Significance
Marine fisheries provide a major global source of protein, feeding billions of people, but they face destabilization in many regions from overexploitation and climate change. Using the most comprehensive dataset of fish diversity and abundance, encompassing over 4,500 surveys from nearshore habitats around the world, we show that biodiversity is among the strongest predictors of reef fish community biomass, comparable in importance to global temperature gradients and human impacts. Importantly, diverse fish communities were more resistant to rising and variable temperature, suggesting that high biodiversity also buffers against changing climate. Maintaining taxonomically and functionally diverse fish communities can thus stabilize fisheries’ yields in a changing ocean.
Keywords: global change, fisheries, functional diversity, macroecology, structural equation model
Abstract
Fishes are the most diverse group of vertebrates, play key functional roles in aquatic ecosystems, and provide protein for a billion people, especially in the developing world. Those functions are compromised by mounting pressures on marine biodiversity and ecosystems. Because of its economic and food value, fish biomass production provides an unusually direct link from biodiversity to critical ecosystem services. We used the Reef Life Survey’s global database of 4,556 standardized fish surveys to test the importance of biodiversity to fish production relative to 25 environmental drivers. Temperature, biodiversity, and human influence together explained 47% of the global variation in reef fish biomass among sites. Fish species richness and functional diversity were among the strongest predictors of fish biomass, particularly for the large-bodied species and carnivores preferred by fishers, and these biodiversity effects were robust to potentially confounding influences of sample abundance, scale, and environmental correlations. Warmer temperatures increased biomass directly, presumably by raising metabolism, and indirectly by increasing diversity, whereas temperature variability reduced biomass. Importantly, diversity and climate interact, with biomass of diverse communities less affected by rising and variable temperatures than species-poor communities. Biodiversity thus buffers global fish biomass from climate change, and conservation of marine biodiversity can stabilize fish production in a changing ocean.
Understanding the controls on marine fish biomass production is central to both sustaining ecosystems and human development goals. Ultimately, the quantity and distribution of biomass in ecosystems is determined by availability of resources and the physical conditions that make life possible. Temperature is a fundamental control on rates of cellular metabolism and biological processes at all levels (1) and, together with the solar energy and mineral nutrients that support plant growth, sets the template for global patterns of biomass production and other biological activities. Superimposed on this bottom-up control are interactions among organisms that mediate biomass production. Top-down control by consumers commonly limits biomass of lower trophic levels below what resources could support (2), with often far-reaching direct and indirect effects on ecosystems (3). Human harvesting is increasingly the dominant top-down control in many ecosystems (4) and, in the ocean, industrialized Homo sapiens has emerged as both a dominant and a keystone predator, strongly reducing fish biomass and transforming marine ecosystems worldwide (5, 6).
Fundamental to the interactions of organisms with one another and with the environment is evolutionary adaptation, which molds populations toward more efficient resource use and, consequently, greater biomass production. In natural, environmentally heterogeneous ecosystems, theory predicts that this adaptation results in communities of many species using a larger fraction of available resources than species-poor communities, and thus that diversity of both traits and species promotes higher total biomass production (7). Meta-analysis of hundreds of experiments supports the positive effect of biodiversity on productivity (8), and suggests that such biodiversity effects are comparable in magnitude to those of other global-change drivers (9). Because resource use efficiency is mediated by functional traits of organisms, functional diversity may be a more direct measure of a community’s capacity for production than species richness (10). As such, theory predicts (11) and experiments confirm (12) that functional differences among species result in diverse communities having more stable community-level production in the face of perturbations. Most of these inferences, however, come from highly controlled, often trophically simplified, and artificial experiments (10), and the influence of biodiversity on productivity in wild ecosystems remains controversial, reflecting a long-running debate over both the importance of biodiversity relative to other global-change drivers, and the difficulty of disentangling their influences using observational data (13, 14).
Resolving the controversy over the contributions of biodiversity to productivity and stability has important implications for conservation and fishery management because the major drivers of biomass production—temperature, resources, fishing, and biodiversity—are changing rapidly alongside growing human population and resource consumption. Biodiversity is declining on average at marine sites impacted by human activity (15), and is decreasing globally at rates orders-of-magnitude above historical background levels (6, 16), with some suggesting that biodiversity loss is already approaching a planetary tipping point beyond which ecosystems may be irreparably compromised (17). This information raises a practical question: how does declining biodiversity affect the resilience of ecosystems to other stressors, specifically climate change and human harvesting?
Quantifying the influence of declining biodiversity on ecosystem services remains a major challenge (18) because interactions among organisms are rarely incorporated in projected models of global change (19), and because most ecosystem services involve chains of biophysical and social processes that have proven difficult to link and quantify (20). Fish biomass production is an important exception to the latter rule in that it is simultaneously a biophysical process and an economically quantifiable ecosystem service. The influence of changing biodiversity on harvestable fish biomass thus represents an unusually direct link from biodiversity to a major ecosystem service. We quantified this relationship using a global dataset of >4,500 surveys of fish assemblages inhabiting shallow, hard bottoms throughout the world ocean collected using standardized protocols (21). We apply path analysis using hierarchical mixed models to ask: How do changing climate, biodiversity, and other drivers influence global reef fish biomass? And how do these factors differ between industrialized temperate regions and the less-developed tropics where people depend more on fish protein?
Results and Discussion
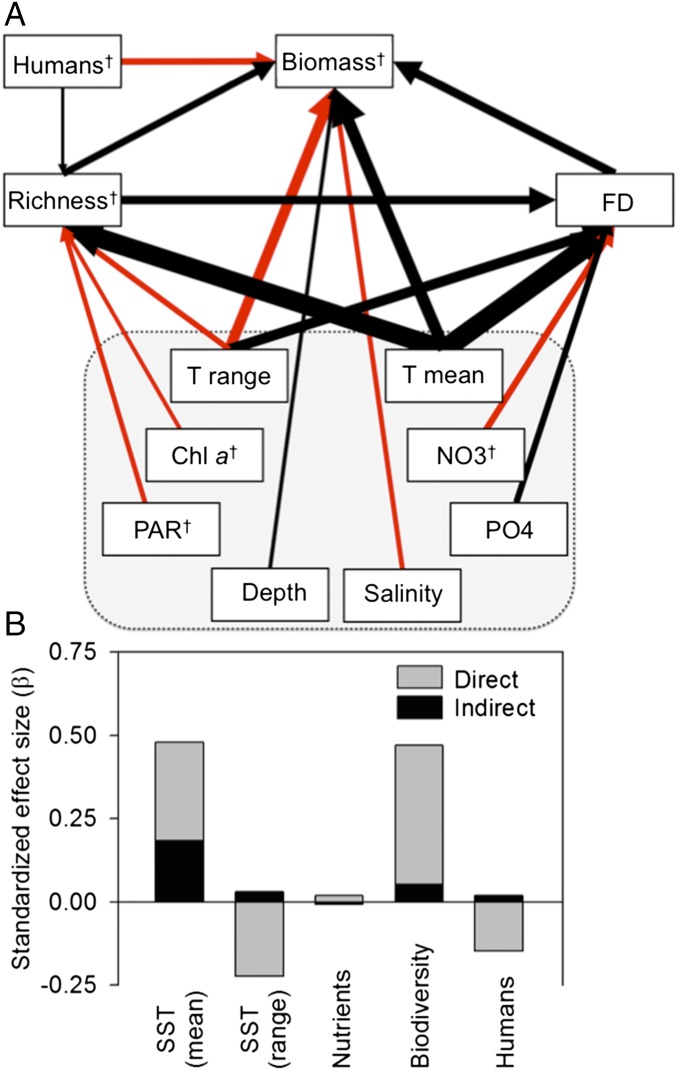
Our results confirm that on a global scale, temperature is the dominant control on reef fish biomass, acting both directly and indirectly by increasing fish species richness and functional diversity (Fig. 1). Temperature is well documented as a fundamental driver of metabolism and production (1) and a strong determinant of species-richness patterns (22). Our analysis unites these results, revealing that roughly one-third of the temperature effect on global reef fish biomass is indirect, acting by boosting fish species and functional diversity, which in turn increases biomass (Fig. 1). The direct effect of temperature on biomass across the global range (Figs. 1 and 2C) primarily reflects strong temperature-dependence of biomass in cool temperate regions (Fig. 3 E and F).
Fig. 1.
(A) Path diagram of factors influencing global reef fish biomass. Black and red paths represent positive and negative influences, respectively. Path thickness is proportional to the standardized regression coefficient (SI Appendix, Table S1). Paths of β < 0.05 are not shown. Gray box surrounds abiotic variables (and chl a), for which paths have been omitted for clarity and are shown in SI Appendix, Fig. S1. †Log10-transformed. FD, functional diversity; T, sea-surface temperature. (B) Summed direct and indirect effects of temperature, mineral nutrients, biodiversity, and human population density.
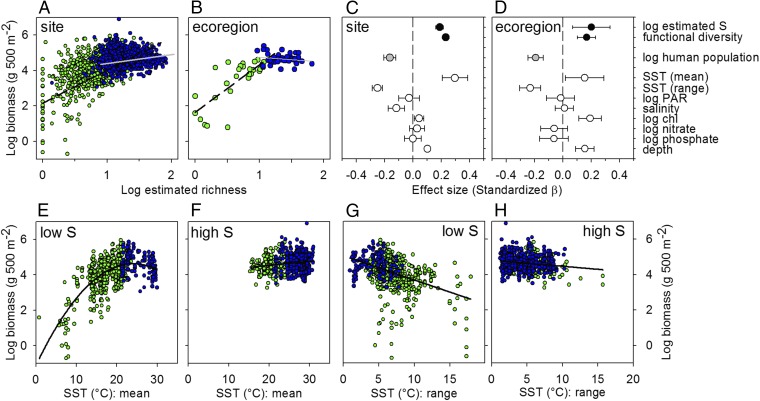
Fig. 2.
Global estimates of the effects of biodiversity and environmental drivers on reef fish community biomass. (A) Log biomass as a function of log estimated richness (corrected for sample coverage) (52). (B) Same as A, but using ecoregion means. (C and D) Effect sizes (standardized partial regression coefficients) of 11 predictors of (log) reef fish biomass from the global hierarchical model among (C) sites and (D) ecoregions. (E and F) Log biomass as a function of mean annual temperature at low- and high-richness sites (relative to median richness), respectively. (G and H) Log biomass as a function of annual temperature range at low- and high-richness sites, respectively. S, estimated richness. Blue and green symbols represent tropical and temperate sites, respectively.
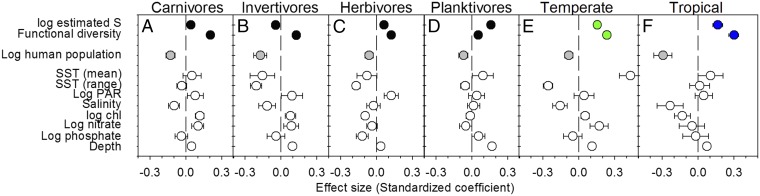
Fig. 3.
Relative influence of biodiversity and environmental factors on log biomass of reef fishes by trophic level (A–D) and latitudinal zone (E and F). Effect sizes are standardized partial regression coefficients (β) from hierarchical linear models estimated separately for each case. Carnivores, top carnivores; invertivores, benthic carnivores; PAR, photosynthetically active radiation.
Our most striking finding is the strong and consistent effect of biodiversity on global reef fish biomass. Species richness and functional diversity both enhanced fish biomass, and were the strongest predictors after temperature, followed closely by human impacts (Figs. 1 and 2C, and SI Appendix, Table S1). These biodiversity components were also the two top predictors of fish biomass in an independent random forest analysis that accounted for 25 other variables, including temperature (SI Appendix, Fig. S2). Biodiversity effects on biomass are difficult to isolate from other influences in observational data, especially the inherent correlation between abundance and richness. Therefore, we probed this result with a series of tests to identify potentially confounding effects of sample size, sampling scale, structural complexity (coral cover), and other potentially spurious correlations. The increase in fish biomass with diversity was robust to all these potential artifacts (SI Appendix). Moreover, comparison of several alternative model fits confirmed at the global level the prior finding from tropical systems (23), that fish biomass scales in a nonsaturating way with richness and is best represented by a decelerating power function (SI Appendix), also matching the pattern seen in recent results from long-term diversity manipulations in grasslands (24). When plotted on log–log axes, it is evident that biomass scales with estimated richness with a shallower slope in the tropics than in temperate sites (Fig. 2 A and B).
Our finding that biodiversity is a major determinant of fish biomass is strengthened by the Reef Life Survey’s unique global scope, standardized methods, integration across trophic levels, and the inclusion in our analyses of a rich suite of environmental covariates. To our knowledge, this represents the most comprehensive observational test yet of diversity effects on biomass in any natural ecosystem, and corroborates previous results from controlled diversity manipulations (8, 9). Importantly, our data show that changing biodiversity is a major control on the globally important ecosystem service of reef fish biomass production, comparable to climate and human impacts. An earlier synthesis of experimental and observational data similarly reached the conclusion that marine fish diversity enhanced fishery catch and stability (25), but that study was widely criticized for using fishery catches as proxies for abundance, extrapolation from small-scale experiments, and failure to account for covarying influences, among other reasons (26, 27). Our analysis accounts for these and other potentially confounding factors, and confirms that biodiversity is indeed a major determinant of global reef fish biomass.
Along with climate and biodiversity, we find that human activities are similarly important as planet-scale environmental gradients in influencing biomass of fish communities on nearshore hard bottoms. Fish biomass declined substantially with proximity to human population in our global analysis (Fig. 1 and 2C), especially in the tropics, where human population was the single strongest predictor of reef fish biomass (Fig. 3F). Because human population is closely correlated with fishing pressure, coastal development, and eutrophication (23), isolating the specific causes of this human impact is challenging. A strong role for fishing as the primary driver is suggested by our finding that human impact was strongest on larger size classes (SI Appendix, Fig. S3), and on top and benthic carnivores (Fig. 3 A and B), which are most prized by fishers (28), as shown previously for reef fishes (29). One possible explanation for the stronger effect of humans in the tropics is that temperate sites have already endured a long history of human impacts and that the smaller effect outside the tropics is only apparent, that is, the result of a shifting baseline. This finding is supported by the high and relatively invariant (log) human population index among temperate (mean ± SD = 4.71 ± 0.90) compared with tropical sites (2.61 ± 2.23, respectively), and by the unstandardized (i.e., raw) effects of human population, which are comparable between temperate (β = −0.072 ± 0.031) and tropical sites (β = −0.058 ± 0.015). Thus, the smaller relative impact of humans at temperate sites (Fig. 3 E and F) probably reflects the rarity of sparsely populated sites, and accordingly smaller gradient in human population outside the tropics. Our results may therefore reflect both strong historical impacts of human activities on temperate fishes, as well as emerging impacts in less-populated tropical sites.
Human influence also appeared to shift ecosystem control from bottom-up to top-down: considering only tropical sites (because all temperate sites had nearby human population), sites far from human influence showed a dominant signal of bottom-up forcing by dissolved phosphate, a key limiting nutrient in oligotrophic waters. In contrast, sites close to human population showed no phosphate effect and instead a modest but highly significant increase in fish biomass with depth (SI Appendix, Fig. S4), a pattern often associated with intensive fishing in shallow waters (30). This apparently human-mediated shift from bottom-up to top-down forcing supports previous analyses (31) and may explain the otherwise surprising weakness of nutrient effects on fish production in the global analysis (Fig. 1), which seems at odds with some regional analyses supporting bottom-up control of fish biomass in pelagic and sediment-bottom habitats (32, 33). The strong impact of humans on global reef fish biomass and trophic control illustrates clearly that human activity has become a pervasive force of marine nature.
All fishes do not contribute equally to the ecosystem service of fish production for human consumption. Thus, evaluating the role of biodiversity in providing this service requires focusing more specifically on preferred targets of fishers. Although many types of fishes are harvested as availability declines, fishers generally and preferentially target large individuals, which also include top carnivores (28). Our data corroborate this behavior, showing that human impacts are strongest on large fishes (>35 cm) (SI Appendix, Fig. S3) and on top and benthic carnivores (Fig. 3). Importantly, biodiversity remains among the strongest predictor of biomass for the largest fishes (SI Appendix, Fig. S3) and for top carnivores (Fig. 3), rivaling or surpassing the effect of temperature at the global scale. In the tropics, biodiversity and human impacts were in fact the only significant predictors of large fish biomass (SI Appendix, Fig. S3D). These effect sizes (partial regression coefficients) account statistically for other predictors in the model, and therefore quantify the effect of biodiversity at a given level of human impact (e.g., harvest). Thus, our global analysis provides strong evidence that maintaining biological diversity enhances the ecosystem service of high-value harvestable fish production.
A frontier in global-change research is understanding how different stressors interact. The link between biodiversity and biomass raises the question of how biodiversity affects responses of fish biomass to ongoing climate change and human impacts. Because biodiversity is strongly controlled by temperature, changing climate is expected to reorganize marine communities, as is already happening (34–36). Our results show that this reorganization has important consequences for fish biomass production because higher diversity buffers fish biomass against expected direct effects of climate. First, although climate variability (temperature range) reduced fish biomass on average, this effect was halved in the richest communities (Fig. 2 G and H and SI Appendix, Table S2), possibly because species-rich communities harbor fishes with a range in thermal niches. The stronger decline in low-richness fish communities is not explained by lower coral cover at those sites (SI Appendix), and remained significant after excluding sites with sea-surface temperature (SST) range >9 °C, beyond the range of most high-richness sites. Second, diverse assemblages had higher mean biomass at higher temperatures (Fig. 2 E and F). Specifically, in low-richness communities, biomass had a hump-shaped relationship to temperature (Akaike Information Criterion confirmed that the quadratic fit was better than linear), increasing over the low range and declining again at the highest temperatures, whereas in high-richness communities fish biomass showed a weaker, linear increase with temperature (Fig. 2 E and F). In short, diversity tends to stabilize fish biomass production against rising and more variable temperatures. This finding suggests that the buffering capacity of biodiversity against climate variability may be a general phenomenon because it has also been documented in grassland plants (37).
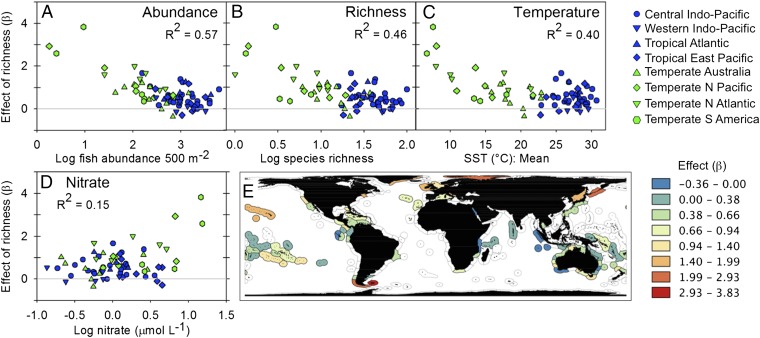
Tropical ecosystems were especially sensitive to human impacts (Fig. 3F) and are considered highly vulnerable to warming-induced reduction in species richness because of strongest warming in tropical (and polar) regions and because many tropical species are near their upper thermal tolerance limits (19, 38). Our analysis suggests, however, that vulnerability of fish biomass to warming should be buffered in tropical communities as a result of their high diversity, which provides resilience to both increasing mean temperatures and increasing climate variability (Fig. 2 E–H and SI Appendix, Table S2). To explore more broadly the sensitivity of marine ecosystems to biodiversity loss, we obtained separate estimates of the dependence of biomass on richness for each of the 68 ecoregions for which estimates could be reliably obtained (39). Fish biomass was most sensitive to changing fish diversity at cooler, nutrient-rich sites also characterized by low richness and abundance (Fig. 4). The relative importance of these influences is difficult to disentangle because they tend to occur together. However, the explanation suggested by theory and prior empirical results is that the naturally low species richness at cooler sites results in low functional redundancy. Thus, at cooler, less diverse sites species fill more unique roles on average (40) and loss of a single species reduces biomass production more than it does at richer sites, where the remaining ecologically similar species can compensate for that loss (41). This finding implies that high-latitude marine ecosystems are especially ecologically vulnerable to climate change, due not only to higher projected invasion rates (19), but also to stronger impacts of species loss on production (Fig. 4) and stronger trophic cascades (31), compared with lower latitudes.
Fig. 4.
Variation in the effect of species richness on biomass as a function of (A–D) environmental drivers and (E) geography. Shown are standardized partial regression coefficients (β) from the hierarchical model, with coefficients estimated separately by ecoregion. P < 0.001 for all relationships.
Although high diversity thus appears to buffer reef fish communities against climate change, diversity provides no such benefit to other impacts of human population. A previous analysis reported a negative interaction between (trophic) diversity and human population density for tropical reef fish biomass (23). In our more geographically comprehensive analysis, we found no interaction between human density and either species richness or multivariate functional diversity (SI Appendix, Table S2). However, fishing pressure and high temperatures act synergistically to increase vulnerability of coral reef fishes (42), potentially explaining our finding of greater sensitivity of reef fish biomass to human impacts in the tropics (Fig. 3). Moreover, many tropical fishes and reef corals appear to be living near the upper limits of their thermal ranges (38, 43). Most forecasts predict widespread loss of coral habitat with warming ocean temperature over the coming century, which will likely have serious negative consequences for associated reef fishes (44). These considerations illustrate that biodiversity can interact differently with different stressors and underscore the importance of reducing overfishing and conserving habitat, including coral reefs, to maintain marine biodiversity and ecosystem services.
In summary, our analysis of global, fishery-independent data provides a uniquely powerful test of the long-debated questions of whether biodiversity promotes greater production and stability in nature, how important those biodiversity effects are relative to other drivers, and how they interact. We find that biodiversity is equally and often more important than water quality, nutrient supply, and human influence in controlling the global distribution of reef fish biomass, and that more diverse fish communities are more resilient to impacts of changing climate. Moreover, both species richness and functional trait diversity contributed roughly equally to fish biomass globally, generalizing local inferences that reef fish trophic interactions (45, 46) and functional diversity (47–49) are key mediators of the community structure and resilience of coral reef ecosystems. Because reef fish biomass provides an important protein source for many people, particularly in the developing world, our results suggest that management to sustain reef fish diversity, of both species and functional types, will also promote higher productivity of fish biomass and higher resilience of that ecosystem service in the face of rising and more variable temperatures.
Materials and Methods
Reef Life Survey.
Standardized quantitative censuses of reef fishes were undertaken by trained recreational SCUBA divers on shallow hard substrate habitats worldwide through the Reef Life Survey program. Data came from 4,556 transects at 1,844 sites, from 55°S to 78°N latitude, and 74 ecoregions and 11 realms (39). Details of fish census methods, data quality, and diver training are in refs. 21, 40, and 50, and an online methods manual (reeflifesurvey.com). Fish counts per 500-m2 transect (2 × 250-m2 blocks) and size estimates were converted to biomass estimates using species-specific length–weight relationships from Fishbase (www.fishbase.org). Where length–weight relationships were described in Fishbase in terms of standard or fork length, equations in Fishbase allowed conversion to total length. Bias in divers’ perception of fish size underwater was corrected using empirical calibrations (51). Length–weight coefficients from similar-shaped close relatives were used for species whose length–weight relationships were not available in Fishbase.
Selection of Variables Used in Models.
As the primary response variable in all of our analyses, we focused on fish biomass rather than productivity because productivity is estimated from biomass and temperature, and is thus a more derived variable. The inclusion of temperature in the equation to estimate productivity also precludes a rigorous test of the effect of temperature on productivity. Biomass is a logical metric of many ecosystem functions performed by fishes because it is tightly linked to many components of metabolism and productivity (23).
From the diver surveys we computed two estimates of fish diversity per transect: species density (richness) and functional trait diversity. An inherent challenge to estimating biodiversity effects on biomass from observational data is the inherent positive correlation between estimated species richness and the number of individuals sampled, obscuring the direction of causality between abundance (or biomass) and richness. We derived estimates of species richness that account for this dependency using fixed-coverage subsampling (52). This approach first computes a rarefaction curve for a given survey by randomly sampling individuals from the survey and calculating for each such subsample the probability that adding a new individual will add a new species; this then allows, by rarefaction (of high-richness samples) or extrapolation (of low-richness samples) a measure of sample completeness: that is, the proportion of the total number of individuals in the community that belong to species represented in the survey (52). Once this process is completed, survey richness is compared at a common (fixed) value of coverage that can be estimated reliably from all samples in the dataset. We used a value of 99% coverage (completeness), meaning that for any survey a new individual fish added to a survey would have a 99% probability of belonging to a species already represented on the survey. This criterion was met by 97% of the surveys in our dataset; the remaining surveys were discarded from the analysis either because they sampled too few individuals or too many individuals of the same species (e.g., a large school of one species) to construct a reliable rarefaction curve. By accounting for the dependence of richness on sample size, the fixed-coverage estimate of richness minimizes the possibility that observed correlations between richness and biomass result simply from larger samples (higher fish densities) capturing more species. This coverage-based estimate of richness yields conservative estimates of the importance of diversity to biomass; fitting the same models with raw number of species recorded per transect produced substantially higher partial coefficients for effect of richness on biomass (SI Appendix, Table S1).
Second, we estimated functional diversity based on data on eight traits and using Rao’s quadratic entropy (Q). Rao’s Q is not constrained to increase with increasing richness (53), and thus can be treated as an independent predictor of diversity in our analyses. Functional diversity was further transformed by 1/(1 – Q) to express it in comparable units to species richness: that is, the effective number of functionally unique species in the sample (54). The eight traits used to calculate functional diversity (SI Appendix, Table S3) came from the database used by Stuart-Smith et al. (40); details of trait assignment and values are provided in that report. Traits were chosen to encompass attributes known to influence functional roles in a fish assemblage, including life history, trophic position, behavior, and habitat associations (SI Appendix, Fig. S5B).
We assembled data on 25 environmental and human-impact variables taken from the Bio-ORACLE dataset (55), a comprehensive, uniform, high-resolution global dataset of geophysical, biotic, and climate rasters. Bio-ORACLE data on SST, photosynthetically active radiation, and surface chlorophyll (chl a) were remotely sensed, taken from monthly level 3 preprocessed data from the Aqua-MODIS and SeaWiFS satellites at a ∼9.2-km spatial resolution. Other water-quality parameters, including dissolved nutrients, were spatially interpolated based on data from in situ surface measurements in the World Ocean Database 2009 (56). An index of human population was calculated by fitting a smoothly tapered surface to each settlement point on a glp00g world population density grid using the quadratic kernel function described by Silverman (57). Populations were screened for a density greater than 1,000 people per 0.04° cell and the search radius was set at 3.959°.
To reduce the list of predictor variables to a more manageable set, we first conducted a random forest analysis to identify those with the most explanatory power (SI Appendix, Fig. S2). Random forest analysis is a machine-learning technique that is insensitive to underlying distributions, collinearity, or interactions among variables, and thus is ideal for ranking closely related or interacting variables (58). Because random forests can be sensitive to overfitting, we pruned the forest to 100 trees based on visual assessment of change in mean-square error with increasing number of trees. We then selected 12 of the top variables to use as the starting point for linear modeling, choosing in decreasing order of explanatory power (percent change in mean-square error of the model based on random permutations of that variable), with the exception that we excluded variables describing local conditions, like algal cover and pollution because these had relatively few measured observations and were largely interpolated. We then subjected the chosen predictor variables to variance inflation factor analysis to assess collinearity. After removing the predictor with the highest value (minimum SST, variance inflation factor = 52.3), the remaining 11 variables had variance inflation factor values < 4 and were retained in the global model. The centering and scaling of predictors used to generate the final linear models and corresponding path coefficients should also alleviate the influence of collinear variables. The final list of predictors retained for model building included: log-estimated species richness, functional diversity, log human population index, mean annual SST, salinity, survey depth, log phosphate concentration, range in annual SST, log chlorophyll concentration, log photosynthetically active radiation, and log nitrate concentration.
Modeling Approach.
We used two general approaches to analyze controls on global reef fish biomass. First, we evaluated controls on fish biomass using hierarchical linear models, including the 11 predictor variables that emerged from our variable selection process described above. Fish biomass was modeled at the site level, with the response variable being mean total biomass of fish per survey at a site; the random effect of site was nested within ecoregion and realm, and the intercept of estimated richness was allowed to vary among ecoregions. This model structure was used in separate analyses of fish biomass at the global level, in temperate versus tropical regions, by trophic level, and for large fish (>35 cm) specifically.
Second, to obtain a more integrated picture of the direct and indirect influences on global fish biomass, we conducted confirmatory path analysis based on piecewise fitting of component hierarchical linear mixed-effects models (59, 60). The global-path model was nearly saturated in the sense that the component model for each endogenous variable included paths from all exogenous variables plus remaining endogenous variables, with the proviso that the model was nonrecursive (i.e., with no reciprocal paths between the same variables). Thus, nitrate and phosphate were modeled as a function of all other abiotic variables plus human population; richness and functional diversity were modeled as a function of all abiotic variables plus human population, and biomass was modeled as a function of all 11 variables (Fig. 1 and SI Appendix, Fig. S1). The overall path model was evaluated using Shipley’s test of directed separation (59), which yields a Fisher’s C statistic that can be compared with a χ2-distribution. If the resulting P value is >0.05, then the model can be said to adequately reproduce the hypothesized causal network.
To examine how the richness–biomass relationship varied geographically, we fit the richness–biomass relationship separately for each of the world’s 68 marine ecoregions (39) for which we have sufficient survey data, using a hierarchical model and allowing the slope of the richness effect to vary by ecoregion. We then extracted the slope estimates representing the richness–biomass relationships among ecoregions and plotted them as a function of sampling effort (number of surveys per ecoregion), ecoregion mean richness, and ecoregion environmental characteristics.
Supplementary Material
Acknowledgments
We thank the many Reef Life Survey divers, researchers, and managers who participated in data collection and provide ongoing expertise and commitment to the program; University of Tasmania staff responsible for Reef Life Survey data management, Antonia Cooper and Just Berkhout; Stuart Kininmonth for providing the population index and mapping; and Doug Rasher and an anonymous reviewer for comments that improved the manuscript. Additional support was provided by the former Commonwealth Environment Research Facilities Program, the Ian Potter Foundation, the Australian Research Council, the Institute for Marine and Antarctic Studies, the Marine Biodiversity Hub, a collaborative partnership supported through the Australian Government’s National Environmental Science Programme, and the Smithsonian Institution. This is contribution 9 from the Smithsonian’s Tennenbaum Marine Observatories Network.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524465113/-/DCSupplemental.
References
- 1.Brown J, Gillooly J, Allen A, Savage V, West G. Toward a metabolic theory of ecology. Ecology. 2004;85(7):1771–1789. [Google Scholar]
- 2.Borer ET, Halpern BS, Seabloom EW. Asymmetry in community regulation: Effects of predators and productivity. Ecology. 2006;87(11):2813–2820. doi: 10.1890/0012-9658(2006)87[2813:aicreo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 4.Darimont CT, Fox CH, Bryan HM, Reimchen TE. Human impacts. The unique ecology of human predators. Science. 2015;349(6250):858–860. doi: 10.1126/science.aac4249. [DOI] [PubMed] [Google Scholar]
- 5.Jennings S, Kaiser MJ. The effects of fishing on marine ecosystems. Adv Mar Biol. 1998;34:201–212. [Google Scholar]
- 6.McCauley DJ, et al. Marine defaunation: animal loss in the global ocean. Science. 2015;347(6219):1255641–1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 7.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: Theoretical considerations. Proc Natl Acad Sci USA. 1997;94(5):1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale BJ, et al. The functional role of producer diversity in ecosystems. Am J Bot. 2011;98(3):572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 9.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 11.McCann KS. The diversity-stability debate. Nature. 2000;405(6783):228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- 12.Gross K, et al. Species richness and the temporal stability of biomass production: a new analysis of recent biodiversity experiments. Am Nat. 2014;183(1):1–12. doi: 10.1086/673915. [DOI] [PubMed] [Google Scholar]
- 13.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75(1):3–35. [Google Scholar]
- 14.Srivastava DS, Vellend M. Biodiversity-ecosystem function research: Is it relevant to conservation? Annu Rev Ecol Evol Syst. 2005;36:267–294. [Google Scholar]
- 15.Elahi R, et al. Recent trends in local-scale marine biodiversity reflect community structure and human impacts. Curr Biol. 2015;25(14):1938–1943. doi: 10.1016/j.cub.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345(6195):401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 17.Rockström J, et al. A safe operating space for humanity. Nature. 2009;461(7263):472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- 18.Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330(6010):1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- 19.Jones MC, Cheung WWL. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J Mar Sci. 2015;72(3):741–752. [Google Scholar]
- 20.Duncan C, Thompson JR, Pettorelli N. The quest for a mechanistic understanding of biodiversity–ecosystem services relationships. Proc Biol Sci. 2015;282(1817):20151348. doi: 10.1098/rspb.2015.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar G, Stuart-Smith R. Ecological effects of marine protected areas on rocky reef communities—A continental-scale analysis. Mar Ecol Prog Ser. 2009;388(1817):51–62. [Google Scholar]
- 22.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466(7310):1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 23.Mora C, et al. Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol. 2011;9(4):e1000606. doi: 10.1371/journal.pbio.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336(6081):589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 25.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 26.Murawski S, Methot R, Tromble G. Biodiversity loss in the ocean: How bad is it? Science. 2007;316(5829):1281–1284, author reply 1281–1284. doi: 10.1126/science.316.5829.1281b. [DOI] [PubMed] [Google Scholar]
- 27.Longhurst A. Doubt and certainty in fishery science: Are we really headed for a global collapse of stocks? Fish Res. 2007;86(1):1–5. [Google Scholar]
- 28.Jennings S, Polunin N. Impacts of predator depletion by fishing on the biomass and diversity of non-target reef fish communities. Coral Reefs. 1997;16(2):81–82. [Google Scholar]
- 29.Soler GA, et al. Reef fishes at all trophic levels respond positively to effective marine protected areas. PLoS One. 2015;10(10):e0140270. doi: 10.1371/journal.pone.0140270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay M. Patterns of fish and urchin grazing on Caribbean coral reefs: Are previous results typical? Ecology. 1984;65(2):446–454. [Google Scholar]
- 31.Boyce DG, Frank KT, Worm B, Leggett WC. Spatial patterns and predictors of trophic control in marine ecosystems. Ecol Lett. 2015;18(10):1001–1011. doi: 10.1111/ele.12481. [DOI] [PubMed] [Google Scholar]
- 32.Ware DM, Thomson RE. Bottom-up ecosystem trophic dynamics determine fish production in the Northeast Pacific. Science. 2005;308(5726):1280–1284. doi: 10.1126/science.1109049. [DOI] [PubMed] [Google Scholar]
- 33.Nixon SW. Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnol Oceanogr. 1988;33(4):1005–1025. [Google Scholar]
- 34.Bates AE, et al. Resilience and signatures of tropicalization in protected reef fish communities. Nat Clim Chang. 2014;4(1):62–67. [Google Scholar]
- 35.Vergés A, et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc Biol Sci. 2014;281(1789):20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poloczanska ES, et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;3(10):919–925. [Google Scholar]
- 37.Isbell F, et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526(7574):574–577. doi: 10.1038/nature15374. [DOI] [PubMed] [Google Scholar]
- 38.Stuart-Smith RD, Edgar GJ, Barrett NS, Kininmonth SJ, Bates AE. Thermal biases and vulnerability to warming in the world’s marine fauna. Nature. 2015;528(7580):88–92. doi: 10.1038/nature16144. [DOI] [PubMed] [Google Scholar]
- 39.Spalding M, et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience. 2007;57(7):573–583. [Google Scholar]
- 40.Stuart-Smith RD, et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature. 2013;501(7468):539–542. doi: 10.1038/nature12529. [DOI] [PubMed] [Google Scholar]
- 41.Thibaut LM, Connolly SR, Sweatman HPA. Diversity and stability of herbivorous fishes on coral reefs. Ecology. 2012;93(4):891–901. doi: 10.1890/11-1753.1. [DOI] [PubMed] [Google Scholar]
- 42.Graham NAJ, et al. Extinction vulnerability of coral reef fishes. Ecol Lett. 2011;14(4):341–348. doi: 10.1111/j.1461-0248.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson GE, Crawley N, Lunde IG. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol. 2009;15(6):1405–1412. [Google Scholar]
- 44.Graham NAJ, et al. Dynamic fragility of oceanic coral reef ecosystems. Proc Natl Acad Sci USA. 2006;103(22):8425–8429. doi: 10.1073/pnas.0600693103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumby PJ, et al. Fishing, trophic cascades, and the process of grazing on coral reefs. Science. 2006;311(5757):98–101. doi: 10.1126/science.1121129. [DOI] [PubMed] [Google Scholar]
- 46.Hughes TP, et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol. 2007;17(4):360–365. doi: 10.1016/j.cub.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 47.Rasher DB, Hoey AS, Hay ME. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology. 2013;94(6):1347–1358. doi: 10.1890/12-0389.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nash KL, Graham N, Jennings S. Herbivore cross-scale redundancy supports response diversity and promotes coral reef resilience. J Appl Ecol. 2015 doi: 10.1111/1365-2664.12430. [DOI] [Google Scholar]
- 49.Adam TC, Burkepile DE, Ruttenberg BI, Paddack MJ. Herbivory and the resilience of Caribbean coral reefs: Knowledge gaps and implications for management. Mar Ecol Prog Ser. 2015;520(2015):1–20. [Google Scholar]
- 50.Edgar GJ, Stuart-Smith RD. Systematic global assessment of reef fish communities by the Reef Life Survey program. Sci Data. 2014;1:140007. doi: 10.1038/sdata.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar GJ, Barrett NS, Morton AJ. Biases associated with the use of underwater visual census techniques to quantify the density and size-structure of fish populations. J Exp Mar Biol Ecol. 2004;308(2):269–290. [Google Scholar]
- 52.Chao A, Jost L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology. 2012;93(12):2533–2547. doi: 10.1890/11-1952.1. [DOI] [PubMed] [Google Scholar]
- 53.Dukát ZB. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J Veg Sci. 2005;16(5):533–540. [Google Scholar]
- 54.Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88(10):2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- 55.Tyberghein L, et al. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob Ecol Biogeogr. 2011;21(2):272–281. [Google Scholar]
- 56.Boyer PT, et al. 2009. Introduction. World Ocean Database 2009, NOASS Atlas NESDIS 66, ed Levitus S (US Government Printing Office, Washington, DC), Vol 1.
- 57.Silverman WB. Density Estimation for Statistics and Data Analysis. Chapman and Hall; Boca Raton, FL: 1986. [Google Scholar]
- 58.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 59.Shipley B. Confirmatory path analysis in a generalized multilevel context. Ecology. 2009;90(2):363–368. doi: 10.1890/08-1034.1. [DOI] [PubMed] [Google Scholar]
- 60.Lefcheck JS. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol Evol. 2015 doi: 10.1111/2041-210X.12512. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.