Abstract
Neisseria meningitidis is the leading cause of bacterial invasive infections in people aged <15 years in the Russian Federation. The aim of this phase III, multicenter, open-label study was to assess the immunogenicity and safety of the quadrivalent meningococcal CRM197-conjugate vaccine MenACWY when administered to healthy Russian subjects aged 2 years and above. A total of 197 subjects were immunized with a single dose of the vaccine, and serogroup-specific serum bactericidal activity was measured pre and 1-month post-vaccination with human complement (hSBA) serum titers. Regardless of baseline serostatus, 1 month after a single dose of MenACWY-CRM197 85% (95%CI, 79–90%) of subjects showed serologic response against serogroup A, 74% (67–80%) against serogroup C, 60% (53–67%) against serogroup W, and 83% (77–88%) against serogroup Y. The percentage of subjects with hSBA titers ≥ 1:8 1 month after vaccination was 89% (83–93%) against serogroup A, 84% (78–89%) against serogroup C, 97% (93–99%) against serogroup W, and 88% (82–92%) against serogroup Y. Comparable results were obtained across all subjects: children (2 to 10 years), adolescents (11 to 17 years), and adults (≥18 years). The MenACWY-CRM197 vaccine showed an acceptable safety profile and was well tolerated across all age groups, with no serious adverse events or deaths reported during the study. In conclusion, a single dose of meningococcal MenACWY-CRM197 vaccine is immunogenic and has an acceptable safety profile, provides a broad protection against the most frequent epidemic serogroups, and is a suitable alternative to currently available unconjugated monovalent or bivalent polysaccharide vaccines in Russia.
Keywords: meningococcal, quadrivalent vaccine, CRM197-conjugate, immunogenicity, safety, adult, children, adolescent, Russia
Introduction
Neisseria meningitidis is a gram-negative bacterium present as a commensal in the human upper respiratory tract. In a small proportion of healthy carriers, the pathogen can enter the bloodstream and become invasive, causing meningitis, septicemia or a combination of both.1 In approximately 10% of cases, invasive meningococcal disease (IMD) is a life threatening condition that after an abrupt onset of symptoms rapidly progresses to death within 24–48 h despite use of vigorous supportive care and appropriate antibiotics.2 Additionally, 12% to 19% of survivors develop long-term neurologic sequelae such as hearing loss, brain damage, and learning disabilities.1,3
Approximately 1.2 million cases of IMD occur annually worldwide, resulting in about 135 000 deaths per year.4 Incidence rates are higher among children, in whom protective antibodies have not yet developed, in teenagers, and in young adults, with an increasing probability of developing severe disease with age.5,6
Twelve different serogroups of Neisseria meningitidis have been identified to date,7 but the vast majority (>90%) of invasive meningococcal infections and epidemics are caused by organisms expressing one of the serogroup A, B, C, X, W-135, or Y capsular polysaccharide.2 The incidence and serogroup distribution of meningococcal disease are highly variable between countries and change over seasons and years: serogroups B and C predominate in Europe, Australia, and New Zealand; serogroups A, C, and W-135 are most common in Asia and Africa; serogroups B, C, and Y prevail in Canada, US, the Caribbean, and Latin America, and serogroup X has caused sporadic and clustered meningitis cases in sub-Saharan Africa.4,8
In the Russian Federation, N. meningitidis is the pathogen most commonly associated with invasive bacterial infections in children <15 y.9 A trend toward a decline in the incidence of generalized forms of meningococcal infection has been observed since 2005,10,11 and the incidence of IMD in 2012 was 0.88 cases per 100 000.12 Geographical differences exist, and whereas the annual incidence among regions ranges from <1 to 2 cases per 100 000 inhabitants, in some areas it can be as high as 2 and 2.5.12 The highest incidence of IMD is observed among infants ≤2 y of age,10-12 with serological group B as the most frequently isolated serogroup in sporadic meningococcal cases;10 serogroups A and C predominate in Russia during epidemic periods, although the presence of W-135 and Y serogroups has been documented too.10-12
Immunization is an important defense against meningococcal disease. For serogroup B, a multicomponent vaccine (Bexsero™, Novartis Vaccines) has been recently approved in the European Union, Canada, and Australia, and strain specific vaccines are also used to control local outbreaks.13,14 Existing vaccines against meningococci of serogroups A, C, W-135, and Y include capsular polysaccharide vaccines (PS), and vaccines in which the meningococcal polysaccharide antigens are chemically conjugated to a carrier protein. Although PS vaccines elicit a protective immunogenic response, conjugate vaccines are preferred to PS because they induce higher antibody responses, particularly in children <2 y of age, and can elicit an anamnestic response.15,16
MenACWY-CRM197, (Menveo®, Novartis Vaccines) is a quadrivalent meningococcal conjugate vaccine indicated to prevent invasive meningococcal disease caused by N. meningitidis antigens A, C, W-135, and Y. It is comprised of capsular polysaccharides from serogroups A, C, W-135, and Y conjugated to a non-toxic mutant of diphtheria toxin CRM197 as the carrier protein. MenACWY-CRM197 is licensed for active immunization in the EU, Canada, and Australia to prevent invasive meningococcal disease in people aged ≥2 y, and it has also been authorized for use in those aged from 2 mo to 55 y in the US.
Phase II and III clinical trials have shown that MenACWY-CRM197 is highly immunogenic and well-tolerated in infants and toddlers,17-22 children,23,24 adolescents,25-29 and adults.30-32
The primary objective of this phase III study was to assess the immunogenicity of a single injection of MenACWY-CRM197 vaccine in healthy subjects aged ≥2 y from the Russian Federation, where there are no quadrivalent meningococcal conjugate vaccines currently licensed. The secondary objective was to examine the safety profile of the vaccine, and to assess outcomes in 3 different age groups: children, adolescents, and adults.
Results
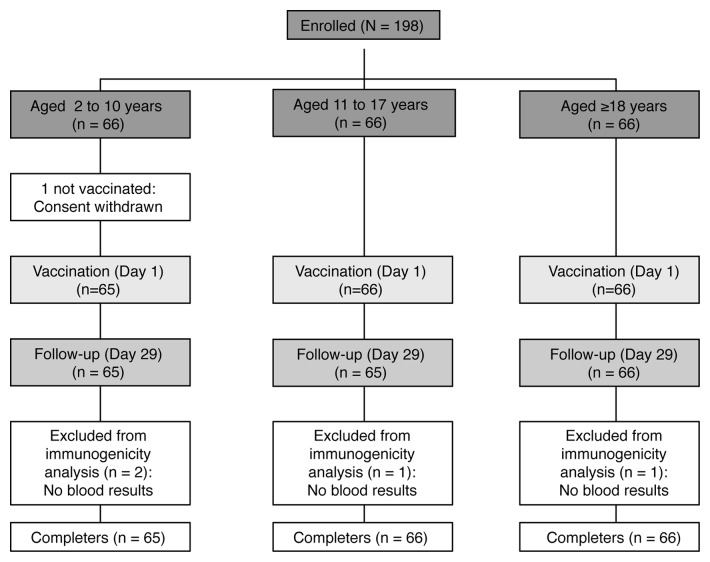
All enrolled subjects completed the study except for 1 subject from the 2 to 10 y age group for whom consent was withdrawn prior to vaccination (Fig. 1). Out of the 197 vaccinated subjects, 2 in the 2 to 10 y age group and 1 in each of the 2 other groups were excluded from the immunogenicity analyses because blood samples were not taken or because serogroup results were missing (Fig. 1). Demographic characteristics of the overall group and individual age-groups of subjects enrolled in the study are shown in Table 1. All subjects were of Caucasian origin, and the mean age of the overall study population was 19.6 (± 15.9) y, with a higher proportion of females (55%), a trend observed for all age groups except for adolescents, where 56% of enrolled subjects were male.
Figure 1. Study flow diagram and subject disposition.
Table 1. Summary of demographic characteristics of the enrolled population, overall and by age group.
| By age group | ||||
|---|---|---|---|---|
|
Overall (≥2 y) n = 198 |
Children (2 to 10 y) n = 66 |
Adolescents (11 to 17 y) n = 66 |
Adults (≥18 y) n = 66 |
|
| Age, mean ± SD (y) | 19.6 ± 15.9 | 6 ± 2.7 | 13.8 ± 2.1 | 38.8 ± 12.5 |
|
Gender, n Male Female |
90 (45%) 108 (55%) |
31 (47%) 35 (53%) |
37 (56%) 29 (44%) |
22 (33%) 44 (67%) |
| Weight, mean ± SD (kg) | 51.3 ± 23.4 | 25.6 ± 10.2 | 55.1 ± 13.1 | 73.3 ± 14.6 |
|
Height, mean ± SD (cm) Ethnic origin, n Caucasian |
152.0 ± 25.9 198 (100%) |
121.6 ± 19.2 66 (100%) |
164.3 ± 12.5 66 (100%) |
170.2 ± 8.6 66 (100%) |
Immunogenicity
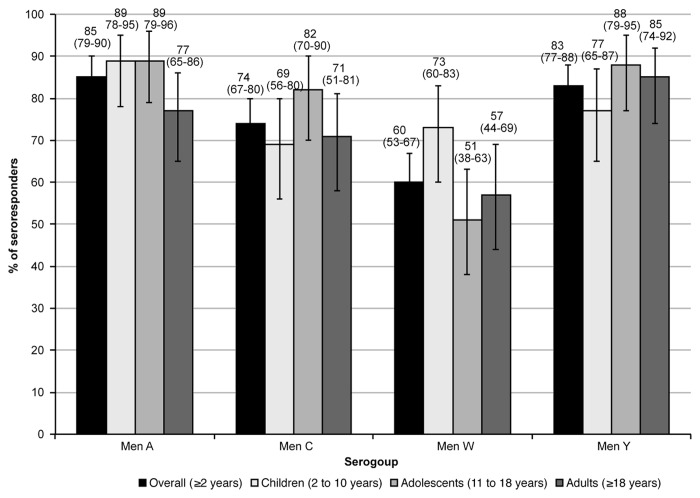
One month after a single dose of MenACWY-CRM197, 85% of the overall population showed serologic response against serogroup A, 74% against serogroup C, 60% against serogroup W, and 83% against serogroup Y (Table 2 and Fig. 2). Among subjects with pre-vaccination serum bactericidal assay using human complement (hSBA) titers < 1:4, a large proportion achieved protective hSBA titers ≥ 1:8 after immunization (88%, 77%, 93%, and 87% against serogroups A, C, W, and Y, respectively) (Table 2). The percentage of participants with pre-vaccination hSBA titers ≥ 1:4 that achieved seroresponse was 53%, 68%, 37%, and 70% against serogroup A, C, W, Y, respectively. When stratified by age group, the percentage of seroresponders was comparable within serogroups: between 77% and 89% against serogroup A, between 69% and 82% against serogroup C, between 51% and 73% against serogroup W, and between 77% and 88% against serogroup Y (Table 2 and Fig. 2).
Table 2. Percentage (95% CI) of subjects with seroresponse 1 mo after MenACWY-CRM197 vaccination by pre-vaccination status, overall and by age group.
| Seroresponse (%) By age group |
|||||
|---|---|---|---|---|---|
|
Serogroup |
Sample size (N of children; adolescents; adults) |
Seroresponse (%) Overall (≥2 y) |
Children (2 to 10 y) |
Adolescents (11 to 17 y) |
Adults (≥18 y) |
| Men A | |||||
| Seronegative (hSBA < 1:4) | 176 (61; 60; 55) | 88 (82–92) | 89 (78–95) | 92 (82–97) | 84 (71–92) |
| Seropositive (hSBA ≥ 1:4) | 17 (2; 5; 10) | 53 (28–77) | 100 (16–100) | 60 (15–95) | 40 (12–74) |
| Overall | 193 (63; 65; 65) | 85 (79–90) | 89 (78–95) | 89 (79–96) | 77 (65–86) |
| Men C | |||||
| Seronegative (hSBA < 1:4) | 126 (51; 46; 29) | 77 (69–84) | 71 (56–83) | 83 (69–92) | 79 (60–92) |
| Seropositive (hSBA ≥ 1:4) | 66 (11; 19; 36) | 68 (56–79) | 64 (31–89) | 79 (54–94) | 64 (46–79) |
| Overall | 192 (62; 65; 65) | 74 (67–80) | 69 (56–80) | 82 (70–90) | 71 (58–81) |
| Men W | |||||
| Seronegative (hSBA < 1:4) | 80 (42; 19; 19) | 93 (84–97) | 93 (81–99) | 95 (74–100) | 89 (67–99) |
| Seropositive (hSBA ≥ 1:4) | 112 (20; 46; 46) | 37 (28–46) | 30 (12–54) | 33 (20–48) | 43 (29–59) |
| Overall | 192 (62; 65; 65) | 60 (53–67) | 73 (60–83) | 51 (38–63) | 57 (44–69) |
| Men Y | |||||
| Seronegative (hSBA < 1:4) | 151 (54; 49; 48) | 87 (80–92) | 78 (64–88) | 96 (86–100) | 88 (75–95) |
| Seropositive (hSBA ≥ 1:4) | 40 (7; 16; 17) | 70 (53–83) | 71 (29–96) | 63 (35–85) | 76 (50–93) |
| Overall | 191 (61; 65; 65) | 83 (77–88) | 77 (65–87) | 88 (77–95) | 85 (74–92) |
Seroresponse was defined as the percentage of subjects with post-vaccination hSBA ≥ 1:8 in subjects with a pre-vaccination hSBA < 1:4, and at least a 4-fold increase in post-vaccination hSBA in subjects with pre-vaccination hSBA ≥ 1:4.
Figure 2. Percentage (95%CI) of subjects who achieved seroresponse 1 mo after immunization with MenACWY-CRM197. Seroresponse was defined as the percentage of subjects with post-vaccination hSBA ≥ 1:8 in subjects with a pre-vaccination hSBA < 1:4, and at least a 4-fold increase in post-vaccination hSBA in subjects with pre-vaccination hSBA ≥ 1:4.
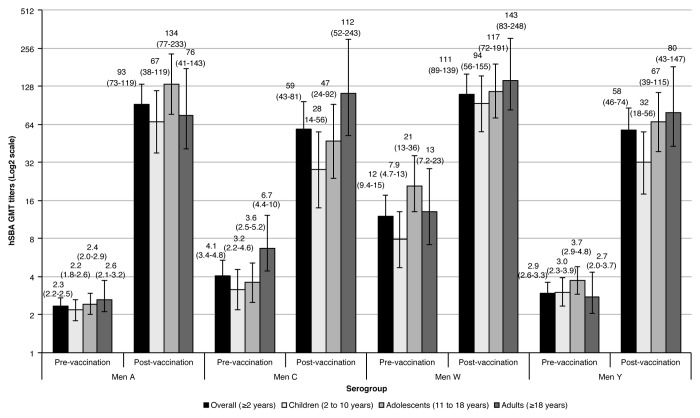
In the overall population, pre-vaccination hSBA geometric mean titers (GMTs) were low for serogroups A, C, and Y (2.35, 4.07, and 2.93, respectively), and relatively higher against serogroup W (12) (Fig. 3). This same trend was observed for all age groups. Four weeks after MenACWY-CRM197 vaccination, GMT titers showed a large increase across all serogroups in the overall study population, with the highest increase corresponding to serogroup A (40-fold), followed by serogroup Y (20-fold), serogroup C (14-fold), and the lowest increase observed against serogroup W (9.33-fold). When assessed by age group, GMTs against serogroup A were again the ones with the highest increase from baseline (GMR between 30 and 55), with the largest difference observed for the adolescent group. Against serogroup C, the post-vaccination group ratios (GMRs) varied between 7.16 and 25, and between 11 and 29 against serogroup Y; for both serogroups, the largest difference was observed for adults. Changes from baseline were lower against serogroup W, being identical for adults and children (11-fold increase), and lower for the adolescent group (6.48-fold increase).
Figure 3. hSBA GMT titers before and 1 mo after MenACWY-CRM197 vaccination.
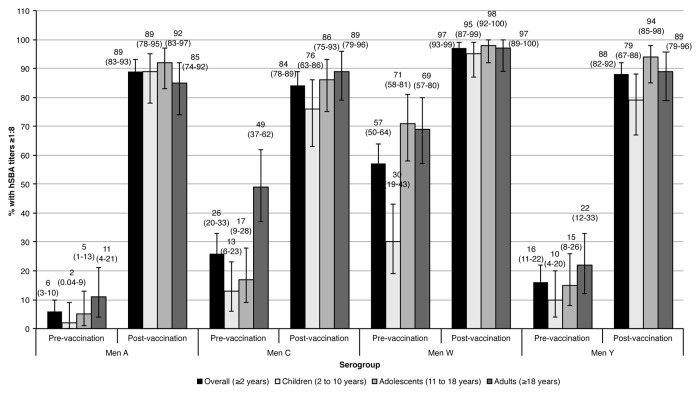
The percentage of subjects with hSBA titers ≥ 1:8 in the overall study cohort before vaccination was low against serogroups A, C, and Y (6%, 26%, and 16%, respectively), and higher against serogroup W (57%) (Fig. 4). Four weeks after vaccination, the percentages increased against serogroups A, C, W, and Y (89%, 84%, 97%, and 88%, respectively). Across age groups, younger children had the lowest baseline percentages against all serogroups compared with adolescents and adults. After vaccination, all age groups showed a substantial rise in the percentage of subjects with hSBA titers ≥ 1:8 against all serogroups: for children, adolescents and adults, 89%, 92%, and 85% against serogroup A, 76%, 86%, and 89% against serogroup C, 95%, 98%, and 97% against serogroup W, and 79%, 94%, and 89% against serogroup Y, respectively.
Figure 4. Percentage (95%CI) of subjects with hSBA titers ≥ 1:8 1 mo after MenACWY-CRM197 vaccination.
Safety and tolerability
Among participants under 5 y, 74% experienced a local reaction within 7 d of MenACWY-CRM197 vaccination, which was comparable to subjects over 6 y of age (64%) (Tables 3 and 4). Among children less than 5 y of age, the most common local adverse event (AE) was injection site tenderness (41%), which was also the most commonly observed local reaction in the subgroup of children under 3 y of age (39%) (Table 3). There were no severe local AEs in any subjects. Systemic AEs were less frequent among participants 2 to 3 y of age (39%) than in the 4 to 5 y age group (67%). Injection site pain was the most commonly reported local AE in the overall population ≥6 y (48%), and occurred at a similar frequency across all age subgroups (42% in children 6 to 10 y old, and 50% in adolescents and adults) (Table 4). In the study cohort older than 6 y of age, headache, malaise, and myalgia were the most common systemic reactions (25%, 20%, and 19%, respectively).
Table 3. Summary of subjects 2 to 5 y of age experiencing local and/or systemic solicited reactions within a week of a single dose of MenACWY-CRM197, overall and by age group.
| Overall | By age group | ||
|---|---|---|---|
| Children (2 to 5 y) n = 27 |
Children (2 to 3 y) n = 18 |
Children (4 to 5 y) n = 9 |
|
| Any reaction, n (%) | 20 (74) | 13 (72) | 7 (78) |
|
Any local reactions, n (%) Reactions, n (%) Tenderness, any Tenderness, severe |
16 (59) 11 (41) 0 |
10 (56) 7 (39) 0 |
6 (67) 4 (44) 0 |
|
Erythema, any Erythema > 100 mm |
7 (26) 0 |
5 (28) 0 |
2 (22) 0 |
|
Induration, any Induration > 100 mm |
5 (19) 0 |
3 (17) 0 |
2 (22) 0 |
|
Any systemic reactions, n (%) Reactions, n (%) Change in eating habits Sleepiness Irritability Vomiting Diarrhea Rash, any Rash, urticarial Body temperature (≥38 °C – Fever) Other reactions, n (%) Body temperature ≥ 40 °C Use of analgesics/antipyretics |
13 (48) 1 (4) 9 (33) 7 (26) 1 (4) 3 (11) 1 (4) 0 1 (4) 0 2 (7) |
7 (39) 1 (6) 5 (28) 3 (17) 0 2 (11) 1 (6) 0 1 (6) 0 0 |
6 (67) 0 4 (44) 4 (44) 1 (11) 1 (11) 0 0 0 0 2 (22) |
Table 4. Summary of subjects ≥6 y of age experiencing local and/or systemic solicited reactions within a week of a single dose of MenACWY-CRM197, overall and by age group.
|
Overall (≥ 6 y) n = 170 |
Children (6 to 10 y) n = 38 |
By Age group Adolescents (11 to 17 y) n = 66 |
Adults (≥18 y) n = 66 |
|
|---|---|---|---|---|
| Any reaction, n (%) | 108 (64) | 22 (58) | 42 (64) | 44 (67) |
|
Local reactions, any, n (%) Reactions, n (%) Injection site pain, any Injection site pain, severe |
90 (53) 82 (48) 5 (3) |
18 (47) 16 (42) 2 (5) |
33 (50) 33 (50) 0 |
39 (59) 33 (50) 3 (5) |
|
Erythema, any Erythema, >100 mm |
30 (18) 3 (2) |
7 (18) 1 (3) |
12 (18) 1 (2) |
11 (17) 1 (2) |
|
Induration, any Induration, >100 mm |
23 (14) 3 (2) |
4 (11) 1 (3) |
11 (17) 1 (2) |
8 (12) 1 (2) |
|
Systemic reactions, any, n (%) Reactions, n (%) Chills, any |
68 (40) 16 (9) |
12 (32) 3 (8) |
30 (45) 5 (8) |
26 (39) 8 (12) |
| Chills, severe | 1 (1) | 1 (3) | 0 | 0 |
| Nausea, any | 13 (8) | 1 (3) | 7 (11) | 5 (8) |
| Nausea, severe | 2 (1) | 0 | 1 (2) | 1 (2) |
|
Malaise, any Malaise, severe |
34 (20) 6 (4) |
6 (16) 1 (3) |
15 (23) 3 (5) |
13 (20) 2 (3) |
|
Myalgia, any Myalgia, severe Arthralgia, any Arthralgia, severe Headache, any Headache, severe Rash, any Rash, urticarial Body temperature (≥38 °C- Fever) 38 °C-38.4 °C |
33 (19) 5 (3) 15 (9) 0 43 (25) 2 (1) 4 (2) 1 (1) 5 (3) 1 (1) |
5 (13) 2 (5) 1 (3) 0 7 (18) 1 (3) 1 (3) 0 2 (5) 1 (3) |
19 (29) 0 7 (11) 0 18 (27) 1 (2) 0 0 2 (3) 0 |
9 (14) 3 (5) 7 (11) 0 18 (27) 0 3 (5) 1 (2) 1 (2) 0 |
| 38.5 °C-38.9 °C | 3 (2) | 1 (3) | 1 (2) | 1 (2) |
|
Other reactions, n (%) 39 °C-39.4 °C 39.5 °C-39.9 °C |
0 1 (1) |
0 0 |
0 1 (2) |
0 0 |
|
≥40 °C Use of analgesics/antipyretics |
0 19 (11) |
0 7 (18) |
0 6 (9) |
0 6 (9) |
Unsolicited AEs after MenACWY-CRM197 immunization occurred in 17% of the overall population aged ≥2 y, with an average 10% of the cases considered to be potentially related to the study vaccination: 3% in the 2–10 y age group, 9% in the adolescent group, and 18% in the adult group. A total of 8 subjects (4% of the overall sample population) experienced AEs such as infections, pyrexia, headache, cough, or erythema that required medical attention, the majority of them in the age subgroup of children 2 to 5 y (5 subjects). None of the subjects reported serious AEs (SAE), and there were no premature withdrawals due to AEs.
Discussion
In this phase-III multicenter, open-label study conducted in the Russian Federation, where there are no quadrivalent meningococcal conjugate vaccines currently licensed, immunization with a single dose of MenACWY-CRM197 elicited a robust immune response. The percentage of subjects in the overall population and in each age subgroup who achieved seroresponse 1 mo after vaccination was high against all antigens regardless of baseline seropositivity. This strong immune response was also demonstrated by the percentage of subjects with a protective post-vaccination hSBA titer ≥ 1:8. Finally, GMTs pre-vaccination increased 40-fold against serogroup A, 14-fold against serogroup C, 9.33-fold against serogroup W, and 20-fold against serogroup Y in the overall population.
The results obtained in children (2 to 10 y) 1 mo after vaccination were similar to previous studies conducted in the USA and Canada.19,23 The rate of seroresponders aged 2 to 10 y in the Halperin study ranged from 61% to 74% across all serogroups, which is in accordance with the 69% to 89% observed for this same age subgroup in our study. Moreover, the percentage of children with meningococcal antibody hSBA titers ≥ 1:8 1 mo after immunization in our study (76% to 95% of subjects across serogroups) was also similar to the percentage of children achieving hSBA titers ≥ 1:4 in these 2 previous trials (68–95% across serogroups).19,23
For the adolescent age subgroup (11 to 18 y), our results were similar to studies conducted in the US25,26 and Costa Rica.28 In these previous studies, the range of seroresponse in adolescent age groups ranged between 75% and 84%,25,28 in our study it was between 82% to 89% for serogroups A, C, and Y, but was lower for serogroup W (51% vs. 75–81% in previous studies)25,28 in accordance with the high baseline titer observed in the current study. Additionally, the majority of subjects (86% to 98% across serogroups) achieved hSBA titers ≥ 1:8 after 1 mo in our study, which is in accordance with the 75% and 99% range seen in the 3 earlier trials.25,26,28
In the adults, our results are in agreement with previous trials conducted in the US30 and Latin America.32 The observed percentage of responders 1 mo post-vaccination in these 2 studies was between 50% and 86% across serogroups,30,32 which is similar to the observed 57% and 85% in our study. In addition, the percentage of subjects who achieved an hSBA titer ≥ 1:8 was between 69% and 98% across groups in the 2 previous trials,30,32 and between 85% and 97% in ours.
From an epidemiological perspective, one interesting result of the present study was the high proportion of Russian subjects with pre-vaccination hSBA titers ≥ 1:8 against serogroup W (30% in the children’s subpopulation, 71% in adolescents, and 69% in adults). However, the percentage of subjects with protective titers 1 mo after vaccination was high for all age subgroups (95% of children, 98% of adolescents, and 97% of adults). The percentage of seropositive children at baseline is in accordance with data from a pivotal study conducted in the US and Canada (NCT00616421), which found that 35% of children had pre-vaccination hSBA titers ≥ 1:8, which increased to 90% at day 29. It is also in agreement with the Halperin study conducted in the US, which showed 39% of children with baseline hSBA titers ≥ 1:4.23 In the adolescent and adult population, another pivotal study conducted in the US found that 40% of adolescents, and 65% of adults had pre-vaccination hSBA titers ≥ 1:8 (NCT00450437), and 96% and 94% of subjects had protective titers 1 mo post-vaccination. Interestingly, 2 additional trials conducted in Taiwan and Korea obtained similar results (NCT01410474 and NCT01274897, respectively). In the Taiwan study, the percentage of subjects with baseline hSBA titers ≥ 1:4 was 49% for the 2 to10 y group, and 71% for the 11 to 18 y group, and seroprotective levels at day 29 were achieved by 93% and 99% of subjects, respectively. In the Korean study, 89% of subjects aged 11 to 59 y had pre-vaccination hSBA titers ≥ 1:4, and seroprotection at day 29 was achieved by 98% of subjects. Reasons for high percentages of seropositive subjects against serogroup W (as also observed in Taiwan and Korea) may include nasal colonization, cross-reacting antibodies induced by non-meningococcal bacteria,33 and an increased prevalence in the carriage of W-135 following the widespread use of MenA/C vaccines, as previously reported in Africa.34 The rates of N. meningitidis in interepidemic periods are between 10% and 35% in healthy individuals, and increase in overcrowded settings or confined populations such university students, household contacts of meningitis cases or military recruits, where carriage rates higher than 70% have been described.35 Active immune sensitization in response to carriage of low virulence meningococcal strains in the nasopharynx has been previously described, and found to correlate positively with increasing age.33,35 Although W-135 is considered a low-virulence meningococcal serogroup, it has been responsible for multinational outbreaks in 2000 that started in the Eastern Mediterranean region after the Hajj pilgrimage season, and recent invasive cases in France which have been related to a history of travel to the Western African belt.36,37 The incidence of IMD due to this serogroup in the Russian Federation is relatively low, estimated to be about 0.8% of all laboratory confirmed cases.10 However, naturally immunized subjects cannot be considered free of risk because although pre-existing titers are in general protective, subjects may still develop the disease.38
The safety and tolerability profile after MenACWY-CRM197 vaccination was also similar to data previously reported by other studies, and comparable within the corresponding age subgroups.2,19,23,25,26,28,29 Most of the solicited adverse events in our study were mild or moderate in intensity, and were resolved within 7 d of vaccination. There were no differences in the rates or type of local reactions across age groups; systemic reactions were observed in a similar proportion of children, adolescents, and adults (39% to 45%), and were less frequent among subjects 6 to 10 y of age (32%).
The present trial has shortcomings that must be acknowledged. First, the study enrolled a relatively small sample size; this was calculated based on the results from previous pivotal studies conducted in US, EU, Canada, and other countries that evaluated the safety and reactogenicity of MenACWY-CRM197,39 and was considered to be adequate to observe the expected immune response. Second, the open-label design did not include a vaccine comparator or a placebo arm, but previous phase II and III trials have compared MenACWY-CRM197 to unconjugated quadrivalent PS vaccines and other available quadrivalent conjugated vaccines, and it was found to be non-inferior, and well tolerated with an acceptable safety profile.39 Third, and inherent to all pre-licensing trials, the limited sample size and short-term follow-up prevents an accurate assessment of rare, delayed, or infrequent adverse events after immunization that will need to be evaluated through post-license safety monitoring.40 However, considering that several previous studies have evaluated the safety and reactogenicity of MenACWY-CRM197, some of them with concomitant administration of other vaccines, new safety concerns are not expected. Finally, we did not evaluate the long-term persistence of antibody levels, but previous studies conducted in the US have reported that a single dose of MenACWY-CRM197 in children 2 to 10 y of age maintained a broad response after 1 y of immunization, and in adolescents immune response has been shown to persist up to 2 to 5 y after vaccination.13,27,29
The only registered quadrivalent meningococcal vaccines for use in the Russian Federation are unconjugated PS, and among conjugated vaccines, only monovalent ones against serogroup C. Given the impossibility of predicting future serogroup distributions by geographical area, season, or year, quadrivalent vaccines against A, C, W-135, and Y serogroups allow a broad-based protection that can lead to a reduction in disease and IMD incidence, particularly in populations at high-risk. Among available quadrivalent preparations, conjugated vaccines are recommended over PS vaccines because they elicit a stronger immunogenic response, especially in children <2 y of age. Moreover, they are able to elicit immunologic memory and long-term protection, decrease oropharyngeal carriage, and have the potential to result in herd immunity.15,16
In summary, the results of the present study show that a single dose of quadrivalent MenACWY-CRM197 conjugate vaccine is able to elicit a robust immunogenic response, and has a good safety and tolerability profile in the healthy Russian population above 2 y of age. In addition, these results were confirmed across a full spectrum of age ranges including children, adolescents, and adults. This study shows that this quadrivalent meningococcal conjugate vaccine provides a broad protection against the most frequent epidemic serogroups, making it a useful alternative with an acceptable safety profile to currently available unconjugated PS monovalent or bivalent vaccines in Russia.
Methods
Study design and objectives
Novartis study V59_50 (ClinicalTrials.gov identifier NCT01725217) was a phase III, multicenter, open-label, single-arm study in healthy subjects ≥2 y of age conducted between November 2012 and March 2013 at 4 sites in the Russian Federation. The local institutional review boards approved the protocol. Written informed consent was obtained from each subject ≥18 y of age, written assent was obtained from subjects 11 to 18 y of age, and the parent/legal representative provided written informed consent in the case of subjects 2 to 18 y of age. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
We enrolled healthy subjects stratified into 3 different age groups: 2 to 10 y, 11 to 17 y, and 18 y and above. Subject disposition and study design are illustrated in Figure 1. The objectives of the study were to evaluate the immunogenicity of a single injection of MenACWY-CRM197 29 d after primary immunization in the overall sample and by age group, to provide an indication of any age-specific variation in the immune response to the administered vaccine, and to assess the safety profile following MenACWY-CRM197 vaccination.
Study subjects
A total of 198 healthy subjects ≥2 y of age of both sexes were enrolled in the trial. Good health was determined by medical history, physical examination, and the clinical judgment of the investigator. Main exclusion criteria were previously suspected or confirmed disease caused by N. meningitidis, exposure to an individual with culture-proven N. meningitidis infection within 60 d before enrolment, previous immunization with a meningococcal vaccine or a vaccine containing meningococcal antigen(s), receipt of any investigational drug or live vaccine within 28 d or any inactivated vaccine within 14 d of enrolment, except for Influenza vaccine if administered up to 15 d prior to study vaccination, and at least 15 d after study vaccination. We further excluded individuals who had experienced a significant acute infection within the 7 d prior to enrolment, fever (defined as body temperature ≥38 °C) within 3 d prior to enrolment, who had any serious acute, chronic, or progressive disease, had epilepsy or any progressive neurological disease or history of Guillain–Barré syndrome, who had a history of any anaphylaxis, serious vaccine reaction, or allergy to any vaccine components including diphtheria toxin (CRM197), who had a known or suspected acquired or congenital immune dysfunction, or were known to have a bleeding diathesis or any condition that may be associated with a prolonged bleeding time.
Vaccines and vaccinations
All subjects received a single dose (0.5 mL) of MenACWY-CRM197 (Menveo®, Novartis Vaccines) administered by intramuscular injection, preferably in the deltoid area of their non-dominant arm. The vaccine consisted of 10 μg of lyophilized meningococcal serogroup A capsular polysaccharide, and 5 μg of capsular polysaccharide of serogroups C, W-135, and Y, conjugated to CRM197.
Immunogenicity
To evaluate the immune response, a blood sample was obtained on day 1 before administration of the study vaccine (pre-vaccination), and 28 d after immunization (day 29). The elicited immunogenicity was measured by hSBA against the 4 meningococcal serogroups (MenA, MenC, MenW, and MenY), and was performed at Novartis Vaccines laboratory in Marburg, Germany.17
The immunogenicity of each serogroup was measured through hSBA response and hSBA GMTs obtained after back transformation of the mean of the logarithmically transformed (base 10) titers at baseline, and 1 mo post-vaccination. Seroresponse was defined as the percentage of subjects with post-vaccination hSBA ≥ 1:8 in subjects with a pre-vaccination hSBA < 1:4, and at least a 4-fold increase in post-vaccination hSBA in subjects with pre-vaccination hSBA ≥ 1:4.
Safety
Vaccinated subjects were observed for approximately 30 min after each immunization to monitor for immediate adverse reactions. The safety profile of the study vaccine was evaluated from days 1 through 7 for selected solicited local and systemic AEs and for unsolicited AEs. Solicited local AEs were tenderness, erythema, and induration for children 2 to 5 y, and pain, erythema, and induration for subjects aged above 6 y. Systemic solicited AEs were fever (body temperature ≥ 38 °C), change in eating habits, sleepiness, irritability, vomiting, diarrhea, rash, and other reactions (use of antipyretics or analgesics) for the group 2 to 5 y, and fever, chills, nausea, malaise, myalgia, arthralgia, headache, rash, and other reactions (use of antipyretics or analgesics) for subjects older than 6 y. Events were considered as severe if the subject was unable to perform normal daily activities. All SAEs, medically attended AEs, and premature withdrawals due to AEs were recorded throughout the study.
Statistical methods
Endpoints for each serogroup were descriptively reported for the overall study population and by age group. We calculated the numbers, percentages, and 95% confidence intervals (CI) of the percentage of subjects who achieved seroresponse, and who had pre- and post-vaccination hSBA titers ≥ 1:8. Pre- and post-vaccination GMTs and their associated 95% CIs were also calculated for each serogroup overall and by age group. Immunogenicity analyses were performed in the full analysis set (FAS), defined as subjects who received the study vaccination and provided evaluable serum samples with assay results available, for at least one serogroup, at baseline and at day 29. Safety data were summarized providing the frequency and proportion of participants reporting an event, and were conducted for all subjects who received the study vaccine and provided any safety data.
Glossary
Abbreviations:
- AE
adverse event
- CI
confidence interval
- GMT
geometric mean titer
- GMR
GMT ratio
- hSBA
serum bactericidal activity assay with human complement
- FAS
full analysis set
- IMD
invasive meningococcal disease
- PS
polysaccharide vaccine
- SAE
serious adverse event
Disclosure of Potential Conflicts of Interest
N.I., S.K., L.N-B., and A.A. have no conflicts of interest. M.B., E.T., C.B., and A.K.A. are permanent employees of Novartis Vaccines.
Acknowledgments
The study was funded by Novartis Vaccines. We acknowledge Dr Mònica Gratacòs (CHC-Europe) and Dr Debaditya Das (Novartis Vaccines) for providing support in the manuscript preparation, revision, and editing.
References
- 1.Pace D, Pollard AJ. . Meningococcal disease: clinical presentation and sequelae. Vaccine 2012; 30:Suppl 2 B3 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.062; PMID: 22607896 [DOI] [PubMed] [Google Scholar]
- 2.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. . Meningococcal disease. N Engl J Med 2001; 344:1378 - 88; http://dx.doi.org/ 10.1056/NEJM200105033441807; PMID: 11333996 [DOI] [PubMed] [Google Scholar]
- 3.Edwards MS, Baker CJ. . Complications and sequelae of meningococcal infections in children. J Pediatr 1981; 99:540 - 5; http://dx.doi.org/ 10.1016/S0022-3476(81)80250-8; PMID: 7277093 [DOI] [PubMed] [Google Scholar]
- 4.Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. . Global epidemiology of invasive meningococcal disease. Popul Health Metr 2013; 11:17; http://dx.doi.org/ 10.1186/1478-7954-11-17; PMID: 24016339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. . Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184 - 91; http://dx.doi.org/ 10.1086/649209; PMID: 20001736 [DOI] [PubMed] [Google Scholar]
- 6.Tan LK, Carlone GM, Borrow R. . Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med 2010; 362:1511 - 20; http://dx.doi.org/ 10.1056/NEJMra0906357; PMID: 20410516 [DOI] [PubMed] [Google Scholar]
- 7.Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al. . Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis 2013; 19:566 - 73; http://dx.doi.org/ 10.3201/eid1904.111799; PMID: 23628376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. . The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30:Suppl 2 B26 - 36; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.032; PMID: 22178525 [DOI] [PubMed] [Google Scholar]
- 9.Kaijalainen T, Kharit SM, Kvetnaya AS, Sirkiä K, Herva E, Parkov OV, Nohynek H. . Invasive infections caused by Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae among children in St Petersburg, Russia. Clin Microbiol Infect 2008; 14:507 - 10; http://dx.doi.org/ 10.1111/j.1469-0691.2008.01967.x; PMID: 18318743 [DOI] [PubMed] [Google Scholar]
- 10.Titova L, Samodova O, Buzinov R, Gordienko T. . Epidemiology Of Meningococcal Infection In Arkhangelsk Oblast. Epinorth 2011; 11:10 - 5 [Google Scholar]
- 11.Koroleva IS, Maxina TA, Zakroeva IM, Beloshitsky GV, Lytkina IN, Pyaeva AP. Epidemiology Of Invasive Meningococcal Disease In Moscow, 2005-2010. Meningitis & Septicaemia Conference. London: Meningitis Research Foundation, 2011:39. [Google Scholar]
- 12.Koroleva I, Beloshitskij G, Zakroeva I, Melnikova A, Koroleva M, Shipulin G, Mironov K. Invasive Meningococcal Disease In Russian Federation. 12th EMGM. Loipersdorf, Austria, 2013. [Google Scholar]
- 13.Black SB, Plotkin SA. . Meningococcal disease from the public health policy perspective. Vaccine 2012; 30:Suppl 2 B37 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.074; PMID: 22607897 [DOI] [PubMed] [Google Scholar]
- 14.O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. . A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs 2014; 74:15 - 30; http://dx.doi.org/ 10.1007/s40265-013-0155-7; PMID: 24338083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec 2011; 86:521 - 39; PMID: 22128384 [PubMed] [Google Scholar]
- 16.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC). . Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:RR-2 1 - 28; PMID: 23515099 [PubMed] [Google Scholar]
- 17.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, et al. . Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 2008; 299:173 - 84; http://dx.doi.org/ 10.1001/jama.2007.29-c; PMID: 18182599 [DOI] [PubMed] [Google Scholar]
- 18.Perrett KP, Snape MD, Ford KJ, John TM, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, Anemona A, et al. . Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J 2009; 28:186 - 93; http://dx.doi.org/ 10.1097/INF.0b013e31818e037d; PMID: 19209097 [DOI] [PubMed] [Google Scholar]
- 19.Halperin SA, Diaz-Mitoma F, Dull P, Anemona A, Ceddia F. . Safety and immunogenicity of an investigational quadrivalent meningococcal conjugate vaccine after one or two doses given to infants and toddlers. Eur J Clin Microbiol Infect Dis 2010; 29:259 - 67; http://dx.doi.org/ 10.1007/s10096-009-0848-8; PMID: 20033465 [DOI] [PubMed] [Google Scholar]
- 20.Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. . Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J 2012; 31:64 - 71; http://dx.doi.org/ 10.1097/INF.0b013e31823dce5c; PMID: 22094635 [DOI] [PubMed] [Google Scholar]
- 21.Klein NP, Shepard J, Bedell L, Odrljin T, Dull P. . Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine administered concomitantly with measles, mumps, rubella, varicella vaccine in healthy toddlers. Vaccine 2012; 30:3929 - 36; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.080; PMID: 22504039 [DOI] [PubMed] [Google Scholar]
- 22.Nolan TM, Nissen MD, Naz A, Shepard J, Bedell L, Hohenboken M, Odrljin T, Dull PM. . Immunogenicity and safety of a CRM-conjugated meningococcal ACWY vaccine administered concomitantly with routine vaccines starting at 2 months of age. [Forthcoming] Hum Vaccin Immunother 2014; 10:280 - 9; http://dx.doi.org/ 10.4161/hv.27051; PMID: 24220326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. . Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2-10 years of age. Vaccine 2010; 28:657 - 63; http://dx.doi.org/ 10.1016/j.vaccine.2009.10.104; PMID: 19895922 [DOI] [PubMed] [Google Scholar]
- 24.Halperin SA, Gupta A, Jeanfreau R, Klein NP, Reisinger K, Walter E, Bedell L, Gill C, Dull PM. . Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age. Vaccine 2010; 28:7865 - 72; http://dx.doi.org/ 10.1016/j.vaccine.2010.09.092; PMID: 20943209 [DOI] [PubMed] [Google Scholar]
- 25.Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull PM, V59P13 Study Group. . Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis 2009; 49:e1 - 10; http://dx.doi.org/ 10.1086/599117; PMID: 19476428 [DOI] [PubMed] [Google Scholar]
- 26.Jackson LA, Jacobson RM, Reisinger KS, Anemona A, Danzig LE, Dull PM. . A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J 2009; 28:86 - 91; http://dx.doi.org/ 10.1097/INF.0b013e31818a0237; PMID: 19116603 [DOI] [PubMed] [Google Scholar]
- 27.Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. . Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 2010; 6:881 - 7; http://dx.doi.org/ 10.4161/hv.6.11.12849; PMID: 21339701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. . Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine 2010; 28:3171 - 9; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.045; PMID: 20189491 [DOI] [PubMed] [Google Scholar]
- 29.Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. . Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J 2013; 32:e170 - 7; http://dx.doi.org/ 10.1097/INF.0b013e318279ac38; PMID: 23114372 [DOI] [PubMed] [Google Scholar]
- 30.Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. . Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol 2009; 16:1810 - 5; http://dx.doi.org/ 10.1128/CVI.00207-09; PMID: 19812260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, Ceddia F. . Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol 2010; 17:537 - 44; http://dx.doi.org/ 10.1128/CVI.00436-09; PMID: 20164251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, Karsten A, Dull PM. . Safety and immunogenicity of an investigational quadrivalent meningococcal CRM(197) conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis 2010; 14:e868 - 75; http://dx.doi.org/ 10.1016/j.ijid.2010.03.017; PMID: 20655261 [DOI] [PubMed] [Google Scholar]
- 33.Goldschneider I, Gotschlich EC, Artenstein MS. . Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med 1969; 129:1327 - 48; http://dx.doi.org/ 10.1084/jem.129.6.1327; PMID: 4977281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gold R. . Epidemiology of meningococcal disease in light of recent Hajj-associated outbreaks. Clin Infect Dis 2003; 36:684 - 6; http://dx.doi.org/ 10.1086/367863; PMID: 12627351 [DOI] [PubMed] [Google Scholar]
- 35.Yazdankhah SP, Caugant DA. . Neisseria meningitidis: an overview of the carriage state. J Med Microbiol 2004; 53:821 - 32; http://dx.doi.org/ 10.1099/jmm.0.45529-0; PMID: 15314188 [DOI] [PubMed] [Google Scholar]
- 36.Wilder-Smith A, Goh KT, Barkham T, Paton NI. . Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis 2003; 36:679 - 83; http://dx.doi.org/ 10.1086/367858; PMID: 12627350 [DOI] [PubMed] [Google Scholar]
- 37.Parent du Chatelet I, Barboza P, Taha MK. . W135 invasive meningococcal infections imported from Sub-Saharan Africa to France, January to April 2012. Euro Surveill 2012; 17:17; PMID: 22687826 [PubMed] [Google Scholar]
- 38.Greenwood BM, Greenwood AM, Bradley AK, Williams K, Hassan-King M, Shenton FC, Wall RA, Hayes RJ. . Factors influencing susceptibility to meningococcal disease during an epidemic in The Gambia, West Africa. J Infect 1987; 14:167 - 84; http://dx.doi.org/ 10.1016/S0163-4453(87)92052-4; PMID: 3106507 [DOI] [PubMed] [Google Scholar]
- 39.Cooper B, DeTora L, Stoddard J. . Menveo®): a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y. Expert Rev Vaccines 2011; 10:21 - 33; http://dx.doi.org/ 10.1586/erv.10.147; PMID: 21162617 [DOI] [PubMed] [Google Scholar]
- 40.Rosenthal KL. Mcvittie LD. The Clinical Testing Of Preventive Vaccines. In: Mathieu M, Ed. Biological Development: A Regulatory Overview. Waltham, Ma: Paraxel, 1993:119-30. [Google Scholar]