Abstract
Social behavior is a basic behavior mediated by multiple brain regions and neural circuits, and is crucial for the survival and development of animals and humans. Two neuropsychiatric disorders that have prominent social behavior abnormalities are autism spectrum disorders (ASD), which is characterized mainly by hyposociability, and Williams syndrome (WS), whose subjects exhibit hypersociability. Here, we review the unique properties of social behavior in ASD and WS, and discuss the major theories in social behavior in the context of these disorders. We conclude with a discussion of the research questions needing further exploration to enhance our understanding of social behavior abnormalities.
Introduction to social behavior
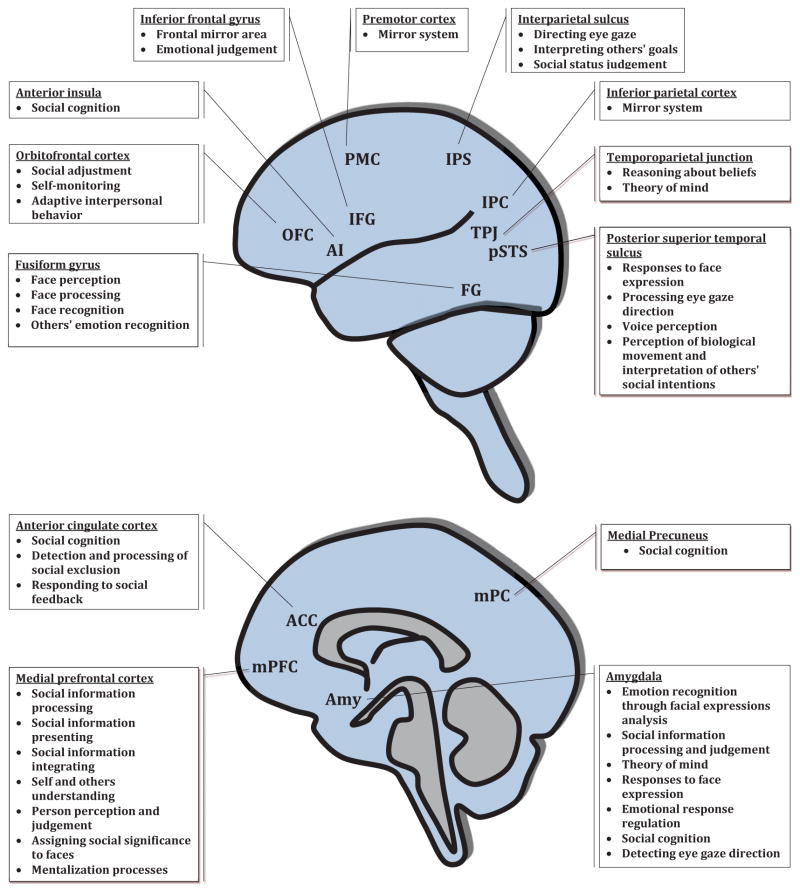
One of the most complicated behaviors humans and animals can perform is social behavior, which takes place between conspecifics and results in social relationships. Social behavior is based on the ability to properly communicate with others; individuals must sense, process and, interpret social cues, as well as respond with appropriate behaviors. These functions are mediated by brain areas comprising the “Social brain” 1, in particular, the medial prefrontal cortex (mPFC), amygdala, anterior insula, anterior cingulate cortex (ACC), inferior frontal gyrus (IFG) and the superior temporal sulcus (STS) (Figure 1).
Figure 1.
Brain regions of high relevancy to social behavior, participating in the different aspects of social behavior and their contribution to social behavior.
Two neuropsychiatric developmental disorders, autism spectrum disorder (ASD) and Williams syndrome (WS), result in contrasting abnormalities in social behavior 2; while ASD is characterized by social avoidance and lack of interest in social interactions, WS is characterized by uninhibited social interactions and overfriendliness. Although the unique opposing social behavior phenotypes of ASD and WS offer an opportunity to study neurobiological mechanisms of social abnormalities, the heterogeneity of ASD symptoms and genetics makes it complicated to directly compare the contrasted social behaviors. On the other hand, the well-characterized genetic information of WS and its unique behavioral phenotype make the study of its neurogenetics more accessible, and could help to understand the relationship among genes, neural circuitry, physiology, and social behavior. In this review, we compare and contrast the symptoms, genetics, and related clinical findings of these two disorders with the hope that further cross-comparative studies will uncover underlying neurobiological mechanisms of social behavior abnormality.
Contrasting social behavior abnormalities in ASD and WS
Autism spectrum disorders
ASDs are a group of heterogeneous neurodevelopmental disorders characterized according to the DSM-5 by: i) deficits in social communication and social interaction; and ii) stereotyped, repetitive behavior 3 with narrow restricted interests 4, often accompanied by sensory abnormalities and language development delay or absence. These symptoms must be present in early childhood and impede the individual’s everyday activity. Autism, from the Greek words autos (“self”) and ismos (“action”), was described initially by Kanner in 1943 5 as a congenital lack of interest in other people. Nowadays, ASD affects 1 in 68 children in the United States 6 (but see 7), with approximately 5 times as many boys affected as girls 8.
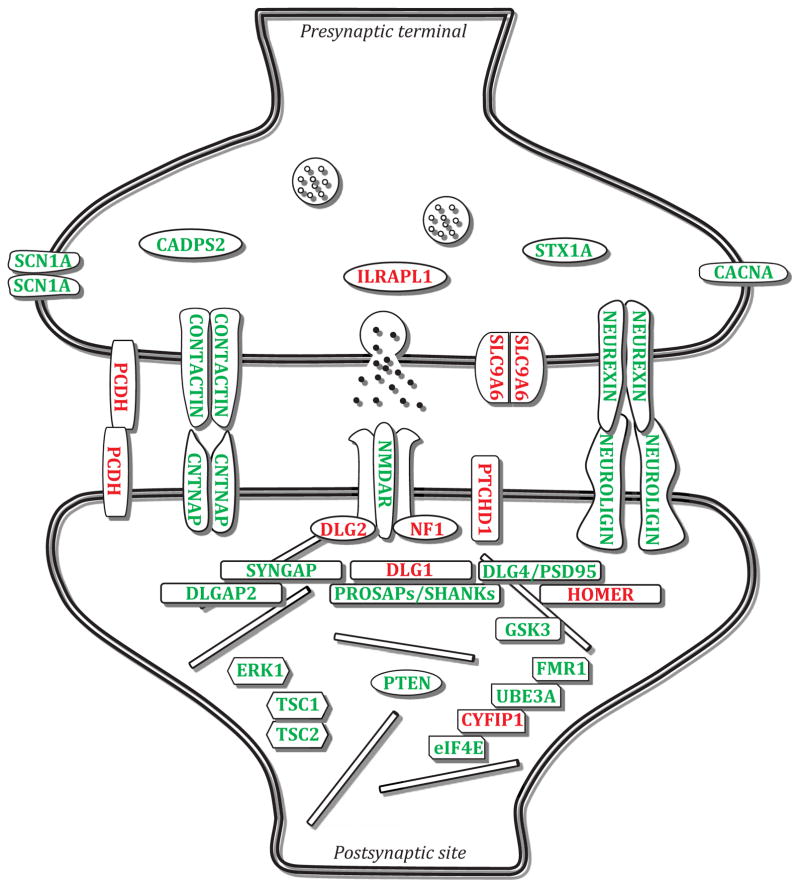
ASD is one of the most heritable common psychiatric disorders, indicating that genetics play a central role in ASD etiology. Nevertheless, the genetic contribution to pathophysiology is challenging to explore because of incomplete penetrance, a large number of susceptibility genes, and complex gene-environment interactions. While genome-wide association studies (GWAS) have yet to yield replicable common variants for autism, possibly due to small sample sizes, studies of copy number variants (CNVs) and single nucleotide polymorphisms (SNPs) have provided gene candidates for further study 9–14. Many of the ASD-linked genes encode synaptic proteins 15 at glutamatergic synapses (Figure 2, Table 1 and Table 2), most of them acting postsynaptically16, indicating that excitatory synaptic dysfunction may be a key pathophysiology of ASD. However, our current understanding of the molecular architecture of inhibitory synapses is very limited, hence further studies on the basic biology of inhibitory synapses may shed new lights on etiology and pathology of ASD.
Figure 2.
Autism-related genes encoding synaptic proteins at the glutamatergic synapse. These synaptic proteins participate in the formation, stabilization and function of the synapse. Proteins in which a mutation in their encoding genes affects social behavior are marked in green, while those with no direct evidence are marked in red.
Table 1.
Autism-related genes and their effects on social behavior of mouse models
| Gene | SFARI Category | Social Interaction | Ultrasonic Vocalization | Other | References |
|---|---|---|---|---|---|
| Neuronal cell adhesion molecules, important for synapse development and function | |||||
| Shank3 | Syndromic | Decreased in Shank3b-KO, decreased in Shank3b het, decreased in Shanke4-9-KO | Decreased in het and female Shanke4-9-KO, increased in male Shanke4-9-KO | Decreased social sniffing in het, impaired social novelty in Shank3b-KO | Peca et al., 2011, Bozdagi et al., 2010, Wang et al., 2011 |
| Shank2 | Strong candidate | Decreased in Shank2-KO, Decreased in Shank2 het | Decreased in Shank2-KO | Schmeisser et al., 2012, Won et al., 2012 | |
| Nrxn1 | Strong Candidate | Normal in KO | ND | Impaired nest building in KO | Etherton et al., 2009 |
| Cntnap2 | Syndromic | Decreased in KO | Decreased in KO | Impaired nest building in KO | Penagarikano et al., 2011 |
| Ptchd1 | Strong Candidate | ND | ND | ND | ND |
| Cntn4 | Strong Candidate | ND | ND | ND | ND |
| Pcdh19 | Syndromic | ND | ND | ND | ND |
| Scaffolding proteins | |||||
| Syngap1 | High Confidence, Syndromic | Decreased in het | ND | Lack of social memory, increased social isolation in het | Guo et al., 2009 |
| Shank3 | High Confidence, Syndromic | Decreased in Shank3b-KO, decreased in Shank3b het, decreased in Shanke4-9-KO | Decreased in het and female Shanke4-9-KO, increased in male Shanke4-9-KO | Decreased social sniffing in het, impaired social novelty in Shank3b-KO | Peca et al., 2011, Bozdagi et al., 2010, Wang et al., 2011 |
| Shank2 | Strong candidate | Decreased in Shank2-KO, Decreased in Shank2 het | Decreased in Shank2-KO | ND | Schmeisser et al., 2012, Won et al., 2012 |
| Neurotransmission | |||||
| Cacana1C | Syndromic | Decreased in het | Decreased in het | ND | Bader et al., 2011 |
| Scn1a | Syndromic | Decreased in het | ND | ND | Han et al., 2012 |
| Grip1 | Strong Candidate | Increased in Grip1/Grip2 double KO | ND | ND | Mejias et al., 2011 |
| Gabrb3 | Strong Candidate | Decreased in KO | ND | Impaired nest building in KO | DeLorey et al., 2008 |
| Slc9a6 | Syndromic | ND | ND | ND | ND |
| Affecting genes expression and protein synthesis | |||||
| Adnp | High Confidence | Decreased in het | ND | Impaired social memory in het | Malishkevich et al., 2015, Vulih-Shultzman et al., 2007 |
| Fmr1 | Syndromic | Decreased in KO toward C3H ovariectomized female, increased in KO toward a wild type or KO mouse | Normal in KO toward a wild type female, decreased in KO toward Fmr1-KO female, increased in KO | Increased social anxiety in KO, decreased social novelty in KO, normal behavior of KO in the partition test | Mines et al., 2010, McNaughton et al., 2008, Mineur et al., 2006, Qin et al., 2011, Spencer et al., 2005, Spencer et al., 2011, Rotschafer et al., 2011 |
| Mecp2 | Syndromic | Intact social recognition but increased social interaction with novel and familiar partners in mutant mice with Mecp2 deficiency in GABAergic neurons. FVB.129F1 Mecp2+/− spend less time investigating a novel mouse compared with wild-type animals. 129.B6F1 Mecp2+/− mice show no social preference | Impaired nest building in mutant mice with Mecp2 deficiency in GABAergic neurons | Chao et al., 2010, Samaco et al., 2012 | |
| Pten | High Confidence, Syndromic | Decreased in mutant mice with PTEN-deletion from the cereberum and the hippocampus, decreased in male and female het, decreased in female Pten and Slc6a4 het, decreased in mutant mice with Pten-deletion from dopaminergic neurons, increased in cytoplasm-predominant Pten germline male KI mice | ND | Impaired nest building in mutant mice with Pten-deletion from the cerebrum and the hippocampus, impaired social novelty in mutant mice with Pten-deletion from the cerebellum and the hippocampus, impaired social novelty in male and female het, impaired social novelty in male mice with Pten-deletion from dopaminergic neurons, impaired social habituation in male het | Clipperton-Allen et al., 2015, Kwon et al., 2006, Clipperton-Allen et al., 2014, Tilot et al., 2014, Page et al., 2009 |
| Tbr1 | High Confidence | Decreased in het | Decreased in het | ND | Huang et al., 2014 |
| Tsc1 | Syndromic | Decreased in het and mutant mice with Tsc1-deletion from cerebellar Purkinje cells, decreased in het mice | Increased in het and mutant mice with Tsc1-deletion from cerebellar Purkinje cells | Impaired social novelty in het and mutant mice with Tsc1-deletion from cerebellar Purkinje cells, impaired nest building in het mice, normal social dominance in het mice | Tsai et al., 2012, Goorden et al., 2007, Sato et al., 2012 |
| Tsc2 | Syndromic | Decreased in het and mutant mice with Tsc2-deletion from cerebellar Purkinje cells, decreased in het mice, decreased in dominant-negative Tsc2 mice | ND | Impaired social novelty in het and mutant mice with Tsc2-deletion from cerebellar Purkinje cells, normal social dominance in het mice | Reith et al., 2013, Sato et al., 2012, Chévere-Torres et al., 2013 |
| Ube3a | Syndromic | Decreased in transgenic mice with Ube3a triplication | Decreased in transgenic mice with Ube3a duplication | Normal social novelty in transgenic mice with Ube3a duplication, impaired social novelty in transgenic mice with Ube3a triplication | Jin et al., 2011 |
Note: Presented are genes from the “Syndromic” “High Confidence” and “Strong candidate” categories from the Gene Score Module on the SFARI website that were shown to affect social behavior in mouse models. Genes are grouped based on their functionality.
Deficient social behavior in ASDs
Although ASDs are heterogeneous in etiology and symptoms, a common central feature is social behavior deficit unrelated to cognitive dysfunction 4. Part of the deficit includes an impairment in social interaction, such as the inability to initiate social interactions or develop relationships, lack of social or emotional reciprocity, lack of interest in others’ emotions 17, communication deficits including impaired speech development and poor expressive language 18, impairment in nonverbal social interaction, and lack of interest in sharing enjoyment and interests with others 19.
The earliest evidence of impaired social behavior that arises during the course of ASD is impaired selective attention and lack of innate preference of newborns for human voice 20 and face 21 over other sounds and visual stimuli. Autistic infants demonstrate impaired joint attention 22, the ability to share eye-gaze focus on an object following the alert of one individual to the other by pointing or eye-gazing. In typical older children, the increased ability to communicate verbally with others results in more complex social behavior, including shared play and interactions with other children; these abilities, impaired in children with ASD 23, emphasize the profound differences between a typical and autistic child, and are one of the major alerts for testing the child for ASD. These social behavior deficits continue in adults with ASDs, impairing their normal behavior.
Williams syndrome
Williams syndrome (WS or Williams-Beuren syndrome, WBS) is a rare multisystemic neurodevelopmental genetic disorder named after Dr. John C.P. Williams who was the first to describe the syndrome in 1961 24. Physically, WS is associated with cardiovascular difficulties, growth abnormalities, connective tissue and endocrine abnormalities, and specific “elfin” facial and physical anomalies. Mentally, WS is associated with central and unique cognitive and personality profiles, independent of IQ, which include overfriendliness (frequently termed as “Cocktail party personality”), increased empathy, mental retardation 25, strength in verbal and language skills 26, weaknesses in visual-spatial skills 27, increased musical interest and emotional reactivity to music 28, and elevated anxiety derived from fear and specific phobias 29.
WS prevalence is between 1/7,500 30 to 1/20,000 individuals 31, and is caused by a hemizygous deletion at 7q11.23 of about 25 genes 32. These genes are part of the WS chromosome region (WSCR), estimated to be about 1.6 megabases, the typical deletion in ~95% of subjects. The other ~5% of subjects have longer deletions of ~1.84 megabases 33, 34, and other extremely rare types of deletions 35–37.
Interestingly, individuals with one or two extra copies of the WSCR genes due to WSCR duplication (Dup7) have an autistic-related phenotype characterized by developmental impairments, poor eye contact, anxiety disorder, repetitive behavior, hyposocial behavior and severe expressive-language delay, which is the most commonly reported feature of Dup7 38–42, although the range of these phenotypes is bigger and much less studied than in WS. Overall, these phenotypes suggest that WSCR genes are dosage-dependent and may affect language skills and development.
Hypersociability in WS
Although characterized by multiple physiological and mental features, the hypersociability phenotype is a striking feature of WS and seemingly opposite to ASDs. This unique social behavior, is the reason why, in one of the first studies to characterize WS subjects, they were described as individuals who “love everyone, are loved by everyone, and are very charming” 43.
In WS, the gregarious personality is characterized by a consistent increased interest and approach to strangers 44, overfriendliness that is positively correlated with age 45, 46, and excessive empathy but poor social judgment ability. One of the main reasons suggested for the hypersociability in WS is the substantial attention bias towards any kind of social stimuli, with a special interest in human faces 47 but see 48, opposite to ASDs.
The distinctive intense gazing pattern begins at infancy and continues throughout development 49. While processing faces, individuals with WS demonstrate atypical patterns, with increased focus on faces and eyes 47, which lasts longer than in the control group 50.
Toddlers and young children with WS continue showing higher sociability behavior, as measured by parental ratings of their child’s social behavior 51, and by the high engagement in dyadic, face-to-face interactions compared to control children52. Hypersociability persists in older children 45 and into adulthood, in which a longitudinal study found improved yet still abnormal social and adaptive functioning 53.
Another difficulty for WS subjects is accurate perception of emotions. In particular, individuals with WS demonstrate difficulties in detecting social fear signals given through facial expressions and voices 54, and show less arousal in response to angry faces 55 compared to non-impaired controls. Additionally, individuals with WS tend to have greater attention bias for positive than negative facial expression 56, and rate happy faces 57 and unfamiliar faces 58 as more approachable than control subjects.
A key factor that affects the cognitive phenotype in WS subjects is the location of the shorter atypical microdeletions. In cases where the atypical deletion included the usual telomeric breakpoint, which includes genes GTF2I 59 and GTF2IRD1 from the general transcription factor 2I gene family, a classic behavioral and neurodevelopmental phenotype was found 60, 61. But, in cases that GTF2I and GTF2IRD1 genes were not deleted, only a mild behavioral and neurodevelopmental phenotype was found 37, 62, 63, suggesting that GTF2I and GTF2IRD1 deletion is important in the etiology of the behavioral and neurodevelopmental phenotype of WS.
GTF2I encodes TFII-I, a highly conserved and ubiquitously expressed multifunctional transcription factor that contains DNA-binding I-repeat domains, leucine zipper and a nuclear localization signal 64. TFII-I regulates gene expression through interactions with tissue-specific transcription factors and complexes related to chromatin-remodeling 65. As GTF2I and GTF2IRD1 genes are in close proximity to each other, most of the WSCR deletions include both genes; however, GTF2I deletion has been shown to be more important for the social behavior phenotype of WS. For example, by comparing the social behavior phenotype in rare cases of microdeletions that spared GTF2I to those with the full WSCR deletion, Dai et al. 66 showed that the behavioral phenotype of the patient with the spared GTF2I was less social. Similarly, in individuals with different microdeletions that spared GTF2I, Morris et al. 62 found that no mental retardation or intellectual difficulties were evident, while the WS cognitive profile was evident. GTF2I was also suggested to be highly involved in other neurobehavioral impairments of WS subjects 35. In contrast, a patient with haploinsufficiency for GTF2IRD1, who had normal GTF2I expression levels, demonstrated normal social behavior but a delay in language acquisition 67.
In mice, homozygous deletion of Gtf2i causes embryonic lethality and severe developmental impairments 68, including neural tube disclosure and exencephaly. Heterozygous deletion of Gtf2i in mice results in impaired social habituation to an unfamiliar mouse, leading to increased time spent investigating the unfamiliar mouse as compared to WT mice 69. In the three-chamber social interaction and recognition test, Gtf2i heterozygous mice spend significantly more time exploring an unfamiliar mouse than a novel object, compared to WT mice 69.
Currently, it is not known how transcriptional dysregulation, resulting from GTF2I-deletion, can lead to the hypersocial phenotype in WS, and there is no clear overlap in transcriptional dysregulations between WS and ASD. A recent iPSC-based (induced pluripotent stem cells) study found that in the pluripotent state GTF2I is already responsible for 10–20% of the transcriptional dysregulation in disease-relevant pathways in WS and Dup7 70. It is therefore possible that such transcriptional dysregulation, as a result of GTF2I deletion, could result in impaired development of neural circuits that are crucial for normal social behavior from the very earliest time points.
Etiology of social behavior abnormalities
Although the etiology of social behavior abnormalities in ASD and WS is still unclear, researchers have identified many associated anatomical and physiological changes. Because of limitations inherent in studying human subjects, basic molecular and cellular research in animal models is crucial to better understand mechanisms underlying social behavior. Indeed findings from these studies have led to the development of several theories that relate to social behavior. However, humans and animals have evolved under different evolutionary pressures. This evolutional divergence has led to the fact that while molecular and cellular functions are largely comparable, social behaviors are much harder to compare. This is due to differences between animals and humans in the complexity of social behaviors as well as the underlying motivations. Moreover, the sensory cues that lead to social response in these two groups are substantially different and hence relay on the proper function of different neural circuits.
We chose to focus on three key theories, representing the physiological, functional and systemic aspects of the theories in the field of social behavior. Since social behavior has been highly studied in the frame of ASD, these theories relate mainly to ASD rather than WS.
Social cognition in human studies
To properly perform social behavior, an individual needs to acquire, process, store and use social input from the environment in order to decide on and perform proper social actions in response, the sum of which is called social cognition. Social cognition also relates to the process of understanding others or one’s own thoughts, mental states and feelings (“Theory of mind”, or mentalisation) 71. This process is impaired in children with ASD 72, and may result in impaired social information analysis and abnormal responses 73. These functions involve mainly the functionality of cortical brain regions (Figure 1). Hence, cortical dysfunction might lead to cognitive dysfunction in general, and specifically social cognition and sensory integration impairments. Importantly, why social cognition is specifically impaired in subjects with otherwise normal cognition is still unknown.
Cortical dysfunction can be the result of improper development of the cortex; in early stages of development, genes determine and regulate the formation of the brain: its cells, synapses and neural circuits. However, later on, the complex interaction between genes and the subject’s environment may lead to alterations in brain development that will result in an inability to respond to the environment 74. Genetic mutations can lead, for example, to improper synapse formation or imbalanced cellular activity between GABAergic and glutamatergic neurons. This may result in lack of proper development and function of inhibitory circuits that are essential for balanced neural activity during critical periods, and in brain regions and circuits essential for social behavior 75. Due to lack of early experience-dependent development, impaired development of primary sensory circuits, for example, could lead to further impairments in more complex functions, governed by higher-order neural circuits that develop later. Consequently, the social brain does not receive proper stimulation and experience with integrating and processing social-related inputs, nor with the execution of social decisions and actions, leading to social disabilities.
Following this logic, and focusing for example on the need to properly process social-related information, improper function of cortical and sub-cortical brain regions results in sensory integration and multi-sensory processing problems, and indeed, sensory abnormalities are found in 90% of children with ASD 76. Not properly integrating and processing the social information around them, overstimulated subjects may have difficulties in changing their attention to social-related information, resulting in improper social orientation that causes behavioral deficits. Overwhelmed by stimuli, autistic subjects might therefore tend to perform repetitive movements that return them to their “safe zone” and relieve their anxiety.
Because the vast majority of ASD and WS research is focused on toddler subjects and older, prenatal and early postnatal processes responsible for early development deficits are less understood (for review, see 77). Consequently, it is difficult to differentiate between causes and effects - whether a primary disruption of brain development leads to social abnormalities, or whether an improper interaction with the environment leads to undeveloped social-related brain regions. Thus, more research needs to be done during infancy and followed up in a longitudinal manner, as this will also enable, earlier diagnosis, earlier intervention, and identification of earlier-acting mechanisms. This can be addressed by studying infants at high familial risk for ASD as part of prospective longitudinal studies 78.
A recent longitudinal MRI study examined the morphology of the corpus callosum in infants at high-risk for ASD, as compared to low-risk controls. The findings from this study showed significantly increased corpus callosum area and thickness in children that were later diagnosed with autism spectrum disorder starting at 6 months of age 79. Additional longitudinal MRI study on the development of white matter pathways in infants at high-risk for ASD found higher fractional anisotropy in 6 months-old ASD subjects, followed by blunted developmental trajectories, resulting in lower fractional anisotropy by 24 months 80. Another study suggested that an increased cortical surface area, resulting from an increased rate of brain growth prior to age 2, is responsible for the brain enlargement in ASD children 81. More specifically, this enlargement in ASD toddlers is attributed to a generalized cerebral cortical enlargement, with an excessive temporal lobe white matter enlargement 81. Yet another longitudinal MRI study also found cerebral enlargement in ASD toddlers, including both of gray and white matter, with the highest degree of enlargement in frontal, temporal, and cingulate cortices 82. Interestingly, a different study on 6 months-old toddlers at high-risk and their low-risk controls, did not find significant differences in intracranial, cerebrum, cerebellum, or lateral ventricle volume or head circumference 83. Additionally, young autistic boys had decreased volumes of white matter and the dorsolateral region of the frontal cortex as compared with control subjects, suggesting a delayed development of these regions 84.
Imaging studies in adult ASD patients support changes particularly in mPFC. MRI study found that ASD subjects have decreased mPFC activation during mentalising, and weaker functional connectivity of the mPFC to other brain regions as compared to the control subjects 85. These findings suggest that ASD subjects use different neural circuits and patterns of activation than control subjects to analyze emotions of themselves and of others. The mPFC was also demonstrated to be involved in joint attention in ASD subjects 86; a lack of signal differentiation and atypical pattern of dorsal mPFC activation was found in ASD subjects compared to controls during a task that required joint attention. Lastly, abnormal long-range connectivity was demonstrated in ASD subjects due to altered development of the integrity of white matter in multiple brain regions (for review, see 87), in addition to local connectivity impairments 88, 89. However, the cellular mechanisms underlying these axonal disorganizations are not fully known.
In support of the imaging findings, histological examination of frontal cortex of ASD subjects has found abnormal neuronal morphology 90 and reduced minicolumns 91, suggesting that improper development of this cortical area might play a role in impaired social input integration.
Dysfunction of frontal lobes is also related to the WS hypersociability profile, as those regions have a role in regulating and suppressing actions that are socially inappropriate. The relatively low intelligence of WS patients presents a challenge to compare cortical function between WS subjects and their control groups, emphasizing the importance of selecting subjects with comparable levels of intelligence. Examining WS subjects with normal-intelligence, Meyer-Lindenberg et al., showed abnormal activity of the prefrontal cortex, including the orbitofrontal cortex (OFC), as a function of task, as compared to normal controls 92. Additionally, Meyer-Lindenberg et al., found relatively reduced task-based connectivity between OFC and the amygdala 92. Functionally, lesions of the OFC were associated with social disinhibition, suggesting that OFC abnormal activity in WS subjects might be responsible for the disinhibition of social approach. Deficits in regulating actions were suggested to be responsible for the high social approach behaviors of WS subjects, resulting in poor social response inhibition due to frontal lobe dysfunction 93, 94. Indeed, Porter et al. showed similarities in social approachability suppression in WS subjects and subjects with frontal lobe damage 94. Both types of subjects express impulsive social approach behavior and verbalize inappropriate thoughts, likely due to poor response-inhibition 94. This was also noted in a recent study in children with WS demonstrating that the frontal lobe-controlled response-inhibition skill was the strongest indication for social approach behavior 95. Lastly, abnormal cortical activity in WS subjects was observed in the right OFC, showing an opposite pattern of OFC activation in response to positive and negative emotional faces 96. Moreover, this study showed reduced activation of right amygdala in response to negative faces as compared to typically developing controls 96.
Cortical dysfunction revealed by animal studies
Although the neurophysiological substrates for social behavior abnormalities are unknown, human and animal model studies speculate that one potential theory that might explain the physiological mechanism of social behavior abnormalities might be the excitatory/inhibitory (E/I) neuronal activity imbalance 97. Changes in the E/I balance can result in hyper- 98 or hypoactivation of specific brain regions 99, and lead to dysfunction. For example, elevated excitatory activity specifically in the mouse mPFC results in impaired social behavior 100, and, consistent with the E/I imbalance theory, elevated activation of inhibitory cells rescues the social deficits 100.
On a genetic level, the association of genes to social behavior is not straightforward, despite multiple animal models showing synaptic or circuit dysfunction that is accompanied with social behavior abnormalities. For instance, E/I imbalance can occur in cortical regions due to mutations in synaptic proteins such as Shank 98, 101, a family of key PSD proteins located in glutamatergic synapses that, together with other postsynaptic proteins (SAPAP and PSD-95), forms a postsynaptic scaffolding complex (Figure 2) 102–104. While ASD is considered a polygenic disorder in most cases, recent studies showed that genetic disruption of Shank2 and Shank3 in mice results in substantial physiological and biochemical alterations at synapses that may contribute to impaired social behavior 99, 105–110.
The importance of E/I balance in the cortex was also demonstrated in a mouse model of Rett Syndrome111. Methyl CpG binding protein 2 (MeCP2) regulates the expression of many genes by acting as a transcriptional activator and repressor, and mutations in MECP2 are known as the primary cause of Rett syndrome. Importantly, specific deletion of Mecp2-deficiency from either all GABAergic neurons in the nervous system (using Viaat-Cre mice) or a specific subset of GABAergic neurons in the forebrain (using Dlx5/6–Cre mice), resulted in mice with features of Rett syndrome and ASD (Table 2) 112. Deletion of Mecp2 resulted in a reduced inhibitory quantal size demonstrating that specific disruption of inhibitory signaling was sufficient to recapitulate ASD behaviors 112.
As stated before, social cognition relies on proper sensing and integration of sensory and social-related input, and indeed sensory abnormalities are highly common in autistic subjects. Recently, two studies on mouse models of ASD demonstrated the importance of the inhibitory system in sensory input processing and integration. Impaired maturation of the inhibitory system in the insula cortex of BTBR mice results in decreased inhibitory neurotransmission and increased levels of excitatory neurotransmission, affecting multisensory integration 98. Of clinical relevance, treatment with benzodiazepine, a positive modulator of GABAergic transmission, early in postnatal development, but not in later age, rescues the impairment 98. Furthermore, GABA-B agonist R-Baclofen has also been shown to reverse social deficits in BTBR mice 113. In another study, impaired function of the inhibitory system affected sensory input processing in the somatosensory barrel cortex of juvenile mice with a R451C substitution in Nlgn3 114, a postsynaptic protein important for trans-synaptic cell-adhesion (Figure 2). Cellot et al. recently showed that R451C mutation affects the release probability of GABA from parvalbumin-expressing interneurons, impairing their modulation of principal cells in layer IV somatosensory barrel cortex. This leads to a shift in E/I balance and, furthermore, affects the generation of cortical gamma rhythms associated with high cognitive functions such as social behavior.
Currently, neurobiological knowledge of the role of synaptic signaling in WS is extremely limited. Therefore, it would be of high interest to study the developmental abnormalities at molecular and cellular levels that lead to cortical dysfunction in WS.
The Amygdala Theory
The amygdala, an almond-shaped region comprised of at least 13 nuclei with unique functions, is part of the limbic system. Highly connected to brain regions responsible for sensory input and autonomic systems, the amygdala takes part in central functions and processes that are crucial for proper social behavior and emotional processing, and hence is suggested as a central component of the “Social brain” (Figure 1). The amygdala’s roles in social behavior include the processing of emotional reactions, anxiety, recognizing social emotion from faces, memory processing, and visual social stimuli processes. The amygdala also has a central role in eye-gazing and face recognition 115, such that subjects with complete amygdala lesions show impaired eye-contact, similar to autistic subjects 116. In highly-functioning autistic subjects, an impaired ability was found in recognizing social information from faces, similar to subjects with focal bilateral amygdala damage 117. Additionally, impaired social judgment was demonstrated in subjects with amygdala lesions 118, while, on the other hand, deep-brain stimulation of the amygdala improved social behavior in an autistic boy 119.
Anatomically, autistic children have larger right and left amygdala volumes than control children, although this difference is gone by adolescence 120. An increased amygdala volume was found also in WS subjects 121, 122, together with a positive correlation between right amygdala volume and the approachability of faces 122. These findings support the notion that abnormalities in amygdala development and function may contribute to deficits in social judgment, emotional information processing, and face expression perception, leading to abnormal emotional reaction and social behavior abnormalities in ASD and WS subjects. Current knowledge is still contradictory, and the opposite social behaviors in ASD and WS offer a research approach to link the function of the amygdala and its effects on social behavior.
Abnormal amygdala activity in response to faces has been found in both ASD and WS imaging studies. Hyperactivation of the amygdala was demonstrated when autistic subjects looked at faces, as compared to controls 123. Furthermore, autistic subjects gazed more away than towards the eyes of a presented face, as compared to controls, with a greater amygdala response in ASD subjects while fixating on the eyes rather than the mouth 124. This suggests that in ASD subjects, the amygdala response to faces has a negatively-valenced overarousal response. However, some other studies showed hypoactivation of the amygdala of ASD subjects while interpreting emotional states by viewing human eyes 125 or while processing human fearful faces 126. In WS subjects, amygdala reactivity to fearful faces, presented as negative social stimuli, was drastically attenuated compared with controls 127, and strikingly, WS subjects showed no amygdala activation in a face-discrimination task 128. The attenuated amygdala activity in WS may result in deficient processing of social-related information, leading to high approachability to strangers’ faces 58. In contrast, higher amygdala reactivity was observed in WS participants in response to happy faces presented as positive social stimuli 127.
The common hyperactivation of the amygdala in the two disorders, but to opposite stimuli, demonstrates the complexity of amygdala functionality and its relevance to social behavior. In autistic subjects aversive-related amygdala activation was observed while eye-gazing, resulting in eye-contact avoidance. In subjects with WS, appetitive-related amygdala activation was observed, perhaps serving to functionally increase attention and processing of happy faces. It might be that different subpopulations of neurons, such as glutamatergic or GABAergic, are active in response to the stimuli in these disorders, resulting in the contrasting behavioral phenotypes. Indeed, a recent study showed that in the medial amygdala, a brain region modulating innate social behavior, inhibitory neurons played an important role in controlling social behavior, while excitatory neurons modulated repetitive asocial behavior 129.
When presented with non-social scenes 130, or threatening scenes, but not threatening faces, WS subjects showed increased amygdala activation and abnormal activation of prefrontal regions linked to the amygdala as compared to controls 92. Indeed, the amygdala-prefrontal circuitry has been shown to be important in the proper representation of emotional salience of a stimulus (for review, see 131). Normally, the amygdala’s output activity is attenuated by the regulation of mPFC excitatory neurons that project and regulate inhibitory neurons in the basolateral amygdala (BLA) or intercalated cells around the BLA, that inhibit output from the central nucleus of the amygdala (CeA) 132. Impairments in this circuitry lead to impaired detection of danger, resulting in lower levels of fear and hypersocial behavior, as demonstrated in human and animal models 133, 134. Interestingly, OFC-amygdala connectivity was functionally disconnected and impaired in WS subjects 92, suggesting that impaired prefrontal-regulated inhibition of the amygdala is responsible for the dissociated fear in those subjects, demonstrating high non-social fear along with low social-related fear. A recent study identified the deficits in the structural integrity of prefrontal-amygdala white matter pathways as the primary cause of this pathology 135. These findings suggest that increased amygdala activation may play a role in non-social scenarios and the increased generalized anxiety and phobias associated with WS.
The overfriendliness in WS subjects coexists with non-social anxiety and phobias, suggesting they have specifically social-related lower levels of anxiety. Indeed, WS and social anxiety disorder (SAD) have multiple opposite characteristics in many areas, including general social drive, specific approach to unfamiliar people, social behavior in an unfamiliar social environment, and attention to faces and eye-gaze (for review, see 136). Functionally, in WS subjects, hypoactivation of limbic regions was detected during facial emotion processing when compared to control subjects, while SAD subjects demonstrated hyperactivation, in addition to hyperactivation in medial frontal regions 136. This suggests that neural circuits that govern general fear are more functionally separated than those related to social fear, and that the latter is oppositely affected in ASD and WS.
Lastly, the amygdala also regulates anxiety, making a simple interpretation of the discussed findings difficult. A direct correlation between anxiety levels and social impairment was observed in the case of autistic subjects 137 as well as WS subjects 138. However in the case of WS, subjects demonstrate hypersociability along with high anxiety levels. While similar amygdala MRI abnormalities are found in both disorders, the social behavioral phenotype is opposite, suggesting that either subcircuits in the amygdala or other brain regions, either upstream or downstream to the amygdala, play a role in the opposite social behavior phenotype.
Overall, future studies are needed to better determine the amygdala’s valence and function in social behavior, to define the interplay between impaired social behavior and anxiety, and to study whether the different amygdala functions rely on different nuclei that might be oppositely affected in ASD and WS. Since imaging and manipulating the different nuclei of the amygdala is technically difficult in humans, animal models for ASD and WS are valuable research tools to dissect these questions.
The Social Motivation Theory
The “Social motivation theory” suggests that impaired motivation to engage in reciprocal social interaction leads to the autistic-like social deficit 139. Three key brain regions are related to “Social motivation” and are all highly connected neuroanatomically: orbital and ventromedial regions of the prefrontal cortex, the amygdala, and the ventral striatum. Supporting this theory, children with ASD have a reduced frontostriatal response to social but not monetary rewarded learning 140. However, other studies also found that the deficit in reward processing in ASD subjects was attributed not only to social reward, but also to a more general deficit of the reward system 141. It is therefore important to determine whether in ASD the impairment is specifically in social motivation and not in general motivation, and to study the interplay between the two.
Perhaps one of the most studied molecular mechanisms related to the modulation of social behavior is oxytocin, a neuropeptide synthesized in the hypothalamus, released by the pituitary and affecting the central nervous system 142. Oxytocin is involved in increasing the degree of approach behavior, social recognition, social memory, the recognition of others’ emotions, emotional information processing, maternal behavior and reducing social fear and anxiety.
Recent studies tested whether oxytocin signaling in mice takes part in the reward aspect of social interaction 143. Oxytocin was found to be an enforcement signal in social behavior, acting in medium spiny neurons (MSNs) of the nucleus accumbens (NAc), where it modifies excitatory synaptic transmission by evoking presynaptic long-term depression 143. When fully abolished in mice, oxytocin was demonstrated to be necessary for social memory, and oxytocin-null mice demonstrated social amnesia that was rescued upon exogenous oxytocin administration 144.
Additional recent studies in mice support the role of reward circuitry in social behavior demonstrating that the ventral tegmental area (VTA), a major source of dopamine in the reward circuitry, is highly active during social interaction 145. Bidirectional control of dopaminergic cells of the VTA modulated social behavior in opposite directions145. Additionally, activation of the VTA-NAc projection increased social interaction, while VTA-mPFC activation did not affect social interaction, and postsynaptic NAc D1 MSNs were shown to be responsible for social behavior regulation 145. Finally, in a study on social attachment in monogamous voles, it was shown that dopamine transmission specifically in the rostral shell of the NAc promotes pair-bond formation, with D1-like receptor activation decreasing and D2-like receptor activation increasing pair-bond formation 146.
Future directions
Recent development of genome-editing techniques such as TALEN 147 and CRISPR 148 will allow us to develop better animal models of disease, such as primates 149, for social behavioral studies. In particular, the common marmoset, a small New-World monkey with rapid reproduction cycles, could become the next generation of genetically engineered models for brain-disorder research 150. Common marmosets are small (~350g), reach sexual maturity at 12–16 months, give birth twice a year, and produce 2–3 offspring with each birth. Importantly, marmosets are evolutionarily much closer to humans than rodents in brain structure and function. Furthermore, marmosets are very social and communicative and can perform some higher cognitive tasks developed for macaque monkeys. Because of the complexity of genetics in ASD, starting with monogenic causes of ASD, such as Shank3 and CHD8, would be beneficial. For WS, Gtf2i would be an excellent candidate for genetic manipulation in marmosets based on the knowledge from both human and mouse studies. Together, these enabling technologies and new models will likely push the field forward in a significant way in the next few years.
Acknowledgments
The authors gratefully acknowledge L. McGrath, F. Dobie, P. Monteiro, A. Krol and Y. Mei for insightful comments on the manuscript. Research in the laboratory of Guoping Feng was supported by the Poitras Center for Affective Disorders Research at MIT, Stanley Center for Psychiatric Research at Broad Institute of MIT and Harvard, National Institute of Health (NIMH), Nancy Lurie Marks Family Foundation, Simons Foundation Autism Research Initiative (SFARI), and Simons Center for the Social Brain at MIT. Boaz Barak was supported by postdoctoral fellowships from the Simons Center for the Social Brain at MIT and the Autism Science Foundation.
Footnotes
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671–8. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couture SM, et al. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med. 2010;40:569–79. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esbensen AJ, Seltzer MM, Lam KS, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. J Autism Dev Disord. 2009;39:57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V) Washington: 2013. [Google Scholar]
- 5.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 6.Investigators A.a.D.D.M.N.S.Y.P. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network 11 Sites United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 7.Elsabbagh M, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai MC, et al. Cognition in Males and Females with Autism: Similarities and Differences. PLoS ONE. 2012;7:e47198. doi: 10.1371/journal.pone.0047198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma D, et al. A genome-wide association study of autism reveals a common novel risk locus at 5p14.1. Ann Hum Genet. 2009;73:263–73. doi: 10.1111/j.1469-1809.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merikangas AK, et al. The phenotypic manifestations of rare genic CNVs in autism spectrum disorder. Mol Psychiatry. 2015;20:1366–72. doi: 10.1038/mp.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders SJ, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–33. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss LA, Arking DE, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–8. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen RK, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nat Med. 2015;21:185–91. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peca J, Feng G. Cellular and synaptic network defects in autism. Curr Opin Neurobiol. 2012;22:866–72. doi: 10.1016/j.conb.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigman MD, Kasari C, Kwon JH, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Dev. 1992;63:796–807. [PubMed] [Google Scholar]
- 18.Lord C, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 19.Bauminger N, Shulman C, Agam G. Peer interaction and loneliness in high-functioning children with autism. J Autism Dev Disord. 2003;33:489–507. doi: 10.1023/a:1025827427901. [DOI] [PubMed] [Google Scholar]
- 20.Mills M, Melhuish E. Recognition of mother’s voice in early infancy. Nature. 1974;252:123–4. doi: 10.1038/252123a0. [DOI] [PubMed] [Google Scholar]
- 21.Volkmar FR, Mayes LC. Gaze behavior in autism. Development and Psychopathology. 1990;2:61–69. [Google Scholar]
- 22.Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. J Autism Dev Disord. 1990;20:115–28. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- 23.Black M, Freeman BJ, Montgomery J. Systematic observation of play behavior in autistic children. J Autism Child Schizophr. 1975;5:363–71. doi: 10.1007/BF01540682. [DOI] [PubMed] [Google Scholar]
- 24.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–8. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- 25.Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am J Med Genet C Semin Med Genet. 2010;154C:229–48. doi: 10.1002/ajmg.c.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman I, Yam A, Bellugi U, Mills D. Language and sociability: insights from Williams syndrome. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mervis CB, Robinson BF, Pani JR. Visuospatial construction. Am J Hum Genet. 1999;65:1222–9. doi: 10.1086/302633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dykens EM, Rosner BA, Ly T, Sagun J. Music and anxiety in Williams syndrome: a harmonious or discordant relationship? Am J Ment Retard. 2005;110:346–58. doi: 10.1352/0895-8017(2005)110[346:MAAIWS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Dykens EM. Anxiety, fears, and phobias in persons with Williams syndrome. Dev Neuropsychol. 2003;23:291–316. doi: 10.1080/87565641.2003.9651896. [DOI] [PubMed] [Google Scholar]
- 30.Stromme P, Bjornstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17:269–71. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- 31.Pober BR. Williams-Beuren syndrome. N Engl J Med. 2010;362:239–52. doi: 10.1056/NEJMra0903074. [DOI] [PubMed] [Google Scholar]
- 32.Korenberg JR, et al. VI. Genome structure and cognitive map of Williams syndrome. J Cogn Neurosci. 2000;12(Suppl 1):89–107. doi: 10.1162/089892900562002. [DOI] [PubMed] [Google Scholar]
- 33.Bayes M, Magano LF, Rivera N, Flores R, Perez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet. 2003;73:131–51. doi: 10.1086/376565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edelmann L, et al. An atypical deletion of the Williams-Beuren syndrome interval implicates genes associated with defective visuospatial processing and autism. J Med Genet. 2007;44:136–43. doi: 10.1136/jmg.2006.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonell A, et al. Partial 7q11.23 deletions further implicate GTF2I and GTF2IRD1 as the main genes responsible for the Williams-Beuren syndrome neurocognitive profile. J Med Genet. 2010;47:312–20. doi: 10.1136/jmg.2009.071712. [DOI] [PubMed] [Google Scholar]
- 36.Fusco C, et al. Smaller and larger deletions of the Williams Beuren syndrome region implicate genes involved in mild facial phenotype, epilepsy and autistic traits. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howald C, et al. Two high throughput technologies to detect segmental aneuploidies identify new Williams-Beuren syndrome patients with atypical deletions. J Med Genet. 2006;43:266–73. doi: 10.1136/jmg.2005.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beunders G, et al. A triplication of the Williams-Beuren syndrome region in a patient with mental retardation, a severe expressive language delay, behavioural problems and dysmorphisms. J Med Genet. 2010;47:271–5. doi: 10.1136/jmg.2009.070490. [DOI] [PubMed] [Google Scholar]
- 39.Depienne C, et al. Autism, language delay and mental retardation in a patient with 7q11 duplication. J Med Genet. 2007;44:452–8. doi: 10.1136/jmg.2006.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malenfant P, et al. Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. J Autism Dev Disord. 2012;42:1459–69. doi: 10.1007/s10803-011-1389-4. [DOI] [PubMed] [Google Scholar]
- 41.Sanders SJ, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somerville MJ, et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med. 2005;353:1694–701. doi: 10.1056/NEJMoa051962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation. 1962;26:1235–40. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- 44.Gosch A, Pankau R. Social-emotional and behavioral adjustment in children with Williams-Beuren syndrome. Am J Med Genet. 1994;53:335–9. doi: 10.1002/ajmg.1320530406. [DOI] [PubMed] [Google Scholar]
- 45.Klein-Tasman BP, Mervis CB. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev Neuropsychol. 2003;23:269–90. doi: 10.1080/87565641.2003.9651895. [DOI] [PubMed] [Google Scholar]
- 46.Gosch A, Pankau R. Personality characteristics and behaviour problems in individuals of different ages with Williams syndrome. Dev Med Child Neurol. 1997;39:527–33. doi: 10.1111/j.1469-8749.1997.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 47.Riby D, Hancock PJ. Looking at movies and cartoons: eye-tracking evidence from Williams syndrome and autism. J Intellect Disabil Res. 2009;53:169–81. doi: 10.1111/j.1365-2788.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 48.Dodd HF, Porter MA, Peters GL, Rapee RM. Social approach in pre-school children with Williams syndrome: the role of the face. J Intellect Disabil Res. 2010;54:194–203. doi: 10.1111/j.1365-2788.2009.01241.x. [DOI] [PubMed] [Google Scholar]
- 49.Mervis CB, et al. Attentional characteristics of infants and toddlers with Williams syndrome during triadic interactions. Dev Neuropsychol. 2003;23:243–68. doi: 10.1080/87565641.2003.9651894. [DOI] [PubMed] [Google Scholar]
- 50.Doherty-Sneddon G, Riby DM, Calderwood L, Ainsworth L. Stuck on you: face-to-face arousal and gaze aversion in Williams syndrome. Cogn Neuropsychiatry. 2009;14:510–23. doi: 10.1080/13546800903043336. [DOI] [PubMed] [Google Scholar]
- 51.Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend” hypersociability in young children with Williams syndrome. Am J Med Genet A. 2004;124A:263–73. doi: 10.1002/ajmg.a.20416. [DOI] [PubMed] [Google Scholar]
- 52.Laing E, et al. Atypical development of language and social communication in toddlers with Williams syndrome. 2002. [Google Scholar]
- 53.Elison S, Stinton C, Howlin P. Health and social outcomes in adults with Williams syndrome: findings from cross-sectional and longitudinal cohorts. Res Dev Disabil. 2010;31:587–99. doi: 10.1016/j.ridd.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Plesa-Skwerer D, Faja S, Schofield C, Verbalis A, Tager-Flusberg H. Perceiving facial and vocal expressions of emotion in individuals with Williams syndrome. Am J Ment Retard. 2006;111:15–26. doi: 10.1352/0895-8017(2006)111[15:PFAVEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Plesa Skwerer D, et al. A multimeasure approach to investigating affective appraisal of social information in Williams syndrome. J Neurodev Disord. 2011;3:325–34. doi: 10.1007/s11689-011-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dodd HF, Porter MA. I see happy people: Attention bias towards happy but not angry facial expressions in Williams syndrome. Cogn Neuropsychiatry. 2010;15:549–67. doi: 10.1080/13546801003737157. [DOI] [PubMed] [Google Scholar]
- 57.Frigerio E, et al. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia. 2006;44:254–9. doi: 10.1016/j.neuropsychologia.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Bellugi U, Adolphs R, Cassady C, Chiles M. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport. 1999;10:1653–7. doi: 10.1097/00001756-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 59.Bayarsaihan D, et al. Genomic organization of the genes Gtf2ird1, Gtf2i, and Ncf1 at the mouse chromosome 5 region syntenic to the human chromosome 7q11.23 Williams syndrome critical region. Genomics. 2002;79:137–43. doi: 10.1006/geno.2001.6674. [DOI] [PubMed] [Google Scholar]
- 60.Botta A, et al. Expression analysis and protein localization of the human HPC-1/syntaxin 1A, a gene deleted in Williams syndrome. Genomics. 1999;62:525–8. doi: 10.1006/geno.1999.5987. [DOI] [PubMed] [Google Scholar]
- 61.Heller R, Rauch A, Luttgen S, Schroder B, Winterpacht A. Partial deletion of the critical 1.5 Mb interval in Williams-Beuren syndrome. J Med Genet. 2003;40:e99. doi: 10.1136/jmg.40.8.e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris CA, et al. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A. 2003;123A:45–59. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- 63.van Hagen JM, et al. Contribution of CYLN2 and GTF2IRD1 to neurological and cognitive symptoms in Williams Syndrome. Neurobiol Dis. 2007;26:112–24. doi: 10.1016/j.nbd.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Hinsley TA, Cunliffe P, Tipney HJ, Brass A, Tassabehji M. Comparison of TFII-I gene family members deleted in Williams-Beuren syndrome. Protein Sci. 2004;13:2588–99. doi: 10.1110/ps.04747604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roy AL. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene. 2001;274:1–13. doi: 10.1016/s0378-1119(01)00625-4. [DOI] [PubMed] [Google Scholar]
- 66.Dai L, et al. Is it Williams syndrome? GTF2IRD1 implicated in visual-spatial construction and GTF2I in sociability revealed by high resolution arrays. Am J Med Genet A. 2009;149A:302–14. doi: 10.1002/ajmg.a.32652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tassabehji M, et al. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005;310:1184–7. doi: 10.1126/science.1116142. [DOI] [PubMed] [Google Scholar]
- 68.Enkhmandakh B, et al. Essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development. Proc Natl Acad Sci U S A. 2009;106:181–6. doi: 10.1073/pnas.0811531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakurai T, et al. Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions. Autism Res. 2011;4:28–39. doi: 10.1002/aur.169. [DOI] [PubMed] [Google Scholar]
- 70.Adamo A, et al. 7q11.23 dosage-dependent dysregulation in human pluripotent stem cells affects transcriptional programs in disease-relevant lineages. Nat Genet. 2015;47:132–41. doi: 10.1038/ng.3169. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher PC, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 72.Colle L, Baron-Cohen S, Hill J. Do children with autism have a theory of mind? A non-verbal test of autism vs. specific language impairment. J Autism Dev Disord. 2007;37:716–23. doi: 10.1007/s10803-006-0198-7. [DOI] [PubMed] [Google Scholar]
- 73.Embregts P, van Nieuwenhuijzen M. Social information processing in boys with autistic spectrum disorder and mild to borderline intellectual disabilities. J Intellect Disabil Res. 2009;53:922–31. doi: 10.1111/j.1365-2788.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 74.Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson SB, Valakh V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron. 2015;87:684–98. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- 77.Yirmiya N, Charman T. The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics. J Child Psychol Psychiatry. 2010;51:432–58. doi: 10.1111/j.1469-7610.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- 78.Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolff JJ, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138:2046–58. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolff JJ, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hazlett HC, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–76. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schumann CM, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hazlett HC, et al. Brain volume findings in 6-month-old infants at high familial risk for autism. Am J Psychiatry. 2012;169:601–8. doi: 10.1176/appi.ajp.2012.11091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 85.Murdaugh DL, et al. Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One. 2012;7:e50064. doi: 10.1371/journal.pone.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redcay E, et al. Atypical brain activation patterns during a face-to-face joint attention game in adults with autism spectrum disorder. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Travers BG, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dajani DR, Uddin LQ. Local brain connectivity across development in autism spectrum disorder: A cross-sectional investigation. Autism Res. 2015 doi: 10.1002/aur.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venkataraman A, Duncan JS, Yang DY, Pelphrey KA. An unbiased Bayesian approach to functional connectomics implicates social-communication networks in autism. Neuroimage Clin. 2015;8:356–66. doi: 10.1016/j.nicl.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bailey A, et al. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 91.Buxhoeveden DP, et al. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathol Appl Neurobiol. 2006;32:483–91. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 92.Meyer-Lindenberg A, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–3. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- 93.Mobbs D, et al. Frontostriatal dysfunction during response inhibition in Williams syndrome. Biol Psychiatry. 2007;62:256–61. doi: 10.1016/j.biopsych.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 94.Porter MA, Coltheart M, Langdon R. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. 2007;45:2839–49. doi: 10.1016/j.neuropsychologia.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Little K, et al. Heterogeneity of social approach behaviour in Williams syndrome: The role of response inhibition. Res Dev Disabil. 2013;34:959–67. doi: 10.1016/j.ridd.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 96.Mimura M, et al. A preliminary study of orbitofrontal activation and hypersociability in Williams Syndrome. J Neurodev Disord. 2010;2:93–98. doi: 10.1007/s11689-009-9041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baroncelli L, et al. Brain plasticity and disease: a matter of inhibition. Neural Plast. 2011;2011:286073. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK. Sensory Integration in Mouse Insular Cortex Reflects GABA Circuit Maturation. Neuron. 2014;83:894–905. doi: 10.1016/j.neuron.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peca J, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee J, et al. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front Cell Neurosci. 2015;9:94. doi: 10.3389/fncel.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim E, et al. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–78. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boeckers TM, Bockmann J, Kreutz MR, Gundelfinger ED. ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem. 2002;81:903–10. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 104.Takeuchi M, et al. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–51. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 105.Bozdagi O, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmeisser MJ, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 107.Wang X, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Won H, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–5. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 109.Yang M, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–41. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y, et al. Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects. Neuron. 2015 doi: 10.1016/j.neuron.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Banerjee A, Castro J, Sur M. Rett syndrome: genes, synapses, circuits, and therapeutics. Front Psychiatry. 2012;3:34. doi: 10.3389/fpsyt.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chao HT, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silverman JL, et al. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40:2228–39. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cellot G, Cherubini E. Reduced inhibitory gate in the barrel cortex of Neuroligin3R451C knock-in mice, an animal model of autism spectrum disorders. Physiol Rep. 2014;2 doi: 10.14814/phy2.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fried I, MacDonald KA, Wilson CL. Single Neuron Activity in Human Hippocampus and Amygdala during Recognition of Faces and Objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 116.Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. 2007;27:3994–7. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–40. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 118.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 119.Sturm V, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2012;6:341. doi: 10.3389/fnhum.2012.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haas BW, Sheau K, Kelley RG, Thompson PM, Reiss AL. Regionally specific increased volume of the amygdala in Williams syndrome: Evidence from surface-based modeling. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martens MA, Wilson SJ, Dudgeon P, Reutens DC. Approachability and the amygdala: insights from Williams syndrome. Neuropsychologia. 2009;47:2446–53. doi: 10.1016/j.neuropsychologia.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 123.Dalton KM, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. J Neurosci. 2012;32:9469–76. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baron-Cohen S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 126.Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 127.Haas BW, et al. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J Neurosci. 2009;29:1132–9. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paul BM, et al. Amygdala response to faces parallels social behavior in Williams syndrome. Soc Cogn Affect Neurosci. 2009;4:278–85. doi: 10.1093/scan/nsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong W, Kim DW, Anderson DJ. Antagonistic Control of Social versus Repetitive Self-Grooming Behaviors by Separable Amygdala Neuronal Subsets. Cell. 2014;158:1348–61. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Munoz KE, et al. Abnormalities in neural processing of emotional stimuli in Williams syndrome vary according to social vs. non-social content. Neuroimage. 2010;50:340–6. doi: 10.1016/j.neuroimage.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 132.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–47. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 134.Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–63. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Avery SN, Thornton-Wells TA, Anderson AW, Blackford JU. White matter integrity deficits in prefrontal-amygdala pathways in Williams syndrome. Neuroimage. 2012;59:887–94. doi: 10.1016/j.neuroimage.2011.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Binelli C, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64C:205–217. doi: 10.1016/j.neuropsychologia.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 137.White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29:216–29. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Riby DM, et al. The interplay between anxiety and social functioning in williams syndrome. J Autism Dev Disord. 2014;44:1220–9. doi: 10.1007/s10803-013-1984-7. [DOI] [PubMed] [Google Scholar]
- 139.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16:231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kohls G, et al. Atypical brain responses to reward cues in autism as revealed by event-related potentials. J Autism Dev Disord. 2011;41:1523–33. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- 142.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 143.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–84. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 145.Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aragona BJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 147.Zhang F, et al. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 2011;29:149–53. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cong L, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Niu Y, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156:836–43. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 150.Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012;17:336–40. doi: 10.1016/j.siny.2012.07.002. [DOI] [PubMed] [Google Scholar]