Abstract
Hepatitis B (HB) infection caused by Hepatitis B virus (HBV) is the most common liver disease in the world. HB vaccine, when administered in conjunction with alum adjuvants, induces Th2 immunity that confers protection against HBV. However, currently available vaccine formulations and adjuvants do not elicit adequate Th1 and CTL responses that are important for prevention of maternal transmission of the virus. Microspheres synthesized from poly (D, L-lactide-co-glycolide) (PLGA) or poly (D, L-lactide) (PLA) polymers have been considered as promising tools for in vivo delivery of antigens and drugs. Here we describe PLA microspheres synthesized by premix membrane emulsification method and their application in formulating a new microsphere based HB vaccine. To evaluate the immunogenicity of this microsphere vaccine, BALB/c mice were immunized with microsphere vaccine and a series of immunological assays were conducted. Results of Enzyme-linked ImmunoSpot (ELISPOT) assays revealed that the number of interferon-gamma (IFN-γ)-producing splenocytes and CD8+ T cells increased significantly in the microsphere vaccine group. Microsphere vaccine group showed enhanced specific cell lysis when compared with HB surface antigen (HBsAg) only group in 51Cr cytotoxicity assays. Moreover, microsphere vaccine elicited a comparable level of antibody production as that of HB vaccine administered with alum adjuvant. We show that phagocytosis of HBsAg by dendritic cells is more pronounced in microsphere vaccine group when compared with other control groups. These results clearly demonstrate the potential of using PLA microspheres as effective HB vaccine adjuvants for an enhanced Th1 immune response.
Keywords: CTL, HBsAg, IFN-γ, anti-HBs, microspheres, polylactide
Introduction
Hepatitis B virus (HBV) remains a global problem despite the availability of an effective hepatitis B (HB) vaccine. Some of the causes for the persistence of the virus include establishment of a stable host cell (hepatocyte)–virus relationship, lack of virus specific drugs, and a large population of carriers. Although these reasons explain in part the high global disease burden of this virus, the precise reason underlying the unusually high incidences of HBV infections worldwide continues to elude the scientific community. At present, approximately 350 million people around the world are HBV carriers and represent a permanent source of infection. About 1 million people die from HBV-associated hepatoma and hepatic cirrhosis every year.1 Currently available HB vaccine contains recombinant hepatitis B surface antigen, which when administered in conjunction with alum adjuvant confers protection against HBV infection by inducing a potent, predominantly humoral immune response.
Although factors governing the clearance of HBV in the acute self-limited HBV infection or the persistence of the virus in chronic infection are not clearly understood, it appears that the disease activity correlates closely with the strength of virus-specific T cell immune responses. The humoral antibody response to viral envelope antigens, which is modulated by Th2 cell response, contributes to the clearance of circulating virus, while the Th1-type cellular immune responses to the envelope, nucleocapsid, and polymerase antigens eliminate infected cells.2 A weak HBV-specific T cell immune response may result in the persistence of HBV infection, whereas strong polyclonal and multi-specific T cell immune responses are associated with self-limited acute infection.3 So vaccines effective in inducing humoral and cellular immune responses may have greater prospects in the prophylaxis and therapy of HBV infection.
Microspheres synthesized from poly (D,L-lactide) (PLA) or poly (D,L-lactide-co-glycolide) (PLGA) polymers are both biodegradable and biocompatible. These 2 polymers have been deemed safe for use as suture material and for controlled release drug delivery systems.4 Studies employing PLA or PLGA microspheres to elicit immune responses have been widely conducted. Microspheres encapsulated with tetanus toxoid, hepatitis B core antigen (HBcAg), B cell epitopes, and DNA have been studied since early 1990s.5-7 These studies have clearly demonstrated the ability of microspheres to enhance immune responses induced by antigens and peptides. However, some drawbacks have limited the progress of microencapsulation technology. In particular, low levels of loading of the antigen and distortion of the epitopes have been shown to decrease the efficacy of the application. Therefore, instead of microencapsulation, several studies now show that microspheres adsorbed with antigens on the surface are more efficient in inducing potent immune responses.8
In this study, PLA microspheres synthesized by premix membrane emulsification method were used to produce a new microsphere based vaccine containing HB surface antigen (HBsAg). BALB/c mice immunized with this vaccine developed HBsAg-specific cellular and humoral immune responses. The potential of this new vaccine in inducing both cellular and humoral immune responses are discussed.
Results
Characterization of PLA microspheres
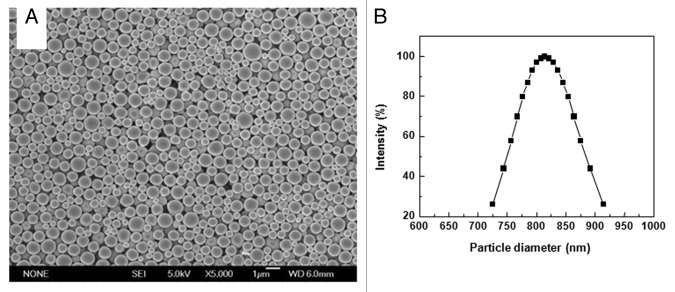
PLA microspheres prepared by the premix membrane emulsification method were examined using scanning electron microscope (SEM). The microspheres exhibited a spherical morphology with a smooth outer surface (Fig. 1A). Except for the differences in size, there were no obvious differences in the morphology. Notably, no cavities or depressions were observed on the surface of the microspheres. Dynamic light scattering analysis of the PLA microsphere preparation revealed that the distribution of the microspheres was uniform. There were no signs of aggregation. The diameter of the microspheres ranged from 700 nm to 950 nm with a mean diameter of about 800 nm (Fig. 1B). These results are in agreement with the SEM picture of the microspheres.
Figure 1. SEM photographs and size distribution of PLA microspheres. (A) Microsphere suspension was spotted on aluminum foil and dried naturally. Surface morphology of PLA microspheres was observed by SEM (B) Microspheres suspended in distilled water were examined by dynamic light scattering technology for size distribution.
Microsphere vaccines elicit enhanced IFN-γ secretion and specific lysis in splenocytes
To evaluate the potential of PLA microspheres in enhancing HBsAg induced specific IFN-γ secretion, we measured the number of IFN-γ secreting cells resulting from the priming of mice with microsphere vaccines containing varying concentrations of microspheres. ELISPOT assays were conducted on splenocytes harvested 7 d post immunization of mice. After 20 h of in vitro re-stimulation, splenocytes from mice immunized with 1 mg or 400 μg microspheres and 4 μg HBsAg exhibited significantly (P < 0.05) higher numbers of IFN-γ secreting cells compared with mice immunized with 4 μg HBsAg alone. This enhancement in the number of IFN-γ secreting cells was dependent on the concentration of microspheres in the vaccine (Fig. 2)
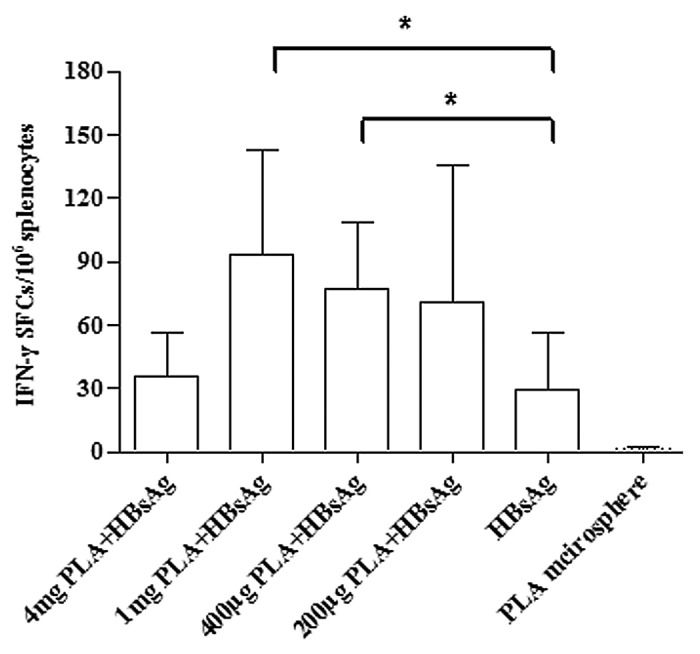
Figure 2. PLA microsphere enhances HBsAg induced IFN-γ secretion. BALB/c mice were subcutaneously immunized with microsphere vaccine containing different doses of microspheres. Formulation for each mouse contained 4 μg HBsAg except for PLA microsphere group. Splenocytes were isolated from vaccinated mice on day 7 post immunization and the number of IFN-γ secretion cells were counted using ELISPOT assays. Data are presented as mean spot forming cells (SFC) with standard deviation for 5 mice per group. Statistical significance was determined by one-way ANOVA and Bonferroni post test between groups. *P < 0.05.
It is usually difficult for the host to eliminate intracellular pathogens such as Mycobacteria and HBV.9,10 Antigen-specific CTL activity is known to play an essential role in clearing these pathogens. To evaluate the impact of PLA microspheres on antigen-specific CTL activity, we performed 51Cr release assays. T cells obtained from mice immunized with microsphere vaccines showed an increased percentage of specific cell lysis. As the E:T ratio increased, the specific lysis activity increased for both microsphere vaccine group and HBsAg only group. The results of the 51Cr release assays clearly demonstrate the ability of PLA microspheres to enhance antigen-specific cellular immune responses.
CD8+ T cells play a pivotal role in cellular immune response against intracellular pathogens, including viruses. To find out if PLA microspheres have a stimulatory effect on the ability of CD8+ T cells to secrete IFN-γ, we first separated CD8+ T cells from mice splenocytes. After re-stimulation with MHC I peptide, significant differences (P < 0.05) were observed in number of IFN-γ secreting CD8+ T cells between microsphere vaccine group and HBsAg only group. Splenocytes treated in parallel under identical conditions also showed a significantly higher number of IFN-γ spot-forming cells (SFCs) in microsphere vaccine group when compared with the HBsAg only group, independently corroborating previous observation of the ability of PLA microspheres to enhance IFN-γ secretion (Fig. 2). Interestingly, the HB vaccine group was as potent as the PLA microsphere vaccine group in enhancing the stimulation of CD8+ T cells for secreting IFN-γ (Fig. 4).
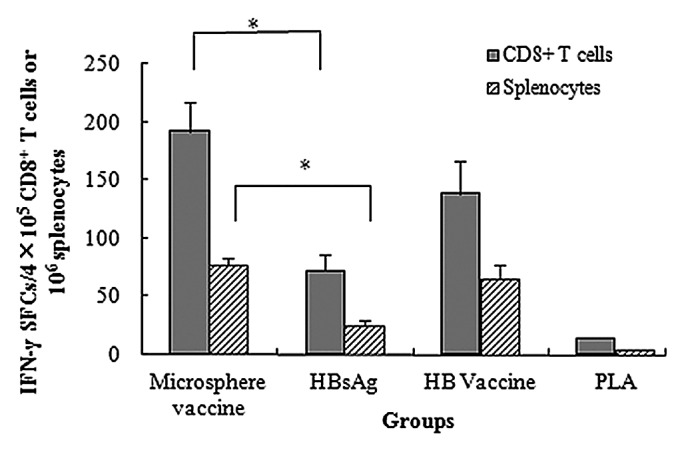
Figure 4. HBsAg-specific IFN-γ production in splenocytes and CD8+ T cells. BALB/c mice were vaccinated with different vaccine formulations. At day 7, splenocytes and CD8+ T cells were isolated for enumeration of IFN-γ secreting cells with ELISPOT assays. Results are shown as the mean value of IFN-γ SFCs observed in 4 × 105 CD8+ T cells or 106 splenocytes. PLA mcirosphere vaccine group shows significantly higher IFN-γ secretion level than aqueous HBsAg group in both splenocytes and CD8+ T cells (*P < 0.05).
Microsphere vaccines facilitate HBsAg-specific humoral response
Serum collected from mice immunized with one dose of different formulations containing 4 μg HBsAg at 14, 30, 60, 90, 180, and 360 d post-immunization were analyzed for presence of anti-HBs antibodies using chemiluminescent microparticle immunoassay. As seen in Figure 5, HB vaccine induced a rapid, high-level, and long-lasting antibody response, while the response levels of microsphere vaccine and HBsAg only groups were relatively low. Mice immunized with recombinant HBsAg only exhibited low seroconversion ratio (Table 1) and the antibody titer decreased gradually after 90 d post immunization. Microsphere vaccine elicited higher antibody titer than HBsAg only and the antibody titer remained constant from 180 to 360 d post immunization with no obvious decrease. Interestingly, lyophilization had a negative effect on the antibody production induced by microsphere vaccine. As seen in Figure 6, anti-HBs titer generated by freeze-dried microsphere vaccine decreased dramatically when compared with microsphere vaccine group. Thus, lyophilization seems detrimental to immunogenicity of microsphere vaccines.
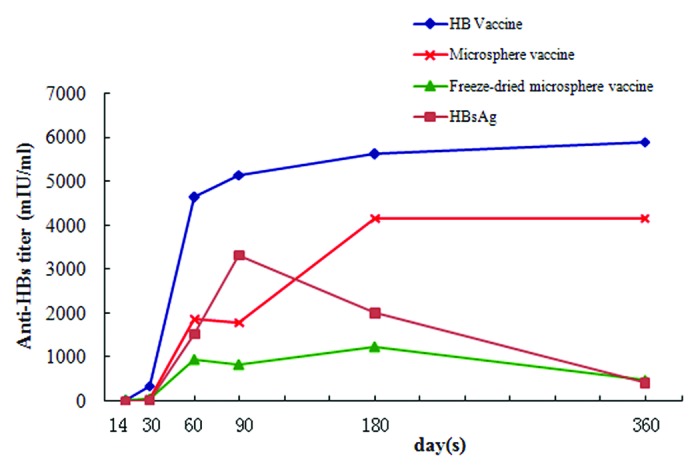
Figure 5. Anti-HBs titers in the sera of mice immunized with different formulations. Groups of animals were immunized subcutaneously with a single dose (containing 4 μg HBsAg) of these formulations. Serum samples were collected at different time points and analyzed using chemiluminescent microparticle immunoassay for anti-HBs titers.
Table 1. Anti-HBs seroconversion ratio in the sera of mice immunized with different formulations (n = 10).
| Groups | Seroconversion ratio | |||||
|---|---|---|---|---|---|---|
| 14 d | 30 d | 60 d | 90 d | 180 d | 360 d | |
| Vaccine | 1/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Microsphere vaccine | 1/10 | 8/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Freeze-dried microsphere vaccine |
1/10 | 8/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| HBsAg | 0/10 | 5/10 | 7/10 | 8/10 | 7/10 | 5/10 |
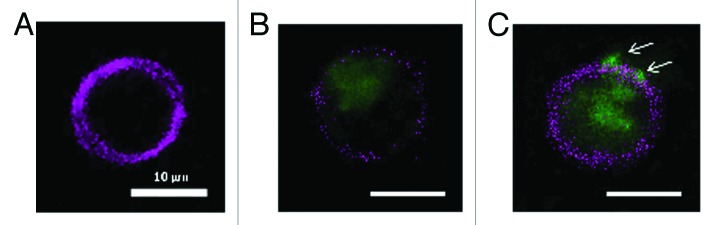
Figure 6. Uptake study in murine DC, stimulated with FITC-labeled HBsAg and microsphere vaccine for 6 h in vitro. (A) APC labeled DC; (B) FITC-labeled HBsAg (blue) phagocytosed by DC; (C) Phagocytosis of microsphere vaccine adsorbed with FITC-labeled HBsAg; DCs isolated from immunized BALB/c mice with magnetic microbeads were co-cultured with microsphere vaccine with FITC-labeled HBsAg at 37 °C for 6 h. DCs were labeled with APC-CD11c mAb and washed followed by fluorescence microscopy. White arrows: microspheres adsorbed with FITC-labeled HBsAg on the surface of DC.
PLA microspheres aid phagocytosis of HBsAg by dendritic cells (DCs) and particle size affects the immune response of microsphere vaccine
To visualize microspheres uptake by DCs, murine DCs isolated using magnetic beads were incubated with FITC-labeled HBsAg or microsphere adsorbed FITC-labeled HBsAg for 6 h. Fluorescence microscopy showed that microspheres were first adsorbed on the surface of DCs, and then endocytosed into the cytoplasm. During 6 h of in vitro stimulation, it was easier for microspheres with a diameter of about 800 nm to be adsorbed and phagocytosed by DCs than for HBsAg protein molecule. More FITC-labeled HBsAg adsorbed on the surface of microspheres were endocytosed into DCs, which helps explain the enhancement in cellular and humoral immune responses of microsphere vaccine (Fig. 6).
Microspheres with different particle sizes were used to evaluate the contribution of microspheres to cytokine secretion. As is shown in Figure 7, microspheres with a diameter of 350 nm demonstrated significantly higher HBsAg-specific IFN-γ production (P < 0.05). Microspheres with diameters of 1 μm or larger could also induce the secretion of HBsAg-specific IFN-γ, but with a relatively lower potency.
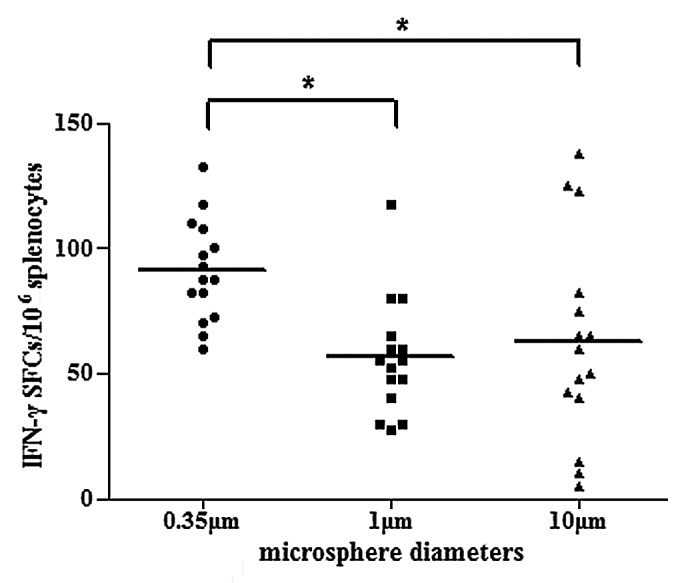
Figure 7. Induction of HBsAg-specific IFN-γ secretion in mice immunized with PLA microsphere vaccine containing microspheres of different diameters. BALB/c mice (n = 15) were immunized with different formulations of microsphere vaccines varying in particle size. At day 7, splenocytes were isolated for ELISPOT assays to evaluate the frequency of occurrences of IFN-γ secreting T cells in splenocytes. Results are shown as the number of spots observed in 106 splenocytes (*P < 0.05).
Discussion
Currently available systems for in vivo delivery of drugs and vaccines are not optimal. Therefore, development of new delivery systems has been a topic of intense research. There has been long standing interest in the use of biodegradable polymers for the delivery of antigens or drugs. Way back in 1970s, Preis et al. used polymers encapsulated with bovine serum albumin for animal immunizations, and showed that vaccine formulations containing polymers had a comparable protective effect as that of freund’s complete adjuvant.11 In recent years, PLA and PLGA microspheres have been investigated intensely because they offer, among other benefits, 2 important advantages over other polymers—biocompatibility and biodegradability. Despite these clear advantages offered by polylactide microspheres, other limitations have restricted the development of microsphere vaccines. For instance, studies focused on encapsulation of antigens or DNA in microspheres for the purpose of controlled release have encountered problems of low loading rate of antigens onto the microspheres.12-14 In addition shear forces and organic solvents present in the loading process distort or destroy the structure of the antigen or change its antigenicity. These drawbacks pose major hurdles in the development of microsphere encapsulation technology. Some of these challenges have been circumvented by adsorbing antigens onto the surface of microspheres rather than attempting to encapsulate them.15 Microspheres with surface-adsorbed antigens offer advantages in not only preserving the antigenicity of antigens, but they are also amenable to scalable, reproducible, and robust manufacturing processes, which would facilitate commercial production of the vaccine. Systemic and mucosal immune responses have been reported for immunization with surface-adsorbed PLGA microspheres containing certain antigens.16,17 Microspheres used in this study were prepared using premix membrane emulsification method. This method produced remarkably homogenous particles (Fig. 1) that could evoke potent immune responses under a variety of experimental conditions tested, underscoring the utility of PLA microspheres in vaccine preparations.
Although factors which result in the clearance of HBV in the acute self-limited HBV infection or the persistence of the virus in chronic infection are not clearly understood, it appears that the disease activity correlates closely with the strength of virus-specific T cell immune response.2,16,18,19 HBV-specific T-cell responses, especially CTLs, play a role in the control of HBV infection in children born to HBsAg-positive mothers after immunoprophylactic treatment.20 The aim of the present study was to evaluate the immune effects of a new microsphere vaccine, which could provide an effective means to combat chronic hepatitis B infection. Since the adsorbed antigen is much easier to release from the surface of the microsphere rather than from encapsulated microspheres used in controlled-release formulations, the microsphere vaccine formulated in this study could evoke a much quicker immune response. We prepared microsphere vaccine by mixing microspheres and recombinant HBsAg, which got adsorbed onto the surface of microspheres. Microsphere vaccines with different ratios of microspheres and HBsAg were examined for IFN-γ secretion. Compared with HBsAg only group, microsphere vaccine containing 1 mg and 400 μg PLA microspheres showed a significant increase in IFN-γ SFCs. Further studies performed using isolated CD8+ T cells and splenocytes for evaluating microsphere vaccine induced IFN-γ secretion confirmed the benefits of microsphere based vaccine. Moreover, CTL responses have been traditionally implicated in eradicating HBV infection.2,17 In this study, PLA microspheres enhanced HBsAg induced specific CTL lysis, which could play a role in HBV clearance in HBV infection. Our results clearly show that immunization with PLA microsphere vaccine induces robust cellular immune responses in mice, underscoring its value as a candidate vaccine for chronic HB immunotherapy.
Humoral response against viral envelope antigens, which is modulated by Th2 cells, contributes to the clearance of circulating virus, while the Th1-type cellular immune responses against the envelope, nucleocapsid, and polymerase antigens eliminates infected cells.2,8 Alum contained in commercial HB vaccine is an excellent adjuvant in inducing humoral immune response. Results of this study show that one dose of PLA microsphere vaccine elicited anti-HBs seroconversion in 8/10 mice 30 d post immunization, and no obvious decline was observed in the antibody titer 1 y post immunization. The titer of anti-HBs in microsphere vaccine group persisted at the level of 2000 to 4000 mIU/mL. Although this level is lower than that of HB vaccine group, it is still well above the level recommended for adequate protection.
Size and surface character of microspheres influence microsphere uptake by immune cells.21 Delivery of microspheres into APCs contributes to the immune response induced by specific antigens.22 In this study, we observed that microsphere vaccine facilitates the phagocytosis of HBsAg into DCs in vitro, which is consistent with the results of enhanced cytokine secretion and CTL lysis induced by PLA microspheres in ELISPOT and CTL assays. The reason for enhanced phagocytosis might lie in the fact that PLA microspheres probably mimic a kind of microparticle that is more liable to be phagocytosed compared with small protein molecules. This results in much larger and more efficient delivery of the adsorbed HBsAg into APCs, which contributes to the enhancement of cell-mediated immune responses. Significant differences in the ability to enhance IFN-γ secretion were observed in PLA microsphere vaccines containing different sizes of the microspheres. Vaccines containing smaller microspheres (0.35 μm diameter) were the most potent in inducing an immune response. This is in agreement with the results of a previous study in which smaller microparticles adsorbed with DNA were more effective in inducing a humoral response.23 Thus, optimization of the size of the microspheres is an important criteria for development of an effective PLA microsphere based vaccine.
In summary, this study evaluates the potential of biodegradable PLA microspheres for use in vaccines for the induction of an immune response against HBsAg. Results of our studies show that HBsAg absorbed on PLA microspheres can evoke potent humoral responses. Homogenous distribution of microspheres in solution, scalability, and reproducibility make PLA microsphere based vaccine delivery for protection against HBV infections a commercially viable option.
Materials and Methods
PLA, recombinant HBsAg and HB vaccine
PLA was purchased from the Institute of Medical Instrument. Recombinant HBsAg was expressed in Hansenula polymorpha and produced by Hualan Biological Engineering Inc. The recombinant antigen was purified to >99.0% purity using High-Performance Liquid Chromatography (HPLC). Aluminum hydroxide adjuvant containing HB vaccine was kindly provided by Hualan Biological Engineering Inc.. The concentration of HBsAg and AL3+ were 20 μg/mL and 0.5 mg/mL, respectively.
Preparation and characterization of PLA microspheres
PLA microspheres were prepared as described previously.24 Briefly, in a two-step procedure, coarse emulsions were first prepared by low-speed stator homogenization and then poured into the premix reservoir. Microspheres were synthesized by extruding the coarse emulsions through SPG membrane under high pressure. The microspheres were stirred overnight to evaporate organic solvent and the resulting PLA microspheres were washed 3 times using PBS and stored until further use. The surface morphology of PLA microspheres was examined by SEM. The mean size was measured by using dynamic light scattering technique.
Preparation of microsphere vaccine
Aqueous HBsAg bulk was diluted with PBS to different concentrations. Fixed amount of PLA microspheres were added to this liquid recombinant HBsAg. The mixture was gently shaken at 25 °C overnight. Further dilutions for immunization were performed using PBS.
Animal and Immunization
Female BALB/c mice (H-2d) aged 6- to 8-wk were purchased and bred in the animal facilities of National Institutes for Food and Drug Control (NIFDC). Aqueous HBsAg bulk was diluted to 40 μg/mL. Diluted aqueous HBsAg and microsphere vaccine formulations containing 40 μg/mL HBsAg were administered subcutaneously into the back of the mice with a volume of 100 μL each. To ensure an equal content of HBsAg in HB vaccine group, mice were vaccinated subcutaneously with 200 μL of the formulation.
Cell isolation, purification of CD8+ T cells and CD11c+ DCs
BALB/c mice were sacrificed 7 d post-immunization and spleens were removed aseptically, ground gently, filtered through a sterile 400-grid wire mesh, and flushed with normal saline. Cells were separated by loading cell suspension gently onto Nycoprep (Axis-Shield) density gradient and centrifuging at 600× g for 20 min at room temperature. Splenocytes at the interphase were collected, washed twice with RPMI 1640 (Hyclone), and suspended in complete RPMI 1640 (RPMI 1640 supplemented with 2 mM L-glutamine, 10 mM HEPES, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% heat-inactivated fetal bovine serum). CD8+ T cells were further purified by positive selection using anti-CD8 (Ly-2) microbeads (Miltenyi Biotec) and cells expressing MHC II molecules were purified using MHC II (la) microbeads as antigen presenting cells. CD11c+ DCs used in phagocytic assay were purified using CD11c (N418) microbeads. The resulting CD8+ T cells and DCs were routinely >85% positive for CD8 or CD11c as determined by flow cytometry.
ELISPOT assays
Splenocytes were harvested at different time points according to the immunization protocols. PVDF 96-well plates (Millipore, MA) which were coated overnight at 4 °C with 5 μg/mL purified anti-mouse IFN-γ monoclonal antibody were flooded with 100 μL 4 × 106 cells /mL splenocytes or 2 × 106 cells/ml CD8+ T cells and 100 μL HBsAg S28–39 peptide (IPQSLDSWWTSL, final concentration: 10 μg/mL) in each well. Then they were incubated at 37 °C, in a 5% CO2 and humidified incubator for 20 h. After incubation the plates were manipulated according to BDTM ELISPOT Set (BD Biosciences) Instruction Manual. SFCs were enumerated with ImmunoSpotTM system (Cellular Technology Ltd.).
Cytotoxic T lymphocytes assays
In vitro re-stimulation and chromium release assays were performed as previously described.25 Briefly, P815 cells (kind gift from CDC, China) labeled with 51Cr were co-cultured with splenocytes at 37 °C and 5% CO2 for 6 h. After centrifugation, 100 μL supernatant was collected for gamma radiation counting. The percentage of specific release was calculated as ([experimental release – spontaneous release] / [total release – spontaneous release]) × 100%. Total release was measured by incubating target cells with Triton X-100. Spontaneous release counts were always less than 10% of the total counts. Data shown are the mean of triplicate cultures.
Evaluation of in vivo anti-HBs production
Antibodies against HBsAg were detected with automated chemiluminescent microparticle immunoassay, Abbott ARCHITECT® anti-HBs. The anti-HBs value equal to or greater than 10 mIU/mL was considered as positive seroconversion. The numbers of serum anti-HBs conversions and titer of each positive serum were calculated.
Phagocytic uptake study
DCs were seeded in 24-well plate at 3 × 105 cells /well, and 50 μL FITC-labeled HBsAg (containing 2 μg HBsAg) or 500 μg microspheres containing 2 μg FITC-labeled HBsAg in 50 μL were added in each well as stimulus. To determine the difference in the 2 stimulus induced phagocytosis, DCs were stained with APC-labeled anti-CD11c mAb (Miltenyi Biotec). Phagocytosis was observed with fluorescence microscope.
Statistical analysis
Statistical analysis was performed by one-way ANOVA and Bonferroni post test using GraphPad Prism software (GraphPad Software) and P < 0.05 was considered statistically significant.
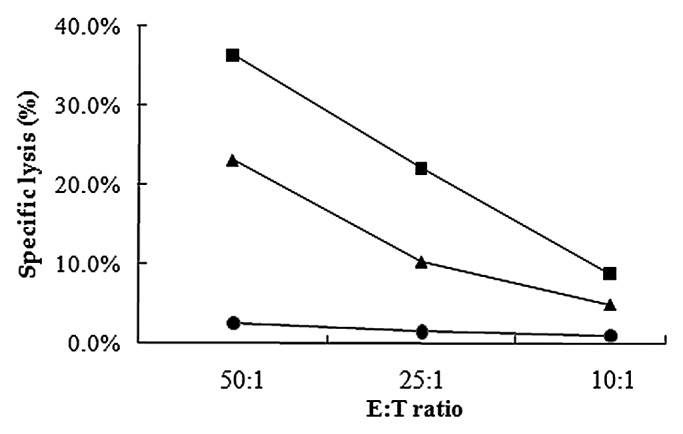
Figure 3.HBsAg specific cytolytic activities of splenocyts from mice immunized with one dose of PLA microsphere vaccine. Splenocytes isolated from mice immunized with different formulations were co-cultured with target cells P815 which were labeled with 51Cr for 6 h. Specific lysis was measured with different E:T ratio. Line with squares: PLA microsphere vaccine; triangles: aqueous HBsAg; dots: PLA microsphere.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the National Science and Technology Key Projects on ‘Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment’ (2012ZX10004701).
References
- 1.Hilleman MR. . Overview of the pathogenesis, prophylaxis and therapeusis of viral hepatitis B, with focus on reduction to practical applications. Vaccine 2001; 19:1837 - 48; http://dx.doi.org/ 10.1016/S0264-410X(00)00364-9; PMID: 11228353 [DOI] [PubMed] [Google Scholar]
- 2.Chisari FV, Ferrari C. . Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995; 13:29 - 60; http://dx.doi.org/ 10.1146/annurev.iy.13.040195.000333; PMID: 7612225 [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa T, Kono D, Chung J, Fowler P, Theofilopoulos A, Kakumu S, Chisari FV. . Polyclonality and multispecificity of the CTL response to a single viral epitope. J Immunol 1998; 161:5842 - 50; PMID: 9834062 [PubMed] [Google Scholar]
- 4.O’Hagan DT, Singh M. . Microparticles as vaccine adjuvants and delivery systems. Expert Rev Vaccines 2003; 2:269 - 83; http://dx.doi.org/ 10.1586/14760584.2.2.269; PMID: 12899577 [DOI] [PubMed] [Google Scholar]
- 5.Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. . Biodegradable and biocompatible poly(DL-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect Immun 1991; 59:2978 - 86; PMID: 1879922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. . Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 2006; 117:78 - 88; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02268.x; PMID: 16423043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Men Y, Tamber H, Audran R, Gander B, Corradin G. . Induction of a cytotoxic T lymphocyte response by immunization with a malaria specific CTL peptide entrapped in biodegradable polymer microspheres. Vaccine 1997; 15:1405 - 12; http://dx.doi.org/ 10.1016/S0264-410X(97)00047-9; PMID: 9302752 [DOI] [PubMed] [Google Scholar]
- 8.Otten G, Schaefer M, Greer C, Calderon-Cacia M, Coit D, Kazzaz J, Medina-Selby A, Selby M, Singh M, Ugozzoli M, et al. . Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J Virol 2003; 77:6087 - 92; http://dx.doi.org/ 10.1128/JVI.77.10.6087-6092.2003; PMID: 12719603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuñiga J, Torres-García D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. . Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol 2012; 2012:193923; http://dx.doi.org/ 10.1155/2012/193923; PMID: 22666281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee WM. . Hepatitis B virus infection. N Engl J Med 1997; 337:1733 - 45; http://dx.doi.org/ 10.1056/NEJM199712113372406; PMID: 9392700 [DOI] [PubMed] [Google Scholar]
- 11.Preis I, Langer RS. . A single-step immunization by sustained antigen release. J Immunol Methods 1979; 28:193 - 7; http://dx.doi.org/ 10.1016/0022-1759(79)90341-7; PMID: 469267 [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Li XM, McGee JP, Zamb T, Koff W, Wang CY, O’Hagan DT. . Controlled release microparticles as a single dose hepatitis B vaccine: evaluation of immunogenicity in mice. Vaccine 1997; 15:475 - 81; http://dx.doi.org/ 10.1016/S0264-410X(97)00225-9; PMID: 9160514 [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Li XM, Wang H, McGee JP, Zamb T, Koff W, Wang CY, O’Hagan DT. . Immunogenicity and protection in small-animal models with controlled-release tetanus toxoid microparticles as a single-dose vaccine. Infect Immun 1997; 65:1716 - 21; PMID: 9125552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta RK, Singh M, O’Hagan DT. . Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Deliv Rev 1998; 32:225 - 46; http://dx.doi.org/ 10.1016/S0169-409X(98)00012-X; PMID: 10837646 [DOI] [PubMed] [Google Scholar]
- 15.Otten G, Schaefer M, Greer C, Calderon-Cacia M, Coit D, Kazzaz J, Medina-Selby A, Selby M, Singh M, Ugozzoli M, et al. . Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J Virol 2003; 77:6087 - 92; http://dx.doi.org/ 10.1128/JVI.77.10.6087-6092.2003; PMID: 12719603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaganathan KS, Vyas SP. . Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine 2006; 24:4201 - 11; http://dx.doi.org/ 10.1016/j.vaccine.2006.01.011; PMID: 16446012 [DOI] [PubMed] [Google Scholar]
- 17.Thomas C, Rawat A, Hope-Weeks L, Ahsan F. . Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm 2011; 8:405 - 15; http://dx.doi.org/ 10.1021/mp100255c; PMID: 21189035 [DOI] [PubMed] [Google Scholar]
- 18.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. . Viral clearance without destruction of infected cells during acute HBV infection. Science 1999; 284:825 - 9; http://dx.doi.org/ 10.1126/science.284.5415.825; PMID: 10221919 [DOI] [PubMed] [Google Scholar]
- 19.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. . Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 1996; 4:25 - 36; http://dx.doi.org/ 10.1016/S1074-7613(00)80295-2; PMID: 8574849 [DOI] [PubMed] [Google Scholar]
- 20.Komatsu H, Inui A, Sogo T, Hiejima E, Tateno A, Klenerman P, Fujisawa T. . Cellular immunity in children with successful immunoprophylactic treatment for mother-to-child transmission of hepatitis B virus. BMC Infect Dis 2010; 10:103; http://dx.doi.org/ 10.1186/1471-2334-10-103; PMID: 20423521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomazic-Jezic VJ, Merritt K, Umbreit TH. . Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res 2001; 55:523 - 9; http://dx.doi.org/; PMID: 11288080 [DOI] [PubMed] [Google Scholar]
- 22.Singh M, Briones M, Ott G, O’Hagan D. . Cationic microparticles: A potent delivery system for DNA vaccines. Proc Natl Acad Sci U S A 2000; 97:811 - 6; http://dx.doi.org/ 10.1073/pnas.97.2.811; PMID: 10639162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foged C, Brodin B, Frokjaer S, Sundblad A. . Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm 2005; 298:315 - 22; http://dx.doi.org/ 10.1016/j.ijpharm.2005.03.035; PMID: 15961266 [DOI] [PubMed] [Google Scholar]
- 24.Wei Q, Wei W, Lai B, Wang LY, Wang YX, Su ZG, Ma GH. . Uniform-sized PLA nanoparticles: preparation by premix membrane emulsification. Int J Pharm 2008; 359:294 - 7; http://dx.doi.org/ 10.1016/j.ijpharm.2008.03.027; PMID: 18457929 [DOI] [PubMed] [Google Scholar]
- 25.Schirmbeck R, Melber K, Mertens T, Reimann J. . Antibody and cytotoxic T-cell responses to soluble hepatitis B virus (HBV) S antigen in mice: implication for the pathogenesis of HBV-induced hepatitis. J Virol 1994; 68:1418 - 25; PMID: 8107205 [DOI] [PMC free article] [PubMed] [Google Scholar]