Abstract
G-protein coupled dopamine and metabotropic glutamate receptors (mGlu) can modulate neurotransmission during Parkinson's disease (PD)-like neurodegeneration. PET imaging studies in a unilateral dopamine denervation model (6-OHDA) showed a significant inverse correlation of presynaptic mGlu4 and postsynaptic mGlu5 expression in the striatum and rapidly declining mGlu4 and enhanced mGlu5 expression in the hippocampus during progressive degeneration over time. Immunohistochemical studies verified the decreased mGlu4 expression in the hippocampus on the lesion side but did not show difference in mGlu5 expression between lesion and control side. Pharmacological MRI studies showed enhanced hemodynamic response in several brain areas on the lesion side compared to the control side after challenge with mGlu4 positive allosteric modulator or mGlu5 negative allosteric modulator. However, mGlu4 response was biphasic having short enhancement followed by negative response on both sides of brain. Studies in mGlu4 expressing cells demonstrated that glutamate induces cooperative increase in binding of mGlu4 ligands – especially at high glutamate levels consistent with in vivo concentration. This suggests that mGlu allosteric modulators as drug candidates will be highly sensitive to changes in glutamate concentration and hence metabolic state. These experiments demonstrate the importance of the longitudinal imaging studies to investigate temporal changes in receptor functions to obtain individual response for experimental drugs.
Keywords: metabotropic glutamate receptor, mGlu4, mGlu5, Parkinson's disease, positron emission tomography, pharmacological magnetic resonance imaging
Graphical abstract
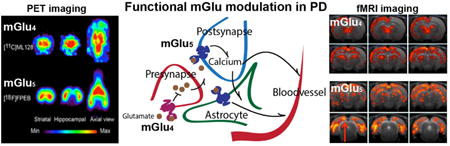
1. Introduction
Recent advances in imaging techniques have enabled investigation of the interactions between superfamily of G protein coupled receptors, including dopamine (DA) and metabotropic glutamate receptors (mGlu), and their associated neurotransmitters; dopamine and glutamate. These receptors, and their modulation in different pathologies including Parkinson's disease (PD), may provide important therapeutic targets.
The primary pathophysiological change that leads to the motor symptoms of PD is a loss of dopaminergic neurons in the substantia nigra pars compacta that modulates the function of the striatum and other basal ganglia structures (Niethammer et al., 2013). The dopamine transporter (DAT) is a membrane-spanning protein, which pumps the neurotransmitter dopamine out of the synapse to cytosol from which it is further transported to vesicles for storage and release. For in vivo imaging of DAT function we have previously introduced a series of tropanes (Brownell et al., 1996; Meltzer et al., 1993). Imaging of dopamine function is common in PD and PD models, however far fewer studies have been performed to image changes in mGlu receptors in PD and PD models.
Based on functional mechanisms glutamate receptors are divided into ionotropic and metabotropic glutamate receptors. mGlus function through second messengers and have been target for drug development especially after development of allosteric modulators as drug candidates binding to specific mGlus (Conn et al., 2014). Of the eight mGlu subtypes mGlu4 and mGlu5 have received a lot of attention for drug development. mGlu4 is expressed presynaptically with high density in the striatopallidal synapse (Bogenpohl et al., 2013; Bradley et al., 1999) within the indirect pathway of the basal ganglia. mGlu4 interacts with the Gai/o subunit of G-protein which couples negatively with adenylate cyclase to inhibit cyclic adenosine monophosphate dependent signal pathways (Ferraguti et al., 1999). mGlu5 has opposite roles and it is mainly expressed postsynaptically, and coupled with G-protein to stimulate downstream effectors and tyrosine kinases, such as adenyl cyclase, phospholipase C-β, mitogen-activated protein kinase, and phosphoinositide 3 kinase (Conn and Pin, 1997; Niswender and Conn, 2010; Sheffler et al., 2011). mGlu5 also has an astrocytic localization (He et al., 2012).
G-protein coupled mGlus modulate intracellular signal transductions and thus regulate the cell physiology (Gubellini et al., 2004; Niswender and Conn, 2010; Pin and Duvoisin, 1995). Since current PD treatments are based on the replacement of dopamine function and suffer greatly from unwanted side-effects including dyskinesia, the mGlu signaling has become a potential target for drug discovery for treatment of neurological dysfunctions like PD (Johnson et al., 2009). Previous clinical and preclinical studies have shown that dopamine denervation leads to upregulation of the expression of postsynaptic dopamine receptors, which even increases the negative side-effects seen with current dopamine replacement therapy (Aubert et al., 2005; Nguyen et al., 2000; Sanchez-Pernaute et al., 2007). So far relatively little is known about the specific functions, signaling, biodistribution and pharmacology of different mGlu4 and mGlu5 subtypes in PD and PD models (Conn and Pin, 1997; Johnson et al., 2009). However, a number of publications support a role for mGlus in development of PD related motor and cognitive dysfunctions (Gasparini et al., 2013; Johnson et al., 2009; Nicoletti et al., 2011).
We explored the functional role of mGlu4 positive allosteric modulator (PAM) and mGlu5 negative allosteric modulator (NAM) in the 6-OHDA rat model of PD during fifteen months of progressive degeneration taking advantage of high sensitivity positron emission tomographic imaging (PET) and used our recently developed novel specific radiopharmaceuticals [11C]ML128 (N-(4-Chloro-3-[11C]methoxyphenyl)-2-picolinamide) for mGlu4 (Kil et al., 2013) and [18F]FPEB (3-fluoro-5-[(pyridin-3-yl)ethynyl] benzonitrile) for mGlu5 (Wang et al., 2007).
The 6-OHDA lesion model provides a means to test the effects of mGlu stimulation on the control and on the dopamine denervated side simultaneously. After the last PET imaging studies we investigated the effects of mGlu4 PAM and mGlu5 NAM induced regional signal changes recorded as hemodynamic response using the IRON (Increased Relaxivity for Optimized Neuroimaging) technique for measurement of cerebral blood volume (CBV) (Chen et al., 2001; Mandeville, 2012). Regional signal changes were induced by a mGlu4 ligand, DFMPP, (N-(4-chloro-3-methoxyphenyl)-2-picolinamide) which is a PAM and a mGlu5 NAM, MTEP (3-((2-methyl-4-thiazolyl)ethynyl) pyridine). This technique can provide a read-out on receptor functioning, as opposed to receptor numbers as measured by PET (Jenkins, 2012).
2. Materials and Methods
2.1. Animal model
Five male Sprague-Dawley rats (weight between 300 and 450 g) were purchased from Charles River Laboratories (CRL) after lesioning with 6-OHDA and verification with apomorphine. 6-OHDA was delivered unilaterally by stereotactic injection into the substantia nigra on the left side of the rats, leaving the other hemisphere to serve as an internal control.
2.1.1. Animal ethics
All experiments and handlings with the animals were performed in accordance with the National Institutes of Health Guide for the Care and Usage of Laboratory Animals and the usage was approved by the Subcommittee on the Research Animal Care (SRAC) of the Institutional Animal Care and Use Committee (IACUC) for Massachusetts General Hospital (MGH) and Harvard Medical School (HMS).
2.2. In vivo PET imaging studies
2.2.1. Imaging ligands
[11C]ML128: The radiosyntheses of [11C]ML128 was performed as previously published (Kil et al., 2013). In brief, [11C]CO2 produced by cyclotron was reduced to [11C]methane ([11C]CH4) which was converted into [11C]CH3I in gaseous iodination condition. The resultant [11C]CH3I was transferred in a stream of nitrogen and trapped in a reaction vessel containing the precursor. After completion of the [11C]CH3I transfer, the reaction vessel was sealed and heated for 5 min. After heating, the reaction mixture was diluted with 1 mL of HPLC eluent, and injected into radio-HPLC using CH3CN/0.1 M HCO2NH4 solution (45/55) as the mobile phase. The desired product was eluted between 10.4 and 11.0 min. The fraction containing [11C]ML128 was diluted with 20 mL of water and passed through a C18 Sep-Pak Plus cartridge (Waters Corp.) and the cartridge was washed with additional 5 mL of water. The [11C]ML128 was then eluted from cartridge with 1 mL of ethanol and passed through a sterile filter. The final product was diluted with 9 mL of 0.9% saline to make 10% ethanol solution in saline for animal injection.
[18F]FPEB: The synthesis of [18F]FPEB was conducted through a convenient thermal reaction as a highly specific PET radiotracer for mGlu5 (Wang et al., 2007). The precursor and standard compounds were prepared by a coupling reaction catalyzed by palladium. Radiosynthesis of [18F]FPEB was performed using nitro as a leaving group replaced by [18F]fluoride under conventional heating condition.
[11C]CFT: Radiosynthesis of [11C]CFT was performed as previously published (Brownell et al., 1996). 11C-methyliodide was produced in an automated system following proton bombardment of N2 containing 0.5% 02 to yield 11CO2. The 11CO2 was dried under a stream of N2 gas by heating. The AlLiO-11CH3 was treated with hydroiodic acid. The resulting 11C methyl iodide was then transferred as a gas using N2 as carrier to a reaction vial containing N-demethylated precursor in DMSO and the mixture was heated. At last [11C]CFT was purified up to 98%.
2.2.2. Experimental procedures
Experimental imaging studies were initiated one month after the lesioning with 6-OHDA. After a rat was anesthetized by gas (maintained at 1.5-2% isoflurane and oxygen flow of 2 L/min throughout the study), the tail vein was catheterized for administration of radiolabeled ligands. The head was adjusted securely in a stereotaxic head holder equipped with a gas inhalation system. The rat was placed ventrally into the scanner at the center of the imaging field, where the spatial resolution is 1.0 mm for the multimodality scanner, Triumph PET/CT/SPECT, TriFoil, Northridge, CA or 1.8 mm for microPET P4, Concorde Microsystems, Knoxville, TN, USA. The respiration rate, pulse oximetry and heart beat were monitored during the whole imaging time using the physiology system that comes with the scanner. In the P4-scanner physiology was measured via heart rate, pulse oximetry and respiration rate using Vet Ox 4800+ (Loveland, CO) and a transmission imaging was conducted using a rotating cobalt-57 point source before administering radioactivity to create maps for attenuation correction. In the Triumph scanner, an X-ray scanning was done following PET imaging to obtain data for the attenuation correction. PET imaging of dopamine transporter function was done using [11C]CFT (0.8-1 mCi (29.6-37 MBq) i.v., specific activity 1400 mCi/μmol (51.8 GBq/μmol)) to investigate degree of the lesion. Studies on mGlu4 and mGlu5 expression were conducted in the same imaging session. mGlu4 expression was imaged with [11C]ML128 (0.6-0.7mCi (22.2-25.9 MBq) i.v., specific activity 2720 mCi/μmol (100.64 GBq/μmol)) for 60 min followed 2 hours later imaging of mGlu5 expression with [18F]FPEB for 90 min (0.5-0.6mCi (18.5-22.2 MBq)i.v, specific activity 1900 mCi/μmol (70.3 GBq)).
PET imaging studies of dopamine transporter function and expression of mGlu4 and mGlu5 were repeated 4 times during the 14-months follow-up period to investigate progressive degeneration induced changes.
2.2.3. PET imaging and data processing
The progressive degeneration induced changes were investigated based on comparison of the left-right side differences of binding potential (BP) of [11C]CFT in the striatum and [18F]FPEB-BP and [11C]ML128 uptake in the striatum and hippocampus.
In this project, altogether fifty-seven PET imaging studies were conducted in the five 6-OHDA-lesioned rats at time-points 1, 5, 10, and 14 months after the 6-OHDA lesion. At each time-point, a series of PET imaging using [11C]CFT, [11C]ML128 and [18F]FPEB were performed on each animal. Based on the [18F]FPEB images of each animal, an image template was built with regions of interest (ROIs) drawn on striatum, hippocampus and cerebellum. Then [11C]CFT images and [11C]ML128 images were registered to the template image. After auto-registration by home-made scripts in MATLAB, all registered images were double-checked to confirm precise registration to the template images. After registration the time activity curves were extracted for each ROI from images of [11C]ML128, [18F]FPEB and [11C]CFT. The cerebellum was used as a reference region to calculate the BP values for [18F]FPEB and [11C]CFT with SRTM (Simplified Reference Tissue Model (Lammertsma and Hume, 1996). SRTM produced stable results of BP values without arterial blood sampling and time-consuming metabolite measurements. We found, using four different mGlu5 radioligands, that there was no apparent specific uptake in the cerebellum (Zhu et al., 2007). Another paper by Rook et al., (Rook et al., 2015) claimed some 20 % displacement of [18F]FPEB by another, cold mGlu5 ligand. However the time activity curves in the cerebellum in that paper (Fig. 2) show very little specific uptake in the cerebellum other than non-specific uptake that could have been displaced by the cold ligand. Further, mGlu5 expression measured in rats using protein immunostaining and western blots showed very little mGlu5 in cerebellum (Romano et al., 1995). Therefore, we used the cerebellum as a reference region. Because of the expression of mGlu4 in the cerebellum of rats we could not to use it as a reference tissue to analyze mGlu4 imaging data. Instead the uptake of [11C]ML128 in left- and right-sided striatum and hippocampus was calculated as a percent of the injected dose per cc (%ID/cc or %ID/g), using right sides' data as control.
Figure 2. phMRI studies in 6-OHDA rat model of PD after challenge of mGlu4 PAM or amphetamine.
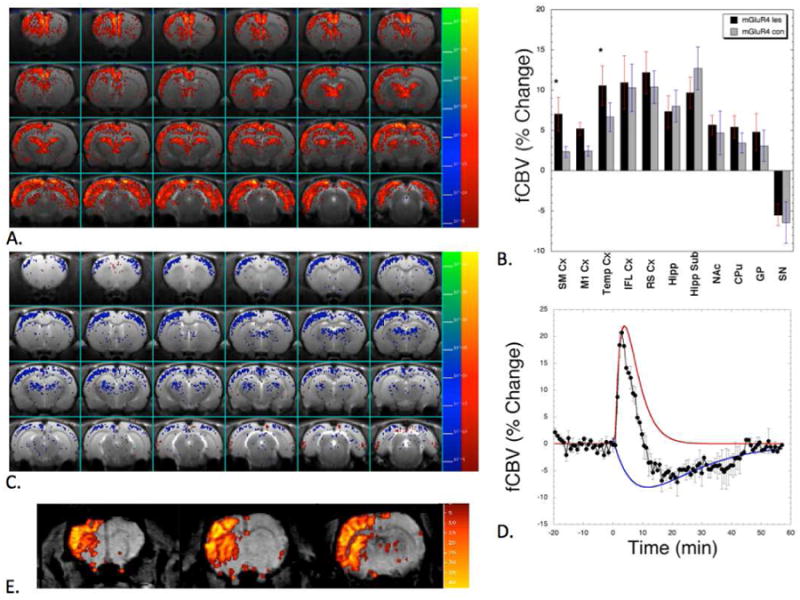
There were positive and negative CBV responses to the injection of the mGlu4 PAM (DFMPP). The initial response was positive as shown in the map (A) which shows a statistical map of the positive CBV response. Note the increased CBV on the lesion side (left) showing cortical upregulation of mGlu4 signaling. In the thalamus, hippocampus infralimbic, medial pre-frontal and retrosplenial cortices there were no differences between the lesion and non-lesion sides (B). For the positive component of the CBV there were significant (paired T-test) differences between the somatosensory and temporal cortices (*, p<0.05). A map of the negative component of the CBV induced by the mGlu4 PAM is shown in (C). The negative component of the CBV was more uniform on the lesion and intact sides and the differences were not significantly different. The decomposition of the CBV time course in the thalamus into the negative (blue) and positive components (red) used to make the maps in (A and C) is shown in (D). (E) shows an opposite CBV response after amphetamine induced dopamine release confirming that the dopamine release is abolished from the left lesion side while the intact right side has normal function. Comparing the images (A) and (E) provides a striking evidence of functional difference between glutamatergic and dopaminergic neurosystems.
After all left- and right-sided BP values of [18F]FPEB and [11C]CFT and tracer uptakes of [11C]ML128 of each rat were obtained the percent differences were calculated to compare the differences of dopamine transporter, mGlu5 and mGlu4 expression. The percentage equation was used to calculate as follows:
2.3. Pharmacological MRI (phMRI) studies
2.3.1. Preparation of animals for phMRI studies
Rats were anesthetized with 1.2% halothane in O2. The tail vein was catheterized for both contrast agent and drug administration. Body temperature was regulated using a circulating warm water blanket set to 37°C. Blood pressure was measured by using non-invasive blood pressure monitoring with an inflating tail cuffs (Kent Scientific, Torrington, CT). All procedures were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.3.2. Reagents in phMRI
Feraheme (paramagnetic iron oxide nanoparticles) was purchased from Amag Pharmaceuticals (Waltham, MA) and used as a contrast agent (10 mg/kg i.v.) to sensitize the images to cerebral blood volume (CBV). DFMPP, an mGlu4 PAM was synthetized in our laboratory and used (10 mg/kg i.p.) in phMRI studies. MTEP (3-((2-methyl-4-thiazolyl)ethynyl) pyridine), a negative allosteric modulator of mGlu5 was purchased from Tocris Bioscience and used in water-solution (10 mg/kg i.v.) as mGlu5 NAM in phMRI studies
2.3.3. Experimental procedures in phMRI
phMRI images were acquired from a 4.7T Oxford magnet with a Bruker console (Billerica, MA) for the mGlu4 (DFMPP) and mGlu5 (MTEP) studies. Rats were maintained at 37°C on 1.2% halothane/O2/Air mixture (30/70%) the same as for preparation. For these studies we followed the same protocol as published earlier for phMRI studies (Chen et al., 2001; Chen et al., 2011; Mandeville et al., 2014). Briefly, a conventional gradient echo sequence with TR/TE of 350/7.5 ms, flip angle of 40 degrees with one average was used to acquire phMRI data using a home-built single-loop transmit/receive coil of 1.5 cm diameter. Twenty-three contiguous coronal slices were obtained for each time point (45 s/image) with in-plane spatial resolution of 0.2 × 0.2 mm2 and a 1 mm slice thickness. We acquired four pre-MION baseline images followed by at least 25 post-MION images for the pre-drug baseline. Then, drugs were injected in the magnet during continuous imaging for another 60 min.
2.3.4. Data Analysis in phMRI
2.3.4.1. Image Co-registration
All images were registered onto the same standard brain template for subsequent averaging across animals. The data were resliced to a thickness of 0.5 mm. Since motion during a functional scan was not a consideration due to anesthesia and the use of a head holder, registration parameters were determined for each functional scan using a single full brain volume that was the average of all time points after MION injection but prior to drug injection. These registration parameters were then applied to all time points in the functional data set. Registration between the functional image set and the standard template was performed by adjusting 12 registration variables in a standard affine transformation as generally applied in human studies. The template images were consistent with the stereotactic coordinate system atlas from Paxinos (Paxinos and Watson, 1986). Then regions of interest (ROIs) can be drawn for any of the regions defined in the atlas.
2.3.4.2. Functional Analysis
Maps of percent CBV changes were obtained by converting signal intensity changes to ΔR2* on a pixel by pixel basis (Chen et al., 2001; Mandeville et al., 2004) and assuming a linear relationship between R2* relaxivity and blood volume. For CBV the transverse relaxation rate (R2*) was measured on a pixel by pixel basis using the standard formulation for gradient echo signal, R2*(t) = -ln(S(t)/S(0))/TE, where TE is the echo time and S(0) is the average value of the signal before MION. Data acquired after injection of MION were converted to percent change in functional CBV (fCBV (V)) using the change in transverse relaxation rate relative to the pre-contrast baseline: fCBV = V(t)/V(0) = R(t)/R(0) −1. Fits were made to the fCBV data using a GLM model and regressors for the positive and negative components using the jip program (http://www.nitrc.org/projects/jip). Then maps were made of p-values for the fits for significant changes of CBV corrected for a false-discovery rate of 5% adjusted for the number of pixels.
2.3.5. Statistics in phMRI
CBV values from regions of interest on the lesioned and intact hemispheres were obtained by integrating the fCBV values over the full width half maximum of the CBV fit to the positive CBV changes. Then these values were compared (lesion vs. intact) using a paired students t-test. Errors reported are standard deviations.
2.4. Immunohistochemical studies
Rat brains were postfixed in 4% paraformaldehyde and cryoprotected in 30% sucrose. The rat brains were sectioned coronally at 30μm using a freezing microtome. Free-floating sections were rinsed with phosphate buffered saline (PBS) with 0.3% TritonX-100 and blocked with 10% normal goat serum in PBS. After blocking brain sections were incubated with mGlu4 (1:500, ab53088, Abcam) and mGlu5 (1:1000, ab76316, Abcam) antibody overnight. The tissue was then rinsed in PBS with 0.3% TritonX-100 and incubated for 1 hour in biotinylated secondary antibody (1:200, Vector Labs, Burlingame, CA, USA). After secondary antibody sections were incubated for 1 hour in ABC (Vector Labs) solution at RT. Following incubation, sections were rinsed with PBS and were visualized by incubating in 3,3′-diaminobenzidine (DAB) substrate kit Vector Labs).
To quantify mGlu4 sections from the lesion and non-lesion side of the hippocampus were counted using the Optical Fractionator probe in the StereoInvestigator software (MBF Bioscience, Williston, VT) for each group (n=3). The region of interest was delineated at 4× magnification. A counting grid of 250×250 μm was placed over the hippocampus. Using 100×100 μm counting frame mGlu4 immunoreactive cells were counted in randomly-placed sampling sites with 40× magnification.
2.5. Binding assay
CHO-K1 cells expressing mGlu4 were used for all binding assay protocols. The binding of [3H]ML128 was measured with increased concentration (0, 10 nM, 100 nM, 1μM, 10 μM and 100 μM) L-Glutamate (Sigma-Aldrich). For the com petitive binding 10 nM of [3H]ML128 was used together with increased concentrations of the DFMPP ranging from 0.01 nM to 10 μM. 50000 freshly harvested CHO CHO-K1 cells expre ssing mGlu4 were transferred to test tubes. The HAMs was used as incubation buffer (10% FBS, 100 units/ml of penicillin/streptomycin and 1mM G418). [3H]ML128 (10 nM) was added to the cell with or without test compound on ice and incubated for 30 min at room temperature. Samples were centrifuged at 1200 rpm for 4 min at +4 °C and wash ed five times with cold Hams cell culture medium in prior of liquid scintillation counting. Samples were lysed by adding 100 μl of 0.5 % NaOH and heating at +56°C for 30 min. Samples were cooled with ice path and transferred to VWR® Solvent-Saver™ scintillation vials. To obtain binding parameters the scintillation liquid (Perkin Elmer, Optima Gold) was added (7 ml) in prior to counting in a Packard TriCarb Model (1 min/vial). Nonspecific binding was determined using 10 μM cold ML128 and specific binding was determined extracting the nonspecific binding from total binding. All measurements were done with three parallels and analyzed with GraphPad Prism software.
3. Results
3.1. Longitudinal PET imaging studies
In the PET studies using [11C]CFT (2β-carbomethoxy-3β-(4-fluorophenyl) tropane) we found that a unilateral nigral administration of 6-OHDA induced a decrease of dopamine transporter function in the striatum by 71± 5% (p<0.001) measured one month after the lesioning followed by progressive degeneration of 1.19±0.07% per month as compared to the control side (Figs. 1A, B; Fig. S.1.). Longitudinal imaging of mGlu4 and mGlu5 showed that presynaptically expressed mGlu4 was enhanced in the striatum on the lesioned side (12.1+/-10.8%, p<0.08) as an early response to 6-OHDA administration while mGlu5 expression at that time was decreased (-12.3+/-4.3%, p<0.005). However, during the following progressive degeneration mGlu4 expression decreased 123 +/- 110% (p<0.03) on the lesion side and mGlu5 expression recovered 46+/-48 % (p not significant) resulting an inverse significant (p<0.01) correlation between mGlu4 and mGlu5 expression in the striatum on the lesion side (Figs. 1 A, C; Figs. S.2., S. 3). In the hippocampus mGlu4 expression was enhanced 18.5+/- 11.6 % (p<0.05) as an early response to 6-OHDA lesioning but declined 102+/- 82 % (p<0.02) during progressive degeneration. mGlu5 expression was enhanced by 3.7 +/- 2.0 % (p<0.02) and did not change significantly during the whole follow up time of 14 months. There were no observable changes in dopamine transporter function or expression of mGlu4 and mGlu5 on the control (right) side during the progressive degeneration.
Figure 1. PET imaging studies of modulation of dopamine transporter function and expression of mGlu4 and mGlu5 in the brain of 6-OHDA lesioned rat during progressive degeneration.
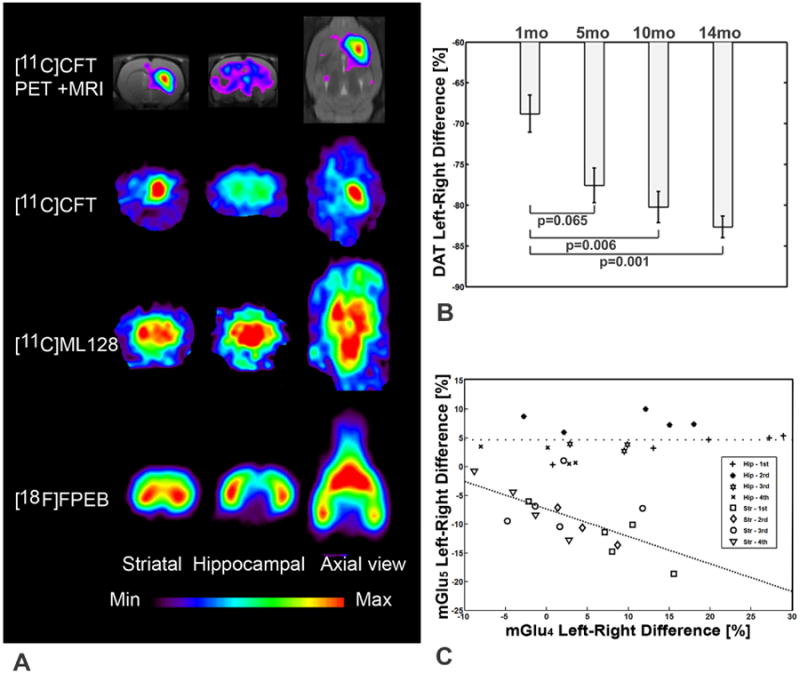
(A) Distribution of [11C]CFT ([11C]2β-carbomethoxy-3β-(4-fluorophenyl) on coronal (striatal and hippocampal level) and midstriatal axial slices shows abolished accumulation on the left (lesion) side. Distribution of [11C]ML128 (N-(4-chloro-3-[11C]methoxyphenyl)-2-picolinamide) on the same level as above shows enhanced accumulation on the left (lesion) side. Distribution of [18F]FPEB (3-fluoro-5-[(pyridin-3-yl)ethynyl] benzonitrile) on the same level as above shows decreased accumulation in the striatum and enhanced accumulation in the hippocampus on the left (lesion) side. (B) Exponential decline of dopamine transporter (DAT) binding of [11C]CFT in the striatum is an indication of progressive degeneration with the rate of 1.19±0.07 % per month. (C) Significant inverse correlation of mGlu4 and mGlu5 expression was observed in the lesioned striatum during progressive degeneration (ValuemGlu5 = (-0.478±0.477) × ValuemGlu4 - 7.34, r=0.647, p<0.01). mGlu4 expression declined in the hippocampus 102+/-82% while mGlu5 expression did not change during progressive degeneration.
3.2. phMRI studies
3.2.1. phMRI studies with mGlu4 PAM
phMRI studies were done using the IRON (Increased Relaxivity for Optimized Neuroimaging) technique to investigate the effects of mGlu4 stimulation on cerebral blood volume (CBV) using mGlu4 PAM, DFMPP. The mGlu4 PAM produced a biphasic pattern of CBV changes with a short increase in CBV followed by a longer decrease in CBV. The pattern of increased and decreased CBV varied somewhat around the brain as shown in the maps (Fig. 2). The increased CBV was highest in the subiculum, thalamus, retrosplenial cortex and infralimbic cortex with the negative CBV being strongest in somatosensory and motor cortices and thalamus. A map of the biphasic responses segregated into positive and negative CBV changes is shown in Fig. 2. Also shown in Fig. 2 are data from another 6-OHDA lesioned animal (not part of the current cohort) following challenge with 2 mg/kg amphetamine showing a robust response to amphetamine on the intact side, and little response on the lesioned side.
3.2.2. phMRI studies with mGlu5 negative allosteric modulator
We also conducted studies with the mGlu5 NAM MTEP. These data showed a pattern that was superficially similar to the PET studies with large uptake in the posterior ventral hippocampal regions and in the anterior striatal and infralimbic cortex region, although the limited spatial resolution of the PET images precludes a more precise comparison with the phMRI. There were also differences noted with the mGlu4 PAM phMRI data. mGlu5 NAM induced a positive CBV change in the substantia nigra compared to a negative change with the mGlu4 PAM. Further, there was much less change in medial prefrontal cortex. There were also significant differences between the lesion and control hemispheres showing a much larger increase on the lesion side in the retrosplenial cortex and a larger increase in somatosensory cortex on the lesion side. There were no other significant differences between the lesioned and control hemispheres with the MTEP. Quantitative values are reported in Fig. 3.
Figure 3. Maps of mGlu5 in 6-OHDA lesioned rats.
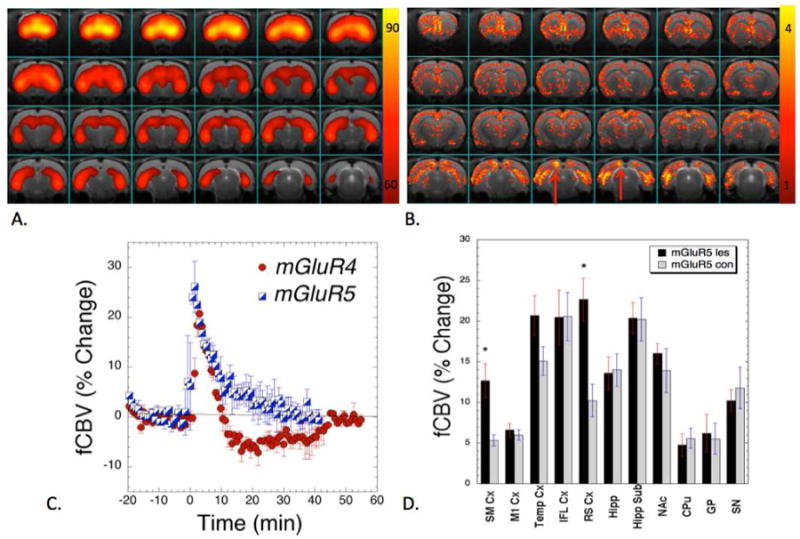
(A) PET image showing the uptake of [18F]FPEB normalized to the percent of the highest uptake in an animal 14 months after lesioning. There is extensive uptake in the medial prefrontal and infralimbic cortex, striatum as well as in the hippocampus. (B) An averaged map of T-values for significant changes due to stimulation with the mGlu5 negative allosteric modulator (MTEP). There are significant upregulations of the response on the lesion side in the SMCx and retrosplenial cortex (shown with the red arrows) compared to the intact side. The infralimbic cortex and hippocampus were relatively symmetric. (C) Comparison of fCBV changes induced by the mGlu5 and mGlu4 ligands showing only positive fCBV changes with the mGlu5 compound. (D) Plot of integrated changes in fCBV on the lesion versus the contralateral side (*, p<0.05; paired T-test).
3.3. Binding studies in mGlu4 expressing CHO cells
The binding density of tritium labeled ML128 was enhanced when the mGlu4 cells were exposed to higher concentrations of glutamate indicating strong positive co-operation between the allosteric binder and glutamate (Fig. 4A). ML128 is known to have PAM capacity to glutamate-activated mGlu4. Similar effects are found with the binding of PAMs and the co-operative factor is usually characteristic for each drug candidate (Fig. 4B). In addition, the cell studies showed for the first time, that the binding site of ML128 and its structural analog DFMPP is the same specific site of mGlu4 (Fig. 4C).
Figure 4. Evidence of cooperative nature of mGlu4 PAM binding site.
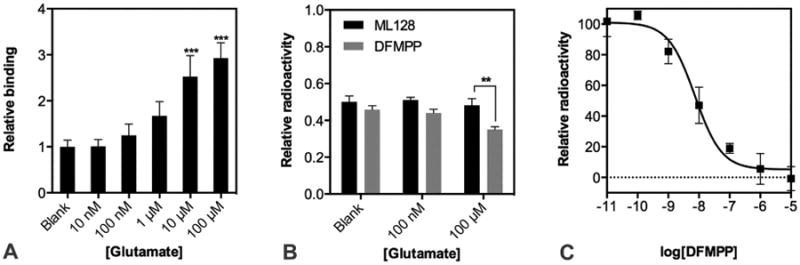
(A) Specific binding of 10nM [3H]ML128 to CHO expressing mGlu4 receptor was determined in the presence of increasing concentration of the natural agonist. Binding of [3H]ML128 without addition of glutamate is set as 1.0 and presented together with SD. (B) The ability of 10 nM cold ML128 or DFMPP to displace the [3H]ML128 (10nM) from its binding site was measured without or increased concentration of natural agonist. The binding of 10 nM [3H]ML128 was set as 1.0 and presented together with SD. (C) Competitive binding assay was done with single dose (10 nM) of hot [3H]ML128 and increased concentration of cold DFMPP. The specific radioactivity of 10 nM [3H]ML128 was set as 100 and presented together with SD. All measurements were performed in three parallels and analyzed with GraphPad Prism.
3.4. Ex vivo studies
After in vivo imaging studies immunohistochemical studies were conducted of mGlu4 and mGlu5 expression. Staining for mGlu4 expression in cortex and hippocampus showed decreased expression on the lesion side (Fig. 5A) supporting the results obtained by PET studies (Fig. 2C). However, immunostaining of mGlu5 expression did not show any significant change in cortex and hippocampus between the lesion and control side.
Figure 5. Representative pictures showing mGlu4 and mGlu5 immunostained rat brain.
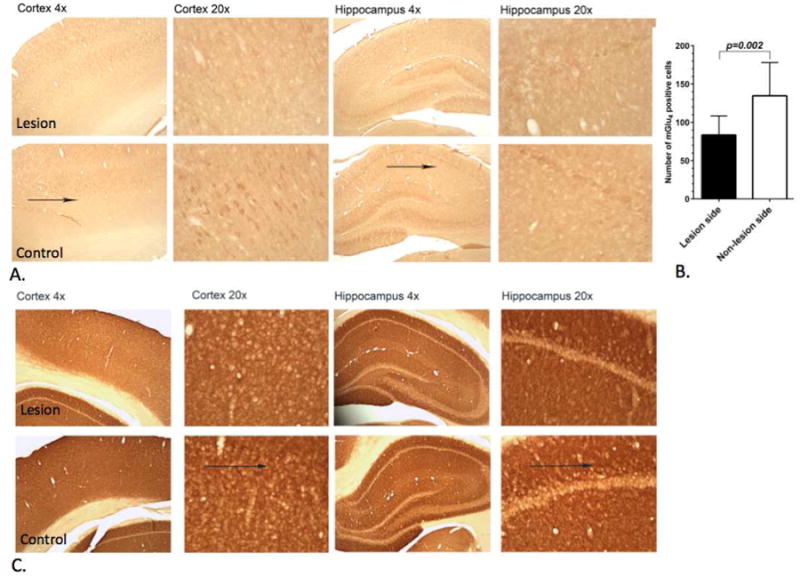
Sections from the lesion and control side of the cortex and hippocampus fourteen months after the unilateral administration of 6-OHDA into the left nigra (magnifications ×4, ×20). (a) Arrows indicate mGlu4 expression area. At that time point mGlu4 expression had slightly decreased in the lesion side in the cortex and hippocampus of rat brain compared to non-lesion side. (B) Quantitative analysis using a paired t-test showed significant (p<0.002) decrease of the mGlu4 expressing cells on the lesion side of the hippocampus. (C) Immunostaining of mGlu5 expression did not show a significant difference between lesion and control side.
4. Discussion
4.1. PET studies of modulation of mGlu4 and mGlu5 expression during neurodegeneration
Our experiments support the result that in PD as well as in experimental PD models, the loss of DA-mediated inhibition in the striatum results in excess excitatory transmission (Johnson et al., 2009). In our studies longitudinal dopaminergic degeneration was investigated with PET imaging studies using [11C]CFT which is a selective and sensitive biomarker for dopamine transporter. These studies showed that unilateral (left side) nigral administration of 6-OHDA induced a decrease in dopamine transporter binding by 71±5% in the lesion side followed by progressive degeneration with a rate of 1.19±0.07% per month (Figs. 1A, B). However, 6-OHDA administration induced an enhanced mGlu4 expression as an early response as well as a decrease of postsynaptic mGlu5 expression in the striatum on the lesion side. PET imaging studies of mGlu4 expression were done with [11C]ML128 and mGlu5 expression with [18]FPEB. After the immediate effect mGlu4 expression gradually declined during progressive dopaminergic degeneration while mGlu5 expression recovered. This is the first study to show inverse modulation of mGlu4 and mGlu5 expression in the striatum as a result of the unilateral nigral administration of 6-OHDA. mGlu4 expression was vulnerable also in the hippocampus. As an early response mGlu4 expression was enhanced 18.5 +/-11.6% in the lesion side as compared to the intact side. However, during the progressive dopaminergic degeneration mGlu4 expression declined in the hippocampus almost as much as in the striatum on the lesion side (102+/-82% vs 123+/-110%). mGlu5 expression was enhanced about four percent in the hippocampus on the lesion side and did not change during the 14 months follow-up time. It should be noted that the observed changes were only on the left side when the 6-OHDA was administered into the left nigra. The [11C]CFT, [11C]ML128 and [18F]FPEB binding did not change on the right intact side in the striatum or hippocampus during the follow-up time. Immunohistochemical studies (Fig. 5) supported PET results of decreased mGlu4 expression on the left lesion side compared to the right control side in the striatum after 14 months of neurodegeneration period.
Our data showed that glutamate levels had a significant effect on drug binding (Fig. 4). However, the extracellular glutamate concentrations are not essential for baseline PET imaging studies in a rat model of PD by two reasons. Firstly, in the PET imaging studies using [11C]ML128 (EC50 of 110 nM) the drug content was 0.5-0.8 nmol/kg and using [18F]FPEB (Kd =0.1-0.15 nM) correspondingly 0.7-0.9 nmol/kg while in phMRI studies the content of DFMPP (EC50 of 80 nM) was 33.3 μmol/kg and MTEP 42.2 μmol/kg. These studies demonstrate that orthosteric glutamate concentration does not effect on the results obtained by PET studies of expression of mGlus. Secondly, the extracellular levels of glutamate have been reported to be about 0.5 – 0.6 M in 6-OHDA lesion rat model. Interestingly, there is no significant difference with extracellular glutamate levels between the lesion and control rats including the striatum area (Robelet et al., 2004). In the PET studies only nanomolar levels of the labeled compounds were used so the results obtained (binding values) are not likely affected by endogenous glutamate (Fig. 4). However, if the allosteric modulators are used with higher doses as drug candidates, it is possible that changes in endogenous glutamate levels could alter drug function. In addition, chronic L-dopa treatment has been shown to increase the extracellular glutamate levels in globulus pallidus synapses. Therefore the increased glutamate levels are more likely be indirectly linked to modification of adaptive properties of glutamate synapses and the glutamate receptors' interactions with other neurotransmitter systems. Additionally, it has been demonstrated that mGlu4 activation could affect excitatory neurotransmission from the STN to the SNc, suggesting that mGlu4 activation could reduce glutamate release onto dying dopaminergic neurons. mGlu4 activation at the striatopallidal area reduces GABA release, which is predicted to normalize basal ganglia output. This leads to the hypothesis that mGlu4 activation may mimic the actions of dopamine in the indirect pathway by inhibiting GABA release at the striatum–globulus pallidus synapse. In addition, the mGlu5 antagonists or NAMs appear particularly promising for the treatment of L-DOPA-induced dyskinesia, a frequent invalidating complication of DA replacement therapy that represents an abnormal form of synaptic plasticity. Finally, pharmacological manipulation of mGlus could open up new modalities of neuroprotection for PD and other degenerative diseases of the nervous system, by decreasing excitotoxicity, modulating signaling pathways or through local translation of trophic factors.
4.2. Allosteric modulators as drug candidates
During the last decade there has been an increasing interest of finding new molecules to target mGlus (Nicoletti et al., 2010; Zhang and Brownell, 2012). Competitive ligands bind to the same orthosteric binding site with Glu in the extracellular Venus flytrap domain in a competitive manner, while other ligands bind to the target protein with non-competitive binding mode (Niswender et al., 2010). All of these compounds may modulate the receptor activity as agonists, antagonists, or positive and negative modulators (Layton, 2005; Niswender et al., 2010). The binding affinities correlate with the agent's ability to stabilize the inactive or active receptor conformation (Kew, 2004). The first functional mGlu compounds were analogs of Glu or glysine derivatives, which were targeting the well characterized orthosteric binding site (Gasparini and Spooren, 2007). However, these ligands suffer from poor receptor selectivity as well as from poor ability to cross the blood brain barrier and leave relatively small amount of possibilities for drug optimization (Carroll, 2008).
During the last two decades there has been a great interest to find non-competitive allosteric modulators to overcome the negative issues with competitive agents (Annoura et al., 1996; Conn et al., 2014; Ritzen et al., 2005). Allosteric binding sites are usually located in seven transmembrane domains, which are distinct from the Glu binding site (Williams and Lindsley, 2005). The structurally more diverse transmembrane domain enabled better receptor sub-type selectivity compared to more homogenous Glu binding site (Kew, 2004). In addtion, the allosteric binding sites expanded the variety of molecular scaffolds in drug design, which led to advanced drug and imaging agents with better blood brain barrier penetration, better sensitivity, higher affinity and overall advanced drug properties and therapeutic effects compared to the classical orthosteric ligands (Conn et al., 2009; Wang et al., 2007; Hamill et al., 2005; Patel et al., 2007).
In this project we used for PET imaging of mGlu4 [11C]ML128 with EC50 of 110 nM and [18F]FPEB to investigate mGlu5 (Kd =0.1-0.15 nM). In phMRI studies we used DFMPP with EC50 of 80 nM.
4.3. phMRI studies of hemodynamic response in 6-OHDA rat model of PD
As seen in Fig. 2, after challenging with positive allosteric modulator of mGlu4 we used a general linear model fitting to map the regionally increased (Fig. 2A) and decreased CBV (Fig. 2C) areas in the 6-OHDA lesioned animals. There were a number of brain regions that showed similar CBV increases on both the control and lesion sides such as the infralimbic and medial prefrontal cortices, hippocampus and restrosplenial cortex. On the other hand there were regions that showed a much larger response on the lesion side including sensorimotor cortex and temporal cortex. The negative CBV changes are mostly confined to cortical and thalamic regions and there were no statistically significant differences between the control and lesion side. A similar map is shown for the negative allosteric modulator of mGlu5, MTEP, in Fig. 3. For comparative purposes, a map of a PET imaging (Fig. 3A) of expression of mGlu5 using a negative allosteric modulator, [18F]FPEB is shown overlaid on the MR image verifying enhanced expression in the prefrontal cortex and hippocampus. Although the phMRI map (Fig. 3B) of MTEP is superficially similar to that of the mGlu4 PAM there are some major differences. First, there is less activation in the medial prefrontal cortex and thalamus compared to mGlu4 agonism consistent with prior studies of the distributions of these receptors (Phillips et al., 1997; Romano et al., 1995). Second, there is a much larger increase in the retrosplenial cortex on the lesion side compared to the intact side unlike for mGlu4 agonism where there is a similar response on both sides. Further, for mGlu4 PAM there is a negative CBV response in the substantia nigra compared to a positive reponse for mGlu5 NAM. There are similar increases on the lesion compared to the intact side in the cortex (SMCx and temporal) as were noted with the mGlu4 PAM. Unlike for the mGlu4 PAM, there were no regions that showed decreased CBV (Figs. 3B, C). Plots of the CBV changes in the intact and lesion sides are shown in Fig. 3D.
The response of an indirect metabolic marker like CBV to mGlu4/5 agonism or antagonism is difficult to predict. mGlu4 agonism can lead to inhibition of adenylate cyclase (Pin and Duvoisin, 1995) but not in all brain regions, as well as decreased glutamate release, however in concert with GABA(A) receptor activation there can be an increase in glutamate release (Antflick and Hampson, 2012). Thus, there is not a simple prediction one can make about the effects of such agonism upon hemodynamic markers. Further, most mGlu4 compounds also show high affinity for mGlu8 (Nicoletti et al., 2011). The inhibition of adenylate cyclase often (but not exclusively) noted for mGlu4 agonism would be predicted to lead to decreased CBV as is seen for dopamine D2/D3 agonists while the increased adenylate cyclase activity caused by D1/D5 agonists leads to increased CBV (Choi et al., 2006; Jenkins, 2012). Therefore, the negative component of the CBV changes noted with the mGlu4 compound may reflect this aspect of the drug. This does not provide an explanation of the increased CBV component noted with mGlu4 agonism, but this may be operating through the inhibition of GABAergic output to the pallidum as noted in both monkeys and rats (Bogenpohl et al., 2013; Valenti et al., 2003). Both the PET and immunohistochemistry show decreased mGlu4 on the lesioned side. The fact that there is an increase in phMRI signal on the lesioned side means that the phMRI is measuring functional output from mGlu4 signaling rather than merely receptor numbers. The fact that this signal increase on the lesioned side is occurring only in sensory and motor cortex is not incongruent with the motor behavioral amelioration produced by mGlu4 PAMs in prior studies of 6-OHDA lesioned rats (Valenti et al., 2003). As we showed in prior studies, there is decreased signal seen with DA signaling in the sensorimotor cortex in the 6-OHDA model (Fig. 2 and (Nguyen et al., 2000)) thus the decreased inhibition of the pallidum may lead to increased cortical signal with mGlu4 stimulation. Thus this remains a topic for future study.
There is good evidence to suggest that MTEP can act as a positive allosteric modulator at mGlu4 in the micromolar range (Mathiesen et al., 2003). mGlu5 does not couple through adenylate cyclase like mGlu4, but rather through phospholipase leading to a cascade of signaling (Pin and Duvoisin, 1995). The mGlu5 agonist, 1S,3R- ACPD, led to constriction of mouse arterioles in cortical slices (He et al., 2012) suggesting that mGlu5 antagonists might increase vasodilatation as noted in the CBV data shown in Fig. 3. We note that this effect may be mediated through astrocytic mGlu5 receptors rather than neuronal mGlu5 receptors (see Fig. 6; and (He et al., 2012)). mGlu5 is upregulated on the cortex on the lesion side in 6-OHDA lesioned animals (Nandhu et al., 2011) even though it appears to be down-regulated on the lesion side on the basal ganglia. Thus, this may explain the increased CBV on the lesioned side in the cortex with the MTEP shown in Fig. 3.
Figure 6. The synaptic interplay of mGlu4, mGluR5 and calcium dependent vasoactive signaling.
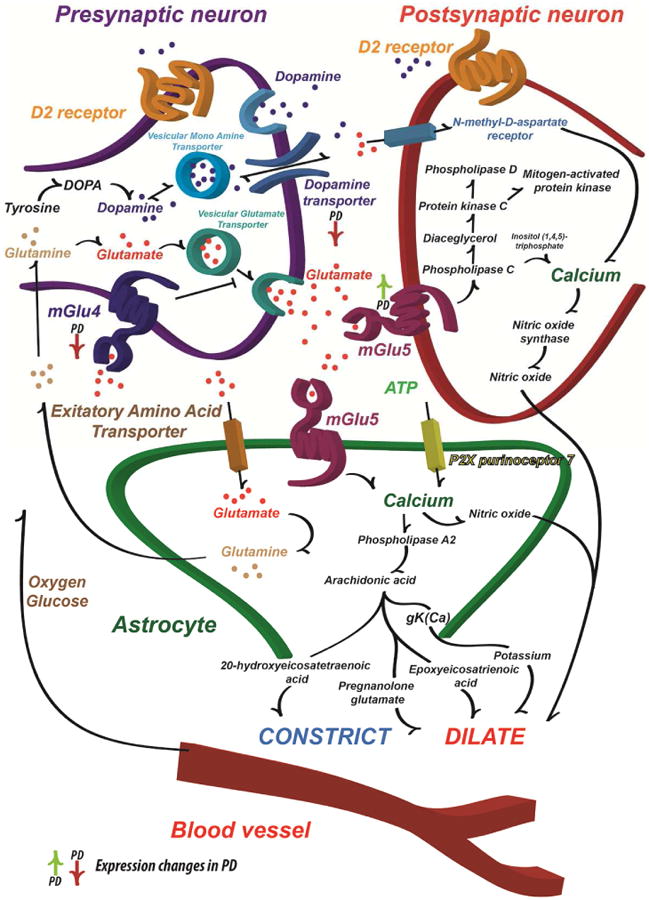
Schematic illustration of the major DA and Glu synaptic effects and how these may influence cerebral blood flow. The primary pathophysiological change that leads to the motor symptoms of PD is the loss of dopaminergic neurons in the substantia nigra pars compacta that modulates the function of the striatum and other basal ganglia structures. The L-DOPA is considered the standard of care for providing symptomatic relief for PD patients. However, long-term L-DOPA treatment leads to the appearance of motor complications (Ahlskog and Muenter, 2001). On the other hand, the excessive Glu signaling in the basal ganglia is one of the hallmarks of the progression of PD. The mGlu (especially mGlu4 and mGlu5) modulators were considered to be one of the best potential drug candidates since the striatum also sends direct projections to the substantia nigra pars compacta, which leads to a parallel increase in Glu release, GABA signaling and contributes to the progressive neuronal degeneration also via an excitotoxic mechanism (Gasparini et al., 2013). The hyperactive Glu stimulus demands energy and couples the hyperactivation tightly with blood flow (Lauritzen et al., 2012). It has been suggested that the astrocytes are able to detect the Glu driven synaptic activity and thus modulate the local blood flow (Attwell et al., 2010). In addition, ATP and its derivative adenosine modulates the blood flow (Latini and Pedata, 2001) as does DA acting on both astrocytes and blood vessels (Choi et al., 2006). Together with dopamine transporter, the synaptic contacts possess a variety of neurotransmitter receptors, including both ionotropic and metabotropic glutamate receptors (Beaulieu and Gainetdinov, 2011). Activation of Glu receptors by synaptically released Glu is known to lead to transient and graded increases in intracellular Ca2+ concentration (Marambaud et al., 2009). Since the Glu signaling is hyperactive in PD, it is assumed that the inhibition of Glu signaling should decrease the PD symptoms, the synaptic activity and eventually lead to decrease of the local blood flow due to decreased energy demand and Ca2+ signaling in pathological site (Attwell et al., 2010; Lauritzen et al., 2012).
The results we obtained provide compelling evidence of mGlu ligand induced blood volume changes in different brain areas in normal and lesion side. Unfortunately, there are no other published studies in PD models to compare our results. Two prior papers used mGlu ligands with ph/fMRI. Sloan et al. showed that the MPEP did not block either BOLD or CBV changes in motor cortex caused by hind-paw stimulation (Sloan et al., 2010) but they did not present results for MPEP alone so it is hard to compare to our data. Simon et al. (Simon et al., 2011) examined mGlu1 and mGlu5 drugs, but they used a fast-spin echo sequence that would be expected to have about 24 times lower sensitivity than the IRON technique that we used, so again it is hard to compare results. Interestingly, our results showed that there was little evidence of a large response in the basal ganglia except in the anterior dorso-medial caudate-putamen a region associated with innervation of motor cortex. Thus, this demonstrates again that we are most likely seeing “signaling” rather than a read-out of receptor numbers using the phMRI. This makes it a complementary tool to the PET studies.
We have previously published on both decreased pre-synaptic dopamine transporters as well as upregulation of dopamine receptors using combined PET and phMRI studies in both non-human primate and rodent models of PD (Brownell et al., 1998a; Brownell et al., 1998b; Chen et al., 1997; Jenkins et al., 2004; Nguyen et al., 2000). Similar to what was observed here, with the non-selective DA agonist apomorphine, we saw an increase in signal on the lesioned side.
A model for interpretation of the hemodynamic changes is outlined below.
5. Conclusions
These are the first studies to demonstrate an inverse correlation of mGlu4 and mGlu5 expression in the striatum during progressive degeneration in the lesion side induced by the unilateral administration of 6-OHDA into the left nigra. As well these are the first studies to demonstrate mGlu4 PAM and mGlu5 NAM induced hemodynamic changes in several brain areas in normal and lesion side also demonstrating higher hemodynamic response on the lesion side. These studies showed that, if the allosteric modulators are used with pharmacological doses as drug candidates, endogenous glutamate level has significant effect on drug response. These experiments also demonstrate the importance of the longitudinal in vivo imaging studies to investigate temporal changes in receptor functions to obtain individual response for experimental drugs.
Supplementary Material
Highlights.
Modulation of mGlu4 and mGlu5 was found on the lesion side of 6-OHDA rat
mGlu4 and mGlu5 have inverse correlation during progressive striatal degeneration
mGlu4 PAM and mGlu5 NAM induced hemodynamic changes enabling phMR imaging
PAM and NAM induced signal changes in phMRI are complementary to PET imaging of mGlus
Endogenous glutamate modulates dose response and binding of allosteric modulators
Acknowledgments
Funding by NIH grants NIBIB-R01EB012864 and NIMH-R01MH91684 to ALB is acknowledged. PP was supported by the Orion Farmos Research Foundation and Kuopio University Foundation.
Footnotes
Author contribution: ALB and AZ designed the study. BGJ, AZ, PP, JKC, DK, NA, AD and ALB conducted the experiments. KEK and ZZ prepared the imaging ligands. BGJ, AZ, JKC and PP analyzed the data. BGJ, AZ, PP and ALB wrote the paper.
Competing financial interest statement: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Antflick JE, Hampson DR. Modulation of glutamate release from parallel fibers by mGlu4 and pre-synaptic GABA(A) receptors. J Neurochem. 2012;120:552–63. doi: 10.1111/j.1471-4159.2011.07611.x. [DOI] [PubMed] [Google Scholar]
- Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert I, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bogenpohl J, et al. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electrophysiological effects of activation in the striatopallidal complex. Neuropharmacology. 2013;66:242–52. doi: 10.1016/j.neuropharm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, et al. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999;407:33–46. [PubMed] [Google Scholar]
- Brownell AL, et al. Cocaine congeners as PET imaging probes for dopamine terminals in normal and MPTP induced parkinsonism in nonhuman primate brain. J Nucl Med. 1996;37:1186–1192. [PubMed] [Google Scholar]
- Brownell AL, et al. In vivo PET imaging in rat of dopamine terminals reveals functional neural transplants. Ann Neurol. 1998a;43:387–390. doi: 10.1002/ana.410430318. [DOI] [PubMed] [Google Scholar]
- Brownell AL, et al. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat Med. 1998b;4:1308–12. doi: 10.1038/3300. [DOI] [PubMed] [Google Scholar]
- Chen YC, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–98. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- Chen YI, et al. Improved mapping of pharmacologically induced neuronal activation using the IRON technique with superparamagnetic blood pool agents. J Magn Reson Imaging. 2001;14:517–24. doi: 10.1002/jmri.1215. [DOI] [PubMed] [Google Scholar]
- Chen YI, et al. Cocaine self-administration leads to alterations in temporal responses to cocaine challenge in limbic and motor circuitry. Eur J Neurosci. 2011;34:800–15. doi: 10.1111/j.1460-9568.2011.07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, et al. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–12. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, et al. Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci. 1999;11:2073–2082. doi: 10.1046/j.1460-9568.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Di Paolo T, Gomez-Mancilla B. Metabotropic glutamate receptors for Parkinson's disease therapy. Parkinsons Dis. 2013;2013:196028. doi: 10.1155/2013/196028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, et al. Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol. 2004;74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- He L, Linden DJ, Sapirstein A. Astrocyte inositol triphosphate receptor type 2 and cytosolic phospholipase A2 alpha regulate arteriole responses in mouse neocortical brain slices. PLoS One. 2012;7:e42194. doi: 10.1371/journal.pone.0042194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG, et al. Mapping dopamine function in primates using pharmacologic magnetic resonance imaging. J Neurosci. 2004;24:9553–60. doi: 10.1523/JNEUROSCI.1558-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): imaging drug action in the brain. Neuroimage. 2012;62:1072–85. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:475–91. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil KE, et al. Radiosynthesis of N-(4-chloro-3-[(11)C]methoxyphenyl)-2-picolinamide ([(11)C]ML128) as a PET radiotracer for metabotropic glutamate receptor subtype 4 (mGlu4) Bioorg Med Chem. 2013;21:5955–62. doi: 10.1016/j.bmc.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–84. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, et al. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62:1040–50. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, et al. Exogenous contrast agent improves sensitivity of gradient-echo functional magnetic resonance imaging at 9.4 T. Magn Reson Med. 2004;52:1272–81. doi: 10.1002/mrm.20278. [DOI] [PubMed] [Google Scholar]
- Mandeville JB. IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage. 2012;62:1000–8. doi: 10.1016/j.neuroimage.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JB, et al. Data collection and analysis strategies for phMRI. Neuropharmacology. 2014;84:65–78. doi: 10.1016/j.neuropharm.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, et al. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–30. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, et al. Substituted 3-phenyltropane analogs of cocaine: synthesis, inhibition of binding at cocaine recognition sites, and positron emission tomography imaging. J Med Chem. 1993;36:855–62. doi: 10.1021/jm00059a010. [DOI] [PubMed] [Google Scholar]
- Nandhu MS, et al. Enhanced glutamate, IP3 and cAMP activity in the cerebral cortex of unilateral 6-hydroxydopamine induced Parkinson's rats: effect of 5-HT, GABA and bone marrow cell supplementation. J Biomed Sci. 2011;18:5. doi: 10.1186/1423-0127-18-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, et al. Detection of the effects of dopamine receptor supersensitivity using pharmacological MRI and correlations with PET. Synapse. 2000;36:57–65. doi: 10.1002/(SICI)1098-2396(200004)36:1<57::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–41. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, et al. Parkinson's disease cognitive network correlates with caudate dopamine. Neuroimage. 2013;78:204–9. doi: 10.1016/j.neuroimage.2013.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Phillips T, et al. Localization of mGluR4 protein in the rat cerebral cortex and hippocampus. Neuroreport. 1997;8:3349–54. doi: 10.1097/00001756-199710200-00031. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Robelet S, et al. Chronic L-DOPA treatment increases extracellular glutamate levels and GLT1 expression in the basal ganglia in a rat model of Parkinson's disease. Eur J Neurosci. 2004;20:1255–66. doi: 10.1111/j.1460-9568.2004.03591.x. [DOI] [PubMed] [Google Scholar]
- Romano C, et al. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–69. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rook JM, et al. Relationship between in vivo receptor occupancy and efficacy of metabotropic glutamate receptor subtype 5 allosteric modulators with different in vitro binding profiles. Neuropsychopharmacology. 2015;40:755–65. doi: 10.1038/npp.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, et al. In vivo evidence of D3 dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiol Dis. 2007;27:220–7. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, et al. Allosteric modulation of metabotropic glutamate receptors. Adv Pharmacol. 2011;62:37–77. doi: 10.1016/B978-0-12-385952-5.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, et al. MRI analysis of mGluR5 and mGluR1 antagonists, MTEP and R214127 in the cerebral forebrain of awake, conscious rats. Neurosci Lett. 2011;505:155–9. doi: 10.1016/j.neulet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Sloan HL, et al. Regional differences in neurovascular coupling in rat brain as determined by fMRI and electrophysiology. Neuroimage. 2010;53:399–411. doi: 10.1016/j.neuroimage.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Valenti O, et al. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J Neurosci. 2003;23:7218–26. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, et al. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61:951–61. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- Zhu A, et al. Evaluation of four pyridine analogs to characterize 6-OHDA-induced modulation of mGluR5 function in rat brain using microPET studies. J Cereb Blood Flow Metab. 2007;27:1623–31. doi: 10.1038/sj.jcbfm.9600461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


