Abstract
Rotavirus may be an important causative agent of acute gastroenteritis (AGE) in the elderly, a population that is particularly vulnerable due to waning immunity. It is estimated that rotavirus may account for 2–5% of adult gastroenteritis hospitalizations in the United States. This is the first study to assess the safety and immunogenicity of the live pentavalent rotavirus vaccine (RV5) in an elderly population. In this study, healthy, independently living adults aged 65–80 years were randomized in a 2:1 ratio to receive three 2-mL oral doses of RV5 or placebo administered 28–42 days apart. All subjects were followed for safety for 42 days post any vaccination and up to 180 days after the final vaccination for clinical adverse events. Immunogenicity of RV5 was measured by serum anti-rotavirus IgA enzyme immunoassay and serum neutralizing antibody responses to human rotavirus serotypes prior to and after each dose. Results of this study demonstrated that RV5 was generally safe and well tolerated in healthy elderly adults, where 9% of placebo and 27% of RV5 recipients experienced a vaccine-related adverse event of mild or moderate intensity. Immune responses (serum anti-rotavirus immunoglobulin A [IgA] and serum neutralizing antibodies against human rotavirus serotypes in the vaccine) were augmented in this population after a single dose of RV5, despite the factors of older age and preexisting antibodies to the virus. Therefore, if vaccination in the elderly is needed, further evaluation of RV5 as a candidate vaccine in this age group may be warranted.
Keywords: Rotavirus gastroenteritis, acute gastroenteritis, elderly, vaccine, pentavalent rotavirus vaccine, RV5, immunogenicity
Introduction
Prior to the introduction of rotavirus vaccines in February 2006, rotavirus was recognized as the leading cause of severe acute gastroenteritis (AGE) in infants and young children worldwide, with nearly every child infected with the virus by the age of 5 y.1,2 Available literature suggests that rotavirus may also be an important causative agent of AGE in the elderly, although the contribution of rotavirus to morbidity and mortality is not completely understood.3-7 This population is potentially vulnerable to additional illnesses because of frailty and waning immunity.5 In adults, rotavirus infection may be asymptomatic or may present with nausea, malaise, headache, abdominal cramping, fever, and diarrhea.5 Furthermore, diarrheal dehydration may lead to cardiovascular complications, which could result in the need for a higher level of care and significantly compromise quality of life.
In 2008, 390 rotavirus-coded hospitalizations for adults ≥ 65 y of age were reported with a median stay of 4 d and median costs of $10 260.8 It is estimated that rotavirus may be responsible for 2–5% of adult gastroenteritis (GE) hospitalizations,5 with advanced age being the most significant mortality risk factor in patients hospitalized for GE.9 Epidemic outbreaks of rotavirus GE have also occurred among adults who live in closed communities such as long-term care facilities.3-6,10-14 Poor host immunity and multiple comorbid disorders may facilitate the spread of infection.5,6,9-12 The aim of the current study was to assess the safety and immunogenicity of RV5 (RotaTeq®, Merck & Co., Inc.) in an elderly population as a preparation for the assessment of a potential public health benefit of the vaccine in this population. RV5 is a live pentavalent vaccine containing 5 human-bovine reassortant rotaviruses, with the WC3 bovine strain as a backbone and viral surface proteins that correspond to human serotypes G1, G2, G3, G4, and P1A[8].15-17
Results
In total, 66 subjects were randomized to the study, 44 in the RV5 group and 22 in the placebo group (Fig. 1). Baseline characteristics of the 2 treatment groups were generally similar with respect to age, sex, and race (Table 1). The mean age for subjects was 70.9 ± 4.3 y in the RV5 group and 71.4 ± 5.0 y in the placebo group. Subject disposition was as follows: 61 subjects completed the 3-dose regimen of RV5 or placebo; 3 withdrew consent; 1 was lost to follow up; and 1 discontinued further study vaccination after dose 2 due to adverse events (AEs). The latter subject reported AEs (GE, rectal hemorrhage, diarrhea, nausea, vomiting) that were judged not to be vaccine related and the subject remained on the study and completed the 180-d safety follow-up thereafter (further details below).
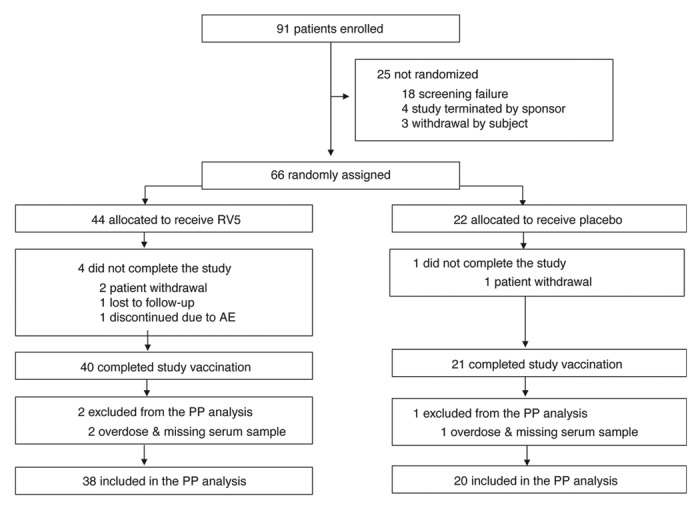
Figure 1. Study disposition. AE, adverse event; PP, per-protocol; RV5, pentavalent rotavirus vaccine, RotaTeq®. Note: Overdose was defined as receiving more than 1 dose within a 12-d period. Missing serum sample refers to a subject missing either baseline or postdose 3 serum sample for immunogenicity testing.
Table 1. Baseline characteristics of subjects.
| RV5 | Placebo | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Subjects in population | 44 | 22 | ||
| Sex | ||||
| Male | 21 | (47.7) | 9 | (40.9) |
| Female | 23 | (52.3) | 13 | (59.1) |
| Age (Years) | ||||
| 65–70 | 26 | (59.1) | 12 | (54.5) |
| 71–80 | 18 | (40.9) | 10 | (45.5) |
| Mean | 70.9 | 71.4 | ||
| SD | 4.3 | 5.0 | ||
| Range | 65–80 | 65–79 | ||
| Race | ||||
| Asian | 4 | (9.1) | 2 | (9.1) |
| Black | 2 | (4.5) | 0 | (0.0) |
| White | 38 | (86.4) | 20 | (90.9) |
| Ethnicity | ||||
| Hispanic or Latino | 6 | (13.6) | 4 | (18.2) |
| Not Hispanic or Latino | 38 | (86.4) | 18 | (81.8) |
RV5, pentavalent rotavirus vaccine, RotaTeq®; SD, standard deviation.
No safety concerns were identified during the internal medical monitoring process. No clinically meaningful differences were found between the 2 treatment groups with regard to the frequency or type of prior and concomitant therapies. While co-administration of influenza vaccine and PNEUMOVAX 23® (Merck & Co., Inc.) was allowed per protocol, no subject received any concomitant vaccination within 30 d prior to or 42 d following any dose of the study vaccine.
The percentage of subjects who reported systemic AEs (Table 2) was numerically larger in the RV5 group than in the placebo group, 59.1% vs. 40.9%, respectively, with a resulting risk difference of 18.2% (95% confidence interval [CI]: –7.4, 41.4). The study was not powered for a difference of this magnitude to become statistically significant. A total of 12 (27%) of participants in the RV5 group reported a vaccine-related adverse event of mild or moderate intensity compared with 2 (9%) of participants in the placebo group. More subjects in the RV5 group reported gastrointestinal disorders than subjects in the placebo group (34.1% vs 18.2%, respectively) (Table 2). The largest differences among the RV5 and placebo group were observed for diarrhea (25.0% vs 9.1%) and nausea (11.4% vs 4.5%). The statistical significance of numerical risk differences and 95% CIs for gastrointestinal disorders, diarrhea, and nausea could not be determined owing to the small number of cases and the power of the study.
Table 2. Summary of systemic adverse events (AEs) days 1 to 42 following any vaccination in the full analysis set.
| RV5 | Placebo | Risk difference vs Placebo | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post Dose 1 | Post Dose 2 | Post Dose 3 | Post Any | Post Dose 1 | Post Dose 2 | Post Dose 3 | Post Any | Post Any | ||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | Estimate (95% CI)d | ||||
| Subjects in population with follow-up | 44 | 41 | 40 | 44 | 22 | 21 | 21 | 22 | ||||||||||||
| With ≥ 1 AE | 19 | (43.2) | 10 | (24.4) | 7 | (17.5) | 26 | (59.1) | 7 | (31.8) | 6 | (28.6) | 4 | (19.0) | 9 | (40.9) | 18.2 (–7.4, 41.4) | |||
| With no AE | 25 | (56.8) | 31 | (75.6) | 33 | (82.5) | 18 | (40.9) | 15 | (68.2) | 15 | (71.4) | 17 | (81.0) | 13 | (59.1) | –18.2 (–41.4, 7.4) | |||
| With vaccine-relateda AEs | 8 | (18.2) | 5 | (12.2) | 2 | (5.0) | 12 | (27.3) | 2 | (9.1) | 2 | (9.5) | 1 | (4.8) | 2 | (9.1) | ||||
| With serious AEs | 1b | (2.3) | 0 | (0.0) | 0 | (0.0) | 1 | (2.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| With serious vaccine-related AEs | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Who died | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Discontinuedc owing to an AE | 0 | (0.0) | 1 | (2.4) | 0 | (0.0) | 1 | (2.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Discontinued owing to a vaccine-related AE | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Discontinued owing to a serious AE | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Discontinued owing to a serious vaccine-related AE | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||||
| Most commonly reported AEs (incidence ≥ 4 subjects in ≥ 1 group) | ||||||||||||||||||||
| Gastrointestinal disorders | 10 | (22.7) | 6 | (14.6) | 2 | (5.0) | 15 | (34.1) | 3 | (13.6) | 1 | (4.8) | 1 | (4.8) | 4 | (18.2) | 15.9 (–8.1, 35.4) | |||
| Diarrhea | 6 | (13.6) | 6 | (14.6) | 1 | (2.5) | 11 | (25.0) | 2 | (9.1) | 1 | (4.8) | 0 | (0.0) | 2 | (9.1) | 15.9 (–5.6, 32.8) | |||
| Nausea | 4 | (9.1) | 1 | (2.4) | 0 | (0.0) | 5 | (11.4) | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) | 1 | (4.5) | 6.8 (–11.7, 20.6) | |||
| General disorders and administration site conditions | 5 | (11.4) | 3 | (7.3) | 1 | (2.5) | 7 | (15.9) | 0 | (0.0) | 2 | (9.5) | 0 | (0.0) | 2 | (9.1) | 6.8 (–13.8, 22.6) | |||
| Fatigue | 5 | (11.4) | 3 | (7.3) | 1 | (2.5) | 7 | (15.9) | 0 | (0.0) | 2 | (9.5) | 0 | (0.0) | 2 | (9.1) | 6.8 (–13.8, 22.6) | |||
| Infections and infestations | 1 | (2.3) | 1 | (2.4) | 2 | (5.0) | 4 | (9.1) | 1 | (4.5) | 2 | (9.5) | 0 | (0.0) | 2 | (9.1) | 0.0 (–19.9, 14.3) | |||
| Injury, poisoning, and procedural complications | 1 | (2.3) | 2 | (4.9) | 1 | (2.5) | 4 | (9.1) | 0 | (0.0) | 1 | (4.8) | 1 | (4.8) | 2 | (9.1) | 0.0 (–19.9, 14.3) | |||
| Musculoskeletal and connective tissue disorders | 5 | (11.4) | 3 | (7.3) | 0 | (0.0) | 7 | (15.9) | 2 | (9.1) | 1 | (4.8) | 0 | (0.0) | 2 | (9.1) | 6.8 (–13.8, 22.6) | |||
| Myalgia | 4 | (9.1) | 1 | (2.4) | 0 | (0.0) | 5 | (11.4) | 1 | (4.5) | 1 | (4.8) | 0 | (0.0) | 1 | (4.5) | 6.8 (–11.7, 20.6) | |||
| Nervous system disorders | 8 | (18.2) | 4 | (9.8) | 1 | (2.5) | 9 | (20.5) | 4 | (18.2) | 2 | (9.5) | 1 | (4.8) | 4 | (18.2) | 2.3 (–20.6, 20.8) | |||
| Headache | 7 | (15.9) | 3 | (7.3) | 1 | (2.5) | 7 | (15.9) | 4 | (18.2) | 2 | (9.5) | 0 | (0.0) | 4 | (18.2) | –2.3 (–24.7, 15.6) | |||
a Determined by the primary investigator to be related to the vaccine; bCerebrovascular accident; cStudy medication withdrawn; dBased on Miettinen and Nurminen method17; AE, adverse event; CI, confidence interval; RV5, pentavalent rotavirus vaccine, RotaTeq®.
No deaths, vaccine-related serious AEs (SAEs), or vaccine-related grade 4 AEs were reported in this study. Two subjects who received RV5 reported nonfatal SAEs including 1 subject with a cerebrovascular accident on day 33 postdose 1 (Table 2) and another subject with coronary artery disease on day 169 postdose 3, neither of which was judged to be vaccine-related by the primary investigator. Both subjects completed the study regimen and 180-d follow-up period postdose 3. As noted earlier, one subject who received RV5 discontinued further study vaccination after experiencing AEs of severe intensity (GE, rectal hemorrhage, diarrhea, nausea, vomiting) 13 d postdose 2. Treatment included loperamide and all AEs resolved within 2 to 4 d without hospitalization. This subject was 70 y old and reported a prior medical history of gastroesophogeal reflex disease (GERD) and rectal hemorrhoids. The AEs were judged by the primary investigator not to be vaccine-related. In the study overall, vaccine-related AEs were of mild or moderate intensity.
To evaluate potential fecal shedding of vaccine virus, investigators collected more than 900 routine stool samples from the 66 subjects. In the RV5 group, 4 subjects had 7 stool samples that were RV-positive (3 subjects postdose 1, 2 subjects postdose 2, and 1 subjects postdose 3) as determined by RV enzyme immunoassay (EIA) compared with 0 subjects in the placebo group. Of note, no RV EIA-positive stool samples were confirmed to have vaccine-strain rotavirus as determined by VP6 genotyping by reverse transcription polymerase chain reaction (RT-PCR). A total of 12 stool samples were collected as potential AGEs from 9 subjects within 15 d after any vaccination. None of these samples tested positive for either wild-type or vaccine-strain rotavirus by EIA and the rotavirus VP6 RT-PCR assay or for norovirus by RT-PCR. Three of the potential AGE samples from 2 subjects were positive for Clostridium difficile.
Results of immunogenicity assays indicated that levels of preexisting antibodies to rotavirus were generally similar between the RV5 and placebo groups with serum antibody titers for anti-rotavirus immunoglobulin A (IgA) and serum neutralizing antibody (SNA) to rotavirus G1, G3, and P1A found to be numerically higher in the RV5 group (Table 3). Following vaccination, the serum anti-rotavirus IgA responses in the RV5 group were numerically higher than those in the placebo group at all postdose time periods. In relationship to baseline titers, the geometric mean fold rise (GMFR) and the percent of subjects achieving a ≥ 3-fold rise in serum anti-rotavirus IgA titer were numerically higher in the RV5 group compared with those in the placebo group. In the RV5 and placebo groups, the IgA post-dose 3 GMFRs were 1.9 and 1.0, respectively, and approximately 27% of the RV5 group achieved a ≥ 3-fold rise in IgA titer compared with 0% of the placebo group (Table 4). The post-vaccination geometric mean titers (GMTs) in SNA to human rotavirus serotypes contained in the vaccine (G1, G2, G3, G4, and P1A[8]) were also numerically higher in the RV5 group for each of the 5 vaccine serotypes compared with those in the placebo group (Table 3). The dose responses based on IgA and SNA titers showed minimal further augmentation of immunological responses with doses 2 and 3 over the responses seen after dose 1.
Table 3. Serum neutralizing antibody (SNA) response to rotavirus serotypes in the per-protocol population.
| RV5 | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | P1A | G1 | G2 | G3 | G4 | P1A | |
| Prevaccination | ||||||||||
| Number of subjectsa | 36 | 19 | ||||||||
| GMT, dilution units (95% CI) |
314.5 (219.2, 451.3) |
62.8 (43.6, 90.5) |
141.8 (95.7, 210.1) |
91.6 (66.1, 126.8) |
230.8 (166.8, 319.4) |
281.4 (152.6, 519.1) |
68.2 (40.6, 114.6) |
90.9 (62.1, 133.0) |
102.7 (65.7, 160.4) |
143.4 (88.9, 231.3) |
| Postdose 1 | ||||||||||
| Number of subjectsb | 28 | 16 | ||||||||
| GMT, dilution units (95% CI) |
506.2 (330.5, 775.4) |
123.4 (81.4, 187.2) |
404.6 (230.6, 710.0) |
199.2 (128.5, 308.6) |
306.4 (219.8, 427.0) |
228.7 (121.0, 432.4) |
56.3 (34.5, 92.0) |
94.5 (59.4, 150.4) |
93.1 (55.4, 156.2) |
126.1 (78.7, 202.0) |
| GMFR, dilution units (95% CI) | 2.0 (1.3, 3.0) | 1.8 (1.2, 2.7) | 2.8 (1.7, 4.9) | 2.2 (1.4, 3.4) | 1.4 (1.0, 1.8) | 1.0 (0.8, 1.1) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.2) |
| Subjects with ≥ 3-fold rise, n (%) | 7 (26.9) | 6 (23.1) | 11 (42.3) | 8 (30.8) | 4 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Postdose 2 | ||||||||||
| Number of subjectsb | 37 | 20 | ||||||||
| GMT, dilution units (95% CI) |
556.1 (389.8, 793.5) |
111.2 (81.6, 151.6) |
330.7 (219.5, 498.3) |
223.9 (154.2, 325.3) |
375.6 (278.4, 506.6) |
226.7 (136.8, 375.6) |
74.3 (46.2, 119.5) |
119.8 (79.6, 180.2) |
110.1 (68.4, 177.5) |
154.0 (98.0, 241.9) |
| GMFR, dilution units (95% CI) | 1.9 (1.3, 2.6) | 1.9 (1.4, 2.6) | 2.3 (1.5, 3.5) | 2.6 (1.8, 3.8) | 1.7 (1.3, 2.2) | 0.8 (0.6, 1.1) | 1.1 (0.8, 1.5) | 1.3 (1.0, 1.5) | 1.1 (0.9, 1.5) | 1.1 (1.0, 1.2) |
| Subjects with ≥ 3-fold rise, n %) | 9 (26.5) | 8 (23.5) | 12 (35.3) | 13 (38.2) | 9 (26.5) | 1 (5.0) | 1 (5.0) | 1 (5.0) | 1 (5.0) | 0 (0.0) |
| Postdose 3 | ||||||||||
| Number of subjectsb | 38 | 18 | ||||||||
| GMT, dilution units (95% CI) |
535.9 (395.9, 725.4) |
127.0 (93.1, 173.3) |
388.0 (264.7, 568.7) | 221.7 (162.9, 301.7) |
327.6 (253.8, 423.0) |
231.6 (140.3, 382.3) | 71.3 (44.5, 114.3) |
102.1 (66.3, 157.3) |
106.9 (64.2, 178.0) |
160.7 (102.3, 252.3) |
| GMFR, dilution units (95% CI) | 1.9 (1.4, 2.6) | 2.1 (1.5, 2.9) | 2.7 (1.7, 4.2) | 2.6 (1.8, 3.6) | 1.5 (1.2, 2.0) | 0.9 (0.7, 1.2) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.4) |
| Subjects with ≥ 3-fold rise, n (95% CI) | 9 (27.3) | 8 (24.2) | 13 (39.4) | 11 (33.3) | 6 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
a Excludes protocol violators and subjects with invalid data based on laboratory determinations; bExcludes protocol violators, subjects with invalid data based on laboratory determinations, subjects with rotavirus-positive antigen enzyme immunoassay results in stool samples, or subjects with samples taken out of specified day range; CI, confidence interval; GMT, Geometric Mean Titer; RV, rotavirus; RV5, pentavalent rotavirus vaccine, RotaTeq®.
Table 4. Serum anti-rotavirus immunoglobulin A (IgA) response in the per-protocol population.
| RV5 | Placebo | |
|---|---|---|
| Geometric Mean Titer (GMT) | ||
| Prevaccination | ||
| Number of subjectsa | 36 | 19 |
| GMT, dilution units (95% CI) | 679.6 (464.5, 994.2) | 578.4 (361.1, 926.4) |
| Postdose 1 | ||
| Number of subjectsb | 28 | 16 |
| GMT, dilution units (95% CI) | 1026.8 (621.6, 1696.2) | 552.8 (314.5, 971.8) |
| Postdose 2 | ||
| Number of subjectsb | 37 | 20 |
| GMT, dilution units (95% CI) | 1187.0 (807.6, 1744.5) | 657.7 (404.9, 1068.5) |
| Postdose 3 | ||
| Number of subjectsb | 38 | 18 |
| GMT, dilution units (95% CI) | 1179.9 (785.5, 1772.2) | 578.5 (349.0, 959.0) |
| Geometric Mean Fold Rise (GMFR) | ||
| Postdose 1 | ||
| Number of subjectsb | 26 | 16 |
| GMFR, dilution units (95% CI) | 1.8 (1.3, 2.6) | 1.0 (0.9, 1.1) |
| Subjects with ≥ 3-fold rise, n (%) | 7 (26.9) | 0 (0.0) |
| Postdose 2 | ||
| Number of subjectsb | 34 | 20 |
| GMFR, dilution units (95% CI) | 1.7 (1.3, 2.2) | 1.0 (0.9, 1.1) |
| Subjects with ≥ 3-fold rise, n (%) | 6 (17.6) | 0 (0.0) |
| Postdose 3 | ||
| Number of subjectsb | 33 | 18 |
| GMFR, dilution units (95% CI) | 1.9 (1.5, 2.4) | 1.0 (0.9, 1.1) |
| Subjects with ≥ 3-fold rise, n (%) | 9 (27.3) | 0 (0.0) |
a Excludes protocol violators and subjects with invalid data based on laboratory determinations;bExcludes protocol violators, subjects with invalid data based on laboratory determinations, subjects with rotavirus-positive antigen enzyme immunoassay results in stool samples, or subjects with samples taken out of specified day range;CI, confidence interval; RV5, pentavalent rotavirus vaccine, RotaTeq®.
Discussion
This is the first study to evaluate the safety and immunogenicity of RV5 in the elderly. The rationale for administering 3 doses of RV5 was based on the approved regimen for use in infants and children.16 No clear immune correlate of vaccine-induced protection against rotavirus disease has been determined. At the time of study initiation, data were not available on the baseline anti-rotavirus antibody titers in adults of this age group. Moreover, the impact of 1, 2, or 3 doses of RV5 on anti-rotavirus antibody titers among elderly subjects was not known.
Our data demonstrate that RV5 was generally safe and well tolerated in this adult population, aged 65 to 80 y living independently outside of long-term care facilities. This was determined based on clinical AEs within 42 d after any dose and 180 d after the final dose of RV5. AEs judged to be vaccine-related AEs were detected more frequently in RV5 than in placebo recipients and were of mild or moderate intensity. There were no serious vaccine-related AEs.
This study also examined serial stool samples and found no confirmed cases of fecal vaccine-virus shedding among the recipients of RV5 in this elderly population, based on initial testing (screening) by EIA and confirmatory testing of EIA-positive samples by VP6 RT-PCR. In comparison, in a large study in infants, the Rotavirus Efficacy and Safety Trial (REST), fecal vaccine-virus shedding in infants who received RV5 was observed in 8.9% (95% CI: 6.2%, 12.3%) postdose 1, 0.0% (95% CI: 0.0%, 1.5%) postdose 2, and 0.3% (95% CI: < 0.1%, 1.4%) postdose 3.19 Testing methods for rotavirus have evolved over time and were not identical in these studies.
In contrast to infants, all subjects in this study had relatively high levels of pre-existing rotavirus antibodies, which is consistent with prior exposure to rotavirus infection in these adults. The vaccine still appeared immunogenic in this population as assessed by pre-designated endpoints {postdose GMTs, GMFRs, and the percent of subjects who achieved a ≥ 3-fold rise in SNA titer response to human rotavirus serotypes contained in the vaccine (G1, G2, G3, G4, and P1A[8])}. These antibody responses were numerically higher in the RV5 group compared with the placebo group and there was an approximately a 2-fold rise or greater over baseline (GMFR) both in the SNA responses (except a GMFR of approximately 1.5 to P1A) and in the IgA responses in the RV5 vaccinees (Table 3). This immunogenicity profile differs from that seen in healthy infants (ie, in REST) where baseline titers were typically low or absent and a higher proportion of seroconversion in SNA was seen to G1, G4, and P1A, as compared with G2 or G3. In this study of RV5 in the elderly, boosted immune responses were observed postdose 1 with little further incremental change after dose 2 or dose 3. In the absence of a clear immune correlate of protection for rotavirus disease, understanding the clinical significance of the observed increase in rotavirus antibody titers in elderly adults following vaccination with RV5 would require efficacy trials.
This study contributes to the current field of knowledge by providing relevant initial data on the safety and immunogenicity of RV5 in the elderly. A limitation of our phase 1 study is the small sample size that provided only descriptive data. Because the study was small and had limited power to detect risk differences, further studies with a larger number of subjects will be needed to fully assess the safety of the vaccine in this population.
Overall, these data from this phase 1 study support the safety of RV5 in elderly subjects aged 65–80 y. Immune responses to rotavirus were augmented in this population after a single dose of RV5, despite the subjects’ older age and the preexisting antibodies to rotavirus. If additional studies support a need for vaccination in the elderly, further evaluation of RV5 as a candidate vaccine in the elderly may be warranted.
Methods
Participants and study design
This was a randomized, double-blind (in-house blind), placebo-controlled, and multicenter trial. The initial stage, phase 1, was completed and was conducted at 8 sites distributed throughout the United States to evaluate the safety and immunogenicity of RV5 in healthy, independently living elderly subjects. The study was conducted from February 3, 2009 to January 12, 2010.
Healthy subjects, aged 65–80 y, who were cognitively competent and independently living outside of a long-term care facility, were eligible to participate in the study with written informed consent. Additional inclusion criteria included laboratory values (hematology and chemistry) within normal limits and negative test results for human immunodeficiency virus, hepatitis B surface antigen, and hepatitis C virus. Cognitive status was determined by the Mini-Mental State Examination questionnaire.20 Subjects were excluded if they had a history of gastrointestinal cancer or surgery; known or suspected immunodeficiency; chronic diarrhea, irritable bowel syndrome, diverticulitis, colitis, or other gastrointestinal reports of unknown cause; fever with an oral temperature ≥ 38.0 °C or clinical evidence of AGE at the time of immunization (of note, dosing was rescheduled after symptoms resolved); or spouse or co-habitants who were immunocompromised or not healthy. Further restrictions during the study period included corticosteroid use, receipt of blood products or other virus vaccines, and participation in other clinical studies. Influenza vaccine and PNEUMOVAX 23® were allowed to be administered concomitantly with the study vaccine. Antacids, proton pump inhibitors, or drugs affecting gut motility were allowed provided the subject’s medical condition requiring such treatment was stable according to the opinion of the study investigator.
The trial was conducted in accordance with the principles of the Declaration of Helsinki and in conformance with Good Clinical Practice standards and applicable national and/or local statutes and regulations regarding ethical committee review, informed consent, and the protection of human subjects participating in biomedical research. The trial was approved by the Western Institutional Review Board (Olympia, WA).
Procedures
Subjects were randomized 2:1 to receive three 2-mL oral doses of RV5 or placebo administered 28–42 d apart. Subjects were considered to have completed the study when they had received 3 doses of RV5 or placebo by study personnel, given blood samples approximately 28–42 d after the last dose, and completed 180 d of safety follow-up after the last dose.
Safety
At each vaccination visit, subjects were given a Vaccination Report Card (VRC) with instructions to record AEs during the 42-d safety follow-up period following each vaccination. The VRC required subjects to record daily oral temperature for 7 d beginning in the evening following each vaccination, as well as episodes of nausea, vomiting, diarrhea, headache, fatigue, myalgia, hematochezia, or potential AGE. All subjects were followed for non-serious AEs (NSAEs) and SAEs in person or by telephone on days 7, 14, 28, and 42 following any vaccination. Subjects were also followed using passive surveillance for SAEs, including vaccine-related SAEs and deaths, from 43 to 180 d following the final vaccination. All AEs were graded based on the US Food and Drug Administration Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials21 in addition to protocol-specified intensity grading as mild, moderate, and severe. Laboratory results were provided to sites via a central laboratory for subjects’ safety monitoring throughout the entire study. Blinded safety data were reviewed at regular intervals during the study by the medical monitoring team.
Routine stool samples were collected from all subjects on day 1 (baseline) and on 5 additional occasions after each vaccination (days 3, 5, 7, 10, and 14) to monitor for possible fecal shedding of vaccine-virus by enzyme EIA rotavirus detection assay and confirmatory rotavirus VP6 RT-PCR. Additionally, whenever there was a potential AGE during the safety follow-up period, a fecal sample was to be collected within 7 and preferably within 3 d of the onset of symptoms and evaluated for rotavirus, Clostridium difficile, and norovirus as described below. Blood and urine samples for safety laboratory tests were collected at the prestudy (screening) visit, which occurred approximately 30–60 d prior to randomization. Safety blood and urine testing was repeated at predose 2, predose 3, and 5 d after each dose to monitor for laboratory AEs.
To screen for rotavirus, all stool samples, routine or from potential AGE episodes, were analyzed using a rotavirus antigen detection EIA.15 A VP6 reverse transcription-polymerase chain reaction (RT-PCR) assay was used as the confirmatory method on EIA-positive stool samples to identify presence of wildtype-virus or vaccine-virus strains.22-24 In addition to the above assays, all AGE stool samples were assayed for Clostridium difficile toxin A and toxin B using a commercially available EIA (Meridian Biosciences, Inc., Cincinnati, OH) according to the manufacturer’s instructions, and assayed for norovirus by RT-PCR as pre-specified in the protocol and previously reported.25
Immunogenicity
For all subjects, 15-mL serum samples were collected on a total of 4 occasions for immunogenicity testing: predose 1 and approximately 28–42 d after dose 1, dose 2, and dose 3, respectively.
Immunogenicity of RV5 was measured by serum anti-rotavirus immunoglobulin A (IgA) EIA and SNA responses to human rotavirus serotypes G1, G2, G3, G4, and P1A[8] prior to dose 1 (baseline) and after each subsequent dose of RV5 or placebo as described elsewhere.15,26-28
Study outcomes
A primary objective of the study was to evaluate the safety of RV5 in healthy adult subjects 65–80 y of age, who were cognitively competent and independently living outside a long-term care facility, with respect to all NSAEs up to 42 d after any dose, and SAEs up to 180 d after the third dose of RV5 or placebo. Analysis of immunogenicity of RV5 after 1, 2, or 3 doses of RV5 or placebo was a co-primary objective of the study. A secondary objective of the study was to summarize fecal vaccine-virus shedding in this population.
Statistical analysis
Safety
A total of 60 subjects (40:20) was planned to be included in the study (part 1). If no SAEs were observed among the 40 subjects in the RV5 group, the study would provide 97.5% confidence that the true SAE rate is < 8.81%. All subjects who received at least 1 dose of RV5 or placebo and had follow-up data were included in safety analyses. AEs of special interest such as fever (≥ 38.0 °C oral or axillary), nausea, vomiting, diarrhea, headache, fatigue, myalgia, or AGE were summarized by treatment group, dose, and across all doses. Risk differences associated with exact 95% CIs and P values for comparisons were determined for AEs of special interest. In addition to AEs of special interest, all those reported in ≥ 4 subjects in any treatment group were evaluated for risk differences associated with exact 95% CIs for comparisons by treatment group and dose. All other AEs, including NSAEs and SAEs, were summarized as frequencies and percentages by treatment group, dose, and type of AE. Any positive fecal shedding of vaccine-virus strains was to be summarized as the number and percent of subjects with fecal vaccine virus shedding by treatment group and sampling day. Of note, CIs and P values on risk differences were calculated using the method proposed by Miettinen and Nurminen.18 No multiplicity adjustments were used for safety analysis.
Immunogenicity
No hypothesis for immunogenicity was tested in this study, the data were only summarized. The primary immunogenicity analyses and summaries of endpoints were based on a per-protocol population, defined as subjects who received at least 1 dose of RV5 or placebo and had at least 1 valid assay result within the study specified time window and were not protocol violators. Additional supportive analysis was performed based on the full analysis set (FAS), defined as subjects who received at least 1 dose of RV5 or placebo and had at least 1 valid assay result without regard to time window and/or protocol violation. Supportive analyses performed on the FAS included GMTs of SNA response to serotypes G1, G2, G3, G4, and P1A[8]; GMTs of serum anti-rotavirus IgA after 1, 2, or 3 doses of RV5 or placebo; and percent of subjects with a ≥ 3-fold increase from baseline of serum anti-rotavirus IgA and SNA responses to serotypes G1, G2, G3, G4, and P1A[8] after 1, 2, or 3 doses of RV5 or placebo.
Glossary
Abbreviations:
- AGE
acute gastroenteritis
- AE
adverse event
- CI
confidence interval
- EIA
enzyme immunoassay
- FAS
full analysis set
- GE
gastroenteritis
- GMFR
geometric mean fold rise
- GMTs
geometric mean titers
- IgA
immunoglobulin A
- NSAEs
non-serious adverse event
- REST
Rotavirus Efficacy and Safety Trial
- RT-PCR
reverse transcription polymerase chain reaction
- RV5
pentavalent rotavirus vaccine, RotaTeq®
- SAE
serious adverse event
- SD
standard deviation
- SNA
serum neutralizing antibody, VRC, Vaccination Report Card
Conflict of Interest
S.H., and J.M. are employees of Merck Sharp and Dohme Corp, a subsidiary of Merck and Co., Inc., who may potentially own stock and/or hold stock options in the company.
J.L., F.S., and M.C. were employees of Merck Sharp and Dohme Corp, a subsidiary of Merck and Co., Inc., at the time this study was conducted and may potentially own stock and/or hold stock options in the company.
A.M. received research support from Merck.
Role of Funding Source
The study was designed by Merck & Co., Inc., which had direct oversight or participation in every stage of the study. All authors had full access to the data after study completion and unblinding and are responsible for the work described in this paper. All authors were involved in at least 1 of the following: conception, design, acquisition, analysis, statistical analysis, interpretation of data, and drafting the manuscript and/or revising the manuscript for important intellectual content. All authors provided final approval of the version to be published.
Trial Registration
The clinical trial registry number for this study (Merck protocol number V260–027) is NCT00836498, available at: http://clinicaltrials.gov/ct2/show/NCT00836498.
Acknowledgments
The authors thank all of the individuals who participated in this study in addition to the principle investigators from each site: Charles P. Andrews, MD; Sabrina A Benjamin, MD; Allen L Chodock, MD; Lucita Cruz, MD; Steven Folkerth, MD; Thomas W Littlejohn III, MD; John W McGettigan Jr, MD; and Alexander V Murray, MD. We would also like to thank Guojun Yuan, PhD and Jin Xu, PhD for statistical analysis and support.
The authors thank Tonya Goodman, Arbor Communications, Inc., Ann Arbor, MI, for manuscript preparation and editorial assistance on behalf of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References
- 1.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. . Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/ 10.1086/605025; PMID: 19817620 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. . Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec 2013; 88:49 - 64; PMID: 23424730 23424730 [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). . Notes from the field: outbreaks of rotavirus gastroenteritis among elderly adults in two retirement communities–Illinois, 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1456; PMID: 22031219 [PubMed] [Google Scholar]
- 4.Edmonson LM, Ebbert JO, Evans JM. . Report of a rotavirus outbreak in an adult nursing home population. J Am Med Dir Assoc 2000; 1:175 - 9; PMID: 12816557 [PubMed] [Google Scholar]
- 5.Anderson EJ, Weber SG. . Rotavirus infection in adults. Lancet Infect Dis 2004; 4:91 - 9; http://dx.doi.org/ 10.1016/S1473-3099(04)00928-4; PMID: 14871633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrie TJ, Lee SHS, Faulkner RS, Ethier J, Young CH. . Rotavirus infection in a geriatric population. Arch Intern Med 1982; 142:313 - 6; http://dx.doi.org/ 10.1001/archinte.1982.00340150113020; PMID: 6277261 [DOI] [PubMed] [Google Scholar]
- 7.Cardemil CV, Cortese MM, Medina-Marino A, Jasuja S, Desai R, Leung J, Rodriguez-Hart C, Villarruel G, Howland J, Quaye O, et al. , Rotavirus Investigation Team. . Two rotavirus outbreaks caused by genotype G2P[4] at large retirement communities: cohort studies. Ann Intern Med 2012; 157:621 - 31; http://dx.doi.org/ 10.7326/0003-4819-157-9-201211060-00006; PMID: 23128862 [DOI] [PubMed] [Google Scholar]
- 8.Lopman BA, Curns AT, Yen C, Parashar UD. . Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980 - 6; http://dx.doi.org/ 10.1093/infdis/jir492; PMID: 21878425 [DOI] [PubMed] [Google Scholar]
- 9.Gangarosa RE, Glass RI, Lew JF, Boring JR. . Hospitalizations involving gastroenteritis in the United States, 1985: the special burden of the disease among the elderly. Am J Epidemiol 1992; 135:281 - 90; PMID: 1546704 [DOI] [PubMed] [Google Scholar]
- 10.Cubitt WD, Holzel H. . An outbreak of rotavirus infection in a long-stay ward of a geriatric hospital. J Clin Pathol 1980; 33:306 - 8; http://dx.doi.org/ 10.1136/jcp.33.3.306; PMID: 6247370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis P, Beby A, Bourgoin A, Lussier-Bonneau MD, Agius G. . [Epidemic of viral gastroenteritis in an elderly community]. Presse Med 1995; 24:356 - 8; PMID: 7899406 [PubMed] [Google Scholar]
- 12.Abbas AM, Denton MD. . An outbreak of rotavirus infection in a geriatric hospital. J Hosp Infect 1987; 9:76 - 80; http://dx.doi.org/ 10.1016/0195-6701(87)90099-5; PMID: 2880904 [DOI] [PubMed] [Google Scholar]
- 13.Lewis DC, Lightfoot NF, Cubitt WD, Wilson SA. . Outbreaks of astrovirus type 1 and rotavirus gastroenteritis in a geriatric in-patient population. J Hosp Infect 1989; 14:9 - 14; http://dx.doi.org/ 10.1016/0195-6701(89)90128-X; PMID: 2570110 [DOI] [PubMed] [Google Scholar]
- 14.Halvorsrud J, Örstavik I. . An epidemic of rotavirus-associated gastroenteritis in a nursing home for the elderly. Scand J Infect Dis 1980; 12:161 - 4; PMID: 6254138 [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. , Rotavirus Efficacy and Safety Trial (REST) Study Team. . Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/ 10.1056/NEJMoa052664; PMID: 16394299 [DOI] [PubMed] [Google Scholar]
- 16.Ciarlet M, Schödel F. . Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine 2009; 27:Suppl 6 G72 - 81; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.107; PMID: 20006144 [DOI] [PubMed] [Google Scholar]
- 17.Matthijnssens J, Joelsson DB, Warakomski DJ, Zhou T, Mathis PK, van Maanen MH, Ranheim TS, Ciarlet M. . Molecular and biological characterization of the 5 human-bovine rotavirus (WC3)-based reassortant strains of the pentavalent rotavirus vaccine, RotaTeq. Virology 2010; 403:111 - 27; http://dx.doi.org/ 10.1016/j.virol.2010.04.004; PMID: 20451234 [DOI] [PubMed] [Google Scholar]
- 18.Miettinen O, Nurminen M. . Comparative analysis of two rates. Stat Med 1985; 4:213 - 26; http://dx.doi.org/ 10.1002/sim.4780040211; PMID: 4023479 [DOI] [PubMed] [Google Scholar]
- 19.RotaTeq [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2012. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. . “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189 - 98; http://dx.doi.org/ 10.1016/0022-3956(75)90026-6; PMID: 1202204 [DOI] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Food and Drug Administration Web site.http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm. Accessed April 08, 2013.
- 22.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606 - 14; http://dx.doi.org/ 10.1016/S0140-6736(10)60889-6; PMID: 20692030 [DOI] [PubMed] [Google Scholar]
- 23.DiStefano DJ, Kraiouchkine N, Mallette L, Maliga M, Kulnis G, Keller PM, Clark HF, Shaw AR. . Novel rotavirus VP7 typing assay using a one-step reverse transcriptase PCR protocol and product sequencing and utility of the assay for epidemiological studies and strain characterization, including serotype subgroup analysis. J Clin Microbiol 2005; 43:5876 - 80; http://dx.doi.org/ 10.1128/JCM.43.12.5876-5880.2005; PMID: 16333070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/ 10.1016/S0140-6736(10)60755-6; PMID: 20692031 [DOI] [PubMed] [Google Scholar]
- 25.Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. . Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods 1999; 83:145 - 54; http://dx.doi.org/ 10.1016/S0166-0934(99)00114-7; PMID: 10598092 [DOI] [PubMed] [Google Scholar]
- 26.Ciarlet M, Sani-Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, Arredondo JL, Schödel F. . Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J 2008; 27:874 - 80; http://dx.doi.org/ 10.1097/INF.0b013e3181782780; PMID: 18756184 [DOI] [PubMed] [Google Scholar]
- 27.Ward RL, Knowlton DR, Zito ET, Davidson BL, Rappaport R, Mack ME, US Rotavirus Vaccine Efficacy Group. . Serologic correlates of immunity in a tetravalent reassortant rotavirus vaccine trial. J Infect Dis 1997; 176:570 - 7; http://dx.doi.org/ 10.1086/514076; PMID: 9291301 [DOI] [PubMed] [Google Scholar]
- 28.Knowlton DR, Spector DM, Ward RL. . Development of an improved method for measuring neutralizing antibody to rotavirus. J Virol Methods 1991; 33:127 - 34; http://dx.doi.org/ 10.1016/0166-0934(91)90013-P; PMID: 1658027 [DOI] [PubMed] [Google Scholar]


