Significance
Environmental challenges in utero perturb fetal growth and alter subsequent adult health outcomes. The role of the placenta is uncertain. We use a genetically modified mouse model of fetoplacental glucocorticoid excess, which exhibits decreased placental vascularity and fetal growth restriction. We show that this model associates with retarded fetal heart development. Strikingly, treatment with pravastatin restores placental vascularity and reverses retarded fetal growth and cardiovascular development. These results highlight the potential of statins to remedy placental vascular insufficiency and enhance fetal outcomes in compromised pregnancy.
Keywords: placenta, 11β-HSD2, glucocorticoids, fetal heart, developmental programming
Abstract
Fetoplacental glucocorticoid overexposure is a significant mechanism underlying fetal growth restriction and the programming of adverse health outcomes in the adult. Placental glucocorticoid inactivation by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) plays a key role. We previously discovered that Hsd11b2−/− mice, lacking 11β-HSD2, show marked underdevelopment of the placental vasculature. We now explore the consequences for fetal cardiovascular development and whether this is reversible. We studied Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− littermates from heterozygous (Hsd11b+/−) matings at embryonic day (E)14.5 and E17.5, where all three genotypes were present to control for maternal effects. Using high-resolution ultrasound, we found that umbilical vein blood velocity in Hsd11b2−/− fetuses did not undergo the normal gestational increase seen in Hsd11b2+/+ littermates. Similarly, the resistance index in the umbilical artery did not show the normal gestational decline. Surprisingly, given that 11β-HSD2 absence is predicted to initiate early maturation, the E/A wave ratio was reduced at E17.5 in Hsd11b2−/− fetuses, suggesting impaired cardiac function. Pravastatin administration from E6.5, which increases placental vascular endothelial growth factor A and, thus, vascularization, increased placental fetal capillary volume, ameliorated the aberrant umbilical cord velocity, normalized fetal weight, and improved the cardiac function of Hsd11b2−/− fetuses. This improved cardiac function occurred despite persisting indications of increased glucocorticoid exposure in the Hsd11b2−/− fetal heart. Thus, the pravastatin-induced enhancement of fetal capillaries within the placenta and the resultant hemodynamic changes correspond with restored fetal cardiac function. Statins may represent a useful therapeutic approach to intrauterine growth retardation due to placental vascular hypofunction.
Low birth weight is associated with an increased risk of cardiometabolic disorders in adulthood (1). Frequently underlying this association is elevated fetal exposure to “stress hormones”—glucocorticoids. Endogenous glucocorticoids (cortisol in humans, corticosterone in rodents) are a key signal in late gestation, which alter developmental trajectories of fetal tissues, predominantly from a proliferative to differentiated state, in preparation for extrauterine life (2). Fetal overexposure to glucocorticoids in humans, primates, and rodents is detrimental for placental and fetal growth and development, and “programs” higher risk of cardiometabolic disease in later life (3–8). Recent data suggest that the detrimental effects of excess glucocorticoids on fetal growth and development result from direct glucocorticoid actions on the placenta and on the fetus itself (9, 10).
The fetus and the placenta are maintained in a low glucocorticoid environment by the abundant expression of fetoplacental 11β-hydroxysteroid dehydrogenase-2 (11β-HSD2), an enzyme that inactivates the much higher levels of glucocorticoids arriving from the maternal circulation (11, 12). In humans and in animal models, placental 11β-HSD2 expression is reduced in adverse situations, including poor maternal nutrition or maternal stress (13–15). Bypass of this protective enzyme, be it through synthetic glucocorticoids that are poor substrates (9, 16), inhibition (by liquorice), or genetic ablation of Hsd11b2 that encodes 11β-HSD2 (10), reduces placental weight. This bypass is accompanied by reduced fetal capillary volume, surface area density, length, and diameter in the placental labyrinth zone. Underlying these placental changes is a striking reduction in placental expression of vascular endothelial growth factor (VEGF)-A (9, 10) a major driver of placental angiogenesis.
Recent evidence suggests that altered placental function, including its hemodynamics, has a direct impact on the development of fetal organs, particularly the heart (17–22). If compromised placental vascular development due to glucocorticoid excess can be rescued, this raises the possibility of a treatment for adverse effects of placental dysfunction on the fetal heart and circulation. We therefore assessed placental and umbilical blood velocity and heart growth and function in Hsd11b2−/− fetuses and then took advantage of the placental VEGF-releasing effects of pravastatin (23) to determine whether it might rescue or ameliorate the effects of fetal glucocorticoid overexposure.
Results
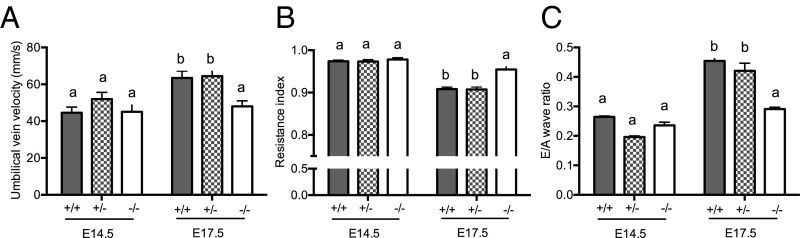
Hsd11b2−/− Fetuses Fail to Show the Normal Gestational Maturation in Umbilical Cord Blood Velocity and Fetal Heart Function.
To evaluate maturational changes in umbilical cord blood velocity and heart function, fetuses of all three genotypes from male and female Hsd11b2+/− matings underwent ultrasound analyses at embryonic day (E)14.5 [maximum of labyrinth zone 11β-HSD2 expression (11, 12) and before fetal adrenal gland steroidogenesis starts (24)], and at E17.5 (as placental 11β-HSD2 falls, around peak fetal plasma glucocorticoid levels, and just before birth, typically E18.5 in Hsd11b2+/− mice; ref. 10). Umbilical vein blood velocity normally increases over gestation, as exemplified by the 1.4-fold increase between E14.5 and E17.5 in wild-type (Hsd11b2+/+) fetuses (Fig. 1A). Although not different from control littermates at E14.5, umbilical vein blood velocity in Hsd11b2−/− fetuses did not undergo the normal gestational increase, such that by E17.5 umbilical vein blood velocity was 24% less than wild type (Fig. 1A). Similarly, the normal gestation decline in umbilical artery resistance (resistance index; RI = systole/[systole+diastole]), apparent in Hsd11b2+/+ and Hsd11b2+/− fetuses (18% decrease between E14.5 and E17.5), did not occur in Hsd11b2−/− fetuses (Fig. 1B). Thus, there was an interaction between gestational age and genotype for both umbilical vein blood velocity and RI. Heart function matures between E14.5 and E17.5, and as the fetal heart becomes more compliant, left ventricle (LV) filling becomes more dependent on passive filling (the E wave) and less dependent on LV filling due to active contraction of the atria (the A wave) (25); this clearly occurs in both Hsd11b2+/+ and Hsd11b2+/− fetuses but did not occur in Hsd11b2−/− fetal hearts (Fig. 1C). In contrast, myocardial performance index, a combined measure of systolic and diastolic function (25), was unaltered by genotype (see Table S1 for myocardial performance index and a breakdown of each of the cardiac components assessed by ultrasound).
Fig. 1.
Umbilical vein velocity (A), umbilical artery resistance index (B), and fetal cardiac E/A wave ratio (C) in Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses at E14.5 and E17.5. Values were normalized for fetal weight and are the mean ± SEM (n = 8 per group). Columns without common notation differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test).
Table S1.
Myocardial performance index of Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses at E14.5 and E17.5
| Performance | E14.5 | E17.5 | ||||
| Hsd11b2+/+ | Hsd11b2+/− | Hsd11b2−/− | Hsd11b2+/+ | Hsd11b2+/− | Hsd11b2−/− | |
| MPI | 0.75 ± 0.05a | 0.75 ± 0.02a | 0.72 ± 0.03a | 0.63 ± 0.03b | 0.64 ± 0.02b | 0.65 ± 0.03b |
| IVCT (ms) | 22 ± 0.3a | 23.4 ± 0.1a | 22.8 ± 0.2a | 18.1 ± 0.2b | 16.9 ± 0.4b | 19.6 ± 1b |
| ET (ms) | 101.4 ± 5 | 97.3 ± 3 | 105.6 ± 5 | 105.3 ± 2 | 98.6 ± 5 | 102.6 ± 4 |
| IVRT (ms) | 26.1 ± 3a | 24.8 ± 2a | 25.9 ± 3a | 22.4 ± 3b | 20.1 ± 1b | 21.7 ± 0.5b |
| EDD (mm/s2) | 1,542 ± 481a | 1,329 ± 312a | 1,376 ± 548a | 3,128 ± 682b | 3,308 ± 384b | 1,365 ± 445a |
| EF (%) | 73.8 ± 3.5a | 76.2 ± 2a | 71.8 ± 1.4a | 85.2 ± 2.1b | 81.3 ± 1.9b | 82.6 ± 1.5b |
Values are the mean ± SEM. Values without common notation (a, b) differ significantly. EDD, early diastolic deceleration; EF, ejection fraction; ET, ejection time; IVCT, isovolumetric contraction time; IVRT, isovolumetric relaxation time; MPI, myocardical performance index.
These functional changes were not due to altered gross morphology of the heart. Thus, at E17.5, there were no differences in overall cardiac volume (Hsd11b2+/+: 3.9 ± 0.1, Hsd11b2+/−: 3.8 ± 0.2, Hsd11b2−/−: 3.4 ± 0.3 mm3) or number of cardiomyocytes (Hsd11b2+/+: 4.1 ± 0.3, Hsd11b2+/−: 4.1 ± 0.2, Hsd11b2−/−: 3.8 ± 0.1 × 106). Perhaps analogously, cardiac function is altered in the absence of gross morphological alteration in mice with cardiomyocyte and vascular smooth muscle-specific deletion of the glucocorticoid receptor (GR) (26).
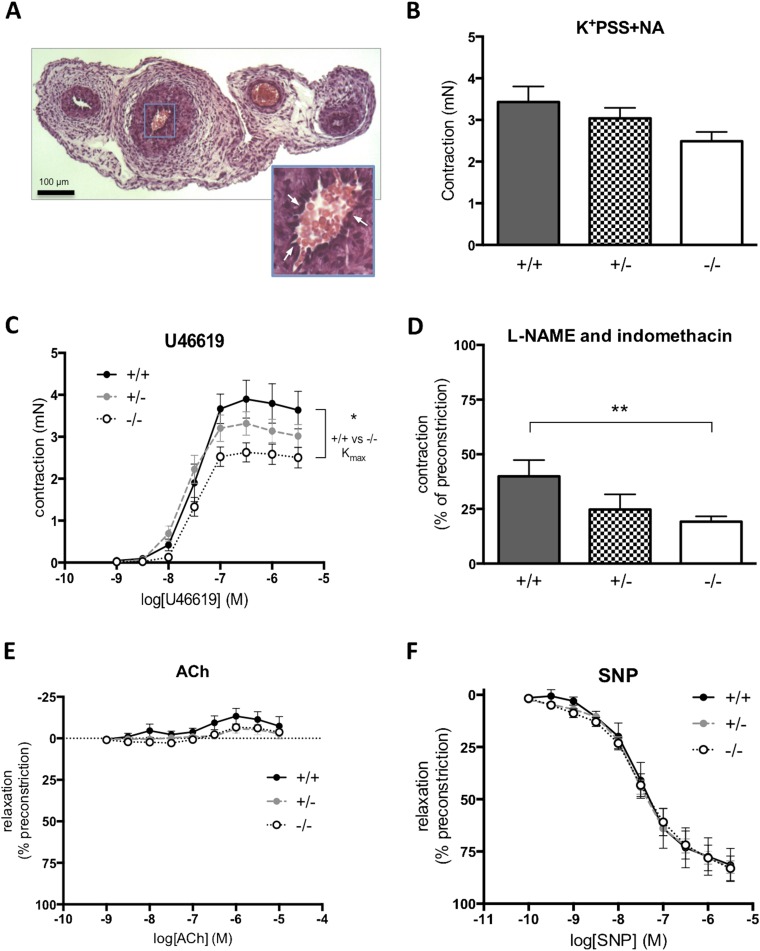
Altered blood velocity in the Hsd11b2−/− umbilical cord prompted us to explore whether this could be attributed to altered umbilical cord structure or function. Histology revealed no significant differences between Hsd11b2+/+ and Hsd11b2−/− in luminal area or wall thickness of the umbilical artery or vein (Table S2). Functionally, isolated umbilical arteries from Hsd11b2−/−mice tended to be more responsive to vasoconstrictors and have lower basal release of endothelium-dependent mediators. With loss of Hsd11b2, there was no significant alteration in maximal contractile response to high potassium (Fig. S1B), whereas the thromboxane agonist, U46619, reduced maximal contractile response (Fig. S1C). The maximal contraction (Kmax) to U46619 was significantly lower in vessels from Hsd11b2−/− compared with controls (2.41 ± 0.24 mN vs. 3.61 ± 0.45 mN, respectively), although the sensitivity to U46619 (EC50) did not differ between genotypes. Basal endothelial function (basal release of nitric oxide and prostacyclin) was explored through contractile response to l-NAME and indomethacin in the presence of an EC50 dose of U46619. l-NAME + indomethacin caused a further 25–50% transient contraction of vessels ∼2 min after addition, returning to baseline within 5 min (Fig. S1D). The contractile response was greatest in the umbilical arteries from control fetuses and lowest in arteries from Hsd11b2−/− (19 ± 2% vs. ±39 ± 7%, P < 0.05). Acetylcholine, an endothelium-dependent vasodilator, did not relax umbilical arteries (Fig. S1). The ability of umbilical arteries to relax to other vasodilators was confirmed by a concentration-dependent relaxation response to the nitric oxide donor drug, sodium nitroprusside (Fig. S1E), with no differences in response between genotypes. This pattern of response concurs with the in vivo findings. Although increased umbilical artery vasoconstriction and reduced endothelium-dependent functions likely contribute to reduced fetal blood supply in 11β-HSD2 null fetuses, the differences between genotypes and magnitude of the changes were modest and other factors are likely also to be involved (i.e., vascular resistance).
Table S2.
Lumen and vessel wall area of umbilical arteries and vein from Hsd11b2+/+ and Hsd11b2−/− fetuses
| Area | Umbilical artery | Umbilical vein | ||
| Hsd11b2+/+ | Hsd11b2−/− | Hsd11b2+/+ | Hsd11b2−/− | |
| Lumen area (μm2) | 4,211 ± 1,265 | 3,624 ± 986 | 36,211 ± 8,246 | 29,425 ± 6,937 |
| Vessel wall area (μm2) | 32,646 ± 1,324 | 28,436 ± 2,534 | 24,328 ± 879 | 18,656 ± 2,289 |
Values are the mean ± SEM.
Fig. S1.
Contractile and vasodilator function of umbilical arteries. (A) H&E-stained cross-section of the umbilical cord. (Scale bar: 100 μm.) (Inset) Higher magnification of the umbilical artery used for myography studies. Arrows indicate the presence of endothelial cell nuclei on the luminal surface of the artery. (B) Maximal contraction of arteries to high potassium physiological saline solution containing noradrenaline (K+PSS+NA) in animal with disrupted Hsd11b2 alleles. (C) Maximum vasodilator response to the thromboxane mimetic U46619 in umbilical arteries from Hsd11b2−/− fetuses (*P < 0.05, unpaired t test of Kmax). (D) Contractile response to inhibition of basal endothelium-dependent relaxation in response to lω-nitro-l-arginine methyl ester (l-NAME) and indomethacin (**P < 0.01, unpaired t test). (E) ACh did not relax umbilicial arteries, and there were no significant differences between genotypes. (F) Vasodilator response to SNP. For B, C, and E, data shown are the mean ± SEM (n = 6, 20, and 9 for Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/−, respectively). For D, data shown are the mean ± SEM (n = 5, 11, and 8 for Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/−, respectively).
Gene Expression Patterns in Hsd11b2−/− Fetal Hearts Reflect Glucocorticoid Overexposure and Earlier Maturation.
To investigate glucocorticoid exposure and probe mechanism underlying altered cardiac function in Hsd11b2−/− fetuses, we measured levels of mRNA encoding glucocorticoid-responsive genes and genes important for contractile function. Cardiac expression of Tsc22d3 (also known as glucocorticoid-induced leucine zipper; GILZ, a mediator of antiinflammatory and perhaps other glucocorticoid actions) expression exhibited a normal gestational increase (26) in Hsd11b2+/+ and Hsd11b2+/− fetuses (Fig. 2A). Hsd11b2−/− fetuses (gestational age and genotype interaction) had elevated levels at E14.5, consistent with higher glucocorticoid exposure in midgestation. Expression of Myh6 (encoding myosin heavy chain-α, MYHCα, the major contractile protein in the adult heart) normally increases between E14.5 and E17.5 (26), as exemplified by the 1.7-fold increase between E14.5 and E17.5 in Hsd11b2+/+ and Hsd11b2+/− fetal hearts (Fig. 2B). Whereas this gestational increase was exaggerated in Hsd11b2−/− fetuses, Myh6 mRNA levels reduced (58%) at E14.5 and increased 1.4-fold at E17.5 compared with Hsd11b2+/+ littermates (Fig. 2B). A similar pattern of expression was observed for the Atp2a2 gene encoding the calcium-handling protein SERCA2a (Fig. 2C). The down-regulation of both Myh6 and SERCA2a genes at E14.5 appears at variance with higher glucocorticoid exposure of Hsd11b2−/− fetuses, predicted to cause early cardiac maturation. This finding raises the possibility that either premature glucocorticoid exposure fails to mimic the normal maturational effects of glucocorticoids upon the heart, or that indirect dysmaturational effects predominate. Secretion of cardiac natriuretic peptide A (ANP; encoded by Nppa) is stimulated by stretch of the myocardium (27) and is considered a marker of cardiomyocyte hypertrophy (28). Its expression increases with gestation, as apparent in Hsd11b2+/+ fetuses (1.8-fold between E14.5 and E17.5) (Fig. 2D). However, neither Hsd11b2−/− nor Hsd11b2+/− fetuses showed this developmental increase in ANP expression in the heart. This finding suggests the Hsd11b2−/− fetal heart tissue is less compliant, as shown by ultrasound in vivo. Thus, overall Hsd11b2−/− fetuses show complex, gene-specific patterns of premature, exaggerated, or reversed maturation of glucocorticoid-sensitive transcripts in the myocardium.
Fig. 2.
Relative levels of Tsc22d3 (A), Myh6 (B), Atp2a2 (C), and Nppa (D) mRNA in hearts of Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses at E14.5 and E17.5. Values are means ± SEM (n = 6–8 per group). Columns without common notation differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test).
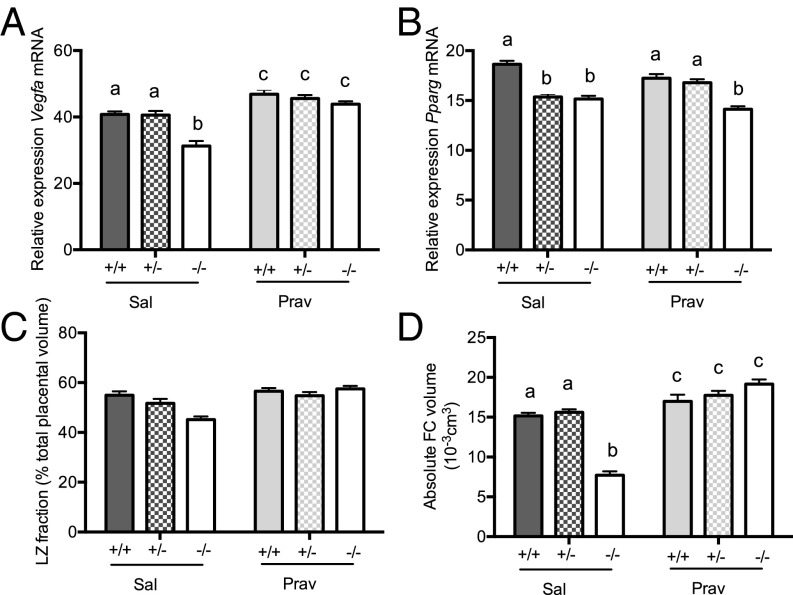
Pravastatin Increases Labyrinth Zone Vegfa Expression and Fetal Capillary Volume in all Genotypes.
To determine whether the adverse effects of glucocorticoid overexposure on the placental vasculature can be overcome and whether this might beneficially impact on fetal heart development, we administered (intraperitoneally) either pravastatin or saline from E6.5 onwards with the aim of stimulating placental vascular endothelial growth factor A (VEGFA) production and, thereby, enhancing vascularization. Consistent with its reported effects on placental VEGF (23), pravastatin up-regulated expression of labyrinth zone Vegfa in all genotypes (Fig. 3A). The increase in Hsd11b2−/− placentas was greater (genotype × treatment), eliminating the genotype difference in placental Vegfa expression. Despite its role in regulating Vegfa expression (29), labyrinth zone Pparg expression levels did not correspond with Vegfa patterns (Fig. 3B); pravastatin had no effect on Pparg mRNA expression, and a reduction in Pparg mRNA was apparent in both saline- and pravastatin-treated Hsd11b2−/− placentas.
Fig. 3.
Placental gene expression and morphology in control and pravastatin-treated Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses. Relative labyrinth zone Vegfa mRNA expression (A), Pparg mRNA expression (B), labyrinth zone (LZ) fraction (C), and fetal capillary (FC) volume (D). Values are the mean ± SEM (n = 6–8 per group). Columns without common notation differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test). FC, fetal capillaries; LZ, labyrinth zone; Prav, pravastatin-treated; Sal, saline-treated.
Corresponding with increased placental Vegfa, placental weight increased with pravastatin (Table 1). Stereological assessment of labyrinth zone volume showed that whereas Hsd11b2−/− saline-treated placentas appeared smaller, this was not statistically significant (Fig. 3C). Furthermore, there was only a trend (P = 0.0536) for labyrinth zone volume increase with pravastatin (Fig. 3C). Detailed investigation of fetal capillary volume provided a clearer insight into placental vascular development. Thus, pravastatin modestly increased the volume of fetal capillaries within the labyrinth zone of Hsd11b2+/+ and Hsd11b2+/− fetuses (Fig. 3D) but completely rescued the deficit in Hsd11b2−/− placentas, with a significant interaction between treatment and genotype. There were no effects of pravastatin on maternal body weight, organ weight, or litter size (Table S3).
Table 1.
E17.5 fetal and placental weights of Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses from saline- (Sal) or pravastatin-treated (Prav) dams
| Weight | Sal (n = 28) | Prav (n = 32) | ||||
| +/+ | +/− | −/− | +/+ | +/− | −/− | |
| Fetal weight (g) | 0.81 ± 0.02a | 0.83 ± 0.021ab | 0.73 ± 0.03c | 0.87 ± 0.01d | 0.85 ± 0.01bd | 0.81 ± 0.01a |
| Placental weight (g) | 0.09 ± 0.03a | 0.09 ± 0.02a | 0.08 ± 0.03a | 0.1 ± 0.03b | 0.1 ± 0.03b | 0.1 ± 0.04b |
Values are the mean ± SEM. Values without common notation (a, b, c, and d) differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test). Prav, pravastatin-treated dams; Sal, saline-treated dams.
Table S3.
Pravastatin treatment does not affect maternal body weight, organ weight, or litter size
| Weight or size | Sal (n = 28) | Prav (n = 32) |
| E17.5 maternal weight (g) | 31.7 ± 1.7 | 32.0 ± 1.9 |
| Brain weight (g) | 0.46 ± 0.01 | 0.47 ± 0.01 |
| Liver weight (g) | 1.63 ± 0.07 | 1.8 ± 0.06 |
| Heart weight (g) | 0.15 ± 0.01 | 0.18 ± 0.01 |
| Left kidney weight (g) | 0.15 ± 0.01 | 0.15 ± 0.01 |
| Litter size | 8.1 ± 0.8 | 9.1 ± 0.5 |
Values are the mean ± SEM. Prav, pravastatin-treated dams; Sal, saline-treated dams.
Pravastatin Strikingly Attenuates Fetal Growth Restriction and Reverses Adverse Umbilical Flow and Cardiac Function in the Hsd11b2−/− Placenta and Fetus.
In saline-treated pregnancies, Hsd11b2−/− fetuses were lighter than littermate controls as reported (10) (Table 1). Pravastatin treatment increased fetal weight across all genotypes, although Hsd11b2−/− remained lighter than their Hsd11b2+/+ and Hsd11b2+/− littermates (Table 1). However, pravastatin ameliorated the growth retardation in Hsd11b2−/− fetuses such that they were the same weight as Hsd11b2+/+ controls.
Pravastatin had a marked effect on placental blood velocity and fetal heart measures. Overall, pravastatin increased umbilical vein blood velocity (Fig. 4A), decreased umbilical artery resistance index (Fig. 4B), and increased fetal cardiac E/A wave ratio (Fig. 4C) in all genotypes. Notably, pravastatin “normalized” the aberrant phenotype of Hsd11b2−/− fetuses such that there were no genotype differences in umbilical vein blood velocity or fetal cardiac E/A ratio in Hsd11b2−/− fetuses from pravastatin-treated dams (Fig. 4 A and C). In contrast, the resistance index remained increased in both saline-treated and pravastatin-treated Hsd11b2−/− fetuses, albeit to a lesser extent in the pravastatin-treated Hsd11b2−/− fetuses compared with saline treated (Fig. 4B).
Fig. 4.
Umbilical vein velocity (A), umbilical artery resistance index (B), and fetal cardiac E/A wave ratio (C) in saline and pravastatin-treated Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses. Values were normalized for fetal weight and are the mean ± SEM (n = 8 per group). Columns without common notation differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test). Prav, pravastatin-treated; Sal, saline-treated.
The effects of pravastatin on cardiac functional changes were not accompanied by gross morphological changes. Thus, there were no differences in overall cardiac volume, ventricular lumen volume, or the ratio of ventricular wall thickness to lumen volume (Table S4).
Table S4.
Pravastatin treatment does not alter overall cardiac volume, ventricular lumen volume or the ratio of ventricular wall thickness to lumen volume of Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses
| Volume | Sal | Prav | ||||
| Hsd11b2+/+ | Hsd11b2+/− | Hsd11b2−/− | Hsd11b2+/+ | Hsd11b2+/− | Hsd11b2−/− | |
| Cardiac volume (mm3) | 3.8 ± 0.4 | 3.6 ± 0.3 | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.8 ± 0.3 | 3.6 ± 0.2 |
| LV lumen volume (mm3) | 0.87 ± 0.16 | 0.6 ± 0.08 | 1.07 ± 0.2 | 1.15 ± 0.1 | 0.87 ± 0.07 | 0.76 ± 0.12 |
| LV wall thickness, Lumen volume | 0.46 ± 0.03 | 0.45 ± 0.02 | 0.49 ± 0.05 | 0.44 ± 0.04 | 0.43 ± 0.05 | 0.45 ± 0.02 |
| RV lumen volume (mm3) | 0.89 ± 0.06 | 0.58 ± 0.18 | 0.88 ± 0.13 | 1.04 ± 0.2 | 0.62 ± 0.1 | 0.79 ± 0.1 |
| RV wall thickness, Lumen volume | 0.42 ± 0.05 | 0.39 ± 0.04 | 0.44 ± 0.06 | 0.43 ± 0.02 | 0.41 ± 0.03 | 0.41 ± 0.04 |
Values are the mean ± SEM. LV, left ventricle; Prav, pravastatin-treated; RV, right ventricle; Sal, saline-treated.
Pravastatin Markedly Alters Fetal Cardiac Ace and Some Collagen mRNAs.
Expression of glucocorticoid-responsive Tsc22d3 mRNA was not altered by pravastatin (Fig. 5A), consistent with increased glucocorticoid exposure and reflecting similar findings from the initial untreated cohort at E17.5 (Fig. 2A). Therefore, the alterations in Hsd11b2−/− fetal heart function are likely independent of direct cardiac glucocorticoid action. Similarly, expression of Mhyc6 and Atp2a2 was unaffected by pravastatin in all genotypes (Fig. 5 B and C). Although there was no effect of pravastatin on cardiac Nppa expression in Hsd11b2+/+ fetuses (Fig. 5D), it increased in pravastatin-treated Hsd11b2−/− and Hsd11b2+/− fetuses. Thus, pravastatin rescued cardiac Nppa expression in Hsd11b2+/− and partially rescued Hsd11b2−/− fetuses. Expression of Ace was decreased in fetal hearts of all genotypes with pravastatin (Fig. 5E), abolishing the genotype difference seen in saline-treated fetuses.
Fig. 5.
Fetal cardiac gene expression in control and pravastatin-treated Hsd11b2+/+, Hsd11b2+/−, and Hsd11b2−/− fetuses. Relative levels of Tsc22d3 (A), Myh6 (B), Atp2a2 (C), Nppa (D), Ace (E), Col1a1 (F), Col3a1 (G), and Col4a1 (H). Values are the mean ± SEM (n = 6–8 per group). Columns without common notation differ significantly (P < 0.05, two-way ANOVA, Tukey’s post hoc test). In the case of Col4a1, *P < 0.05, a t test of corresponding genotype between treatments. Prav, pravastatin-treated; Sal, saline-treated.
Collagen is a key contributor to cardiac wall stiffness. In fetuses from saline-treated dams, there was an increase in the cardiac expression of Col1a1 (which determines rigidity) (30) in Hsd11b2−/− and Hsd11b2+/− fetuses, compared with Hsd11b2+/+ littermates (Fig. 5F). This difference was not evident in fetuses from pravastatin-treated dams. Col3a1, which determines elasticity (30), showed a reciprocal effect; Col3a1 mRNA levels were reduced in hearts of saline-treated Hsd11b2−/− and Hsd11b2+/− fetuses compared with wild-type littermates (Fig. 5G). However, although pravastatin had no effect in Hsd11b2+/+, it increased Col3a1 mRNA levels in Hsd11b2−/− and Hsd11b2+/− fetuses. These expression patterns correspond with the changes in cardiac function. For Col4a1 (Fig. 5H), there was no effect of genotype or treatment, but a significant interaction. Thus, pravastatin increased Col4a1 expression in hearts of Hsd11b2+/+ fetuses by 8.5-fold, but decreased it in Hsd11b2−/− fetuses (68% decrease). Pravastatin did not alter Vegfa and Pparg in the fetal heart. These data demonstrate that although pravastatin does not reverse cardiac glucocorticoid overexposure in Hsd11b2−/− fetuses, it does change key collagens and other endocrine genes in a pattern that corresponds with enhancement of Hsd11b2−/− fetal heart function.
Discussion
Pravastatin treatment dramatically ameliorates the adverse phenotype of Hsd11b2−/− fetuses; placental labyrinth zone morphology, umbilical blood velocity, fetal weight and fetal heart function, and gene expression are, for the most part, normalized. Thus, despite persistently increased placental and fetal glucocorticoid exposure in Hsd11b2−/− fetuses, it is possible to counter these adverse outcomes, including the “intrauterine growth restriction” (IUGR) phenotype. These findings highlight the crucial role of the placenta in informing fetal development and suggest statins as a potential therapy for IUGR with placental vascular insufficiency.
Despite the “maturational” effects of antenatal glucocorticoids, we surprisingly found that Hsd11b2−/− fetuses exhibit delayed or impaired cardiac functional maturation. Whether these changes in fetal heart function alter cardiac function in adulthood will be important to uncover in the future, although in this experimental model, adult heart function is likely to be influenced by the effect of lifelong absence of 11β-HSD2 upon salt regulation, blood pressure, and renal function (31), confounding interpretation. Pravastatin treatment then eradicated the impaired Hsd11b2−/− fetal cardiac maturation in conjunction with normalizing placental vascular parameters. We postulate that placental and umbilical cord hemodynamics could be an important factor directly influencing fetal heart development; intervention is required to demonstrate this. However, recent evidence supports the view that the placenta directly influences the development of specific fetal organs, notably the heart. Thus, human placental size and shape are epidemiologically associated with the incidence of cardiovascular disease in later life (17, 32, 33). Thornburg et al. proposed (18) that because the fetal heart beats directly against the resistance of the placental bed, changes in placental blood velocity must impact on fetal heart development. Placental insufficiency (albeit severe—with absent or reversed diastolic velocity in the umbilical artery) results in increased loading of the right ventricle (19). Importantly, extensive work in genetically modified mouse models has revealed the necessity of a functional placenta for optimal heart development; the cardiac defects exhibited in Pparγ and p38α null embryos are rectified by targeted placental normalization (21, 22, 34). Furthermore, mice with genetic disruption of HOXA13, which is not expressed in the heart but is an important transcriptional regulator of placental Tie2 (and, thus, placental vascular branching) show abnormal placental endothelium that is associated with reduced ventricular wall thickness in the fetal heart (20), presumably occurring secondarily to the placental defect.
Pravastatin, an HMG-CoA reductase inhibitor that reduces cholesterol biosynthesis, is currently contraindicated in pregnancy, because of its potential effects in altering NO bioavailability in the fetal circulation, with detrimental consequences for the fetal brain sparing response to acute hypoxia, as may happen intrapartum (35). However, pravastatin in various mouse models of preeclampsia appears to ameliorate preeclamptic pathology (23, 36), and pravastatin is the subject of a randomized control trial to ameliorate severe preeclampsia (37). Three biological compartments are exposed to pravastatin in our model: (i) the maternal, although our experimental design controls for alteration in maternal physiology because all fetal genotypes are generated within the one pregnancy, (ii) the placental and (iii), the fetal. Restoration of vasculogenesis in preeclamptic placentas following pravastatin has been variously attributed to stimulation of placental VEGF release, soluble Flt-1 (sFlt-1; a VEGF receptor), and placental growth factor (36, 38). Here, pravastatin enhanced labyrinth zone Vegfa expression in all genotypes. Accordingly, fetal capillary volume, umbilical vein velocity, and umbilical resistance index underwent corresponding changes. Pravastatin will doubtless have placental actions beyond Vegfa. Indeed, in human first trimester placental explants, pravastatin inhibits insulin-like growth factor 1 receptor function with adverse implications for trophoblast differentiation (39). With regard to the fetus, the levels of pravastatin achieved within the fetal circulation in this current study are unknown, but earlier studies have demonstrated that transfer of pravastatin in ex vivo human placenta occurs albeit to a limited extent (40, 41). However, it is of interest to note that we observed no induction of Vegfa expression in Hsd11b2−/− fetal heart, suggesting that if pravastatin is eliciting direct effects on the fetus, it may be via different pathways. Although we cannot discount the potential for direct effects of pravastatin on the fetus, the intriguing possibility is thus raised that the changes in cardiac parameters are primarily due to effects of pravastatin on enhancing the placental vasculature, with effects on the fetal heart occurring secondarily.
Further specific investigations are required to dissect this potential placenta–cardiac axis. Placenta-specific removal of Hsd11b2 and manipulation of VEGFA specifically in the placenta will be useful to determine how placental vasculature impacts on fetal heart development and function. Nevertheless, our findings suggest the intriguing possibility that using extrinsic factors to enhance placental vasculature in compromised pregnancies could have beneficial impact on fetal heart development and in IUGR more generally. Indeed, other gestational insults, such as fetal hypoxia, which also cause IUGR and cardiovascular programming can be overcome by administration of vitamin C (42, 43). However, the mechanism is likely different; whereas oxidative stress was attenuated by vitamin C, placental labyrinth zone volume remained unaltered (42, 43).
Overall, these data add to the growing body of evidence that placental vasculature has a key role in fetal development and programming outcomes. Moreover, enhancement of placental vasculature in compromised pregnancies may be beneficial for fetal heart development and in IUGR.
Methods
Animals.
Male and female Hsd11b2+/− mice, congenic on the C57BL/6J background (44), were mated overnight, and the morning of the day the vaginal plug was identified was designated E0.5. The resultant pregnancies were only analyzed if each of the possible offspring genotypes was represented in the litter: Hsd11b2+/+ (“control” littermates), Hsd11b2+/−, and Hsd11b2−/−. This approach controls for alteration in maternal physiology because all fetal genotypes are generated within the one pregnancy. Animals were given standard chow, water, and housing arrangements, and all studies were conducted in the strictest standards of humane animal care under the auspices of the UK Home Office Animals (Scientific Procedures) Act, 1986 and local ethical committee approval.
Two groups of dams were used for this study. Group 1 underwent characterization of changes in placental and umbilical blood velocity and fetal heart development over gestation. A subset of group 1 dams underwent ultrasound analyses at E14.5 or E17.5 (n = 8 at each timepoint). Following imaging, the pregnant dam was euthanized in situ, and scanned fetuses were excised following identification by corroboration of position with the ultrasound images. Fetuses were fixed and umbilical cords were collected for subsequent myography studies. Placental and fetal tissues were collected from a further subset of dams (n = 8 at each timepoint) for gene expression analysis.
Group 2 were injected with either saline (Sal) or 20 μg/kg of pravastatin sodium salt (Prav; Cayman Chemical) intraperitoneally daily from E6.5 onwards. At E17.5, a subset underwent ultrasound analyses and placentas were collected for stereological analysis (n = 8), whereas an additional cohort (n = 6–8) was generated for placental and fetal gene analysis.
Umbilical cords were placed in ice-cold Krebs–Henseleit solution before subsequent myography studies. For RNA extractions, placentas were dissected rapidly over wet ice and separated into junctional and labyrinth zones before freezing on dry ice. Fetal hearts were dissected and immediately frozen on dry ice. For histological investigations, whole placentas, umbilical cords, and fetuses were fixed in formalin and paraffin embedded. Fetal tails were collected in all cases for genotyping and gendertyping by PCR as described (10). However, sex was not taken into account in the final analyses because of an insufficient number of each sex for each possible genotype to reach statistical power.
High-Resolution Ultrasound Analysis.
In vivo ultrasound assessment was performed by using a Vevo 770 ultrasound biomicroscope (Visualsonics) using a RMV707B 30 MHz center frequency transducer. Pregnant mice were scanned as described (26). Fetal-placental units were imaged over a strict 20-min time period, with a minimum of three units being analyzed in each pregnancy. Blood velocity within the umbilical artery, vein, and placenta was measured (45). Fetal hearts were visualized in B-mode and Doppler measurements were undertaken to determine the E/A wave ratio and myocardial performance index (MPI) (26). Images were recorded for offline analysis.
Placental and Umbilical Cord Morphology.
Placental stereological investigations were conducted as described (10). Umbilical cord morphology was ascertained from four cross-sectional hemotoxylin and eosin-stained sections taken from the midline of the umbilical cord, 80 μm apart. The umbilical artery and vein area and perimeter were calculated by manually tracing the outer smooth muscle outline and lumen perimeter by using Nikon NIS Elements Imaging Software v4.10. (Nikon Instruments). All measurements were performed by an observer blind to genotype. Treatment and intraobserver error was <5%.
Cardiac Morphology.
Serial H&E-stained sections were assessed by using Nikon NIS Elements Imaging Software v4.10. (Nikon Instruments). Cardiac tissue volume and cardiomyocyte number were determined by using stereological investigations as described (46). Ventricle wall thickness was assessed by measuring the thickness of the wall at the point perpendicular from the center of the longest axis of the ventricle.
Umbilical Vessel Myography.
The contractile and vasodilator capacity of umbilical vessels was assessed by myography, based on modifications of previously established protocols. Umbilical arteries were carefully dissected, cut into lengths of ∼1.5 mm, then mounted on a wire myograph (610M; Danish Myo Technology) by using 25-μm-diameter wire. Vessels were placed at 2 mN pretension, allowed to equilibrate for 30–60 min, before establishing vessel viability with high K+ physiological saline solution (K+PSS) + noradrenaline (10 μM). Arteries with a contraction of 1 mN or less were excluded from the analysis. Vessels were contracted with increasing doses of thromboxane mimetic (U46619). EC80 of U46619 were chosen to precontract arteries, before carrying out concentration response curves to the endothelium-dependent vasodilator, acetylcholine (ACh), and the endothelium-independent vasodilator, sodium nitroprusside (SNP). To assess basal endothelial activity, vessels were partially precontracted with EC50 U46619, before addition of the eNOS inhibitor, lω-nitro-l-arginine methyl ester (l-NAME; 200 μM), and the cyclooxygenase inhibitor, indomethacin (10 μM). The data from force transducers were processed by a MacLab/4e analog–digital converter and displayed through Chart software, version 3.4.3 (AD Instruments).
Quantitative PCR.
Total RNA was extracted from tissue by using QIAzol Lysis reagent (Qiagen Sciences) as per the manufacturer's instructions. Total RNA (1 μg) was reverse transcribed by using Mouse Moloney leukemia virus reverse transcriptase and random primers (Promega). The cDNA was subsequently purified with Ultraclean PCR Cleanup kit (MoBio Laboratories).
Specific mRNA levels were measured by quantitative RT-PCR on the Rotorgene 6000 system (Corbett Research) using QuantiTect SYBR Green Mastermix (Qiagen Sciences). Primers for Vegfa, peroxisome proliferator-activated receptor-γ (Pparg); glucocorticoid-induced leucine zipper (GILZ, for Tsc22d3); myosin heavy chain 6-α (Myh6); sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (Atp2a2); natriuretic peptide A (Nppa); angiotensin I converting enzyme (Ace); collagen, type I, α-1 (Col1a1); collagen, type III, α-1 (Col3a1); and collagen, type IV, α-1 (Col4a1) were purchased as Qiagen QuantiTect primers with the exception of the internal standards, Tbp, Ppia, and Sdha, which were designed by using Primer-BLAST (www.ncbi.nlm.nih.gov). Primer pairs for all genes are listed in Table S5. Standard curves were generated through 10-fold serial dilution of purified PCR products for each gene with analysis by using Rotorgene 6000 Software. All samples were normalized against Tbp, Sdha, and Ppia by using the GeNorm algorithm (47).
Table S5.
PCR conditions
| Gene | Qiagen QuantiTect name or primer sequence |
| Vegfa | QT00160769 |
| Pparg | QT00100296 |
| Tsc22d3 | QT01552005 |
| Nr3c1 | QT00160349 |
| Nr3c2 | QT00312305 |
| Myh6 | QT00160902 |
| Atp2a2 | QT00149121 |
| Nppa | QT00250922 |
| Ace | QT00100135 |
| Col1a1 | QT 00162204 |
| Col3a1 | QT 01055516 |
| Col4a1 | QT 00287392 |
| Col5a1 | QT 01055474 |
| Sdha | F 5′-TGGGGCGACTCGTGGCTTTC-3′ |
| R 5′-CCCCGCCTGCACCTACAACC-3′ | |
| Ppia | F 5′-AGCATACAGGTCCTGGCATC-3′ |
| R 5′-TTCACCTTCCCAAAGACCAC-3′ | |
| Tbp | F 5′-GGGAGAATCATGGACCAGAA-3′ |
| R 5′-CCGTAAGGCATCATTGGACT-3′ |
Statistical Analysis.
All data are expressed as mean ± SEM, with each litter representing n = 1, with no more than 1 representative pup per litter analyzed. For fetal and placental weights, n = 14–20. Fetal sex was noted but was not taken into account in analyses, including fetal weight, because statistical power was insufficient for analysis by gender and genotype. For ultrasound (n = 8), values were normalized to fetal weight. For heart and umbilical cord morphology and gene expression studies, n = 6–8. Two-way ANOVA followed by Tukey’s post hoc test or one-way ANOVA followed by Tukey’s post hoc test were used as appropriate. P < 0.05 was accepted as statistically significant.
Acknowledgments
This study was supported in part by Wellcome Trust Project Grant WT079009; European Union FP7 collaborative Grant Developmental Origins of Healthy and Unhealthy Ageing 278603 (to M.C.H. and J.R.S.); and The Raine Medical Research Priming Grant (to C.S.W.). E.A.R.-Z. was funded by a studentship from the British Heart Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520356113/-/DCSupplemental.
References
- 1.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2B):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 2.Fowden AL, Forhead AJ. Glucocorticoids as regulatory signals during intrauterine development. Exp Physiol. 2015;100(12):1477–1487. doi: 10.1113/EP085212. [DOI] [PubMed] [Google Scholar]
- 3.Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology. 2006;147(1):599–606. doi: 10.1210/en.2005-0748. [DOI] [PubMed] [Google Scholar]
- 4.Wyrwoll CS, Mark PJ, Mori TA, Waddell BJ. Developmental programming of adult hyperinsulinemia, increased proinflammatory cytokine production, and altered skeletal muscle expression of SLC2A4 (GLUT4) and uncoupling protein 3. J Endocrinol. 2008;198(3):571–579. doi: 10.1677/JOE-08-0210. [DOI] [PubMed] [Google Scholar]
- 5.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: New model for adult hypertension. Lancet. 1993;341(8841):339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27(6):1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 7.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101(10):2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287(5):E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt DP, Mark PJ, Waddell BJ. Glucocorticoids prevent the normal increase in placental vascular endothelial growth factor expression and placental vascularity during late pregnancy in the rat. Endocrinology. 2006;147(12):5568–5574. doi: 10.1210/en.2006-0825. [DOI] [PubMed] [Google Scholar]
- 10.Wyrwoll CS, Seckl JR, Holmes MC. Altered placental function of 11beta-hydroxysteroid dehydrogenase 2 knockout mice. Endocrinology. 2009;150(3):1287–1293. doi: 10.1210/en.2008-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RW, et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137(2):794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 12.Burton PJ, Smith RE, Krozowski ZS, Waddell BJ. Zonal distribution of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 messenger ribonucleic acid expression in the rat placenta and decidua during late pregnancy. Biol Reprod. 1996;55(5):1023–1028. doi: 10.1095/biolreprod55.5.1023. [DOI] [PubMed] [Google Scholar]
- 13.Mairesse J, et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292(6):E1526–E1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell KJ, et al. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37(6):818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS. Foetal and placental 11β-HSD2: A hub for developmental programming. Acta Physiol (Oxf) 2014;210(2):288–295. doi: 10.1111/apha.12187. [DOI] [PubMed] [Google Scholar]
- 16.Vaughan OR, Sferruzzi-Perri AN, Coan PM, Fowden AL. Adaptations in placental phenotype depend on route and timing of maternal dexamethasone administration in mice. Biol Reprod. 2013;89(4):80. doi: 10.1095/biolreprod.113.109678. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJ, et al. The placental origins of sudden cardiac death. Int J Epidemiol. 2012;41(5):1394–1399. doi: 10.1093/ije/dys116. [DOI] [PubMed] [Google Scholar]
- 18.Thornburg KL, O’Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(Suppl):S54–S59. doi: 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol. 2006;28(2):126–136. doi: 10.1002/uog.2832. [DOI] [PubMed] [Google Scholar]
- 20.Shaut CA, Keene DR, Sorensen LK, Li DY, Stadler HS. HOXA13 is essential for placental vascular patterning and labyrinth endothelial specification. PLoS Genet. 2008;4(5):e1000073. doi: 10.1371/journal.pgen.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams RH, et al. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6(1):109–116. [PubMed] [Google Scholar]
- 22.Barak Y, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One. 2010;5(10):e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michelsohn AM, Anderson DJ. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron. 1992;8(3):589–604. doi: 10.1016/0896-6273(92)90285-l. [DOI] [PubMed] [Google Scholar]
- 25.Corrigan N, Brazil DP, Auliffe FM. High-frequency ultrasound assessment of the murine heart from embryo through to juvenile. Reprod Sci. 2010;17(2):147–157. doi: 10.1177/1933719109348923. [DOI] [PubMed] [Google Scholar]
- 26.Rog-Zielinska EA, et al. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22(16):3269–3282. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 27.Rubattu S, Volpe M. The atrial natriuretic peptide: A changing view. J Hypertens. 2001;19(11):1923–1931. doi: 10.1097/00004872-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, et al. Identification of hypertrophy- and heart failure-associated genes by combining in vitro and in vivo models. Physiol Genomics. 2012;44(8):443–454. doi: 10.1152/physiolgenomics.00148.2011. [DOI] [PubMed] [Google Scholar]
- 29.Jozkowicz A, Dulak J, Piatkowska E, Placha W, Dembinska-Kiec A. Ligands of peroxisome proliferator-activated receptor-gamma increase the generation of vascular endothelial growth factor in vascular smooth muscle cells and in macrophages. Acta Biochim Pol. 2000;47(4):1147–1157. [PubMed] [Google Scholar]
- 30.Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J. 1995;16(Suppl C):38–44. doi: 10.1093/eurheartj/16.suppl_c.38. [DOI] [PubMed] [Google Scholar]
- 31.Kotelevtsev Y, et al. Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999;103(5):683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54(2-3):525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother’s body size and placental size predict coronary heart disease in men. Eur Heart J. 2011;32(18):2297–2303. doi: 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okada Y, et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007;25(2):233–237. doi: 10.1038/nbt1280. [DOI] [PubMed] [Google Scholar]
- 35.Kane AD, Herrera EA, Hansell JA, Giussani DA. Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol. 2012;590(2):323–334. doi: 10.1113/jphysiol.2011.217968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumasawa K, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108(4):1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramma W, Ahmed A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol. 2014;101-102:153–160. doi: 10.1016/j.jri.2013.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saad AF, et al. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod Sci. 2014;21(1):138–145. doi: 10.1177/1933719113492207. [DOI] [PubMed] [Google Scholar]
- 39.Forbes K, et al. Statins inhibit insulin-like growth factor action in first trimester placenta by altering insulin-like growth factor 1 receptor glycosylation. Mol Hum Reprod. 2015;21(1):105–114. doi: 10.1093/molehr/gau093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarek J, et al. The transfer of pravastatin in the dually perfused human placenta. Placenta. 2013;34(8):719–721. doi: 10.1016/j.placenta.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Nanovskaya TN, et al. Transplacental transfer and distribution of pravastatin. Am J Obstet Gynecol. 2013;209(4):373.e1–373.e5. doi: 10.1016/j.ajog.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giussani DA, et al. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One. 2012;7(2):e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richter HG, et al. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol. 2012;590(6):1377–1387. doi: 10.1113/jphysiol.2011.226340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes MC, et al. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 2006;26(14):3840–3844. doi: 10.1523/JNEUROSCI.4464-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol. 2006;291(3):H1421–H1428. doi: 10.1152/ajpheart.00031.2006. [DOI] [PubMed] [Google Scholar]
- 46.Corstius HB, et al. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res. 2005;57(6):796–800. doi: 10.1203/01.PDR.0000157726.65492.CD. [DOI] [PubMed] [Google Scholar]
- 47.Vandesompele J, et al. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034.