Significance
Exaggerated beta oscillations within the cortico-basal ganglia-thalamic (CBT) network are putative electrophysiological signatures of bradykinesia and rigidity in Parkinson’s disease (PD). However, it is unclear how exaggerated beta oscillations emerge and how such oscillation patterns are related to PD motor deficits. In this study, we demonstrate that a single cell type, the striatal cholinergic interneuron, mediates the emergence of exaggerated beta oscillations within CBT circuits of normal mice and induces parkinsonian-like motor deficits. Because the striatal cholinergic system is uninhibited by loss of dopamine, these results provide mechanistic insights into the therapeutic effects of anticholinergic drugs in the treatment of PD and highlight the potential for developing beta oscillation-based biomakers for PD.
Keywords: striatum, optogenetics, beta oscillations, coherence, cholinergic interneurons
Abstract
Cortico-basal ganglia-thalamic (CBT) neural circuits are critical modulators of cognitive and motor function. When compromised, these circuits contribute to neurological and psychiatric disorders, such as Parkinson’s disease (PD). In PD, motor deficits correlate with the emergence of exaggerated beta frequency (15–30 Hz) oscillations throughout the CBT network. However, little is known about how specific cell types within individual CBT brain regions support the generation, propagation, and interaction of oscillatory dynamics throughout the CBT circuit or how specific oscillatory dynamics are related to motor function. Here, we investigated the role of striatal cholinergic interneurons (SChIs) in generating beta and gamma oscillations in cortical-striatal circuits and in influencing movement behavior. We found that selective stimulation of SChIs via optogenetics in normal mice robustly and reversibly amplified beta and gamma oscillations that are supported by distinct mechanisms within striatal-cortical circuits. Whereas beta oscillations are supported robustly in the striatum and all layers of primary motor cortex (M1) through a muscarinic-receptor mediated mechanism, gamma oscillations are largely restricted to the striatum and the deeper layers of M1. Finally, SChI activation led to parkinsonian-like motor deficits in otherwise normal mice. These results highlight the important role of striatal cholinergic interneurons in supporting oscillations in the CBT network that are closely related to movement and parkinsonian motor symptoms.
Exaggerated beta oscillations (15–30 Hz) within the cortico-basal ganglia-thalamic (CBT) neural network are putative electrophysiological correlates of bradykinesia and rigidity in Parkinson’s disease (PD) (1–4). Therapies that effectively manage PD motor symptoms, such as dopamine replacement therapy and deep brain stimulation, are associated with a suppression of the exaggerated beta oscillations (4, 5). Beta oscillations are also found in the CBT circuits of patients with other movement-related disorders, such as epilepsy and dystonia (6, 7), and in normal, nonhuman primates (8, 9) and normal rodents (10, 11). Moreover, brief elevations (≤200 ms) of beta oscillations are observed in the basal ganglia of task-performing nonhuman primates and rodents during specific phases of behavioral tasks (10, 12, 13), indicating that beta oscillations may be important for motor and nonmotor functions. In contrast to the regulated temporal variability of beta oscillations in normal motor functions, temporal stability is correlated with the parkinsonian motor symptoms of bradykinesia and rigidity (2). Together, these findings suggest that brief epochs of beta oscillations are a normal aspect of basal ganglia dynamics, their temporal modulation is important for movement regulation, and loss of regulation or uncontrolled expression of beta oscillations may contribute to movement deficits, such as those observed in PD.
Despite the clear link between CBT beta oscillations and movement, the mechanisms underlying their generation remain elusive. Dopamine clearly modulates the generative mechanisms of CBT beta oscillations and beta frequency coherence between CBT structures. A hallmark of PD pathology is chronic reduction of dopamine input to CBT circuits due to midbrain dopaminergic neuron loss. Loss of midbrain dopamine increases beta oscillation power and coherence in PD animal models (14, 15). Similarly, acute reduction of dopamine locally in the striatum increases striatal beta oscillations (16). Systemic dopaminergic drugs decrease beta oscillation power and coherence in PD patients (17), and also increase finely tuned gamma oscillations in the basal ganglia and thalamus, although we know little about the functional significance of these finely tuned gamma oscillations in PD (17, 18). Because of the dense innervation of the striatum by the midbrain dopaminergic system (19), we have recently proposed that dopamine may modulate basal ganglia beta oscillations by acting on the striatum, in particular via the striatal cholinergic system. Dopamine, acting on D2 receptors, provides tonic suppression of acetylcholine (ACh) release in the striatum (20). Under conditions of low striatal dopaminergic tone, such as in PD, increased cholinergic tone may play a more prominent role in modulating striatal dynamics, such as supporting the generation of exaggerated beta oscillations (21–23). Additionally, the striatum contains high levels of cholinergic markers (24), highlighting a prominent role for cholinergic regulation in striatal function.
Recently, we demonstrated that direct local infusion of the cholinergic agonist carbachol into the striatum of normal mice can generate robust beta oscillations within the striatum (23). Using computational approaches, our previous study predicted that increased striatal cholinergic tone could lead to elevated beta oscillations through activation of muscarinic receptors that lead to the suppression of M current in the striatal medium spiny neurons. Here, to further demonstrate the function of the intrinsic striatal cholinergic system in modulating neural dynamics of the CBT network and the related receptor mechanisms, we selectively activated striatal cholinergic interneurons (SChIs) by using optogenetic techniques, while simultaneously recording local field potentials (LFPs) in the striatum and the primary motor cortex (M1), with or without local infusion of selective cholinergic receptor antagonist. We found that brief optogenetic stimulation of SChIs acutely elevated beta oscillations in the striatum and all cortical layers of M1, and gamma oscillations in the striatum and deeper layers of M1. Increased oscillations were accompanied by increased coherence between striatum and M1 in a layer-dependent manner. In addition, local striatal infusion of the muscarinic receptor blocker scopolamine, but not the nicotinic receptor blocker mecamylamine, reduced striatal beta oscillations. Finally, we assessed the effect of SChI stimulation on locomotion and found that activation of SChIs decreased movement, increased immobility, and increased rotation. Together, these results demonstrate that activation of SChIs can generate elevated beta and gamma oscillations and coherence in the cortico-striatal network in a cortical layer-dependent manner via muscarinic receptors, and alter locomotion behavior.
Methods Summary
Technical details are in SI Methods Summary. Briefly, all animal procedures were approved by the Boston University Institutional Animal Care and Use Committee (IACUC). Surgically implanted adult mice were recorded awake, head-fixed. Recordings were made simultaneously in the striatum [stereotactic coordinates 0 anterior-posterior (AP), 2.5–3 medial-lateral (ML), 2–2.5 depth] by using a glass pipette to avoid photoelectric effects during optogenetic laser illumination, and in the primary motor cortex (M1) by using laminar electrodes containing 16 electrode contacts spaced at 100 μm, positioned across the entire cortical depth (coordinates 1.5–2 AP, 1.25–1.5 ML). To optogenetically activate SChIs, a fiber was coupled to a 473-nm laser. All data analysis was performed in MATLAB. Statistics were performed in MATLAB or GraphPad Prism.
SI Methods Summary
Transgenic Mice and Surgeries.
All animal procedures were approved by the Boston University IACUC. A total of 54 adult mice, both males and females, were used in this study. Bacterial artificial chromosome transgenic mice that expressed Cre-recombinase under the control of the ChAT (GM24Gsat) were obtained from Mutant Mouse Regional Resource Center. ChAT transgenic mice were crossed with Ai32 mice (B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/Jjax; Jackson Laboratory) that contained a Lox-Stop-Lox-ChR2-EYFP gene (58). In the double-positive offspring ChAT-ChR2 transgenic mice, ChR2 proteins were selectively expressed in cholinergic neurons. Their littermates positive for the ChR2 gene and negative for the Cre-recombinase gene (thus not expressing ChR2 proteins) were used for control experiments (Ai32 control mice).
Stereotactic surgeries were performed under general isoflurane anesthesia. Custom-made head plates or implants were attached to the skull by using anchor screws and metabond (Parkell; S380) or dental acrylic (Stoelting; 51458). To be able to target the M1 and the striatum during later recordings, we marked the corresponding stereotactic coordinates during the surgery. Analgesic buprenorphine was administered as postoperative care for at least 48 h. All recording and behavioral experiments were performed after the animals recovered from surgery, typically 3–6 d after the surgery.
Electrophysiology Recording and Optogenetics.
All recordings were performed in awake, head-fixed mice. Striatal recording, drug infusion, and optogenetic stimulation were performed through a borosilicate glass electrode (impedance 1–5 mOhm, filled with saline, Warner Instruments; G100F-4) coupled to an optical fiber (200 µm in diameter) and an infusion cannula (200 µm outer diameter). The distances between the tips of the glass electrode, the optical fiber, and the infusion cannula were adjusted to be within 500 µm. The fiber-electrode-cannula bundle was inserted into the dorsal striatum [coordinates 0 AP, 2.5–3 ML, 2–2.5 depth] at a slow speed of 1–2 µm/s. Recordings in the M1 were made with a laminar probe containing 16 electrode contacts (100 µm spacing between contacts, Neuronexus Technologies; A1X16-10mm-100-703-A16), positioned across the entire M1 cortical depth (coordinates 1.5–2 AP, 1.25–1.5 ML). The top electrode contact was positioned just below the brain surface, whereas the deepest contact was approximately 1.5 mm from the brain surface.
Striatal recordings were performed with a Multiclamp 700b amplifier and digitized at 20 kHz by using Digidata1440A. M1 recordings were performed by using a Tucker Davis Technologies (TDT) multichannel recording system (RZ5D). LFPs were low-pass filtered at 3 kHz and then digitized at 3051.75 Hz. Recordings in the striatum and M1 were collected separately, and cross-registered during data analysis by using TTL pulses generated by a Digidata1440A and recorded by both the Digidata1440A and TDT systems. The most superficial electrode contact in M1 was excluded from further analysis because tissue damage at the brain surface during surgery or craniotomy may have impaired the signals collected on these electrode contacts in some animals. We also excluded the three deepest electrode contacts, which were deeper than 1.5 mm from the cortical surfaces and, thus, outside of M1.
Optogenetic laser stimulation was through an optical fiber (200 nm in diameter) coupled to a 473 nm laser (Shanghai laser; BL473T3-100FL). Laser light was pulsed at a Poisson-distributed 40 Hz for a total of 5 s, repeating every 30 s. We also tested the condition where laser light was left on for 5 s. In addition, we tested conditions in which laser stimulation lasted 1 s, either left on for 1 s, or pulsed at 50 or 100 Hz, or Poisson-distributed 4, 9, 20, or 40 Hz. Each pulse lasted 5 ms regardless of the pulse pattern. Laser power at the fiber tip was adjusted to 10 mW.
Cholinergic receptor antagonists or control ACSF (Tocris Bioscience) were infused into the striatum through a custom infusion cannula coupled to the striatal recording electrode as described above. Scopolamine hydrobromide (Sigma Aldrich; S0929, at 100 µg/µL, 0.5 µL), mecamylamine hydrochloride (Sigma Aldrich; M9020, at 10 µg/µL, 0.5 μL), or ACSF (0.5 µL) was infused by using a Harvard Apparatus infusion pump at a rate of 0.1 µL/min over 5 min. Before drug infusion, we performed 30 trials of optogenetic laser stimulation for assessing laser-evoked oscillations without drugs. Recording resumed 20–30 min after drug infusion to allow drug diffusion.
Electrophysiological Data Analysis.
All data analysis were performed by using MATLAB (Mathworks). LFPs were first bandpass filtered between 0.5 and 500 Hz with a second-order Butterworth filter to eliminate low frequency drifts, and then down-sampled to 250 Hz by using MATLAB’s resample function. The resulting LFPs were passed through a 60-Hz notch filter by using MATLAB’s iirnotch. To reduce edge artifacts, 300 ms of data were removed from the beginning and end of every trial for all subsequent analysis.
In awake, head-fixed recordings, there were occasional motion artifacts that influenced spectral analysis. Trials with artifacts were rejected according to the following method. LFPs recorded in each trial were first bandpass filtered with a second-order Butterworth filter from 1 to 100 Hz. Trials where LFPs had a maximum amplitude greater than 7.5 SDs from the mean or a minimum amplitude greater than 5 SDs from the mean were rejected.
At the onset of optogenetic laser stimulation, LFPs exhibited large deflections, which were likely due to synchronized current influx into stimulated neurons and their downstream targets. At the offset of optogenetic laser stimulation, LFPs also exhibited large deflections, likely due to the current efflux from activated neurons. Because these large LFP deflections interfere with spectral analysis, we excluded the 500 ms immediately after laser onset and offset from further analyses. To estimate the power spectrum and coherence for different M1 layers, recordings from different laminar electrode contacts were grouped according to anatomical locations based on the electrode intercontact distance and histological findings. We grouped the recordings into superficial layers (0–500 µm from the cortical surface, electrode contacts 2–6), middle layers (500–800 µm, contacts 7–10), and deep layers (900–1,200 µm, contacts 11–13).
Spectral analysis.
We used the multitaper Fourier method for all spectral analysis, because it reduces noise through spectral smearing by obtaining multiple independent estimates from the data. Spectrums and spectrograms were calculated by using the mtspectrumc and mtspecgramc functions of the Chronux toolbox, respectively (59). There was a frequency smear of ±2 Hz, corresponding to 17–19 tapers, depending on period length. For spectrograms, a sliding 1-s window with 20% overlap and three tapers was chosen. The log normalized power during and after laser illumination was computed from the natural logarithm of the quotient from division by the prelaser baseline power. The power spectrum was calculated by averaging across each electrode group (M1 only) and then across trials for each animal. The population power spectrum was obtained by averaging across animals. For the 1-s stimulation protocols used in Fig. S2, because of the 500-ms data removal immediately after laser onset and offset, we did not have enough time points left for power spectrum analysis during laser stimulation. Hence, we only examined the 1-s period starting 500 ms after laser offset.
Fig. S2.
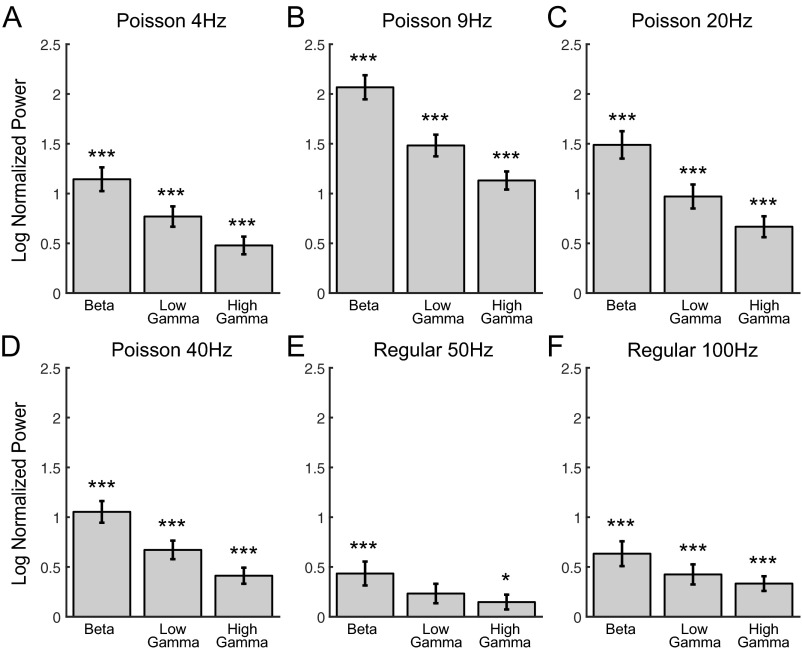
Optogenetic stimulation of SChIs increased striatal beta and gamma oscillatory power by using 1-s-long laser illumination patterns, pulsed at Poisson-distributed 4 Hz (A), Poisson-distributed 9 Hz (B), Poisson-distributed 20 Hz (C), Poisson-distributed 40 Hz (D), regular 50 Hz (E), and regular 100 Hz (F). These stimulation protocols elicited increases in beta (15–30 Hz), low-gamma (30–60 Hz), and high-gamma (60–100 Hz) oscillations in striatum. The bars are the natural logarithm of the quotient of the power during the 1-s period starting 500 ms after laser offset divided by the prelaser baseline power. The error bars represent the SEM. (*P < 0.05, ***P < 0.001, nonparametric signed-rank test).
For statistical testing, alpha was 0.05 and not corrected. For nondrug data, a Wilcoxon signed-rank test was performed against the null hypothesis of no laser-induced power change for each frequency band. Bar plot 95% confidence intervals were estimated with a bootstrap method by using 1,000 resamples with replacement. For the drug infusion bar plots, nonoverlap of the confidence intervals indicated a significant difference in laser-induced power changes preinfusion to postinfusion. For the scopolamine drug infusion bar plots, preinfusion and postinfusion were tested separately by using a bootstrap resampling test against 0 laser-induced power change because scopolamine reduced the prelaser baseline power. For mecamylamine and ACSF, baseline power was not affect by infusion, so preinfusion to postinfusion laser-induced power changes were compared with a paired signed-rank test.
Coherence analysis.
The coherence and coherograms were computed by averaging trials from all animals with Chronux’s coherency and cohgramc functions, respectively. The same frequency smear, window, and resulting tapers from the power analysis were used. For coherence, after a Fisher z-transformation, the prelaser baseline was subtracted from the coherence estimates for the other periods. Population estimates were calculated by averaging across M1 channel groups. The jackknifed 95% confidence intervals were used to test for significance of laser-induced coherence changes at the 0.05 alpha level.
Behavioral Analysis.
A custom-made optical fiber array containing four fibers, each 200 µm in diameter, was constructed to target a large fraction of the dorsal striatum unilaterally (location 1: AP 0.86 and ML 2, location 2: AP 0.5 and ML 2, location 3: AP 0.0 and ML 2.5, location 4: AP −0.46 and ML 2.8, all at dorsal-ventral −2.75) (27). The experimental group consisted of ChAT-ChR2 mice that selectively expressed ChR2 protein in cholinergic neurons. The control group consisted of littermate Ai32 mice, which do not express ChR2 proteins in any cells.
Mice were first acclimatized to an arena (diameter: 15 cm and height: 13 cm), for approximately 20 min/d for 2 d, before being tested with laser illumination. During experiments, mice were connected to the laser through a thin, long optical fiber to ensure free movement. Laser light was pulsed at a Poisson-distributed 40 Hz, identical to that used in the electrophysiology experiments, with a 5-ms pulse width and 10 mW power (measured at the tip of the fiber array before surgical implantation). A Logitech video camera monitored mice at 24–28 Hz from above. Locomotion behavior was assessed 8 min/d, over seven consecutive days.
Locomotion was analyzed offline in MATLAB. The dark mice were first identified against the white arena background by using adaptive thresholding. Morphological functions were then used to segment and locate the mouse head, body, and tail. We then identified the center of the body segment and calculated the movement speed and direction across frames. Immobility periods were defined as periods in which the average movement speed was below 1.5 cm/s, using a half-second moving-average window (34). Rotational behavior was manually quantified by categorizing every completed 360-degree rotation as either ipsilateral or contralateral, relative to the implanted hemisphere. The effect of laser illumination was quantified in 2-min epochs: 2 mins before laser onset (baseline), 2 mins during laser illumination (laser), 2 mins immediately after laser offset (post-laser 1), and 2–4 mins after laser offset (post-laser 2). Each measure of locomotion, including normalized distance traveled, percent time immobile, and movement speed when mobile, was assessed by using Bonferroni-corrected nonparametric paired signed-rank tests to determine the statistical significance of each period compared with the prelaser baseline period.
Histology.
Upon completion of electrophysiological recordings, mice were anesthetized by using pentobarbital and perfused with 4% (vol/vol) paraformaldehyde. The whole brains were postfixed overnight in 4% (vol/vol) paraformaldehyde, equilibrated with 30% (wt/vol) sucrose, and embedded in optimal cutting temperature compound. Coronal brain slices (50 μm thickness) containing the striatum were prepared by using a Cryostat (Microm HM 525). The slices were then stained by using Cresyl violet to identify the locations of striatal and motor cortex electrodes. For fluorescent immunocytochemistry experiments, the slices were blocked by using 10% (vol/vol) donkey serum, permeabilized with 0.2% Triton X solution for 1–2 h, and then incubated with goat anti-choline acetyltransferase primary antibody (1:250; Millipore AB144P) overnight at 4 °C. Brain slices were then washed three times with Tris buffer and incubated with donkey anti-goat secondary antibody (1:200; Life Technologies A11058) for 1–2 h. Finally, the slices were rinsed with Tris buffer and mounted on slides by using Vectorshield mounting agent (Vector Laboratories).
Results
Selective Optogenetic Activation of SChIs Increased Striatal Alpha, Beta, and Gamma Oscillations.
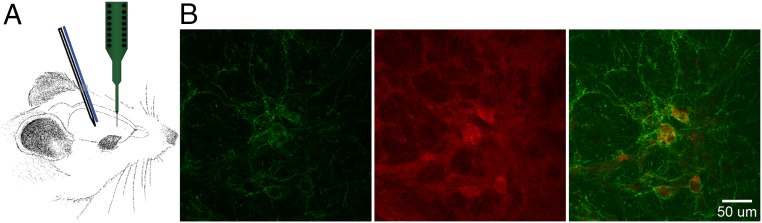
We recently demonstrated that the striatal cholinergic system can support the generation of beta oscillations by using a combination of mathematical modeling and experimental techniques that combined in vivo electrophysiology with local infusion of the nonselective cholinergic agonist carbachol (23). To further explore the ability of intrinsic SChIs in modulating beta oscillations throughout the CBT network, we recorded LFPs in the striatum and the primary motor cortex (M1), while optogenetically activating SChIs in transgenic choline acetyltransferase promoter (Chat)-Channelrhodopsin-2 (ChR2) mice (Fig. 1A). Chat-ChR2 mice were generated by crossing Chat-Cre mice with Cre-regulated ChR2-reporter mice (Ai32), resulting in selective ChR2 expression in Chat-positive SChIs (Fig. 1B). Control groups consisted of Ai32 transgenic littermates that did not express ChR2 protein because of the lack of Cre recombinase expression, despite the presence of ChR2 genes in the genome. An optical fiber coupled to a glass electrode was positioned in the dorsal striatum for simultaneous optogenetic stimulation and LFP recordings. Glass electrodes were used to record LFPs at the site of laser illumination to avoid photoelectric effects that are routinely observed on electrodes made of other materials (Fig. 1A) (25).
Fig. 1.
Experimental setup and protocols. (A) Illustration of the recording configuration. The recording pipette was coupled to an optical fiber and a laminar probe containing 16 electrode contacts, positioned in the M1. (B) A representative image of the striatum showing ChR2-eYFP fluorescence (green; Left), immunofluorescence of ChAT (red; Middle), and colocalization (Right).
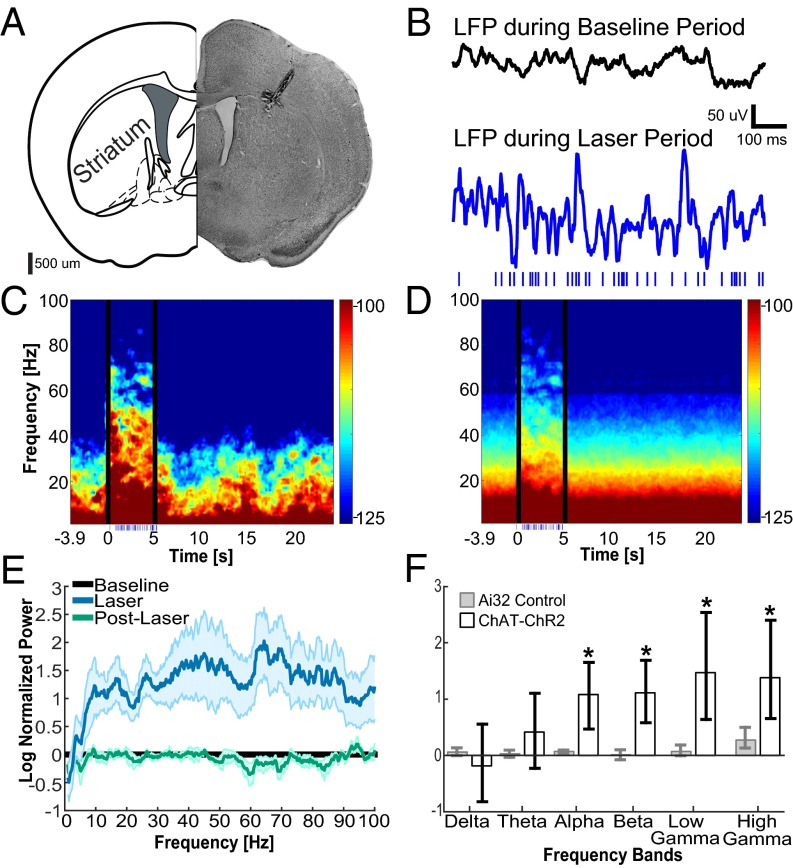
Optogenetic activation of SChIs with a Poisson-distributed 40-Hz laser light pulse train robustly increased oscillations across broad frequencies in the striatum (Fig. 2 C and D). The increase in oscillation power persisted for several hundred milliseconds after laser offset (Fig. 2 C and D), suggesting that direct patterning of SChIs by laser pulses was unlikely to account for the elevated oscillations at these frequencies. To further evaluate the changes at specific frequency bands, we calculated the normalized spectrum before, during, and after laser stimulation. We found that optogenetic stimulation of SChIs robustly increased oscillation power across higher frequency bands conventionally defined as alpha (8–15 Hz), beta (15–30 Hz), low gamma (30–60 Hz), and high gamma (60–100 Hz), but not lower frequency bands of delta (1–4 Hz) or theta (4–8 Hz) (Fig. 2 E and F; n = 7 mice). A similar laser illumination protocol in Ai32 control mice failed to alter oscillation power at any frequency (n = 5 mice, P > 0.05 for all bands, signed-rank test), confirming that the observed changes in Chat-ChR2 mice are due to optogenetic activation of SChIs.
Fig. 2.
Optogenetic activation of SChIs increased striatal alpha, beta, and gamma oscillations. (A) Representative coronal histological section showing an electrode and optical fiber track into the striatum. (B) Representative 1-s LFPs recorded in the striatum before (Top) and during (Bottom) laser stimulation at Poisson-distributed 40 Hz (Bottom; blue dashes indicate the time of laser pulses). (C) Representative spectrogram from one mouse, aligned to laser onset and averaged over all trials. The 500 ms immediately after laser onset and offset were excluded from the corresponding statistics because of strong LFP deflections. (D) Population spectrogram upon optogenetic stimulation of SChIs in ChAT-ChR2 mice (n = 7 mice). (E) Population power spectrum normalized to baseline, across frequencies before (Baseline, black), during (blue), and after laser stimulation (green), in the Chat-ChR2 mice (n = 7 mice). The shaded area around each solid line represents the SEM. (F) Bar plots comparing changes upon laser stimulation in different frequency bands between the Chat-ChR2 experimental group and the Ai32 control group for delta (1–4 Hz), theta (4–8 Hz), alpha (8–15 Hz), beta (15–30 Hz), low gamma (30–60 Hz), and high gamma (60–100 Hz) oscillations. The error bars represent the bootstrapped 95% confidence intervals (*P ≤ 0.05, nonparametric signed-rank test).
To further rule out the possibility that the observed oscillation changes were due to direct activation of SChIs, we tested a set of seven additional laser illumination parameters, including 5-s-long constant laser illumination, 1-s-long fixed interval pulse trains at 50 and 100 Hz, as well as Poisson-distributed pulse trains at 4, 9, 20, and 40 Hz. Because of the large LFP deflections induced by optogenetic stimulation of SChIs during the 1-s stimulation, it was difficult to reliably estimate the power changes during laser stimulation. Thus, we analyzed the 1-s period immediately following laser offset. In general, we observed similar increases in beta and gamma oscillations by using these stimulation patterns (Figs. S1B and S2; n = 5 mice, P ≤ 0.05, signed-rank test). Together, these results demonstrate that direct activation of SChIs generates robust beta and gamma oscillations within the striatum that are independent of the particular optogenetic stimulation pattern and persist for an extended period after the offset of light illumination, suggesting that these oscillations emerge from dynamic network interactions upon an increase in the striatal cholinergic tone.
Fig. S1.
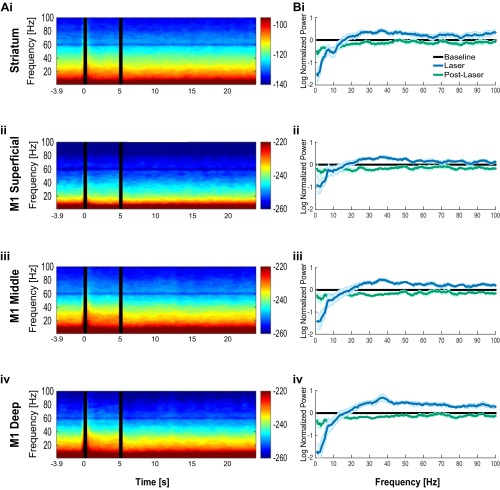
Oscillations induced by optogenetic stimulation of SChIs with a constant, 5-s-long laser illumination pattern. (A) Spectrograms before, during, and after optogenetic stimulation in striatum (i), as well as superficial (ii), middle (iii), and deep (iv) M1 layers. Laser was on at 0–5 s. (B) Normalized spectral power to prelaser baseline period in striatum (i), as well as the superficial (ii), middle (iii), and deep (iv) layers of M1. Constant light illumination patterns elicited increases in beta and gamma oscillations in striatum, as well as all layers of motor cortex. The shaded region represents the SEM.
SChI Activation Leads to Layer-Dependent Beta and Gamma Oscillation Changes in the M1.
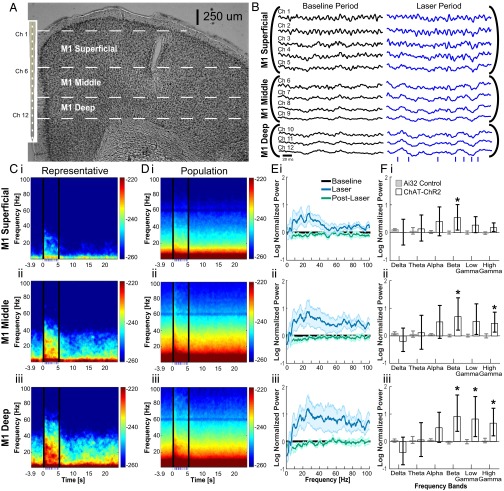
To understand the influence of the oscillations induced by optogenetic stimulation of SChIs on the rest of the CBT network, we performed simultaneous recordings in M1 by using a laminar electrode containing 16 contacts spaced at 100 μm, which can identify cortical layer-dependent changes (Fig. 3 A and B). We did not observe laser-induced photoelectric artifacts on the laminar electrodes in M1. Because they were ∼2 mm away from the striatal laser illumination site, <0.1% of laser light was expected to reach M1 (25). Optogenetic activation of SChIs in the striatum coincided with robust LFP voltage deflections across all cortical depths in M1 (Fig. 3B), and oscillation power consistently increased with cortical depth (Fig. 3C). To compare the difference in LFPs across anatomically defined cortical layers, we averaged the power spectra recorded at different depths, with the first 500 µm corresponding to the superficial layers, 500–800 µm corresponding to the middle layers, and 900–1,200 µm corresponding to the deep layers.
Fig. 3.
SChI activation led to layer-dependent beta and gamma oscillation changes in the M1. (A) Representative coronal section demonstrating the position of laminar electrodes in M1. (B) Representative 200-ms LFPs during the baseline period before laser simulation (black) and during laser stimulation period (blue). (C) Representative power spectrum from an individual mouse for superficial layers (C, i, averaged across Ch1–Ch5), middle layers (C, ii, averaged across Ch6–Ch9), and deep layers (C, iii, averaged across Ch10–Ch12). The 500 ms immediately after laser onset and offset were excluded from the corresponding statistics because of strong LFP deflections. (D) Population spectrograms aligned to laser onset for superficial layers (D, i), middle layers (D, ii), and deep layers (D, iii) (n = 7 mice). Bottom, blue dashes indicate the timing of laser light pulsed at Poisson-distributed 40 Hz, for 5 s. (E) Population spectrum for M1 superficial layers (E, i), middle layers (E, ii), and deep layers (E, iii). (F) Bar plots comparing oscillation powers at different frequencies in superficial (F, i), middle (F, ii), and deep (F, iii) layers for delta (1–4 Hz), theta (4–8 Hz), alpha (8–15 Hz), beta (15–30 Hz), low gamma (30–60 Hz), and high gamma (60–100 Hz). Error bars represent the bootstrapped 95% confidence intervals (*P ≤ 0.05, nonparametric signed-rank test).
Beta frequency power consistently increased in all M1 layers during SChI stimulation. In contrast, gamma frequency power, both high gamma and low gamma, were selectively increased in the deep layers, but not the superficial layers (Fig. 3 D–F). In the middle layers, high gamma power was elevated, but not low gamma power. We observed small but nonsignificant changes in delta, theta, and alpha frequencies across all layers. Together, these results provide direct evidence that SChI activation can enhance beta oscillations in all M1 layers, and gamma oscillations in a cortical layer-dependent manner. These SChI activation-induced beta and gamma oscillations across different M1 layers suggest that beta oscillations can be widely expressed by neuronal ensembles within different M1 cortical layers, whereas gamma oscillations are selectively expressed in deeper layers, although it is unknown how different cell types within each M1 layer uniquely support these oscillations.
SChI Activation Modulates Coherence Between the Striatum and M1.
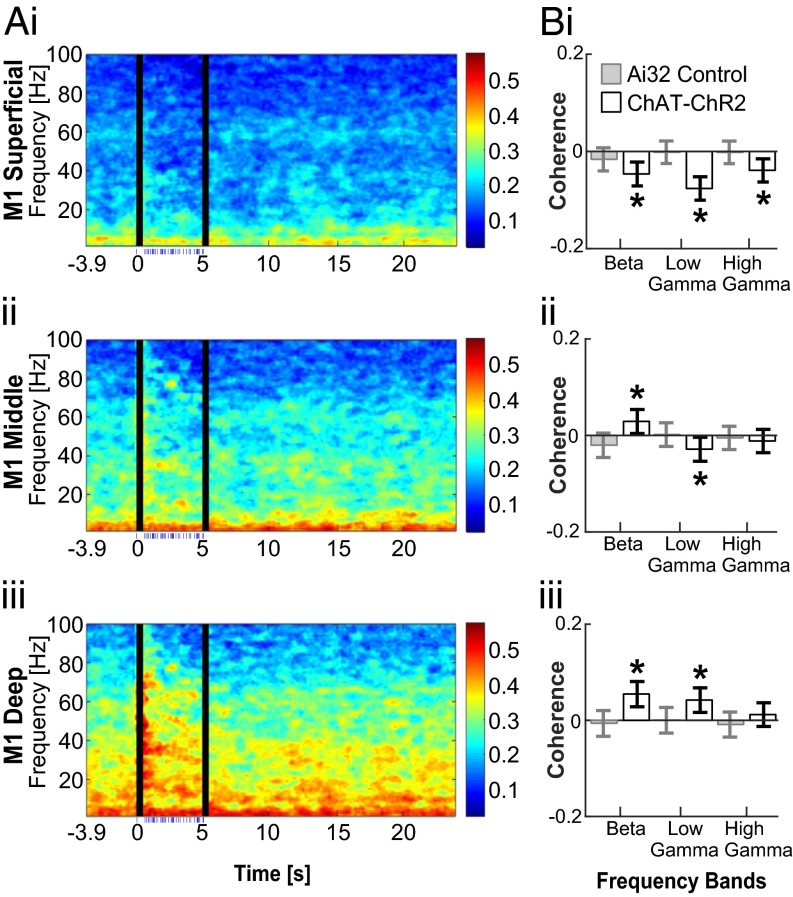
To evaluate the synchronization of oscillations between the striatum and M1 upon optogenetic activation of SChIs, we calculated the coherence of the LFPs recorded in these two structures before and during laser stimulation. We found an increase in coherence during laser stimulation between deep layers of M1 and the striatum at both beta and low gamma frequencies, but not at high gamma frequencies (Fig. 4; P ≤ 0.05, jackknife test). This increased coherence is consistent with the idea that deep M1 layers contribute to M1-striatal coordination through direct axonal projections. However, we note that although the coherence at beta frequencies below 23 Hz is maintained throughout the simulation, coherence at higher beta frequencies dissipates rapidly after the first second of stimulation, suggestive of multiple independent beta oscillations within the 15–30 Hz range defined here.
Fig. 4.
SChI activation modulated coherence between the striatum and M1. (A) Population coherograms show coherence between the striatum and superficial (i), middle (ii), and deep layers (iii) of M1 before, during, and after laser stimulation. Laser stimulation was pulsed at a Poisson-distributed 40 Hz, during 0–5 s (bottom, blue dashes indicate the timing of laser pulses, n = 7 mice). The 500 ms immediately after laser onset and offset were excluded from the corresponding statistics because of strong LFP deflections. (B) Bar plots comparing the coherence between the striatum and M1 superficial (B, i), middle (B, ii), and deep (B, iii) layers before and during laser stimulation for beta (15–30 Hz), low gamma (30–60 Hz), and high gamma (60–100 Hz) frequencies. Error bars indicate the jackknifed 95% confidence interval error bars (*P ≤ 0.05, nonparametric jackknife test).
The lack of coherence changes at high gamma frequencies suggests that the observed increase in high gamma power may be due to increased local neuronal activity within M1 (26). In the middle layer, where both beta and high gamma power increased, we observed a significant increase in coherence at beta frequency, but not at high gamma frequencies (Fig. 4). Considering that middle layers of M1 do not directly connect to the striatum, the observed increase in coherence at beta frequencies are likely coordinated or relayed through other structures, such as the thalamus that directly project to the middle layer, especially layer 4. Surprisingly, despite the increase in superficial layer beta power, activation of SChIs resulted in significant attenuation of coherence at beta and gamma frequencies (Fig. 4; P ≤ 0.05, jackknife test), suggesting that oscillations within the striatum and superficial M1 are independently regulated. In summary, activation of SChIs in the striatum appears to engage multiple independent beta generators that interact in the CBT network in a cortical layer-dependent manner, whereas high gamma oscillations are locally generated.
Striatal Muscarinic Receptors Mediate SChI-Induced Beta and Low Gamma Oscillations in the Corticostriatal Network.
To understand the receptor mechanism by which SChIs engage the striatum and M1 to generate beta and gamma oscillations, we combined optogenetic activation of SChIs with local intracranial infusion of selective cholinergic receptor antagonists. We infused either the muscarinic antagonist scopolamine (100 µg/µL) or the nicotinic antagonist mecamylamine (10 µg/µL) into the striatum, and optogenetically activated SChIs while simultaneously recording LFPs in the striatum and M1.
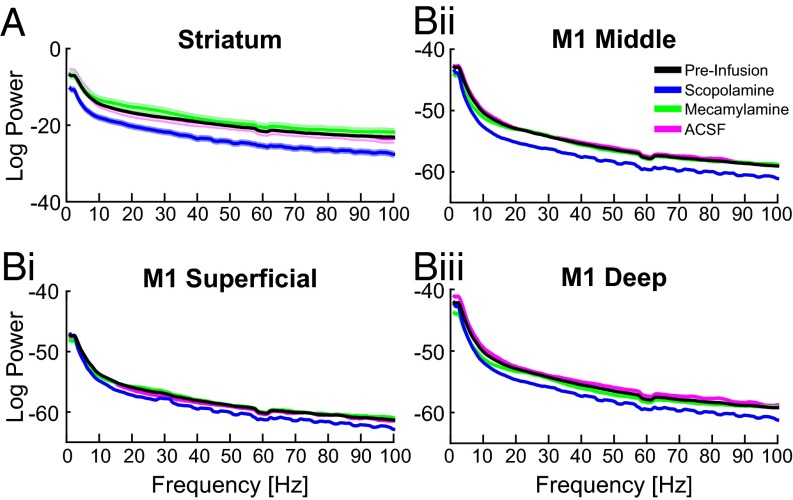
We observed that scopolamine infusion in the striatum broadly decreased oscillation power in the striatum across all frequencies analyzed (approximately from 2 to 100 Hz, Fig. 5A; n = 8 mice; >2 SEM). A similar reduction in oscillation power was also observed in the middle and deep layers of M1, although not at very low frequencies of ∼2–5 Hz, (Fig. 5B; n = 8 mice). Interestingly, no change in power was observed in the superficial layers of M1, suggesting that oscillatory dynamics in the superficial layers are only loosely coupled to the CBT network (Fig. 5 B, i). Together these results demonstrate that intrinsic striatal muscarinic tone is responsible for basal levels of oscillations across broad frequencies in the striatum and deeper layers of M1.
Fig. 5.
Striatal muscarinic receptors modulated basal levels of beta and gamma oscillations in the striatum and deeper layers of M1. After striatal drug infusion, population power spectrums in the striatum (A), and different layers of M1 [superficial (B, i), middle (B, ii), and deep layers (B, iii)]. The shaded area around each solid line represents the SEM. Scopolamine infusion reduced oscillation power in the striatum, middle layers of M1, and deep layers of M1.
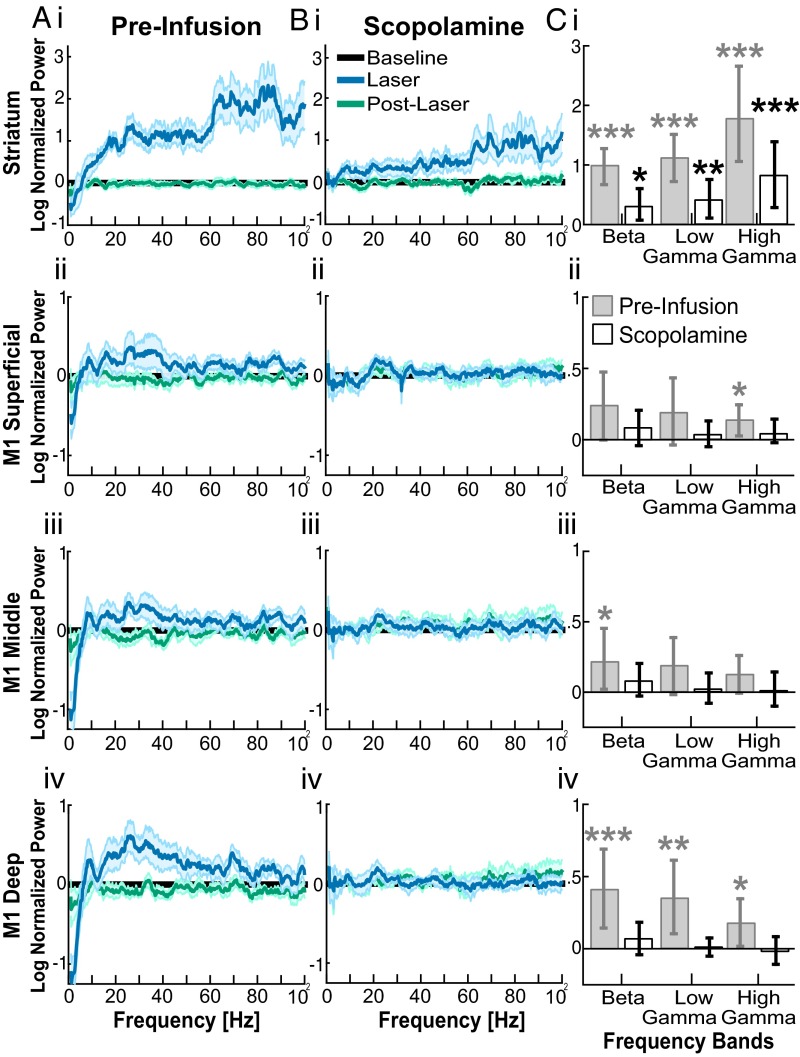
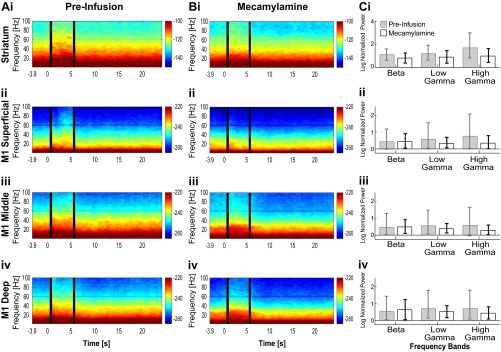
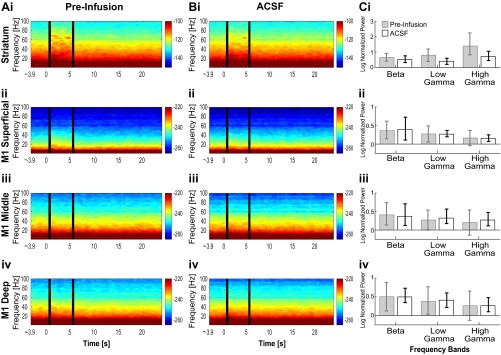
We next tested the effectiveness of optogenetic activation of SChIs in generating oscillations in the presence of muscarinic or nicotinic receptor blockers. Because of the reduction of oscillation power across all frequencies upon scopolamine infusion, we cannot directly compare the effects of optogenetic stimulation in the presence of scopolamine to that observed in the absence of scopolamine. We thus compared the effect of optogenetic activation of SChIs in the presence of scopolamine and found that activation of SChIs in the presence of the muscarinic blocker scopolamine remained effective at inducing beta, low gamma, and high gamma oscillations in the striatum (Fig. 6 B, i and C, i; P ≤ 0.05, bootstrap test). However, the increases were relatively weaker compared with that observed without scopolamine (Fig. 6 A, i). In M1, SChI stimulation in the presence of scopolamine failed to alter beta, low gamma, or high gamma power from the prestimulation baseline in any cortical layer (Fig. 6 B, ii–iv and C, ii–iv; P > 0.05, signed-rank test). Infusion of artificial cerebrospinal fluid (ACSF) or the nicotinic antagonist mecamylamine failed to alter oscillation power at any frequency in the striatum or M1, compared with the preinfusion baseline (Fig. 5) (Fig. S3, n = 6 mice infused with mecamylamine; and Fig. S4, n = 5 mice infused with ACSF; P > 0.05, signed-rank test). Together, these results suggest that cholinergically induced elevation of beta and gamma oscillations in the striatum is mediated by striatal muscarinic receptors, but not by striatal nicotinic receptors. The fact that in the presence of scopolamine, SChI activation only elevated striatal oscillations, but not M1 oscillations, suggests that striatal beta oscillations are independent of phasic beta input from M1, supporting the existence of a basal ganglia generator of beta oscillations. These results further suggest that the elevation in M1 beta oscillations depends on active muscarinic mechanisms in the striatum.
Fig. 6.
Striatal muscarinic receptors mediated SChI-induced beta and gamma oscillations in the striatum and M1. (A and B) Optogenetic stimulation induced changes in the power spectrum before (A) and after (B) scopolamine infusion (n = 8 mice), in the striatum (i), superficial (ii), middle (iii), and deep layers of M1 (iv). The power spectrum during laser stimulation (blue) and after laser stimulation (green) was normalized to the baseline (black). (C) Bar plot comparison of oscillation power changes in beta (15–30 Hz), low gamma (30–60 Hz), and high gamma (60–100 Hz) frequencies, before (gray) and after (white) scopolamine infusion. Error bars are the bootstrapped 95% confidence intervals (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; nonparametric bootstrap test).
Fig. S3.
Oscillations induced by optogenetic stimulation of SChIs, in the presence of the nicotinic receptor antagonist mecamylamine in the striatum (i) as well as superficial (ii), middle (iii), and deep (iv) M1 layers. (A) Preinfusion spectrogram demonstrating increased beta and gamma oscillations in the striatum, as well as elevated beta oscillations in all layers of M1 and gamma oscillations in deeper layers of M1. (B) Postinfusion spectrograms showing increased beta and gamma oscillations in striatum and M1. (C) Bar plot comparing SChI stimulation-induced oscillation changes between preinfusion and postinfusion periods. There were no significant changes in beta and gamma power when comparing premecamylamine with postmecamylamine infusion laser-induced power in the striatum. The error bars represent the 95% confidence intervals (*P < 0.05; ***P < 0.001; nonparametric paired signed-rank test). Beta, 15–30 Hz; low gamma, 30–60 Hz; high gamma, 60–100 Hz.
Fig. S4.
Oscillations induced by optogenetic stimulation of SChIs upon infusion with ACSF in the striatum (i) as well as the superficial (ii), middle (iii), and deep (iv) M1 layers. (A) Preinfusion spectrogram. (B) Postinfusion spectrogram. (C) Bar plot comparing laser-induced oscillation power normalized to the prelaser baseline during preinfusion and postinfusion period. Laser stimulation was effective at inducing beta and gamma oscillations. There were no significant differences between preinfusion and postinfusion in striatum. The error bars represent the 95% confidence intervals. (*P < 0.05; ***P < 0.001; nonparametric paired signed-rank test). Beta, 15–30 Hz; low gamma, 30–60 Hz; high gamma, 60–100 Hz.
Unilateral SChI Activation Decreases Locomotion and Increases Rotation Behavior.
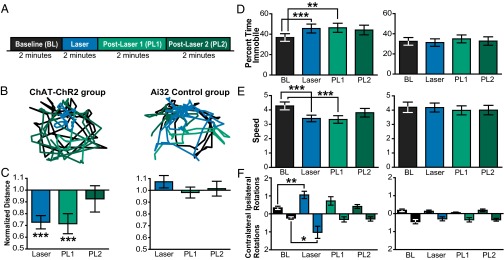
To evaluate whether a transient increase in striatal cholinergic tone is sufficient to alter locomotion, we optogenetically stimulated SChIs in another group of freely moving mice. To optimize the illumination volume, we designed a fiber array containing four optical fibers unilaterally targeting a large fraction of the dorsolateral striatum that receives M1 inputs (27) in Chat-ChR2 mice (n = 5 mice), or control Ai32 mice (n = 5 mice). We monitored the locomotion in a custom arena while optogenetically stimulating SChIs by using the same light illumination pattern at Poisson-distributed 40 Hz, but illuminated for 2 min to better quantify behavioral effects (Fig. 7A). Locomotion was monitored for 8 min/d, over 7 consecutive days.
Fig. 7.
Unilateral SChI activation decreased locomotion and increased rotation behavior. (A) Behavioral optogenetic experimental protocol consists of a baseline period, laser period, and two post-laser periods (2 min per period). (B) Representative positions of a ChAT-ChR2 mouse (Left) and a control Ai32 mouse (Right) before (black trace), during (blue trace), and post-laser periods (green traces) after laser illumination. (C–E) Bar plot comparison of locomotor activity measures, including baseline-normalized distance (C), percent time immobile (D), and movement speed when mobile (E). (F) Bar plot comparison of rotation in the Chat-ChR2 group (Left) and the control Ai32 group (Right) (n = 5 mice for Chat-ChR2 group; n = 5 mice for control Ai32 group). Error bars indicate the SEM (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; Bonferroni-corrected nonparametric paired signed-rank tests).
We first estimated the overall effect of stimulating SChIs on locomotion by calculating the total distance traveled during each 2-min period before, during, and after laser illumination and found that optogenetic activation of SChIs significantly reduced the total distance traveled compared with the pre-laser baseline period (Fig. 7 B and C; P ≤ 0.001, Bonferroni-corrected nonparametric paired signed-rank tests). This reduction persisted for an additional 2 min immediately after laser offset (post-laser 1) (P ≤ 0.001, paired signed-rank test) and returned to baseline in the subsequent 2-min interval (Post-Laser 2) (P > 0.05, paired signed-rank test). Further analysis indicated that locomotion reduction resulted from increased immobility, defined as the percentage of time when movement was smaller than 1.5 cm/s (Fig. 7D; P ≤ 0.001, paired signed-rank test), as well as decreased movement speed (Fig. 7E; P ≤ 0.001, paired signed-rank test). The same optogenetic analysis in control Ai32 mice did not produce any change in locomotion, immobility, or movement speed (n = 5, P > 0.05 for all periods analyzed, paired signed-rank test), confirming that the observed locomotion deficit was due to optogenetic activation of SChIs.
To further assess whether unilateral optogenetic stimulation of SChIs biases movement direction preference, we assessed rotation behavior. Interestingly, we found that stimulation of SChIs in one hemisphere resulted in a significant increase in both ipsilateral and contralateral rotations during the laser period (Fig. 7F; P ≤ 0.01 and P ≤ 0.05, respectively, paired signed-rank test). Similar analysis in control Ai32 mice did not yield any change in rotation behavior (P > 0.05, paired signed-rank tests). In the context of our experimental protocol, these results suggest that optogenetic activation of SChIs is not sufficient to bias movement direction preference in freely moving mice, but instead leads to an overall increase in rotation behavior. In summary, our results suggest that SChIs are critically involved in modulating locomotion, and that a selective increase in striatal cholinergic tone leads to direct motor behavioral deficits.
Discussion
We tested the role of intrinsic physiological striatal ACh release in modulating beta and gamma oscillations within cortico-striatal circuits. Combining optogenetics, pharmacology, electrophysiology, and behavioral assays in mice, we show that SChI activation reliably and reversibly increased alpha, beta, and gamma oscillations in the striatum. In M1, SChI stimulation increased beta power in all layers and gamma power in a layer-dependent manner. SChI-induced striatal and M1 beta and low gamma oscillations critically depended on striatal muscarinic receptors. Furthermore, SChI activation correlated with decreased locomotion and increased rotation behavior. Together, these results demonstrate that SChIs mediate changes in beta and gamma oscillations within the CBT circuit that are relevant to movement and movement deficits.
Activation of SChIs selectively increased higher frequency oscillations in the striatum, including alpha, beta, and gamma oscillations, but not lower frequency theta or delta oscillations. In our previous work, we found beta oscillations were elevated in striatum in response to the local infusion of the cholinergic agonist, carbachol (23). However, this study did not look at gamma oscillations and, thus, more work is needed to determine whether carbachol also elevates gamma frequency rhythms in striatum. In M1, SChI activation increased beta oscillations in all layers, and gamma oscillations only in middle and deep layers. Interestingly, despite the increase in beta, low gamma, and high gamma oscillations in the striatum and M1 deeper layers, the coherence between these two structures remained unchanged at high gamma frequencies during SChI activation, further confirming that these oscillations are supported by distinct mechanisms, with high gamma oscilations likely representing a local increase in neuronal excitability (26). Using conventionally defined frequency bands, we find SChI stimulation increased alpha oscillations (8–15 Hz) in striatum but not in M1. Interestingly, the PD motor symptoms of bradykinesia and rigidity are correlated with elevated beta oscillations in basal ganglia that can extend down to 8 Hz and, thus, includes the alpha frequency range (4, 28).
To determine the contributions of cholinergic receptor subtypes toward beta oscillation generation, we incorporated pharmacological techniques into our present study. Local infusion of scopolamine, a muscarinic receptor antagonist, in the striatum not only reduced oscillation powers across all frequencies analyzed, but also drastically reduced the evoked beta and gamma oscillations upon optogenetic stimulation of SChIs.
In contrast, scopolamine did not alter SChI-induced high gamma oscillations, suggesting a mechanistic separation between the generators of beta/low gamma and high gamma oscillations. In contrast, the nicotinic receptor antagonist mecamylamine failed to change SChI-induced elevation of beta, low gamma, or high gamma oscillations in either the striatum or M1. These results demonstrate that SChIs exert rapid and powerful control over the dynamic generation of beta and gamma oscillations both within the striatum and throughout the CBT network through muscarinic mechanisms.
Beta frequency oscillations are found in the basal ganglia and the cortex of PD patients (29, 30) and PD animal models (15, 29, 31). Beta elevation correlates with the PD motor symptoms of bradykinesia and rigidity. The multiple plastic changes seen throughout the CBT loop due to chronic loss of dopamine are not present in our current experiments, and we do not consider the SChI stimulation state equivalent to the parkinsonian state. Indeed SChI stimulation-induced gamma oscillations and contralateral rotational behavior are not observed in parkinsonian states. Nevertheless, it is interesting that some parkinsonian phenomenology (elevated beta and hypokinetic movement) was evident during SChI stimulation despite normal dopaminergic function and lack of parkinsonian plastic changes. Interestingly, one recent study demonstrated that optogenetic inhibition of SChIs alleviated some parkinsonian motor symptoms in 6-hydroxydopamine (6-OHDA) mice (32). Together, these findings suggest that the CBT network components that modulate beta oscillations in normal states may be operational, but unmodulated, in PD states.
Striatal manipulations often produce alterations in behavior that generally can be classified as either hyperkinetic or hypokinetic. Examples of striatal perturbations resulting in hyperkinesia include inhibition of striatal fast spiking (FS) interneurons (33), optogenetic stimulation of D1 medium spiny neurons (MSNs) (34), and intrastriatal infusion of amphetamine (35). In contrast, hypokinetic movements are produced by optogenetic stimulation of striatal D2 MSNs (34). Here, we demonstrated that stimulation of SChIs also resulted in hypokinetic movements, highlighting a prominent role of SChIs in modulating basal ganglia network dynamics. We note that a recent study reported a lack of movement changes upon optogenetic simulation of SChIs in virally labeled animals (32). It is possible that sufficient activation of SChIs is needed to produce an observable behavioral effect, and our experimental conditions using transgenic mice may allow us to activate sufficient numbers of SChIs to bias behavior.
Interestingly, we observed unexpected changes in rotational behavior in both directions after unilateral SChI stimulation. It is possible that unilateral stimulation of SChIs could engage not only the ipsilateral CBT loop but also the contralateral CBT loop through M1 bilaterally projecting intratelencephalic neurons (36). Alternatively, because both ACh and glutamate are released from SChIs (37) and can potentially activate both D1 and D2 populations of MSNs, it is also possible that the direction of rotation depends on the relative excitation level of these two populations.
Although the source of the exaggerated beta oscillations in PD has not yet been established, we suggest here that it may consist of multiple, interacting sources. At least two independent beta generators are suggested by the distinct time course of coherence between deep layers of M1 and the striatum upon SChI activation, with coherence at higher beta frequencies of ∼22–30 Hz increasing and then rapidly dissipating, and coherence at lower beta frequencies of ∼15–22 Hz staying elevated throughout SChI activation. The dissipating coherence in the high beta frequency range despite consistently elevated beta power suggests at least two independent sources of high frequency beta, possibly one from basal ganglia networks and another in M1. An independent beta generator in M1 is consistent with in vitro work, demonstrating that deep layers of M1 can generate high frequency beta oscillations (∼27 Hz) in layer V in the presence of carbachol and kainate (38). Additionally, we observed decreased coherence between striatum and superficial M1 throughout the entire beta frequency range (15–30 Hz) despite significant increases in beta power in these structures upon SChI stimulation, suggesting an additional source of beta oscillations within the CBT loop. It has been observed that both high and low frequency beta oscillations are elevated in the basal ganglia in parkinsonian states and are correlated with different PD motor symptoms (39). The observed dissociation of different frequency beta oscillations here, in normal mice with elevated striatal cholinergic tone, may indicate possible distinct network sources that generate PD-related beta-band oscillations.
Whereas the literature proposes several putative mechanisms of CBT loop beta oscillation generation, our previous work suggests that networks of striatal MSNs can produce beta oscillations with sufficient MSN excitation (23). Because muscarinic receptors can increase MSN excitability by decreasing the M-current, our computational model predicts that muscarinic receptor blockade will interfere with the beta-producing mechanism in the striatum. This mechanism is supported by the experimental results of the present study demonstrating that striatal muscarinic blockade significantly decreases SChI stimulation-induced beta oscillations in the striatum. If the beta-producing mechanism of high striatal cholinergic tone plays a role in PD, our work suggests that modalities reducing striatal cholinergic tone may be instrumental in alleviating excessive beta oscillations in the parkinsonian CBT loop, along with their correlated motor symptoms. In fact, systemic antimuscarinic drugs were the sole pharmacologic treatment for PD until the late 1960s, when l-dopa was introduced (40), although their clinical use is limited by neuropsychiatric and cognitive side effects (41).
The literature proposes two other origins of pathologic beta oscillations in PD: the subthalamic nucleus (STN)-globus pallidus externa (GPe) circuit (42) and M1 (38). The former hypothesis depends on the presence of plastic changes attributable to chronic loss of dopamine in the STN-GPe network. Such mechanisms may be applicable to the parkinsonian state. However, our results suggest that neither chronic plastic changes nor chronic low dopamine is needed to produce robust beta oscillations within the CBT loop upon SChI stimulation. We note that a similar finding is evident in M1, where robust beta oscillations can emerge in nonparkinsonian M1 slices in the presence of sufficient excitation with carbachol and kainate (38, 43). Excitation of M1 neurons occurs in response to increased D1 MSN spiking and can also occur transiently to a subset of M1 neurons in response to D2 MSN spiking (44). To what extent M1 generated beta oscillations are induced under the conditions of SChI stimulation is an interesting topic of future study. Excitation to M1 during SChI stimulation may depend on the amount and timing of excitation provided to D1 versus D2 MSNs (44).
Recent studies (45, 46) showed that microinfusions of GABAergic antagonists in GPe failed to reduce beta oscillatory activities at the site of infusion in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin-induced Parkinsonian monkeys, although infusion of glutamatergic antagonists in GPe and STN reduced beta oscillations. These results are consistent with the view that the striatum is a source of beta oscillations, whereas the STN-GPe network serves as an amplifier of the striatally generated oscillations. In our framework, mechanisms that decrease the activity of STN or GPe will compromise the beta amplification mechanism and, thus, decrease beta oscillations. Correspondingly, mechanisms that increase the connectivity of STN and GPe (e.g., suppression of GPe lateral inhibition with GABAergic antagonist) may increase beta oscillations. This observation, taken together with the fact that microinfusion of GABAergic antagonists blocks a small amount of striatal inputs to GPe, suggests that substantial change to GPe beta oscillatory activity would not be expected with the microinfusion experiments performed in refs. 45 and 46.
Although much of the oscillatory spectrum in striatum is elevated during SChI stimulation, these elevations do not occur across the entire frequency range and, thus, are not considered a broadband increase in power. In contrast, scopolamine infusion decreased power at all frequencies, as expected with a broadband decrease (Fig. 5A). However, in the presence of scopolamine, SChIs remained as effective as in the absence of scopolomine in elevating high gamma oscillations, but not beta or low gamma, suggesting a mechanistic separation between the generators of these frequencies. Additionally, coherence between striatum and the deep layers of M1 increased for beta and low gamma, but not high gamma, despite increases in power in both structures in all these frequency bands. Thus, beta and low gamma elevations appear to be both selectively elevated and coordinated in the CBT circuit during SChI stimulation, whereas high gamma may represent increased neuronal excitability (26).
SChI activation also produced electrophysiological effects that are not commonly observed in PD, including gamma power (30–100 Hz) elevation in cortex and striatum. Finely tuned gamma oscillations in the range of 60–90 Hz are a consistent feature of basal ganglia and thalamic recordings (47) that are diminished in PD patients in the absence of dopaminergic replacement (17, 18). However, rather than an increase in finely tuned gamma, here we observed a broader-band gamma power increase, which may represent a general increase in neuronal activity (26). Laser-induced striatal high gamma oscillations remained elevated after either muscarinic or nicotinic blockade, suggesting that ACh is not involved in the generation of striatal high gamma oscillations. Glutamate, which is also coreleased from SChIs, may be responsible for the increase in high gamma oscillations during SChI stimulation (37). Striatal low gamma oscillations, however, are more correlated with beta in our current study, consistent with that observed in the basal ganglia of rats (10). While it is unclear how low gamma is generated in the CBT circuits, evidence from the ventral striatum of rats have suggested that low and high gamma have distinct sources, with dopaminergic drugs selectively diminishing the power of low gamma and enhancing the power of high gamma (48). In addition, low and high gamma oscillations are increased at different times during a spatial decision task (49), likely coordinated by different sets of FS interneurons (48, 49). Further work is required to delineate the mechanisms that result in increased M1 low and high gamma oscillatory activity due to increased striatal cholinergic tone.
Interneurons represent a small proportion of striatum, but these neurons can strongly modulate striatal output. The proportion of interneurons in striatum is 4–5% in mouse, >23% in monkey (50), and up to at least 25% in human (50), suggesting a more prominent role for striatal interneurons in nonhuman primates and humans. The majority of GABAergic interneurons in the striatum are parvalbumin-positive FS interneurons, which project primarily to the MSNs. Although FS interneurons are excited by nicotinic agonists (51), optogenetic activation of SChIs failed to increase FS interneuron spiking in one study (52). Additionally, optogenetic stimulation of SChIs in ChAT-ChR2 mice elicits both fast and slow inhibitory postsynaptic potentials in MSNs by nicotinic-mediated GABA release from dopamine projections to striatum (53) and neuropeptide Y-expressing neurogliaform GABAergic interneurons, resulting in inhibition of MSNs (52). However, nicotinic receptors generally quickly desensitize and, thus, we did not expect GABAergic blockade of MSN spiking to continue through the 5 s of stimulation used in our current study. Accordingly, our study reveals that nicotinic receptor blockade by mecamylamine did not change the spectral profile of either beta or gamma oscillations in the striatum or cortex over 5-s periods. This result suggests that SChI-induced GABA release does not exert long-term control over the SChI-induced oscillations along the corticostriatal circuit.
In contrast, we show that SChI-induced elevation of beta and low gamma oscillations depends on muscarinic receptors, because striatal scopolamine infusion lowered SChI-induced spectral power elevations of these oscillations. ACh acts through muscarinic receptors present on MSNs to decrease the activity of KCNQ (M-current) and Kir2.3 channels, thereby increasing MSN excitability (54, 55). In addition, ACh also works through muscarinic receptors to inhibit the release of glutamate from corticostriatal terminals and GABA from striatal FS cells (51, 56). Thus, muscarinic receptor activity diminishes the impact of glutamatergic and GABAergic input on MSNs while simultaneously increasing the excitability of MSNs. Therefore, muscarinic receptor activation could potentially accentuate the intrinsic dynamics of the MSN network.
It is surprising that stimulation of a single interneuron type, SChIs, can reversibly reproduce some of the key electrophysiological and behavioral manifestations of PD in normal mice, including increased beta oscillations in corticostriatal circuits, increased coherence between the cortex and the basal ganglia, and decreased mobility. SChI stimulation also increased rotational behavior, a phenotype often observed in mice rendered parkinsonian by a 6-OHDA lesion (57). These results suggest that the exaggerated beta oscillations in PD may reflect an uncontrolled expression of a normal dynamical state of the CBT network, a state that is directly modulated by SChI excitability.
In summary, we provide evidence supporting the existence of a beta frequency pacemaker within the CBT loop that can be activated by stimulation of striatal cholinergic interneurons via striatal muscarinic receptors. Combined with our findings of behavioral deficits similar to those in PD upon stimulating this pacemaker, our results suggest that the beta oscillations in PD may be an overexpression of normal CBT network dynamics due to a striatal dopamine/ACh imbalance, rather than a de novo oscillation due to a PD-induced network pathology.
Acknowledgments
We thank Kimberley H. Ching for assistance with immunocytochemistry and illustration of recording configuration, Moona Abdulkerim for assistance with behavioral experiments, members of the X.H. laboratory and N.K. laboratory for various technical support and suggestions over the course of the study, and our reviewers for their helpful comments and suggestions. X.H. acknowledges funding from NIH Director’s New Innovator Award 1DP2NS082126, NINDS Grant 1R21NS078660, Pew Foundation, Alfred P. Sloan Foundation, Boston University Biomedical Engineering Department, and Boston University Photonic Center. X.H. and M.M.M acknowledge CRCNS NIH Grant 1R01NS081716, and N.K. acknowledges NSF DMS-1042134-5.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605658113/-/DCSupplemental.
References
- 1.Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17(6):656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Little S, Pogosyan A, Kuhn AA, Brown P. β band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp Neurol. 2012;236(2):383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P, Williams D. Basal ganglia local field potential activity: Character and functional significance in the human. Clinical Neurophysiol. 2005;116(11):2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23(7):1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- 5.Kühn AA, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrojo Ruiz M, Brücke C, Nikulin VV, Schneider GH, Kühn AA. Beta-band amplitude oscillations in the human internal globus pallidus support the encoding of sequence boundaries during initial sensorimotor sequence learning. Neuroimage. 2014;85(Pt 2):779–793. doi: 10.1016/j.neuroimage.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 7.Sochurkova D, Rektor I. Event-related desynchronization/synchronization in the putamen. An SEEG case study. Exp Brain Res. 2003;149(3):401–404. doi: 10.1007/s00221-003-1371-2. [DOI] [PubMed] [Google Scholar]
- 8.Bartolo R, Merchant H. β oscillations are linked to the initiation of sensory-cued movement sequences and the internal guidance of regular tapping in the monkey. J Neurosci. 2015;35(11):4635–4640. doi: 10.1523/JNEUROSCI.4570-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23(37):11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leventhal DK, et al. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73(3):523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43(6):883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Feingold J, Gibson DJ, DePasquale B, Graybiel AM. Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks. Proc Natl Acad Sci USA. 2015;112(44):13687–13692. doi: 10.1073/pnas.1517629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16(8):1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharott A, et al. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci. 2005;21(5):1413–1422. doi: 10.1111/j.1460-9568.2005.03973.x. [DOI] [PubMed] [Google Scholar]
- 15.Mallet N, et al. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28(18):4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa RM, et al. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron. 2006;52(2):359–369. doi: 10.1016/j.neuron.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Brown P, et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci. 2001;21(3):1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempf F, et al. Gamma activity and reactivity in human thalamic local field potentials. Eur J Neurosci. 2009;29(5):943–953. doi: 10.1111/j.1460-9568.2009.06655.x. [DOI] [PubMed] [Google Scholar]
- 19.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBoer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996;317(2–3):257–262. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- 21.Ikarashi Y, Takahashi A, Ishimaru H, Arai T, Maruyama Y. Regulation of dopamine D1 and D2 receptors on striatal acetylcholine release in rats. Brain Res Bull. 1997;43(1):107–115. doi: 10.1016/s0361-9230(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 22.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9(6):832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy MM, et al. Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proc Natl Acad Sci USA. 2011;108(28):11620–11625. doi: 10.1073/pnas.1107748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30(10):545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Han X, et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62(2):191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22(18):8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kühn AA, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215(2):380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Stein E, Bar-Gad I. β oscillations in the cortico-basal ganglia loop during parkinsonism. Exp Neurol. 2013;245:52–59. doi: 10.1016/j.expneurol.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Pollok B, et al. Motor-cortical oscillations in early stages of Parkinson’s disease. J Physiol. 2012;590(13):3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devergnas A, Pittard D, Bliwise D, Wichmann T. Relationship between oscillatory activity in the cortico-basal ganglia network and parkinsonism in MPTP-treated monkeys. Neurobiol Dis. 2014;68:156–166. doi: 10.1016/j.nbd.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurice N, et al. Striatal cholinergic interneurons control motor behavior and basal ganglia function in experimental parkinsonism. Cell Reports. 2015;13(4):657–666. doi: 10.1016/j.celrep.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Gittis AH, et al. Selective inhibition of striatal fast-spiking interneurons causes dyskinesias. J Neurosci. 2011;31(44):15727–15731. doi: 10.1523/JNEUROSCI.3875-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Rebec GV. Neuronal and behavioral correlates of intrastriatal infusions of amphetamine in freely moving rats. Brain Res. 1993;627(1):79–88. doi: 10.1016/0006-8993(93)90751-8. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14(4):278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higley MJ, et al. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6(4):e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamawaki N, Stanford IM, Hall SD, Woodhall GL. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience. 2008;151(2):386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Toledo JB, et al. High beta activity in the subthalamic nucleus and freezing of gait in Parkinson’s disease. Neurobiol Dis. 2014;64:60–65. doi: 10.1016/j.nbd.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Fox SH. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs. 2013;73(13):1405–1415. doi: 10.1007/s40265-013-0105-4. [DOI] [PubMed] [Google Scholar]
- 41.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J Neurosci. 2010;30(37):12340–12352. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lacey MG, et al. Spike firing and IPSPs in layer V pyramidal neurons during beta oscillations in rat primary motor cortex (M1) in vitro. PLoS One. 2014;9(1):e85109. doi: 10.1371/journal.pone.0085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldenburg IA, Sabatini BL. Antagonistic but not symmetric regulation of primary motor cortex by basal ganglia direct and indirect pathways. Neuron. 2015;86(5):1174–1181. doi: 10.1016/j.neuron.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci. 2004;24(33):7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tachibana Y, Iwamuro H, Kita H, Takada M, Nambu A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur J Neurosci. 2011;34(9):1470–1484. doi: 10.1111/j.1460-9568.2011.07865.x. [DOI] [PubMed] [Google Scholar]
- 47.Jenkinson N, Kühn AA, Brown P. γ oscillations in the human basal ganglia. Exp Neurol. 2013;245:72–76. doi: 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Berke JD. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur J Neurosci. 2009;30(5):848–859. doi: 10.1111/j.1460-9568.2009.06843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Meer MA, Redish AD. Low and high gamma oscillations in rat ventral striatum have distinct relationships to behavior, reward, and spiking activity on a learned spatial decision task. Front Integr Nuerosci. 2009;3:9. doi: 10.3389/neuro.07.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graveland GA, Williams RS, DiFiglia M. A Golgi study of the human neostriatum: Neurons and afferent fibers. J Comp Neurol. 1985;234(3):317–333. doi: 10.1002/cne.902340304. [DOI] [PubMed] [Google Scholar]
- 51.Koós T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22(2):529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.English DF, et al. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2011;15(1):123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nelson AB, et al. Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron. 2014;82(1):63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25(32):7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen W, et al. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10(11):1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- 56.Higley MJ, Soler-Llavina GJ, Sabatini BL. Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci. 2009;12(9):1121–1128. doi: 10.1038/nn.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175(2):303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 58.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: A platform for analyzing neural signals. J Neurosci Methods. 2010;192(1):146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]