Abstract
T cellular responses play a significant role in mediating protective immune responses against influenza in humans. In the current study, we evaluated the ability of a candidate virosomal H5N1 vaccine adjuvanted with Matrix MTM to induce CD4+ and CD8+ T cell responses in a phase 1 clinical trial. We vaccinated 60 healthy adult volunteers (at days 0 and 21) with 30 μg haemagglutinin (HA) alone or 1.5, 7.5, or 30 μg HA formulated with Matrix MTM. To evaluate the T cellular responses, lymphocytes were stimulated in vitro with homologous (A/Vietnam/1194/2004 [H5N1]) and heterologous H5N1 (A/Anhui/1/05 or A/Bar-headed Goose/Qinghai/1A/05) antigens. The antigen-specific cytokine responses were measured by intracellular cytokine staining and by multiplex (Luminex) assays. An increase in CD4+ Th1 and Th2 cytokines was detected 21 days after the first vaccine dose. No increase in Th cytokine responses was observed after the second dose, although it is possible that the cytokine levels peaked earlier than sampling point at day 42. Formulation with the Matrix MTM adjuvant augmented both the homologous and cross-reactive cytokine response. Antigen-specific CD8+ T cell responses were detected only in a few vaccinated individuals. The concentrations of Th1 and to a lesser extent, Th2 cytokines at 21 days post-vaccination correlated moderately with subsequent days 35 and 180 serological responses as measured by the microneutralisation, haemagglutination inhibition, and single radial hemolysis assays. Results presented here show that the virosomal H5N1 vaccine induced balanced Th1/Th2 cytokine responses and that Matrix MTM is a promising adjuvant for future development of candidate pandemic influenza vaccines.
Keywords: human, influenza, Matrix M, H5N1, vaccine, cytokine, CD4, CD8, Th1, Th2
Introduction
Highly pathogenic avian influenza (HPAI) viruses continue to pose a significant health threat. This threat is highlighted by recent studies showing that airborne transmission of HPAI viruses could be acquired with a limited number of mutations.1,2 Vaccination is the best measure to prevent influenza, but meta-analyses have highlighted the need for better vaccines.3,4 Due to the poor immunogenicity of candidate influenza H5N1 vaccines, there is a need for developing more effective vaccine and adjuvant formulations.
The immune response against influenza is multifaceted and both the humoral and cell-mediated arms are important. Antibody responses against the virus’ HA protein are the major mediators of protection against influenza. Thus, traditionally vaccine immunogenicity is assessed by haemagglutination inhibition (HI) or microneutralisation (MN) assays that measure virus-specific antibodies. For seasonal influenza vaccines, an HI titer of 1:40 is considered a protective response according to the criteria defined by the EU Committee for Medicinal Products for Human Use (CHMP). However, for pandemic vaccines, an HI or MN titer that correlates with protection has not been defined. There is growing evidence highlighting an important role for T cellular responses in anti-influenza immunity. In both mice and humans, viral clearance is associated with antigen-specific CD8+ T cell responses5,6 and CD4+ T cells are required for maintaining the memory CD8+ T and B cell responses.7 Furthermore, recent studies have shown that pre-existing influenza-specific CD4+ and CD8+ T cells provide protection against heterologous strains by targeting the conserved internal proteins of the influenza virus.8
In response to antigenic exposure, naïve T cells differentiate into specific lineages, Th1, Th2, Th17, and regulatory T cells with distinct effector functions and cytokine profiles. Th1 cells have been shown to facilitate recovery from heterosubtypic influenza virus infection in mice.9,10 Multifunctional Th1 cells (simultaneously producing IL-2, IFN-γ, and TNF-α) have been associated with protection against HIV,11 Leishmania Major,12 and tuberculosis.13 While the importance of these cells has not yet been determined for influenza, they have been described as functionally superior to single cytokine producing CD4+ T cells.14,15 Th2 cytokines IL-4, IL-5, IL-10, and IL-13 enhance B cell proliferation,16 and antibody secreting cell differentiation.17 In mice, an aluminum-based adjuvanted influenza vaccine boosted Th2 responses, and HI titers, but did not confer superior protection to unadjuvanted vaccine18 and IL-4 treatment of influenza-infected mice inhibited anti-influenza responses.19 In another murine model, IgG2a and IgG1 antibodies, as stimulated by Th1 and Th2 cells, respectively had distinct roles for anti-influenza immunity.20 Collectively, these experimental models suggest that anti-influenza immunity is associated with Th1 or balanced Th1/Th2 response. However, further studies, particularly in human clinical trials are needed to better understand precise role of T cells in anti-influenza immunity and in vaccine immunogenicity. In contrast to alum-adjuvanted influenza vaccines that induced a Th2 skewed immune response in humans, clinical trials with emulsion-based influenza H5N1 vaccines showed that more balanced or Th1 type of responses can be elicited.21,22
We have previously reported that a virosomal candidate H5N1 vaccine adjuvanted with Matrix MTM induced balanced Th1/Th2 responses in a mouse model23 and protected the animals against HPAI H5N1 influenza challenge.14 The vaccine was then tested in a phase 1 clinical trial where the HI and single radial hemolysis (SRH) responses fulfilled all the EU CHMP immunogenicity criteria after 2 vaccine doses, even at the lowest antigen dose (1.5 μg HA).24 Notably, the early influenza-specific CD4+ Th1 cell responses predicted subsequent seroprotection after the booster dose.25 In the present study we investigated in detail the quality of the T cell response and found that the Matrix MTM adjuvanted virosomal vaccine induces balanced Th1/Th2 responses in humans and that CD4+ T cell responses are cross-reactive toward antigenically distinct H5N1 virus clades.
Results
The Matrix MTM adjuvanted vaccine induces a balanced Th1/Th2 response
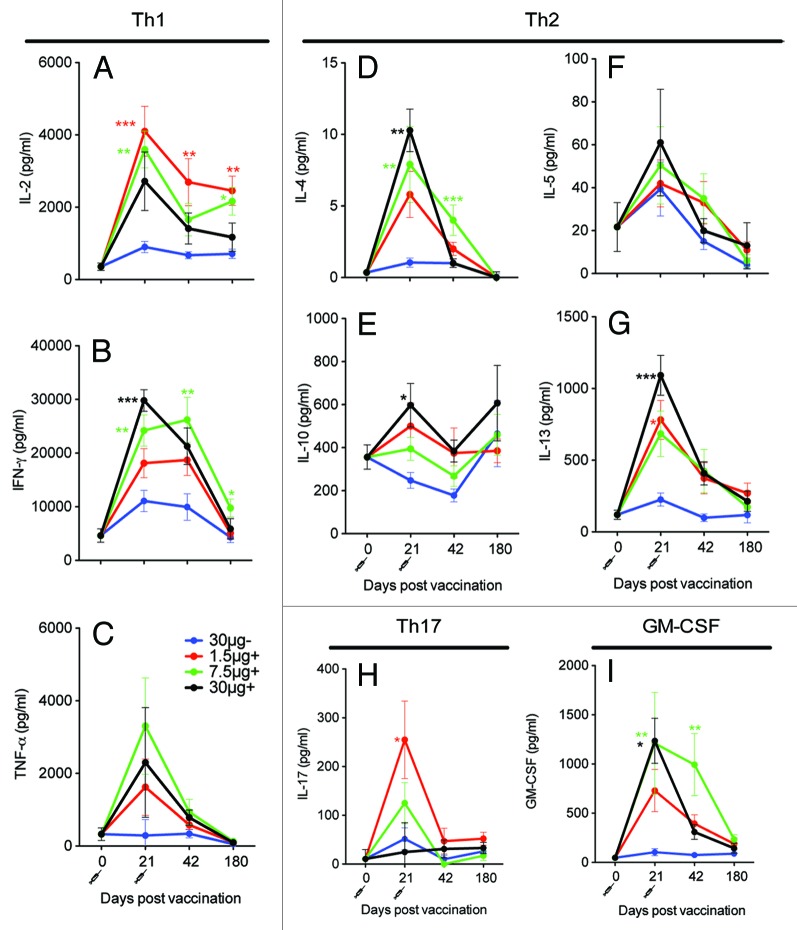
In this study we evaluated T cell responses after H5N1 virosomal vaccine administered alone or in combination with Matrix MTM. PBMCs were isolated pre-vaccination (day 0) and at days 21, 42, and 180 and stimulated with H5N1 NIBRG-14 virosomes. Th1 (IL-2, IFN-γ, and TNF-α), Th2 (IL-4, IL-5, IL-10, and IL-13), Th17 (IL-17) cytokines, and IL-12 and GM-CSF were measured in the supernatant. Low concentrations of all Th1 cytokines were detected pre-vaccination. At day 21 the concentrations of IFN-γ and IL-2 were boosted in all groups, while increased TNF-α concentrations were only observed in the Matrix MTM adjuvanted groups (Fig. 1A–C). The highest concentrations of Th1 cytokines were detected in the Matrix MTM adjuvanted groups and significantly higher concentrations of IFN-γ were observed in the Matrix MTM adjuvanted 30 μg (P < 0.001) and 7.5 μg (P < 0.01) groups than the virosomal alone group at day 21. Significantly higher concentrations of IL-2 were found in the 1.5 μg (P < 0.001) and 7.5 μg (P < 0.01) Matrix MTM adjuvanted groups as compared with the virosomal alone group. For the Th2 cytokines, the highest response was also generally found in the Matrix MTM adjuvanted groups (Fig. 1D–G). Low concentrations of IL-4 (mean 2–10 pg/mL) were measured, but vaccinees receiving 7.5 μg or 30 μg adjuvanted with Matrix MTM had significantly higher IL-4 concentrations than those who were immunized with the virosomal vaccine alone at day 21 (P < 0.001). Similarly, Matrix MTM significantly boosted IL-13 and IL-10 concentrations in the 30 μg group (P < 0.05) at day 21.
Figure 1. The Matrix MTM adjuvanted boosted the Th1 and Th2 cytokine responses. Four groups of 15 vaccinees were given 2 immunizations with 30 μg HA of H5N1 NIBRG-14 virosomal vaccine alone (30 μg-) or increasing doses of virosomal vaccine (1.5, 7.5, or 30 μg HA) in combination with Matrix MTM adjuvant (+). PBMCs were isolated at indicated time-points and stimulated for 72 h with H5N1 virosomes. The supernatant was frozen at –80 °C and later thawed for analysis of Th1 (IL-2, IFN-γ, TNF-α), Th2 (IL-4, IL-5, IL-10, IL-13), and Th17 (IL-17) cytokines and GM-CSF. The mean ± SEM is shown for each vaccine group. *, **, and *** indicate significant differences (P < 0.05, P < 0.01, and P < 0.001, respectively) from the 30 μg- group, One-way ANOVA with the Dunnet post hoc test.
The day 42 cytokine responses were lower than at day 21. The IFN-γ and IL-4 concentrations were significantly higher in the 7.5 μg Matrix MTM adjuvanted group (P < 0.01) and the IL-2 response was significantly higher in the 1.5 μg Matrix MTM adjuvanted groups than in the virosomal vaccine alone group (P < 0.01). The responses generally continued to decline at day 180, but the concentrations of IL-2 and IFN-γ were still significantly higher in the Matrix MTM adjuvanted 7.5 μg groups than in the 30 μg virosomal vaccine alone group (P < 0.05) at day 180. IL-17 was also found at increased levels at 21 days post-vaccination. In contrast, few vaccinees produced IL-17 at day 42. Subjects with high cytokine concentrations at day 21 generally also had the highest concentrations at days 42 and 180 (Table S1). In summary, an increase in Th1, Th2, and Th17 cytokines was observed post-vaccination and higher concentrations of Th1 and Th2 cytokines were observed in the Matrix MTM adjuvanted groups as compared with the virosomal vaccine alone group.
IL-12 mediates a Th1 response,26 but in the present study, no IL-12 was observed (data not shown). The Granulocyte-macrophage colony-stimulating factor (GM-CSF) is another regulator of T cell responses,27 which plays a role in protection from influenza.28 Significantly higher concentrations of GM-CSF were found in the 7.5 μg and 30 μg HA adjuvanted groups than in the non-adjuvanted group at day 21 (P < 0.05) (Fig. 1I).
Cross-reactive T cell responses
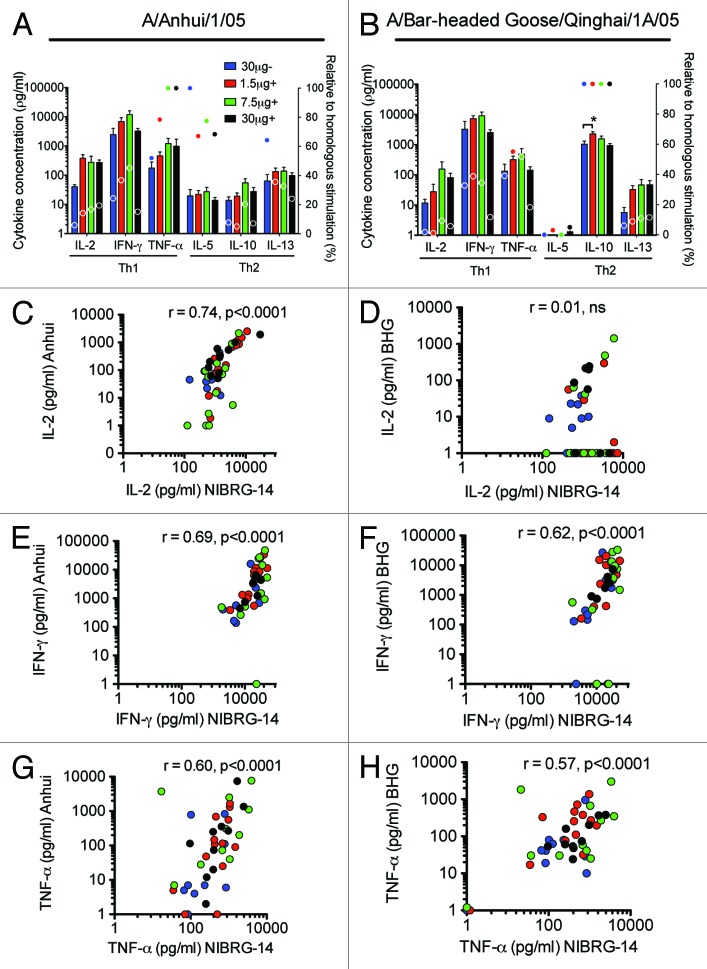
Influenza viruses are undergoing constant antigenic change. Vaccines stimulating cross-reactive T-cells are therefore needed. The cross-reactivity of the cytokine response after vaccination with the H5N1 virosomal vaccine was evaluated by stimulating PBMCs with HA from A/Anhui/1/05 (clade 2.3.4) (Anhui) or A/Bar-headed Goose/Qinghai/1A/05 (Clade 2.2) (BHG) and measuring the production of Th1 and Th2 cytokines by Luminex. Except for IL-4 (data not shown), all the measured cytokines were produced in response to Anhui stimulation, while BHG induced production of all cytokines except IL-4 and IL-5 (Fig. 2A and B). Lower levels of cytokines were observed after heterologous than homologous virus stimulation. There was a correlation between Th1 cytokines produced after stimulation with NIBRG-14 and the heterologous Anhui virus, r = 0.60 for TNF-α to r = 0.74 for IL-2 (both P < 0.0001) (Fig. 2C, E, and G). Significant (P < 0.0001) correlations were also found between the NIBRG-14 and BHG antigens for IFN-γ (r = 0.62) and TNF-α (r = 0.57) but not IL-2 (r = 0.01) (Fig. 2D, F, and G). For the Th2 cytokines, there was no significant correlation between cytokine concentrations after NIBRG-14 and BHG stimulation, but IL-5 and IL-13 responses correlated between NIBRG-14 and Anhui stimulated samples (data not shown).
Figure 2. The cross-reactivity of the Th1 cytokine responses measured by Luminex and correlation with homologous responses. Four groups of 15 vaccinees were given 2 immunizations with 30 μg HA of H5N1 NIBRG-14 virosomal vaccine alone (30 μg-) or increasing doses of virosomal vaccine (1.5, 7.5 or 30 μg HA) in combination with Matrix MTM adjuvant (+). PBMCs were isolated at day 42 into the study and stimulated for 72 h with influenza H5N1 antigen. The homologous response was evaluated toward the NIBRG-14 antigen, using the H5N1 virosomes. The cross-H5 clade response was measured against A/Anhui/1/05 (Anhui) and A/Bar-headed Goose/Qinghai/1A/05 (BHG). The supernatants were frozen at −80 °C and later thawed for analysis of Th1 (IL-2, IFN-γ, TNF-α) and Th2 (IL-4, IL-5, IL-10, IL-13) cytokines. (A and B) Mean cytokine concentrations +SEM after Anhui or BHG stimulation (bars, y-axis) and response in percentage relative to that observed for homologous stimulation as shown in Figure 1 (circles, second y-axis) are shown. *Indicates significant difference (P < 0.05) from the 30 μg- group in a One-way ANOVA with Dunnet’s post hoc test. (C–H) Individual IL-2, IFN-γ, and TNF-α cytokine concentrations after NIBRG-14 stimulation plotted against those after Anhui or BHG stimulation as indicated on the y-axis. The correlation was tested by Spearman rank test and the correlation coefficient (r) and P value are shown on the graphs.
Th1 and CD8+ T cell cytokine responses measured by intracellular cytokine staining
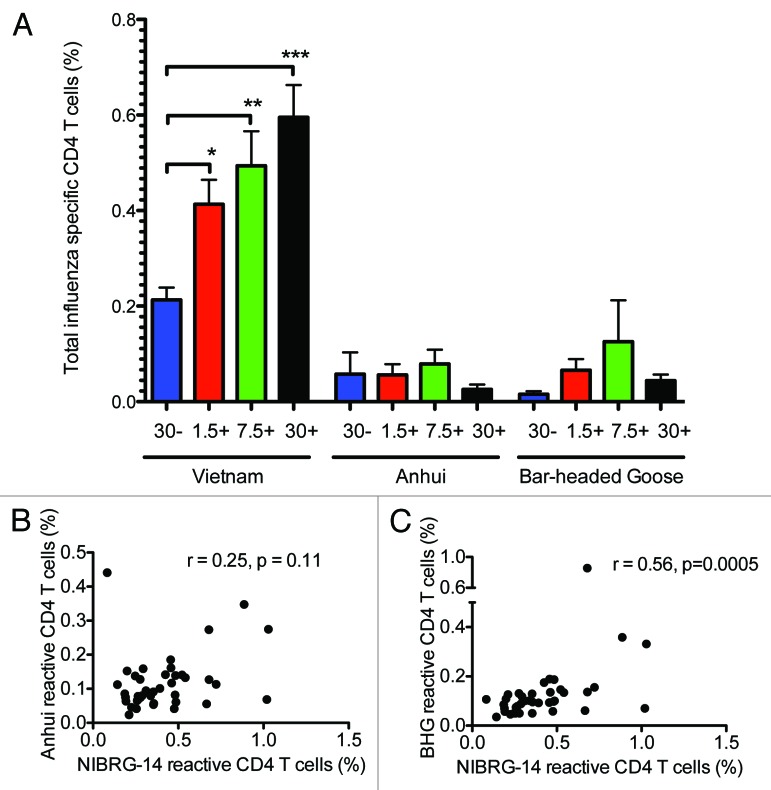
Measuring cytokine responses on a single-cell level allow for phenotyping cytokine producing cells. CD4+ T cells producing one or more of the cytokines IL-2, IFN-γ and TNF-α upon influenza stimulation were measured by flow cytometry at 21 days after the second vaccine dose (day 42). In the 30 μg HA virosomal vaccine alone group, >0.2% of the CD4+ T cells produced one or more of the measured cytokines after NIBRG-14 stimulation (Fig. 3A). Significantly higher frequencies of influenza-specific CD4+ T cells were observed in all the adjuvanted groups (P < 0.05) with frequencies of NIBRG-14 specific cells ranging from 0.4% in the 1.5 μg HA adjuvanted group to 0.6% in the 30μg HA adjuvanted group. In contrast to CD4+ T cell responses, low frequencies of CD8+ T cells producing IL-2, IFN-γ and TNF-α were observed (Fig. S1). The cross-reactive response was measured toward the HA from the Anhui and BHG strains (Fig. 3A). For both strains, low frequencies of influenza-specific CD4+ Th1 cells were observed and there were no significant differences in responses between the adjuvanted and non-adjuvanted groups. Figure 3B and C shows the correlations between NIBRG-14 and Anhui or BHG reactive CD4+ Th1 cells, respectively. There was a significant correlation (r = 0.56, P = 0.0005) between CD4+ T cells reactive toward NIBRG-14 and BHG, but not between NIBRG-14 and Anhui (r = 0.25, P = 0.11).
Figure 3. The Matrix MTM adjuvant boosted the frequencies of Th1 cells specific for the homologous strain, while lower frequencies of cells were cross-reactive toward HA of heterologous clades as measured by intracellular staining. Four groups of 15 vaccinees were given 2 immunizations with 30 μg HA of H5N1 NIBRG-14 virosomal vaccine alone (30 μg-) or increasing doses of virosomal vaccine (1.5, 7.5 or 30 μg HA) in combination with Matrix MTM adjuvant (+). PBMCs were isolated at day 42 into the study and stimulated for 16 h with influenza H5N1 antigen, co-stimulators, brefeldin A, and monensin. The homologous response was evaluated toward the NIBRG-14 antigen (clade1), using the H5N1 virosomes. The cross-H5 clade response was measured against A/Anhui/1/05 (Anhui) (clade 2.3,4) and A/Bar-headed Goose/Qinghai/1A/05 (BHG) (clade 2.2). (A) The mean percentage of influenza-specific CD4+ T cells ± SEM is shown for each vaccine group. *, **, and *** indicate significant differences (P < 0.05, P < 0.01, and P < 0.001, respectively) from the 30 μg- group, One-way ANOVA with Dunnet’s post hoc test. (B and C) Correlation between NIBRG-14 and Anhui/BHG reactive CD4+ T cells. The correlation was tested by Spearman rank test and the correlation coefficient (r) and P value are shown on the graphs.
To compare the cytokine responses measured by the multiplex cytokine assay to ICS, we plotted the cytokine concentrations found at the peak of the cytokine response (day 21) by multiplex cytokine staining against the percentages of influenza-specific CD4+ T cells producing the respective cytokines (Fig. 4). A significant correlation was found for all the measured Th1 cytokines, ranging from r = 0.49 for IL-2 to r = 0.63 for IFN-γ (P < 0.0004).
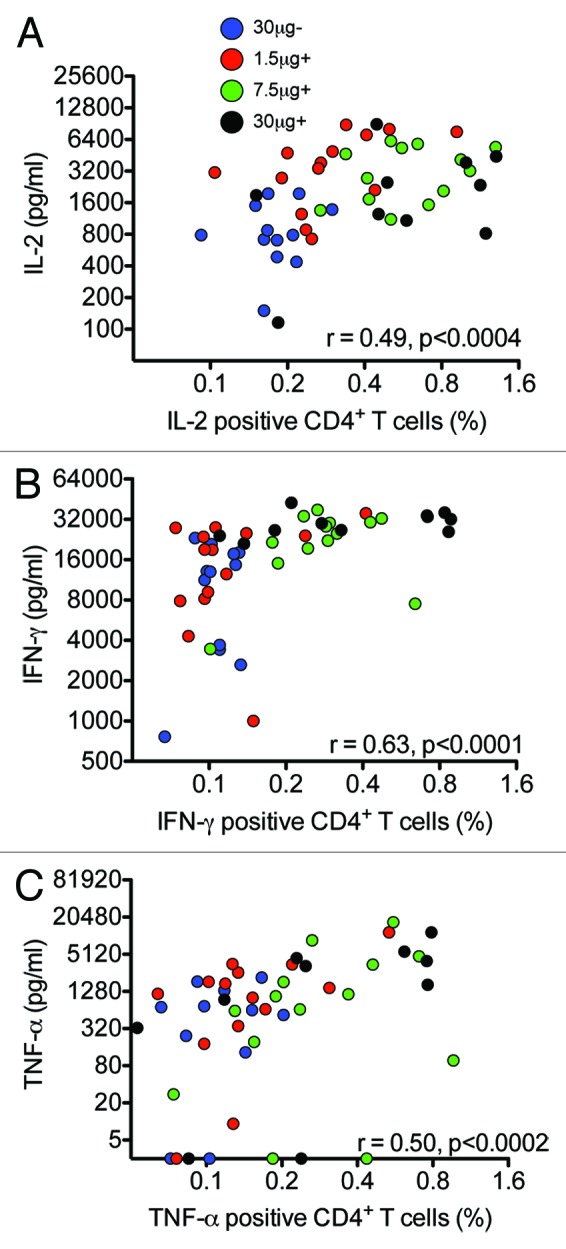
Figure 4. A correlation was observed between cytokine responses measured by multiplex and intracellular cytokine staining. Four groups of 15 vaccinees were given 2 immunizations with 30 μg HA of H5N1 NIBRG-14 virosomal vaccine alone (30 μg-) or increasing doses of virosomal vaccine (1.5, 7.5, or 30 μg HA) in combination with Matrix MTM adjuvant (+). PBMCs were isolated at day 21 into the study and stimulated in vitro with influenza H5N1 virosomes for 16 h for intracellular cytokine staining (ICS) or 72 h for Luminex analysis. ICS was performed on fresh cells, gating on CD3+CD4+ T cells, while the supernatant was frozen at –80 °C and later thawed for analysis of IL-2, IFN-γ, and TNF-α. A to C) Graphs show the correlation between CD4+ T cells displaying the indicated cytokine after ICS (x-axis) plotted against cytokine concentrations measured by Luminex (y-axis). The correlation was tested by Spearman rank test and the correlation coefficient (r) and P value is shown in the graphs.
Correlation of T cellular responses and serology
Previous studies found that vaccine-specific Th1 cells after the first vaccine dose are a good predictor of subsequent seroprotective antibody responses after the second dose25 and later boost.21 Multifunctional Th1 cells represent a distinct phenotype, producing more of each individual cytokine than single-cytokine producers. We evaluated the ability of multifunctional Th1 cells to predict seroprotection (Table 1). In addition to Th1 cytokines, Th2 cytokines may also predict seroprotection. We therefore compared Th1 vs. Th2 cytokines secreted in the supernatant of influenza-stimulated cells for correlation with previously reported serological responses in the HI, SRH, and virus MN assays. A kinetic analysis showed that 2 immunizations were required to elicit antibody titers above the protective threshold in all assays and peak antibody responses were found at day 35 (14 d after the second immunisation).24 We plotted Th1 and Th2 cytokine concentrations from the supernatants of influenza stimulated PBMCs 21 d after the first immunisation against peak day 35 and long-term day 180 antibody responses (Table 2). For the Th1 cytokines, there was a significant correlation between the concentrations of IL-2 at day 21 and MN (r = 0.60), HI (r = 0.36), and SRH (r = 0.42) titers at day 35. A weaker correlation was found between the concentrations of IFN-γ at day 21 and HI, MN, and SRH titers at day 35 (Table 2), while no association was observed between day 21 TNF-α concentrations and HI or SRH titers and only a weak correlation (r = 0.37, P < 0.05) between TNF-α and MN titers. The Th2 cytokines also correlated with serological responses. IL-10 and IL-13 secretion correlated with later HI and MN titers at day 35 (r = 0.35 – 0.44, P < 0.05), but not with SRH titers (Table 2). Cytokine responses at day 21 were not a good predictor for long-term antibody responses at day 180. Overall the concentrations of Th1 cytokines were a better predictor for subsequent seroprotection than Th2 cytokines in the in vitro stimulated PBMCs (Table1).
Table 1.
| Multifunctional Th1 cells |
||
|---|---|---|
| Day 35 | HI | 0.47** |
| MN | 0.41** | |
| SRH | 0.50** | |
| Day 180 | HI | 0.48** |
| MN | 0.43** | |
| SRH | 0.02 |
The table shows the correlation between multifunctional Th1 cells, simultaneously secreting the cytokines IL-2, IFN-γ and TNF-α measured at 21 d after the first vaccine dose by flow cytometry and haemagglutination inhibition (HI), microneutralisation (MN) and single radial hemolysis (SRH) titers measured at the peak of the serological response day 35 (14 d after the second vaccine dose) and at day 180 into the study. The Spearman correlation coefficients are shown in the table. * and ** are significant correlations, P < 0.05 and P < 0.01, respectively.
Table 2.
| T helper 1 cytokines | T helper 2 cytokines | |||||||
|---|---|---|---|---|---|---|---|---|
| IL-2 | IFN-γ | TNF-α | IL-4 | IL-5 | IL-10 | IL-13 | ||
| Day 35 | HI | 0.36* | 0.32* | 0.27 | 0.26 | 0.03 | 0.42** | 0.35* |
| MN | 0.60** | 0.44** | 0.37* | 0.36* | 0.04 | 0.44** | 0.38** | |
| SRH | 0.42** | 0.33* | 0.22 | 0.16 | 0.07 | 0.21 | 0.12 | |
| Day 180 | HI | 0.03 | 0.31* | 0.24 | 0.16 | 0.07 | 0.21 | 0.12 |
| MN | 0.30* | 0.39** | 0.19 | 0.35* | 0.04 | 0.35* | 0.33* | |
| SRH | 0.06 | 0.03 | 0.03 | 0.01 | 0.15 | 0.10 | 0.16 | |
The table shows the correlation between cytokines measured at 21 d after the first vaccine dose in the supernatant of in vitro stimulated PBMCs and haemagglutination inhibition (HI), microneutralisation (MN) and single radial hemolysis (SRH) titers measured at the peak of the serological response day 35 (14 d after the second vaccine dose) and at day 180 into the study. Cytokine concentrations were plotted against serological responses and the Spearman correlation coefficients are shown in the table. * and ** are significant correlations, P < 0.05 and P < 0.01, respectively.
Discussion
Influenza A H5N1 poses a pandemic threat and effective H5N1 vaccines are needed. The correlates of protection for seasonal influenza may not be directly extrapolated to highly pathogenic avian influenza strains, therefore candidate pandemic avian vaccines should undergo additional immunological testing. An important consideration is the vaccine-induced T helper cells. These orchestrate the anti-viral immune response and, based on the cytokines produced, can be defined as Th1 or Th2 skewed and/or involving one or more of the recently defined Th subsets, e.g., Th17. Th cells directly influence other arms of adaptive immunity, such as cytotoxic T lymphocytes and B cells. Furthermore, despite inducing functionally active influenza-specific antibodies, pure Th2 inducing vaccines may be inferior for protection against influenza, at least in mice.18 The present study investigated the T cellular responses after influenza vaccination with H5N1 virosomes alone or in combination with the Matrix MTM adjuvant in man. We found that the vaccines elicited production of Th1, Th2, and Th17 cytokines and that the Matrix MTM adjuvant significantly augmented the cytokine concentrations. This finding in humans confirms the balanced T helper response we observed for these vaccines in murine studies.14,23,29 In murine studies of adjuvanted influenza vaccines, Matrix-M™, induced significantly higher both Th1 and Th2 cytokine response than observed after AS03 or alum formulations.29
GM-CSF production was also elicited upon influenza-stimulation in all Matrix MTM adjuvanted groups, GM-CSF has adjuvant properties30 and the adjuvant effect of Matrix MTM could partly rely on induction of GM-CSF. Similar to the frequencies of influenza-specific CD4+ Th1 cells,25 the concentrations of T helper cytokines in the supernatants from in vitro influenza stimulated cells peaked at day 21 and were not further increased by a second dose of vaccine. However, since we evaluated the cytokine responses at day 42, 3 weeks post the second dose, it is possible that these have peaked prior to the sampling point.
We found that the Matrix MTM adjuvant induced higher frequency of H5N1 specific CD4+ T-cells compared with the unadjuvanted vaccine formulation. This has also previously been shown for oil-in-water adjuvants in previous human studies of H5N1.21,22,31 Similarly, both specific CD4+ T cells and Th1 and Th2 cytokines were increased after immunization with AS03 adjuvanted pandemic H1N1 vaccine.32-34
We also evaluated cytokine production upon stimulation in vitro with heterologous H5 clades (Anhui, clade 2.3.4, and BHG, clade 2.2). As expected, lower concentrations of most cytokines were produced after heterologous than homologous stimulation. The virosomal vaccine may induce T cells specific for epitopes within both the HA and NA, while only epitopes shared between the HA of NIBRG-14 and Anhui/BHG strains were measured in the cross-reactive responses. Surprisingly, 2-fold higher IL-10 concentrations were observed after BHG stimulation than NIBRG-14 stimulation. While BHG antigen may activate pre-existing memory T cells to a higher production of IL-10, it remains possible that the impurities in the BHG antigen preparation stimulates IL-10 production from other IL-10 producing cell types (reviewed in ref. 35). Notably, only a few vaccinees had CD8+ T cell responses to the vaccine on any of the sampling points tested (days 21, 42, and 180). It is possible that the peak in CD8+ T cells was missed, although significant frequencies of influenza-specific CD8+ T cells were observed at 3 weeks after second vaccine dose (day 42) in the pre-clinical studies.14 However, only low CD8+ T cell frequencies reactive toward HA and NA have been observed in humans.22,36 IL-2, IFN-γ, TNF-α, and GM-CSF are produced by CD8+ T cells as well as CD4+ T cells, but the observed low frequencies of cytokine producing CD8+ T cells suggest that most T cell cytokines measured in the supernatant of in vitro activated cells were Th derived.
CD4+ Th1 cytokines were measured both in the supernatant by the Luminex assay and intracellularly by flow cytometry. There was a moderate correlation between the 2 methods (r = 0.49–0.60, P < 0.0004) as reported previously.37,38 The discrepancy may be explained by the difference in stimulation time (16 h for ICS and 72 h for Luminex) and the ICS potentially measuring non-secreted cytokines. Conversely, cytokines in the supernatant may be utilized by other cells and thus not detected in the Luminex assay. ICS can only be used for a limited number of analytes at a time, while the Luminex assay can evaluate many analytes in one setup and is easily standardisable, allowing for high-throughput analysis of vaccine responses.39 It is therefore advantageous to study vaccine-induced cell-mediated responses using both methods.
There is an interest in early identification of vaccine non-responders who could be offered other preventive measures or revaccination in due time. CD4+ Th1 cells detected after one dose of H5N1 vaccine may predict subsequent seroprotection after the second dose,25 a later booster dose21 or revaccination.32 The previous studies did not exclude the possibility that also Th2 cytokine responses would correlate with seroprotection. We compared the Th1 vs. Th2 cytokine responses found after the first immunization to predict serological responses after the second vaccine dose. We found only a moderate correlation between day 21 Th1 or Th2 cytokines and serological MN, HI and SRH responses after the second immunization, but Th1 cytokines showed the strongest correlation. Particularly, day 21 IL-2 concentrations correlated significantly with MN, HI and SRH responses (r = 0.36–0.60, P < 0.05) at day 35 confirming our previous observation where IL-2 responses were associated with antibody responses to a cell-grown H7N1 vaccine.40 However, the relatively modest correlation coefficients imply that more robust early predictors for vaccine response are needed.
We conclude that the virosomal H5N1 vaccine adjuvanted with Matrix MTM adjuvant induces a balanced T helper response with production of both Th1 and Th2 cytokines in humans. The vaccine also induced T cells cross-reactive toward the HA of heterologous H5N1 virus clades, thus emphasizing the promise for this vaccine against divergent influenza H5N1 viruses.
Materials and Methods
Study design
The open label phase I clinical trial (ClinicalTrials.gov NCT00868218) was conducted in accordance with the Helsinki declaration. Study details are described elsewhere.24 Sixty healthy volunteers were vaccinated intramuscularly with either 30 μg HA of virosomal H5N1 vaccine alone or 1.5, 7.5, or 30 μg HA of vaccine with 50 μg of Matrix MTM adjuvant. The vaccine was administered twice, at days 0 and 21 (±1) and blood collected immediately prior to vaccination. The volunteers provided blood samples at days 0, 3, 7, 14, 21, 24, 28, 35, 42, and 180. Peripheral blood mononuclear cells (PBMCs) were isolated from CPT41 and resuspended in lymphocyte medium (RPMI 1640 with L-glutamine, 0.1mM non-essential amino acids, 10 mM Hepes, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone and 10% FBS) prior to use in the multiplex and intracellular cytokine staining (ICS) assays.
Vaccine and adjuvant
The virosomal subunit vaccine was produced by Crucell, The Netherlands; Berna Biotech, Switzerland24 from the NIBRG-14 virus with the haemagglutinin and neuraminidase of A/Vietnam/1194/2004 (H5N1) on the A/Puerto Rico/8/34 (H1N1) backbone and was standardized based on HA content. The Matrix MTM adjuvant was manufactured by Isconova AB, Sweden.24 The adjuvanted vaccine containing 50 μg of Matrix MTM was supplied in pre-filled syringes.
Multiplex cytokine assay
The PBMCs were stimulated (106 cells/200 μL of lymphocyte medium for 72 h) with 10 μg/mL HA of the influenza H5N1 virosomes or HA from A/Anhui/1/05 or A/Bar-headed Goose/Qinghai/1A/05 (produced in tobacco plants by Fraunhofer, a kind gift from Dr Vidadi Yusibov). The supernatant was assessed for GM-CSF, IL-2, IFN-γ, TNF-α, IL-4, IL-5, IL-10, IL-12, IL-13, and IL-17 by a Bio-plex 200 (Bio-Rad) according to the manufacturer’s instructions. Values for unstimulated samples (medium alone) were subtracted for data analysis.
Intracellular cytokine staining
T cells were intracellularly stained for cytokines as described previously.41 Fresh PBMCs were stimulated for 16 h with 10 µg/mL HA of influenza H5N1 NIBRG-14 virosomes or HA from A/Anhui/1/05 or A/Bar-headed Goose/Qinghai/1A/05 in lymphocyte medium containing anti-CD28 (1 µg/mL), anti-CD49d (1 µg/mL) (PharMingen), Brefeldin A (1 µg/mL) and Monensin (0.7 μg/mL) (BD). Cells were stained for CD3, CD4, CD8, IFN-γ, IL-2, and TNF-α using the Cytofix/Cytoperm kit (BD) and analyzed by a BD FACSCanto flow cytometer. The basal cytokine production (non-stimulated cells) was subtracted for data analysis.
Serological assays
The MN assay and a modified HI assay, using horse erythrocytes were performed by the Health Protection Agency (HPA), UK as previously described.42-44 The SRH assay was performed by Istituto Superiore di Sanitá (ISS), Italy.45
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by the EU FP7 Univax (601738), RCN Globvac project (220670), the K.G. Jebsen Centre for Influenza Vaccine Research, and intramurally by the Influenza Centre, University of Bergen. The work in the UK was funded by the Health Protection Agency. We thank the Ministry of Health and Care Services, Norway, Crucell, The Netherlands for providing the vaccine, ISCONOVA, Uppsala, Sweden for providing the Matrix MTM adjuvant, Dr Vidadi Yusibov (Fraunhofer USA) for the H5N1 plant antigens and the staff at the Influenza Centre, Bergen for help with performing experiments.
References
- 1.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, et al. . Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486:420 - 8; PMID: 22722205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. . Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534 - 41; http://dx.doi.org/ 10.1126/science.1213362; PMID: 22723413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterholm MT, Kelley NS, Sommer A, Belongia EA. . Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36 - 44; http://dx.doi.org/ 10.1016/S1473-3099(11)70295-X; PMID: 22032844 [DOI] [PubMed] [Google Scholar]
- 4.Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. . Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010; CD001269; PMID: 20614424 [DOI] [PubMed] [Google Scholar]
- 5.McKinstry KK, Strutt TM, Swain SL. . The potential of CD4 T-cell memory. Immunology 2010; 130:1 - 9; http://dx.doi.org/ 10.1111/j.1365-2567.2010.03259.x; PMID: 20331470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMichael AJ, Gotch FM, Noble GR, Beare PA. . Cytotoxic T-cell immunity to influenza. N Engl J Med 1983; 309:13 - 7; http://dx.doi.org/ 10.1056/NEJM198307073090103; PMID: 6602294 [DOI] [PubMed] [Google Scholar]
- 7.Stambas J, Guillonneau C, Kedzierska K, Mintern JD, Doherty PC, La Gruta NL. . Killer T cells in influenza. Pharmacol Ther 2008; 120:186 - 96; http://dx.doi.org/ 10.1016/j.pharmthera.2008.08.007; PMID: 18801385 [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. . Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274 - 80; http://dx.doi.org/ 10.1038/nm.2612; PMID: 22286307 [DOI] [PubMed] [Google Scholar]
- 9.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. . Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis 1999; 180:579 - 85; http://dx.doi.org/ 10.1086/314952; PMID: 10438342 [DOI] [PubMed] [Google Scholar]
- 10.Graham MB, Braciale VL, Braciale TJ. . Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med 1994; 180:1273 - 82; http://dx.doi.org/ 10.1084/jem.180.4.1273; PMID: 7931062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. . Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 2008; 38:350 - 63; http://dx.doi.org/ 10.1002/eji.200737768; PMID: 18200635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. . Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843 - 50; http://dx.doi.org/ 10.1038/nm1592; PMID: 17558415 [DOI] [PubMed] [Google Scholar]
- 13.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. . Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008; 181:4955 - 64; http://dx.doi.org/ 10.4049/jimmunol.181.7.4955; PMID: 18802099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen G, Major D, Roseby S, Wood J, Madhun AS, Cox RJ. . Matrix-M adjuvanted virosomal H5N1 vaccine confers protection against lethal viral challenge in a murine model. Influenza Other Respir Viruses 2011; 5:426 - 37; http://dx.doi.org/ 10.1111/j.1750-2659.2011.00256.x; PMID: 21668670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. . Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol 2007; 81:8468 - 76; http://dx.doi.org/ 10.1128/JVI.00228-07; PMID: 17553885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangye SG, Ferguson A, Avery DT, Ma CS, Hodgkin PD. . Isotype switching by human B cells is division-associated and regulated by cytokines. J Immunol 2002; 169:4298 - 306; http://dx.doi.org/ 10.4049/jimmunol.169.8.4298; PMID: 12370361 [DOI] [PubMed] [Google Scholar]
- 17.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. . Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol 2004; 5:55 - 63; http://dx.doi.org/ 10.1038/ni1016; PMID: 14647274 [DOI] [PubMed] [Google Scholar]
- 18.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. . Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine 2008; 26:2350 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2008.02.063; PMID: 18400340 [DOI] [PubMed] [Google Scholar]
- 19.Moran TM, Isobe H, Fernandez-Sesma A, Schulman JL. . Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol 1996; 70:5230 - 5; PMID: 8764032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. . Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 2006; 13:981 - 90; http://dx.doi.org/ 10.1128/CVI.00156-06; PMID: 16960108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. . Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877 - 82; http://dx.doi.org/ 10.1073/pnas.0813390106; PMID: 19237568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, Leroux-Roels GG, Van Mechelen M. . H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443 - 54; http://dx.doi.org/ 10.1007/s10875-010-9490-6; PMID: 21174144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhun AS, Haaheim LR, Nilsen MV, Cox RJ. . Intramuscular Matrix-M-adjuvanted virosomal H5N1 vaccine induces high frequencies of multifunctional Th1 CD4+ cells and strong antibody responses in mice. Vaccine 2009; 27:7367 - 76; http://dx.doi.org/ 10.1016/j.vaccine.2009.09.044; PMID: 19781678 [DOI] [PubMed] [Google Scholar]
- 24.Cox RJ, Pedersen G, Madhun AS, Svindland S, Sævik M, Breakwell L, Hoschler K, Willemsen M, Campitelli L, Nøstbakken JK, et al. . Evaluation of a virosomal H5N1 vaccine formulated with Matrix M™ adjuvant in a phase I clinical trial. Vaccine 2011; 29:8049 - 59; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.042; PMID: 21864624 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen GK, Madhun AS, Breakwell L, Hoschler K, Sjursen H, Pathirana RD, Goudsmit J, Cox RJ. . T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. J Infect Dis 2012; 206:158 - 66; http://dx.doi.org/ 10.1093/infdis/jis330; PMID: 22551811 [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. . Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 1993; 260:547 - 9; http://dx.doi.org/ 10.1126/science.8097338; PMID: 8097338 [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. . Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res 2006; 16:126 - 33; http://dx.doi.org/ 10.1038/sj.cr.7310017; PMID: 16474424 [DOI] [PubMed] [Google Scholar]
- 28.Huang FF, Barnes PF, Feng Y, Donis R, Chroneos ZC, Idell S, Allen T, Perez DR, Whitsett JA, Dunussi-Joannopoulos K, et al. . GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med 2011; 184:259 - 68; http://dx.doi.org/ 10.1164/rccm.201012-2036OC; PMID: 21474645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnusson SE, Reimer JM, Karlsson KH, Lilja L, Bengtsson KL, Stertman L. . Immune enhancing properties of the novel Matrix-M™ adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine 2013; 31:1725 - 33; http://dx.doi.org/ 10.1016/j.vaccine.2013.01.039; PMID: 23384754 [DOI] [PubMed] [Google Scholar]
- 30.Pichichero ME. . Improving vaccine delivery using novel adjuvant systems. Hum Vaccin 2008; 4:262 - 70; http://dx.doi.org/ 10.4161/hv.4.4.5742; PMID: 18398303 [DOI] [PubMed] [Google Scholar]
- 31.Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, Icardi G, Dramé M, Roman F, Gillard P. . Immunogenicity profile of a 3.75-mug hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis 2011; 203:1054 - 62; http://dx.doi.org/ 10.1093/infdis/jiq174; PMID: 21450995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathirana RD, Bredholt G, Akselsen PE, Pedersen GK, Cox RJA. . A(H1N1)pdm09 vaccination of health care workers: improved immune responses in low responders following revaccination. J Infect Dis 2012; 206:1660 - 9; http://dx.doi.org/ 10.1093/infdis/jis589; PMID: 22969149 [DOI] [PubMed] [Google Scholar]
- 33.Jul-Larsen Å, Madhun AS, Brokstad KA, Montomoli E, Yusibov V, Cox RJ. . The human potential of a recombinant pandemic influenza vaccine produced in tobacco plants. Hum Vaccin Immunother 2012; 8:653 - 61; http://dx.doi.org/ 10.4161/hv.19503; PMID: 22634440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman F, Clément F, Dewé W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. . Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835 - 43; http://dx.doi.org/ 10.1128/CVI.00480-10; PMID: 21450978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva M, O’Garra A. . The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10:170 - 81; http://dx.doi.org/ 10.1038/nri2711; PMID: 20154735 [DOI] [PubMed] [Google Scholar]
- 36.Lee LY, Ha LA, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, et al. . Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest 2008; 118:3478 - 90; PMID: 18802496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabilan L, Andersson G, Lolli F, Ekre HP, Olsson T, Troye-Blomberg M. . Detection of intracellular expression and secretion of interferon-gamma at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol 1990; 20:1085 - 9; http://dx.doi.org/ 10.1002/eji.1830200521; PMID: 2113474 [DOI] [PubMed] [Google Scholar]
- 38.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. . Detection of intracellular cytokines by flow cytometry. J Immunol Methods 1993; 159:197 - 207; http://dx.doi.org/ 10.1016/0022-1759(93)90158-4; PMID: 8445253 [DOI] [PubMed] [Google Scholar]
- 39.Gijzen K, Liu WM, Visontai I, Oftung F, van der Werf S, Korsvold GE, Pronk I, Aaberge IS, Tütto A, Jankovics I, et al. . Standardization and validation of assays determining cellular immune responses against influenza. Vaccine 2010; 28:3416 - 22; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.076; PMID: 20206285 [DOI] [PubMed] [Google Scholar]
- 40.Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, Höschler K, Saville M, Vogel FR, Barclay W, et al. . A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889 - 97; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.116; PMID: 19368768 [DOI] [PubMed] [Google Scholar]
- 41.Pedersen G, Halstensen A, Sjursen H, Naess A, Kristoffersen EK, Cox RJ. . Pandemic influenza vaccination elicits influenza-specific CD4+ Th1-cell responses in hypogammaglobulinaemic patients: four case reports. Scand J Immunol 2011; 74:210 - 8; http://dx.doi.org/ 10.1111/j.1365-3083.2011.02561.x; PMID: 21438900 [DOI] [PubMed] [Google Scholar]
- 42.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. . Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937 - 43; PMID: 10074505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. . Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937 - 43; http://dx.doi.org/ 10.1016/S0140-6736(00)05066-2; PMID: 11425416 [DOI] [PubMed] [Google Scholar]
- 44.Stephenson I, Wood JM, Nicholson KG, Zambon MC. . Sialic acid receptor specificity on erythrocytes affects detection of antibody to avian influenza haemagglutinin. J Med Virol 2003; 70:391 - 8; http://dx.doi.org/ 10.1002/jmv.10408; PMID: 12767002 [DOI] [PubMed] [Google Scholar]
- 45.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, et al. . MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384; http://dx.doi.org/ 10.1371/journal.pone.0004384; PMID: 19197383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.