Abstract
Chronic HCV is a surreptitious disease currently affecting approximately 3% of the world's population that can lead to liver failure and cancer decades following initial infection. However, there are currently no vaccines available for the prevention of chronic HCV. From patients who acutely resolve HCV infection, it is apparent that a strong and broad cytotoxic T lymphocyte (CTL) response is important in HCV clearance. DNA vaccines are naked plasmid DNA molecules that encode pathogen antigens to induce a pathogen-specific immune response. They are inexpensive to produce and have an excellent safety profile in animals and humans. Additionally, DNA vaccines are able to induce strong CTL responses, making them well-suited for an HCV vaccine. We aimed to maximize vaccine recipients' opportunity to induce a broad T cell response with a novel antigenic sequence, multi-antigen vaccine strategy. We have generated DNA plasmids encoding consensus sequences of HCV genotypes 1a and 1b non-structural proteins NS3/4a, NS4b, NS5a, and NS5b. Rhesus macaques were used to study the immunogenicity of these constructs. Four animals were immunized 3 times, 6 weeks apart, at a dose of 1.0mg per antigen construct, as an intramuscular injection followed by in vivo electroporation, which greatly increases DNA uptake by local cells. Immune responses were measured 2 weeks post-immunization regimen (PIR) in immunized rhesus macaques and showed a broad response to multiple HCV nonstructural antigens, with up to 4680 spot-forming units per million peripheral blood mononuclear cells (PBMCs) as measured by Interferon-γ ELISpot. In addition, multiparametric flow cytometry detected HCV-specific CD4+ and CD8+ T cell responses by intracellular cytokine staining and detected HCV-specific CD107a+/GrzB+ CD8+ T cells indicating an antigen specific cytolytic response 2 weeks PIR compared with baseline measurements. At the final study time point, 6 weeks PIR, HCV-specific CD45RA- memory-like T cells remained detectable in peripheral blood. Data presented in this manuscript support the notion that vaccine immunogenicity studies using a macaque model can be used to depict key anti-HCV nonstructural antigenic cellular immune responses and support the development of DNA-based prophylactic HCV vaccines.
Keywords: DNA Vaccine, consensus antigens, Prophylactic Vaccine, Genotype 1a, Hepatitis C, Genotype 1b
Introduction
Hepatitis C virus (HCV) is responsible for widespread chronic hepatitis, affecting approximately 3% of the world population.1 Even in developed countries with public health education, and advanced sanitation practices, HCV infection persists in the population. A functional cure, or sustained virologic response (SVR) of chronic HCV, can be achieved with medical treatment. Recently, direct-acting antiviral drugs (DAA) have gained approval for clinical use with improved SVR rates of 90% in treatment naive patients with chronic genotype 1or 4 infections.2 Unfortunately, although medical treatments are improving, up to 75% of patients chronically infected with HCV are unaware of their infection, precluding them from treatment until diagnosed.3 Additionally, the cost of new DAA regimens in the US is an order of magnitude greater than the previous standard treatment, which is a financial burden largely placed on tax-payers.4 HCV-related direct health care costs have been projected to reach $9.1 billion by 2024.5 Cost and access currently precludes treatment with new DAA drugs in developing countries where HCV prevalence is greatest. Alternatively, with known risk factor data that would facilitate a selective vaccine strategy, a prophylactic vaccine would be a more cost-effective approach to controlling HCV disease burden, even where the least expensive medical treatment options are available.6 Importantly, there are currently no HCV vaccines approved for clinical use.
Vaccination is considered the most efficacious and cost-effective means to control disease burden worldwide.7 Because up to 25% of patients infected with HCV will spontaneously resolve acute infection by mounting a rapid cell-mediated immune response, developing an efficacious HCV vaccine is a viable strategy to control HCV-associated liver disease.8 Using a non-living DNA vaccine platform along with a highly efficient delivery method, such as in vivo electroporation (EP), vaccine toxicity is virtually eliminated while concomitantly inducing strong vaccine-specific immune responses, as evidenced by multiple non-human primate and clinical trials.9-12 In addition, DNA vaccines are stable at ambient temperature, easy to manipulate, and production is quick and inexpensive, relative to other vaccine platforms. Moreover, DNA can be administered in a series of multiple immunizations to boost immune responses without concerns of interference by pre-existing immunity, which is a limitation of viral vector vaccine platforms.13 Taken together, these characteristics make DNA-based immunizations an attractive vaccine modality for HCV.
At present, the target of an HCV vaccine is achieving an immune response that recapitulates responses observed during acute resolution (AR) of HCV infection. Our vaccine formulation was designed to achieve multiple correlates of resolution. First, a robust and broadly reactive CTL response has been correlated with AR.14 DNA + EP as a vaccine platform is known for eliciting strong CTL responses. Our HCV vaccine contains 4 non-structural HCV antigens, NS3/4A, NS4B, NS5A, and NS5B, which together comprise approximately two-thirds of the entire HCV genome, allowing recipients the opportunity to mount a broad multi-antigenic immune response. Second, in patients infected with HCV, T cell specificity focuses on non-structural HCV proteins during acute infection, whereas T cell specificity during chronic infection trends toward HCV structural proteins.15 Therefore, only non-structural antigens are included in the study vaccine formulation to focus the T cell reactivity on antigens that are associated with AR. Interestingly, studies of patients who are able to demonstrate AR upon primary infection, are able to clear virus more rapidly and show a reduced peak viremia during secondary infection when compared with primary infection, indicating that T cell memory has a protective effect.16-18 Thus, vaccination affords recipients the opportunity to develop a protective HCV-specific memory T cell pool without exposure to the virus. Taken together, these data support the notion that a broad primary immune response to nonstructural HCV antigens would be important host immune response needed for protection against HCV.
Our HCV antigens are synthetic consensus sequences that span genotypes 1a and 1b, which account for 70% of all chronic infections, thereby resulting in antigens that are highly homologous to the most prevalent genotypes encountered in the environment. We have previously demonstrated immunogenicity of the NS3/4A antigen alone in mice and non-human primates,12,19 and the NS4B, NS5A, and NS5B antigens have been studied individually in mice.20 In this study, we have used a non-human primate model to test immunogenicity of our complete formulation of HCV antigens.
The chimpanzee is the only non-human natural host for HCV infection. However, substantial ethical and financial boundaries thwart their use as a model for HCV studies. While the rhesus macaque cannot serve as a challenge model to study HCV infection and vaccine efficacy, these non-human primates have proven themselves invaluable as a useful large mammal model to studying vaccine safety, cellular immunogenicity, and predicting human immune responses. Here, we report that our combination HCV vaccine formulation induces both strong and broad HCV-specific immune responses in non-human primates with immunological determinants common to acute resolution in humans.
Materials and Methods
DNA plasmids, expression, and immunofluorescence
Development of DNA plasmids, pConHCV-NS3/4A, pConHCV-NS4B, pConHCV-NS5A, and pConHCV-NS5B, has been described previously using the pVAX1 backbone.20,21 Hemagglutinin peptide-tagged versions of each construct were used for in vitro expression in human muscle RD cells. RD cells were grown on a glass coverslip to 60–90% confluency. Plasmids were transiently transfected in RD cells with polyethyleneimine (3ug PEI: 1ug DNA). After 24 h, cells were washed with PBS and fixed and permeablized with ice-cold 1:1 methanol/acetone for 20 min. Fixed cells were washed 3x with PBS for 5mins each, followed by 1 h of blocking with BlockAid (Life Techonologies). The primary anti-HA antibody (clone 6E2, Cell Signaling Technology), was diluted 1:100 in BlockAid and incubated on cells at 4 °C, overnight. Following washing with PBS as before, the secondary F(ab')2 anti-mouse IgG eFluor® 570 (eBioscience) was diluted 1:500 in BlockAid and incubated on cells at room temperature for 1 h. After a final PBS wash step, coverslips were applied to glass microscope slides with Fluoroshield with DAPI mounting medium (Sigma-Aldrich). Slides were visualized and captured using a Leica DM5500B.
Immunization schedule and in vivo electroporation delivery
Female rhesus macaques (Macaca mulatta) of Indian origin (n = 4) were immunized at weeks 0, 6, and 12 with 1.0 mg per plasmid construct of pConHCV-NS3/4A, pConHCV-NS4B, pConHCV-NS5A, and pConHCV-NS5B. DNA was formulated in sterile WFI and delivered via IM injection into the quadriceps muscle in a total volume of 0.75 mL per injection followed by in vivo electroporation using the constant current CELLECTRA® device (Inovio Pharmaceuticals, Inc.).
Animal husbandry and specimen collection schedule
Rhesus macaques (Macaca mulatta) were housed at BioQual in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. Animals were allowed to acclimate for at least 30 d in quarantine prior to any immunization and the treatment protocol was IACUC approved.
Blood samples
Animals were anesthetized with Ketamine (0.1 mL/kg) or Telazol (0.06–0.10 mL/kg). The inguinal area was cleaned with alcohol. Blood samples were collected from the femoral vein using the Sarstedt S-Monovette collection system. Once all samples were collected, the needle was removed from the animal and pressure was applied to the injection site to achieve hemostasis. Blood collection tubes were placed on a rocker for gentle agitation and mixing of components.
Isolation of PBMCs from whole blood
Animals were bled 2 wk previous to the first immunization, 2 wk following the final immunization, and additionally, 4 wk following the final immunization. Ten or 20 mL of blood were collected in EDTA tubes, and peripheral blood mononuclear cells (PBMC) were isolated by standard Ficoll-Paque PLUS (GE Healthcare) centrifugation and re-suspended in complete culture R10 medium (RPMI 1640 with 2 mM/L L-glutamine, 25 Mm HEPES, 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 55 μM/L β-mercaptoethanol). Red blood cells (RBC) were lysed with ammonium chloride-potassium (ACK) lysis buffer (Cambrex BioScience).
Rhesus IFN-gamma ELISpot enzyme-linked immunospot assay (ELISpot)
IFN-γ ELISpot was performed as previously described to determine antigen specific IFN-γ secretion from immunized animals. Briefly, ELISpot 96-well plates (Millipore) were coated with anti-human [clone GZ-4] (Mabtech, Cincinnati, OH) interferon-gamma (IFN-γ capture antibody and incubated overnight at 4 °C. The following day, plates were washed with PBS and blocked for 2 h with R10. 2 × 105 macaque PBMCs from each animal were added per well and stimulated overnight at 37 °C, 5% CO2 in the presence of R10 (negative control), Concanavalin A (positive control), or specific peptide antigens for each construct. Peptide pool antigens are made of 15-mer peptides overlapping by 8 amino acids and spanning the entire length of each construct (pConHCV-NS3/4A, pConHCV-NS4B, pConHCV-NS5A, and pConHCV-NS5B) Peptides were synthesized by Genscript (Piscatway, NJ), resupended in DMSO and pooled into different stimulation pools at a concentration of 2 ug/mL/peptide.9,17 After 24 h of stimulation, the cells were washed and incubated overnight at 4 °C with anti-human IFN-γ biotinylated detector antibody (Clone 7-B6-1) (Mabtech). Plates were washed, and streptavidin-alkaline phosphatase (R&D Systems) was added to each well and incubated for 2h at room temperature. After washing, 5-Bromo-4-Chloro-3′ Indolylphosphate p-Toluidine Salt (BCIP) and Nitro Blue Tetrazololium Chloride (NBT) chromogen (R&D Systems) was added. The plates were then rinsed with water and dried. Spots were counted by an automated ELISpot reader (CTL Limited, Inc.) and raw values were multiplied by the dilution factor so that data are represented as spot forming units (SFU) per million PBMCs. Antigen-specific responses were determined by subtracting the number of spots in the negative control wells from each antigen-stimulated well.
Antibodies for PBMC flow cytometry
Surface and intracellular fluorescently-labeled monoclonal antibodies (mAbs) include: (1) anti-CD3 APC.Cy7 [SP34–2], anti-CD8 FITC [SK1], anti-IL-2 PE [MQ1–17H12], anti-TNFα AlexaFluor 700 [MAb11], anti-CD107a PE.Cy7 [H4A3], anti-CD14 PacBlue [M5E2], and anti-CD16 PacBlue [3G8] (BD Biosciences); (2) anti-CD4 PerCP.Cy5.5 [OKT4], anti-IFNγ BrilliantViolet 650 [4S.B4], and anti-T-bet APC [4B10] (Biolegend); (3) anti-CD45RA Q-Dot605 [MEM-56], anti-Granzyme B PE.TexasRed [GB11], anti-CD19 PacBlue [SJ25-C1] and LIVE/DEAD Fixable Aqua Dead Cell Stain Kit for 405 nm excitation (Life Technologies); (4) anti-CD27 PE.Cy5 [O323] (eBioscience).
Flow cytometry staining protocol for PBMCs
PBMCs were isolated from rhesus macaques and cryopreserved. Samples were thawed and rested overnight in R10 medium at 2x106 cells/mL. Cells were then stimulated for 5.5 h with: (1) HCV genotype 1a/1b consensus peptide pools specific for NS3/4A (2) NS4B, (3) NS5A, (4) NS5B, (5) R10 (negative) or (6) Staphylococcal Entertoxin B (SEB, positive). An amount of 1 uL/mL GolgiPlug (brefeldin A) and 0.7 uL/mL GolgiStop (monensin) (BD Biosciences) were added 1 h after stimulation start.
Following stimulation, cells were washed with phosphate-buffered saline (PBS) and incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain for 7 min at 37 °C. Pooled surface stain antibodies were added to cells in R10 and incubated for 30 min in the dark at 4 °C. Cells were washed with PBS and permeabilized with a FoxP3 Buffer set (eBioscience) as per the manufacturer's recommendations for 45 min in the dark at 4 °C. Cells were washed with Permeabilization/Wash buffer (eBioscience) and pooled intracellular stain antibodies were added to cells in Permeabilization/Wash buffer for 1 h in the dark at 4 °C. Cells were washed with Permeabilization/Wash buffer and fixed in Stabilizing Fixative (BD Bioscience).
For each sample, up to 1.25 × 106 events were collected on a modified LSRII flow cytometer (BD Immunocytometry Systems) configured for detection of 18 fluorescent parameters. Data was analyzed using FlowJo software (Treestar, Inc.). Responses from the negative controls were subtracted from the antigenic stimulations prior to graphing. Data was graphed using GraphPad Prism (v. 6.0, GraphPad Software).
Statistical analysis
Statistical comparisons were performed using Prism v6 (GraphPad Software, Inc.). Due to sample size, nonparametricity could not be determined. All data were assumed parametric; therefore, statistical differences in non-human primates before and after immunization were assessed by paired Student’s T test, whereby P < 0.05 was considered significant.
Results
HCV vaccine strategy, construct expression
HCV is a rapidly mutating and highly propagative virus, for which an effective vaccine remains elusive. We approached the challenge of an HCV vaccine with a strategy to maximize recipients' opportunity to mount a broad HCV-specific immune response using a consensus antigen approach with a formulation of multiple HCV antigens. Our HCV vaccine formulation is composed of 4 human-optimized synthetic genotype 1a/1b consensus HCV antigen plasmids: NS3/4A, NS4B, NS5A, and NS5B (Fig.1B). Together these antigens comprise approximately 62% of the entire HCV genome (Fig. 1A). Because T cell responses are critical for acute resolution, our antigens are non-structural genes that only express intracellularly during natural infection. Hemagglutinin (HA) peptide-tagged versions of each plasmid were used for in vitro expression studies to test antigen expression in human muscle-derived RD cells. RD cells were transiently transfected with an HCV plasmid, or empty control vector (pVAX), and stained with anti-HA-tag mAb (Fig. 1C). These data demonstrate that each HCV plasmid is capable of transfecting human muscle cells and express detectable HCV protein antigens required to elicit an immune response.
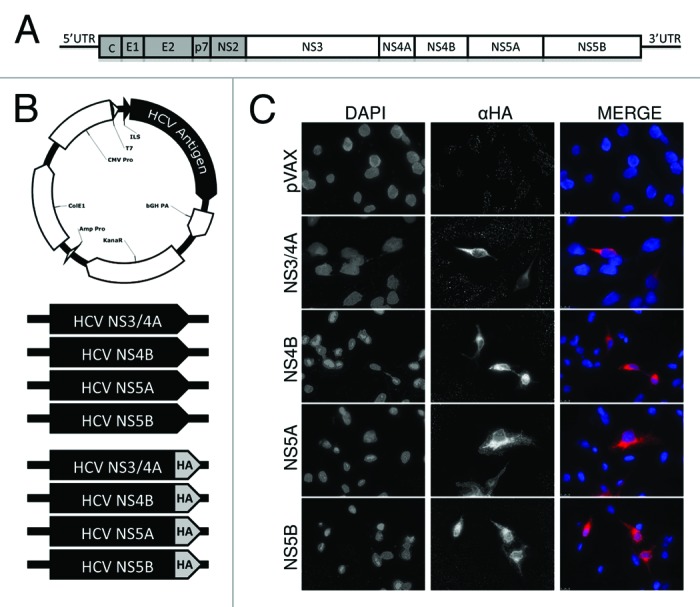
Figure 1. HCV antigen plasmid constructs and expression in human muscle RD cells.(A) Schematic representing the HCV genome illustrating the non-structural (NS) genes used as antigens in experiments (white blocks). (B) Schematic representing the HCV antigenic plasmids. Plasmids were constructed using pVAX backbone (empty vector), with expression driven by the CMV promoter and N-terminal IgE leader sequence (ILS). Four plasmids used for immunizations contained synthetic human codon-optimized HCV genotype 1a/1b consensus antigens: NS3/4A, NS4B, NS5A, NS5B. Identical plasmids with an additional C-terminal hemagglutinin (HA) tag were used for simplified staining in expression experiments. (C) Expression of HCV antigens by immunofluorescence staining of RD cells. Cells were stained with an anti-HA mAB 24 h after transfection with HA-tagged constructs.
Immunization and phlebotomy schedule
We have previously shown immunogenicity of NS3/4A in mice and NHPs,21 and individually NS4B, NS5A, and NS5B in mice.20 Combining all 4 plasmids in a single vaccine formulation was chosen for this study to increase breadth of HCV-specific immune responses. To test immunogenicity of the combination HCV vaccine formulation, 4 rhesus macaques were immunized thrice intramuscularly (I.M.), separated by 6 wk, with 1.0 mg of each plasmid. Each injection was immediately followed by in vivo electroporation (E.P.) at the site of injection to enhance DNA uptake into cells. Peripheral blood samples were collected 2 wk previous to the first immunization, to measure baseline levels of HCV antigen reactivity, and then 2 wk post-immunization regimen (PIR), and finally 6 wk PIR (Fig. 2). Peripheral blood mononuclear cells (PBMCs) were used to study HCV-specific T cells elicited by our vaccine since previous work has established that HCV-specific T cells in blood mirror those in the liver during acute infection, validating the use of PBMCs as a marker for intrahepatic T cell activity.22
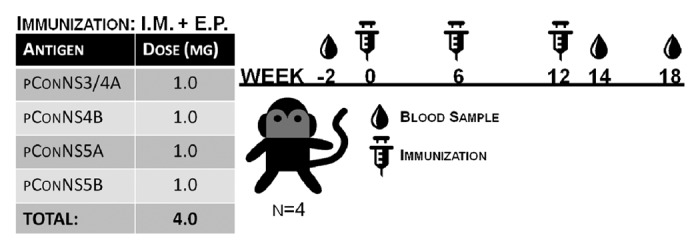
Figure 2. Non-human primate immunization schedule. Four rhesus macaques were immunized with 1.0mg of each, NS3/4A, NS4B, NS5A, NS5B plasmids followed by in vivo electroporation, 3 times, at 6 wk intervals. Blood was collected previous to first immunization (Baseline), and then 2 wk following the final immunization (2 wk PIR), and a final blood collection 6 wk post-immunization regimen (6 wk PIR).
Immunization induces strong and broad HCV-specific Interferon-γ responses
Immunogenicity was monitored through the use of quantitative Interferon-γ (IFNγ) ELISpot assays (Fig. 3). While all constructs demonstrated immunogenicity in the context of vaccination, the immunodominant domains of each antigen varied among the outbred animals, which is consistent with what is seen in the genetically diverse human population, and highlights the importance of a multi-antigenic vaccine strategy. At 2 wk post 3rd immunization, antigen-specific IFNγ responses were above background in 3/4 animals with total responses ranging from 170 to 4680 spot-forming units/106 PBMCs (SFU), and in one animal, NHP902, did not exceed its baseline response in the IFNγ ELISpot assay. Antigen-specific IFNγ reactivity against peptides of HCV NS3/4A, NS4B, NS5A, and NS5B were variable between animals and ranged from undetectable to 1783 SFU, 3 to 726 SFU, 100 to 796 SFU, and 66 to 2000 SFU, respectively. These data illustrate that, in an outbred heterogeneous group of non-human primates, our HCV vaccine can induce PBMCs of substantial magnitude that are broadly reactive to regions spanning all 4 HCV antigens.
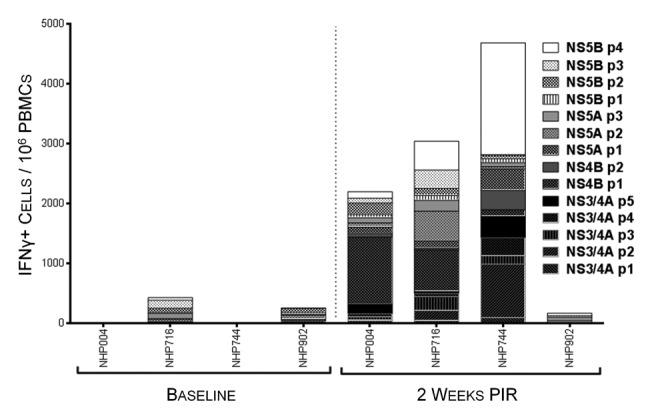
Figure 3. HCV-specific interferon-γ responses detectable in peripheral blood 2 wk following immunization. IFNγ ELIspot assays were performed on NHP PBMCs isolated at baseline and 2 wk post-immunization regimen (PIR) by stimulating cells overnight with antigen-matched overlapping linear peptide pools. HCV-specific IFNγ responses, as spot-forming units (IFNγ+ cells)/million PBMCs, are represented as stacked responses, demonstrating specificity across multiple regions of multiple antigens for individual NHPs.
HCV-specific effector CD4+ and CD8+ T lymphocytes are elicited by HCV immunization
In order to further study the IFNγ responses induced by HCV immunization by identifying PBMC populations responsible for IFNγ production, as well as detect other effector cytokines produced in response to antigen stimulation, we performed intracellular staining assays (ICS) and acquired cell phenotype data by flow cytometry at 2 wk PIR (Fig. 4). PBMCs were stimulated ex vivo with antigen-matched peptides in the presence of golgi transport inhibitors and immunofluorescently stained for T cell markers (CD3, CD4, and CD8), intracellular cytokines (IFNγ; tumor necrosis factor-α, TNFα; and interleukin-2, IL-2), and cytolytic markers (surface CD107a, LAMP-1; and granzyme B, GrzB). From acquired flow cytometry data, CD3+/CD4-/CD8+ T cells (putative cytotoxic T lymphocyte; CTL) and CD3+/CD8-/CD4+ T cells (Type 1 helper T lymphocytes; Th1) were analyzed for HCV-specific cytokine responses. T lymphocytes were included in the reported HCV-specific frequencies if they stained positive for one or more effector cytokines: IFNγ, which is an aforementioned correlate of acute resoultion; TNFα, which is another important pro-inflammatory and antiviral effector cytokine; IL-2, which is necessary for the proliferation and survival of T cells; or, exhibited a cytolytic phenotype (CD107a+/GrzB+, which indicates a cell is both producing the cytolytic enzyme GrzB and actively degranulating), in response to HCV peptide stimulation. Total cytokine responses were predominately due to HCV-specific CD8+ CTL. However, HCV-specific CD4+ Th1 cells were also detectable. NHP004 and NHP744 demonstrated Th1 and CTL reactivity to all 4 HCV antigens with total 2.24% and 2.87% Th1, and 5.38% and 5.21% CTL HCV-specific frequencies, respectively (Fig. 4). Cytokine responses were detectable but limited to less than 4 antigens in NHP716 and NHP902 with total 1.05% and 1.61% Th1, and 0.96% and 1.02% CTL HCV-specific frequencies, respectively. Such variable T cell reactivity profiles further highlights the importance of a multi-antigenic vaccine in diverse populations. Altogether, with the exception of TNFα production in CD4+ T cells, cytokine secretion in response to HCV peptide stimulation was significantly (p<0.05) increased 2 weeks PIR compared to baseline measurements for each HCV antigen (Fig. 5). Taken together, these data illustrate that HCV immunization elicits both peripheral HCV-specific Th1 and CTL that react to one or more of the 4 HCV antigens within our HCV vaccine formulation.
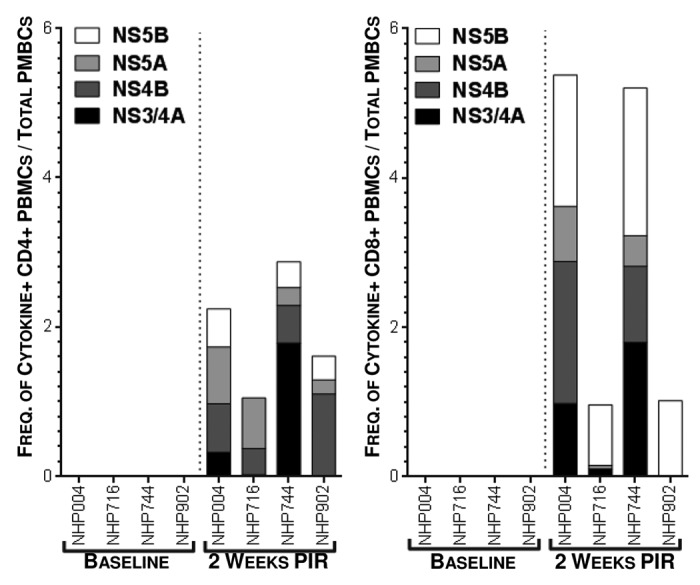
Figure 4. Detectable frequencies of HCV-specific peripheral T lymphocytes are induced by immunization. Cytokine production, by intracellular cytokine staining, following antigen-matched peptide stimulation revealed HCV-specific T cells in peripheral blood 2 wk PIR, compared with baseline, by flow cytometric analysis. CD3+/CD4+/CD8- (Th1) or CD3+/CD8+/CD4- (CTL) cells were counted as cytokine-positive if they produced one or more of IFNγ, TNFα, or IL-2. Total cytokines responses are represented as stacked responses to demonstrate breadth across multiple antigens (■, NS3/4A; ■, NS4B; ■, NS5A; ■, NS5B).
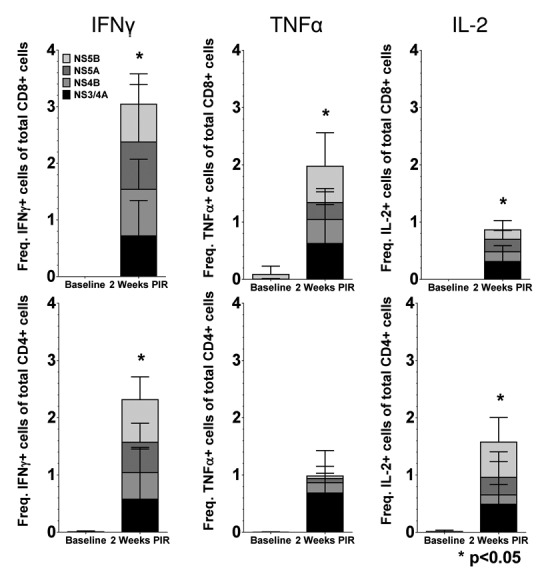
Figure 5. Cytokine production of HCV-specific T cells following immunization. Mean frequencies of cytokine-positive Th1 and CTL cells stacked by HCV antigen at 2 wk PIR compared with baseline (■, NS3/4A; ■, NS4B; ■, NS5A; ■, NS5B). Total responses were denoted as significant by paired Student t test, if responses to each individual antigen were significant (P < 0.05), as compared with baseline samples.
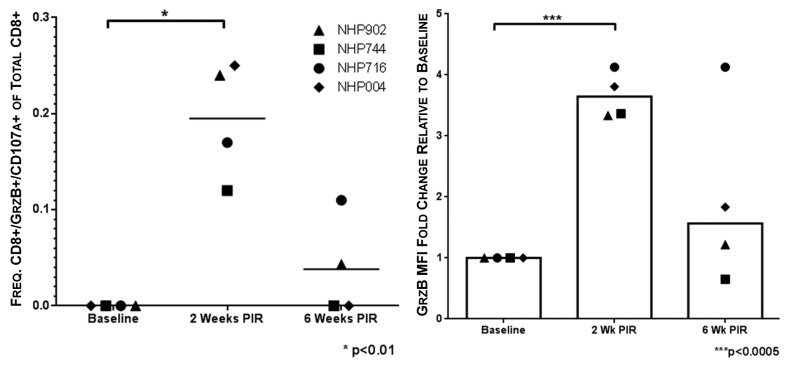
Cytolytic CD8+ T cells are associated with acute resolution of HCV infection.23 Given their importance in protection from chronic disease, we aimed to elicit HCV-specific cytolytic CTL with our vaccine. To measure cytolytic CTL frequencies, PBMCs were stimulated with HCV peptides in the presence of anti-CD107a antibody, such that in the event CTL degranulation, the granule membrane-associated CD107a would be exposed on the cell surface and labeled. Subsequent to peptide stimulation CTL were further stained intracellularly for GrzB. CTL stained double-positive for CD107a and GrzB were considered putative CTL with cytolytic potential. Animals showed statistically significant (P < 0.01) HCV-specific cytolytic CTL responses at 2 wk PIR (0.20 ± 0.06%/total CD8+) as compared with pre immunization frequencies. At the final time point of the study, 2 of 4 NHP maintained detectable HCV-specific cytolytic CTL frequencies (0.11% and 0.04%/total CD8+) (Fig. 6A). Additionally, at 2 wk PIR, GrzB mean fluorescence intensity (MFI) of total CD8+ T cells increased significantly by 3.6-fold, indicating greater GrzB production per cell in response to HCV peptide stimulation. By 6 wk PIR, GrzB MFI returned to a modest increase above baseline (Fig. 6B). These data indicate that our vaccine elicits circulating HCV-specific CTL that demonstrate cytolytic activity.
Figure 6. Vaccine-induced HCV-specific cytolytic CTL remain detectable 6 wk beyond immunization. Putative cytolytic CTL, as defined by positive staining for both surface CD107a and intracellular granzyme B (GrzB), were induced by HCV immunization at 2 wk PIR in all NHPs. Cytolytic responses remained detectable 6 wk PIR in NHP716 and NHP 902 (●, NHP716; ◆, NHP004; ▲, NHP902; ■, NHP744).
HCV immunization gives rise to circulating effector memory-like CTL
We characterized PBMCs of immunized NHPs for HCV-specific CTL exhibiting a memory-like phenotype by negative staining for CD45RA, and observing CD27 staining to differentiate effector memory-like (EM; CD45RA-/CD27-) from central memory-like (CM; CD45RA-/CD27+) CTL, at the final immune analysis time point, 6 wk PIR. HCV peptide stimulation of PBMCs from immunized animals induced detectable cytokine secretion and cytolytic activity within the EM and CM CTL population in all animals (Fig. 7). IFNγ, TNFα, and IL-2 production was induced in up to 9.4%, 10.1%, and 1.9% of EM CTL in response to HCV peptide stimulation, respectively, and up to 9.8%, 9.1%, and 0.8% of CM CTL, respectively. Cytolytic degranulation in response to HCV peptide stimulation was detectable in frequencies of up to 5.5% total EM CTL, and 3.4% total CM CTL. Taken together, at the final time point of this study, HCV-specific CTL that exhibit a phenotype similar to memory T cells, including CTL demonstrating cytolytic activity, remained detectable in circulation of all NHP studied.
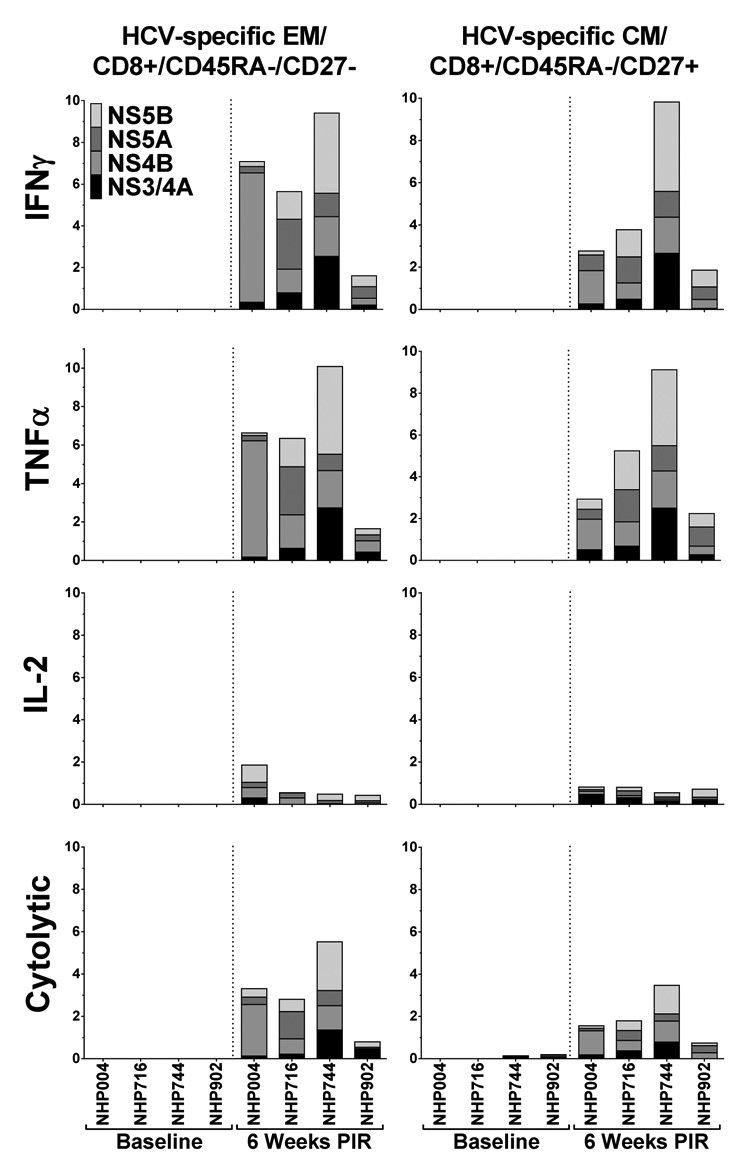
Figure 7. HCV-specific memory T cells persist in circulation. At the final timepoint of the study, 6 wk PIR, cytokine-secreting and putative cytolytic (surface-CD107a+/GrzB+) memory-like CD8+ T cells remained detectable in the blood, as compared with baseline samples, in response to HCV-peptide stimulation. Memory-like CD8+ T cells were characterized as effector (EM; CD45RA-/CD27-) or central (CM; CD45RA-/CD27+) memory phenotypes.
Discussion
Hepatitis C virus is a major cause of chronic liver disease worldwide, which is responsible for 350 000 deaths annually, and the leading indication for liver transplantation. Although new DAAs are improving medical treatment of HCV infection in wealthy nations, a safe and effective prophylactic HCV vaccine has remained elusive since the non-A, non-B hepatitis virus was first cloned and characterized in 1989.24 A vaccine approach still remains a feasible strategy to control HCV-associated disease because 25% of acute infections are self-limiting, demonstrating that the human immune system is capable of controlling HCV. Numerous studies in chimpanzees and humans have established the importance of early, strong, and broadly-reactive T cell responses against non-structural viral proteins in the clearance of acute HCV infection.8,14,25 These immunological correlates of acute resolution provide benchmarks for HCV vaccine development. The importance of an early T cell response is highlighted by patients who resolve multiple HCV infections. In such cases subsequent infections are cleared more rapidly with reduced peak viremia than primary infection.18 Inducing immune memory with a vaccine allows a more rapid recall response upon viral exposure compared with virus-naïve hosts. Additionally, immune priming within the liver during HCV exposure drives expansion of regulatory T cells in naïve animals, which favors chronicity upon HCV infection.26 Conversely, immune priming in the periphery with an adenovirus/DNA combined platform, has elicited protective functional T cells exhibiting dampened coinhibitory markers, such as PD-1, in the liver using the chimpanzee challenge model, compared with unimmunized animals.27
Various vaccine platforms have been tested in pre-clinical and clinical studies, namely DNA and viral vectors, due to the necessity of eliciting CD8+ T cell responses. Viral vectors encoding HCV non-structural proteins from a genotype 1b sequence was safe and immunogenic in a promising phase I clinical trial.28 However, adenoviral vectors limit the breadth of T cell reactivity to an encoded antigen compared with DNA.29 Whereas, immunization with DNA elicits broad T cell responses specific to both immunodominant and immunorecessive epitopes of encoded viral antigens.30 Importantly, naked DNA alone is poorly immunogenic, but the addition of in vivo electroporation has enhanced DNA immunogenicity to magnitudes suitable for clinical applications.31 Hence, to achieve a strong and broad HCV-specific CTL response, a synthetic DNA vaccine in combination with in vivo electroporation technology was chosen due to the impressive magnitude of immune responses generated in previous pre-clinical and clinical studies using the synthetic DNA + EP vaccine platform.9,10,20,21,32,33
The breadth of a T cell response by an HCV vaccine can be maximized for any given recipient by formulating a vaccine with multiple antigens. To that end, we have included HCV antigens that comprise 62% of the entire HCV genome, and 90% of the nonstructural HCV genes, in a single vaccine formulation. Additionally, we approached the diverse genetic landscape of HCV viruses found in the human population by synthesizing consensus genotype 1a/1b antigens for our vaccine, thereby including the most common antigen sequences that account for 70% of all chronic HCV infections. Together, we have developed an HCV vaccine aimed at maximizing any recipients' likelihood of achieving established immunological benchmarks associated with resolution of acute HCV infection.
As in natural viral infection, DNA vaccines produce intracellular protein antigens de novo, as shown by expression of each antigen following transfection of a human muscle cell line. This characteristic of DNA + EP vaccines leads to strong antigen-specific CTL responses.34 Thus, DNA is an ideal platform for inducing the early and strong T cell benchmarks associated with viral clearance. We have previously characterized the immunogenicity of a genotype 1a/1b HCV consensus NS3/4A vaccine in NHPs.12,21 However, the importance of a broadly-reactive T cell response prompted us to formulate the multiantigenic HCV vaccine used in the present study. We have previously shown in mice that each antigen alone is immunogenic.20,21 For this study, we immunized 4 NHPs with a combination of 1.0 mg of HCV 1a/1b consensus NS3/4A, NS4B, NS5A, and NS5B, I.M., followed by in vivo electroporation, and analyzed the resulting T cell responses 2 wk PIR and 6 wk PIR. By both IFNγ ELIspot and ICS analyses, we have demonstrated broad T cell responses that span all 4 HCV antigens. In the outbred animals that model genetic diversity in the human population, the importance of a multiantigenic approach is evident, in that individuals mounted T cell responses to differing degrees against each antigen, and differing regions within each antigen. For example, while both NHPs revealed broad T cell reactivity, the N-terminal region of NS4B dominated the IFNγ response of NHP004, whereas NS5B was a minor antigen. Conversely, NHP744 mounted a strong IFNγ response to the C-terminal region of NS5B, while reactivity against NS4B peptides was modest.
Similarly, we were able to detect Th1 cytokine production in response to peptide stimulation of multiple HCV antigens within both CD4+ and CD8+ T cell populations by multiparametric flow cytometry. Importantly, immunization gave rise to IFNγ-secreting HCV-specific CD8+ T cells, which is an immunological determinant highly associated with acute HCV clearance.35 Upon recruitment of HCV-specific T cells to the liver late in acute infection, HCV replication is strongly inhibited due to IFNγ secretion. This is an effective means of controlling HCV replication without concomitant tissue damage, but lysis of infected cells is necessary to eliminate infection.36 By 2 wk PIR, all NHPs elicited significant frequencies of circulating cytolytic CTL, and significantly increased GrzB production within the CD8+ population, as measured by surface CD107a and GrzB expression, and GrzB MFI, respectively. Further, at the final time point of the study, 6 wk PIR, 2 NHPs maintained cytolytic CTL frequencies detectable above the assay background threshold with a moderate increase in GrzB staining intensity. Finally, HCV-specific memory T cells have been shown to expand and control secondary infection in human acute resolvers.18 Studies in chimpanzees have revealed that circulating HCV-specific memory CD8+ T cells are critical for protection against reinfection.23 At the final time point of the present study, NHP memory-like CD45RA- CD8+ T cells were analyzed for HCV reactivity. Following in vitro peptide stimulation, high frequencies of EM and CM CD8+ T cells produced antiviral cytokines and secreted GrzB in response to all 4 HCV antigens.
Taken together, the DNA HCV vaccine presented in this study has demonstrated strong immunogenicity, broad T cell reactivity, and T cell phenotypes closely associated with protection against chronic HCV infection. We believe the historical safety profile of DNA products in the clinic, together with the immunological benchmarks of HCV clearance achieved in a relevant non-human primate model, supports clinical investigation of this HCV vaccine. Thus, the HCV antigen plasmids used in this study will be tested in clinical trials (ClinicalTrials.gov Identifier: NCT02027116).
Disclosure of Potential Conflicts of Interest
D.B.W. has grant funding, participates in industry collaborations, has received speaking honoraria, and fees for consulting. This service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments or stock/stock options and in the interest of disclosure therefore he notes potential conflicts associated with this work with in particular Inovio where he serves on the SAB as well as with Pfizer, Bristol Myers Squibb, Merck, Aldevron, Roche, Ferring Pharma, and possibly others. Licensing of technology from his laboratory has created over 150 jobs in the private sector in the biotech/pharma industry. The other authors declare no competing financial interests.
Acknowledgments
This work is supported by grants funded through the PA Department of Health non-formula grant program (4100051718) and a Ruth L. Kirschstein National Research Service Award (5T32MH079785 to BL). This work was supported in part by NIH grants awarded to Dr Weiner.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. . Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333 - 42; http://dx.doi.org/ 10.1002/hep.26141; PMID: 23172780 [DOI] [PubMed] [Google Scholar]
- 2.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. . Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878 - 87; http://dx.doi.org/ 10.1056/NEJMoa1214853; PMID: 23607594 [DOI] [PubMed] [Google Scholar]
- 3.Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C, The National Academies Press, 2010. [PubMed] [Google Scholar]
- 4.Stepanova M, Kanwal F, El-Serag HB, Younossi ZM. . Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology 2011; 53:737 - 45; http://dx.doi.org/ 10.1002/hep.24131; PMID: 21319199 [DOI] [PubMed] [Google Scholar]
- 5.Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. . Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013; 57:2164 - 70; http://dx.doi.org/ 10.1002/hep.26218; PMID: 23280550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massad E, Coutinho FA, Chaib E, Burattini MN. . Cost-effectiveness analysis of a hypothetical hepatitis C vaccine compared to antiviral therapy. Epidemiol Infect 2009; 137:241 - 9; http://dx.doi.org/ 10.1017/S0950268808000873; PMID: 18631422 [DOI] [PubMed] [Google Scholar]
- 7.Plotkin SA, Orenstein WA, Offit PA. Vaccines, 6th edn Elsevier Saunders: Philadelphia? Pa., 2013. [Google Scholar]
- 8.Walker CM. . Adaptive immunity to the hepatitis C virus. Adv Virus Res 2010; 78:43 - 86; http://dx.doi.org/ 10.1016/B978-0-12-385032-4.00002-1; PMID: 21040831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012 Oct 10;4(155):155ra138 [DOI] [PMC free article] [PubMed]
- 10.Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al. , NIAID HIV Vaccine Trials Network. . Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013; 208:818 - 29; http://dx.doi.org/ 10.1093/infdis/jit236; PMID: 23840043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraro B, Talbott KT, Balakrishnan A, Cisper N, Morrow MP, Hutnick NA, Myles DJ, Shedlock DJ, Obeng-Adjei N, Yan J, et al. . Inducing humoral and cellular responses to multiple sporozoite and liver-stage malaria antigens using exogenous plasmid DNA. Infect Immun 2013; 81:3709 - 20; http://dx.doi.org/ 10.1128/IAI.00180-13; PMID: 23897618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang Kuhs KA, Ginsberg AA, Yan J, Wiseman RW, Khan AS, Sardesai NY, O’Connor DH, Weiner DB. . Hepatitis C virus NS3/NS4A DNA vaccine induces multiepitope T cell responses in rhesus macaques mimicking human immune responses [corrected]. [corrected] Mol Ther 2012; 20:669 - 78; http://dx.doi.org/ 10.1038/mt.2011.188; PMID: 21952169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutzler MA, Weiner DB. . DNA vaccines: ready for prime time?. Nat Rev Genet 2008; 9:776 - 88; http://dx.doi.org/ 10.1038/nrg2432; PMID: 18781156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann-Haefelin C, Thimme R. . Adaptive immune responses in hepatitis C virus infection. Curr Top Microbiol Immunol 2013; 369:243 - 62; http://dx.doi.org/ 10.1007/978-3-642-27340-7_10; PMID: 23463204 [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Vaughan K, Greenbaum J, Peters B, Law M, Sette A. . A meta-analysis of the existing knowledge of immunoreactivity against hepatitis C virus (HCV). PLoS One 2012; 7:e38028; http://dx.doi.org/ 10.1371/journal.pone.0038028; PMID: 22675428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. . Hepatitis C virus reinfection in injection drug users. Hepatology 2006; 44:1139 - 45; http://dx.doi.org/ 10.1002/hep.21376; PMID: 17058216 [DOI] [PubMed] [Google Scholar]
- 17.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, et al. . Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009; 200:1216 - 26; http://dx.doi.org/ 10.1086/605947; PMID: 19764883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. . Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010; 138:315 - 24; http://dx.doi.org/ 10.1053/j.gastro.2009.09.017; PMID: 19782080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang Kuhs KA, Toporovski R, Ginsberg AA, Olsen AL, Shedlock DJ, Morrow MP, Yan J, Wells RG, Weiner DB. . Peripheral immunization induces functional intrahepatic hepatitis C specific immunity following selective retention of vaccine-specific CD8 T cells by the liver. Hum Vaccin 2011; 7:1326 - 35; http://dx.doi.org/ 10.4161/hv.7.12.18279; PMID: 22108033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang Kuhs KA, Toporovski R, Yan J, Ginsberg AA, Shedlock DJ, Weiner DB. . Induction of intrahepatic HCV NS4B, NS5A and NS5B-specific cellular immune responses following peripheral immunization. PLoS One 2012; 7:e52165; http://dx.doi.org/ 10.1371/journal.pone.0052165; PMID: 23284919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang KA, Yan J, Draghia-Akli R, Khan A, Weiner DB. . Strong HCV NS3- and NS4A-specific cellular immune responses induced in mice and Rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Vaccine 2008; 26:6225 - 31; http://dx.doi.org/ 10.1016/j.vaccine.2008.07.052; PMID: 18692108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin EC, Capone S, Cortese R, Colloca S, Nicosia A, Folgori A, Rehermann B. . The kinetics of hepatitis C virus-specific CD8 T-cell responses in the blood mirror those in the liver in acute hepatitis C virus infection. J Virol 2008; 82:9782 - 8; http://dx.doi.org/ 10.1128/JVI.00475-08; PMID: 18667501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. . Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 2003; 197:1645 - 55; http://dx.doi.org/ 10.1084/jem.20030239; PMID: 12810686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. . Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989; 244:359 - 62; http://dx.doi.org/ 10.1126/science.2523562; PMID: 2523562 [DOI] [PubMed] [Google Scholar]
- 25.Terilli RR, Cox AL. . Immunity and hepatitis C: a review. Curr HIV/AIDS Rep 2013; 10:51 - 8; http://dx.doi.org/ 10.1007/s11904-012-0146-4; PMID: 23180007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Veerapu NS, Shin EC, Biancotto A, McCoy JP, Capone S, Folgori A, Rehermann B. . Subinfectious hepatitis C virus exposures suppress T cell responses against subsequent acute infection. Nat Med 2013; 19:1638 - 42; http://dx.doi.org/ 10.1038/nm.3408; PMID: 24270546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SH, Shin EC, Capone S, Caggiari L, De Re V, Nicosia A, et al. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012 Oct;143(4):1048-60.e4 [DOI] [PMC free article] [PubMed]
- 28.Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012 Jan 4;4(115):115ra1 [DOI] [PMC free article] [PubMed]
- 29.Schirmbeck R, Reimann J, Kochanek S, Kreppel F. . The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol Ther 2008; 16:1609 - 16; http://dx.doi.org/ 10.1038/mt.2008.141; PMID: 18612271 [DOI] [PubMed] [Google Scholar]
- 30.Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. . Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol 1997; 71:2715 - 21; PMID: 9060624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. . Synthetic DNA Vaccines: Improved Vaccine Potency by Electroporation and Co-Delivered Genetic Adjuvants. Front Immunol 2013; 4:354; http://dx.doi.org/ 10.3389/fimmu.2013.00354; PMID: 24204366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J, Corbitt N, Pankhong P, Shin T, Khan A, Sardesai NY, Weiner DB. . Immunogenicity of a novel engineered HIV-1 clade C synthetic consensus-based envelope DNA vaccine. Vaccine 2011; 29:7173 - 81; http://dx.doi.org/ 10.1016/j.vaccine.2011.05.076; PMID: 21651948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, Parkinson R, Wu L, Sidhu MK, Phillipson-Weiner R, et al. . Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol 2008; 180:7969 - 79; http://dx.doi.org/ 10.4049/jimmunol.180.12.7969; PMID: 18523260 [DOI] [PubMed] [Google Scholar]
- 34.Sardesai NY, Weiner DB. . Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421 - 9; http://dx.doi.org/ 10.1016/j.coi.2011.03.008; PMID: 21530212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. . Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A 2002; 99:15661 - 8; http://dx.doi.org/ 10.1073/pnas.202608299; PMID: 12441397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heim MH. . Innate immunity and HCV. J Hepatol 2013; 58:564 - 74; http://dx.doi.org/ 10.1016/j.jhep.2012.10.005; PMID: 23063572 [DOI] [PubMed] [Google Scholar]