Significance
Staphylococcus aureus is a major cause of life-threatening bacterial infection. A significant risk factor for infection is nasal carriage. Previously, we reported spontaneous mutations during carriage associated with infection, including loss-of-function of the gene repressor of surface proteins (rsp). Here we use genomic screens, experimental assays, and molecular examination of rsp mutants from patients to understand how rsp is involved in infection; we find it has far-reaching effects on gene regulation. Paradoxically, rsp mutants exhibited attenuated toxicity and reduced disease severity early in experimental infection, without sacrificing the ability to cause abscesses and bloodstream infection. This work reveals a complex relationship between correlates of disease in the laboratory and in patients, demonstrating that life-threatening disease can be associated with reduced severity early in infection.
Keywords: Staphylococcus aureus, bloodstream infection, rsp, SSR42, toxicity regulator
Abstract
Staphylococcus aureus is a major bacterial pathogen, which causes severe blood and tissue infections that frequently emerge by autoinfection with asymptomatically carried nose and skin populations. However, recent studies report that bloodstream isolates differ systematically from those found in the nose and skin, exhibiting reduced toxicity toward leukocytes. In two patients, an attenuated toxicity bloodstream infection evolved from an asymptomatically carried high-toxicity nasal strain by loss-of-function mutations in the gene encoding the transcription factor repressor of surface proteins (rsp). Here, we report that rsp knockout mutants lead to global transcriptional and proteomic reprofiling, and they exhibit the greatest signal in a genome-wide screen for genes influencing S. aureus survival in human cells. This effect is likely to be mediated in part via SSR42, a long-noncoding RNA. We show that rsp controls SSR42 expression, is induced by hydrogen peroxide, and is required for normal cytotoxicity and hemolytic activity. Rsp inactivation in laboratory- and bacteremia-derived mutants attenuates toxin production, but up-regulates other immune subversion proteins and reduces lethality during experimental infection. Crucially, inactivation of rsp preserves bacterial dissemination, because it affects neither formation of deep abscesses in mice nor survival in human blood. Thus, we have identified a spontaneously evolving, attenuated-cytotoxicity, nonhemolytic S. aureus phenotype, controlled by a pleiotropic transcriptional regulator/noncoding RNA virulence regulatory system, capable of causing S. aureus bloodstream infections. Such a phenotype could promote deep infection with limited early clinical manifestations, raising concerns that bacterial evolution within the human body may contribute to severe infection.
The bacterium Staphylococcus aureus constitutes a major pathogen causing an array of diseases including deep abscesses, endocarditis, sepsis, and necrotizing pneumonia (1). The toll of severe disease and mortality inflicted by S. aureus, the ongoing rise in multiple antibiotic-resistant strains, and the prolonged hospital stays it causes make it one of the most important human pathogens (2, 3).
Despite much effort, the determinants of S. aureus virulence remain incompletely understood. It is known that S. aureus can secrete a wide range of proteins, including adhesins (4), nucleases (5, 6), complement control proteins (7–9), and multiple toxins, which interfere with host immune function. Toxins elicit cytotoxicity toward a variety of cells ranging from epithelial cells to leukocytes (1, 4, 10), and their secretion is associated with lethality in some disease models (11–14). Additionally, some bacterial lineages, such as USA300, display high levels of toxicity, which may be linked to their evolutionary success (13, 15).
S. aureus asymptomatically colonizes the anterior nares of one-third of the human population, and this bacterial reservoir represents a source for invasive infection (1, 16). However, bacterial isolates from blood differ phenotypically from those from the nares, exhibiting decreased cytotoxicity (17) and reduced hemolysis (18). This finding is surprising because carried isolates represent the source for most human disease, and invasive and carried isolates are closely related genetically (19). One possible explanation for the low-hemolysis phenotype of the bloodstream isolates involves their carrying mutations in transcription factors. For example, a major regulator of S. aureus cytotoxicity and hemolysis, accessory gene regulator (agr), is known to be mutated in a proportion of bacteria recovered from within human host cells (20–22). Such mutants have also been noted among hospital-derived isolates of virulent clones of S. aureus (23). They exhibit prolonged intracellular residence due to attenuated cytotoxicity and consequent delays in initiation of host cell death (24–26).
However, other genetic mechanisms might also control the induction of an attenuated cytotoxic state. One candidate for such a role was suggested by a study of a patient with long-term nasal S. aureus carriage. Within this population, isolates with reduced cytotoxicity evolved through a loss-of-function mutation in the gene repressor of surface proteins (rsp), a gene encoding an AraC-family transcriptional regulator. The occurrence of this mutation accompanied the progression to a fatal bacteremia (27) and caused a reduction in the cytotoxicity of the nasal S. aureus population (17).
Here, we used an unbiased genome-wide screen for staphylococcal genes involved in prolonged intracellular survival. We show that rsp and the long noncoding RNA (ncRNA) SSR42 were by far the most significantly recovered genes from the screen. We demonstrate that rsp controls SSR42 expression, is required for normal cytotoxicity and hemolytic activity, is required for lethality in experimental infection, and is induced by hydrogen peroxide. Crucially, inactivation of rsp preserves bacterial dissemination, because it neither affects formation of deep abscesses in mice nor survival in human blood. Thus, we have identified a pleiotropic transcriptional regulator/ncRNA virulence regulatory system that controls hemolysis and cytotoxicity and a low-cytotoxic phenotype that plays a central role in invasive S. aureus infection. This study provides an important demonstration of how within-host bacterial evolution can radically alter bacterial phenotypes pertinent to disease severity and outcome.
Results
Rsp and the ncRNA SSR42 Are Required for Intracellular Cytotoxicity and Hemolysis of S. aureus.
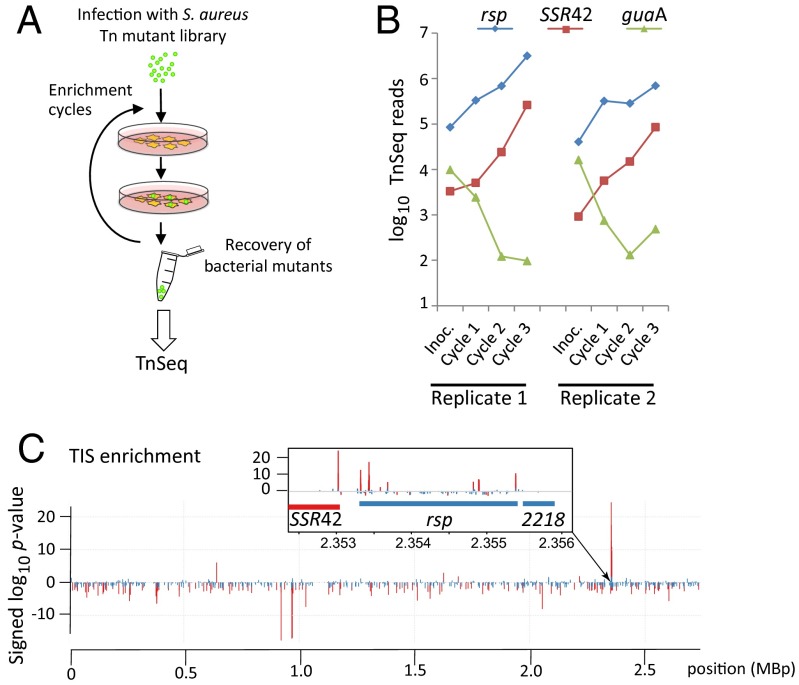
To identify genes mediating prolonged intracellular survival (perhaps due to attenuated cytotoxicity) in S. aureus, we used an unbiased genome-wide approach: We generated a transposon mutant library pool comprising ∼25,000 independent mutants within the highly cytotoxic isolate S. aureus 6850 (28). We then screened it for transposon mutants that were recovered from epithelial (HeLa) cells after internalization as described in Materials and Methods. Changes of frequencies of transposon insertion sites (TIS) in the recovered bacterial pools were compared with those of the inoculum by TIS deep sequencing, hereafter referred to as TnSeq (Fig. 1A and SI Appendix, Fig. S1A).
Fig. 1.
A genome-wide screen for noncytotoxic S. aureus identifies rsp and SSR42. (A) HeLa cells were infected with a mariner transposon mutant library of S. aureus 6850. Viable bacteria were recovered from host cells 8 h after infection and were used to reinfect epithelial cells in three consecutive enrichment cycles. Pools of recovered bacteria and the respective inoculum were analyzed by TnSeq. (B) Sequence reads from transposons within the genes encoding rsp (blue) and SSR42 (red) were strongly enriched in noncytotoxic mutants (P < 0.001). By contrast, transposon insertions in genes such as the drug target guaA (75) (green) were significantly depleted. (C) Genome-wide significance (signed log10 P values) of changes in TIS frequencies demonstrate that the locus encoding rsp and SSR42 is most significantly enriched (Inset). Positive and negative values on y axis, respectively, indicate enrichment and depletion in TIS reads compared with the inoculum. Significant changes (adjusted P < 0.05) are highlighted in red.
We found that mutants in the rsp locus and the ncRNA SSR42 located directly upstream of rsp were significantly enriched in the intracellular fraction (Fig. 1 B and C, Table 1, and SI Appendix, Fig. S1 and Dataset S1) (adjusted P values 3.6 × 10−4 and 2.4 × 10−9). Replication of rsp mutants in vitro and within HeLa cells did not differ significantly compared with wild-type bacteria (SI Appendix, Fig. S1 B and C). We therefore excluded differences in intracellular growth as a reason for the frequent recovery of rsp mutants. We also excluded differential gentamicin susceptibility as an explanation for the enhanced survival of rsp mutants observed in the screen (SI Appendix, Fig. S1D).
Table 1.
TnSeq screening results of S. aureus Himar1 transposon mutant library in epithelial cells
| Mutant* | P value† | Product |
| Inactivated genes enriched in intracellular S. aureus‡ | ||
| ssr42 | <10−6 | Small stable RNA (SSR) 42 |
| rsp | 0.0004 | AraC-type transcriptional regulator |
| geh | 0.037 | Glycerol ester hydrolase |
| ruvA | 0.045 | Holliday junction DNA helicase RuvA |
| hemL | 0.050 | Glutamate-1-semialdehyde aminotransferase |
| Inactivated genes depleted in intracellular S. aureus | ||
| guaA | 0.0003 | Bifunctional GMP synthase |
| 0220 | 0.0004 | Transmembrane efflux pump protein, putative |
| pbuX | 0.0004 | Xanthine permease, putative |
| purM | 0.003 | Phosphoribosylformyl glycinamidine cyclo-ligase PurM |
| 1920 | 0.008 | ATP-dependent RNA helicase, DEAD box family, putative |
| 2160 | 0.008 | Phosphosugar-binding transcriptional regulator, putative |
Gene or locus IDs according to NCBI GenBank accession no. CP006706.1 (i.e., 0181 represent RSAU_000181).
The P values were corrected for multiple testing and genes/loci showing P < 0.05 were reported as significantly increased or decreased. For further details, see SI Appendix, Table S1.
Trend followed by the mutant in the given genes/loci throughout the intracellular passages of screening.
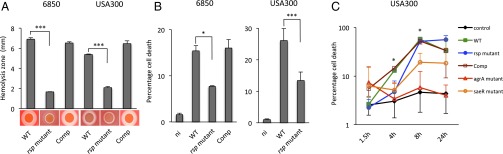
We therefore generated targeted mutants of rsp to study its contribution to virulence. In S. aureus 6850, we deleted the complete ORF, leaving the adjacent ncRNA as well as downstream ORFs intact (SI Appendix, SI Materials and Methods). Furthermore, we transduced the insertional mutation within rsp, NE1304, into a clean genomic background of S. aureus USA300 (SI Appendix, Table S5). Hemolysis and cytotoxicity are hallmarks of S. aureus virulence, and both are regulated by the agr quorum sensing system. However, we found that hemolysis on sheep blood agar plates was also strongly rsp-dependent (Fig. 2A). We also noted that cytotoxicity toward epithelial cells was rsp-dependent (Fig. 2B). We observed enhanced cytotoxicity and hemolysis in rsp complementants relative to wild-type, likely because of enhanced rsp expression in complementants [relative expression level was 11.99 ± 5.16 (mean ± SD), 95% CI 6.57–17.41)] relative to wild-type, as determined by quantitative RT-PCR (qRT-PCR).
Fig. 2.
S. aureus rsp mutants are less hemolytic and show altered kinetics of cytotoxicity. (A) Hemolysis by S. aureus is drastically reduced in an rsp mutant but is readily restored to wild-type (WT) levels by expressing rsp in trans (Comp) in both S. aureus backgrounds, 6850 and USA300. Statistical analysis was performed by one-way ANOVA and Tukey’s post hoc analysis. ***P < 0.001. (B) Host cell cytotoxicity assayed at 4 h after infection is significantly reduced in rsp mutants compared with wild-type (WT) and complemented mutants (Comp) in infected HeLa epithelial cells for both S. aureus strains, 6850 and USA300. ni, uninfected control. Statistical significance was determined by one-way ANOVA. *P < 0.05; ***P < 0.001. (C) Mutation within S. aureus rsp delays pathogen-induced cytotoxicity. HeLa cells were left uninfected (control) or infected with S. aureus wild-type (USA300 WT), an isogenic rsp mutant, and a complemented mutant (USA300 Comp) along with mutants within the global regulators agrA and saeR (SI Appendix, Table S1). Kinetics of cytotoxicity were monitored over time by propidium iodide staining and flow cytometry, here depicted on the y axis using a log scale. Statistical analysis at each time point was performed by one-way ANOVA and Tukey’s post hoc analysis. *P < 0.05 (rsp mutant compared with wild-type).
To analyze the kinetics of cytotoxicity, we infected HeLa with wild-type, isogenic rsp mutants, as well as complemented mutants, and compared with strains deficient in either agr or sae, both global regulators of S. aureus virulence. We determined intracellular cytotoxicity at 1.5, 4, 8, and 24 h after infection. agr and sae mutants were strongly attenuated over the course of infection (Fig. 2C). The rsp mutant was attenuated at 4 and 8 h after infection compared with the wild-type (P = 0.015 and 0.029, respectively), but appeared to display similar cytotoxicity after 24 h (P = 0.192) (Fig. 2C). However, rsp mutation neither influences internalization of S. aureus by HeLa cells nor phagosomal escape (SI Appendix, Fig. S2), both of which have been shown to be associated with cytotoxicity (29–31). Thus, our data suggest that rsp-defective S. aureus remain within the host cell longer and delay pathogen-induced cell death.
rsp Is Required for Lethality in Murine Infection, but Not for Abscess Formation.
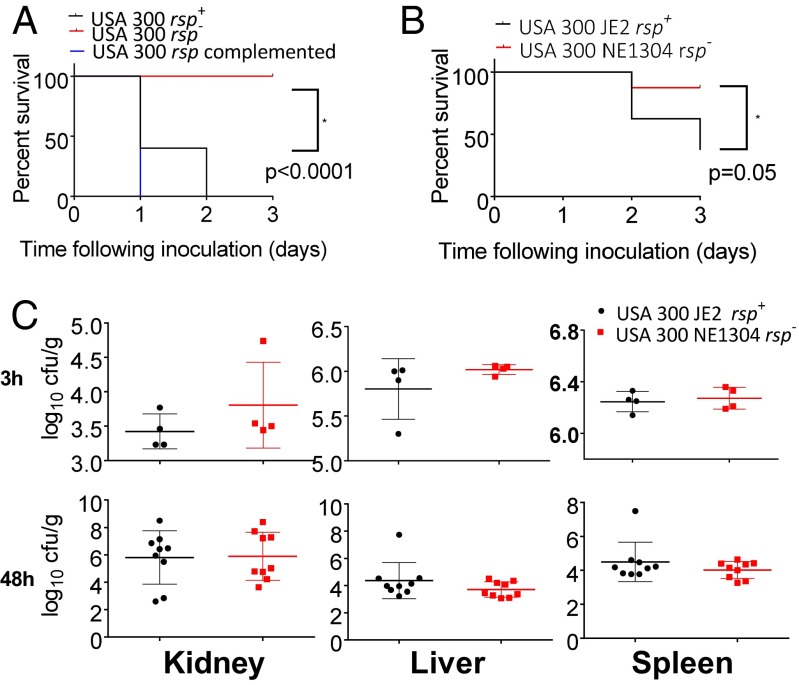
Because in vitro toxicity has been linked to severe outcome in acute mouse infection models (32), we investigated whether rsp altered progression of experimental S. aureus infection. Despite recovering rsp mutants from human bloodstream infection, we observed reduced lethality in a lung-challenge model when comparing survival of mice infected with either rsp mutant, their respective wild-type, or complemented strains. Remarkably, all mice infected with rsp mutants survived for 3 d (Fig. 3A), whereas mortality was 100% at day 2 in the USA300 background and reached 40% in the 6850 background (SI Appendix, Fig. S3A) (P < 0.0001 and P = 0.01 for USA300 and S. aureus 6850, respectively).
Fig. 3.
rsp mutants exhibit reduced lethality in mouse models but are capable of forming deep abscesses. (A) In a murine pneumonia model, infected mice survived when challenged with lethal doses of rsp mutants of strain USA300 LAC*, whereas wild-type and complemented strains were virulent (n = 10). The comparison shown is by log-rank test between wild-type and rsp mutant organisms. (B) In intravenous infections, mice were challenged with S. aureus USA300 JE2 or its rsp mutant. Significantly enhanced lethality was seen in the wild-type relative to the mutant. (C) Bacterial counts in kidney, liver, and spleen were comparable 3 and 48 h after intravenous infection. Shown is the number of colony-forming units (cfu) per gram of tissue.
A model in which abscesses form after intravenous administration of S. aureus (33) supported this observation (Fig. 3B). Starting from day 2 after infection, clinical severity scores increased in the mice infected with wild-type bacteria compared with the group infected with the rsp mutant (SI Appendix, Fig. S3 B and C); severity scores on day 2 differed (P = 0.04) and on day 3 (P = 0.0002). Mice challenged with rsp wild-type bacteria also lost more weight (SI Appendix, Fig. S3) (day 2 difference, P = 0.01) and, as in the pulmonary model, survival was significantly reduced compared with the rsp mutant (P = 0.05) (Fig. 3B). These results show that rsp influences bacterially induced lethality in vivo and that this observed mortality occurred in the first days after experimental infection.
However, 3 h after injection of the USA300 strain, or its rsp insertion mutant, viable bacteria were detectable in multiple tissues, with high concentrations in liver and spleen, but with low renal concentrations of both strains (Fig. 3C). By 48 h, both wild-type and rsp mutant bacteria showed increased bacterial load (Fig. 3C) and had clear histological evidence of abscess formation (SI Appendix, Fig. S4). Compatible with the similar bacterial loads, the numbers of abscesses identified histologically were similar. Their architecture also appeared similar (SI Appendix, Fig. S4). This finding indicates that rsp inactivation does not inhibit bacterial dissemination from the blood, survival, or proliferation in tissues in mice.
rsp Mutants Are Isolated from the Human Bloodstream and Survive in Human Blood.
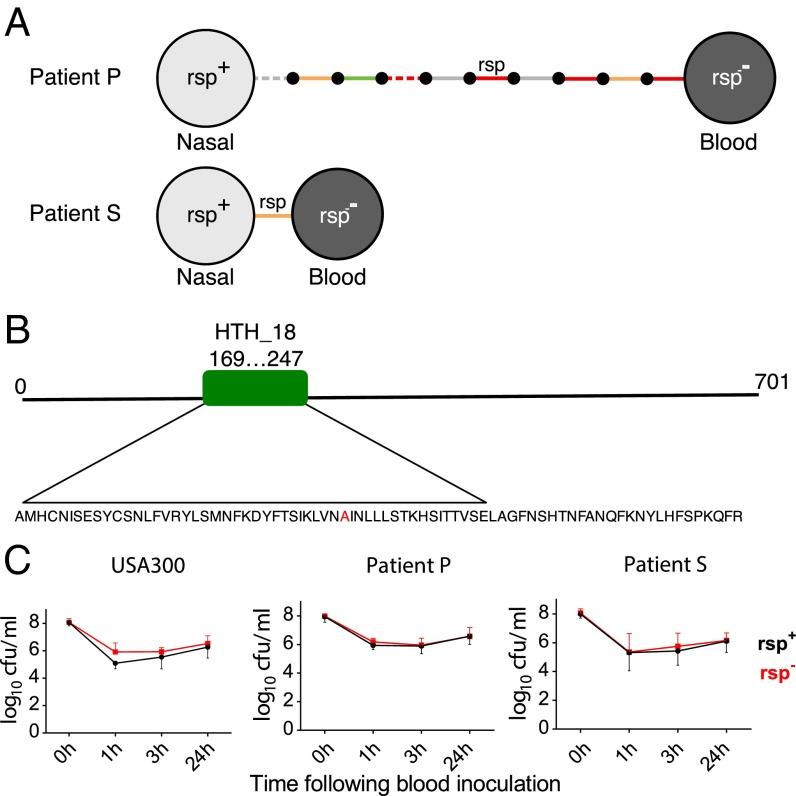
In a previous longitudinal study of asymptomatic S. aureus carriage, one patient, designated patient P, was recruited and was admitted to hospital with a S. aureus bloodstream infection 15 mo after joining the study. Bloodstream isolates recovered from this patient differed by a small number of mutations from the ancestor (Fig. 4A), one of which caused a stop codon in rsp, as described (27). Subsequently, we identified a second patient, patient S, who was treated for a S. aureus bloodstream infection at a hospital in Oxfordshire, United Kingdom, with a nasal swab subsequently taken as part of routine surveillance. The bloodstream isolate differed from the nasal isolate by only one mutation (Fig. 4A), located in the DNA binding domain of rsp (Fig. 4B), which is predicted to abrogate DNA binding (SI Appendix, SI Materials and Methods). The nasal isolate carried the common (wild-type) allele, so we considered it to be the ancestor.
Fig. 4.
Low hemolytic rsp mutants are recovered from patients and occur naturally. (A) S. aureus from bloodstream infections carry mutations in rsp. Two bloodstream isolates were obtained from patients P and S and compared with their respective carried strains. Isolates are represented by light (rsp+) and dark (rsp−) gray circles. Intergenic (gray), synonymous (green), nonsynonymous (orange), and nonsense (red) SNPs and indels are represented by solid and dashed lines, respectively. Small black circles represent hypothetical intermediate genotypes. The ordering of mutations along the branch in patient P is arbitrary. Remarkably, only a single mutation (A204P) separated the bacteremic from a carriage isolate in patient S. (B) Rsp is highly conserved in S. aureus and contains a helix-turn-helix domain (amino acids 169–247). The observed substitution in the patient S rsp− isolate (indicated in red) occurs in the center of this domain, substituting an alanine with a proline and thereby predicted to disrupt the 3D structure of the DNA binding region. (C) Bacterial survival with the same strains used in Fig. 3, as well as with pairs of clinical isolates (A), was assessed after inoculation into human blood from three healthy donors. Bacterial survival was measured at three different time points (1, 3, and 24 h). There was no significant difference in blood survival observed between the rsp mutant (black lines) and wild-type bacteria (red lines). Statistical significance was determined by general linear modeling, modeling counts at each time point as a function of rsp genotype, and genetic background of the organism.
Compatible with murine deep abscess formation after intravenous challenge, we observed that bacterial survival in human blood was similarly rsp-independent. We inoculated wild-type or rsp mutant bacteria into whole blood drawn from healthy human donors and quantified viable bacterial counts over time (Fig. 4C). The studied isolates included the highly cytotoxic S. aureus background of strain JE2 (34, 35), a member of the epidemic, highly pathogenic methicillin-resistant S. aureus (MRSA) USA300 lineage (ST-8), as well as the common ST-15 (patient P) and ST-59 (patient S) lineages (SI Appendix, Table S1). Rsp-associated differences in bacterial survival in human blood were not observed (Fig. 4C). Thus, the enhanced early cytotoxicity observed in rsp wild-type organisms appears dispensable for bloodstream survival and dissemination after intravenous challenge.
rsp Is a Global Regulator of S. aureus Immune Modulators and Toxins.
The spontaneous evolution of rsp loss-of-function mutations found in human bloodstream infections, and the rsp mutants’ capability to survive ex vivo in human blood, demonstrate that they are not avirulent in humans. However, this observation raises questions as to whether, in the absence of rsp, S. aureus might elaborate an alternative set of virulence proteins other than toxins. Rsp is a transcription regulator; hence, we tested this hypothesis by analysis of differential transcription and protein expression between wild-type strains and rsp mutants in three genetic backgrounds of S. aureus isolates.
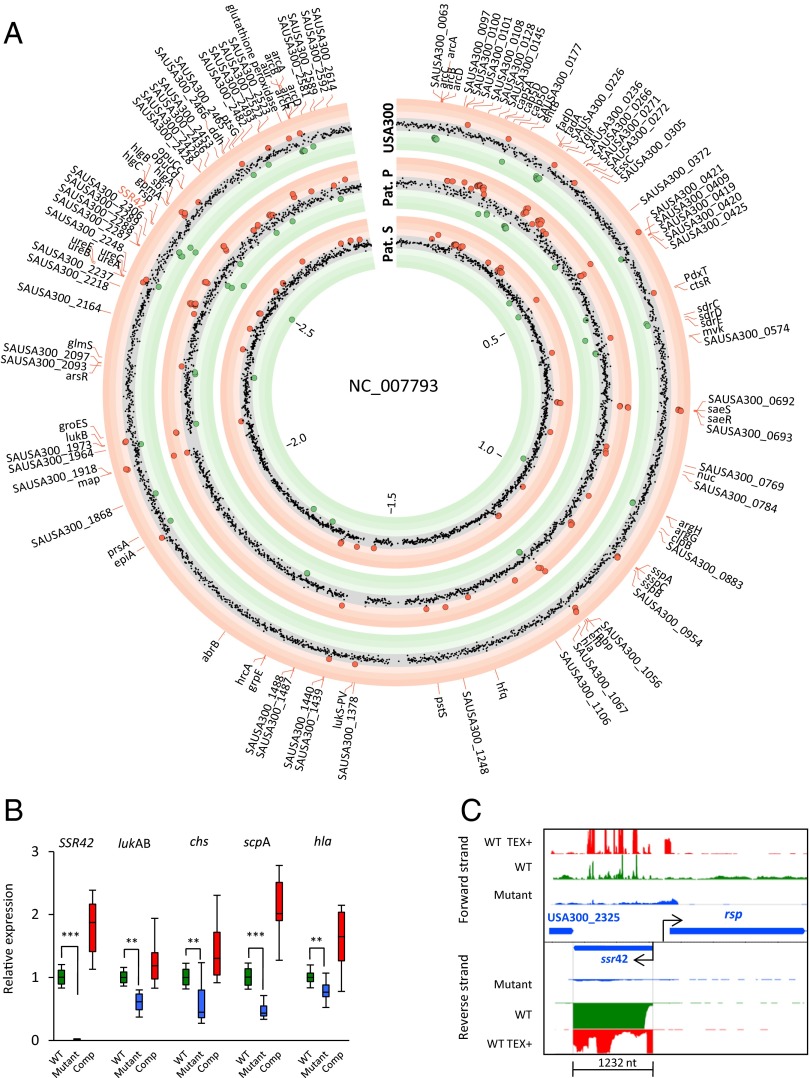
Initially, we studied bacteria from the stationary phase of growth to minimize growth-phase specific differences between strains. In USA300 and patient P and patient S strain backgrounds, we found transcription to differ between loss-of-function mutants (rsp−) and wild-type (rsp+) isolates in ∼30% of the 2,368 genes present in all three strains, using a statistical model designed to detect consistent effects of rsp mutations across strains (SI Appendix, SI Materials and Methods). Transcription was similar across genetic backgrounds (ρ between 0.67 and 0.79), indicating broadly consistent effects of the rsp defects studied (Fig. 5A and SI Appendix, Fig. S5). However, interactions between rsp genotype and genetic background were also evident (Dataset S2).
Fig. 5.
rsp is a global regulator of S. aureus virulence. S. aureus gene expression was measured in stationary growth phase, showing the transcripts of the rsp mutant relative to the wild-type. (A) Effects of rsp mutation on gene transcription were determined by DESeq2 fitting of three separate models, one for each of three S. aureus backgrounds, estimating effects per gene from RNA-seq data. Concentric red rings indicate log2 fold induction, and green rings repression by the mutant relative to the wild-type. Differentially regulated genes are shown as large dots (red, up-regulated in mutant relative to wild-type; green, down-regulated in mutant relative to wild-type) within each strain background (USA300 and patients P and S); see also SI Appendix, Fig. S5. (B) Transcriptomic profiling revealed several rsp-dependently transcribed genes, including the ncRNA SSR42, as well as the virulence factor genes lukAB, chs, scpA, and hla. We verified the relative expression of these factors during exponential growth phase by comparing S. aureus USA300 wild-type (WT; green), its isogenic rsp mutant (blue), and a complemented mutant (Comp; red), by qRT-PCR. Similar results were obtained for stationary phase cultures as well as for S. aureus 6850 (SI Appendix, Fig. S6). Box plots show the median and quantiles, with whiskers indicating the range of the data. Statistical analysis was performed by one-way ANOVA and Tukey’s post hoc analysis. **P < 0.01; ***P < 0.001. (C) The transcriptome landscape at the rsp locus demonstrates rsp-dependent transcription of the ncRNA SSR42. RNA-seq of wild-type S. aureus USA300 (WT; green histograms) and its isogenic rsp mutant (blue histograms) demonstrated a loss of SSR42 transcription in the absence of rsp. TSS of the wild-type S. aureus USA300 were enriched by treatment with Terminator-5′ phosphate-dependent EXonuclease (WT TEX+; red histograms; SI Appendix, SI Materials and Methods), because this enzyme degrades RNA lacking a physiological 5′ terminus. Subsequent RNA-seq revealed that SSR42, which is situated immediately upstream of rsp, is transcribed in antiparallel direction and is 1,232 nt long.
Among the genes up-regulated in rsp mutants (highly regulated genes in Table 2 and all results in SI Appendix, Fig. S2), we found a strong enrichment for involvement in pathogenesis (P = 10−5.0), such as map (21.38-fold), a reported immunomodulatory molecule (36); nuc (20.8-fold), a nuclease capable of lysing neutrophil extracellular traps (6); the Ig-binding protein sbi (37) (21.22-fold); and capsule biosynthesis genes (≥21.0-fold), whose product impedes phagocytosis (38). Genes influenced by rsp also include reported complement inhibitors such as extracellular proteases sspABC (39) (≥ 20.8-fold), the extracellular fibrinogen binding protein efb (40) (22.08-fold), complement regulator bind ing protein sdrE (41)(≥ 20.9-fold), and the protease aureolysin aur (42) (21.2-fold). Genes associated with adhesion to squamous cells and colonization were found to be down-regulated (sdrCD) (43) (2−0.87 and 2−0.23-fold, respectively) (SI Appendix, Fig. S2).
Table 2.
The 20 most up- and down-regulated genes in rsp mutant compared with wild-type
| Gene* | Fold-change | Product |
| Up-regulated in rsp mutant | ||
| 0693 | 6.00 | Putative lipoprotein |
| 0692 | 5.82 | Conserved hypothetical protein |
| 0409 | 5.10 | Conserved hypothetical protein |
| 1056 | 4.53 | Conserved hypothetical protein |
| efb | 4.23 | Fibrinogen-binding protein |
| saeR | 4.06 | DNA-binding response regulator SaeR |
| saeS | 4.00 | Sensor histidine kinase SaeS |
| 1918 | 3.36 | Truncated β-hemolysin |
| hlgA | 3.34 | Gamma-hemolysin component A |
| ureC | 3.23 | Urease, α-subunit |
| 0108 | 3.20 | Antigen, 67 kDa |
| 1052 | 3.182 | Fibrinogen-binding protein |
| ureB | 3.160 | Urease, β-subunit |
| 0274 | 3.117 | Conserved hypothetical protein |
| 0278 | 3.095 | Conserved hypothetical protein |
| 0273 | 3.031 | Putative membrane protein |
| hlgC | 3.010 | Gamma-hemolysin component C |
| 2524 | 3.010 | Conserved hypothetical protein |
| 0272 | 2.868 | Conserved hypothetical protein |
| 0238 | 2.848 | Transcriptional antiterminator, BglG family |
| lukA (G) | 2.828 | Leukocidin LukA/G |
| Down-regulated in rsp mutant | ||
| 2493 | 0.56 | Conserved hypothetical protein |
| 2311 | 0.56 | Conserved hypothetical protein |
| entB | 0.55 | Isochorismatase |
| sdrC | 0.55 | SdrC protein |
| grpE | 0.54 | Cochaperone GrpE |
| hrcA | 0.53 | Heat-inducible transcription repressor |
| 2310 | 0.53 | Conserved hypothetical protein |
| arsR | 0.51 | Arsenical resistance operon repressor |
| 2245 | 0.49 | Staphylococcal accessory regulator R |
| 0372 | 0.48 | Putative lipoprotein |
| 0225 | 0.48 | Putative acyl-CoA acetyltransferase FadA |
| fadD | 0.470 | Acyl-CoA dehydrogenase FadD |
| 0226 | 0.463 | 3-hydroxyacyl-CoA dehydrogenase |
| arcA | 0.460 | Arginine deiminase |
| fadE | 0.454 | Acyl-CoA synthetase FadE |
| 0229 | 0.444 | Putative acyl-CoA transferase FadX |
| 2453 | 0.435 | ABC transporter, ATP-binding protein |
| 2306 | 0.435 | ABC transporter, ATP-binding protein |
| 2307 | 0.297 | ABC transporter, permease protein |
| 0179 | 0.255 | Putative d-isomer specific 2-hydroxyacid dehydrogenase |
| SSR42 | 0.004 | Small stable RNA 42 |
Gene symbol or last four digits of locus tag (i.e., 0238 represents SAUSA300_0238).
Additionally, we studied transcription in the USA300 background using RNA sequencing (RNA-seq) and qPCR in both exponential and stationary growth phases (Fig. 5B, SI Appendix, Fig. S6, and Datasets S2 and S3). Comparing these results, we noted that some important Rsp targets such as α-hemolysin (hla) demonstrated decreased transcription in rsp mutants during exponential growth, but increased transcript levels during stationary phase (Table 3). This finding indicates that some rsp effects may be modified by quorum-sensing mechanisms.
Table 3.
Exemplar genes which show expression discordance between stationary and exponential growth phase
| Gene | Fold-change | Product | |
| Exponential | Stationary | ||
| hla | 0.48 | 2.57 | α-Hemolysin |
| coa | 0.05 | 1.16 | Coagulase |
| chs | 0.06 | 1.37 | Chemotaxis-inhibiting protein CHIPS |
| sbi | 0.38 | 2.33 | IgG-binding protein SBI |
| lukA(G) | 0.26 | 2.828 | Leukocidin A/G |
| lukB(H) | 0.19 | 2.713 | Leukocidin B/H |
Rsp Influences Abundance of Secreted Proteins.
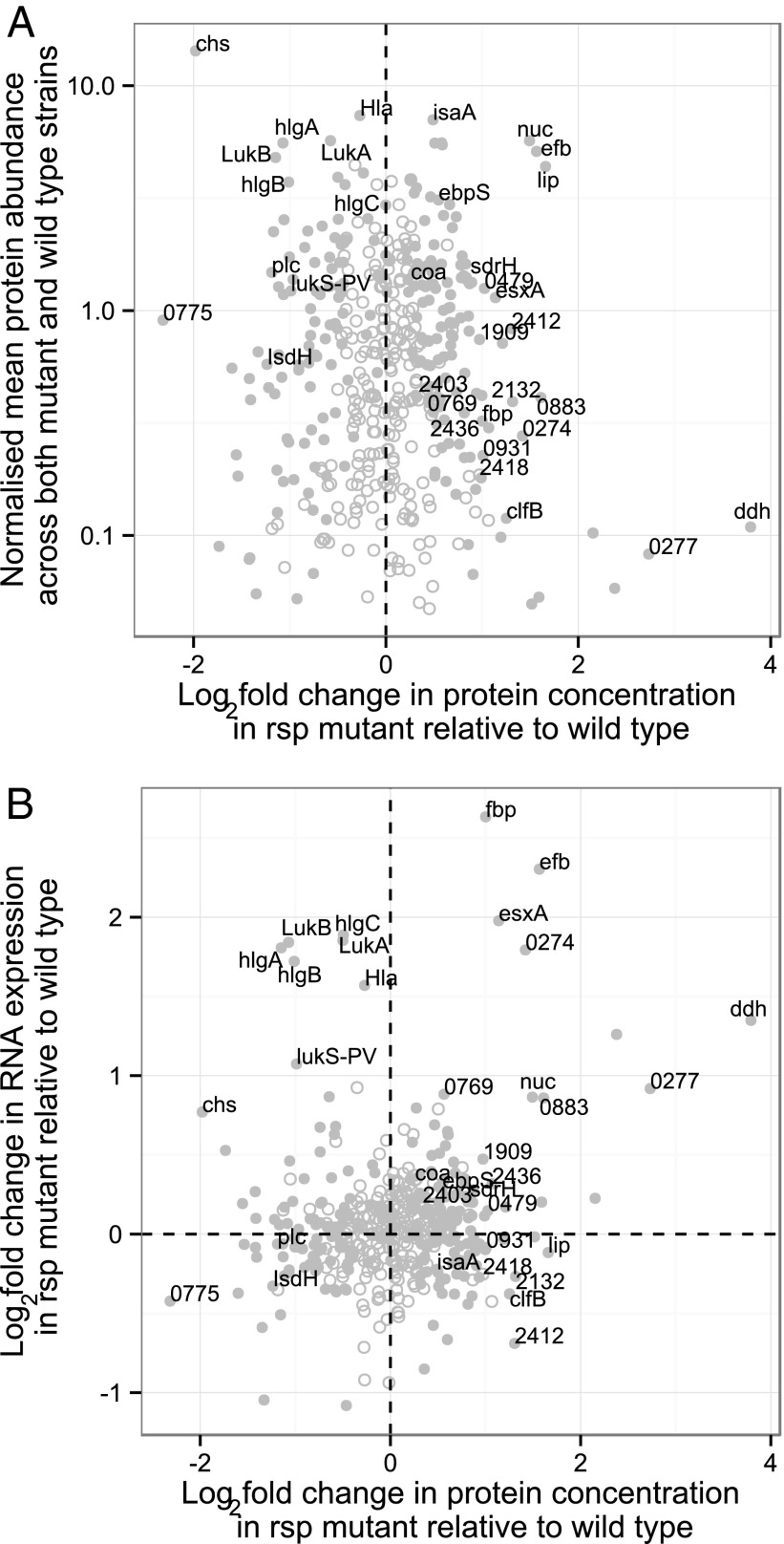
Noting that Rsp affected gene transcription of many secreted proteins (Table 2 and SI Appendix, Fig. S6), we tested whether the effect of Rsp on transcripts was detectable at a protein level in the supernatants of S. aureus strains ST-8 (USA300), ST-15 (patient P), and ST-59 (patient S). We compared protein abundances (Dataset S4) with intracellular RNA levels in stationary phase (Fig. 6). For these analyses, cells were grown in α-MEM, because this medium afforded enhanced sensitivity of detection over growth in tryptone soy broth (SI Appendix, Fig. S7). A high proportion (113 of 636; 18%) of the proteins detected in the supernatant of any of the strains analyzed were affected by rsp mutation. As predicted from functional assays, toxins [α-hemolysin Hla, γ hemolysin components HlgA-C, the Panton-Valentine Leukocidin LukS, and LukAB (also known as LukGH (44)] had decreased abundances in rsp mutant organisms, as did the neutrophil chemotaxis inhibitor CHIPS (chs gene product) (Fig. 6A). However, for a subset of genes, including the toxins hla, lukB, and hlgB, we noted stationary-phase RNA abundances of these genes to be significantly increased in rsp mutant organisms (Fig. 6B). This discordance between transcript and protein levels, which has been previously observed in the context of hla expression and translation, suggests the existence of posttranscriptional control(s) (35) on toxin secretion.
Fig. 6.
Complex rsp-dependent control mechanisms influence toxin production. MS was performed on the supernatant of stationary phase culture of USA300 S. aureus wild-type and an rsp mutant. Fold-change in the protein concentration in rsp mutant relative to wild-type organisms (x axis) was compared with protein abundance in both wild-type and mutant organisms (A), and fold-change of RNA expression in rsp mutant relative to wild-type organisms (B), as derived from RNA-seq performed at the same time point. Each dot represents a gene, and numbers denote locus identifiers (e.g., 0274 refers to SAUSA300_0274). Open circles indicate that the effect of rsp is not significant (P > 0.05) for this gene, whereas filled symbols indicate that the effect of rsp is significant on that gene. The dotted lines indicate the condition where rsp mutation has no effect. In B, the upper right quadrant identifies genes that are increased in RNA as well as protein level. The upper left quadrant shows genes with increased RNA expression, but lower levels of protein in stationary phase. A number of important toxins, including Hla, HlgA, HlgC, and LukB, fall within this category.
Thus, Rsp has pleiotropic effects on the bacterial cell, inducing some virulence factors (such as toxins) and significantly reducing the concentrations of others known to be involved in pathogenesis and complement evasion, including the ESAT-6 homolog EsxA, nuclease (36), the complement control protein Efb (40), and lipase (Fig. 6).
Rsp Controls, and Is Adjacent to, the Highly Transcribed RNA SSR42.
Having demonstrated that Rsp controls an extensive set of genes enriched in virulence factors, we sought to explore mechanisms by which Rsp might exert its effects. We precisely mapped transcription start sites (TSS) in wild-type and rsp mutants in a USA300 background. This finding showed that SSR42 is situated directly upstream of rsp and is transcribed in an antiparallel orientation in a highly rsp-dependent manner (Fig. 5C). SSR42 expression was almost completely lost in the absence of rsp in exponential growth (Fig. 5 B and C). RNA-seq in the stationary growth phase showed that, in wild-type bacteria of USA300, P, and S background, SSR42 comprised 6.4 ± 1.9% (mean ± SD) of RNA mapping to the genome compared with 0.02 ± 0.01% in rsp mutants. We showed SSR42 to be longer than previously noted (45) at 1,232 nt (Fig. 5C), and as such to be the longest nonribosomal ncRNA identified in S. aureus.
rsp and Its Targets Are Induced by Hydrogen Peroxide.
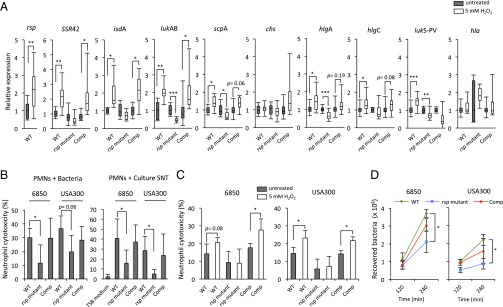
Given the widespread effects of rsp, we decided to investigate mechanisms of induction of the rsp regulon. Using the S. aureus Transcriptome Meta-Database (46), we observed extensive overlap between rsp-regulated genes and genes differentially regulated in response to challenge with hydrogen peroxide or azurophilic granules, both secretions from neutrophils (47). For example, 73 of 113 significantly peroxide-regulated genes were also rsp-regulated. Because neutrophils are one of the first cells recruited to invading bacteria and produce bactericidal reactive oxygen species (ROS) (48), we tested the hypothesis that rsp might be activated when bacteria encountered the antimicrobial ROS, hydrogen peroxide. Supporting this idea, we observed that rsp was rapidly up-regulated in S. aureus after hydrogen peroxide treatment (22.24-fold) (Fig. 7A). A subset of rsp target genes was similarly induced by ROS in a clearly rsp-dependent manner, which included SSR42 (22.14-fold), isdA (21.99-fold), lukAB (22.03-fold), scpA (21.43-fold), hlgA (21.48-fold), hlgC (21.31-fold), and lukS-PVL (21.50-fold). Induction of the general stress protein 20U (dps), which is known to react to oxidative stress (47), is also partially rsp-dependent (SI Appendix, Fig. S8A). By contrast, the genes hla (21.20-fold) and chs (21.06-fold) were not significantly altered in the given time (Fig. 7A). Similar trends were seen in a different strain background (SI Appendix, Fig. S8B). This finding indicates that rsp-dependent regulatory pathways for early gene induction respond to peroxide challenge.
Fig. 7.
rsp effects are induced by hydrogen peroxide and regulate cytotoxicity toward PMNs. (A) Upon 10-min exposure to 5 mM hydrogen peroxide, transcription of rsp and its targets is up-regulated in exponentially growing wild-type S. aureus LAC* (WT). This response to hydrogen peroxide was rsp-dependent and rescued in complemented mutants (Comp). Values indicate expression levels of peroxide-challenged bacteria (open bars) relative to untreated controls (filled bars). Statistical analysis was performed by pairwise t test. *P < 0.05; **P < 0.01; ***P < 0.001. (B) Bacterial infection of PMN with wild-type, rsp mutant, and complementants, as well as treatment with bacterial culture supernatant for 4 h, demonstrated that host cell death levels were significantly decreased and complementable in rsp mutants. Statistical significance was determined by one-way ANOVA and Tukey’s post hoc analysis. *P < 0.05; **P < 0.01; ***P < 0.001. (C) S. aureus culture supernatants collected from wild type, rsp mutant, and complementants after peroxide challenge exhibit increased cytotoxic potential in an Rsp-dependent manner. Human PMNs were intoxicated with the supernatants, and cytotoxicity was determined by LDH release. The vertical axis indicates the percentage of neutrophil cell death compared with complete cell lysis (positive control). Statistical analysis was performed by pairwise t test. *P < 0.05. (D) S. aureus (strains 6850 and USA300) rsp wild-type (WT), rsp mutant, and complementants (Comp) were used to infect neutrophils. Intracellular bacteria were recovered 2 and 4 h after infection, and number of viable bacteria was determined. Statistical analysis at each time point was performed by one-way ANOVA and Tukey’s post hoc analysis. *P < 0.05 (rsp mutant compared with wild type).
Because a fraction of rsp-regulated gene products are related to immune evasion and neutrophil killing (49), we examined the cytotoxic potential of both bacteria and bacterial supernatants toward neutrophils. rsp-dependent neutrophil killing was evident in both investigated strain backgrounds (Fig. 7B). Furthermore, neutrophil cell death increased in an rsp-dependent manner, when intoxicated with bacterial supernatants from cultures that were challenged with hydrogen peroxide (Fig. 7C). Thus, hydrogen peroxide, which is also produced by neutrophils, induces an rsp-dependent response that is directed against leukocytes.
To determine whether the ability of S. aureus to infect and reside within neutrophils was rsp-dependent, we incubated two strains of S. aureus with primary human neutrophils and quantified intracellular S. aureus by plating. After 2 h of incubation, rsp mutants of both genetic backgrounds were present and viable in the neutrophils at similar amounts, indicating similar capability to infect and reside in a viable state. Both strains grew within neutrophils over the next 2 h, but counts were significantly higher for wild-type than for rsp mutants (P = 0.02), compatible with rapid bacterial replication within neutrophils, as well as neutrophil lysis (Fig. 7 B and D), being facilitated by rsp (Fig. 7D).
Thus, rsp-dependent mechanisms detect oxidative stress, increase bacterial replication in neutrophils, shorten intracellular bacterial residence, and produce mediators that kill neutrophils and epithelial cells. They also increase mortality in experimental infection, but are dispensable for bloodstream infection and abscess formation.
Discussion
We have shown that naturally occurring loss-of-function mutants in S. aureus enact global regulatory changes in gene expression that reduce bacterial toxicity and prolong bacterial residence inside mammalian cells, while maintaining the ability to survive, proliferate, and cause disseminated infection within the human body.
Specifically, we have characterized the pleiotropic transcription factor rsp (50), a member of the AraC family of transcriptional regulators (AFTR). We found that Rsp regulates the duration of S. aureus residence inside cells, cytotoxicity toward epithelial cells and neutrophils, and lethality in animal models of acute S. aureus infection (Table 4). Genome-wide screening suggests that loss-of-function mutants in rsp and the ncRNA SSR42 have the strongest influence among S. aureus genes on prolonging intracellular residence of S. aureus. The prolonged intracellular residence in the host cell cytoplasm is associated with a delayed cytotoxicity that differs in kinetics from that seen in mutants of the key virulence regulators, agrA and saeR mutants (Fig. 2B). rsp expression is not required for dissemination of S. aureus from the blood or for deep abscess formation, and we have identified and characterized rsp loss-of-function mutants found in human bacteremia. Thus, our data suggest that S. aureus can adopt an attenuated cytotoxic phenotype, with prolonged intracellular residence (51–53), which permits effective dissemination of the organism with few initial symptoms, followed by deep abscess establishment. The attenuated toxicity phenotype was not noted in isolates from skin and soft tissue infection (17), suggesting that such attenuated toxicity and intracellular survival may be particularly important in bloodstream infection, as opposed to other forms of infection, such as skin and soft tissue infection. Our findings that rsp contributed to lethality in pulmonary disease is also supported by a recent study of cutaneous S. aureus disease (54). In both models, toxins have been shown to be key mediators of disease (13, 55).
Table 4.
Effect of rsp mutation on S. aureus
| Pathway/effect/phenomenon | rsp wild-type | rsp mutants |
| Intracellularity | Rapid host cell lysis after endocytosis | Prolonged intracellular residence after endocytosis |
| Cytotoxicity | Cytotoxicity (epithelial cells, neutrophils) | Reduced cytotoxicity |
| Hemolysis | Normal α-toxin hemolysis | Strongly reduced α-toxin hemolysis |
| SSR42 expression | SSR42 expression (6% of mRNA) | Absent SSR42 expression |
| Virulence | Lethality (murine sepsis and pneumonia) | Reduced lethality |
| Peroxide response | Virulence response to peroxide | Reduced peroxide response |
| Source | Identified in nasal carriage isolates | Identified in bloodstream isolates |
We demonstrated that Rsp and SSR42 represent a regulatory system consisting of a protein and ncRNA in S. aureus. Structurally, it consists of the two adjacent genes, in antiparallel localization and with two distinct TSS (Fig. 5C). SSR42 is an Rsp target, as evidenced by the absence of transcription after rsp inactivation by transposon insertion, ORF deletion, point mutation in DNA binding domains, or translational termination, even though SSR42 comprises ∼5% of nonribosomal RNA in wild-type cells. Some of the Rsp effects are mediated by SSR42, because SSR42-dependent production of the Rsp targets, α-toxin, has been demonstrated (45). Synergy of SSR42-mediated effects with direct effects of Rsp itself, such the recently demonstrated binding of Rsp to the agr promoter, may also occur (54).
We found that rsp transcription was induced by hydrogen peroxide, which is produced by neutrophils in vivo upon stimulation. This finding suggests a model in which rsp fulfills an environment-sensing role: On encountering phagocytes, it initiates a specific response that consists of virulence factors that target phagocyte functions, including α and γ hemolysins, lukAB (lukGH), and the Panton–Valentine leukocidin. This model is compatible with the function of other AFTR members, which, in other bacterial genera, regulate carbon metabolism, stress responses, and virulence in response to changing environmental conditions such as antibiotic use and stress (56, 57). In S. aureus, the AFTRs rbf, rsr, and aryK promote biofilm formation (58), modulate sarR and agr in a skin infection model (59), and potentiate toxin expression and virulence (60), respectively.
Curiously, we have observed that a subset of genes (Fig. 7) attenuated by rsp cannot be readily complemented by supplying rsp in trans. Explanations for this phenomenon could be due to: (i) the highly complex virulence regulatory cross-talk in S. aureus; (ii) the involvement of posttranscriptional mechanisms, as we detected by comparing transcriptomic and proteomic data (Fig. 6); or (iii) the requirement for an SSR42-dependent cis-interaction, and thus will require further study.
In summary, our results provide new evidence that a S. aureus regulatory system involving the rsp transcription factor is subject to spontaneously occurring loss-of-function mutations during evolution within the human body. These knockout mutants display attenuated lethality in the initial stages of experimental infection, but still invade deep tissues, causing severe disease. Although rsp loss-of-function stands alone as an interesting mechanism, it has wider significance as an example of how within-host bacterial evolution affects key regulatory pathways, thus influencing disease progression and clinical outcome.
Materials and Methods
Transposon Mutant Library Generation and TnSeq.
S. aureus strain 6850 was transformed with plasmid pBTn, and mutagenesis was performed as described (61). TnSeq DNA libraries were generated, and Illumina-specific adaptors were ligated to the fragments, and these were enriched for TIS by PCR. After sequencing on the Illumina Hi-Seq 2500 platform, sequences were mapped (62) to the S. aureus 6850 genome (63), and differences in frequencies between the samples were detected with DEseq2 (64). For further details, see SI Appendix, SI Materials and Methods.
Infection Screens with S. aureus Transposon Mutant Libraries.
For in vitro cell death screens, HeLa cell monolayers were infected for 1 h with pooled mutant libraries of S. aureus 6850 at a multiplicity of infection (MOI) of 1. Extracellular bacteria were removed by using 20 μg/mL Lysostaphin (AMBI) and 100 μg/mL gentamicin (GIBCO) for 30 min. The infected cells were further incubated for 8 h in RPMI medium containing 100 µg/mL gentamicin to inhibit extracellular growth of bacteria that were released by host cell disruption. The bacteria were recovered (output) by hypoosmotic rupture of the HeLa cells using sterile water and plated onto tryptone soy agar plates; including the inoculum (input), this process completed one cycle of infection. Thereby, the screening process selected for intracellular noncytotoxic bacteria, because if the bacteria killed the epithelial cells or escaped extracellularly, they were killed by gentamicin and thus were not recovered on the agar plates. A three-cycle infection method was adopted to enrich the subsequent effects. The output from one cycle was used as input for the next cycle. All three outputs and the input were subjected to TnSeq (see above).
Clinical Samples.
S. aureus strains were isolated from two patients with concomitant nasal carriage and bloodstream infection. Patient P was recruited to a previously reported longitudinal study of asymptomatic carriage among adults attending general practices in Oxfordshire, U.K., developing a S. aureus bloodstream infection 15 mo after joining the study (27). Patient S was treated for a S. aureus bloodstream infection at a hospital in Oxfordshire, with a nasal swab subsequently taken as part of routine surveillance. Microbiological processing was performed as described (27). DNA was extracted by using a commercial kit (FastDNA; MP Biomedicals).
Genome Sequencing, Assembly, and Variant Calling.
We used the Illumina HiSeq 2000 platform with 96-fold multiplexing, read lengths of 100 or 150 bp, insert sizes of 200 bp, and mean depth of 125 reads. As described (27), we used Velvet (65) to assemble reads into contigs de novo for each genome. We used Stampy (66) to map the reads of each isolate against MRSA252 (67) and a host-specific draft genome assembled by Velvet. We used xBASE (68) to annotate the draft genome assemblies. SAMtools (69) and Picard (broadinstitute.github.io/picard/) were used to call single-nucleotide polymorphisms (SNPs) from mapping, which we filtered by using published criteria (27). We additionally used Cortex (70) to detect SNPs and indels.
RNA Extraction, RNA-Seq, and Real-Time RT-PCR.
Bacterial mRNA was extracted by using TRIzol (71) or RNeasy (QIAGEN), and reverse transcription was performed according to manufacturer’s guidelines (QIAGEN/Superscript II; Invitrogen). For determination of TSS, processed transcripts were depleted by using Terminator 5′-phosphate-dependent exonuclease (TEX) kit (Epicentre) as described (72). The cDNA was sequenced on the Illumina HiSeq 2000/2500 platforms, and reads were adapter-removed, trimmed, and mapped to the respective bacterial genomes. DESeq2 (64) was used to analyze differential gene expression.
Proteomics.
Proteins were precipitated from bacterial culture supernatant, resolubilized, and digested with trypsin. Desalted peptides were separated on a Dionex Ultimate 3000 UPLC system (Thermo Scientific) and introduced to a TripleTOF 5600 mass spectrometer (AB Sciex) by electrospray ionization. Collision-induced dissociation-fragment data were converted to MASCOT format, and MS/MS spectra were interpreted with PEAKS (73). The reference protein database used for identification is provided in Dataset S5. Statistics was performed with DESeq2 (64).
Infection Experiments.
HeLa cells were seeded in tissue culture microwell plates (Corning) or in µ-Plate ibiTreat (ibidi) and infected with S. aureus at a MOI of 10. The extracellular bacteria were removed by treatment with Lysostaphin and gentamicin for 30 min and further incubated with gentamicin. Cell death was measured by staining with Annexin-V/PI/7-AAD. Human neutrophils were infected with S. aureus (74) at a MOI of 10. Cell death was measured by lactate dehydrogenase (LDH) assay, and bacterial titers were enumerated to see intraphagosomal survival.
Female BALB/c mice 8 wk of age were administered S. aureus either intravenously through the lateral tail vein (sepsis model) or intranasally (pneumonia model), with previously titrated bacterial suspensions. Weight and clinical score were determined. Animals were killed, and bacterial titers were enumerated from organs by plating.
Ethical Framework.
Animal studies were either approved by the local government of Franconia, Germany (approval nos. 2531.01-06/12 and 2532-2-155) and performed in strict accordance with the guidelines for animal care and experimentation of German Animal Protection Law or were approved under the Animal (Scientific Procedures) Act 1986 (Project license 30/2825) and were approved by the University of Oxford Animal Care and Ethical Review Committee. Both sites conformed to Directive 2010/63/EU of the European Union. Work with human neutrophils was approved by the Ethics Commission of the University of Wuerzburg (Code 2015091401). Patient P isolates were obtained during participation in a study of S. aureus carriage in Oxfordshire. This study was approved by Oxfordshire Research Ethics Committee B (approval reference 08/H0605/102 granted September 2, 2008) and obtained individual written consent from all participants. Patient S isolates were collected from routine clinical samples. Ethical approval for sequencing S. aureus isolates from routine clinical samples and linkage to patient data without individual patient consent in Oxford and Brighton in the U.K. was obtained from Berkshire Ethics Committee (10/H0505/83) and the U.K. National Information Governance Board [8-05(e)/2010].
For additional experimental details, please see SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Michael Otto for pBTn and Alexander Keller for help with DNA techniques. Mass spectrometry analysis was performed in the Target Discovery Institute Mass Spectrometry Laboratory led by Benedikt M. Kessler. We also thank the Network on Antimicrobial Resistance in Staphylococcus aureus Program supported by NIAID/NIH Contract HHSN272200700055C for making the JE2 mutant library available. The research leading to these results was supported by European Union Seventh Framework Program Grants 601783 (BELLEROPHON project) (to D.H.W.) and 316655 (VACTRAIN) (to C.L.); and German Science Foundation Transregional Research Collaborative TRR34 (www.dfg.de) Projects C11 (to S.D., A.-C.W., T.R., and M.J.F.) and Z3 (to K.O.), and within Grant FR1504/2-1 (to S.B. and M.J.F.). This work was also supported by the Oxford National Institute for Health Research Biomedical Research Centre (D.H.W. and C.S.R.) and by Wellcome Trust Core Funding Grant 090532/Z/09/Z. D.J.W. is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and Royal Society Grant 101237/Z/13/Z.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE67448 and GSE67424).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520255113/-/DCSupplemental.
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Dantes R, et al. Emerging Infections Program–Active Bacterial Core Surveillance MRSA Surveillance Investigators National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee BY, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clin Microbiol Infect. 2013;19(6):528–536. doi: 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berends ET, et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342(6160):863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6(2):132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JA. Molecular mechanisms of complement evasion: Learning from staphylococci and meningococci. Nat Rev Microbiol. 2010;8(6):393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
- 9.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonzo F, 3rd, Torres VJ. The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol Mol Biol Rev. 2014;78(2):199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillet Y, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 12.Inoshima I, et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17(10):1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: Alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13(12):1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 14.Rose HR, et al. Cytotoxic virulence predicts mortality in nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 2015;211(12):1862–1874. doi: 10.1093/infdis/jiu554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 16.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Study Group Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 17.Laabei M, et al. Evolutionary trade-offs underlie the multi-faceted virulence of Staphylococcus aureus. PLOS Biol. 2015;13(9):e1002229. doi: 10.1371/journal.pbio.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nozohoor S, et al. Virulence factors of Staphylococcus aureus in the pathogenesis of endocarditis. A comparative study of clinical isolates. Zentral Bakteriol. 1998;287(4):433–447. doi: 10.1016/s0934-8840(98)80182-5. [DOI] [PubMed] [Google Scholar]
- 19.Bode LG, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 20.Soong G, et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. MBio. 2015;6(2):e00289-15. doi: 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shopsin B, et al. Prevalence of agr dysfunction among colonizing Staphylococcus aureus strains. J Infect Dis. 2008;198(8):1171–1174. doi: 10.1086/592051. [DOI] [PubMed] [Google Scholar]
- 22.Traber KE, et al. agr function in clinical Staphylococcus aureus isolates. Microbiology. 2008;154(Pt 8):2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLeo FR, et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci USA. 2011;108(44):18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuchscherr L, et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3(3):129–141. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinka J, et al. Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation. Int J Med Microbiol. 2014;304(8):1038–1049. doi: 10.1016/j.ijmm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Tuchscherr L, Löffler B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr Genet. 2016;62(1):15–17. doi: 10.1007/s00294-015-0503-0. [DOI] [PubMed] [Google Scholar]
- 27.Young BC, et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci USA. 2012;109(12):4550–4555. doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vann JM, Proctor RA. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and inoculum-dependent damage to endothelial cell monolayers. Infect Immun. 1987;55(9):2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haslinger-Löffler B, et al. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005;7(8):1087–1097. doi: 10.1111/j.1462-5822.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 30.Menzies BE, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66(12):5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayles KW, et al. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66(1):336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75(2):1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng AG, et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fey PD, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4(1):e00537–e12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery CP, Boyle-Vavra S, Daum RS. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One. 2010;5(12):e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee LYMY, et al. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J Clin Invest. 2002;110(10):1461–1471. doi: 10.1172/JCI16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. The Sbi protein is a multifunctional immune evasion factor of Staphylococcus aureus. Infect Immun. 2011;79(9):3801–3809. doi: 10.1128/IAI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17(1):218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jusko M, et al. Staphylococcal proteases aid in evasion of the human complement system. J Innate Immun. 2014;6(1):31–46. doi: 10.1159/000351458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch TK, et al. Staphylococcus aureus proteins Sbi and Efb recruit human plasmin to degrade complement C3 and C3b. PLoS One. 2012;7(10):e47638. doi: 10.1371/journal.pone.0047638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharp JA, et al. Staphylococcus aureus surface protein SdrE binds complement regulator factor H as an immune evasion tactic. PLoS One. 2012;7(5):e38407. doi: 10.1371/journal.pone.0038407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laarman AJ, et al. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol. 2011;186(11):6445–6453. doi: 10.4049/jimmunol.1002948. [DOI] [PubMed] [Google Scholar]
- 43.Corrigan RM, Miajlovic H, Foster TJ. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009;9:22. doi: 10.1186/1471-2180-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ventura CL, et al. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One. 2010;5(7):e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison JM, et al. Characterization of SSR42, a novel virulence factor regulatory RNA that contributes to the pathogenesis of a Staphylococcus aureus USA300 representative. J Bacteriol. 2012;194(11):2924–2938. doi: 10.1128/JB.06708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagarajan V, Elasri MO. SAMMD: Staphylococcus aureus microarray meta-database. BMC Genomics. 2007;8:351. doi: 10.1186/1471-2164-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palazzolo-Ballance AM, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180(1):500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 48.Kehl-Fie TE, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10(2):158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: A biological tug of war. Annu Rev Microbiol. 2013;67:629–650. doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- 50.Lei MG, Cue D, Roux CM, Dunman PM, Lee CY. Rsp inhibits attachment and biofilm formation by repressing fnbA in Staphylococcus aureus MW2. J Bacteriol. 2011;193(19):5231–5241. doi: 10.1128/JB.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koziel J, et al. Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors. PLoS One. 2009;4(4):e5210. doi: 10.1371/journal.pone.0005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9(3):215–222. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 53.Prajsnar TK, et al. A privileged intraphagocyte niche is responsible for disseminated infection of Staphylococcus aureus in a zebrafish model. Cell Microbiol. 2012;14(10):1600–1619. doi: 10.1111/j.1462-5822.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li T, et al. AraC-type regulator Rsp adapts Staphylococcus aureus gene expression to acute infection. Infect Immun. 2015;84(3):723–734. doi: 10.1128/IAI.01088-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao F, et al. Proteomic identification of saeRS-dependent targets critical for protective humoral immunity against Staphylococcus aureus skin infection. Infect Immun. 2015;83(9):3712–3721. doi: 10.1128/IAI.00667-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Tauschek M, Robins-Browne RM. Control of bacterial virulence by AraC-like regulators that respond to chemical signals. Trends Microbiol. 2011;19(3):128–135. doi: 10.1016/j.tim.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Alekshun MN, Levy SB. Alteration of the repressor activity of MarR, the negative regulator of the Escherichia coli marRAB locus, by multiple chemicals in vitro. J Bacteriol. 1999;181(15):4669–4672. doi: 10.1128/jb.181.15.4669-4672.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cue D, et al. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol. 2009;191(20):6363–6373. doi: 10.1128/JB.00913-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamber S, et al. The staphylococcus-specific gene rsr represses agr and virulence in Staphylococcus aureus. Infect Immun. 2010;78(10):4384–4391. doi: 10.1128/IAI.00401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chua KY, et al. Hyperexpression of α-hemolysin explains enhanced virulence of sequence type 93 community-associated methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2014;14:31. doi: 10.1186/1471-2180-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, et al. Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob Agents Chemother. 2009;53(10):4200–4210. doi: 10.1128/AAC.00428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remmele CW, et al. Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res. 2014;42(16):10579–10595. doi: 10.1093/nar/gku762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraunholz M, et al. Complete genome sequence of Staphylococcus aureus 6850, a highly cytotoxic and clinically virulent methicillin-sensitive strain with distant relatedness to prototype strains. Genome Announc. 2013;1(5):e00775-13. doi: 10.1128/genomeA.00775-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zerbino DR, Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lunter G, Goodson M. Stampy: A statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 2011;21(6):936–939. doi: 10.1101/gr.111120.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holden MT, et al. Complete genomes of two clinical Staphylococcus aureus strains: Evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA. 2004;101(26):9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaudhuri RR, et al. xBASE2: A comprehensive resource for comparative bacterial genomics. Nucleic Acids Res. 2008;36(Database issue):D543–D546. doi: 10.1093/nar/gkm928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, et al. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iqbal Z, Caccamo M, Turner I, Flicek P, McVean G. De novo assembly and genotyping of variants using colored de Bruijn graphs. Nat Genet. 2012;44(2):226–232. doi: 10.1038/ng.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lasa I, et al. Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci USA. 2011;108(50):20172–20177. doi: 10.1073/pnas.1113521108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma CM, et al. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464(7286):250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, et al. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteom. 2012;11(4):M111.010587. doi: 10.1074/mcp.M111.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pang YY, et al. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2(6):546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulhbacher J, et al. Novel riboswitch ligand analogs as selective inhibitors of guanine-related metabolic pathways. PLoS Pathog. 2010;6(4):e1000865. doi: 10.1371/journal.ppat.1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.