Abstract
Background
The role of branched-chain amino acids (BCAAs) in cardiovascular disease (CVD) remains poorly understood. We hypothesized that baseline BCAA concentrations predict future risk of CVD and that a Mediterranean Diet (MedDiet) intervention may counteract this effect.
Methods
We developed a case-cohort study within the “PREvención con DIeta MEDiterránea” (PREDIMED), with 226 incident CVD cases and 781 non-cases. We used LC-MS/MS to measure plasma BCAAs (leucine, isoleucine and valine), both at baseline and after 1-year follow-up. The primary outcome was a composite of incident stroke, myocardial infarction, or cardiovascular death.
Results
After adjustment for potential confounders, baseline leucine and isoleucine concentrations were associated with higher CVD risk: the hazard ratios (HRs) for the highest vs. lowest quartile were 1.70 (95% confidence interval, 1.05–2.76) and 2.09 (1.27–3.44), respectively. Stronger associations were found for stroke. For both CVD and stroke, we found higher HRs across successive quartiles of BCAAs in the control group than in the MedDiet groups. Using stroke as the outcome, a significant interaction (P=0.009) between the baseline BCAA score and the intervention with MedDiet was observed. No significant effect of the intervention on 1-yr changes in BCAAs nor any association between 1-year changes in BCAAs and CVD were observed.
Conclusions
Higher concentrations of baseline BCAAs were associated with increased risk of CVD, especially stroke, in a high cardiovascular risk population. A Mediterranean-style diet had a negligible effect on 1-year changes in BCAAs, but it may counteract the harmful effects of BCAAs on stroke.
Keywords: Cardiovascular disease, Stroke, Branched-chain amino acids, Leucine, Isoleucine, Valine, Mediterranean Diet
Introduction
Although compelling evidence has demonstrated that adherence to the Mediterranean diet (MedDiet) is effective for primary CVD prevention (1), the biological mechanisms underpinning this effect are not well understood (2). Recently, metabolomics applied to the field of nutrition has offered great potential to provide new insights into underlying mechanisms (3). Several studies have found an association between the concentrations of select small molecule metabolites in peripheral blood and CVD risk (4–7). Among various metabolites, branched-chain amino acids (BCAAs) have been found to predict obesity, insulin resistance, diabetes, and CVD outcomes.
BCAAs, including leucine, isoleucine, and valine, are a subgroup of amino acids derived from the diet that are essential for normal growth and function at the cell and organism levels (8). The relevance of the metabolism of these amino acids to coronary heart disease remains poorly understood (8), but increased circulating concentrations of BCAAs may be explained by an obesity-related decline in their catabolism in adipose tissue (9). Using a case-cohort study conducted in older participants at high cardiovascular risk from the PREDIMED trial, we sought to address: 1) whether baseline BCAA concentrations predict future risk of CVD; 2) whether the MedDiet interventions counteracts the presumed deleterious effects of BCAAs on CVD risk; and 3) whether the beneficial effect of the MedDiet on CVD is partially explained by its effects on plasma BCAA concentrations.
Methods
We designed an unstratified case-cohort study within the “PREvención con DIeta MEDiterránea” (PREDIMED) trial (“www.predimed.es”). The protocol, design, methods, and primary results of the PREDIMED trial have been reported in detail elsewhere (1,10). Briefly, 7,447 participants were randomly assigned to a Mediterranean diet supplemented with 1 liter per week of extra-virgin olive oil for participants and their families (MedDiet+EVOO), a Mediterranean diet supplemented with 30 grams of mixed nuts (15 g of walnuts, 7.5 g of hazelnuts, and 7.5 g of almonds) per day (MedDiet+nuts), or a control diet consisting of advice to reduce the intake of all types of fat (control group).
The present study comprises a random selection of 794 participants (approximately 10%) from the eligible PREDIMED cohort at baseline with available EDTA plasma samples, together with all incident cases of CVD during the follow-up with available samples (samples were unavailable for 57 out of the 288 incident CVD cases occurring in the PREDIMED study). We excluded 18 participants because metabolite values were not available (n=3), or because extreme outlying values of metabolite concentrations (baseline or after 1-year follow-up) were detected in the initial multidimensional scaling analysis (n=15). Of the 970 participants included in our analyses, 781 were in the subcohort (including 37 overlapping cases) and 226 were CVD cases (Supplemental Figure 1). Of the CVD cases, 177 experienced a stroke (112 ischemic and 5 hemorrhagic strokes). In addition, 916 participants of the 970 also had a 1-year follow-up sample and were included in the 1-year change analyses. Medical conditions and risk factors were collected using a questionnaire during the first screening visit.
Study samples and metabolite profiling
Fasting blood samples were collected at baseline and yearly thereafter during follow-up. After an overnight fast, plasma EDTA tubes were collected and aliquots were coded and kept refrigerated until they were stored at −80°. In June 2014, pairs of samples (baseline and first-year visits from each participant) were randomly ordered and shipped on dry ice to the Broad Institute of Harvard and MIT for the metabolomics analyses.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) techniques developed at the Broad Institute were used to quantitatively profile amino acids, biogenic amines, and other polar plasma metabolites (6,7). Pooled reference samples and standard reference samples were inserted in the analytical queue every twenty samples. The raw data were processed using MultiQuant software (AB SCIEX) to integrate chromatographic peaks and the data were visually inspected to ensure the quality of signal integration.
Clinical assessment
The PREDIMED primary endpoint was a composite of non-fatal acute myocardial infarction, non-fatal stroke, or cardiovascular death. Information on primary endpoints was collected by study physicians who were blinded to the intervention and from other sources of information, such as the National Death Index (1). This anonymized information was sent to the Clinical Endpoint Committee which adjudicated the events blinded to the intervention group.
Statistical analysis
Individual BCAA values were normalized and scaled to multiples of 1 SD using the rank-based inverse normal transformation. We fitted weighted Cox regression models using Barlow weights to account for the over-representation of cases, as recommended for case-cohort designs (11). We calculated hazard ratios (HR) and their 95% confidence intervals (95% CI) for the composite CVD endpoint, and also separately for stroke, which was the most frequently occurring event of the cardiovascular composite endpoint. Follow-up time was calculated from the date of enrollment to the date of diagnosis of CVD for cases, and to the date of the last visit or the end of the follow-up period for non-cases (December 1, 2010). We fitted crude models, age-, sex-, and intervention group-adjusted models, and multivariable models. Multivariate-adjusted models were additionally adjusted for smoking status (never/current/former), body mass index (kg/m2), leisure-time physical activity (metabolic equivalent task [MET]s-min/day), and family history of premature coronary heart disease (yes/no). In a secondary analysis, we additionally adjusted for diabetes, hypertension and dyslipidemia because these risk factors were likely to be intermediate biomarkers in the causal pathway between BCAAs and risk of CVD. We calculated a baseline BCAA score as the weighted sum of normalized values of leucine, isoleucine, and valine by inverse normal transformation. The weights corresponded to the respective coefficients from the multivariable Cox regression model fitted with each individual metabolite (7).
The individual BCAAs and the BCAA score were also analyzed according to quartiles of their distributions. Quartile cutpoints were generated based on the distributions of BCAAs among non-cases. We conducted tests of linear trend by examining an ordinal score based on the median value in each quartile of BCAAs in the multivariable models.
We conducted joint analyses and interactions tests for the BCAA score and the intervention groups (MedDiet+EVOO and MedDiet+nuts vs. control group). We additionally conducted stratified analyses by diabetes status at baseline, sex and age group (<70 yrs vs. ≥70 yrs). The likelihood ratio test was used to assess the significance of interaction between stratifying variables and the BCAA score.
We also examined the associations between 1-year changes in individual BCAAs and the overall BCAA score on CVD risk (using as outcome only cases occurring after 1-year follow-up). With respect to individual metabolites, we first calculated the difference between baseline and 1-year concentrations, then normalized this difference using the inverse normal transformation. For 1-year changes in the BCAA score, we summed 1-year changes of the 3 metabolites and subsequently normalized the sum.
We conducted several sensitivity analyses. First, we additionally adjusted for total fat (g/d), protein (g/d), alcohol (g/d), fiber (g/d) and total energy intake (kcal/d). Second, we adjusted for baseline adherence to the Mediterranean dietary pattern, using a previously validated 14-item score (12). Finally, we additionally adjusted for plasma concentrations of other amino acids that were correlated with the BCAA score.
All statistical analyses were performed using Stata version 13.1 (Stata Corp).
Results
We included 970 participants, 226 CVD cases and 744 non-cases followed for a median of 4.6 years. Table 1 shows characteristics of participants in the random subcohort and in all CVD cases (with 37 subjects overlapping). As expected, as a result of random selection, the characteristics of the subcohort were very similar to those obtained in the full PREDIMED cohort (1). A higher intake of protein, fats, alcohol and total energy but a lower intake of carbohydrates and fiber were found in participants with higher concentrations of BCAAs (Supplemental Table 1).
Table 1.
Baseline participant characteristics in the random subcohort and of the cases
| Subcohorta | Cases | Pb | |
|---|---|---|---|
| n | 781 | 226 | |
|
| |||
| Age (years) | 67.2 (6) | 69.5 (6.5) | <0.001 |
| Sex (% women), | 56.7 | 40.3 | <0.001 |
| Intervention group, % | |||
| MedDiet+EVOO | 37.0 | 35.4 | 0.122 |
| MedDiet+nuts | 33.2 | 28.8 | |
| Control | 29.8 | 35.8 | |
| Family history of premature CHD, % | 24.8 | 19.5 | 0.064 |
| Hypertension, % | 83.5 | 82.3 | 0.627 |
| Dyslipidemia, % | 73.4 | 58.0 | <0.001 |
| Diabetes, % | 47.1 | 64.6 | <0.001 |
| Smoking, % | |||
| Never | 62.1 | 46.0 | <0.001 |
| Former | 25.5 | 33.6 | |
| Current | 12.4 | 20.4 | |
| Waist circumference, cm | 99.9 (9.9) | 101.5 (10.7) | 0.028 |
| Body mass index, kg/m2 | 29.7 (3.6) | 29.6 (3.8) | 0.704 |
| Physical activity, METs/d | 259 (259) | 237 (238) | 0.164 |
| Education, % | |||
| Elementary or lower | 76.2 | 80.1 | 0.388 |
| Secondary or higher | 23.8 | 19.9 | |
| Total energy intake, kcal/d | 2337 (618) | 2363 (688) | 0.572 |
| Leucine intake, g/d | 7.5 (2.0) | 7.4 (2.2) | 0.667 |
| Isoleucine intake, g/d | 4.6 (1.3) | 4.6 (1.3) | 0.940 |
| Valine intake, g/d | 5.1 (1.3) | 5.1 (1.5) | 0.746 |
| Score for adherence to Mediterranean dietc | 8.8 (1.9) | 8.5 (1.8) | 0.005 |
Abbreviation: EVOO, Extra-virgin olive oil; CHD, coronary heart disease; MET, metabolic equivalent. Values are mean (SD) or percentage.
37 cases are included in the randomly selected subcohort.
To avoid the problem inherent to the fact that some cases were included both in the subcohort and the cases series we compared the observed means (or proportions) in the cases vs. the expected values (expected=mean or proportion observed in the subcohort)
This score is based on the 14-item dietary screener.33
Baseline BCAAs and risk of CVD and stroke
Table 2 shows the associations between baseline individual BCAAs (leucine, isoleucine, and valine) and both CVD and stroke. Leucine and isoleucine, but not valine, were associated with CVD (model 2). Baseline concentrations of individual BCAAs were more strongly associated with stroke than with the composite CVD endpoint. Each 1-SD increase in baseline leucine, isoleucine, and valine was associated with 45% (95%CI, 13–85%), 51% (95%CI, 17–95%), and 37% (95%CI, 8–73%) relative increases in the risk of stroke, respectively (Table 2). Similarly, we found a stronger association for the outcome limited to stroke than for the overall CVD composite outcome in the comparison between extreme quartiles of BCAAs: after multivariate adjustment, the estimated HR for incident stroke in the top versus the bottom quartile was 2.17 (95%CI, 1.17–4.03) for leucine, 2.85 (95%CI, 1.49–5.45) for isoleucine, and 1.91 (95%CI, 1.02–3.57) for valine. These associations were attenuated and no longer significant except for isoleucine after adjusting for diabetes, dyslipidemia and hypertension (Supplemental Table 2).
Table 2.
Incident Composite CVD and Stroke by Baseline Plasma BCAA Concentrationsa (Leucine, Isoleucine, and Valine) in the PREDIMED Trial, 2003–2010: Observed Event Rates and Hazard Ratios.
| Composite CVD (226 cases, 754 non-cases) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Leucine | Isoleucine | Valine | ||||
| Crude | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Per SD | 1.25 (1.07–1.45) | 0.004 | 1.36 (1.17–1.58) | <0.001 | 1.18 (1.00–1.37) | 0.043 |
|
| ||||||
| Model 1b | ||||||
| Per SD | 1.19 (1.01–1.41) | 0.039 | 1.29 (1.09–1.53) | 0.004 | 1.13 (0.96–1.34) | 0.140 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.55 (0.96 – 2.50) | 1.44 (0.86 – 2.41) | 1.42 (0.89 – 2.25) | |||
| Q3 | 1.32 (0.82 – 2.14) | 1.72 (1.05 – 2.81) | 1.22 (0.76 – 1.95) | |||
| Q4 | 1.71 (1.06 – 2.75) | 2.11 (1.29 – 3.45) | 1.42 (0.90 – 2.23) | |||
| P for trend | 0.060 | 0.002 | 0.238 | |||
|
| ||||||
| Model 2c | ||||||
| Per SD | 1.19 (1.00–1.42) | 0.044 | 1.29 (1.08–1.54) | 0.005 | 1.12 (0.95–1.33) | 0.183 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.57 (0.97–2.55) | 1.44 (0.85–2.42) | 1.42 (0.88–2.28) | |||
| Q3 | 1.31 (0.80–2.14) | 1.71 (1.04–2.83) | 1.18 (0.73–1.91) | |||
| Q4 | 1.70 (1.05–2.76) | 2.09 (1.27–3.44) | 1.40 (0.88–2.24) | |||
| P for trend | 0.072 | 0.003 | 0.290 | |||
| Stroke (117 cases, 744 non-cases)
| ||||||
|---|---|---|---|---|---|---|
| Leucine | Isoleucine | Valine | ||||
| Crude | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| Per SD | 1.38 (1.11–1.72) | 0.003 | 1.45 (1.17–1.81) | 0.001 | 1.34 (1.08–1.66) | 0.008 |
|
| ||||||
| Model 1b | ||||||
| Per SD | 1.44 (1.13–1.84) | 0.004 | 1.51 (1.17–1.94) | 0.001 | 1.36 (1.08–1.72) | 0.009 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.36 (0.72–2.57) | 1.59 (0.81–3.14) | 1.69 (0.91–3.14) | |||
| Q3 | 1.41 (0.75–2.64) | 1.61 (0.82–3.18) | 1.67 (0.89–3.14) | |||
| Q4 | 2.17 (1.17–4.03) | 2.84 (1.48–5.47) | 1.90 (1.04–3.49) | |||
| P for trend | 0.010 | 0.001 | 0.065 | |||
|
| ||||||
| Model 2c | ||||||
| Per SD | 1.45 (1.13–1.85) | 0.003 | 1.51 (1.17–1.95) | 0.001 | 1.37 (1.08–1.73) | 0.009 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.35 (0.71–2.57) | 1.60 (0.82–3.13) | 1.69 (0.91–3.16) | |||
| Q3 | 1.39 (0.73–2.63) | 1.60 (0.81–3.17) | 1.66 (0.87–3.17) | |||
| Q4 | 2.17 (1.17–4.03) | 2.85 (1.49–5.45) | 1.91 (1.02–3.57) | |||
| P for trend | 0.011 | 0.001 | 0.071 | |||
Abbreviations: BCAA, branched-chain amino acid; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MedDiet, Mediterranean diet intervention group supplemented with either extra-virgin olive oil or nuts; PREDIMED, Prevencion con dieta mediterranea.
An inverse normal transformation was applied to raw values.
Model 1: Adjusted for age (years), sex (male, female), and intervention group (MedDiet+EVOO, MedDiet+nuts).
Model 2: Adjusted as for Model 1, plus body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), and family history of premature coronary heart disease (yes, no).
Per 1-SD increase in the BCAA score, a 46% higher risk (HR=1.46, 95% CI, 1.05–2.01) of incident CVD was observed in the multivariable model (Table 3). In stratified analyses by intervention groups, higher HRs across BCAA score quartiles in the control group compared with the MedDiet groups, for both CVD and stroke, were observed. The direct association between baseline BCAA concentrations and CVD became weaker and no longer statistically significant after additionally adjusting for diabetes, dyslipidemia and hypertension. Also the association with stroke was attenuated after adjusting for these additional factors (Supplemental Table 3). Similarly, no association was found within the two subgroups after stratifying by diabetes status (Supplemental Table 4).
Table 3.
Incident Composite CVD and Stroke by Baseline Plasma Branched-Chain Amino Acid Scorea in the PREDIMED trial, 2003–2010: Observed Event Rates and Hazard Ratios.
| Composite CVD
| ||||||
|---|---|---|---|---|---|---|
| Overall | Both MedDiet Groups | Control Group | ||||
| Subcohort, n | 781 | 548 | 233 | |||
| Cases, n | 226 | 145 | 81 | |||
|
| ||||||
| Crude | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| Per SD | 1.60 (1.21–2.13) | 0.001 | 1.66 (1.16–2.37) | 0.006 | 1.48 (0.93–2.35) | 0.101 |
|
| ||||||
| Model 1 | ||||||
| Per SDb | 1.46 (1.07–2.01) | 0.018 | 1.52 (1.02–2.27) | 0.038 | 1.32 (0.80–2.19) | 0.278 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.19 (0.73–1.92) | 0.72 (0.40–1.30) | 2.54 (1.04–6.19) | |||
| Q3 | 1.24 (0.78–1.97) | 0.86 (0.49–1.51) | 2.41 (0.99–5.88) | |||
| Q4 | 1.45 (0.91–2.32) | 1.44 (0.84–2.46) | 1.45 (0.57–3.70) | |||
| P for trend | 0.114 | 0.114 | 0.828 | |||
|
| ||||||
| Model 2 | ||||||
| Per SDc | 1.46 (1.05–2.01) | 0.024 | 1.58 (1.05–2.37) | 0.029 | 1.28 (0.77–2.14) | 0.346 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 1.20 (0.74–1.94) | 0.75 (0.41–1.37) | 2.73 (1.11–6.76) | |||
| Q3 | 1.22 (0.76–1.97) | 0.86 (0.47–1.55) | 2.44 (0.98–6.08) | |||
| Q4 | 1.43 (0.89–2.31) | 1.53 (0.88–2.67) | 1.40 (0.53–3.67) | |||
| P for trend | 0.145 | 0.082 | 0.966 | |||
| Stroke
| ||||||
|---|---|---|---|---|---|---|
| Overall | Both MedDiet Groups | Control Group | ||||
| Subcohort, n | 767 | 539 | 228 | |||
| Cases, n | 117 | 71 | 46 | |||
|
| ||||||
| Crude | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| Per SD | 1.38 (1.12–1.69) | 0.002 | 1.31 (1.16–2.37) | 0.051 | 1.50 (1.08–2.08) | 0.015 |
|
| ||||||
| Model 1 | ||||||
| Per SDb | 1.42 (1.13–1.79) | 0.003 | 1.37 (1.01–1.86) | 0.043 | 1.52 (1.05–2.18) | 0.025 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 0.99 (0.52–1.88) | 0.80 (0.37–1.72) | 1.67 (0.53–5.23) | |||
| Q3 | 1.39 (0.77–2.50) | 0.93 (0.44–1.98) | 2.37 (0.78–7.22) | |||
| Q4 | 1.70 (0.94–3.09) | 1.68 (0.82–3.43) | 1.97 (0.64–6.12) | |||
| P for trend | 0.048 | 0.146 | 0.227 | |||
|
| ||||||
| Model 2 | ||||||
| Per SDc | 1.43 (1.13–1.80) | 0.003 | 1.40 (1.03–1.90) | 0.034 | 1.51 (1.06–2.16) | 0.022 |
| Across quartiles | ||||||
| Q1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Q2 | 0.97 (0.51–1.85) | 0.81 (0.37–1.78) | 1.69 (0.54–5.27) | |||
| Q3 | 1.37 (0.75–2.49) | 0.94 (0.42–2.10) | 2.46 (0.8–7.56) | |||
| Q4 | 1.69 (0.92–3.10) | 1.77 (0.84–3.72) | 2.03 (0.65–6.36) | |||
| P for trend | 0.053 | 0.120 | 0.211 | |||
Abbreviations as in Table 1.
An inverse normal transformation was applied to raw values of leucine, isoleucine, and valine, and a weighted sum of these 3 values was computed to calculate the BCAA score
Model 1: Adjusted for age (years), sex (male, female), and intervention group (except in analyses by intervention group).
Model 2: Adjusted as for model 1, plus body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day) and family history of premature coronary heart disease (yes, no).
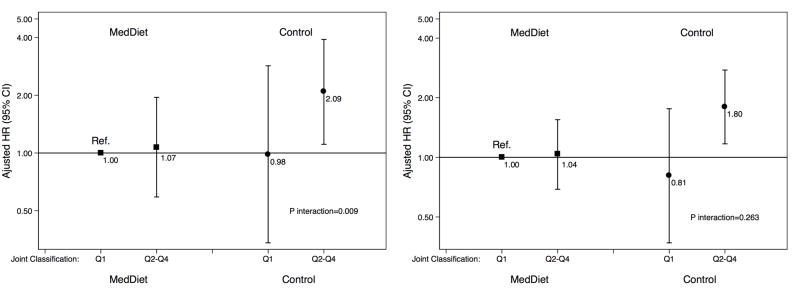
In a joint classification of BCAA score and intervention groups, the reference category corresponded to those participants in the MedDiet groups who were in the lowest quartile of the BCAA score. We found a positive and significantly higher risk of CVD in the higher quartiles of the BCAA score of the control group (HR for Q1 vs. Q2–Q4=1.80; 95%CI, 1.17–2.76) (Figure 1A) (P for interaction = 0.058) compared to the lowest quartile of the BCAA score of the MedDiet groups. The interaction was stronger in joint analyses limited to stroke (Figure 1B) (P for interaction = 0.009). Specifically, after excluding participants in the MedDiet+EVOO group, those in the MedDiet+nuts group attenuated the increased risk of stroke associated with higher baseline concentrations of BCAA (P for interaction = 0.014). We did not find any statistically significant interaction between MedDiet+EVOO and baseline concentrations of BCAA after excluding those participants in the MedDiet+nuts group (P for interaction = 0.60).
Figure 1. Multivariate-adjusted Hazard Ratios (95%CI) of Incident CVD and Quartiles of BCAA Score at Baseline, Stratified by Intervention group (MedDiet versus Control Group).
Panel A) Composite CVD. Panel B) Stroke. An inverse normal transformation was applied to raw values of leucine, isoleucine, and valine and a weighted sum of these 3 values was computed to calculate the BCAA score. The hazard ratios are adjusted for age (years), sex (male/female), intervention group, body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), and family history of premature coronary heart disease.
P for interaction with 2 degrees of freedom between each MedDiet intervention group (EVOO and nuts) (binary, yes/no) and the BCAA score (continuous), with 2 cross-product terms (EVOO*BCAA and NUTS*BCAA). Abbreviations: BCAA, branched-chain amino acid; CI, confidence interval; HR, hazard ratio; MedDiet, Mediterranean diet intervention groups.
Additional adjustment for dietary variables (energy-providing macronutrients and total energy intake) and for baseline adherence to Mediterranean diet the results did not materially change (HR per SD=1.49; 95% CI: 1.07–2.09 and 1.49; 95% CI: 1.07–2.07, respectively). We observed stronger associations between BCAA and CVD after adjusting for baseline plasma concentrations of amino acids correlated with the BCAA score: the HRs per SD were 1.85 (95%CI 1.13–3.03), 1.67 (95% CI 1.18–2.36), and 1.81 (95%CI 1.22–2.72) when additionally adjusted for phenylalanine/tyrosine, arginine and GABA, respectively.
We did not observe any statistically significant interaction between baseline BCAAs and sex or age on composite CVD or stroke. In sensitivity analyses, we assessed the effect of the MedDiet intervention on the overall CVD composite outcome and also limited to stroke, stratified by baseline concentrations of BCAA score (Supplemental Table 5). The cardioprotective effect of the MedDiet interventions appeared more pronounced among participants with lower baseline BCAA scores, although the interaction was not statistically significant.
Associations between 1-yr changes in BCAAs and the effect of MedDiet intervention on the risk of CVD and stroke
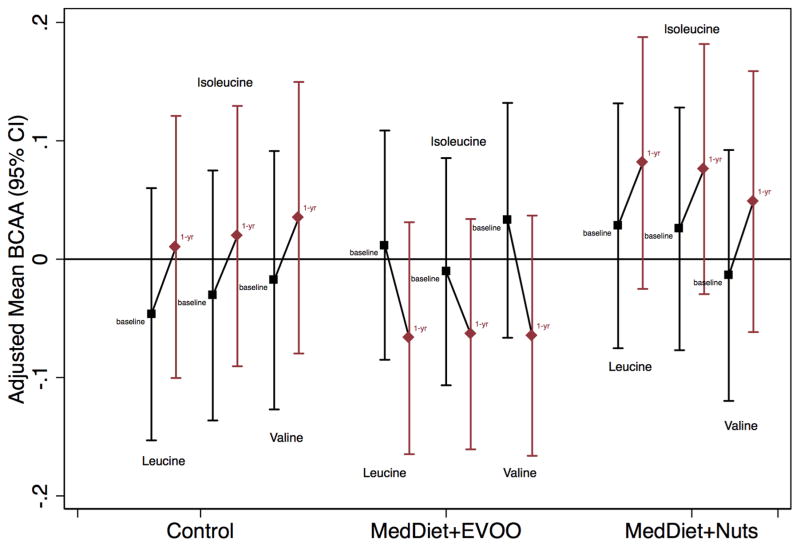
Figure 2 shows adjusted mean values (and 95% CI) for each metabolite at baseline and after 1-yr follow-up by intervention group. A non-significant decrease in individual BCAAs was observed only in the MedDiet+EVOO group.
Figure 2. Changes in Leucine, Isoleucine, and Valine after 1 Year of Intervention, by Intervention Group.
Changes are adjusted for age (years), sex (male, female), and body mass index (kg/m2). Abbreviations: BCAA, branched-chain amino acid; EVOO, extra virgin olive oil; MedDiet, Mediterranean diet intervention group. *Baseline means comparison between intervention groups
Changes in individual BCAAs were not associated with the risk of CVD (Supplemental Table 6). Analyses limited to stroke yielded similar results. Similarly, we did not observe any significant association between 1-yr changes in BCAA score and the risk of CVD or stroke (Supplemental Table 7), nor did we observe any association between the intervention and 1-yr changes in the BCAA score (P=0.101 and 0.729 for the MedDiet+EVOO group and MedDiet+nuts group, respectively).
The magnitude of effects of the MedDiet intervention on CVD or stroke was unchanged after adjusting for baseline and 1-yr change in BCAA score (Supplemental Table 8).
Discussion
In this case-cohort study of 970 participants from the PREDIMED trial, we found that baseline circulating BCAAs were positively associated with CVD and they were even more strongly associated with stroke. Our results suggest that the MedDiet interventions may counteract the deleterious associations between baseline BCAAs and risk of CVD and especially of stroke.
Our results are consistent with several previous studies of baseline BCAAs and CVD (4,6, 13), however our study represents the first longitudinal assessment with repeated measurements of BCAA concentrations. Shah, et al (4) found a direct cross-sectional association between a principal component-derived factor with BCAAs and related catabolites and prevalent myocardial infarction. They also showed an association between this BCAA-related factor and coronary artery disease, an association which was also replicated in a nested case-control study (13). Another case-control study observed a positive association between a score of three amino acids at baseline (tyrosine, phenylalanine, and isoleucine) and the risk of CVD (6).
Interestingly, despite the increased CVD risk observed for baseline BCAAs, we found no association between 1-yr changes in BCAAs and CVD risk. Further, our study was able to assess the interaction with a dietary intervention and our results suggest that the reduction in CVD risk in participants receiving a MedDiet intervention was not explained by 1-yr changes in BCAAs.
The health effect of BCAAs remains controversial. On the one hand, BCAA-rich diets or diets supplemented with BCAAs could improve metabolic health (14) and they could have benefits in patients with heart failure (15). On the other hand, several studies have shown detrimental associations between high plasma values of BCAAs and cardiometabolic diseases (16), especially obesity (17), insulin resistance (18), diabetes (7), and carotid intima-media thickness (19). Potential mechanisms underlying the effects of BCAAs are yet to be elucidated. High concentrations of BCAAs could lead to insulin resistance but alternatively these high values could also represent a biomarker of a metabolic dysregulated status rather than an initiating event in the causal chain leading from dietary exposures to insulin resistance (14). Current research suggests that the rise in circulating BCAAs is driven in part by an obesity-related decline in their catabolism in adipose tissue (9). This defective BCAA catabolism leads to an accumulation of BCAAs and their intermediate products, inducing oxidative stress (20). Specifically, leucine may have a role in the inhibition of nitric oxide (NO) synthesis in endothelial cells and this reduced bioavailability contributes to the development of endothelial dysfunction (21). Moreover, higher concentrations of BCAAs can lead to an increased activity of mTOR, and, consequently, induce alterations in protein turnover, lipid/glucose/nucleotide metabolism, and autophagy regulation in the heart (20).
With the exception of isoleucine, the associations became attenuated and non-significant when we additionally adjusted for diabetes or stratified by diabetic status. A similar change was observed when adjusting for hypertension and dyslipidemia (Supplemental Tables 2–3). However, we did not adjust our main estimates for type 2 diabetes because this would probably represent an overadjustment, given that type 2 diabetes may represent an intermediate step in the causal pathway.
A novel finding of our study is that a Mediterranean diet may offset the increased risk associated with high baseline concentrations of BCAAs on CVD. Given that the PREDIMED participants were at high risk of CVD at baseline, it seems plausible that BCAAs concentrations were already abnormally high. Previous studies reported beneficial effects of a Mediterranean-style diet on obesity (22), diabetes (23), and cardiovascular risk factors (24). In our study, the MedDiet interventions did not appear to significantly change BCAA concentrations, but participants with higher baseline BCAA scores had higher CVD risk, notably in the control group. These data and previous findings provide additional evidence to support the hypothesis that an intervention with a Mediterranean diet may counteract disease risk through changes in classical cardiovascular risk factors, but not directly on the potentially detrimental metabolic effects of increased concentrations of BCAAs. The benefits of a Mediterranean dietary pattern on stroke, and more specifically the potential protective effect of extra-virgin olive oil and nuts, are well known (25–27). Our interpretation is that the overall Mediterranean pattern is more relevant to explain the risk reduction than just one of its components (2).
Both basic science and epidemiologic studies are needed to increase our knowledge about the biological processes affected by altered BCAA homeostasis and its consequences on the development of CVD (8). It is largely unknown how both systemic and local BCAA catabolism is impaired when the heart is under pathological stressors or during the acute phase of a CVD event (20). In line with the stronger association found in our analyses limited to stroke, BCAAs are known to have a unique role in the brain. One hypothesis is that plasma BCAAs may provoke an increase in brain BCAAs and a decline in aromatic amino acids and this may have functional consequences such as altered hormonal function and blood pressure (28). Moreover, nuts are rich in arginine and its anti-atherogenic effect is in part connected with the arginine-NO pathway (29). This effect may counteract the inhibition of leucine on NO synthesis from L-arginine (21). However, one recent study found that diminished concentrations of BCAAs are present in patients with ischemic stroke (30), and therefore, more research is needed to fully understand these complex biological mechanisms.
In both the control and the MedDiet+nuts intervention arms we observed increased BCAA concentrations after one year of dietary intervention in contrast to the MedDiet+EVOO group where a reduction was observed. However, none of these changes were statistically significant. This may seem counterintuitive given that a reduced risk of CVD was obtained with the two MedDiet interventions and therefore cast doubts on the use of changes in BCAAs as a relevant biomarker of the dietary interventions. On the other hand, given that the PREDIMED intervention was not specifically designed to modify the profile of amino acid intake, this result is not unexpected. The most likely explanation for our findings is that the MedDiet interventions did not appear to directly affect changes in BCAA concentrations, but they may nevertheless counteract the deleterious associations between baseline BCAAs and risk of CVD and especially of stroke, potentially via downstream pathways or alternative protective mechanisms (2).
Several studies have assessed the association between dietary intakes and BCAA concentrations (31–37). Two studies reported an immediate increase of plasma BCAA concentrations after consuming a Western-style diet (31,32). However, another study found increased BCAA content in a dietary pattern rich in fish (e.g., tuna), soybeans, cheese, chicken, and turkey (33). Further research is needed to know the optimal quantity and quality of protein and amino acids intake to improve cardiometabolic health, especially among older people (34). In contrast to previous findings, a recent study found no correlation between long-term dietary intakes (estimated both with food frequency questionnaire and dietary records) and plasma concentrations of several amino acids, including BCAAs (35). These authors suggested that dietary intakes are probably not a major determinant of plasma BCAAs concentrations (35). However, since dietary protein from both animals and plants is an important source of BCAAs, dietary intakes of protein quality and quantity should still be considered (36). Our results may suggest that high baseline concentrations of BCAAs associated with higher cardiovascular risk might be mainly a biomarker of the underlying metabolic dysfunctions that can be partially independent of the quantity of BCAAs ingested with diet.
Strengths in our study include the use of major cardiovascular events as main outcome, the prospective evaluation of the association between BCAAs plasma concentrations and incident CVD, and the adjustment for multiple potential confounders associated with CVD within a well-characterized primary prevention trial. Several limitations should also be considered. First, we cannot rule out that BCAA concentrations were not equally distributed between missing and non-missing samples in cases but this number was relatively low (we assessed 226 of the original 288 cases). Second, while we only included 10% of non-cases from the PREDIMED study, the case-cohort design retains randomization, maximizes the efficiency of a high-throughput metabolomic profiling, and enables the extension of our results to the full cohort. Third, our results may not be generalizable to other populations because all the study participants lived in a Mediterranean country and were at high cardiovascular risk.
In conclusion, our results indicate a direct association between higher concentrations of BCAAs at baseline and increased risk of CVD. This association was even stronger with stroke, but a Mediterranean-style diet appeared to offset the risk associated with increased BCAAs, especially when the diet was enriched with nuts. Our findings also suggest that the Mediterranean diet has a negligible effect of on 1-year changes in BCAAs; therefore, it likely exerts its cardioprotective effects via alternative pathophysiological processes.
Supplementary Material
Acknowledgments
We are very grateful to all the participants for their enthusiastic collaboration, the PREDIMED personnel for their excellent assistance, and the personnel of all affiliated primary care centers.
Abbreviations and acronyms
- BCAA
branched-chain amino acid
- CI
confidence interval
- CVD
cardiovascular disease
- EVOO
extra-virgin olive oil
- FFQ
food frequency questionnaire
- MedDiet
Mediterranean diet
- MET
metabolic equivalent task
- PREDIMED
“PREvención con DIeta MEDiterránea” (Prevention with Mediterranean Diet)
Footnotes
This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.
Financial disclosures: Grants Received: E. R. (California Walnut Commission) and J. S-S, M.A.M-G (International Nut and Dried Fruit Foundation). None of the other authors have a relevant conflict of interest to report related to this research.
References
- 1.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-González MA, Salas-Salvadó J, Estruch R, Corella D, Fitó M, Ros E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog Cardiovasc Dis. 2015;58:50–60. doi: 10.1016/j.pcad.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis MC, Hu FB. Systems epidemiology: a new direction in nutrition and metabolic disease research. Curr Nutr Rep. 2013:2225–35. doi: 10.1007/s13668-013-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–14. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 5.Shah SH, Sun J, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–50. doi: 10.1016/j.ahj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engström G, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34:1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc Res. 2011;90:220–3. doi: 10.1093/cvr/cvr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell metabolism. 2012;15:606–14. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ros E, Covas MI, Fiol M, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–85. doi: 10.1093/ije/dyq250. [DOI] [PubMed] [Google Scholar]
- 11.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 12.Schroder H, Fito M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–5. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232:191–6. doi: 10.1016/j.atherosclerosis.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carubelli V, Castrini AI, Lazzarini V, Gheorghiade M, Metra M, Lombardi C. Amino acids and derivatives, a new treatment of chronic heart failure? Heart Fail Rev. 2015;20:39–51. doi: 10.1007/s10741-014-9436-9. [DOI] [PubMed] [Google Scholar]
- 16.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62:961–9. doi: 10.1016/j.metabol.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wurtz P, Soininen P, Kangas AJ, Rönnemaa T, Lehtimäki T, Kähönen M, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–55. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang R, Dong J, Zhao H, Li H, Guo H, Wang S, et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS One. 2014;9:e99598. doi: 10.1371/journal.pone.0099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Wang Y. Branched Chain Amino Acids in Heart Failure. In: Rajendram R, Preedy VR, Patel VB, editors. Branched Chain Amino Acids in Clinical Nutrition. Vol. 2. New York: Springer; 2015. pp. 81–8. [Google Scholar]
- 21.Yang Y, Wu Z, Meininger CJ, Wu G. l-Leucine and NO-mediated cardiovascular function. Amino Acids. 2015;47:435–47. doi: 10.1007/s00726-014-1904-y. [DOI] [PubMed] [Google Scholar]
- 22.Buckland G, Bach A, Serra-Majem L. Obesity and the Mediterranean diet: a systematic review of observational and intervention studies. Obes Rev. 2008;9:582–93. doi: 10.1111/j.1467-789X.2008.00503.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwingshackl L, Missbach B, König J, Hoffmann G. Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18:1292–9. doi: 10.1017/S1368980014001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grosso G, Mistretta A, Frigiola A, Gruttadauria S, Biondi A, Basile F, et al. Mediterranean diet and cardiovascular risk factors: a systematic review. Crit Rev Food Sci Nutr. 2014;54:593–610. doi: 10.1080/10408398.2011.596955. [DOI] [PubMed] [Google Scholar]
- 25.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M. Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case-control, cohort and intervention studies. Br J Nutr. 2014;112:248–59. doi: 10.1017/S0007114514000713. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Xu G, Wei Y, Zhu W, Liu X. Nut consumption and risk of stroke. Eur J Epidemiol. 2015;30:189–96. doi: 10.1007/s10654-015-9999-3. [DOI] [PubMed] [Google Scholar]
- 28.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005;135:1539S–46S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- 29.Cooke JP, Tsao P, Singer A, Wang BY, Kosek J, Drexler H. Anti-atherogenic effect of nuts: is the answer NO? Arch Intern Med. 1993;153:896, 899, 902. [PubMed] [Google Scholar]
- 30.Kimberly WT, Wang Y, Pham L, Furie KL, Gerszten RE. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke. 2013;44:1389–95. doi: 10.1161/STROKEAHA.111.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López AM, Noriega LG, Diaz M, Torres N, Tovar AR. Plasma branched-chain and aromatic amino acid concentration after ingestion of an urban or rural diet in rural Mexican women. BMC Obes. 2015;2:8. doi: 10.1186/s40608-015-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badoud F, Lam KP, Perreault M, Zulyniak MA, Britz-McKibbin P, Mutch DM. Metabolomics reveals metabolically healthy and unhealthy obese Individuals differ in their response to a caloric challenge. PLoS One. 2015;10:e0134613. doi: 10.1371/journal.pone.0134613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagao K, Jinzu H, Noguchi Y, Bannai M. Impact of Dietary Essential Amino Acids in Man. In: Rajendram R, Preedy VR, Patel VB, editors. Branched Chain Amino Acids in Clinical Nutrition. Vol. 1. New York: Springer; 2015. pp. 3–12. [Google Scholar]
- 34.Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–59. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki M, Ishihara J, Takachi R, Todoriki H, Yamamoto H, Miyano H, et al. Validity of a Self-Administered Food-Frequency Questionnaire for Assessing Amino Acid Intake in Japan: Comparison With Intake From 4-Day Weighed Dietary Records and Plasma Levels. J Epidemiol. 2015 Aug 15; doi: 10.2188/jea.JE20150044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tome D. Criteria and markers for protein quality assessment - a review. Br J Nutr. 2012;108(Suppl 2):S222–9. doi: 10.1017/S0007114512002565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.