Abstract
It is well established that mechanical ventilation can injure the lung, producing an entity known as ventilator-induced lung injury (VILI). There are various forms of VILI, including volutrauma (i.e., injury caused by overdistending the lung), atelectrauma (injury due to repeated opening/closing of lung units), and biotrauma (release of mediators that can induce lung injury or aggravate pre-existing injury, potentially leading to multiple organ failure). Experimental data in the pediatric context are in accord with the importance of VILI, and appear to show age-related susceptibility to VILI, although a conclusive link between use of large Vts and mortality has not been demonstrated in this population. The relevance of VILI in the pediatric intensive care unit population is thus unclear. Given the physiological and biological differences in the respiratory systems of infants, children, and adults, it is difficult to directly extrapolate clinical practice from adults to children. This Critical Care Perspective analyzes the relevance of VILI to the pediatric population, and addresses why pediatric patients might be less susceptible than adults to VILI.
Keywords: mechanical ventilation, ventilator-induced lung injury, pediatrics, animal studies, human studies
Mechanical ventilation is one of the most common indications for admission to a pediatric intensive care unit (PICU), with up to 64% of admitted children requiring ventilation for at least 24 hours (1, 2). Mechanical ventilation is life saving, but numerous experimental and clinical studies have shown that it can induce lung injury, leading to potentially irreversible structural and functional damage (3–8), a concept known as ventilator-induced lung injury (VILI) (6, 9). Major mechanisms underlying VILI include: (1) barotrauma and volutrauma due to alveolar overdistension; (2) atelectrauma due to ventilation at low lung volumes; and (3) biotrauma with release of mediators in the lung (7) that can have local and systemic consequences. The importance of VILI in adults was underscored by a landmark clinical trial demonstrating that a lung-protective strategy using low Vt (i.e., 6 ml/kg predicted body weight [PBW]) was associated with improved survival compared with a high Vt (i.e., 12 ml/kg PBW) in patients with acute respiratory distress syndrome (ARDS) (10, 11).
The practice of using low-Vt mechanical ventilation in children has been readily adopted since the publication of these trials (12), despite the many differences in physiological and immune systems between children and adults (13). However, the clinical impact of using such a strategy in the pediatric population has not been well investigated. This Critical Care Perspective addresses the current practice of bedside management in ventilated pediatric patients, and analyzes the differences between pediatric and adult patients with respect to the susceptibility to VILI.
Animal Studies Relevant to the Pediatric Context
Mechanical ventilation has been shown experimentally to induce inflammatory responses in preterm fetal sheep and baboons (14–16). Similarly, in pediatric rodent models, mechanical ventilation can induce VILI in healthy lungs and exacerbate lung injury when Vts greater than or equal to 20 ml/kg are used (17–20). Cannizzaro and coworkers (17) found that ventilation with Vt of 20 ml/kg with or without positive end-expiratory pressure (PEEP) was deleterious by two separate mechanisms (i.e., loss of lung volume without PEEP and overstretching of lung units when inadequate PEEP was applied). Ventilation with Vt of 25 ml/kg of LPS-primed lungs from newborn rats resulted in a synergistic effect on cytokine responses and led to more severe histological lung injury (20).
Other than the inflammatory responses, ventilation with a Vt greater than or equal to 20 ml/kg also reduced pulmonary expression of genes and proteins, such as vascular endothelial growth factor -A or platelet-derived growth factor–A, that are critical for lung growth and development, resulted in increased elastin synthesis coupled with increased elastase activity, epithelial apoptosis, and reduced lung abundance of proteins, such as lysyl oxidases and fibrillins regulating elastic fiber elasticity (18, 21). Of note, a recent study employing 4- to 8-day-old healthy rats reported a transient improvement in compliance after 3 hours of mechanical ventilation with Vt 40 ml/kg without PEEP; however, compliance alone is not an adequate measure of lung injury (19).
Young Animals Are Less Susceptible Than Older Animals to VILI
Given that the weight of experimental evidence suggests that mechanical ventilation with supraphysiologic Vts is also injurious in pediatric animal models, it is interesting to compare the occurrence of VILI in pediatric and adult animal models (Table 1). Copland and coworkers (22) were the first to compare the effects of supraphysiologic Vt ventilation on pulmonary injury and cytokine messenger RNA expression in an in vivo model of healthy newborn (i.e., 5–8 d old) and adult (i.e., 3–4 mo old) rats. The rats were randomized to receive no mechanical ventilation or ventilation using a Vt of 25 or 40 ml/kg without PEEP. A low-Vt group (i.e., Vt < 10 ml/kg) was not included. After 3 hours of mechanical ventilation (MV), adult rats displayed a more profound decrease in respiratory system compliance, greater numbers of inflammatory cells in alveoli or interalveolar septa, and more cytokine messenger RNA responses including IL-1β, macrophage inflammatory protein (MIP)-2, IL-6, IL-10, and TNF-α, as compared with newborns.
Table 1.
Summary of Key Findings of Experimental Studies Investigating Differences between Younger (Pediatric) and Older (Adult) Animals
| Reference | Animal Model | Main Findings |
|---|---|---|
| 22 | Newborn (5–8 d) vs. adult (3–4 mo) | High Vt (25 and 40 ml/kg) MV for 3 h resulted in less inflammation and changes of compliance in newborns than in adult rats. |
| 24 | Infant (26 d), juvenile (5 wk), and adult (12 wk) rats | MV with Vt 30 ml/kg for 1 h or Vt dictated by baseline TLC in an ex vivo lung model resulted in less injury in infant than in adult rats. |
| 26 | Newborn (5–8 d) vs. adult rats | MV at Vt 30 ml/kg for 1 h resulted in increased coagulopathy in newborns than in adult rats. |
| 25 | Juvenile (21 d) vs. adult (16 wk) BALB/c mice | MV with Vt 15 ml/kg for 3 h after nebulization of LPS induced less lung injury in juvenile than in adult mice. |
Definition of abbreviations: MV = mechanical ventilation; TLC = total lung capacity.
This study would appear to show that adult lungs are more susceptible to VILI than newborns, but it is important to understand that the injurious stimulus was likely different in both groups. As in many studies, the authors used Vts that were based on body weight. This approach makes sense as a means of normalizing Vt as long as lung size is proportional to body weight. However, the relationship between lung volume and body weight varies greatly between the immature lung and the mature lung. For example, the ratio of FRC to body weight averages about 47 ml/kg in the newborn rat compared with about 13 ml/kg in the adult rat (23). In addition, the ratio of total lung capacity (TLC) to body weight is significantly larger in infant than in adult rats (24). As a consequence, lung stresses would be different between infant and adult rats for a given Vt normalized to body weight. Hence, the data of Copland and coworkers cannot be taken to indicate greater susceptibility to lung injury in the adult compared with the infant, as the injurious stimulus, and hence lung stress, was, in fact, greater in the adult rats.
Kornecki and coworkers (24) used an ex vivo nonperfused model of healthy lungs obtained from rats of three different age groups (infant [26 d]; juvenile [5 wk]; adults [12 wk]). After 1 hour of MV with a Vt of 30 ml/kg, there was a more profound reduction in TLC and histological evidence of lung injury in the adult compared with infant rat lungs. These data are subject to the same criticisms as for the Copland study, as Vt was normalized to body weight (22). However, Kornecki and colleagues also used Vts that were normalized to baseline TLC (i.e., Vt set at 50% of TLC), which would address this criticism, and found greater susceptibility to injury in the adult rats. Based upon their findings, the authors concluded that the age-related susceptibility to VILI could be explained by intrinsic properties of the developing lung. Smith and coworkers (25) examined the impact of 3 hours of MV (Vt of 15 ml/kg) in juvenile (21 d) and adult (16 wk) C57BL/6 mice treated with aerosolized LPS. Juvenile mice showed a significantly lower number of polymorphonuclear cells and lower total protein content in bronchoalveolar lavage. There was also a lower concentration of IL-1β and IL-6 in whole infant lung homogenates.
However, the age-related susceptibility to VILI has been challenged by one group of investigators. Chan and colleagues (26) observed increased coagulopathy—a finding commonly seen in pulmonary inflammatory diseases—in newborn (i.e., 5–8 d) compared with adult (age unspecified; weight, 300 g) rats after 1 hour of MV with Vt 30 ml/kg. Furthermore, newborn rats demonstrated higher concentrations of Factor Xa in lung lavage material than adult rats. This suggests that injurious MV may also be associated with deleterious effects in the newborn rat, although their findings have not as yet been confirmed by others.
Is VILI Relevant to the Pediatric ICU?
It thus appears that the development of VILI is not limited only to adults, and there appears to be an age-dependent relationship. The obvious question is whether VILI is clinically relevant in the pediatric ICU using the range of Vt and PEEP levels commonly used. Plötz and colleagues (27) observed profound pulmonary inflammatory responses in 12 infants without pre-existing lung injury who received 2 hours of elective ventilation using a Vt of 10 ml/kg. However, no control group was included in this study, so it is possible that their observations may be explained by other factors related to the procedure for which they required ventilation.
To date, there is a lack of randomized, controlled clinical trials assessing low versus high Vts in children with ARDS. However, two groups of investigators compared the impact of changes in ventilation practice on outcomes in pediatric patients (Table 2) (28, 29). Albuali and coworkers (28) compared the bedside management of patients with ARDS in two periods before (n = 79 patients) and after (n = 85 patients) the ARMA trial. The mean Vt significantly decreased between the two periods, from 10.2 (±1.7) to 8.1 (±1.4) ml/kg measured body weight. Multivariate analysis showed an independent association between Vt and mortality (e.g., lower Vt led to lower mortality: odds ratio [OR] = 1.59; 95% confidence interval [CI] = 1.20–2.10) after adjusting for disease severity, ventilator settings, and the use of high-frequency oscillatory ventilation. Briassoulis and coworkers (29) performed a similar study, including patients with ARDS, status asthmaticus, or bronchiolitis. After the introduction of a low–volume–pressure ventilation strategy, the mean (±SE) Vt decreased significantly, from 12.1 9 (±0.7) to 9.0 (±0.5) ml/kg. The mean peak inspiratory pressure (PIP) also decreased from 34 (±1.5) to 29.6 (±1.2) mm Hg. These changes were associated with a significant decrease in mortality, likely linked to decreased lung stretch. It may very well be that limiting inspiratory pressures is more beneficial. The findings by Briassoulis and colleagues are in line with two other pediatric observational studies showing a relationship between high PIP and mortality (30, 31).
Table 2.
Summary of Key Findings of Human Studies Exploring the Association between Vt and Mortality
| Reference | Study Design | Age (yr) | Disease (N) | P:F Ratio | Mortality* (%) | Vt (ml/kg) | Main Findings |
|---|---|---|---|---|---|---|---|
| 33 | Prospective, two center—4 yr | <18 | ALI (320; 67% ARDS) | 161 ± 74† | 22 | 10 ± 4.9‡ | No association between Vt and mortality |
| 30 | Prospective, multicenter—1 yr | <16 | ALI (117) | Not reported | 35 | 8.0‡ | A negative relationship between Vt and mortality was observed, indicating lower mortality with higher Vt Higher peak inspiratory pressures were significantly associated with mortality |
| 31 | Retrospective, single center—7 yr | <18 | AHRF (389; 48% ALI/ARDS) | 138 (83–192)§ | 20 | 6–10 ± 7‡ | No association between Vt and mortality in patients managed with a lung-protective ventilation strategy, irrespective of lung injury score Higher peak inspiratory pressures were significantly associated with mortality |
| 34 | Retrospective, multicenter—1 yr | <16 | AHRF (461; 11.2% ALI, 66.3% ARDS) | 115 (76–168)§ | 41.6 (AHRF) 43.5 (ARDS) |
8.8‡ | No difference in mortality between low and high Vt at a cutoff of either 7 or 8 ml/kg |
| 28 | Retrospective, single center—2 periods (P1 and P2) of 4 yr | <17 | ALI (164; 79.2% ARDS) | 153 ± 59.9 (P1), 139.2 ± 53.1 (P2)† | 28 | 10.2 ± 1.7 (period 1) vs. 8.1 ± 1.4 (period 2) | High Vt was independently associated with mortality (OR = 1.59; 95% CI = 1.20–2.10) after adjusting for disease severity, ventilator settings, and use of HFOV |
Definition of abbreviations: ALI = acute lung injury; AHRF = acute hypoxemic respiratory failure; ARDS = acute respiratory distress syndrome; CI = confidence interval; HFOV = high-frequency oscillatory ventilation; OR = odds ratio; P1 = period 1; P2 = period 2; P:F = PaO2:FiO2.
Overall mortality rate.
Mean ± SD.
Median.
Median (interquartile range).
These studies would suggest that lower Vts are beneficial; however, there are a number of caveats. First, given the before–after design of these studies, other factors related to medical care may have improved over time, and may have contributed to the improved mortality (32). Second, observational clinical studies examining the relationship between Vt and mortality have reported opposing results (Table 1) (30, 31, 33–35). Two of these studies (enrolling 320 and 117 patients) were designed as prospective studies in patients with acute lung injury according to the North American–European Consensus Conference criteria (30, 33). Mortality rates ranged between 22 and 35%. Flori and colleagues (33) did not identify an independent association between Vt and mortality. Erickson and colleagues (30) reported a negative relationship between either the highest Vt (OR = 0.79; 95% CI = 0.77–0.94) or median Vt (OR = 0.82; 95% CI = 0.67–0.99) and mortality, irrespective of age or after controlling for disease severity (i.e., lowest PaO2:FiO2 ratio). This suggests lower mortality with higher Vt. Similar findings were made in a number of retrospective studies (31, 34, 35). Particularly noteworthy is the study from the Chinese Pediatric Intensive Care Network (34). They reported data from 461 patients diagnosed with acute hypoxemic respiratory failure; 306 of these patients developed ARDS. The median PaO2/FiO2 was 115 (25–785; interquartile range = 76–168) indicating a severely ill population. Mortality rates were very high (46.1%). The median Vt was 8.8 ml/kg (25–75; interquartile range = 6.7–10.4). Survival rates were comparable between patients managed with a Vt of 8 ml/kg or less and greater than 8 ml/kg. These observations mirror the three negative adult randomized trials, where, in the protective, arm patients received Vts of roughly 7 ml/kg compared with roughly 10 ml/kg in the traditional arm (36). Third, many investigators have studied heterogeneous patient populations with respect to age and disease severity, ranging from severe ARDS to its milder variant, acute hypoxemic respiratory failure. Many studies included children as old as 17 years, who likely can be viewed as adults in terms of susceptibility to VILI, making it difficult to sort out the possible age-related susceptibility to VILI. Fourth, the gap between low and high Vt used may have been too small to pick up any difference. Finally, the actual Vt delivered may be lower than what is reported in some patients due to the use of uncuffed endotracheal tubes or inaccurate measurements from the endotracheal tube. Measurement of Vt at the ventilator may not take the compliance of the ventilator circuit into account, especially in young children (37).
Possible Explanations for the Age-related Susceptibility to VILI
Mismatch between Lung Volumes and Bodyweight
As discussed previously here, part of the explanation for the apparent differences in VILI in relation to age may relate to the systematic bias when normalizing Vt to body weight. This approach is problematic in the context of adults with widely different body habitus (e.g., lean versus obese) and in infants/children as they develop, because the lung increases at a different rate than does body weight, especially in infants. To address this issue, in adult studies, investigators have used Vts normalized to PBW (e.g., ARDS Network studies), because lung size varies with PBW (38). Similarly, in children, increases in TLC relate to height, and, as such, it has been recommended that lung volumes should be normalized to height in children over 1 month of age (39, 40). This suggests that, for pediatric animal studies, the Vt should be calculated based on some measure related to lung size. The study by Kornecki and colleagues (24) is the only one that has partially addressed this issue. However, in their study, larger Vts were still more injurious in the adult than in the infant lung, despite setting Vt based on baseline TLC (24). This warrants further study.
Structural/Biological Differences
There may be structural/biological factors that could explain the differences in VILI as a function of age (Figure 1). The elastin concentration of the infant lung increases 10-fold over the first 20 days of life and less rapidly thereafter, whereas the collagen concentration increases linearly from infancy to childhood (41, 42). Thus, differences in elastic properties of the lungs may account for the differences in lung strain. Another factor may be related to how mechanical forces are sensed and the subsequent host response. Mechanotransduction is the conversion of mechanical stimuli into biochemical and molecular cellular responses (43). VILI is the result of mechanical forces acting on lung structures during MV (3). Possible targets of these physical forces include, among others, the epithelial and endothelial cells, and the extracellular matrix (44). The exact mechanisms leading to mechanotransduction during ventilation are yet to be fully elucidated, but include mechanosensors, such as extracellular matrix signaling pathways, stretch-activated ion channels, the activation of mitogen-activated protein kinase cascades, and other transcription factors (45, 46). Nuclear factor (NF) -κB, an important transcription factor of inflammation, is activated in alveolar macrophages and alveolar type II cells in response to ventilation (47). Fetal lung cells most likely share at least some of these pathways, although no studies have addressed possible differences between the pediatric and adult lung (48, 49). Interestingly, age-dependent differences in NF-κB have been described in animal models, showing less inflammation in neonatal mice after exposure to hyperoxia or LPS (50, 51).
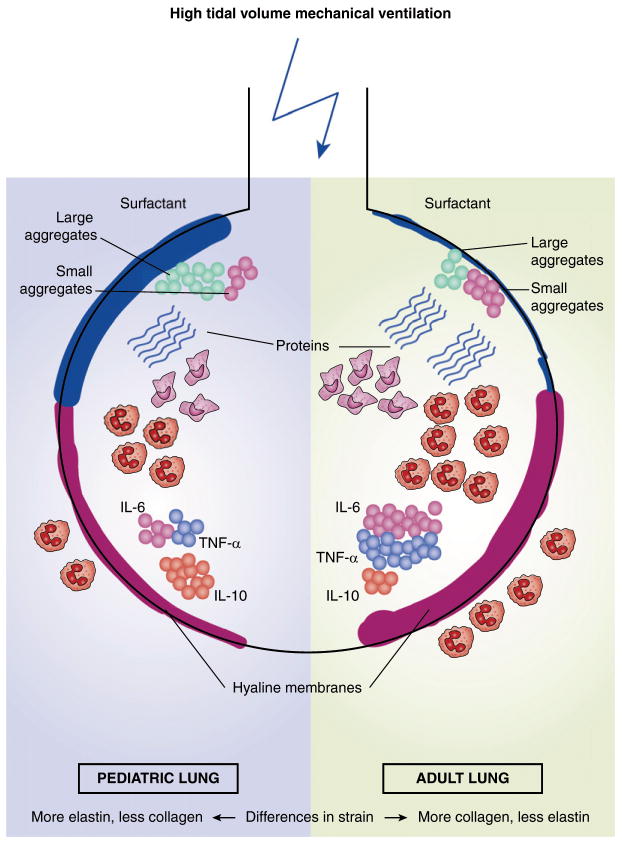
Figure 1.
This simplified illustration of an alveolus summarizes the differences in ventilator-induced lung injury (VILI) between the pediatric (left) and the adult lung (right). Injurious, high Vt ventilation may lead to an increased surfactant production in the pediatric compared with the adult lung, with higher levels of large aggregates. Next, there may be a greater degree of alveolar and interstitial infiltration by polymorphonuclear leukocytes, production of proinflammatory mediators, such as IL-6 and TNF-α, a decreased production of anti-inflammatory mediators, including IL-10, protein leakage, and more hyaline membrane formation in the adult lung. It may also be surmised that a fixed Vt causes different lung strain between the pediatric and the adult lung. Elastin and collagen levels differ significantly between the pediatric and the adult lung.
Endogenous surfactant is composed of two forms: superior large aggregates (LAs) and nonfunctional, inferior small aggregates (52). Ventilation with Vt up to 15 ml/kg resulted in increased conversion from LAs to small aggregates in adult rabbits, thereby partially contributing to VILI (53). On the other hand, short-term effects of mechanical ventilation included increased stimulation of surfactant secretion in various adult models, suggesting a transient beneficial effect (54). A time-dependent increase in surfactant LAs has also been observed in newborn rats after ventilation with Vt of 40 ml/kg for 180 minutes, although the total surfactant pool initially increased, but decreased after 60 minutes (19). These findings suggest that the effects of stress on surfactant production may not just be species dependent, but also age and time dependent. Thus, it is possible that differences in surfactant kinetics may explain the difference between pediatric and animal models, although this warrants further study.
Differences in Immune Response
There are important differences in the immune response between infants and adults that are likely relevant (55). Mechanical ventilation can trigger a complex array of pro- and anti-inflammatory mediators involving innate immunity, and enhance the production of specific cytokines, such as TNF-α (56). The presence of TNF-α may be necessary to augment expression of proinflammatory genes (57). The Toll-like receptor (TLR) 4 signaling pathway may be involved in this ventilator-induced inflammation, even if there are no infectious agents present (58). TLRs are essential components of the innate immune system, which not only recognize microbial products, such as LPS, but also degradation products released from damaged tissue (59). Although the TLR pattern-recognition receptor system is well developed in newborns, the TLR-mediated innate immune responses are remarkably low during early life (60). Compared with adults, diminished TNF-α responses in neonates with various stimuli, including TLR ligands, has been reported (61, 62). In addition, less proinflammatory cytokine is produced by neonatal immune cells compared with adults after stimulation with TLR ligands (63). This is in line with the observation that the transcriptional response of mainly the TLR-4 pathway was much higher in adult mice than in juvenile mice after 3 hours of mechanical ventilation with Vt of 15 ml/kg (25). In general, the immune system of a child under 1 year of age is relatively immature, including broad deficits in both innate and adaptive immunity (64). For instance, the response to LPS in infants is age dependent, reaching the adult response at about 6–9 months (65). Neonatal polymorphonuclear cells and monocytes function to a lesser degree compared with adults, with decreased chemotactic responses for as long as 1–2 years (66–69). The levels of soluble plasma proteins that have a role in innate immunity are lower in newborns than in adults, including complement components and acute-phase products (70). Thus, innate immunity is less developed compared with adults, and the full capacity of the innate immune system is not reached until the teenage years (55).
Adaptive immunity is also different between young children and adults. At birth, the immune system has a strong T helper 2, anti-inflammatory predisposition, with peripheral mononuclear cells having little ability to produce TNF-α or other proinflammatory mediators compared with adults (71, 72). In addition, at birth, T cells are biased toward regulatory T cells, thereby potentially suppressing the innate immune response (73, 74). This anti-inflammatory predisposition may persist during early childhood (75). Barsness and coworkers (76, 77) demonstrated, in six children aged 2 months to 8 years, that peritoneal macrophages had both a pro-and anti-inflammatory response after stimulation with LPS or IL-1B, in contrast with adults, where only a proinflammatory response was seen. Furthermore, there was skewing toward a more anti-inflammatory response in the children.
In aggregate, all of this suggests that (injurious) mechanical ventilation does not activate the innate immunity in infants or young children to the extent that it does in adults.
Clinical Implications and Directions for Future Research
The development of VILI in mechanically ventilated children, as well as the differential response to injurious mechanical ventilation between children and adults, has only begun to be explored. At present, there are no recommendations related to an optimal Vt that can be supported by rigorous evidence, and our review does not provide any definitive answers. The physiologic Vt in humans, rats, and mice ranges between 5 and 7 ml/kg ideal bodyweight (13, 78, 79). However, many of the experimental studies have used supraphysiologic Vt to study the effects on lung injury, making it difficult to extrapolate the observed differences between pediatric subjects and adults into clinical practice. However, one group of investigators has shown that a Vt in the physiological range exacerbated pre-existing lung injury (80). We therefore propose that VILI is a definite entity in the pediatric population, but that it’s propensity increases with increasing age following a yet-unknown function, and reaches susceptibility similar to adults at a yet-unknown age. Based on this proposition, we would provide broad-brush recommendations for clinical practice. Given this uncertainty, it seems reasonable to use data obtained in adults (i.e., aiming for a Vt within the physiological range, while limiting plateau pressure to 30 cm H2O in patients who do not have a stiff chest wall) (81). Vt greater than 10 ml/kg, a value similar to what has previously been proposed for pediatric patients with ARDS, should be avoided (82). Measurement of the Vt should be near the endotracheal tube, especially in small children.
We propose that future studies should focus on two objectives. The first is to improve our understanding of the various pathophysiological mechanisms of VILI in the pediatric lung, as well as why there appear to be differences between pediatric and adult animal models. This suggests that specific age-appropriate models need to be established. Second, randomized controlled trials should be designed to test the best approach for providing lung-protective ventilation in a well defined group of children with moderate-to-severe ARDS according to the Berlin definition (83–85). Producing the pediatric counterpart of the ARMA trial would be difficult, due to the required sample size in a disease that is not very common (85), but would provide much needed clarity.
At a Glance Commentary.
Scientific Knowledge on the Subject
Supraphysiologic Vt ventilation induces ventilator-induced lung injury (VILI) in adults.
What This Study Adds to the Field
There is a lack of data from randomized, controlled clinical trials addressing the relevance of VILI in pediatric patients with acute respiratory distress syndrome. Observational data from the pediatric population are insufficient to answer the question about the relevance of VILI to children. Experimental data demonstrate an age-related susceptibility to VILI, although the pathophysiological mechanisms are not completely understood. One factor may be the difference in the immune response after mechanical ventilation–induced stretch.
Acknowledgments
Supported by a grant from the Ter Meulen Fund, Dutch Royal Academy of Sciences (M.C.J.K.), and by grants from the Canadian Institute for Health Research (H.Z. and A.S.S.).
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Farias JA, Frutos F, Esteban A, Flores JC, Retta A, Baltodano A, Alía I, Hatzis T, Olazarri F, Petros A, et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med. 2004;30:918–925. doi: 10.1007/s00134-004-2225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randolph AG, Meert KL, O’Neil ME, Hanson JH, Luckett PM, Arnold JH, Gedeit RG, Cox PN, Roberts JS, Venkataraman ST, et al. Pediatric Acute Lung Injury and Sepsis Investigators Network. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–1340. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 3.dos Santos CC, Slutsky AS. The contribution of biophysical lung injury to the development of biotrauma. Annu Rev Physiol. 2006;68:585–618. doi: 10.1146/annurev.physiol.68.072304.113443. [DOI] [PubMed] [Google Scholar]
- 4.Plötz FB, Slutsky AS, van Vught AJ, Heijnen CJ. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med. 2004;30:1865–1872. doi: 10.1007/s00134-004-2363-9. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med. 2006;32:24–33. doi: 10.1007/s00134-005-2817-8. [DOI] [PubMed] [Google Scholar]
- 7.Terragni PP, Filippini C, Slutsky AS, Birocco A, Tenaglia T, Grasso S, Stripoli T, Pasero D, Urbino R, Fanelli V, et al. Accuracy of plateau pressure and stress index to identify injurious ventilation in patients with acute respiratory distress syndrome. Anesthesiology. 2013;119:880–889. doi: 10.1097/ALN.0b013e3182a05bb8. [DOI] [PubMed] [Google Scholar]
- 8.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 9.Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet. 2003;361:332–340. doi: 10.1016/S0140-6736(03)12329-X. [DOI] [PubMed] [Google Scholar]
- 10.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 11.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 12.Turner DA, Arnold JH. Insights in pediatric ventilation: timing of intubation, ventilatory strategies, and weaning. Curr Opin Crit Care. 2007;13:57–63. doi: 10.1097/MCC.0b013e32801297f9. [DOI] [PubMed] [Google Scholar]
- 13.Jeffries HE, Martin LD. Respiratory phsyiology. In: Wheeler DS, Wong HR, Shanley TP, editors. The respiratory tract in paediatric criticall illness and injury. London: Springer-Verlag; 2009. pp. 1–12. [Google Scholar]
- 14.Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;300:L232–L241. doi: 10.1152/ajplung.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGreal EP, Chakraborty M, Winter VT, Jones SA, Coalson JJ, Kotecha S. Dynamic expression of IL-6 trans-signalling molecules in the lungs of preterm baboons undergoing mechanical ventilation. Neonatology. 2011;100:130–138. doi: 10.1159/000322148. [DOI] [PubMed] [Google Scholar]
- 17.Cannizzaro V, Zosky GR, Hantos Z, Turner DJ, Sly PD. High tidal volume ventilation in infant mice. Respir Physiol Neurobiol. 2008;162:93–99. doi: 10.1016/j.resp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice: prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol. 2008;294:L3–L14. doi: 10.1152/ajplung.00362.2007. [DOI] [PubMed] [Google Scholar]
- 19.Martinez F, Lewis J, Copland I, Engelberts D, Kavanagh BP, Post M, Schurch S, Belik J. Mechanical ventilation effect on surfactant content, function, and lung compliance in the newborn rat. Pediatr Res. 2004;56:19–25. doi: 10.1203/01.PDR.0000128980.82797.29. [DOI] [PubMed] [Google Scholar]
- 20.Roth-Kleiner M, Ridsdale R, Cao L, Kuliszewski M, Tseu I, McKerlie C, Post M. Lipopolysaccharide exposure modifies high tidal volume ventilation–induced proinflammatory mediator expression in newborn rat lungs. Pediatr Res. 2007;61:191–196. doi: 10.1203/01.pdr.0000252437.51779.21. [DOI] [PubMed] [Google Scholar]
- 21.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 22.Copland IB, Martinez F, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med. 2004;169:739–748. doi: 10.1164/rccm.200310-1417OC. [DOI] [PubMed] [Google Scholar]
- 23.Fisher JT, Mortola JP. Statics of the respiratory system in newborn mammals. Respir Physiol. 1980;41:155–172. doi: 10.1016/0034-5687(80)90049-3. [DOI] [PubMed] [Google Scholar]
- 24.Kornecki A, Tsuchida S, Ondiveeran HK, Engelberts D, Frndova H, Tanswell AK, Post M, McKerlie C, Belik J, Fox-Robichaud A, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;171:743–752. doi: 10.1164/rccm.200408-1053OC. [DOI] [PubMed] [Google Scholar]
- 25.Smith LS, Gharib SA, Frevert CW, Martin TR. Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol. 2010;43:475–486. doi: 10.1165/rcmb.2009-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan A, Jayasuriya K, Berry L, Roth-Kleiner M, Post M, Belik J. Volutrauma activates the clotting cascade in the newborn but not adult rat. Am J Physiol Lung Cell Mol Physiol. 2006;290:L754–L760. doi: 10.1152/ajplung.00339.2005. [DOI] [PubMed] [Google Scholar]
- 27.Plötz FB, Vreugdenhil HA, Slutsky AS, Zijlstra J, Heijnen CJ, van Vught H. Mechanical ventilation alters the immune response in children without lung pathology. Intensive Care Med. 2002;28:486–492. doi: 10.1007/s00134-002-1216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albuali WH, Singh RN, Fraser DD, Seabrook JA, Kavanagh BP, Parshuram CS, Kornecki A. Have changes in ventilation practice improved outcome in children with acute lung injury? Pediatr Crit Care Med. 2007;8:324–330. doi: 10.1097/01.PCC.0000269390.48450.AF. [DOI] [PubMed] [Google Scholar]
- 29.Briassoulis G, Venkatamaran S, Vasilopoulos A, Sianidou L, Papadatos J. Influence of low volume–pressure limited ventilation on outcome of severe paediatric pulmonary diseases. Minim Invasive Ther Allied Technol. 1999;8:377–384. [Google Scholar]
- 30.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B Paediatric Study Group; Australian and New Zealand Intensive Care Society. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 31.Khemani RG, Conti D, Alonzo TA, Bart RD, III, Newth CJ. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med. 2009;35:1428–1437. doi: 10.1007/s00134-009-1527-z. [DOI] [PubMed] [Google Scholar]
- 32.Weisberg HI. Bias and causation. Hoboken: John Wiley & Sons, Inc; 2010. Sources of bias. [Google Scholar]
- 33.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 34.Hu X, Qian S, Xu F, Huang B, Zhou D, Wang Y, Li C, Fan X, Lu Z, Sun B Chinese Collaborative Study Group for Pediatric Respiratory Failure. Incidence, management and mortality of acute hypoxemic respiratory failure and acute respiratory distress syndrome from a prospective study of Chinese paediatric intensive care network. Acta Paediatr. 2010;99:715–721. doi: 10.1111/j.1651-2227.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 35.Silva DC, Shibata AR, Farias JA, Troster EJ. How is mechanical ventilation employed in a pediatric intensive care unit in Brazil? Clinics (Sao Paulo) 2009;64:1161–1166. doi: 10.1590/S1807-59322009001200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 37.Cannon ML, Cornell J, Tripp-Hamel DS, Gentile MA, Hubble CL, Meliones JN, Cheifetz IM. Tidal volumes for ventilated infants should be determined with a pneumotachometer placed at the endotracheal tube. Am J Respir Crit Care Med. 2000;162:2109–2112. doi: 10.1164/ajrccm.162.6.9906112. [DOI] [PubMed] [Google Scholar]
- 38.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 39.Stocks J, Quanjer PH Official Statement of The European Respiratory Society. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 40.Thorsteinsson A, Larsson A, Jonmarker C, Werner O. Pressure–volume relations of the respiratory system in healthy children. Am J Respir Crit Care Med. 1994;150:421–430. doi: 10.1164/ajrccm.150.2.8049825. [DOI] [PubMed] [Google Scholar]
- 41.Brody JS, Therlbeck WM. Development, growth, and aging of the lung. In: Fishman JP, editor. Handbook of physiology: the respiratory system—mechanics of breathing. Bethesda: American Physiological Society; 1986. pp. 355–386. [Google Scholar]
- 42.Nardell EA, Brody JS. Determinants of mechanical properties of rat lung during postnatal development. J Appl Physiol. 1982;53:140–148. doi: 10.1152/jappl.1982.53.1.140. [DOI] [PubMed] [Google Scholar]
- 43.dos Santos CC, Slutsky AS. Mechanotransduction, ventilator-induced lung injury and multiple organ dysfunction syndrome. Intensive Care Med. 2000;26:638–642. doi: 10.1007/s001340051217. [DOI] [PubMed] [Google Scholar]
- 44.Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 2008;34:631–639. doi: 10.1007/s00134-007-0964-9. [DOI] [PubMed] [Google Scholar]
- 45.Uhlig U, Uhlig S. Ventilation-induced lung injury. Compr Physiol. 2011;1:635–661. doi: 10.1002/cphy.c100004. [DOI] [PubMed] [Google Scholar]
- 46.Rocco PR, Dos Santos C, Pelosi P. Pathophysiology of ventilator-associated lung injury. Curr Opin Anaesthesiol. 2012;25:123–130. doi: 10.1097/ACO.0b013e32834f8c7f. [DOI] [PubMed] [Google Scholar]
- 47.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-κB and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–716. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- 48.Liu M, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J Appl Physiol (1985) 2000;89:2078–2084. doi: 10.1152/jappl.2000.89.5.2078. [DOI] [PubMed] [Google Scholar]
- 49.Liu M, Tanswell AK, Post M. Mechanical force–induced signal transduction in lung cells. Am J Physiol. 1999;277:L667–L683. doi: 10.1152/ajplung.1999.277.4.L667. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest. 2004;114:669–678. doi: 10.1172/JCI19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-κB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med. 2007;175:805–815. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136:426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y, Veldhuizen RA, Yao LJ, McCaig LA, Bartlett AJ, Lewis JF. Ventilation strategies affect surfactant aggregate conversion in acute lung injury. Am J Respir Crit Care Med. 1997;155:493–499. doi: 10.1164/ajrccm.155.2.9032184. [DOI] [PubMed] [Google Scholar]
- 54.Chander A, Fisher AB. Regulation of lung surfactant secretion. Am J Physiol. 1990;258:L241–L253. doi: 10.1152/ajplung.1990.258.6.L241. [DOI] [PubMed] [Google Scholar]
- 55.Ygberg S, Nilsson A. The developing immune system—from foetus to toddler. Acta Paediatr. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 56.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc. 2007;4:77–84. doi: 10.1513/pats.200608-149JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183:471–482. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- 58.Villar J, Cabrera NE, Casula M, Flores C, Valladares F, Díaz-Flores L, Muros M, Slutsky AS, Kacmarek RM. Mechanical ventilation modulates TLR4 and IRAK-3 in a non-infectious, ventilator-induced lung injury model. Respir Res. 2010;11:27. doi: 10.1186/1465-9921-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 60.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 62.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, III, Hajjar AM, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis DB, Wilson CB. Developmental immunology and role of host defenses in fetal and neonatal susceptibility to infection. In: Remington JS, Klein JO, Wilson CB, editors. Infectious diseases of the fetus and newborn infant. Philadelphia: Elsevier Saunders; 2006. pp. 87–210. [Google Scholar]
- 67.Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol. 2011;90:74–81. doi: 10.1016/j.jri.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 68.Fox SE, Lu W, Maheshwari A, Christensen RD, Calhoun DA. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine. 2005;29:135–140. doi: 10.1016/j.cyto.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 69.Maródi L, Csorba S, Nagy B. Chemotactic and random movement of human newborn monocytes. Eur J Pediatr. 1980;135:73–75. doi: 10.1007/BF00445897. [DOI] [PubMed] [Google Scholar]
- 70.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 71.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 72.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of Toll-like receptor 4–mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J, Yang X, Auh SL, Kim KD, Tang H, Fu YX. Do adaptive immune cells suppress or activate innate immunity? Trends Immunol. 2009;30:8–12. doi: 10.1016/j.it.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr Opin Infect Dis. 2013;26:213–218. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- 75.Wood JH, Partrick DA, Johnston RB., Jr The inflammatory response to injury in children. Curr Opin Pediatr. 2010;22:315–320. doi: 10.1097/MOP.0b013e328338da48. [DOI] [PubMed] [Google Scholar]
- 76.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, Banerjee A, McIntyre RC., Jr IL-1beta induces an exaggerated pro- and anti-inflammatory response in peritoneal macrophages of children compared with adults. Pediatr Surg Int. 2004;20:238–242. doi: 10.1007/s00383-003-1118-y. [DOI] [PubMed] [Google Scholar]
- 77.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC., Jr Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg. 2004;39:912–915. doi: 10.1016/j.jpedsurg.2004.02.009. discussion 912–915. [DOI] [PubMed] [Google Scholar]
- 78.Mitzner W, Brown R, Lee W. In vivo measurement of lung volumes in mice. Physiol Genomics. 2001;4:215–221. doi: 10.1152/physiolgenomics.2001.4.3.215. [DOI] [PubMed] [Google Scholar]
- 79.Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- 80.Sly PD, Nicholls PK, Berry LJ, Hantos Z, Cannizzaro V. High tidal volume ventilation does not exacerbate acid-induced lung injury in infant rats. Respir Physiol Neurobiol. 2013;189:129–135. doi: 10.1016/j.resp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 81.van Kaam A. Lung-protective ventilation in neonatology. Neonatology. 2011;99:338–341. doi: 10.1159/000326843. [DOI] [PubMed] [Google Scholar]
- 82.Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med. 2009;37:2448–2454. doi: 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- 83.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 84.De Luca D, Piastra M, Chidini G, Tissieres P, Calderini E, Essouri S, Medina Villanueva A, Vivanco Allende A, Pons-Odena M, Perez-Baena L, et al. Respiratory Section of the European Society for Pediatric Neonatal Intensive Care (ESPNIC). The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med. 2013;39:2083–2091. doi: 10.1007/s00134-013-3110-x. [DOI] [PubMed] [Google Scholar]
- 85.Kneyber MC, Rimensberger PC. The need for and feasibility of a pediatric ventilation trial: reflections on a survey among pediatric intensivists. Pediatr Crit Care Med. 2012;13:632–638. doi: 10.1097/PCC.0b013e31824fbc37. [DOI] [PubMed] [Google Scholar]