Abstract
Objective
This study aims to assess the cost-effectiveness of hepatitis A immunization in Indonesia, including an explicit comparison between one-dose and two-dose vaccines.
Methods
An age-structured cohort model based on a decision tree was developed for the 2012 Indonesia birth cohort. Using the model, we made a comparison on the use of two-dose and one-dose vaccines. The model involved a 70-year time horizon with 1-month cycles for children less than 2 years old and annually thereafter. Monte Carlo simulations were used to examine the economic acceptability and affordability of the hepatitis A vaccination.
Results
Vaccination would save US$ 3 795 148 and US$ 2 892 920 from the societal perspective, for the two-dose and one-dose vaccine schedules, respectively, in the context of hepatitis A treatment. It also would save 8917 and 6614 discounted quality-adjusted-life-years (QALYs), respectively. With the vaccine price of US$ 3.21 per dose, the implementation of single dose vaccine would yield an incremental cost-effectiveness ratio (ICER) of US$ 4933 per QALY gained versus no vaccination, whereas the two-dose versus one-dose schedule would cost US$ 14 568 per QALY gained. Considering the 2012 gross-domestic-product (GDP) per capita in Indonesia of US$ 3557, the results indicate that hepatitis A vaccination would be a cost-effective intervention, both for the two-dose and one-dose vaccine schedules in isolation, but two-dose vaccination would no longer be cost-effective if one-dose vaccination is a feasible option. Vaccination would be 100% affordable at budgets of US$ 71 408 000 and US$ 37 690 000 for the implementation of the two-dose and one-dose vaccine schedules, respectively.
Conclusions
The implementation of hepatitis A vaccination in Indonesia would be a cost-effective health intervention under the market vaccine price. Given the budget limitations, the use of a one-dose-vaccine schedule would be more realistic to be applied than a two-dose schedule. The vaccine price, mortality rate and discount rate were the most influential parameters impacting the ICERs.
Keywords: hepatitis A, cost-effectiveness, vaccine, immunization, Indonesia
Introduction
Approximately 1.4 million cases of hepatitis A virus (HAV) infection occur annually worldwide and almost half of those cases are reported in Asia.1 HAV is primarily transmitted from person to person by the fecal-oral route and the ingestion of contaminated foods or drinks.2 As the World Gastroenterology Organization (WGO) reported that poor hygiene and poor sanitation pose the greatest risk related to HAV infection,3 the incidence rate of HAV infection in a country is inversely related to its wealth.2 In Asia, the endemicity levels of HAV infection vary considerably between countries.4 Several countries still have a high endemicity level (e.g., India, Bangladesh, and Pakistan), other countries are intermediate in level (e.g., Uzbekistan, Kazakhstan, and Azerbaijan) or low (e.g., Indonesia, China, and Thailand).5 Additionally, 3 high-income countries in Asia (Japan, South Korea, and Singapore) are classified into the very low endemicity level.5
Despite the relatively low endemicity of HAV infection in Indonesia, a substantial proportion of adolescents and adults may be susceptible to infection due to social developments, such as globalization, migration, and travel patterns.6 In particular, as a middle-income country with continuously improving sanitation, it has been reported that fewer children in Indonesia are infected by HAV in early childhood than earlier.7 Yet, this condition paradoxically may lead to a higher disease incidence, since HAV disease primarily manifests itself in older age groups. In the context of hepatitis A prevention, it has been emphasized that the most effective way is through vaccination, which has been implemented in several countries and has reduced hepatitis A cases significantly.8 Also in Indonesia, where transmission occurs primarily from person to person in the general community and hepatitis A outbreaks periodically happen, control of hepatitis A may be achieved through a widespread vaccination program.
Until now, an economic evaluation on hepatitis A vaccination has not yet been conducted in Indonesia. It is important to know whether potential favorable cost-effectiveness may exist within the context of the Indonesian government perspective to justify full inclusion of the hepatitis A vaccine into the national immunization program (NIP). The objective of this study is to assess the cost-effectiveness of hepatitis A vaccination in Indonesia, including an explicit comparison between the one-dose and two-dose vaccine schedules.
Results
Baseline analyses
Assuming a vaccine coverage of 80% and vaccine efficacies of 93% (first dose) and 95% (second dose), vaccination of 4 200 000 infants9 would reduce HAV infection by 452 834 and 322 207 cases when using the two-dose and one-dose vaccine schedules, respectively. In particular, the two-dose vaccine schedule would reduce hepatitis A cases by 247 694 (65.0%), 148 670 (65.0%), 56 064 (68.7%), and 406 (59.8%) for mild, moderate, severe, and fatal cases, respectively. The one-dose vaccine schedule would reduce hepatitis A cases by 174 157 (45.7%), 104 579 (45.7%), 43 224 (53.0%), and 247 (36.3%) for mild, moderate, severe, and fatal cases, respectively. Hepatitis A vaccination would save 8917 and 6614 discounted quality-adjusted-life years (QALYs) for the two-dose and one-dose vaccine schedules, respectively. Furthermore, it also would save US$ 3 795 148 and US$ 2 892 920 from the societal perspective for both schedules, respectively, in the context of hepatitis A treatment (Table 1A). The cost-effectiveness values from all perspectives are shown in Table 1B. With a vaccine price of US$ 3.21 per dose, the implementation of hepatitis A vaccine from the healthcare perspective would yield incremental cost-effectiveness ratios (ICERs) at US$ 7510 and US$ 5025 per QALY gained for the two-dose and one-dose vaccine schedules, respectively. From the societal perspective, it would yield ICERs at US$ 7421 and US$ 4933 per QALY gained for both schedules. Considering the 2012 gross-domestic-product (GDP) per capita in Indonesia of US$ 3557,10 the results confirmed that hepatitis A vaccination using the two-dose and one-dose vaccine schedules would be cost-effective interventions since the ICERs were between 1 and 3 times GDP per capita.11 Additionally, the ICERs of the two-dose over the one-dose schedule were US$ 14 648 and US$ 14 568 per QALY gained from the healthcare and societal perspectives, respectively.
Table 1A. Results from both vaccination strategies.
| Vaccine | Without vaccination |
With vaccination |
Difference |
|---|---|---|---|
| Two-dose vaccine schedule Number of cases a Mild Moderate Severe Death Cost of illness Healthcare perspective b ,c Societal perspective b ,c Cost of vaccination program Acquisition cost b Administration cost b Total vaccination cost b QALYs lost b |
692 424 381 347 228 808 81 590 679 $ 4 441 405 $ 5 604 793 0 0 0 13 896 |
239 590 133 653 80 138 25 526 273 $ 1 437 763 $ 1 809 645 $ 62 859 401 $ 7 107 260 $ 69 966 661 4980 |
452 834 247 694 148 670 56 064 406 $ 3 003 642 $ 3 795 148 ($ 62 859 401) ($ 7 107 260) ($ 69 966 661) 8917 |
| One-dose vaccine schedule Number of cases a Mild Moderate Severe Death Cost of illness Healthcare perspective b, c Societal perspective b ,c Cost of vaccination program Acquisition cost b Administration cost b Total vaccination cost b QALYs lost b |
692 424 381 347 228 808 81 590 679 $ 4 441 405 $ 5 604 793 0 0 0 13 896 |
370 217 207 190 124 229 38 366 432 $ 2 155 823 $ 2 711 873 $ 31 914 096 $ 3 608 398 $ 35 522 494 7282 |
322 207 174 157 104 579 43 224 247 $ 2 285 582 $ 2 892 920 ($ 31 914 096) ($ 3 608 398) ($ 35 522 494) 6614 |
Note: a Undiscounted; b Discounted; c Costs are excluding vaccination cost
Table 1B. Cost effectiveness results.
| Cost effectiveness of vaccination | One-dose | Two-dose |
|---|---|---|
| Vs no vaccination Net cost per QALY gained (healthcare) a Net cost per QALY gained (societal) a |
US$ 5025 US$ 4933 |
US$ 7510 US$ 7421 |
| Vs one-dose vaccine schedule Net cost per QALY gained (healthcare) a Net cost per QALY gained (societal) a |
US$ 14 648 US$ 14 568 |
Note: a Discounted
Univariate, probabilistic sensitivity, and affordability analyses
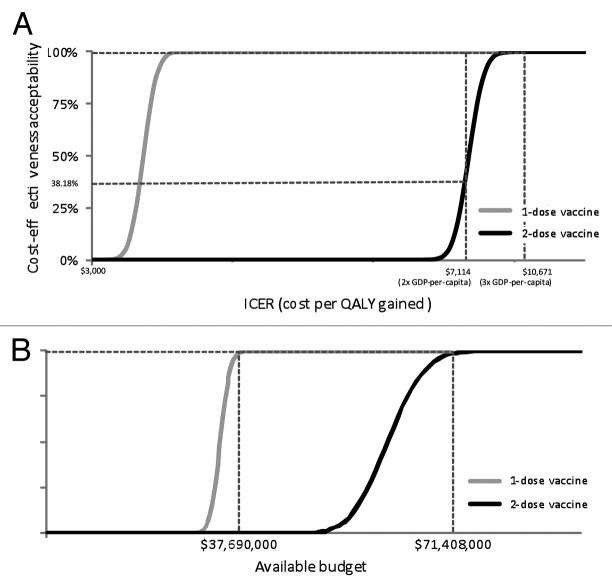
The effects of varying input parameters on the ICERs are shown in a tornado chart (Fig. 1). For the schedule using 2 administrations, the result confirmed that the vaccine price, mortality rate and discount rate provide most impact on the ICERs. The cost-effectiveness acceptability curves (CEACs) from the societal perspective showed that at the threshold ICER of US$ 7114 (2 times GDP per capita), the probability for the implementation of hepatitis A vaccination to be cost-effective would be 38.18% and 100% for two-dose and one-dose vaccine schedules, respectively. If a threshold ICER of US$ 10 671 (3 times GDP per capita) were used, the probability for the implementation of hepatitis A vaccination to be cost-effective would be 100% for both vaccine schedules (Fig. 2A). The affordability curves related to the required budget for vaccination from the healthcare perspective, are shown in Figure 2B. At budgets of US$ 71 408 000 and US$ 37 690 000 for the implementation of the two-dose and one-dose vaccine schedules, the implementation of hepatitis A vaccination would be 100% affordable.
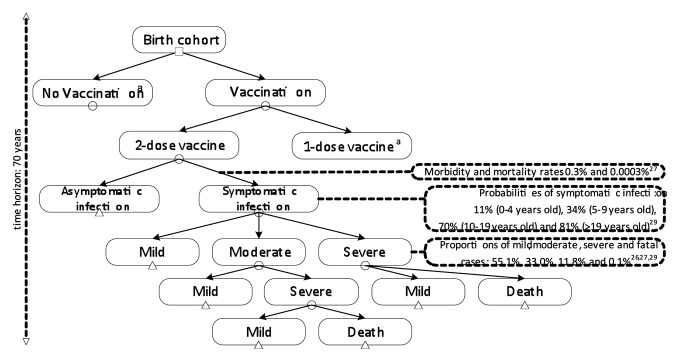
Figure 1. Decision analytic model.aSame branches with 2-dose vaccine are applied
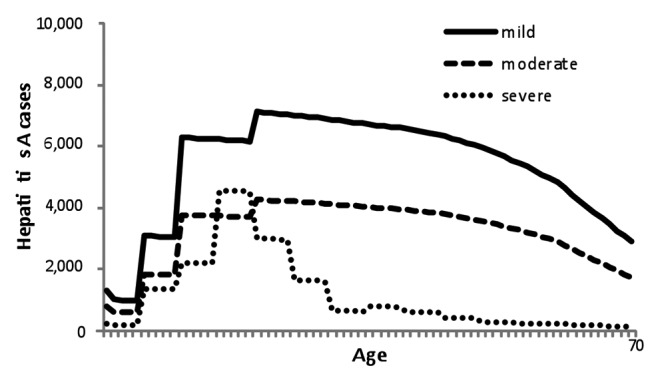
Figure 2. Age-specific hepatitis A-associated case.
Discussion
As a consequence of the improvement in hygiene and sanitary conditions, which are coupled with the economic rising of Indonesia from a low-income country into a middle-income country, the incidence of HAV infection has gradually declined. Without vaccination, HAV causes 692 424 cases in Indonesia where the disease acquisition occurs in adulthood rather than childhood as a typical of hepatitis A case in a low endemicity country.12 Applying a vaccine coverage at 80%, vaccination of 4 200 000 infants would reduce HAV-cases by 452 834 and 322 207 for vaccination with the two-dose and one-dose vaccine schedules, respectively. Also, the cost-effectiveness analyses yielded ICERs from the societal perspective at US$ 7421 and US$ 4933 per QALY gained for both vaccine schedules. Our finding that the implementation of universal hepatitis A immunization could be cost-effective even in a low endemicity country, such as Indonesia, is linear with a previous study.13 It could be emphasized that incidence was only one of the major determining factors for the cost-effectiveness of universal hepatitis A vaccination. Even in very low endemic countries, such as Canada and certain parts of the United States, universal vaccination could be a cost-effective intervention.14 However, in other very low endemic countries, for instance in Belgium and Australia, it was shown that two-dose universal childhood hepatitis A vaccination was not cost-effective, using dynamic and static models, respectively.15,16 These results are mainly influenced by estimated disease incidence, vaccine price, the schedule and the inclusion of societal cost.14 In particular, another finding that the implementation of the one-dose vaccine schedule would be more cost-effective intervention compared with the two-dose vaccine schedule is in line with a previous study in Argentina.17 This further warrants future attention on the implementation of the one-dose vaccine schedule, especially to control community-wide outbreaks since a single dose of hepatitis A vaccine has been proven an effective strategy if vaccination was started early and applied with high coverage. Additionally, compared with the two-dose vaccine schedule, the one-dose vaccine schedule is cheaper and easier to be implemented. Yet, in high-risk groups (such as children with chronic liver disease and immune-compromised individuals) for hepatitis A, a two-dose vaccine schedule is still preferred.8 In the context of health economic perspective, however, the implementation of the one-dose vaccine schedule would be more realistic to be implemented in Indonesia. Related to the sensitivity analyses, the results in this study reconfirmed the results from several previous studies that the vaccine price,18-20 mortality rate,21 and discount rate,14,18,22,23 were the most influential parameters impacting the ICERs in the implementation of hepatitis A vaccination. However, the dominant role of the vaccine price might lead the small difference between the ICERs from the healthcare and societal perspectives.24
This study is the first economic evaluation study on hepatitis A immunization in Indonesia. Yet, we do not present the first economic analysis on that matter in South East Asia Region (SEAR). Compared with previous studies in Thailand,19,25 our study has some significant differences in the process of analysis. First, we explicitly compared the two-dose and one-dose vaccine schedules in a cost-effectiveness study in order to investigate the difference on the cost-effectiveness results by performing the ICERs of both vaccines over without vaccination, while 2 previous studies used only one vaccine schedule in their cost-benefit analyses. Also, we performed the ICERs of the two-dose over one-dose vaccine schedules. Second, we adopted both the healthcare and societal perspectives in our study. However, the healthcare perspective is relevant for assisting decision makers in the health sector only, while the societal perspective is often preferred to reflect the full public health impact. Third, we performed an age-structured cohort model based on a decision tree by dividing the outpatient cases into 2 different levels: mild (requiring home treatment) and moderate cases (requiring general practitioner treatment), and considering the annual decline of infection incidence and the annual loss of vaccine protection that would render results that are more precise and valid.
Nevertheless, several limitations were found in this study. The first and main limitation is that we use a static model rather than a dynamic model, which has the ability to incorporate the effect of herd immunity. In general, the static model tends to over-estimate the cost-effectiveness result. Notably, it seems likely that there would be an even more favorable cost-effectiveness if we took herd immunity into account. Next to the ability to incorporate the epidemiology of hepatitis A and the development of herd immunity, the disadvantage of a dynamic model is the requirement for data, which are currently scarce in Indonesia. Particularly, the age specific force of infection is difficult to be estimated as it requires serial seroprevalence data and social contact data. The second limitation is the lack of vaccine efficacy data against disease for different levels of severity and the lack of empirical data on vaccine effectiveness against infection on the one-dose versus two-dose protection over time. The third limitation is the lack of specific local data related to the proportion of incidence for all levels of severity. In this study, we derived those numbers from international data. Yet, we varied these estimates extensively in multiple sensitivity analyses. Finally, we applied treatment costs from a 2006 study on estimated unit costs related to HAV infection due to poor sanitation in Indonesia and these costs were inflated to 2012 price levels. Obviously, hepatitis A vaccination would be more cost-effective when the treatment costs are higher, and vice versa.
Our study provides information for policy makers in Indonesia to justify full inclusion of the hepatitis A vaccine into the NIP. With the market price of US$ 3.21 per dose, vaccinating using both the two-dose and one-dose vaccine schedules could be a cost-effective intervention according to the World Health Organization’s (WHO’s) criteria for cost-effectiveness. Furthermore, when we took uncertainties into account, the implementation of universal hepatitis A immunization would not be affordable when the budget does not exceed US$ 71 408 000 and US$ 37 690 000 for the two-dose and one-dose vaccine schedules, respectively. In fact, the Indonesian government spent approximately US$ 68 million for NIP activities in 2011.26 Compared with the total Indonesian government health budget for the whole mandatory immunization program (hepatitis B, BCG (bacille Calmette–Guérin), diphtheria-pertussis-tetanus, measles, and polio), the required investment by the Indonesian government for universal hepatitis A vaccination would be unrealistic without external support. A solution that could be applied to reduce the vaccine price is through financial aids from international organizations. However, saving funds could enhance implementation of further vaccination programs in a country with limited vaccination budget, such as Indonesia. In particular, the implementation of the one-dose vaccine schedule could be considered since it has been proven to be the most cost-effective intervention in this study. Also, using the combined hepatitis A/B vaccine instead of monovalent vaccine could be considered to reduce the administration costs since the combined hepatitis A/B vaccine has been proven as a highly immunogenic and well-tolerated in a previous study.27 Hopefully, this study helps the Indonesian government in making regulation to reduce the incidence of HAV infection in Indonesia, in line with WHO’s goal on the implementation of universal vaccination.
Methods
Model
In this study, we used the Indonesia 2012 birth cohort of 4 200 000 infants9 in an age-structured cohort model based on a decision tree. The model involves a 70-y time horizon (the average life expectancy in Indonesia)28 with 1-mo cycles for children less than 2-y-old and annually thereafter. Differing from several previous studies13,19,25 in Asia, we made a comparison of the use of a two-dose vs. a one-dose vaccine schedule. The model was run in Microsoft Excel 2010 and @Risk 4.5.4 was used in probabilistic sensitivity analysis (Fig. 3).
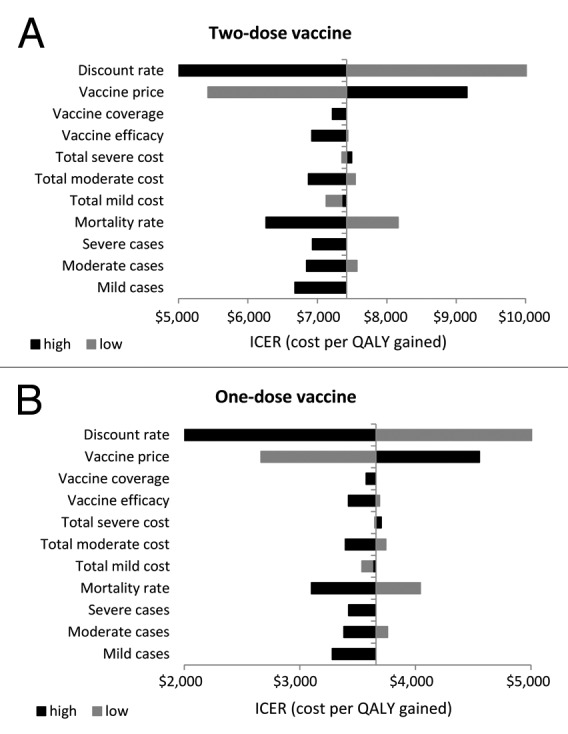
Figure 3. Univariate sensitivity analysis from the societal perspective.
Incidence of HAV infection
We classified HAV infection into 4 levels of severity which are generally used for global assessments: mild (home treatment), moderate (general practitioner treatment), severe (hospitalization), and fatal cases.29 From the World Bank’s report30 in 2006 on economic impacts of sanitation in Indonesia and considering the annual incidence of HAV infection declining linearly at an average annual rate of 2% as the result of socioeconomic improvement,13 we obtained the number of hepatitis A cases in 2012 (mild, moderate, severe, and fatal cases) by considering the morbidity and mortality rates of 0.3211% and 0.0003%.30 We estimated the total number of severe cases by applying the ratio of hospitalization (severe) and outpatient visit (mild-moderate) at 11.8%:88.2% according to a study by Zhuang et al.13 For the number of severe cases in each age group, we applied data from a study on hepatitis A cases at one of biggest public hospitals in Indonesia during 2011.31 Furthermore, we estimated that moderate cases would make up 37.5% and mild cases 62.5% from outpatient visit cases based on a study by Buma et al.29 Several data from previous studies related to the age-specific probabilities of symptomatic infection,32 hospitalization rate,31 and case fatality rate13 were used to estimate mild-moderate, severe, and fatal cases in various age groups. For economic consequences, we only consider symptomatic infections since asymptomatic infections were assigned no costs and excluded from further follow-up for disease outcomes.13 As the liver transplant in acute hepatitis patients with fulminant liver failure is very rare in Indonesia, we did not take this into account (Fig. 4).
Figure 4. (A) Cost-effectiveness acceptability curves from the societal perspective. (B) Affordability curves from the healthcare perspective.
Vaccine characteristics
Hepatitis A vaccine would be given in a two-dose schedule at 12 and 18 mo of age and in a one-dose schedule at 12 mo of age. We applied vaccine efficacy at 93% and 95% for the first and second dose, based on vaccine immunogenicity and safety studies.33-35 Furthermore, we assumed that with the two-dose vaccine schedule, vaccine protection would annually decline by 0.31% within the first 10 y and 0.62% thereafter according to the expert panel opinion.36 In the one-dose vaccine schedule, vaccine protection would annually decline by 1.62% within the first 10 y and 2.67% thereafter.36 Vaccine coverage in this study was assumed to be 80% for both the two-dose and one-dose vaccine schedules, according to a previous hepatitis B study37 conducted in Indonesia (Table 2).
Table 2. Parameters used in the model.
| Parameters | Baseline | Distribution | References |
|---|---|---|---|
| Vaccine coverage Vaccine efficacy 1st dose 2nd dose Annual loss of vaccine protection 1-dose schedule (1–10 y) 1-dose schedule (>10 y) 2-dose schedule (1–10 y) 2-dose schedule (>10 y) Probability of symptomatic infection 0–4 5–9 10–19 20+ Hepatitis A hospitalization rate 0–4 5–9 10–14 15–19 20–24 25–29 30–34 35–39 40–44 45–49 50+ Hepatitis A case fatality rate 1–14 15–39 40+ Hepatitis A cases Mild Moderate Severe Death Age-dependent hepatitis A related proportion of mild, moderate, severe and fatal cases Utility losses Mild Moderate Severe Death Total healthcare costs per case (US$) Mild Moderate Severe Total societal costs per case (US$) Mild Moderate Severe Vaccination cost (US$) Vaccine price (per dose) Administration cost (per dose) Discount rate |
80% 93% 95% 1.62% 2.67% 0.31% 0.62% 11% 34% 70% 81% 1.05% 8.42% 13.68% 28.42% 18.95% 10.53% 4.21% 5.26% 4.21% 3.16% 2.11% 0.030% 0.054% 0.436% 381,347 228,808 81,590 679 0.01885 0.02474 0.03888 1.00000 8.77 17.53 25.82 11.31 20.08 36.24 3.21 0.36 3% |
Normal (95%CI; 76.64–83.36%) Normal (95%CI; 89.10–96.90%) Normal (95%CI; 91.01–98.99%) NA NA NA NA Normal (95%CI; 380 137–382 556) Normal (95%CI; 227 871–229 745) Normal (95%CI; 81 030–82 150) Normal (95%CI; 628–730) Dirichlet Triangular (using 25% lower and upper) Gamma (5.06–15.81) Gamma (25.20–55.80) Gamma (59.50–114.30) Gamma (9.24–25.03) Gamma (34.10–71.60) Gamma (124.50–215.80) Triangular (using 25% lower and upper) Triangular (using 25% lower and upper) 0–5% |
37 13 36 31 32 13 13,29–32; calculated 13,29–32; calculated 13,29,38; calculated 30,39; calculated 27,33; calculated 37,39 9,39 13 |
NA, not applicable
QALY (quality-adjusted-life-year) losses
To estimate QALY losses, we applied data from several previous studies with estimated durations of illness at 16, 21, and 33 d for mild, moderate, and severe cases, respectively,28 and disutility scores at 0.43 for the state lived with hepatitis A.38 Based on those data, we estimated QALY losses, e.g., mild cases at 0.01885 (16 x 0.43 / 365 d).39 We applied the same method for estimating QALY losses for moderate and severe cases. We did not consider caregiver QALY losses in our study (Table 2).
Hepatitis A costs
Differing from 2 previous studies in SEAR,19,24 the analysis in this study was viewed from 2 perspectives: healthcare and societal. We only considered direct medical cost in the healthcare perspective, while in the societal perspective, we considered both direct and indirect costs. We derived our cost estimations from a 2006 study on estimated unit costs related to disease due to poor sanitation in Indonesia.30 Healthcare costs due to HAV infection related mild, moderate and severe cases were estimated from informal outpatient care/home treatment (e.g., self-treatment cost), formal outpatient care/general practitioner treatment (e.g., medical direct costs) and formal inpatient care/hospitalization (e.g., medication, diagnostic, registration, and other medical direct costs) sources, respectively.30 These healthcare costs were compiled from the information on disease rates, treatment-seeking rates, treatment practices, and unit costs, which were applied country-wide based on the available costing studies conducted in Indonesia.30 For societal costs, we additionally took direct non-medical costs (e.g., transportation) and indirect costs (e.g., productivity loss)9 into account. Vaccine price and administration cost per dose were applied at US$ 3.2137 and US$ 0.36,9 respectively, based on previous studies in Indonesia. All results from the analyses were converted to 2012 US$ by using purchasing power parities (PPPs)40 and all costs were discounted with a yearly rate of 3% (Table 2).
Analytic methods
ICER= (Total cost with vaccination – Total cost of without vaccination)/(Total QALY gained without vaccination – Total QALY gained with vaccination)
The ICER was calculated to measure the outcomes from both perspectives in relation to the WHO’s definition on cost-effectiveness of universal vaccinations according to the GDP per capita: (1) highly cost-effective (less than one GDP per capita); (2) cost-effective (between 1 and 3 times GDP per capita); and (3) cost-ineffective (more than 3 times GDP per capita).11 We performed both univariate and probabilistic sensitivity analyses (PSA). Univariate sensitivity analyses were performed to investigate the effects of different input parameters primarily by varying each parameter with ± 25% while keeping other parameters constant. PSA were performed by running 5000 Monte Carlo simulations. The results of the PSA were presented in CEACs by using 2 thresholds: 2 times GDP per capita and 3 times GDP per capita. We evaluated affordability of vaccinations related to the required budget (vaccination and treatment costs) from the healthcare perspective, based on the distribution of incremental costs and health gains from the same 5000 Monte Carlo simulations.
Glossary
Abbreviations:
- HAV
hepatitis A virus
- WGO
World Gastroenterology Organization
- NIP
national immunization program
- QALY
quality-adjusted-life-year
- ICER
incremental cost-effectiveness ratio
- GDP
gross domestic product
- CEACs
cost-effectiveness acceptability curves
- SEAR
South East Asia Region
- WHO
World Health Organization
- BCG
bacille Calmette-Guérin
- PPPs
purchasing power parities
- PSA
probabilistic sensitivity analyses
Disclosure of Potential Conflicts of Interest
M.J.P. received grants, honoraria, and travel stipends from various pharmaceutical companies, inclusive those interested in the subject matter of this paper. The rest of the authors have no relevant affiliations or financial involvement with any organizations or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.David AM, Steering Committee for Prevention and Control of Infectious Diseases. . Hepatitis A outbreaks--methods of intervention in South-East Asian countries. Int J Infect Dis 2004; 8:201 - 9; http://dx.doi.org/ 10.1016/j.ijid.2003.09.005; PMID: 15234323 [DOI] [PubMed] [Google Scholar]
- 2.Luyten J, Beutels P. . Costing infectious disease outbreaks for economic evaluation: a review for hepatitis A. Pharmacoeconomics 2009; 27:379 - 89; http://dx.doi.org/ 10.2165/00019053-200927050-00003; PMID: 19586076 [DOI] [PubMed] [Google Scholar]
- 3.World Gastroenterology Organization. Management of acute viral hepatitis. 2007: 1-23. Available from: http://www.worldgastroenterology.org/assets/downloads/en/pdf/guidelines/02_acute_hepatitis.pdf
- 4.Barzaga BN. . Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine 2000; 18:Suppl 1 S61 - 4; http://dx.doi.org/ 10.1016/S0264-410X(99)00467-3; PMID: 10683551 [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen KH, Wiersma ST. . Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010; 28:6653 - 7; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.037; PMID: 20723630 [DOI] [PubMed] [Google Scholar]
- 6.Luyten J, Van de Sande S, de Schrijver K, Van Damme P, Beutels P. . Cost-effectiveness of hepatitis A vaccination for adults in Belgium. Vaccine 2012; 30:6070 - 80; http://dx.doi.org/ 10.1016/j.vaccine.2012.07.049; PMID: 22858555 [DOI] [PubMed] [Google Scholar]
- 7.Vranckx R, Alisjahbana A, Deville W, Meheus A. . Hepatitis A antibodies in Indonesian neonates and children. Int J Infect Dis 1997; 2:31 - 3; http://dx.doi.org/ 10.1016/S1201-9712(97)90008-4 [DOI] [Google Scholar]
- 8.Suwantika AA, Yegenoglu S, Riewpaiboon A, Tu HA, Postma MJ. . Economic evaluations of hepatitis A vaccination in middle-income countries. Expert Rev Vaccines 2013; 12:1479 - 94; http://dx.doi.org/ 10.1586/14760584.2013.851008; PMID: 24168129 [DOI] [PubMed] [Google Scholar]
- 9.Wilopo SA, Kilgore P, Kosen S, Soenarto Y, Aminah S, Cahyono A, Ulfa M, Tholib A. . Economic evaluation of a routine rotavirus vaccination programme in Indonesia. Vaccine 2009; 27:Suppl 5 F67 - 74; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.040; PMID: 19931723 [DOI] [PubMed] [Google Scholar]
- 10.The World Bank. GDP per capita. Available from the website: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD
- 11.Walker DG1, Hutubessy R, Beutels P. . . WHO guide for standardization of economic evaluations of immunization programmes. Vaccine 2010; 28:2356 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.035; PMID: 19567247 [DOI] [PubMed] [Google Scholar]
- 12.Pham B, Duval B, De Serres G, Gilca V, Tricco AC, Ochnio J, Scheifele DW. . Seroprevalence of hepatitis A infection in a low endemicity country: a systematic review. BMC Infect Dis 2005; 5:56; http://dx.doi.org/ 10.1186/1471-2334-5-56; PMID: 16001978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuang GH, Pan XJ, Wang XL. . A cost-effectiveness analysis of universal childhood hepatitis A vaccination in China. Vaccine 2008; 26:4608 - 16; http://dx.doi.org/ 10.1016/j.vaccine.2008.05.086; PMID: 18597903 [DOI] [PubMed] [Google Scholar]
- 14.Anonychuk AM, Tricco AC, Bauch CT, Pham B, Gilca V, Duval B, John-Baptiste A, Woo G, Krahn M. . Cost-effectiveness analyses of hepatitis A vaccine: a systematic review to explore the effect of methodological quality on the economic attractiveness of vaccination strategies. Pharmacoeconomics 2008; 26:17 - 32; http://dx.doi.org/ 10.2165/00019053-200826010-00003; PMID: 18088156 [DOI] [PubMed] [Google Scholar]
- 15.Beutels P, Luyten J, Lejeune O, Hens N, Blicke J, De Schrijver K, Van de Sande S, Van Herck K, Van Damme P. Evaluation of universal and targeted hepatitis A vaccination programs in Belgium. Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE); 2008. 176p. KCE reports 98A (D/2008/http://dx.doi.org/ 10.273/88). Available from the website: https://kce.fgov.be/sites/default/files/page_documents/d20081027388.pdf. [DOI]
- 16.Beutels P, MacIntyre CR, McIntyre P. Cost-effectiveness of childhood hepatitis A vaccination in Australia. Paper presented at 5th World Congress of the International Health Economics Association, Barcelona, Spain, 9-12 July 2005.
- 17.Ellis A, Rüttimann RW, Jacobs RJ, Meyerhoff AS, Innis BL. . Cost-effectiveness of childhood hepatitis A vaccination in Argentina: a second dose is warranted. Rev Panam Salud Publica 2007; 21:345 - 56; http://dx.doi.org/ 10.1590/S1020-49892007000500002; PMID: 17761046 [DOI] [PubMed] [Google Scholar]
- 18.Valenzuela MT, Jacobs RJ, Arteaga O, Navarrete MS, Meyerhoff AS, Innis BL. . Cost-effectiveness of universal childhood hepatitis A vaccination in Chile. Vaccine 2005; 23:4110 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2005.03.021; PMID: 15964479 [DOI] [PubMed] [Google Scholar]
- 19.Teppakdee A, Tangwitoon A, Khemasuwan D, Tangdhanakanond K, Suramaethakul N, Sriratanaban J, Poovorawan Y. . Cost-benefit analysis of hepatitis a vaccination in Thailand. Southeast Asian J Trop Med Public Health 2002; 33:118 - 27; PMID: 12118439 [PubMed] [Google Scholar]
- 20.Sartori AMC, de Soárez PC, Novaes HMD, Amaku M, de Azevedo RS, Moreira RC, Pereira LM, Ximenes RA, Martelli CM. . Cost-effectiveness analysis of universal childhood hepatitis A vaccination in Brazil: regional analyses according to the endemic context. Vaccine 2012; 30:7489 - 97; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.056; PMID: 23107593 [DOI] [PubMed] [Google Scholar]
- 21.O’Connor JB, Imperiale TF, Singer ME. . Cost-effectiveness analysis of hepatitis A vaccination strategies for adults. Hepatology 1999; 30:1077 - 81; http://dx.doi.org/ 10.1002/hep.510300422; PMID: 10498662 [DOI] [PubMed] [Google Scholar]
- 22.Quezada A, Baron-Papillon F, Coudeville L, Maggi L. . Universal vaccination of children against hepatitis A in Chile: a cost-effectiveness study. Rev Panam Salud Publica 2008; 23:303 - 12; http://dx.doi.org/ 10.1590/S1020-49892008000500002; PMID: 18510790 [DOI] [PubMed] [Google Scholar]
- 23.Lopez E, Debbag R, Coudeville L, Baron-Papillon F, Armoni J. . The cost-effectiveness of universal vaccination of children against hepatitis A in Argentina: results of a dynamic health-economic analysis. J Gastroenterol 2007; 42:152 - 60; http://dx.doi.org/ 10.1007/s00535-006-1984-x; PMID: 17351805 [DOI] [PubMed] [Google Scholar]
- 24.Freiesleben de Blasio B, Flem E, Latipov R, Kuatbaeva A, Kristiansen IS. . Dynamic modeling of cost-effectiveness of rotavirus vaccination, Kazakhstan. Emerg Infect Dis 2014; 20:29 - 37; http://dx.doi.org/ 10.3201/eid2001.130019; PMID: 24378188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soogarun S, Wiwanitkit V. . Vaccinating Thai adolescents against hepatitis A: is it cost-effective?. Southeast Asian J Trop Med Public Health 2002; 33:Suppl 3 145 - 8; PMID: 12971496 [PubMed] [Google Scholar]
- 26.SABIN. Indonesia: Government expenditures on routine immunization, vaccines and health. Available from: http://www.sabin.org/sites/sabin.org/files/Indonesia.pdf
- 27.Murdoch DL, Goa K, Figgitt DP. . Combined hepatitis A and B vaccines: a review of their immunogenicity and tolerability. Drugs 2003; 63:2625 - 49; http://dx.doi.org/ 10.2165/00003495-200363230-00008; PMID: 14636084 [DOI] [PubMed] [Google Scholar]
- 28.The World Bank. Life expectancy at birth. Available from: http://data.worldbank.org/indicator/SP.DYN.LE00.IN
- 29.Buma AH, Beutels P, van Damme P, Tormans G, van Doorslaer E, Leentvaar-Kuijpers A. . An economic evaluation of hepatitis A vaccination in Dutch military personnel. Mil Med 1998; 163:564 - 7; PMID: 9715622 [PubMed] [Google Scholar]
- 30.Water and Sanitation Program East Asia and The Pacific (WSP-EAP), The World Bank. Economic impacts of sanitation in Indonesia. 2008: 1-89. Available from the website: http://www.wsp.org/sites/wsp.org/files/publications/esi_indonesia.pdf
- 31.Gaol DPL. The overview of hepatitis A at Hasan Sadikin Hospital Bandung period 1 January 2011-31 December 2011. Bachelor thesis. Maranatha Christian University, Faculty of Medicine; 2013. [Google Scholar]
- 32.Bauch CT, Anonychuk AM, Pham BZ, Gilca V, Duval B, Krahn MD. . Cost-utility of universal hepatitis A vaccination in Canada. Vaccine 2007; 25:8536 - 48; http://dx.doi.org/ 10.1016/j.vaccine.2007.10.001; PMID: 17996339 [DOI] [PubMed] [Google Scholar]
- 33.Ren A, Feng F, Ma J, Xu Y, Liu C. . Immunogenicity and safety of a new inactivated hepatitis A vaccine in young adults: a comparative study. Chin Med J (Engl) 2002; 115:1483 - 5; PMID: 12490092 [PubMed] [Google Scholar]
- 34.Yin WD. . The Healive™ inactivated domestic hepatitis A vaccine: production and application. Chin J Vaccines Immun 2004; 10:174 - 7 [Google Scholar]
- 35.Liu CB, Ren YH, Zhang YC, Wu WT, Li SP, Kang WX, et al. . The study on immunogenicity, safety and vaccination schedule of a new inactivated hepatitis A vaccine in Chinese children. Chin J Vaccines Immun 2002; 8:1 - 3 [Google Scholar]
- 36.Jacobs RJ, Meyerhoff AS, Zink T. . Hepatitis A immunization strategies: universal versus targeted approaches. Clin Pediatr (Phila) 2005; 44:705 - 9; http://dx.doi.org/ 10.1177/000992280504400809; PMID: 16211195 [DOI] [PubMed] [Google Scholar]
- 37.Levin CE, Nelson CM, Widjaya A, Moniaga V, Anwar C. . The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ 2005; 83:456 - 61; PMID: 15976897 [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs RJ, Moleski RJ, Meyerhoff AS. . Valuation of symptomatic hepatitis a in adults: estimates based on time trade-off and willingness-to-pay measurement. Pharmacoeconomics 2002; 20:739 - 47; http://dx.doi.org/ 10.2165/00019053-200220110-00003; PMID: 12201793 [DOI] [PubMed] [Google Scholar]
- 39.Postma MJ, Jit M, Rozenbaum MH, Standaert B, Tu HAT, Hutubessy RC. . Comparative review of three cost-effectiveness models for rotavirus vaccines in national immunization programs; a generic approach applied to various regions in the world. BMC Med 2011; 9:84; http://dx.doi.org/ 10.1186/1741-7015-9-84; PMID: 21740545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The World Bank. PPP conversion factor. Available from the website: http://data.worldbank.org/indicator/PA.NUS.PPP?page=