Abstract
Rotavirus (RV) is the most common etiological agent causing acute gastroenteritis (GE) in children aged <5 years. This cross-sectional, hospital-based surveillance study (NCT01201252) was designed to investigate RVGE disease burden. It was conducted from July 2009–July 2010 at 3 referral hospitals in the United Arab Emirates (UAE). Children who had been hospitalized for acute GE were enrolled with informed consent. Stool samples were tested for RV using enzyme immunoassay and RV-positive samples were further typed using reverse transcriptase-polymerase chain reaction and reverse hybridization to determine the G and P types. GE data were collected from medical charts and GE severity was assessed through clinical examination. Treatment and outcome were prospectively recorded. Among 6323 children hospitalized due to any reason, 771 (12.2%) presented acute GE and were enrolled, of whom 758 (98.3%) were included in the final analysis. Acute GE and RVGE accounted for 12.0% (758/6323) and 6.0% (381/6323) of all hospitalizations, respectively. RVGE accounted for 50.3% (381/758) of GE hospitalizations and predominantly affected, children younger than 2 years (66.1%; 252/381). The severity of GE before hospitalization was significantly associated with RV-positive status (P = 0.0031). The majority (>95%) of children received intravenous hydration during hospitalization. RVGE occurred throughout the year, with a subtle winter peak in February 2010 (63.6%; 56/88). G1WTP[8]WT was the most commonly detected RV strain (56.3%) in 268 analyzed samples. RV was a major cause of GE-hospitalizations in children under 5 years in the UAE; the highest number of RVGE cases was observed in children younger than 2 years.
Keywords: rotavirus, gastroenteritis, United Arab Emirates, epidemiology, diarrheal hospitalizations
Introduction
Rotavirus (RV) has been established as the single most important cause of worldwide acute gastroenteritis (GE), resulting in about 453 000 deaths and approximately 2.4 million hospital admissions among children younger than 5 y each year.1-3 In 2009, data from a global RV surveillance network, including 10 countries from the Eastern Mediterranean region, estimated that approximately 38% children (range 14–54%) younger than 5 y who had been hospitalized for diarrhea were RV-positive.4 Furthermore, an epidemiological study on the prevalence and seasonality of RV, conducted in 1994 in pediatric wards in the United Arab Emirates (UAE), reported that approximately 21% of acute GE hospitalizations in children younger than 10 y were attributable to RV; the highest proportion (89.3%) occurred in children under 5 y of age.5
Literature on RV disease burden has shown that the incidence of RV is similar in low and high-income countries. Nevertheless, diarrhea-related mortality is low in high-income countries where there is better access to health-care and generally better-nourished populations.6 This highlights that although routine strategies, such as improved sanitation, are effective in reducing the diarrheal disease associated with bacterial and parasitic agents, they may have less impact upon RV disease burden.7 Indeed, the World Health Organization (WHO) recommends RV vaccination as the most effective strategy to prevent RV-related morbidity and mortality.8
Two live, oral RV vaccines have been commercially available since 2006: Rotarix™ (GlaxoSmithKline) and RotaTeq® (Merck and Co. Inc.). Thereafter, considerable reductions in RV-attributable morbidity and mortality, and all-cause diarrhea have been recorded in the high and middle-income countries where RV vaccination was introduced.9-14
The UAE introduced a RV vaccine into its national immunization schedule in June 2013.15 However, as there were no recent data on the local disease burden or circulating RV strains, it would be difficult to measure the impact of vaccination. This hospital-based surveillance study was therefore designed to estimate the proportion of RVGE among GE hospitalizations in young children, as well as assessing: the proportion of acute GE and RVGE among all hospitalizations; GE episode severity; disease management according to RV status; RVGE seasonality and the prevalence of RV strains.
Results
Demographic characteristics
A total of 6323 children were admitted to any of the 3 hospitals between July 2009 and July 2010. Of these, 799 were screened for acute GE and 771 (12.2%) were enrolled in our study; 28 children were not eligible and the reasons for not including them were not captured. Thirteen children were excluded from the final analysis due to at least one of the following: stool samples collected outside the specified time frame of 4–10 d after GE onset (n = 7); greater than 5 y (n = 2); informed consent provided after enrollment (n = 5).
The final analysis therefore included 758 (98.3%) children with a median age of 17 mo (range 0–59 mo); 55.5% (421/758) were male and 99.5% (754/758) lived in the same city as their admitting hospital.
Hospitalization due to acute GE and RVGE
Among all hospitalizations, acute GE and RVGE accounted for 12.0% (95% CI: 11.2–12.8; 758/6323) and 6.0% (95% CI: 5.4–6.6; 381/6323), respectively. The proportion of acute GE hospitalizations attributable to RVGE was 50.3% (95% CI: 46.6–53.9; 381/758).
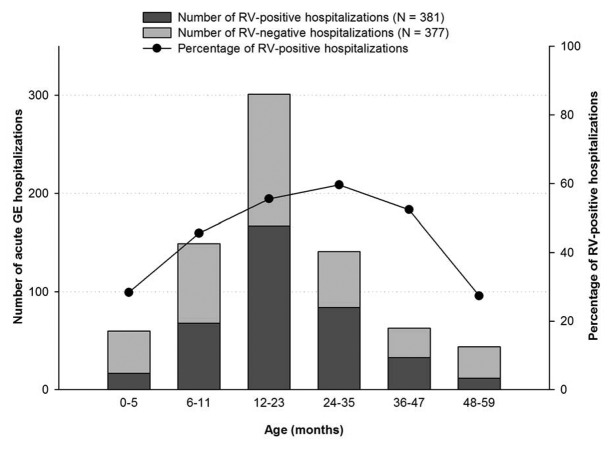
Of the 381 RV-positive children, 88.1% (336) were younger than 3 y (Fig. 1). The highest number of RVGE hospitalizations occurred in children between 12 and 23 mo of age (55.5%; 167/301) and the lowest number was in those aged 48 to 59 mo (27.3%; 12/44).
Figure 1. Age distribution of children hospitalized with acute GE and RVGE (n = 758). Footnote: Note, N: Number of children included in the final analysis.
Clinical characteristics
Before hospitalization, 42.3% (161/381) of RV-positive children and 31.0% (117/377) of RV-negative children had suffered a severe episode of GE, as assessed by the Vesikari scale (Table 1). Exploratory analysis showed that the association between RV-positive status and GE severity was statistically significant (P = 0.0031). Vomiting was reported in 363/381 (95.3%) of RV-positive and 324/377 (85.9%) of RV-negative children (Table 1).
Table 1. Clinical characteristics by RV status (n = 758).
| Symptoms and signs | Number (%) of children | |
|---|---|---|
| RV-positive 381 (50.3) |
RV-negative 377 (49.7) |
|
| Severity before hospitalization* | ||
| Mild (< 7) | 51 (13.4) | 72 (19.1) |
| Moderate (7–10) | 169 (44.4) | 188 (49.9) |
| Severe (≥ 11) | 161 (42.3) | 117 (31.0) |
| Symptoms before hospitalization | ||
| Diarrhea | 367 (96.3) | 345 (91.5) |
| Vomiting | 363 (95.3) | 324 (85.9) |
| Fever | 209 (54.9) | 212 (56.2) |
| Duration of diarrhea before hospitalization | ||
| 1–4 d | 335 (91.3) | 303 (87.8) |
| 5 d | 22 (6.0) | 15 (4.3) |
| ≥ 6 d | 10 (2.7) | 27 (7.8) |
| Treatment received during hospitalization | ||
| Oral rehydration | 361 (94.8) | 330 (87.5) |
| Intravenous rehydration | 377 (99.0) | 359 (95.2) |
| Antibiotics | 39 (10.2) | 72 (19.1) |
| Outcome at discharge | ||
| Recovered | 337 (99.0) | 370 (98.1) |
| Ongoing | 4 (1.0) | 5 (1.3) |
| Unknown | 0 (0.0) | 1 (0.3) |
Severity of RVGE based on 20-point Vesikari scale, N, Number of children in the final analysis.
The mean (± standard deviation) duration of hospitalization was 3.19 (±1.34) days for RV-positive children and 3.45 (±1.90) days for RV-negative children. The majority (>95%) of children received intravenous hydration during hospitalization irrespective of their RV status (Table 1). No deaths occurred during the study period and nearly all (>98%) children had recovered at discharge.
RV strain distribution
Of 268 (70.3%) RV-positive stool samples available for typing, G1WT was the most commonly detected G type (60.4%; 162) and P[8]WT was the most commonly detected P type (81.3%; 218) in all the 3 hospitals. The most prevalent RV type combinations detected were G1WTP[8]WT (56.3%; 151/268) and G9P[8]WT (12.7%; 34/268) (Fig. 2).
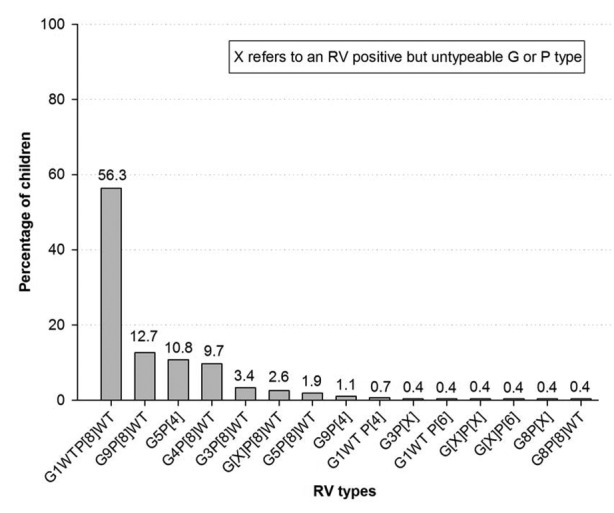
Figure 2. Distribution of RV strains in RV-positive stool samples (n = 268). Footnote: Note, N: Number of RV positive children; WT: Wild type.
Seasonal distribution
RVGE occurrence was highest between February and April 2010 and peaked during February 2010 (63.6%; 56/88; Fig. 3). P[8]WT was prevalent throughout the year. G1WT accounted for most cases throughout the year except during July 2010, when G2 was detected in all the RV-positive stool samples.
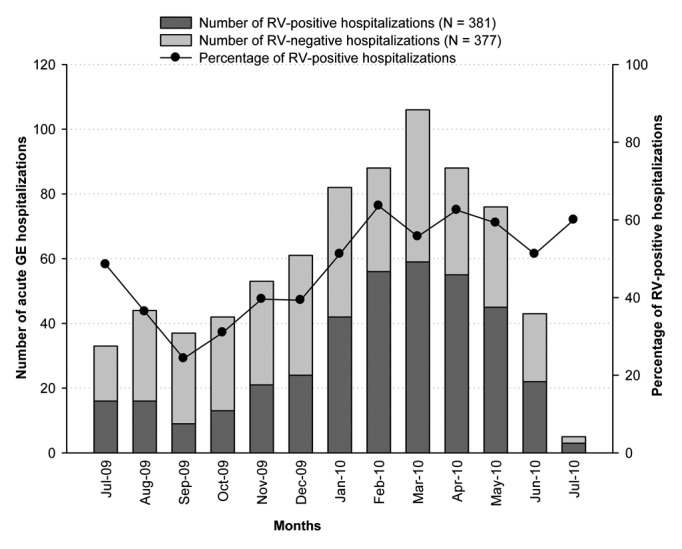
Figure 3. Seasonal distribution of hospitalization for acute GE and RVGE (n = 758). Footnote: Note 1: N, Number of children included in the final analysis. Note 2, Jul-09 and Jul-10 do not account for full months.
Discussion
This hospital-based, 1-y surveillance study found that RV was a major cause of acute GE in children younger than 5 y in the UAE, and accounted for 50.3% of all acute GE-related hospitalizations. These findings are consistent with recent estimates for RVGE of 16–61% in children under 5 y from Middle Eastern and North African countries,16 but higher than the 21% previously reported for the pediatric population in the UAE.5 This could be due to factors such as the improved sensitivity of the EIA test used in the current study (100% sensitivity and 97% specificity for RV),17 the inclusion of all children hospitalized for acute GE and the active surveillance methodology followed in the study.
As previously observed in the Eastern Mediterranean region18-21 and Europe,22 we found the burden of RVGE to be the highest in children younger than 2 y. More specifically, RVGE was found predominantly in the 7–12 mo age group in a study conducted in 1994 in UAE (34.8%).5
As previously described, we found an association between GE severity on the Vesikari scale and RV-positive status.23-26 The average hospital stay for children with RVGE in our study was 3.2 d, compared with 2.6 d previously reported by Howidi et al. in the UAE.27
As previously reported for Middle Eastern countries,16 G1WTP[8]WT was the most commonly detected RV strain in our study. However, RV strain distribution has been shown to vary greatly among the Middle Eastern countries, for example, in Iran, G4P[8] is the most common RV strain,18 while a varied RV strain distribution is observed in Oman.28 Indeed, WHO surveillance data across the Eastern Mediterranean region indicated substantial strain diversity in the RV types circulating in this region.29
RVGE hospitalizations occurred throughout the year with a subtle peak in winter. This has also been observed in Eastern Mediterranean countries including Iran,18 Oman28 and Saudi Arabia.30 In the present study, hospitalizations among RV-negative children were higher than RV-positive children during Jul-09 to Dec-09. Previous studies have shown that several microorganisms including viruses other than RV, bacteria and protozoa cause GE in children.31 These microorganisms might be responsible for the increased number of hospitalizations among RV-negative children in the UAE. However, stool samples in our study were tested only for RV and other potential causes of GE were not systematically assessed.
Some of the limitations of our study were that we did not capture the reasons for excluding 28 children who were screened, but not enrolled. We only considered children hospitalized for acute GE and did not collect data from children treated in emergency rooms or as out-patients. We did not explore potential regional differences although none have been previously described for the UAE. Although we were only able to type 70.3% of the RV positive samples, our estimates do help understand the RV-attributable fraction of GE that can be linked to the direct costs of hospitalization.
A major strength of our study was that it was conducted according to the WHO generic protocol for RVGE surveillance in children younger than 5 y.32 RV typing was also performed in a single certified laboratory. This was the first cross-sectional, multicenter, hospital-based surveillance in the UAE to assess the disease burden associated with GE and RVGE hospitalization, and the only study to have assessed RV strain prevalence. The number of subjects with acute GE enrolled in the study was high and thus minimized the potential for selection bias. Furthermore, since the study was conducted in 3 large pediatric referral hospitals across the country, the findings are likely to be representative of the whole population.
The incidence of RV disease is similar in both industrialized and developing countries, which suggests that improvements in water supply, hygiene, and sanitation may not adequately control the disease.3 Hence additional interventions such as implementing effective immunization against RV into the routine vaccination programs is an important public health measure to be considered for UAE. Indeed, the neighboring countries of Bahrain, Iraq and Morocco have already introduced RV vaccine in their immunization schedules in accordance with the WHO recommendations.33 Our study data might be useful as a reference baseline for policy makers to take an informed decision about the introduction of universal RV vaccination in the UAE, thus providing a measurable benefit to the health of younger children, the population mainly affected by RVGE.
Data from this cross-sectional, multicenter, hospital-based surveillance study showed RV to be a major cause of acute GE-related hospitalizations in children younger than 5 y in the UAE, and particularly among children under 2 y of age. These data may assist policy makers in setting priorities for and assessing the impact of introducing preventive interventions in the UAE.
Patients and Methods
Study design
This cross-sectional, multicenter, hospital-based, observational study was conducted from 2nd July 2009 to 19th July 2010 at 3 referral pediatric hospitals in the UAE: Al-Qassimi Hospital, the largest hospital in Sharjah, serving a population of approximately 800 000; Sheikh Khalifa Medical City, a tertiary hospital in Abu Dhabi with dedicated pediatric wards and a catchment of approximately 800 000 and Al-Ain Hospital in Al-Ain, one of the largest hospitals serving a population of approximately 60 000. The study was undertaken in accordance with the WHO generic protocol for hospital-based surveillance to estimate the burden of RVGE in children less than 5 y of age.32
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the national independent ethics committee and the research and ethics committees of the participating hospitals. Written informed consent was obtained from parents/guardians of all children before enrollment.
Case definition
Acute GE was defined according to the WHO as ‘three or more loose stools and/or two or more vomiting episodes within a 24 h period’, with the onset of symptoms ≤14 d before admission.32 The severity of each case was categorized according to the 20-point Vesikari scale, where a score ≥11 was considered to be severe.34
Inclusion and exclusion criteria
All children under 5 y of age, who were hospitalized due to acute GE, were enrolled. Children with possible nosocomial infections (onset of GE 48 h after admission) were excluded from the study.
Data collection
Demographic and GE episode symptom data were collected for all enrolled children and their clinical signs were recorded following medical examination. The duration of hospitalization and disease outcome were prospectively recorded.
Laboratory analyses
Stool samples were collected from all children and were tested on-site for the presence of RV using an enzyme immunoassay (EIA; Premier™ Rotaclone®; Meridian Bioscience).35 All RV-positive samples were further tested at the DDL Diagnostic Laboratory, The Netherlands, by reverse transcriptase-polymerase chain reaction for VP7 and VP4 genes, followed by a reverse hybridization assay and direct sequencing analysis, if necessary, to determine G and P types.36 If the specific VP4 or VP7 genes could not be amplified and were untypeable, possibly due to low viral load or the presence of aberrant viral variants, the G or the P types were denoted ‘X’.
Statistical analyses
All statistical analyses were performed using the statistical analysis system (SAS) version 9.2 (SAS Institute Inc.).
As data on population risk were not available, it was not possible to determine sample size estimates using statistical methods. The target enrollment therefore considered all children under 5 y of age who were hospitalized for acute GE at the referral hospitals during a 1-y period. The proportion of RVGE hospitalizations among acute GE hospitalizations and the proportion of acute GE and RVGE hospitalizations among all hospitalizations due to any cause were calculated with exact 95% Clopper-Pearson confidence intervals (CI)37 using ProcStatXact 7.0. Statistical comparisons between age, GE severity and duration of hospitalization, with RV status (positive vs. negative) were performed using the chi-square test (exploratory analysis). All analyses were descriptive and results were presented for the overall population, by center and by RV status.
Glossary
Abbreviations:
- CI
confidence interval
- EIA
enzyme immunoassay
- GE
gastroenteritis
- RV
rotavirus
- RVGE
rotavirus gastroenteritis
- UAE
United Arab Emirates
- WHO
World Health Organization
Disclosure of Potential Conflicts of Interest
K.G. and R.D. are both employed by the GlaxoSmithKline group of companies. R.D. declares to have stock options. L.J.V.D. is employed by and is shareholder of DDL Diagnostic Laboratory, which received a fee from GlaxoSmithKline Biologicals for rotavirus genotyping and MH’s institution, received a grant for conducting the study. M.H., G.B. and H.Y. have no competing interests to declare.
Acknowledgments
The authors thank Devi Priya and Shruti Bapna (GlaxoSmithKline Vaccines) for medical writing, Abdelilah Ibrahimi (XPE Pharma and Science on behalf of GlaxoSmithKline Vaccines) for publication coordination, and Julia Donnelly (freelance publication manager on behalf of GlaxoSmithKline Vaccines) for support in copy editing. The authors would also like to acknowledge Dr Ahmad Khalafalla and Runa Mithani for the coordination and monitoring of the study sites (both employed by GlaxoSmithKline group of companies).
Financial Disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analyses. GlaxoSmithKline Biologicals SA also paid all costs associated with the development and the publication of the present manuscript.
Trademark Statement
Rotarix is a trademark of the GlaxoSmithKline group of companies
Rotateq is a registered trademark of Merck and Co. Inc.
Rotaclone is a registered trademark of Meridian Bioscience, USA.
Ethical Statements
The trial was registered with clinicaltrials.gov (identifier NCT01201252) and was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice which has its origins in the Declaration of Helsinki. All participants provided written and informed consent.
Author Contributions
M.H. and G.B. were the coordinating investigators and together with H.Y. were responsible for the conduct of the study. K.G. contributed to the study design and performed the statistical analysis. L.J.V.D. was responsible for R.V. genotyping, and contributed to the analysis of the results. R.D. managed the study at GlaxoSmithKline Vaccines and contributed to the analysis, interpretation and critically reviewed the study report. All authors had access to the data and participated in the drafting, review and approval of the manuscript. The corresponding author took final responsibility for submitting the manuscript.
References
- 1.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. . Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/ 10.1086/605025; PMID: 19817620 [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. . Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/ 10.3201/eid0905.020562; PMID: 12737740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. . 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136 - 41; http://dx.doi.org/ 10.1016/S1473-3099(11)70253-5; PMID: 22030330 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). . Rotavirus surveillance --- worldwide, 2009. MMWR Morb Mortal Wkly Rep 2011; 60:514 - 6; PMID: 21527889 [PubMed] [Google Scholar]
- 5.Ijaz MK, Alharbi S, Uduman SA, Cheema Y, Sheek-Hussen MM, Alkhair AR, Shalabi AG, Ijaz SS, Bin-Othman SA, Sattar SA, et al. . Seasonality and prevalence of rotavirus in Al-Ain, United Arab Emirates. Clin Diagn Virol 1994; 2:323 - 9; http://dx.doi.org/ 10.1016/0928-0197(94)90002-7; PMID: 15566778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tate JE, Patel MM, Steele AD, Gentsch JR, Payne DC, Cortese MM, Nakagomi O, Cunliffe NA, Jiang B, Neuzil KM, et al. . Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9:395 - 407; http://dx.doi.org/ 10.1586/erv.10.17; PMID: 20370550 [DOI] [PubMed] [Google Scholar]
- 7.Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. . Rotavirus vaccines: current prospects and future challenges. Lancet 2006; 368:323 - 32; http://dx.doi.org/ 10.1016/S0140-6736(06)68815-6; PMID: 16860702 [DOI] [PubMed] [Google Scholar]
- 8.WHO. . Rotavirus vaccine and intussusception:report from an expert consultation. Wkly Epidemiol Rec 2011; 86:317 - 21; PMID: 21800466 [PubMed] [Google Scholar]
- 9.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. . Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/ 10.1086/652403; PMID: 20402596 [DOI] [PubMed] [Google Scholar]
- 10.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. . Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362:299 - 305; http://dx.doi.org/ 10.1056/NEJMoa0905211; PMID: 20107215 [DOI] [PubMed] [Google Scholar]
- 11.Yen C, Armero Guardado JA, Alberto P, Rodriguez Araujo DS, Mena C, Cuellar E, Nolasco JB, De Oliveira LH, Pastor D, Tate JE, et al. . Decline in rotavirus hospitalizations and health care visits for childhood diarrhea following rotavirus vaccination in El Salvador. Pediatr Infect Dis J 2011; 30:Suppl S6 - 10; http://dx.doi.org/ 10.1097/INF.0b013e3181fefa05; PMID: 21048524 [DOI] [PubMed] [Google Scholar]
- 12.Quintanar-Solares M, Yen C, Richardson V, Esparza-Aguilar M, Parashar UD, Patel MM. . Impact of rotavirus vaccination on diarrhea-related hospitalizations among children < 5 years of age in Mexico. Pediatr Infect Dis J 2011; 30:Suppl S11 - 5; http://dx.doi.org/ 10.1097/INF.0b013e3181fefb32; PMID: 21183834 [DOI] [PubMed] [Google Scholar]
- 13.Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, Coronado L, Patel MM, Parashar UD, Cortese MM. . Reduction of diarrhea-associated hospitalizations among children aged < 5 Years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J 2011; 30:Suppl S16 - 20; http://dx.doi.org/ 10.1097/INF.0b013e3181fefc68; PMID: 21183835 [DOI] [PubMed] [Google Scholar]
- 14.Braeckman T, Van Herck K, Raes M, Vergison A, Sabbe M, Van Damme P. . Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J 2011; 30:Suppl S21 - 4; http://dx.doi.org/ 10.1097/INF.0b013e3181fefc51; PMID: 21183836 [DOI] [PubMed] [Google Scholar]
- 15.Introduction of Rotavirus vaccine into the HAAD immunization schedule, 2013: [http://www.haad.ae/HAAD/LinkClick.aspx?fileticket=mTpWfMzPf4A%3D&tabid=183]. Accessed on 07 Feb, 2014. [Google Scholar]
- 16.Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM. . Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect Dis 2011; 11:9; http://dx.doi.org/ 10.1186/1471-2334-11-9; PMID: 21214934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manual of rotavirus detection and characterization methods, 2009: [http://whqlibdoc.who.int/hq/2008/who_ivb_08.17_eng.pdf]. Accessed on 07 Feb, 2014. [Google Scholar]
- 18.Eesteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M, Yaghini F, El Mohamady H, Patel M, Klena JD, et al. . Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis 2009; 200:Suppl 1 S244 - 7; http://dx.doi.org/ 10.1086/605050; PMID: 19821714 [DOI] [PubMed] [Google Scholar]
- 19.Ghazi HO, Khan MA, Telmesani AM, Idress B, Mahomed MF. . Rotavirus infection in infants and young children in Makkah, Saudi Arabia. J Pak Med Assoc 2005; 55:231 - 4; PMID: 16045090 [PubMed] [Google Scholar]
- 20.Nafi O. . Rotavirus gastroenteritis among children aged under 5 years in Al Karak, Jordan. East Mediterr Health J 2010; 16:1064 - 9; PMID: 21222424 [PubMed] [Google Scholar]
- 21.Malek MA, Teleb N, Abu-Elyazeed R, Riddle MS, Sherif ME, Steele AD, Glass RI, Bresee JS. . The epidemiology of rotavirus diarrhea in countries in the Eastern Mediterranean Region. J Infect Dis 2010; 202:Suppl S12 - 22; http://dx.doi.org/ 10.1086/653579; PMID: 20684691 [DOI] [PubMed] [Google Scholar]
- 22.Giaquinto C, van Damme P, REVEAL Study Group. . Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis 2010; 42:142 - 7; http://dx.doi.org/ 10.3109/00365540903380495; PMID: 19916900 [DOI] [PubMed] [Google Scholar]
- 23.Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al. , Rotavirus Study Group. . Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393 - 400; http://dx.doi.org/ 10.1542/peds.2008-2088; PMID: 19254975 [DOI] [PubMed] [Google Scholar]
- 24.Benhafid M, Rguig A, Trivedi T, Elqazoui M, Teleb N, Mouane N, Maltouf AF, Parashar U, Patel M, Aouad RE. . Monitoring of rotavirus vaccination in Morocco: establishing the baseline burden of rotavirus disease. Vaccine 2012; 30:6515 - 20; http://dx.doi.org/ 10.1016/j.vaccine.2012.08.058; PMID: 22959990 [DOI] [PubMed] [Google Scholar]
- 25.Benhafid M, Youbi M, Klena JD, Gentsch JR, Teleb N, Widdowson MA, Elaouad R. . Epidemiology of rotavirus gastroenteritis among children <5 years of age in Morocco during 1 year of sentinel hospital surveillance, June 2006-May 2007. J Infect Dis 2009; 200:Suppl 1 S70 - 5; http://dx.doi.org/ 10.1086/605048; PMID: 19817617 [DOI] [PubMed] [Google Scholar]
- 26.Kurugöl Z, Geylani S, Karaca Y, Umay F, Erensoy S, Vardar F, Bak M, Yaprak I, Ozkinay F, Ozkinay C. . Rotavirus gastroenteritis among children under five years of age in Izmir, Turkey. Turk J Pediatr 2003; 45:290 - 4; PMID: 14768791 [PubMed] [Google Scholar]
- 27.Howidi M, Al Kaabi N, El Khoury AC, Brandtmüller A, Nagy L, Richer E, Haddadin W, Miqdady MS. . Burden of acute gastroenteritis among children younger than 5 years of age--a survey among parents in the United Arab Emirates. BMC Pediatr 2012; 12:74; http://dx.doi.org/ 10.1186/1471-2431-12-74; PMID: 22708988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Awaidy SA, Bawikar S, Al Busaidy S, Baqiani S, Al Abedani I, Varghese R, Abdoan HS, Al Abdoon H, Bhatnagar S, Al Hasini KS, et al. . Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J Infect Dis 2009; 200:Suppl 1 S248 - 53; http://dx.doi.org/ 10.1086/605339; PMID: 19817605 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC). . Rotavirus surveillance--worldwide, 2001-2008. MMWR Morb Mortal Wkly Rep 2008; 57:1255 - 7; PMID: 19023263 [PubMed] [Google Scholar]
- 30.Kheyami AM, Nakagomi T, Nakagomi O, Dove W, Hart CA, Cunliffe NA. . Molecular epidemiology of rotavirus diarrhea among children in Saudi Arabia: first detection of G9 and G12 strains. J Clin Microbiol 2008; 46:1185 - 91; http://dx.doi.org/ 10.1128/JCM.02244-07; PMID: 18234870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott EJ. . Acute gastroenteritis in children. BMJ 2007; 334:35 - 40; http://dx.doi.org/ 10.1136/bmj.39036.406169.80; PMID: 17204802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Generic protocol for (i) hospital-based surveillance to estimate the burden of rotavirus among children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children: field test version, 2002. Accessed on 07 Feb, 2014. Available from: http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.15.pdf.
- 33.WHO vaccine-preventable diseases: monitoring system. 2013 global summary: Accessed 07 May, 2014 Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules?schedulecriteria%5Bregion%5D%5B%5D=EMRO&schedulecriteria%5Bdummy%5D=&schedulecriteria%5Bvaccine%5D%5B%5D=ROTAVIRUS&schedulecriteria%5BOK%5D=OK].
- 34.Ruuska T, Vesikari T. . Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259 - 67; http://dx.doi.org/ 10.3109/00365549009027046; PMID: 2371542 [DOI] [PubMed] [Google Scholar]
- 35.Sligo F, Jameson A, Comrie M. . New Zealand Polynesian women’s access to information about cervical screening. J Manag Med 1998; 12:361 - 9, 321; http://dx.doi.org/ 10.1108/02689239810245407; PMID: 10351261 [DOI] [PubMed] [Google Scholar]
- 36.van Doorn LJ, Kleter B, Hoefnagel E, Stainier I, Poliszczak A, Colau B, Quint W. . Detection and genotyping of human rotavirus VP4 and VP7 genes by reverse transcriptase PCR and reverse hybridization. J Clin Microbiol 2009; 47:2704 - 12; http://dx.doi.org/ 10.1128/JCM.00378-09; PMID: 19553575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clopper CJ, Pearson ES. . The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934; 26:404 - 13; http://dx.doi.org/ 10.1093/biomet/26.4.404 [DOI] [Google Scholar]