Abstract
‘Ex vivo’ gene therapy using viral vectors to overexpress BMP-2 is shown to heal critical-sized bone defects in experimental animals. To increase its safety, we constructed a dual-expression lentiviral vector to overexpress BMP-2 or luciferase and an HSV1-tk analog, Δtk (LV-Δtk-T2A-BMP-2/Luc). We hypothesized that administering ganciclovir (GCV) will eliminate the transduced cells at the site of implantation. The vector-induced expression of BMP-2 and luciferase in a mouse stromal cell line (W-20-17 cells) and mouse bone marrow cells (MBMCs) was reduced by 50% compared with the single-gene vector. W-20-17 cells were more sensitive to GCV compared with MBMCs (90–95% cell death at 12 days with GCV at 1 µg ml−1 in MBMCs vs 90–95% cell death at 5 days by 0.1 µg ml−1 of GCV in W-20-17 cells). Implantation of LV-Δtk-T2A-BMP-2 transduced MBMCs healed a 2 mm femoral defect at 4 weeks. Early GCV treatment (days 0–14) postoperatively blocked bone formation confirming a biologic response. Delayed GCV treatment starting at day 14 for 2 or 4 weeks reduced the luciferase signal from LV-Δtk-T2A-Luc-transduced MBMCs, but the signal was not completely eliminated. These data suggest that this suicide gene strategy has potential for clinical use in the future, but will need to be optimized for increased efficiency.
Keywords: suicide gene therapy, bone repair, lentiviral vector
INTRODUCTION
Enhanced bone regeneration is often required in clinical scenarios involving massive bone loss. Current treatments (for example, autologous bone grafting or recombinant BMP-2) have limitations in terms of availability, cost and local adverse effects.1–4 ‘Ex vivo’ gene therapy has been successfully used to heal critical-sized defects in rodent models.5–7 Previously, we have used BMP-2-producing bone marrow cells created via adenoviral or lentiviral gene transfer to heal critical-sized femoral defects in rats.8–11 As gene therapy for bone repair would be used to treat non-lethal conditions, any increased morbidity or mortality would not be acceptable. Insertional mutagenesis and emergence of replication competent viral particles remain areas of concern with respect to lentiviral vectors; therefore, the safety of this approach needs careful evaluation before it can be used clinically.
Herpes simplex virus thymidine kinase (HSV-tk) is an enzyme that phosphorylates a number of antiviral prodrugs such as ganciclovir (GCV) to toxic metabolites that can kill virally infected cells. Clinically, GCV is frequently used for the treatment of CMV infections in immune-compromised hosts such as HIV/AIDS and organ transplant patients.12–14 Viral thymidine kinases differ significantly from the host counterpart enzymes, hence the relative specificity of the antiviral drugs for the viral-infected cells.15 This property of virus-specific enzymes has been utilized to minimize host side effects and develop ‘suicide’ gene approaches primarily for the treatment of malignancies in many organs including the brain, thyroid, lung, stomach, prostate and breast.16–20 In order to potentially enhance the safety of ‘ex vivo’ gene therapy, we developed a suicide gene therapy approach using a dual-gene expression lentiviral vector encoding either BMP-2 or luciferase and a truncated form of the tk called delta tk (Δtk) to eliminate the implanted transduced cells by GCV administration. The Δtk is known to retain the functional properties of the enzyme without causing sterility in transgenic males,21 and its smaller size is advantageous in the construction of a bicistronic vector. Our hope was that the dual-gene expression vector encoding BMP-2 and Δtk would produce sufficient BMP-2 to heal a critical-sized bone defect. We constructed a luciferase vector as well to document the transduced cell response to GCV administration. We determined the effects of GCV administration on the bone quality using LV-BMP-2-transduced cells to heal a 2-mm critical-sized femoral defect. The effects of early GCV administration on in vivo bone formation was assessed in the hind-limb muscle pouch and in 2-mm femoral defect models by implantation of the dual-vector-transduced cells overexpressing Δtk and BMP-2. Finally, we examined the in vivo efficacy of delayed GCV administration for 2 and 4 weeks in eliminating the dual-transduced cells overexpressing Δtk and Luc after implantation in the mouse femoral defect model.
RESULTS
Expression of BMP-2 and thymidine kinase by LV-Δtk-T2A-BMP-2-transduced cells
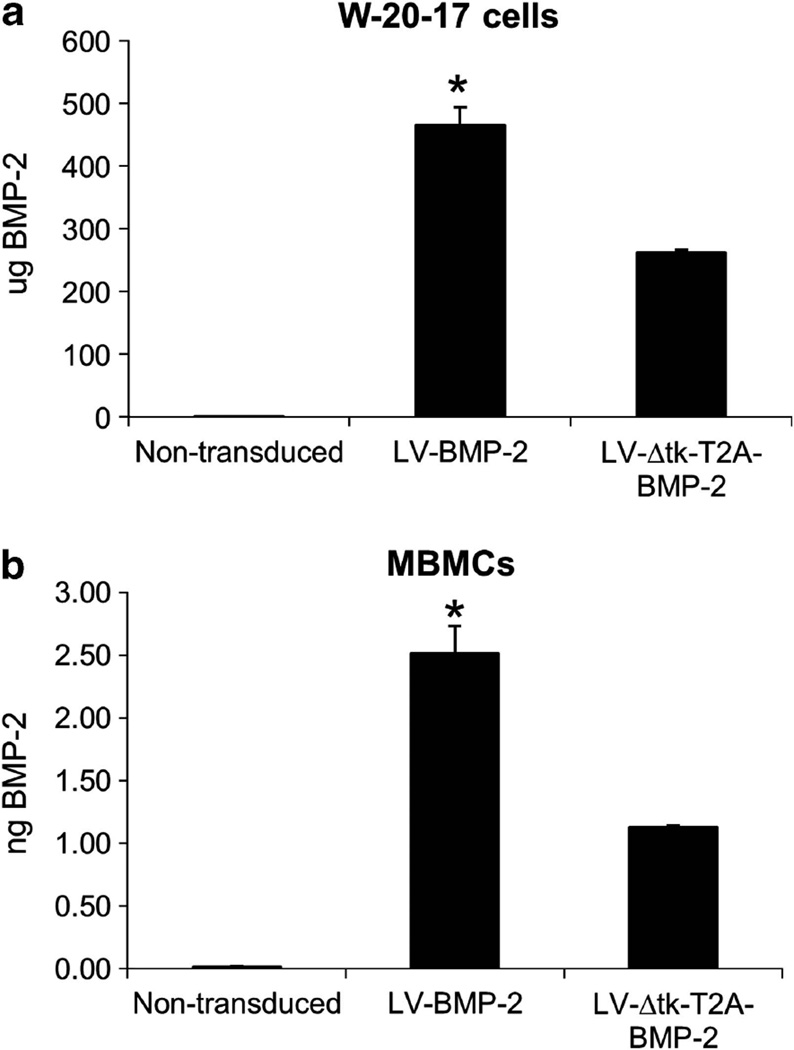
As shown in Figure 1, we constructed a new lentiviral vector that has the ability to concomitantly yet independently produce BMP-2 or luciferase and the Δtk suicide gene (LV-Δtk-T2A-BMP-2/Luc). Both the W-20-17 cells, a mouse bone marrow stromal cell line and mouse bone marrow cells (MBMCs) that were transduced with LV-Δtk-T2A-BMP-2 produced almost half the amount of BMP-2 (56% and 45%, respectively) made by the LV-BMP-2-transduced cells under the same culture conditions. The W-20-17 cells were highly proliferative and reached confluence in 3–4 days. A total of 1 × 106 W-20-17 cells transduced with LV-BMP-2 produced 465 ± 28 µg BMP-2 in 24 h, whereas LV-Δtk-T2A-BMP-2 cells made 261 ± 4.5 µg giving a ratio of 56%, P < 0.05. BMP-2 production by 1 × 106 transduced MBMCs was on average 5.4 × 10−6 times lower than W-20-17 cells. The ratio of BMP-2 made by LV-BMP-2-transduced MBMCs relative to LV-Δtk-T2A-BMP-2 cells was about 45% (2.51 ± 0.2 vs 1.3 ± 0.01 ng BMP-2, P < 0.05) (Figure 2).
Figure 1.
Structure of the constructed bicistronic lentiviral vector used to overexpress Δtk and BMP-2/Luc. LTR, long terminal repeat, Ψ, packaging signal, RRE, Rev-responsive element, cppt, central polypurine tract.
Figure 2.
Comparison of BMP-2 production by (a) W-20-17 and (b) MBMCs transduced with LV-BMP-2 and LV-Δtk-T2A-BMP-2. The W-20-17 cells were highly proliferative and reached confluence in 3–4 days. A total of 1 × 106 cells transduced with LV-BMP-2 made 465 ± 28 µg BMP-2 in 24 h, whereas LV-Δtk-T2A-BMP-2 cells only made 261 ± 4.5 µg giving a ratio of 56%. BMP-2 production by 1 × 106 transduced MBMCs was on average 5.4 × 10−6 times lower than W-20-17 cells. The ratio of BMP-2 made by LV-BMP-2-transduced MBMCs relative to LV-Δtk-T2A-BMP-2 cells was about 46% (2.51 ± 0.2 vs 1.3 ± 0.01 ng BMP-2). Non-transduced cells were used as negative control. Data expressed as mean ± s.e., n = triplicate experiment, *P < 0.05 compared with LV-Δtk-T2A-BMP-2 cells.
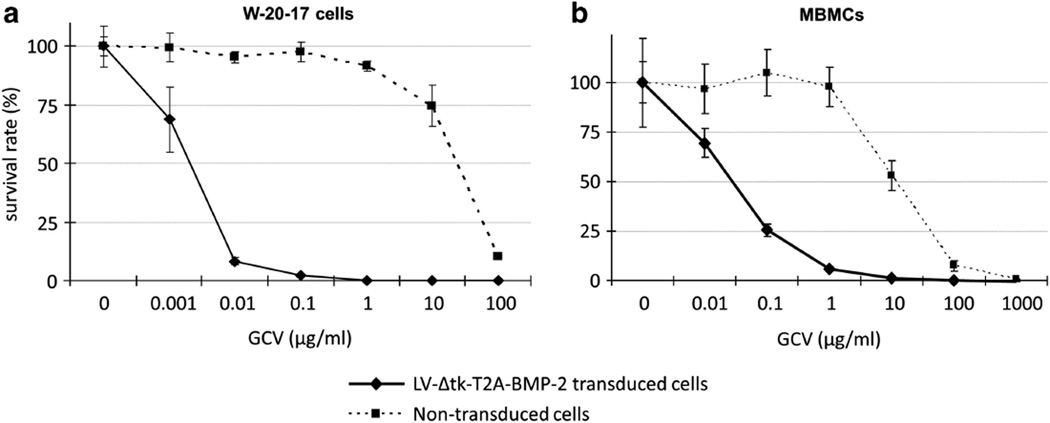
After 5 days of treatment with different doses of GCV ranging from 0.001 to 100 µg ml−1, W-20-17 cells transduced with LV-Δtk-T2A-BMP-2 were killed entirely by GCV at 0.1, 1, 10 and 100 µg ml−1, and only 8% survived at 0.01 µg ml−1. Significant GCV toxicity to non-transduced W-20-17 cells started at 10 µg ml−1 where 25% of the cells did not survive after 5 days. Treatment with GCV at 100 µg ml−1 was lethal to 90% of non-transduced W-20-17 cells (Figure 3a). Higher doses and longer duration of GCV treatment were required for cell killing in MBMCs. After 12 days of treatment with GCV at doses ranging from 0.01 to 1000 µg ml−1, LV-Δtk-T2A-BMP-2-transduced MBMCs were entirely killed by 10, 100 and 1000 µg ml−1 of GCV, and 95% were killed by 1 µg ml−1 of GCV. At 0.01 and 0.1 µg ml−1 of GCV, 70% and 25% of the transduced MBMCs survived, respectively. Non-transduced MBMCs treated with 10 µg ml−1 of GCV showed a 50% cell death, and higher doses were lethal to the vast majority of the non-transduced cells (Figure 3b).
Figure 3.
Cell viability assay comparing in vitro cytotoxicity of GCV on the (a) W-20-17 and (b) MBMCs transduced with LV-Δtk-T2A-BMP-2. The W-20-17 cells were more sensitive to the GCV cell killing effect, as they were mostly killed at 5 days with relatively lower GCV concentrations compared with MBMCs that had to be treated for 12 days in culture. Near total eradication of transduced cells started to occur at GCV dose of 1 µg ml−1, which was almost 100 times higher than the minimum required dose for the near total elimination of the transduced W-20-17 cells. Non-transduced cells were used as controls, and both cell types showed significant cytotoxic response to GCV at 100 µg ml−1.
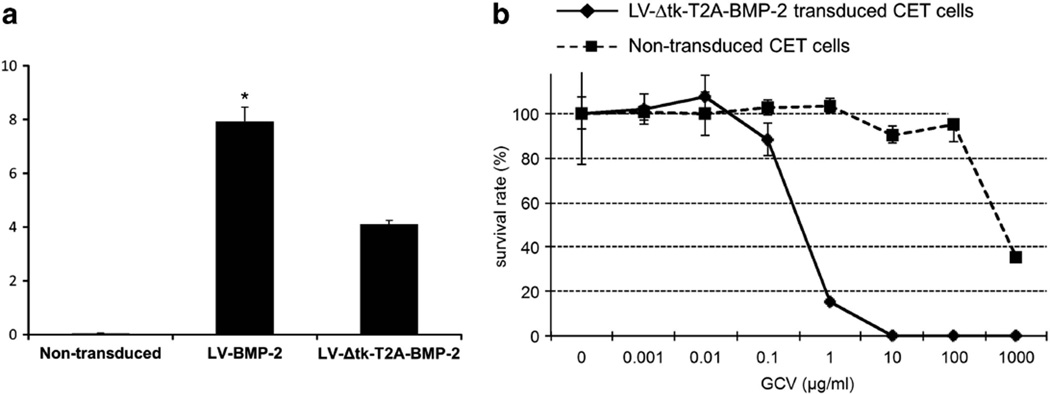
We also performed similar in vitro experiments to measure the BMP-2 expression and to evaluate the cell killing of the GCV treatment in a human bone marrow mesenchymal stem cell line, CET cells. We found an approximate 50% reduction in the transgene expression in the CET cells transduced with the dual-gene expression vector compared with the single-gene expression vector (Figure 4a). Similar to the MBMCs, after 12 days of treatment with GCV at doses ranging from 0.01 to 1000 µg ml−1, LV-Δtk-T2A-BMP-2-transduced CET cells were entirely killed by 10, 100 and 1000 µg ml−1 of GCV, and 85% were killed by 1 µg ml−1 of GCV. At 0.01 and 0.1 µg ml−1 of GCV, 100% and 85% of the transduced CET cells survived, respectively (Figure 4b). Overall, the CET cells were slightly less responsive to the GCV treatment than the MBMCs.
Figure 4.
(a) Comparison of BMP-2 production by CET cells transduced with LV-BMP-2 and LV-Δtk-T2A-BMP-2. There was a 50% decline in gene expression in the cells transduced with the dual-expression vector compared with the single-gene expression vector, *P < 0.05 compared with LV-Δtk-T2A-BMP-2 cells. (b) Cell viability assay comparing in vitro cytotoxicity of GCV on the CET cells transduced with LV-Δtk-T2A-BMP-2. The cells were less responsive than the MBMCs to the toxic effects of the GCV at 12 days of culture.
Similar results were obtained when using LV-Δtk-T2A-Luc-transduced cells instead of LV-Δtk-T2A-BMP-2-transduced cells in the gene expression and the cell viability assays (data not shown).
The effect of GCV treatment on bone formation induced by LV-BMP-2-transduced cells
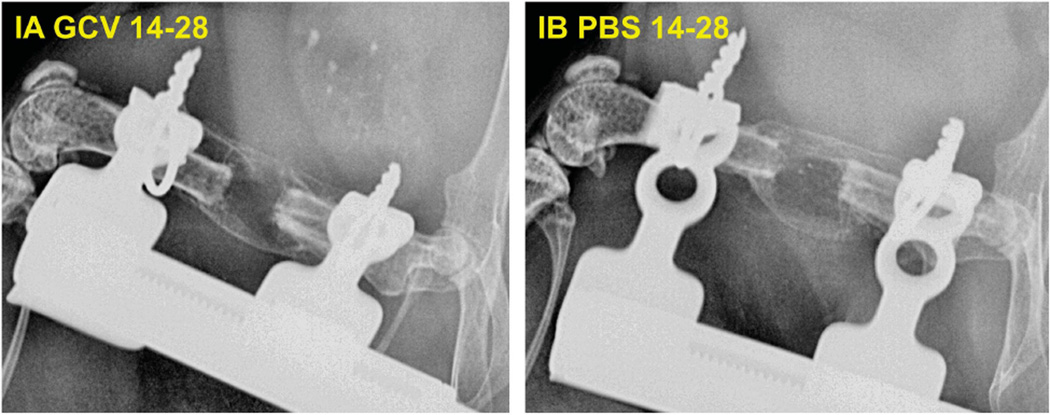
To evaluate the effects of GCV treatment on the quality of bone formation, we placed 2 × 106 LV-BMP-2-transduced MBMSCs in the 2-mm defects in six mice and treated three mice with GCV from days 14 to 28 (group IA) and three controls with Phosphate Buffered Saline (PBS) from days 14 to 28 (group IB). We performed x-rays at day 14 and 28. All the defects were healed in both groups (Figure 5).
Figure 5.
Comparison of defect healing under the effect of GCV administration for 2 weeks from days 14 to 28 in group IA (GCV treated) vs group IB (control PBS treated). A total of 2 × 106 MBMCs transduced with LV-BMP-2 were placed in the defects. GCV treatment did not cause any significant delay in bone healing, as all the defects in both groups were healed at 4 weeks.
In vivo inhibition of bone formation by early GCV administration from days 0 to 14
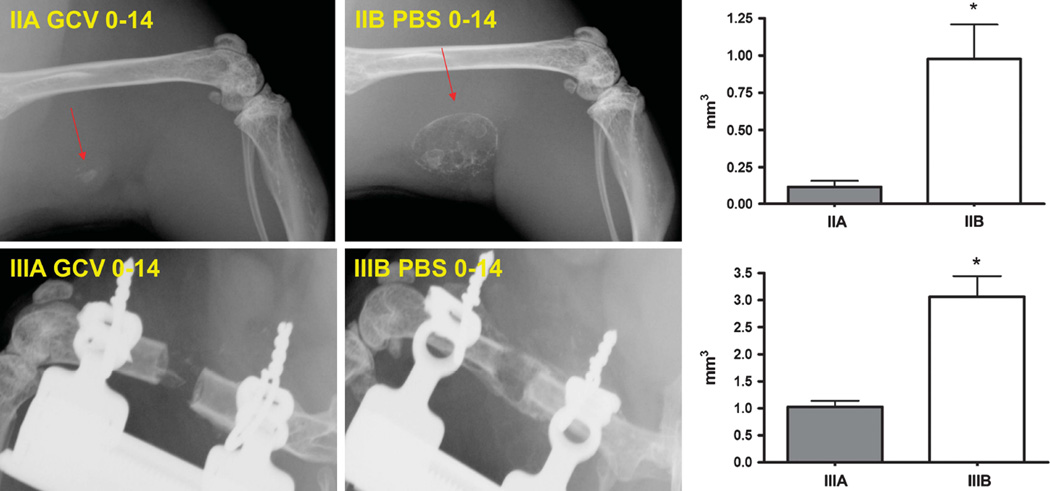
To demonstrate the efficacy of the LV-Δtk-T2A-BMP-2-transduced cells to induce osteogenesis and retain the ability to respond to GCV in vivo, 2 × 106 MBMCs were transduced with LV-Δtk-T2A-BMP-2 and were implanted in the hamstring muscle pouch in the hindlimb of syngeneic recipient mice. The experimental group (group IIA) received GCV treatment from days 0 to 14, and the control group (group IIB) received PBS injections for the same time period. On the X-rays, new bone formation was detectable as early as 2 weeks postoperatively in group IIB (Figure 6). In contrast, very scant amounts of bone were seen in group IIA at 2 and 4 weeks postoperatively. Quantitative measurement of bone volume in the muscle pouch using µ-CT demonstrated that, at 4 weeks after the procedure, the average bone volume in group IIB was 8.5 times larger than group IIA (0.98 ± 0.2 mm3 vs 0.11 ± 0.04 mm3, P < 0.05) (Figure 6).
Figure 6.
Comparison of in vivo BMP-2 production in GCV-treated and control animals that received GCV or PBS injections from 0 to 14 days after surgery. A total of 2 × 106 LV-Δtk-T2A-BMP-2 transduced MBMCs were implanted in the muscle pouch or the 2-mm defects. Top panel (group IIA and IIB) shows the X-rays obtained at 4 weeks postoperatively in the muscle pouch model, and bottom panel (groups IIIA and IIIB) shows that obtained at 4 weeks postoperatively of the femoral defect models. Early GCV treatment resulted in significant inhibition of bone formation and lack of defect healing. Arrows point to the bony mass formed in the muscle pouch. µ-CT-based new bone volumes of the final specimens at 4 weeks show significant reductions in bone formation as a result of GCV treatment. Data expressed as mean ± s.e., n = 3 animals per group, *P < 0.05 compared with the experimental group.
Next, we evaluated the effects of early GCV treatment on the transduced MBMCs implanted in the 2-mm femoral defect model. We implanted 2 × 106 MBMCs transduced with LV-Δtk-T2A-BMP-2 in the 2-mm femoral defect, and GCV (group IIIA) or PBS (group IIIB) was administered from days 0 to 14. This resulted in the healing of all the defects at 4 weeks postoperatively in group IIIB, but in group IIIA (GCV treated animals) none of the defects healed. There was only scattered bone formation that failed to bridge the gap (Figure 6). µ-CT analysis at 4 weeks demonstrated that the new bone volume in group IIIB was three times larger, on average, than group IIIA animals (3.06 ± 0.4 mm3 vs 1.03 ± 0.1 mm3, P < 0.05) (Figure 6).
In vivo killing effect on the transduced cells by delayed GCV administration
We evaluated the effects of delayed GCV administration on transduced cell viability for 2 and 4 weeks starting on day 14 postoperatively in two separate studies. 5 × 106 LV-Δtk-T2A-Luc-transduced MBMCs were implanted in the 2-mm femoral defect. In the experimental group (group IVA), GCV was administered for 2 weeks from days 14 to 28, and in the control group (group IVB) PBS injections were given for the same period of time. Before starting GCV treatment, there was no significant difference in Luc expression (total flux, photon/s) detected in the femoral defects between the treatment and control groups. Treatment with GCV for 2 weeks from days 14 to 28 (group IVA) was associated with a significant decrease of 62% in the luciferase expression at 4 weeks compared with the baseline at 2 weeks (9.93E + 05 ± 1.22E + 05 photon/s at 2 weeks vs 3.82E + 05 ± 4.29E + 04 photon/s at 4 weeks, P < 0.05). In contrast, the control animals did not show a significant decrease in the luciferase expression in the 2-week period from days 14 to 28. At 4 weeks after the surgery, GCV treatment resulted in a 56% decrease in the signal intensity in group IVA compared with group IVB animals (3.82E + 05 ± 4.29E + 04 photon/s vs 8.60E + 05 ± 2.27E + 05 photon/s, P = 0.08), but the difference was not statistically significant (Table 1).
Table 1.
In vivo killing effect on the transduced cells by delayed GCV administration starting from day 14 for 2 and 4 weeks
| Groups | 2w | 4w | 6w |
|---|---|---|---|
| IVA, GCV day 14–28 | 9.93E + 05 ± 1.22E + 05 | 3.82E + 05 ± 4.29E + 04 (56%) | NA |
| IVB, PBS day 14–28 | 8.48E + 05 ± 1.43E + 05 | 8.60E + 05 ± 2.27E + 05 (−3 %) | NA |
| VA, GCV day 14–42 | 6.17E + 06 ± 1.46E + 06 | 1.80E + 06 ± 4.69E + 05* (70.8 %) | 1.31E + 06 ± 4.59E + 05* (78.8 %) |
| VB, PBS day 14–42 | 5.17E + 06 ± 1.79E + 06 | 2.44E + 06 ± 1.10E + 06 (52.9 %) | 1.58E + 06 ± 8.40E + 05 (69.6 %) |
Abbreviations: GCV, ganciclovir; NA, not applicable.
Luciferase expression is presented as total flux (p/s) ± s.e.m. with the average decline in expression compared with the 2-week time point in parentheses.
P < 0.05 compared with 2w, ANOVA.
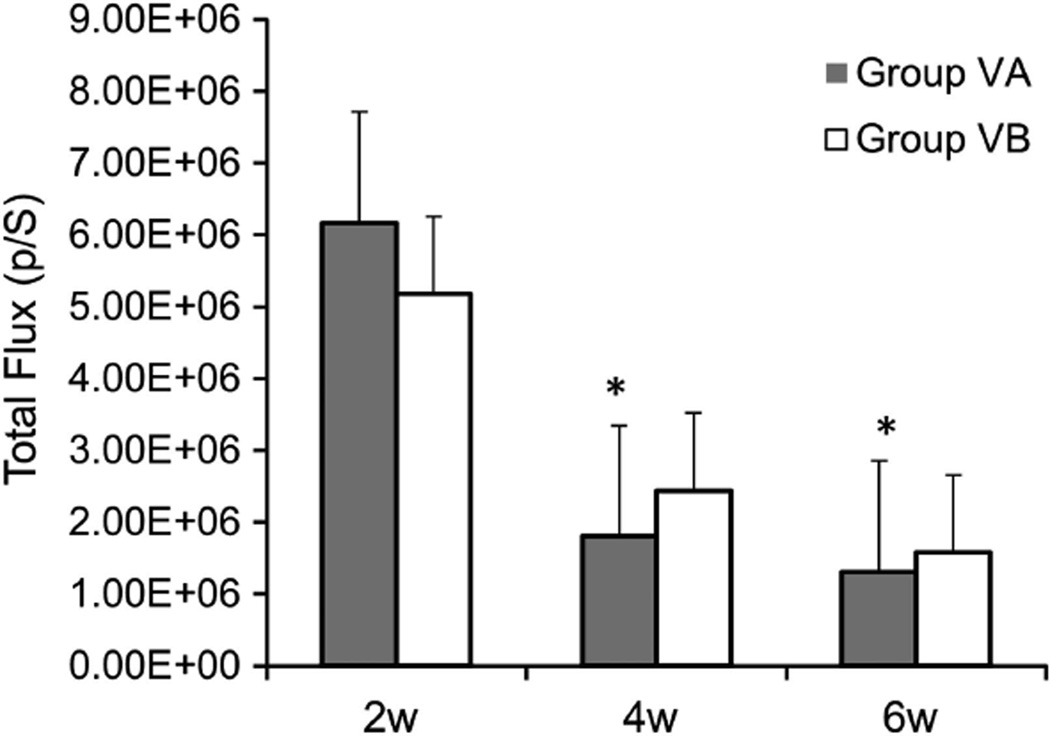
In a subsequent experiment, the treatment with GCV (group VA) or PBS (group VB) was given for 4 weeks from days 14 to 42. We hypothesized that two more weeks of GCV treatment may result in improved cell killing effect and a further decline in the luciferase signal levels. Comparison of the luciferase signal levels at 2, 4 and 6 weeks revealed no significant differences between group VA and group VB (Figure 7, Table 1). Similar to the previous experiment, there was a tendency towards decreased luciferase expression over time from 2 to 6 weeks. We observed a significant decline in luciferase expression in group VA at 4 and 6 weeks compared with 2 weeks (70.8% and 78.8% decrease at 4 and 6 weeks, respectively, P < 0.05 ANOVA), whereas in group VB the decline in luciferase expression at 4 and 6 weeks compared with 2 weeks did not reach statistical significance (52.9% and 69.6% decrease at 4 and 6 weeks, respectively, P > 0.05 ANOVA) (Figure 7, Table 1).
Figure 7.
Comparison of real-time luciferase gene expression in groups VA and VB using the CCCD imaging, Total flux (p/s) was measured in a region of interest around the defect site. Data expressed as mean ± s.e., *P < 0.05 compared with 2 weeks.
DISCUSSION
We have demonstrated successful use of a dual-gene expression lentiviral vector system overexpressing BMP-2 and Δtk in an ex vivo gene therapy strategy for bone repair. We used a self-cleaving 2A peptide sequence (T2A) to achieve adequate expression of both target genes in the transduced cells. We observed healing of a critical-sized bone defect in mouse femurs using mouse bone marrow cells transduced with the dual-expression vector that overexpressed BMP-2. We also demonstrated in vitro and in vivo cell toxicity upon administration of GCV in W-20-17 and MBMCs. Early administration of GCV from days 0 to 14 after implantation of transduced cells in the mouse defect was effective in blocking bone formation, indicating the presence of Δtk-mediated cell toxicity. The effect of late administration of GCV starting from day 14 for 2 and 4 weeks was evaluated by in vivo quantitation of luciferase expression by the transduced cells. We noted a decline in luciferase expression by 2 and 4 weeks of treatment compared with baseline, but total eradication of the transduced cells was not achieved.
A variety of viral vector strategies (retroviral, adenoviral, lentiviral and AAV) using BMP-2 cDNA are available to promote bone repair.22 Each has certain advantage and disadvantages. The delivery of BMP-2 by means of integrating retroviral and lentiviral vectors provides sustained but low levels of the protein locally that may enhance healing. However, the integration of the viral vectors in the genome of the cells and incorporation of the cells in the bone may increase the overall risk of unwanted long-term adverse reactions.23 Therefore, there is interest in developing non-integrating vectors, such as adenovirus or adeno-associated virus. However, these vectors have their own limitations and may be associated with poor transduction efficiencies, immune reactions to adenovirus and diffusion of the free virus expressing BMP-2 to other tissues such as the liver and lungs.24,25
Optimal tissue engineering approaches may require expression of more than one gene of interest. Insertion of internal ribosomal entry site (IRES) between genes of interest is a commonly used technology to construct bicistronic or multicistronic vectors.26–28 However, the efficacy of this strategy has been shown to be limited by the large size of the IRES sequence and marked decrease in expression of the transgene downstream of the IRES.29 Unpublished preliminary data from our laboratory established that constructing a dual-gene expression vector to overexpress luciferase and BMP-2 resulted in decreased BMP-2 production and subsequent non-union (data not shown). This limitation was reported to be overcome by viral ‘self-cleaving’ 2A peptides of 18–22 amino-acid length.30,31 The first 2A peptide was described in foot and mouth disease virus, a member of picornavirus family by Ryan et al.32 The mechanism of self-cleaving is thought to involve ribosomal skip in the synthesis of a glycyl-prolyl peptide bond of the 2A sequence resulting in separation of 2A and its immediate downstream peptide.33 Four different 2A peptides have been widely used in biomedical research: foot and mouth disease virus 2A (F2A); equine rhinitis A virus 2A (E2A); porcine teschovirus-1 2A (P2A) and Thoseaasigna virus 2 A (T2A). The former three viruses belong to the picornavirus family, and the latter is an insect virus. P2A and T2A showed the highest cleavage efficiency, consistently more than 50% in human, zebrafish and mouse cell lines.30 We demonstrated that the 2A peptide can be successfully utilized to construct LV-based dual-gene expression vectors to overexpress BMP-2 or luciferase and Δtk. Our in vitro results showed that this system reduces gene expression by almost 50% compared with a single-gene vector, but the BMP-2 production was still sufficient to heal a critical-sized defect. The luciferase expression was also sufficient to be detected in the femoral defect, but an increased number of cells was used to attain a sustained and detectable signal. To our knowledge, this is the first report demonstrating the use of bicistronic vectors to promote bone repair. As a result of the ribosomal skip of the glycyl-prolyl bond, the cleaved-off peptide downstream of 2A sequence has an extra proline at its N-terminus.34 We demonstrated that the cleaved-off BMP-2 was functionally active by means of ELISA and documentation of bone healing in the mouse femoral defect and muscle pouch models.
Suicide gene therapy has mainly been used to develop novel treatment regimens to treat malignancies alone or in combination with other modalities.16–20 The two most frequently used enzyme/pro-drugs are HSV-tk/GCV and cytosine deaminase/5-flourocytosine (CD/5-FC).35 HSV-tk/GCV system has also been used to enhance the safety of allogeneic bone marrow transplantation and prevention of the graft versus host disease (GVHD).36,37 Typically, the target cells are transduced with a vector containing the enzyme sequence, and the pro-drug is administered systemically. The pro-drug is subsequently turned into a toxic metabolite that can exert direct and indirect (bystander) cytotoxic effects. Bystander cytotoxicity refers to the cell killing effect of the toxic metabolites on the adjacent non-transduced cells. In the HSV-tk/GCV system, the establishment of gap junctions is critical for bystander cell killing effect on non-transduced tumor cells.38,39 In fact, overexpression of connexin in low gap junction HT-29 colorectal tumor cell line resulted in enhanced cytotoxicity of HSV-tk/GCV suicide gene therapy.40 The CD/5-FC bystander effect is less reliant on gap junctions, as the toxic metabolite (5-FU) can freely cross the plasma membrane.41,42 Synergistic cytotoxicity exists with using 5-FC and GCV in double suicide gene therapy. Typically, this is achieved by using CD/TK fusion genes under various tissue specific promoters.43
We observed a much higher sensitivity to GCV in W-20-17 cells compared with MBMCs, which could be related to a number of factors including higher transduction efficiency, the levels of tk expression, the rate of cell multiplication, different cell sensitivity to GCV and different density of gap junctions between the two cell lines. Although the W-20-17 cells were more sensitive to the Δtk/GCV system, we used the mouse bone marrow cells for the in vivo studies because this would more closely simulate the clinical application of gene therapy in humans. Our results suggest that the responsiveness to the GCV is influenced by the cell type, and further investigation of the optimal cell type to be used for ex vivo gene therapy is necessary.
Early GCV treatment blocked the bone formation very effectively, which indicates a biological response to cytotoxic effects of the Δtk/GCV system in both the hind limb and defect models. This toxic effect may be secondary to the direct toxicity or the bystander effect on either the transduced cells or the progenitor cells that are normally recruited to the defect site in response to the BMP-2. We confirmed that treatment with GCV does not negatively affect bone formation when the transduced cells do not overexpress the thymidine kinase gene.
A weakness of our study is that we used the LV-Δtk-T2A-Luc-transduced cells in the experiments assessing the response to the delayed GCV treatment. We did not use the LV-Δtk-T2A-BMP-2-transduced cells to study the efficacy of delayed GCV treatment because this is not technically feasible, as the bone is already healed before the treatment has started. Therefore, we used the LV-Δtk-T2A-Luc-transduced cells as a surrogate marker to evaluate the effects of delayed GCV treatment.
Previously, we had demonstrated stable gene expression up to 12 weeks in LV-Luc-transduced rat bone marrow cells implanted in SCID mouse hind limb muscle pouch and radial defect models44 and at least 8 weeks in LV-Luc-transduced rat bone marrow cells implanted in femoral defects of syngeneic rats.10 In this study, we observed a trend of decreased luciferase signal intensity associated with delayed GCV treatment for 2 and 4 weeks. The controls did not show any significant signal changes over the course of the treatment, although a trend for decreased signal was also observed. A head to head comparison of signal intensity at 4 and 6 weeks after surgery between the GCV-treated and control groups showed a trend towards a decreased signal in response to treatment, but the difference did not reach a statistical significance after 2 or 4 weeks of GCV treatment. Taken together, we showed that GCV treatment was associated with diminished luciferase expression, but the signal was not completely eradicated. In addition, 4 weeks of treatment with GCV was not effective in further reducing the signal intensity. However, early GCV treatment was accompanied by very strong inhibition of bone formation. The reasons for the more consistent response observed in early GCV administration compared with late administration are not clear but may be secondary to the differences in the biological environment and tissue availability of GCV.
When using suicide gene therapy techniques in the treatment of cancers, tumor shrinkage or slowing the growth of tumor has been observed, but total eradication of the tumor cells has been challenging.45–47 Although we did not completely eliminate the transduced cells with this suicide strategy, a reduction in the cell number after bone repair has been completed should enhance the safety of ex vivo gene therapy.
In summary, we present evidence, for the first time, of the successful use of a 2A peptide-based bicistronic lentiviral vector overexpressing Δtk and BMP-2 in promoting bone repair. We also demonstrated cytotoxic effects of early and late GCV treatment in a mouse defect model. Clinically, the suicide approach should be used after the process of bone healing is completed. Further experiments are needed to optimize suicide gene therapy to ensure the safety of ex vivo gene therapy for bone repair. These may include increasing connexin expression in the bone marrow cells to enhance the bystander cell toxicity or using a TK/CD fusion construct for double suicide gene therapy.40,43
MATERIALS AND METHODS
Vector construction
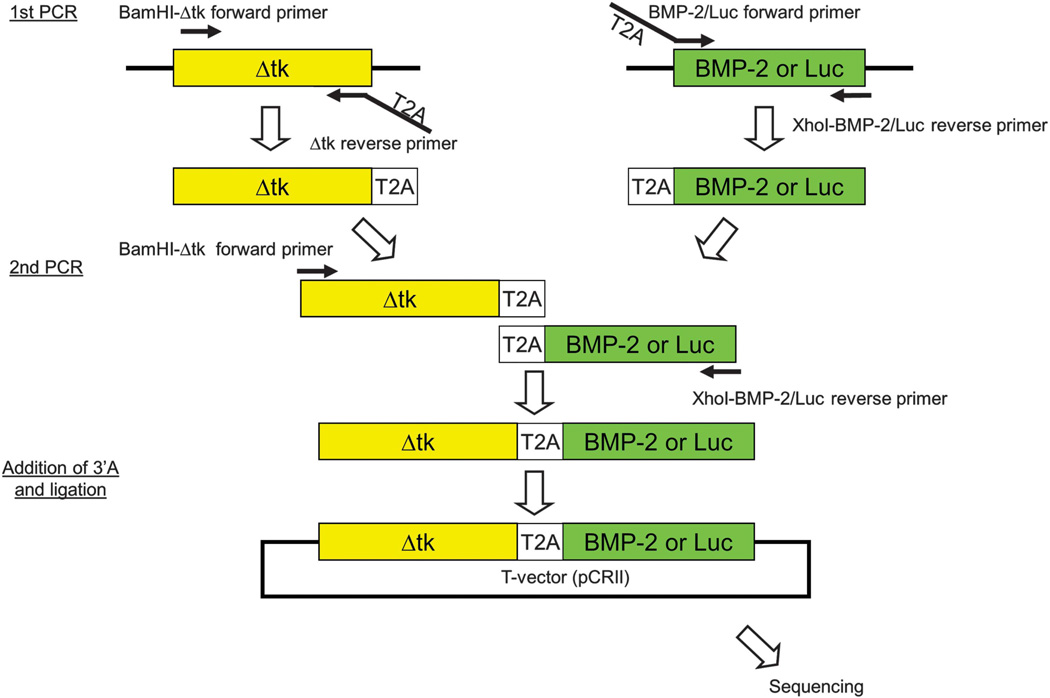
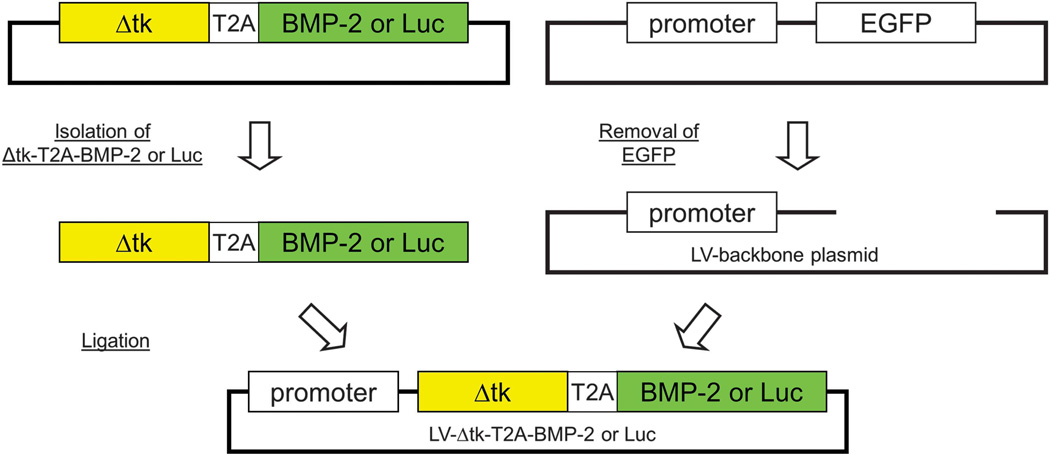
In brief, the Δtk-T2A-BMP-2 and Δtk-T2A-Luc cDNA were created by overlap PCR and were cloned into a commercially available T vector system, pCRII-TOPO (Invitrogen, Carlsbad, CA, USA) for sequencing (Figure 8). After confirmation of no point mutations or frame shifts in the PCR amplicons, Δtk-T2A-BMP-2 or Δtk-T2A-Luc cDNA were isolated from pCRII-TOPO and inserted into lentiviral backbone plasmid (Figure 9).
Figure 8.
Steps involved in overlap PCR for the construction of the Δtk-T2A-BMP-2/Luc. The two-step overlap PCR technique involved amplification of Δtk-T2A and T2A-BMP-2/Luc sequences separately in the first PCR followed by creation of the Δtk-T2A-BMP-2/Luc sequence in the second PCR. The Δtk-T2A-BMP-2/Luc was inserted into a T-vector system for sequence verification.
Figure 9.
Steps involved in the insertion of Δtk-T2A-BMP-2/Luc sequence in the backbone of the lentiviral vector. Δtk-T2A-BMP-2/Luc was isolated from the T-vector and inserted downstream of the RhMLV promoter in the lentiviral backbone plasmid after removing EGFP gene from SIN18-RhMLV-E plasmid.
Plasmids of dual-gene expression lentiviral vectors containing Δtk, a truncated form of the herpes thymidine kinase gene,21,48 and BMP-2 or luciferase genes were constructed (pLV-Δtk-BMP-2 or pLV-Δtk-Luc). The Δtk was connected to the BMP-2 or Luc cDNA by a self-cleaving T2A sequence for post-translational splicing. A two-step overlap extension PCR technique was used to construct this sequence. In the first step of the PCR, Δtk-T2A was amplified by AccuPrime Pfx DNA polymerase (Invitrogen, Carlsbad, CA, USA) using Δtk forward primer (5′-CGC GGA TCC AAG CTT ATG CCC ACG CTA CT-3′), which contained a BamHI restriction site and Δtk reverse primer, which included the complementary T2A sequence (5′-AGG GCC GGG ATT CTC CTC CAC GTC ACC GCA TGT TAG AAG ACT TCC TCT GCC CTC GTT AGC CTC CCC CAT CTC CC-3′). The stop codon of the Δtk sequence was eliminated in the reverse primer to avoid termination of translation. Col2.3-Δtk-ClaPa plasmid48 was used as PCR template. T2A-BMP-2 was amplified using BMP-2 forward primer, which included the T2A sequence at its 5′ end (5′-GAG GGC AGA GGA AGT CTT CTA ACA TGC GGT GAC GTG GAG GAG AAT CCC GGC CCT ATG GTG GCC GGG ACC CGC TG-3′) and BMP-2 reverse primer including XhoI restriction site (5′-CCG CTCGAG CTA GCG ACA CCC ACA ACC CT-3′). The pGEM-T-E-BMP-29 was used as a PCR template. These PCR products were run on an agarose gel and purified by the QIAEX II Gel Extraction Kit (Qiagen, Valencia, CA, USA).
In the second step of PCR, purified Δtk-T2A and T2A-BMP-2 served as templates. Δtk forward primer and BMP-2 reverse primer were used to create the full-length Δtk-BMP-2 linked by T2A. 3′ adenosine overhang was added to the final PCR amplicon and cloned into the T-vector system pCR II-TOPO (Invitrogen). No point mutation or frame shifts were found in the amplified Δtk-T2A-BMP-2 by sequencing analysis (QuickLane DNA sequencing service, Agencourt Bioscience-Beckman Coulter Genomics, Danvers, MA, USA). Δtk-T2A-BMP-2 was isolated from the T-vector and inserted downstream of the RhMLV promoter in the lentiviral backbone plasmid after removing EGFP gene from SIN18-RhMLV-E plasmid.9,49
Δtk-T2A-Luc cDNA was created in the same way. Luc forward primer including T2A sequence (5′-GAG GGC AGA GGA AGT CTT CTA ACA TGC GGT GAC GTG GAG GAG AAT CCC GGC CCT ATG GAA GAC GCC AAA AAC AT -3′) and Luc reverse primer (5′- CCG CTC GAG TTA CAC GGC GAT CTT TCC GC -3′) were used instead of the BMP-2 primers.
Viral transduction
MBMCs were collected from the femurs and tibias of 8-week-old male BL/6 mice and maintained in culture for 4–5 days. Overnight transduction of W-20-17 (a mouse bone marrow stromal cell line), MBMCs and CET Human Bone Marrow Mesenchymal Stem Cells (Thermo Scientific, Waltham, MA, USA) with dual- and single-gene expression vectors was carried out at a multiplicity of infection of 25 as previously described.9,44,50 Transgene expression: fresh media was added 24 h after the transduction, and the assessment of BMP-2 production (by ELISA) or luciferase expression (by luciferase assay) was carried out 24 h after the addition of the fresh media. BMP-2 ELISA: transduced W-20-17, CET cells or MBMCs were incubated in fresh media for another 24 h after transduction. The culture media of transduced cells were collected for analysis of in vitro BMP-2 production. The BMP-2 amount during a 24-h period was quantified by using the ELISA kit (Quantikine, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The BMP-2 production was standardized by cell number and reported as nanogram BMP-2/day by 1 × 106 cells. Luciferase assay: the luciferase activity in the transduced cells was evalutated by using a commercial luciferase assay system (Promega, Madison, WI, USA) on the following day of transduction completion. 1 × 106 viral-transduced W-20-17, CET cells or MBMCs were used for the assay. The cells were treated with 900 µg ml−1 of the manufacturer’s cell culture lysis reagent (Promega). The treated cells and reagent were collected using a cell scraper and centrifuged at 12 000 g for 15 s, then the supernatant/cell lysate was used for the assay. Luminescence was measured by using the Synergy HT Microplate Reader (BioTek, Winooski, VT, USA).
Cell viability assay
After overnight transduction, transduced cells were split onto a 96-well assay plate (Coster flat bottom black plate, Corning Life Science, Lowell, MA, USA) (2 × 103 W-20-17, or 5 × 103 MBMCs. W-20-17 cells were cultured for 5 days, and MBMCs and CET cells were cultured for 12 days in 100 µl of media containing serial concentrations of GCV (0.001, 0.01, 0.1, 1, 10, 100 or 1000 µg ml−1). The culture media was replaced every 3 days. To evaluate cell survival rate, living cell number was quantified by using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, San Luis Obispo, CA, USA) using a Synergy HT Microplate Reader (BioTek). Cell survival rate was expressed as the ratio of ATP activity of surviving cells to the ATP activity of the cells cultured without GCV.
Animal studies
All animal studies were performed after approval by the Animal Care Committee in our institution. MBMCs were collected from the femurs and tibias of 8-week-old male BL/6 mice and maintained in culture for 1 week. Table 2 demonstrates the animal groups utilized in this study. A total of 2 × 106 LV-BMP-2 or LV-Δtk-T2A-BMP-2-transduced MBMCs were mounted on a piece of collagen sponge and transplanted in the surgically created hind limb muscle pouch or 2-mm femoral defects of 14-week-old male BL/6 mice. In brief, the mice were anesthetized with 1.5–2% isoflurane using a nose cone, and the left leg was shaved and sterile prepped. For creation of the hind limb muscle pouch, a small incision was made on the lateral side of the thigh, and a pouch was made by a 5-mm muscle splitting incision over the hamstring muscle. The 2-mm femoral defect surgery included a lateral incision over the iliotibial band followed by circumferential exposure of the femur and application of an external fixator device, which was secured by thin steel wires. The 2-mm defect was created using a fine 0.5 mm oscillating saw. The desired number of cells was then placed in the muscle pouch/defect, and the muscle and skin were closed in separate layers. Adequate pain relief was given by buprenorphine until 48 h postoperatively. The experimental group received GCV (100 mg kg−1 per day i.p.) and the control group received PBS injection (n = 3 mice per group). The animals with defects that were treated with LV-BMP-2-transduced cells received GCV or PBS from days 14 to 28 to evaluate the effects of GCV on the quality of bone formation. X-rays were taken on days 14 and 28 to conform bone healing. The treatment and control groups that had LV-Δtk-T2A-BMP-2-transduced MBMCs received GCV or PBS from days 0 to 14, respectively. Both plain radiography and µ-CT were used to evaluate bone formation in these groups. A total of 5 × 106 LV-Δtk-T2A-Luc-transduced MBMCs were transplanted in the mouse femoral defects for the assessment of delayed administration of GCV. The experimental group was treated with GCV, and the control group animals received PBS from days 14 to 28 (n = 8 mice per group). Treatment with GCV or PBS was carried out to day 42 in five mice per group to evaluate the effect of longer duration of the treatment. In vivo bioluminescent imaging by a Xenogen-IVIS CCCD optical system (Xenogen IVIS, Alameda, CA, USA) was done to detect in vivo luciferase expression 12 min after i.p. injection of luciferin (15 mg kg−1) (Table 2).
Table 2.
Study Groups
| Groups | Type of surgery | Implanted cells | No. per group | Treatment |
|---|---|---|---|---|
| IA | 2-mm defect | 2 × 106 LV-BMP-2 | 3 | GCV, day 14–28 |
| IB | 2-mm defect | 2 × 106 LV-BMP-2 | 3 | PBS, day 14–28 |
| IIA | Hind limb muscle pouch | 2 × 106 LV-Δtk-T2A-BMP-2 | 3 | GCV, day 0–14 |
| IIB | Hind limb muscle pouch | 2 × 106 LV-Δtk-T2A-BMP-2 | 3 | PBS, day 0–14 |
| IIIA | 2-mm defect | 2 × 106 LV-Δtk-T2A-BMP-2 | 3 | GCV, day 0–14 |
| IIIB | 2-mm defect | 2 × 106 LV-Δtk-T2A-BMP-2 | 3 | PBS, day 0–14 |
| IVA | 2-mm defect | 5 × 106 LV-Δtk-T2A-Luc | 4 | GCV, day 14–28 |
| IVB | 2-mm defect | 5 × 106 LV-Δtk-T2A-Luc | 4 | PBS, day 14–28 |
| VA | 2-mm defect | 5 × 106 LV-Δtk-T2A-Luc | 5 | GCV, day 14–42 |
| VB | 2-mm defect | 5 × 106 LV-Δtk-T2A-Luc | 5 | PBS, day 14–42 |
Statistical analysis
Data were expressed as mean ± s.e.m., and student t-test was used for comparison of means between two variables and one-way ANOVA was used for comparison of means between three or more variables with significance at P < 0.05.
ACKNOWLEDGEMENTS
This study was supported by NIH grant 1R01AR057076-01A1 to JRL.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Anderson DW, Burton DC, Jackson RS. Postoperative cervical myelopathy and cord compression associated with the use of recombinant bone morphogenetic protein-2 in posterior cervical decompression, instrumentation, and arthrodesis: a report of two cases. Spine. 36:E682–E686. doi: 10.1097/BRS.0b013e3181f8f4d3. [DOI] [PubMed] [Google Scholar]
- 2.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Eufinger H, Leppanen H. Iliac crest donor site morbidity following open and closed methods of bone harvest for alveolar cleft osteoplasty. J Craniomaxillofac Surg. 2000;28:31–38. doi: 10.1054/jcms.2000.0105. [DOI] [PubMed] [Google Scholar]
- 4.Singh K, Phillips FM, Kuo E, Campbell M. A prospective, randomized, double-blind study of the efficacy of postoperative continuous local anesthetic infusion at the iliac crest bone graft site after posterior spinal arthrodesis: a minimum of 4-year follow-up. Spine. 2007;32:2790–2796. doi: 10.1097/BRS.0b013e31815b7650. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Therapy. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 6.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Therapy. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 7.Alden TD, Varady P, Kallmes DF, Jane JA, Jr, Helm GA. Bone morphogenetic protein gene therapy. Spine. 2002;27(16) Suppl 1:S87–S93. doi: 10.1097/00007632-200208151-00016. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–917. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama O, An DS, Kung SP, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–398. doi: 10.1016/j.ymthe.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–931. doi: 10.1016/j.bone.2007.12.216. [DOI] [PubMed] [Google Scholar]
- 11.Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. ‘Same day’ ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther. 2011;19:960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A. Antiviral Treatment of Cytomegalovirus Infection. Infect Disord Drug Targets. 2011;11:475–503. doi: 10.2174/187152611797636640. [DOI] [PubMed] [Google Scholar]
- 13.Henderly DE, Jampol LM. Diagnosis and treatment of cytomegalovirus retinitis. J Acquir Immune Defic Syndr. 1991;4(Suppl 1):S6–S10. [PubMed] [Google Scholar]
- 14.Jacobson MA, O’Donnell JJ. Approaches to the treatment of cytomegalovirus retinitis: ganciclovir and foscarnet. J Acquir Immune Defic Syndr. 1991;4(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 15.Brown DG, Visse R, Sandhu G, Davies A, Rizkallah PJ, Melitz C, et al. Crystal structures of the thymidine kinase from herpes simplex virus type-1 in complex with deoxythymidine and ganciclovir. Nat Struct Biol. 1995;2:876–881. doi: 10.1038/nsb1095-876. [DOI] [PubMed] [Google Scholar]
- 16.Cihova M, Altanerova V, Altaner C. Stem Cell Based Cancer Gene Therapy. Mol Pharm. 2011;8:1480–1487. doi: 10.1021/mp200151a. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Gao Y, Pu K, Ma L, Song X, Liu Y. All-trans retinoic acid enhances bystander effect of suicide-gene therapy against medulloblastomas. Neurosci Lett. 2011;503:115–119. doi: 10.1016/j.neulet.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Ye L, Chen X, Peng J, Zhang X, Yi H, et al. Combination gene therapy using VEGF-shRNA and fusion suicide gene yCDglyTK inhibits gastric carcinoma growth. Exp Mol Pathol. 2011;91:745–752. doi: 10.1016/j.yexmp.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 20.Spitzweg C, Morris JC. Gene therapy for thyroid cancer: current status and future prospects. Thyroid. 2004;14:424–434. doi: 10.1089/105072504323150732. [DOI] [PubMed] [Google Scholar]
- 21.Salomon B, Maury S, Loubiere L, Caruso M, Onclercq R, Klatzmann D. A truncated herpes simplex virus thymidine kinase phosphorylates thymidine and nucleoside analogs and does not cause sterility in transgenic mice. Mol Cell Biol. 1995;15:5322–5328. doi: 10.1128/mcb.15.10.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharmaceut Design. 2013;19:3466–3473. doi: 10.2174/1381612811319190012. [DOI] [PubMed] [Google Scholar]
- 23.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthopaedic Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 25.Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–595. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12:1755–1785. doi: 10.1261/rna.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licursi M, Christian SL, Pongnopparat T, Hirasawa K. In vitro and in vivo comparison of viral and cellular internal ribosome entry sites for bicistronic vector expression. Gene Ther. 2011;18:631–636. doi: 10.1038/gt.2011.11. [DOI] [PubMed] [Google Scholar]
- 28.Ngoi SM, Chien AC, Lee CG. Exploiting internal ribosome entry sites in gene therapy vector design. Curr Gene Ther. 2004;4:15–31. doi: 10.2174/1566523044578095. [DOI] [PubMed] [Google Scholar]
- 29.Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, et al. Correction of multi-gene deficiency in vivo using a single ’self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22:589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 32.Ryan MD, King AM, Thomas GP. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991;72(Pt 11):2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- 33.Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Gani D, et al. Analysis of the aphthovirus 2A/2B polyprotein ’cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ’skip’. J Gen Virol. 2001;82(Pt 5):1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 34.Luke G, Escuin H, De Felipe P, Ryan M. 2A to the fore - research, technology and applications. Biotechnol Genet Eng Rev. 2010;26:223–260. doi: 10.5661/bger-26-223. [DOI] [PubMed] [Google Scholar]
- 35.Altaner C. Prodrug cancer gene therapy. Cancer Lett. 2008;270:191–201. doi: 10.1016/j.canlet.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Ciceri F, Bonini C, Gallo-Stampino C, Bordignon C. Modulation of GvHD by suicide-gene transduced donor T lymphocytes: clinical applications in mismatched transplantation. Cytotherapy. 2005;7:144–149. doi: 10.1080/14653240510018136. [DOI] [PubMed] [Google Scholar]
- 37.Ciceri F, Bordignon C. Suicide-gene-Transduced donor T-cells for controlled graft-versus-host disease and graft-versus-tumor. Int J Hematol. 2002;76:305–309. doi: 10.1007/BF02982688. [DOI] [PubMed] [Google Scholar]
- 38.Matuskova M, Hlubinova K, Pastorakova A, Hunakova L, Altanerova V, Altaner C, et al. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2009;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 2000;60:3989–3999. [PubMed] [Google Scholar]
- 40.Nicholas TW, Read SB, Burrows FJ, Kruse CA. Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol Histopathol. 2003;18:495–507. doi: 10.14670/HH-18.495. [DOI] [PubMed] [Google Scholar]
- 41.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuriyama S, Masui K, Sakamoto T, Nakatani T, Kikukawa M, Tsujinoue H, et al. Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res. 1998;18:3399–3406. [PubMed] [Google Scholar]
- 43.Boucher PD, Im MM, Freytag SO, Shewach DS. A novel mechanism of synergistic cytotoxicity with 5-fluorocytosine and ganciclovir in double suicide gene therapy. Cancer Res. 2006;66:3230–3237. doi: 10.1158/0008-5472.CAN-05-3033. [DOI] [PubMed] [Google Scholar]
- 44.Feeley BT, Conduah AH, Sugiyama O, Krenek L, Chen IS, Lieberman JR. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res. 2006;24:1709–1721. doi: 10.1002/jor.20229. [DOI] [PubMed] [Google Scholar]
- 45.Hassan W, Sanford MA, Woo SL, Chen SH, Hall SJ. Prospects for herpes-simplex-virus thymidine-kinase and cytokine gene transduction as immunomodulatory gene therapy for prostate cancer. World J Urol. 2000;18:130–135. doi: 10.1007/s003450050185. [DOI] [PubMed] [Google Scholar]
- 46.Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 47.Shalev M, Kadmon D, Teh BS, Butler EB, Aguilar-Cordova E, Thompson TC, et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol. 2000;163:1747–1750. [PubMed] [Google Scholar]
- 48.Visnjic D, Kalajzic I, Gronowicz G, Aguila HL, Clark SH, Lichtler AC, et al. Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res. 2001;16:2222–2231. doi: 10.1359/jbmr.2001.16.12.2222. [DOI] [PubMed] [Google Scholar]
- 49.Kung SK, An DS, Chen IS. A murine leukemia virus (MuLV) long terminal repeat derived from rhesus macaques in the context of a lentivirus vector and MuLV gag sequence results in high-level gene expression in human T lymphocytes. J Virol. 2000;74:3668–3681. doi: 10.1128/jvi.74.8.3668-3681.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]