Fig. 1.
Glycosylation reactions catalyzed by Kdo transferases.
Here, we report the structure and function of a prototype β-Kdo transferase. The enzyme acts as the chain-terminating component of the multidomain WbbB protein, which is involved in biosynthesis of the OPS in Raoultella terrigena. The protein possesses a GT-B fold that shows Rossmann-folds of reduced numbers of α/β repeats, as well as additional novel structural features that place it in a new family of GTs, assigned as family GT99 in the CAZy database (2).
Results
β-Kdo-Transferases in the Synthesis of Bacterial Glycans.
In the biosynthesis of the conserved glycolipid anchor for many Gram-negative capsules, the KpsS enzyme adds the first Kdo residue to the lipid acceptor and this product is then extended by KpsC, which contains duplicated domains with predicted β-Kdo GT activity. Definitive biochemical assignment of KpsS and KpsC as β-Kdo GTs in the CPS assembly (5) facilitated bioinformatic assignments of other candidates from O-antigen and CPS biosynthesis confined to Gram-negative bacteria. A search of the Bacterial Carbohydrate Structure Database for β-Kdo–containing glycans resulted in <40 unique glycan structures, and some could be correlated with existing sequence data in GenBank. Combining the structural data on bacterial polysaccharides with sequenced gene clusters for their synthesis allowed prediction of functionally related β-Kdo GTs (Fig. 2, Fig. S1, and Table S1), although overall shared sequence similarity was low (Table S2). Details of the basis for the functional assignments are given in Materials and Methods.
Fig. 2.
β-Kdo–containing bacterial glycans and the gene clusters associated with their biosynthesis. Only those gene clusters encoding enzymes central to the experimental approach are shown; others are given in Fig. S1. The analysis is confined to examples with known gene clusters and unambiguous polysaccharide structures. For all structures except CPS of Burkholderia oklahomensis, O-acetylation and O-propionylation are nonstoichiometric. In multidomain proteins, the conserved domains identified using the NCBI Conserved Domain Search service are indicated. β-Kdo GTs are shown in green, other GTs are represented in blue, acyltransferases (AT) in yellow, nucleotide sugar precursor synthesis genes in pink, and PS export machinery genes in magenta. For the WbbB protein from R. terrigena, the percentage of amino acid identity/similarity is indicated. The organization of O-antigen biosynthesis gene cluster of K. pneumoniae O12 is identical to that of R. terrigena, with WbbB sharing 72% identity and 83% similarity.
Table S1.
β-Kdo GTs representatives used in phylogenetic analysis
| No. | Bacteria | Protein | Abbreviation | Amino acid | Domain | GenBank |
| 1 | Raoultella terrigena | WbbB | Rt_WbbB | 401 | 1–401 | AAQ82931 |
| 2 | Klebsiella pneumoniae O12 | WbbB | Kp_WbbB | 398 | 1–398 | BAN08508 |
| 3 | Aeromonas hydrophila O11 | WbbB | Ah_WbbB | 391 | 1–391 | AKL88477 |
| 4 | Serratia marcescens O4 | WbbK | Sm_WbbK | 396 | 1–396 | AAC00183 |
| 5 | Providencia alcalifaciens O36 | WpaF | Pa_WpaF | 409 | AEB61498 | |
| 6 | Cronobacter sakazakii O6 | WepM | Cs_WepM | 413 | AFI60286 | |
| 7 | Actinobacillus pleuropneumoniae 5a | Cps5B | Ap_Cps5B | 526 | AAC26631 | |
| 8 | Escherichia coli K12 | Orf3 | Ec_Orf3 | 537 | AMA19673 | |
| 9 | Escherichia coli K14 | Orf2 | Ec_Orf2 | 476 | 286–761 | AMA19676 |
| 10 | Sinorhizobium meliloti 1021 | RkpZ1 | Sm_RkpZ1 | 432 | CAC48977 | |
| 11 | Sinorhizobium meliloti 1021 | RkpZ2 | Sm_RkpZ2 | 343 | CAC48963 | |
| 12 | Sinorhizobium meliloti 1021 | SM_b20824 | SM_b20824 | 382 | 397–778 | CAC48964 |
| 13 | Sinorhizobium meliloti Rm41 | RkpZ | Sm_RkpZ | 440 | CAB62159 | |
| 14 | E. coli RS218 serogroup K1 | KpsS | Ec_KpsS | 401 | KIE82101 | |
| 15 | E. coli RS218 serogroup K1 | KpsC | Ec_KpsC-N | 302 | 1–302 | KIE82100 |
| 16 | E. coli RS218 serogroup K1 | KpsC | Ec_KpsC-C | 373 | 303–675 | KIE82100 |
| 17 | Neisseria meningitidis | KpsS (LipB) | Nm_KpsS | 419 | AEQ62063 | |
| 18 | Neisseria meningitidis | KpsC (LipA) | Nm_KpsC-N | 326 | 1–326 | AEQ62064 |
| 19 | Neisseria meningitidis | KpsC (LipA) | Nm_KpsC-C | 378 | 328–704 | AEQ62064 |
| 20 | Haemophilus influenzae | KpsS (HcsB) | Hi_KpsS | 406 | AAQ12663 | |
| 21 | Haemophilus influenzae | KpsC (HcsA) | Hi_KpsC-N | 302 | 1–302 | AAQ12662 |
| 22 | Haemophilus influenzae | KpsC (HcsA) | Hi_KpsC-C | 378 | 303–680 | AAQ12662 |
| 23 | Campylobacter jejuni | KpsS | Cj_KpsS | 394 | EIB21594 | |
| 24 | Campylobacter jejuni | KpsC | Cj_KpsC-N | 295 | 1–295 | EIB21593 |
| 25 | Campylobacter jejuni | KpsC | Cj_KpsC-C | 394 | 296–689 | EIB21593 |
| 26 | Cronobacter sakazakii | KpsS | Cs_KpsS | 409 | AFK00904 | |
| 27 | Cronobacter sakazakii | KpsC | Cs_KpsC-N | 304 | 1–304 | AFK00903 |
| 28 | Cronobacter sakazakii | KpsC | Cs_KpsC-C | 374 | 305–678 | AFK00903 |
| 29 | Sinorhizobium fredii USDA 257 | Cps10C | Sf_Cps10C | 510 | AFL51445 | |
| 30 | Burkholderia oklahomensis EO147 | DM82_2445 | Bo_2445 | 398 | AIO67090 | |
| 31 | Neisseria meningitidis serogroup E | CseA | Nm_CseA | 398 | CCP19687 | |
| 32 | Actinobacillus pleuropneumoniae 10 | Cps10C | Ap_Cps10C | 480 | AEB33789 | |
| 33 | Sinorhizobium fredii USDA 205 | N181_28220 | Sf_28220 | 510 | KSV81166 |
Table S2.
Pairwise sequence alignment of WbbB1–401 with 33 proteins (domains) from the phylogenetic analysis, using the “BLAST 2 sequences” tool
| Abbreviation | % Identity | % Similarity | Overlap (aa) | E value | QXXXD, HP |
| Rt_WbbB | 100 | 100 | 401 | 0 | + |
| Kp_WbbB | 67 | 80 | 401 | 0 | + |
| Sm_WbbK | 43 | 61 | 384 | 4e-110 | + |
| Ah_WbbB | 45 | 60 | 381 | 6e-108 | + |
| Sf_28220 | 28 | 42 | 208 | 1e-12 | + |
| Ec_Orf3 | 25 | 40 | 299 | 1e-10 | + |
| Pa_WpaF | 26 | 40 | 282 | 3e-10 | + |
| Sf_Cps10C | 27 | 43 | 199 | 7e-10 | + |
| Sm_RkpZ | 27 | 48 | 125 | 1e-09 | + |
| Bo_2445 | 31 | 44 | 110 | 2e-09 | + |
| Ap_Cps10C | 24 | 41 | 197 | 5e-09 | + |
| Cs_WepM | 34 | 54 | 68 | 2e-08 | — |
| Cj_KpsC-C | 29 | 47 | 110 | 2e-08 | + |
| Nm_KpsC-C | 32 | 47 | 107 | 3e-08 | + |
| Ec_KpsC-C | 30 | 43 | 122 | 7e-08 | + |
| Cs_KpsC-C | 31 | 43 | 120 | 8e-08 | + |
| Hi_KpsC-N | 27 | 41 | 77 | 2e-07 | HP |
| Ec_KpsC-N | 26 | 42 | 76 | 1e-06 | HP |
| Ec_KpsS | 27 | 44 | 116 | 4e-06 | + |
| Cs_KpsC-N | 26 | 43 | 163 | 5e-06 | + |
| Cj_KpsS | 59 | 66 | 27 | 1e-05 | — |
| Nm_CseA | 23 | 38 | 254 | 2e-05 | + |
| Hi_KpsC-C | 29 | 43 | 107 | 2e-05 | + |
| Sm_Rkpz1 | 27 | 37 | 116 | 2e-05 | + |
| Cs_KpsS | 41 | 58 | 34 | 5e-05 | — |
| Ec_Orf2 | 42 | 60 | 33 | 1e-04 | — |
| Sm_Rkpz2 | 30 | 42 | 109 | 1e-04 | + |
| Cj_KpsC-N | 21 | 42 | 109 | 1e-04 | + |
| Hi_KpsS | 25 | 47 | 69 | 8e-04 | — |
| Nm_KpsS | 25 | 41 | 111 | 0.002 | D, HP |
| SM_b20824 | 22 | 35 | 229 | 0.015 | + |
| Nm_KpsC-N | 21 | 35 | 259 | 0.034 | + |
| Ap_Cps5B | 34 | 50 | 32 | 0.093 | — |
The protein abbreviations, accession numbers and amino acids range for multidomain proteins are given in Table S1. The last column identifies whether the conserved QXXXD and HP motifs are present (or not) in the aligned region; in some cases single motifs or residues were found and these are specifically indicated.
In general, the glycans containing β-Kdo fell into three functional categories: (i) capsule linkers, (ii) repeat units of CPSs and OPSs, and (iii) nonreducing terminal residues in a group of OPSs synthesized in a particular chain-terminating strategy. Multiple sequence alignments were prepared using the identified β-Kdo GTs (domains) along with homologs of KpsS and the individual domains of KpsC from a number of pathogens (Fig. S2 and Table S1). Because of variations in amino acid sequences and protein length (ranging from 295 to 326 aa for N-terminal KpsC domains, to 537 aa for E. coli K12 Orf3) the alignments introduced multiple gaps. Amino acid sequence variations reflect not only evolutionary relationships between proteins but also the differences in acceptor substrates (e.g., lysophosphatidylglycerol, another Kdo residue, hexoses, 6-deoxyhexoses, and HexNAc) and linkage specificities. Elimination of poorly aligned and divergent regions using GBlocks resulted in 68 of the original 906 positions that fulfill the GBlocks relaxed parameters (12), and these positions were used to calculate a maximum-likelihood phylogenetic tree (Fig. 3).
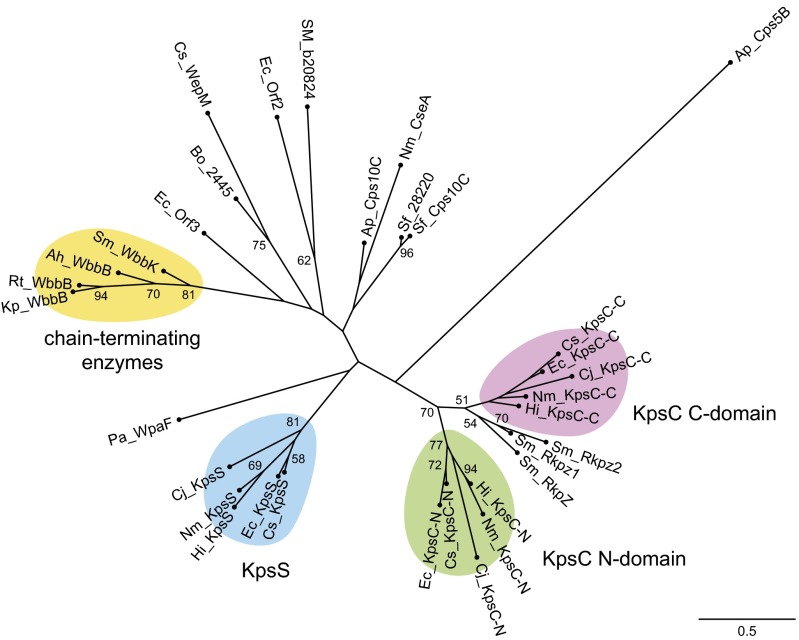
Fig. 3.
Unrooted phylogenetic tree of β-Kdo GTs. Kdo-transferase domains in multidomain proteins were separated based on the conserved domains identified using the NCBI Conserved Domain Search, disordered regions in Phyre2 secondary structure models, and biochemical data (see SI Materials and Methods for details). Multiple sequence alignment was performed with MAFFT followed by curation in GBlocks (Fig. S2). The maximum-likelihood tree was calculated using PhyML. Bootstrap values above 50% are shown adjacent to nodes and are based on 1,000 replications. The accession numbers of the proteins are given in Table S1.
Known and predicted polymerizing β-Kdo GTs (KpsC and RkpZ homologs) form a well-define clade, with each of KpsC domain grouping in separate subclades with counterparts from different bacteria. All analyzed KpsS proteins also group together, with higher similarity occurring between sequences from closely related species. Despite the difference in the chemical nature of acceptor substrates, KpsS enzymes are more closely related to single-addition β-Kdo GTs participating in CPS and O-antigen synthesis. The high sequence divergence between these GTs (i.e., long terminal branches) precludes deeper phylogenetic analysis. Four chain-terminating enzymes group together, as might be expected from the similar roles of product structures. R. terrigena WbbB (WbbBRt) and Klebsiella pneumoniae WbbB (WbbBKpO12) are the most closely related, reflecting taxonomic closeness of recently delineated Raoultella and Klebsiella genera (13). Each of these enzymes possess multiple GT modules with different predicted specificities. For example, WbbBRt is a large (1,106-aa residue) enzyme that we predict to be solely responsible for the synthesis of the capped OPS repeat unit domain. At the C terminus of WbbBRt/Kp, there are two GT domains sharing homology with GT1 and GT25 families, presumably one each for transfer of Rha and GlcNAc. The N terminus contains the candidate β-Kdo GT domain and this activity is verified below.
In Vitro Analysis of the Activity of the β-Kdo GT Domain of WbbBRt.
The β-Kdo GT domain from the R. terrigena WbbB protein was selected as a model for biochemical and structural characterization. To generate a construct expressing only the β-Kdo GT domain, WbbBRt was examined using Phyre2 for regions of predicted disordered sequence that might mark the separation of its three GT domains. The β-Kdo GT and the central GT1 domains are separated by a region with strong predicted propensity to form a parallel coiled-coil domain according to COILS (14) prediction. The coiled-coil segment might function as a molecular ruler in regulation of OPS chain length, analogous to a similar role in WbdD, an OPS chain-terminating dual methyltransferase/kinase in E. coli serotype O9a (15, 16). The break point after Ser401 was selected, which is within a small disordered region and is right before coiled-coil segment. A truncated polypeptide comprising amino acids 2–401 was cloned and overexpressed in E. coli BL21 (DE3). WbbB2–401 was soluble and the added C-terminal His6-tag facilitated purification to apparent homogeneity using nickel affinity chromatography. Purified WbbB2–401 migrated on SDS/PAGE in accordance with the theoretical molecular mass of a monomer of 46,047 Da (Fig. S3A). The molecular mass of 120 kDa estimated by size-exclusion chromatography is consistent with a dimer or a trimer (Fig. S3B).
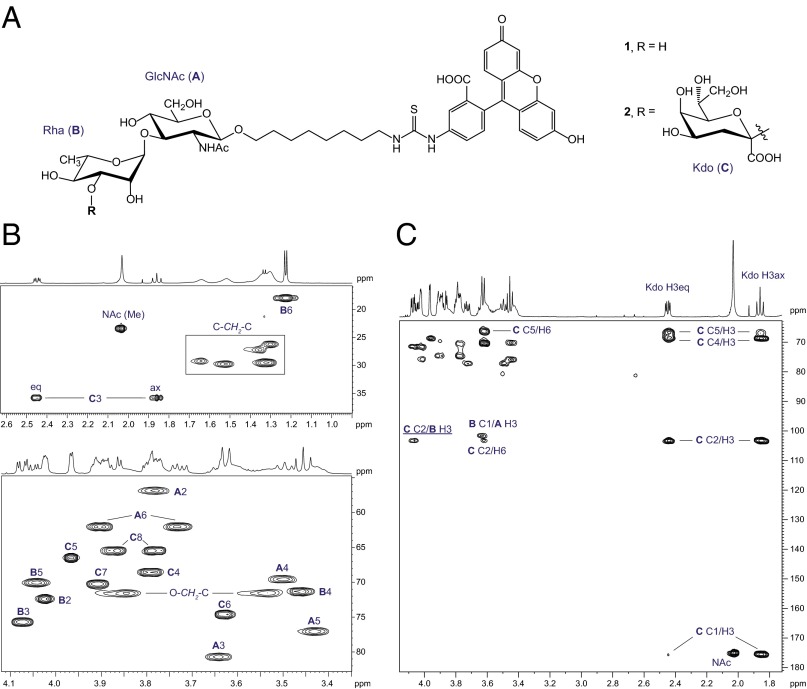
The activity of WbbB2–401 was assayed in vitro using a synthetic acceptor (1) comprising a R. terrigena OPS disaccharide repeat unit [α-l-Rha-(1→3)-β-d-GlcNAc] linked to a fluorescein tag (Fig. 4A). This structure represents the terminal disaccharide that is capped by a β-Kdo residue in the native OPS (17). The nucleotide-sugar donor of Kdo residues, CMP-β-Kdo, is unstable (18), and was generated in situ using CTP, Kdo and purified CMP-Kdo synthethase (KdsB) from E. coli (19). The reaction products were analyzed by thin-layer chromatography (TLC) and liquid chromatography–mass spectrometry (LC-MS) on a reverse-phase C18 column (Fig. S4 A and B). In the absence of enzyme, two minor (photo)degradation products of the substrate were observed on TLC. Accordingly, in the LC-MS profile the signal corresponding to substrate ion [M+H]+ at m/z 884.31 was accompanied by two minor peaks at m/z 868.33 and 1197.39. The observed in-source fragmentation of these ions, as well as MS/MS data, indicated that the structural heterogeneity of acceptor arises from the fluorescein moiety (Fig. S4 C and D), not the carbohydrate. WbbB2–401 catalyzed apparent complete conversion of all acceptor species into products that migrated slower on TLC. The major peak in the LC-MS profile eluted at 7.3 min and showed a [M+H]+ ion peak at m/z 1104.36. This result represents a gain of 220.05 u compared with 1, which corresponds to one added Kdo residue. The presence of ions at m/z 1088.38 and 1417.44 indicated that WbbB2–401 also transferred a Kdo residue to both degraded acceptors. As expected, no product was observed when the substrate was incubated in the absence of either KdsB or WbbB2–401.
Fig. 4.
NMR spectroscopic analysis of product 2. (A) Structures of synthetic acceptor 1 and the product 2, obtained in vitro using WbbB2–401. (B) Parts of 1H,13C HSQC spectrum. Numbers refer to H/C pairs in sugar residues designated as A (GlcNAc), B (Rha), and C (Kdo). O-CH2-C and C-CH2-C signals belong to eight-carbon methylene linker. (C) Part of 1H,13C HMBC spectrum. The interresidue correlation between Kdo C-2 and Rha H-3 (underlined) demonstrates Kdo-(2→3)-Rha linkage.
WbbB2–401 Adds a Single β-Kdo Residue to Position O-3 of Rhamnose.
To determine the position and anomeric configuration of ketosidic linkage of Kdo, the products from a scaled up reaction were purified using a Sep-Pak C18 cartridge. The structure 2 was established by a combination of 1H NMR, proton-coupled 13C NMR, 2D 1H,1H COSY, total correlation spectroscopy (TOCSY), rotating-frame nuclear Overhauser effect spectroscopy (ROESY), 1H,13C heteronuclear single-quantum coherence (HSQC), and heteronuclear multiple band correlation (HMBC) experiments (NMR chemical shifts are given in Table S3). In addition to the signals for fluorescein tag, GlcNAc, and Rha residues, the HSQC spectrum of 2 (Fig. 4B) contained two methylene signals (δC 36.0, δH 1.86, 2.45), two HOCH2-C signals (δC 65.6, δH 3.78, 3.87), and four other signals in the region δC/δH 66.6−74.7/3.63−3.97, consistent with the presence of a single Kdo residue. The signal at δC 36.0 is characteristic of Kdop C-3 and indicated pyranosidic form (20) [C-3 of Kdof, the furanose form, is at δ ∼45 ppm (21)]. Correlations from H-3 to H-5 were traced in the COSY spectrum, and the signals for the corresponding carbon atoms from C-3 to C-5 were assigned based on HSQC data. The coherence transfer was interrupted after H-5, because of a small 3J5,6 coupling constant typical of Kdo. Signals for H-6 and C-6 were identified based on H-5/H-6 correlation observed in the ROESY spectrum, as well as C-5/H-6 and C-6/H-4 correlations in the HMBC spectrum (Fig. 4C). The remaining assignments were completed by tracing the coherence transfer of H-6 to the exocyclic protons H-7 and H-8 and confirmed by HMBC data. The signals for Kdo C-1 and C-2 were found from correlations H-3ax/C-1 at δ 1.86/175.5 and H-3ax/C-2 at δ 1.86/103.3 in the HMBC spectrum. The β-anomeric configuration was inferred based on 3JC-1,H-3ax coupling constant of 4.6 Hz, characteristic for trans-diaxial orientation of C-1 and H-3ax (22), as well as the relatively large difference between chemical shifts of H-3ax and H-3eq of 0.59 ppm (20, 23). The 13C NMR chemical shifts of Kdop were indicative of its terminal position (23), whereas the Rha C-3 signal was shifted downfield evidently because of a mild α-glycosylation effect. The Kdo-(2→3)-Rha linkage was unambiguously confirmed by the correlation between Kdo C-2 and the Rha H-3 at δ 103.3/4.07 in the HMBC spectrum (Fig. 4C). Thus, the identity of the reaction product 2 confirmed the predicted terminal Kdo at position O-3 of Rha in a structure identical to the native glycan.
Table S3.
1H and 13C NMR data for the carbohydrate moiety of product 2 (δ, ppm)
| Sugar residue | H-1 C-1 | H-2 C-2 | H-3 (3ax, 3eq) C-3 | H-4 C-4 | H-5 C-5 | H-6 (6a, 6b) C-6 | H-7 C-7 | H-8a, 8b C-8 |
| →3)-β-d-GlcpNAc-(1→ | 4.48 | 3.78 | 3.64 | 3.50 | 3.43 | 3.73, 3.90 | ||
| 102.0 | 57.0 | 80.8 | 69.7 | 77.2 | 62.1 | |||
| →3)-α-l-Rhap-(1→ | 4.88 | 4.02 | 4.07 | 3.46 | 4.04 | 1.23 | ||
| 101.6 | 72.5 | 75.8 | 71.5 | 70.2 | 18.1 | |||
| β-Kdop-(2→ | 1.86, 2.45 | 3.79 | 3.97 | 3.63 | 3.91 | 3.78, 3.87 | ||
| 175.5 | 103.3 | 36.0 | 68.7 | 66.6 | 74.7 | 70.4 | 65.6 |
Chemical shifts for NAc group are at δH 2.03, δC 23.5 (CH3) and 175.0 (CO). 1H NMR chemical shifts are given in italics.
The WbbB2–401 Structure Reveals a Novel Variant of the Canonical GT-B Fold.
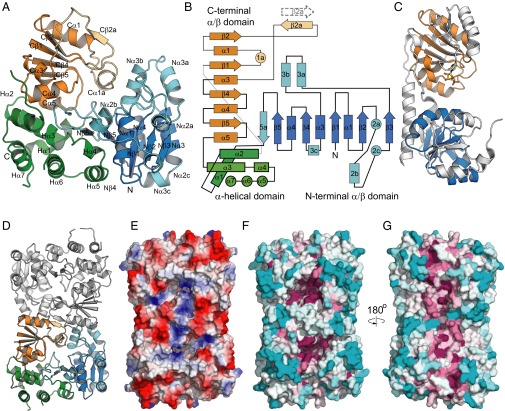
We determined the structure of WbbB2–401 in two crystal forms: as a ligand-free protein in P212121 (2.3 Å resolution) and as a cytidine monophosphate (CMP) complex in C2221 (2.1 Å resolution). WbbB2–401 is built around two α/β domains with recognizable Rossmann-fold topology, and therefore broadly meets the criteria of conforming to a GT-B–fold. However, comparison of the WbbB2–401 structure with a canonical GT-B enzyme, WaaA (an α-Kdo GT; PDB ID code 2XCU) (24) shows that the organization of WbbB is profoundly different (Fig. 5 A–C). Rather than the usual six or seven parallel β-strands for each domain, the α/β repeats are reduced in number, with five parallel β-strands (plus one antiparallel) for the N-terminal, and four β-strands for the C-terminal Rossmann-fold domain. The N-terminal Rossmann-fold domain is supplemented by the insertion of extended loops and additional α-helices, principally into the Nβ2–Nβ3 and Nβ3–Nβ4 loops. WbbB also includes an additional α-helical domain made from five α-helices contributed by the C-terminal portion of the chain, and two helices formed by the linker between the N- and C-terminal α/β domains. Instead of forming the two-lobed structure divided by a clear cleft typical of GT-B enzymes, the Rossmann-fold domains in WbbB occupy opposite corners of a roughly trapezoidal protein, with the remaining two corners filled by internal extensions of the Rossmann-fold domains and the α-helical domain. The Rossmann-fold domains of WbbB2–401 are therefore considerably rearranged relative to WaaA, with the N-terminal α/β domain being rotated and displaced some 25 Å from the position it occupies in WaaA. The interactions between these elements are extensive, suggesting minimal capacity for the large-scale interdomain movements usually characteristic of the functional cycle of GT-B enzymes.
Fig. 5.
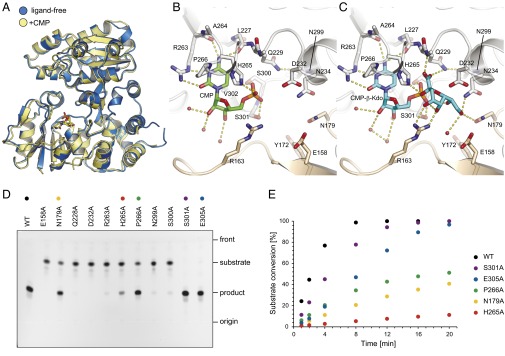
Structure of WbbB2–401. (A) Organization of the protomer. The protomer has three distinct domains: an N-terminal α/β domain (blue), a C-terminal α/β domain (orange), and an α-helical domain (green). Elements that do not correspond to the Rossmann-fold architecture are shown in a lighter shade. Secondary structure elements are numbered in analogy to the six-stranded α/β domain of canonical GT-B proteins. Helices are numbered by the preceding β-strand; elements with letter suffixes denote elements inserted into the standard Rossmann-fold. (B) Topology of WbbB2–401. Secondary structural elements are colored the same as in A. (C) Structure of WaaA (PDB ID code 2XCU) for comparison. WaaA is an α-Kdo transferase with a canonical GT-B organization. Elements of the N-terminal and C-terminal α/β domains that have corresponding elements in WbbB are shown in blue and orange, respectively. Image orientation places the C-terminal α/β domain in a similar orientation to A. Note that in WbbB2–401 the α-helical domain occupies the approximate space occupied in WaaA by the N-terminal α/β domain, which is rotated and displaced. (D) Structure of the WbbB2–401 dimer, viewed down the twofold axis. The second protomer is colored white. (E) Electrostatic surface of the WbbB2–401 CMP complex, showing the substrate binding face. (F) Sequence conservation mapped onto the surface. A multiple sequence alignment of WbbB homologs was mapped onto the WbbB2–401 CMP structure using Consurf. Magenta denotes the most conserved residues, cyan denotes the most variable. The two clusters of absolutely conserved residues represent the substrate binding and catalytic sites of each chain. (G) Sequence conservation on the WbbB2–401 face distal to the CMP binding site. The conserved central vertical band of residues likely mediate interactions with the other domains of WbbB in the complete protein.
Searching the PDB with DALI finds no proteins with global resemblance to WbbB2–401, whereas the conserved core of the individual α/β-fold domains show weak resemblance (rmsd values around 3.5 Å) to many proteins, including various GTs. The α-helical domain shows no significant resemblance to anything else in the PDB. Although its ancestry among GT-B enzymes remains apparent, WbbB represents an unprecedentedly radical departure from the typical GT-B organization. On the basis of its sequence and structural novelty, WbbB2–401 was assigned to a new family of GTs, family GT99.
Oligomeric Organization of WbbB2–401.
Analysis of both crystal structures with PISA (25) indicates that the WbbB2–401 forms a dimer. In the ligand-free structure, this dimer occupies the asymmetric unit, whereas in the CMP costructure, the two chains each represent half of a distinct dimer straddling a crystallographic symmetry axis. The interface is made up from Cα1a (C-terminal α/β domain) packing on the Nβ1–Nα1 and Nα3a–Nα3b loops and the N-terminal end of Nα3b (N-terminal α/β domain); in addition, residues 270–272 in the long Cβ2–Cα3 loop interact as a short antiparallel strand, Cβ2a (Fig. 5 B and D). The interface buries 1,440 Å2, or 9% of the total surface buried per subunit, and with a predicted dissociation energy of 9.6 kcal/mol. Gel-filtration analysis is consistent with this dimer being present in solution (Fig. S3B).
WbbB2–401 is one domain of a larger protein that includes two other candidate GT domains in the C-terminal part of the protein. The β-Kdo GT domain is separated from the other GT domains by a 70-aa region with low sequence identity to other proteins, followed by the 90-aa predicted parallel coiled-coil domain. The organization of the dimer places the C termini at opposite corners of the molecule on the same face as the active site, suggesting that the linker may be required to bridge the C termini of WbbB2–401 to the N-terminal end of the coiled-coil. The presence of a wide medial strip of surface-exposed conserved residues on the face opposite the CMP binding site possibly reflects the position of this linker joining WbbB2–401 to the coiled-coil domain (Fig. 5G).
Active Site Organization and CMP Binding.
Cocrystallization trials with 1 mM CMP yielded a new crystal form not seen with the ligand-free protein. Examination of the electron density showed that CMP binding occurs with good occupancy in both active sites in the asymmetric unit. In common with other GT-B enzymes, the nucleotide, CMP, binds to the C-terminal α/β domain. Superposition of the ligand-free and CMP-bound structures shows that CMP binding induces fairly small movements (<2 Å) that are localized primarily within the C-terminal α/β domain; this region partakes in the dimeric interface, and CMP binding also somewhat alters the conformation of the dimer (Fig. 6A). This finding is in contrast with other GT-B enzymes, where nucleotide binding typically results in a large rotation (on the order of 20°) of the two domains, closing the catalytic cleft (Fig. S5A), but is consistent with the extensive interactions between all three domains, which seems to produce a more rigid platform than is typically seen for GT-B enzymes.
Fig. 6.
CMP binding to WbbB2–401 and in vitro activity of WbbB2–401 and site-directed mutants. (A) Superposition of ligand-free (blue) and CMP bound (yellow) of WbbB2–401. Local movement in the C-terminal α/β domain accompanies CMP binding; note that this movement occurs at the dimeric interface. (B) Details of CMP binding site. (C) Model of CMP-β-Kdo in the binding site. The ligand was placed by superimposing the CMP portion of the molecule, then positioning the sugar ring by torsions around the P–O and O–C2 bonds. Potential hydrogen bonds are shown in dashed lines. (D) The reaction mixtures were incubated for 20 min and analyzed by TLC. Of six mutants showing little or no activity on TLC, E158A and D232 were completely inactive, whereas others retained residual activity (<3% conversion after 20 min). (E) Time course of Kdo-transferase reaction. The percentage of conversion was determined using LC-MS by integrating the peak areas of the substrate and the product in the merged extracted ion chromatograms.
Although GT-B–fold proteins appear to have no absolutely conserved residues, there are several features identified as being conserved across multiple families. These features include a glutamate residue that forms hydrogen bonds with the O2 and O3 groups of ribose, a glycine-rich loop that coordinates the phosphate ion, and a (D/E)(D/E)G motif between Nβ5 and Nα5 that may contribute the general base (Fig. S5B) (26, 27). WbbB2–401 lacks all of these substrate-interacting motifs. Instead, the cytosine ring of CMP is sandwiched between Val302 and Pro266, whereas the Watson and Crick face makes hydrogen bonds with the carbonyl oxygen and guanidinyl group of Arg263 (Fig. 6B). The amine also forms a hydrogen bond with the carbonyl oxygen of Leu227. The phosphate group forms hydrogen bonds with His265, as well as Ser300 (Oγ) and Ser301 (both N and Oγ) at the N terminus of helix Cα4. His265 and Pro266 (the HP motif) are invariant in all analyzed β-Kdo GTs (Fig. S2) and aligned structurally with highly conserved HP motifs of CMP-sialic acid-dependent sialyltransferases. H265A has a more severe catalytic defect than P266A; however, neither of the mutations abolishes enzyme activity completely (Fig. 6 D and E). The ribose hydroxyl O2 and O3 do not form hydrogen bonds directly with the protein, but instead interact with structured water molecules. This CMP binding mode is similar to what is seen in GT-B sialyltransferases of CAZy families GT52 and GT80. For example, Pasteurella multocida multifunctional sialyltransferase (GT80; PDB ID code 2EX1) coordinates CMP using homologous (K/R)XPH and SS motifs to mediate identical interactions to bind both the base and phosphate group (27) (Fig. S5). Note that this resemblance seems localized to the CMP binding pocket; although GT80 sialyltransferases also show insertion of extra α-helices, these occur at topologically distinct sites. Overall, these similarities are unlikely to be convergent, leading us to conclude that sialyltransferases are likely the most closely related previously characterized relatives of β-Kdo transferases.
The CMP binding site is part of a larger conserved pocket that is formed at the conjunction of the three domains. Immediately adjacent to the CMP binding pocket is an extended, conserved, basic pocket that is a good candidate for Kdo binding (Fig. 5 E and F). This pocket is formed mostly by elements of the C-terminal α/β domain, as well as the β-hairpin motif linking the N-terminal α/β domain and the α-helical domain. Arg163 repositions itself in the CMP costructure so the guanidine group stacks on the ribose C4 and C5. This residue is then well positioned to donate one or two hydrogen bonds to β-Kdo, as well as stabilize the negative charges associated with the carboxylate and phosphate groups. The rest of the pocket is lined by hydrogen bonding groups, including Glu158, Tyr172, Asn179, Gln228, Asp232, and Asn234. E158A was found be completely inactive, whereas N179A shows significant catalytic defects. Gln228 and Asp232 are of special note as together they form a QXXXD motif that is conserved across all identified β-Kdo transferases (Fig. S2). Asp232 is positioned 4.4 Å from the nearest phosphate oxygen. The D232A mutant proved completely inactive, whereas the Q228A mutant was severely compromised in activity (Fig. 6D).
Modeling potential CMP-β-Kdo binding modes suggests that interactions with critical residues such as Gln228 and Asn179 can only be achieved if the Kdo ring is inserted edgewise into the cleft between Asp232 and Arg163; binding here positions the carboxylate group between His265 and Asp232, allowing it to form hydrogen bonds with Gln228Nε and Val229N. Placing the Kdo ring in a boat conformation (shown in Fig. 6C) seems to maximize interactions with the pocket. In CMP-β-Kdo, this conformation is stabilized by internal hydrogen bonds (between O5, O7, and a phosphate oxygen) and closely resembles the conformation argued to explain the unusually high CMP-β-Kdo autohydrolytic reaction rate (18). In this boat conformation, O4 and O5 can form hydrogen bonds with Arg163, whereas O8 forms hydrogen bonds with Asn179 and Asn234. Interestingly, in this model the anomeric carbon atom is completely buried from solvent, whereas the Asp232 carboxylate is 3.2 Å away, and in line with the C2–O2 bond that is broken during the reaction.
The active site is extended from the CMP binding site into a 20 Å long groove (Fig. 5 E and F), which may accommodate the long-chain acceptor molecule during substrate binding. The location of the acceptor-binding site is uncertain, although the presence of an extended exposed hydrophobic patch (Trp20, Trp54, Leu64, Ile159, and Phe348) contributed mainly by the N-terminal Rossmann-fold domain presents a reasonable candidate. These residues are, however, too distant to interact with the terminal residue of the acceptor. Of note here is Glu158, which appears absolutely required for activity, but is located too far from CMP to do more that form a single hydrogen bond with the donor. Possibly, this residue plays an important role in acceptor positioning or activation.
Discussion
The recent discovery of β-Kdo transferases (KpsC and KpsS) involved in the assembly of a conserved β-Kdo oligosaccharide linker in a large family of CPSs provided a foundation for a survey of homologs in bacterial strains known to contain β-Kdo as a component of their surface glycans. This approach allowed identification of new potential β-Kdo transferases, all being members of the pfam05159 family. The phylogenetic analysis reflects highly divergent amino acid sequences of identified GTs (Fig. 3). When the search was performed without limitations of the glycan structural data (i.e., PSI-BLAST search against the nonredundant protein sequences database) the KpsC and KpsS homologs were confined to Proteobacteria and one species of green sulfur bacteria. The predicted function for some β-Kdo GTs are supported by functional complementation (28) and in vitro biochemical studies (5); however, the structures of enzymatic products were not completely characterized (5). Although the overall sequence similarities between the β-Kdo transferases are low (Table S2), as reflected in the pylogenetic tree (Fig. 3), there is substantially more conservation in the putative catalytic sites (Fig. S2). Divergence in the flanking sequence presumably reflects the differences in acceptor molecules. We consider these proteins to be GT99 members in the absence of detailed structural or mechanistic data that would indicate otherwise.
We successfully delineated the N-terminal β-Kdo GT domain from the modular R. terrigena GT and demonstrated that its coiled-coil region and two other GT domains are not required for either folding or in vitro activity of WbbB2–401. In vivo, the β-Kdo GT domain of WbbB transfers a Kdo residue to the nonreducing end of a long polysaccharide chain. In the E. coli O9a prototype for this type of OPS-assembly system, a nonreducing terminal methylphosphate serves as chain-terminator, creating a modal distribution of long O-antigen chains (15, 16) as well as a signal to export OPS to the periplasmic side of the inner membrane. However, in E. coli O9a, different proteins dictate polymerization and chain termination, whereas the GT99, GT1-like, and GT25-like modules in WbbB are consistent with it performing both functions in R. terrigena. The involvement of β-Kdo transferases (KpsC and KpsS) in the assembly of a conserved capsule linker in a large family of CPSs found in important Gram-negative pathogens (5, 10) has stimulated interest in whether these enzymes offer a new potential therapeutic target for small-molecule inhibitors. A solved structure for a β-Kdo GT prototype provides an important asset in such strategies.
To our knowledge, the crystal structure of WbbB2–401 provides the first insight into this new GT99 family. The complex of WbbB2–401 with CMP reveals a characteristic donor recognition HP motif that is involved in coordinating the cytosine ring; this interaction is also conserved among different bacterial sialyltransferase families (29). A detailed evaluation of the donor substrate binding site is challenging because of the instability of CMP-Kdo, which undergoes rapid hydrolysis to CMP and Kdo (18). However, relative activity assays showed that the H265A and P266A mutants maintain reduced catalytic activity. Another bacterial “sialyl motif” S(S/T) (30, 31) is reduced to a single invariant Ser residue (S300 in WbbBRt) in most β-Kdo GTs, although the four closest WbbB homologs also possess an adjacent Ser residue. Mutation S300A dramatically decreases enzyme activity, whereas S301A only has a slight catalytic defect. β-Kdo transferases diverge, however, from the sialyltransferases in possessing an invariant QXXXD motif. Mutagenesis of these residues severely impedes activity, suggesting an essential role for this motif in WbbB2–401’s mechanism.
The mechanisms used by retaining GTs have proved difficult to pin down. Initially, in an analogy with retaining glycosyl hydrolases, it was thought that these enzymes would use a double-displacement mechanism, wherein a protein carboxylate group attacks the anomeric carbon as a nucleophile, displacing the nucleotide leaving group. The resulting covalent intermediate is resolved by the incoming acceptor displacing the carboxylate in a second SN2 reaction, restoring the original anomeric configuration. Although this mechanism remains at least partially supported by available data for family GT6 (32, 33), most candidate acidic groups in retaining GTs have alternative roles in substrate binding, are weakly conserved, or are too distant from the anomeric carbon to allow them to readily act as nucleophiles (1). As a result, recent attention has focused on the alternative SNi “internal return” mechanisms. Here, the incoming acceptor is positioned adjacent to the phosphate group; dissociation of the phosphate-donor bond forms an oxocarbenium ion, and transfer of a proton from the acceptor hydroxyl to the phosphate-leaving group allows the deprotonated acceptor to recombine with the oxocarbenium ion. Anomeric configuration is retained because the acceptor and nucleotide are positioned on the same face of the donor (1).
WbbB2–401 is unique among known retaining GTs in that the donor sugar is a ketose. Although the C2-carboxylate group should contribute substantially to stabilizing an oxocarbenium ion transition state (34), Kdo’s bulky carboxylate group may impede the acceptor sugar from closely approaching the anomeric carbon while the nucleotide is present. Modeling potential donor binding modes suggests that the anomeric carbon is likely to be wholly buried in the active site, leaving little room for the acceptor to approach with a geometry suitable for SNi mechanisms. The same model predicts that Asp232—a residue indispensable for WbbB2–401 activity and one of six residues absolutely conserved among all β-Kdo transferases—may be ideally positioned (about 3.2 Å from the anomeric carbon, and in line with the nucleotide leaving group) to attack C2 as a nucleophile (Fig. 6C). It is also worth noting that WbbB2–401 likely evolved from an inverting sialyltransferase family that uses a conserved histidine to accelerate the reaction, most likely by protonating the leaving group (35, 36). WbbB2–401 similarly requires His265 for maximal activity. Because SNi mechanisms require no such general acid (as the phosphate group acquires a proton directly from the acceptor), this suggests that the WbbB2–401 reaction mechanism retains considerable SN2-like characteristic. Together, these observations imply that retaining β-Kdo transferases are at least strong candidates for using a glycosyl hydrolase-like double-displacement mechanism.
Materials and Methods
Bacterial Strains and Growth Conditions.
Bacterial cultures were grown with aeration at 37 °C in Luria Broth Base (LB) (Invitrogen) supplemented with kanamycin (50 μg/mL−1) when necessary. R. terrigena ATCC 33257 K− was a gift from U. Mamat (Research Centre Borstel, Leibnitz Centre for Medicine and Biosciences, Borstel, Germany). E. coli O4:K12:H1 was obtained from Wyeth-Lederle Vaccines. E. coli O15:K14:H4 (strain SSI 85370) was purchased from Cedarlane. E. coli strain CWG1217 [F−, mcrA, Δ(mrr-hsdRMS-mcrBC), ϕ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, rpsL(Strr), endA1, Δ(wzx-glf-wbbHIJK)] used for cloning is a derivative of strain TOP10 from Invitrogen that lacks part of the K-12 wb* gene cluster. Protein expression was performed in E. coli BL21 (DE3) [F– ompT gal dcm hsdSB(rB− mB−) λ(DE3)] or the methionine auxotroph E. coli B834 (DE3) [F− ompT gal dcm hsdSB(rB− mB−) met λ(DE3)].
Sequencing of CPS Gene Clusters.
Primers OL1052 and OL1053, based on the kpsS and kpsT genes, respectively, were used to amplify the region 2 of CPS gene cluster in E. coli K14 with the Phusion DNA polymerase. This process took advantage of the observation that the serotype-specific CPS biosynthesis gene cassettes (named region 2) usually lie between two conserved gene blocks with kpsS and kpsT as the flanking genes (Fig. 2). The PCR product was sequenced by primer walking on both strands. However, with E. coli serotype K12, only a short 2,810-bp PCR product was obtained, which contained two insertion elements, insA and insB, indicating that serotype-specific genes were relocated in this isolate.
E. coli K12 genomic DNA was sequenced using Illumina MiSeq technology but the CPS gene cluster region was represented as several contigs. The E. coli K12 CPS biosynthesis genes showed 100% identity to those from E. coli KTE195 (GenBank accession no. ASUJ01000047.1), the serotype of which is not indicated. The region 2 genes of E. coli KTE195 are located in the correct position on the chromosome but are flanked by two pairs of insA and insB sequences. We used an intergenic region sequence between flanking IS elements and region 2 to designed new primers OL1078 and OL1079 and successfully amplified the relocated region 2 in the E. coli K12 serotype reference strain. The sequence was confirmed by primer walking. Custom oligonucleotide primers (Sigma) are listed in Table S4.
Table S4.
Primer sequences
| Primer | Sequence (5′→ 3′) | Plasmid | |
| Amplification of R. terrigena WbbB2–401* | |||
| OL1025 | GATCCCATGGGCCTGGCTGTATTTTTACCTCC | pWQ812 | |
| OL1026 | GATCCTCGAGGGAAATGGCTTCATTTATCAATG | ||
| Site-directed mutagenesis† | |||
| OL1054 | E159 | GCAGCGGGCCAATCGCGATGTGCATCACC | pWQ813 |
| OL1055 | E159 | GGTGATGCACATCGCGATTGGCCCGCTGC | |
| OL1056 | N180A | CTTCGGTCCCGCCAGCGACGCCGGCAAAGT | pWQ814 |
| OL1057 | N180A | ACTTTGCCGGCGTCGCTGGCGGGACCGAAG | |
| OL1058 | Q229A | GTCATCCTCAACCGCAAGCACGACGCCGGCC | pWQ815 |
| OL1059 | Q229A | GGCCGGCGTCGTGCTTGCGGTTGAGGATGAC | |
| OL1060 | D233A | GGCGATCAGATTGGAGGCATCCTCAACCTGAAG | pWQ816 |
| OL1061 | D233A | CTTCAGGTTGAGGATGCCTCCAATCTGATCGCC | |
| OL1062 | R264A | ACCCGGATGCGCGGCCACCAGGATGTCC | pWQ817 |
| OL1063 | R264A | GGACATCCTGGTGGCCGCGCATCCGGGT | |
| OL1064 | H266A | CAGGCTACCCGGAGCCGCGCGCACCAGG | pWQ821 |
| OL1065 | H266A | CCTGGTGCGCGCGGCTCCGGGTAGCCTG | |
| OL1066 | P267A | TGGTGCGCGCGCATGCGGGTAGCC | pWQ824 |
| OL1067 | P267A | GGCTACCCGCATGCGCGCGCACCA | |
| OL1068 | N300A | CTCAAGACCGACGCTTGAGGCAATGGTGAAGACTTCGTTGC | pWQ825 |
| OL1069 | N300A | GCAACGAAGTCTTCACCATTGCCTCAAGCGTCGGTCTTGAG | |
| OL1070 | S301A | AAGACCGACGCTTGCGTTAATGGTGAAGACTTCGTT | pWQ826 |
| OL1071 | S301A | AACGAAGTCTTCACCATTAACGCAAGCGTCGGTCTT | |
| OL1072 | S302A | CTCAAGACCGACGGCTGAGTTAATGGTGAAGACTTCGTTG | pWQ827 |
| OL1073 | S302A | CAACGAAGTCTTCACCATTAACTCAGCCGTCGGTCTTGAG | |
| OL1074 | E306A | GCCGGTTAATATTGCCGCAAGACCGACGCTTGA | pWQ828 |
| OL1075 | E306A | TCAAGCGTCGGTCTTGCGGCAATATTAACCGGC | |
| Amplification of E. coli CPS gene cluster region 2 | |||
| OL1052 | GATCGTGGTCACAGACTCTATCG | ||
| OL1053 | AGATTGAGAATTTGACGAAGTC | ||
| OL1078 | ATGATTTAGCATCCTAAAACC | ||
| OL1079 | CATGGTCTGTAATCATATCG | ||
The introduced NcoI and XhoI restriction sites are underlined.
The second column indicates the introduced amino acid substitution. Site-specific mutations are underlined.
Bioinformatic Assignment of β-Kdo GTs.
The assignments for the candidate β-Kdo GTs (Table S1) involved integrated assessment of the GT requirements imposed by the glycan structure, organization of genetic loci responsible for biosynthesis (Fig. 2 and Fig. S1), and multiple sequence alignments of the proteins (Fig. S2 and Table S2) (see SI Materials and Methods for details). Glycan structures containing β-Kdo were retrieved from the Bacterial Carbohydrate Structure Database (37).
Multiple Sequence Alignment and Phylogenetic Analysis.
The proteins and domains used in alignment are listed in Table S1. The protein secondary structure was modeled using the Phyre2 server (38). The coiled-coil segment was predicted using COILS (14). WbbBRt was aligned with its homologs WbbBKpO12, WbbBAhO11, and WbbKSmO4 to identify the break points in their sequences corresponding to Ser401 in WbbBRt. E. coli Orf2 and Sinorhizobium meliloti SM_b20824 domains were separated based on disordered regions in Phyre2 model and the conserved domains identified using the National Center for Biotechnology Information (NCBI) Conserved Domain Search service. N. meningitidis KpsC1–325 and KpsC327–704 were shown to be active as separate polypeptides in vivo (5). Multiple-sequence alignment of KpsC homologs was performed to identify the break points corresponding to Pro326 in N. meningitidis KpsC. A total of 33 proteins and separated domain sequences were aligned using MAFFT (39) with the iterative refinement method E-INS-i and BLOSUM62 scoring matrix. Poorly aligned and divergent regions were eliminated using GBlocks running under relaxed parameters (12). Phylogenetic analysis was performed using the PhyML 3.0 server (40) (LG matrix; SPR tree improvement) with 1,000 bootstrap replicates. The multiple-sequence alignment and the tree were visualized with ESPript (41) and FigTree, respectively.
Overexpression and Purification of WbbB2–401 and Its Mutants.
WbbB2–401-His6 and its mutants were expressed in E. coli BL21 (DE3). Overnight cultures were used to inoculate 250 mL LB media at 1/100 dilution. Cultures were grown at 37 °C until OD600 ∼0.2 was reached and then placed at 18 °C. Protein expression was induced at OD600 ∼0.6 by adding isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM and cultures were grown at 18 °C overnight. Cells were harvested by centrifugation at 5,000 × g for 10 min and either used for immediate protein purification or kept at −80 °C until needed. The cell pellet was resuspended in 25 mL buffer A (50 mM Tris⋅HCl, 250 mM NaCl, pH 7.4) containing 10 mM imidazole and supplemented with 1/2 Complete mini EDTA-free protease inhibitor tablets (Roche). The cells were lysed by ultrasonication for a total of 3 min 30 s, with 30% amplitude in pulses (10 s on/15 s off). The suspension was cleared by successive centrifugation steps at 4,000 × g for 10 min and 100,000 × g for 1 h at 4 °C. The WbbB2–401-His6 protein was purified from the supernatant using 2 mL Ni-NTA Agarose resin columns (Qiagen). The column was washed sequentially with 10 mL of binding buffer, 20 mL of buffer A containing 25 mM imidazole, and eluted with 10 mL of buffer A containing 250 mM imidazole; 1-mL fractions were collected. Protein was exchanged into imidazole-free buffer A using a PD-10 desalting column (GE Lifesciences). Protein purity was assessed by SDS/PAGE analysis using 10% (wt/vol) acrylamide resolving gels, and the concentration was estimated from A280 values and a theoretical extinction coefficient of 40,800 M-1⋅cm−1 predicted by ProtParam (42). Folding of the mutant proteins was confirmed by a differential-scanning fluorescence method (43) using the Protein Thermal Shift Dye kit (ThermoFisher). Proteins were stored in buffer A containing 10% glycerol at −80 °C.
Overexpression and Purification of Selenomethionine-Labeled WbbB2–401.
WbbB2–401-His6 was expressed in the methionine auxotrophic strain E. coli B834 (DE3). A starter culture was grown at 37 °C overnight in LB media. Cells from the overnight culture were collected by centrifugation (4,000 × g for 10 min) and resuspended in cold sterile water. The cell suspension was used to inoculate 750 mL selenomethionine minimal media (44) supplemented with l-SeMet (50 µg/mL) to achieve a final OD600 0.3. The culture was then grown at 18 °C until OD600 ∼0.6 and protein expression was induced with IPTG to a final concentration of 0.5 mM. The culture was then incubated at 18 °C for 24 h and protein purification followed the procedure described above.
Overexpression and Purification of KdsB.
Plasmid pJKB72 encoding His6-tagged CMP-Kdo synthethase (KdsB) from E. coli K-12 was a gift from H. Brade (Research Centre Borstel, Leibnitz Centre for Medicine and Biosciences, Borstel, Germany). KdsB was expressed in E. coli LE392 and purified essentially as described previously (45).
SDS/PAGE.
SDS/PAGE was performed using 10% resolving gels in Tris-glycine buffer, and the gels were stained with Simply Blue SafeStain (ThermoFisher).
FPLC.
Purified WbbB2–401 (0.2 mg at 2 mg/mL) was eluted through a Superose 6 HR 10/30 size-exclusion column (GE Healthcare) with buffer A (50 mM Tris⋅HCl, 250 mM NaCl, pH 7.4) at 0.5 mL/min. Elution profiles of freshly purified WbbB2–401 (immediately after elution from the Ni-NTA Agarose column in buffer A containing 250 mM imidazole) and after buffer exchange and storage for 5 d at 4 °C were essentially identical. Molecular mass was estimated from a standard curve derived from the standards β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), BSA (66 kDa), ovalbumin (44 kDa), and carbonic anhydrase (29 kDa).
Synthesis of Acceptor 1.
The route used for the synthesis of acceptor disaccharide 1 is provided in SI Materials and Methods (Fig. S6). The N-phthaloyl protected glucosamine derivative 3 (46) was converted to the corresponding glycosyl bromide and coupled to 8-azido-1-octanol in the presence of N-iodosuccinimide, silver triflate, and 2,4,6-collidine giving the desired product 4, in 92% yield. Deacetylation of 4 by treatment with sodium methoxide and then protection of the C4 and C6 hydroxyl groups as a benzylidene acetal afforded acceptor 5 in 79% yield over the two steps. Glycosylation of 5 with rhamnose donor 6 (47) gave disaccharide 7 in 72% yield. Full deprotection of sugar moiety was smoothly carried out by first ethylenediamine-mediated cleavage of the phthalimide protecting group and acetyltion of the resulting amine to give disaccharide 8 in 91% over the two steps. Next, acid hydrolysis of the acetal-protecting groups in 8 and then acetylation of liberated hydroxyl groups produced, over the two steps, a 91% yield of compound 9. The acetylation step was done to facilitate purification of the compound. Removal of the O-acetate protecting groups in 9 afforded a 92% yield of compound 10. Hydrogenation of the azido group led to primary amine 11, which was obtained in 84% yield. Finally, 11 was treated with FITC to generate 1 in 46% yield.
In Vitro Determination of WbbB2–401 Activity.
In vitro reactions were performed in PCR tubes at 37 °C. The acceptor concentration was estimated by measuring absorbance at 490 nm in 0.01 M NaOH, using extinction coefficient 88,000 M−1⋅cm−1 (48). To optimize the amount of enzymes in a coupled assay, the reactions (20 µL) contained 50 mM Hepes (pH 8.0), 10 mM MgCl2, 2 mM Kdo, 5 mM CTP, 0.17 mM synthetic acceptor, and variable amounts of KdsB (0.5–4 μg) and WT WbbB2–401 (1-4 μg). The reaction was initiated by addition of KdsB. The reaction was stopped by spotting 1 µL on TLC plate (Silicagel 60 F254) after 1, 2, 4, 8, 12, 16, and 20 min and the TLC plate was developed in freshly prepared chloroform-methanol-water-acetic acid mixture (25:15:4:2, vol/vol). The products were visualized using a hand-held UV lamp.
The activities of site-directed mutants were examined under conditions where CMP-Kdo is expected to be in excess. The reaction was scaled up to 80 µL and contained all of the components at the same concentrations as above, with 2 µg KdsB and 4 µg WbbB2–401. Ten-microliter aliquots were withdrawn after 1, 2, 4, 8, 12, 16, and 20 min. For mutants showing little or no activity after 20 min in TLC analysis, the reaction mixture was incubated for longer time and aliquots were withdrawn after 20, 60, and 120 min. The reactions were quenched by incubating at 98 °C for 5 min. Aliquots were kept on ice until the end of experiment and centrifuged at 13,000 × g for 5 min. An equal amount of water was added to the samples and 1-µL aliquots were analyzed by LC-MS (see SI Materials and Methods for details). The assay was performed in triplicate for WT and each of WbbB2–401 mutants shown in Fig. 6E. The percentage of conversion was determined by integrating the peak areas of the substrate and the product in the merged extracted ion chromatograms using MassHunter Workstation Software (Agilent).
Purification of Reaction Product for Structural Analysis.
Reaction mixtures (9 × 100 µL) containing 50 mM Hepes (pH 8.0), 10 mM MgCl2, 2 mM Kdo, 5 mM CTP, 0.34 mM synthetic acceptor, 4 µg KdsB, and 8 µg WbbB2–401 were incubated at 37 °C for 30 min. Completion of reaction was confirmed by TLC as described above. The reaction mixtures were pooled, diluted with water up to 10 mL, and loaded to a Sep-Pak C18 column, previously washed with 10 mL acetonitrile and 20 mL water. The column was washed with 20 mL water and the product was eluted in 50% (vol/vol) acetonitrile-water mixture and dried using a Speed-Vac.
NMR Spectroscopy.
NMR studies were performed at the NMR Centre in the University of Guelph Advanced Analysis Center. The product 2 was deuterium-exchanged by dissolving in 99.9% D2O and drying using a Speed-Vac. NMR spectra were obtained at 30 °C in 99.96% D2O using a Bruker Avance II 600 MHz spectrometer equipped with a cryoprobe. Internal sodium 3-trimethylsilylpropanoate-2,2,3,3-d4 (δH = 0 ppm, δC = − 1.6 ppm) served as a reference. Two-dimensional experiments were performed using standard Bruker software, and the Bruker TopSpin 2.1 program was used to acquire and process the NMR data. Mixing times in TOCSY and ROESY experiments were set up to 100 and 200 ms, respectively. The HMBC experiment was optimized for the JH,C coupling constant 8 Hz.
WbbB2–401 Structure Determination.
All crystallization experiments were conducted in a sitting-drop configuration at room temperature, by mixing protein in a 1:1 ratio with well solution, and then conducting vapor diffusion against ∼100 μL of the same well solution. Selenomethionine-labeled WbbB2–401 protein was crystallized by using 1 μL of 10 mg/mL protein and a well solution of 0.2 M MgCl2, 0.1 M Tris⋅HCl pH 8.5, 25% polyethylene glycol 4000, 10 mM Tris(2-carboxyethyl)phosphine (TCEP). Crystals grew as prisms (200 mm) after three days. For the CMP complex, protein was concentrated to 10 mg/mL in a buffer that included 20 mM Tris⋅HCl pH 8.0, 150 mM NaCl, 1 mM TCEP, 1 mM EDTA, and 1 mM CMP. Crystals were grown using a well solution of 0.2 M NaCl, 0.1 M Bis-Tris pH 5.5, and 25% PEG 3350. Prismatic crystals up to 150 μm in length were observed within 14 d. Both crystal forms were cryoprotected with paratone-N oil before freezing in liquid nitrogen for data collection at 100 K. Data were collected for the selenomethionyl protein crystals at the Canadian Light Source beamline CLS08B1 at a wavelength of 0.97836 Å; crystals diffracted to 2.3 Å. The structure was determined using single anomalous scattering. Anomalous substructure searching with Phenix autosol found 12 anomalous scattering centers, with an overall figure of merit 0.231; autotracing in Phenix was able to then correctly trace most the structure. Manual rebuilding in Coot (49) and refinement in Phenix (50) was used to complete the structure. Ramachandran statistics for the ligand-free structure were 96.8% favored, 2.8% allowed, and 0.4% outliers (as determined by Molprobity). Data for CMP-complex crystals were collected at the Canadian Light Source, beamline CLS08ID at wavelength of 0.97949 Å, with diffraction to 2.1 Å. The structure of the CMP complex crystals was determined using molecular replacement in Phaser (51) in Phenix, with the complete ligand-free protomer as a search model. Ramachandran statistics for the CMP costructure were 98.1% favored, 1.8% allowed, and 0.1% outliers. In both structures, several residues in the loops between Cα5 and Hα3, and between Hα6 and Hα7 are disordered. Data collection and refinement statistics are shown in Table 1. Structural figures were prepared in Pymol.
Table 1.
Data collection, phasing and refinement statistics
| Data collection and statistics | Sel Met (apo) | CMP complex |
| Data collection | ||
| Space group | P212121 | C2221 |
| Cell dimensions | ||
| a, b, c (Å) | 82.93, 82.93, 120.71 | 92.75, 159.39, 120.04 |
| Resolution (Å) | 50–2.3 (2.36–2.3)* | 50–2.1 (2.15–2.10) |
| Rsym | 0.073 (0.93) | 0.094 (0.64) |
| I/σI | 19.1 (2.2) | 13.3 (2.85) |
| Completeness (%) | 99.8 (100) | 100 (100) |
| Redundancy | 5.8 (5.8) | 7.2 (7.5) |
| Refinement | ||
| Resolution (Å) | 41.5–2.3 | 50–2.1 |
| No. reflections | 37,670 | |
| Rwork/Rfree | 0.186/0.229 | 0.169/0.211 |
| No. atoms | ||
| Protein | 6,211 | 6,187 |
| Ligand/ion | 1 | 42 |
| Water | 562 | 836 |
| B-factors | ||
| Protein | 33.2 | 24.1 |
| Ligand/ion | 42.9 | 11.6 |
| Water | 34.3 | 31.7 |
| Rms deviations | ||
| Bond lengths (Å) | 0.003 | 0.005 |
| Bond angles (°) | 0.636 | 0.852 |
A single crystal was used in each case to determine the structure.
Values in parentheses are for highest-resolution shell.
SI Materials and Methods
General DNA Methods.
Genomic DNA was prepared using the PureLink Genomic DNA Mini Kit (Invitrigen). Plasmid DNA was purified using the PureLink Plasmid Miniprep Kit (Invitrogen), and DNA fragments from PCRs and restriction digestions were purified using the PureLink PCR purification kit (Invitrogen). Restriction endonucleases and T4 DNA ligase (New England Biolabs) were used according to the manufacturer’s instructions. Custom oligonucleotide primers (Sigma) and recombinant plasmid constructs used in this study are listed in Table S4.
The DNA fragment encoding amino acid residues 2−401 of WbbB from Raoultella terrigena was amplified by PCR using Pwo DNA Polymerase (Roche Applied Science) and the OL1025/OL1026 primer pair. The PCR product was digested with NcoI/XhoI and ligated into pET28a(+) to construct pWQ812. Single amino acid substitutions were introduced by PCR using KOD Hot Start DNA polymerase (Novagen) and pWQ812 as the template. PCR products were digested with DpnI and the digestion mixture was used to transform Escherichia coli CWG1217 to generate the plasmids pWQ813-pWQ817, pWQ821, and pWQ824-pWQ828. All of the constructs were confirmed by DNA sequencing performed in the Genomics Facility of the Advanced Analysis Center at the University of Guelph.
Bioinformatic Assignment of β-Kdo GTs.
Group 2 CPSs are synthesized by sequential sugar addition to the nonreducing terminus of the growing glycan in the cytoplasm and the completed CPS is exported by machinery that includes an ATP-binding cassette (ABC) transporter (11, 52). E. coli isolates with group 2 capsules, Neisseria meningitidis, Haemophilus influenzae, and Campylobacter jejuni possess homologs of KpsS and KpsC and the conserved structure of the glycolipid product was confirmed in E. coli and N. meningitidis (10). In addition, β-Kdo is present in CPS repeat units of several E. coli K-(CPS) antigen serotypes (53). These CPS have di- or trisaccharide repeat units containing Kdo and d-ribose, l-rhamnose (l-Rha), or d-GalNAc. Genome sequence data were unavailable for these strains, so we sequenced the region of capsular gene clusters in E. coli K12 and K14 responsible for CPS repeat-unit synthesis and identified a candidate gene in each serotype encoding a predicted β-Kdo that add a Kdo residue to O-3 of Rha and O-6 of GalNAc, respectively. Similarly, in Actinobacillus pleuropneumoniae serotype 5a (54), Cps5B is the candidate for GT transferring β-Kdo to position O-6 of GlcNAc. A. pleuropneumoniae serotype 5b differs only by the presence of a side-chain β-Glc attached to Kdo, and the Cps5B proteins from serotypes 5a and 5b share 99% identity.
The plant symbiont, Sinorhizobium meliloti Rm1021 uses a related ABC transporter-dependent pathway to produce a low-molecular-mass CPS composed of 10–20 residues of β-(2→7)-linked Kdo, but the precise structure of the lipid terminus is not established (28, 55). The rkp gene cluster encodes two KpsC homologs (RkpZ1 and RkpZ2) and a homolog of KpsS (SM_b20824), found as the N-terminal domain of a multidomain GT. RkpZ1 and RkpZ2 are both required for CPS production. A functional homolog is encoded by rkpZ in S. melilotti Rm41 (28). The Sinorhizobium fredii US Department of Agriculture (USDA) 257 CPS cluster directs production of a glycan with a disaccharide repeat unit and encodes enzymes for the synthesis of GDP-mannose precursor (ManB and ManC), a GT1 family enzyme (Cps10D), and a putative β-Kdo GT (Cps10C). The S. fredii USDA 205 CPS repeat unit contains Gal instead of Man, but the organization of corresponding CPS biosynthesis locus is similar to that of strain USDA 257, with the N181_28225 (CpsD) and N181_28220 (CpsC) homologs in these strains sharing 90% and 86% identity, respectively. Although there is no other obvious β-Kdo GT candidates in USDA 205 genome, the function of N181_28220 remains unclear and it may transfer Kdo to position 3 of Man rather than Gal.
Some bacteria use Wzy-dependent glycan-assembly mechanisms for CPSs or OPSs. In these pathways, undecaprenyl diphosphate-linked repeat units are synthesized in the cytoplasm, flipped to the periplasm, then polymerized in a blockwise fashion by the pathway-defining Wzy polymerase (reviewed in refs. 56 and 57). Burkholderia oklahomensis CPS is the only listed example of a Wzy-dependent (“group 1” CPS) and genes encoding the corresponding genes for initiating galactose-1-phosphate transferase (WbaP), Kdo GT and two other GTs were found. β-Kdo is found in the OPS repeat units of Providencia alcalifaciens O36 (58) and Cronobacter sakazakii O6 (59), where it is attached to 6-deoxy-l-talose (6d-l-Tal) and d-Glc, respectively. Both OPS are synthesized via Wzy-dependent pathway. In these pathways, synthesis is initiated by an N-acetylglucosamine-1-phosphate transferase (WecA) encoded by a gene outside the OPS biosynthesis cluster. The anticipated additional GT genes were found within each cluster, along with those encoding the polymerase and exporter. WpaF from P. alcalifaciens and WepM from C. sakazakii are predicted β-Kdo GTs; they belong to pfam05159 and showed up to 35% identity with KpsS from other bacteria.
Some O-PSs are assembled by sequential GT reactions to create a completed Und-PP–linked O-PS that is then exported by an ABC transporter. There are two strategies in these examples that differ in the presence or absence of a nonreducing terminal residue, distinct from a component of the repeat-unit structure, which serves as a chain terminator and an export signal (60). R. terrigena ATCC 33257 and Klebsiella pneumoniae O12 provide examples that have identical OPS repeat-unit domain structures built of →4)-l-Rha-(1→3)-d-GlcNAc-(1→ disaccharide repeats, capped by a single β-linked Kdo residue (17, 23). In K. pneumoniae O12 Kdo was found to be attached at position O-3 of Rha, and the same is expected in R. terrigena given the high sequence conservation of the genetic loci. A similar structure that differs only in nonstoichiometric O-acetylation of Rha has been recently reported in OPS from Aeromonas hydrophila O11 (61). Finally, a structurally related OPS from Serratia marcescens O4 differs in one sugar residue and glycosidic linkages (i.e., 4-substituted Glc versus 3-substituted GlcNAc) and also possess O-acetylation at Rha (62). The presence of terminal Kdo in OPS from A. hydrophila O11 and S. marcescens O4 was not reported, but the glycosidic linkage of Kdo is particularly labile under mild acid hydrolysis conditions routinely used to obtain delipidated LPS. All of these bacteria possess OPS-biosynthesis gene clusters with similar organization and some conserved contents, including rml genes for synthesis of dTDP-l-Rha, wzm and wzt encoding ABC-transporter, and wbbL encoding a putative monofunctional rhamnosyltransferase (63). R. terrigena WbbB (WbbBRt) is a 1,106-aa multidomain enzyme that we predict to be solely responsible for synthesis of the capped repeat unit domain. At the C terminus there are two GT domains sharing homology with GT1 and GT25 families, presumably one each for transfer of Rha and GlcNAc. The N terminus contains a candidate β-Kdo GT domain. The same three domains are present in WbbBKpO12 and WbbBAhO11, as expected from the similar O-PS structures and they share 72% and 39% (overall) amino acid identity, respectively, with WbbBRt. In contrast, WbbBRt and WbbKSmO4 shared 40% identity and this was confined to the putative N-terminal β-Kdo GT domain (residues 1‒455 in WbbBRt and 1‒447 in WbbKSmO4). The divergence in the C-terminal regions of these proteins is in agreement with different sugar and linkage types in their repeat units.
General Synthetic Methods.
All reagents were purchased from commercial sources and were used without further purification. TLC was performed on Silica Gel 60 F254 with detection under UV or charring with a solution of p-anisaldehyde in H2SO4, AcOH and EtOH. Flash-column chromatography was carried out on Silica Gel 60 (40–60 μm). 1H and 13C NMR spectra were recorded with Varian 500 MHz NMR spectrometers and were referenced to residual proton signals in the deuterated solvents: CDCl3: 7.26 ppm (1H) and 77.16 ppm (13C), CD3OD: 3.31 ppm (1H) and 49.00 ppm (13C), D2O 4.79 (1H) ppm, or external CH3CN for D2O: 1.49 ppm (13C). NMR peak assignments of 1H and 13C were based on COSY and HSQC. Optical rotations were measured at the sodium D line (589 nm) in a microcell (1 dm, 1 mL) on a Perkin-Elmer 241 polarimeter and are expressed in units of degree⋅mL/(g⋅dm). High-resolution electrospray ionization (ESI)-MS spectra were recorded by Agilent Technologies 6220 oaTOF using samples dissolved in methanol, methanol–toluene 3:1 or dichloromethane.
8-Azidooctyl 3,4,6-triO-acetyl-2-deoxy-2-phthalimido-β-d-glucopyranoside (4).
To a solution of 3 (2.28 g, 4.78 mmol) in CH2Cl2 (20 mL), HBr/AcOH (12 mL) was added at 0 °C. The reaction mixture was stirred for 6 h at 0 °C, then diluted with CH2Cl2 and washed with ice-cold satd aq NaHCO3 three times. The organic layer was dried over Na2SO4 and concentrated to give the corresponding glycosyl bromide. The crude residue was dissolved in CH2Cl2 (15 mL) and added to a mixture of 8-azido-1-octanol (2.45 g, 14.3 mmol), 2,4,6-collidine (1.26 mL, 9.55 mmol) and 4 Å MS (5 g) in CH2Cl2 (15 mL) at –15 °C under an Ar atmosphere. AgOTf (2.45 g, 9.55 mmol) was added to the mixture at –15 °C and then mixture was stirred overnight at –15 °C. The reaction mixture was then neutralized with triethylamine and filtered through Celite. The filtrate was washed with satd aq NaHCO3 and brine, and then dried over Na2SO4 and concentrated. The residue was purified by chromatography (30:1 toluene–acetone) to give 4 (2.75 g, 92%) as a colorless oil. Rf 0.62 (1:1 n-hexane–EtOAc); [α]D = +16.9 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.86–7.83 (m, 2H, Ar), 7.75–7.72 (m, 2H, Ar), 5.77 (dd, 1H, J = 9.0, 10.7 Hz, H-4), 5.35 (d, 1H, J = 8.5 Hz, H-1), 5.16 (app t, 1H, J = 9.3 Hz, H-3), 4.34–4.28 (m, 2H, H-2, H-6a), 4.16 (dd, 1H, J = 2.3, 12.1 Hz, H-6b), 3.87–3.79 (m, 2H, H-5, OCHHCH2 a), 3.42 (dt, 1H, J = 6.4, 9.7 Hz, OCHHCH2 b), 3.17 (t, 2H, J = 7.1 Hz, CH2N3), 2.10 (s, 3H, CH3CO), 2.02 (s, 3H, CH3CO), 1.85 (s, 3H, CH3CO), 1.48–1.34 (m, 4H, CH2 x2), 1.19–0.96 (m, 8H, CH2 x4); 13C NMR (125 MHz, CDCl3, δC): 170.8, 170.3, 169.6, 134.4, 131.5, 123.7, 98.3 (C-1), 71.9 (C-5), 70.9 (C-3), 70.2 (OCH2CH2), 69.2 (C-4), 62.2 (C-6), 54.8 (C-2), 51.5 (CH2N3), 29.2 (CH2), 29.03 (CH2), 29.00 (CH2), 28.8 (CH2), 26.6 (CH2), 25.8 (CH2), 20.9 (CH3CO), 20.7 (CH3CO), 20.6 (CH3CO); ESI-MS m/z calculated for C28H36N4O10Na 611.2324, found 611.2315 [M+Na]+.
8-Azidooctyl 4,6-O-benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranoside (5).
To a solution of 4 (2.57 g, 4.37 mmol) in MeOH (40 mL), 3 M NaOMe in MeOH (29.1 μL, 87.3 μmol) was added and the mixture was stirred for 0.5 h at room temperature. The reaction mixture was then neutralized with Amberlite IR-120 (H+ form), then filtered through cotton and evaporated. After the deacetylated sugar was dissolved in DMF (20 mL), benzaldehyde dimethyl acetal (0.983 mL, 6.56 mmol) and 10-camphorsulfonic acid (203 mg, 0.874 mmol) were added. The reaction mixture was heated to 40 °C and was stirred overnight before being cooled to room temperature. The acid was neutralized by the addition of triethylamine and the mixture was coconcentrated with toluene. The residue was purified by column chromatography (5:1 toluene–EtOAc) to give compound 5 (1.90 g, 79%) as a colorless oil. Rf 0.43 (2:1 n-hexane–EtOAc); [α]D = –32.0 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.87–7.85 (m, 2H, Ar), 7.74–7.71 (m, 2H, Ar), 7.51–7.49 (m, 2H, Ar), 7.40–7.35 (m, 3H, Ar), 5.57 (s, 1H, CHPh), 5.25 (d, 1H, J = 8.5 Hz, H-1), 4.62 (dd, 1H, J = 8.6, 10.4 Hz, H-3), 4.38 (dd, 1H, J = 4.3, 10.4 Hz, H-6a), 4.23 (dd, 1H, J = 8.5, 10.4 Hz, H-2), 3.85–3.79 (m, 2H, H-6a, OCHHCH2 a), 3.67–3.58 (m, 2H, H-4, H-5), 3.42 (dt, 1H, J = 6.6, 9.6 Hz, OCHHCH2 b), 3.18 (t, 2H, J = 7.0 Hz, CH2N3), 2.61 (br s, 1H, OH), 1.49–1.26 (m, 4H, CH2 x2), 1.20–0.97 (m, 8H, CH2 x4); 13C NMR (125 MHz, CDCl3, δC): 137.1, 134.2, 131.8, 129.5, 128.5, 126.4, 123.6, 102.1 (CHPh), 99.1 (C-1), 82.5 (C-4), 70.1 (OCH2CH2), 68.9 (C-6), 68.8 (C-3), 66.3 (C-5), 56.8 (C-2), 51.5 (CH2N3), 29.3 (CH2), 29.1 (CH2), 29.0 (CH2), 28.9 (CH2), 26.6 (CH2), 25.8 (CH2). ESI-MS m/z calculated for C29H34N4O7Na 573.2320, found 673.2315 [M+Na]+.
8-Azidooctyl 2,3-O-isopropylidene-4-O-levlinoyl-α-l-rhamnopyranosyl-(1→3)-4,6-O- benzylidene-2-deoxy-2-phthalimido-β-d-glucopyranoside (7).
A mixture of 6 (78.4 mg, 0.192 mmol), 5 (88.2 mg, 0.160 mmol), and 4 Å MS (300 mg) in CH2Cl2 (2 mL) was stirred under an Ar atmosphere for 1 h at room temperature. The mixture was cooled to 0 °C, then NIS (64.8 mg, 0.288 mmol) and AgOTf (14.8 mg, 57.6 μmol) were added. The reaction mixture was stirred for 1 h at 0 °C, then triethylamine was added. The mixture was filtered through Celite and washed with 10% (wt/vol) aq Na2S2O3, satd aq NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by column chromatography (2:1 n-hexane–EtOAc) to give 7 (96.5 mg, 72%) as a pale yellow oil. Rf 0.53 (1:1 n-hexane–EtOAc); [α]D = –29.7 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.86–7.84 (m, 2H, Ar), 7.76–7.73 (m, 2H, Ar), 7.48–7.46 (m, 2H, Ar), 7.36–7.32 (m, 3H, Ar), 5.53 (s, 1H, CHPh), 5.26 (d, 1H, J = 8.5 Hz, H-1), 4.72 (s, 1H, H-1′), 4.64 (dd, 1H, J = 9.0, 9.9 Hz, H-3), 4.58 (dd, 1H, J = 7.9, 10.2 Hz, H-4′), 4.39 (dd, 1H, J = 3.7, 10.4 Hz, H-6a), 4.26 (dd, 1H, J = 8.6, 10.2 Hz, H-2), 3.98 (dd, 1H, J = 5.5, 7.7 Hz, H-3′), 3.83–3.77 (m, 4H, H-6b, H-2′, H-5′, OCHHCH2 a), 3.66–3.64 (m, 2H, H-4, H-5), 3.40 (dt, 1H, J = 7.6, 9.6 Hz, OCHHCH2 b), 3.16 (t, 2H, J = 7.0 Hz, CH2N3), 2.78–2.71 (m, 1H, CH2CHHCOO), 2.63–2.44 (m, 3H, CH2CHHCOO, CH2CH2CO), 2.12 (s, 3H, CH3CO), 1.48–1.23 (m, 7H, CH2 x2, CH3C), 1.15 (quint, 2H, J = 7.5 Hz, CH2), 1.09–0.91 (m, 9H, CH2 x3, CH3C), 0.60 (d, 3H, J = 6.3 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 206.2, 171.9, 137.1, 134.5, 131.4, 129.3, 128.2, 126.4, 123.7, 109.5 (C(CH3)2), 102.1 (CHPh), 98.8 (C-1), 97.5 (C-1′), 80.7 (C-4), 75.9 (C-2′), 75.5 (C-3′), 74.49 (C-4′), 74.47 (C-3), 68.8 (OCH2CH2), 68.5 (C-6), 66.5 (C-5), 64.6 (C-5′), 56.9 (C-2), 51.4 (CH2N3), 37.9 (CH2CH2COO), 29.8 (CH3CO), 29.2 (CH2), 28.94 (CH2), 28.91 (CH2), 28.8 (CH2), 27.9 (CH2CH2CO), 27.4 (C(CH3)2), 26.5 (CH2), 26.1 (C(CH3)2), 25.7 (CH2), 16.2 (C-6′). ESI-MS m/z calculated for C43H54N4O13Na 857.3580, found 857.3577 [M+Na]+.
8-Azidooctyl 4-O-acetyl-2,3-O-isopropylidene-α-l-rhamnopyranosyl-(1→3)-2-acetamido-4,6-O- benzylidene-2-deoxy-β-d-glucopyranoside (8).
A mixture of 7 (66.6 mg, 79.8 μmol) and ethylenediamine (2.0 mL) in n-BuOH (2.0 mL) was stirred at 80 °C overnight. The reaction mixture was cooled to room temperature and then coconcentrated with toluene. The residue was dissolved in pyridine (5.0 mL) and Ac2O (3.0 mL) was added. The mixture was stirred at room temperature overnight, then treated with MeOH (3 mL) and coconcentrated with toluene. The residue was diluted with CH2Cl2 and washed with satd aq NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by column chromatography (3:1 toluene–acetone) to give 8 (50.0 mg, 91%) as a white amorphous solid. Rf 0.44 (3:1 toluene–acetone); [α]D = –48.8 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.45–7.43 (m, 2H, Ar), 7.31–7.30 (m, 3H, Ar), 6.00 (d, 1H, J = 8.8 Hz, NH), 5.45 (s, 1H, CHPh), 5.10 (s, 1H, H-1′), 4.70 (dd, 1H, J = 7.7, 10.5 Hz, H-4′), 4.68 (d, 1H, J = 8.3 Hz, H-1), 4.33 (dd, 1H, J = 4.8, 10.4 Hz, H-6a), 4.18 (app t, 1H, J = 9.7 Hz, H-3), 4.15–4.11 (m, 2H, H-2′, H-3′), 3.88 (app dq, 1H, J = 6.3, 10.3 Hz, H-5′), 3.82–3.74 (m, 3H, H-2, H-6b, OCHHCH2 a), 3.58–3.48 (m, 2H, H-4, H-5), 3.42 (dt, 1H, J = 6.8, 9.5 Hz, OCHHCH2 b), 3.25 (t, 2H, J = 6.9 Hz, CH2N3), 2.03 (s, 3H, CH3CO), 2.00 (s, 3H, CH3CO), 1.61–1.54 (m, 4H, CH2 x2), 1.49 (s, 3H, CH3C), 1.44–1.30 (m, 11H, CH2 x4, CH3C), 0.58 (d, 3H, J = 6.3 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 170.5, 170.3, 137.3, 129.2, 128.2, 126.5, 109.7 (C(CH3)2), 102.0 (CHPh), 100.9 (C-1), 97.7 (C-1′), 80.1 (C-4), 76.1 (C-2′), 75.7 (C-3′), 75.6 (C-3), 74.6 (C-4′), 70.0 (OCH2CH2), 68.9 (C-6), 66.4 (C-5), 64.4 (C-5′), 57.6 (C-2), 51.6 (CH2N3), 29.6 (CH2), 29.3 (CH2), 29.2 (CH2), 28.9 (CH2), 27.7 (C(CH3)2), 26.8 (CH2), 26.6 (C(CH3)2), 25.9 (CH2), 23.5 (CH3CO), 21.1 (CH3CO), 16.4 (C-6′). ESI-MS m/z calculated for C34H50N4O11Na 713.3368, found 713.3367 [M+Na]+.
8-Azidooctyl 2,3,4-triO-acetyl-α-l-rhamnopyranosyl-(1→3)-2-acetamido-4,6-di-O-acetyl-2- deoxy-β-d-glucopyranoside (9).
A mixture of 8 (50.0 mg, 72.4 μmol) and 80% (vol/vol) AcOH aq. (5.0 mL) was stirred for 6 h at 60 °C. The reaction mixture was cooled to room temperature and coconcentrated with toluene. The residue was dissolved in pyridine (5 mL), then Ac2O (3.0 mL) was added. The reaction mixture was stirred for 3 d at room temperature, then MeOH (3 mL) was added and then mixture was and coconcentrated with toluene. The residue was purified by column chromatography (5:1 toluene–acetone) to give 9 (48.2 mg, 91%) as a white amorphous solid Rf 0.47 (2:1 toluene–acetone); [α]D = +7.3 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 6.16 (d, 1H, J = 6.8 Hz, NH), 5.20 (dd, 1H, J = 2.2, 3.0 Hz, H-2′), 5.16 (d, 1H, J = 8.2 Hz, H-1), 5.15 (dd, 1H, J = 3.1, 10.4 Hz, H-3′), 5.03 (app t, 1H, J = 10.1 Hz, H-4′), 4.96 (app t, 1H, J = 10.1 Hz, H-4), 4.72 (d, 1H, J = 1.9 Hz, H-1′), 4.55 (app t, 1H, J = 9.9 Hz, H-3), 4.24 (dd, 1H, J = 4.9, 12.2 Hz, H-6a), 4.06 (dd, 1H, J = 2.2, 12.2 Hz, H-6b), 3.89–3.16 (m, 2H, H-5′, OCHHCH2 a), 3.63 (ddd, 1H, J = 2.4, 4.9, 10.0 Hz, H-5), 3.42 (dt, 1H, J = 6.7, 9.7 Hz, OCHHCH2 b), 3.25 (t, 2H, J = 7.0 Hz, CH2N3), 2.96 (app dt, 1H, J = 8.2, 9.1 Hz, H-2), 2.11 (s, 3H, CH3CO), 2.06 (s, 6H, CH3CO x2), 2.03 (s, 3H, CH3CO), 1.99 (s, 3H, CH3CO), 1.96 (s, 3H, CH3CO), 1.61–1.55 (m, 4H, CH2 x2), 1.35–1.24 (m, 8H, CH2 x4), 0.58 (d, 3H, J = 6.3 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 171.8, 170.9, 170.7, 170.3, 170.1, 169.8, 100.5 (C-1), 98.9 (C-1′), 80.5 (C-3), 71.6 (C-5), 70.5 (C-4), 70.4 (C-4′), 70.3 (OCH2CH2), 69.60 (C-2′), 69.56 (C-3′), 68.0 (C-5′), 62.5 (C-6), 59.4 (C-2), 51.6 (CH2N3), 29.6 (CH2), 29.3 (CH2), 29.2 (CH2), 28.9 (CH2), 26.8 (CH2), 25.9 (CH2), 23.5 (CH3CO), 21.3 (CH3CO), 21.0 (CH3CO), 20.9, (CH3CO x3), 17.5 (C-6′); ESI-MS m/z calculated for C32H50N4O15Na 753.3165, found 753.3155 [M+Na]+.
8-Azidooctyl α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranoside (10).
To a solution of 9 (39.8 mg, 54.5 μmol) in MeOH (2.0 mL), 3.0 M NaOMe in MeOH (66.7 μL) was added. After stirring overnight at room temperature, the reaction mixture was neutralized with Amberlite IR-120 (H+ form), then filtered through cotton and concentrated to give 10 (26.2 mg, 92%) as a white amorphous solid. [α]D = –44.6 (c 1.0, MeOH); 1H NMR (500 MHz, CD3OD, δH): 4.79 (s, 1H, H-1′), 4.42 (d, 1H, J = 8.5 Hz, H-1), 3.94 (app dq, 1H, J = 6.2, 9.6 Hz, H-5′), 3.90–3.86 (m, 2H, H-6a, OCHHCH2 a), 3.77–3.68 (m, 3H, H-2, H-2′, H-6b), 3.64 (dd, 1H, J = 3.3, 9.5 Hz, H-3′), 3.57 (app t, 1H, J = 9.7 Hz, H-3), 3.46 (dt, 1H, J = 6.6, 9.6 Hz, OCHHCH2 b), 3.37 (t, 2H, J = 9.7 Hz, H-4, H-4′), 3.30–3.26 (m, 3H, H-5, CH2N3), 1.98 (s, 3H, CH3CO), 1.61–1.52 (m, 4H, CH2 x2), 1.38–1.26 (m, 8H, CH2 x4), 1.24 (d, 3H, J = 6.3 Hz, H-6′); 13C NMR (125 MHz, CD3OD, δC): 173.3, 103.2 (C-1′), 102.3 (C-1), 83.8 (C-3), 77.9 (C-5), 73.8 (C-4), 72.6 (C-2′), 72.1 (C-3′), 70.8 (OCH2CH2), 70.5 (C-5′), 70.4 (C-4′), 62.7 (C-6), 56.9 (C-2), 52.4 (CH2N3), 30.6 (CH2), 30.33 (CH2), 30.26 (CH2), 29.9 (CH2), 27.8 (CH2), 27.0 (CH2), 23.0 (CH3CO), 17.9 (C-6′). ESI-MS m/z calculated for C22H40N4O10Na 543.2637, found 543.2639 [M+Na]+.
8-Aminooctyl α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranoside (11).
To a solution of 10 (13.6 mg, 26.1 μmol) in H2O (1.0 mL) under Ar atmosphere, Pd/C (12.0 mg) was added. The flask was then flushed with H2 gas and the mixture was stirred overnight under a H2 atmosphere at room temperature. The reaction mixture was the filtered through Celite to remove Pd/C and the filtrate was concentrated. The residue was purified by reverse-phase chromatography [C18 Sep-pak, 20% (vol/vol) CH3CN] to afford 11 (10.9 mg, 84%) as a white amorphous solid. [α]D = –39.2 (c 1.0, H2O); 1H NMR (500 MHz, D2O, δH): 4.88 (d, 1H, J = 1.3 Hz, H-1′), 4.57 (d, 1H, J = 8.6 Hz, H-1), 4.01 (app dq, 1H, J = 6.2, 9.8 Hz, H-5′), 3.97–3.91 (m, 2H, H-6a, OCHHCH2 a), 3.82–3.75 (m, 4H, H-2, H-6b, H-2′, H-3′), 3.67–3.60 (m, 2H, H-3, OCHHCH2 b), 3.55–3.44 (m, 2H, H-4, H-5, H-4′), 3.03–2.92 and 2.50 (m, 2H, CH2NH2), 2.08 (s, 3H, CH3CO), 1.70–1.57 (m, 4H, CH2 x2), 1.47–1.35 (m, 8H, CH2 x4), 1.26 (d, 3H, J = 6.2 Hz, H-6′); C13 NMR (125 MHz, D2O, δC): 174.8, 102.0 (C-1′), 101.1 (C-1), 82.1 (C-3), 76.6 (C-5), 72.5 (C-4), 71.4 (C-2′), 71.2 (OCH2CH2), 70.8 (C-3′), 69.6 (C-5′), 69.1 (C-4′), 61.4 (C-6), 56.1 (C-2), 48.2 (CH2NH2), 29.2 (CH2), 28.84 (CH2), 28.76 (CH2), 28.1 (CH2), 26.3 (CH2), 26.2 (CH2), 25.7 (CH2), 22.8 (CH3CO), 17.1 (C-6′). ESI-MS m/z calculated for C22H43N2O10 495.2839, found 495.2809 [M+H]+.
8-Aminooctyl α-l-rhamnopyranosyl-(1→3)-2-acetamido-2-deoxy-β-d-glucopyranoside FITC Conjugate (1).
To a solution of 11 (10.9 mg, 22.0 μmol) in DMF (1.0 mL) containing 0.1% N,N-diisopropylethylamine, fluorescein isothiocyanate (10.3 mg, 26.4 μmol) was added. The reaction mixture was stirred overnight at room temperature protected from light and was then concentrated. The residue was purified by reverse-phase chromatography [C18 Sep-pak, 20% (vol/vol) MeOH in H2O] and size-exclusion chromatography (Sephadex LH-20, MeOH) to give 1 (9.0 mg, 46%) as an orange amorphous solid. [α]D = –27.0 (c 0.9, MeOH); 1H NMR (500 MHz, CD3OD, δH): 7.95 (s, 1H, Ar), 7.72 (d, 1H, J = 7.6 Hz, Ar), 7.19 (d, 1H, J = 8.2 Hz, Ar), 7.09 (d, 2H, J = 9.2 Hz, Ar), 6.66 (d, 2H, J = 2.1 Hz, Ar), 6.62 (dd, 2H, J = 2.3, 9.0 Hz, Ar), 4.84 (s, 1H, H-1′), 4.42 (d, 1H, J = 8.5 Hz, H-1), 3.93 (app dq, 1H, J = 6.2, 9.6 Hz, H-5′), 3.89–3.86 (m, 2H, H-6a, OCHHCH2 a), 3.77–3.68 (m, 3H, H-2, H-6b, H-2′), 3.65–3.57 (m, 4H, H-3, H-3′, CH2N), 3.46 (dt, 1H, J = 6.7, 9.6 Hz, OCHHCH2 b), 3.37 (app t, 2H, J = 9.7 Hz, H-4, H-4′), 3.28 (ddd, 1H, J = 2.0, 5.9, 9.6 Hz, H-5), 1.99 (s, 3H, CH3CO), 1.68–1.65 (m, 2H, CH2), 1.55 (br s, 2H, CH2), 1.38–1.25 (m, 8H, CH2 x4), 1.23 (d, 3H, J = 6.2 Hz, H-6′); 13C NMR (125 MHz, CD3OD, δC): 173.4, 132.2, 103.9, 103.3 (C-1′), 102.3 (C-1), 83.9 (C-3), 77.9 (C-5), 73.8 (C-4), 72.6 (C-2′), 72.1 (C-3′), 70.8 (OCH2CH2), 70.6 (C-5′), 70.4 (C-4′), 62.7 (C-6), 56.9 (C-2), 30.6 (CH2), 30.4 (CH2), 30.3 (CH2), 30.0 (CH2), 27.9 (CH2), 27.0 (CH2), 23.1 (CH3CO), 17.9 (C-6′). ESI-MS m/z calculated for C43H52N3O15S 882.3125, found 882.3124 [M–H]–.
LC-MS.
LC-MS analyses were performed on an Agilent 1200 HPLC liquid chromatograph interfaced with an Agilent UHD 6530 Q-Tof mass spectrometer at the Mass Spectrometry Facility of the Advanced Analysis Centre, University of Guelph. A C18 column (Agilent Poroshell 120, EC-C18 50 mm × 3.0 mm 2.7 µm) was used for chromatographic separation with the following solvents: water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The mobile phase gradient was as follows: initial conditions were 10% B hold for 1 min; increasing to 100% B in 29 min; column wash at 100% B for 5 min; 20 min re-equilibration. The flow rate was maintained at 0.4 mL/min. The mass spectrometer electrospray capillary voltage was maintained at 4.0 kV and the drying gas temperature at 250 °C with a flow rate of 8 L/min. Nebulizer pressure was 30 psi and the fragmenter was set to 160. Nitrogen was used as both nebulizing and drying gas. The mass-to-charge ratio was scanned across the m/z range of 50–1,500 m/z in 4 GHz (extended dynamic range) positive ion mode. The acquisition rate was set at two spectra per second. The instrument was externally calibrated with the ESI TuneMix (Agilent). The sample injection volume was 1 µL.
Supplementary Material
Acknowledgments
This work was supported by operating funding from the Canadian Institutes of Health Research (C.W.); Discovery Grants from the National Science and Engineering Research Council of Canada (to M.S.K., T.L.L., and C.W.); and GlycoNet (the Canadian Glycomics Network, National Centres of Excellence Program) (to M.S.K, T.L.L., and C.W.). T.L.L. and C.W. are recipients of Canada Research Chairs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession numbers for region 2 of CPS gene clusters sequences of Escherichia coli K12 and K14 are KU314824 and KU314825, respectively). The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (coordinates for the Raoultella terrigena WbbB2–401 and for WbbB2–401 in complex with CMP are PBD ID codes 5FA0 and 5FA1, respectively).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603146113/-/DCSupplemental.
References
- 1.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 2.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker JL, Çelik E, DeLisa MP. Expanding the glycoengineering toolbox: The rise of bacterial N-linked protein glycosylation. Trends Biotechnol. 2013;31(5):313–323. doi: 10.1016/j.tibtech.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Koeller KM, Wong CH. Synthesis of complex carbohydrates and glycoconjugates: Enzyme-based and programmable one-pot strategies. Chem Rev. 2000;100(12):4465–4494. doi: 10.1021/cr990297n. [DOI] [PubMed] [Google Scholar]
- 5.Willis LM, Whitfield C. KpsC and KpsS are retaining 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci USA. 2013;110(51):20753–20758. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth KM, Marchant A. Conservation of the 2-keto-3-deoxymanno-octulosonic acid (Kdo) biosynthesis pathway between plants and bacteria. Carbohydr Res. 2013;380:70–75. doi: 10.1016/j.carres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7.York WS, Darvill AG, McNeil M, Albersheim P. 3-Deoxy-d-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res. 1985;138(1):109–126. [Google Scholar]
- 8.Becker B, Hård K, Melkonian M, Kamerling JP, Vliegenthart JF. Identification of 3-deoxy-manno-2-octulosonic acid, 3-deoxy-5-O-methyl-manno-2-octulosonic acid and 3-deoxy-lyxo-2-heptulosaric acid in the cell wall (theca) of the green alga Tetraselmis striata Butcher (Prasinophyceae) Eur J Biochem. 1989;182(1):153–160. doi: 10.1111/j.1432-1033.1989.tb14811.x. [DOI] [PubMed] [Google Scholar]
- 9.Becker B, Lommerse JPM, Melkonian M, Kamerling JP, Vliegenthart JFG. The structure of an acidic trisaccharide component from a cell wall polysaccharide preparation of the green alga Tetraselmis striata Butcher. Carbohydr Res. 1995;267(2):313–321. [Google Scholar]
- 10.Willis LM, et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci USA. 2013;110(19):7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013;378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt M, Bollet C, Carta A, Rousselier P. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int J Syst Evol Microbiol. 2001;51(Pt 3):925–932. doi: 10.1099/00207713-51-3-925. [DOI] [PubMed] [Google Scholar]
- 14.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 15.Hagelueken G, et al. A coiled-coil domain acts as a molecular ruler to regulate O-antigen chain length in lipopolysaccharide. Nat Struct Mol Biol. 2015;22(1):50–56. doi: 10.1038/nsmb.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke BR, et al. In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide: The chain-length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan. J Biol Chem. 2011;286(48):41391–41401. doi: 10.1074/jbc.M111.295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens K, et al. Antiserum against Raoultella terrigena ATCC 33257 identifies a large number of Raoultella and Klebsiella clinical isolates as serotype O12. Innate Immun. 2010;16(6):366–380. doi: 10.1177/1753425909350057. [DOI] [PubMed] [Google Scholar]
- 18.Lin CH, Murray BW, Ollmann IR, Wong CH. Why is CMP-ketodeoxyoctonate highly unstable? Biochemistry. 1997;36(4):780–785. doi: 10.1021/bi962055c. [DOI] [PubMed] [Google Scholar]
- 19.Gronow S, Brabetz W, Brade H. Comparative functional characterization in vitro of heptosyltransferase I (WaaC) and II (WaaF) from Escherichia coli. Eur J Biochem. 2000;267(22):6602–6611. doi: 10.1046/j.1432-1327.2000.01754.x. [DOI] [PubMed] [Google Scholar]
- 20.Lenter M, Jann B, Jann K. Structure of the K16 antigen from Escherichia coli O7:K16:H-, a Kdo-containing capsular polysaccharide. Carbohydr Res. 1990;197:197–204. doi: 10.1016/0008-6215(90)84142-h. [DOI] [PubMed] [Google Scholar]
- 21.Ahrens R, Jann B, Jann K, Brade H. Structure of the K74 antigen from Escherichia coli O44:K74:H18, a capsular polysaccharide containing furanosidic beta-KDO residues. Carbohydr Res. 1988;179:223–231. doi: 10.1016/0008-6215(88)84120-x. [DOI] [PubMed] [Google Scholar]
- 22.Unger FM, Stix D, Schulz G. The anomeric configurations of the two ammonium (methyl 3-deoxy-d-manno-2-octulopyranosid)onate salts (methyl α- and β-ketopyranosides of KDO) Carbohydr Res. 1980;80(1):191–195. [Google Scholar]
- 23.Vinogradov E, et al. Structures of lipopolysaccharides from Klebsiella pneumoniae. Eluicidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J Biol Chem. 2002;277(28):25070–25081. doi: 10.1074/jbc.M202683200. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt H, et al. Structural and mechanistic analysis of the membrane-embedded glycosyltransferase WaaA required for lipopolysaccharide synthesis. Proc Natl Acad Sci USA. 2012;109(16):6253–6258. doi: 10.1073/pnas.1119894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Wrabl JO, Grishin NV. Homology between O-linked GlcNAc transferases and proteins of the glycogen phosphorylase superfamily. J Mol Biol. 2001;314(3):365–374. doi: 10.1006/jmbi.2001.5151. [DOI] [PubMed] [Google Scholar]
- 27.Ni L, et al. Cytidine 5′-monophosphate (CMP)-induced structural changes in a multifunctional sialyltransferase from Pasteurella multocida. Biochemistry. 2006;45(7):2139–2148. doi: 10.1021/bi0524013. [DOI] [PubMed] [Google Scholar]
- 28.Sharypova LA, Chataigné G, Fraysse N, Becker A, Poinsot V. Overproduction and increased molecular weight account for the symbiotic activity of the rkpZ-modified K polysaccharide from Sinorhizobium meliloti Rm1021. Glycobiology. 2006;16(12):1181–1193. doi: 10.1093/glycob/cwl042. [DOI] [PubMed] [Google Scholar]
- 29.Freiberger F, et al. Biochemical characterization of a Neisseria meningitidis polysialyltransferase reveals novel functional motifs in bacterial sialyltransferases. Mol Microbiol. 2007;65(5):1258–1275. doi: 10.1111/j.1365-2958.2007.05862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Ichikawa M, Takakura Y. Conserved amino acid sequences in the bacterial sialyltransferases belonging to Glycosyltransferase family 80. Biochem Biophys Res Commun. 2008;365(2):340–343. doi: 10.1016/j.bbrc.2007.10.201. [DOI] [PubMed] [Google Scholar]
- 31.Romanow A, et al. Dissection of hexosyl- and sialyltransferase domains in the bifunctional capsule polymerases from Neisseria meningitidis W and Y defines a new sialyltransferase family. J Biol Chem. 2014;289(49):33945–33957. doi: 10.1074/jbc.M114.597773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monegal A, Planas A. Chemical rescue of alpha3-galactosyltransferase. Implications in the mechanism of retaining glycosyltransferases. J Am Chem Soc. 2006;128(50):16030–16031. doi: 10.1021/ja0659931. [DOI] [PubMed] [Google Scholar]
- 33.Soya N, Fang Y, Palcic MM, Klassen JS. Trapping and characterization of covalent intermediates of mutant retaining glycosyltransferases. Glycobiology. 2011;21(5):547–552. doi: 10.1093/glycob/cwq190. [DOI] [PubMed] [Google Scholar]
- 34.Horenstein BA. Quantum mechanical analysis of an α-carboxylate-substituted oxocarbenium ion. Isotope effects for formation of the sialyl cation and the origin of an unusually large secondary 14C isotope effect. J Am Chem Soc. 1997;119(5):1101–1107. [Google Scholar]
- 35.Ni L, et al. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism. Biochemistry. 2007;46(21):6288–6298. doi: 10.1021/bi700346w. [DOI] [PubMed] [Google Scholar]
- 36.Lin LY-C, et al. Structure and mechanism of the lipooligosaccharide sialyltransferase from Neisseria meningitidis. J Biol Chem. 2011;286(43):37237–37248. doi: 10.1074/jbc.M111.249920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toukach PV. Bacterial carbohydrate structure database 3: Principles and realization. J Chem Inf Model. 2011;51(1):159–170. doi: 10.1021/ci100150d. [DOI] [PubMed] [Google Scholar]
- 38.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guindon S, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 41.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42(Web Server issue):W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasteiger E, et al. Protein identification and analysis tools on the ExPASy server. In: Walker J, editor. Proteomics Protocols Handbook. Humana Press; Totowa, NJ: 2005. pp. 571–607. [Google Scholar]
- 43.Phillips K, de la Peña AH. The combined use of the Thermofluor assay and ThermoQ analytical software for the determination of protein stability and buffer optimization as an aid in protein crystallization. Curr Protoc Mol Biol. 2011;Chapter 10:Unit10.28. doi: 10.1002/0471142727.mb1028s94. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero SA, Hecht HJ, Hofmann B, Biebl H, Singh M. Production of selenomethionine-labelled proteins using simplified culture conditions and generally applicable host/vector systems. Appl Microbiol Biotechnol. 2001;56(5-6):718–723. doi: 10.1007/s002530100690. [DOI] [PubMed] [Google Scholar]
- 45.Frirdich E, Vinogradov E, Whitfield C. Biosynthesis of a novel 3-deoxy-D-manno-oct-2-ulosonic acid-containing outer core oligosaccharide in the lipopolysaccharide of Klebsiella pneumoniae. J Biol Chem. 2004;279(27):27928–27940. doi: 10.1074/jbc.M402549200. [DOI] [PubMed] [Google Scholar]
- 46.Akiya S, Osawa T. Nitrogen-containing sugars. VI. On the N, N-phthaloyl derivatives of D-glucosamine. Chem Pharm Bull (Tokyo) 1960;8(7):583–587. [Google Scholar]
- 47.Song G, Liu H, Zhang W, Geng M, Li Y. Synthesis and biological evaluation of cytotoxic activity of novel anthracene L-rhamnopyranosides. Bioorg Med Chem. 2010;18(14):5183–5193. doi: 10.1016/j.bmc.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 48.Klonis N, Sawyer WH. Spectral properties of the prototropic forms of fluorescein in aqueous solution. J Fluoresc. 1996;6(3):147–157. doi: 10.1007/BF00732054. [DOI] [PubMed] [Google Scholar]
- 49.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 50.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 51.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev. 2010;74(3):341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jann B, Ahrens R, Dengler T, Jann K. Structure of the capsular polysaccharide (K19 antigen) from uropathogenic Escherichia coli O25:K19:H12. Carbohydr Res. 1988;177:273–277. doi: 10.1016/0008-6215(88)85064-x. [DOI] [PubMed] [Google Scholar]
- 54.Ward CK, Lawrence ML, Veit HP, Inzana TJ. Cloning and mutagenesis of a serotype-specific DNA region involved in encapsulation and virulence of Actinobacillus pleuropneumoniae serotype 5a: Concomitant expression of serotype 5a and 1 capsular polysaccharides in recombinant A. pleuropneumoniae serotype 1. Infect Immun. 1998;66(7):3326–3336. doi: 10.1128/iai.66.7.3326-3336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraysse N, et al. Sinorhizobium meliloti strain 1021 produces a low-molecular-mass capsular polysaccharide that is a homopolymer of 3-deoxy-D-manno-oct-2-ulosonic acid harboring a phospholipid anchor. Glycobiology. 2005;15(1):101–108. doi: 10.1093/glycob/cwh142. [DOI] [PubMed] [Google Scholar]
- 56.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 57.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ovchinnikova OG, et al. Localization and molecular characterization of putative O antigen gene clusters of Providencia species. Microbiology. 2012;158(Pt 4):1024–1036. doi: 10.1099/mic.0.055210-0. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, et al. Genetic analysis of the Cronobacter sakazakii O4 to O7 O-antigen gene clusters and development of a PCR assay for identification of all C. sakazakii O serotypes. Appl Environ Microbiol. 2012;78(11):3966–3974. doi: 10.1128/AEM.07825-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenfield LK, Whitfield C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr Res. 2012;356:12–24. doi: 10.1016/j.carres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 61.Merino S, Canals R, Knirel YA, Tomás JM. Molecular and chemical analysis of the lipopolysaccharide from Aeromonas hydrophila strain AH-1 (Serotype O11) Mar Drugs. 2015;13(4):2233–2249. doi: 10.3390/md13042233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oxley D, Wilkinson SG. Structural studies of glucorhamnans isolated from the lipopolysaccharides of reference strains for Serratia marcescens serogroups O4 and O7, and of an O14 strain. Carbohydr Res. 1988;175(1):111–117. doi: 10.1016/0008-6215(88)80161-7. [DOI] [PubMed] [Google Scholar]
- 63.Izquierdo L, Merino S, Regué M, Rodriguez F, Tomás JM. Synthesis of a Klebsiella pneumoniae O-antigen heteropolysaccharide (O12) requires an ABC 2 transporter. J Bacteriol. 2003;185(5):1634–1641. doi: 10.1128/JB.185.5.1634-1641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.