Abstract
Background. No immunogenicity data has been reported after a single dose of the quadrivalent HPV vaccine (qHPV-Gardasil®) and no data are available on co-administration of this vaccine with the HAV/HBV vaccine (Twinrix-Junior®). Two pre-licensure studies reported similar anti-HPV but lower anti-HBs titers when co-administering HPV and HBV vaccines.
Objectives. To assess the immunogenicity of the qHPV and HAV/HBV vaccine when co-administered (Group-Co-adm) or given one month apart (Group-Sep) and to measure the persistence of HPV antibodies three years post-second dose of qHPV vaccine in both study groups.
Methods. 416 9–10 year-old girls were enrolled. Vaccination schedule was 0–6 months. Anti-HAV and anti-HBs were measured in all subjects 6 months post-first dose and 1 month post-second dose. Anti-HPV were measured 6 months post-first dose in Group-Co-adm and in all subjects 1 and 36 months post-second dose.
Results. Six months post-first dose: 100% of subjects had detectable anti-HAV and 56% and 73% had detectable anti-HBs in Group-Co-Adm and Group-Sep, respectively. In Group-Co-adm 94, 100, 99 and 96% had detectable antibodies to HPV 6, 11, 16 and 18, respectively. One month post-second dose of qHPV and HAV/HBV vaccine, in both study groups 99.5–100% of subjects had an anti-HAV titer ≥ 20IU/L, 97.5–97.6% an anti-HBs level ≥ 10IU/L, and 100% had an anti-HPV titer ≥ 3LU. Thirty-six months post-second dose of qHPV all but four subjects (99%) had antibodies to HPV18 and 100% had antibodies to HPV6, 11 and 16. The great majority (97–100%) had an anti-HPV titer ≥ 3 LU. Post-second dose administration of qHPV and HAV/HBV, no meaningful difference was observed in the immune response in the two study groups to any component of vaccines.
Conclusions. The results indicate that qHPV and HAV/HBV can be given during the same vaccination session. Two doses of of qHPV and HAV/HBV vaccines induce a strong immune response. Three years post-second dose of qHPV, the great majority of subjects had antibodies to HPV types included in the vaccine. A two-dose schedule for pre-adolescents might be a reasonable alternative to the currently approved three-dose schedules.
Keywords: HPV, Hepatitis A and B, Immunogenicity, Two doses, Vaccination
Introduction
The approved schedules for human papilloma virus (HPV) vaccines (qHPV (Gardasil®) and bHPV (Cervarix®)) include three doses administered at 0, 1–2 and 6 m. The first two doses given within a relatively short period of time are expected to prime the immune system and the third is expected to confer long-lasting immunity.1 When given according to this schedule to 16–45 y old females, HPV vaccines have been shown to be highly immunogenic and protect against persistent infection, anogenital warts and precancerous abnormalities.2-10 Post-licensure trials with bHPV showed that > 98% of 15–25 y old females developed antibodies to HPV 16 and HPV 18 which remain detectable up to 76 mo after a single vaccine dose.3 In addition, a significant increase in antibody titers resulted from a second dose of vaccine and high clinical efficacy was demonstrated in individuals who received less than three vaccine doses.4-6 Finally, in preadolescents and adolescents, both HPV and combined HAV/HBV vaccines are more immunogenic than in young adults7-10 and therefore it is possible that preadolescents may require less than three doses to ensure long-term protection.11-14
In most Canadian provinces and in some other countries, HPV and hepatitis B virus (HBV) vaccines are co-administered to school age children. Two studies have shown that the co-administration of these vaccines result in similar levels of seroprotection against HBV, although anti-hepatitis B surface antigen (anti-HBs) geometrical mean titers (GMTs) were 30–60% lower when the vaccines were co-administered compared with those who received the vaccines separately.15,16 In the province of Quebec, the qHPV vaccine and the combined hepatitis A and hepatitis B vaccine (HAV/HBV vaccine (Twinrix-Junior®)) are co-administered in a two-dose schedule (0, 6 mo) to 9–10 y old girls and it was deemed necessary to assess the potential interference of vaccine co-administration on the levels of induced antibody to each vaccine.
The main objectives of this study were to compare the immunogenicity of qHPV and HAV/HBV vaccine when administered simultaneously or one month apart and to assess the persistence of type-specific HPV antibodies 36 mo post-second dose of qHPV vaccine.
Methods
Population and study design
This was an open-label, randomized, single center clinical trial. In 2008–2009, subjects were randomized 1:1 (by using SAS Institute software; blocks of 25 subjects per study group were generated) to receive qHPV and combined HAV/HBV vaccines during the same vaccination session (Group Co-adm; co-administration of qHPV and HAV/HBV; two separate injections in opposite deltoids) or one month apart starting with the HPV vaccine (Group Sep; separate administration of qHPV and HAV/HBV). Nine to ten year-old girls eligible for provincial school-based HPV and HAV/HBV vaccination were invited to participate. The exclusion criteria included: previous HPV, HAV or HBV vaccination; a history of an illness which results in immunodeficiency; immunosuppressive therapy; a coagulopathy and/or the use of anticoagulant therapy; a history of having received immunoglobulin or other blood derived products during previous 6 mo; participation in another clinical trial during last 6 mo; or a history of an anaphylactic reaction to a component included in the study vaccines.
The sample size was calculated based on previously reported data on seroconversion rates after two doses of qHPV and HAV/HBV vaccine.8,17,18 A sample size of 145 subjects per group was estimated to be sufficient to assess non-inferiority between the two groups assuming a seroconversion rate for HPV and HAV/HBV of 98% with a power of at least 80% and a level of significance of 5%. This sample size was also estimated to be sufficient to detect a difference of 0.33 (small to moderate size effect) between the GMTs responses. Based on an anticipated 5% loss of subjects per year,19,20 an enrolment of 200 subjects per study group was necessary to ensure that 145 evaluable subjects per study group would complete the second phase of the study in 2012.
The study was approved by the Research Ethics Board of the Laval University Hospital Center and by the Ethics Board of Centre de santé et de services sociaux de la Vieille-Capitale. Written informed consent was obtained from the parents or legal guardians, and an assent from participating children. This study is registered with ClinicalTrials.gov, NCT01456715.
Vaccine administration and blood sampling
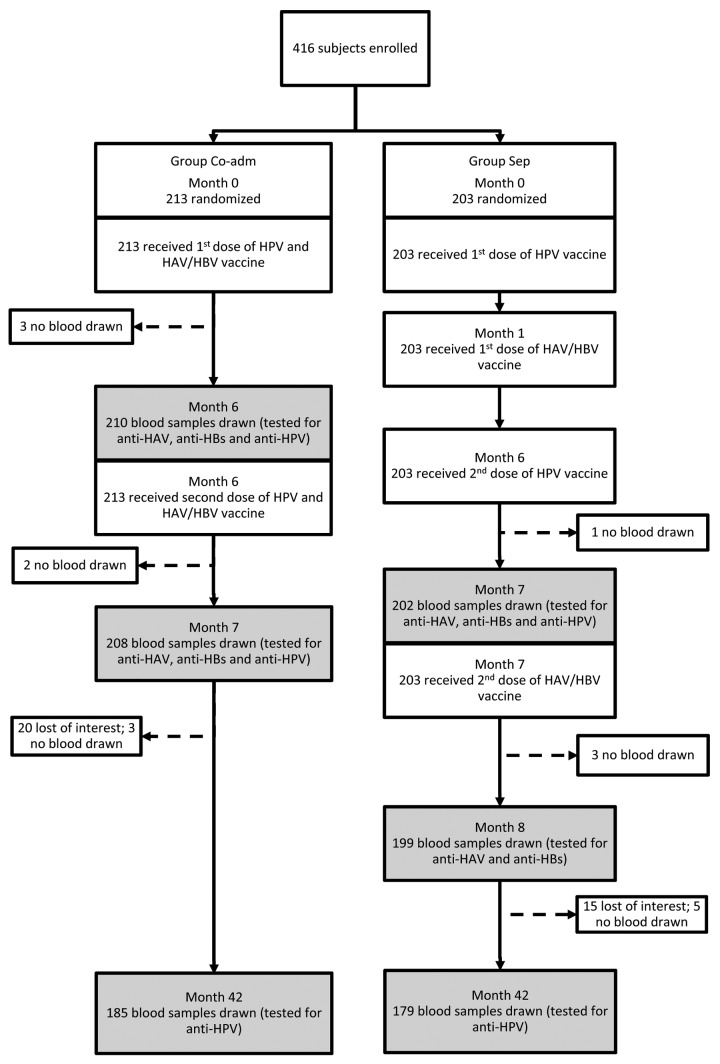
One commercial lot of qHPV (NG31270) and one of combined HAV/HBV vaccine (AHABB118AD) were used in this study. Both vaccines were provided by the Quebec Ministry of Health and Social Services. The same vaccines lots were used in provincial school-based immunization program. Vaccines were administered intramuscularly in the deltoid at dosages recommended by the manufacturers. Blood samples were collected 6 mo post-first dose of HAV/HBV and qHPV vaccine in Group Co-adm and 6 mo post-first dose of HAV/HBV vaccine in Group Sep. Blood samples were also collected in both study groups 1 and 36 mo post-second dose of each vaccine administration (Fig. 1). This study design was chosen in order: 1) to optimize participant retention in the study (aiming at a maximum of three blood samples collected from each participant); 2) to generate immunogenicity data post-first dose of qHPV (in Group Co-adm); 3) to be able to make comparisons of immune response observed in the two study groups six months post-first dose of combined HAV/HBV vaccine; and 4) to be able to compare immunogenicity data observed in the two study groups one month post-second dose administration (for anti-HPV, anti-HAV and anti-HBs). Given that in a previous study9 we assessed the long-term persistence of immunity after two doses of the same combined HAV/HBV vaccine administered to the same age group according to the same schedule, it was decided to test blood samples collected 36 mo post-second dose for the presence of anti-HPV only. Limited financial resources available were also taken into consideration when designing this study. Ten milliliters of blood were collected at each study time point.
Figure 1. Participants flowchart
Laboratory procedures
Quantitative HPV type specific antibody levels were measured using the Luminex Total IgG assay. The assay performance was validated using 140 blinded specimens which were previously tested by the Merck cLIA, Merck TIgG and a HPV 16 and 18 pseudovirus assay implemented by the BC Centre for Disease Control, British Columbia, Canada.21 HPV antibody titers are reported in Luminex Units (LU). Anti-HBs was measured by the MONOLISA Anti-HBs EIA and MONOLISA Anti-HBs Calibrator Kit (Bio-Rad Laboratories). Anti-HAV was measured by HAV Total Assay EIA (BIO-Rad Laboratories). Commercial assays were performed using the manufacturers recommended protocols. Samples displaying signals at the upper limit of the respective assay’s linear range were diluted in specimen dilution buffer and retested to determine the final antibody concentration. Positive and negative quality control sera were included in each assay run to monitor inter- and intra-run performance and assure the quantitative validity of the results.
Data analysis
We assessed the proportion of subjects with detectable anti-HPV, anti-HBs and anti-HAV in samples collected in the two study groups at the different study time points with 95% Clopper-Pearson confidence intervals. An anti-HBs titer equal or greater than 10 IU/L was considered seroprotective.22
There is no consensus regarding the levels of anti-HPV that are seroprotective. We selected a threshold of 3 LU because it correlated best with results obtained with the three assays which were used to cross validate the Luminex assay (r > 0.9). Similarly, there is no consensus regarding anti-HAV seroprotective levels. We arbitrarily used the threshold of 20 IU/L as a proxy for seroprotection for anti-HAV23-25 and defined a priori for all study vaccines an anamnestic response as a ≥ 4-fold rise in antibody level. For subjects with anti-HBs titers under 10 IU/L, a level above this threshold was also required to be considered as an anamnestic response. Log-transformed antibody levels were used for geometrical mean titers (GMTs) calculation. To allow GMTs calculation, samples with undetectable antibodies were assigned the arbitrary value of 1 IU/L. Fisher’s exact test was used for the comparison of antibody distribution and Wilcoxon test was used for continuous variables. All statistics were two-tailed. P values of 0.05 or less were considered significant. Bonferroni correction was used to counteract multiple comparisons. SAS Institute software version 9.2 (Cary, NC, USA) was used for statistical analysis. All available serological results were included in the Total Vaccinated Cohort (TVC) analysis. The results of subjects who had two serological results available in 2008–2009 and of those who had three serological results available in 2011–2012 were included in the According to Protocol (ATP) analysis.
Results
Study participation
Among the 671 potential participants contacted by a research nurse, 420 agreed to participate and 416 met the study inclusion criteria and were enrolled. The baseline socio-demographic characteristics of the study subjects at the first visit are presented in Table 1. The study flowchart is presented in Figure 1.
Table 1. Baseline socio-demographic characteristics of the subjects.
| Characteristic | Group Co-adm | Group Sep |
|---|---|---|
| Female | 213 (100%) | 203 (100%) |
| Age (years) | ||
| Mean | 9.6 | 9.6 |
| Median | 9.5 | 9.6 |
| Range | 9.0–10.8 | 9.0–10.9 |
| Body mass index | 17.3 | 17.4 |
| Race/ethnicity | ||
| Caucasian | 98.6% | 98.4% |
The differences between the TVC and ATP immunogenicity results were minimal and not statistically significant. We present here the According to Protocol results only.
HAV immunogenicity results
Six months post-first dose of HAV/HBV vaccine all subjects (100%) had detectable anti-HAV levels and 67.1% (Group Co-adm) and 72.9% (Group Sep) an anti-HAV of ≥ 20 IU/L. Anti-HAV GMTs were 30.5 and 38.2 IU/L in Group Co-adm and Group Sep, respectively (all P > 0.05) (Table 2).
Table 2. Anti-HAV and anti-HBs seropositivity/seroprotection rates and GMTs (ATP analysis).
| Results | 6 mo after 1st dose of HAV/HBV vaccine* | 1 mo after 2nd dose of HAV/HBV vaccine | ||
|---|---|---|---|---|
| Group Co-adm n = 207 (95%CI) |
Group Sep n = 199 (95%CI) |
Group Co-adm n = 207 (95%CI) |
Group Sep n = 199 (95%CI) |
|
| Anti-HAV + | 100% (98.3–100) |
100% (98.2–100) |
100% (98.2–100) |
100% (98.2–100) |
| Anti-HAV ≥ 20IU/L | 67.1% (60.3–73.4) |
72.9% (66.1–78.8) |
100% (98.2–100) |
99.5% (97.2–99.9) |
| Anti-HAV GMTs |
30.5 IU/L (27.1–34.3) |
38.2 IU/L (33.4–43.6) |
2962 IU/L (2598–3379) |
2129 IU/L (1808–2507) |
| Anti-HBs + | 56.5% (49.5–63.4) |
72.9% (66.1–78.9) |
98.1% (95.1–99.5) |
99.0% (96.4–99.9) |
| Anti-HBs ≥ 10IU/L | 43.5% (36.6–50.5) |
59.3% (52.1–66.2) |
97.6% (94.5–99.2) |
97.5% (94.2–99.2) |
| Anti-HBs GMTs |
7.3 IU/L (5.8–9.2) |
12.5 IU/L (9.8–15.8) |
1701 IU/L (1339–2159) |
2005 IU/L (1585–2536) |
HAV/HBV vaccine – Twinrix-Junior®
One month post-second dose of HAV/HBV vaccine, 99.5–100% of subjects had an anti-HAV level of ≥ 20 IU/L. A 56–97-fold increase of anti-HAV GMTs was observed post-second dose administration (Table 2). An anamnestic response post-second dose was observed in 98.5–99.5% of subjects.
HBV immunogenicity results
Results for anti-HBs in the two study groups were different post-first dose of HAV/HBV vaccine but not post-second dose. With separate administration of the first dose of HAV/HBV and HPV vaccine, there was a higher proportion of subjects with detectable anti-HBs (72.9% vs. 56.5%; P < 0.0001) or a seroprotective anti-HBs level (59.3% vs. 43.5%; P = 0.02). The GMTs were 12.5 IU/L and 7.3 IU/L in Group Sep and Group Co-adm, respectively (P = 0.053) (Table 2). One month post-second dose, no statistically significant difference persisted between the two study groups (all P > 0.05). In both study groups, 98.1–99% of subjects had detectable anti-HBs and 97.5–97.6% a seroprotective anti-HBs level. A 158–234-fold increase of anti-HBs GMTs were observed post-second dose administration (Table 2). An anamnestic response was observed in 97.0- 97.6% of subjects.
HPV Immunogenicity results
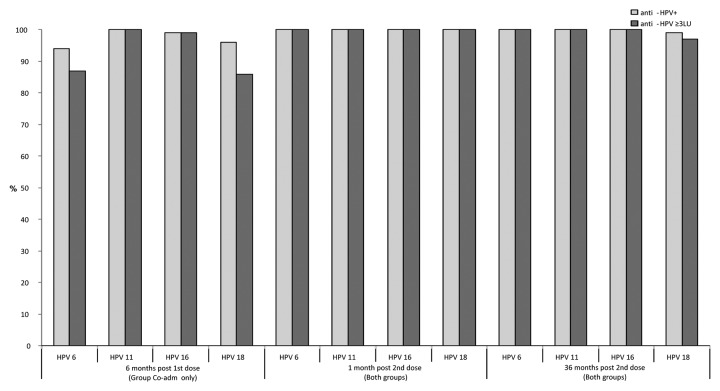
Six months post-first dose of qHPV vaccine administration (Group Co-adm) and before the second dose, 94%, 100%, 99% and 96% had detectable antibodies and 87%, 100%, 99%, and 86% had an anti-HPV titer ≥ 3 LU to HPV 6, 11, 16 and 18, respectively. The GMTs were 11, 71, 42 and 12 LU for HPV 6, HPV 11, HPV 16 and HPV 18, respectively (Table 3).
Table 3. Anti-HPV GMTs at different study time points (LU*; 95% CI; ATP analysis).
| GMTs | 6 mo after 1st dose of qHPV vaccine** | 1 mo after 2nd dose of qHPV vaccine | 36 mo after 2nd dose of qHPV vaccine | ||
|---|---|---|---|---|---|
| Group Co-adm n = 207 |
Group Co-adm n = 207 |
Group Sep n = 199 |
Group Co-adm n = 182 |
Group Sep n = 177 |
|
| Anti-HPV 6 | 11 (10–14) |
1103 (989–1230) |
1220 (1094–1362) |
77 (66–91) |
85 (72–101) |
| Anti-HPV 11 | 71 (62–81) |
3897 (3575–4248) |
4136 (3773–4533) |
308 (268–353) |
336 (291–388) |
| Anti-HPV 16 | 42 (36–49) |
3287 (2977–3628) |
3543 (3224–3893) |
286 (243–336) |
330 (283–384) |
| Anti-HPV 18 | 12 (10–14) |
887 (791–994) |
993 (890–1109) |
45 (37–55) |
60 (50–71) |
LU- Luminex Units; **qHPV vaccine - Gardasil®
One month post-second dose of qHPV vaccine, all subjects (100%) in both study groups had an antibody titer ≥ 3 LU to all 4 HPV types included in the vaccine. A 55 to 100-fold increase of GMTs was observed post-second dose administration when compared with pre-second dose (6 mo post-first dose). No statistically significant difference was observed in anti-HPV seropositivity rates or GMTs in the two study groups (Table 3). A ≥ 4-fold antibody level increase post-second dose administration was observed in 98–99% of subjects. The 6 subjects who did not have at least a 4-fold anti-HPV titer increase already had high titers pre-second dose. All 17 subjects with undetectable antibodies 6 mo post-first dose showed an anamnestic response post-second dose, with antibody titers varying from 79 to 2901 LU.
Thirty-six months post-second dose of qHPV vaccine all but four subjects (99%) had detectable antibodies to HPV 18 and all (100%) had detectable antibodies to HPV 6, 11 and 16. The great majority (97–100%) had also an anti-HPV equal or greater than 3 LU (Fig. 2). GMTs varied from 45 to 336 LU depending on the HPV type (Table 3). These GMTs were 11 to 18-fold lower when compared with those observed one month post-second dose.
Figure 2. Proportion of subjects with detectable HPV antibodies and with anti-HPV titer ≥ 3 LU
Discussion
To our knowledge, this is the first clinical trial assessing the potential interference when co-administering qHPV and combined HAV/HBV vaccine. For anti-HBs seroconversion/seroprotection and anti-HBs GMTs, our results are consistent with those previously reported.12,13 Namely, post-first dose of vaccine lower seroprotection/seroconversion rates and lower anti-HBs GMTs were observed when the two vaccines were co-administered when compared with administration of the two vaccines one month apart. However, in our study the above mentioned differences were no longer statistically significant post-second dose administration. It is of note that the GMTs observed one month post-second dose in the co-administration group was 170-fold higher than the recognized seroprotective threshold for hepatitis B.22 Similarly, the anti-HAV GMTs in both study groups were more than 100-fold higher than the antibody titers cited in several publications as seroprotective.23-25 Thus, the differences in anti-HAV and anti-HBs GMTs observed one month post-second dose of vaccine should have no clinical significance.
This study is also the first to assess the presence of antibodies after a single dose of the qHPV vaccine. Six months after a single dose of qHPV vaccine, the great majority of subjects (94–100%; n = 207) had detectable anti-HPV to genotypes included in the vaccine and subjects without detectable antibodies demonstrated an anamnestic response post-second dose of vaccine. The high magnitude of the immune response to the second dose of qHPV (55–100-fold increase in the GMTs) indicates excellent priming after a single dose of vaccine. Given these results, preadolescent girls vaccinated with qHPV appear well protected by two-doses administered at 0 and 6 mo and, in absence of the immediate risk of being infected the second dose of the three doses schedule (0, 2, 6 mo) does not seem necessary. The kinetics of antibodies observed during the first three years post-second dose is similar to those reported after three doses in older females in which vaccine efficacy was demonstrated.8,26 Additionally, the pattern of antibody kinetics in our study is consistent with that reported recently in another Canadian study which compared the immunogenicity of two-dose schedule (0–6 mo; given at the age of 9–13) with that observed after three-dose schedule (0–2-6 mo; given at the age of 9–13 or 16–26)27 and with those observed in a study conducted in Vietnam with alternative three-dose schedules (0–2-6; 0–3-9; 0–6-12 and 0–12–24 mo; with the start of vaccination at the age of 11–13).13 Although direct comparison of results among these three studies cannot be done because of different study designs, different populations and different laboratory assays used, the high consistency of results reported 32–36 mo after vaccination with two or three doses of qHPV vaccine are encouraging.
In our study, 4 out of 366 tested subjects (1.1%) had no detectable antibodies to HPV18- 36 mo post-second dose. This is also consistent with previous studies’ results observed after two or three doses of qHPV vaccine when using total IgG assay21,27,28 which has a higher sensitivity when compared with Merck cLIA assay, especially for the detection of antibodies to HPV18.21,
As data from previous follow-up studies indicate an excellent protection even in individuals with very low or undetectable post-vaccination antibodies,29,30 the excellent persistence of antibodies in preadolescent girls vaccinated three years earlier with two doses of qHPV suggests that their protection will be retained. At present, the duration of HPV vaccine induced protection remains unknown. It is unclear if a booster dose of HPV vaccine will be required to maintain protection in women who have received either two or three doses of vaccine. However, if two doses of HPV could provide similar protection to a three dose schedule, the resources saved by eliminating a dose could be applied to further extend existing HPV immunization programs. Additionally, the use of two doses schedule makes the program more attractive financially, less health resource demanding and allows diminishing the number of vaccination related injections.
This study has some limitations. First, immunosuppressed individuals were excluded from this study and the observed immune response should not be extrapolated to this population. Second, this was a single-center open-label study. However, participants were randomly assigned to receive the two vaccines during the same vaccination session or one month apart. Thus, the open-label design should not have impacted the immunogenicity results observed in two study groups. Third, we used a 0–6 mo vaccination schedule and the results might be different when using other vaccination schedules, especially schedules with short intervals between doses (e.g., 0–1 or 0–2 mo; when a “prime-prime” effect is expected). Forth, virtually all subjects in this study were Caucasians and we cannot exclude that immunogenicity of the alternative schedule we used might differ in non-Caucasians. However, studies conducted with the HPV vaccines in Asia and Africa do not indicate any meaningful difference in immune response in these populations and in the Caucasian population.31-33 Additionally, only girls were eligible to participate in this study. The decision regarding study population was based on girls only eligibility to publicly funded provincial HPV immunization program. Another limitation is that we measured anti-HPV titers post-first dose only in subjects belonging to the Group Co-adm. Thus, we cannot exclude that different results might have been observed after a single dose of qHPV given alone. However, based on previous studies results which showed no interference for HPV components when HPV vaccines were co-administered with HBV vaccines12,13 our hypothesis is that when given alone the first dose of qHPV should be at least as immunogenic as when given in co-administration. Finally, in this study the presence of antibody and the antibody levels were measured 6 mo post-first dose and by doing so we may have underestimated the proportion of subjects who seroconverted and the GMTs that were present 4–6 wk post-first dose. This hypothesis is supported by the magnitude of immune response to the second dose of vaccine observed in subjects with no detectable antibody 6 mo post-first dose.
In conclusion, qHPV and combined HAV/HBV can be given during the same vaccination session. A two-dose schedule (0, 6 mo) induces a strong immune response to all components included in these two vaccines. This schedule was therefore chosen for the Quebec publicly funded school-based immunization program in preadolescents.34 For qHPV, WHO Strategic Advisory Group of Experts also recently recommended for consideration a two-dose schedule for preadolescent girls.35
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest in relation to this study to be declared.
Acknowledgments
This study was financially supported by the Quebec Ministry of Health and Social Services and is part of the Quebec HPV immunization program evaluation plan. We are grateful to all study participants and their parents or guardians, to our research coordinators, research nurses and research technicians. We are thankful to our colleagues from the Laboratoire de Santé Publique du Québec and from the BC Centre for Disease Control, Carole Dagenais, René Lamirande, Darrel Cook and Simon Dobson for their contribution in the realization of this study.
References
- 1.Plotkin SA, Orenstein WA, Offit PA. Vaccines. Sixth Edition ed. Philadelphia: Saunders Elsevier; 2012. [Google Scholar]
- 2.Villa LL. . Prophylactic HPV vaccines: Reducing the burden of HPV-related diseases. Vaccine 2006; 24:S-23 - 8; PMID: 16194583 [DOI] [PubMed] [Google Scholar]
- 3.GlaxoSmithKline. Cervarix - Efficacity and Immunogenicity. GSKVaccinesDirect.com 2013; https://www.gskvaccines.ca/gsk.ca/CA/htdocs/products/CERVARIX/product_info_efficacy.htm. Accessed July 10th, 2013.
- 4.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, González P, Solomon D, Jiménez S, Schiller JT, et al. , CVT Vaccine Group. . Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011; 103:1444 - 51; http://dx.doi.org/ 10.1093/jnci/djr319; PMID: 21908768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einstein MH, Baron M, Levin MJ, Chatterjee A, Fox B, Scholar S, Rosen J, Chakhtoura N, Meric D, Dessy FJ, et al. , HPV-010 Study Group. . Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum Vaccin 2011; 7:1343 - 58; http://dx.doi.org/ 10.4161/hv.7.12.18281; PMID: 22048173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, et al. , CVT Group. . Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013; 6:1242 - 50; http://dx.doi.org/ 10.1158/1940-6207.CAPR-13-0203; PMID: 24189371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. , HPV Vaccine Adolescent Study Investigators Network. . Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health 2007; 40:564 - 71; http://dx.doi.org/ 10.1016/j.jadohealth.2007.02.015; PMID: 17531764 [DOI] [PubMed] [Google Scholar]
- 8.Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. . Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793 - 802; http://dx.doi.org/ 10.1001/jama.2013.1625; PMID: 23632723 [DOI] [PubMed] [Google Scholar]
- 9.Gilca V, Dionne M, Boulianne N, Murphy D, De Wals P, De Serres G. Immunogenicity of two peadiatric doses of Twinrix and Recombivax-HB and the effect of a booster dose given seven years later. Poster presentation. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases. Belgium, Brussels”, June 9-13. 2009.
- 10.Van Damme P, Van der Wielen M. . Combining hepatitis A and B vaccination in children and adolescents. Vaccine 2001; 19:2407 - 12; http://dx.doi.org/ 10.1016/S0264-410X(00)00464-3; PMID: 11257370 [DOI] [PubMed] [Google Scholar]
- 11.Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES 3rd, Kollmann TR. . Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30:3572 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.051; PMID: 22469863 [DOI] [PubMed] [Google Scholar]
- 12.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al. . Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin 2011; 7:1374 - 86; http://dx.doi.org/ 10.4161/hv.7.12.18322; PMID: 22048171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. . Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 Years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. J Infect Dis 2013; 208:1325 - 34; http://dx.doi.org/ 10.1093/infdis/jit363; PMID: 23901077 [DOI] [PubMed] [Google Scholar]
- 14.Van Damme P, Leroux-Roels G, Law B, Diaz-Mitoma F, Desombere I, Collard F, Tornieporth N, Van Herck K. . Long-term persistence of antibodies induced by vaccination and safety follow-up, with the first combined vaccine against hepatitis A and B in children and adults. J Med Virol 2001; 65:6 - 13; http://dx.doi.org/ 10.1002/jmv.1094; PMID: 11505437 [DOI] [PubMed] [Google Scholar]
- 15.Wheeler CM, Bautista OM, Tomassini JE, Nelson M, Sattler CA, Barr E, Protocol 11 study Investigators. . Safety and immunogenicity of co-administered quadrivalent human papillomavirus (HPV)-6/11/16/18 L1 virus-like particle (VLP) and hepatitis B (HBV) vaccines. Vaccine 2008; 26:686 - 96; http://dx.doi.org/ 10.1016/j.vaccine.2007.11.043; PMID: 18164106 [DOI] [PubMed] [Google Scholar]
- 16.Schmeink CE, Bekkers RL, Josefsson A, Richardus JH, Berndtsson Blom K, David MP, Dobbelaere K, Descamps D. . Co-administration of human papillomavirus-16/18 AS04-adjuvanted vaccine with hepatitis B vaccine: randomized study in healthy girls. Vaccine 2011; 29:9276 - 83; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.037; PMID: 21856349 [DOI] [PubMed] [Google Scholar]
- 17.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, et al. , Protocol 016 Study Group. . Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006; 118:2135 - 45; http://dx.doi.org/ 10.1542/peds.2006-0461; PMID: 17079588 [DOI] [PubMed] [Google Scholar]
- 18.Duval B, Gîlca V, Boulianne N, Deceuninck G, Rochette L, De Serres G. . Immunogenicity of two paediatric doses of monovalent hepatitis B or combined hepatitis A and B vaccine in 8-10-year-old children. Vaccine 2005; 23:4082 - 7; http://dx.doi.org/ 10.1016/j.vaccine.2004.07.022; PMID: 15963363 [DOI] [PubMed] [Google Scholar]
- 19.Gilca V, De Serres G, Boulianne N, Murphy D, De Wals P, Ouakki M, Trudeau G, Massé R, Dionne M. . Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine 2013; 31:448 - 51; http://dx.doi.org/ 10.1016/j.vaccine.2012.11.037; PMID: 23206974 [DOI] [PubMed] [Google Scholar]
- 20.Gilca V, De Serres G, Boulianne N, Murphy D, Ouakki M, De Wals P, Trudeau G, Massé R, Dionne M. . Long-term persistence of immunity after vaccination of pre-adolescents with low doses of a recombinant hepatitis B vaccine. Hum Vaccin Immunother 2013; 9:1685 - 90; http://dx.doi.org/ 10.4161/hv.25015; PMID: 23744506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, McNeil S, Money D, Dionne M, Palefsky J, et al. . Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014; 32:624 - 30; http://dx.doi.org/ 10.1016/j.vaccine.2013.09.007; PMID: 24055350 [DOI] [PubMed] [Google Scholar]
- 22.European, Consensus, Group. Are booster immunisations needed for lifelong hepatitis B immunity?. Lancet 2000; 355:561 - 5; http://dx.doi.org/ 10.1016/S0140-6736(99)07239-6; PMID: 10683019 [DOI] [PubMed] [Google Scholar]
- 23.Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, Bell BP, McMahon BJ. . Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis 2008; 198:1776 - 82; http://dx.doi.org/ 10.1086/593335; PMID: 18976095 [DOI] [PubMed] [Google Scholar]
- 24.Wolters B, Müller T, Ross RS, Clauberg R, Werfel U, Roggendorf H, Siggelkow C, Hausen T, Roggendorf M. . Comparative evaluation of the immunogenicity of combined hepatitis A and B vaccine by a prospective and retrospective trial. Hum Vaccin 2009; 5:248 - 53; http://dx.doi.org/ 10.4161/hv.5.4.7051; PMID: 19276678 [DOI] [PubMed] [Google Scholar]
- 25.Bian GL, Ma R, Dong HJ, Ni HX, Hu FJ, Chen YR, Chen JQ, Zhou SY, Lin YX, Xu GZ. . Long-term clinical observation of the immunogenicity of inactivated hepatitis A vaccine in children. Vaccine 2010; 28:4798 - 801; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.096; PMID: 20471440 [DOI] [PubMed] [Google Scholar]
- 26.Schwarz TF, Spaczynski M, Schneider A, Wysocki J, Galaj A, Schulze K, Poncelet SM, Catteau G, Thomas F, Descamps D. . Persistence of immune response to HPV-16/18 AS04-adjuvanted cervical cancer vaccine in women aged 15-55 years. Hum Vaccin 2011; 7:958 - 65; http://dx.doi.org/ 10.4161/hv.7.9.15999; PMID: 21892005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. . Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793 - 802; http://dx.doi.org/ 10.1001/jama.2013.1625; PMID: 23632723 [DOI] [PubMed] [Google Scholar]
- 28.Brown B, Blas M, Cabral A, Carcamo C, Gravitt P, Halsey N. . Randomized trial of HPV4 vaccine assessing the response to HPV4 vaccine in two schedules among Peruvian female sex workers. Vaccine 2012; 30:2309 - 14; http://dx.doi.org/ 10.1016/j.vaccine.2012.01.058; PMID: 22306855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al. . HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 2008; 26:6844 - 51; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.073; PMID: 18930097 [DOI] [PubMed] [Google Scholar]
- 30.Bonanni P, Boccalini S, Bechini A. . Efficacy, duration of immunity and cross protection after HPV vaccination: a review of the evidence. Vaccine 2009; 27:Suppl 1 A46 - 53; http://dx.doi.org/ 10.1016/j.vaccine.2008.10.085; PMID: 19480962 [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa H, Ebihara K, Tanaka Y, Noda K. . Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Sci 2013; 104:465 - 72; http://dx.doi.org/ 10.1111/cas.12106; PMID: 23331518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL, Zhang L, Wang W, Zhong X, Saah AJ. . Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double-blind, placebo-controlled trial in Chinese males and females. Vaccine 2012; 30:4284 - 91; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.079; PMID: 22433961 [DOI] [PubMed] [Google Scholar]
- 33.Watson-Jones D, Brown J, Baisley K, Balthazar B. Kapiga S. [Internet]. Impact of malaria and helminth infections on HPV vaccine innunogenicity in Tanzanian females. 28th International Papillomavirus Conference - Puerto Rico CLINICAL SCIENCE New vaccine trials (Phase I-III; preventive and therapeutic trials) Page 209. Available from http://www.hpv2012pr.org/HPV2012_PUERTO_RICO_Abstract%20Clinical_Sciences.pdf Accessed on December 2, 2013.
- 34.Ministère de la Santé et des Services sociaux. Virus du papillome humain [En ligne] http://www.msss.gouv.qc.ca/sujets/santepub/vaccination/index.php?programme-de-vaccination-contre-le-vph (Page accédée le 5 juin 2014).
- 35.World Health Organisation. Immunization, Vaccines and Biologicals - Summary of the SAGE April 2014 meeting. [On line] http://www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en/ (Page accessed June 5, 2014).