Significance
Microphthalmia-associated transcription factor (MITF) is a master regulator of survival of the melanocyte lineage, exerting its effects through a cascade of transcriptional activation by interacting with a consensus DNA-binding sequence on the promoters of target genes. This study found that MITF regulates expression of bromodomain plant homeodomain finger transcription factor (BPTF), which has been recently shown to promote melanoma cell growth and metastasis, and serves as a prognostic marker of survival associated with melanoma. These results suggest that MITF directs its prosurvival program in melanoma cells, at least in part by activating BPTF expression, providing additional insight into the MITF signaling cascade. The participation of BPTF in the MITF pathway suggests a critical role for BPTF in maintaining melanoma cell survival, further supporting its role in melanoma progression.
Keywords: melanoma, signaling cascade, oncogenes
Abstract
Microphthalmia-associated transcription factor (MITF) plays a critical and complex role in melanocyte transformation. Although several downstream targets of MITF action have been identified, the precise mechanisms by which MITF promotes melanocytic tumor progression are incompletely understood. Recent studies identified an oncogenic role for the bromodomain plant homeodomain finger transcription factor (BPTF) gene in melanoma progression, in part through activation of BCL2, a canonical target of MITF signaling. Analysis of the BPTF promoter identified a putative MITF-binding site, suggesting that MITF may regulate BPTF expression. Overexpression of MITF resulted in up-regulation of BPTF in a panel of melanoma and melanocyte cell lines. shRNA-mediated down-regulation of MITF in melanoma cells was accompanied by down-regulation of BPTF and BPTF-regulated genes (including BCL2) and resulted in reduced proliferative capacity of melanoma cells. The suppression of cell growth mediated by MITF silencing was rescued by overexpression of BPTF cDNA. Binding of MITF to the BPTF promoter was demonstrated using ChIP analysis. MITF overexpression resulted in direct transcriptional activation of BPTF, as evidenced by increased luciferase activity driven by the BPTF promoter. These results indicate that BPTF transduces key prosurvival signals driven by MITF, further supporting its important role in promoting melanoma cell survival and progression.
Microphthalmia-associated transcription factor (MITF) is known to play a key role in melanocyte biology and progression. MITF performs these functions by activating transcription of many genes through interaction with the consensus DNA-binding sequence (CACGTG, termed E box) present on the promoters of target genes. MITF controls expression of several proteins required for melanin synthesis, including tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), and dopachrome tautomerose (DCT) (1–3). In addition, MITF plays a critical role in melanocyte survival and serves as a lineage-specific oncogene in melanoma, as evidenced by its amplification in a subset of melanomas (4–6). MITF expression is regulated by several factors, including SOX-10, CREB, PAX3, LEF1, and ATF2 (7, 8). MITF in turn regulates a plethora of genes, with its complex role in melanoma biology, as its overexpression has been shown to result in both proproliferative and antiproliferative stimuli (4, 5, 9, 10). Accordingly, verified targets of MITF include both genes that promote cell survival and block apoptosis, (e.g., CDK2, BCL2, DIAPH1, and TBX2), and genes that block cell cycle progression (e.g., CDKN2A and p21Cip1). Thus, although several downstream targets of MITF action have been identified, the precise mechanisms by which MITF promotes melanocytic tumor progression are still poorly understood.
Recently, we identified a role for the bromodomain plant homeodomain finger transcription factor (BPTF) gene in melanoma progression (11). BPTF is the largest subunit of the nucleosome-remodeling factor, a key member of the imitation switch (ISWI) family of ATP-dependent chromatin-remodeling factors. shRNA-mediated silencing of BPTF resulted in suppression of the proliferative and metastatic capacity of melanoma cells, along with induction of the apoptotic phenotype. BPTF exerted its oncogenic effects in melanoma, at least in part, by activation of several genes, including BCL2, BCL-XL, and CCND2. Here we explore the role of BPTF in the MITF signal transduction pathway, and demonstrate activation of BPTF by MITF in melanoma cells.
Results
MITF Is Not Regulated by BPTF.
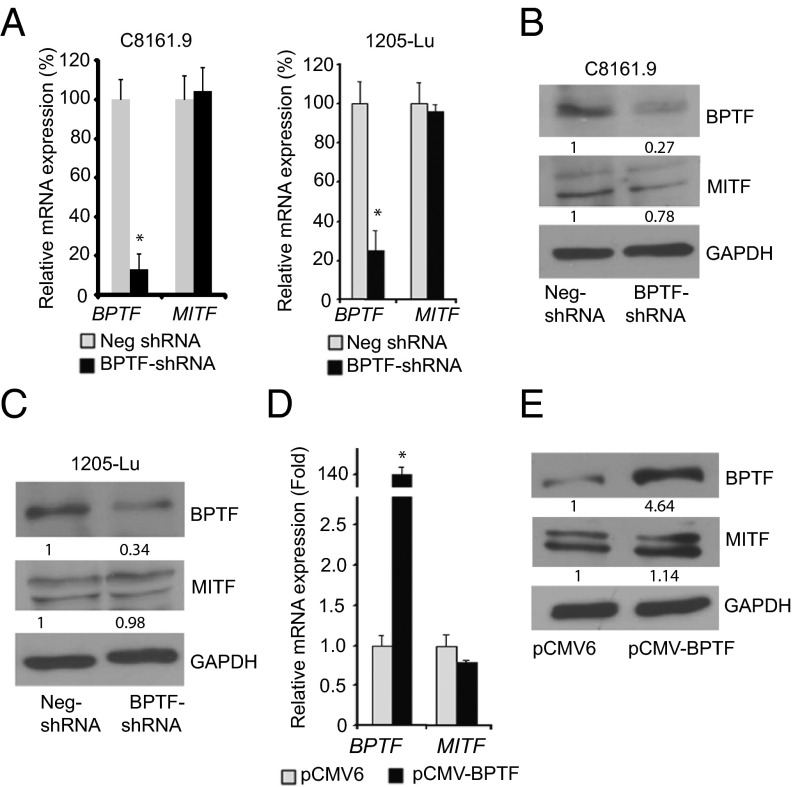
Given that BCL2 is regulated by both MITF and BPTF, we explored whether BPTF regulates MITF expression. Stable, shRNA-mediated suppression of BPTF expression in C8161.9 and 1205-Lu melanoma cells had no effect on MITF expression at the RNA (Fig. 1A) and protein levels (Fig. 1 B and C), respectively. Furthermore, overexpression of BPTF did not affect MITF expression in 1205-Lu cells (Fig. 1 D and E). These results indicate that BPTF does not regulate expression of MITF.
Fig. 1.
Effects of suppression of BPTF by a specific anti-BPTF shRNA on MITF expression at the RNA (A) and protein (B and C) levels in C8161.9 and 1205–Lu melanoma cells. (D and E) Effects of overexpression of BPTF cDNA on MITF RNA or protein expression in 1205-Lu melanoma cells. In A and D, data presented reflect mean ± SEM of three replicates. *P < 0.05.
Effects of Regulation of MITF Expression on BPTF Levels.
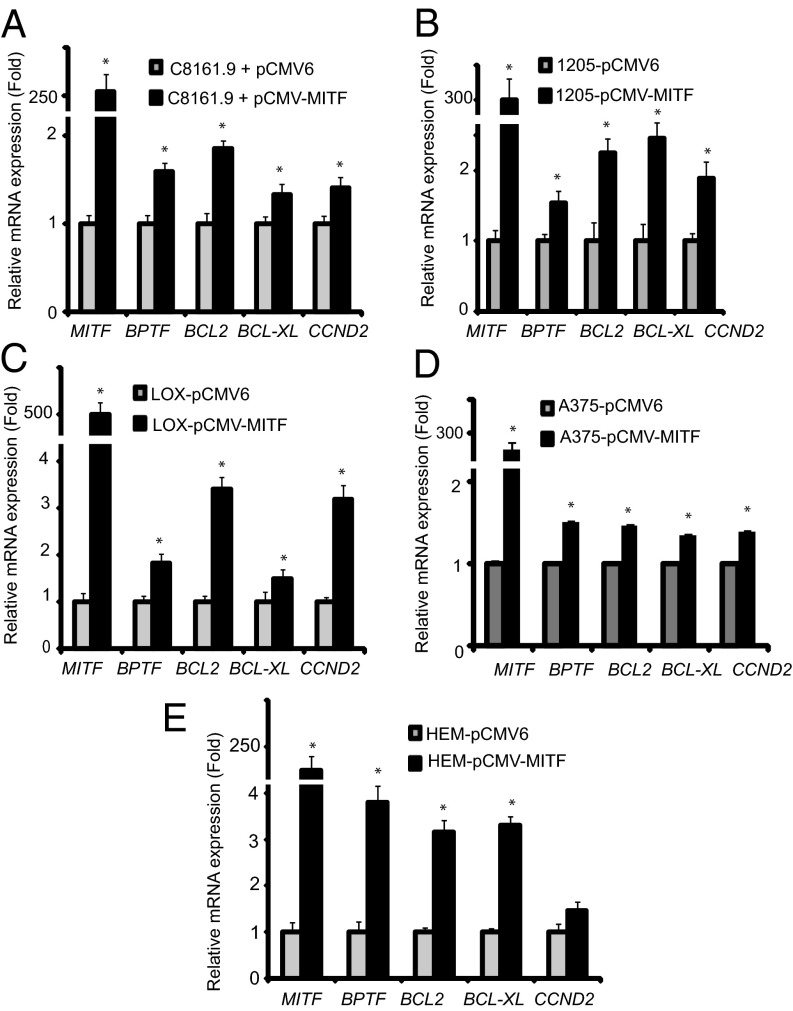
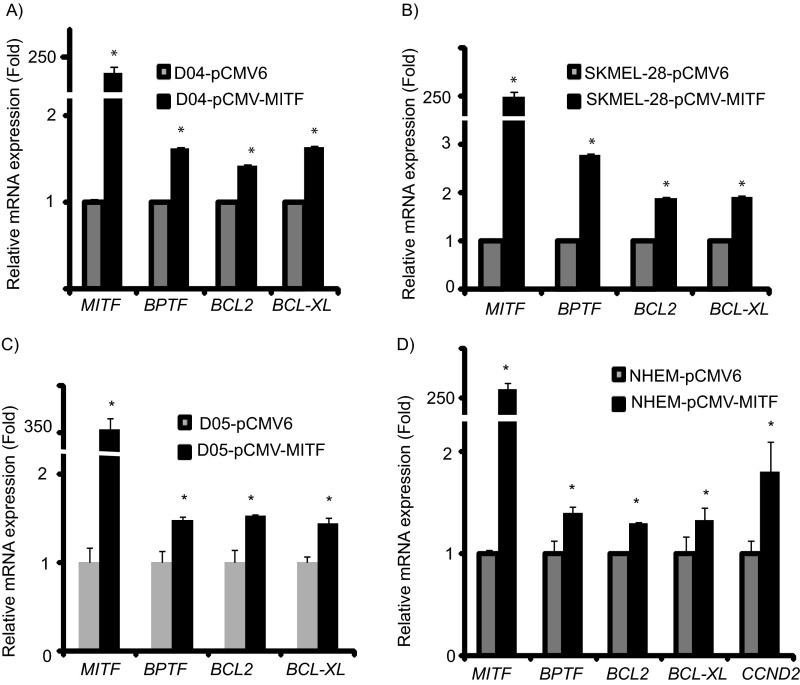
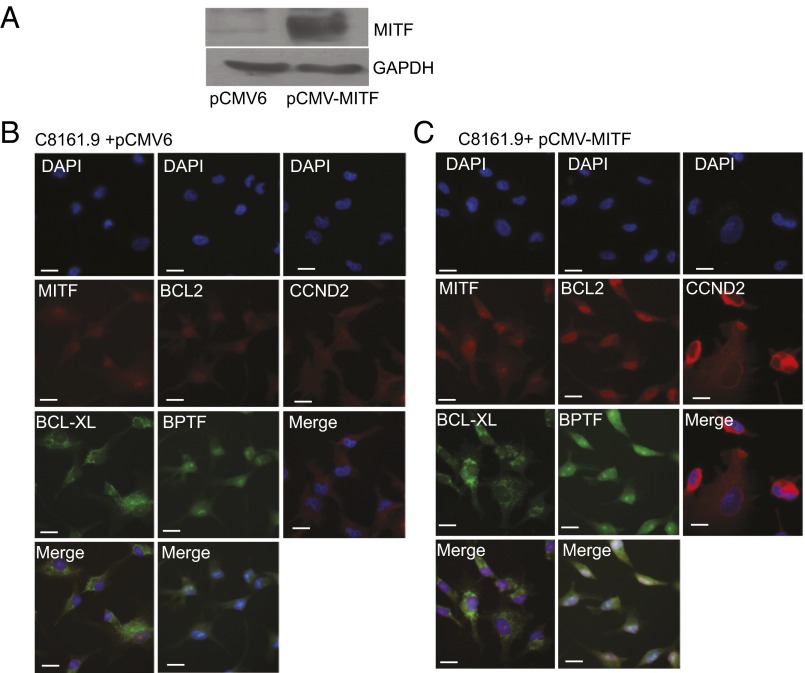
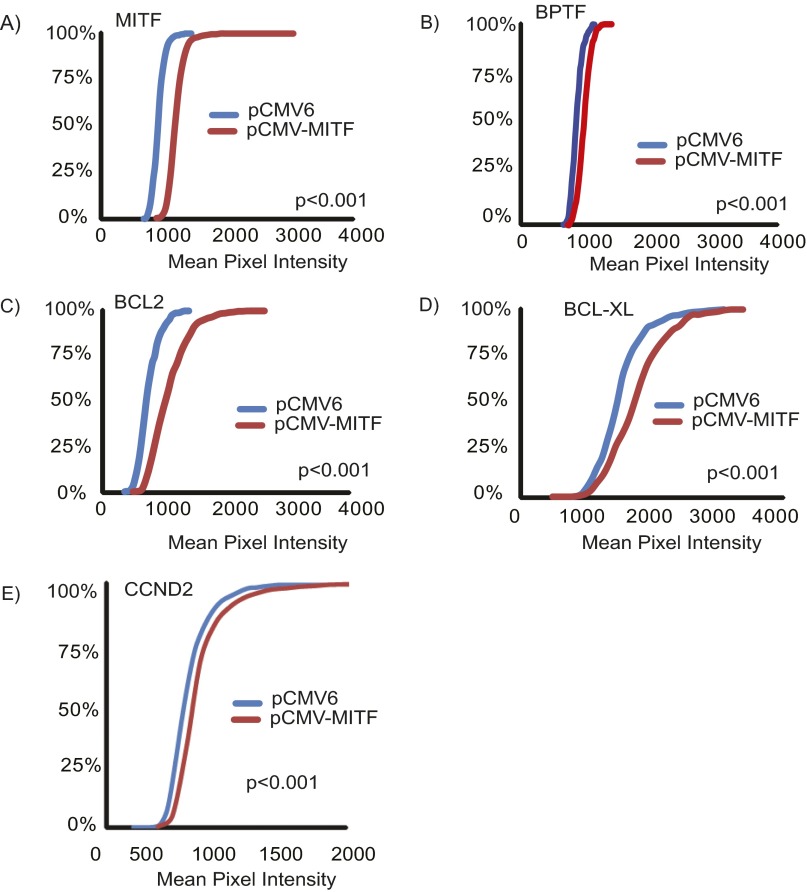
We then assessed whether MITF could regulate BPTF expression. We initially examined the consequences of overexpression of MITF cDNA to determine its effects on expression of BPTF and of BPTF-regulated genes. Overexpression of MITF in a panel of melanoma and melanocyte cell lines (Fig. 2 and Fig. S1) significantly up-regulated the expression of BPTF and downstream prosurvival genes—including BCL2, BCL-XL, and CCND2—at the RNA level. Overexpression of MITF cDNA (Fig. 3) significantly up-regulated expression of BPTF protein in C8161.9 cells, along with that of BCL2, BCL-XL, and CCND2, compared with overexpression of control pCMV6 vector (Fig. 3 B and C; quantification of immunofluorescence shown in Fig. S2). These results were confirmed by overexpressing MITF cDNA in 1205-Lu melanoma cells (Fig. S3; quantification of immunofluorescence shown in Fig. S4). These results indicate that MITF regulates expression of BPTF, along with that of the BPTF-regulated genes BCL2, BCL-XL, and CCND2.
Fig. 2.
Effects of overexpression of MITF cDNA in four different melanoma cell lines (A–D) and the HEM human melanocyte cell line (E) on expression of BPTF, BCL2, BCL-XL, and CCND2. In all panels, data presented reflect mean ± SEM of three replicates. *P < 0.05.
Fig. S1.
Effects of overexpression of MITF cDNA in three different melanoma cell lines (A–C) and the human melanocyte NHEM cell line (D) on expression of BPTF, BCL2, BCL-XL, and CCND2. The expression level of CCND2 was undetectable in D04, SKMEL-28, and D05 cells. In all panels data presented reflect mean ± SEM of three replicates. *P < 0.05.
Fig. 3.
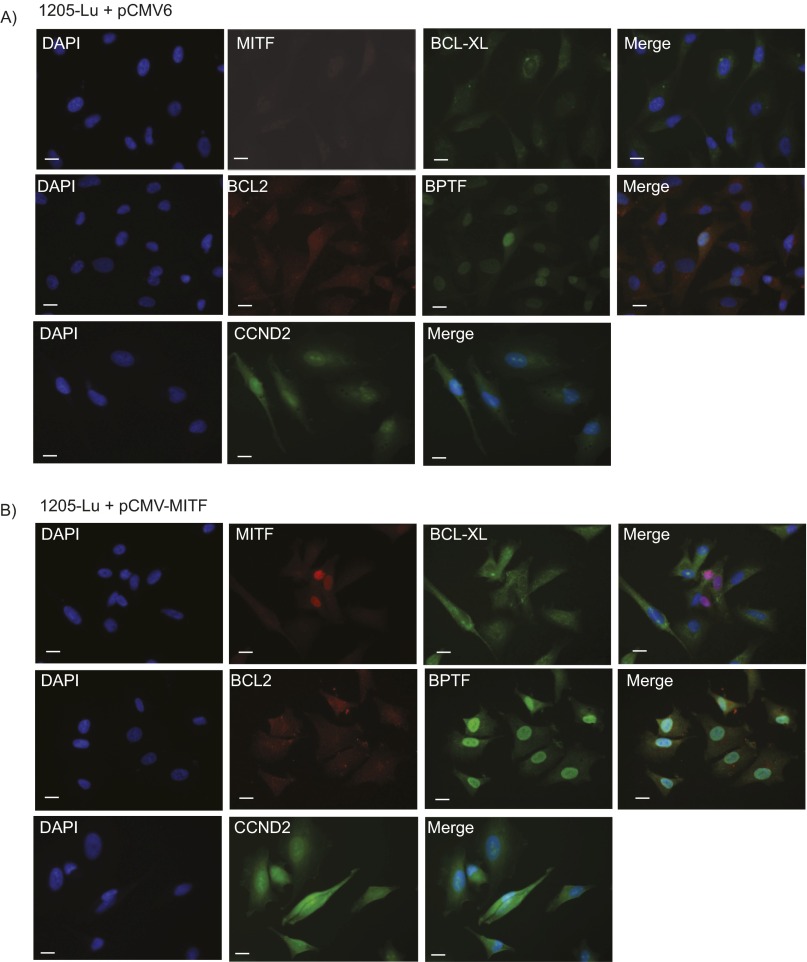
Overexpression of MITF cDNA and its effects on expression of BPTF and BPTF-regulated genes. (A) Western analysis of MITF and GAPDH expression following overexpression of MITF cDNA in HEK293 cells. (B) Immunofluorescence analysis of BPTF, BCL2, BCL-XL, and CCND2 proteins following overexpression of a control vector in C8161.9 cells. (C) Immunofluorescence analysis of BPTF, BCL2, BCL-XL, and CCND2 proteins following overexpression of MITF cDNA. (Scale bars, 20 μm.)
Fig. S2.
(A–E) Quantification of immunofluorescence staining for MITF, BPTF, BCL2, BCL-XL and CCND2 in C8161.9 cells following overexpression of pCMV6 vector and MITF cDNA vector.
Fig. S3.
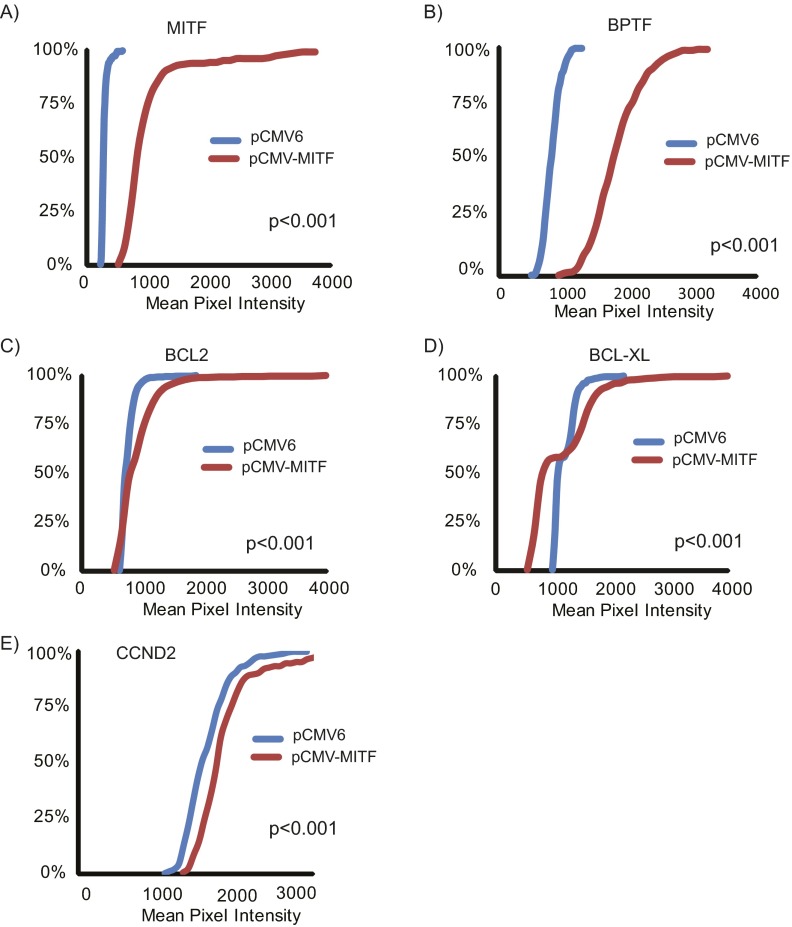
Effects of MITF cDNA overexpression in 1205-Lu melanoma cells. Expression of BPTF, BCL2, BCL-XL, and CCND2 protein following overexpression of pCMV6 control vector (A) and MITF cDNA vector (B) in 1205-Lu melanoma cell lines as determined by immunofluorescence. (Scale bars, 20 μm.)
Fig. S4.
(A–E) Quantification of immunofluorescence staining for MITF, BPTF, BCL2, BCL-XL, and CCND2 in 1205-Lu cells following overexpression of pCMV6 vector and MITF cDNA vector.
Effects of Silencing of MITF Expression in Melanoma Cells.
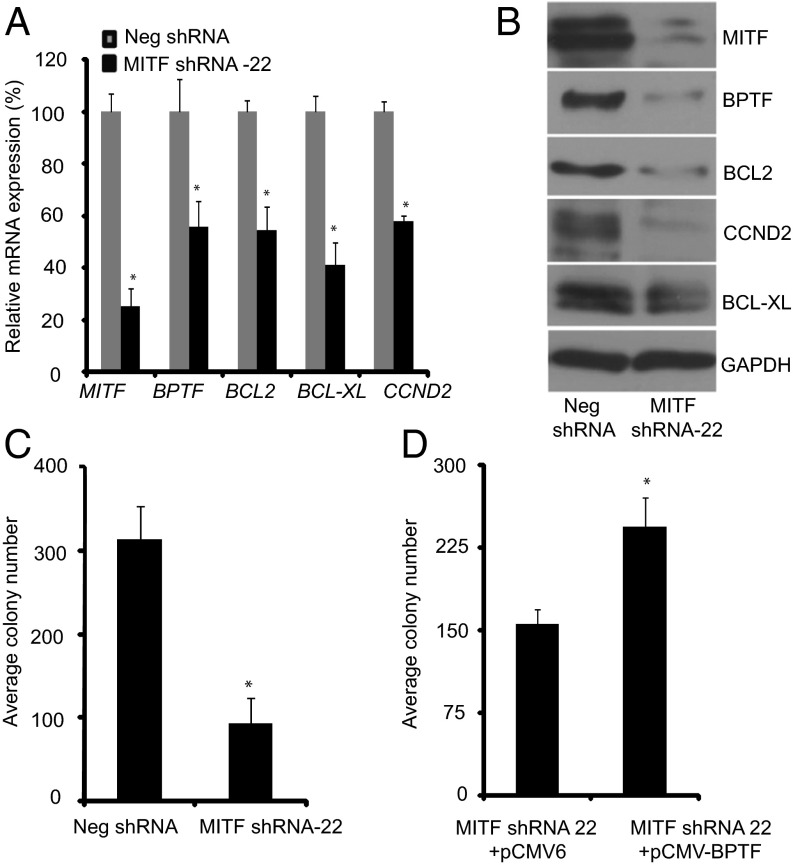
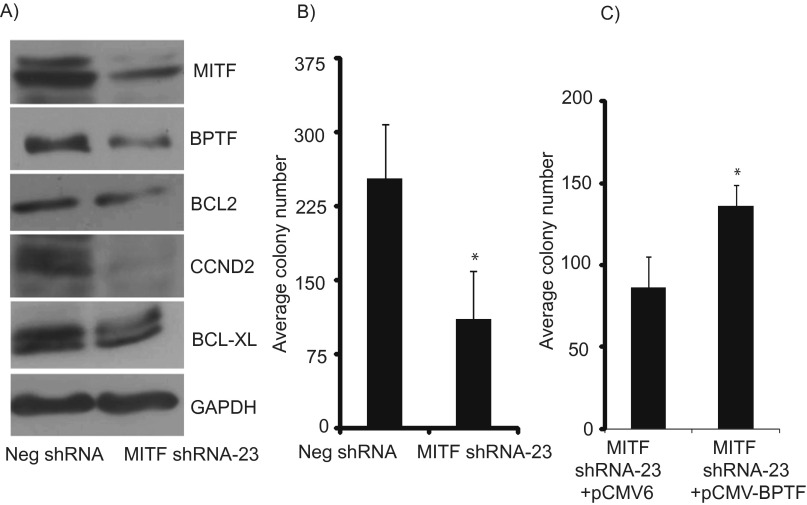
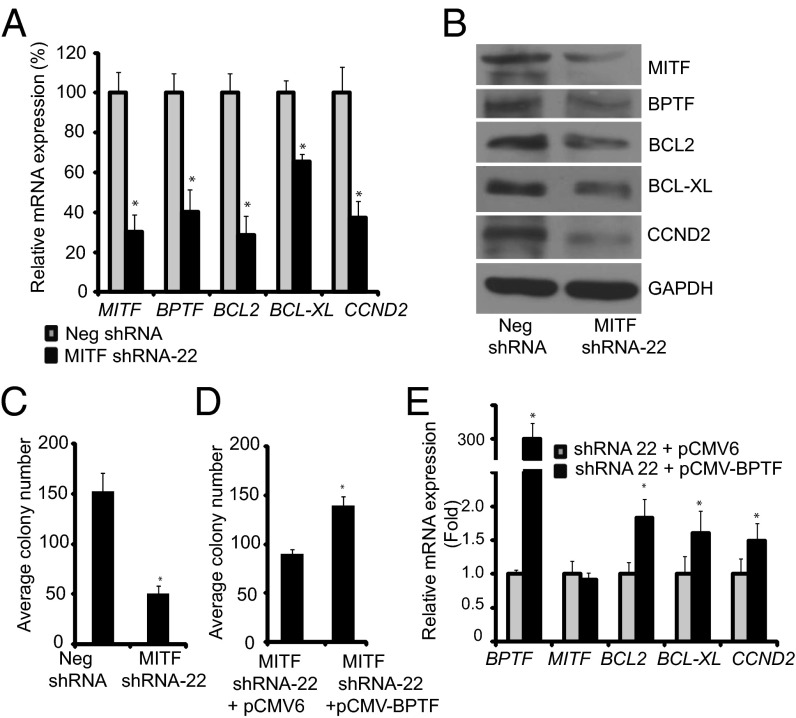
To further investigate the effects of modulation of MITF expression on that of BPTF, we stably suppressed MITF expression in C8161.9 melanoma cells using two specific shRNAs (#22 and #23) targeting human MITF. Silencing of MITF expression following overexpression of shRNA-22 significantly suppressed BPTF expression, along with that of BCL2, BCL-XL, and CCND2, both at the RNA and protein levels (Fig. 4 A and B). Suppression of MITF expression resulted in substantially decreased proliferative capacity of melanoma cells, as evidenced by reduced colony formation ability of anti-MITF shRNA-expressing cells (Fig. 4C). Overexpression of BPTF cDNA in C8161.9 melanoma cells significantly reversed the suppression of tumor cell proliferation mediated by expression of anti-MITF shRNA (Fig. 4D). Similar results were obtained when MITF was targeted by another specific shRNA (#23) in C8161.9 cells (Fig. S5). To confirm these results, MITF expression was suppressed in 1205-Lu melanoma cells, and similar effects were observed on expression of target genes and on colony formation (Fig. 5 A–D). Overexpression of BPTF cDNA in anti-MITF shRNA-expressing 1205-Lu cells had no effect on MITF RNA levels, but did result in up-regulation of expression of BCL2, BCL-XL, and CCND2 (Fig. 5E).
Fig. 4.
Effects of suppression of MITF expression in C8161.9 cells. (A) Expression of MITF, BPTF, BCL2, BCL-XL, and CCND2 RNA following stable expression of anti-MITF shRNA #22 in C8161.9 cells. (B) Expression of MITF, BPTF, BCL2, BCL-XL, and CCND2 protein following stable expression of anti-MITF shRNA #22 in C8161.9 cells. (C) Assay of C8161.9 colony formation following suppression of MITF expression. (D) Effects of overexpression of BPTF cDNA or vector control on colony forming capacity of C8161.9 cells expressing anti-MITF shRNA. In A, C, and D data presented reflect mean ± SEM of three replicates. *P < 0.05.
Fig. S5.
Effects of suppression of MITF expression on expression of BPTF and BPTF-regulated genes. (A) Expression of BPTF, BCL2, BCL-XL, and CCND2 protein following stable expression of anti-MITF shRNA #23 in C8161.9 cells. (B) Assay of C8161.9 colony formation following suppression of MITF expression. (C) Effects of overexpression of BPTF cDNA on colony forming capacity of C8161.9 cells in anti-MITF shRNA-expressing cells. *P < 0.05.
Fig. 5.
Effects of suppression of MITF expression in 1205-Lu melanoma cells. (A) Expression of MITF, BPTF, BCL2, BCL-XL, and CCND2 RNA following stable expression of anti-MITF shRNA #22 in 1205-Lu cells. (B) Expression of MITF, BPTF, BCL2, BCL-XL, and CCND2 protein following stable expression of anti-MITF shRNA #22 in 1205-Lu cells. (C) Assay of 1205-Lu colony formation following suppression of MITF expression. (D) Effects of overexpression of BPTF cDNA on colony forming capacity of 1205-Lu cells expressing anti-MITF shRNA. (E) Effects of overexpression of BPTF cDNA on expression of the BPTF, BCL2, BCL-XL, and CCND2 in 1205-Lu cells expressing anti-MITF shRNA. In A, C, D, and E data presented reflect mean ± SEM of three replicates. *P < 0.05.
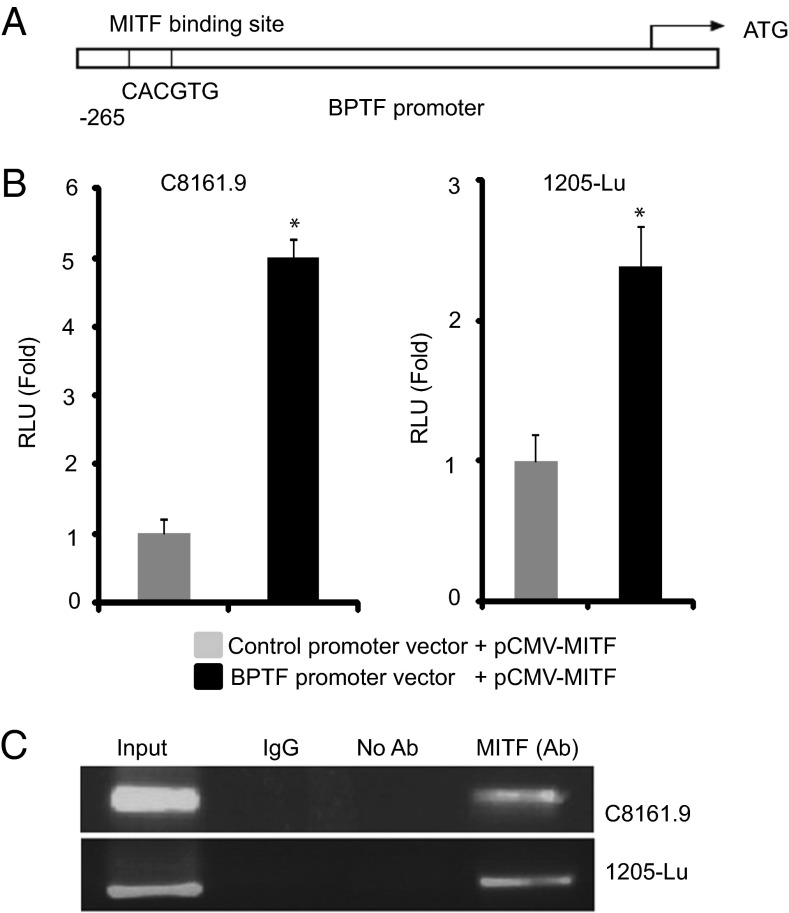
MITF Binds to the BPTF Promoter.
To understand the mechanism by which MITF regulates BPTF, we analyzed the BPTF promoter and identified a putative consensus MITF-binding site (CACGTG) 265 bp from the transcriptional initiation site (Fig. 6A). To determine if MITF activates the BPTF promoter, cotransfection of MITF cDNA along with a vector encoding the luciferase gene driven by the BPTF promoter significantly up-regulated BPTF transcriptional activity, as evidenced by increased reporter gene expression compared with the control promoter vector (Fig. 6B). Finally, to determine whether the transcriptional activation of BPTF by MITF occurs through a direct interaction of MITF with the BPTF promoter, ChIP analysis was performed in two melanoma cell lines (C8161.9 and 1205-Lu) using the anti-MITF antibody. A specific PCR band was obtained when primers specific for the BPTF promoter were used, confirming the binding of MITF to the BPTF promoter (Fig. 6C). These results indicate that MITF binds directly to the BPTF promoter to activate its transcription and to transduce several of its prosurvival signals.
Fig. 6.
Interaction of MITF with the BPTF promoter. (A) The BPTF promoter harbors a putative MITF binding site. (B) Assay of luciferase activity showing effects of overexpression of MITF cDNA compared with vector control on BPTF promoter activity. (C) ChIP analysis using an anti-MITF antibody and primers specific for the BTPF promoter, indicating the binding of MITF to the BPTF promoter. *P < 0.05.
Association Between Expression Levels of MITF and BPTF in Melanoma Tissues.
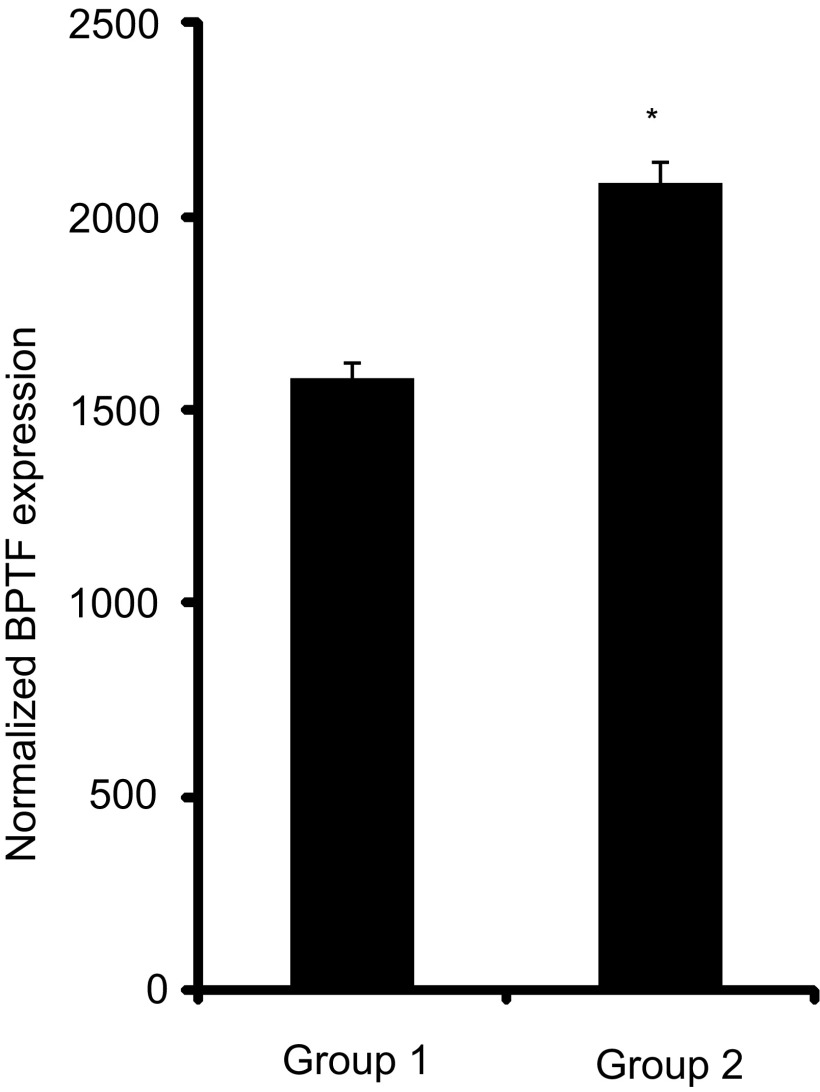
Finally, we assessed the potential association between expression levels of MITF and BPTF in human melanoma specimens using The Cancer Genome Atlas (TCGA) analysis of cutaneous melanoma (cancergenome.nih.gov). Normalized RNA expression levels for MITF and BPTF were available for 473 cases. Initially, a significant association was identified between expression of MITF and BPTF. Specifically, BPTF expression was elevated in samples with high MITF expression (i.e., above the median) and reduced in samples with low MITF expression (i.e., below the median, P < 0.0001). In addition, given that MITF positively regulates BPTF expression, we hypothesized that melanomas with higher MITF expression should also exhibit higher levels of BPTF expression. The mean expression level of BPTF in melanomas with high MITF expression (i.e., above the median) was significantly higher than melanomas with low MITF expression (i.e., below the median) (Fig. S6) (P < 0.0001).
Fig. S6.
Mean normalized expression of BPTF in melanoma samples from TCGA database expressing either low MITF (group 1) or high MITF (group 2). *P < 0.0001.
Discussion
Recent studies identified an oncogenic role for BPTF in melanoma progression, and described a role both in the prediction and promotion of melanoma distant metastasis (11). BPTF mediated its proproliferative and pro-oncogenic effects by regulating the expression of prosurvival genes, such as BCL2, BCL-XL, and CCND2. The antiapoptotic BCL2 protein plays important roles in promoting melanocytic survival and as a potential therapeutic target (12, 13). The BCL2 gene is a direct target of the MITF protein, because an E-box motif on the BCL2 promoter has been shown to be a principal mechanism by which MITF activates BCL2 (14). Our studies suggest the activation of BPTF by MITF as an additional mechanism that potentiates BCL2 expression in melanoma cells.
MITF mediates several discrete functions in the melanocyte lineage, including the regulation of differentiation, as well as proliferation and survival (15). Because BCL2 is controlled by both MITF and BPTF [and was identified as a BPTF-regulated gene (11)], it was important to investigate whether these two genes are involved in activating each other. The overexpression of BPTF cDNA in melanoma cell lines had no effect on MITF expression, thereby ruling out the regulation of MITF by BPTF. In contrast, regulation of MITF expression, whether by overexpression or by shRNA-mediated silencing, produced significant effects on expression of BPTF and BPTF-regulated genes. Specifically, MITF overexpression in a panel of melanoma and melanocyte cell lines up-regulated BPTF expression, along with that of BCL2, BCL-XL, and CCND2, indicating a role for MITF in regulating BPTF. The regulation of BPTF by MITF was further supported by suppression of MITF expression using two separate shRNAs in two melanoma cell lines, demonstrating down-regulation of BPTF, BCL2, BCL-XL, and CCND2 at the RNA and protein levels.
MITF silencing resulted in significant suppression of melanoma cell proliferation. MITF’s control of melanoma cell proliferation has been previously explained by its activation of various genes that are involved in cell growth, such as TBX2, CDK2, and BIRC7 (16-18). Importantly, forced expression of BPTF cDNA rescued the inhibition of melanoma colony formation caused by MITF depletion, suggesting that MITF directs its prosurvival program in the melanocytic lineage, at least in part, by activating BPTF expression. These results suggest that down-regulation of BPTF is possibly one of the key events to produce melanoma growth arrest following MITF suppression. Importantly, these results add BPTF to the list of known oncogenic stimuli induced by MITF, and suggest that BPTF transduces some of the key prosurvival signals mediated by MITF.
Intriguingly, analysis of the BPTF promoter revealed the presence of a consensus MITF-binding site (CACGTG) upstream of the transcription initiation site. ChIP analysis demonstrated binding of MITF to the BPTF promoter. In addition, MITF regulated transcriptional activity of the BPTF promoter, as evidenced by increased luciferase expression driven by the BPTF promoter. These results describe the mechanism by which MITF controls BPTF expression to mediate its oncogenic effects in melanoma cells. Finally, our hypothesis was further supported by the TCGA analysis of human melanoma specimens, in which there was a significant association between expression levels of MITF and BPTF. Importantly, melanomas with high MITF expression were found to express significantly higher levels of BPTF than melanomas with low MITF expression.
Recently, BPTF was shown to be essential for differentiation of murine melanocytic stem cells (19). In addition, MITF and BPTF were shown to coregulate overlapping gene-expression programs in melanoma cells and melanocytes, supporting our results. Our studies extend these results by demonstrating that MITF binds directly to the BPTF promoter, regulates its expression, and transduces certain proproliferative signals via BPTF.
Our prior analysis showed that BPTF activates the MAP kinase pathway by regulating ERK expression at the transcriptional level (11). Taken together, these studies demonstrate that BPTF is involved in multiple signaling pathways critical to melanoma proliferation and survival. Additional studies will be required to determine the role played by BPTF in promoting the progression of tumors lacking MITF activation. However, given that MITF is a master regulator of melanocyte lineage survival, these studies provide important additional insights into the MITF signaling cascade in melanoma. In addition, the participation of BPTF in the MITF pathway suggests a critical role for BPTF in maintaining melanoma cell survival, and provides further support for its principal role in melanoma progression and as a potential target for therapy.
Materials and Methods
Cell Culture.
C8161.9 cells (obtained from Danny Welch, University of Kansas, Kansas City, KS) were grown in DMEM/F12 with 5% (vol/vol) FBS. 1205-Lu cells were purchased from Coriell Institute, and grown in TU 2% (vol/vol) media. LOX cells (kindly provided by Oystein Fodstad, Oslo University Hospital, Oslo), A375, HEK293T, and SKMEL-28 cells (purchased from ATCC), and D05 and D04 cells (kindly provided by Boris Bastian, University of California, San Francisco) were grown in RPMI with 5% (vol/vol) FBS (Life Technologies). Normal human melanocytes (NHEM) were purchased from Lonza, and were grown in MGM-4 media; the human melanocyte line (HEM) was purchased from PromoCell, and grown in melanocyte growth media. All cell lines were grown at 37 °C in an atmosphere containing 5% CO2.
Plasmids and Transfection.
Plasmids pCMV6-BPTF, pCMV6-MITF, and pCMV6 (control vector) were purchased from Origene Technologies. The Lentiviral pLKO1-based shRNA vector set (five clones) targeting human MITF (RHS4533_EG4286) was purchased from Openbiosystems, along with its control, and used for human cell lines, including anti-MITF shRNA-22 and shRNA-23 used in this analysis. The pLightswitch BPTF promoter vector and the pLightswitch control vector were purchased from SwitchGear Genomics. Transient transfections were carried out using Lipofectamine-2000 (Life Technologies) according to the manufacturer’s protocol.
RNA Extraction and Quantitative RT-PCR.
RNA from cell lines was extracted using the RNeasy mini kit (Qiagen) using the manufacturer’s protocol. RNA concentration was measured by the NanoDrop 1000 spectrophotometer (ThermoFischer Scientific). cDNA synthesis was performed by using 1 µg of RNA using the iScript cDNA synthesis kit (Bio-Rad) following the manufacturer’s instructions. mRNAs were assayed using the TaqMan Gene Expression Assays in accordance with the manufacturer's instructions (Applied Biosystems). TaqMan probes for BPTF, MITF, HPRT1, CCND2, BCL-XL, and BCL2 were purchased from Applied Biosystems. Gene-expression levels were quantified using the 7500 Fast Real Time Sequence detection system Software (Applied Biosystems). Comparative real-time PCR was performed in triplicate, including no-template controls. Relative expression was calculated using the comparative Ct method.
Colony Formation.
For the colony formation assay, 600 cells per group were plated in triplicates in a six-well plate. Cells were allowed to grow till visible colonies appeared, stained with Crystal violet, washed with PBS, and counted.
Lentiviral Transduction and Stable Cell Generation.
Selected shRNAs cloned into the pLKO1-vector were cotransfected into HEK293T cells along with expression vectors containing the GAG/POL, REV, and VSVG genes. Lentiviruses were harvested 48 h after transfection. Subconfluent human melanoma cells were infected with each harvested lentivirus in the presence of 8 µg/mL of polybrene, and were selected in 1 µg/mL of puromycin at 48 h postinfection, in their respective culture medium.
Western Blot Analysis.
Western blot analysis was performed as described previously (20, 21). Target proteins were detected by using specific antibodies against MITF (1:500; Thermo Scientific, MS-771-P), BPTF (1:1,000; Bethyl Laboratories, A300-973A), BCL2 (sc-7382; 1:1,000) and GAPDH (sc-365062; 1:1,000) from Santa Cruz Biotechnology, and BCL-XL (2764; 1:1,000) from Cell Signaling Technology.
Luciferase Activity.
Luciferase activity was measured by using the Dual Luciferase Assay (Promega) 48 h after transfection as per the manufacturer’s instructions. Each reporter plasmid was transfected at least three times (on different days) and each sample was assayed in triplicate.
ChIP Analysis.
ChIP analysis was performed using the EZ-ChIP kit (Upstate Biotechnology) according to the manufacturer’s directions and as previously described (22). The sequence of primers used for PCR is: F- 5′ CATTTCGCGAGCTGCGCG 3′, and R-5′ GTGGAGGGAGAAGGCTCAAT 3′.
Immunofluorescence.
Immunofluorescence assays were performed and quantitated as described previously (23, 24). Antibodies to MITF, BPTF, BCL-2, BCL-XL, and BCL2 were used to detect the individual proteins.
Statistical Analysis.
All quantified data represent an average of at least triplicate samples or as indicated. Error bars represent SEM. Statistical significance was determined by the Student’s t test; two-tailed P < 0.05 was considered significant. The association between expression levels of MITF and BPTF was analyzed using the χ2 test.
Acknowledgments
This work was supported by US Public Health Service Grant CA114337 (to M.K.-S.), the Kay Charitable Trust, and the T. Robert and Katherine Burke Fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606027113/-/DCSupplemental.
References
- 1.Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14(12):7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolotto C, et al. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol. 1998;18(2):694–702. doi: 10.1128/mcb.18.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yavuzer U, et al. The Microphthalmia gene product interacts with the retinoblastoma protein in vitro and is a target for deregulation of melanocyte-specific transcription. Oncogene. 1995;10(1):123–134. [PubMed] [Google Scholar]
- 4.Levy C, Khaled M, Fisher DE. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene. 2003;22(20):3035–3041. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- 6.Garraway LA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436(7047):117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 7.Huber WE, et al. A tissue-restricted cAMP transcriptional response: SOX10 modulates alpha-melanocyte-stimulating hormone-triggered expression of microphthalmia-associated transcription factor in melanocytes. J Biol Chem. 2003;278(46):45224–45230. doi: 10.1074/jbc.M309036200. [DOI] [PubMed] [Google Scholar]
- 8.Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: Regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107(1):1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DC. How to make a melanoma: What do we know of the primary clonal events? Pigment Cell Melanoma Res. 2008;21(1):27–38. doi: 10.1111/j.1755-148X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Goding CR. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14(14):1712–1728. [PubMed] [Google Scholar]
- 11.Dar AA, et al. The role of BPTF in melanoma progression and in response to BRAF-targeted therapy. J Natl Cancer Inst. 2015;107(5):djv034. doi: 10.1093/jnci/djv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaoka A, et al. Anti-cell death activity promotes pulmonary metastasis of melanoma cells. Oncogene. 1997;14(24):2971–2977. doi: 10.1038/sj.onc.1201147. [DOI] [PubMed] [Google Scholar]
- 13.Bedikian AY, et al. Oblimersen Melanoma Study Group Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 14.McGill GG, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109(6):707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 15.Haq R, Fisher DE. Biology and clinical relevance of the micropthalmia family of transcription factors in human cancer. J Clin Oncol. 2011;29(25):3474–3482. doi: 10.1200/JCO.2010.32.6223. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68(19):7872–7881. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- 17.Du J, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6(6):565–576. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Dynek JN, et al. Microphthalmia-associated transcription factor is a critical transcriptional regulator of melanoma inhibitor of apoptosis in melanomas. Cancer Res. 2008;68(9):3124–3132. doi: 10.1158/0008-5472.CAN-07-6622. [DOI] [PubMed] [Google Scholar]
- 19.Koludrovic D, et al. Chromatin-remodelling complex NURF is essential for differentiation of adult melanocyte stem cells. PLoS Genet. 2015;11(10):e1005555. doi: 10.1371/journal.pgen.1005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dar AA, et al. miRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. J Biol Chem. 2011;286(19):16606–16614. doi: 10.1074/jbc.M111.227611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dar AA, et al. The role of miR-18b in MDM2-p53 pathway signaling and melanoma progression. J Natl Cancer Inst. 2013;105(6):433–442. doi: 10.1093/jnci/djt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majid S, et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis. 2009;30(4):662–670. doi: 10.1093/carcin/bgp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dar AA, Belkhiri A, El-Rifai W. The aurora kinase A regulates GSK-3beta in gastric cancer cells. Oncogene. 2009;28(6):866–875. doi: 10.1038/onc.2008.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Raamsdonk CD, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]