Abstract
Background
As multidrug-resistant (MDR) pathogens continue to emerge, there is a substantial amount of pressure to identify new drug candidates. Carboxyl polyethers, also referred to as polyether antibiotics, are a unique class of compounds with outstanding potency against a variety of critical infectious disease targets including protozoa, bacteria and viruses. The characteristics of these molecules that are of key interest are their selectivity and high potency against several MDR etiological agents.
Objective
Although many studies have been published about carboxyl polyether antibiotics, there are no recent reviews of this class of drugs. The purpose of this review is to provide the reader with an overview of the spectrum of activity of polyether antibiotics, their mechanism of action, toxicity and potential as drug candidates to combat drug-resistant infectious diseases.
Conclusion
Polyether ionophores show a high degree of promise for the potential control of drug-resistant bacterial and parasitic infections. Despite the long history of use of this class of drugs, very limited medicinal chemistry and drug optimization studies have been reported, thus leaving the door open to these opportunities in the future. Scifinder and PubMed were the main search engines used to locate articles relevant to the topic presented in the present review. Keywords used in our search were specific names of each of the 88 compounds presented in the review as well as more general terms such as polyethers, ionophores, carboxylic polyethers and polyether antibiotics.
Keywords: antibacterial, antibiotics, antifungal, antimalarial, antiviral, carboxylic polyether, drug resistance, ionophore, polyethers
1. Introduction
Polyether antibiotics or carboxyl ionophores [1,2] represent a unique class of compounds reported as potent antibiotics that belong to the larger family of naturally occurring ionophores. First used in 1967, the term ionophore refers to the molecule’s ability to bind a metal ion and facilitate its transport across cellular membranes. This chemo-physiological property has made polyether ionophores a useful tool in the study of cation transport mechanisms and has been the rationalization for their biological activities [1,3].
Polyether antibiotics are believed to affect their targeted cells by means of the modification of the permeability of cellular membranes to cationic metal species. Polyether ionophores possess distinctive structural features that are crucial to their ability to interact with metal species. These features include an exterior alkyl backbone that confers their lipophilic characteristics and an oxygen-rich internal cavity that can bind metal ions [2,4,5].
Naturally occurring ionophores are grouped into three categories with regard to the mechanism through which membrane permeability is altered. The first group is the mobile carriers that bind the metal species in an internal polar cavity thus shielding their charge. The second group is the quasi-ionophores. These molecules form trans-membrane hydrophilic channels through which metal ions flow through the membrane. The last group consists of the neutral ionophores that facilitate the diffusion of cations across membranes according to the membrane potential [2,7].
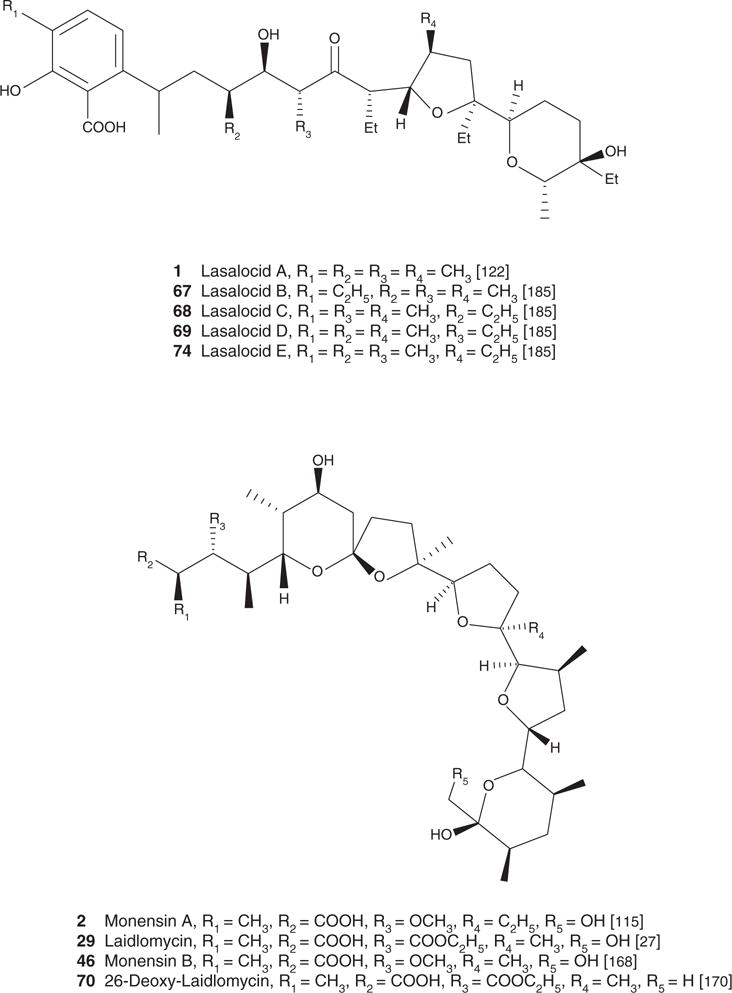
Naturally occurring carboxyl ionophores are relatively high in molecular weight, 500 – 1,000 amu, and are lipid-soluble compounds that display a high affinity for cations such as K+, Na+, Ca2+, thus yielding mobile cation complexes that can freely travel across biological membranes [1]. Their affinity for specific metal ions differs by the electrostatic neutralization of the cation and by the position of the charged carboxylate and its ability to form an ionic or quasi-ionic bond with a cation. With the exception of lasalocid (1), all naturally occurring carboxyl ionophores possess an affinity for Na+ and K+. Lasalocid (1) forms dimers and divalent complexes with Ca2+ and Mg2+. This metal complex formation results in a paracyclic complex as a result of the head-to-tail hydrogen bonding.
The normal physiologic steady state of most living cells is dependent on the establishment of intracellular and extracellular concentration gradients of Na+ and K+. Intracellular concentrations of K+ are higher than that of Na+ and extracellular concentrations are respectively reversed [6]. These ion concentration gradients are essential for cell function. Carboxyl ionophores can easily penetrate cellular membranes owing to their lipophilic properties [6,8,17] and perturb the intracellular cation balance.
Owing to the intrinsic necessity of establishing trans-membrane electrical potentials for cell metabolism by means of the establishment of ion gradients, carboxyl ionophores present significant biological interest not only for their therapeutic utility but also for the possibility of using these chemo-physiological dynamics in combination with traditional or current therapeutics [3,8,9].
A total of 53 bacteria of Streptomycetaceae family are reported to produce carboxyl ionophores. These microorganisms belong to three genera, Streptomyces, Actinomadura and Dactylosporangium. Streptomyces sp are the main producers of these types of compounds and ~ 50% of the carboxyl ionophores known at present are derived from two Streptomyces species (Streptomyces hygrocropicus and Streptomyces albus) [1]. Industrially, these highly biologically active molecules are produced by several microorganisms at low cost and in a predictable manner; thus their industrial production is well established via industrial fermentation and represents an asset in replacing or augmenting existing therapies owing to the establishment of resistance [3,10,11].
2. Biological activity
Polyether antibiotics show a broad spectrum of bioactivity including antibacterial, antifungal, antiparasitic, antiviral and tumor cell cytotoxicity. In addition to the previously mentioned biological activities, several studies have investigated the potential herbicidal [12] and anti-inflammatory activities [13] of these highly active molecules as well as their effects on the CNS and immunoregulatory systems (Table 1) [14,15].
Table 1.
Overview of reported bioactive naturally occurring polyethers (from 1967 – 1994*), sources and their reported in vivo and in vitro testing.
| Name (acronyms), molecular formula, molecular weight | Organism from which it was isolated | In vitro activities | Tested in vivo | Availability of crystal structure | Discovery year, journal, authors |
|---|---|---|---|---|---|
| Lasalocid A (1), C34H54O8, 590.79 | Streptomyces lasaliensis NRRL 3382 | Antiparasitic [40,64,68,85], antiviral [88], antibacterial [1,119], antifungal [1,31] | Antimicrobial and/or antiparasitic effect [32,67,113,120,121] | [122] | [122] |
| Monensin A (2), C36H62O11, 670.88 | Streptomyces cinnamonensis ATCC 15413 | Antiparasitic [38,40,65,76,85,111], antiviral [88,89,112], antibacterial [30,168], antifungal [1], antiproliferative/apoptotic [42,168] | Antimicrobial and/or antiparasitic effect [40,66,67,76,68,113,168], immunoregulatory effect [52], cardiovascular effect [99,114], Antiviral [134] | [115] | [115] |
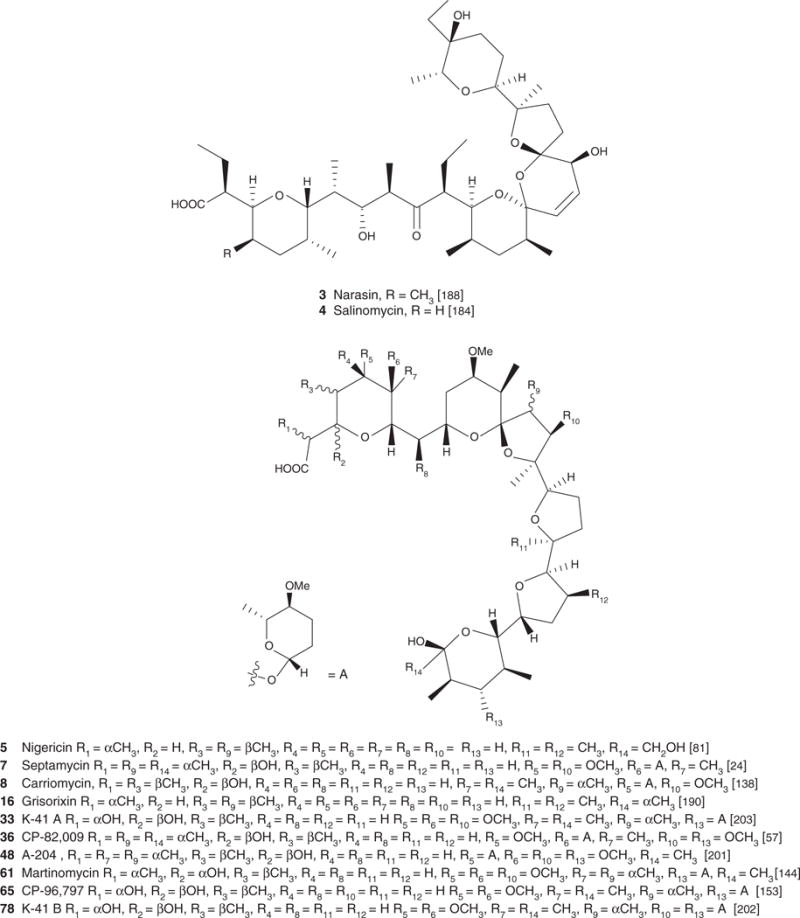
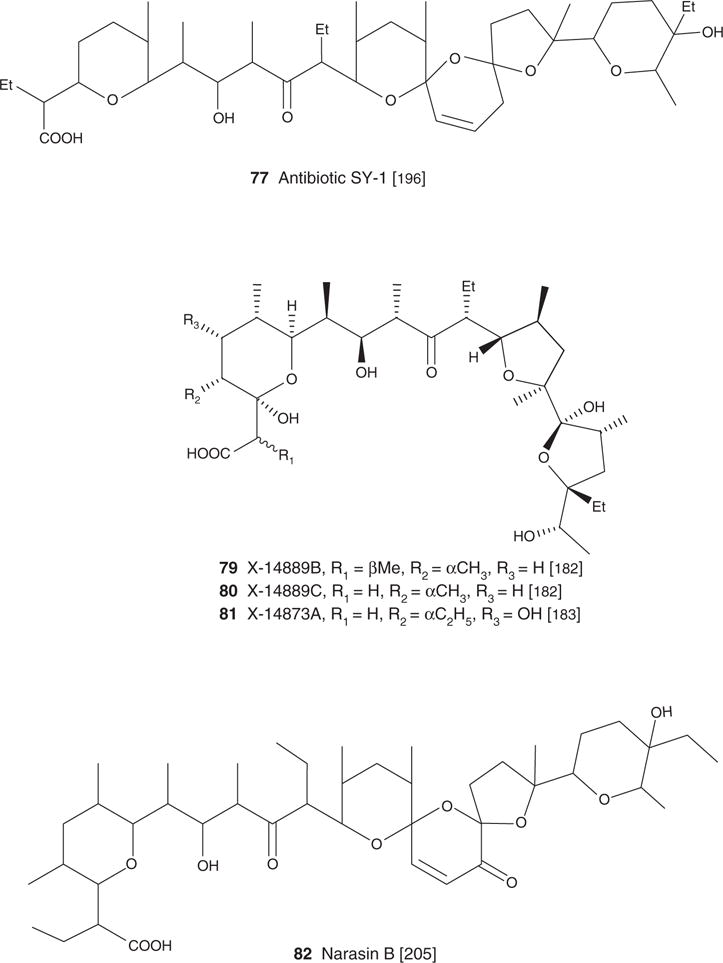
| Narasin A (3), C43H72O11, 765.03 | Streptomyces aureofaciens NRRL 5758 | Antibacterial [1,30,188], antifungal [1], antiparasitic [38,40,85] | Antiviral [134], antiparasitic [188] | [188] | |
| Salinomycin (4), C42H70O11, 751.00 | Streptomyces lasaliensis | Antiparasitic [38,68,85], antiviral [88], antibacterial [1,30], antifungal [1] | Antimicrobial and/or antiparasitic effect [49,66,67,88,113,116,118], immunoregulatory effect [52,106] | [184] | |
| Nigericin (5), C40H68O11, 724.97 | Streptomyces hygrocopicus | Antiparasitic [40,65,75], antiviral [88,112], antibacterial [1], antifungal [1], antiproliferative/apoptotic [42,80,97] | Antimicrobial and/or antiparasitic effect [40,41,72,78], effect on CNS tissues [14,79], antitumor [80], antiherbicidal/insecticidal [12], Antiviral [134] | [81] | [81] |
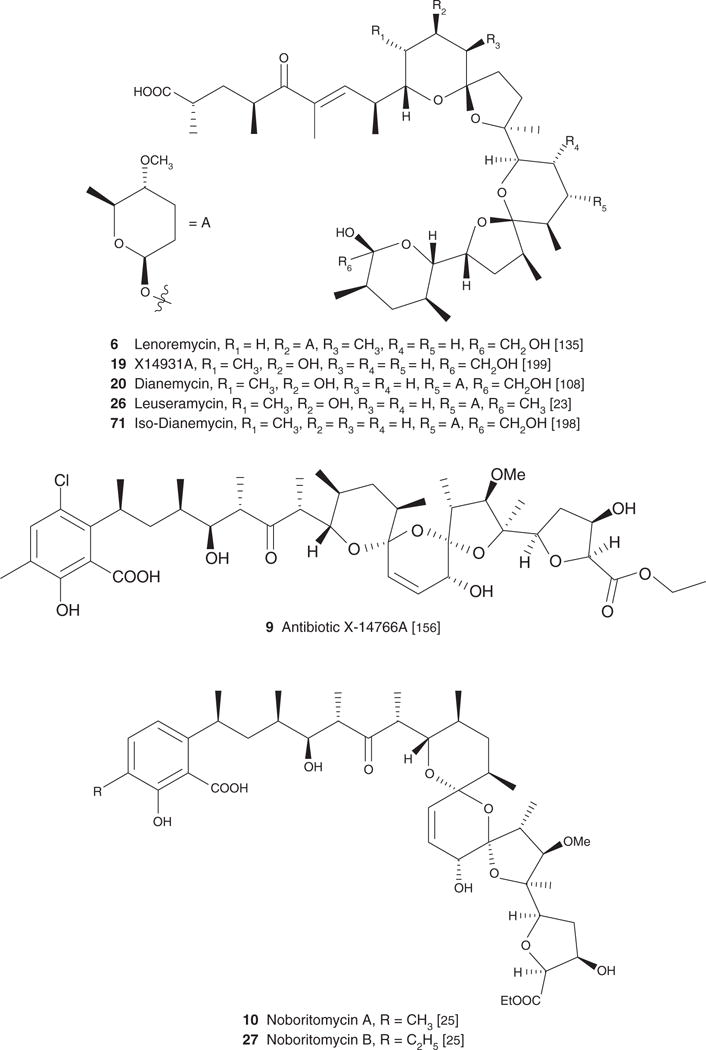
| Lenoremycin (6), C47H77O13Na, 873.10 | Antibacterial, antifungal [1] [135] | [135] | [135] | ||
| Septamycin (7), C48H82O16, 915.16 | Streptomyces hygroscopicus NRRL 5678 | Antibacterial, antifungal [1,24], antiproliferative/apoptotic [24] | Antiparasitic [24,195], Antiviral [134] | [24] | |
| Carriomycin (8), C47H80O15, 885.15 | Streptomyces hygroscopicus | Antiproliferative/apoptotic [42], antibacterial, antifungal [43] | Antimicrobial and/or antiparasitic effect [136,137] | [138] | [138] |
| Antibiotic X-14766A (9), C43H62C1O14Na, 861.40 | Streptomyces malachitofuscus subsp. downeyi | Antibacterial antifungal [155] | [156] | [156] | |
| Noboritomycin A (10), C43H63O14Na, 826.96 | Streptomyces Noboritoensis NRRL 8123 | Antibacterial [25] | Antibacterial [25] | [25] | |
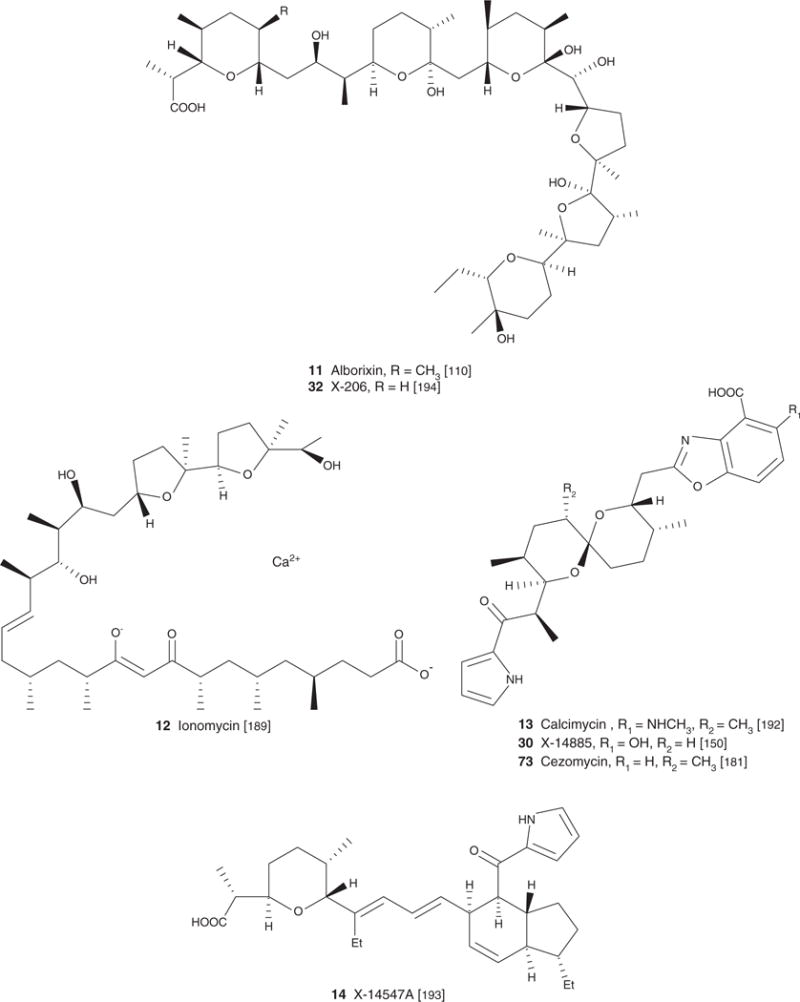
| Alborixin (11), C48H84O14, 85.18 | Streptomyces albus | Antibacterial [1], antiviral [88], antiparasitic [38,85,109], anticocicidal [111] | Antimicrobial and/or antiparasitic effect [40,64,69], cardiovascular effect [103] | [110] | [110] |
| Ionomycin (12), C41H70CaO9, 747.07 | Streptomyces conglobatus ATCC 31005 | Antiparasitic [38], antibacterial, antifungal [1,189], Antiproliferative/apoptotic [98,129] | Immunoregulatory effect [53,55,126–128], effect on CNS tissues [15] | [189] | |
| Calcimycin (A23187) (13), C29H37N3O6, 523.62 | Streptomyces Chartreusensis | Antiparasitic [38], antibacterial, antifungal [1,29] | [192] | [192] | |
| X14547 A (14) 439.98 | Antiparasitic [38] | [193] | |||
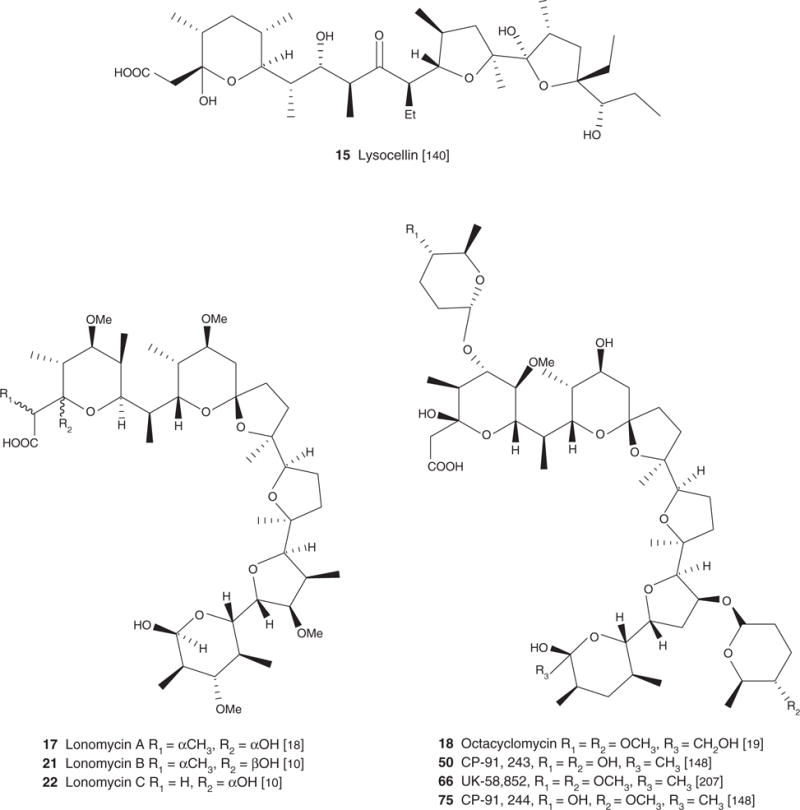
| Lysocellin (15), C34H59O10Na.1/2H2O, 659.81 | Streptomyces cacaoi | Antiproliferative/apoptotic [42], antibacterial [17] | Antimicrobial and/or antiparasitic effect [137] | [140] | [140] |
| Grisorixin (16), C40H68O10, 708.96 | Streptomyces griseus | Antiparasitic [38], antibacterial [11] Antimicrobial |
Antimicrobial and/or antiparasitic effect [85], Cardiovascular effect [102,103,130] | [28] | [28] |
| Lonomycin A (17), C44H76O14, 829.07 | Streptomyces hygrocopicus | Antibacterial [18], antiproliferative/apoptotic [42], antiparasitic [38,40] | Antimicrobial and/or antiparasitic effect [125], cardiovascular effect [105] | [18] | |
| Octacyclomycin (18), C52H88O19, 1,016.24 | Streptomyces sp. | Antibacterial, antifungal, antiproliferative/apoptotic [19] | [19] | ||
| X-14931A (19), C40H66O11, 722.95 | Antibacterial [16,199], antifungal [199] | Antiparasitic [199] | [199] | [199] | |
| Dianemycin (20), C47H78O14, 867.12 | Antiviral [88], antibacterial [8], antiproliferative/apoptotic [42] | Anti-inflammatory [13] | [108] | [108] | |
| Lonomycin B (21), C44H75O14Na, 851.07 | Streptomyces ribosidifi cus TM-481 | Antibacterial and antifungal [10] | [10] | ||
| Lonomycin C (22), C43H73O14Na, 837.04 | Streptomyces ribosidifi cus TM-481 | Antiproliferative/apoptotic [42], antibacterial [10] | [10] | ||
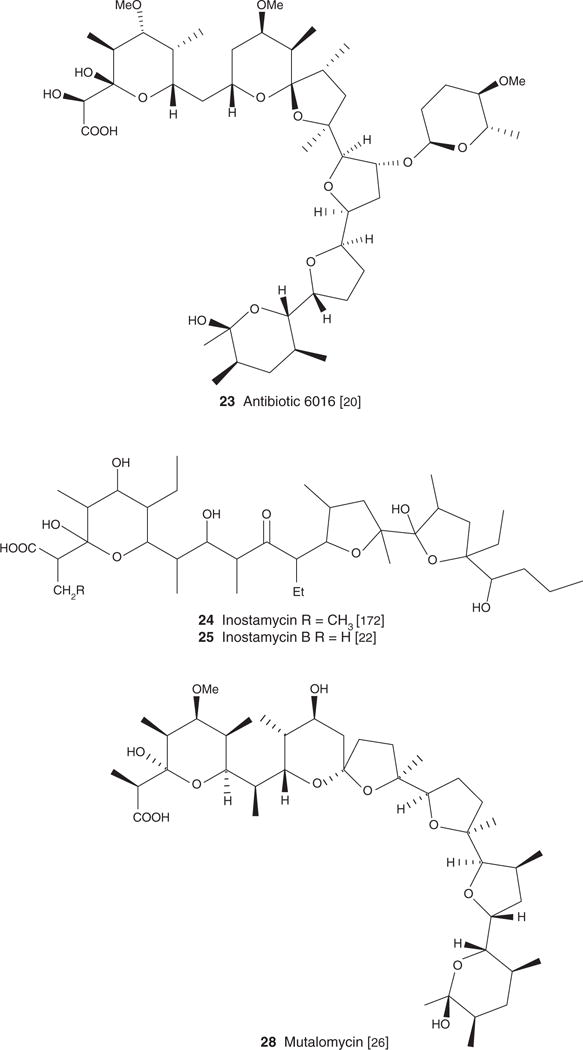
| Antibiotic 6016 (23), C46H78O16, 887.10 | Streptomyces albus | Antibacterial, antiparasitic [20] | [91] | [91] | |
| Inostamycin (24), C38H68O11, 700.94 | Streptomyces sp. MH816-AF15 | Antiproliferative/apoptotic [21], Antibacterial [22] | [171] | [171] | |
| Inostamycin B (25), C37H66O11, 687.01 | Streptomyces sp. MH816-AF15 | Antibacterial, antifungal [22] | [22] | ||
| Leuseramycin (26), C47H78O13, 851.10 | Streptomyces hygroscopicus TM-531 | Antibacterial, antifungal [23] | [23] | ||
| Noboritomycin B (27), C44H65O14Na, 840.99 | Streptomyces noboritoensis NRRL 8123 | Antibacterial [25] | Antiparasitic [25] | [25] | |
| Mutalomycin (28) S-11743 C41H69O12, 754.99 | Streptomyces mutabilis | Antiparasitic [26] | [26] | ||
| Laidlomycin (29) AB-78 C37H62O12, 698.88 | Streptoverticillum eurocidium | antiproliferative/apoptotic [27,42] Antibacterial [27,30,119], antiviral [92] |
[27] | ||
| X-14885 (30), C27H31N2O7 Na·H2O, 536.57 | Streptomyces sp. X-14885 | Antibacterial, antifungal [150] | [150] | [150] | |
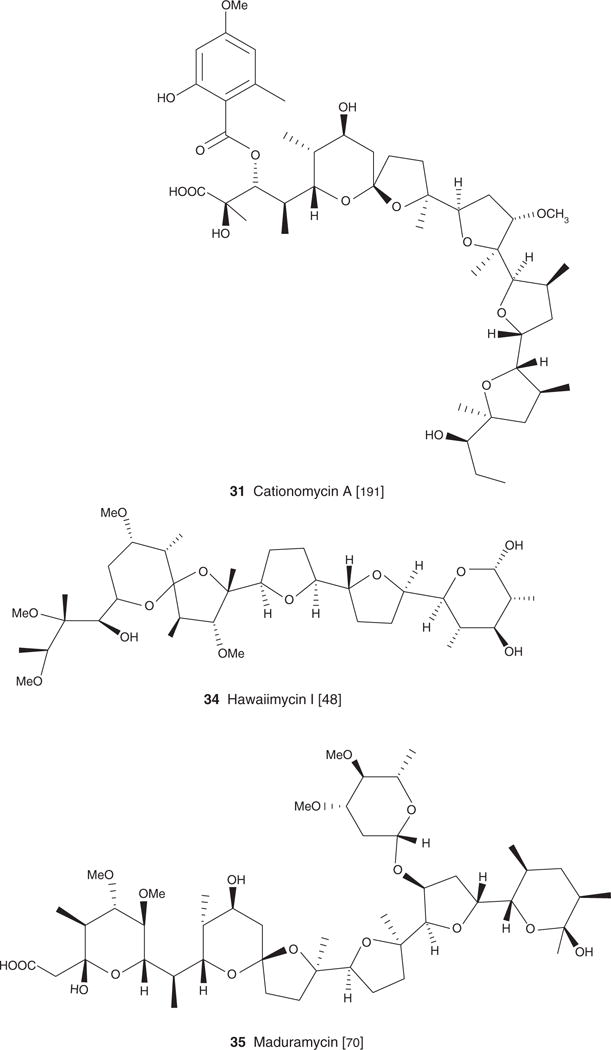
| Cationomycin A (31), C45H70O15, 851.03 | Actinomadura azurea sp. Nov | Antiparasitic [38], antibacterial [1,191] | Antiparasitic [191] | [191] | |
| AntibioticX-206 (32), C47H82O14H2O, 889.15 | Streptomyces sp. | Antiparasitic [39] | Antimicrobial and/or antiparasitic effect [39,132,133] cardiovascular effect [131] | [194] | [194] |
| K-41-A (33), C48H82O18, 947.15 | Streptomyces gypseum NRRL 11168 | Antiparasitic [139], antibacterial [203] | Antiherbicidal/insecticidal [141] | [142] | [203] |
| Hawaiimycin I (34), C36H64O12Na, 711.42 | Streptomyces sp. | Antiparasitic [48] | [48] | ||
| Maduramycin (35) C47H80O17, 917.13 | Actinomadura rubra | Antibacterial [70] Antiparasitic [85,157] |
Antimicrobial and/or antiparasitic effect [50,67,69] | [70] | |
| CP-82,009 (36), C49H84O17, 945.20 | Actinomadura sp. (ATCC 53676) | Antibacterial [57] | Antiparasitic [57] | [57] | [57] |
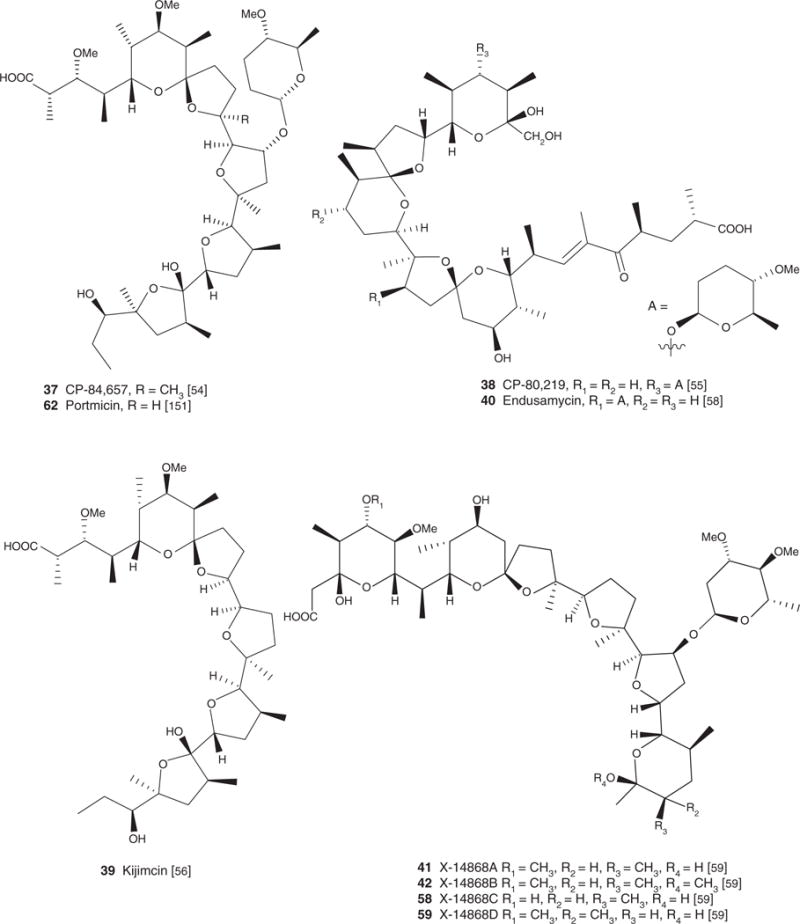
| CP-84,657 (37), C45H78O14, 843.09 | Actinomadura sp. | Antibacterial [54] | Antiparasitic [54] | [54] | [54] |
| CP-80,219 (38), C47H78O14, 867.13 | Streptomyces hygroscopicus | Antibacterial [55] | Antiparasitic [55] | [55] | [55] |
| Kijimicin (39), C37H64O11, 684.90 | Actinomadura sp. MI215-NF3 | Antibacterial [56], antiviral [86–88], antiparasitic [82] | Antiparasitic [56] | [56] | [56] |
| Endusamycin (40), C47H77O14Na, 889.17 | Streptomyces endus subsp. aureus (ATCC39574) | Antibacterial [58] | Antiparasitic [58] | [58] | [58] |
| X-14868A (41), C47H80O17, 917.13 | Nocardia X-14868 | Antibacterial, antifungal [59] | Antiparasitic [59] | [59] | |
| X-14868B (42), C48H82O17, 931.15 | Nocardia X-14868 | Antibacterial, antifungal [59] | Antiparasitic [59] | [59] | |
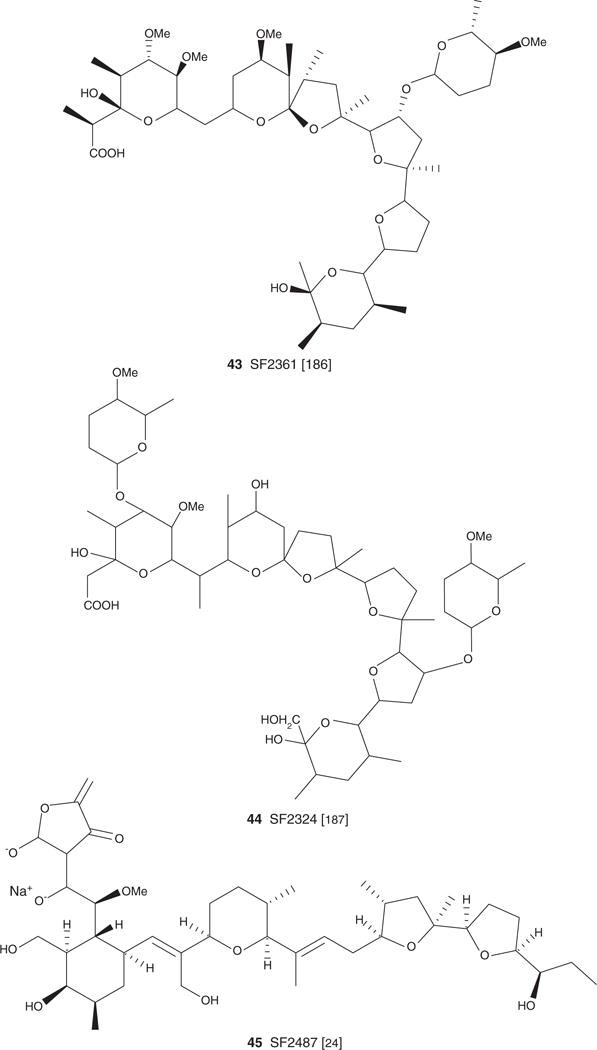
| SF 2361 (43), C48H82O16, 915.16 | Actinomadura sp. SF-2361 | Antiviral [88], antiproliferative/apoptotic [42] | [186] | ||
| SF2324 (44), C52H88O19, 1017.25 | Antiviral [88,120], antiproliferative/apoptotic [42] | [187] | |||
| SF 2487 (45), C42H63O12Na, 805.00 | Actinomadura sp SF2487 | Antibacterial [124], antiviral [88,124], antiproliferative/apoptotic [42], antiparasitic [82] | [124] | [124] | |
| Monensin B (46) C35H60O11 656.84 | Streptomyces cinnamonensis ATCC 15413 | Antiviral [1] | [166] | [200] | |
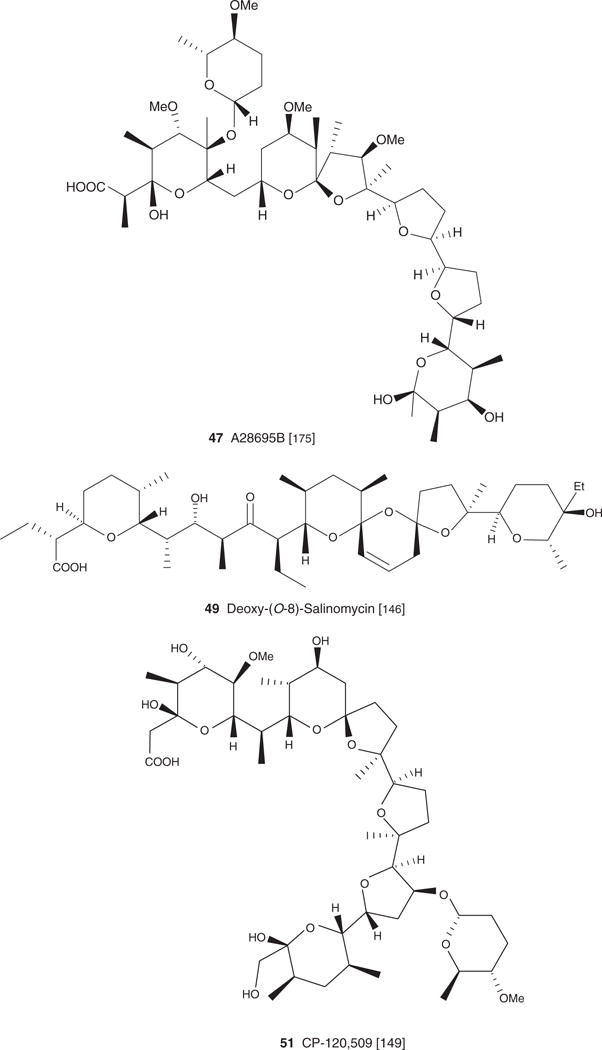
| A 28695B (47) C48H82O17Na, 953.54 | Streptomyces albus NRRL 3883 | Antiparasitic [195], Antiviral [134] | [195] | ||
| A-204 (48) C49H84017, 944.12 | Streptomyces albus | Antiviral [1] | [201] | [201] | |
| Deoxy-(0-8)-salinomycin (49), C42H70010, 735.00 | Streptomyces albus ATCC 21838 | Antibacterial, antifungal [146] | [146] | [146] | |
| CP-91,243 (50), C50H83O18Na, 995.10 | Actinomadurra roseorufa | Antibacterial, antiparasitic, antifungal [148] | [148] | ||
| CP-120,509 (51), C45H76O17, 889.10 | Actinomadura roseorufa | Antibacterial, antifungal [149] | Antiparasitic [149] | [149] | [149] |
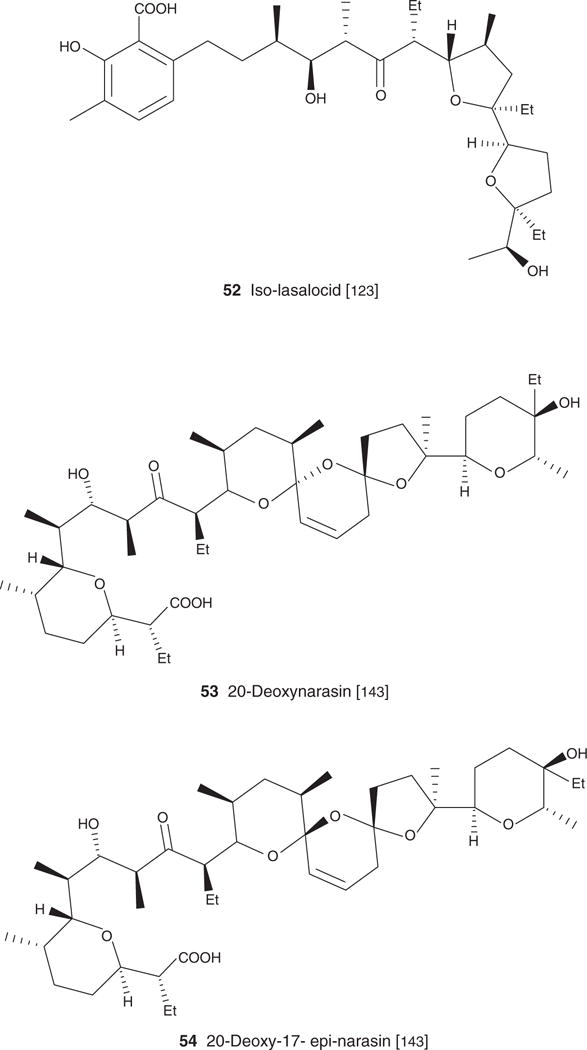
| Iso-lasalocid (52), C34H54O8, 590.79 | Streptomyces lasaliensis | Antibacterial [123] | [123] | [123] | |
| 20-Deoxynarasin (53), C43H72O10, 743.00 | Streptomyces aureofaciens NRRL 11181 | Antibacterial, antiviral [143] | Antiparasitic [143] | [143] | |
| 20-deoxy-epi-17-narasin (54), C42H70O10, 735.01 | Streptomyces aureofaciens NRRL 11181 | Antibacterial, antiviral [143] | Antiparasitic [143] | [143] | |
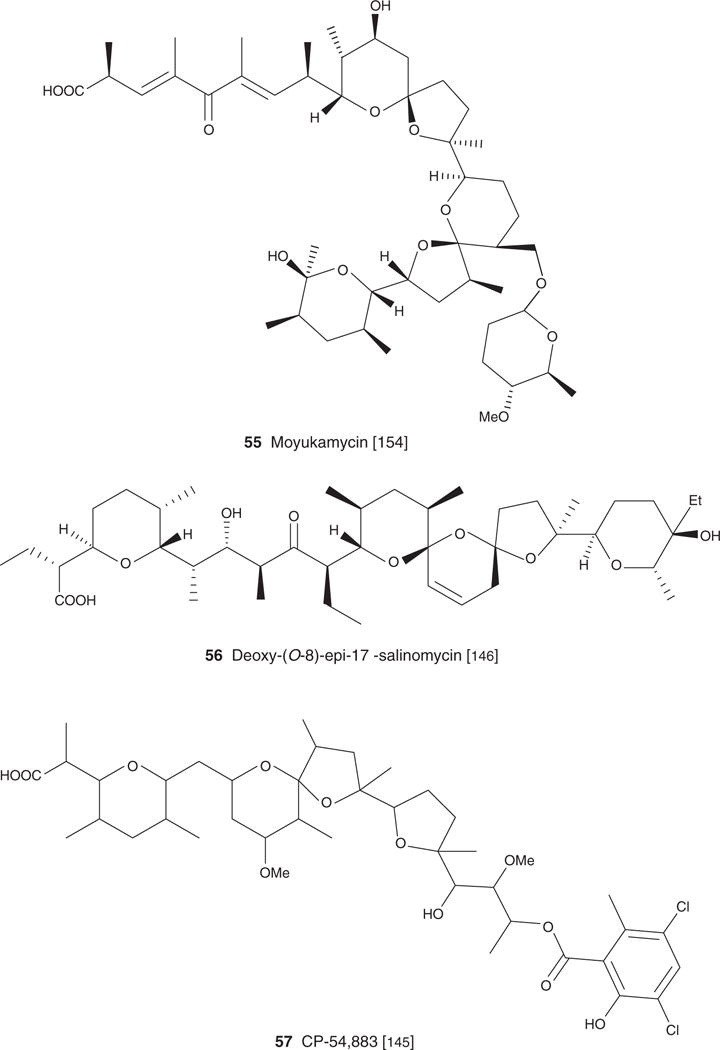
| Moyukamycin (55), C47H75O13Na, 871.10 | Streptomyces hygroscopicus TM-581 (FERM-BP 274) | Antibacterial, antifungal [154] | [154] | ||
| (Deoxy-(O-8)3)-epi-l7 salinomycin (56), C42H70O10, 735.01 | Streptomyces albus ATCC 21838 | Antibacterial, antifungal [146] | Hypertension [147] | [146] | [146] |
| CP-54,883 (57), C41H61O12Cl2, 817.83 | Actinomadura routienii Huang sp. | Antibacterial, antiparasitic [145] | [145] | ||
| X-14868C (58), C46H78O17, 903.10 | Nocardia X-14868 | Antibacterial, antifungal [59] | Antiparasitic [59] | [59] | |
| X-14868D (59), C47H80O17, 917.13 | Nocardia X-14868 | Antibacterial, antifungal [59] | [59] | ||
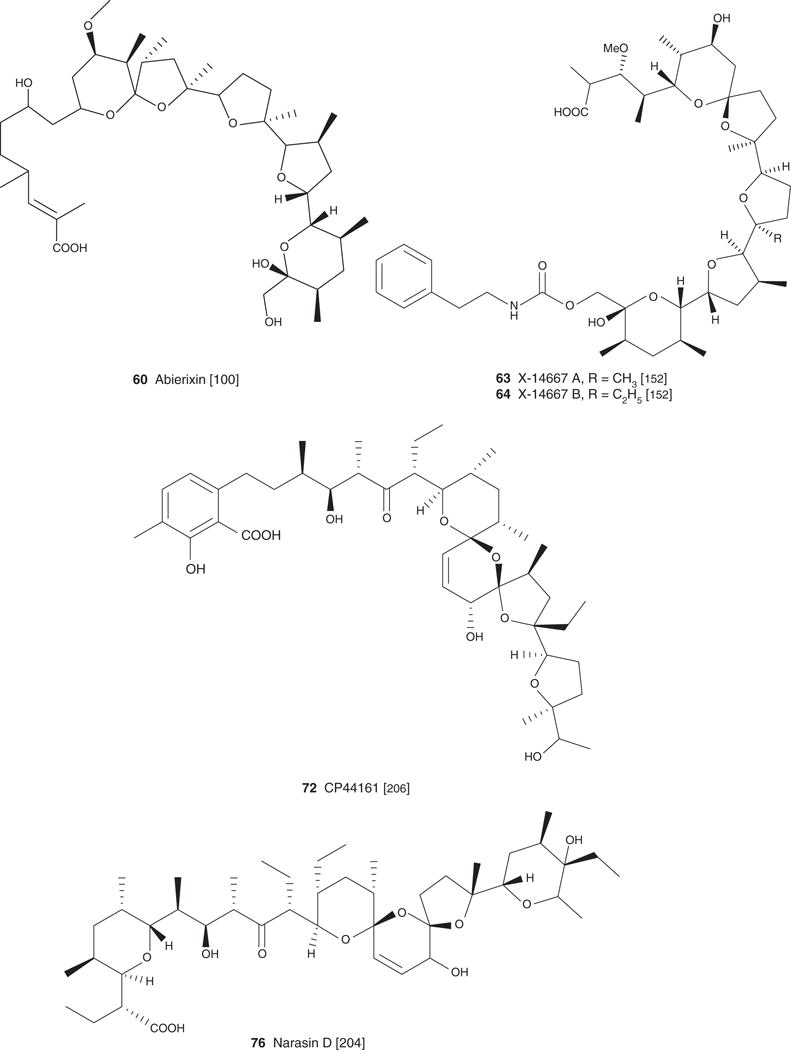
| Abierixin (60), C40H68O11, 724.96 | Streptomyces albus NRRL B-1865 | Antibacterial, antifungal [100] | Antiparasitic [100] | [100] | |
| Martinomycin (61), C49H84O17, 967.50 | Streptomyces salvialis | Antibacterial [144] | Antiherbicidal/insecticidal [144] | [144] | |
| Portmicin (62), C44H76O14, 829.07 | Nocardiopsis sp. No. 6270 | Antibacterial, antifungal [151] | Antiparasitic [151] | [151] | |
| X-14667 A (63), C44H69NO12, 804.02 | Streptomyces cinnamonensis | Antibacterial, antifungal [152] | [152] | ||
| X-14667 B (64), C45H71NO12, 818.04 | Streptornyces cinnamonensis | Antibacterial, antifungal [152] | [152] | ||
| CP-96,797 (65), C47H80O17, 917.15 | Streptomyces sp. | Antibacterial, antifungal [153] | Antiparasitic [153] | [153] | [153] |
| UK-58, 852 (66) C52H88O18 001.24 | Actinomadura roseorufa Huang sp. nov., ATCC 39697 | Antiparasitic, antibacterial [207] | [207] |
The most recent report on the discovery of these molecules that we came across.
2.1 Antibacterial
The antibacterial potential of carboxyl ionophores is widely documented with reports on the discovery of these compounds typically mentioning their activity against Gram-positive and Gram-negative bacteria, as well as some fungi. From these reports it can easily be gathered that carboxyl polyethers are molecules of outstanding potency against numerous pathogenic bacteria (drug-sensitive and drug-resistant). The fact that their potency against serious threats such as Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) rivals that of clinically used drugs such as vancomycin and oxacillin adds to their potential utility. At this point it is difficult to fully assess the true value of these molecules as potential therapeutic agents simply because, on the basis of the reports gathered here, none of these molecules have been assayed in an animal model infected with these bacteria. Thus at this stage all that can be summarized from the reports presented later is that carboxyl polyethers are selective and potent antibacterial agents in vitro. They are appealing as antibacterial agents because of their ability to inhibit growth of MRSA and VRE at very low concentrations, both of which are major public health threats.
2.1.1 Activities against drug-sensitive bacteria strains
A similar inhibitory pattern emerged from reports on the antibacterial potential of carboxyl polyethers with respect to a strong selectivity towards Gram-positive bacteria [1,5,10,8,16,17–19,20,21]. Monensin (2), narasin (3), salinomycin (4), nigericin (5), lenoremycin (6), septamycin (7), carriomycin (8), X-14766A (9), noboritomycin A (10), alborixin (11), ionomycin (12), A23187 (13), X-14547A (14), lysocellin (15) and lasalocid (1) were all evaluated against eight Gram-positive bacteria (cocci, rods, filamentous) as well as against seven Gram-negative bacteria, and four fungal species. All but ionomycin (12) showed potent activity against Gram-positive bacteria (MIC varying in the range 0.006 – 12.5 μg/ml). In contrast, not one of these compounds displayed activity against the tested Gram-negative bacteria (MIC > 100 μg/ml). Several other carboxyl ionophores are reported as having a similar biological activity profile against Gram-negative, and Gram-positive bacteria. These are grisorixin (16) [11], lonomycin (17) [18], octacyclomycin (18) [19], X-14931A (19) [16], dianemycin (20) [8], lonomycin B (21) [10] and C (22) [10], antibiotic 6016 (23) [20], inostamycin (24) [22], inostamycin B (25) [22] and leuseramycin (26) [23].
Not all carboxyl polyethers show this selectivity towards Gram-positive bacteria. Compounds such as septamycin (7), noboritomycin A (10) and B (27) stand out for their ability to target not only Gram-positive bacteria but also Gram-negative bacteria. The reported MIC of septamycin (7) against Escherichia coli was 0.1 μg/ml [24]. Noboritomycin A (10) and B (27) inhibited the growth of Neisseria pharyngi with an MIC of 0.01 μg/ml [25]. The MIC of mutalomycin (28) against the same pathogen was 0.3 μg/ml [26].
Not all carboxyl ionophores possess significant activity against Gram-positive bacteria. For example, laidlomycin (29) was reported as inactive against Staphylococcus aureus [27].
Although in general anaerobic bacteria are more resistant to carboxyl polyethers, few Clostridium sp, Eubacterium sp, Propionibacterium sp and Peptococcus sp are reported extremely sensitive to compounds such as nigericin (5), monensin (2), dianemycin (20), lysocellin (15), lasalocid A (1) and A23187 (13) with MIC values as low as 0.049 μg/ml in many cases [1].
Carboxyl ionophores have broad-spectrum activity against Gram-positive but not Gram-negative bacteria. Guyot et al. [28] attributed this overall differential selectivity to the presence of an outer membrane in Gram-negative bacteria. The outer membrane is believed to be impermeable to hydrophobic compounds. These authors came to this conclusion while using autoradiography; they could show that only Bacillus cereus, not E. coli, could incorporate the radiolabeled calcimycin (13) [29].
2.1.2 Activities against multidrug-resistant strains of bacteria
Carboxyl polyethers were reported as potent agents against a variety of multidrug-resistant (MDR) strains of pathogenic bacteria. Lycocellin (15), for instance, was reported as active against a streptomycin-, erythromycin-, chloramphenicol- and penicillin-resistant strain of S. aureus, MIC 4 μg/ml [17]. The MIC for the Na+ salt of the antibiotic No 6016 (23) against a similar strain was reported to be 1.56 μg/ml [20]. Noboritomycin A (10) and B (27) are also reported as being active against several resistant strains: penicillin-resistance strains of S. aureus, MIC 0.01 μg/ml, tetracycline-resistance strains of Micrococcus sp, MIC 0.01 μg/ml, aminoglycoside-resistant strains of Streptococcus faecalis, MIC 0.01 μg/ml and macrolide-resistant strains of Sarcina lutea, MIC 0.01 μg/ml [25]. Septamycin (7) (Na+ salt) was also reported effective against these same strains: penicillin-resistant strain of S. aureus, MIC 0.31 μg/ml, tetracycline-resistant strain of Micrococcus sp, MIC 0.31 μg/ml, aminoglycoside-resistant strain of Streptococcus faecalis, MIC 0.1 μg/ml and macrolide-resistant strain of Sarcina lutea, MIC 0.31 μg/ml [24].
In 2007, Yoo et al. [30] published a report on the potential of several carboxylic polyethers, laidomycin (29), monensin (2), salinomycin (4) and narasin (3), to inhibit two resistant bacterial strains, VRE and MRSA [30]. The MIC of all tested carboxyl ionophores against MRSA and VRE varied from 0.5 to 4 μg/ml and from 8 to 16 μg/ml, respectively, narasin being the most potent in both cases. These compounds were more potent than vancomycin and oxacillin. The MIC of vancomycin against VRE was 64 μg/ml and that of oxacillin against MRSA was > 32 μg/ml [30].
2.2 Antifungal
In general, fungi are more resistant to carboxyl ionophores. In several reports, MIC values were greater than the highest tested concentration [1,11,17–19].
There are some exceptions: several species of fungi such as Paecilomyces variotii, Candida albicans, Saccharomyces cerevisiae and Penicillium digitatum are reported as moderately sensitive to some carboxylic polyethers, for example, grisorixin (16) [11], monensin (2) [1], narasin (3) [1], salinomycin (4) [1], lenoremycin (6) [1], carriomycin (8) [1], alborixin (11) [1], A23187 (13) [1], dianemycin (20) [11], lysocellin (45) [1], X-14931A (48) [16] and leuseramycin (26) [23], with MIC values varying in the range of 20 – 100 μg/ml. P. variotii, C. albicans, S. cerevisiae and P. digitatum were quite sensitive to several other carboxyl polyethers such as lysocellin (15) [1], A23187 (13) [1], lenoremycin (6) [1] and nigericin (5) [1]. The MIC values for these compounds were < 2 μg/ml.
More recent studies about the antifungal capacities of carboxyl ionophores focused on opportunistic infections of Pneumocystis carinii [31,32]. HIV patients who develop AIDS have a particular need for a new, effective and prophylactic therapy to pneumocytosis. Lasalocid (1) and nigericin (5) (Figure 1), two common carboxyl ionophores, were shown to have a significant inhibitory effect on the growth of P. carinii in vitro. Lasalocid (1) at 0.05 μg/ml induced a 91.3 and 92.0% reduction in cyst and trophozoite concentrations, respectively. Nigericin (5) at a concentration of 0.05 μg/ml was 100% effective in reducing both morphologies of the fungi, although cytolytic effects on the cells monolayer were observed [31]. Lasalocid (1) was tested in vivo and showed promising activity in prophylactic use including the ability to prevent the occurrence of pneumonitis in corticosteroid-immunosuppressed Sprague–Dawley rats in a dose-dependent manner. The dosage of lasalocid (1) 10 mg/kg/day was protective for 80% of infected rats whereas the dose of 20 mg/kg/day was reported to be safe and nontoxic. All rats from this group were protected from the disease [32].
Figure 1. Structure of carboxyl polyethers.
Scifinder and PubMed were the main search engines used to locate articles relevant to the topic presented in the present review. Keywords used in our search were specific names of each of the 88 compounds presented in the review as well as more general terms such as polyethers, ionophores, carboxylic polyethers and polyether antibiotics.
2.3 Antiparasitic
2.3.1 Plasmodium sp
The emergence and spread of resistant strains of the malarial parasites to several of the antimalarial drugs marketed at present, specifically chloroquine, constitute a serious public health threat in countries all around the world, most of which are poor [33–36]. To address the emerging resistant strains, the simultaneous use of more than one drug known to have different mechanisms of action (MOAs) is considered an important change in the approach to fighting malaria. Such an approach slows the development of resistance to an individual drug, thus limiting the spread of resistant strains [37]. The success of combination therapy is dependent on the availability of molecules with unique MOA. In recent years, a great deal of effort has been dedicated to the discovery of new antimalarial agents. Among the potential candidates reported thus far are carboxyl ionophores. From the reports we gathered, it emerges that these molecules stand out as potential antimalarial drug candidates for several reasons.
First, carboxyl ionophores are reported as potent in vitro antimalarial agents, with IC50 values in the nanomolar and picomolar range [38,39]. Carboxyl ionophores are typically organized into three subclasses. The first category includes compounds specific to monovalent cations such as alborixin (11), lonomycin A (17), nigericin (5), grisorixin (16), narasin A (3), salinomycin (4), cationomycin (31) and monensin A (2) (Figure 1). The second includes molecules specific to divalent cations such as calcimycin (13), X14885 A (30), X14547A (14) (Figure 1). The third includes molecules that show less specificity towards monovalent or divalent cations, that is, lycocellin (15), lasalocid A (1) and ionomycin (12). Of these three subgroups, carboxyl ionophores specific to mono valent cations are reported as the most potent, apart from cationomycin (31) having IC50 = 35 μg/ml, with IC50 values in the range of 0.6 – 6.5 μg/ml (Alborixin, IC50 = 0.6 μg/ml; lonomycin, IC50 = 1.4 μg/ml; nigericin, IC50 = 1 μg/ml; grisorixin, IC50 = 0.9 μg/ml; narasin A, IC50 = 1 μg/ml; salinomycin, IC50 = 2.8 μg/ml) [38,39].
Second, carboxyl ionophores are also reported to have potent activity in vivo. Gumilla et al. [40] evaluated the in vivo antimalarial potential of alborixin (11), lonomycin A (17), nigericin (5), narasin (3) and monensin A (2) using rats infected with Plasmodium chabaudi and Plasmodium vinckei petteri [40]. Alborixin (11), lonomycin A (17), nigericin (5), narasin (3) and monensin A (2) showed significant in vivo antimalarial activity: decreasing the parasitemia by ≥ 70% by day 5 in P. chabaudi and P vinckei petteri infected mice. Overall, the ED50 varied between 0.4 and 4.1 mg/kg. Carboxyl ionophores that are specific to monovalent ions including alborixin (11), lonomycin A (17), nigericin (5), narasin (3) and monensin A (2) seemed to be the most potent of all the three subclasses in vivo as was the case in vitro. The therapeutic index using the intraperitoneal route of administration for lonomycin A (17), nigericin (5), narasin A (3), monensin A (2) and lasalocid A (1) ranged from 2 to 6 [40]. Adovelande and Schrevel also assessed the in vivo antimalarial activity of monensin (2) and nigericin (5) using P. vincke petteri infected mice. The ED50 and ED90 values for monensin (2) were 1.1 and 3.5 mg/kg, respectively. The ED50 and ED90 for nigericin (5) were 1.8 and 4.6 mg/kg, respectively. Monensin (2) seemed more effective at curing mice than nigericin (5); 100% of mice that received 10 mg/kg of monensin (2) were cured whereas only one-third of mice that received this dose of nigericin (5) were cured [41].
Third, several studies reported that these compounds could act in a selective manner and that the level of toxicity varied from one subclass of carboxyl ionophores to another. Gumila et al. [42] reported the in vitro differential ionophore activity between Plasmodium falciparum and normal mammalians cells, Human Jurkat lymphoblasts and U937 macrophage cell lines. It seemed that mammalian cells were only affected by compounds such as nigericin (5), alborixin (11), lonomycin (17), narasin (3) and monensin (2) at concentrations that are significantly, at least 35-fold, higher than the IC50 against the malarial parasite [38]. Whereas the IC50 of narasin (3), monensin (2) and nigericin (5) against the malarial parasite varied in the range 0.6 – 1.0 μg/ml, their MV50 against U937 macrophage varied between 23.5 and 305 μg/ml and their LV50, LD50 against Jurkat lymphoblast, between 45 and 500 μg/ml [38]. These authors also reported on the in vivo toxicity that seemed to be dependent on the subclass of the molecule, that is, its specificity towards monovalent or divalent cation species. Gumilla et al. [40] assessed the acute and subacute toxicity of alborixin (11), lonomycin A (17), nigericin (5), narasin (3) and monensin A (2) using rats infected with P. chabaudi and P. vinckei petteri [40]. Alborixin displayed the highest acute toxicity, LD50 = 1 mg/kg, after intraperitoneal administration. Overall, carboxyl ionophores that are specific monovalent-ion complexing, that is, alborixin (11), lonomycin (17), nigericin (5), narasin (3) and monensin A (2), seemed to be more toxic with LD50 values between 4 and 30 mg/kg. The LD50 values for polyethers specific to divalent metal ions on the other hand varied in the range of 30 – 80 mg/kg [40]. In similar findings, Berger et al. [43] found the acute toxicity of lasalocid A (1) and nigericin (5) to be 2.5 and 40 mg/kg, respectively.
Fourth, two carboxyl ionophores were reported to be significantly more potent than chloroquine. Adovelande and Schrevel assessed the antimalarial activity of monensin (2) and nigericin (5) alongside chloroquine and showed that in vitro these were, respectively, 25- and 30,000-fold more potent [41].
Fifth, several carboxyl ionophores were reported as having outstanding activity against chloroquine-resistant strains. Otoguro et al. [44,39] published two reports in 2001 and 2002 in which the effects of several carboxyl ionophores were evaluated against chloroquine-resistant and sensitive strains of P. falciparum alongside several drugs marketed at present. These antimalarial agents were artesumate, artemisin, chloroquine, pyrimethamanine, amodiaquine and quinine trimethamine trimethoprim [44,39]. These studies revealed that carboxyl ionophores such as X-206 (32), lonomycin A (17), nigericin (5), narasin (3), salinomycin (4), dianemycin (20), monensin (2) and lysocellin (15) were extremely potent against chloroquine-resistant strains of P. falciparum with IC50 values varying in the range 0.15 – 6.4 nM. These molecules seemed to be more potent than the standard drugs mentioned earlier. The IC50 values for these drugs varied from 7.6 to values > 100,000 nM. Artemether (IC50 = 7.6 μg/ml) was the most potent of these compounds. The cytotoxicity against mammalian cells, human diploid embryonic cell, was also assessed. In this study several ionophores, lasalocid A (1), octocyclomycin (18) and X-206 (32) displayed high selectivity indexes. The highest selective index was that of X-206 (32), that is, 3,673 against chloroquine-resistant strain and 1,080 against chloroquine-sensitive strain. These compounds showed higher potency and selectivity towards chloroquine-resistant strains when compared to chloroquine-sensitive strain [39]. Last, the authors performed a comparative assessment of the in vivo effect of X-206 (32), artesunate and artemether in Plasmodium berghei infected mice. X-206 (32) showed a narrow therapeutic window and had an ED50 = 0.53 mg/kg, the lowest evaluated. It was found to be toxic when > 3 mg/kg. Berger et al. [43] estimated the acute toxicity of X-206 (32) to be 11 mg/kg. In 2002, these authors reported another compound, K-41 (33), active in vitro against a chloroquine-resistant strain of P. falciparum. K-41 (33) (IC50 = 8.5 nM) showed activity similar to that of artemether and artesunate and was more potent than artemisinin, chloroquine and pyrimethamine. K-41 (33) had a moderate selectivity index in vivo; it was active orally against chloroquine-resistant strains, the ED50 (7.0 mg/kg) was close to that of artemether, chloroquine and artesunate. As far as acute toxicity of K-41 (33) is concerned an LD50 > 100 mg/kg was reported [44].
Finally, carboxyl ionophores affect the malaria parasite by means of a mechanism that is distinct from that of drugs available at present. Although there is some conjecture over this subject, some authors believe this MOA could be similar to that of chloroquine and others believe it is completely different [38,40,41]. It is generally accepted that carboxyl ionophores act via anti-transport of cation with H+ after inserting themselves in cellular membranes [2]. Adovelande and Schrevel believe that this could result in the alkalination of the food vacuole of the parasite in a similar manner as chloroquine [41]. Chloroquine is reported to act in two ways: by increasing the internal pH of the food vacuole and by binding to ferriprotoporphyrin thus preventing its crystallization into hemozoin. This will then accumulate to a toxic level inside the vacuole [41,45,46]. The mechanism proposed by Gumilla et al. [38] is different from that of chloroquine [38]. According to these authors, the anti-transport of cations with H+ cannot justify the outstanding potency of these compounds, for the simple reason that changes that occur in the plasma membrane of infected erythrocytes reduce the efficiency of ion shuttling across the membrane. These authors believe that carboxyl ionophores do not merely insert themselves in plasma membrane but are completely internalized [38,47]. Once inside the cell and in close proximity with the parasite, they can inhibit its development either by altering its ion content or by disturbing the preestablished ionic gradient between the host and parasite cytosol [38]. The parasite is reportedly more sensitive during the shizont stage [40].
A polyether isolated from the marine Streptomyces sp. H-668 and named hawaiimycin-I (34) (Figure 1) [48] reinforced the critical role of certain key structural features including the terminal carboxylic group on the bioactivity of carboxyl ionophores. Hawaiimycin-I (34) is extremely close structurally to a set of carboxyl ionophores reported as potent antimalarial [40,44], that is, monensin A (1), K-41 (33), nigericin (5), lonomycin (17) and grisorixin (16). These molecules share a unique skeleton:5 interconnected furan and pyran rings. The key difference with hawaiimycin-I (34), established to have only a weak antimalarial activity (IC50 = 100 – 200 ng/ml against P. falciparum) [48], is the absence of the terminal carboxylic group. Crystal structures of this type of compounds reveal that the terminal carboxylic moieties can either be implicated in ion coordination or be involved in hydrogen bonding with distant OH groups [2,5].
Toxicity concerns remain the primary hindrance in the development of carboxyl polyethers into antimalarial drugs. Apart from this issue, it can be gathered from the literature that carboxyl polyethers possess a great potential as antimalarial drug candidates and leads for further optimization. Carboxyl polyethers owe their outstanding potency, in vitro and in vivo, to their unique MOA. Studies published by Gumilla et al. [40] illustrate that it is possible through semisynthetic modification of these molecules to generate more desirable drug candidates. These authors reported that the therapeutic index could be improved in the case of monensin and lasalocid A; the therapeutic index of monensin A (2) is 5 p.o. but that of its synthetic analogue, monensin A (2) methyl ether, is 12 i.p. The therapeutic index of lasalocid A (1) is 3.3 i.p. but that of its analogue, 5-bromo lasalocid A (1), is 22 i.p. [40].
2.3.2 Eimeria sp
Eimeria sp are apicomplexan protozoan parasites and etiological agents of coccidiosis, a disease affecting cattle and poultry. In an attempt to control these diseases, anticoccidial drugs are generally added to the feed of these animals. Several carboxylic polyethers are among the drugs that are now approved for use as control therapeutics for coccidiosis. The first approved application of veterinary carboxyl ionophores was in the prophylactic and therapeutic treatment of coccidiosis in poultry [49,50,51]. Carboxylic ionophores are reported to also have a positive impact on the growth of these animals [52,53]. Maduramicin (35), salinomycin (4), narasin (3), lasalocid (1) and monensin (2) are the most prevalent of the feed additives marketed at present [1,52,53,54]. Several other carboxyl ionophores are also reported to have anticoccidial utility in chickens and turkeys. Although these are the most common additives used now, there are several other carboxyl ionophores reported to be more effective than additives marketed at present [49–59].
Dirlam et al. [57] performed a comparative study of the anticoccidial activity of CP-82,009 (36), salinomycin (4) and maduramicin (35) in chickens infected with several pathogenic strains of Eimeria: Eimeria tenella, Eimeria necatrix, Eimeria acervulina, Eimeria maxima and Eimeria brunette. CP-82,009 (36) at doses of 5 or 10 mg/kg of feed showed a broader spectrum and tolerance [57]. These same authors reported in 1990 that another carboxyl polyether, CP-84,657 (37) had outstanding anticoccidial potential. This agent at a dosage ≤ 5 mg/kg cured chickens infected with different types of Eimeria sp. CP-84,657 (37) was more potent than several molecules, such as salinomycin (4), narasin (3), lasalocid (1) and monensin (2), marketed at present [54]. CP-80,219 (38) was reported to have anticoccidial effects against E. tenella in chickens at dosages of 30 and 120 mg/kg of feed [55].
Kijimicin (39), tested along with monensin (2) and salinomycin (4) in chicken infected with E. tenella, was more effective than several marketed drugs. Its anticoccidial index (ACI) was 156.8 versus 144.1 for monensin (2) sodium salt and 127.1 for salinomycin (4) sodium salt [56].
Endusamycin (40) showed a protective effect on chicken infected with E. tenella and E. acervulina at doses of 10 – 40 mg/kg of feed [58]. The toxicity of this compound in rats was analyzed; when administered to rats orally, the LD50 was 7.5 mg/kg orally [58].
Liu et al. [59] reported that X-14868A (41) and X-14868B (42) were potent anticoccidial agents for chickens infected with E. tenella. Doses of 7 and 15 mg/kg of X-14868A (41) and X-14868B (42), respectively, were estimated to be protective quantities against coccidiosis. These agents are more potent than well-known coccidiostats agents. For example, monensin (2) required doses are 98 – 121 mg/kg, lasalocid (1) required doses are 75 – 125 mg/kg and salinomycin (4) doses are 60 – 100 mg/kg [59].
Smith and Strout suggested an MOA for the coccidiocidal effect of carboxyl ionophores [51]. They suggested that anticoccidial agents act either by inhibiting the development of intracellular parasites or by inducing destruction of intracellular sporoizoids [51].
2.3.3 Cryptosporidium parvum
Cryptosporidium parvum infections in immunocompetent individuals are generally self-limiting. In immunocompromised patients, particularly in AIDS patients, these infections are severe and are becoming increasingly prevalent [60–62]. Although many drugs are used for the clinical treatment of C. parvum, the effective treatments of infected AIDS patients are limited [60,61]. The screening of several anticoccidial molecules has led to the in vitro and in vivo examination of several carboxyl ionophores [63,64–68]. Monensin (2), maduramicin (35), salinomycin (4), alborixin (11) and lasalocid (1) were all evaluated for their respective activity against C. parvum [63,64–66,69,70]. When compared with control anticoccidial agents, monensin (2) reduced the development of C. parvum by > 90% [65]. Treatment with maduramicin (35) and alborixin (11) of severe combined immune deficient (SCID) mice inoculated with 106 oocysts (bovine) oral gavage, beginning 4 weeks post infection at 3 mg/kg/day for 3 weeks, resulted in a 96% reduction in fecal parasite load (p < 0.003) with maduramicin (16) [69]; this correlated with significant reductions in parasite loads in cross-sections of small intestine tissue (p < 0.000002) and colon (p < 0.000006). Alborixin (12) showed less effective results with oocyst reduction at 71% after 3 weeks [69]. Although some toxicity was observed, illustrated by some weight loss, the significant anticryptosporidial activity was overwhelming over this moderate toxicity [69].
2.3.4 Toxoplasma gondii
A parasite that infects a wide variety of organisms, T. gondii is best known for its neurological effects and its particularly hearty cystic stage [71–74]. T. gondii’s most deleterious activity is its induction of toxoplasmic encephalitis, thus causing fatal CNS lesions. This is an especially opportunistic pathogen in AIDS patients [73–77]. Treatment for toxoplasmic encephalitis is typically a combination therapy of pyrimethamine and sulfadiazine. Unfortunately, this type of chemotherapy is not effective against the cystic stage of T. gondii, thus reactivation of the bradyzoites is chronic, especially in the CNS [14,78]. Previous data has demonstrated that several polyether antibiotics are highly active against the tachyzoite stage of T. gondii [74,76,79–81]. A double in vitro and in vivo study on the bradyzoites stage revealed under immunofluoresence and electron microscopy that very low dosages, 0.0001 μg/ml, of monensin (2) significantly altered the cytological physiology, that is, swollen cysts and large numbers of vacuoles, of T. gondii [76]. Temporal, in vivo mouse brain T. gondii bradyzoites, evaluations of a 6- and 48-h exposure yielded significant antiparasitic activity [57,82]. Six-hour single dose treatment of 0.1 μg/ml of monensin (2) effectively eliminated the viability or lysed the cells completely. In a similar 48-h trial with concentrations of as little as 0.0001 μg/ml, the bradyzoites were swollen or lysed, and at a concentration of 0.01 μg/ml all parasites seemed permanently altered beyond viability under electron microscopic evaluation [76]. Apart from carboxyl ionophores antibiotics only two other drugs have shown activity against the bradyzoites stage, atovaquone [83] and 2′–3′-dideoxyinosine (ddi) [84]. In vitro and in vivo tests have shown that these other molecules take a significantly longer time to have the same activity as monensin (2) [57,74,81,82,]. Monensin (2) is lethal to cystic forms of T. gondii, which is rapid and independent of the metabolic activity of the bradyzoites [83].
2.3.5 Neospora canium
The coccidian parasite N. canium is often misdiagnosed as T. gondii. Owing to its recent discovery, few evaluations of antiparasitic therapeutics have been evaluated, although of these few evaluations, predictable results of the antimicrobial effects of naturally occurring carboxyl polyethers were illustrated [85]. To examine the inhibition of tachyzoite multiplication, a 2-day treatment monoclonal antibody-based enzyme immunoassay (EIA), and a 5-day cell culture flask (CCF) treatment and a lesion-based assay were developed. In this study, 43 chemotherapeutics, sulfonamides, dihydrofolate reductase/thymidylate synthase inhibitors, macrolides, lincosamides, pentamidine analogues, eight miscellaneous antiprotozoal and six carboxyl polyethers were evaluated for use in treating Neospora canium infections. This assay determined that five out of the six ionophores evaluated, that is, lasalocid (1), maduramicin (35), narasin (3), monensin (2) and salinomycin (4), caused 100% reduction in tachyzoite induced lesions at concentrations of 0.000001 μg/ml in the CCF assay, which was the lowest concentration examined. Alborixin (11) was toxic to host cells at these concentrations [85].
2.4 Antiviral activity
From the literature reviewed, it can be gathered that carboxyl polyethers possess several features that could qualify them as promising anti-HIV-1 leads. Although none of the reports reviewed assessed the potency of these molecules alongside clinically used drugs the reported IC50 values against HIV-1 targets are, however, significant. These molecules are also reported to have the potential to inhibit the replication of the virus in key cells such as CD4-expressing lymphoid cells and mononuclear phagocytes. In addition, carboxyl polyethers have proven to be effective against acute as well as chronic infections in vitro. The effective dose and toxicity level varied from molecule to molecule but few molecules have proven effective at nontoxic concentrations. As a group, carboxyl polyethers do not belong to any of the class of anti-HIV drugs known at present. Some carboxyl polyethers such as monensin (2) and kijimicin (39) were evaluated as protease inhibitors, with respect to their interference with posttranslational modifications of HIV-1 proteins. It is well established that HIV-1, which has an extremely high mutation rate, easily develops resistance to protease inhibitors by altering the active site of the targeted protease enzyme. Whether carboxyl polyethers that interfere with posttranslational modifications of HIV-1 proteins will share this fate is not well established at this time owing to the limited understanding of the exact sequence of events through which compounds such as monensin (2) and kijimicin (39) impair posttranslational modification. None of the studies reviewed investigated the effectiveness of these carboxyl polyethers in an animal model.
2.4.1 Activity against HIV-I-AIDS
Several reports describe the potential of carboxyl ionophores, monensin (2), salinomycin (4), lasalocid (1), nigericin (5), dianemycin (20), alborixin (11), kijimicin (39), SF2361 (43), SF2324 (44), SF2487 (45) and laidomycin (29) as anti-HIV agents [86–91].
Nakamura et al. [88] assessed the effect of 10 carboxyl ionophores, monensin (2), salinomycin (4), lasalocid (1), nigericin (5), dianemycin (20), alborixin (11), kijimicin (39), SF2361 (43), SF2324 (44) and SF2487 (45), on the replication of HIV-1. All but SF2324 (44) showed a dose-dependent inhibition of the virus replication. Apart from SF2324 (44), the concentration that inhibited reverse transcription activity, viral replication, by 50%, EC50, varied in the range 0.40 ± 0.040 to 7.12 ± 4.4 μg/ml. The IC50 concentration that decreases cell viability by 50% of SF2324 as its EC50 in H9 cells, primary infection, was determined to be > 100 μg/ml, the highest tested concentration. The IC50 of the remaining compounds varied in the range 6.98 ± 2.9 to 78 ± 4.7 μg/ml (Table 2). For these compounds an inhibitory effect at nontoxic dose was observed. The ratio IC50/EC50 varied in the range 3.18 – 76.72 μg/ml (Table 2). The chronically infected cells, U937, were sensitive to all but SF2487, but a higher cytotoxicity was observed as shown by the low IC50/EC50 ratio, which varied here from 1.18 to 8.32 μg/ml (Table 2) [86,88].
Table 2.
HIV-1 infectivity and cytotoxicity.
| Compounds | Acute infection (H9)
|
Chronic (U937)
|
||||
|---|---|---|---|---|---|---|
| EC90 (μg/ml) | IC50 (μg/ml) | IC50/EC90 | EC90 (μg/ml) | IC50 (μg/ml) | IC50/EC90 | |
| Monensin (2) | 4.22 ± 2.6 | 13.44 ± 2.8 | 3.18 | 0.5 ± 0.25 | 0.59 ± 0.19 | 1.18 |
| Salinomycin (4) | 0.4 ± 0.04 | 6.98 ± 2.9 | 17.63 | 0.09 ± 0.02 | 0.28 ± 0.05 | 3.14 |
| Lasalocid (1) | 2.37 ± 2.9 | 48.80 ± 4.7 | 20.63 | 0.2 ± 0.2 | 1.35 ± 0.35 | 6.92 |
| Nigericin (5) | 0.68 ± 0.46 | 14.46 ± 8.0 | 21.26 | 0.05 ± 0.33 | 0.4 ± 0.007 | 8.32 |
| Dianemycin (20) | 7.12 ± 4.4 | 78.40 ± 4.7 | 11.01 | 1.1 ± 0.14 | 1.1 ± 0 | 1.00 |
| SF2361 (43) | 0.64 ± 0.36 | 49.10 ± 4.6 | 76.72 | 1.35 ± 0.49 | 2.1 ± 0.14 | 1.56 |
| SF2324 (44) | > 100 | > 100 | > 1.00 | 3.7 ± 0.71 | 10.1 ± 0.14 | 2.73 |
| SF2487 (45) | 0.49 ± 0.18 | 7.8 ± 1.3 | 15.92 | 0.03 ± 0.15 | 1.85 ± 0.28 | 64.91 |
| Alborixin (11) | 2.5 ± 1.1 | 13.50 ± 3.1 | 5.4 | 0.12 ± 0.04 | 0.80 ± 0.28 | 6.53 |
| Kijimicin (39) | 1.63 ± 1.9 | 18.62 ± 11 | 11.41 | 0.1 ± 0.007 | 0.55 ± 0.07 | 5.79 |
| Dextran Sulfate | < 1 | > 100 | > 100 | NT | ||
From Nakamura et al., 1992.
EC50: Concentration that inhibits reverse transcription activity by 50% (viral replication); IC50: Concentration that decreases cell viability by 50%.
It is believed that all carboxyl ionophores do not share a common MOA against HIV. These compounds target different stages of the HIV infective cycle, that is, the pre- and post-absorption steps [86–90]. Nakamura et al. [88], using a time of addition design, exposure of H9 cells to these molecules before, during and after viral absorption, partitioned these agents in two main groups on the basis of their MOA. The first group, believed to inhibit viral absorption was made of lasalocid (1), dianemycin (20), SF2361 (43), SF2324 (44), SF2487 (45) and alborixin (11). The second group, believed to interfere with post-viral adsorption events, was made of monensin (2), salinomycin (4), lasalocid (1), nigericin (5), Dianemycin (20), alborixin (11), kijimicin (39), SF2361 (43), SF2324 (44) and SF2487 (45) [88]. Yamauchi et al. [87] working specifically with kijimicin (39), tested at concentrations of 0.08 – 10.0 μg/ml confirmed its lack of ability at interfering with the absorption step, as well as early stage of the replication, such as integration. It was proposed that kijimicin (39) acted by decreasing the infectivity of the virus, which was possibly accomplished by means of incomplete glycosylation of gp120 [87].
A dose-dependent study of the effect of monensin (2) on chronically infected MOLT-3/HTLV-IIIB revealed a marked inhibitory effect on the proteolytic processing of gpl60 to gpl20, after 7 h of exposure at 10 μM [89]. This effect was specific as no effect on the protein level of gag proteins Pr53gag p24 was noted. No effect on the expression of tat, vif and nef gene products was observed either. Monensin (2) also showed a marked reduction in the formation of syncytia at concentration > 3 nM when MOLT-3/HTLV-IIIB cells were co-cultured with CEM cell. Both effects were reversible if the cells were washed free of monensin (2) [89]. All these findings were later confirmed by a second group [90]. Laidlomycin (29) was also reported to induce a dose-dependent inhibition of HIV replication. The MOA of laidlomycin (29) was proposed to be via inhibition of gp120 expression. Laidlomycin (29) also induced an inhibitory effect on syncithium formation at concentrations as low as 1 μg/ml [92].
2.4.2 Activity against other types of viruses
Many carboxyl ionophores are reported to be active against several other DNA and RNA viruses: monensin (2), monensin B (46), nigericin (5), narasin (3), lasalocid (1), X-206 (32), septamycin (7), A 28695B (47) and A204 (48) were tested against transmissible gasteroenteritis coronavirus, Newcastle disease virus (NDV), infectious canine hepatitis virus and infectious bovine rhinotracheitis virus [1]. All these agents showed a significant activity against transmissible gasteroenteritis coronavirus, MIC ranging from 0.005 to 0.25 μg/ml. Nigericin (5), narasin (3) and A28695B (47) are effective against NDV with MIC ranging from 0.02 to 2.0 μg/ml. Only septamycin (7) was active against infectious canine hepatitis virus (MIC = 0.32 μg/ml). Monensin B (46), nigericin (5), narasin (3), X-206 (32), septamycin (7) and A204 (48) were effective against the infectious bovine rhinotracheitis virus, MIC ranging from 0.005 to 0.08 μg/ml [1].
2.5 Cytotoxicity and antiproliferative
Resistance to chemotherapy is a common clinical problem in patients with cancer. During treatment tumor cells or neoplastic cells are often found to be refractory to a variety of drugs with different structures or modes of actions. These cancers are referred to as MDR forms. Most multidrug resistance is caused by trans-membrane xenobiotic transport proteins belonging to the superfamily of ATP-binding cassette (ABC) transporter efflux pumps [93,94].
Carboxyl polyethers are interesting antineoplastic drug candidates for several reasons and show potent antiproliferative activity in vitro and in vivo. Some carboxyl polyethers such as nigericin (5) have proven to be selectively cytotoxic inhibiting DNA replication of tumor cells in vivo. Another feature that makes carboxyl polyethers even more appealing as antineoplastic drug candidates is their ability to reverse multidrug resistance in human carcinoma. Carboxyl polyethers are reported as chemosensitizing agents. These molecules selectively increase the sensitivity of cancerous cells, but not normal cells, to several cytotoxic agents of which the clinically used anticancer drug paclitaxel [95].
Kawada et al. [42] evaluated the effects of several carboxyl ionophores on colchicine resistance in human carcinoma MDR KB-C410 [42]; these compounds were inostamycin (24), lysocellin (15), laidlomycin (29), monensin (2), dianemycin (20), leuseramycin (26), nigericin (5), lonomycin A&C (17, 22), carriomycin (8), deoxy-salinomycin, antibiotic 6016 (23), SF2324 (44), SF2361 (43) and SF2487 (45). It was discovered that the cancer cells responded better to colchicine when some polyethers were added simultaneously. Laidlomycin (29), monensin (2), dianemycin (20) and leuseramycin (26) led to > 100-fold potentiation of colchicine cytotoxicity. The most active compound was laidlomycin (29); at 0.3 and 1 μg/ml, it potentiated cytotoxicity of KB-C4 cells by ~ 725-fold [42]. Compared to tumor cells, the potentiation of normal cells was negligible; the ratio of IC50 in the absence of polyether versus the IC50 in presence of polyether only varied in the range 0.4 – 2.4 μg/ml. Inostamycin (24) was also reported to have a chemosensitizing effect on paclitaxel in Ms-1 cell, small cell lung carcinoma. This effect was specific to these types of cells and to paclitaxel, as no potentiation was noted with other tested agents, driamycin, vinblastine, methotrexate, cisplatin, etoposide and camptothecin [95]. Inostamycin (24) was also reported to reverse multidrug resistance in these cell lines. Using radiolabeled vinblastine, Kawada et al. [96] established that MDR cells treated with inostamycin (24) (0.5 – 2 μg/ml) accumulated the drug. Inostamycin (24) (1 μg/ml) acted by inhibiting drug efflux [96].
Baibakov et al. [80] and Margolis et al. [97] studied the antineoplastic MOA of nigericin (5) and established that nigericin (5) was a unique type of cytostatic agent. The compound (5) acts not by interfering with spindle microtubules or transcription/translation processes directly, but rather by altering intracellular pH [80,97]. Nigericin (5) showed antiproliferative potential, as a cytostatic agent, in Ehrlich ascites carcinoma cells. The proposed MOA related to its ability to acidify the intracellular environment, a consequence of its H+/K+ anti-transport ability [97]. It is believed that by decreasing the pH DNA synthesis is halted. Near to 100% inhibition of the DNA synthesis was achieved at 0.5 μM nigericin (5), with the pH of the incubation medium being 7.0 [97]. An additional study utilizing an in vivo method such as tridimensional histocultures of human lung tissues, with normal cells present in the same histoculture, determined that this antineoplastic effect of nigericin (5) was selective. Nigericin (5) 10−6 M showed no effect, either on the histology of normal cells or on their DNA synthesis. However, in cancerous tissues the same concentration of nigericin (5) halted DNA synthesis in 67% of the cases, induced pyknotic nuclei and dystropic alteration in 27% of tumor exposed to nigericin (5) and necrosis in 13% of cases. Another carboxylic polyether, ionomycin (12), is also reported to possess antineoplastic properties. It induced inhibition of the growth of a human bladder cancer cell line, HT1367, in vitro in a dose concentration gradient, 0.1 – 100 μg/ml, and time dependent, 10 μM of ionomycin (12), manner. The MOA here is reported to be the induction of apoptosis by means of the downregulation of Bcl-2 and upregulation of Bax. Ionomycin (12) was also effective in vivo, as it inhibited HT1367 tumor’s growth [98].
2.6 Cardiovascular effects
Several carboxyl polyethers were reported to show ionotropic, chronotropic and hemodynamic properties. Because of the critical role of Ca2+ in cardiovascular contractile systems, this was predictable at least for those carboxyl polyethers that are selective for this ion, lasalocid (1) and A23187 (13). It is well established that that lasalocid (1), also known as X-537A, is a positive ionotropic agent. In canine studies, lasalocid (1) is able to increase the contractility index of the heart by approximately threefold at 2 mg/kg [3,7]. X-537A is also reported as a positive ionotropic and chronotropic agent in dogs. In barbiturated dogs, at dosage 2 mg/kg, it caused a threefold increase in the contractility as well as a modest rise in aortic pressure and heart rate. It also induced a drop in total peripheral resistance followed by a drastic increase in blood flow through the coronary arteries of the left ventricle [3]. The exact MOA through which these carboxyl polyethers induce contractility of cardiac muscle is still unclear. Initially, it was believed that this could be the result of their ability to shuttle Ca2+ and catecholamine across membranes. But this was refuted after the discovery that carboxyl polyethers specific to monovalent cations were able to induce, even more effectively, this type of response. Nonetheless, it is believed that catecholamine is partially involved because the effects of these molecules on the cardiac muscle is suppressed by β-adrenergic inhibitors.
Several other carboxyl ionophores were reported to possess a cardiovascular effect. Monensin (2) reportedly induced an increase of arterial blood pressure, myocardial contractility and total peripheral resistance when intravenously administered to anesthetized cats at a dose of 0.075 – 0.375 mg/kg [99]. Similar effect was observed when 0.125 – 2.00 mg/kg were administered via the same route to anesthetized dogs [99]. Monensin (2) was also effective on isolated organs; it showed a vasoconstrictor effect on isolated rabbit aortic strips at concentration ≤ 7 μg/ml and a myocardial stimulation effect in isolated rabbit hearts [99].
Three other carboxyl ionophores, grisorixin (16), alborixin (11), lonomycin A (17), are reported to have similar effects. Grisorixin (16) induced a coronary vasodilator effect in dogs at doses of 60 μg/ml; this was associated with a marked increase in the coronary blood flow, which reached a maximum at this concentration. At doses of 125 – 500 g/kg further inotropic and hypertensive effects were observed [101]. Grisorixin (16) was also reported to have a cardio-tonic effect on isolated perfused rat hearts [102]. In guinea pigs, grisorixin (16) and alborixin (11) 4 mg/kg/min i.v. are reported to increase blood pressure. The MOA is believed to be linked to their intrinsic ionophorous potential, via the ability of the molecules to alter the blood concentration of cations such as K+ and Na+ [103]. Moins et al. [104] working with anesthetized dog reported similar findings, as 2 mg/kg of grisorixin (16) and 1 mg/kg of alborixin increased the ventricular contractile force and systolic and diastolic arterial pressures. This was associated with an increase in the plasma concentration of K+ followed by a decrease in the concentration of Na+ [104]. Owing to the reports of compounds such as grisorixin (11) inducing the release of catecholamine, the physiological response associated to the release of catecholamine was also proposed as a possible justification of the cardio vascular effects [101]. Lonomycin A (17), another carboxyl polyether, was also reported to induce a coronary vasodilatation effect in dogs. Its MOA was proposed to be the stimulation of the Na+, K+-ATPase activity [105].
As stressed by Pressman, and despite their toxicity, carboxyl polyethers are important drug leads for control of various cardiovascular conditions. Their value lies in their outstanding ionotropic, chronotropic and hemodynamic potential. These molecules could prove life-saving tools in controlling conditions such as failure owing to myocardial infraction and other similar forms of shock. Carboxyl polyethers such as lasalocid (1) (1 mg/kg) were proven effective at restoring dogs in cardiogenic shock. Similar data were reported for salinomycin (4) (0.15 mg/kg). According to Pressman, the narrower the transport spectrum of a molecule, the fewer the undesirable effects that are produced [3].
2.7 Other biological activities of carboxyl polyethers: immunoregulatory, herbicidal, anti-inflammatory
2.7.1 Immunoregulatory
Two measures are usually adopted to protect poultry against a variety of disease such as Angara disease virus (ADV) and NDV and promote growth. These include vaccination and addition of substances such as antibiotics, coccidiostat and vitamins to feed. Among additives approved at present are several carboxyl polyethers, lasaloicid (1), monensin (2), salinomycin (4) and manduramycin (35). There are some recent concerns about these additives and the possible association with vaccine failure in poultry. Few reports have actually proven that therapeutic agents such as cyclophosphamide and corticosteroids possess immunomodulatory properties.
Munir et al. in 1994 and 2007 studied the immunomodulatory effects of salinomycin (4) and monensin (2); specifically the effects of these molecules on the protective immune response in NDV and ADV vaccinated chickens. These authors established that unlike cyclophosphamide, salinomycin (4), at a dosage of 0.1 g/kg, did not adversely affect the bursal, splenic, thymic and liver weight gain [106]. It was also established that NDV vaccinated chickens that received salinomycin (4) 60 mg/kg of feed had higher antibiotic titers at days 14, 21, 28, 35, 42 compared to unmedicated chickens [52,106] or vaccinated chickens that were medicated with monensin (2) 12 mg/kg instead [52]. These salinomycin medicated chickens also gained significantly more weight, p < 0.05, compared to all the other groups evaluated. These chickens were challenged with both viruses ADV and NDV after vaccination. No post-ADV or post-NDV challenge mortality was observed in vaccinated chickens that received salinomycin (4) and no clinical sign of the disease was observed [52,106].
From these studies, it seemed that far from causing vaccine failure, carboxyl polyether such as salinomycin (4) actually seem to have a boosting effect on the anti-NDV and anti-ADV immune response [52,106].
Another carboxylic polyether reported as having immunoregulatory potential was ionomycin (12), a calcium-specific ionophore. Ionomycin (12) interferes with physiological processes by increasing the intracellular level of calcium, an effect that could have several implications on key cells of the immune system such as neutrophils and macrophages. They exert their action by production of microbicidal species such as superoxide anion via an NADPH-dependent process known as ‘respiratory burst’ in response to in situ triggers such as inflammatory agents. Ca2+ is documented as an enhancer of NADPH oxidase activity. Finkel et al. [107] reported that ionomycin (12) at a concentration of 1 – 10 nM can prime neutrophils to release approximately sevenfold more superoxide anion during stimulation. Ionomycin (12) was also reported to induce T cell activation [107].
2.7.2 Herbicidal
It has been reported that biological activities of nigericin (6) also possess herbicidal properties. A concentration-dependent study of the effect of nigericin (5) on the growth of the radicle of garden cress seed showed a dose-dependent inhibitory effect. A 50% reduction in the elongation was achieved at 1.3 – 2.0 μg/ml, the maximum inhibition was reported at ~ 3.33 μg/ml. Nigericin (5) did not induce browning or necrosis of tissues in the process [12].
2.7.3 Anti-inflammatory
Dianemycin (20) was recently reported as having topical anti-inflammatory potential on several animal models for cutaneous inflammation. In a croton-oil induction of ear edema, dianemycin (20) had a comparable activity to prednisolone, a potent steroidal anti-inflammatory with an ED50 of 0.8 versus 0.4 mg/ear. No anti-inflammatory activity was observed in the UV-induced erythema test or in the delayed type sensitivity test unlike the control, prenisolone. Additionally, no acute toxicity was observed with topical application ≤ 10 mg/ear [13].
3. Toxicity
Acute toxicity of the naturally occurring polyether ionophores is relatively specific to the organism that it is affecting. It is clear that minimal exposure to these very active compounds initiates some type of physiological response [7]. In the case of higher organisms and specifically mammals and birds, these effects are almost equally diverse [1]. For example, a common poisoning scenario is a horse consuming a small quantity of feed prepared with a ruminant’s polyether antibiotic and dying rather quickly or wild turkeys consuming anticoccidial chicken feed and dying. Although the chirality of the central carboxylate of the molecules can predict inonophoric specificity, the respective LD50 acute toxicity values for individual species of molecules are equally diverse and unpredictable [79]. For example, LD50 ± SE values for an acute oral dose of monensin (2) were: mouse (m) 70.0 ± 9.0 mg/kg, (f) 96.0 ± 12.0 mg/kg; rat (m) 40.1 ± 3.0 mg/kg, (f) 28.6 ± 3.8 mg/kg; dog (m) > 20.0 mg/kg, (f) > 10.0 mg/kg; rabbit 41.7 ± 3.6 mg/kg; monkey > 160.0 mg/kg; chicken 200.0 mg/kg; cattle 26.4 mg/kg; sheep 11.9 ± 1.2 mg/kg; goat 26.4 ± 4.0 mg/kg; swine 16.7 ± 3.57; horse 2 – 3 mg/kg; trout > 1000.0 mg/kg [79]. “Dogs given a daily oral doses of 0, 1.25, 2.5, 5, or 7.5 mg/kg of monensin (2) for 1 year survived with no evidence of toxicity in the two lowest dose groups … Four generations of rats were continuously maintained on diets containing 0, 33, 50, or 80 ppm of monensin (2). Except for a decrease in body weight gain, there were no compound-related effects on reproduction or observed teratogenic effects” [79]. These same dosing conditions were evaluated for a 2-year period, with respect to the skeletal and cardiac muscles, with no increase in chronic lesions or neoplasms being detected [79]. Despite these maverick chemo-physical qualities of the ionophores, considerable numbers of toxicology studies have attempted to illustrate/translate the specific toxicology and pharmacology of the most common polyether ionophores to human health utilization [7].
4. Cardiac toxicity
Whether a monovalent or divalent ionophore is used, these molecules will disrupt the Ca2+ concentrations, either forming Ca2+ complexes or depolarizing the membrane potential, changing the H+ concentrations in the mitochondria [4]. It is clear that Ca2+ is an essential ion in the physiological activities of composite functionality in the entire human body’s daily functions. Cardiac function is especially sensitive to the roles of Ca2+ owing to the intrinsic functions of the ion and its role in muscular contractions; so, expectedly, potent effects of polyether antibiotics have been observed in mammalian cardiac studies [3,56,80–82,108]. Canine studies are accepted as the translative animal model for human cardiac response and for the effects of carboxylic polyether ionophores. These studies have shown that as little as 2 μg/kg i.v. can induce coronary dilation [108,109]. The concern for clinical applications of these ionophores is that persons with coronary heart’s disease who have coronary arterial dilation, as part of the hearts own auto regulatory response to occlusion of coronary arterial vessels, will dilate the affected vessels further in the presence of an indiscriminate vasodilator. The unaffected vesicles would dilate further and the already maximally dilated obstructed vessels would divert the blood supply away from the ischemic regions of the myocardium. This phenomenon is called ‘coronary steal’ and has been observed in the administration of the drug dipyridamole, a thrombus inhibitor and chronic vasodilator [103,110]. As a large number of the population is affected by coronary heart disease these concerns have validity, although this phenomena was experimentally evaluated and the coronary steal was not observed [110].
Only recently have the in vitro tools been available for cardiac toxicity evaluations with respect to interaction on the hERG (human Ether-a-go-go Related Gene) gene and the associated Kv11.1 potassium ion channel that repolarizes the IKr current in the cardiac action potentials. The polyether antibiotics have not yet been critically evaluated in hERG assays. The drug interactions related to hERG ion channel were not mandated by ICH guidelines until November 2005 (mandate #CHMP/ICH/423/02). Consequently, the effects of carboxyl polyether ionophores on the QTc with respect to the interaction with hERG have not yet been evaluated.
5. Conclusions
Carboxyl polyethers are broad spectrum antibiotics. They are active against a wide range of biological targets: bacterial, fungal, protozoan, viral, neoplastic and cardiovascular. A few authors have also reported carboxyl polyethers as immunomodulating agents. In vitro, these molecules have proven to be effective at very low concentrations. The malaria parasite (P. falciparum) is especially sensitive to several of these compounds with IC50 values in the nanomolar range. Based on the reports in this review it can be argued that carboxyl polyethers are highly selective. Unlike Gram negative bacteria or fungi for instance, Gram positive bacteria tend to be extremely sensitive to these antibiotics. Moreover, the IC50 values of several of these molecules against mammalian cells tend to be significantly higher, when compared to the IC50 against targeted etiological agents.
Despite these interesting features the only current application for carboxyl polyethers is in veterinary medicine, where they are used as controls for coccidiosis and feed absorption efficiency. The main obstacle for the use of carboxyl polyethers as drugs to control human diseases seems to be the issue of toxicity. With reductions in toxicity carboxyl polyethers possess some interesting qualities against serious threats such as MRSA and VRE and have proven to be more effective (in vitro) than drugs that are currently used clinically, such as vancomycin and oxacillin. They have also shown to be effective against several other resistant bacterial strains including penicillin resistance strains of S. aureus, tetracycline resistant strain of Micrococcus sp, aminoglycoside resistant strain of Streptococcus faecalis, and macrolides resistant strain of Sarcina lutea. Carboxyl polyethers have also proven effective at namomolar range against chloroquine resistant strains of P. falciparum and are more potent against these resistant strains than several currently marketed antimalarial drugs. Their mechanism of action against the malaria parasite appears uniquely different from that of the currently utilized drugs. This makes them possible candidates for use in combination therapy.
6. Expert opinion
On the basis of our assessment of the literature, polyether ionophores show a high degree of promise for the potential control of drug-resistant bacterial and parasitic infections. Many of these infectious diseases fall under the umbrella of neglected disease with limited options for therapeutic intervention. Furthermore, recent decades have emphasized the importance of combination therapies for the long-term control of disease and mitigation of resistance. The long history of the utilization of polyether ionophores as growth promoting antibiotics by the agricultural industry validates the potential cost-effective use of these drug products for other applications. The polyether antibiotics seem to provide reasonable oral availability, half-life, metabolic and general stability, absence of a UV sensitizing chromophore combined with significant potency. Despite the long history of use of the class, very limited medicinal chemistry and drug optimization studies have been reported, leaving the door open to these opportunities in the future. In many instances, the potency and in vivo efficacy for the control of bacterial and parasitic infections is truly remarkable. However, concerns about toxicity need to be addressed with further optimization and preclinical evaluation. On the basis of the available literature, there is still insufficient data to clearly classify these molecules as either too toxic or safe as a class. Several carboxyl polyethers have indeed been shown to be too toxic in animal models for malaria but the results cannot be generalized to the entire class. Using an in vivo model for cancer, nigericin (5) was recently reported to be highly selective in targeting cancerous cells, broadening the scope of possible utility for this class [80]. As a result, an organized and carefully orchestrated identification and optimization of leads using in vitro models followed by an in vivo assessment of toxicity and efficacy would seem to yield viable drug candidates for the control of drug-resistant infectious diseases and cancer with a reasonable understanding of pharmacokinetics, stability and cost of goods. The reported toxicity for monensin (2) of > 160.0 mg/kg (LD50) in primates and frequent utilization of this drug in agriculture makes the polyether class an attractive group of molecules for optimization and development of human health applications for the control of drug-resistant diseases such as malaria and MRSA.
Acknowledgments
Declaration of interest
Dion A Kevin II is supported by the MS/AL SeaGrant and National Institutes of Health. Damaris AF Meujo is supported by a fellowship from MMV (Medicine for Malaria Venture) and Triton Biopharma. Mark T Hamann is funded by NIH, NOAA/SeaGrant, MMV, Kraft Foods, and is a cofounder and significant shareholder of Triton Biopharma.
Bibliography
- 1.Westley JW. Polyether Antibiotics: Naturally Occurring Acid Ionophores, Edition. Marcel Dekker Inc; New York: 1982. p. 1. [Google Scholar]
- 2.Pressman BC. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- 3.Pressman BC, Deguzman NT. Biological applications and evolutionary origins of ionophores. Adv Exp Med Biol. 1977;84:285–300. doi: 10.1007/978-1-4684-3279-4_14. [DOI] [PubMed] [Google Scholar]
- 4.Lutz WK, Winker FK, Dunitz JD. Crystal Structure of the Antibiotic Monensin Similarities and Differences between Free Acid and Metal Complex. Helv Chim Acta. 1972;54(4):1103–8. doi: 10.1002/hlca.19710540419. [DOI] [PubMed] [Google Scholar]
- 5.Dorkov P, Pantcheva IN, Sheldrick WS, et al. Synthesis, structure and antimicrobial activity of manganese(II) and cobalt(II) complexes of the polyether ionophore antibiotic Sodium Monensin A. J Inorg Biochem. 2008;102(1):26–32. doi: 10.1016/j.jinorgbio.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrandt J, Meingassner JG, Mieth H. Mode of action of the anticoccidial agent septamycine. Zentralbl Veterinarmed B. 1978;25(3):186–93. [PubMed] [Google Scholar]
- 7.Pressman BC, Fahim M. Pharmacology and toxicology of the monovalent carboxylic ionophores. Annu Rev Pharmacol Toxicol. 1982;22:465–90. doi: 10.1146/annurev.pa.22.040182.002341. [DOI] [PubMed] [Google Scholar]
- 8.Hamill RL, Hoehn MM, Pittenger GE, et al. Dianemycin, an antibiotic of the group affecting ion transport. J Antibiot (Tokyo) 1969;22(4):161–4. doi: 10.7164/antibiotics.22.161. [DOI] [PubMed] [Google Scholar]
- 9.Stern PH. Ionophores. Chemistry, physiology and potential applications to bone biology. Clin Orthop Relat Res. 1977;122:273–98. [PubMed] [Google Scholar]
- 10.Mizutani T, Yamagishi M, Hara H, et al. Lonomycins B and C, two new components of polyether antibiotics. Fermentation, isolation and characterization. J Antibiot (Tokyo) 1980;33(11):1224–30. doi: 10.7164/antibiotics.33.1224. [DOI] [PubMed] [Google Scholar]
- 11.Gachon P, Kergomard A, Staron T, Esteve C. Grisorixin, an ionophorous antibiotic of the nigericin group. I. Fermentation, isolation, biological properties and structure. J Antibiot (Tokyo) 1975;28(5):345–50. doi: 10.7164/antibiotics.28.345. [DOI] [PubMed] [Google Scholar]
- 12.Heisey RM, Putnam AR. Herbicidal effects of geldanamycin and nigericin, antibiotics from Streptomyces hygroscopicus. J Nat Prod. 1986;49(5):859–65. doi: 10.1021/np50047a016. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJK, Kim HP, Park BK, et al. Topical anti-inflammatory activity of dianemycin isolated from Streptomyces sp. MT 2705-4. Arch Pharmacal Res. 1997;20(4):372–4. doi: 10.1007/BF02976203. [DOI] [PubMed] [Google Scholar]
- 14.Parfenova H, Haffner J, Leffler CW. Phosphorylation-dependent stimulation of prostanoid synthesis by nigericin in cerebral endothelial cells. Am J Physiol. 1999;277:C728–38. doi: 10.1152/ajpcell.1999.277.4.C728. [DOI] [PubMed] [Google Scholar]
- 15.Mccollum AT, Jafarifar F, Chan R, Guttmann RP. Oxidative stress inhibits ionomycin-mediated cell death in cortical neurons. J Neurosci Res. 2004;76(1):104–9. doi: 10.1002/jnr.20059. [DOI] [PubMed] [Google Scholar]
- 16.Westley JW, Liu CM, Sello LH, et al. Isolation and characterization of antibiotic X-14931A, the naturally occurring 19-deoxyaglycone of dianemycin. J Antibiot (Tokyo) 1984;37(7):813–5. doi: 10.7164/antibiotics.37.813. [DOI] [PubMed] [Google Scholar]
- 17.Ebata E, Kasahara H, Sekine K, Inoue Y. Lysocellin, a new polyether antibiotic. I. Isolation, purification, physico-chemical and biological properties. J Antibiot (Tokyo) 1975;28(2):118–21. doi: 10.7164/antibiotics.28.118. [DOI] [PubMed] [Google Scholar]
- 18.Omura S, Shibata M, Machida S, Sawada J. Isolation of a new polyether antibiotic, lonomycin. J Antibiot (Tokyo) 1976;29(1):15–20. doi: 10.7164/antibiotics.29.15. [DOI] [PubMed] [Google Scholar]
- 19.Funayama S, Nozoe S, Tronquet C, et al. Isolation and structure of a new polyether antibiotic, octacyclomycin. J Antibiot (Tokyo) 1992;45(10):1686–91. doi: 10.7164/antibiotics.45.1686. [DOI] [PubMed] [Google Scholar]
- 20.Kusakabe Y, Mitsuoka S, Omuro Y, Seino A. Antibiotic no. 6016, a polyether antibiotic. J Antibiot (Tokyo) 1980;33(12):1437–42. doi: 10.7164/antibiotics.33.1437. [DOI] [PubMed] [Google Scholar]
- 21.Baba YUH, Tsukuda M, Mochimatsu I, et al. Cytostatic effect of inostamycin, an inhibitor of cytidine 5′-diphosphate 1,2-DIACYL-sn-GLYCEROL (CDP-DG): inositol transferase, on oral squamous cell carcinoma cell lines. Cell Biol Int. 2001;25(7):613–20. doi: 10.1006/cbir.2000.0706. [DOI] [PubMed] [Google Scholar]
- 22.Odai H, Shindo K, Odagawa A, et al. Inostamycins B and C, new polyether antibiotics. J of Antibiot. 1994;47(8):939–41. doi: 10.7164/antibiotics.47.939. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani T, Yamagishi M, Kara H, et al. Studies on the ionophorous antibiotics. XXIV. Leuseramycin, a new polyether antibiotic produced by Streptomyces hygroscopicus. J Antibiot. 1980;33(2):137–43. doi: 10.7164/antibiotics.33.137. [DOI] [PubMed] [Google Scholar]
- 24.Keller-juslen C, King HD, Von Wartburg A. Septamycin, a polyether antibiotic. Taxonomy, fermentation, isolation and characterization. J Antibiot. 1975;28(11):854–9. doi: 10.7164/antibiotics.28.854. [DOI] [PubMed] [Google Scholar]
- 25.Keller-Juslen C, King HD, Kuhn M, et al. Noboritomycins A and B, new polyether antibiotics. J Antibiot (Tokyo) 1978;31(9):820–8. doi: 10.7164/antibiotics.31.820. [DOI] [PubMed] [Google Scholar]
- 26.Fehr T, King HD, Kuhn M. Mutalomycin, a new polyether antibiotic taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo) 1977;30(11):903–7. doi: 10.7164/antibiotics.30.903. [DOI] [PubMed] [Google Scholar]
- 27.Kitame F, Utsushikawa K, Koama T, et al. Laidlomycin, a new antimycoplasmal polyether antibiotic. J Antibiot (Tokyo) 1974;27(11):884–8. doi: 10.7164/antibiotics.27.884. [DOI] [PubMed] [Google Scholar]
- 28.Alleaume MH. Crystal structure of the thallium salt of the antibiotic grisorixin. J Chem Soc Chem Commun. 1972;3:175–6. [Google Scholar]
- 29.Guyot JJ, Jeminet G, Prudhomme M, et al. Interaction of the calcium ionophore A. 23187 (calcimycin) with Bacillus cereus and Escherichia coli. Lett Appl Microbiol. 1993;16(4):192–5. [Google Scholar]
- 30.Yoo JC, Kim JH, Ha JW, et al. Production and biological activity of laidlomycin, anti-MRSA/VRE antibiotic from Streptomyces sp. CS684. J Microbiol. 2007;45(1):6–10. [PubMed] [Google Scholar]
- 31.Cirioni O, Giacometti A, Barchiesi F, Scalise G. In vitro activity of lytic peptides, inhibitors of ion transport systems and ionophorous antibiotics against Pneumocystis carinii. J Antimicrob Chemother. 1998;42(2):141–5. doi: 10.1093/jac/42.2.141. [DOI] [PubMed] [Google Scholar]
- 32.Oz HS, Hughes WT, Rehg JE. Efficacy of lasalocid against murine Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1997;41(1):191–2. doi: 10.1128/aac.41.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delfini LF. The first case of Plasmodium falciparum resistant to chloroquine treatment discovered in the Republic of Afghanistan. Trans R Soc Trop Med Hyg. 1989;83(3):316. doi: 10.1016/0035-9203(89)90486-0. [DOI] [PubMed] [Google Scholar]
- 34.Schapira A, Beales PF, Halloran ME. Malaria: living with drug resistance. Parasitol. 1993;9(5):168–74. doi: 10.1016/0169-4758(93)90140-b. [DOI] [PubMed] [Google Scholar]
- 35.Geyer M. Antimalarial drug combination therapy report of a who technical consultation world health organization, geneva WHO. 2001. Copyright 2001 by Roll Back Malaria/World Health Organization. [Google Scholar]
- 36.Trape JF, Pison G, Preziosi MP, et al. Impact of chloroquine resistance on malaria mortality. C R Acad Sci III. 1998;321:689–97. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 37.Kassankogno Y, et al. Combination therapy for uncomplicated malaria in africa? liaison bulletin of the malaria programme WHO/AFRO. 2001;4(1):1–4. [Google Scholar]
- 38.Gumila C, Ancelin ML, Jeminet G, et al. Differential in vitro activities of ionophore compounds against Plasmodium falciparum and mammalian cells. Antimicrob Agents Chemother. 1996;40(3):602–8. doi: 10.1128/aac.40.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otoguro K, Kohana A, Manabe C, et al. Potent antimalarial activities of polyether antibiotic, X-206. J Antibiot (Tokyo) 2001;54(8):658–63. doi: 10.7164/antibiotics.54.658. [DOI] [PubMed] [Google Scholar]
- 40.Gumila C, Ancelin ML, Delort AM, et al. Characterization of the potent in vitro and in vivo antimalarial activities of ionophore compounds. Antimicrob Agets Chemother. 1997;41(3):523–9. doi: 10.1128/aac.41.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adovelande J, Schrevel J. Carboxylic ionophores in malaria chemotherapy: the effects of monensin and nigericin on Plasmodium falciparum in vitro and Plasmodium vinckei petteri in vivo. Life Sci. 1996;59(20):309–15. doi: 10.1016/s0024-3205(96)00514-0. [DOI] [PubMed] [Google Scholar]
- 42.Kawada M, Sumi S, Umezawa K, et al. Circumvention of multidrug resistance in human carcinoma KB cells by polyether antibiotics. J Antibiot (Tokyo) 1992;45(4):556–62. doi: 10.7164/antibiotics.45.556. [DOI] [PubMed] [Google Scholar]
- 43.Imada A, Nozaki Y, Hasegawa T, et al. Carriomycin, a new polyether antibiotic produced by Streptomyces hygroscopicus. J Antibiot (Tokyo) 1978;31(1):7–14. doi: 10.7164/antibiotics.31.7. [DOI] [PubMed] [Google Scholar]
- 44.Otoguro K, Aki I, Ui Hideaki, et al. In vitro and in vivo antimalarial activities of the monoglycoside polyether antibiotic, K-41 against drug resistant strains of Plasmodia. J Antibiot. 2002;55(9):832–34. doi: 10.7164/antibiotics.55.832. [DOI] [PubMed] [Google Scholar]
- 45.Fitch COY. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life science. 2004;74(16):1957–72. doi: 10.1016/j.lfs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Reeves DC, Liebelt DA, Lakshmanan V, et al. Chloroquine-resistant isoforms of the Plasmodium falciparum chloroquine resistance transporter acidify lysosomal pH in HEK293 cells more than chloroquine-sensitive isoforms. Mol Biochem Parasitol. 2006;150(2):28899. doi: 10.1016/j.molbiopara.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovac LV, Poliachova Horvath Ionophores and intact cells. I Valinomycin and nigericin act preferentially on mitochondria and not on the plasma membrane of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982;721:341–8. doi: 10.1016/0167-4889(82)90088-x. [DOI] [PubMed] [Google Scholar]
- 48.Na M, Meujo DAF, Kevin D, et al. A New Antimalarial Polyether from a Marine Streptomyces sp. H668. Tet Lett. 2008 doi: 10.1016/j.tetlet.2008.08.052. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conway DP, Johnson JK, Guyonnet V, et al. Efficacy of semduramicin and salinomycin against different stages of Eimeria tenella and E. acervulina in the chicken. Vet Parasitol. 1993;45(3–4):215–29. doi: 10.1016/0304-4017(93)90077-z. [DOI] [PubMed] [Google Scholar]
- 50.Cerruti Sola SLA, Agostini A, Castagnaro M. Efficacy of maduramicin against turkey coccidiosis in battery: a clinical and pathological study. Schweizer Archiv fur Tierheilkunde. 1996;138(4):201–6. [PubMed] [Google Scholar]
- 51.Smith CK, 2nd, Strout RG. Eimeria tenella: effect of narasin, a polyether antibiotic on the ultrastructure of intracellular sporozoites. Exp Parasitol. 1980;50(3):426–36. doi: 10.1016/0014-4894(80)90045-4. [DOI] [PubMed] [Google Scholar]
- 52.Munir K, Muneer MA, Tiwari A, et al. Effects of polyether ionophores on the protective immune responses of broiler chickens against Angara disease and Newcastle disease viruses. Vet Res Commun. 2007;31(7):909–29. doi: 10.1007/s11259-007-0030-7. [DOI] [PubMed] [Google Scholar]
- 53.Dahlgren C, Karlsson A. Ionomycin-induced neutrophil NADPH oxidase activity is selectively inhibited by the serine protease inhibitor diisopropyl fluorophosphate. Antioxid Redox Signal. 2002;4(1):17–25. doi: 10.1089/152308602753625816. [DOI] [PubMed] [Google Scholar]
- 54.Dirlam JP, Belton AM, Bordner J, et al. CP-84,657, a potent polyether anticoccidial related to portmicin and produced by Actinomadura sp. J Antibiot (Tokyo) 1990;43(6):668–79. doi: 10.7164/antibiotics.43.668. [DOI] [PubMed] [Google Scholar]
- 55.Dirlam JP, Presseau-Linabury L, Koss DA. The structure of CP-80, 219, a new polyether antibiotic related to dianemycin. J Antibiot. 1990;43(6):727–30. doi: 10.7164/antibiotics.43.727. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi Y, Nakamura H, Ogata R, et al. Kijimicin, a polyether antibiotic. J Antibiot (Tokyo) 1990;43(4):441–3. doi: 10.7164/antibiotics.43.441. [DOI] [PubMed] [Google Scholar]
- 57.Dirlam JP, Belton AM, Bordner J, et al. CP-82, 009, a potent polyether anticoccidial related to septamycin and produced by Actinomadura. J Antibiot. 1992;45(3):331–40. doi: 10.7164/antibiotics.45.331. [DOI] [PubMed] [Google Scholar]
- 58.Oscarson JR, Bordner J, Celmer WD, et al. Endusamycin, a novel polycyclic ether antibiotic produced by a strain of Streptomyces endus subsp. aureus. J Antibiot (Tokyo) 1989;42(1):37–48. doi: 10.7164/antibiotics.42.37. [DOI] [PubMed] [Google Scholar]
- 59.Liu CM, Hermann TE, Downey A, et al. Novel polyether antibiotics X-14868A, B, C, and D produced by a Nocardia. Discovery, fermentation, biological as well as ionophore properties and taxonomy of the producing culture. J Antibiot (Tokyo) 1983;36(4):343–50. doi: 10.7164/antibiotics.36.343. [DOI] [PubMed] [Google Scholar]
- 60.Flanigan TP. Human immunodeficiency virus infection and cryptosporidiosis: protective immune responses. Am J Trop Med Hyg. 1994;50(5 Suppl):29–35. [PubMed] [Google Scholar]
- 61.Fujikawa H, Miyakawa H, Iguchi K, et al. Intestinal cryptosporidiosis as an initial manifestation in a previously healthy Japanese patient with AIDS. J Gastroenterol. 2002;37(10):840–3. doi: 10.1007/s005350200138. [DOI] [PubMed] [Google Scholar]
- 62.Lim YA, Rohela M, Sim BL, et al. Prevalence of cryptosporidiosis in HIV-infected patients in Kajang Hospital, Selangor. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 4):30–3. [PubMed] [Google Scholar]
- 63.Blagburn BL, Sundermann CA, Lindsay DS, et al. Inhibition of Cryptosporidium parvum in neonatal Hsd:(ICR)BR Swiss miceby polyether ionophores and aromatic amidines. Antimicrob Agents Chemother. 1991;35(7):1520–3. doi: 10.1128/aac.35.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You X, Schinazi RF, Arrowood MJ, et al. In-vitro activities of paromomycin and lasalocid evaluated in combination against Cryptosporidium parvum. J Antimicrob Chemother. 1998;41(2):293–6. doi: 10.1093/jac/41.2.293. [DOI] [PubMed] [Google Scholar]
- 65.Mcdonald V, Stables R, Warhurst DC, et al. In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrob Agents Chemother. 1990;34(8):1498–500. doi: 10.1128/aac.34.8.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzipori SR, Campbell I, Angus KW. The therapeutic effect of 16 antimicrobial agents on Cryptosporidium infection in mice. Aust J Exp Biol Med Sci. 1982;60(Pt 2):187–90. doi: 10.1038/icb.1982.20. [DOI] [PubMed] [Google Scholar]
- 67.Folz SD, Lee BL, Nowakowski LH, Conder GA. Anticoccidial evaluation of halofuginone, lasalocid, maduramicin, monensin and salinomycin. Vet Parasitol. 1988;28(1–2):1–9. doi: 10.1016/0304-4017(88)90013-1. [DOI] [PubMed] [Google Scholar]
- 68.Woods KM, Nesterenko MV, Upton SJ. Efficacy of 101 antimicrobials and other agents on the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;90(6):603–15. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]
- 69.Mead JR, You X, Pharr JE, et al. Evaluation of maduramicin and alborixin in a SCID mouse model of chronic cryptosporidiosis. Antimicrob Agents Chemother. 1995;39(4):854–8. doi: 10.1128/aac.39.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleck WF, Strauss DG, Meyer J, Porstendorfer G. Fermentation, isolation, and biological activity of maduramycin: a new antibiotic from Actinomadura rubra. Z Allg Mikrobiol. 1978;18(6):389–98. doi: 10.1002/jobm.3630180602. [DOI] [PubMed] [Google Scholar]
- 71.Soete M, Fortier B, Camus D, Dubremetz JF. Toxoplasma gondii: kinetics of bradyzoite-tachyzoite interconversion in vitro. Exp Parasitol. 1993;76(3):259–64. doi: 10.1006/expr.1993.1031. [DOI] [PubMed] [Google Scholar]
- 72.Fruth IA, Arrizabalaga G. Toxoplasma gondii: induction of egress by the potassium ionophore nigericin. Int J Parasitol. 2007;37(14):1559–67. doi: 10.1016/j.ijpara.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carrada-bravo T. Toxoplasmosis. A public health problem. Advances and perspectives. Bol Med Hosp Infant Mex. 1983;40(7):353–62. [PubMed] [Google Scholar]
- 74.Wong SY, Remington JS. Biology of Toxoplasma gondii. Aids. 1993;7(3):299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Couzinet S, Dubremetz JF, David L, Prensier G. Toxoplasma gondii: activity of the polyether ionophorous antibiotic nigericin on tachyzoites in cell culture. Exp Parasitol. 1994;78(4):341–51. doi: 10.1006/expr.1994.1037. [DOI] [PubMed] [Google Scholar]
- 76.Couzinet S, Dubremetz JF, Buzoni-Gatel D, et al. In vitro activity of the polyether ionophorous antibiotic monensin against the cyst form of Toxoplasma gondii. Parasitol. 2000;12:359–65. doi: 10.1017/s0031182099006605. [DOI] [PubMed] [Google Scholar]
- 77.Ricketts AP, Pfefferkorn ER. Toxoplasma gondii: susceptibility and development of resistance to anticoccidial drugs in vitro. Antimicrob Agents Chemother. 1993;37(11):2358–63. doi: 10.1128/aac.37.11.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahmoudi N, Garcia-Domenech R, Galvez J, et al. New active drugs against liver stages of Plasmodium predicted by molecular topology. Antimicrob Agents Chemother. 2008;52(4):1215–20. doi: 10.1128/AAC.01043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oh JH, O’malley KL, Krajewski S, et al. Bax accelerates staurosporine-induced but suppresses nigericin-induced neuronal cell death. Neuroreport. 1997;8(8):1851–6. doi: 10.1097/00001756-199705260-00012. [DOI] [PubMed] [Google Scholar]
- 80.Baibakov BAF, Frank G, Margolis L, et al. Antitumor effect of potassium/hydrogen antiporter nigericin on human lung carcinoma grown in in vivo-like histocultures. Int J Oncol. 1993;3(6):1127–9. doi: 10.3892/ijo.3.6.1127. [DOI] [PubMed] [Google Scholar]
- 81.Steinrauf LK, Pinkerton M, Chamberlin JW. The structure of nigericin. Biochem Biophys Res Commun. 1968;33(1):29–31. doi: 10.1016/0006-291x(68)90249-0. [DOI] [PubMed] [Google Scholar]
- 82.Tomio T, Arisuke W, Munekazu I, et al. JP2001278787. Antimalarial agents containing kijimicin. 2001
- 83.Huskinson-mark J, Araujo FG, Remington JS. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J Infect Dis. 1991;164(1):170–1. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- 84.Sarciron ME, Petavy PL, Peyron AF. Alterations of Toxoplasma gondii Induced by 2′,3′-Dideoxyinosine In vitro. J Parasitol. 1998;84(5):1055–9. [PubMed] [Google Scholar]
- 85.Lindsay DS, Rippey NS, Cole RA, et al. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am J Vet Res. 1994;55(7):976–81. [PubMed] [Google Scholar]
- 86.Nakamura M, Ohno T, Kunimoto S, et al. Kijimicin: an inhibitor of human immunodeficiency virus in acutely and chronically infected cells. J Antibiot (Tokyo) 1991;44(5):569–71. doi: 10.7164/antibiotics.44.569. [DOI] [PubMed] [Google Scholar]
- 87.Yamauchi T, Nakamura M, Honma H, et al. Mechanistic effects of kijimicin on inhibition of human immunodeficiency virus replication. Mol Cell Biochem. 1993;119(1–2):35–41. doi: 10.1007/BF00926851. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura M, Kunimoto S, Takahashi Y, et al. Inhibitory effects of polyethers on human immunodeficiency virus replication. Antimicrob Agents Chemother. 1992;36(2):492–4. doi: 10.1128/aac.36.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pal R, Gallo RC, Sarngadharan MG. Processing of the structural proteins of human immunodeficiency virus type 1 in the presence of monensin and cerulenin. Proc Natl Acad Sci USA. 1988;85(23):9283–6. doi: 10.1073/pnas.85.23.9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robin L, Dewar MB, Vasudevachari V, et al. Biosynthesis and Processing of Human Immunodeficiency Virus Type 1 Envelope Glycoproteins: Effects of Monensin on Glycosylation and Transport. J Virol. 1989;63(6):2452–6. doi: 10.1128/jvi.63.6.2452-2456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Otake NT, Ogita H, Nakayama H, et al. X-Ray crystal structure of the thallium salt of antibiotic-6016, a new polyether ionophore. J Chem Soc Chem Comm. 1978:875–876. [Google Scholar]
- 92.Nakamura M, Kunimoto S, Kawashima H, et al. Inhibitory effect of laidlomycin on human immunodeficiency virus replication. J Antibiot (Tokyo) 2000;53(9):975–8. doi: 10.7164/antibiotics.53.975. [DOI] [PubMed] [Google Scholar]
- 93.Lage H. ABC-transporters: implications on drug resistance from Microorganisms to human cancers. Int J Antimicrob Agents. 2003;22(3):188–9915. doi: 10.1016/s0924-8579(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 94.Gyemant N, Tanaka M, Molnar P, et al. Reversal of multidrug resistance of cancer cells in vitro: modification of drug resistance by selected carotenoids. Anticancer Res. 2006;26(1A):367–74. [PubMed] [Google Scholar]
- 95.Simizu S, Tanabe K, Tashiro E, et al. Potentiation of paclitaxel cytotoxicity by inostamycin in human small cell lung carcinoma, Ms-1 cells. Jpn J Canc Res Gann. 1998;89(9):970–6. doi: 10.1111/j.1349-7006.1998.tb00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawada M, Umezawa K. Long-lasting Accumulation, Department of Applied Chemistry, Faculty of Science and Technology, Keio University, Yokohama. Jpn J Canc Res Gann. 1991;82(10):1160–4. doi: 10.1111/j.1349-7006.1991.tb01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Margolis LB, Novikova IY, Rozovskaya IA, Skulachev VP. K+/H+-antiporter nigericin arrests DNA synthesis in Ehrlich ascites carcinoma cells. Proc Natl Acad Sci USA. 1989;86:6626–9. doi: 10.1073/pnas.86.17.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyake H, Hara I, Yamanaka K, et al. Calcium ionophore, ionomycin inhibits growth of human bladder cancer cells both in vitro and in vivo with alteration of Bcl-2 and Bax expression levels. J Urol. 1999;162(3):916–21. doi: 10.1097/00005392-199909010-00090. [DOI] [PubMed] [Google Scholar]
- 99.Atef M, Youssef S, Eld AH, et al. Some cardiovascular effects of monensin. Tieraerztliche Wochenschrift. 1986;93(2):81–4. [PubMed] [Google Scholar]
- 100.David L, Leal Ayala H, Tabet JC. Abierixin, a new polyether antibiotic. Production, structural determination and biological activities. J Antibiot (Tokyo) 1985;38(12):1655–63. doi: 10.7164/antibiotics.38.1655. [DOI] [PubMed] [Google Scholar]
- 101.Moins N, Gachon P, Maublant J, Duchene-Marullaz P. Hemodynamic effects of grisorixin, a monocarboxylic ionophore. Arch Internationales de Pharmacodynamie et de Therapie. 1982;260(1):104–14. [PubMed] [Google Scholar]
- 102.Chollet-debord F, Moins N, Renoux M, Gachon P. Effects of the ionophore grisorixin on myocardial function and metabolism in isolated perfused working rat heart under normoxic and hypoxic conditions. Can J Physiol Pharmacol. 1986;64(5):631–40. doi: 10.1139/y86-105. [DOI] [PubMed] [Google Scholar]
- 103.Gachon P, Moins N. The cardiovascular effects of two monocarboxylic inophores, grisorixin and alborixin, in anesthetized guinea-pigs. Arzneimittelforschung. 1980;30(9):1502–7. [PubMed] [Google Scholar]
- 104.Moins N, Gachon P, Duchene-Marullaz P. Effects of two monocarboxylic ionophores, grisorixin and alborixin, on cardiovascular function and plasma cation concentrations in the anesthetized dog. J Cardiovasc Pharmacol. 1979;1(6):659–71. doi: 10.1097/00005344-197911000-00007. [DOI] [PubMed] [Google Scholar]
- 105.Tsuchida K, Kaneko K, Aihara H, Chiba S. Cardiovascular effects of an ionophorous antibiotic, lonomycin A, in anesthetized dogs. Jpn J Pharmacol. 1985;38(1):109–12. doi: 10.1254/jjp.38.109. [DOI] [PubMed] [Google Scholar]
- 106.Munir KM, Khan MA. Immunomodulatory effects of salinomycin sodium in broiler chickens. Pakistan Vet J. 1994;14(4):171–9. [Google Scholar]
- 107.Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143(4):1283–9. [PubMed] [Google Scholar]
- 108.Czerwinski EW, Steinrauf LK. Structure of the antibiotic dianemycin. Biochem Biophys Res Commun. 1971;45(5):1284–7. doi: 10.1016/0006-291x(71)90157-4. [DOI] [PubMed] [Google Scholar]
- 109.Delhomme C, Kergomard A, Kergomard G, Staron T. Alborixin, a new antibiotic ionophore: taxonomy, isolation and biological properties. J Antibiot (Tokyo) 1976;29(7):692–5. doi: 10.7164/antibiotics.29.692. [DOI] [PubMed] [Google Scholar]
- 110.Alleaume MB, Farges B, Gachon C, et al. T. X-ray structure of alborixin, a new antibiotic ionophore. J Chem Soc Chem Com. 1975;11:4112. [Google Scholar]
- 111.Folz SD, Nowakowski LH, Lee BL, et al. Anticoccidial activity of alborixin. J Parasitol. 1987;73(1):29–35. [PubMed] [Google Scholar]
- 112.Crance JM, Gratier D, Guimet J, Jouan A. Inhibition of sandfly fever Sicilian virus (Phlebovirus) replication in vitro by antiviral compounds. Res Virol. 1997;148:353. doi: 10.1016/s0923-2516(97)89132-7. 365. [DOI] [PubMed] [Google Scholar]
- 113.Rehg JE. Anticryptosporidial activity of lasalocid and other ionophorous antibiotics in immunosuppressed rats. J Infect Dis. 1993;168(6):1566–9. doi: 10.1093/infdis/168.6.1566. [DOI] [PubMed] [Google Scholar]
- 114.Gurbanov KG, Kovalev GV, Paperno AA. Experimental study of the cardiac and hemodynamic effects of the carboxylic ionophore monensin. Farmakologiya i Toksikologiya (Moscow) 1990;53(3):17–19. [PubMed] [Google Scholar]
- 115.Agtarap A, Chamberlin JW, Pinkerton M, Steinrauf L. The structure of monensic acid, a new biologically active compound. J Am Chem Soc. 1967;89(22):5737–9. doi: 10.1021/ja00998a062. [DOI] [PubMed] [Google Scholar]
- 116.Johansen CH, Bjerrum L, Pedersen K. Impact of salinomycin on the intestinal microflora of broiler chickens. Acta Vet Scand. 2007;49(1):30. doi: 10.1186/1751-0147-49-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujita T, Majima H, Itoh T, Sano H. Combined effect of salinomycin and feeding on whole body glucose kinetics in sheep fed a high-concentrate diet. Reprod Nutr Dev. 2006;46(5):503–14. doi: 10.1051/rnd:2006035. [DOI] [PubMed] [Google Scholar]
- 118.Kyriakis SC, Sarris K, Kritas SK, et al. The effect of salinomycin on the control of Clostridium perfringens type-A infection in growing pigs. Zentralbl Veterinarmed B. 1995;42(6):355–9. doi: 10.1111/j.1439-0450.1995.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 119.Edrington TS, Callaway TR, Varey PD, et al. Effects of the antibiotic ionophores monensin, lasalocid, laidlomycin propionate and bambermycin on Salmonella and E. coli O157: H7 in vitro. J Appl Microbiol. 2003;94(2):207–13. doi: 10.1046/j.1365-2672.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 120.Brasseur P, Lemeteil D, Ballet JJ. Anti-cryptosporidial drug activity screened with an immunosuppressed rat model. J Protozool. 1991;38(6):230S–1S. [PubMed] [Google Scholar]
- 121.Lemeteil D, Roussel F, Favennec L, et al. Assessment of candidate anticryptosporidial agents in an immunosuppressed rat model. J Infect Dis. 1993;167(3):766–8. doi: 10.1093/infdis/167.3.766. [DOI] [PubMed] [Google Scholar]
- 122.Johnson SM, Herrin J, Liu SJ, Paul IC. The crystal and molecular structure of the barium salt of an antibiotic containing a high proportion of oxygen. J Am Chem Soc. 1970;92(14):4428–35. doi: 10.1021/ja00717a047. [DOI] [PubMed] [Google Scholar]
- 123.Westley JW, Blount JF, Evans RH, Jr, et al. Biosynthesis of lasalocid II X-ray analysis of a naturally occurring isomer of lasalocid A. J Antibiot. 1974;27(8):597–604. doi: 10.7164/antibiotics.27.597. [DOI] [PubMed] [Google Scholar]
- 124.Hatsu M, Sasaki T, Miyadoh S, et al. SF2487, a new polyether antibiotic produced by Actinomadura. J Antibiot (Tokyo) 1990;43(3):259–66. doi: 10.7164/antibiotics.43.259. [DOI] [PubMed] [Google Scholar]
- 125.Miyagami T, Takei Y, Matsumoto Y, et al. An in vitro study on the toxoplasmacidal activity of lonomycin A in host cells. J Antibiot (Tokyo) 1981;34(2):218–23. doi: 10.7164/antibiotics.34.218. [DOI] [PubMed] [Google Scholar]
- 126.Zhao JZ, Yaoying LI, Zeng Haixian, et al. Mechanisms underlying induction of IL-2 secretion by PDB plus ionomycin in CD4+CD25+ T cells from cord blood and adult peripheral blood. Zhongguo Bingli Shengli Zazhi. 2006;22(6):1133–7. [Google Scholar]
- 127.Takeuchi TT, Yoshimoto Kensei. WO2006030802. Human T cell culture in the presence of TPA, ionomycin and anti-CD3 antibody for screening BAFF suppressor or inhibitor. 2006
- 128.Holmes AG, Watt MJ, Carey AL, Febbraio MA. Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism. 2004;53(11):1492–5. doi: 10.1016/j.metabol.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 129.Park HJ, Makepeace CM, Lyons JC, Song CW. Effect of intracellular acidity and ionomycin on apoptosis in HL-60 cells. Eur J Cancer. 1996;32A(3):540–6. doi: 10.1016/0959-8049(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 130.Moins N, Gachon P, Maublant J. Myocardial imaging in dogs treated with grisorixin: relationship between thallium-201 uptake and coronary blood flow. J Nucl Med. 1982;23(4):330–6. [PubMed] [Google Scholar]
- 131.Westley J. US4058620. Polyether Antibiotics, For Improving, Cardiovascular Function. :1977.
- 132.Strout RGO. Eimeria Tenella. Screening of chemotherapeutic compounds in cell cultures. Exp Parasitol. 1973;33(3):477–85. doi: 10.1016/0014-4894(73)90115-x. [DOI] [PubMed] [Google Scholar]
- 133.Gorman MH, Robert L. US3627883. Treating coccidiosis with antibiotic X206. 1971
- 134.Gale C, McDouglas LR. US 3995027;1976
- 135.Blount JFE, Ralph H, Jr, LIU, et al. X-ray structure of Ro 21-6150, a polyether antibiotic related to Dianemycin. J Chem Soc Chem Com. 1975;20:853–5. [Google Scholar]
- 136.Matsuno TH, Yamazaki Fumio. Anticoccidial activity of a new polyether antibiotic, carriomycin, in battery trial. Takeda. 1982;43(3/4):192–3. [Google Scholar]
- 137.Williams RD, Carl Power. EP250134. Method for combating swine dysentery. 1987
- 138.Nakayama HO, Miyamae Noboru, Hiroshi, et al. Studies on the ionophorous antibiotics. Part 14. Crystal and molecular structure of the thallium salt of carriomycin. J Chem Soc, Perkin Transactions 2. Physic Org Chem. 1979(3):293–5. [Google Scholar]
- 139.Omura SO, Yamada Kazuhiko. JP2003335667. Polyether antibiotic K-41 as an antimalarial. 2003
- 140.Otake NM, Koenuma H, Kinashi S, Sato Y. The crystal and molecular structure of the silver salt of lysocellin, a new polyether antibiotic J.C.S. Chem Comm. 1975:92–93. [Google Scholar]
- 141.Ishiguro T. JP53020420. inventor Antibiotic K-41 as acaricide. 1978
- 142.Shiro MN, Nagashima Hiroshi, et al. X-ray determination of the structure of the polyether antibiotic K-41. Journal of the Chemical Society Chem Comm. 1978;16:682–3. [Google Scholar]
- 143.Nakatsukasa WM, Marconi GG, Neuss N, Hamill RL. US4141907. Desoxynarasin antibiotics. 1979
- 144.Bernan VS, Montenegro DA, Goodman JJ, et al. Martinomycin, a new polyether antibiotic produced by Streptomyces salvialis. I. Taxonomy, fermentation and biological activity. J Antibiot (Tokyo) 1994;47(12):1434–41. doi: 10.7164/antibiotics.47.1434. [DOI] [PubMed] [Google Scholar]
- 145.Cullen WP, Celmer WD, Chappel LR, et al. CP-54, 883 a novel chlorine-containing polyether antibiotic produced by a new species of actinomadura: taxonomy of the producing culture, fermentation, physico-chemical and biological properties of the antibiotic. J Antibiot. 1987;40(11):1490–5. doi: 10.7164/antibiotics.40.1490. [DOI] [PubMed] [Google Scholar]
- 146.Westley John W, Blount John F, Evans RH, Jr, et al. C-17 epimers of deoxy-(O-8)-salinomycin from Streptomyces albus (ATCC 21838) J Antibiot. 1977;30(7):610–12. doi: 10.7164/antibiotics.30.610. [DOI] [PubMed] [Google Scholar]
- 147.Liu Chao-Min, Westley J. US4137241. Deoxy-(0-8)-epi-17-salinomycin. 1979
- 148.Dirlam JP, Cullen WP, Huang LH, et al. CP-91,243 and CP-91,244, novel diglycoside polyether antibiotics related to UK-58,852 and produced by mutants of Actinomadura roseorufa. J Antibiot (Tokyo) 1991;44(11):1262–6. doi: 10.7164/antibiotics.44.1262. [DOI] [PubMed] [Google Scholar]
- 149.Dirlam JP, Bordner J, Chang SP, et al. The isolation and structure of CP-120,509, a new polyether antibiotic related to semduramicin and produced by mutants of Actinomadura roseorufa. J Antibiot. 1992;45(9):1544–8. doi: 10.7164/antibiotics.45.1544. [DOI] [PubMed] [Google Scholar]
- 150.Westley JW, Liu CM, Blount JF, et al. Isolation and characterization of a novel polyether antibiotic of the pyrrolether class, antibiotic X-14885A. J Antibiot (Tokyo) 1983;36(10):1275–8. doi: 10.7164/antibiotics.36.1275. [DOI] [PubMed] [Google Scholar]
- 151.Kusakabe Y, Takahashi N, Iwagaya Y, Seino A. Portmicin, a new antibiotic. J Antibiot (Tokyo) 1987;40(2):237–8. doi: 10.7164/antibiotics.40.237. [DOI] [PubMed] [Google Scholar]
- 152.Liu C, Hermann TE, Liu M, et al. Novel polyether antibiotics X-14667A and X-14667B from Streptomyces cinnamonensis subsp. urethanofaciens. Discovery, fermentation, biological as well as ionophore properties and taxonomy of the producing culture. J Antibiot (Tokyo) 1981;34(10):1241–8. doi: 10.7164/antibiotics.34.1241. [DOI] [PubMed] [Google Scholar]
- 153.Dirlam JP, Bordner J, Cullen WP, et al. The structure of CP-96,797, a polyether antibiotic related to K-41A and produced by Streptomyces sp. J Antibiot. 1992;45(7):1187–9. doi: 10.7164/antibiotics.45.1187. [DOI] [PubMed] [Google Scholar]
- 154.Nakayama H, Seto H, Otake N, et al. Studies on the ionophorous antibiotics. XXVIII. Moyukamycin, a new glycosylated polyether antibiotic. J Antibiot. 1985;38(10):1433–6. doi: 10.7164/antibiotics.38.1433. [DOI] [PubMed] [Google Scholar]
- 155.Liu CM, Hermann TE, Prosser BL, et al. X-14766A, a halogen containing polyether antibiotic produced by Streptomyces malachitofuscus subsp. downeyi ATCC 31547. Discovery, fermentation, biological properties and taxonomy of the producing culture. J Antibiot (Tokyo) 1981;34(2):133–8. doi: 10.7164/antibiotics.34.133. [DOI] [PubMed] [Google Scholar]
- 156.Westley JW, Evans RH, Jr, Sello, et al. Isolation and characterization of the first halogen containing polyether antibiotic X-14766A, a product of Streptomyces malachitofuscus subsp. downeyi. J Antibiot (Tokyo) 1981;34(2):139–47. doi: 10.7164/antibiotics.34.139. [DOI] [PubMed] [Google Scholar]
- 157.Caffarel-mendez S, Demuynck C, Jeminet G. [In vitro study of various ionophore antibiotics and some of their derivatives. II. Characterization of the ionophore properties of the compounds in a model system for Na+ and K+ ions] Reprod Nutr Dev. 1987;27(5):921–8. [PubMed] [Google Scholar]
- 158.Leblanc D, Morin A, Daigneault J. Comparative effect of salinomycin and monensin on Streptococcus bovis strain ATCC 9809. Microbios. 1993;76(306):41–5. [PubMed] [Google Scholar]
- 159.Sanyal SN, Sharma P, Kanwar SS. Oxidative effects of Na+-specific ionophore monensin on the rat epididymis. Drug Chem Toxicol. 2007;30(4):411–23. doi: 10.1080/01480540701524136. [DOI] [PubMed] [Google Scholar]
- 160.Euzeby J. The epidemiology of human cryptosporidiosis. Bull Acad Natl Med. 2002;186(5):837–47. [PubMed] [Google Scholar]
- 161.Gomez L, Jouany JP. Effects of lasalocid and cationomycin on the evolution of certain parameters in the blood plasma of sheep. Arch Tierernahr. 1994;46(3):283–93. doi: 10.1080/17450399409381778. [DOI] [PubMed] [Google Scholar]
- 162.Griffin T, Rybak ME, Recht L, et al. Potentiation of antitumor immunotoxins by liposomal monensin. J Natl Cancer Inst. 1993;85(4):292–8. doi: 10.1093/jnci/85.4.292. [DOI] [PubMed] [Google Scholar]
- 163.Recht LD, Raso V, Davis R, Salmonsen R. Immunotoxin sensitivity of Chinese hamster ovary cells expressing human transferrin receptors with differing internalization rates. Cancer Immunol Immunother. 1996;42(6):357–61. doi: 10.1007/s002620050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caridha D, Yourick D, Cabezas M, et al. Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin. Antimicrob Agents Chemother. 2008;52(2):684–93. doi: 10.1128/AAC.00874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carson J, Statham P. The inhibition by ionophores in vitro of an Enterococcus-like pathogen of rainbow trout, Oncorhynchus mykiss. Vet Microbiol. 1993;36(3–4):253–9. doi: 10.1016/0378-1135(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 166.Walba D, Hermsmeier M, Haltiwanger C, et al. Crystal structures of monensin B lithium and silver salts. J Org Chem. 1986;51(2):245–7. [Google Scholar]
- 167.Mitani M, Umetsu T, Yamanishi T. Studies on the ionophorous antibiotics. VIII. Effects of monovalent and divalent cation ionophores on blood platelets. J Antibiot (Tokyo) 1977;30(3):239–43. doi: 10.7164/antibiotics.30.239. [DOI] [PubMed] [Google Scholar]
- 168.Haney ME, Jr, Hoehn Monensin, a new biologically active compound. Antimicrob Agents Chemother. 1967:349–52. doi: 10.1128/AAC.7.3.349. [DOI] [PubMed] [Google Scholar]
- 169.Mitani M, Koenuma M, Otake N. Studies on the ionophorous antibiotics. XII. Effects of ionophore lysocellin on cation distribution and respiration in mitochondria. J Antibiot (Tokyo) 1977;30(10):829–35. doi: 10.7164/antibiotics.30.829. [DOI] [PubMed] [Google Scholar]
- 170.Grafe U, Schlegel R, Stengel C, et al. Isolation and structure of 26-deoxylaidlomycin, a new polyether antibiotic from Streptoverticillium olivoreticuli. J Basic Microbiol. 1989;29(3):149–55. [Google Scholar]
- 171.Imoto MK, Umezawa Y, Takahashi H, et al. Takeuchi: Isolation and structure determination of inostamycin, a novel inhibitor of phosphatidylinositol. Natl Prod. 1990;53:825–9. [Google Scholar]
- 172.Finkel TH, Pabst MJ, Suzuki H, et al. Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J Biol Chem. 1987;262(26):12589–96. [PubMed] [Google Scholar]
- 173.Vial HJ, Thuet MJ, Philippot JR. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D-3 derivatives. Mol Biochem Parasitol. 1982;5:189–98. doi: 10.1016/0166-6851(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 174.Liu CM, Westley JW, Palleroni NJ, et al. X-14934A, a novel polyether antibiotic produced by Streptomyces. Program and Abstracts of the 27th Intersci Conf on Antimicrob. :272. Agents Chemother No. 1007. [Google Scholar]
- 175.Dorman DE, Hamill RL, Occolowitz JL, et al. Structure of polyether antibiotic A28695B. J Antibiot (Tokyo) 1980;33(2):252–5. doi: 10.7164/antibiotics.33.252. [DOI] [PubMed] [Google Scholar]
- 176.Smith II, Strout Ck. Eimeria tenella: Accumulation and retention of anticoccidial ionophores by extracellular sporozoites. Exp Parasitol. 1979;48(3):325–30. doi: 10.1016/0014-4894(79)90115-2. [DOI] [PubMed] [Google Scholar]
- 177.Carter GT, Schlingmann G, Kenion GB, et al. Martinomycin, a new polyether antibiotic produced by Streptomyces salvialis. II. Isolation and structure determination. J Antibiot (Tokyo) 1994;47(12):1549–53. doi: 10.7164/antibiotics.47.1549. [DOI] [PubMed] [Google Scholar]
- 178.Tsuji N, Terui Y, Nagashima K, et al. New polyether antibiotics, A-130B and A-130C. J Antibiot (Tokyo) 1980;33(1):94–7. doi: 10.7164/antibiotics.33.94. [DOI] [PubMed] [Google Scholar]
- 179.Available from: http://chemdb.niaid.nih.gov/struct_search/class/class_many.asp?class=POLYETHERS
- 180.David L, Ayala Leal, Tabet H. Abierixin, a new polyether antibiotic. Production, structural determination and biological activities. J Antibiot. 1985;38(12):1655–63. doi: 10.7164/antibiotics.38.1655. [DOI] [PubMed] [Google Scholar]
- 181.Kergomard DL. A Production by controlled biosynthesis of a novel ionophore antibiotic, cezomycin (demethylamino A23187) J Antibiot. 1982;35(10):1409–11. doi: 10.7164/antibiotics.35.1409. [DOI] [PubMed] [Google Scholar]
- 182.Westley JW, Cm LIU, Blount JF, et al. Isolation and characterization of four polyether antibiotics, X-14889A, B, C, and D, closely related to lysocellin and the ferensimycins. J Antibiot. 1993;46(2):280–6. doi: 10.7164/antibiotics.46.280. [DOI] [PubMed] [Google Scholar]
- 183.Westley JW, Liu CM, Blount JF, et al. Isolation and characterization of three novel polyether antibiotics and three novel actinomycins as cometabolites of the same Streptomyces sp. X-14873, ATCC 31679. J Antibiot (Tokyo) 1986;39(12):1704–11. doi: 10.7164/antibiotics.39.1704. [DOI] [PubMed] [Google Scholar]
- 184.Kinashi H, Otake N, Yonehara H, et al. The structure of salinomycin, a new member of the polyether antibiotics. Tet Lett. 1973;49:4955–8. [Google Scholar]
- 185.Westley JW, Benz W, Donahue J, et al. Biosynthesis of lasalocid. III. Isolation and structure determination of four homologs of lasalocid A. J Antibiot (Tokyo) 1974;27(10):744–53. doi: 10.7164/antibiotics.27.744. [DOI] [PubMed] [Google Scholar]
- 186.Tadashi N, Yukio T, Yuko K, et al. JP61260888. Novel antibiotic substance SF-2361, production and used thereof. 1986
- 187.Toru Sasaki. JP60130394. A New Antibiotic SF2324 Produced by Actinomadura. 1985
- 188.Berg DH, Hamill RL. The isolation and characterization of narasin, a new polyether antibiotic. J Antibiot (Tokyo) 1978;31(1):1–6. doi: 10.7164/antibiotics.31.1. [DOI] [PubMed] [Google Scholar]
- 189.Liu WC, Slusarchyk DS, Astle G, et al. Ionomycin, a new polyether antibiotic. J Antibiot (Tokyo) 1978;31(9):815–9S. doi: 10.7164/antibiotics.31.815. [DOI] [PubMed] [Google Scholar]
- 190.Gachon P, Kergomard A. Grisorixin, an ionophorous antibiotic of the nigericin group. II. Chemical and structural study of grisorixin and some derivatives. J Antibiot (Tokyo) 1975;28(5):351–7. doi: 10.7164/antibiotics.28.351. [DOI] [PubMed] [Google Scholar]
- 191.Nakamura G, Kobayashi K, Sakurai T, et al. sp J Antibiot (Tokyo) 1981;34(11):1513–4. doi: 10.7164/antibiotics.34.1513. [DOI] [PubMed] [Google Scholar]
- 192.Chaney MO, Demarco PV, Jones ND, Occolowitz JL. Structure of A23187, a divalent cation ionophore. J Am Chem Soc. 1974;96:1932–3. doi: 10.1021/ja00813a047. [DOI] [PubMed] [Google Scholar]
- 193.Westley JW, Evan RH, et al. Structure of antibiotic X- 14547 a carboxylic acid polyether produced by Streptomyces antibiotics. J Am Chem Soc. 1978;100:6784–8786. [Google Scholar]
- 194.Blount JF, Westley JW. X-ray crystal and molecular structure of the antibiotic X-206. Chem Commun. 1975;533 [Google Scholar]
- 195.US3839558. Hamill and Hoehn Antibiotics A28695 A and A28695 B and a process for production thereof. 1974
- 196.Akira S, Yukio M, Kiyoshi T, et al. US4138496. SY-1 substance. 1979
- 197.Walter NM, Gary GM, Norbert N. US4204039. Process for producing deoxynarasin antibiotics. 1978
- 198.Hauske JR, Kostek G. Structure elucidation of a new polyether antibiotic iso-dianemycin. J of Org Chem. 1989;54(14):3500–4. [Google Scholar]
- 199.Westley JW, Liu C, Sello LH, et al. Isolation and characterization of antibotic A-14931A, the naturally occurring 19-deoxyaglycone of dianemycin. J of Antibiot. 1984;37(7):813–15. doi: 10.7164/antibiotics.37.813. [DOI] [PubMed] [Google Scholar]
- 200.Gorman M, Chamberlin JW, Hamill RL. Compounds related to monensin. Antimicr Agent Chemoth. 1967:363–8. [PubMed] [Google Scholar]
- 201.Jones ND, Chaney MO, Chamberlin JW, et al. Chen Structure of A204A, a new polyether antibiotic. J Am Chem Soc. 1973;95:3399–3400. doi: 10.1021/ja00791a062. [DOI] [PubMed] [Google Scholar]
- 202.Tsuji N, Nagashima K, Terui Y, Tori K. Structure of K-41B, a new diglycoside polyether antibiotic. J Antibiot (Tokyo) 1979;32(2):169–72. doi: 10.7164/antibiotics.32.169. [DOI] [PubMed] [Google Scholar]
- 203.Tsuji N, Nagashima K, Kobayashi M, et al. Two new antibiotics, A-218 and K-41. Isolation and characterization. J Antibiot (Tokyo) 1976;29(1):10. doi: 10.7164/antibiotics.29.10. [DOI] [PubMed] [Google Scholar]
- 204.Nakatsukasa Walter, Hamill M, Robert L. US4110435. Antibiotic A-28086 factor D. 1978
- 205.Occolowitz JL, Berg DH, Debono M, Hamill RL. The structure of narasin and a related ionophore. Biomed Mass Spec. 1976;3(6):272–7. doi: 10.1002/bms.1200030604. [DOI] [PubMed] [Google Scholar]
- 206.Celmer WD, Cullen WP, Moppett CE, et al. US4,081,532. :1978.
- 207.Ruddock J, Cornish DR, Cullen WP, et al. EP0169011. 1986


