Significance
The hygiene, or “old friends,” hypothesis proposes that lack of exposure to immunoregulatory microorganisms in modern urban societies is resulting in an epidemic of inflammatory disease, as well as psychiatric disorders in which chronic, low-level inflammation is a risk factor. An important determinant of immunoregulation is the microbial community occupying the host organism, collectively referred to as the microbiota. Here we show that stress disrupts the homeostatic relationship between the microbiota and the host, resulting in exaggerated inflammation. Treatment of mice with a heat-killed preparation of an immunoregulatory environmental microorganism, Mycobacterium vaccae, prevents stress-induced pathology. These data support a strategy of “reintroducing” humans to their old friends to promote optimal health and wellness.
Keywords: anxiety, chronic psychosocial stress, fear, microbiota, posttraumatic stress disorder
Abstract
The prevalence of inflammatory diseases is increasing in modern urban societies. Inflammation increases risk of stress-related pathology; consequently, immunoregulatory or antiinflammatory approaches may protect against negative stress-related outcomes. We show that stress disrupts the homeostatic relationship between the microbiota and the host, resulting in exaggerated inflammation. Repeated immunization with a heat-killed preparation of Mycobacterium vaccae, an immunoregulatory environmental microorganism, reduced subordinate, flight, and avoiding behavioral responses to a dominant aggressor in a murine model of chronic psychosocial stress when tested 1–2 wk following the final immunization. Furthermore, immunization with M. vaccae prevented stress-induced spontaneous colitis and, in stressed mice, induced anxiolytic or fear-reducing effects as measured on the elevated plus-maze, despite stress-induced gut microbiota changes characteristic of gut infection and colitis. Immunization with M. vaccae also prevented stress-induced aggravation of colitis in a model of inflammatory bowel disease. Depletion of regulatory T cells negated protective effects of immunization with M. vaccae on stress-induced colitis and anxiety-like or fear behaviors. These data provide a framework for developing microbiome- and immunoregulation-based strategies for prevention of stress-related pathologies.
Immunoregulation, indicated by a balanced expansion of effector T-cell populations and regulatory T cells (Treg), is known to be driven by microbial signals, mainly by organisms with which mammals coevolved, including: (i) the commensal microbiota, which have been altered by the Western lifestyle, including a diet that is commonly low in microbiota-accessible carbohydrates (1, 2); (ii), pathogens associated with the “old infections” that were present throughout life in evolving human hunter-gatherer populations (3); and (iii) organisms from the natural environment with which humans were inevitably in daily contact (and so had to be tolerated by the immune system) (4). Immunoregulation is thought to be compromised in modern high-income settings due to reduced contact with these three categories of organisms (4–6). A failure of immunoregulation, attributable to reduced exposure to the microbial environment within which the mammalian immune system evolved, is thought to be one factor contributing to recent increases in stress-related and chronic inflammatory disorders in high-income countries (1, 3, 4). Results from both preclinical and clinical studies are consistent with the idea that inadequate immunoregulation also increases risk for development of stress-related psychiatric disorders (4, 7, 8).
Consistent with the hypothesis that subjects with stress-related psychiatric disorders, such as posttraumatic stress disorder (PTSD), suffer from a failure of immunoregulation, PTSD is associated with decreases in Treg (9), an increased proinflammatory milieu (10), autoimmunity (11), and exaggerated symptoms of inflammatory bowel disease (IBD) (11, 12). Prospective studies have demonstrated that elevated plasma concentrations of C-reactive protein predict subsequent PTSD symptoms (7). Furthermore, prospective studies of gene networks identify enrichment of innate immune responses and IFN signaling (types I and II) as putative causal signatures for PTSD development (13).
Trauma and stressor exposure can alter the composition of the gut microbiome (14) and, consequently, the homeostatic balance between the gut microbiota and mucosal immune system, with important consequences for enteric infections, mucosal inflammation, bacterial translocation (15), as well as emotional behavior, including anxiety-like behavior (16). Glucocorticoid hormones, important mediators of physiologic responses to stress, increase the abundance of pathobionts, decrease IgA (which normally inhibits bacterial adherence to intestinal epithelial cells), increase bacterial adherence over twofold, and increase bacterial translocation to mesenteric lymph nodes (17, 18). Furthermore, stress-induced decreases in an individual’s microbial diversity are thought to increase vulnerability to infectious pathology (15). Meanwhile, orally administered probiotics with immunoregulatory and antiinflammatory properties have been shown to induce anxiolytic and antidepressant-like effects in animal models (6, 16). It remains unclear whether these beneficial effects of probiotics are due to their ability to prevent stress-induced decreases in microbial diversity, their immunoregulatory effects, or both.
Previous studies have demonstrated that probiotics can have antiinflammatory effects in rodent models of chronic inflammation, including colitis, following either mucosal or subcutaneous administration (19, 20), and in some cases these effects are observed using heat-killed preparations (20). Subcutaneous injections of heat-killed preparations of immunoregulatory bacteria may have some advantages, including long-term duration of antiinflammatory and immunoregulatory effects, lasting up to 12 wk following administration (21).
If inadequate immunoregulation and subsequent chronic low-grade inflammation are risk factors for development of stress-related psychiatric disorders, pretreatment with an immunoregulatory agent would be expected to be protective. However, the potential for immunoregulatory approaches to prevent stress-related psychiatric disorders has not been tested. Therefore, in the current study, we evaluated the potential for immunization with a heat-killed preparation of Mycobacterium vaccae to prevent chronic psychosocial stress-induced pathophysiology, including spontaneous colitis, exaggeration of chemically induced colitis, and exaggerated anxiety- and fear-like behaviors. M. vaccae is an abundant soil saprophyte, a microorganism that lives on dead or decaying organic matter, with immunoregulatory properties (22). A heat-killed preparation of the organism modulates dendritic cell function (23) and induces Treg and secretion of antiinflammatory cytokines, including IL-10 and transforming growth factor β (22).
Results
M. vaccae Increases Proactive Coping.
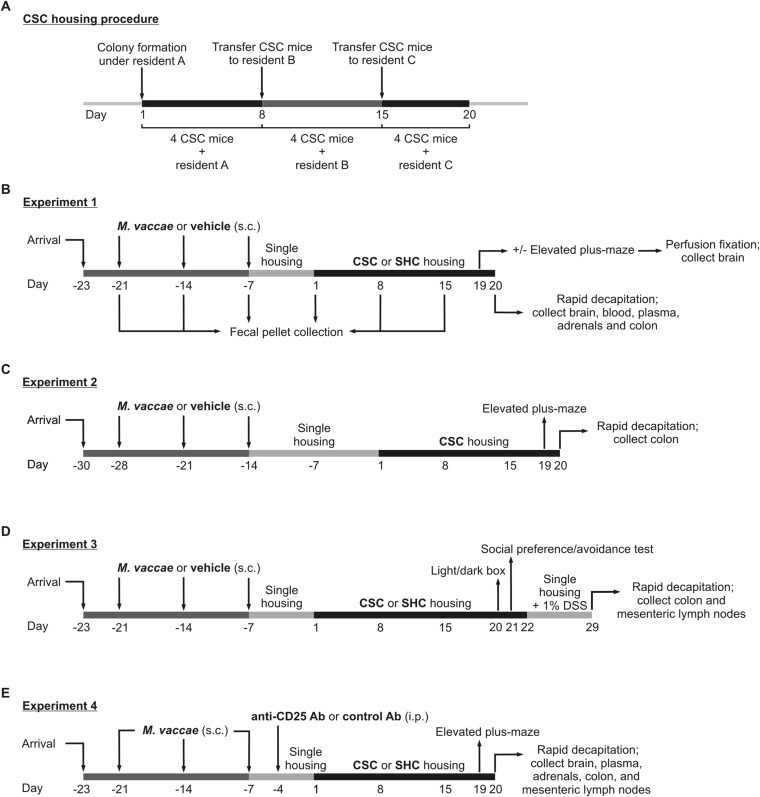
Reactive, as opposed to proactive, coping behavior may increase the risk of developing stress-related disorders in humans (24) and anxiety- and depressive-like responses in rodents (25). Here we quantified reactive versus proactive coping responses during exposure to the chronic subordinate colony housing (CSC) procedure (26) (Exp. 1) (for details, see SI Materials and Methods; Fig. S1 A and B). Briefly, we immunized male C57BL/6NCrl mice with either vehicle or a heat-killed preparation of M. vaccae [National Collection of Type Cultures (NCTC) 11659; 0.1 mg, subcutaneously] (Fig. S2A) on days ‒21, ‒14, and ‒7. On day 1, mice were assigned to the single-housed control (SHC) group or CSC group, with four CSC mice being housed together with a dominant male for 19 consecutive days. We assessed stress coping behaviors of M. vaccae- or vehicle-immunized mice during 2 h of CSC exposure on days 1, 8, and 15, effects of preimmunization with M. vaccae on CSC-induced changes in the gut microbiome on days –21, –14, –7, 1, 8, and 15, anxiety-like behavior on the elevated plus-maze (EPM) on day 19, and pathophysiology on day 20.
Fig. S1.
Diagrammatic illustration of the chronic subordinate colony housing (CSC) procedure and experimental timelines of Exps. 1–4. (A) Diagrammatic illustration of the CSC procedure, used in Exps. 1–4. (B) Experimental timeline from Exp. 1. (C) Experimental timeline from Exp. 2. (D) Experimental timeline from Exp. 3. (E) Experimental timeline from Exp. 4. Day 1 is defined as the first day of the CSC exposure; day –1 is defined as the day before CSC exposure.
Fig. S2.
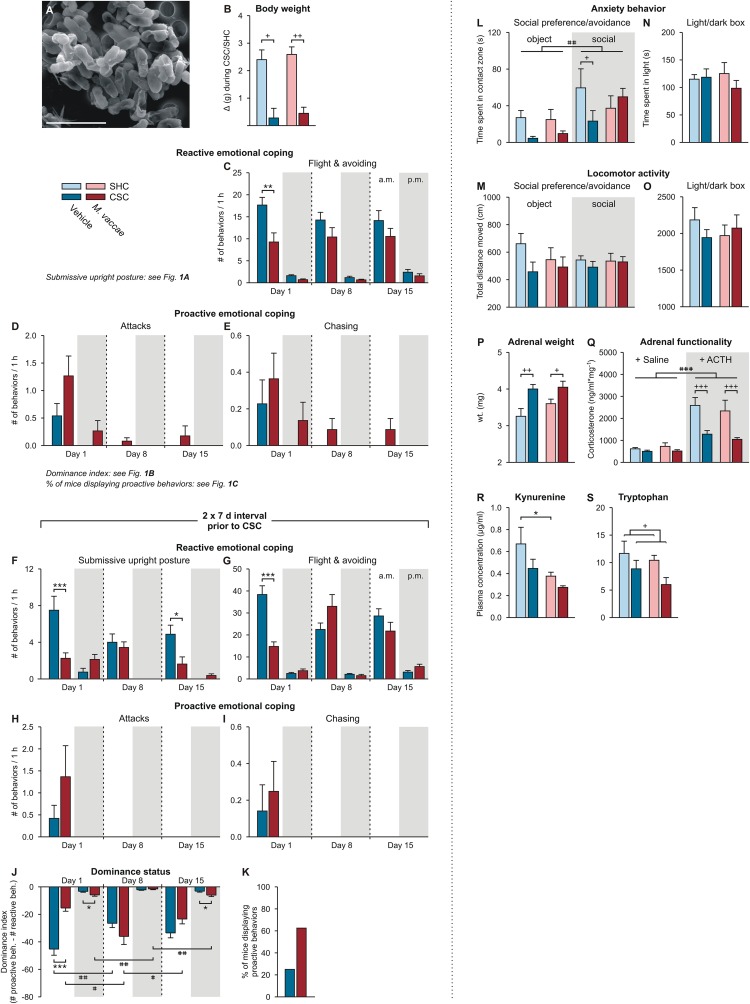
Behavioral and physiological data of Exps. 1–3. (A) Scanning electron microscopy image illustrating the heat-killed preparation of M. vaccae. (Scale bar, 3 µm.) (B) Effects of M. vaccae immunization on body weight gain during the 19-d single-housed control condition, or CSC exposure in Exp. 1. (C–E) Effects of M. vaccae or vehicle immunization on stress coping behavior during CSC exposure in Exp. 1, where CSC onset was initiated 1 wk following the final immunization with M. vaccae or vehicle. (C) Effects of M. vaccae immunization before CSC onset on the number of flight and avoiding behaviors, relative to vehicle-immunized controls, by CSC mice during the first hour (1000–1100 hours; white background) of CSC exposure on day 1 and during the first hour following exposure to a novel dominant male aggressor on days 8 and 15. Also shown are behavioral responses in the evening (1700–1800 hours; gray background) of days 1, 8, and 15. (D and E) The number of attacks (D) and number of chasing behaviors (E). (F–K) Effects of M. vaccae or vehicle immunization on stress coping behavior during CSC exposure in Exp. 2, where CSC onset was initiated 2 wk following the final immunization with M. vaccae or vehicle. Effects of M. vaccae immunization before CSC onset on the number of (F) submissive upright postures, (G) flight and avoiding behaviors, (H) number of attacks, and (I) number of chasing behaviors. (J) Dominance index. (K) Percent of vehicle- and M. vaccae-immunized mice displaying proactive behaviors during the 19-d CSC procedure. (L–Q) Behavioral end points from Exp. 3. (L) Time spent in the contact zone during the object and social phases of the social preference/avoidance test (day 21). (M) Locomotor activity during the SPAT. (N) Time spent in the light compartment during the light/dark box test (day 20). (O) Locomotor activity during the LDB test. (P–S) Additional physiologic end points from Exp. 1, collected on day 20. (P and Q) Effects of M. vaccae immunization and CSC on adrenal weight and functionality. (P) Adrenal weight measured on day 20. (Q) In vitro adrenal functionality assessed by corticosterone secretion following stimulation with saline or adrenocorticotropic hormone on day 20. (R and S) Blood samples were collected on day 20 and plasma (R) l-kynurenine and (S) l-tryptophan concentrations were measured using high-performance liquid chromatography with electrochemical detection. Bars represent means; error bars represent +SEM. Significance was assessed by (B, N–P, R, and S) two-factor ANOVA, (C–J) LMM, conducted separately for AM and PM time points, (K) Fisher’s exact test, (L and M) LMM, and (Q) three-factor ANOVA. *P < 0.05, **P < 0.01, ***P < 0.01, between-subjects effects of M. vaccae versus vehicle, Fisher’s least significant difference (LSD) tests; +P < 0.05, ++P < 0.01, +++P < 0.001, between-subjects effects of CSC versus SHC; #P < 0.05, ##P < 0.01, within-subjects effects of time, paired t tests using Bonferroni correction. (Q) ###P < 0.001, main effect of ACTH versus saline (three-factor ANOVA). The number of independent data points (N) in each of the graphs (B–S) and sample size (n) for each group are as follows: (B) N = 63; vehicle/SHC, 9; vehicle/CSC, 22; M. vaccae/SHC, 9; M. vaccae/CSC, 23. (C) N = 46; vehicle, 23; M. vaccae, 23. (D and E) N = 46; vehicle, 22–23; M. vaccae, 22–23. (F, H, and I) N = 16; vehicle, 7–8; M. vaccae, 7–8. (G, J, and K) N = 16; vehicle, 8; M. vaccae, 8. (L–O) N = 30; vehicle/SHC, 8; vehicle/CSC, 7 (object), 8 (social); M. vaccae/SHC, 6 (object), 7 (social); M. vaccae/CSC, 7 (object), 8 (social). (P and Q) N = 34; vehicle/SHC, 9; vehicle/CSC, 8; M. vaccae/SHC, 9; M. vaccae/CSC, 8. (R) N = 30; vehicle/SHC, 9; vehicle/CSC, 7; M. vaccae/SHC, 9; M. vaccae/CSC, 5. (S) N = 30; vehicle/SHC, 9; vehicle/CSC, 7; M. vaccae/SHC, 9; M. vaccae/CSC, 6.
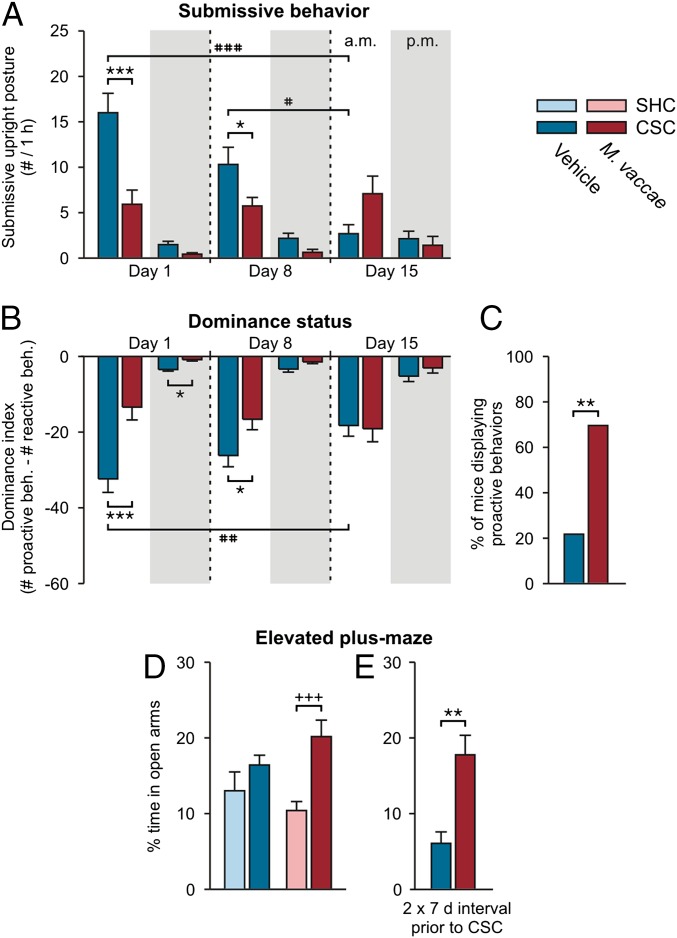
M. vaccae immunization did not affect body weight gain before CSC exposure (vehicle, 6.4 ± 0.3 g; M. vaccae, 6.9 ± 0.3 g; Student’s t test, P > 0.05) and did not affect CSC-induced reduction in body weight gain (Fig. S2B). However, immunization with M. vaccae decreased the number of submissive upright posture displays (Fig. 1A) [linear mixed model (LMM) for AM behavior, M. vaccae × time, F(2, 93.0) = 9.6, P < 0.001]. These effects were particularly evident during the first hour of CSC exposure on day 1, when M. vaccae-immunized mice showed a 63% reduction in the amount of submissive upright posture relative to vehicle-injected mice (Fig. 1A). Whereas 95.7% of vehicle-injected mice were defeated, as measured by displaying at least one submissive upright posture, only 65.2% of M. vaccae-immunized mice were defeated during the first hour on day 1 (Fisher’s exact test, P < 0.05). M. vaccae-immunized mice also showed reduced numbers of flight and avoiding behaviors (Fig. S2C) [LMM for AM behavior, M. vaccae, F(1, 131.5) = 10.8, P < 0.01]. There were no differences in the number of times experimental CSC mice attacked or chased the resident male (Fig. S2 D and E). M. vaccae-treated mice had a higher dominance index [the sum of proactive behaviors (attacks, chasing) minus the sum of reactive behaviors (submissive upright postures, flight, avoiding)] (Fig. 1B) [LMM for AM behavior, M. vaccae × time, F(2, 90.2) = 4.5, P < 0.05]. Overall, during the 19-d CSC procedure, 69.6% of M. vaccae-immunized mice displayed at least one proactive behavior, whereas only 21.7% of vehicle-treated mice did so (Fig. 1C) (Fisher’s exact test, P < 0.01).
Fig. 1.
Immunization with heat-killed M. vaccae induces proactive stress coping during chronic subordinate colony housing exposure and anxiolytic or fear-reducing behavioral responses on day 19. (A) Number of submissive upright posture displays (10–11:00 AM, white background; 5–6:00 PM, gray background) on days 1, 8, and 15 of CSC. (B) Dominance index. (C) Percent of vehicle- and M. vaccae-immunized mice displaying proactive behaviors during the 19-d CSC procedure. (D and E) Anxiety-like or fear-reducing behavior as measured on the elevated plus-maze on day 19 in (D) Exp. 1 or (E) Exp. 2. Bars represent means; error bars represent +SEM (A and C–E) or −SEM (B). Significance was assessed by linear mixed model analysis, conducted separately for AM and PM time points (A and B), Fisher’s exact test (C), two-factor ANOVA (D), and Student’s t test (E). *P < 0.05, **P < 0.01, ***P < 0.001, (A and B) between-subjects effects of M. vaccae versus vehicle, Fisher’s least significant difference (LSD) tests; (C) Fisher’s exact test; (E) Student’s t test. +++P < 0.01, (D) between-subjects effects of SHC versus CSC, Fisher’s LSD test; #P < 0.05, ##P < 0.01, ###P < 0.001, (A and B) within-subjects effects of time, paired t tests using Bonferroni correction. The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (A and B) N = 46; vehicle, 22–23; M. vaccae, 22–23. (C) N = 46; vehicle, 23; M. vaccae, 23. (D) N = 47; vehicle/SHC, 7; vehicle/CSC, 15; M. vaccae/SHC, 9; M. vaccae/CSC, 16. (E) N = 16; vehicle/CSC, 8; M. vaccae/CSC, 8.
A nearly identical pattern of behavior was observed during CSC exposure when the interval between the final immunization and CSC exposure was extended to 2 wk (Figs. S1C and S2 F–K). Immunization with M. vaccae decreased the number of submissive upright posture displays (Fig. S2F) [LMM, M. vaccae, F(1, 37.6) = 14.9, P < 0.001]. M. vaccae-immunized mice also showed reduced numbers of flight and avoiding behaviors (Fig. S2G) [LMM for AM behavior, M. vaccae × time, F(2, 28.8) = 10.5, P < 0.001]. There were no differences in the number of times experimental CSC mice attacked or chased the resident male (Fig. S2 H and I). M. vaccae-treated mice had a higher dominance index (Fig. S2J) [LMM for AM behavior, M. vaccae × time, F(2, 30.2) = 14.8, P < 0.0001]. Overall, during the 19-d CSC procedure, 62.5% of M. vaccae-immunized mice displayed at least one proactive behavior, whereas only 25.0% of vehicle-treated mice did so (Fig. S2K) (Fisher’s exact test, P = 0.14).
Together, these data demonstrate that immunization with M. vaccae induced a long-lasting shift toward a more proactive coping response (27), characterized by decreased submissive, flight, and avoiding behaviors, during chronic psychosocial stress that, based on previous studies in rodents and humans, may decrease vulnerability to the development of more persistent anxiety- and depressive-like symptoms (24, 25).
When tested on day 19, following the 19-d CSC procedure, CSC exposure had anxiolytic or fear-reducing effects in M. vaccae-immunized mice but not vehicle-immunized mice, as measured by time spent on the open arms of the EPM (Fig. 1D and Table S1) [two-factor ANOVA, M. vaccae × CSC, F(1, 43) = 2.3, P = 0.13; CSC, F(1, 43) = 10.1, P < 0.01]. M. vaccae-immunized, CSC-exposed mice spent more time exploring the aversive open arms of the EPM relative to M. vaccae-immunized, SHC mice. In Exp. 2, when a 2-wk interval was used between the final immunization with M. vaccae and the onset of the CSC procedure, M. vaccae immunization induced a strong anxiolytic response when CSC-exposed mice were tested on the EPM on day 20 (Fig. 1E, Fig. S1C, and Table S1) [Student’s t test, t(1, 14) = 3.9, P < 0.01]. In contrast to our previous data (28), CSC exposure did not increase anxiety-like behavior in vehicle-treated mice (Fig. 1D), probably representing a floor effect (vehicle-treated mice spent very little time exploring the open arms); vehicle-treated mice in the current study received multiple subcutaneous injections and were older at the time of testing, relative to previous studies. These differences may explain the high baseline anxiety in vehicle-immunized mice.
Table S1.
EPM behavior, tph2 mRNA expression, and slc6a4 mRNA expression data from Exps. 1, 2, and 4
| Vehicle | M. vaccae | |||
| Data in experiments | SHC | CSC | SHC | CSC |
| Elevated plus-maze data | ||||
| Exp. 1 | ||||
| Entries in open arms (n) | 11.57 ± 1.78 | 11.33 ± 0.96 | 9.33 ± 0.93 | 12.31 ± 1.08 |
| Entries in closed arms (n) | 18.67 ± 1.69 | 19.92 ± 1.40 | 20.44 ± 1.02 | 21.50 ± 1.33 |
| Percent entries in open arms (%) | 35.32 ± 2.60 | 36.37 ± 1.82 | 30.89 ± 1.67 | 35.65 ± 1.75 |
| †Time spent in open arms (s) | 36.68 ± 7.32 | 42.21 ± 3.39 | 26.75 ± 3.17 | 50.87 ± 5.46++ |
| ‡Time spent in closed arms (s) | 226.43 ± 5.90 | 214.36 ± 3.12 | 228.91 ± 4.69 | 203.34 ± 6.95++ |
| Percent time spent in open arms (%) | 13.02 ± 2.48 | 16.42 ± 1.28 | 10.41 ± 1.18 | 20.16 ± 2.18++ |
| Latency to first entry of open arms (s) | 19.23 ± 1.48 | 12.87 ± 3.90 | 33.25 ± 11.55 | 16.41 ± 3.14 |
| §Full entries of open arms (n) | 0.50 ± 0.34 | 1.42 ± 0.36 | 0.38 ± 0.26 | 1.56 ± 0.50 |
| Exp. 2 | ||||
| Entries in open arms (n) | 4.50 ± 1.24 | 8.00 ± 0.89* | ||
| Entries in closed arms (n) | 10.13 ± 1.77 | 14.00 ± 0.62 | ||
| Percent entries in open arms (%) | 24.28 ± 6.12 | 35.34 ± 2.80 | ||
| Time spent in open arms (s) | 15.88 ± 3.92 | 46.04 ± 6.80** | ||
| Time spent in closed arms (s) | 256.09 ± 10.25 | 213.09 ± 7.54** | ||
| Percent time spent in open arms (%) | 6.06 ± 1.53 | 17.76 ± 2.58** | ||
| Latency to first entry of open arms (s) | 141.33 ± 38.58 | 30.72 ± 11.96* | ||
| Full entries of open arms (n) | 0.00 ± 0.00 | 1.63 ± 0.56* | ||
| M. vaccae | ||||
| +Control antibody | +Anti-CD25 antibody | |||
| SHC | CSC | SHC | CSC | |
| Exp. 4 | ||||
| ¶Entries in open arms (n) | 4.29 ± 0.84 | 7.00 ± 1.15 | 2.80 ± 0.97 | 2.14 ± 0.40** |
| Entries in closed arms (n) | 10.57 ± 2.23 | 11.50 ± 1.89 | 9.80 ± 0.86 | 8.00 ± 2.25 |
| ‖Percent entries in open arms (%) | 28.86 ± 1.49 | 38.42 ± 4.05 | 20.38 ± 5.54 | 20.96 ± 4.69** |
| ††Time spent in open arms (s) | 21.47 ± 4.86 | 55.24 ± 12.51 | 13.85 ± 4.45 | 10.51 ± 2.73*** |
| ‡‡Time spent in closed arms (s) | 236.69 ± 12.92 | 195.99 ± 16.06+ | 251.15 ± 13.30 | 249.59 ± 10.23 |
| Percent time spent in open arms (%) | 8.60 ± 1.99 | 22.24 ± 4.94++ | 5.47 ± 1.93 | 3.88 ± 0.99*** |
| Latency to first entry of open arm (s) | 123.24 ± 40.74 | 33.83 ± 12.01 | 129.77 ± 38.02 | 133.61 ± 35.74 |
| §§Full entries of open arms (n) | 0.86 ± 0.40 | 2.83 ± 0.65++ | 0.60 ± 0.24 | 0.29 ± 0.18*** |
| Vehicle | M. vaccae | |||
| SHC | CSC | SHC | CSC | |
| tph2 mRNA expression in subdivisions of the DR and MnR | ||||
| Exp. 1 | ||||
| rDRD | 1.87 ± 0.11 | 1.84 ± 0.19 | 2.37 ± 0.06** | 2.42 ± 0.18** |
| cDRD | 1.94 ± 0.06 | 1.89 ± 0.09 | 1.94 ± 0.08 | 1.80 ± 0.15 |
| rDRV | 2.59 ± 0.13 | 2.55 ± 0.15 | 2.86 ± 0.23 | 2.60 ± 0.15 |
| cDRV | 2.54 ± 0.14 | 2.34 ± 0.10 | 2.50 ± 0.10 | 2.16 ± 0.20 |
| DRVL/VLPAG | 1.38 ± 0.04 | 1.36 ± 0.04 | 1.40 ± 0.05 | 1.24 ± 0.07 |
| DRC | 1.43 ± 0.05 | 1.62 ± 0.12 | 1.56 ± 0.06 | 1.38 ± 0.06 |
| DRI | 1.36 ± 0.05 | 1.35 ± 0.03 | 1.47 ± 0.08 | 1.33 ± 0.05 |
| Entire DR, average | 1.72 ± 0.04 | 1.74 ± 0.03 | 1.84 ± 0.06 | 1.66 ± 0.04 |
| rMnR | 0.92 ± 0.03 | 1.02 ± 0.05 | 1.09 ± 0.10 | 1.13 ± 0.07 |
| cMnR | 0.71 ± 0.03 | 0.83 ± 0.02 | 0.81 ± 0.06 | 0.86 ± 0.06 |
| M. vaccae | ||||
| +Control antibody | +Anti-CD25 antibody | |||
| SHC | CSC | SHC | CSC | |
| Exp. 4 | ||||
| rDRD | 3.24 ± 0.17 | 3.32 ± 0.16 | 3.36 ± 0.06 | 3.21 ± 0.27 |
| cDRD | 3.10 ± 0.28 | 2.80 ± 0.24 | 2.68 ± 0.18 | 3.07 ± 0.25 |
| rDRV | 3.60 ± 0.09 | 3.35 ± 0.27 | 3.43 ± 0.23 | 3.59 ± 0.33 |
| cDRV | 3.08 ± 0.22 | 3.10 ± 0.17 | 3.05 ± 0.18 | 2.83 ± 0.27 |
| DRVL/VLPAG | 2.07 ± 0.10 | 2.13 ± 0.11 | 2.16 ± 0.13 | 2.16 ± 0.16 |
| DRC | 2.87 ± 0.17 | 2.39 ± 0.04 | 2.62 ± 0.05 | 2.74 ± 0.12 |
| DRI | 1.91 ± 0.08 | 1.92 ± 0.09 | 2.23 ± 0.13* | 2.13 ± 0.17* |
| Entire DR, average | 2.50 ± 0.05 | 2.37 ± 0.04 | 2.46 ± 0.06 | 2.49 ± 0.09 |
| rMnR | 1.40 ± 0.05 | 1.85 ± 0.01++ | 1.70 ± 0.05** | 1.68 ± 0.04 |
| cMnR | 1.23 ± 0.07 | 1.29 ± 0.06 | 1.21 ± 0.07 | 1.30 ± 0.08 |
| Vehicle | M. vaccae | |||
| SHC | CSC | SHC | CSC | |
| slc6a4 mRNA expression in subdivisions of the DR and MnR | ||||
| Exp. 1 | ||||
| rDRD | 3.78 ± 0.22 | 2.9 ± 0.21++ | 3.55 ± 0.29 | 3.13 ± 0.37 |
| cDRD | 3.46 ± 0.24 | 3.69 ± 0.20 | 3.72 ± 0.52 | 3.23 ± 0.41 |
| rDRV | 3.87 ± 0.44 | 3.28 ± 0.17 | 4.14 ± 0.37 | 3.11 ± 0.25+ |
| cDRV | 5.34 ± 0.98 | 4.68 ± 0.77 | 6.44 ± 1.23 | 4.40 ± 0.58 |
| DRVL/VLPAG | 2.43 ± 0.22 | 2.17 ± 0.08 | 2.37 ± 0.34 | 2.12 ± 0.04 |
| DRC | 2.17 ± 0.09 | 1.85 ± 0.15+ | 2.29 ± 0.12 | 2.23 ± 0.11* |
| DRI | 2.51 ± 0.23 | 2.33 ± 0.13 | 2.17 ± 0.19 | 2.17 ± 0.07 |
| Entire DR, average | 3.11 ± 0.23 | 2.75 ± 0.12 | 3.34 ± 0.32 | 2.65 ± 0.13 |
| rMnR | 1.78 ± 0.21 | 1.5 ± 0.25 | 1.97 ± 0.24 | 1.52 ± 0.90 |
| cMnR | 1.72 ± 0.12 | 1.5 ± 0.05 | 2.15 ± 0.35 | 1.52 ± 0.10+ |
| M. vaccae | ||||
| +Control antibody | +Anti-CD25 antibody | |||
| SHC | CSC | SHC | CSC | |
| Exp. 4 | ||||
| rDRD | 1.76 ± 0.15 | 1.82 ± 0.17 | 1.68 ± 0.20 | 1.82 ± 0.12 |
| cDRD | 1.52 ± 0.17 | 1.55 ± 0.13 | 1.62 ± 0.15 | 1.51 ± 0.09 |
| rDRV | 2.42 ± 0.25 | 1.87 ± 0.14 | 1.75 ± 0.23* | 1.95 ± 0.12 |
| cDRV | 1.56 ± 0.13 | 1.61 ± 0.14 | 1.48 ± 0.11 | 1.45 ± 0.16 |
| DRVL/VLPAG | 1.09 ± 0.05 | 1.09 ± 0.09 | 1.03 ± 0.09 | 1.04 ± 0.08 |
| DRC | 1.19 ± 0.11 | 1.12 ± 0.08 | 0.96 ± 0.08 | 1.02 ± 0.06 |
| DRI | 1.13 ± 0.09 | 1.08 ± 0.13 | 0.98 ± 0.08 | 0.88 ± 0.05 |
| Entire DR, average | 1.35 ± 0.08 | 1.29 ± 0.09 | 1.19 ± 0.10 | 1.22 ± 0.06 |
| rMnR | 1.09 ± 0.07 | 1.10 ± 0.08 | 0.97 ± 0.08 | 1.13 ± 0.13 |
| cMnR | 0.80 ± 0.09 | 0.88 ± 0.09 | 0.74 ± 0.05 | 0.82 ± 0.09 |
+P < 0.05, ++P < 0.01, CSC versus SHC, EPM, Exp. 1, Exp. 4, Fisher's least significant difference tests; tph2 mRNA expression, Exp. 4, Fisher's least significant difference tests; slc6a4 mRNA expression, Exp. 1, Fisher's least significant difference tests; *P < 0.05, **P < 0.01, ***P < 0.001, vehicle versus M. vaccae, EPM, Exp. 2, Student's t tests; control antibody versus anti-CD25 antibody, EPM, Exp. 4, Fisher's least significant difference tests; vehicle versus M. vaccae, tph2 mRNA expression, Exp. 1, Fisher's least significant difference tests; control antibody versus anti-CD25 antibody, tph2 mRNA expression, Exp. 4, Fisher's least significant difference tests; vehicle versus M. vaccae, slc6a4 mRNA expression, Exp. 1, Fisher's least significant difference tests.
Two-factor ANOVA, CSC, F(1, 40) = 9.2, P < 0.01.
Two-factor ANOVA, CSC, F(1, 40) = 8.9, P < 0.01.
Two-factor ANOVA, CSC, F(1, 38) = 4.6, P < 0.05.
Two-factor ANOVA, anti-CD25 antibody, F(1, 21) = 13.6, P < 0.01.
Two-factor ANOVA, anti-CD25 antibody, F(1, 21) = 10.2, P < 0.01.
Two-factor ANOVA, anti-CD25 antibody × CSC, F(1, 21) = 6.9, P < 0.05.
Two-factor ANOVA, anti-CD25 antibody, F(1, 21) = 6.6, P < 0.05.
Two-factor ANOVA, anti-CD25 antibody × CSC, F(1, 21) = 7.6, P < 0.05.
In Exp. 3, CSC exposure had an anxiogenic effect in the social preference/avoidance test, decreasing time spent in the contact zones of the novel object and novel conspecific (Figs. S1D and S2L) [LMM, CSC, F(1, 43.3) = 7.3, P < 0.05]. There was an overall preference for social contact, relative to the novel object (Fig. S2L) [LMM, social, F(1, 45.8) = 11.1, P < 0.01]. There were no effects of M. vaccae, or M. vaccae × CSC interactions, on conflict anxiety in the social preference/avoidance test, and there were no effects of either M. vaccae immunization or CSC exposure on locomotor activity (Fig. S2M). There were no effects of either M. vaccae immunization or CSC exposure on conflict anxiety or locomotor activity in the light/dark box test (Figs. S1D and S2 N and O).
Consistent with previous studies, CSC exposure increased adrenal weight (Figs. S1B and S2P) and in vitro adrenal insensitivity to adrenocorticotropic hormone (ACTH) (Figs. S1B and S2Q). M. vaccae immunization did not prevent these effects. These data suggest that CSC exposure was physically and/or psychologically stressful for both vehicle- and M. vaccae-immunized groups.
Persistent Effects of M. vaccae Immunization on Brain Serotonergic Systems.
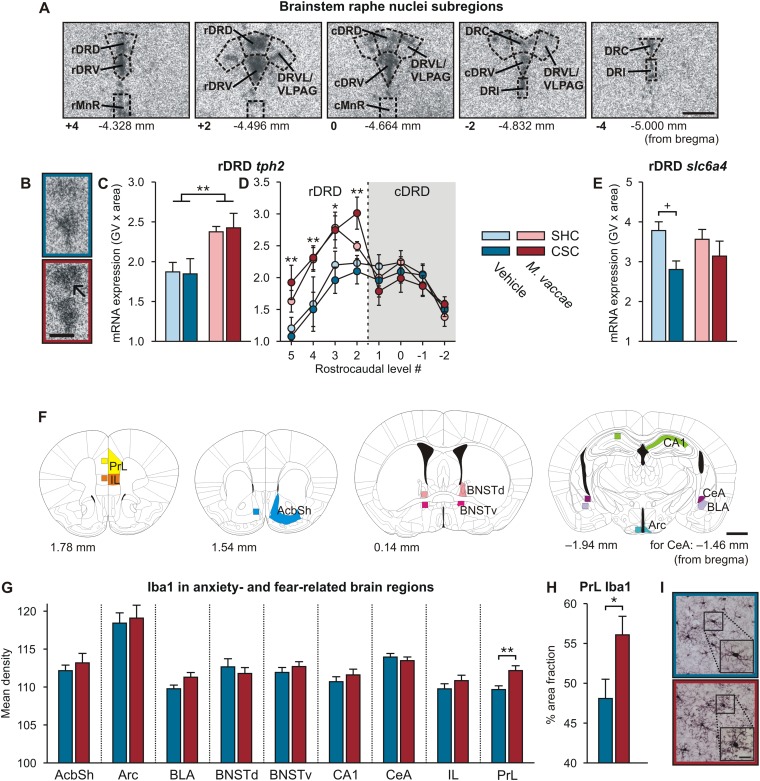
Because chronic exercise alters brain serotonergic gene expression (29–31) and because this may be relevant to the stress resistance effects of chronic exercise, we examined the effects of CSC exposure and M. vaccae immunization on serotonergic gene expression in the brainstem raphe nuclei. Specifically, we analyzed expression of tph2, encoding tryptophan hydroxylase 2, the rate-limiting enzyme in the biosynthesis of serotonin, and slc6a4, encoding solute carrier family 6 (neurotransmitter transporter), member 4, the high-affinity, low-capacity, sodium-dependent serotonin transporter. Immunization with M. vaccae increased tph2 mRNA expression selectively in the rostral region of the dorsal raphe nucleus, dorsal part (rDRD) (Fig. S3 A–D and Table S1) [LMM, M. vaccae × region, F(8, 715.6) = 7.4, P < 0.001].
Fig. S3.
M. vaccae administration has persistent effects on brain serotonergic systems and microglial density in the brain. (A–E) M. vaccae immunization has long-term effects on brain serotonergic systems. In situ hybridization histochemistry was used to measure tph2 and slc6a4 mRNA expression at multiple rostrocaudal levels of the dorsal and median raphe nuclei in mice from Exp. 1. (A) Photomicrographs illustrate representative tph2 mRNA expression at multiple rostrocaudal levels within the dorsal raphe nucleus. (B) Representative photomicrographs illustrating autoradiographic images of tph2 hybridization signal in vehicle- (Top) and M. vaccae- (Bottom) immunized mice. The arrow indicates the rostral dorsal raphe nucleus, dorsal part (rDRD). (C) tph2 mRNA expression averaged across all rostrocaudal levels of the rDRD of each mouse. (D) tph2 mRNA expression at specific rostrocaudal levels of the rDRD and caudal dorsal raphe nucleus, dorsal part (cDRD). (E) Expression of slc6a4 mRNA expression, encoding the high-affinity, sodium-dependent serotonin transporter, averaged across all rostrocaudal levels of the rDRD of each mouse. Values represent means; error bars represent SEM. *P < 0.05, **P < 0.01, (C and D) two-factor ANOVA, M. vaccae main effect. +P < 0.05, (E) CSC versus SHC, Fisher’s least significant difference tests. cDRV, caudal dorsal raphe nucleus, ventral part; cMnR, caudal median raphe nucleus; DRC, dorsal raphe nucleus, caudal part; DRI, dorsal raphe nucleus, interfascicular part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; GV, gray value; rDRV, rostral dorsal raphe nucleus, ventral part; rMnR, rostral median raphe nucleus. Numbers at the lower left of each photomicrograph in A indicate experimenter-designated rostrocaudal levels and correspond to those in D. Numerical values below each photograph in A indicate rostrocaudal levels in mm bregma. [Scale bars, 500 µm (A) and 250 µm (B)] The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (C and D) N = 31; vehicle/SHC, 8; vehicle/CSC, 8; M. vaccae/SHC, 7; M. vaccae/CSC, 8. (E) N = 31; vehicle/SHC, 8; vehicle/CSC, 9; M. vaccae/SHC, 6; M. vaccae/CSC, 8. (F–I) Effects of M. vaccae immunization, in CSC mice, on microglial density. (F) Illustration of anxiety- and fear-related brain regions that were sampled for measurement of ionized calcium-binding adapter molecule 1 (Iba1) immunostaining in M. vaccae and vehicle-immunized, CSC-exposed mice in Exp. 1. Numbers to the lower left of each illustration indicate rostrocaudal coordinates in mm bregma. Colored shadings on the right of each illustration indicate the brain regions studied. Colored boxes on the left of each illustration indicate the region sampled for densitometric measurement of Iba1 immunostaining. (G) Mean density measurements of Iba1 immunostaining in the nine regions studied in M. vaccae- and vehicle-immunized, CSC-exposed mice. (H) Detailed cumulative densitometric threshold analysis of Iba1 immunostaining in the prelimbic cortex (PrL). (I) Representative photomicrographs of Iba1 immunostaining in the PrL of a vehicle-immunized (Top) and M. vaccae-immunized (Bottom), CSC-exposed mouse. Boxes indicate regions shown at higher magnification (lower right of each image). Values represent means; error bars represent SEM. *P < 0.05, **P < 0.01, Student’s t test. AcbSh, nucleus accumbens, shell region; Arc, arcuate nucleus of the hypothalamus; BLA, basolateral amygdaloid nucleus, anterior part; BNSTd, bed nucleus of the stria terminalis, lateral division, dorsal part; BNSTv, bed nucleus of the stria terminalis, ventral part; CA1, CA1 field of the dorsal hippocampus; CeA, central amygdaloid nucleus; IL, infralimbic cortex. [Scale bars, 1 mm (F) and 20 µm (I).] The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (G and H) N = 29; vehicle/CSC, 15; M. vaccae/CSC, 14.
Immunization with M. vaccae also prevented a stress-induced decrease in slc6a4 mRNA expression, also in the rDRD (Fig. S3E) [two-factor ANOVA, CSC, F(1, 27) = 6.5, P < 0.05], further supporting long-term effects of M. vaccae immunization on this subset of serotonergic neurons. Broader implications of these findings include the capacity for bioimmunomodulatory approaches to alter gene expression patterns in highly specific neural systems in the brain across a long timescale, at least 4 wk, effects that may influence stress coping strategies and stress resilience.
Influence of M. vaccae Immunization on Microglia.
Given the potential role of inflammatory mediators in determining behavioral coping responses to psychosocial stressors (32) and recent findings that the gut microbiota and peripheral immune activity continuously control maturation and function of microglia in the central nervous system (CNS) (33, 34), we investigated the effects of M. vaccae immunization on microglial density. To determine whether M. vaccae immunization altered the number or morphological properties of microglia within the brain, we performed immunohistochemical staining of ionized calcium-binding adapter molecule 1 (Iba1), a 17-kDa actin-binding protein specifically and constitutively expressed in microglia (35, 36) that is an immunohistochemical marker for both ramified and activated microglia (35, 37). Iba1 immunostaining was evaluated in brain regions implicated in control of anxiety and fear states (Fig. S3F). Because we were interested in effects of M. vaccae on activated microglia, we conducted analyses using CSC-exposed mice only. The density and morphology of microglia were analyzed using image analysis. We found that, among CSC-exposed mice, immunization with M. vaccae selectively increased microglial density in the prelimbic part of the medial prefrontal cortex (PrL) (Fig. S3 G and I) [Student’s t test, t(1, 24) = 2.4, P < 0.05], which plays an important role in fear expression (38) and provides the main cortical input to the dorsal raphe nucleus controlling stress-induced anxiety states (39). There were no effects in the infralimbic part (IL) of the medial prefrontal cortex or other regions studied. Detailed cumulative densitometric threshold analysis revealed that there was 8% more Iba1 immunostaining in the PrL in M. vaccae-immunized CSC-exposed mice compared with vehicle-immunized CSC-exposed mice (Fig. S3 H and I) [Student’s t test, 56.1 ± 2.3 versus 48.1 ± 2.4, respectively, t(1, 24) = 3.1, P < 0.01]. These data confirm the long-term effects of M. vaccae immunization on the microglial phenotype in the PrL, a forebrain structure critical for control of fear expression, in CSC-exposed mice.
Stress Promotes Colitogenic Dysbiosis.
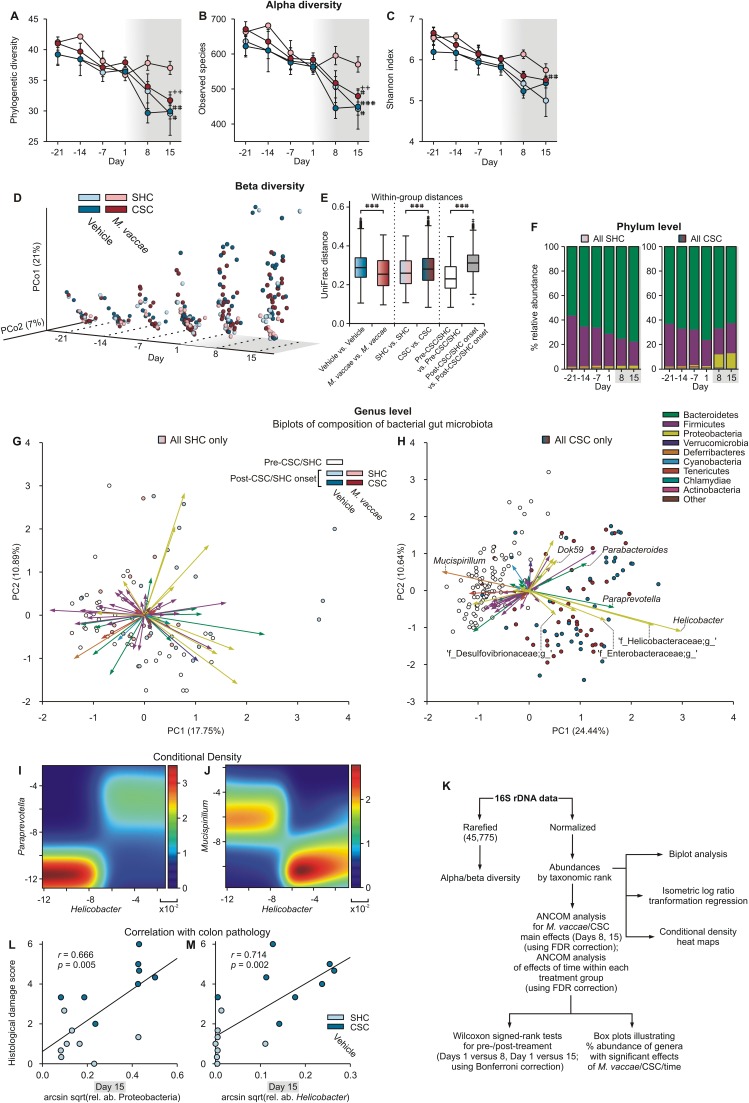
Given that stress alters the composition of the gut microbiome (14) and, consequently, the homeostatic balance between the gut microbiota and mucosal immune system, with important consequences for mucosal inflammation (15) as well as emotional behavior, including anxiety-like behavior (16), we conducted next-generation sequencing to characterize the effects of stress on the composition of the gut microbiome. Furthermore, we investigated whether or not prior immunization with M. vaccae had any impact on stress-induced changes in the gut microbiota. In microbial ecology, there are two principal measures of species diversity, with α-diversity assessing diversity within a sample and β-diversity assessing diversity between samples. There were strong overall declines in α-diversity over time, particularly evident at the onset of the CSC procedure (Fig. S4 A–C) [LMM, day, phylogenetic diversity, F(5, 28.1) = 11.0, P < 0.0001; observed species, F(5, 28.5) = 10.9, P < 0.0001; Shannon index, F(5, 35.9) = 13.9, P < 0.0001], suggesting that the CSC procedure was stressful for all mice, which were housed in the same room. As observed with other stressors (15), CSC exposure increased β-diversity, relative to SHC conditions (Fig. S4 D and E). Based on analysis of all samples across all time points, α-diversity was higher and β-diversity was lower in M. vaccae-immunized mice compared with vehicle-immunized mice (Fig. S4 A–E) [LMM, α-diversity, M. vaccae, phylogenetic diversity, F(1, 26.9) = 5.9, P < 0.05; observed species, F(1, 31.1) = 5.4, P < 0.05; Shannon index, F(1, 37.0) = 11.8, P < 0.01; M. vaccae × CSC × day, phylogenetic diversity, F(16, 36.1) = 2.1, P < 0.05; observed species, F(16, 36.1) = 2.1, P < 0.05; Shannon index, F(16, 47.5) = 1.3, P = 0.27], indicating that M. vaccae immunization had a stabilizing effect on the gut microbiota throughout the study, consistent with recent studies demonstrating that host adaptive immunity modulates the gut microbiota (40). In line with these findings, multiple linear regression showed that 11% of the variation in the gut microbiota was explained by the histological damage score in the colon, reflecting intestinal immune activation.
Fig. S4.
M. vaccae fails to prevent expansion of colitogenic microbes. (A–K) Analysis of gut microbial community structure and microbial composition in mice from Exp. 1. (A–C) α-Diversity of gut microbial communities as measured by (A) phylogenetic diversity, (B) number of observed species, and (C) Shannon index over time. Analyses were performed on 16S rRNA V4 amplicon data, with a rarefaction depth of 45,775 reads per sample (the smallest sequencing depth that we did not filter out; three samples had <1,000 reads and were excluded from analysis). Gray shading indicates the onset of the CSC procedure immediately following collection of fecal samples on day 1. (D) β-Diversity was calculated using unweighted UniFrac (conclusions with weighted UniFrac were unchanged). Principal coordinate 1 (PCo1) vs. PCo2 are displayed along with an explicit time axis (axis pointing to the right). The proportion of variance explained by each principal coordinate axis is denoted in the corresponding axis label; PCo1 explains 21% of the variability and PCo2 explains 7%. (E) Distance comparison plot with box plots illustrating distances within and between sample groupings using the β-diversity distance matrix. The bottoms and tops of boxes indicate the first and third quartiles, respectively; whiskers indicate the 1.5 interquartile range (IQR) beyond the upper and lower quartiles. Values outside the whiskers are indicated by “+.”###P < 0.001. Differences within and between group distances using Student’s t tests with Bonferroni correction. (F) Stacked vertical bar charts illustrating the percent relative abundance of different phyla across days based on analysis of all samples from SHC (Left) and CSC (Right) mice. (G and H) Biplots illustrating genus-level gut microbial community analysis (open and closed circles) and composition analysis (vectors) using principal component analysis of center logratio transformed and standardized data from (G) SHC- and (H) CSC-exposed mice. The samples are indicated with individual symbols, according to pre-CSC onset (open symbols; days –21, –14, –7, and 1) or post-CSC onset (closed symbols; days 8 and 15); the distance between symbols approximates the dissimilarity of their microbial communities, as measured by Euclidean distance. PCA axes 1 and 2 explain (G) 18.64% and (H) 35.08% of the variation. Vectors point in the direction of the greatest increase of values for the corresponding genus across all time points. The angle between the arrows indicates approximated correlation (>90° indicates a negative correlation). See Table S2 for identification of genera corresponding to each vector. (I and J) Conditional density plots illustrating representative positive correlations (I; Helicobacter versus Paraprevotella) and negative correlations (J; Helicobacter versus Mucispirillum) between specific genera, consistent with the biplot analysis. (K) Flow chart illustrating the methods used for analysis of M. vaccae/CSC and time effects on gut microbial community structure and microbial composition. (L and M) Relative abundances of (L) Proteobacteria and (M) Helicobacter on day 15 in vehicle-immunized mice exposed to either SHC or CSC conditions were transformed using the arcsin square root transformation and plotted against the histological damage to the colon. One SHC sample was an extreme outlier for relative abundance of both Proteobacteria and Helicobacter and was removed for this analysis. (A–C) Symbols represent means; error bars represent ±SEM. +P < 0.05, ++P < 0.01 (A–C) between-subjects effects of SHC versus CSC, Mann–Whitney U test; #P < 0.05, ##P < 0.01, ###P < 0.001 (A–C) within-subjects effects of time, relative to day 1, Wilcoxon signed-rank tests with Bonferroni correction. The numbers of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (A–J) N = 62; vehicle/SHC, 10; vehicle/CSC, 21; M. vaccae/SHC, 9; M. vaccae/CSC, 22. (L and M) N = 16; vehicle/SHC, 8; vehicle/CSC, 8.
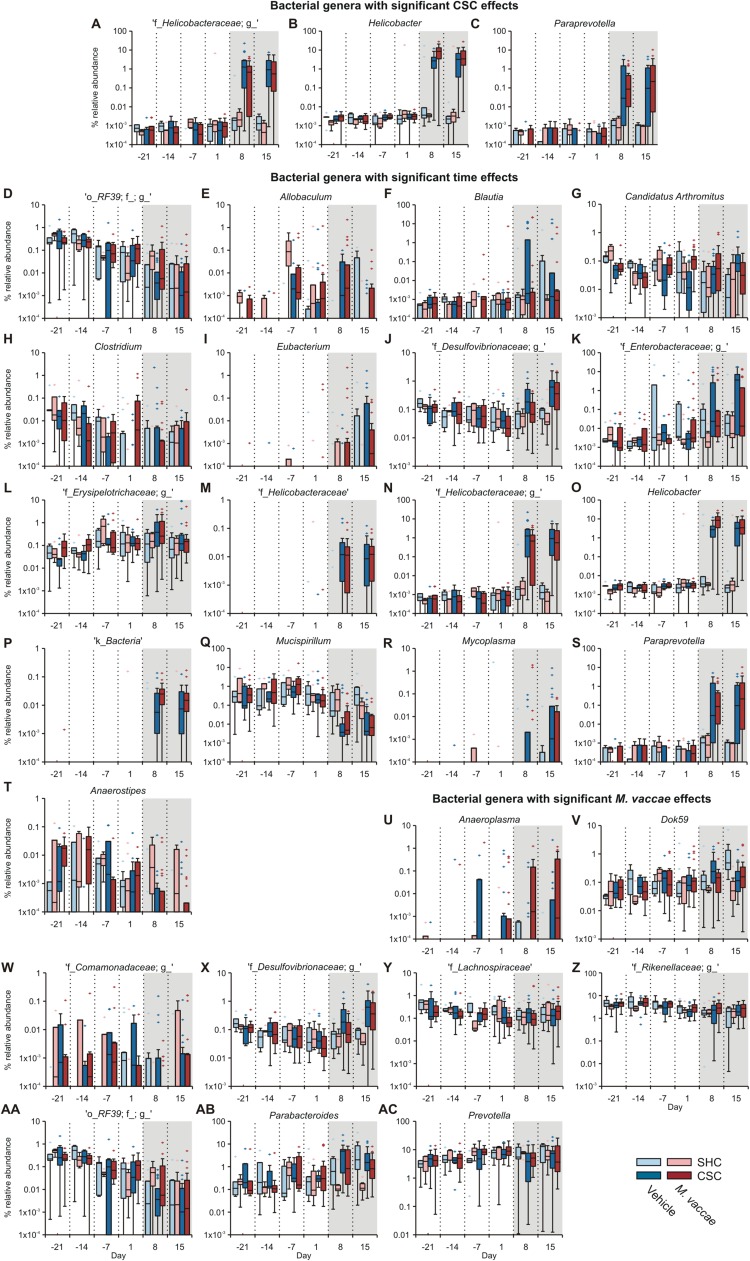
Detailed analysis of the microbial composition, conducted using analysis of composition of microbiomes (ANCOM) (41), revealed main effects of CSC to increase the abundance of Proteobacteria (percent relative abundances are plotted in Fig. S4F), including Helicobacter, and an unidentified genus of Helicobacteraceae, as well as Paraprevotella (Bacteroidetes) on day 8 or 15 [ANCOM, false discovery rate (FDR)-adjusted P < 0.05] (Figs. S4 G–J and S5 A–C and Table S2). Changes in microbial community structure over time were evaluated using ANCOM over all six time points, followed, when appropriate, by Wilcoxon signed-rank tests comparing days 1 versus 8 and days 1 versus 15, using Bonferroni correction (Fig. S4K). Consistent with the analysis of the main effects of CSC exposure above, time-dependent increases in Helicobacter, two unidentified genera of Helicobacteraceae, and Paraprevotella were seen on days 8 and 15 in both vehicle-immunized and M. vaccae-immunized CSC mice but not SHC mice (Fig. S5 M–O and S and Table S3). In addition, decreases in Mucispirillum were observed on days 8 and 15 in both vehicle-immunized and M. vaccae-immunized CSC mice but not SHC mice (Fig. S5Q and Table S3). Furthermore, we observed a main effect of M. vaccae to stabilize the abundance of several genera on day 8 or 15, including an unidentified genus of Desulfovibrionaceae (Proteobacteria) (Fig. S5X). Our data are consistent with a CSC-induced gut dysbiosis and a shift toward a gut microbiota with increased potential for inflammation (Fig. S4H). Psychological stress increases Helicobacter abundance through glucocorticoid actions on glucocorticoid receptors (18), and previous studies have found that Helicobacter abundance predicts intestinal inflammation scores specifically in mice with impaired immunoregulation (IL-10−/− mice; r = 0.58) (42). Consistent with these previous findings, relative abundances of both Proteobacteria and Helicobacter predicted histological damage to the colon in our study (Fig. S4 L and M). Meanwhile, expansion of Paraprevotella, as observed in our study (Figs. S4 H and I and S5S), has been associated with multiple murine models of experimental autoimmunity (43), consistent with a stress-induced autoimmune-like response to dietary, microbiota, or self-antigens in the absence of adequate immunoregulation. Finally, decreases in the abundance of Mucispirillum over time, as observed in our study in both vehicle/CSC and M. vaccae/CSC mice (Figs. S4 H and J and S5Q and Table S3), have been identified as a biological signature of gut infection (44). A decline of Mucispirillum is associated with early disruption of the colonic surface mucus layer and a prolonged delay to recovery after the period of pathogen clearance (44).
Fig. S5.
Gut microbiota data of Exp. 1, showing main effects of M. vaccae, CSC, and time on gut microbial composition. (A–C) Main effects of CSC on gut microbial composition. Main effects of CSC exposure, relative to SHC, were analyzed separately on days 8 and 15 using ANCOM (significant at FDR 0.05). Main effects of CSC, relative to SHC controls, were observed for the following genera. (A) An unidentified genus within the Helicobacteraceae family on days 8 and 15. (B) Helicobacter on days 8 and 15. (C) Paraprevotella (Bacteroidetes) on day 15. Gray shading indicates the onset of the CSC procedure immediately following collection of fecal samples on day 1. (D–T) Effects of time, within specific treatment groups, on gut microbial composition. Box plots illustrate main effects of time, based on analysis of all six time points, using ANCOM (significant at FDR 0.05). For significant post hoc comparisons, based on Wilcoxon signed-rank tests comparing day 1 with day 8 and day 1 with day 15, see Table S3. Effects of time were observed for the following genera. (D) An unidentified genus in the order RF39 (Tenericutes; vehicle/SHC, M. vaccae/SHC, M. vaccae/CSC). (E) Allobaculum (Firmicutes; vehicle/CSC). (F) Blautia (Firmicutes; vehicle/CSC). (G) Candidatus Arthromitus (Firmicutes; vehicle/CSC). (H) Clostridium (Firmicutes; vehicle/CSC). (I) [Eubacterium] (Firmicutes; vehicle/CSC, M. vaccae/CSC). (J) An unidentified genus in the family Desulfovibrionaceae (Proteobacteria; vehicle/CSC, M. vaccae/CSC). (K) An unidentified genus in the family Enterobacteraceae (Proteobacteria; vehicle/CSC). (L) An unidentified genus in the family Erysipelotrichaceae (Firmicutes; vehicle/CSC). (M) An unidentified genus in the family Helicobacteraceae (Proteobacteria; vehicle/CSC, M. vaccae/CSC). (N) A second unidentified genus in the family Helicobacteraceae (Proteobacteria; vehicle/CSC, M. vaccae/CSC). (O) Helicobacter (Proteobacteria; vehicle/CSC, M. vaccae/CSC). (P) An unidentified genus in the kingdom Bacteria (vehicle/CSC, M. vaccae/CSC). (Q) Mucispirillum (Deferribacteres; vehicle/CSC, M. vaccae/CSC). (R) Mycoplasma (Tenericutes; vehicle/CSC, M. vaccae/CSC). (S) Paraprevotella (Bacteroidetes; vehicle/CSC, M. vaccae/CSC). (T) Anaerostipes (Firmicutes; M. vaccae/CSC). Gray shading indicates the onset of the CSC procedure immediately following collection of fecal samples on day 1. (U–AC) Main effects of immunization with M. vaccae, relative to immunization with vehicle, were analyzed separately on days 8 and 15 using ANCOM (significant at FDR 0.05). We observed a main effect of M. vaccae to stabilize the abundance of several genera on day 8 and/or 15, including Dok59, an unidentified genus of Desulfovibrionaceae, and Parabacteroides. Main effects of M. vaccae, relative to vehicle, were observed for the following genera. (U) Anaeroplasma (Tenericutes) on day 8. (V) Dok59 (Proteobacteria) on day 8. (W) An unidentified genus in the family Comamonadaceae (Proteobacteria) on day 8. (X) An unidentified genus in the family Desulfovibrionaceae (Proteobacteria) on day 8. (Y) An unidentified genus in the family Lachnospiraceae (Firmicutes) on day 8. (Z) An unidentified genus in the family Rikkenellaceae (Bacteroidetes) on day 8. (AA) An unidentified genus in the order RF39 (Tenericutes) on day 8. (AB) Parabacteroides (Bacteroidetes) on days 8 and 15. (AC) Prevotella (Bacteroidetes) on day 8. Gray shading indicates the onset of the CSC procedure immediately following collection of fecal samples on day 1. Data are expressed as percent relative abundance, which is the normalized proportion of genera counts, on a log scale. The bottoms and tops of boxes indicate the first and third quartiles, respectively; whiskers indicate 1.5 IQR beyond the upper and lower quartiles. Values outside the whiskers are indicated by +. The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: N = 62; vehicle/SHC, 10; vehicle/CSC, 21; M. vaccae/SHC, 9; M. vaccae/CSC, 22.
Table S2.
Principal component axis 1 and principal component axis 2 coordinates, degrees, and radius of all biplot vectors in Fig. S4
| Genus | PCA1 | PCA2 | Degrees | Radius |
| SHC biplot (Fig. S4G) | ||||
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__[Ruminococcus] | −0.75 | −0.04 | −176.80 | 0.75 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcus | −0.91 | −0.13 | −171.61 | 0.92 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__ | −0.83 | −0.14 | −170.39 | 0.84 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__AF12 | −0.49 | −0.14 | −163.71 | 0.51 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Coprococcus | −0.50 | −0.15 | −163.49 | 0.52 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Paraprevotellaceae]; g__[Prevotella] | −0.65 | −0.19 | −163.37 | 0.68 |
| p__Tenericutes; c__Mollicutes; o__RF39; f__; g__ | −0.56 | −0.36 | −147.31 | 0.67 |
| p__Firmicutes; c__Bacilli; o__Bacillales; f__Alicyclobacillaceae; g__Alicyclobacillus | −0.07 | −0.05 | −143.99 | 0.08 |
| p__Deferribacteres; c__Deferribacteres; o__Deferribacterales; f__Deferribacteraceae; g__Mucispirillum | −0.92 | −0.67 | −143.89 | 1.14 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__; g__ | −0.67 | −0.61 | −137.75 | 0.91 |
| p__Cyanobacteria; c__4C0d-2; o__YS2; f__; g__ | −0.35 | −0.39 | −131.98 | 0.52 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Prevotellaceae; g__Prevotella | −0.45 | −0.92 | −116.16 | 1.02 |
| p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Lactobacillaceae; g__Lactobacillus | −0.15 | −0.53 | −105.57 | 0.55 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__ | −0.07 | −0.55 | −97.59 | 0.55 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__S24-7; g__ | 0.09 | −0.23 | −67.69 | 0.24 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__Clostridium | 0.09 | −0.22 | −67.13 | 0.24 |
| Unassigned | 0.10 | −0.15 | −58.06 | 0.18 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales | 0.35 | −0.40 | −48.94 | 0.53 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__Candidatus Arthromitus | 0.11 | −0.11 | −45.57 | 0.16 |
| p__Verrucomicrobia; c__Verrucomicrobiae; o__Verrucomicrobiales; f__Verrucomicrobiaceae; g__Akkermansia | 0.70 | −0.71 | −45.28 | 1.00 |
| p__Proteobacteria; c__Alphaproteobacteria; o__Rickettsiales; f__mitochondria | 0.05 | −0.05 | −43.88 | 0.08 |
| p__Proteobacteria; c__Betaproteobacteria; o__Methylophilales; f__; g__ | 1.21 | −1.06 | −41.21 | 1.60 |
| p__Proteobacteria; c__Alphaproteobacteria; o__RF32; f__; g__ | 0.95 | −0.60 | −32.37 | 1.12 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__Coprobacillus | 0.88 | −0.52 | −30.74 | 1.02 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__ | 0.34 | −0.19 | −29.37 | 0.39 |
| p__Proteobacteria; c__Betaproteobacteria; o__Rhodocyclales; f__Rhodocyclaceae; g__Dok59 | 1.16 | −0.53 | −24.49 | 1.28 |
| p__Chlamydiae; c__Chlamydiia; o__Chlamydiales; f__Chlamydiaceae; g__ | 0.34 | −0.13 | −21.45 | 0.36 |
| p__Proteobacteria; c__Betaproteobacteria; o__Burkholderiales; f__Comamonadaceae; g__ | 0.21 | −0.07 | −17.40 | 0.22 |
| p__Cyanobacteria; c__Chloroplast; o__Streptophyta; f__; g__ | 0.10 | −0.02 | −13.36 | 0.11 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Porphyromonadaceae; g__Parabacteroides | 1.56 | −0.30 | −10.73 | 1.59 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__RF16; g__ | 0.75 | 0.02 | 1.28 | 0.75 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnobacterium | 0.27 | 0.01 | 1.29 | 0.27 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptococcaceae; g__rc4-4 | 0.13 | 0.01 | 2.86 | 0.13 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Anaerostipes | 0.46 | 0.03 | 3.35 | 0.46 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidaceae; g__Bacteroides | 0.68 | 0.10 | 8.71 | 0.69 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Blautia | 1.06 | 0.19 | 10.26 | 1.08 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__Allobaculum | 0.15 | 0.03 | 12.90 | 0.16 |
| p__Tenericutes; c__Mollicutes; o__Anaeroplasmatales; f__Anaeroplasmataceae; g__Anaeroplasma | 0.16 | 0.07 | 24.67 | 0.18 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae | 0.42 | 0.25 | 30.55 | 0.49 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptostreptococcaceae; g__ | 0.09 | 0.06 | 33.46 | 0.11 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__[Eubacterium] | 0.24 | 0.20 | 39.54 | 0.32 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae | 0.06 | 0.05 | 43.39 | 0.08 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Pseudomonadales; f__Pseudomonadaceae; g__Pseudomonas | 0.07 | 0.07 | 44.54 | 0.09 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae | 0.39 | 0.44 | 48.97 | 0.59 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae; g__ | 0.83 | 1.08 | 52.25 | 1.36 |
| k__Bacteria | 0.08 | 0.13 | 58.67 | 0.15 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae; g__ | 0.40 | 1.36 | 73.75 | 1.42 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae; g__Helicobacter | 0.52 | 1.87 | 74.50 | 1.94 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Paraprevotellaceae]; g__Paraprevotella | 0.09 | 0.58 | 81.28 | 0.58 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptococcaceae; g__ | 0.01 | 0.34 | 87.60 | 0.34 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Epulopiscium | −0.05 | 0.22 | 101.84 | 0.22 |
| p__Proteobacteria; c__Deltaproteobacteria; o__Desulfovibrionales; f__Desulfovibrionaceae; g__ | −0.28 | 0.53 | 117.58 | 0.60 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae | −0.24 | 0.40 | 120.85 | 0.47 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__[Mogibacteriaceae]; g__ | −0.20 | 0.22 | 131.18 | 0.30 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Dorea | −0.35 | 0.40 | 131.26 | 0.53 |
| p__Firmicutes; c__Clostridia; o__Clostridiales | −0.28 | 0.28 | 135.54 | 0.40 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae | −0.39 | 0.35 | 137.60 | 0.52 |
| p__Tenericutes; c__Mollicutes; o__Mycoplasmatales; f__Mycoplasmataceae; g__Mycoplasma | −0.14 | 0.09 | 147.62 | 0.17 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__ | −0.46 | 0.17 | 159.61 | 0.49 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Dehalobacteriaceae; g__Dehalobacterium | −0.62 | 0.14 | 167.01 | 0.63 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Odoribacteraceae]; g__Odoribacter | −1.29 | 0.25 | 169.07 | 1.31 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__; g__ | −0.84 | 0.07 | 174.90 | 0.84 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Oscillospira | −1.23 | 0.08 | 176.35 | 1.23 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__ | −0.76 | 0.04 | 176.88 | 0.76 |
| p__Proteobacteria; c__Deltaproteobacteria; o__Desulfovibrionales; f__Desulfovibrionaceae; g__Desulfovibrio | −0.63 | 0.03 | 177.05 | 0.63 |
| CSC biplot (Fig. S4H) | ||||
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__ | −0.32 | 0.00 | −179.93 | 0.32 |
| p__Tenericutes; c__Mollicutes; o__RF39; f__; g__ | −1.18 | −0.08 | −176.21 | 1.19 |
| k__Bacteria | −0.37 | −0.04 | −174.38 | 0.37 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae | −0.53 | −0.06 | −173.72 | 0.53 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae | −0.19 | −0.03 | −172.35 | 0.19 |
| p__Proteobacteria; c__Deltaproteobacteria; o__Desulfovibrionales; f__Desulfovibrionaceae; g__Desulfovibrio | −1.12 | −0.37 | −161.52 | 1.18 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptococcaceae; g__ | −0.21 | −0.08 | −159.77 | 0.23 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Ruminococcus | −0.74 | −0.30 | −157.80 | 0.80 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Rikenellaceae; g__AF12 | −0.63 | −0.27 | −156.77 | 0.69 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__ | −0.81 | −0.41 | −152.95 | 0.91 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Ruminococcaceae; g__Oscillospira | −0.90 | −0.51 | −150.35 | 1.03 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Dehalobacteriaceae; g__Dehalobacterium | −0.75 | −0.44 | −149.38 | 0.87 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Pseudomonadales; f__Pseudomonadaceae; g__Pseudomonas | −0.08 | −0.05 | −147.96 | 0.09 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__[Mogibacteriaceae]; g__ | −0.48 | −0.30 | −147.60 | 0.57 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__; g__ | −0.75 | −0.51 | −145.80 | 0.91 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Paraprevotellaceae]; g__[Prevotella] | −0.27 | −0.22 | −140.84 | 0.35 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__[Ruminococcus] | −0.69 | −0.60 | −138.77 | 0.91 |
| Unassigned | −0.16 | −0.15 | −136.25 | 0.22 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__ | −0.44 | −0.43 | −135.63 | 0.62 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Odoribacteraceae]; g__Odoribacter | −1.10 | −1.12 | −134.48 | 1.57 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Dorea | −0.55 | −0.68 | −128.97 | 0.87 |
| p__Tenericutes; c__Mollicutes; o__Anaeroplasmatales; f__Anaeroplasmataceae; g__Anaeroplasma | −0.17 | −0.28 | −122.04 | 0.33 |
| p__Firmicutes; c__Clostridia; o__Clostridiales | −0.17 | −0.52 | −108.68 | 0.55 |
| p__Firmicutes; c__Bacilli; o__Bacillales; f__Alicyclobacillaceae; g__Alicyclobacillus | −0.02 | −0.13 | −99.98 | 0.13 |
| p__Proteobacteria; c__Betaproteobacteria; o__Burkholderiales; f__Comamonadaceae; g__ | 0.04 | −0.13 | −71.16 | 0.13 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Epulopiscium | 0.21 | −0.33 | −57.52 | 0.39 |
| p__Proteobacteria; c__Deltaproteobacteria; o__Desulfovibrionales; f__Desulfovibrionaceae; g__ | 0.53 | −0.67 | −51.46 | 0.85 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae | 0.94 | −0.56 | −31.02 | 1.09 |
| p__Proteobacteria; c__Gammaproteobacteria; o__Enterobacteriales; f__Enterobacteriaceae; g__ | 1.51 | −0.81 | −28.31 | 1.71 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae; g__ | 2.45 | −0.94 | −20.92 | 2.63 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae; g__Helicobacter | 2.99 | −1.10 | −20.26 | 3.19 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__[Paraprevotellaceae]; g__Paraprevotella | 1.67 | −0.46 | −15.29 | 1.73 |
| p__Chlamydiae; c__Chlamydiia; o__Chlamydiales; f__Chlamydiaceae; g__ | 0.10 | −0.02 | −9.13 | 0.10 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae | 0.09 | 0.01 | 5.83 | 0.09 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Anaerostipes | 0.20 | 0.08 | 22.47 | 0.22 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Porphyromonadaceae; g__Parabacteroides | 1.14 | 0.74 | 33.17 | 1.36 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Bacteroidaceae; g__Bacteroides | 0.46 | 0.34 | 36.39 | 0.57 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Blautia | 1.33 | 1.08 | 39.24 | 1.71 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__ | 0.43 | 0.66 | 56.67 | 0.79 |
| p__Proteobacteria; c__Betaproteobacteria; o__Rhodocyclales; f__Rhodocyclaceae; g__Dok59 | 0.55 | 0.84 | 56.69 | 1.00 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__RF16; g__ | 0.26 | 0.45 | 60.16 | 0.52 |
| p__Proteobacteria; c__Alphaproteobacteria; o__RF32; f__; g__ | 0.49 | 0.88 | 60.95 | 1.00 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__Coprobacillus | 0.41 | 0.95 | 66.77 | 1.03 |
| p__Proteobacteria; c__Betaproteobacteria; o__Methylophilales; f__; g__ | 0.48 | 1.37 | 70.76 | 1.45 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__Allobaculum | 0.10 | 0.31 | 71.83 | 0.33 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptostreptococcaceae; g__ | 0.03 | 0.11 | 75.29 | 0.11 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__Candidatus Arthromitus | 0.02 | 0.11 | 79.71 | 0.12 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__ | 0.04 | 0.27 | 81.06 | 0.27 |
| p__Tenericutes; c__Mollicutes; o__Mycoplasmatales; f__Mycoplasmataceae; g__Mycoplasma | 0.00 | 0.03 | 85.07 | 0.03 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Lachnobacterium | 0.02 | 0.23 | 85.07 | 0.23 |
| p__Verrucomicrobia; c__Verrucomicrobiae; o__Verrucomicrobiales; f__Verrucomicrobiaceae; g__Akkermansia | 0.03 | 0.81 | 87.63 | 0.81 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Peptococcaceae; g__rc4-4 | 0.00 | 0.14 | 90.62 | 0.14 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales | −0.01 | 0.41 | 91.73 | 0.41 |
| p__Firmicutes; c__Erysipelotrichi; o__Erysipelotrichales; f__Erysipelotrichaceae; g__[Eubacterium] | −0.02 | 0.30 | 94.46 | 0.30 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Lachnospiraceae; g__Coprococcus | −0.08 | 0.34 | 102.93 | 0.34 |
| p__Cyanobacteria; c__4C0d-2; o__YS2; f__; g__ | −0.34 | 0.70 | 115.88 | 0.77 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__; g__ | −0.21 | 0.31 | 123.70 | 0.37 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__Prevotellaceae; g__Prevotella | −0.21 | 0.19 | 138.52 | 0.28 |
| p__Firmicutes; c__Clostridia; o__Clostridiales; f__Clostridiaceae; g__Clostridium | −0.05 | 0.03 | 146.36 | 0.05 |
| p__Firmicutes; c__Bacilli; o__Lactobacillales; f__Lactobacillaceae; g__Lactobacillus | −0.04 | 0.02 | 152.73 | 0.05 |
| p__Proteobacteria; c__Epsilonproteobacteria; o__Campylobacterales; f__Helicobacteraceae | −0.20 | 0.09 | 154.61 | 0.22 |
| p__Bacteroidetes; c__Bacteroidia; o__Bacteroidales; f__S24-7; g__ | −0.39 | 0.18 | 154.74 | 0.43 |
| p__Cyanobacteria; c__Chloroplast; o__Streptophyta; f__; g__ | −0.29 | 0.11 | 159.75 | 0.30 |
| p__Deferribacteres; c__Deferribacteres; o__Deferribacterales; f__Deferribacteraceae; g__Mucispirillum | −1.72 | 0.52 | 163.30 | 1.79 |
| p__Proteobacteria; c__Alphaproteobacteria; o__Rickettsiales; f__mitochondria | −0.32 | 0.00 | 179.70 | 0.32 |
Table S3.
Comparisons of taxa relative abundances between samples of day 1 and day 8 and between day 1 and day 15
| Days 1–8 | Days 1–15 | ||
| Genus | Bonferroni P values† | Genus | Bonferroni P values |
| Vehicle/SHC | Vehicle/SHC | ||
| o__RF39; f__; g__ | 0.0218* | o__RF39; f__; g__ | 0.0077** |
| M. vaccae/SHC | M. vaccae/SHC | ||
| o__RF39; f__; g__ | 0.0284* | o__RF39; f__; g__ | 0.4413 |
| Vehicle/CSC | Vehicle/CSC | ||
| Allobaculum | 0.2939 | Allobaculum | 0.1348 |
| Blautia | 0.0092** | Blautia | 0.0078** |
| Candidatus Arthromitus | 0.5377 | Candidatus Arthromitus | 0.0022** |
| Clostridium | 0.1075 | Clostridium | 0.1348 |
| [Eubacterium] | 0.2694 | [Eubacterium] | 0.0221* |
| f__Desulfovibrionaceae; g__ | 0.0190* | f__Desulfovibrionaceae; g__ | 0.0050** |
| f__Enterobacteriaceae; g__ | 0.0847 | f__Enterobacteriaceae; g__ | 0.0056** |
| f__Erysipelotrichaceae; g__ | 0.0234* | f__Erysipelotrichaceae; g__ | 0.4280 |
| f__Helicobacteraceae | 0.0020** | f__Helicobacteraceae | 0.0029** |
| f__Helicobacteraceae; g__ | 0.0005*** | f__Helicobacteraceae; g__ | 0.0004*** |
| Helicobacter | 0.0005*** | Helicobacter | 0.0005*** |
| k__Bacteria | 0.0029** | k__Bacteria | 0.0020 ** |
| Mucispirillum | 0.0099** | Mucispirillum | 0.0008*** |
| Mycoplasma | 0.1153 | Mycoplasma | 0.0098** |
| Paraprevotella | 0.0006*** | Paraprevotella | 0.0018** |
| M. vaccae/CSC | M. vaccae/CSC | ||
| [Eubacterium] | 0.4986 | [Eubacterium] | 0.1988 |
| f__Desulfovibrionaceae; g__ | 0.0059** | f__Desulfovibrionaceae; g__ | 0.0005*** |
| f__Helicobacteraceae | 0.0020** | f__Helicobacteraceae | 0.0009*** |
| f__Helicobacteraceae; g__ | 0.0005*** | f__Helicobacteraceae; g__ | 0.0003*** |
| Helicobacter | 0.0003*** | Helicobacter | 0.0003*** |
| k__Bacteria | 0.0004*** | k__Bacteria | 0.0004*** |
| Mucispirillum | 0.0047** | Mucispirillum | 0.0009*** |
| Mycoplasma | 0.1796 | Mycoplasma | 0.0147* |
| o__RF39; f__; g__ | 0.3081 | o__RF39; f__; g__ | 0.0126* |
| Paraprevotella | 0.0005*** | Paraprevotella | 0.0004*** |
Wilcoxon signed-rank test, following significant effects of time based on ANCOM analysis, of time effects within each treatment group; *P < 0.05, **P < 0.01, ***P < 0.001.
M. vaccae Prevents Stress-Induced Colitis.
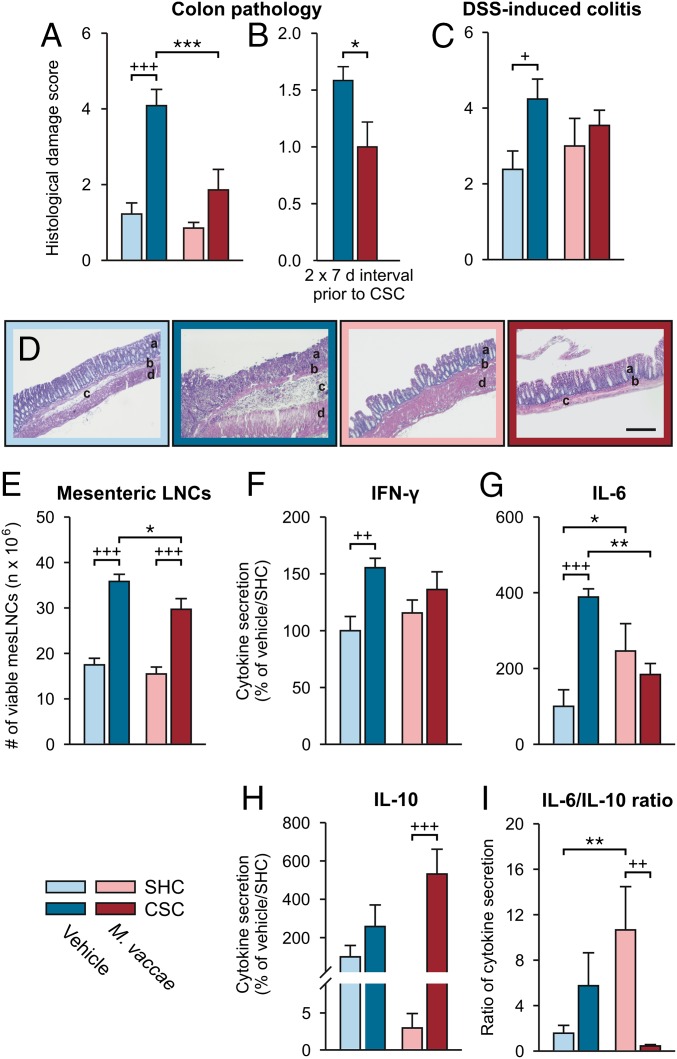
Evidence suggests that anxiety and depression are more common in IBD patients and that the symptoms of these conditions are more severe during periods of active disease (45). Oral or intraperitoneal administration of immunomodulatory bacterial products have been shown to both prevent and treat experimental colitis in animal models (46, 47), suggesting that these substances can act through gut-dependent and gut-independent mechanisms to attenuate chemically induced colitis. Chronic subordinate colony housing exposure reproducibly induces spontaneous colitis and aggravates chemically induced colitis (26). Importantly, although immunization with M. vaccae did not prevent the CSC-induced increase in colitogenic Helicobacter spp. (Figs. S4H and S5 B and M–O and Table S3), it did prevent CSC-induced spontaneous colitis [Fig. 2 A and B; Exp. 1, two-factor ANOVA, M. vaccae × CSC, F(1, 29) = 6.7, P < 0.05; Fig. 2B, Exp. 2, Student’s t test, t(1, 14) = 5.4, P < 0.05], and decreased plasma concentrations of kynurenine, a biomarker of inflammation (48) (Fig. S2R) [LMM, M. vaccae, F(1, 24) = 5.6, P < 0.05], suggesting increased immunoregulation (49). In contrast, CSC exposure decreased tryptophan concentrations (Fig. S2S) [two-factor ANOVA, CSC, F(1, 27) = 4.705, P < 0.05], whereas M. vaccae immunization had no effect.
Fig. 2.
M. vaccae prevents chronic CSC-induced spontaneous colitis and CSC-induced aggravation of chemically induced colitis. (A and B) Colonic histological damage scores reflecting CSC-induced spontaneous colitis on day 20 of (A) Exp.1 and (B) Exp. 2. (C–I) CSC-induced aggravation of chemically induced colitis on day 29 of Exp. 3. (C) Colonic histological damage scores following SHC or CSC conditions, followed by administration of DSS (1%; days 22–29) in drinking water. (D) Photomicrographs from hematoxylin and eosin-stained colon sections. (Scale bar, 200 µm.) a, lamina mucosa; b, lamina muscularis mucosa; c, lamina submucosa; d, lamina muscularis externa. (E) Number of viable mesenteric lymph node cells (mesLNCs). (F–H) IFN-γ (F), IL-6 (G), and IL-10 (H) secretion from mesLNCs stimulated with anti-CD3 antibody in vitro. (I) IL-6/IL-10 ratio. Bars represent means; error bars represent +SEM. Significance was assessed using (A, C, and E–I) two-factor ANOVA and (B) Student’s t test. Post hoc comparisons were made using Fisher’s LSD tests. *P < 0.05, **P < 0.01, ***P < 0.001, between-subjects effects of vehicle versus M. vaccae, within the same CSC/SHC condition; +P < 0.05, ++P < 0.01, +++P < 0.001, between-subjects effects of SHC versus CSC, within the same drug condition. The number of independent data points (N) in each of the graphs (A–C and E–I) and sample size (n) for each group are as follows: (A) N = 33; vehicle/SHC, 9; vehicle/CSC, 8; M. vaccae/SHC, 9; M. vaccae/CSC, 7. (B) N = 16; vehicle/CSC, 8; M. vaccae/CSC, 8. (C) N = 30; vehicle/SHC, 7; vehicle/CSC, 7; M. vaccae/SHC, 8; M. vaccae/CSC, 8. (E, F, and H) N = 28; vehicle/SHC, 6; vehicle/CSC, 7; M. vaccae/SHC, 7; M. vaccae/CSC, 8. (G and I) N = 27; vehicle/SHC, 6; vehicle/CSC, 6; M. vaccae/SHC, 7; M. vaccae/CSC, 8.
Similarly, M. vaccae pretreatment prevented the CSC-induced aggravation of dextran sulfate sodium (DSS; 1% for 7 d)-induced colitis (Exp. 3) (Fig. 2 C–I and Fig. S1D) [two-factor ANOVA, CSC, F(1, 26) = 4.6, P < 0.05]. Of note, in the model of DSS-induced colitis, M. vaccae immunization attenuated the CSC-induced increase in the number of viable mesenteric lymph node cells (Fig. 2E) [two-factor ANOVA, M. vaccae, F(1, 24) = 4.8, P < 0.05; CSC, F(1, 24) = 76.4, P < 0.0001] and attenuated the CSC-induced IFN-γ (Fig. 2F) [two-factor ANOVA, CSC, F(1, 24) = 8.5, P < 0.01] and IL-6 secretion [Fig. 2G; two-factor ANOVA, M. vaccae × CSC, F(1, 24) = 14.9, P < 0.01]. Chronic subordinate colony housing exposure increased IL-10 secretion from anti-CD3–stimulated mesenteric lymph node cells assessed in vitro (Fig. 2 H and I) [two-factor ANOVA, CSC, F(1, 24) = 12.7, P < 0.01], an effect that was only evident in M. vaccae-immunized mice, indicating an antiinflammatory bias in M. vaccae-immunized, CSC-exposed mice. Overall, these data suggest that, in the absence of adequate immunoregulation, CSC exposure led to activation of the host immune response toward Helicobacter spp., other elements of the CSC-induced colitogenic microbiota, or dietary or self-antigens, resulting in colitis. Furthermore, these data suggest that immunization with M. vaccae restored immunoregulation and prevented colitogenic effects of CSC, despite stress-induced development of a gut microbiota with colitogenic potential. Based on these findings, immunization with M. vaccae, or similar bioimmunomodulatory approaches, may be useful for prevention of chronic stress/repeated trauma-induced inflammation and subsequent development of somatic and mental disorders.
Treg Dependence of M. vaccae Effects.
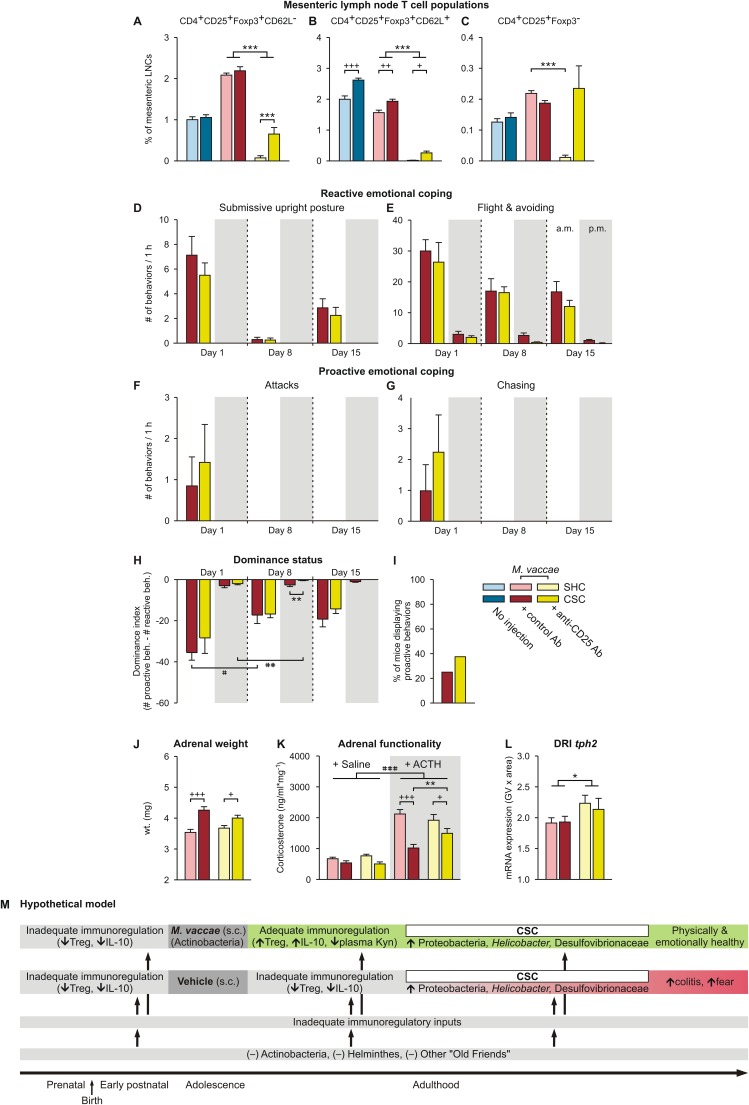
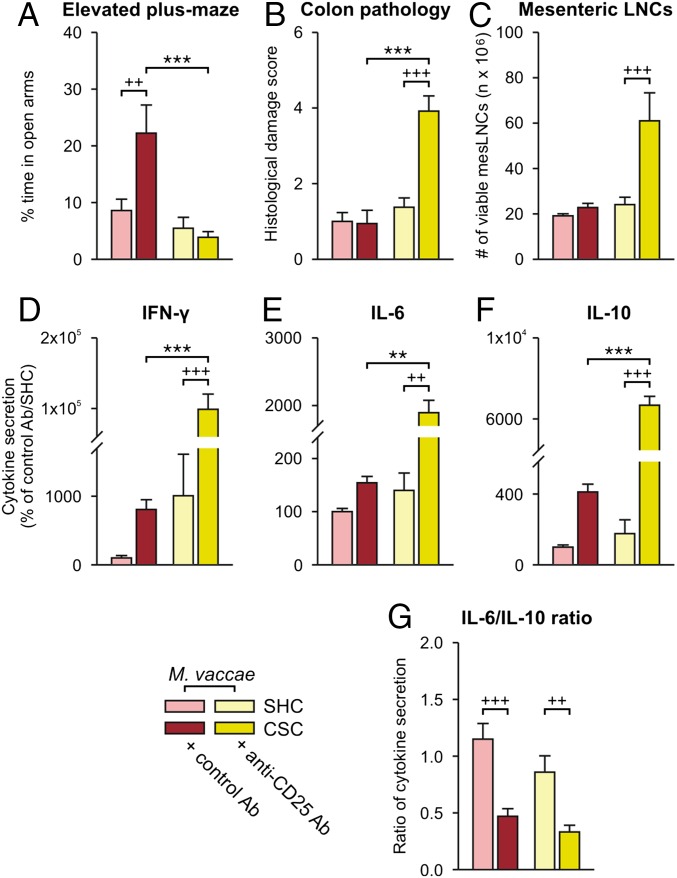
Psychosocial stress decreases Treg in mice and humans (50, 51), and Treg depletion increases anxiety- and depressive-like behaviors in mice (52). Likewise, PTSD subjects (9) and subjects with major depressive disorder (53) have reduced Treg, which is reversed 1 y following effective narrative exposure therapy (54) or treatment with antidepressants, respectively (53). Given these findings and studies showing that CSC reduces Treg in peripheral lymph nodes (26) and M. vaccae immunization induces Treg (22), we investigated a potential role for Treg in the stress-protective effects of M. vaccae (Exp. 4) (Fig. S1E). Here, we were primarily interested in the effects of depletion of Treg in M. vaccae-immunized mice. Therefore, all mice were immunized with M. vaccae and on day –4 treated intraperitoneally with either anti-CD25 antibody [PC-61.5.3; administration of this anti-CD25 antibody is an effective means of depleting Treg in mice in vivo (55, 56)] or control antibody [rat IgG1 isotype control; anti-horseradish peroxidase (HRPN)] (Fig. S1E; for confirmation of the efficacy of Treg depletion, see Fig. S6 A–C). As before, we assessed dominant–subordinate interactions during CSC exposure. On day 19 of CSC, mice were tested on the EPM, and then, on day 20, mice were euthanized for collection of adrenals, colon, and mesenteric lymph nodes.
Fig. S6.
Data of Exp. 3 showing physiologic and behavioral responses to chronic psychosocial stress and a hypothetical model for mechanisms underlying the promotion of stress-resilience effects following M. vaccae immunizations. (A–C) Effects of M. vaccae, CSC, and anti-CD25 antibody treatment on T helper cells. (A–C) Effects of anti-CD25 antibody, versus control antibody, administration on day –4 on mesenteric lymph node T-cell populations were evaluated using flow cytometry on day 20. (Left) Data for uninjected controls, for comparison. CD4+CD25+FoxP3+ regulatory T cells restrain the immune responses to self-antigens, pathogens, allergens, and commensal microorganisms. (A) CD62L− Treg. (B) CD62L+ Treg. (C) CD4+CD25+Foxp3− effector T cells. (A–C) Treatment with anti-CD25 antibodies successfully depleted CD25+ cells in mesenteric lymph nodes of M. vaccae-immunized SHC mice (>95% depletion in all cases). However, in M. vaccae-immunized CSC-exposed mice, a considerable number of CD25+ cells were still detectable. (A and B) CD4+CD25+FoxP3+CD62L− Treg (A) as well as CD4+CD25+FoxP3+CD62L+ Treg (B) were reduced only by 70.3% and 86.5%, respectively, presumably because the 19-d CSC exposure induced Treg following the initial depletion, a depletion that remains evident in SHC mice. CD25 is also expressed on CD4+ effector cells. For CD4+CD25+FoxP3−CD62L− effector cells, the bias in efficiency of depletion in M. vaccae-immunized SHC versus CSC mice was even more prominent. (C) Whereas CD4+CD25+FoxP3−CD62L− cells were successfully depleted in SHC mice (95.5% depletion), no reduction was seen in M. vaccae-immunized CSC mice (126.3% of control values). CSC exposure in M. vaccae-immunized mice either prevented CD25+ cells from depletion or activated a yet unknown pathway that led to a specific increase in CD25+ T helper cells. The latter is in line with our previous observations that CSC induced unspecific T-cell activation leading to increased production of effector cytokines by in vitro activated T cells (132). (D–I) Effects of anti-CD25 antibody treatment on reactive versus proactive coping responses in M. vaccae-immunized mice. (D) Effects of treatment with an anti-CD25 antibody, relative to treatment with a control antibody, on day –4, on the number of submissive upright posture displays, during the first hour (1000–1100 hours; white background) of CSC exposure on day 1 and during the first hour following exposure to a novel dominant male aggressor on days 8 and 15. Also shown are behavioral responses in the evening (0500–0600 hours; gray background) of days 1, 8, and 15. (E–G) Effects of anti-CD25 antibody or control antibody treatment on the number of (E) flight and avoiding behaviors, (F) attacks, and (G) chasing behaviors during CSC exposure. (H) Dominance index scores (number of proactive behaviors minus the number of reactive behaviors) of anti-CD25– and control antibody-treated mice during the 19-d CSC procedure. (I) Percent of anti-CD25– and control antibody-treated mice that displayed proactive behaviors (attack, chase) during the 19-d CSC procedure. (J) Adrenal weight measured on day 20. (K) In vitro adrenal functionality measured on day 20. (L) tph2 mRNA expression in the interfascicular part of the dorsal raphe nucleus (DRI). (M) Hypothetical model illustrating how M. vaccae immunization counteracts disease vulnerability. Inadequate immunoregulatory inputs during the pre- and postnatal periods, during adolescence, as well as during adulthood, led to increased vulnerability to stress-induced spontaneous colitis and exaggerated stress-induced fear responses. Immunization with M. vaccae restores immunoregulation that protects against stress-induced spontaneous colitis and exaggerated fear responses, despite a stress-induced transition to a colitogenic microbial gut milieu. Kyn, l-kynurenine. Bars represent means; error bars represent (A–G and J–L) +SEM or (H) −SEM. Data were analyzed using (A–C, J, and L) two-factor ANOVA for between-subjects designs or by (D–H) LMM, conducted separately for AM and PM time points, (I) Fisher’s exact test, or (K) three-factor ANOVA. Post hoc comparisons were made using Fisher’s LSD tests. *P < 0.05, **P < 0.01, ***P < 0.001, anti-CD25– versus control antibody-treated mice; +P < 0.05, ++P < 0.01, +++P < 0.001, CSC versus SHC; #P < 0.05, ##P < 0.01, ###P < 0.001, within-subjects effects of (H) time, paired t tests using Bonferroni correction, or (K) main effect of ACTH. The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (A–C) N = 48; uninjected/SHC, 8; uninjected/CSC, 8; vehicle/SHC, 8; vehicle/CSC, 8; M. vaccae/SHC, 8; M. vaccae/CSC, 8. (D–L) N = 32; vehicle/SHC, 8; vehicle/CSC, 8; M. vaccae/SHC, 8; M. vaccae/CSC, 8; vaccae/CSC, 8.
Among M. vaccae-immunized mice, treatment with anti-CD25 antibody had no effect on stress coping behaviors during CSC (Fig. S6 D–I). Treatment of M. vaccae-immunized mice with anti-CD25 antibody had no effect on tph2 or slc6a4 mRNA expression in the rDRD (Table S1), suggesting that both the effects of M. vaccae on behavioral coping strategies during CSC exposure and brain tph2 and slc6a4 mRNA expression are independent of Treg. Interestingly, treatment with the anti-CD25 antibody, which would be expected to increase proinflammatory signaling in the periphery, increased tph2 mRNA expression in the interfascicular part of the dorsal raphe nucleus (DRI) (Fig. S6L) [two-factor ANOVA, anti-CD25, F(1, 22) = 4.7, P < 0.05], a subpopulation of serotonergic neurons that we have shown previously is activated by acute proinflammatory stimuli (57, 58).
Stress-induced adrenal hypertrophy was evident in M. vaccae-immunized CSC mice treated with either control or anti-CD25 antibody (Fig. S6J) [two-factor ANOVA, CSC, F(1, 27) = 29.7, P < 0.0001], as was the stress-induced adrenal ACTH insensitivity (Fig. S6K) [three-factor ANOVA, CSC, F(1, 52) = 33.2, P < 0.0001], consistent with results from Exp. 1, indicating that CSC exposure was aversive for all animals and that M. vaccae does not affect CSC-induced changes in hypothalamic–pituitary–adrenal (HPA) axis function.
Anti-CD25 antibody treatment of M. vaccae-immunized, CSC-exposed mice prevented the permissive effect of M. vaccae immunization on CSC-induced reductions in anxiety, as assessed by the percentage of time spent on the open arms of the EPM (Fig. 3A; Table S1) [two-factor ANOVA, anti-CD25 × CSC, F(1, 21) = 7.4, P < 0.05], suggesting that the anxiolytic or fear-reducing effects of M. vaccae are dependent on Treg. As expected, there was also no CSC-induced colitis in M. vaccae-immunized mice pretreated with control antibody, whereas M. vaccae-immunized, CSC-exposed mice pretreated with anti-CD25 antibody responded to CSC exposure with development of spontaneous colitis (Fig. 3 B–G) [based on anti-CD25 × CSC interactions using two-factor ANOVAs for histological damage score: F(1, 23) = 14.6, P < 0.01; number of viable mesenteric lymph node cells: F(1, 27) = 6.2, P < 0.05; and anti-CD3–stimulated cytokine secretion from mesenteric lymph node cells in vitro, IFN-γ: F(1, 28) = 20.8, P < 0.0001; IL-6: F(1, 27) = 4.9, P < 0.05; and IL-10: F(1, 26) = 8.6, P < 0.01]. In addition, there was a main effect of CSC to reduce the IL-6/IL-10 ratio, due to the exaggerated release of IL-10 in these M. vaccae-immunized, CSC-exposed mice (Fig. 3G) [CSC, F(1, 28) = 31.8, P < 0.0001]. Together, these data support the hypothesis that stress-protective effects of M. vaccae to prevent colitis and promote anxiolytic/fear-reducing responses are dependent on activation of Treg and an antiinflammatory bias, whereas the shift toward a more proactive emotional coping response to stress is not. Alternatively, brain mechanisms driving proactive emotional coping responses may already be established by the time of Treg depletion, and are not reversible or are reversed over a longer time frame. A diagrammatical illustration of the overall hypothetical model is presented in Fig. S6M.
Fig. 3.
Depletion of regulatory T cells prevents stress-protective effects of M. vaccae. (A) Anxiety- or fear-like behavior on the elevated plus-maze (day 19; Exp. 4). (B–G) CSC-induced spontaneous colitis measured on day 20. (B) Colonic histological damage scores. (C) Number of viable mesLNCs. (D–F) Secretion of (D) IFN-γ, (E) IL-6, and (F) IL-10 from mesLNCs stimulated with anti-CD3 antibody in vitro (control antibody/SHC group: 100%). (G) IL-6/IL-10 ratio. Bars represent means; error bars represent +SEM. Significance was assessed using two-factor ANOVA. Post hoc comparisons were made using Fisher’s LSD tests. **P < 0.01, ***P < 0.001, between-subjects effects of control versus anti-CD25 antibody, within the same CSC condition; ++P < 0.01, +++P < 0.001, between-subjects effects of SHC versus CSC, within the same antibody condition. The number of independent data points (N) in each of the graphs and sample size (n) for each group are as follows: (A) N = 28; M. vaccae/SHC/control Ab, 7; M. vaccae/CSC/control Ab, 7; M. vaccae/SHC/anti-CD25 Ab, 6; M. vaccae/CSC/anti-CD25 Ab, 8. (B) N = 23; M. vaccae/SHC/control Ab, 5; M. vaccae/CSC/control Ab, 6; M. vaccae/SHC/anti-CD25 Ab, 5; M. vaccae/CSC/anti-CD25 Ab, 7. (C–G) N = 29–31; M. vaccae/SHC/control Ab, 8; M. vaccae/CSC/control Ab, 7; M. vaccae/SHC/anti-CD25 Ab, (C, E, and G) 8, (D) 6, (F) 7; M. vaccae/CSC/anti-CD25 Ab, 8.
SI Materials and Methods
Animals.
All studies used male C57BL/6NCrl mice (Charles River) weighing 19‒22 g. Mice were housed in groups of four or five in polycarbonate mouse cages [width (W) × depth (D) × height (H): 27 cm × 42 cm × 15 cm] during the 2-wk period of Mycobacterium vaccae or vehicle injections (for details, see below) (Fig. S1). Adolescence in mice consists of early adolescence [prepubescent or juvenile, postnatal day (pnd) 21–34], middle adolescence (periadolescent, pnd 34–46), and late adolescence (pnd 46–59) time periods (85). Consequently, because 19- to 22-g male C57BL/6NCrl mice are ∼5 wk of age (supplier information), these mice were in the middle-adolescence period (pnd 35) upon arrival and initial treatment with M. vaccae or vehicle, late adolescence (pnd 59) at the start of the chronic subordinate colony housing (CSC) stressor, and adulthood (pnd ≥78) at the time of behavioral testing. After the last immunization, all experimental mice were individually housed in polycarbonate mouse cages (W × D × H: 21 cm × 27 cm × 14 cm) for 1 wk (or 2 wk; Exp. 2) before exposure to CSC or single-housed control (SHC) conditions started. Male offspring (weighing 30–35 g) from the mating of a high anxiety-related behavior female mouse (kindly provided by R. Landgraf, Max Planck Institute of Psychiatry, Munich, Germany) and a C57BL/6NCrl male mouse were used as dominants. All mice were kept under standard laboratory conditions (12-h light/dark cycle, lights on at 0600 hours, 22 °C, 60% humidity) and had free access to tap water and standard mouse diet (ssniff Spezialdiäten). All experimental protocols were approved by the Committee on Animal Health and Care of the local government, and were performed according to international guidelines on the ethical use of animals.
General Experimental Procedures for the CSC Paradigm.
Day 1 is defined as the first day of the CSC exposure. During the CSC procedure, four experimental CSC mice (intruder mice) were housed together with a larger dominant male (resident) for 19 consecutive days, to induce chronic psychosocial stress/traumatization. Before the CSC procedure, the future dominant males were tested for their aggressive behavior. Males that started to injure their opponents by excessive and harmful bites during testing were not used in further tests. To avoid habituation during the chronic stressor exposure, intruder mice were moved to the cage of a novel dominant aggressor male on days 8 and 15 at 1000 hours.
Unless otherwise specified, after arrival (day –23), experimental mice were housed in groups of four or five mice per cage and received subcutaneous injections of either M. vaccae (n = 97, Exps. 1–4; note: All intruder mice of Exp. 2 received injections of M. vaccae; i.e., there were no vehicle-injected mice in Exp. 2) or vehicle (n = 66, Exps. 1–4) on days –21, –14, and –7 (Exps. 1, 3, and 4) or –28, –21, and –14 (Exp. 2). Immediately after the final immunization injection on day –7 or –14, all experimental mice were housed individually for 1 wk (Exps. 1, 3, and 4) or 2 wk (Exp. 2). In Exp. 4, we performed intraperitoneal injections of either anti-CD25 (PC-61.5.3; n = 16) or control antibody (rat IgG1, HRPN; n = 16) (both from Bio X Cell) on day –4. Note that, in Exp. 4, an additional set of SHC (n = 8) and CSC (n = 8) mice was used, which was neither injected with M. vaccae or vehicle nor with anti-CD25 or control antibody, as uninjected controls.
General Experimental Procedures for the CSC Paradigm.
The general experimental procedures for the CSC paradigm are illustrated in Fig. S1A. On day 1, experimental M. vaccae- and vehicle-treated mice were exposed to CSC [19 d; Exp. 1 (M. vaccae, n = 24; vehicle, n = 24; one mouse of each group died during the experiment); Exp. 2 (M. vaccae, n = 8; vehicle, n = 8); Exp. 3 (M. vaccae, n = 8; vehicle, n = 8; one vehicle-CSC mouse died during the experiment); Exp. 4 (M. vaccae/anti-CD25 antibody, n = 8; M. vaccae/control antibody, n = 8; uninjected CSC, n = 8)] or kept as single-housed controls [19 d; Exp. 1 (M. vaccae, n = 9; vehicle, n = 10); Exp. 3 (M. vaccae, n = 8; vehicle, n = 8); Exp. 4 (M. vaccae/anti-CD25 antibody, n = 8; M. vaccae/control antibody, n = 8; uninjected SHC, n = 8)]. Based on our previous data indicating pronounced social hierarchy effects on physiological and behavioral parameters when group housing nonfamiliar same-size male conspecifics, single housing and not group housing is considered to represent the most appropriate housing condition for controls in this paradigm (28, 86).
Exp. 1 design.
The design of Exp. 1 is illustrated in Fig. S1B. The behavior of both CSC (M. vaccae- and vehicle-immunized) and resident dominant aggressor mice was recorded during CSC (2 h/d; see below for details) on days 1, 8, and 15 for each CSC arena for subsequent quantification of proactive and reactive behaviors shown by each individual CSC and resident mouse. Fresh fecal pellets were collected from each mouse on days –21, –14, –7, 1, 8, and 15 for analysis of gut microbial communities. Fecal samples were collected from each mouse immediately before immunizations with vehicle or M. vaccae (days –21, –14, and –7), immediately before mice were placed with the dominant aggressor (days 1, 8, and 15), or from time-matched SHC mice on days 1, 8, and 15. Pellets were frozen individually in sterile Eppendorf tubes and stored at –80 °C until analyzed. At this point, frozen stool pellets were shipped to the University of Colorado Boulder for analysis. In Exp. 1, a subset of experimental mice (vehicle-SHC, n = 9; vehicle-CSC, n = 15; M. vaccae-SHC, n = 9; M. vaccae-CSC, n = 16) was tested on the EPM from 1800 to 2100 hours on day 19. Afterward, mice were single-housed for 90 min and then perfused for quantification of Iba1 immunohistochemical staining of microglia. For comparison, another subset of experimental mice (vehicle-CSC, n = 8; M. vaccae-CSC, n = 7) was single-housed between 1800 and 2100 hours on day 19 (without EPM exposure) and perfused 90 min later. Because we were interested in the effects of M. vaccae on activated microglia, we conducted analyses using CSC-exposed mice only. Analysis of mice either exposed or not exposed to the EPM revealed no differences in Iba1 immunostaining, and so data from the two groups were pooled for analysis of the effects of M. vaccae immunization on Iba1 immunostaining. The remaining experimental mice in Exp. 1 (vehicle-SHC, n = 9; vehicle-CSC, n = 8; M. vaccae-SHC, n = 9; M. vaccae-CSC, n = 8) were tested on the EPM from 1800 to 2100 hours on day 19. Afterward, CSC mice were put back into their respective CSC colony and SHC mice were kept singly. All of these remaining experimental mice were killed between 0800 and 1000 hours on day 20 for assessment of the physiological parameters detailed below. Fresh-frozen brain tissue was used for assessment of tph2 and slc6a4 mRNA expression in different subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR).
Exp. 2 design.
The design of Exp. 2 is illustrated in Fig. S1C. In Exp. 2, all experimental mice (vehicle-CSC, n = 8; M. vaccae-CSC, n = 8) were tested on the EPM from 0700 to 1200 hours on day 19. After EPM testing, CSC mice were put back in their respective CSC arena. All experimental mice were killed between 0800 and 1000 hours of day 20 for assessment of the physiological parameters detailed below.
Exp. 3 design.
The design of Exp. 3 is illustrated in Fig. S1D. In Exp. 3, all experimental mice (vehicle-SHC, n = 8; vehicle-CSC, n = 7; M. vaccae-SHC, n = 8; M. vaccae-CSC, n = 8) were tested in the light/dark box test (LDB) on day 20, and in the social preference/avoidance test (SPAT) on day 21, always from 0700 to 1200 hours. After LDB and SPAT testing, CSC mice were put back in their respective CSC colony and SHC mice were kept singly. On day 22, CSC mice were single-housed and all mice received 1% DSS in their drinking water for 1 wk (one vehicle-SHC mouse died) and were killed between 0800 and 1100 hours of day 29 for assessment of colitis severity.
Exp.4 design.
The design of Exp. 4 is illustrated in Fig. S1E. In Exp. 4, all experimental mice (M. vaccae/control antibody-SHC, n = 8; M. vaccae/control antibody-CSC, n = 7; M. vaccae/anti-CD25 antibody-SHC, n = 8; M. vaccae/anti-CD25 antibody-CSC, n = 8; uninjected SHC, n = 8; uninjected CSC, n = 8) were tested on the EPM from 0700 to 1200 hours on day 19. Afterward, CSC mice were put back in their respective CSC colony and SHC mice were kept singly. All experimental mice were killed between 0800 and 1000 hours of day 20 for assessment of physiological and immunological parameters as well as tph2 and slc6a4 mRNA expression in different subregions of the DR and MnR.
General Procedures for Immunization with M. vaccae and Vehicle.
Experimental mice received either subcutaneous immunization with 0.1 mg whole heat-killed M. vaccae suspension [10 mg/mL solution; strain NCTC 11659, batch ENG 1, provided by Bio Elpida diluted to 1 mg/mL in 100 µL sterile borate-buffered saline (BBS)] using 21-gauge needles or injections of 100 µL of the vehicle, BBS. The dose used in these experiments (0.1 mg) was 1/10 of the dose used in human studies (1 mg) (4–6) and identical to the dose used in previous studies in mice (7, 8).
Treatment with Anti-CD25 or Control Antibody.
We injected experimental mice of Exp. 4, which were all immunized subcutaneously with M. vaccae on days –21, –14, and –7, with 1 mg of either anti-CD25 antibody (intraperitoneal PC-61.5.3; n = 16) or control antibody (intraperitoneal rat IgG1, HRPN; n = 16) (both from Bio X Cell) on day –4 of the experimental protocol.
Induction of DSS Colitis.
On day 22 (as described in detail above), an acute colitis was induced in SHC and CSC mice of Exp. 3 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC) by administering 1% DSS (50 kDa; cat. no.16011036, ICN Biomedicals) in drinking water ad libitum for 7 d (days 22–29).
Behavioral Observation During CSC Exposure.
To confirm the intended dominant (resident)/subordinate (CSC mice) hierarchy within each CSC arena and to assess possible treatment (M. vaccae vs. vehicle and anti-CD25 antibody versus control antibody) effects on the behavioral coping strategy (proactive versus reactive coping) of experimental mice during 19 d of CSC exposure, mice of each CSC colony were videotaped for 1 h in the morning (1000–1100 hours; immediately after formation of the CSC groups) and in the evening (1700–1800 hours; 7 h following CSC group formation) on days 1, 8, and 15. Their defensive behavior was individually analyzed in terms of reactive emotional behaviors (submissive upright behavior, flight, avoiding) and proactive emotional coping behaviors (attacking, chasing) (87). The occurrence of all different behaviors was scored individually for each of the five mice forming one colony (four CSC mice plus one resident mouse), and a dominance index was calculated (the sum of proactive behavioral elements minus the sum of reactive behavioral elements) for each. The mouse with the highest dominance index within each colony was assessed as dominant; in all cases this was the resident. Furthermore, the percentage of CSC mice showing any kind of proactive behavior toward the resident in their CSC colony was calculated for both M. vaccae- and vehicle-treated CSC mice.
Body Weight Gain.
To assess treatment (M. vaccae and/or CSC) effects on body weight gain, all experimental mice were weighed on days –23, –21,–14, –7, 1, 3, 8, 10, 15, and 17 (Exps. 1–4), day 19 (Exps. 1, 2, and 4), day 20 (Exp. 3 only), and days 22 and 29 (Exp. 3 only). Afterward, delta body weight gain between day –23 and day 1 (the pre-CSC period; Exps. 1, 3, and 4), between day –30 and day 1 (the pre-CSC period; Exp. 2), between day 1 and day 19 (time of CSC exposure; Exps. 1, 2, and 4) or day 1 and day 20 (time of CSC exposure; Exp. 3), and between day 22 and day 29 (time of DSS treatment; Exp. 3 only) was calculated for each individual mouse. Data reported represent the mean + SEM of delta body weight gain of all experimental mice per treatment group.
Behavioral Testing.
Elevated plus-maze test.
To assess treatment (M. vaccae and/or CSC and/or anti-CD25 antibody) effects on anxiety-related behavior with a fear component, experimental mice from Exp. 1 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC), Exp. 2 (vehicle-CSC, M. vaccae-CSC), and Exp. 4 (M. vaccae/control antibody-SHC, M. vaccae/anti-CD25 antibody-SHC, M. vaccae/control antibody-CSC, M. vaccae/anti-CD25 antibody-CSC) were exposed to the EPM test for 5 min on day 19 as previously described (26, 28). EPM testing took place between 1800 and 2100 hours under dim red light conditions (Exp. 1) or between 0700 and 1200 hours with 130 lx on the open and 30 lx on the closed arms of the maze (Exps. 2 and 4). The mouse EPM consists of two open (6 cm × 30 cm) and two closed (6 cm × 30 cm) arms radiating from a central platform (6 cm × 6 cm) to form a plus-shaped figure elevated 130 cm above the floor. The open arm edges were 0.3 cm in height to avoid falling. Each mouse was placed on the central platform facing a closed arm. The time spent on the open and closed arms was recorded by means of a video/computer setup to allow calculation of the percentage of time spent on the open arms of the maze. The number of entries into the closed arms of the maze was taken as an indicator of locomotor activity. The maze was cleaned thoroughly before each test. Data shown represent the mean + SEM of all experimental mice per treatment group.
Light/dark box test.
To assess treatment (M. vaccae and/or CSC) effects on conflict anxiety-related behavior, all experimental mice from Exp. 3 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC) were tested in the LDB on day 20 between 0700 and 1200 hours, as described previously, with some modifications. The LDB consisted of a brightly lit (W × L × D: 27 cm × 27 cm × 27 cm; 350 lx) and a dark (W × L × D: 27 cm × 18 cm × 27 cm; 50 lx) compartment, separated by a partition wall that had a small opening (6 cm in width and 6 cm high) at floor level. For habituation, mice were individually placed in the dark box, with the opening of the partition wall closed, for 30 s. After this time period the partition wall was opened and mice were allowed to freely explore the arena for 5 min. The time spent in the light box and the total distance traveled, indicative of general locomotion, were analyzed using EthoVision XT (v5.0.216; Noldus Information Technology). The LDB was cleaned thoroughly before each test. CSC mice were directly taken from the colony cages for LDB testing. After testing, CSC mice were put back in their respective colony and SHC mice were kept singly.
Social preference/avoidance test.
To assess treatment (M. vaccae and/or CSC) effects on neophobia and social avoidance behavior, all experimental mice from Exp. 3 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC) were exposed to the SPAT on day 21 between 0700 and 1200 hours. Briefly, the experimental mouse was placed in the SPAT box (W × L × D: 27 cm × 45 cm × 27 cm; light intensity: 10–40 lx) for 30 s to habituate to the unfamiliar environment before a small empty wire mesh cage (W × L × D: 6.5 cm × 10 cm × 5 cm) was introduced to the SPAT box for 150 s. This initial period gives an indication of neophobia, as it is exposure to a novel object. Afterward, this empty cage was exchanged for an identical cage containing an unfamiliar male mouse for another 150 s. Time spent in the 8-cm broad contact zone around the wire mesh cage and the total distance traveled, indicative of general locomotion, were recorded using EthoVision XT (v5.0.216; Noldus Information Technology) during both 150-s trials. The box was cleaned thoroughly before every test.
Euthanasia and Tissue Collection.
Perfusion fixation for immunohistochemical staining of Iba1.
To assess treatment (M. vaccae) and EPM exposure effects on Iba1 immunostaining in CSC mice in Exp. 1, one subset of experimental mice (vehicle-CSC+EPM, M. vaccae-CSC+EPM) was exposed to the EPM (5 min) on day 19 and one subset (vehicle-CSC−EPM, M. vaccae-CSC−EPM) was kept as a non–EPM-exposed control. Because we were interested in the effects of M. vaccae on activated microglia, we conducted analysis using CSC-exposed mice only. After 90 min of single housing following the test procedure all mice were deeply anesthetized with an overdose of CO2 anesthesia and transcardially perfused with 30 mL of 0.05 M PBS (pH 7.4) followed by 30 mL of 4% (wt/vol) paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH 7.4). Brains were then removed and postfixed for 12 h in 4% paraformaldehyde in PB (pH 7.4). Afterward, brains were transferred to 25% sucrose in 0.1 M PB (pH 7.4) until they sank to the bottom of the sucrose solution, snap-frozen in ice-cold isopentane, and stored at –80 °C until sectioning for Iba1 immunohistochemistry. At this point, frozen brains were shipped to the University of Colorado Boulder for analysis using immunohistochemistry. Serial coronal sections (six alternate sets, 30-µm) were sliced using a cryostat (Leica CM1900; –22 °C) and stored at –20 °C in a cryoprotectant solution (270 mL ethylene glycol, 160 mL glycerol, 202 mL 0.2 M Na2HPO4⋅7H2O, 48 mL 0.2 M NaH2PO4⋅H2O, and 320 mL dH2O) until immunohistochemical analysis. Analysis of mice either exposed or not exposed to the EPM revealed no differences in Iba1 immunostaining, and so data from the two groups were pooled for analysis of the effects of M. vaccae immunization on Iba1 immunostaining.
End points using fresh frozen tissues.
To assess treatment (M. vaccae or CSC) effects on other physiological parameters, another set of experimental mice in Exp. 1 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC), which were exposed to the EPM (5 min) on the evening of day 19, was rapidly euthanized by decapitation under CO2 anesthesia between 0800 and 1000 hours on day 20. All mice in Exps. 2 and 4 were killed on day 20, whereas mice in Exp. 3 were killed on day 29, again by decapitation under CO2 anesthesia between 0800 and 1000 hours.
Trunk Blood Collection.
Blood collection for each mouse was finished within 3 min after entering the animal room. Trunk blood was collected in EDTA-coated tubes (41.1504.005; Sarstedt) on ice and centrifuged at 4 °C (5,000 × g, 10 min). Plasma samples were stored at –20 °C until assayed.
Determination of Plasma Kynurenine and Tryptophan Concentrations.
Plasma concentrations of l-kynurenine and l-tryptophan were measured at the University of Colorado Boulder using high-performance liquid chromatography with electrochemical detection. Plasma was thawed on ice and 50 µL was diluted with 50 µL 2 M perchloric acid. The diluted plasma was filtered with a Microcon 10-kDa Centrifugal Filter Unit with Ultracel-10 Membrane (MRCPRT010; EMB Millipore) and centrifuged at 12,000 × g for 5 min at 4 °C. The supernatant was loaded onto an ESA model 542 autosampler with ESA model 582 HPLC system. We used a mobile phase of 10% methanol (34885-2L-R), 70 mM K2H2PO4 (7778-77-0), and 1 mM 1-octanesulfonic acid (5324-84-5) (pH 3.5). The flow rate was 1 mL/min. Five microliters of supernatant was injected using a partial fill and flush volume of 20 µL. Until injection, the plasma was kept at 4 °C throughout all procedures. The sample was separated on a 250 mm × 4.6 mm 5-µm Ultrasphere ODS column (235329; Hichrom) at room temperature. l-tryptophan and l-kynurenine concentrations were measured using an ESA Coulochem II electrochemical detector. The guard cell was set to 500 mV with 100 µA gain, the first electrode was set to 500 mV with 2 µA gain, and the second (detection) electrode was set to 900 mV with 200 nA gain. Chromatograms were analyzed using EZChrom Elite software (Agilent Technologies), and peak heights were used to calculate concentrations based on known standards.
Determination of Adrenal Weight.
To assess treatment (M. vaccae, CSC, or anti-CD25 antibody) effects on adrenal weight in Exps. 1 and 4, the left and right adrenal glands of each nonperfused (Exp. 1) experimental mouse were removed after decapitation on day 20, pruned of fat, and weighed. Data represent the mean + SEM of the sum of the left and right absolute adrenal weight of all experimental mice per treatment group.
Adrenocorticotropic Hormone Stimulation of Adrenal Explants in Vitro.
To assess treatment (M. vaccae, CSC, or anti-CD25 antibody) effects on adrenal adrenocorticotropic hormone (ACTH) responsiveness, basal and ACTH-induced corticosterone (CORT) secretion from adrenals of all experimental mice killed on day 20 was quantified in Exps. 1 and 4. Adrenal in vitro stimulation was performed as described recently (88). Briefly, after weighing, left and right adrenals were stored in ice-cold Dulbecco’s modified Eagle medium (12634-028; DMEM/F-12; Life Technologies) containing 0.1% BSA until all mice were killed and adrenals were removed. Afterward, each left and right adrenal gland was cut into halves, each half containing cortical and medullary tissue. Next, adrenal halves were weighed and preincubated in 200 µL DMEM/F-12 for 4 h (37 °C, 95% O2, 5% CO2). Afterward, culture medium was replaced and each half of one adrenal was incubated with either medium containing saline (basal) or medium containing ACTH (100 nM) for 6 h at 37 °C (95% O2, 5% CO2). Finally, supernatants were carefully removed and stored at –20 °C until they were analyzed using a commercially available CORT ELISA (RE52211, IBL International). CORT concentrations were calculated in relation to the weights of the respective adrenal explants (i.e., relative CORT secretion) and expressed as ng/mL⋅mg−1. To illustrate treatment effects (M. vaccae, CSC, or anti-CD25 antibody) on in vitro adrenal CORT secretion on a more organismal level, relative CORT secretion from the left and right adrenal gland of each mouse was summed (total CORT secretion). Data shown represent the mean + SEM of total CORT (basal and ACTH-stimulated) secretion of all experimental mice per treatment group.
Determination of the Histological Damage Score of the Colon.
To assess treatment (M. vaccae, CSC, or anti-CD25 antibody) effects on histological markers of the distal part of the intestinal tract in Exps. 1–4, the colon of all experimental mice killed on day 20 (Exps. 1, 2, and 4) or day 29 (Exp. 3) was removed and mechanically cleaned. Afterward, 1 cm of the distal third of the colon was cut longitudinally, laid on a filter paper, and fixed in 10% formalin overnight. The next day the fixed tissue was embedded in paraffin and cut longitudinally. Three 3-µm hematoxylin/eosin-stained sections taken at 100-μm distances from each other were evaluated by histological scoring performed by an investigator blind to treatment. For statistics, each individual score represented the mean of the three sections. Histology was scored as follows:
Epithelium: 0, normal morphology; 1, loss of globlet cells; 2, loss of globlet cells in large areas; 3, loss of crypts; 4, loss of crypts in large areas.
Infiltration: 0, no infiltration; 1, infiltrate around crypt base; 2, infiltrate reaching to lamina muscularis mucosae; 3, extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant edema; 4, infiltration of the lamina submucosa.
The total histological damage score of each mouse represents the sum of the epithelium and infiltration scores and ranges from 0 to 8. Data shown represent the mean + SEM of the histological damage score of all experimental mice per treatment group. The colonic tissue of three mice of the M. vaccae/control antibody-SHC group and of one mouse of the M. vaccae/control antibody-CSC group (Exp. 4) were lost during paraffin embedding.
Isolation and Incubation of Mesenteric Lymph Node Cells.
To assess treatment (M. vaccae, CSC, or anti-CD25 antibody) effects on anti-CD3–stimulated cytokine secretion from isolated lymph node cells in Exps. 3 and 4, mesenteric lymph nodes were harvested under sterile conditions from all experimental mice killed on day 20 (Exp. 4) or day 29 (Exp. 3) and collected on ice in cell-culture medium [RPMI-1640 supplemented with 10% FBS (A15-151; PAA), 100 U/mL penicillin and 100 µg/mL streptomycin (P11-010; PAA), and 3 × 10−5 M β-mercaptoethanol (M6250; Sigma-Aldrich)]. Lymph nodes were mechanically disrupted and filtered through a cell strainer (70-µm nylon, Falcon, 352350; Becton Dickinson Biosciences). Afterward, cells were washed three times in cell-culture medium and adjusted to a concentration of 106 cells per mL. Two × 105 (200 µL) lymph node cells were transferred to wells of a 96-well plate and stimulated by precoating the wells with 200 µL of 2.5 µg/mL anti-CD3 antibody. Four wells containing 2 × 105 lymph node cells were prepared for each mouse. After incubation for 24 h (37 °C, 5% CO2), IFN-γ, IL-6, and IL-10 concentrations were measured in the supernatants by ELISA [EM10012 (IFN-γ), EM2IL62 (IL-6), EM2IL102 (IL-10); Thermo Fisher Scientific) using two wells per mouse. Data among duplicate/quadruplicate measures of each mouse were averaged. Data shown represent the mean + SEM of all experimental mice per treatment group. IFN-γ secretion data from one mouse of the M. vaccae/anti-CD25 antibody-SHC group (Exp. 3) had to be excluded due to technical problems.
tph2 and slc6a4 in Situ Hybridization Histochemistry.
To assess treatment (M. vaccae, CSC, or anti-CD25 antibody) effects on tph2 and slc6a4 mRNA expression in different subregions of the DR in Exps. 1 and 4, in situ hybridization histochemistry was performed as previously described (89). After decapitation, brains of all experimental mice were removed, snap-frozen in isopentane, cooled on dry ice for 2–4 s, and then put in aluminum foil on dry ice until all animals were euthanized. Afterward, brains were stored at –80 °C before being shipped to the University of Colorado Boulder for subsequent in situ hybridization. Frozen brains were blocked into forebrain and hindbrain pieces with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately −3.40 mm bregma) using a mouse brain matrix (RBM-2000C; ASI Instruments) to ensure a consistent coronal plane of sectioning. Thereafter, brains were individually wrapped in aluminum foil and stored at –80 °C until sectioning for tph2 and slc6a4 in situ hybridization histochemistry. Brains were sectioned throughout the midbrain and pons using a Leica 1900 cryostat (North Central Instruments; five alternate sets of microscope slides with 12-µm sections) and stored at ‒80 °C.
tph2.
An oligonucleotide probe complementary and specific to tph2 mRNA (5′-TCC GTC CAA ATG TTG TCA GGT GGA TCC AGC CTC ACA ATG GTG GTC-3′; Integrated DNA Technologies) was radiolabeled with [35S]deoxyadenosine-5′-(alpha-thio)-triphosphate (dATP; NEG034H001MC; PerkinElmer) using the enzyme terminal transferase (Promega) to create a DNA oligonucleotide probe complementary to bases 489–533 of rat tph2 mRNA. The probe was cleaned (QIAquick Nucleotide Removal Kit; 28304; Qiagen) and used in an in situ hybridization histochemistry assay as described previously (89). Air-dried slides were apposed to a BioMax MR autoradiography film (871 5187; Carestream Health) along with 14C standards (ARC0146C; American Radiolabeled Chemicals) for 26 d. Data shown represent the mean + SEM.
slc6a4.
An oligonucleotide complementary and specific to slc6a4 mRNA (5′-ACT GCA GAG TAC CCA TTG GAT ATT TGG CTA GGC TCT GCC CTG TCC GCT GT-3′; Integrated DNA Technologies) was radiolabeled with [35S]dATP; NEG034H001MC; PerkinElmer) using the enzyme terminal transferase (Promega) to create a DNA oligonucleotide probe complementary to bases 207–256 of rat slc6a4 mRNA. The probe was cleaned (QIAquick Nucleotide Removal Kit; 28304; Qiagen) and used in an in situ hybridization histochemistry assay as described previously (90). Air-dried slides were apposed to BioMax MR autoradiography film (871 5187; Carestream Health) along with 14C standards (ARC0146C; American Radiolabeled Chemicals) for 24 d. Data shown represent the mean + SEM.
Semiquantitative Analysis of Gene Expression.
Digital autoradiography images were analyzed with ImageJ (NIH) by an experimenter blind to the treatment groups to measure gray value × area (as a measure of tph2 and slc6a4 mRNA expression) using matrices in the shape of each subdivision of the brainstem dorsal raphe nucleus (DR). Area (mm2) was defined as the area (within each matrix) that fell above a gray value threshold that was kept consistent throughout analysis. A total of 12 rostrocaudal sections, designated levels +6 to −5, containing seven major subdivisions of the DR, were analyzed [rostral aspect of the dorsal raphe nucleus, dorsal part (rDRD), −4.303 to −4.554 mm bregma; rostral aspect of the dorsal raphe nucleus, ventral part (rDRV), −4.303 to −4.544 mm bregma; caudal aspect of the dorsal raphe nucleus, dorsal part (cDRD), −4.604 to –4.724 mm bregma; caudal aspect of the dorsal raphe nucleus, ventral part (cDRV), −4.604 to −4.784 mm bregma; dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray region (DRVL/VLPAG), −4.544 to −4.784 mm bregma; dorsal raphe nucleus, caudal part (DRC), −4.784 to −4.964 mm bregma; dorsal raphe nucleus, interfascicular part (DRI), −4.784 to −4.964 mm bregma]. An average, above-threshold mean gray value was computed for the DRVL/VLPAG using values from both the left and right hemispheres. The background mean gray value of each image, measured in the surrounding midbrain tegmentum devoid of tph2 and slc6a4 mRNA expression, was subtracted from each value. Throughout the 12 rostrocaudal levels, values for each of the seven DR subdivisions were averaged, and average tph2 or slc6a4 mRNA expression levels in the entire DR were also calculated for each mouse. Data shown represent the mean + SEM.
Iba1 immunohistochemistry.
Brains were immunostained for Iba1, a 17-kDa actin-binding protein specifically and constitutively expressed in microglia (35, 36) that is an immunohistochemical marker for both ramified and activated microglia (35, 37). Sections from perfusion-fixed brain tissue were gently shaken on an orbital shaker throughout the protocol. Sections were rinsed twice for 15 min each time in 0.1 M phosphate buffer and then rinsed in 3% hydrogen peroxide (H2O2) in 0.1 M PB for 30 min. Sections were then incubated in 0.1 M PB containing 3% normal horse serum. The tissue was then incubated with the primary antiserum (anti-rabbit Iba1; 019-19741; lot MNH4488; Wako Pure Chemical Industries; 1:10,000) in 0.1 M PB containing 1% normal horse serum, 0.1% BSA, 0.3% Triton X-100, and 0.01% sodium azide for ∼72 h at 4 °C. Sections were rinsed twice for 15 min each time in 0.1 M PB and then incubated in an affinity-purified secondary antibody (biotinylated donkey anti-rabbit IgG; Jackson ImmunoResearch; 711-065-152; lot 106974; 1:200) in 0.1 M PB containing 2% normal horse serum, 0.1% BSA, and 0.2% Triton X-100 for 2 h. Sections were again rinsed twice for 15 min in 0.1 M PB followed by incubation with an avidin–biotin–peroxidase complex (PK-6106; Vectastain Elite ABC Kit; Vector Laboratories) in 0.1 M PB containing 2% normal horse serum, 0.1% BSA, and 0.2% Triton X-100 for 90 min. Tissue was rinsed three times for 15 min each time in 0.1 M PB before being placed in a peroxidase chromogen substrate solution consisting of 0.05% 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich; D9015), 0.004% ammonium chloride, 0.2% d-glucose, and 0.02% nickel ammonium sulfate and preincubation for 20 min followed by addition of 0.1% glucose oxidase directly into each well. Finally, the tissue was rinsed twice for 15 min in 0.1 M PB to stop the reaction and then stored in 0.1 M PB containing 0.01 M sodium azide at 4 °C until mounting. For mounting of brain sections onto microscope slides, brain sections were rinsed briefly in distilled water with 0.15% gelatin (gelatin from porcine skin, type A; G-2625; lot 60H0477; Sigma-Aldrich) and then mounted on microscope slides and left overnight to dry. Sections were dehydrated through an ethanol series (70%, 95%, and 100% for 2 min each) and cleared in xylenes for 5 min. Coverslips were mounted on the slides using Entellan mounting medium (14802; Electron Microscopy Sciences).
Analysis of microglial density and morphology.
Microglial density and morphology were analyzed using image analysis of Iba1-immunostained sections. Sections were examined using a Nikon 90i upright microscope using a 20× objective lens (200× total magnification) with a Nikon DS-Fi1 digital camera linked to a computer with NIS Elements Advanced Research imaging software (Coherent Scientific). Results were analyzed by an experimenter blind to treatment conditions. Quantitative analysis of Iba1-immunoreactive (Iba1-ir) material was conducted in eight brain regions proposed to be involved in anxiety-related behavior, as well as in the arcuate nucleus, a region with a reduced blood–brain barrier. The regions selected were: 1.78 mm bregma: infralimbic cortex (IL), prelimbic cortex (PrL); 1.54 mm bregma: nucleus accumbens, shell region (AcbSh); 0.14 mm bregma: bed nucleus of the stria terminalis, lateral division, dorsal part (BSTLD), bed nucleus of the stria terminalis, lateral division, ventral part (BSTLV); –1.46 mm bregma: central amygdaloid nucleus (Ce); –1.94 mm bregma: arcuate nucleus of the hypothalamus (Arc), basolateral amygdaloid nucleus, anterior part (BLA), CA1 field of the dorsal hippocampus (CA1).
The anatomical locations of each of the nine brain regions were determined using a standard adult mouse brain atlas (91). Schematic representations of the anatomical location of each region and its corresponding region of interest (ROI) examined are illustrated in Fig. S3. One section from each mouse at each anatomical level was sampled. Quantifications were completed bilaterally. Because the immunohistochemistry was conducted in free-floating sections and the brain hemispheres were not marked for identification before sectioning, it was not possible to differentiate the left from the right hemisphere in this study. As suggested in a recent review, both estimated cell count and the total density of Iba1-ir material were quantified (92). This was performed in an initial analysis using NIS Elements thresholding procedures, where only pixels within a specified range of size and/or density were included for analysis. For density, parameters for the threshold of detection were set such that both the soma and the processes were detected, while avoiding detection of background pixels (density range: 137–255 to 125–255 across regions).
Following identification of treatment (M. vaccae) effects in the PrL, but not other ROIs, a more thorough analysis was conducted in the PrL as follows. Thresholding analysis used MATLAB (v2013b; MathWorks) images were converted into 8-bit (256-color) grayscale and then further processed to create two specific datasets. The first was the pixel-intensity spectra, which reflect the number of pixels within each image that occur at each of the 256 pixel intensities (0, black; 256, white). The second dataset created was the cumulative threshold spectra, which reflect the cumulative number of pixels that occur at or below each of the 256 pixel intensities. Cumulative data, derived from the data used to generate the intensity histograms, were expressed as a percentage of the overall number of pixels present within a given image. The cumulative threshold spectra operate in a manner very similar to a standard single-point threshold analysis except for the fact that information is provided on all potential points at which an image could be thresholded and therefore provide a much more comprehensive view of between-group differences.
For analysis of microglial morphology, digital reconstructions of all microglia present in the given ROI were created using MATLAB 2013b. A variety of image-processing functions were called from MATLAB’s image-processing toolbox to analyze these reconstructions. Specifically, we examined the total area of the cells (soma plus processes), maximum axis length, convex area (as defined by the area defined by a set X of points in the Euclidean space that is the smallest convex set that contains X), and mean intensity of Iba1 immunostaining. As there were no differences in microglial density or morphology of microglia in mice exposed to the EPM and those that were not, data were pooled from both groups of mice.
Assessment of the Severity of DSS-Induced Colitis.
To assess treatment (M. vaccae and/or CSC) effects on the severity of DSS-induced colitis, all experimental mice of Exp. 3 (vehicle-SHC, vehicle-CSC, M. vaccae-SHC, M. vaccae-CSC) were euthanized following 1 wk of DSS (1%) treatment between 0800 and 1100 hours on day 29. One vehicle-SHC mouse died during DSS treatment and was excluded from analysis.
The colon of all experimental mice was removed and mechanically cleaned. Afterward, 1 cm of the distal third of the colon was treated as described in detail above for quantification of the histological damage score. Moreover, mesenteric lymph nodes from each experimental mouse were individually harvested under sterile conditions. Subsequently, mesenteric lymph node cells were isolated, counted individually for each mouse, and treated as described in detail above for quantification of anti-CD3–stimulated IFN-γ, IL-6, and IL-10 secretion.
Fluorescence-Activated Cell-Sorting Analysis of Mesenteric Lymph Node Cells.
To assess the effects of antibody-mediated depletion (Exp. 3; M. vaccae/control antibody-SHC, M. vaccae/anti-CD25 antibody-SHC, M. vaccae/control antibody-CSC, M. vaccae/anti-CD25 antibody-CSC, uninjected SHC, uninjected CSC) on subsets of CD25+ T cells, flow cytometric analysis of isolated mesenteric lymph node cells of all mice was performed as described previously (93). Briefly, cell isolation was performed exactly as described above (Isolation and Incubation of Mesenteric Lymph Node Cells) and in ref. 28. Cell-surface staining was performed using rat anti-mouse CD4 (clone RM4-5; eBioscience), rat anti-mouse CD25 (clone PC61; eBioscience), and rat anti-mouse CD62L (clone MEL-14; BD Biosciences) antibodies. For detection of intracellular Foxp3, the Treg staining kit (eBioscience) was applied according to the manufacturer’s instructions using isotype-matched phycoerythrin (PE)-labeled IgG2a as control (BD Biosciences). Data were collected on an LSR II flow cytometer (BD Biosciences) and analyzed using DIVA software (BD Biosciences). All staining profiles were based on live-gated cells, as determined by forward and sideward scatter properties. Exclusion of doublet cells was performed by side scatter (SSC) area − SSC width gating. Cells were further subdivided into different subpopulations according to CD4, CD25, Foxp3, and CD62L expression, and the percentage of specific subtypes with respect to a single cell gate is given in Fig. S6.
Scanning Electron Microscopy.
Scanning electron microscopy images of the heat-killed preparation of M. vaccae (NCTC 11659) (Fig. S2A) were conducted as follows. The samples were postfixed in a mixture of 1% osmium tetroxide (OsO4) in 0.1 M phosphate buffer (pH 8) for 1 h, rinsed quickly in distilled water, and deposited on a filter with a porosity of 1 μm. Samples were dehydrated by the method of bypassing the critical point and metallized with gold palladium. Observations were performed on a Quanta 250 FEG (FEI) scanning electron microscope.
Ultra-High-Throughput Microbial Community Analysis on the Illumina MiSeq Platform.
PCR and sequencing were performed using a modified version of the protocol presented in ref. 94, adapted for the Illumina MiSeq. Briefly, the V4 region of the 16S ribosomal RNA (rRNA) gene was amplified with region-specific primers that included the Illumina flow cell adapter sequences. The reverse amplification primer also contained a 12-base barcode sequence that supports pooling of up to 2,167 different samples in each lane. After cluster formation on a MiSeq instrument, the amplicons were sequenced with custom primers. These sequencing primers were designed to be complementary to the V4 amplification primers to avoid sequencing of the primers, and the barcode is read using a third sequencing primer in an additional cycle. The amplification primers were adapted from the published protocol (94) to include nine extra bases in the adapter region of the forward amplification primer that support paired-end sequencing on the MiSeq. The amplification and sequencing primers additionally contain a new pad region to avoid primer–dimer formation with the modified adapter.
Quality filtering of reads was applied as described previously (94). Reads were truncated at their first low-quality base (defined by an “A” or “B” quality score). Reads shorter than 75 bases were then discarded, as were reads whose barcode did not match an expected barcode. In addition to the experimental samples, the MiSeq run also contained a control library made from phiX174, which, in this run, accounted for 47% of reads. The phiX control was used because of the limited sequence diversity among the 16S amplicons.
DNA encoding rRNA (16S) sequences was extracted using the EMP protocol (95). Sequences were barcoded and multiplexed for sequencing on a MiSeq platform. After sequencing, raw reads were quality-filtered and demultiplexed using the QIIME 1.8 (96) script split_libraries_fastq.py, leaving 35,741,149 reads, with samples having on average 121,569 reads. Sequences were clustered at 97% similarity into operational taxonomic units (OTUs) using an open reference picking approach (97) with clustering and taxonomy assignment via UCLUST (98) using the Greengenes August 2013 reference set (99, 100). Sequences were aligned with PyNAST (101), and phylogeny was constructed with FastTree v2.1.3 (102). Default parameters for pick_open_reference_otus.py were used, which filters out singleton OTUs and OTUs that represent less than 0.0005% of the total sequence count.
Statistical Analyses.
General statistical methods.
For statistical comparisons, the software package SPSS (v21.0), R, as well as customized Python scripts were used. Except where noted, statistical outliers were identified using Grubbs’s test (103) and were removed from the analysis. Due to experimental challenges (including technical error), it is common for neuroscientists to apply Grubbs’s method for dealing with potentially extreme observations (104, 105). Accordingly, we applied Grubbs’s method to deal with extreme observations. No outliers were removed in the analysis of gut microbial communities or composition. Comparisons of two independent samples were made using Student’s t tests. Analyses of multifactor designs were conducted using multifactor ANOVA. For analyses of experimental end points with repeated measures, data were analyzed using a linear mixed model (LMM). A survey of covariance structures was performed by examining the –2 restricted log-likelihood value (i.e., an information criterion function used to measure goodness of fit) and the number of parameters (i.e., a measure of parsimony) to determine the covariate structure that best fit the model. When appropriate, post hoc pairwise comparisons were made using Fisher’s protected least-significant difference (LSD) tests, false-discovery rate (FDR), or Bonferroni correction, as indicated in the text. A Fisher’s exact test was used to test for differences in categorical dependent variables. Data are presented as mean + SEM. A two-tailed α-level of 0.05 was used for all tests.
Analysis of treatment effects on microbial community structure and microbial composition.
Analysis of treatment effects on gut microbial community structure was carried out with QIIME 1.8, R, as well as customized Python scripts. Except where noted, the raw OTU table was rarefied to 45,775 sequences per sample to correct for differential sequencing effort. Although rarefaction is a conservative approach that limits power for discovery of differences, it is essential to correct for different library sizes for α- and β-diversity analysis. α-Diversity was calculated with QIIME, using phylogenetic diversity, the observed number of species, and Shannon entropy (in bits) (106).
β-Diversity was calculated using weighted and unweighted UniFrac (107) and visualized using EMPeror (108). β-Diversity was evaluated with UniFrac, a community dissimilarity metric based on the fraction of unique branch length observed in pairs of communities in a common phylogenetic tree (107, 109). The phylogenetic distance (UniFrac distance) is calculated as the fraction of unshared branch lengths between the pair of communities. Unweighted and weighted UniFrac distance matrices served as inputs for clustering analyses and significance tests aimed at determining which variable explained the greatest amounts of variation in the taxonomic composition of the gut bacterial communities (110). Unweighted UniFrac distances compare microbial communities within a phylogenetic context based on the presence/absence of members, whereas weighted UniFrac also incorporates relative abundance information (110). UniFrac-based principal coordinate analysis (PCoA) aids in the exploration of potential factors that might explain the groupings of similar communities. PCoA, visualized with EMPeror (108), was used to map the UniFrac distance matrix onto a set of orthogonal axes capturing the greatest amount of variation in all of the samples tested. Distances between samples on a PCoA plot reflect the corresponding dissimilarities in their community membership (unweighted UniFrac) or community structure (weighted UniFrac).
Statistical analyses of microbial composition were computed with R and Python. 16S rDNA data were normalized by taking the number of species in each sample and dividing by the total number of species. Relative abundances were determined for the genus level. Normalized relative abundances at the genus level were used to prepare biplots to visualize the effects of treatment on microbial community structure (described in detail below).
Biplot Analysis.
To explore correlations among the different genera present in the samples, compositional biplot analyses were performed. A multiplicative zero replacement technique was used to determine pseudocounts for zeros (111). A center log transformation (112) was conducted to transform the data, and a singular value decomposition was performed to obtain a two-rank approximation of the genera and the samples. The vectors correspond to different genera—the angle between any two lines approximates the correlation between any two genera. The points are samples and the Euclidean distance between them approximates the dissimilarity between each other. The direction of a vector corresponds to the variance of the samples explained by that genus.
Conditional Density Estimation.
To validate the correlations among the genera shown in the biplot, a conditional density estimator was used. For a pair of genera, zero abundance data points were disregarded and their abundances were log-transformed. A conditional kernel density estimation was then applied on the log-transformed abundances (113).
Multiple Linear Regression.
To analyze how colon damage affects microbial communities, a multiple linear regression was conducted. A multiplicative zero replacement strategy was used to replace zeros with small pseudocounts (111), and an isometric logratio transformation (ilr) (114) was used to transform the genera abundances from the Aitchison simplex onto the real space. The multiple linear regression was conducted with these transformed data. This regression showed that 11% of the variation in the gut microbiota was explained by the histological damage score.
Analysis of Composition of Microbiomes.
Effects of M. vaccae and CSC on abundances by taxonomic rank were analyzed using analysis of composition of microbiomes (ANCOM) and an FDR of 0.05, and a Kruskal–Wallis test was used within ANCOM to test for significant differences. ANCOM analysis for M. vaccae/CSC main effects was conducted on days 8 and 15 only. ANCOM analysis of effects of time within each treatment group was conducted, followed, when appropriate, by Wilcoxon signed-rank tests to identify differences between day 1 versus day 8 and day 1 versus day 15, using Bonferroni correction. Although our design was a repeated measurement design, data on very few animals were available at all time points. For example, in the M. vaccae/CSC group, microbiome data were available on only 3 out of 23 animals at all six time points. Similarly, microbiome data on only 4 out of 23 animals in the vehicle/CSC group were available at all six time points. Thus, for all practical purposes, these data can be regarded as independent sample data rather than longitudinal data. Hence, we applied the independent sample ANCOM to analyze the time course microbiome data.
The CSC Model as a Model of Repeated Psychosocial Traumatization or PTSD.
Here we used the CSC model in adult male C57BL/6NCrl mice. The CSC model involves introducing four experimental mice into the home cage of a larger, dominant male mouse for 19 d; on days 8 and 15 of the procedure the experimental mice are moved to the cage of a novel dominant male, resulting in reestablishment of dominant/subordinate hierarchies. Thus, the CSC paradigm combines repeated/chronic, psychological, and social aspects of stress/trauma and, therefore, represents a potent animal model to mimic the type of health-compromising stressors/traumata faced by humans (high face validity). In support, mice exposed to the CSC procedure share multiple pathophysiological outcomes with PTSD (high predictive validity), a stress-related mental disorder diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders 5th Ed. when the following criteria are fulfilled: reexperiencing of aversive details of the traumatic events, avoiding of trauma-related external reminders, negative cognitions and mood, and hyperarousal (115). In accordance, CSC mice are repeatedly reexposed to the psychosocial trauma of social defeat, and show social deficits toward unfamiliar male conspecifics (3), persisting negative emotions (e.g., fear and anxiety) (116, 117), as well as indications of increased arousal, including increased locomotor activity during the active phase of the light cycle (117) and elevated plasma norepinephrine (7). Moreover, PTSD is associated with gastrointestinal pathology (118), an association that may be due to acute trauma exposure rather than to PTSD per se (119). The CSC exposure induces development of spontaneous colonic inflammation as measured by increased: (i) histological damage score (28, 120, 121), (ii) bacterial translocation as a consequence of disturbed colonic barrier functions (120, 121), (iii) numbers of colonic macrophages and dendritic and T cells (121, 122), and (iv) proinflammatory cytokine secretion from isolated lamina propria mononuclear (121, 122) and mesenteric lymph node cells (28). Furthermore, CSC exposure results in an aggravation of DSS-induced colitis, a model of inflammatory bowel disease (IBD) (123). As observed in PTSD (124), CSC exposure is associated with basal hypocorticism (125) that, in the case of CSC exposure, may be due to adrenal ACTH nonresponsiveness (28, 88). Both conditions are associated with a flattened diurnal cortisol/corticosterone rhythm (28, 126), increased dexamethasone suppression of ACTH (127, 128), and increased HPA axis reactivity toward novel stressors (88, 129). An important finding in both PTSD (9, 130, 131) and the CSC model (132) is a reduction in the percentage of Treg in plasma or peripheral lymph nodes, respectively, likely contributing to the overall increased inflammatory state. Of note, the suppression of Treg in PTSD is reversed 1 y following effective narrative exposure therapy (54). Based on the above findings, the CSC paradigm seems to be an adequate animal model for PTSD (133), complex PTSD (C-PTSD) (134), and research domains outlined in the National Institute of Mental Health Research Domain Criteria Project (RDoC) relevant to these disorders.
Discussion
The results support the conclusion that immunization with a heat-killed preparation of M. vaccae increases resilience to stress-related pathologies in part through the induction of Treg and an antiinflammatory bias. Immunization with M. vaccae decreased submissive behavioral displays, as well as flight and avoiding behaviors, during an initial encounter with a dominant male aggressor. Following exposure to the chronic psychosocial stressor for 19 d, mice immunized with M. vaccae, but not vehicle-immunized mice, responded with decreased anxiety- or fear-like behaviors when tested on the EPM. These behavioral responses were associated with altered gene expression in serotonergic systems previously implicated in stress resilience and changes in microglial density in brain structures implicated in control of fear expression. Immunization with M. vaccae also prevented stress-induced spontaneous colitis and stress-induced exaggeration of chemically induced colitis, a model of IBD. The effects of immunization with M. vaccae on anxiety- or fear-like behaviors in stressed mice, as well as its effects on stress-induced exaggeration of spontaneous colitis, were prevented by depletion of Treg. In contrast, there were no effects of Treg depletion on M. vaccae-induced changes in behavioral responses to a dominant aggressor, suggesting multiple different mechanisms through which immunization with M. vaccae alters stress-related behavior. Together, these data are consistent with the hypothesis that immunization with M. vaccae can prevent stress-induced exaggeration of colonic inflammation and downstream effects on anxiety- and fear-like behavior. As such, immunization with heat-killed preparations of immunoregulatory bacteria may have utility in prevention of anxiety and affective disorders and their medical comorbidity, including exaggerated autoimmunity (11, 59) and exaggerated symptoms of IBD (12, 11, 59).
When challenged with a dominant aggressor 1–2 wk following the final immunization with M. vaccae, mice showed a robust decrease in submissive upright posture and decreases in reactive behavioral coping responses, such as flight and avoiding behaviors, relative to vehicle-immunized controls. Previous studies have shown that a reactive emotional coping strategy during social defeat, as measured by a short latency to display submissive postures, predicts vulnerability to subsequent development of anxiety- and depressive-like behavioral responses (25, 27, 60, 61) and that inflammatory factors within the CNS drive the vulnerability to depressive-like behavioral responses in individuals with reactive coping responses (32). Thus, the decreased submissive behaviors in M. vaccae-immunized mice are consistent with a stress-resilient behavioral phenotype. The mechanisms underlying the shift in behavioral strategy during psychosocial stress are not clear, but do not seem to depend on Treg. Of potential importance, M. vaccae-immunized mice had altered tph2 and slc6a4 mRNA expression specifically in the dorsal parts of the rostral to midrostrocaudal dorsal raphe nucleus, a subregion of the dorsal raphe nucleus that has previously been implicated in stress resilience (62). The effects of immunization on tph2 mRNA expression were observed in both SHC and CSC mice and were therefore independent of the stress-induced differences in peripheral inflammation.
Factors that are known to influence individual variability in stress coping behaviors during psychosocial stress include endocrine factors, such as testosterone, or increased sympathetic reactivity, which are both associated with a more proactive behavioral coping strategy (27). Interestingly, the microbiome generally (63) and the probiotic Lactobacillus reuteri specifically have been shown to increase testosterone in mice, effects that could be mimicked by interfering with IL-17A signaling, a proinflammatory cytokine (64). Immunoregulatory microbes act on regulatory dendritic cells, which in turn bias T-cell differentiation toward Treg and away from IL-17–producing T helper 17 (Th17) cells (65, 66). Testosterone in turn increases serotonin transporter mRNA expression and binding in rats and humans in brain regions innervated by the rostral dorsal raphe nucleus (67, 68), an effect consistent with our finding that immunization with M. vaccae prevented stress-induced decreases in slc6a4 mRNA expression specifically in the rostral dorsal raphe nucleus. In addition, testosterone increases the neuronal firing rates of serotonergic neurons in the dorsal raphe nucleus (69). The effects of testosterone on serotonin transporter mRNA, binding, and serotonergic neuronal firing are thought to be dependent on aromatization of testosterone to 17β-estradiol (68, 69); 17β-estradiol induces anxiolytic effects on the EPM, in association with increases in tph2 mRNA expression, specifically in the dorsal part of the dorsal raphe nucleus (70), as observed in our studies. Thus, immunoregulatory microbes can increase plasma testosterone concentrations, and testosterone or its metabolite, 17β-estradiol, replicates the effects of M. vaccae immunization on behavioral coping strategies during social defeat, performance on the EPM, and slc6a4 and tph2 mRNA expression in the dorsal raphe nucleus. Future studies should explore these potential endocrine mechanisms, as well as their interactions with the microbiome–gut–brain and behavior axis.
The effects of M. vaccae immunization to decrease submissive behavioral displays and decrease flight and avoiding behaviors were long-lasting, evident at least 1–2 wk following the final immunization. Meanwhile, the effects of M. vaccae immunization to induce anxiolytic responses (measured on day 19) and prevent CSC-induced exaggeration of colitis (measured on day 20) were observed 4–5 wk following the final immunization. As demonstrated using anti-CD25 antibody experiments, the effects of M. vaccae immunization to induce anxiolytic responses and prevent stress-induced exaggeration of colitis appear to be dependent on Treg. This timeline is consistent with previous studies demonstrating a long half-life of Treg in C57BL/6 mice, estimated to be 27 d (71). Long-lasting protection following immunization with the same heat-killed preparation of M. vaccae has been observed in a murine model of allergic airway inflammation, where protective effects last up to 12 wk (21). Persistent effects of M. vaccae in this model may depend in part on Treg derived from memory T cells (72), as mice are repeatedly exposed to the allergen antigen, ovalbumin. It is likely, however, that repeated immunization would be necessary in adults to induce persistent immunoregulatory effects.
The stress-protective effects of M. vaccae immunization appear to be independent of the changes in the diversity or community structure of the gut microbiota. Consistent with previous studies, exposure of mice to chronic psychosocial stress resulted in decreased diversity of gut microbial communities, as measured by α-diversity, and altered gut microbial community structure. Stress-induced changes in gut microbial communities were driven by expansion of Helicobacter spp., which have been shown to induce colitis in hosts with impaired immunoregulation, such as IL-10−/− mice, through induction of IFN-γ and IL-12 (73, 74). Importantly, preinoculation with immunoregulatory L. reuteri and Lactobacillus paracasei reduced intestinal inflammation in Helicobacter hepaticus-challenged mice, despite failing to alter the quantity of H. hepaticus in cocolonized mice (75). L. reuteri primes dendritic cells to drive the development of Treg, through interactions with the C-type lectin DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) (65). Our results parallel these previous studies, in that Helicobacter spp. relative abundance was correlated with colitis scores in vehicle-treated mice, whereas M. vaccae-immunized CSC mice, characterized by abrogated release of IFN-γ and IL-6, together with exaggerated release of IL-10 from freshly isolated mesenteric lymph node cells stimulated in vitro, were protected against both development of spontaneous colitis and aggravation of chemically induced colitis in a murine model of IBD.
The stress-protective effects of M. vaccae immunization also appear to be independent of the changes in the functionality of the HPA axis. Glucocorticoid hormones, acting at glucocorticoid receptors, have been shown to induce expansion of Helicobacter spp. (18). These findings are consistent with other studies showing that glucocorticoid hormones decrease IgA (which normally inhibits bacterial adherence to intestinal epithelial cells), increase bacterial adherence to the intestinal epithelium over twofold, and increase bacterial translocation to mesenteric lymph nodes (17). Mice exposed to the CSC procedure experience a profound activation of the HPA axis, with persistent adrenal hypertrophy that is evident within 24 h and persists through days 2, 7, 14, and 20 of the CSC procedure, exaggerated ACTH and corticosterone release to novel stressors, and in vitro adrenal insufficiency, as measured by decreased sensitivity to ACTH (26). Glucocorticoid insensitivity of effector immune cells, as indicated by glucocorticoid insensitivity of lipopolysaccharide-stimulated splenocytes and anti-CD3–stimulated T cells, may contribute to the exaggerated inflammatory responses in CSC mice (26). Immunization with M. vaccae had no effect on the CSC-induced adrenal hypertrophy or the in vitro adrenal insensitivity, suggesting that, although HPA axis changes may contribute to stress-induced exaggerations of inflammation, they do not mediate the protective effects of M. vaccae immunization.
As previously demonstrated for allergic airway inflammation (22), the beneficial effects of M. vaccae on colitis and measures of anxiety appear to be dependent on induction of Treg and enhanced immunoregulation. The effects of CSC exposure on colitis and anxiety may be linked, as DSS-induced colitis (76–78), or chronic gastrointestinal inflammation induced by Trichuris muris infection, is sufficient to induce anxiety-like behavioral responses. This effect may be mediated in part by afferent signaling of the vagus nerve, as prior vagotomy prevents the anxiogenic effects of DSS-induced colitis (77), although this is not the case for T. muris infection (79). Bacteria belonging to the phylum Actinobacteria, specifically M. vaccae (80), and probiotics including Bifidobacterium breve 1205 (81), Bifidobacterium longum 1714 (81), and B. longum NCC3001, have been shown to decrease anxiety-like behaviors in mice. A number of probiotics belonging to the phylum Firmicutes also have been shown to decrease anxiety-like behaviors in mice, including Lactobacillus helveticus ROO52 (82) and Lactobacillus rhamnosus (JB-1) (83). Anxiolytic effects of B. longum 1714 (81) and L. rhamnosus (JB-1) (83) are prevented by vagotomy, suggesting that signaling via the vagus nerve may be particularly important in the anxiolytic effects of some probiotics administered via the mucosal route. In our study, immunization with M. vaccae reduced spontaneous colitis and stress-induced exaggeration of chemically induced colitis and, in CSC mice, induced anxiolytic effects, effects that may involve both vagus-dependent and vagus-independent mechanisms.
In conclusion, these data suggest that exposure to environmental microorganisms, administration of probiotics with immunoregulatory actions, or immunoregulation-promoting immunizations with heat-killed preparations of these organisms or antigens derived from these organisms may confer health benefits, including mental health benefits in subjects with stress-related psychiatric disorders, such as PTSD and major depression, at least partly through prevention of stress-induced intestinal inflammation. From a broader perspective, immunoregulatory approaches such as that demonstrated here may prove useful in both prevention and treatment of stress-related psychiatric disorders, such as anxiety and affective disorders, in which inadequate immunoregulation, resulting in chronic, low-grade inflammation, is a risk factor. Although not specifically addressed here, immunoregulatory approaches may also prove useful in prevention of neurodevelopmental and other somatic and neuropsychiatric disorders in which elevated inflammation contributes to disease vulnerability (84). Lack of exposure to “old friends” through reduced contact with healthy soils and healthy animals in modern urban settings may partly explain the increased rates of immune-mediated diseases, including psychiatric disorders, in these settings. Restoring exposure to these old friends, through immunization or other routes, may decrease inflammation-associated disease vulnerability in modern urban societies.
Materials and Methods
For detailed materials and methods, see SI Materials and Methods. All experimental protocols were approved by the Committee on Animal Health and Care of the government of Oberpfalz, Germany, and were performed according to international guidelines on the ethical use of animals.
Acknowledgments
The authors thank L. Beninson, W. Craig, N. Grunwald, and S. Peters for excellent technical and experimental assistance. This study was supported in part by a 2010 NARSAD Young Investigator Award (to C.A.L.); German Research Foundation Grant RE 2911/5-1 (to S.O.R.); the University of Colorado Boulder (C.A.L.); Award NSF-IOS 0845550 (to C.A.L.); and donations through the CU Foundation. The research of S.D.P. was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES101744-04).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the European Bioinformatics Institute database (accession no. ERP015380), and https://qiita.ucsd.edu/study/description/1634.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600324113/-/DCSupplemental.
References
- 1.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20(5):779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Hertzen L, et al. Helsinki alert of biodiversity and health. Ann Med. 2015;47(3):218–225. doi: 10.3109/07853890.2015.1010226. [DOI] [PubMed] [Google Scholar]
- 3.Atherton JC, Blaser MJ. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J Clin Invest. 2009;119(9):2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014;177(1):1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnmacht C, et al. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349(6251):989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 6.Sefik E, et al. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science. 2015;349(6251):993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eraly SA, et al. Marine Resiliency Study Team Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommershof A, et al. Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav Immun. 2009;23(8):1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Lindqvist D, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 11.O’Donovan A, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77(4):365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cámara RJ, Gander ML, Begré S, von Känel R. Swiss Inflammatory Bowel Disease Cohort Study Group Post-traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterol. 2011;2(1):2–9. doi: 10.1136/fg.2010.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breen MS, et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 2015;20(12):1538–1545. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey MT. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv Exp Med Biol. 2014;817:255–276. doi: 10.1007/978-1-4939-0897-4_12. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 17.Alverdy J, Aoys E. The effect of glucocorticoid administration on bacterial translocation. Evidence for an acquired mucosal immunodeficient state. Ann Surg. 1991;214(6):719–723. doi: 10.1097/00000658-199112000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo G, et al. Psychological stress enhances the colonization of the stomach by Helicobacter pylori in the BALB/c mouse. Stress. 2009;12(6):478–485. doi: 10.3109/10253890802642188. [DOI] [PubMed] [Google Scholar]
- 19.Sheil B, et al. Is the mucosal route of administration essential for probiotic function? Subcutaneous administration is associated with attenuation of murine colitis and arthritis. Gut. 2004;53(5):694–700. doi: 10.1136/gut.2003.027789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laudanno O, Vasconcelos L, Catalana J, Cesolari J. Anti-inflammatory effect of bioflora probiotic administered orally or subcutaneously with live or dead bacteria. Dig Dis Sci. 2006;51(12):2180–2183. doi: 10.1007/s10620-006-9175-4. [DOI] [PubMed] [Google Scholar]
- 21.Zuany-Amorim C, et al. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J Immunol. 2002;169(3):1492–1499. doi: 10.4049/jimmunol.169.3.1492. [DOI] [PubMed] [Google Scholar]
- 22.Zuany-Amorim C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8(6):625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 23.Le Bert N, Chain BM, Rook G, Noursadeghi M. DC priming by M. vaccae inhibits Th2 responses in contrast to specific TLR2 priming and is associated with selective activation of the CREB pathway. PLoS One. 2011;6(4):e18346. doi: 10.1371/journal.pone.0018346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant RA, Marosszeky JE, Crooks J, Baguley I, Gurka J. Coping style and post-traumatic stress disorder following severe traumatic brain injury. Brain Inj. 2000;14(2):175–180. doi: 10.1080/026990500120826. [DOI] [PubMed] [Google Scholar]
- 25.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: Role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langgartner D, Füchsl AM, Uschold-Schmidt N, Slattery DA, Reber SO. Chronic subordinate colony housing paradigm: A mouse model to characterize the consequences of insufficient glucocorticoid signaling. Front Psychiatry. 2015;6:18. doi: 10.3389/fpsyt.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koolhaas JM, et al. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23(7):925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 28.Reber SO, et al. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: Implications and mechanisms. Endocrinology. 2007;148(2):670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood BN, et al. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57(5):559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood BN, et al. Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loughridge AB, Greenwood BN, Day HE, McQueen MB, Fleshner M. Microarray analyses reveal novel targets of exercise-induced stress resistance in the dorsal raphe nucleus. Front Behav Neurosci. 2013;7:37. doi: 10.3389/fnbeh.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood SK, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78(1):38–48. doi: 10.1016/j.biopsych.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed Z, et al. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55(7):687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 36.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224(3):855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 37.Vinet J, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation. 2012;9:27. doi: 10.1186/1742-2094-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maier SF. Behavioral control blunts reactions to contemporaneous and future adverse events: Medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress. 2015;1:12–22. doi: 10.1016/j.ynstr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. Host adaptive immunity alters gut microbiota. ISME J. 2015;9(3):770–781. doi: 10.1038/ismej.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandal S, et al. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassett SA, et al. Changes in composition of caecal microbiota associated with increased colon inflammation in interleukin-10 gene-deficient mice inoculated with Enterococcus species. Nutrients. 2015;7(3):1798–1816. doi: 10.3390/nu7031798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin P, et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS One. 2014;9(8):e105684. doi: 10.1371/journal.pone.0105684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belzer C, et al. Dynamics of the microbiota in response to host infection. PLoS One. 2014;9(7):e95534. doi: 10.1371/journal.pone.0095534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm Bowel Dis. 2009;15(7):1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 46.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo H, et al. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health Study. Am J Epidemiol. 2016;183(4):249–258. doi: 10.1093/aje/kwv242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin HM, et al. Metabolomic analysis identifies inflammatory and noninflammatory metabolic effects of genetic modification in a mouse model of Crohn’s disease. J Proteome Res. 2010;9(4):1965–1975. doi: 10.1021/pr901130s. [DOI] [PubMed] [Google Scholar]
- 50.Freier E, et al. Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology. 2010;35(5):663–673. doi: 10.1016/j.psyneuen.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Wu W, et al. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. 2014;63(12):1883–1892. doi: 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SJ, et al. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One. 2012;7(7):e42054. doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grosse L, et al. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl) 2016;233(9):1679–1688. doi: 10.1007/s00213-015-3943-9. [DOI] [PubMed] [Google Scholar]
- 54.Morath J, et al. The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: Evidence from a randomized controlled trial. J Psychiatr Res. 2014;54:1–10. doi: 10.1016/j.jpsychires.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 55.Long TT, Nakazawa S, Onizuka S, Huaman MC, Kanbara H. Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. Int J Parasitol. 2003;33(2):175–183. doi: 10.1016/s0020-7519(02)00261-8. [DOI] [PubMed] [Google Scholar]
- 56.Taylor MD, et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174(8):4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 57.Hollis JH, Evans AK, Bruce KP, Lightman SL, Lowry CA. Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain Behav Immun. 2006;20(6):569–577. doi: 10.1016/j.bbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Lowry CA, et al. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146(2):756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12(6):638–646. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID. Low inborn anxiety correlates with high intermale aggression: Link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav. 2007;51(1):11–19. doi: 10.1016/j.yhbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Wood SK, Bhatnagar S. Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiol Stress. 2015;1:164–173. doi: 10.1016/j.ynstr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 2011;39(3):140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markle JG, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 64.Poutahidis T, et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9(1):e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smits HH, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115(6):1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 66.Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. doi: 10.3389/fcimb.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kranz GS, et al. High-dose testosterone treatment increases serotonin transporter binding in transgender people. Biol Psychiatry. 2015;78(8):525–533. doi: 10.1016/j.biopsych.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63(2):241–247. doi: 10.1016/s0169-328x(98)00281-2. [DOI] [PubMed] [Google Scholar]
- 69.Robichaud M, Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol. 2005;17(3):179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 70.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163(2):705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dépis F, Kwon HK, Mathis D, Benoist C. Unstable FoxP3+ T regulatory cells in NZW mice. Proc Natl Acad Sci USA. 2016;113(5):1345–1350. doi: 10.1073/pnas.1524660113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vukmanovic-Stejic M, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116(9):2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kullberg MC, et al. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66(11):5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burich A, et al. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281(3):G764–G778. doi: 10.1152/ajpgi.2001.281.3.G764. [DOI] [PubMed] [Google Scholar]
- 75.Peña JA, et al. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73(2):912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hassan AM, et al. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Front Behav Neurosci. 2014;8:386. doi: 10.3389/fnbeh.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bercik P, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163(6):1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bercik P, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139(6):2102–2112.e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 80.Matthews DM, Jenks SM. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav Processes. 2013;96:27–35. doi: 10.1016/j.beproc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26(11):1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 82.Ohland CL, et al. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38(9):1738–1747. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi GB, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 86.Singewald GM, Nguyen NK, Neumann ID, Singewald N, Reber SO. Effect of chronic psychosocial stress-induced by subordinate colony (CSC) housing on brain neuronal activity patterns in mice. Stress. 2009;12(1):58–69. doi: 10.1080/10253890802042082. [DOI] [PubMed] [Google Scholar]
- 87.Reber SO, Neumann ID. Defensive behavioral strategies and enhanced state anxiety during chronic subordinate colony housing are accompanied by reduced hypothalamic vasopressin, but not oxytocin, expression. Ann N Y Acad Sci. 2008;1148:184–195. doi: 10.1196/annals.1410.003. [DOI] [PubMed] [Google Scholar]
- 88.Uschold-Schmidt N, Nyuyki KD, Füchsl AM, Neumann ID, Reber SO. Chronic psychosocial stress results in sensitization of the HPA axis to acute heterotypic stressors despite a reduction of adrenal in vitro ACTH responsiveness. Psychoneuroendocrinology. 2012;37(10):1676–1687. doi: 10.1016/j.psyneuen.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Donner NC, et al. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety. 2012;29(4):307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic; San Diego: 2005. [Google Scholar]
- 92.Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: What are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–171. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 93.Lechner A, et al. Protective immunity and delayed type hypersensitivity reaction are uncoupled in experimental Leishmania major infection of CCR6-negative mice. Microbes Infect. 2007;9(3):291–299. doi: 10.1016/j.micinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caporaso JG, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Navas-Molina JA, et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 99.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonald D, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6(3):610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caporaso JG, et al. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26(2):266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Price MN, Dehal PS, Arkin AP. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- 104.Verma SP, Díaz-González L, Rosales-Rivera M, Quiroz-Ruiz A. Comparative performance of four single extreme outlier discordancy tests from Monte Carlo simulations. ScientificWorldJournal. 2014;2014:746451. doi: 10.1155/2014/746451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rousselet GA, Pernet CR. Improving standards in brain-behavior correlation analyses. Front Hum Neurosci. 2012;6:119. doi: 10.3389/fnhum.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61(1):1–10. [Google Scholar]
- 107.Lozupone C, Knight R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience. 2013;2(1):16. doi: 10.1186/2047-217X-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamady M, Lozupone C, Knight R. Fast UniFrac: Facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 2010;4(1):17–27. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73(5):1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin-Fernandez JA, Barcelo-Vidal C, Pawlowsky-Glahn V. Dealing with zeros and missing values in compositional data sets using nonparametric imputation. Math Geol. 2003;35(3):253–278. [Google Scholar]
- 112.Pawlowsky-Glahn V, Egozcue JJ, Tolosana-Delgado R. Lecture Notes on Compositional Data Analysis. Universitat de Girona; Girona, Spain: 2007. [Google Scholar]
- 113.Krishnaswamy S, et al. Conditional density-based analysis of T cell signaling in single-cell data. Science. 2014;346(6213):1250689. doi: 10.1126/science.1250689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, Barcelo-Vidal C. Isometric logratio transformations for compositional data analysis. Math Geol. 2003;35(3):279–300. [Google Scholar]
- 115.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Arlington, VA: 2013. 5th Ed. [Google Scholar]
- 116.Korte SM, De Boer SF. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463(1–3):163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 117.Slattery DA, et al. Behavioural consequences of two chronic psychosocial stress paradigms: Anxiety without depression. Psychoneuroendocrinology. 2012;37(5):702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Boscarino JA. Diseases among men 20 years after exposure to severe stress: Implications for clinical research and medical care. Psychosom Med. 1997;59(6):605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 119.Husarewycz MN, El-Gabalawy R, Logsetty S, Sareen J. The association between number and type of traumatic life experiences and physical conditions in a nationally representative sample. Gen Hosp Psychiatry. 2014;36(1):26–32. doi: 10.1016/j.genhosppsych.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Reber SO. Stress and animal models of inflammatory bowel disease—An update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37(1):1–19. doi: 10.1016/j.psyneuen.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 121.Reber SO, et al. Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain Behav Immun. 2011;25(6):1153–1161. doi: 10.1016/j.bbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 122.Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: Research and clinical implications. Psychosom Med. 1999;61(3):378–386. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 123.Reber SO, Obermeier F, Straub RH, Veenema AH, Neumann ID. Aggravation of DSS-induced colitis after chronic subordinate colony (CSC) housing is partially mediated by adrenal mechanisms. Stress. 2008;11(3):225–234. doi: 10.1080/10253890701733351. [DOI] [PubMed] [Google Scholar]
- 124.Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42(3):503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149(6):2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- 126.Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005;162(5):998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 127.Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. Am J Psychiatry. 2004;161(8):1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]
- 128.Füchsl AM, Langgartner D, Reber SO. Mechanisms underlying the increased plasma ACTH levels in chronic psychosocially stressed male mice. PLoS One. 2013;8(12):e84161. doi: 10.1371/journal.pone.0084161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Kloet CS, et al. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40(6):550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Wieck A, Grassi-Oliveira R, Hartmann do Prado C, Teixeira AL, Bauer ME. Neuroimmunoendocrine interactions in post-traumatic stress disorder: Focus on long-term implications of childhood maltreatment. Neuroimmunomodulation. 2014;21(2–3):145–151. doi: 10.1159/000356552. [DOI] [PubMed] [Google Scholar]
- 131.Zhou J, et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 2014;9(4):e94075. doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmidt D, et al. Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain Behav Immun. 2010;24(7):1097–1104. doi: 10.1016/j.bbi.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 133.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1:9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Herman JL. Complex PTSD: A syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377–391. [Google Scholar]