Abstract
Little is known about the population structure of social microorganisms, yet such studies are particularly interesting for the ways that genetic variation impacts their social evolution. Dictyostelium, a eukaryotic microbe widely used as a developmental model, has a social fruiting stage in which some formerly independent individuals die to help others. To assess genetic variation within the social amoeba Dictyostelium purpureum, we sequenced ~4000 base pairs of ribosomal DNA (rDNA) from 37 isolates collected in Texas, Virginia, and Japan. Our analysis showed extensive genetic variation between populations and clear evidence of phylogenetic structure. We identified three major phylogenetic groups that were more different than other accepted species pairs. Tests using pairs of clones showed that both sexual macrocyst and asexual fruiting body formation were influenced by genetic divergence. Macrocysts were less likely to form between pairs of clones from different groups than from the same group. There was also a correlation between the genetic divergence of a pair of clones and their degree of mixing within fruiting bodies. These observations suggest that cryptic species might occur within D. purpureum and, more importantly, reveal how genetic variation impacts social interactions.
Keywords: Cellular slime molds, cryptic species, macrocysts, population structure, rDNA gene tree, social evolution
Group-living organisms like the social insects have provided a wealth of insights into social evolution. Recent work has extended the study of social evolution to microbes (Crespi 2001; West et al. 2006). Most of these behaviors are likely to be influenced by the genetic distribution of individual cells or their population structure. If genetically identical cells occur in close proximity in the wild and form social groups, then cooperation benefits close relatives. However, if nonrelatives co-occur in nature on a very small scale and are likely to interact, then cheating or conflict can arise within the social group.
The life cycle of social amoebae presents an opportunity to study the dynamics of group formation and social living. Widely used as a model for the study of multicellular development, these eukaryotic microbes possess an unusual social life cycle in which they achieve multicellularity via aggregation of cells, rather than through a single-cell bottleneck as in most multicellular organisms. Social amoebae usually exist as single-celled, amoeboid predators on bacteria. However, when sufficient densities of social amoebae starve, thousands of individual amoebae aggregate to form a multicellular slug that differentiates into a fruiting body (Bonner 1967). This fruiting body is composed of two basic cell types, the fertile spores and the dead stalk cells, which hold aloft the spores for dispersal by passing invertebrates (Huss 1989). Thus, stalk cells, much like social insect workers, forgo their own reproduction to help others reproduce, and it is this level of cooperation for which a better understanding of group formation is needed.
An alternative to asexual fruiting body formation is sexual mating or macrocyst formation, which also happens under starving conditions but additionally requires darkness and high moisture (Raper 1984; Kessin 2001). Haploid amoeboid cells of two mating types fuse to form a giant cell (i.e., zygote) that engulfs thousands of other amoebae nearby (Raper 1984; Kessin 2001). Then, the macrocyst forms, produces a cellulose wall, undergoes meiosis, and eventually releases offspring. This sexual stage has been studied in several dictyostelid species (Clark et al. 1973; Chang and Raper 1981; Raper 1984; Kessin 2001), and depending on the species, has been found to occur between strains of the same (i.e., homothallic) as well as different (i.e., heterothallic) mating types.
Fruiting body morphology and development have traditionally been used to classify the dictyostelids. However, a recent study reconstructed the phylogeny of the Dictyostelia using molecular data and showed a surprising amount of genetic differentiation among dictyostelid species: as much as from hydra to human (Schaap et al. 2006). This work allows a macroevolutionary analysis of the origins of social traits. Our study has a similar motivation at a more microevolutionary level. We seek to understand how much variation might occur among clones previously assigned to the same species and how this is related to social interactions. Schaap et al. (2006) included two clones of several species in their analysis, and these were sometimes quite divergent, for example in Dictyostelium purpureum. Genetic divergence at this level is likely to determine the suitability of partners for both sexual and cooperative interactions, although in different ways. In particular, interactions between species are fundamentally different from those within species. Accordingly, the objectives of this study were (1) to examine intraspecific genetic variation of the social amoeba D. purpureum by sequencing regions of the nuclear ribosomal DNA (rDNA), (2) to examine the potential for macrocyst formation (the sexual stage) among differently related clones, and (3) to determine whether the genetic distance between pairs of clones correlates with their degree of mixing within individual fruiting bodies given that kin discrimination has been documented to occur in this species (Mehdiabadi et al. 2006).
For both asexual fruiting body formation and sexual macrocyst formation, we predicted that distantly related pairs ought to be less compatible than closely related pairs. However this prediction is complicated by the fact that macrocysts usually have the additional constraint that they only occur between different mating types. With sex, selection often favors associating with more distantly related clones. However, if clones are too distantly related (i.e., cryptic species), then reproductive incompatibility is likely to occur. For altruistic behavior, if clones can recognize relatives (i.e., exhibit kin recognition), selection should favor associating with closely related clones within the species (Hamilton 1964a,b; Mehdiabadi et al. 2006; West et al. 2007).
Methods
SAMPLES, DNA PREPARATION, AND SEQUENCING
We used 37 D. purpureum isolates from five locations around the United States and Japan: (1) Two isolates from Mountain Lake, Virginia (37°21′N, 80°31′W), (2) Two isolates from Japan (35°25′N, 138°41′E), (3) 22 isolates from Houston, Texas (29°46′N, 95°27′W), and the remaining 11 clones from suburbs within 30 miles of Houston, (4) eight isolates from Pasadena, Texas (29°35′N, 95°4′W) and (5) three isolates from Webster, Texas (29°32′N, 95°9′W). Each isolate was frozen as a pure culture (i.e., development of fruiting bodies arising from a single spore) for permanent storage. In addition, we included a published sequence of D. purpureum (GenBank accession number DQ340386). Samples of Dictyostelium discoideum (GenBank accession number X00601.1), Dictyostelium citrinum (GenBank accession number DQ340385.1), and Dictyostelium macrocephalum (GenBank accession number AM168049) served as outgroups.
We used standard methods for clonal growth, DNA extraction, and sequencing (see Supporting material). Primer pairs used to amplify the rDNA are found in the Supporting material. Sequences have been deposited in GenBank under accession numbers FJ424826–FJ424862.
PHYLOGENETIC AND SEQUENCE ANALYSES
We assembled contigs for individual clones in SeqMan (Lasergene ver. 7.0; DNASTAR Inc., Madison, WI) and aligned sequences using ClustalW (Thompson et al. 1994) in BioEdit version 7.0.0 (Hall 1999). We then grouped individual clones into haplotypes (i.e., isolates with identical gene sequences; Table 1).
Table 1.
Dictyostelium purpureum unique haplotypes. Symbols refer to geographic locations of clones as shown in Figure 1.
| Haplotype number | Isolates belonging to that haplotype |
|---|---|
| 1 | QSpu4 ● |
| 2 | QSpu36 ○, QSpu37 ○ |
| 3 | QSpu6 ● |
| 4 | QSpu23 ■ |
| 5 | QSpu26 ■, QSpu27 ■ |
| 6 | QSpu28 ■ |
| 7 | QSpu2 ●, QSpu22 ● |
| 8 | QSpu3 ●, QSpu5 ●, QSpu15 ●, QSpu24 ■ |
| 9 | QSpu16 ● |
| 10 | QSpu25 ■, QSpu35 □ |
| 11 | QSpu1 ● |
| 12 | QSpu21 ● |
| 13 | QSpu33 ▴ |
| 14 | QSpu29 ■ |
| 15 | QSpu30 ■ |
| 16 | QSpu13 ●, QSpu17 ●, QSpu31 ▴, QSpu32 ▴ |
| 17 | QSpu18 ● |
| 18 | QSpu9 ●, QSpu12●, QSpu19 ● |
| 19 | QSpu7 ●, (possibly QSpu10 ●) |
| 20 | QSpu8 ●, (possibly QSpu10 ●), QSpu11● QSpu14●, QSpu20●, QSpu34 □ |
| 21 | D. purpureum reference sequence (GenBank accession number: DQ340386) |
To reconstruct the rDNA gene tree, we used Bayesian, maximum-likelihood, maximum-parsimony, and neighbor-joining approaches. For the Bayesian tree, we used MrBayes (Huelsenbeck and Ronquist 2001) to estimate a phylogenetic tree of unique haplotypes based on the GTR + I + Γ substitution model. Four Metropolis-coupled Markov chains were run for 250,000 burn-in generations followed by 1.75 × 106 generations of data collection. We used GARLI (Zwickl 2006) to infer the maximum-likelihood bootstrap tree with 1000 bootstrap replicates under the GTR + I + Γ substitution model. We generated the maximum-parsimony bootstrap tree using PAUP* (Swofford 2002) with 1000 bootstrap replicates, tree bisection-reconnectiion (TBR) branch swapping, 100 random addition sequences per replicate, no limit to MaxTrees, and gaps treated as missing data. The neighbor-joining tree of unique haplotypes was constructed using MEGA4 (Tamura et al. 2007). Bootstrap values were based on 1000 replicates.
We estimated evolutionary divergence between various phylogenetic groups by calculating the number of base differences per site using MEGA4 (Tamura et al. 2007). All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option). There were a total of 5264 positions in the final dataset for 38 D. purpureum sequences.
To test for population differentiation, we calculated FST with the rDNA sequence data using an analysis of molecular variance approach (Excoffier et al. 1992) implemented in Arlequin 2.0 (Schneider et al. 2000).
MATING EXPERIMENTS
We performed one 12-clone and two 11-clone round robin macrocyst experiments, in which each clone was paired with other clones as well as with itself. Thus, 210 trials were carried out. We assigned clones to experiments based on their position in the gene tree and their geographical location; each of the three experiments consisted of clones that spanned the entire tree and clones collected from both the same and different geographical locations (see Supporting Tables S1–S3). In addition, we repeated Experiments 1 (see Supporting Table S1) and 2 (see Supporting Table S2) to confirm the repeatability of our results. Details on mating experiments are shown in the Supporting material.
We predicted that paired clones that are more closely related to one another should mate more readily (i.e., produce macrocysts in a short amount of time) than paired clones that are more distantly related, unless they are of the same sex in which case we did not expect successful mating. In some cases, one replicate produced macrocysts between clones and the other replicate did not (at one week of scoring: 1/144; at two weeks of scoring or later: 13/144). We considered a pair as capable of forming macrocysts if they did so in any replicate. If replicate experiments had macrocysts form at different times (6/144), we used the earliest time in our Fisher’s exact test analysis and the averaged time in our correlation analyses.
We also tested whether genetic distance of a given pair of clones correlates with their degree of mixing in fruiting bodies by sequencing isolates from pairwise experiments reported in Mehdiabadi et al. (2006). This study showed strong kin discrimination in 12 of 14 experiments: individual fruiting bodies were predominantly clonal, although cells of two D. purpureum isolates were plated (in the absence of bacteria) in equal proportions at the start of pairwise experiments. We conducted correlation analyses in the same way as for the macrocyst data.
Results
MOLECULAR GENE TREE
We identified 20 unique rDNA haplotypes from the 37 sequenced isolates (Table 1, Fig. 1). Of the 5263 sites aligned in all samples (including outgroups), 809 were variable and 517 were parsimony informative. Bayesian analysis of the unique haplotypes produced the phylogenetic tree shown in Figure 1. We identified three major groups of haplotypes within D. purpureum. The deepest bifurcation separates groups A and B from C (Fig. 1). Almost all of the Pasadena clones, three Houston clones, and one Webster clone form group A, resulting from nine haplotypes for 10 samples. Group B comprises the two Japan isolates and one Houston haplotype (QSpu4), which clearly differed in rDNA sequence relative to other Houston clones. Group C includes most of the Houston clones, two Webster clones, two Virginia clones, and two Pasadena clones for a total of nine haplotypes for 24 samples.
Figure 1.
Bayesian tree of 21 unique haplotypes of D. purpureum. Published sequences of D. macrocephalum, D. citrinum, and D. discoideum served as outgroups. Houston clone QSpu10 is not shown, but could belong to either haplotype number 19 or 20. Nodes with posterior probabilities below 50% are not shown. Letters refer to specific phylogenetic groups (see text) and symbols refer to geographic locations of clones.
The results of the maximum-likelihood, maximum-parsimony, and neighbor-joining analyses were similar to those of the Bayesian analyses. These trees had the same basic topology as the Bayesian-gene tree and there were similar levels of nodal support. However, there were two differences: (1) The Japan clones branched off more basally on the neighbor-joining tree (data not shown) compared to the Bayesian, maximum-likelihood (data not shown), and maximum-parsimony (data not shown) trees, and (2) group B is a well-supported clade in the Bayesian tree, but not as well resolved within the larger subclade, which includes Group A, in the maximum-likelihood and maximum-parsimony trees.
We measured genetic distances between major phylogenetic groups by calculating the number of base differences per site and found large genetic distances between the major phylogenetic groups, larger than some recognized species pairs. The average genetic distance between D. purpureum and outgroup taxa is 0.098, 0.036 between outgroups D. discoideum and D. citrinum, and ranged from 0 to 0.054 within D. purpureum. Group A is fairly divergent from the rest of the other clones; this group’s average pairwise sequence divergence from Group B is 0.040 (range: 0.032–0.047), and is 0.050 (range: 0.038–0.054) from Group C. Similarly, average between-group genetic distances are just as high between Group B and Group C (0.045; range: 0.032–0.049).
To understand whether this level of genetic variability between the major phylogenetic groups of D. purpureum is extraordinarily high, we took a subset of our rDNA sequence data (1878 bp of the small subunit rDNA) that corresponded to the sequence used to reconstruct the phylogeny of the Dictyostelia and calculated the pairwise genetic distance between taxa that are recognized as closely related but distinct species (Schaap et al. 2006). For five closely related species pairs, pairwise genetic distances were as follows: (1) D. citrinum (OH494) and D. dimigraformum (AR5b): 0.009; (2) D. clavatum (TNS-C-189) and D. longosporum (TNS-C-109): 0.003 (3) D. brunneum (WS700) and D. giganteum (WS589): 0.004; (4) D. mucoroides (TNS-C-114) and D. phaerocephalum (GR11): 0.001; and (5) D. capitatum (91HO-50) and D. pseudobrefeldianum (91HO-8): 0.003. After recalculating the average between-group sequence divergence for the major D. purpureum phylogenetic groups (only using the same rDNA sequence data as that calculated for the above species pairs), we found that the major clades within D. purpureum are more divergent than all five species pairs and are more than twice as distinct as four of them (Group A vs. Group B: 0.011; Group B vs. Group C: 0.012; and Group A vs. Group C: 0.013).
Although clones from Houston are represented in all three groups, the sequence data reveal some level of population structure in this species (Fig. 1), and the overall FST estimate (the measure of differentiation between subpopulations in the total population) shows highly significant population differentiation for D. purpureum (FST: 0.458, P < 0.0001). For pairwise comparisons between all five populations, we found that population pairwise FSTs were significant for Pasadena, TX versus Houston, TX (FST: 0.469, P < 0.0001), Pasadena, TX versus Japan (FST: 0.686, P = 0.019), and Houston, TX versus Japan (FST: 0.733, P = 0.007). Other comparisons were not significant (data not shown).
MATING EXPERIMENTS
These findings of high genetic variation and phylogenetic structure suggest that D. purpureum may actually consist of multiple, reproductively isolated populations. To test the hypothesis that groups A, B, and C represent two or three cryptic species, we performed pairwise matings with clones from the same and different groups in three sets of experiments (see Supporting Tables S1–S3).
In all three experiments, we found that only two clones were (weakly) homothallic (i.e., self-compatible) and 32 clones were heterothallic (i.e., compatible only with others; see Supporting Tables S1–S3), which confirmed previously published findings that this species consists of both homothallic and heterothallic strains (Clark et al. 1973; Nickerson and Raper 1973). In addition, nine of the 34 clones did not form macrocysts in any combination (i.e., incompatible against all tested; see Supporting Tables S1–S3). In each of the three experiments, we had a total of 12 of 66, 19 of 78, and 2 of 66 pairwise combinations of clones form macrocysts, respectively (see Supporting Tables S1–S3). However, excluding matings with homothallic strains, we found 17% (17/99) of “within” group matings were successful and 2% (2/88) of “between” group matings were successful. This resulted in at least six mating types for this species: at least six in each of Experiments 1 and 2 and four in Experiment 3. We defined mating types to be mutually exclusive (i.e., for example, in Experiment 2, QSpu34 and QSpu16 are considered the same mating type because both mate with QSpu5 but no other clones; see Supporting Table S2). We also investigated whether the time to macrocyst formation correlated with genetic divergence between pairs of clones but found no significant relationship (nonparametric Spearman Rank Correlation test: Z = 0.141, P = 0.8875) despite nonindependence of the data. Nevertheless, the data confirmed our hypothesis: there were significantly more successful “within” than “between” group matings for groups A–C whether we analyze macrocyst formation after the standard one week of scoring (within: 8/99, between: 0/88; Fisher’s exact test, P = 0.0074) or after four weeks of scoring (within: 17/99, between: 2/88; Fisher’s exact test, P = 0.0011).
GENETIC DISTANCE AND COOPERATION
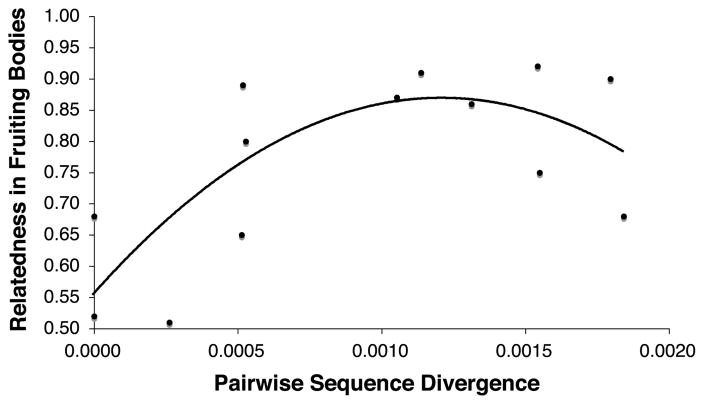
To test whether the degree of mixing between pairs of clones correlates with genetic distance, we examined whether genetic divergence of the clones explains the propensity to cooperatively make fruiting bodies. For the 14 pairwise mixing experiments performed in Mehdiabadi et al. (2006), we did not find a significant relationship between a pair of clones’ genetic divergence (as determined by rDNA sequencing) and relatedness within fruiting bodies (nonparametric Spearman rank correlation test: Z = 1.621, P = 0.1051). However, if we restrict our test to only the 13 pairs involving clones from within the major groups A–C; that is, only pairs presumably within a “species” (one pair involved clones from different groups, B vs. C), we find a significant association. Removal of that point makes the data normal (Shapiro-Wilk W test, P > 0.05). We find that a quadratic regression is significant and a better fit than a linear model (general linear model: y = −214,411x2 + 519.26x + 0.5537; likelihood-ratio test = 5.35, P = 0.021). Furthermore, we still find a significant positive relationship between a pair of clones’ genetic divergence and relatedness within fruiting bodies when using nonparametric tests, indicating the result is not sensitive to the assumption of normality (Fig. 2; nonparametric Spearman Rank Correlation test: Z = 1.861, one tailed P-value = 0.0314).
Figure 2.
Relationship between genetic distance of a given pair of clones and the average relatedness in fruiting bodies arising from their pairwise mixture for 13 within phylogenetic group experiments (see Mehdiabadi et al. 2006). Genetic distance or pairwise sequence divergence represents the number of base differences per site from analysis between sequences. Methods on calculations of relatedness in fruiting bodies are reported in Mehdiabadi et al. (2006). Relatedness of 0.50 denotes complete mixing between a pair of clones, whereas a relatedness of 1.00 signifies complete sorting between a pair of clones.
Discussion
The monophyletic group of clones we sequenced was clearly differentiated from the outgroup. Within D. purpureum there are three distinct groups, which are as distant as some closely related but distinct Dictyostelium species. We found this phylogenetic structure in D. purpureum had some basis in geography. The two samples collected from Virginia were from Group C. The two Japanese isolates shared the same haplotype (Fig. 1). Although most samples from Houston, Texas were found in Group C, there were exceptions, including the QSpu4 haplotype, which was in Group B and QSpu1, QSpu6, and QSpu21, which were in Group A. The genetic variation among the Houston samples was as high as the genetic variation found between taxa that are recognized as closely related but distinct species.
Genetic differentiation in D. purpureum is further supported by our finding of reduced sexual compatibility between members of the three groups. However, there were also several inconsistent and ambiguous results, which are not uncommon for such macrocyst studies (see Clark et al. 1973; Erdos et al. 1975; Chang and Raper 1981). First, apparently homothallic clones did not produce macrocysts in all pairwise experiments, though they should have. This could be due to imperfect environmental conditions for all possible pairwise matings. It is also possible the isolates that some homothallic clones were paired with in their unsuccessful matings produced inhibitory compounds to prevent macrocyst formation (Lewis and O’Day 1979). This could provide an explanation for why QSpu2 formed macrocysts in almost all combinations except those with the Japan clones (see Supporting Table S2). Second, as previous work has shown in various dictyostelids, we also found that several strains did not form macrocysts in any mating combination (Clark et al. 1973) and that there were a few inconsistent mating reactions between replicates (Chang and Raper 1981).
Clones that are more diverged may also be less likely to form chimeric fruiting bodies in contrast to genetically different clones within species, at least for D. discoideum (Strassmann et al. 2000; Queller et al. 2003; Ostrowski et al. in press). We have demonstrated that D. purpureum preferentially forms groups with its own clonemates and forms predominantly clonal-fruiting bodies (Mehdiabadi et al. 2006). In that study, the degree of mixing between pairs of clones differed in the 14 experiments (Mehdiabadi et al. 2006). We can now show that within the three lineages, more closely related clones are more likely to mix (Fig. 2). Similar patterns have also been found using microsatellite loci in another dictyostelid, D. discoideum (Ostrowski et al. in press). Here, we found a curvilinear relationship between genetic distance and the degree of mixing within fruiting bodies for D. purpureum. Such a relationship suggests that only close relatedness matters, and that beyond this threshold of relatedness, isolates exclude one another in fruiting bodies to about the same degree.
Such studies on social microorganisms are particularly interesting for the ways that genetic variation impacts social interactions (Vos and Velicer 2008). Two related but different responses are shown in this study. First, macrocyst formation was less likely to occur between pairs of clones from different groups than between pairs from the same group. This sexual incompatibility seems consistent with the presence of cryptic species in D. purpureum, or at least with partially isolated lineages. Second, D. purpureum shows another kind of social incompatibility. More distant clones are less likely to cooperate in social development. This could be another manifestation of incompatibility through isolation, but in this case the effect is seen at a much finer genetic scale, as might be expected if the effect is driven by kin selection for restricting altruism to close relatives.
Supplementary Material
Acknowledgments
We thank members of the Strassmann–Queller laboratory for help with collecting samples, especially C. Jack, T. Edwards, W. Massie, and S. Rodriguez. We also thank S. Brady for help with phylogenetic analyses; D. Matute for statistical advice; and P. Abbot, J. Coyne, S. Kalla, S. West, and two anonymous reviewers for helpful comments and discussion. This material is based on work supported by the National Science Foundation (NSF) Program under Grant EF-0626963. NJM was supported by an NSF Postdoctoral Fellowship DBI-0301415 in Microbial Biology and a Keck Postdoctoral Fellowship in Computational Biology (National Library of Medicine Grant 5T15 LM07093).
Footnotes
The following supporting information is available for this article:
Table S1. Results of macrocyst experiment 1 for 11 clones (symbols under clone names refer to their geographical location; see Fig. 1 legend).
Table S2. Results of macrocyst experiment 2 for 12 clones (symbols under clone names refer to their geographical location; see Fig. 1 legend).
Table S3. Results of macrocyst experiment 3 for 11 clones (symbols under clone names refer to their geographical location; see Fig. 1 legend).
Supporting Information may be found in the online version of this article.
(This link will take you to the article abstract).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting informations supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
LITERATURE CITED
- Bonner JT. The cellular slime molds. 2. Princeton Univ. Press; Princeton, NJ: 1967. [Google Scholar]
- Chang MT, Raper KB. Mating types and macrocyst formation in Dictyostelium rosarium. J Bacteriol. 1981;147:1049–1053. doi: 10.1128/jb.147.3.1049-1053.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Francis D, Eisenberg R. Mating types in cellular slime molds. Biochem Biophys Res Commun. 1973;52:672–678. doi: 10.1016/0006-291x(73)90765-1. [DOI] [PubMed] [Google Scholar]
- Crespi BJ. The evolution of social behavior in microorganisms. Trends Ecol Evol. 2001;16:178–183. doi: 10.1016/s0169-5347(01)02115-2. [DOI] [PubMed] [Google Scholar]
- Erdos GW, Raper KB, Vogen LK. Sexuality in the cellular slime mold Dictyostelium giganteum. Proc Natl Acad Sci USA. 1975;72:970–973. doi: 10.1073/pnas.72.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour I. J Theor Biol. 1964a;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. J Theor Biol. 1964b;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huss MJ. Dispersal of cellular slime moulds by two soil invertebrates. Mycologia. 1989;81:677–682. [Google Scholar]
- Kessin RH. Dictyostelium—evolution, cell biology, and the development of multicellularity. Cambridge Univ. Press; Cambridge, U.K: 2001. [Google Scholar]
- Lewis KE, O’Day DH. Evidence for a hierarchical mating system operating via pheromones in Dictyostelium giganteum. J Bacteriol. 1979;138:251–253. doi: 10.1128/jb.138.1.251-253.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdiabadi NJ, Jack C, Talley-Farnham T, Platt TG, Shaulsky G, Queller DC, Strassmann JE. Kin preference in a social microbe. Nature. 2006;442:881–882. doi: 10.1038/442881a. [DOI] [PubMed] [Google Scholar]
- Nickerson AW, Raper KB. Macrocysts in the life cycle of the Dictyosteliaceae. I. Formation of the macrocysts. Am J Bot. 1973;60:190–197. [Google Scholar]
- Ostrowski EA, Katoh M, Shaulsky G, Queller DC, Strassmann JE. Kin discrimination increases with genetic distance in a social amoeba. PLoS Biology. doi: 10.1371/journal.pbio.0060287. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller DC, Foster KR, Fortunato A, Strassmann JE. Cooperation and conflict in the social amoeba, Dictyostelium discoideum. In: Kikuchi T, Kubo T, Higashi S, editors. Social insects and sociogenetics. Hokkaido Univ. Press; Sapporo: 2003. pp. 173–200. [Google Scholar]
- Raper KB. The dictyostelids. Princeton Univ. Press; Princeton, NJ: 1984. [Google Scholar]
- Schaap P, Winckler T, Nelson M, Alvarez-Curto E, Elgie B, Hagiwara H, Cavender J, Milano-Curto A, Rozen DE, Dingermann T, et al. Molecular phylogeny and evolution of morphology in the social amoebas. Science. 2006;314:661–663. doi: 10.1126/science.1130670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excoffier L. Arlequin: A software for population genetics data analysis version 2.0. Genetics and Biometry Lab, Dept. of Anthropology, Univ. of Geneva; 2000. [Google Scholar]
- Strassmann JE, Zhu Y, Queller DC. Altruism and social cheating in the social amoeba, Dictyostelium discoideum. Nature. 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods) version 4. Sinauer Associates; Sunderland, MA: 2002. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Clustal-W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M, Velicer GJ. Isolation by distance in the spore-forming soil bacterium Myxococcus xanthus. Curr Biol. 2008;18:386–391. doi: 10.1016/j.cub.2008.02.050. [DOI] [PubMed] [Google Scholar]
- West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microbes. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Ann Rev Ecol Evol Syst. 2007;38:53–77. [Google Scholar]
- Zwickl DJ. PhD dissertation. Univ. of Texas; Austin: 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.