Significance
Endocytosis is crucial for sustained synaptic transmission. During high-frequency neurotransmission, endocytosis recycles vesicular components rapidly and may promote the clearance of used SNAREs and other membrane proteins from release sites. We report that tissue-specific dynamin-1 deletion significantly reduces synaptic depression during bursts of the high-frequency stimulation at the mature calyx of Held in mice. This effect is contrary to the expected consequence of reduced recycling and cannot be explained by the commonly known mechanisms underlying short-term depression. Rather, the data imply that endocytosis may have a rapid, retrograde effect on transmitter release (e.g., through alterations of release site clearance) during high rates of synaptic vesicle fusion. Our finding indicates a role of dynamin-1 in high-frequency synaptic transmission and short-term plasticity.
Keywords: short-term plasticity, release site clearance, dynamin, bulk endocytosis, actin
Abstract
Dynamin is a large GTPase with a crucial role in synaptic vesicle regeneration. Acute dynamin inhibition impairs neurotransmitter release, in agreement with the protein’s established role in vesicle resupply. Here, using tissue-specific dynamin-1 knockout [conditional knockout (cKO)] mice at a fast central synapse that releases neurotransmitter at high rates, we report that dynamin-1 deletion unexpectedly leads to enhanced steady-state neurotransmission and consequently less synaptic depression during brief periods of high-frequency stimulation. These changes are also accompanied by increased transmission failures. Interestingly, synaptic vesicle resupply and several other synaptic properties remain intact, including basal neurotransmission, presynaptic Ca2+ influx, initial release probability, and postsynaptic receptor saturation and desensitization. However, acute application of Latrunculin B, a reagent known to induce actin depolymerization and impair bulk and ultrafast endocytosis, has a stronger effect on steady-state depression in cKO than in control and brings the depression down to a control level. The slow phase of presynaptic capacitance decay following strong stimulation is impaired in cKO; the rapid capacitance changes immediately after strong depolarization are also different between control and cKO and sensitive to Latrunculin B. These data raise the possibility that, in addition to its established function in regenerating synaptic vesicles, the endocytosis protein dynamin-1 may have an impact on short-term synaptic depression. This role comes into play primarily during brief high-frequency stimulation.
Sustained neurotransmission requires synaptic vesicle (SV) recycling. The reformation of functional SVs, in general, requires several seconds or even longer (1, 2) and is thus several orders of magnitude slower than vesicle release at active zones (AZs). Vesicle fusion can reach rates of up to a few thousand vesicles per second in many types of nerve terminals, such as ribbon synapses (3,000/s) (3), cerebellar basket cell terminals (5,000/s) (4), and the calyx of Held (6,000/s) (5–7). Because release sites are limited in number, synapses need to reuse them rapidly in succession during repetitive stimulation, and this requirement may be more restrictive than that of SV availability (8). AZs are composed of a meshwork of evolutionarily conserved scaffold proteins including RIM, Munc-13, RIM-BP, α-liprin, ELKS, and Ca2+ channels (9–11), but their dynamic properties are poorly understood (10). During high-frequency release, vesicle membrane components (12) and soluble N-Ethylmaleimide sensitive factor (NSF) attachment protein receptor (SNARE) proteins that occupy the release sites need to be cleared rapidly so that new vesicles can dock and prime for new rounds of exocytosis. The recovery of release sites may become rate-limiting during high rates of transmitter release (8). However, direct experimental testing is challenging due to the transient nature of this process. The molecular mechanism underlying site clearance and functional recovery are unclear. Tight exo/endocytosis coupling (13, 14) may contribute to the reavailability of release sites by promoting clearance of vesicle components from release sites during high synaptic activity (8). Refractoriness of release sites during high-frequency release had already been considered early on by Bernhard Katz (15). It was further supported by evidence for ultrastructural changes in AZs after stimulation (16, 17) and by studies on the temperature-sensitive dynamin mutant shibire in Drosophila (18) and on the perturbations of other proteins involved in endocytosis (19–21).
The calyx of Held is a fast central synapse in the auditory brainstem, and it spontaneously fires action potentials (APs) at ∼70 Hz [ranging between 0.4 and 174 Hz in postnatal day 32 (P32) mice] (22) or ∼30 Hz (median frequency in P13–28 mice) (23) in vivo. It can follow AP input at up to ∼500 Hz under sound stimulation in anesthetized mice (23, 24). The calyx of Held may reuse its release sites at least 3–5 times per second during transient high-frequency stimulation (8). This makes it an ideal model synapse for the study of rapid processes during endocytosis and release site clearance.
Dynamin is a large guanosine triphosphatase (GTPase) with multiple domains interacting with other molecules, and it is critical for membrane fission during vesicle trafficking (25). Among three dynamin isoforms (encoded by Dnm1, Dnm2, and Dnm3) in the mammalian brain, dynamin-1 is the most abundant (26). The conventional deletion of dynamin-1 causes early lethality in mice and accumulation of clathrin-coated endocytic intermediates at nerve terminals (26, 27). Direct presynaptic recordings from the calyx of Held in conventional dynamin-1 KO animals reveal a significant impairment of the slow form of endocytosis and a reduction of presynaptic Ca2+ currents (28). Further deletion of dynamin-3 exacerbates dynamin-1 KO phenotypes (29) and leads to striking synaptic facilitation (30). The spontaneous Dnm1 mutation (R256L) in dogs causes an exercise-induced collapse syndrome (31), and fitful mice carrying a Dnm1 mutation exhibit epilepsy (32). The shibire mutation paralyzes Drosophila at the restrictive temperature due to synaptic vesicle depletion at neuromuscular junctions (33). It also displays a rapid enhancement of synaptic depression within 20 ms of stimulation presumably arising from rapid obstruction of release sites (18).
Here, we examine how dynamin loss affects fast synaptic transmission at the mature calyx of Held (P16–20) by tissue-specific Dnm1 gene deletion. To our surprise, the loss of dynamin-1 decreased short-term synaptic depression during brief high-frequency stimulation. This effect depends on the high-frequency stimulation and actin polymerization. Interestingly, the vesicle resupply rate, along with other properties of synaptic transmission, remains intact in conditional knockout (cKO) synapses. These data call for roles of dynamin in short-term synaptic plasticity in addition to its known function in regenerating new SVs. Mechanisms, which imply more efficient release site clearance in cKO than control during brief high-frequency stimulation, are discussed.
Results
Tissue-Specific Dynamin-1 Ablation Does Not Affect Basal Neurotransmission at the Mature Calyx of Held.
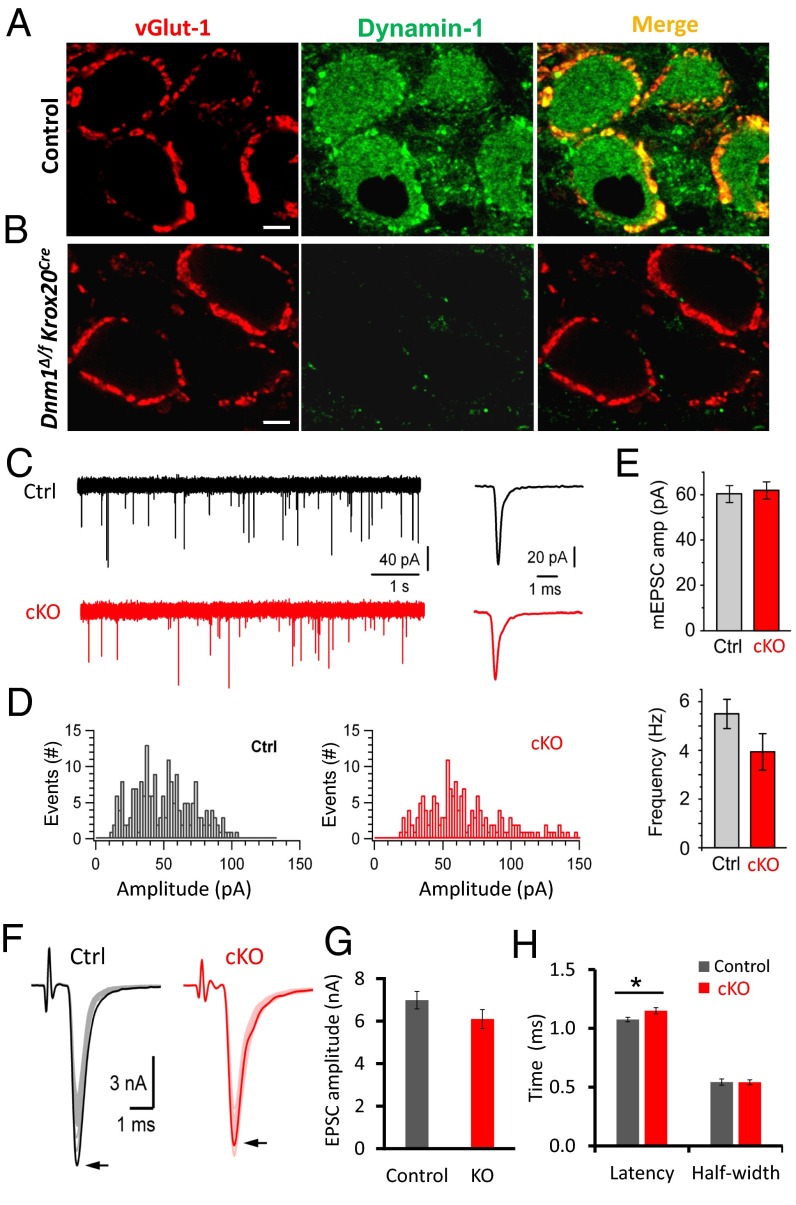
Dynamin-1 was selectively ablated in mouse auditory brainstem by crossing Dnm1f/f (34) and Krox20Cre (35, 36). These conditional dynamin-1 knockout mice (Dnm1Δ/f Krox20Cre, hereafter named cKO) were viable, fertile, and without notable outward defects. This contrasts with the conventional dynamin-1 KO mice (26) and their out-breeding KO strain (crossing with CD-1) (28) that showed a much shorter life span (<2 wk) and smaller (∼1/3 or less) body size and weight than that of the control. The morphology of the calyx of Held in cKO mice was indistinguishable from control (Fig. 1B, Left) with comparable, well-developed presynaptic structures at P20. Immunostaining showed abundant fluorescence of a synaptic marker vesicular glutamate transporter 1 (vGlut-1) in both control and cKO synapses, but dynamin-1 fluorescence nearly disappeared in cKO synapses (Fig. 1B, Middle), suggesting efficient dynamin-1 deletion in the cKO calyx of Held.
Fig. 1.
Tissue-specific ablation of dynamin-1 has no major impact on the morphology of the calyx of Held and on basal synaptic transmission. (A and B) Immunofluorescence of the mature calyx of Held (P20) from a control and a cKO brain slice. Antibodies against the vesicular glutamate transporter-1 (red) and dynamin-1 (green, polyclonal antibody) were used. Note the disappearance of dynamin-1 fluorescence in cKO. (Scale bars: 5 µm.) (C, Left) Representative mEPSCs from MNTB neurons (P16–20). (Right) The averaged mEPSC traces from a control (222 events) and a cKO (n = 171 events) cell shown on the left. (D) mEPSC amplitude distribution from a control and a cKO synapse. (E) Amplitude and frequency of mEPSCs in both control (n = 15 synapses; total events: 4,414) and cKO groups (n = 17 synapses; total events: 3,614). (F) Representative control and cKO EPSCs evoked by 30 APs at 1 Hz. Arrows indicate the first EPSC peak. (G and H) EPSC amplitudes (G) and kinetics (H) from both control (n = 35) and cKO calyx of Held (n = 50) (P < 0.05 for the latency, Student’s t test).
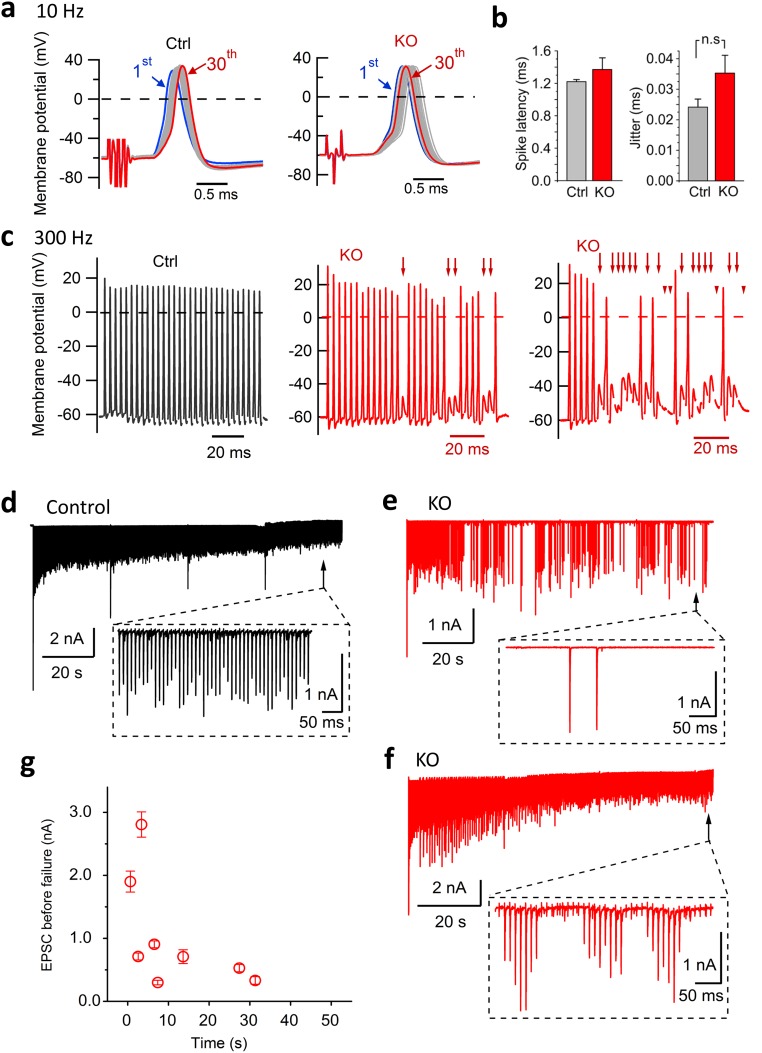
We first monitored the spontaneous miniature excitatory postsynaptic currents (mEPSCs) in the medial nucleus of trapezoid body (MNTB) neurons. The mEPSCs in cKO showed similar kinetics and amplitudes as those in control (control: −60.3 ± 3.7 pA, n = 15 synapses; cKO: −61.7 ± 3.8 pA, n = 17 synapses). The mEPSC frequency in cKO (cKO: 3.9 ± 0.7 Hz, n = 17) was slightly smaller than that of control (5.5 ± 0.6 Hz, n = 15; P = 0.076) (Fig. 1 C–E). The initial EPSCs induced by APs had comparable amplitudes (Fig. 1 F–H, control: −6.9 ± 0.4 nA, n = 35 synapses; cKO: −5.9 ± 0.5 nA, n = 50 synapses, P = 0.14) and half-widths (543 ± 26.8 μs, n = 35 for control; 541 ± 20.6 μs, n = 50 for cKO; P = 0.96) between the two groups. The EPSC latency in cKO was slightly longer than control (1.07 ± 0.02 ms for control; 1.15 ± 0.03 ms for cKO; P < 0.05). These findings demonstrate that basal transmission in a circuit of the central nervous system is nearly intact in the absence of dynamin-1, suggesting that dynamin-1 is not essential for low levels of synaptic activity. The remaining levels of vesicle recycling may be supported by dynamin-2 and -3 in the calyx of Held. These data are consistent with the nearly intact basal neurotransmission observed in conventional KO synapses (28). However, the conventional KO results in somewhat smaller presynaptic Ca2+ current and correspondingly smaller EPSCs; such changes were not observed in cKO mice.
The Lack of Dynamin-1 Increases Steady-State Transmitter Release and Reduces Short-Term Depression During High-Frequency Stimulation.
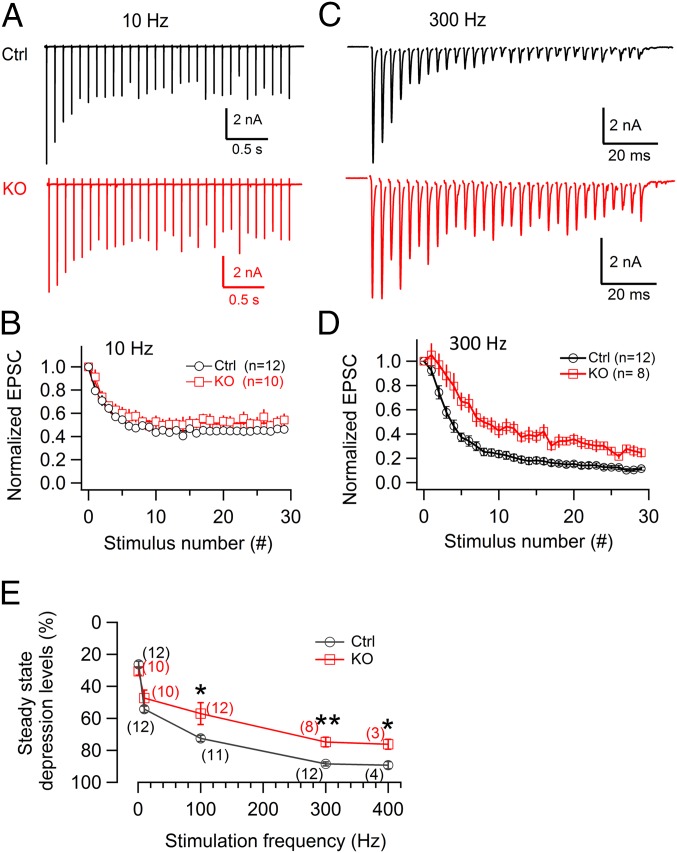
The calyx of Held can follow AP stimulation over a wide range of frequencies in vivo (23). We therefore examined the changes in EPSCs during AP stimulation at different frequencies in MNTB neurons. The steady-state depression levels were calculated based on the average value of the last five normalized EPSCs. We define depression as the complement to steady-state release that is expressed as a percentage of the first EPSC. In response to the low-frequency AP stimulation (30 APs at 10 Hz, Fig. 2 A and B), cKO synapses showed moderate depression (47.3 ± 5.0%, n = 10) similar to that in controls (54.2 ± 2.1%, n = 12; P = 0.23). However, in response to 300-Hz APs, cKO synapses showed much less depression than controls (Fig. 2 C and D), and steady-state release was larger in cKO. The average depression was significantly less in cKO (74.8 ± 3.03%, n = 8 synapses) than in control (88.5 ± 1.23%, n = 12 synapses; P = 0.0016) (Fig. 2D). This effect developed very rapidly during high-frequency stimulation so that depression curves diverged within a few AP stimuli (within ∼10 ms) (Fig. 2D). Furthermore, this effect strongly depended on the stimulation frequency (Fig. 2E), as shown by the separation of the two traces at 100 Hz or higher.
Fig. 2.
Lack of dynamin-1 causes a relative increase in steady-state transmitter release and less synaptic depression during high-frequency stimulation. (A) Representative control and cKO EPSC trains in response to 30 APs at 10 Hz. (B) Average short-term depression (STD) curves at 10 Hz from control (n = 12) and cKO (n = 10) synapses (τ = 0.27 s for both groups) showed no difference in STD. (C) As in A but in response to APs at 300 Hz. Note the larger EPSCs at the end of the train in cKO. (D) Average STD curves at 300 Hz in cKO (τfast = 7.9 ms, τslow = 74 ms; n = 8 synapses) and control (τfast = 12.2 ms, τslow = 123 ms; n = 12 synapses). The less depression in cKO compared with control was evident within a few stimuli (∼10 ms) as their respective depression curves started separating. (E) The average STD levels in control and cKO synapses were plotted over different stimulation frequencies. Note that the difference in STD between control and cKO is frequency-dependent. The steady-state depression levels were calculated as the average value of the last five normalized EPSCs in each train from individual synapses; the number in each parentheses indicates the cell number recorded for that frequency. For any single data point in E, the numbers of animals that we used were from a minimum of three to four mice to a maximum of seven to eight mice.
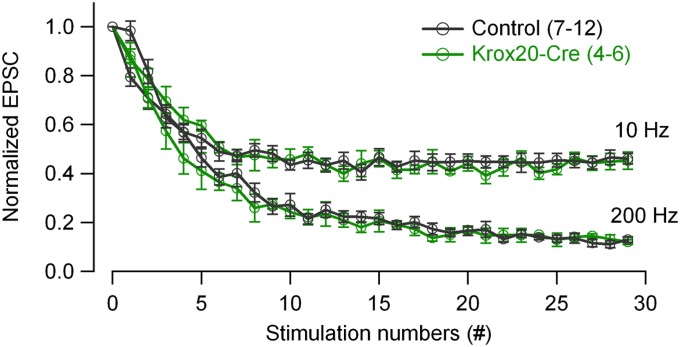
The reduced synaptic depression in cKO observed here is consistent with the weaker depression that is observed at synapses lacking syndapin-1 (37), a critical dynamin-interacting partner. Along the same line, dynamin-1 and -3 double knockout synapses even exhibit strong facilitation, which is largely accounted for by a pronounced decrease in release probability and vesicle number (30). On the other hand, the reduction in depression in cKO synapses was unexpected because acute dynamin GTPase inactivation (18) or inhibition (20, 21) enhanced synaptic depression. An involvement of the Krox20Cre gene in the depression phenotype of cKO synapses can be ruled out because the Krox20Cre/+ gene alone did not change short-term synaptic depression (Fig. S1).
Fig. S1.
The mature calyx of Held in Krox20Cre/+ mice showed equal short-term depression as in control. Average short-term depression curves under both 10 and 200 Hz were shown for control (n = 12 and 7 synapses at 10 and 200 Hz, respectively) and Krox20Cre/+ mice (n = 6 and 4 synapses at 10 and 200 Hz, respectively) (P16–20). The overlap of control and Krox20Cre/+ average curves indicates that the Krox20Cre gene alone has no effect on short-term depression at the calyx of Held and that the reduced depression in cKO synapses at higher frequencies (Fig. 2) were not due to the genetic background of Krox20Cre/+ mice.
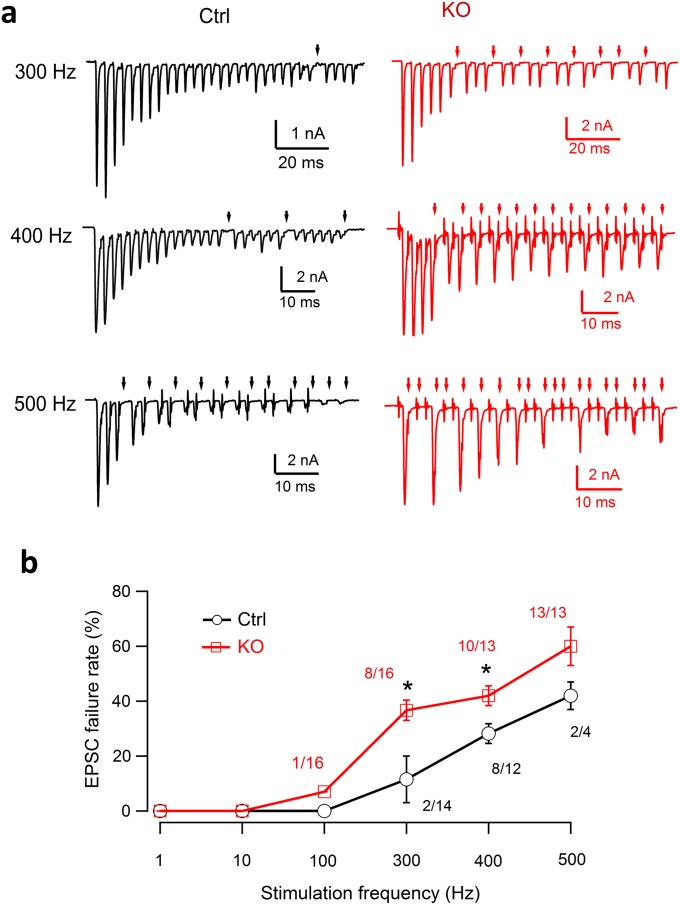
We also noted that synaptic transmission in cKO was more prone to fail than in control during high-frequency stimulation (Fig. S2). Further characterization of AP transmission revealed that the cKO calyx of Held could faithfully transmit APs at low frequencies (Fig. S3 A and B), but failed frequently at high frequencies (Fig. S3C). This was unexpected because the mature calyx of Held is known as a high-fidelity synapse (22, 38, 39). The transmission failures in cKO depended on both stimulation frequency (Fig. S2 A and B) and duration (Fig. S3 E and F). Interestingly, they occurred before synaptic vesicle depletion as indicated by the complete and sudden (rather than gradual) EPSC disappearance (Fig. S3 E and F, Insets). Further loose-patch recordings, in which local current signals from presynaptic and postsynaptic sites can be distinguished (Fig. S4A), revealed more frequent presynaptic AP failures than quantal failures in cKO synapses (Fig. S4 B and C).
Fig. S2.
Transmission failure and frequency dependence at the calyx of Held from control and cKO. (A) Representative EPSC trains from control (black) and cKO (red) mature calyx of Held in response to 30 APs at 300, 400, and 500 Hz. Note the more frequent failures at the cKO synapses than at the control at the same frequency. (B) The larger fraction of synapses with transmission failures and the higher failure rate at variable frequencies. Note that the fraction of synapses with a failure, as indicated by the numbers adjacent to each mark (the number of synapses with a failure/the total synapse number recorded), was higher than control. The failure rate, calculated from the number of EPSC failures in the total APs in each trace, was also significantly higher in cKO. Only synapses with failures were analyzed here for both control and cKO synapses.
Fig. S3.
Decrease in neurotransmission fidelity at the mature calyx of Held from cKO mice. (A) Postsynaptic APs (30 overlapping events) recorded in MNTB neurons elicited by presynaptic stimulation at 10 Hz. (B) Average spike latency (1.22 ± 0.02 ms in control, n = 5 synapses; 1.37 ± 0.14 ms in cKO, n = 5 synapses; P = 0.32) and jitter (24 ± 2.5 µs in control, n = 5 synapses; 35 ± 5.6 µs in KO, n = 4 synapses; P = 0.09). (C) As in A but at 300 Hz stimulation. Note the frequent AP failures in the cKO synapses. Arrows indicate postsynaptic AP failures; arrowheads indicate complete lack of membrane-potential changes. (D) Representative EPSCs in response to sustained APs at a control MNTB neuron. EPSCs were evoked by presynaptic fiber stimulation for 2 min (100 Hz, 4 × 30 s). (Inset) EPSCs (arrow) on an expanded timescale. (E and F) Same as D but from individual cKO synapses. Note the frequent, complete failure of neurotransmission and the large EPSC amplitude immediately before the failure. (Insets) Expanded views at the end of stimulation (arrows). (G) The amplitude of EPSCs immmediately before the transmission failures at individual cKO synapses (n = 8). The average EPSC amplitude of five EPSCs immediately before the first failure in each recording was shown; none of five control cells had a failure within this time window and are not shown.
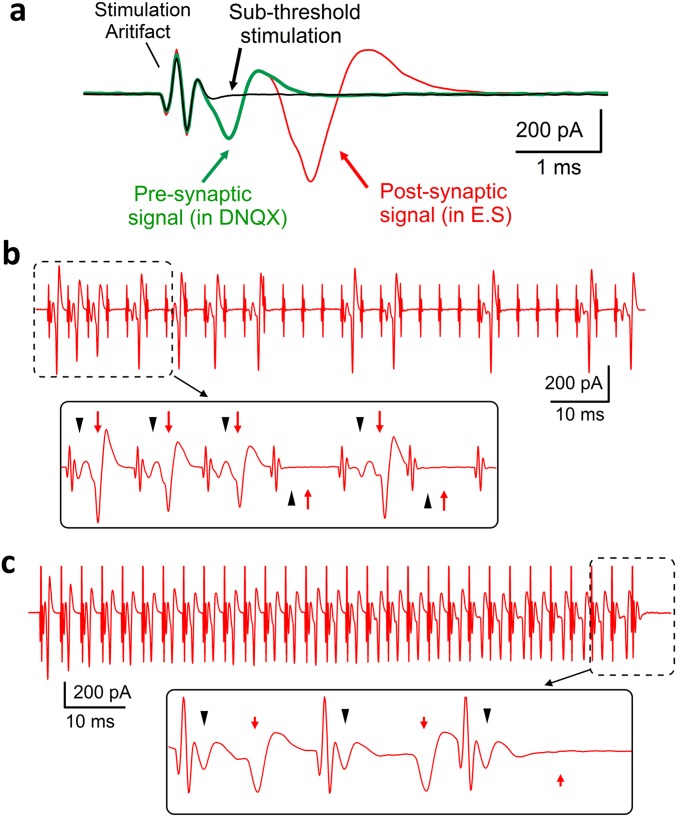
Fig. S4.
Pronounced presynaptic AP failures at the calyx of Held in dynamin-1 cKO. (A) Isolation of local electric signals generated from presynaptic and postsynaptic activities at the calyx of Held using a cell-attached, loose voltage-clamp. A representative example was shown to identify the signal arising from postsynaptic APs [blocked after acute 6,7-dinitroquinoxaline-2,3-dione (DNQX) application], presynaptic AP (identified by subthreshold stimulation), stimulation artifacts, and their combination at the same synapse. (B) A train of 30 EPSCs (300 Hz) recorded in a dynamin-1 cKO calyx of Held showed pronounced failures, many of which had no signals from presynaptic APs (arrowheads) and consequently from postsynaptic APs (arrow). (Inset) Expanded view of the boxed region. (C) Another example recorded from a cKO synapse. Note the selective disappearance of the postsynaptic signal at the last stimulation (Inset) with the presynaptic AP signal intact.
The Lack of Dynamin-1 Does Not Significantly Affect the Common Factors Underlying Synaptic Depression.
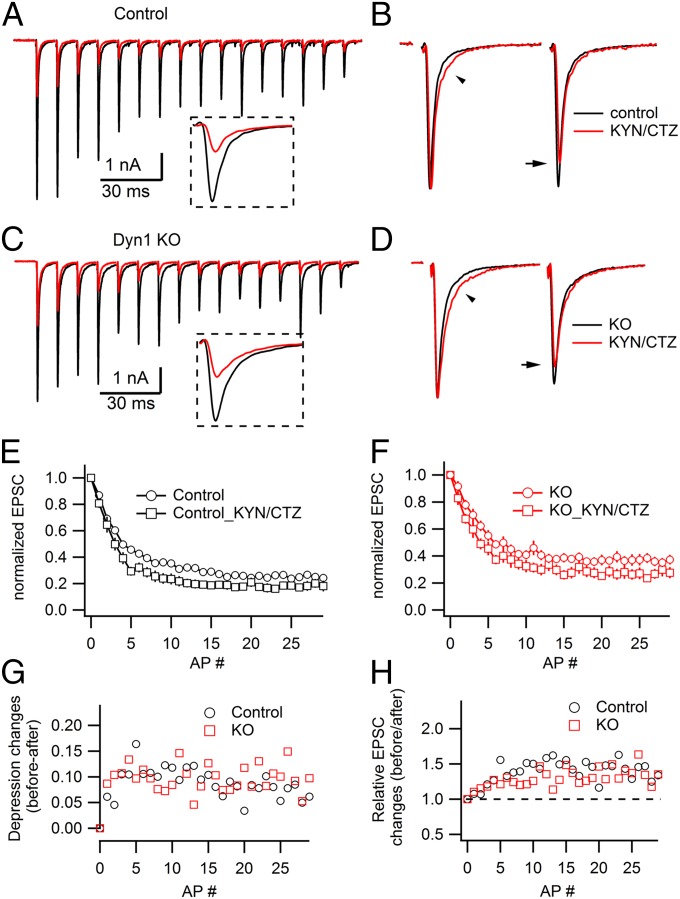
To further explore the mechanism underlying the altered short-term depression in cKO synapses, we examined whether other factors known to influence short-term depression (STD) (40–43) account for the results. First, the changes in postsynaptic receptor saturation and desensitization are known to contribute to synaptic depression in the juvenile calyx of Held (44, 45). We used 1 mM kynurenic acid (KYN) and 100 μM cyclothiazide (CTZ) to reduce AMPA receptor saturation and desensitization, respectively. We found similar effects of these drugs in both control and cKO synapses. Normalized EPSCs before and after KYN + CTZ application nearly overlapped, with only slightly slower EPSC decay under KYN + CTZ (Fig. 3 B and D). These results speak against a major role of AMPA receptor saturation and desensitization in the mature calyx of Held in both control and cKO, in agreement with the previous study (45). Under this condition, synaptic depression was slightly enhanced to an equal extent in both control and cKO (Fig. 3 E–H).
Fig. 3.
Postsynaptic receptor saturation and desensitization do not contribute to the smaller STD at mature cKO calyx of Held. (A) EPSC changes before (black) and after (red) the application of 1 mM KYN and 100 µM CTZ during a train of 30 APs at 100 Hz in a control synapse. (Inset) Enlarged view of the first EPSCs under two conditions. (B) The first two normalized EPSCs showed substantial overlap before and after KYN/CTZ exposure. Note the slightly slower EPSC decay kinetics (arrowhead) and a minor decrease in the second EPSC peak (arrow) after applying KYN/CTZ (red). (C and D) Similar to A and B, but in a cKO synapse. (E and F) Average changes of STD with and without 1 mM KYN and 100 µM CTZ in control (E) (n = 21 and 9 synapses without and with KYN/CTZ, respectively) and cKO synapses (F) (n = 35 and 10 synapses without and with KYN/CTZ, respectively). Both groups showed a small but significant decrease of STD (P < 0.05). (G and H) Equal contribution of postsynaptic factors to 100 Hz STD in both control and cKO groups.
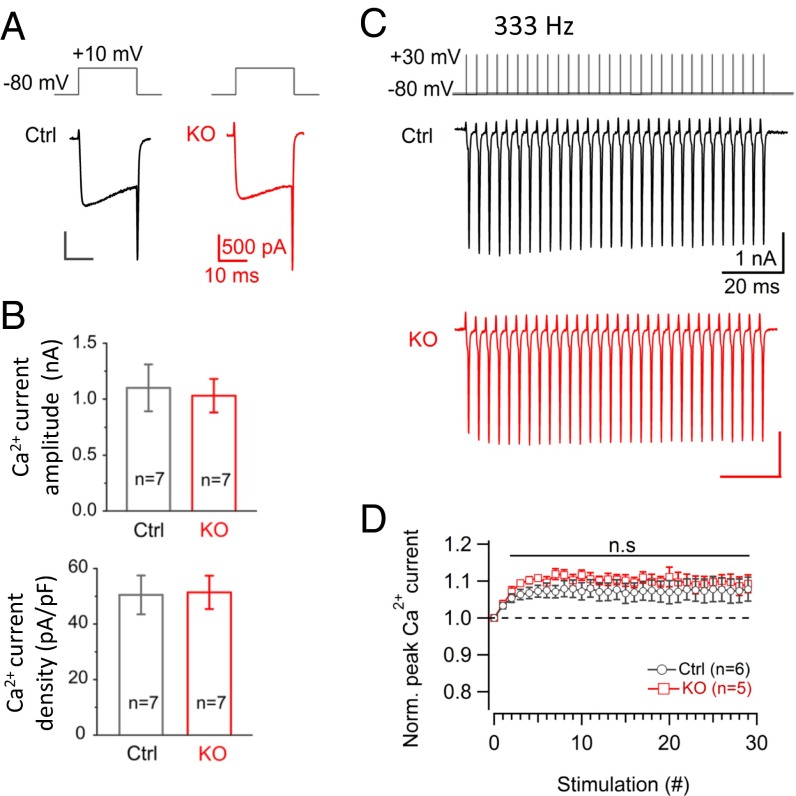
Second, a decrease in presynaptic Ca2+ influx through voltage-gated Ca2+ channels might effectively decrease synaptic depression (46–48) because of the supralinear relationship between intracellular Ca2+ and transmitter release (7, 49). A recent study suggests the presence of homeostatic regulation of presynaptic Ca2+ influx at the neuromuscular junction (50). To explore this possibility, we performed direct presynaptic whole-cell recordings at the P13–15 calyx of Held. There was no difference in Ca2+ current amplitude and density between control and cKO synapses (Fig. 4 A and B). A relevant question is activity-dependent Ca2+ current facilitation because it modulates neurotransmission even with undistinguishable Ca2+ influx under basal conditions (51). We thus applied 30 AP-like (AP-L) pulses (1 ms, +30 mV, 333 Hz) to mimic afferent AP activity (Fig. 4C). Both control and cKO synapses showed significant Ca2+ current facilitation, but there was no difference in their amplitudes between control (1.07 ± 0.031) and cKO (1.1 ± 0.021 for the average of last 10 points; P = 0.58) (Fig. 4D). This excludes the potential involvement of the changes in presynaptic Ca2+ influx and activity-dependent facilitation in the STD phenotype in cKO synapses.
Fig. 4.
Presynaptic Ca2+ influx and Ca2+ current facilitation in cKO synapses are comparable to control. (A) Representative presynaptic Ca2+ currents evoked by a single depolarizing pulse (+10 mV, 20 ms) at a calyx of Held from control and cKO mice (P13–15). (B) Ca2+ current amplitude and density between control and cKO synapses (n = 7 in each group). (C) The 333-Hz, AP-like 30 pulses (top, +30 mV, 1 ms) triggered a small but significant facilitation of presynaptic Ca2+ current both in control and in cKO synapses. (D) A comparable amplitude of Ca2+ current facilitation in control (n = 6) and in cKO (n = 5, P = 0.62 and 0.58 for the last 5- and 10-point averages, respectively) synapses.
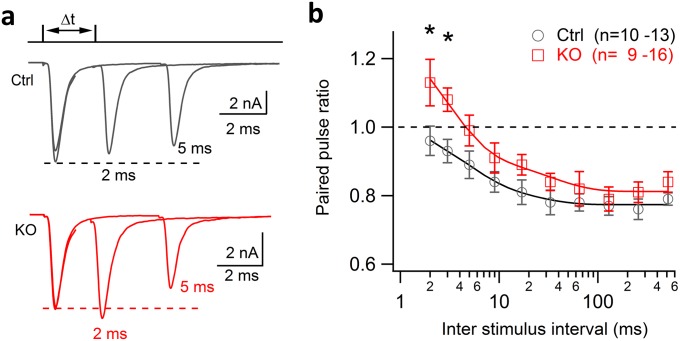
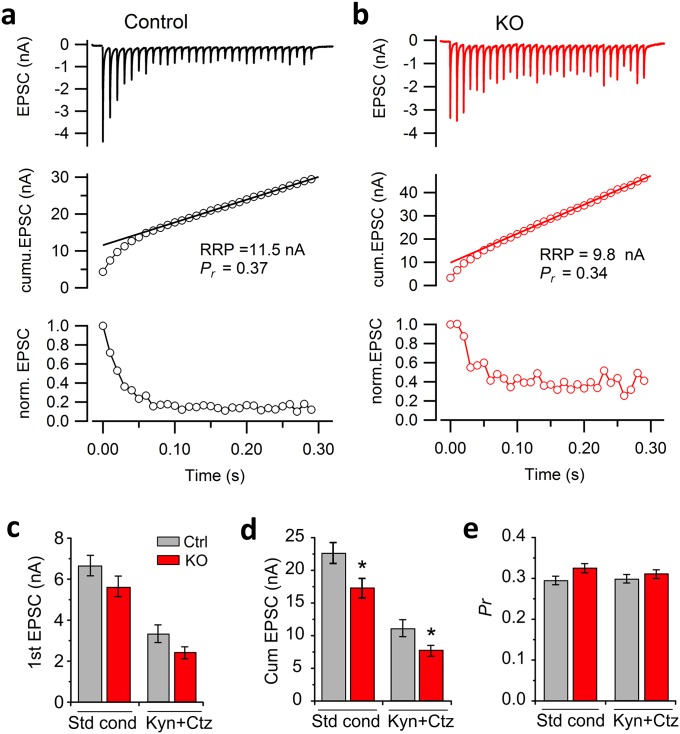
Third, a decrease in release probability (Pr) can cause less synaptic depression or even facilitation after the loss of dynamin-1, as we showed previously in dynamin-1, -3 double KO synapses (30), the terminals of which are largely vesicle-depleted and have accumulated abundant clathrin-coated profiles (29). We used two different approaches to assess a putative Pr change in the cKO calyx of Held. First, we examined the paired-pulse ratio (PPR) of EPSCs as a Pr indicator. We observed no significant difference in PPRs between control and cKO over a wide range of frequencies up to 200 Hz (Fig. S5). At 300 Hz or above, PPRs in cKO changed from depression to facilitation, indicating a selective enhancement of transmitter release under the high-frequency stimulation. It is noteworthy that the PPR is an indirect indicator of Pr that may have certain limitations (52). We therefore estimated Pr using an alternative approach by calculating the ratio of the first EPSC amplitude divided by its own readily releasable pool (RRP) in each synapse. The RRP size was measured using 100 Hz train stimulation in the presence of KYN + CTZ (Fig. S6) (44). We found no significant difference in Pr between control and cKO synapses, despite the fact that the RRP size was slightly smaller in cKO than in control (Fig. S6). Given the limitations of this method of estimating RRP (53), this method may underestimate RRP size, particularly in cKO which has the enhanced steady-state release. To accurately measure RRP, we turned to direct presynaptic capacitance measurements with strong depolarization (50 ms, 0 mV). This experiment demonstrated comparable RRP sizes between control and cKO (Fig. 7 A and B). These results, plus equal sizes of AP evoked EPSCs between two groups, suggest a similar Pr in cKO compared with control. Thus, an alteration in initial Pr appears unlikely to be the major factor that accounts for the reduced depression in cKO synapses, but the contribution of minor Pr decrease cannot be ruled out at very high frequencies (≥300 Hz).
Fig. S5.
Paired-pulse ratios at the mature calyx of Held from control and cKO mice. (A) EPSCs induced by paired APs at different intervals (Δt = 2, 3, 5, 9, 18, 36, 72, 144, and 288 ms) in a control (Top) and a cKO synapse (Bottom). Synapses were rested for at least 20 s between each trial. (B) Average changes of PPRs at different frequencies from control and cKO synapses. Note that there was no significant difference in PPRs between control and cKO groups over a wide range of frequency, but PPRs switched from depression to facilitation selectively at higher frequencies (>200 Hz).
Fig. S6.
The size of readily releasable vesicle pool and release probability estimated by a train of APs at 100 Hz. (A and B) RRP size estimation in control and in cKO calyces. EPSCs were elicited by 30 APs at 100 Hz and recorded from MNTB neurons in an ES containing 1 mM KYN and 100 µM CTZ (Top), and RRP size was estimated as the initial value in a linear back-extrapolation of the cumulative EPSC peak amplitude (Middle). Short-term depression curves for the respective control and cKO synapses are shown at the Bottom. (C–E) The average amplitude of the first EPSC (C), RRP size (D), and synaptic release probability (Pr) (E) without and with KYN/CTZ. Pr was estimated as the fraction of the first EPSC amplitude divided by the estimated RRP size (n = 9 and 10 for control and cKO synapses, respectively).
Fig. 7.
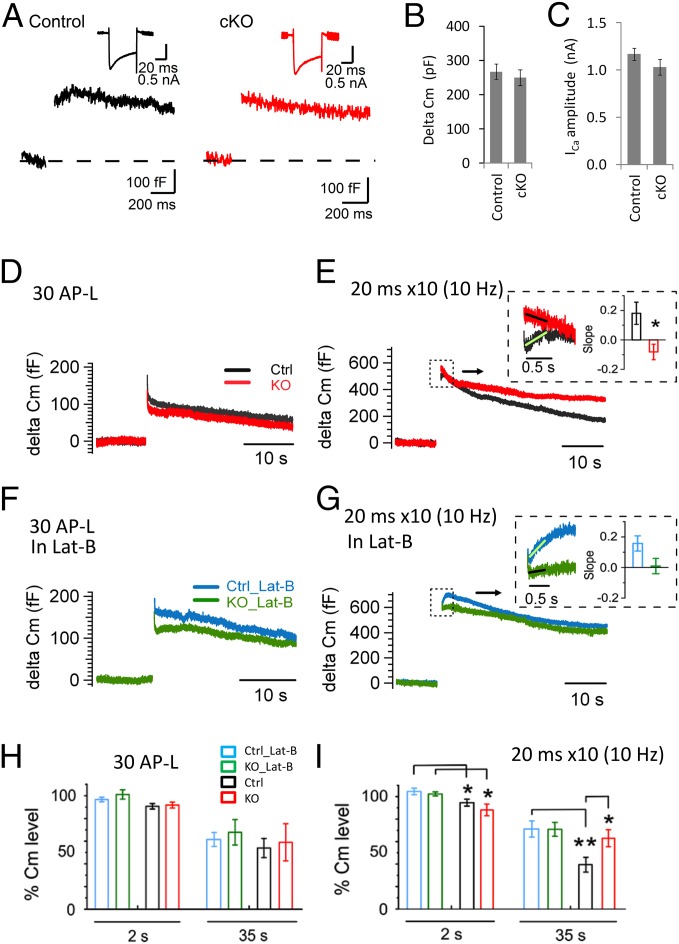
Exocytosis and endocytosis examined by capacitance measurements at the calyx of Held under the normal condition and in the presence of Latrunculin-B. (A) A typical Cm trace induced by a 50-ms depolarization (0 mV) at P8–10 calyx of Held. (B and C) Average delta Cm (control: 270 ± 22 fF, n = 12; cKO: 250 ± 23 fF, n = 16; P = 0.58) and presynaptic Ca2+ currents in control (1.17 ± 0.06 nA, n = 12) and cKO synapses (1.03 ± 0.08 nA, n = 16; P = 0.21). (D) Average presynaptic Cm changes at the calyx of Held (P8–10) under mild stimulation (30 AP-like pulses at 333 Hz, +30 mV for 1 ms). (E) Cm changes under strong stimulation (10 × 20 ms, 0 mV, 100-ms interval). (Inset) An expanded view of the average Cm traces within 1 s after stimulation (control slope: 0.18 ± 0.07 fF, n = 7; cKO slope: −0.08 ± 0.05, n = 5; P = 0.017). (F) Average Cm changes after the mild stimulation in the presence of Lat-B (15 μM). (G) Average Cm changes after strong stimulation in the presence of Lat-B (15 μM). (Inset) The expanded view of Cm changes and slopes in control and in cKO (control: 0.16 ± 0.05, n = 6; cKO: 0.0053 ± 0.05, n = 7; P = 0.052). (H) Summary of Cm decays after mild stimulation (30 AP-L at 333 Hz) under the normal condition and in Lat-B for control and cKO. The data showed the relative Cm levels remaining at 2 s (control: 90.5 ± 2.4%, n = 7; cKO: 91.5 ± 2.6%, n = 8; P = 0.8) and at 35 s from the end of stimulation (control: 53.9 ± 8.4%, n = 6; cKO: 58.8 ± 11.5%, n = 6; P = 0.75). In the presence of Lat-B, the Cm levels at 2 s (control: 97.0 ± 2.0%, n = 7; cKO: 101.2 ± 4.1%, n = 8; P = 0.35) and at 35 s (control: 61.5 ± 6.1%, n = 7; cKO: 67.8 ± 11.2%; P = 0.64). (I) As in H but after strong stimulation (20-ms depolarization × 10 at 10 Hz). The Cm levels after strong stimulation at 2 s (control: 94.4 ± 3.1%, n = 7; cKO: 88.0 ± 5.2%, n = 5; P = 0.33) and at 35 s (control: 39.4 ± 6.7%, n = 7; KO: 62.9 ± 7.6%, n = 5; P = 0.04). In the presence of Lat-B, the Cm levels at 2 s (control: 105 ± 2.9%, n = 6; cKO: 102.3 ± 1.8%, n = 7; P = 0.53) and at 35 s (control: 71.2 ± 7.3%, n = 6; cKO: 70.9 ± 6.2%, n = 7; P = 0.98). Student’s t test was used for statistical comparison between the two groups in H and I.
Vesicle Resupply Is Intact After Brief High-Frequency APs in the Absence of Dynamin-1.
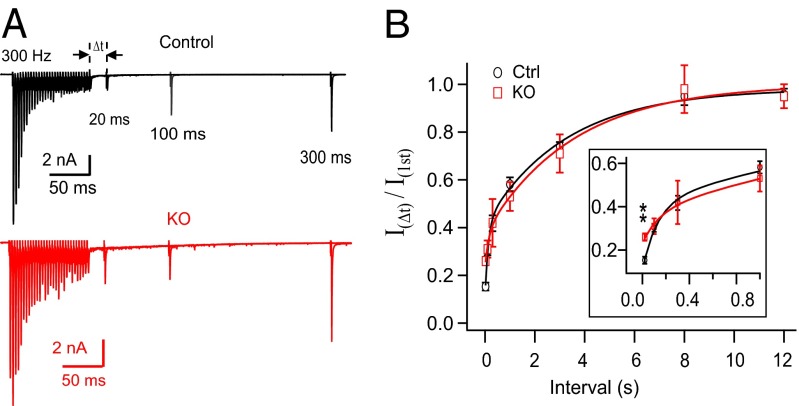
The balance between vesicle pool depletion and rapid vesicle replenishment during high-frequency stimulation is crucial for short-term plasticity. Genetic perturbation of endocytic proteins often leads to a delay in vesicle resupply, as shown in mutants of endophilin (54–56), synaptojanin (57, 58), and dap160/intersectin-1 (59). Intersectin-1 KO synapses in mice showed deficient vesicle replenishment even without a detectable change in capacitance decays (60). We tested the vesicle resupply rate by examining the recovery of transmission evoked by an AP at different intervals following each high-frequency stimulation (30 APs at 300 Hz) (Fig. 5A). Transmission recovery was measured as the ratio of the test EPSC amplitude relative to the first EPSC in each train. Consistent with the two phases of Ca2+-dependent recovery (61), transmission recovered with a double-exponential time course, with no significant difference between control (τfast = 0.13 s, 30%; τslow = 3.3 s, 57%) and cKO synapses (τfast = 0.15 s, 14%; τslow = 3.5 s, 63%) (Fig. 5B). This indicates a normal rate of vesicle resupply following brief high-frequency stimulation in cKO, which is not surprising given the presence of a large pool of recycling vesicles in this type of synapses (40, 62, 63). It is noteworthy that the recovery curve in cKO started with a significantly higher initial level (0.26 ± 0.017, n = 5) than in control (0.15 ± 0.016, n = 12, P < 0.01) (Fig. 5A). This is consistent with less depression at steady state in cKO synapses (Fig. 2C).
Fig. 5.
Synaptic transmission recovery from the short-term depression is intact in the absence of dynamin-1. (A) Synaptic transmission at different time intervals (Δt = 0.02, 0.1, 0.3, 1, 3, 8, 12 s) after the 300-Hz AP train. Synapses were allowed to rest for at least 20 s between trials. (B) The time course of the transmission recovery curves from STD in control (τfast = 0.13 s, 30%; τslow = 3.3 s, 57%; n = 12) and in cKO (τfast = 0.15 s, 14%; τslow = 3.5 s, 63%; n = 5) synapses were similar. Only synapses without failure were analyzed for both control and cKO groups. (Inset) The recovery curves at expanded time scales to show the significantly higher starting level of recovery in cKO synapses.
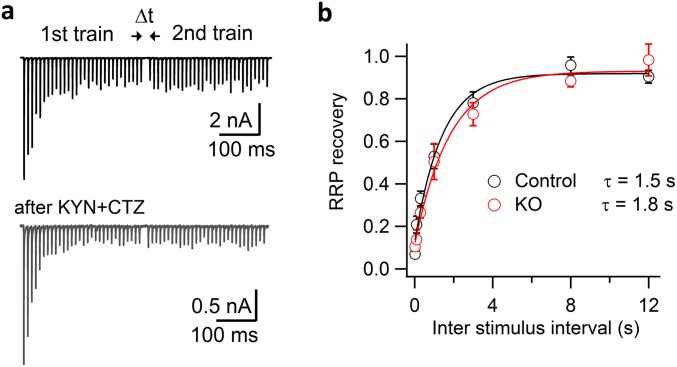
As an alternative estimate of vesicle resupply, we measured the RRP recovery rate with a pair of 100-Hz trains at different time intervals (Δt) in the presence of 1 mM KYN and 100 μM CTZ (Fig. S7A). We found that RRP recovery was unchanged in cKO synapses (τ = 1.5 s) compared with control (τ = 1.3 s) (Fig. S7B). These data are consistent with intact vesicle resupply (Fig. 5) and also agree with the intact RRP recovery after dynamin inhibition by pipette infusion of 0.3 mM GTPγS (64). Therefore, a lack of dynamin-1 does not affect the vesicle resupply to AZs following brief high-frequency stimulation at the mature calyx of Held.
Fig. S7.
Recovery of readily releasable vesicle pool at the calyx of Held in control and cKO. (A) Representative paired EPSC trains (30 EPSCs in each) recorded in a control synapse at 100 Hz and separated at different time intervals (Δt = 0.02, 0.1, 0.3, 1, 3, 8, and 12 s). Both 1 mM KYN and 100 µM CTZ were added in the ES during recordings. RRP size at different times was estimated as in Fig. S6. (B) RRP recovery curves in control (τ = 1.5 s, n = 6) and in cKO (τ = 1.8 s, n = 4) synapses were unaltered; the recovery fraction was estimated as the ratio of RRPs derived from the second train divided by the one from the first train at different time intervals.
Actin Depolymerization Has a Stronger Effect on Synaptic Depression in cKO than in Control Synapses.
It is known that dynamin regulates actin function through direct and indirect interactions (65) and that the loss of dynamin strongly enhances actin reorganization (34, 66). Therefore, actin may differentially contribute to endocytosis, as well as transmitter release, in cKO and control synapses. Indeed, previous studies of ultrastructure reported an increase in bulk endocytosis—a fast and efficient membrane retrieval mechanism primarily induced during very intense stimulation—in synapses lacking dynamin-1 (67). In addition, it was shown that actin plays an important role in bulk and ultrafast endocytosis (68–72). These observations raise the possibility that an actin-dependent increase in bulk endocytosis in cKO could make release site clearance more efficient than in control during high-frequency release. If this is the case, inhibition of actin polymerization and bulk endocytosis is expected to slow down endocytosis and release site clearance and thereby accelerate the depression.
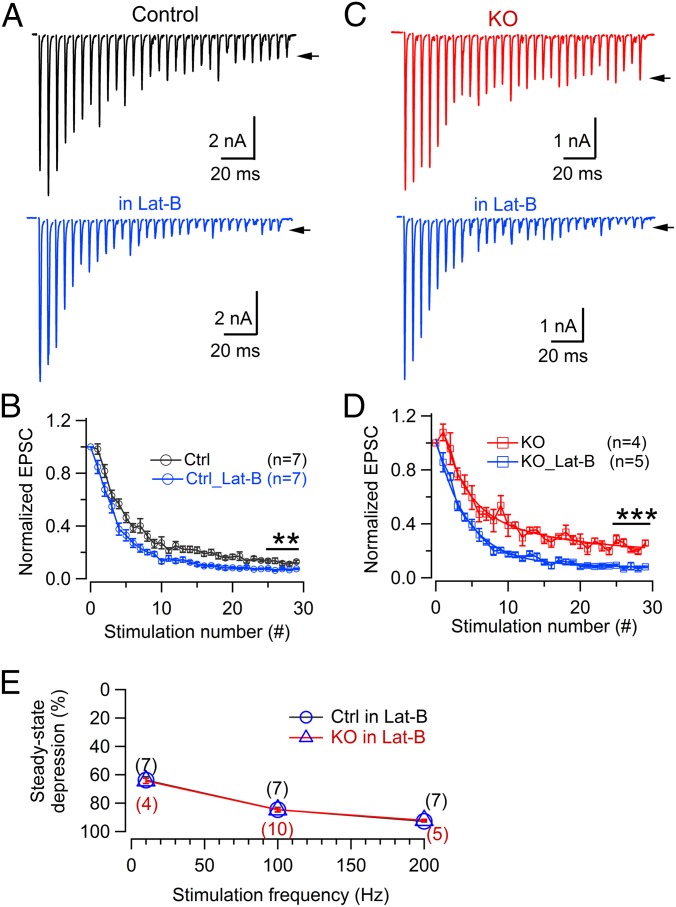
To test this idea, we acutely inhibited actin polymerization using Latrunculin-B (Lat-B, 15 μM) (73) in brain slices. Lat-B caused a significant decrease of steady-state release after a 200-Hz AP train (Fig. 6 A and B) in control, without significantly changing the initial EPSC amplitude (−9.3 ± 1.4 nA with Lat-B, −8.45 ± 1.2 nA without Lat-B; n = 7 each; P = 0.64). This is consistent with a previous study (73). However, Lat-B in cKO produced a much greater effect on synaptic depression at 200 Hz and brought synaptic depression to the same level as in control (7.2 ± 0.5%, n = 7 for control; 7.8 ± 0.8%, n = 5 for cKO; P = 0.46) (Fig. 6 B and D). Lat-B abolished the difference in the depression levels between control and cKO at both low and high frequencies (Fig. 6E). These data, together with normal transmission recovery, are consistent with the possibility that we mentioned above.
Fig. 6.
Disruption of actin polymerization eliminates the difference in STD between control and cKO synapses during high-frequency stimulation. (A and B) Latrunculin-B (15 μM) marginally enhanced steady-state depression in control synapses at 200 Hz (30 APs) without significantly changing EPSC amplitude. Two recordings shown in A were from different terminals in control mice; arrows indicate the peak of the last EPSC. Note the small STD difference (0.13 ± 0.01, no Lat-B, n = 7 synapses; 0.072 ± 0.005, with lat-B, n = 7 synapses; P = 0.006). (C and D) The Lat-B effect in cKO synapses. Note that steady-state depression under the normal condition in cKO (0.24 ± 0.02, n = 4 synapses) comes down strongly in the presence of Lat-B (0.078 ± 0.008, n = 5 synapses; P < 0.001), reaching a level that is comparable to controls. (E) Steady-state depression levels in the presence of Lat-B (calculated as the average of the last 5 points in each train of individual recording) at different stimulation frequencies. The number in each parentheses indicates the synapse number recorded at that frequency; three to seven mice were used for each data point.
The Properties of Presynaptic Endocytosis in the Calyx of Held from Dynamin-1 cKO Mice.
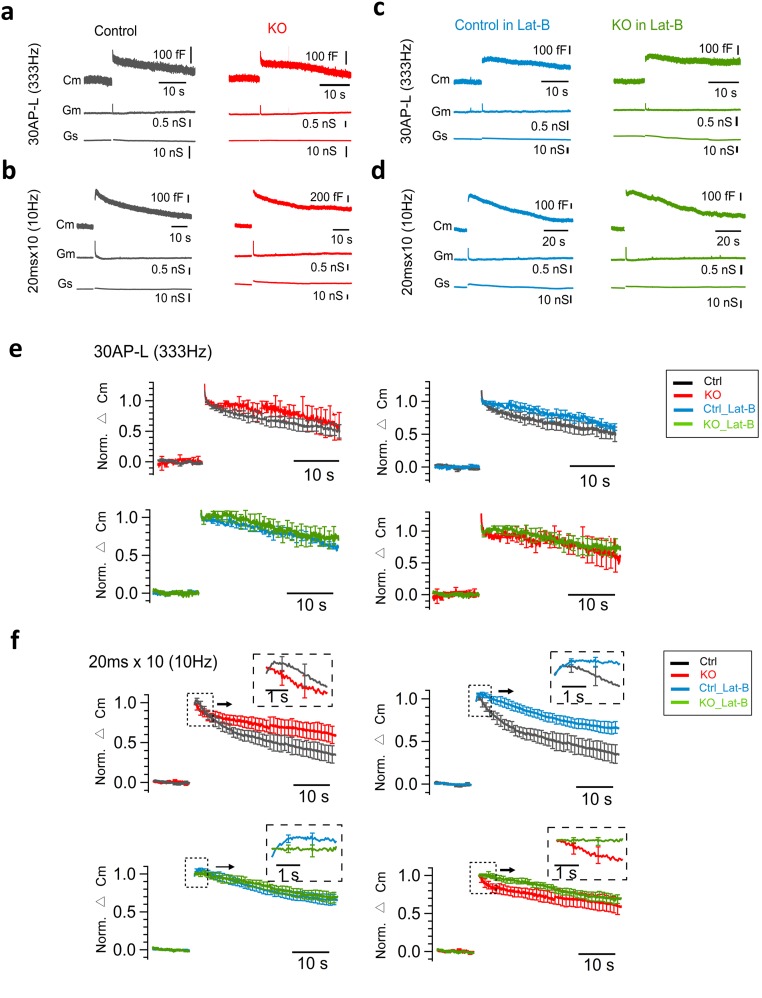
We performed direct presynaptic membrane capacitance (Cm) recordings and observed a large Cm increase at P8–10 calyx of Held. After a 50-ms depolarization (0 mV, with 0.2 mM Cs–EGTA in the pipette solution), the amplitudes of the Cm increases and presynaptic Ca2+ currents were comparable between control and cKO synapses (P = 0.58 and 0.21, respectively) (Fig. 7 A–C), suggesting similar sizes of RRPs between the two groups. Next, we monitored the dynamics of endocytosis after different stimulation protocols and characterized the Cm levels of a fast phase (at 2 s) and a slow phase (at 35 s) (with 0.5 mM Cs–EGTA in the pipette solution). We first applied mild stimulation using 30 AP-L pulses at 333 Hz to mimic high-frequency AP activity. Both control and cKO synapses showed moderate exocytosis followed by similar levels of Cm recovery at 2 s and at 35 s (Fig. 7 D and H). After strong stimulation with depolarizing pulses (10 × 20 ms, 0 mV, at 10 Hz); however, we observed a large Cm increase (524 ± 54 fF, n = 7 for control; 555 ± 62 fF, n = 5 for KO; P = 0.84) (Fig. 7E). Again, the decay was biphasic with the second phase significantly slower in cKO than in controls (Cm level at 35 s in control: 39.4 ± 6.7%, n = 7; KO: 62.9 ± 7.6%, n = 5; P = 0.04) (Fig. 7 E and I). The fast phase of Cm decay (at 2 s) in cKO tended to be faster (decreased to 88.0 ± 5.2% of its peak, n = 5) than in control (to 94.4 ± 3.1%, n = 7; P = 0.33). These data agree well with our previous work showing a pronounced impairment in the second Cm decay phase after strong but not after mild stimulation (28), as well as impaired clathrin-coated endocytosis under the electron microscope in dynamin-1 KO terminals (26, 28, 67). Remaining dynamin-2 and -3 may be sufficient to support low levels of endocytosis and transmission in the absence of dynamin-1.
It was reported that bulk endocytosis as observed by electron microscopy is coupled in time to strong stimulation (67, 74). We reasoned that Cm changes immediately after strong stimulation may capture, at least a part of, bulk endocytosis. We therefore focused on Cm changes within half a second after depolarization. It is known that Cm changes in this time window can be affected significantly by many factors, such as gating currents (75, 76), high levels of asynchronous release after strong stimulation, and/or other processes irrelevant to transmitter release (77, 78). Therefore, they are often disregarded in Cm studies of endocytosis, including in our previous work (28). On the other hand, direct comparison of these signals between control and cKO under the same experimental conditions may still provide useful information on the fast endocytosis because these factors are expected to be equally present in both groups. We found that initial Cm decreases right after weak stimulation (30 AP-L at 333 Hz) were similar between control and cKO (Fig. 7D), confirming equal contributions of these various factors under the same stimulation condition. Cm transients immediately after strong stimulation (10-Hz, 20-ms train), however, were clearly different in cKO, compared with control (Fig. 7E, Inset). Most cKO synapses (four of five cells) showed a net decrease in initial Cm (cKO slope: −0.08 ± 0.05, n = 5) during half a second after the train (Fig. 7E, Inset), contrasting with transient Cm increases (control slope: 0.18 ± 0.07 fF, n = 7; P = 0.017) observed in the majority of control synapses (five of seven).
Next, we examined the role of actin depolymerization induced by Lat-B (15 μM) on Cm changes. With mild stimulation (30 AP-L at 333 Hz), Lat-B slightly increased the exocytosis and showed a trend of slowing down the Cm decay at 2 s in both control and cKO, although differences were not statistically significant (Fig. 7 F and H and Fig. S8 A, C, and E). With the strong stimulation (10 × 20 ms at 10 Hz), however, Lat-B nearly abolished the fast Cm decay (Fig. 7G, Inset and Fig. S8 B, D, and F) and significantly reduced the membrane retrieval during the first 2 s in both control (P = 0.035) and cKO synapses (P = 0.048) (Fig. 7I) (compared with the normal conditions without Lat-B; Fig. 7E, Inset and Fig. S8 B and F). The slow phase (at 35 s) was significantly slower in control (P = 0.0084), but not in cKO synapses (P = 0.43, compared with those without Lat-B) (Fig. S8 B, D, and F). We closely examined initial Cm changes within half a second after stimulation and compared the responses between conditions with and without Lat-B (Fig. 7 E and G, Insets). We found that Lat-B abolished the fast Cm decrease and led to a nearly flat or even increasing Cm trace in cKO. There was only a minor difference in Cm changes in control between conditions with and without Lat-B application. Cm slopes between control (0.16 ± 0.05, n = 6) and cKO (0.0053 ± 0.05, n = 7; P = 0.052) in the presence of Lat-B were comparable. Despite the technical limitations, these data indicate that actin-dependent fast endocytosis (e.g., bulk endocytosis) may become important under strong stimulation to balance the rapid exocytosis load and that Lat-B has different effects on Cm decays between cKO and control.
Fig. S8.
Cm changes in response to mild and strong stimulations with and without Latrunculin-B. (A and B) Representative Cm recordings from control and cKO. Cm, Gm, and Gs traces are shown in parallel in response to 30 AP at 333-Hz stimulation (A) and 10 × 20 ms at 10 Hz (B). (C and D) Same as in A and B, but in the presence of Lat-B (15 μM). Note the different effect of Lat-B on the Cm trace immediately after depolarization. (E) Comparison of the normalized Cm traces between control and cKO with and without Lat-B under 30 AP at 333 Hz. There is no significant change among groups. (F) As in E, but under 10 × 20 ms at 10-Hz stimulation. Note that Lat-B has a different effect on Cm trace changes at different time windows between control and cKO (F, Right panels). The fast Cm decreases in cKO were nearly abolished by Lat-B (Right Lower). (Insets) Expanded view of Cm traces immediately after depolarization.
Discussion
We examined synaptic transmission at the mature calyx of Held using tissue-specific dynamin-1 KO and found an unexpected reduction in synaptic depression during brief high-frequency AP stimulation. This reduction in synaptic depression in cKO depends on neither vesicle availability to AZs nor other common factors underlying synaptic depression, but it does rely on actin polymerization. Actin depolymerization differentially affects the short-term depression in control and cKO, as well as endocytosis. Our finding indicates an important role of dynamin-1 in repetitive transmitter release and short-term synaptic depression in native brain circuitry, in addition to its established role in regenerating and supplying new synaptic vesicles for long-term neurotransmission.
We have systematically characterized synaptic transmission in the cKO calyx of Held, including mEPSC amplitude and frequency, evoked EPSCs, action potential waveform, presynaptic Ca2+ influx and facilitation, postsynaptic receptor saturation and desensitization, RRP size, and vesicle resupply rate. These factors are largely intact in cKO synapses and unlikely to account for the reduction of synaptic depression observed in cKO.
One simple explanation for this phenotype is a Pr decrease in the cKO calyx of Held. A larger PPR at frequencies above 300 Hz appears to support this idea (Fig. S5). However, PPRs were not statistically different over a wide range of frequencies (<300 Hz) despite their tendency to be slightly higher. Other assessments of release probability (using the ratio of single AP-evoked EPSC divided by the RRP) pointed toward a similar initial Pr in the two groups. These observations suggest that a Pr change in cKO synapses, if it exists, should be very small. Therefore, a Pr decrease in cKO appears unlikely to be the major factor to account for the depression phenotype in cKO. Nevertheless, a contribution of a Pr change cannot be ruled out at very high frequencies (e.g., >300 Hz).
Another possible explanation for the decreased depression in cKO is an enhanced release site clearance at AZs. This process might rapidly become rate-limiting during high rates of transmitter release (8, 15). Rapid local membrane remodeling and protein sorting/diffusion may facilitate site clearance at the subsecond timescale through fast membrane retrieval (long before slow endocytosis comes into play). Multiple forms of fast endocytosis that have high efficiency in retrieving membrane, such as bulk endocytosis and ultrafast endocytosis, have been documented (2, 14, 28, 67, 74, 79, 80). It has also been reported that bulk endocytosis as observed by electron microscopy increases significantly (approximately twofold) during intense stimulation in the absence of dynamin-1 (27, 67). It is likely that an up-regulated actin-dependent mechanism (e.g., bulk endocytosis) in cKO promotes release site clearance during high-frequency stimulation, leading to enhanced transmitter release and reduced depression. If so, the availability of release sites might rapidly emerge as a rate-limiting step during brief high-frequency release in the calyx of Held. Future work using novel techniques, such as fast superresolution imaging (21, 81) or pulse-chase flash-freezing electron microscopy (14), is required to investigate the local membrane dynamics, protein sorting, and diffusion at the millisecond timescale. Nanometer resolution imaging of AZs under high rates of transmitter release conditions needs to be achieved. New approaches that can selectively regulate fast endocytosis are also needed to address this fundamental question.
The greater effect of Lat-B on synaptic depression in cKO compared with control (Fig. 6) correlates well with its larger effect on the inhibition of the Cm decrease immediately after a depolarization in cKO (Fig. 7 E and G, Insets), indicating that both processes may be related through an actin-dependent mechanism. Dynamin can regulate actin function through direct and indirect interactions (65, 82), and actin is important for bulk (68–70) and ultrafast endocytosis (72). Interestingly, dynamin KO leads to alteration of actin polymerization and reorganization in other types of cells (34, 66). A role for actin in vesicle recruitment has also been reported (73, 83, 84). Similarly, Lat-B caused an apparent enhancement in short-term depression in our study. This effect can be explained by inadequate vesicle recruitment to AZs and/or insufficient clearance of SV components (e.g., SNARE proteins) from AZs; the latter may prevent the rapid reavailability of the limited release sites for new rounds of vesicle docking and priming during high rates of release. We favor the latter interpretation under our experimental conditions because the vesicle resupply rate in cKO is comparable to that in the control (Fig. 5 and Fig. S7).
The role of dynamin in short-term synaptic depression has been assessed in previous work with different results, using the shibire mutant in Drosophila (18) or dynasore (20, 21). It is unclear how these differences come out. A common property in previous dynamin inhibition studies is that GTPase-inactivated dynamin is not physically removed from synapses. Dominant-negative effects of these inactivated dynamin molecules and/or some off-target effects of inhibitors (85) may explain, at least in part, the discrepancy. For example, the inactivated dynamin contains multiple interacting domains that may sequester their interaction partners (such as endophilin and syndapin) in clathrin-coated pits (CCPs) and result in a different endocytic defect. This effect is highlighted by a study showing that the accumulated CCPs on the plasma membrane (PM) in shibire mutants are converted into large bulk cisternae following the further photo-inactivation of dynamin (all of the domains) (86). The dynamin inhibitors or else inactivation can have off-target effects. For example, dynasore affects actin polymerization (in dynamin-1, -2, and -3 triple KO cells) (85), intracellular Ca2+ levels, mEPSC frequency (87), and bulk endocytosis possibly through actin perturbation or syndapin sequestration (68, 85).
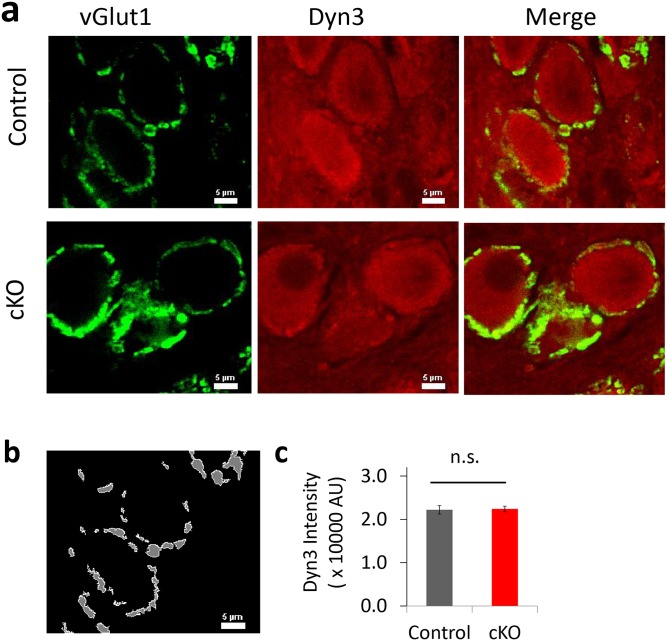
Developmental compensation appears unlikely to account for the results of cKO because compensation should weaken or mask the phenotype (i.e., no changes in depression), but we observed the opposite (i.e., significantly less depression). In addition, the cKO mice were outwardly normal, and their nerve terminals showed no compensatory dynamin-3 increases (Fig. S9). The puncta redistribution of dynamin-3 observed in neuronal cultures (26) was not evident in the cKO calyx of Held.
Fig. S9.
Dynamin 3 fluorescence in the calyx of Held in cKO mice. (A) Representative images of double-color immunohistochemistry of the calyx of Held at P20 control and cKO mice. Antibodies against vGlut1 and dynamin 3 are shown in green and red with the same settings for imaging acquisition and display. (B) An example of the presynaptic regions defined by the vGlut1 fluorescence threshold (white) that were used for the quantitative analysis of dynamin 3 levels. (C) No significant change in anti-dynamin 3 fluorescence levels in the presynaptic terminals between control and cKO (n = 30–50 calyces for each group).
Discrepancies between dynamin KO and dynamin inhibition have been consistently reported in other studies. For example, synaptic ultra-structures are very different after stimulation between shibire mutants (nearly empty terminals with abundant CCPs on the PM) (88) and dynamin KO (abundant large vacuoles and some CCPs) (27, 29, 67). Likewise, a spontaneous dynamin-1 point mutation in fitful mice causes enhanced depression (32) with a phenotype similar to shibire, albeit less severe, highlighting the discrepancy between dynamin-1 inhibition and deletion (KO) in the same species. Remarkably, despite these discrepancies, our findings and previous studies (18, 20, 21) point to a potential regulatory effect of the same process during exocytosis and endocytosis coupling, implying that alterations in release site clearance may be an overlooked mechanism for modulation of short-term plasticity during high-frequency transmitter release.
Materials and Methods
Generation of Conditional Dynamin-1 KO Selectively in Mouse Auditory Brainstem.
The tissue-specific dynamin-1 conditional KO mice were generated by crossing the Dnm1f/f mice (34) and the Krox20Cre mice (36). The KO mice were viable and fertile with similar body weight and size, which contrasts with the short life span (<2 wk) and much smaller body size (1/3 of the control size or less at P12) of the conventional dynamin-1 KO mice after out-breeding with the CD1 strain (28). Littermates carrying no Krox20Cre were used as controls unless otherwise specified. All animal care and use were approved by Animal Care and Use Committees at the University of Wisconsin–Madison. See SI Materials and Methods for details.
Immunofluorescence Staining and Confocal Imaging.
Mouse brains (P18–20) were fixed and cut into 40- to 60-µm slices. Immunohistochemistry and confocal fluorescence imaging were performed as described previously (66). See SI Materials and Methods for details.
Brain Slices, Patch Clamp, and Presynaptic Capacitance Measurements.
Brain slices (180–200 µm) containing MNTBs were prepared from P16–20 mice unless otherwise specified, and patch clamp recordings were made as previously described (44). The extracellular solution (ES, in mM): 120 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 25 glucose, 3 myoinositol, 2 Na-Pyruvate, 0.4 (l-)ascorbic acid (pH 7.4 with 95% O2 and 5% CO2 bubbling). Strychnine–HCl (2 µM), 10 µM bicuculline, and 50 µM D-AP5 were routinely included in extracellular solution for EPSC recordings. EPSCs were evoked by a bipolar electrode, and no offline Rs correction was applied. Cm was measured using the Sine+DC technique under whole-cell patch clamp as described previously (28, 66). Cm levels (%) after endocytosis recovery in fast and slow phases were calculated at 2 and 35 s, respectively. For details of EPSC recordings and Cm measurements, see SI Materials and Methods. All experiments were conducted at room temperature (20–22 °C).
Data Analysis and Statistics.
Electrophysiology data were analyzed with IgorPro (WaveMetrics). Values were presented as mean ± SEM, statistical analyses were performed using two-tail Student’s t test, and significance level was set at P < 0.05, denoted with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001). See SI Materials and Methods for details.
SI Materials and Methods
Generation of Conditional Dynamin-1 KO Selectively in Mouse Auditory Brainstem.
The tissue-specific dynamin-1 conditional KO mice were generated by crossing the Dnm1f/f mice (34) in which exons 2–4 were flanked by lox-P sites and the Krox20Cre mice (36). The Krox20Cre strain carries a Cre-encoding sequence at the locus of Krox20 (also known as EGR2), a zinc-finger transcriptional factor gene expressed primarily in the auditory brainstem including the calyx-generating globular bushy cells in the ventral cochlear nucleus. We obtained Dnm1f/+ Krox20Cre/+ mice in the first generation, and then these mice were mated with Dnm1f/f and gave rise to Dnm1f/Δ Krox20+/Cre, Dnm1f/+ Krox20+/Cre, Dnm1f/Δ Krox20+/+, and Dnm1f/+ Krox20+/+ due to germline recombination (36). Dnm1f/Δ Krox20+/Cre mice were further crossed with Dnm1f/f to obtain the conditional dynamin-1 KO (Dnm1f/Δ Krox20+/Cre), in which Cre expression deletes the floxed Dnm1 gene selectively in Krox20Cre-positive cells from embryonic day 9–10 (E9–10, expression onset of Krox20Cre) onward (36). Littermates carrying no Krox20Cre were used as controls unless otherwise specified. The KO mice were viable and fertile, with similar body weight and size, which contrasts with the short life span (<2 wk) and much smaller body size (1/3 of the control size or less at P12) of the conventional dynamin-1 KO mice after out-breeding with the CD1 strain (28). Animal use and care were approved by the animal care and use committee at the University of Wisconsin–Madison.
Immunofluorescence Staining and Confocal Imaging.
Mouse brains on P18–20 were dissected and fixed overnight with 4% (wt/vol) paraformaldehyde + 4% (wt/vol) sucrose in 0.12 M sodium phosphate buffer. Brain regions containing the calyx of Held were cut into 40- to 60-µm sections using a microtome (VT1200, Leica) and placed on glass slides. Tissue sections were permeabilized and blocked with 0.4% TritonX-100 + 3% (wt/vol) BSA + 2% (vol/vol) goat serum for 1 h and incubated with primary antibodies for 2 h. After a thorough wash, sections were incubated with fluorescent dye-conjugated secondary antibodies for 50 min and sealed with Fluoromount-G (Southern Biotech, 0100–01) and coverslips. Antibodies against dynamin-1 (Epitomics, 1851–1; rabbit, 1:200), vesicle glutamate transporter-1 (Millipore, Ab5905; guinea pig, 1:800) and the secondary antibodies conjugated with Alexa Fluorophore 488 and 561 (Invitrogen) were used for immunohistochemistry.
Fluorescence images were taken under a spinning disk confocal microscope with a 100× objective (APO 100× oil, N.A. 1.49). Samples were excited with different lasers; emission fluorescence was filtered with proper band-pass filters that match with emission wave length and was collected by an EMCCD camera (iXon ×3 DU897, Andor) (66). Images were acquired with the same imaging settings (e.g., laser intensity, exposure time, EM gain) and displayed at the same contrast levels for control and cKO synapses using NIS-Elements AR imaging software (version 4.12.01, built 888).
Brain Slices, Patch Clamp, and Presynaptic Capacitance Measurements.
Brain slices (180–200 µm) containing the MNTB were prepared from P16–20 mice unless otherwise specified, and patch clamp recordings were made as previously described (44). Postsynaptic patch pipettes had resistances of 3–5 MΩ. The series resistance (Rs) was compensated by 60–80% during recording so that the remaining Rs after compensation was no more than 3 MΩ (and only recordings with Rs < 15 MΩ were analyzed). Holding membrane potential (Vh) was set at −80 mV, and data were acquired at 20 kHz in most cases. All experiments were conducted at room temperature (20–22 °C).
EPSCs were evoked by a bipolar electrode placed on the calyx input axon fibers between MNTB and the brain slice midline. No offline Rs correction was applied. The ES (in mM) was composed of the following: 120 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 25 glucose, 3 myoinositol, 2 Na-Pyruvate, 0.4 (l-) ascorbic acid (pH 7.4, with 95% O2 and 5% CO2 bubbling). Freshly prepared 2 µM strychnine–HCl, 10 µM bicuculline, and 50 µM D-AP5 were routinely included in extracellular solution for EPSC recordings. mEPSC were extracted from the spontaneous EPSCs recorded at resting condition (no TTX). The 0.1 mM cyclothiazide and 1 mM kynurenic acid were added to the ES to measure the RRP. The patch pipette solution [intracellular solution (IS), in mM] for postsynaptic recordings was composed of the following: 137 Cs–gluconate, 10 Hepes, 20 TEA–Cl, 5 Na2–phosphocreatinine, 4 Mg-ATP, 0.3 Na2GTP, 5 Cs–EGTA, and 2 QX-314 (pH = 7.2). For presynaptic current-clamp recordings, IS contained (in mM): 137 K-gluconate, 10 Hepes, 5 Na2–phosphocreatinine, 4 Mg–ATP, 0.3 Na2GTP, 0.2 K–EGTA (pH = 7.2). Latrunculin-B (15 μM, ab144291, Abcam) was added to the ES during the recording, and experiments were performed between 10 and 60 min of continuous perfusion of Latrunculin-B. D-AP5 was from Tocris, Latrunculin-B from Abcam, and the other chemicals were from Sigma.
Presynaptic Ca2+ current recordings (Fig. 4) were performed at P13–15 mice, in which IS contained the following: 137 Cs–gluconate, 10 Hepes, 20 TEA–Cl, 5 Na2–phosphocreatinine, 4 Mg–ATP, 0.3 Na2GTP, and 0.5 Cs–EGTA. The same IS was applied for endocytosis measurements at the P8–11 calyx of Held (Fig. 7 D–I), except in Fig. 7 A–C, where Cs–EGTA was 0.2 mM for RRP evaluation. Cm was measured using the Sine+DC technique under whole-cell patch clamp as described previously (28, 66). Cm signals were recorded starting 10 ms after each depolarization to gain more information about the fast endocytosis. Exocytosis was analyzed as the Cm increase at 400 ms after each pulse unless otherwise specified because Cm signals before that point are strongly affected by the other factors irrelevant to transmitter release (75, 76). The average Cm curves were calculated across individual Cm traces, and error bars were calculated from resampled Cm traces at a low frequency for clarity (Fig. S8). To assess the membrane recovery dynamics after exocytosis, Cm traces were normalized after the first 400 ms following the stimulation, and the Cm levels (%) after endocytosis recovery in fast and slow phases were calculated at 2 and 35 s, respectively, from the end of depolarization. Because Cm recovery under strong depolarization in control and in cKO showed a distinctive pattern immediately after depolarization, we performed a linear fitting of Cm traces between 50 and 450 ms after depolarization and measured their slope to assess the rate of net Cm changes (Fig. 7 E and G, Insets).
Data Analysis and Statistics.
Electrophysiology data were analyzed with IgorPro (WaveMetrics) and Origin (OrginLab). For depression analysis in both control and cKO synapses, only the recordings containing no transmission failures were analyzed. Fiber stimulation artifacts were blanked for clarity in some figures. Steady-state levels of STD were calculated as the average value of the last five normalized EPSC peaks to the first EPSC peak in each train. Values were presented as mean ± SEM, and n represents the number of synapses recorded unless otherwise indicated. Each data point for statistics was collected from multiple animals (at least three to five mice), and intra-animal variance was comparable to interanimal variance. Statistical analyses were performed using two-tail Student’s t test (normality of data were confirmed), and significance level was set at P < 0.05, denoted with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001).
Acknowledgments
We thank Patrick Charnay and Ralf Schneggenburger for providing Krox20Cre mice; Pietro DeCamilli and Shaw Ferguson for Dnm1f/f mice and for very helpful discussions; Charles Wollitz, Jaffna Mathiaparanam, Elizabeth Westrick, and Juliana Maedke for technical support; and Meyer Jackson, Ed Chapman, Tim Gomez, and Erwin Neher for reading the manuscript and helpful suggestions. This work is supported by National Institutes of Health Grants R01DK093953 (to X.L.) and P30NS069271 and Brain Research Foundation Grant BRFSG201407 (to X.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520937113/-/DCSupplemental.
References
- 1.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4(9):a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27(44):11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371(6497):513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 4.Sakaba T. Two Ca(2+)-dependent steps controlling synaptic vesicle fusion and replenishment at the cerebellar basket cell terminal. Neuron. 2008;57(3):406–419. doi: 10.1016/j.neuron.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406(6798):889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 6.Bollmann JH, Sakmann B, Borst JG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289(5481):953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 7.Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435(7041):497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- 8.Neher E. What is rate-limiting during sustained synaptic activity: Vesicle supply or the availability of release sites. Front Synaptic Neurosci. 2010;2:144. doi: 10.3389/fnsyn.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gundelfinger ED, Fejtova A. Molecular organization and plasticity of the cytomatrix at the active zone. Curr Opin Neurobiol. 2012;22(3):423–430. doi: 10.1016/j.conb.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Curr Opin Neurobiol. 2011;21(1):144–150. doi: 10.1016/j.conb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Südhof TC. The presynaptic active zone. Neuron. 2012;75(1):11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12(3):127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe S, et al. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 2013;504(7479):242–247. doi: 10.1038/nature12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz B. Neural transmitter release: From quantal secretion to exocytosis and beyond. The Fenn Lecture. J Neurocytol. 1996;25(12):677–686. doi: 10.1007/BF02284834. [DOI] [PubMed] [Google Scholar]
- 16.Heuser JE, et al. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuser JE, Reese TS. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981;88(3):564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki F, Hazen M, Ordway RW. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nat Neurosci. 2000;3(9):859–860. doi: 10.1038/78753. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki F, et al. The DISABLED protein functions in CLATHRIN-mediated synaptic vesicle endocytosis and exoendocytic coupling at the active zone. Proc Natl Acad Sci USA. 2011;108(25):E222–E229. doi: 10.1073/pnas.1102231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63(2):216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Hua Y, et al. Blocking endocytosis enhances short-term synaptic depression under conditions of normal availability of vesicles. Neuron. 2013;80(2):343–349. doi: 10.1016/j.neuron.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci. 2009;29(44):13770–13784. doi: 10.1523/JNEUROSCI.3285-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonntag M, Englitz B, Kopp-Scheinpflug C, Rübsamen R. Early postnatal development of spontaneous and acoustically evoked discharge activity of principal cells of the medial nucleus of the trapezoid body: An in vivo study in mice. J Neurosci. 2009;29(30):9510–9520. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp-Scheinpflug C, Tolnai S, Malmierca MS, Rübsamen R. The medial nucleus of the trapezoid body: Comparative physiology. Neuroscience. 2008;154(1):160–170. doi: 10.1016/j.neuroscience.2008.01.088. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson SM, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316(5824):570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi M, et al. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci USA. 2008;105(6):2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou X, Paradise S, Ferguson SM, De Camilli P. Selective saturation of slow endocytosis at a giant glutamatergic central synapse lacking dynamin 1. Proc Natl Acad Sci USA. 2008;105(45):17555–17560. doi: 10.1073/pnas.0809621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raimondi A, et al. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70(6):1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lou X, et al. Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses. Proc Natl Acad Sci USA. 2012;109(8):E515–E523. doi: 10.1073/pnas.1121626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson EE, et al. A canine DNM1 mutation is highly associated with the syndrome of exercise-induced collapse. Nat Genet. 2008;40(10):1235–1239. doi: 10.1038/ng.224. [DOI] [PubMed] [Google Scholar]
- 32.Boumil RM, et al. A missense mutation in a highly conserved alternate exon of dynamin-1 causes epilepsy in fitful mice. PLoS Genet. 2010;6(8):e1001046. doi: 10.1371/journal.pgen.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JH, Saito K, Ikeda K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J Cell Biol. 1983;96(6):1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17(6):811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca²+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69(2):304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voiculescu O, Charnay P, Schneider-Maunoury S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis. 2000;26(2):123–126. doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Koch D, et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. 2011;30(24):4955–4969. doi: 10.1038/emboj.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: Developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20(24):9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaughlin M, van der Heijden M, Joris PX. How secure is in vivo synaptic transmission at the calyx of Held? J Neurosci. 2008;28(41):10206–10219. doi: 10.1523/JNEUROSCI.2735-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: Lessons from a large synapse. Trends Neurosci. 2002;25(4):206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- 41.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 42.Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21(2):269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutta Roy R, Stefan MI, Rosenmund C. Biophysical properties of presynaptic short-term plasticity in hippocampal neurons: Insights from electrophysiology, imaging and mechanistic models. Front Cell Neurosci. 2014;8:141. doi: 10.3389/fncel.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28(33):8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taschenberger H, Leão RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36(6):1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- 46.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59(6):882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46(4):633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura T, Yamashita T, Saitoh N, Takahashi T. Developmental changes in calcium/calmodulin-dependent inactivation of calcium currents at the rat calyx of Held. J Physiol. 2008;586(9):2253–2261. doi: 10.1113/jphysiol.2007.142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59(6):861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Müller M, Davis GW. Transsynaptic control of presynaptic Ca²⁺ influx achieves homeostatic potentiation of neurotransmitter release. Curr Biol. 2012;22(12):1102–1108. doi: 10.1016/j.cub.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanou E, Sullivan JM, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2016;113(4):1062–1067. doi: 10.1073/pnas.1524636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Branco T, Staras K. The probability of neurotransmitter release: Variability and feedback control at single synapses. Nat Rev Neurosci. 2009;10(5):373–383. doi: 10.1038/nrn2634. [DOI] [PubMed] [Google Scholar]
- 53.Neher E. Merits and limitations of vesicle pool models in view of heterogeneous populations of synaptic vesicles. Neuron. 2015;87(6):1131–1142. doi: 10.1016/j.neuron.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 54.Milosevic I, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72(4):587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123(3):521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 56.Schuske KR, et al. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40(4):749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- 57.Verstreken P, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40(4):733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 58.Di Paolo G, et al. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431(7007):415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 59.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43(2):193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 60.Sakaba T, et al. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proc Natl Acad Sci USA. 2013;110(20):8266–8271. doi: 10.1073/pnas.1219234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature. 1998;394(6691):384–388. doi: 10.1038/28645. [DOI] [PubMed] [Google Scholar]
- 62.de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J Neurosci. 2003;23(31):10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu X, Zhu Q, Sun J. Quantitative analysis of vesicle recycling at the calyx of Held synapse. Proc Natl Acad Sci USA. 2015;112(15):4779–4784. doi: 10.1073/pnas.1424597112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J Neurosci. 2009;29(35):11038–11042. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orth JD, McNiven MA. Dynamin at the actin-membrane interface. Curr Opin Cell Biol. 2003;15(1):31–39. doi: 10.1016/s0955-0674(02)00010-8. [DOI] [PubMed] [Google Scholar]
- 66.Fan F, et al. Dynamin 2 regulates biphasic insulin secretion and plasma glucose homeostasis. J Clin Invest. 2015;125(11):4026–4041. doi: 10.1172/JCI80652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, et al. A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis. eLife. 2014;3:e01621. doi: 10.7554/eLife.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nguyen TH, et al. Actin- and dynamin-dependent maturation of bulk endocytosis restores neurotransmission following synaptic depletion. PLoS One. 2012;7(5):e36913. doi: 10.1371/journal.pone.0036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shupliakov O, et al. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci USA. 2002;99(22):14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23(4):1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gormal RS, Nguyen TH, Martin S, Papadopulos A, Meunier FA. An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells. J Neurosci. 2015;35(4):1380–1389. doi: 10.1523/JNEUROSCI.3228-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe S, et al. Clathrin regenerates synaptic vesicles from endosomes. Nature. 2014;515(7526):228–233. doi: 10.1038/nature13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee JS, Ho WK, Lee SH. Actin-dependent rapid recruitment of reluctant synaptic vesicles into a fast-releasing vesicle pool. Proc Natl Acad Sci USA. 2012;109(13):E765–E774. doi: 10.1073/pnas.1114072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clayton EL, Evans GJ, Cousin MA. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 2008;28(26):6627–6632. doi: 10.1523/JNEUROSCI.1445-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kilic G, Lindau M. Voltage-dependent membrane capacitance in rat pituitary nerve terminals due to gating currents. Biophys J. 2001;80(3):1220–1229. doi: 10.1016/S0006-3495(01)76098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13(5):1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita T, Hige T, Takahashi T. Vesicle endocytosis requires dynamin-dependent GTP hydrolysis at a fast CNS synapse. Science. 2005;307(5706):124–127. doi: 10.1126/science.1103631. [DOI] [PubMed] [Google Scholar]
- 78.Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25(50):11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: Faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98(6):3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- 80.Alabi AA, Tsien RW. Perspectives on kiss-and-run: Role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. doi: 10.1146/annurev-physiol-020911-153305. [DOI] [PubMed] [Google Scholar]
- 81.Huang F, et al. Video-rate nanoscopy using sCMOS camera-specific single-molecule localization algorithms. Nat Methods. 2013;10(7):653–658. doi: 10.1038/nmeth.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Menon M, Schafer DA. Dynamin: Expanding its scope to the cytoskeleton. Int Rev Cell Mol Biol. 2013;302:187–219. doi: 10.1016/B978-0-12-407699-0.00003-0. [DOI] [PubMed] [Google Scholar]
- 83.Lee JS, Ho WK, Neher E, Lee SH. Superpriming of synaptic vesicles after their recruitment to the readily releasable pool. Proc Natl Acad Sci USA. 2013;110(37):15079–15084. doi: 10.1073/pnas.1314427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakaba T, Neher E. Involvement of actin polymerization in vesicle recruitment at the calyx of Held synapse. J Neurosci. 2003;23(3):837–846. doi: 10.1523/JNEUROSCI.23-03-00837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park RJ, et al. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126(Pt 22):5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kasprowicz J, Kuenen S, Swerts J, Miskiewicz K, Verstreken P. Dynamin photoinactivation blocks Clathrin and α-adaptin recruitment and induces bulk membrane retrieval. J Cell Biol. 2014;204(7):1141–1156. doi: 10.1083/jcb.201310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Douthitt HL, Luo F, McCann SD, Meriney SD. Dynasore, an inhibitor of dynamin, increases the probability of transmitter release. Neuroscience. 2011;172:187–195. doi: 10.1016/j.neuroscience.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9(11):3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]