Significance
We conducted a genome-wide screen to identify bacterial factors required for Vibrio parahaemolyticus, an important cause of seafood-borne gastroenteritis, to survive in vitro and colonize the mammalian intestine. Our analysis revealed uncharacterized components of a horizontally acquired type III secretion system linked to virulence (T3SS2) and hundreds of genes that likely contribute to colonization independent of T3SS2. Our work revealed that toxR, a conserved gene in vibrios that governs expression of horizontally acquired virulence factors in Vibrio cholerae, was critical for expression of T3SS2. Thus, expression of disparate virulence-linked elements, acquired via lateral gene transfer in independently evolved pathogenic vibrios, is controlled by a common ancestral transcription factor.
Keywords: Vibrio parahaemolyticus, transposon-insertion sequencing, type III secretion, bacterial pathogenesis, pathogen evolution
Abstract
Vibrio parahaemolyticus is the most common cause of seafood-borne gastroenteritis worldwide and a blight on global aquaculture. This organism requires a horizontally acquired type III secretion system (T3SS2) to infect the small intestine, but knowledge of additional factors that underlie V. parahaemolyticus pathogenicity is limited. We used transposon-insertion sequencing to screen for genes that contribute to viability of V. parahaemolyticus in vitro and in the mammalian intestine. Our analysis enumerated and controlled for the host infection bottleneck, enabling robust assessment of genetic contributions to in vivo fitness. We identified genes that contribute to V. parahaemolyticus colonization of the intestine independent of known virulence mechanisms in addition to uncharacterized components of T3SS2. Our study revealed that toxR, an ancestral locus in Vibrio species, is required for V. parahaemolyticus fitness in vivo and for induction of T3SS2 gene expression. The regulatory mechanism by which V. parahaemolyticus ToxR activates expression of T3SS2 resembles Vibrio cholerae ToxR regulation of distinct virulence elements acquired via lateral gene transfer. Thus, disparate horizontally acquired virulence systems have been placed under the control of this ancestral transcription factor across independently evolved human pathogens.
The gram-negative γ-proteobacterium Vibrio parahaemolyticus thrives in either pathogenic or symbiotic association with marine organisms and as a planktonic bacterium (1). This facultative human pathogen, abundant in aquatic environments, was first isolated following a food poisoning outbreak in 1952 and has emerged as the leading cause of seafood-associated gastroenteritis worldwide and a blight on global aquaculture (2, 3). Sequencing of the V. parahaemolyticus genome and the development of animal models of infection have demonstrated a critical role for type III secretion in V. parahaemolyticus virulence (4, 5).
Pathogenic isolates of V. parahaemolyticus encode two type III secretion systems (T3SSs), which are multiprotein structures that mediate the translocation of bacterial effector proteins directly into eukaryotic cells (4, 6). All V. parahaemolyticus strains encode a T3SS on the large chromosome (T3SS1), and the vast majority of clinical isolates, but few environmental isolates possess a horizontally acquired pathogenicity island (VPaI-7) encoding a second T3SS (T3SS2) and one or more pore-forming toxins (TDH) (7). Studies using the infant rabbit model of V. parahaemolyticus infection, which recapitulates manifestations of human gastrointestinal disease (e.g., profuse diarrhea, enteritis, epithelial disruption), revealed that although T3SS1 and TDH are dispensable for intestinal colonization and pathogenesis, colonization and pathology are dependent on T3SS2, consistent with the epidemiological association between T3SS2 and pathogenicity (5). Furthermore, T3SS2 gene expression is induced during intestinal colonization, likely in response to bile, which promotes production of V. parahaemolyticus T3SS2 regulator B (VtrB) (8, 9).
V. parahaemolyticus is presumed to use many bacterial factors, in addition to T3SS2, to survive and proliferate within the human gastrointestinal tract. Putative colonization factors have been reported using a murine orogastric model of V. parahaemolyticus infection; however, the relevance of these findings to human disease is unclear because T3SS2 is not required for colonization of the mouse intestine (10). Genome-wide analysis of V. parahaemolyticus colonization factors has not been performed in any host; however, for other pathogens, passage of transposon-insertion libraries through infection models has enabled highly effective, largely unbiased, and genome-wide identification of factors required for fitness in vivo (11).
We used transposon-insertion sequencing (TIS) to categorize genetic loci based on contributions to V. parahaemolyticus viability in vitro and in vivo (11). We discovered genes and regulatory networks that contribute to intestinal colonization independent of the few known V. parahaemolyticus virulence factors. We identified uncharacterized components of T3SS2 and found that ToxR, an ancestral Vibrio transcription factor previously thought to be dispensable for regulation of T3SS2 gene expression (9), is, in fact, critical for V. parahaemolyticus intestinal colonization and T3SS2 activity. ToxR is also an important regulator of horizontally acquired virulence genes in Vibrio cholerae, where it controls an unrelated set of virulence elements acquired via lateral gene transfer (12). Thus, pathogenic vibrios have independently linked ToxR, which is responsive to signals encountered in the intestine, to control of critical virulence factors.
Results
TIS Identifies Genes Required for Viability in Vitro.
We generated a high-density transposon-insertion library in a spontaneous streptomycin-resistant mutant (SmR) of V. parahaemolyticus RIMD 2210633, a sequenced clinical isolate of the pandemic O3:K6 serotype, using a Mariner-based transposon, which inserts at TA dinucleotides without additional sequence constraints (4, 13). The relative abundance of individual insertion mutants within such a library reflects each mutant’s fitness (11). Sequencing-based characterization of the V. parahaemolyticus library identified at least 179,373 distinct mutants with an average of ∼20 reads per insertion site (Fig. S1). Plotting the frequency of genes relative to the percentage of TA sites disrupted per gene revealed two distinct populations (Fig. 1A). The major peak, representing genes tolerant of transposon insertion, is centered at ∼75% of TA sites disrupted, whereas the minor peak consists of genes with a lower frequency of disruption, many of which are likely required for bacterial viability (14).
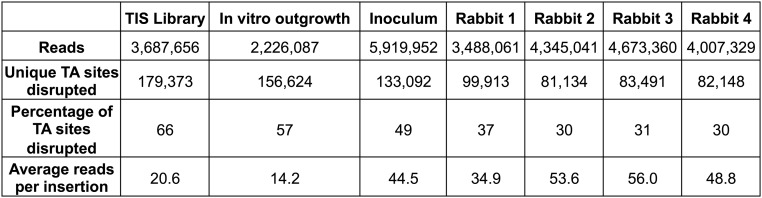
Fig. S1.
TIS statistics. The number of reads mapped to TA sites in the V. parahaemolyticus genome, the number of unique TA sites disrupted, the percentage of TA sites disrupted, and the average number of mapped reads per insertion site are reported for each analysis of the V. parahaemolyticus transposon-insertion library.
Fig. 1.
EL-ARTIST gene classifications. (A) Distribution of percentage disruption for genes with 10+ TA sites. Genes classified as underrepresented (UR), regional (R), and neutral (N) are represented within each bin and in aggregate. (B) Transposon-insertion profiles of representative underrepresented, regional, and neutral genes. The vertical axis indicates the number of reads mapped to a TA site along the horizontal axis.
We used EL-ARTIST, a TIS analysis pipeline, to categorize genes as “underrepresented” or “neutral” (Fig. 1B) based on the insertion profile across each locus relative to genomic context (14). Neutral loci are presumed to be dispensable for V. parahaemolyticus growth in vitro, in contrast to underrepresented loci, which lack corresponding insertion mutants. The analysis also identified “regional” loci (Fig. 1B), which lack transposon insertions across part, but not all, of a locus. All 4,831 annotated protein-coding genes were analyzed (Dataset S1); however, we excluded 372 genes with fewer than 10 TA sites to promote statistical confidence. Most V. parahaemolyticus genes (3,898 genes) were classified as neutral, whereas far fewer genes (565 genes) were classified as regional or underrepresented (Fig. 1A).
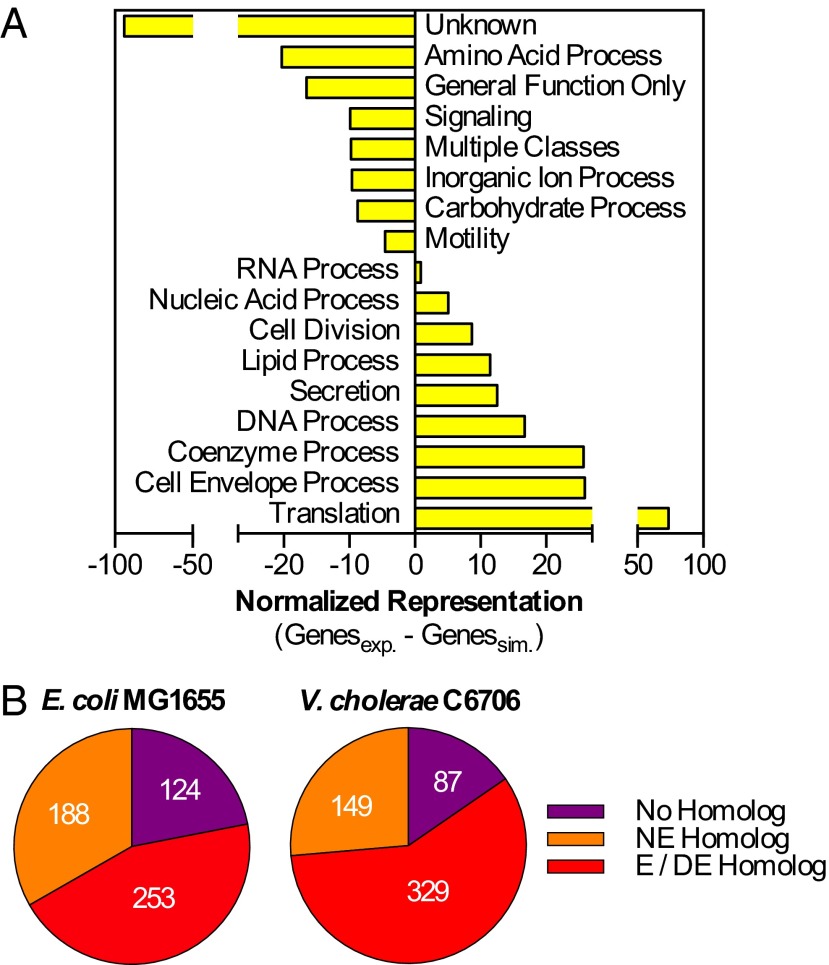
Gene set enrichment analysis using the cluster of orthologous group (COG) database indicated that the 565 nonneutral genes were disproportionately associated with a subset of biological processes (Fig. 2A and Dataset S2). The distribution of COGs among nonneutral genes closely paralleled the distribution of COGs associated with essential and domain-essential genes identified in a TIS assessment of genes required for V. cholerae viability in vitro (14) (Fig. S2 and Dataset S2B). Interspecies similarities in classification were also observed in gene-by-gene comparisons of the 565 V. parahaemolyticus nonneutral genes and homologs in Escherichia coli and V. cholerae, organisms in which the genes required for viability have been extensively characterized (Fig. 2B and Dataset S2C). Many nonneutral V. parahaemolyticus genes had homologs among the 287 protein-coding genes absent from the Keio collection (a proxy for the E. coli essential gene set), and most are reportedly essential or domain-essential in V. cholerae (15). Still, a notable fraction of the 565 genes had homologs that were individually dispensable for E. coli or V. cholerae viability in vitro or lacked homologs in these organisms. A small subset of E. coli essential genes (34 genes) was neutral in V. parahaemolyticus (Dataset S2D) and contained genes similar to those from a comparable analysis of V. cholerae, which may be indicative of Vibrio-specific adaptations in the associated processes (14). The 565 nonneutral genes likely encompassed all or almost all genes required for V. parahaemolyticus growth in vitro. Notably, this set of genes contained loci that have been deleted in previous studies, suggesting that the distribution of insertion mutants within a transposon-insertion library can reflect multiple factors, not solely the viability of each insertion mutant, and that our analysis likely overestimates the number of nonneutral loci in vitro.
Fig. 2.
Bioinformatic analysis of regional and underrepresented genes. (A) Abbreviated COG terms with statistically significant, differential representation in the regional and underrepresented datasets (exp.) relative to random sampling of the V. parahaemolyticus genome (sim.). (B) Status of E. coli and V. cholerae homologs of the V. parahaemolyticus genes classified as regional and underrepresented. E/DE, essential or domain-essential; NE, nonessential.
Fig. S2.
Additional analysis of datasets. COG terms associated with V. parahaemolyticus regional or underrepresented genes (yellow) compared with COG terms associated with V. cholerae domain-essential or essential genes (red) (14). *Adjusted-P < 0.05, a COG term with statistically significant, differential representation in either dataset (exp.) relative to random sampling of the organism’s genome (sim.).
Identification of Genes Required for Intestinal Colonization.
We used the V. parahaemolyticus SmR library to identify genes required for colonization of the mammalian gastrointestinal tract. Infant rabbits were orogastrically inoculated with 109 cfu of the library and euthanized at the onset of disease (5). Transposon-insertion sites were sequenced for in vivo passaged libraries recovered from the distal small intestine (Fig. S1), the primary site of intestinal pathology (5). We compared the passaged library with the inoculum using a modified version of the Con-ARTIST pipeline, which identifies changes in transposon-insertion profiles following growth under a selective condition while compensating for stochastic changes in mutant representation due to experimental bottlenecks (16). Plots of the percentage of TA sites disrupted per gene revealed a leftward shift in a representative passaged library relative to the inoculum (Fig. 3A), indicative of an infection bottleneck. The number of unique mutants recovered from individual animals (80,000–100,000 mutants) reflects the minimum size of this bottleneck (Fig. S1), which is comparable to the size of the bottleneck observed for V. cholerae infection of the infant rabbit intestine (16–19). Consequently, our analyses were restricted to the 3,744 genes (Dataset S3A) found to have sufficient representation within the inoculum to enable discrimination between stochastic loss attributable to the infection bottleneck and loss due to negative selection (Materials and Methods). Due to this requirement, loci previously classified as underrepresented were not assayed in vivo.
Fig. 3.
Identification of conditionally depleted genes. (A) Distribution of percentage disruption for genes subjected to statistical analysis (Materials and Methods) in the bacterial inoculum (Inoculum) and a representative passaged library (Distal Small Intestine). Genes classified subsequently as conditionally depleted (blue) and all other genes queried (orange) are represented within each bin. (B) Transposon-insertion profiles of the neutral locus of vscN1 and of vscN2, a representative conditionally depleted gene. The vertical axis indicates the number of reads mapped to a TA site along the horizontal axis for the simulated inoculum and a representative passaged library (Distal SI).
Two hundred thirty V. parahaemolyticus genes were classified as “conditionally depleted” following colonization of the infant rabbit distal small intestine. Conditionally depleted loci displayed a robust, statistically significant, and reproducible reduction in the relative abundance of corresponding mutants in vivo (Fig. 3), suggesting that they are required for, or make a significant contribution to, intestinal colonization. Interestingly, only 34 of the 230 conditionally depleted genes were found to be up-regulated in previous in vivo transcriptional profiling, and the majority encode T3SS2 genes (8) (Fig. S3A and Dataset S4A). The 230 conditionally depleted genes include structural components of T3SS2, but no corresponding T3SS1 genes, consistent with evidence that T3SS2, but not T3SS1, is required for colonization (5) (Fig. 3B). Of 29 T3SS2 genes sufficiently represented in the inoculum to permit analysis, 22 were conditionally depleted, including 10 structural components; vopV (VPA1357), an effector protein required for colonization; vtrB (VPA1348), a transcriptional regulator; and 10 uncharacterized genes encoded in the T3SS2 gene cluster (6, 20–24) (Fig. 4A and Fig. S3B). The remaining seven genes were primarily T3SS2 effector proteins previously shown to be dispensable for infection.
Fig. S3.
Conditionally depleted genes: expression and T3SS2 components. (A) Overlap between V. parahaemolyticus conditionally depleted genes and transcriptional profiling in vivo (8) for genes assessed in both studies (orange and blue), conditionally depleted genes (blue), and genes not queried in our analysis (black). (B) Conditionally depleted genes encoded in the T3SS2 gene cluster are listed along with functions inferred from previous reports or HHPred predictions.
Fig. 4.
Bioinformatic analysis of conditionally depleted genes. (A) Predicted structural schematic of T3SS2 indicating components that are conditionally depleted (blue), not queried in our analysis (black), or lacking known V. parahaemolyticus homologs (gray with dashed lines). (B) COG terms with statistically significant, differential representation in the conditionally depleted dataset (exp.) relative to random sampling of the 3,744 genes queried (sim.).
Relative to the 3,744 genes analyzed in vivo, conditionally depleted genes were disproportionately associated with a subset of biological processes (Fig. 4B), suggesting functions that may be particularly important for V. parahaemolyticus fitness in vivo. However, depletion of these mutants may also reflect general fitness defects that become evident during expansion of the library in vivo. To explore the latter possibility, we assessed relative fitness during competitive growth of the library in vitro and found that some in vivo conditionally depleted genes exhibited reduced fitness in vitro as well (Dataset S3C), including a subset with homologs that are required for the optimal fitness of V. cholerae in vitro and in vivo (16–18) (Dataset S4B). Our analysis also identified many in vivo conditionally depleted genes that appeared dispensable for optimal fitness in vitro. Because such genes can offer insight into processes required for in vivo survival, we performed further study of a subset of the genes that appear to contribute specifically to fitness in vivo.
Validation of Selected Genes Required for Fitness in Vivo.
We generated deletion mutants for 10 genes, including eight not previously implicated in V. parahaemolyticus infection of the infant rabbit gastrointestinal tract and two T3SS2 structural components [vscN2 (VPA1338) and vopD2 (VPA1361)] required for V. parahaemolyticus infection (5, 22). As a negative control, we deleted the T3SS1 gene vscN1 (VP1668) (5) (Fig. 3B). We barcoded mutants with unique sequence tags stably integrated into a neutral locus, enabling enumeration of individual mutant frequencies within a complex population. A mixture of all mutants was competed against an excess of differentially tagged V. parahaemolyticus SmR in vitro and in vivo (Fig. 5A), and the competitive index of each deletion strain (a measure of relative fitness) was calculated from changes in individual tag frequencies. Most deletion strains exhibited in vitro fitness indistinguishable from the negative control (Fig. 5A), but the competitive indices of purL and cvpA were slightly, although significantly, lower. Importantly, all candidate deletion strains displayed in vivo competitive indices significantly lower than the in vivo competitive index of ∆vscN1, indicating in vivo attenuation relative to the negative control. Because our analysis controlled for the duration of population expansion without regard for potentially different in vitro and in vivo growth rates, these data do not preclude the possibility that the purL and cvpA mutants are attenuated in vivo solely due to the growth defects observed in vitro, provided that there was a substantially greater number of bacterial generations during in vivo growth. Collectively, these data validate the in vivo screen and suggest that our approach yielded a high confidence dataset that constitutes the most thorough dissection to date of the genetic requirements for V. parahaemolyticus intestinal colonization.
Fig. 5.
Targeted validation of conditionally depleted genes. (A) Competitive indices of deletion mutants following coinfection of the distal small intestine (blue circles) or in vitro outgrowth (white squares). Deletion strains display in vivo competitive indices significantly lower (adjusted-P < 0.05) than the negative control (orange circles). Horizontal lines indicate the geometric mean of independent measurements. (B) T3SS2 activity of deletion strains (****P < 0.0001). vscN1 was deleted from all strains to eliminate T3SS1-mediated cytotoxicity.
Given the importance of T3SS2 for V. parahaemolyticus infection of the infant rabbit intestine, we investigated whether the in vivo attenuation of the validated mutants reflected a defect in T3SS2 activity. We measured each mutant’s T3SS2-dependent cytotoxicity against cultured HT-29 colonic epithelial cells, an established readout of T3SS2 activity (25) (Fig. 5B). As expected, deletion of vscN2 or vopD2 abolished T3SS2-mediated cytotoxicity, but seven of the deletion strains displayed cytotoxicity levels equivalent to the ∆vscN1-positive control. Consequently, the in vivo attenuation of these seven mutants appears attributable to T3SS2-independent processes.
The seven genes are associated with processes not previously linked to V. parahaemolyticus infection of the intestinal tract. VP0231, a predicted UDP-galactose phosphate transferase involved in lipopolysaccharide biosynthesis, and purL (VP0666), an enzyme required for de novo synthesis of purine nucleotides, are thought to contribute to cell envelope biogenesis and nucleic acid metabolism, respectively (Dataset S4A); these processes are important for intestinal colonization by other enteric pathogens (16–18). cvpA (VP2186) is required for colicin V production and biofilm formation in E. coli and for V. cholerae survival in vivo, but it is unclear how this gene contributes to V. parahaemolyticus colonization (16–18, 26). The genes tamA (VP0307) and tamB (VP0308) encode components of a translocation and assembly module (Tam) found to mediate the assembly of autotransporters and the virulence of multiple pathogens (27). Finally, disruption of exsD (VP1698), which encodes a negative regulator of T3SS1 gene transcription, results in constitutive T3SS1 expression, suggesting that overexpression of T3SS1 genes in vivo impairs V. parahaemolyticus colonization (28).
In contrast to the seven mutants with unimpaired cytotoxicity, a toxR (VP0820) mutant lacked T3SS2-mediated cytotoxicity in vitro. ToxR is a transmembrane transcription factor present in all V. parahaemolyticus strains and in diverse Vibrio species. V. cholerae ToxR is this pathogen’s master regulator of virulence factor expression; it modulates production of cholera toxin and the toxin coregulated pilus (TCP) that is required for intestinal colonization (12). A cotranscribed factor, ToxS, may augment the activity of V. cholerae ToxR through a direct interaction that protects ToxR from proteolytic degradation (29). In a mouse model of V. parahaemolyticus infection, ToxRS has been proposed to contribute to disease through regulation of the outer membrane protein OmpU; however, our data indicate that OmpU is dispensable for V. parahaemolyticus colonization (10) (Dataset S3A). Disruption of V. parahaemolyticus toxS (VP0819) impaired colonization of the intestine but had no effect on T3SS2 activity in vitro (Fig. 5), a phenotypic divergence that suggests toxR may contribute to in vivo fitness both in conjunction with, and independent of, toxS. Notably, V. parahaemolyticus ToxR has not previously been implicated in T3SS2 gene expression.
ToxR Is Required for Induction of vtrB.
T3SS2 gene transcription depends on VtrA (VPA1332) and VtrB, two ToxR-like transcription factors encoded within the T3SS2 gene cluster in VPaI-7 (24). In vitro, VtrA- and VtrB-dependent T3SS2 expression is induced by crude bile, which promotes VtrA induction of the vtrB promoter through an unknown mechanism (9). VtrB then induces transcription of the full complement of T3SS2 genes (9, 24). We hypothesized that toxR might be required for the expression of these regulatory factors in V. parahaemolyticus. Consistent with this hypothesis, we found that deletion of toxR abolished bile-dependent induction of vtrB transcription (Fig. 6A); however, toxR was not required for production of VtrA (Fig. 6B). Interestingly, overexpression of VtrA restored vtrB induction and T3SS2 protein expression in the toxR mutant (Fig. 6A and Fig. S3B). Thus, our data suggest that toxR augments the activity of VtrA, specifically its induction of vtrB transcription. Finally, we observed that plasmid-based expression of VtrB restored the toxR mutant’s production of VopD2 and T3SS2-mediated cytotoxicity to the level of the positive control (Fig. 7). Collectively, these data are consistent with a model in which ToxR augments the ability of VtrA to induce vtrB transcription.
Fig. 6.
toxR is required for induction of vtrB transcription. (A) Relative abundance of mRNA transcripts in the presence or absence of crude bile. ND, none detected. (B) VtrA-His6 expression in the presence or absence of crude bile via immunoblotting (∼29 kDa). Due to elevated VtrA-His6 levels, less lysate was run for samples containing pvtrA-his6. IPTG, isopropyl β-d-1-thiogalactopyranoside; α-RNAP, α-RNA polymerase; wt, wild type.
Fig. 7.
Expression of vtrB bypasses the requirement for toxR. (A) Immunoblotting of the T3SS2 component VopD2 in ∆toxR strains grown in crude bile and exogenously expressing vtrB (pvtrB) or an unrelated gene (pcyA). (B) T3SS2 activity in ∆toxR strains grown in crude bile and exogenously expressing pvtrB or pcyA (****P < 0.0001). vscN1 was deleted from all strains to eliminate T3SS1-mediated cytotoxicity. ND, none detected.
Discussion
We harnessed the power of TIS to identify genes and regulatory networks that enable V. parahaemolyticus survival in vitro and in the intestine. We defined 565 genes likely required for the viability of this pandemic cause of seafood-borne enteritis, including loci homologous to essential genes in related bacteria and potential V. parahaemolyticus-specific essential genes. The latter could enable the design of targeted antimicrobials, reducing the use of broad-spectrum antibiotics in commercial aquaculture, which is currently plagued by this pathogen. The 230 stringently defined conditionally depleted genes, which are required for V. parahaemolyticus fitness in vivo, encompassed components of the pathogen’s principal virulence factor (T3SS2) and numerous mediators of biological processes not previously linked to V. parahaemolyticus colonization in any model of infection. We also discovered that ToxR, an ancestral transmembrane transcription factor necessary for the expression of horizontally acquired virulence genes in V. cholerae, is critical for V. parahaemolyticus infection. Unexpectedly, ToxR proved necessary for induction of T3SS2 gene expression in V. parahaemolyticus; thus, as in V. cholerae, it governs expression of the pathogen’s key virulence factor.
All 10 of the previously annotated T3SS2 structural components assayed in our study were conditionally depleted (Fig. S3A). Consistent with a previous report, a T3SS2 effector-encoding gene (vopV) was required for intestinal colonization (23). Additionally, 10 conditionally depleted genes encoded within the T3SS2 gene cluster lacked prior annotation; these genes may encode components of the secretion apparatus, given that many components of the general T3SS machinery have not yet been defined for T3SS2 (6). BLAST analysis did not identify sequence homology between any of the 10 genes and known T3SS components; however, structure-based prediction (HHpred) revealed that VPA1351 bears structural homology to the inner membrane ring of the Salmonella enterica (serovar Typhimurium) SPI-1 T3SS, whereas VPA1352 and VPA1365 likely encode T3SS chaperones (6, 30) (Fig. S3A). The uncharacterized gene VPA1350 may correspond to a needle-associated component of T3SS2 (31). Notably, the other six genes are conserved in the T3SS found in V. cholerae AM-19226, and may correspond to unidentified components of the T3SS2 structural apparatus in this organism as well; however, we cannot rule out the possibility that these genes encode T3SS2 effector proteins or factors that contribute to in vivo fitness through T3SS2-independent processes (32).
Our genome-wide approach identified numerous V. parahaemolyticus genes outside of VPaI-7 that contribute to intraintestinal survival independent of T3SS2. Given their putative function in autotransporter assembly, the requirement for tamA and tamB in V. parahaemolyticus intestinal colonization is somewhat surprising, in that no autotransporter proteins have been reported in a Vibrio species. V. parahaemolyticus may produce noncanonical autotransporters that are individually or collectively required for colonization. Alternatively, the function of Tam in vibrios may not be restricted to insertion of autotransporters into the outer membrane, as previously postulated (33). Tam proteins contribute to the pathogenesis of several gram-negative bacteria, and TamB is required for colonization of the squid light organ by the commensal Vibrio fischeri, suggesting that these membrane proteins play a potentially broad role in diverse host–microbe interactions (33, 34). Interestingly, only tamB was required for V. cholerae colonization of the infant rabbit gastrointestinal tract (Dataset S4B), highlighting the variation in processes underlying disease linked to enteropathogenic vibrios.
ToxR is another conditionally depleted locus encoded outside of VPaI-7 that contributes to intestinal colonization. Our data, which we are unable to reconcile with a contradictory report from Gotoh et al. (9), indicate that ToxR is required for induction of the key T3SS2 regulator, vtrB. Consequently, T3SS2 expression can be restored in a toxR mutant via either complementation with ToxR (Fig. S4) or exogenous expression of VtrB. Importantly, toxR-dependent T3SS2 expression is not an artifact of ∆vscN1 deletion or the streptomycin-resistant parent strain because an identical toxR mutation constructed in the wild-type background yields the same phenotype (Fig. S4). The toxR mutant’s inability to activate bile-dependent T3SS2 gene expression could by itself account for the mutant’s in vivo attenuation. However, ToxR may also contribute to virulence via regulation of additional pathways, potentially in conjunction with the cotranscribed conditionally depleted gene toxS. This regulatory paradigm is similar to ToxR-dependent virulence gene regulation in V. cholerae, although a distinct set of virulence elements is involved. In V. cholerae, ToxR forms a disulfide-linked complex with a related protein, TcpP, that governs transcription of the virulence regulator toxT. ToxR’s regulatory role can be bypassed by overexpression of either TcpP or ToxT (35). The V. parahaemolyticus regulator VtrA lacks periplasmic cysteine residues, precluding disulfide bond formation like that required for TcpP–ToxR interaction in V. cholerae; however, as with TcpP, exogenous expression of VtrA overcomes the requirement for toxR, restoring vtrB transcription and T3SS2 protein expression. Additional studies are needed to identify the precise means by which ToxR enables virulence gene expression. Collectively, our data suggest that V. parahaemolyticus ToxR augments VtrA induction of the vtrB promoter, a posttranslational regulatory mechanism that bears striking similarity to that observed in V. cholerae regulation of distinct virulence elements.
Fig. S4.
toxR is required for T3SS2 expression in wild-type and SmR V. parahaemolyticus. T3SS2 expression was assessed for strains grown in the presence or absence of crude bile via immunoblotting against VopD2 (a component of T3SS2). (A) Wild-type (Wt) V. parahaemolyticus, the SmR parent strain, isogenic toxR mutants constructed in either background, and mutant strains exogenously expressing toxR (ptoxR) all grown in 1 μM isopropyl β-d-1-thiogalactopyranoside (IPTG). (B) ∆toxR strains exogenously expressing toxR (ptoxR), vtrA (pvtrA-his6), or vtrB (pvtrB). α-RNAP, α-RNA polymerase.
A critical step in the evolution of pathogenic V. parahaemolyticus and V. cholerae involved acquisition of foreign DNA by nonpathogenic precursor strains. For V. parahaemolyticus, acquisition of T3SS2 appears to have enabled colonization of the human intestine. For V. cholerae, sequential acquisition of the TCP biogenesis genes and the cholera toxin genes ctxAB allowed this organism to thrive in the small intestine and promote its own dissemination (12). Strikingly, V. cholerae and V. parahaemolyticus appear to have independently linked control of disparate horizontally transmitted elements critical for infection to the same ancestral transcription factor, ToxR. This strategy may have facilitated the evolution of these two highly successful pathogens because ToxR is responsive to signals encountered in the host small intestine (36). Furthermore, the common route of acquisition for these distinct virulence elements, lateral gene transfer, suggests a broader role for ToxR in the regulation of foreign DNA.
Materials and Methods
Transposon (16) and animal infection studies (5) were performed as previously described with modifications outlined in SI Materials and Methods. Detailed descriptions of all strains (Dataset S5), reagents, and methodologies used in this study are provided in SI Materials and Methods.
The animal protocols for these studies were reviewed and approved by the Harvard Medical Area Standing Committee on Animals (Institutional Animal Care and Use Committee protocol 04308, Animal Welfare Assurance of Compliance A3431-01). All animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH (37) and the Animal Welfare Act of the United States Department of Agriculture.
SI Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
All strains and plasmids used in this study are listed in Dataset S5. A spontaneous streptomycin-resistant mutant of V. parahaemolyticus RIMD 2210633, V. parahaemolyticus SmR, was used for all experiments, and mutations were constructed in the SmR background except when specified otherwise. Strains were cultured at 37 °C in LB or on LB-agar plates unless otherwise specified. Antibiotics and additional chemicals were used at the following concentrations: 0.04% crude bile; 50 μg/mL carbenicillin (Cb); 5 μg/mL and 20 μg/mL chloramphenicol (Cm) for V. parahaemolyticus and E. coli, respectively; 5 μg/mL gentamicin (Gm); 1 μg/mL isopropyl β-d-1-thiogalactopyranoside; 50 μg/mL kanamycin (Km); and 200 μg/mL streptomycin (Sm). Isogenic mutants of V. parahaemolyticus were constructed, as previously described, via standard allelic exchange using derivatives of the pDM4 suicide vector carrying DNA sequences flanking the gene(s) targeted for deletion, with either Sm or Cb selection for V. parahaemolyticus (31). Plasmids were propagated in E. coli DH5-α, and E. coli SM10λPir was used to deliver allelic exchange vectors and the pYB742 transposon vector via conjugation (13). The vtrA, cloned into the SmaI site (this study), and vtrB were expressed from the pTAC promoter of pMMB207 (31).
For introduction of barcodes into V. parahaemolyticus, a 1,168-bp fragment of VPA0179 that included 76 bp of the intergenic region between VPA0179 and VPA0180 was amplified and cloned into SacI and XbaI digested pGP704. The resulting plasmid (pSoA178) was amplified with a mixture of primers containing ∼30 bp of random sequence. The resulting fragment was cloned into a SacI and XbaI digested pGP704 derivative, where the ampicillin resistance gene (bla) was exchanged with a Gm resistance gene (aacC1). The resulting plasmid mix pSoA179 was then transformed into E. coli SM10λPir. Individual colonies carrying unique tag sequences were isolated and used as donors to deliver pSoA179 barcoded derivatives into V. parahaemolyticus SmR and the 11 deletion strains. Thirteen barcodes were independently integrated into SmR, and three barcodes were independently integrated into each isogenic mutant via homologous recombination in the intergenic locus between VPA0179 and VPA0180, which was tolerant of transposon insertion in vitro and in vivo.
For creation of a chromosomal epitope-tagged vtrA, a nucleotide sequence encoding a His6 epitope tag was introduced before the stop codon of vtrA. Because insertion of the tag disrupted the annotated ORF of VPA1333, the vector used to generate the chromosomal vtrA-His6 strain reconstituted the VPA1333 ORF immediately downstream of the native vtrA stop codon.
Transposon-Insertion Library Construction.
The transposon-insertion library was created using E. coli SM10λPir + pYB742 as a donor to conjugate the pYB742 (13) transposon vector into V. parahaemolyticus SmR (recipient). One-milliliter overnight cultures of donor and recipient strains were pelleted, washed, and combined in 100 μL of LB. Conjugation mixtures were spotted onto 0.45-μm HA filters, placed on LB-agar plates, and incubated at 37 °C overnight. The conjugation reactions were each resuspended in 2 mL of LB and spread on two 245 × 245 mm2 plates of LB-agar [3% (wt/vol) sodium chloride] with Cb + Km + Sm and grown at 37 °C overnight. Approximately 7 million colonies were scraped from plates of the independent conjugation mixtures, pooled, and transferred into LB with 80% (vol/vol) glycerol for storage at −80 °C.
In Vitro and in Vivo Transposon Studies.
For the outgrowth experiment, an aliquot of the frozen V. parahaemolyticus transposon-insertion library was thawed in LB for 3 h at 37 °C, and ∼109 cells were then expanded in vitro in LB at 37 °C through serial passaging at 3-h intervals for a total of 6 h. After 6 h, ∼1010 cells were spread on LB-agar [3% (wt/vol) sodium chloride] with Cb + Km + Sm and grown at 37 °C overnight to recover viable transposon-insertion mutants.
Mixed-gender litters of 2-d-old New Zealand White infant rabbits, cohoused with a lactating mother (Pine Acre Rabbitry) were treated with cimetidine (50 mg/kg, s.c. injection; Hospira) 3 h before inoculation. Bacterial samples were thawed in LB for 3 h at 37 °C, collected by centrifugation, resuspended in sodium bicarbonate [2.5% (wt/vol) in water, pH 9], and adjusted to 2 × 109 cfu/mL by OD600 measurement. Rabbits were inoculated orogastrically with 500 μL of the V. parahaemolyticus transposon-insertion library using a size 4 French catheter. Portions of the inoculum were diluted serially to determine the precise dose, and ∼1010 cfu was spread on LB plates [3% (wt/vol) sodium chloride] with Cb + Km + Sm to generate a reference input library for subsequent statistical analyses. Infant rabbits were monitored periodically for manifestations of disease and euthanized at the onset of visible diarrhea (∼24 h postinfection). The entire intestinal tract was removed from infected rabbits, and 3-cm sections of the proximal small intestine, distal small intestine, cecum, and midcolon were collected. Intestinal sections were homogenized in 1 mL of sterile PBS using a minibeadbeater-16 (BioSpec Products, Inc.). Homogenates were plated directly on LB [3% (wt/vol) sodium chloride] with Cb + Km + Sm to recover viable transposon-insertion mutants and serially diluted to enumerate bacterial burden.
Characterization of Transposon-Insertion Libraries.
Characterization of the transposon-insertion library was performed as previously described (14). The genomic DNA flanking sites of transposon insertion were isolated and sequenced, and reads >15 nt were mapped to the V. parahaemolyticus RIMD 2210633 genome [Reference Sequence (RefSeq) database: chromosome 1, NC_004603.1; chromosome 2, NC_004605.1], allowing for no mismatches. Sequence reads that failed to align at a TA site in the V. parahaemolyticus genome were discarded, and reads mapping to multiple TA sites were randomly distributed between the multiple sites. The number of reads at each TA site was tallied, datasets were normalized for origin proximity, and transposon-insertion profiles were depicted using Artemis. The two V. parahaemolyticus chromosomes were analyzed independently using EL-ARTIST (16). Chromosome 1 classifications were obtained using hidden Markov model analysis following sliding window (SW) training (10 TA sites; P < 0.005). Chromosome 2 classifications were obtained using SW analysis (10 TA sites; P < 0.005). For both chromosomes, genetic loci with fewer than 10 TA sites were retroactively excluded from classification. The terms neutral, underrepresented, and regional are used in place of the standard EL-ARTIST classifications: “nonessential,” “essential,” and “domain-essential.”
Con-ARTIST analysis was used to compare the library inoculum with libraries recovered from the distal small intestines of four infected animals (16). After simulation of the infection bottleneck, we modified the Con-ARTIST Mann–Whitney U (MWU) test to exclude from consideration any TA sites that (i) lie outside the central 80% of an annotated ORF or (ii) lack at least one mapped read in the simulated control dataset. To meet standards of statistical significance, we only considered loci with at least five TA sites and required a statistically significant (average MWU: P < 0.05) reduction in relative abundance of fourfold or greater (average fold change < 0.25). To meet standards of reproducibility, we required that a locus exceed these standards of statistical significance in at least three of the four independent analyses. Loci that met the aforementioned standards of statistical significance and reproducibility were classified as “conditionally depleted.” Loci that contained at least five independent insertion mutants across all four samples but did not meet the standards of statistical significance and reproducibility were classified as “tested.” Any loci for which the standards of statistical significance or reproducibility were technically unattainable (i.e., fewer than five TA sites disrupted in one or more of the simulated control datasets) are reported as “no data.”
In Vitro and in Vivo Competition Experiments.
Overnight cultures of barcoded strains were pooled so that the total number of barcoded SmR cells was approximately equal to the number of barcoded mutant cells. Bacterial samples were collected by centrifugation, resuspended in sodium bicarbonate [2.5% (wt/vol) in water, pH 9], and adjusted to 2 × 109 cfu/mL by OD600 measurement. A portion of the pool was spread on LB-agar with Sm + Gm and grown overnight, serving as an input control and allowing for the enumeration of individual tag frequencies in the inoculum. Approximately 109 cfu of the pool was transferred to 25 mL of LB and cultured at 37 °C for the duration of the in vivo competition experiment (∼24 h), and this in vitro outgrowth was performed in triplicate. Another portion of the pool was used to inoculate infant rabbits that were monitored, euthanized, and processed as described above. The bacterial burden was determined in both intestinal homogenates as well as in vitro cultures by colony-forming unit plating of serial dilutions. The sequence tags in the inoculum and in each sample were amplified, sequenced, and analyzed as described previously, with a similarity threshold of 0.9 (19).
To determine the relative fitness of each mutant, we compared two strains (the wild type and a mutant) in a population. Each strain consists of several subpopulations with distinct tags: wild type has 13 different tags (x), and each mutant has three different tags (y) with frequencies fwt,x and fmut,y, respectively. When both expand exponentially from a time point t0 to a sampling time point ts, their relative fitness in terms of offspring per generation is proportional to with CI as the competitive index. In our case, fwt,x,i,0 and fmut,y,j,0 are the frequencies of the tag in the inoculum. Each inoculum was measured in triplicate, and tag frequencies in each replicate are referred to as i (wild type) and j (mutant). The terms fwt,x,s and fmut,y,s describe the frequencies at the sampling time point in the animal host. Because the inoculum was measured in triplicate, each mutant strain was tagged with three individual tags, and the SmR was tagged with 13, we have a total of measurements for the CI. The 95% confidence interval of the CI in single animal hosts (intrahost variance) was determined by bootstrapping from these 351 measurements. For each strain and each tag, we sampled bacteria from three in vitro competitions and the distal small intestine of six rabbits. For a combined estimate of CIs in the sample population, a random-effects meta-analysis was performed using the metafor package in the statistical software package R (version 3.0.2). The pooled rate proportions and 95% confidence intervals were calculated using the estimates, and the variance of CIs in each animal was determined by bootstrapping and corrected for multiple testing using the Benjamini–Hochberg procedure.
Bioinformatics Analysis.
Homology was determined using BLAST against V. cholerae N16961 and E. coli MG1655 (E value < 1−10). Genes required for V. cholerae infection and fitness were defined as those genes reported in at least one of three transposon-based studies of V. cholerae infection (16–18). Gene set enrichment analysis was performed using bootstrapping and a 95% confidence interval corrected for multiple testing using the Benjamini–Hochberg procedure. Predicted amino acid sequences of conditionally depleted genes encoded within VPaI-7 were uploaded to HHPred for sequence- and structure-based homology searches (30).
T3SS2 Cytotoxicity Experiments, Gene Expression, and Immunoblotting.
For cytotoxicity experiments, all mutants were constructed in the ∆vscN1 (SmR) background to eliminate T3SS1-mediated cytotoxicity. Twenty microliters of overnight cultures was transferred to 2 mL of LB and cultured at 37 °C for 3 h in the presence or absence of 0.04% crude bile. These midexponential-phase cultures were used for assays of T3SS2 cytotoxicity, gene expression, and immunoblotting. Strains cultured in LB + 0.04% crude bile were used to infect HT29 cells at a multiplicity of infection of 1 in DMEM with 5% FBS, and cytotoxicity was measured via quantification of lactate dehydrogenase release at 4 h postinfection. Statistical comparisons of T3SS2-mediated cytotoxicity were performed using an ordinary one-way ANOVA corrected for multiple comparisons. All trends are representative of at least three biological replicates.
Quantitative RT-PCR was used to measure the levels of gene transcripts in the ∆vscN1 (SmR) strain or additional mutations constructed in this background. One milliliter of each midexponential-phase culture was pelleted, and total RNA was extracted with TRIzol reagent. DNase treatment, cDNA synthesis, and quantitative PCR amplifications were carried out as previously described (8). The amplification data were analyzed by the ∆∆Ct (threshold cycle) method using the VPr001 transcript as an internal control. All trends are representative of at least three biological replicates.
Using mutations constructed in wild-type, SmR, or SmR ∆vscN1 background, protein samples were obtained from 1 mL of midexponential-phase cultures pelleted and resuspended in 80 μL of culture supernatants (to retain secreted proteins). Protein samples were separated by NuPAGE Bis-Tris gel electrophoresis and transferred onto nitrocellulose membranes. Immunoblots were performed with antisera directed against His6 (rabbit α-His6 GTX30501; GeneTex), RNA polymerase (mouse α-RNAP-α; Santa Cruz Biotechnology), and VopD2 (rabbit α-VopD2) (22). Secondary antibodies conjugated to horseradish peroxidase and Supersignal West Pico and Femto chemiluminescent substrates (Thermo Scientific) were used to detect protein levels, with imaging performed on the Chemidoc Touch Imaging System (Biorad). All trends are representative of at least three biological replicates.
Supplementary Material
Acknowledgments
We thank Drs. Tetsuya Iida and Toshio Kodoma for providing reagents, Jennifer Pena for technical assistance, and members of the M.K.W. laboratory for helpful discussions. This work was supported by NIH Grants R37 AI-042347 (to M.K.W.), F31 AI-120665 (to T.P.H.), and F32 GM108355-02 (to M.C.C.); the Howard Hughes Medical Institute (M.K.W.); Northern Norway Regional Health Authority Grants SFP1293-16 (to S.A.) and A67600 (to P.A.z.W.); Research Council of Norway Grant 249979/RU (to S.A.); and Bill & Melinda Gates Foundation Grant OPP1111658 (to P.A.z.W.). C.J.B. is supported by a Pew Latin American Fellowship and a Becas Chile Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601718113/-/DCSupplemental.
References
- 1.McCarter L. The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol. 1999;1(1):51–57. [PubMed] [Google Scholar]
- 2.Letchumanan V, Chan K-G, Lee L-H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol. 2014;5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang KF, Lightner DV. Homologues of insecticidal toxin complex genes within a genomic island in the marine bacterium Vibrio parahaemolyticus. FEMS Microbiol Lett. 2014;361(1):34–42. doi: 10.1111/1574-6968.12609. [DOI] [PubMed] [Google Scholar]
- 4.Makino K, et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V cholerae. Lancet. 2003;361(9359):743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie JM, et al. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog. 2012;8(3):e1002593. doi: 10.1371/journal.ppat.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portaliou AG, Tsolis KC, Loos MS, Zorzini V, Economou A. Type III Secretion: Building and Operating a Remarkable Nanomachine. Trends Biochem Sci. 2015;41(2):175–189. doi: 10.1016/j.tibs.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Boyd EF, et al. Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus. BMC Microbiol. 2008;8(1):110. doi: 10.1186/1471-2180-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livny J, et al. Comparative RNA-Seq based dissection of the regulatory networks and environmental stimuli underlying Vibrio parahaemolyticus gene expression during infection. Nucleic Acids Res. 2014;42(19):12212–12223. doi: 10.1093/nar/gku891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh K, et al. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS One. 2010;5(10):e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitaker WB, Parent MA, Boyd A, Richards GP, Boyd EF. The Vibrio parahaemolyticus ToxRS regulator is required for stress tolerance and colonization in a novel orogastric streptomycin-induced adult murine model. Infect Immun. 2012;80(5):1834–1845. doi: 10.1128/IAI.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barquist L, Boinett CJ, Cain AK. Approaches to querying bacterial genomes with transposon-insertion sequencing. RNA Biol. 2013;10(7):1161–1169. doi: 10.4161/rna.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007;75(12):5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaichi Y, et al. High-resolution genetic analysis of the requirements for horizontal transmission of the ESBL plasmid from Escherichia coli O104:H4. Nucleic Acids Res. 2015;43(1):348–360. doi: 10.1093/nar/gku1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao MC, et al. High-resolution definition of the Vibrio cholerae essential gene set with hidden Markov model-based analyses of transposon-insertion sequencing data. Nucleic Acids Res. 2013;41(19):9033–9048. doi: 10.1093/nar/gkt654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato J, Hashimoto M. Construction of consecutive deletions of the Escherichia coli chromosome. Mol Syst Biol. 2007;3:132. doi: 10.1038/msb4100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard JR, et al. ARTIST: high-resolution genome-wide assessment of fitness using transposon-insertion sequencing. PLoS Genet. 2014;10(11):e1004782. doi: 10.1371/journal.pgen.1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, Waldor MK, Mekalanos JJ. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe. 2013;14(6):652–663. doi: 10.1016/j.chom.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamp HD, et al. Gene fitness landscapes of Vibrio cholerae at important stages of its life cycle. PLoS Pathog. 2013;9(12):e1003800. doi: 10.1371/journal.ppat.1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abel S, et al. Sequence tag-based analysis of microbial population dynamics. Nat Methods. 2015;12(3):223–226. doi: 10.1038/nmeth.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park K-SS, et al. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect Immun. 2004;72(11):6659–6665. doi: 10.1128/IAI.72.11.6659-6665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, et al. The hydrophilic translocator for Vibrio parahaemolyticus, T3SS2, is also translocated. Infect Immun. 2012;80(8):2940–2947. doi: 10.1128/IAI.00402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama T, et al. Identification of two translocon proteins of Vibrio parahaemolyticus type III secretion system 2. Infect Immun. 2008;76(9):4282–4289. doi: 10.1128/IAI.01738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiyoshi H, et al. VopV, an F-actin-binding type III secretion effector, is required for Vibrio parahaemolyticus-induced enterotoxicity. Cell Host Microbe. 2011;10(4):401–409. doi: 10.1016/j.chom.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Kodama T, et al. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region. PLoS One. 2010;5(1):e8678. doi: 10.1371/journal.pone.0008678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama T, et al. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell Microbiol. 2007;9(11):2598–2609. doi: 10.1111/j.1462-5822.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 26.Hadjifrangiskou M, et al. Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J Bacteriol. 2012;194(22):6195–6205. doi: 10.1128/JB.01012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selkrig J, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19(5):506–510, S1. doi: 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Konkel ME, Call DR. Regulation of type III secretion system 1 gene expression in Vibrio parahaemolyticus is dependent on interactions between ExsA, ExsC, and ExsD. Virulence. 2010;1(4):260–272. doi: 10.4161/viru.1.4.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almagro-Moreno S, Root MZ, Taylor RK. Role of ToxS in the proteolytic cascade of virulence regulator ToxR in Vibrio cholerae. Mol Microbiol. 2015;98(5):963–976. doi: 10.1111/mmi.13170. [DOI] [PubMed] [Google Scholar]
- 30.Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(Web Server issue):W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, et al. A Vibrio parahaemolyticus T3SS effector mediates pathogenesis by independently enabling intestinal colonization and inhibiting TAK1 activation. Cell Reports. 2013;3(5):1690–1702. doi: 10.1016/j.celrep.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dziejman M, et al. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc Natl Acad Sci USA. 2005;102(9):3465–3470. doi: 10.1073/pnas.0409918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks JF, 2nd, et al. Global discovery of colonization determinants in the squid symbiont Vibrio fischeri. Proc Natl Acad Sci USA. 2014;111(48):17284–17289. doi: 10.1073/pnas.1415957111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selkrig J, et al. Conserved features in TamA enable interaction with TamB to drive the activity of the translocation and assembly module. Sci Rep. 2015;5:12905. doi: 10.1038/srep12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan SJ, et al. Formation of an Intramolecular Periplasmic Disulfide Bond in TcpP Protects TcpP and TcpH from Degradation in Vibrio cholerae. J Bacteriol. 2015;198(3):498–509. doi: 10.1128/JB.00338-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrington DA, et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.