Abstract
This review provides the first comprehensive overview of the use of both nanoparticles and nanofibers for topical drug delivery. Researchers have explored the use of nanotechnology, specifically nanoparticles and nanofibers, as drug delivery systems for topical and transdermal applications. This approach employs increased drug concentration in the carrier, in order to increase drug flux into and through the skin. Both nanoparticles and nanofibers can be used to deliver hydrophobic and hydrophilic drugs and are capable of controlled release for a prolonged period of time. The examples presented provide significant evidence that this area of research has—and will continue to have — a profound impact on both clinical outcomes and the development of new products.
Keywords: Nanoparticles, Electrospun fiber mats, Nanofibers, Skin, Topical, Drug delivery
1. Introduction
1.1. Skin structure
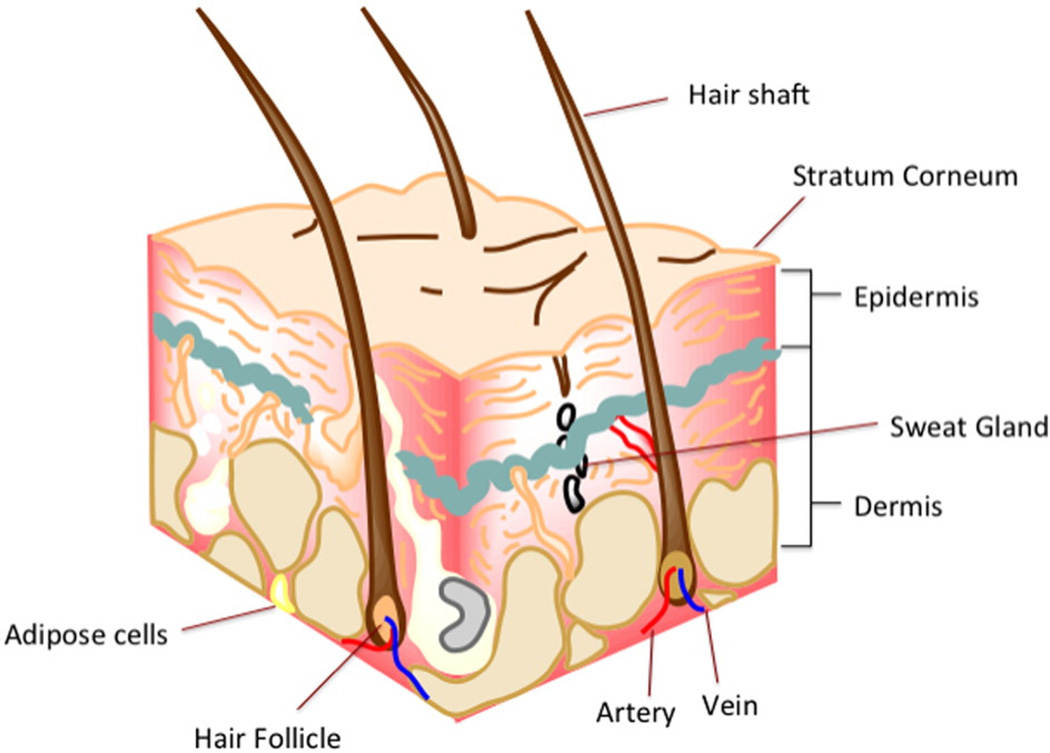
The skin is the largest organ of the human body and performs several critical functions: It protects the body from the external environment, serving as both a first line of defense against the entry of chemicals and microorganisms as well as providing a barrier to the loss of fluids and salts and helps regulate body temperature. Human skin can vary widely in structure and thickness, but is usually about 1.5 mm thick and consists of three layers (Fig. 1) [1]: (i) The epidermis consists of the stratum corneum and the viable epidermis. The stratum corneum (SC) is the outermost layer of the skin. It is largely responsible for the barrier function of the skin and the poor absorption of drugs. It is comprised of a 10–20 µm thick matrix of dehydrated and dead corneocytes (terminally differentiated keratinocytes) that are embedded in highly ordered lipid layers [2]. The viable epidermis (usually about 0.06–0.8 mm thick) is immediately under the SC and represents the first layer of living cells. It consists of about 4–5 layers of dermal fibroblasts and keratinocytes. (ii) Below the epidermis is the dermis, usually a 0.3–5 mm thick layer that contains connective tissue, sweat glands, hair follicles, and a network of capillaries, lymphatic vessels and nerve endings. (iii) The deepest layer of the skin is the subcutaneous tissue or hypodermis, which is composed of loose, white, fibrous connective tissue in which fat provides cushioning [3].
Fig. 1.
A schematic of the structure of the skin illustrating the stratum corneum, the epidermis and the dermis. The hypodermis is not included in this diagram. Image created using Chem Bio Draw Ultra version 14.
1.2. Drug delivery and the skin
The large surface area of the skin (nearly 20 square feet) makes it a potentially interesting route for drug delivery. Topical drug delivery is primarily intended for a local effect, where it can (i) potentially eliminate the need for systemically administered drug therapies, (ii) minimize the total dose required to reach the targeted site (skin), and (iii) reduce off-target adverse effects. Some examples of topical dermatologic products include cutaneous anti-fungal treatments, sunscreens, keratolytic agents, local anesthetics, antiseptics and anti-inflammatory agents for skin diseases such as psoriasis. Transdermal drug delivery, on the other hand, is intended for a systemic drug effect with the skin merely being the drug's entry portal into the body. For example, transdermal patches are used to deliver nicotine for smoking cessation, buprenorphine and fentanyl for chronic pain, and hyoscine (also known as scopolamine) for motion sickness.
In both topical and transdermal drug delivery, drug permeation through the SC is regulated by Fick's second law [4]:
| (1) |
where J is the flux, D is the diffusion coefficient of the drug, C is the drug concentration in the carrier, P is the drug partition coefficient, and L is the thickness of the SC. Beyond the SC, the applied drug must penetrate the various layers of the skin, encountering both lipophilic and hydrophilic domains as it travels toward the dermis. Human skin equivalents with lipid and permeability profiles closer to human skin have been developed as alternatives to human cadaver and animal skin to characterize in vitro drug permeation [5]. Depending on the drug's properties and the method of delivery, the drug may remain local or traverse into the dermis, where, if hydrophilic, it may be rapidly absorbed into the circulation via capillaries. The capillary bed extends into the upper layers of the dermis right underneath the dermato-epidermal junction.
In general, small molecules (both lipophilic and hydrophilic) can penetrate the SC without assistance or traverse the epidermis through shunt pathways created by sweat glands and hair shafts. In contrast, the delivery of larger molecules, such as peptides, proteins, siRNA, or DNA, through the skin continues to be a major research challenge. Therefore, the scientific community has been studying ways to facilitate and enhance the penetration of a wide range of drugs through the SC (for topical delivery) or to assist drug molecules to traverse into the dermis (for transdermal delivery). Some of these strategies are briefly described below.
Chemical enhancers, such as fatty acids, surfactants, esters, alcohols, polyalcohols, pyrrolidones, amines, amides, sulfoxides, terpenes, alkanes and phospholipids [3,6], can be used to enhance topical drug penetration by: (i) perturbing the highly ordered structure of the SC; (ii) solubilizing and extracting the keratin and/or lipid components of the SC; (iii) fluidizing the crystalline structure and dissolving the lipids of the SC; or (iv) improving the partitioning of a drug, co-enhancer or solvent into the SC [3,6,7]. Unfortunately, chemical penetration enhancers tend to be skin irritants, due to their disruptive effect on the lipid structures of the skin. Usually, the amount of enhancer required to achieve pharmacologically effective drug penetration rates is higher than the concentration tolerated by skin [6]. For this reason, the utility of chemical penetration enhances has so far been limited.
Physical enhancers use physical methods to temporarily compromise the integrity of the skin's barrier function, such as hydrating the skin via occlusion, sonophoresis, laser and or thermal ablation, electroporation, radiofrequency treatment, iontophoresis, magnetophoresis, high pressure jets, and microneedle arrays [6,8]. Although some of these technologies have been translated into FDA-approved products for topical and transdermal drug delivery, physical enhancers are invasive, may cause longer-term damage to the skin's barrier properties, and may produce tingling, irritation and burning sensations [9].
As an alternative, researchers have explored the use of nanotechnology, specifically nanoparticles and nanofibers, as drug delivery systems for topical and transdermal applications. This approach increases the drug concentration in the vehicle or formulation (Cv), and as a result, increases drug flux (see eq. (1)). Both nanoparticles and nanofibers can be used to deliver hydrophobic and hydrophilic drugs and are capable of controlled release for a prolonged period of time. In this review, we provide an overview of nanoparticles and nanofibers that have been developed for topical drug delivery applications. While nanoparticle and nanofibers drug delivery systems have been reviewed before separately [10–12], as far as the authors are aware this is the first comprehensive review that combines nanofiber and nanoparticle systems. Together, these systems have significantly impacted the treatment of many prevalent dermatologic conditions and have collectively generated substantial commercial revenues [8]. The examples of nanoparticles and nanofibers presented herein as topical or transdermal drug delivery systems, provide significant evidence that this area of research has — and will continue to have — a profound impact on both clinical outcomes and the commercialization of these technologies.
2. Nanoparticles for topical therapy
Nanoparticles (also sometimes referred to as nanocarriers) have been investigated for various biomedical applications for over a decade. In general, the use of nano-sized particles offers several advantages over other drug delivery systems. They are used to (i) enhance the solubility of highly hydrophobic drugs; (ii) provide sustained and controlled release of encapsulated drugs; (iii) increase the stability of therapeutic agents by chemical or physical means; (iv) deliver higher concentrations of drugs to target areas due to an Enhanced Permeation and Retention (EPR) effect; and (v) provide targeted treatments when modified with cell-specific ligands. Drug-loaded nanoparticles often accumulate in hair follicles and thereby facilitate the penetration of drug molecules through the superficial layers of the SC, followed by drug release into the deeper layers of the skin. The most commonly used nanoparticles for topical and/or transdermal drug delivery are polymeric nanoparticles, nano-emulsions, lipid-based nanoparticles (liposomes and solid-lipid nanoparticles), metal nanoparticles and dendrimers. The following section discusses the different types of nanoparticles and their potential uses in various topical therapeutic applications (see Table 1 for a summary).
Table 1.
Types of nanoparticles and their clinical uses as topical drug delivery vehicles.
| Application | Drug | Type of nanoparticle | Reference |
|---|---|---|---|
| Acne | Azelaic acid | PLGA | [71] |
| Adapalene | SLN, Eudragit® EPO | [182,183] | |
| Isotretinoin | SLN, Niosome, liposome | [184–186] | |
| Tretinoin | Chitosan-SLN, Lipid-core-polymeric nanocapsules, lipid based | [187,188,189] | |
| Benzoyl peroxide | SLN, Niosome, liposome | [190,191] | |
| Retinoic acid | SLN | [192] | |
| Cyproterone acetate | Lipid, SLN | [193] | |
| Triclosan | Eudragit® E 100 | [194] | |
| Clindamycin | Liposome | [195] | |
| Salicylic acid | Liposome | [196] | |
| Lauric acid | Liposome | [197] | |
| Finasteride | Liposome | [198] | |
| Tea oil | Liposome | [199] | |
| Erythromycin | Niosome | [200] | |
| Sphingosome | SLN | [201] | |
| Target Microorganism | Type of nanoparticle | Reference | |
| Antibacterial |
E. coli, B. subtilis, S. aureus, methicillin-resistant coagulase-negative staphylococci, vancomycin-resistant Enterococcus faecium, ESBL-positive K. pneumonia, S. typhi, Vibri cholera |
Silver | [76,202,203] |
| MRSA, VRE, E. coli, Pseudomonas aeruginosa | Gold | [204,205] | |
| E. coli, B. subtilis, B. megaterium | Magnesium oxide | [82] | |
| E. coli, S. aureus, Listeria monocytogenes | Titanium dioxide | [206] | |
|
E. coli 0157:H7, B. subtilis, Pseudomonas fluorescens, L. monocytogenes, Salmonella enteritidis, S. aureus, S. typhimurium |
Zinc oxide | [207] | |
| B. subtilis | Copper oxide | [83] | |
| Antiviral | HIV-1, Influenza virus, Herpes Simplex virus, Respiratory syncytial virus, Monkey pox virus |
Silver | [208–211] |
| HIV and several strains of influenza virus | Gold | [208,212] | |
| Antifungal | Trichophyton mentagrophytes and Candida species | Silver | [213,214] |
| Candida albicans Biofilms | Titanium dioxide | [215] | |
| Drug | Type of nanoparticle | Reference | |
| Skin cancer | Cisplatin | Lipids | [216] |
| Hyaluronic acid nanoparticles | [217] | ||
| Hexadentate-poly (dl-lactic-co-glycolic acid) nanoparticles | [218] | ||
| PAMAM dendrimer | [219] | ||
| Human ferritin | [220] | ||
| Vincristine | Lipids | [221] | |
| Doxorubicin | Lipids | [222] | |
| SLN | [223] | ||
| Hyaluronic acid nanoparticles | [224] | ||
| Poly-l-lysine dendrimer | [225] | ||
| Paclitaxel | N-isopropylacrylamide/vinyl pyrrolidone based nanoparticles | [226] | |
| Disease | Type of nanoparticle | Reference | |
| Skin inflammation | Eczema/psoriasis | SLN | [227] |
| Lipid nanoparticles | [228] | ||
| Polymeric nanospheres | [31] | ||
| Dermatitis | Zinc oxide | [229] | |
| Hydrocortisone-loaded chitosan nanoparticles | [230] | ||
| Hydrocortisone-loaded poly(ε-caprolactone) | [231] | ||
| Stevens–Johnson syndrome/Toxic epidermal necrolysis | Nanocrytalline silver dressing | [232] | |
| Chronic skin inflammation | Polymer-lipid | [233] | |
| Ammonium glycyrrhizinate-loaded niosomes | [234] | ||
| Curcumin | [235] | ||
| Intended therapeutic effect | Type of nanoparticle | Reference | |
| Cosmetics | Sunscreen | Zinc oxide | [236] |
| Magnesium oxide | [237] | ||
| Titanium dioxide | [238] | ||
| Octyl or ethylhexyl methoxycinnamate (OMC) with SLN and PLGA | [118,239] | ||
| Skin care, wounds, antioxidants | Vitamin E with polymer | [172] | |
| Antioxidants/anti-aging | Coenzyme Q10 with liposome | [117] | |
| Intended therapeutic effect | Type of nanoparticle | Reference | |
| Ocular | Glaucoma | Nano-emulsion | [240] |
| Liposomes | [241] | ||
| SLN | [242] | ||
| Polymeric nanoparticles | [243] | ||
| Dendrimers | [243] | ||
| Ocular inflammation | Liposomes | [244] | |
| SLN | [244] | ||
| Polymeric nanoparticles | [245] | ||
| Chronic dry eye | Nano-emulsion | [244] | |
| Liposomes | [246] | ||
| SLN | [247] | ||
| Eye cancer | Polymeric nanoparticles | [248] | |
| Intraocular infections | SLN | [249] | |
| Corneal neovascularization | Nanoparticles | [250] | |
| Wound healing | Gold nanoparticle | [251] | |
| Copper oxide nanoparticle | [252] | ||
| Cerium oxide nanoparticles | [62] | ||
| Polymeric nanoparticles | [253] | ||
| Liposomes | [254] |
2.1. Natural polymeric nanoparticles
Although a wide range of natural polymers have been used to prepare nanoparticles, chitosan-based nanoparticles have been the most extensively studied for topical drug delivery applications [13]. In addition, a few publications report on the use of alginic acid and albumin-derived nanoparticles for topical drug delivery, which are also briefly described in this section. Chitosan is a linear polysaccharide derived from chitin, which is found in the exoskeleton of crustaceans. The amino group of chitosan readily undergoes protonation in acidic to neutral conditions, resulting in a net positive charge. This makes chitosan water-soluble and act as a bioadhesive, since the positively-charged chitosan can bind to negatively-charged mucoproteins. This interaction helps prolong the circulation time of chitosan-bound drugs, leading to enhanced drug bioavailability [14]. Chitosan nanoparticles can be prepared using (i) ionic cross-linking, (ii) covalent cross-linking [15], (iii) precipitation [16], (iv) polymerization [17], or (v) self-assembly methods [18], with sizes ranging from 20 to 800 nm dependent on the method of preparation. Chitosan-drug complexes are generally formed via electrostatic interactions between cationic chitosan and anionic drugs [19,20]. For example, Cheng et al. [19] prepared chitosan nanoparticles linked with antisense oligonucleotide to produce particles with a size of 102.6 ± 12.0 nm, zeta potential of 1.45 ± 1.75 mV, and an encapsulation efficiency of 87.6 ± 3.5%. Kim et al. [20] increased the solubility of retinol by more than 1600-fold by encapsulating it into chitosan nanoparticles. Drug delivery via chitosan nanoparticles can be controlled by the degradation of the polymer, which can be tuned by varying the molecular weight and degree of deacetylation of chitosan. As a result, chitosan nanoparticles have been shown to provide sustained release from days to months [21]. In addition, chitosan nanoparticles can be formulated as pulsatile delivery vehicles, where release of the payload is dependent on the physiological needs of the patient; for example, the release of insulin from glucose-sensitive gels in response to varied glucose concentrations has been demonstrated in vitro [22].
Alginate is an anionic polysaccharide derived from algae. Although alginate-derived nanoparticles are not extensively studied as topical drug delivery vehicles, two reports have been recently published in the literature. Ibrahim et al. prepared brimonidine-loaded alginate nanoparticles and showed that they provide a sustained release without an initial burst in vitro and provided an improvement on the intraocular pressure lowering effects when compared to marketed brimonidine tartrate eye drops in vivo [23]. Friedman et al. explored the use of nanoparticles synthesized from chitosan and alginate for the delivery of antimicrobial agents against Propionibacterium acnes for the topical treatment of acne and showed superior antimicrobial activity as compared to benzoyl peroxide alone in vitro [24].
Albumin is a non-toxic, biocompatible and biodegradable carrier protein used in drug delivery. Albumin nanoparticles have gained considerable attention as drug delivery vehicles due to their high binding capacity. Despite this, it is our finding that there is only one report in the literature on the use of albumin nanoparticles for topical drug delivery. Das et al. showed that aspirin-loaded albumin nanoparticles provide prolonged release in vitro for 72 h and hold promise as a topical treatment for diabetic retinopathy [25].
2.2. Synthetic polymeric nanoparticles
Natural polymers sometimes lack batch-to-batch consistency, making it difficult to prepare particles with reproducible characteristics and drug delivery profiles [26]. On the other hand, synthetic polymers can be supplied with high purity and batch-to-batch reproducibility, for preparing nanoparticles with more consistent drug release profiles. Synthetic polymeric nanoparticles are predominantly used to deliver lipophilic, small molecule drugs [26]. Since volatile organic solvents are often used during the preparation of synthetic polymeric nanoparticles, it is challenging to maintain the biological activity of peptides and proteins during the nanoparticle manufacturing process. Tyrosine-derived polymers, aliphatic polyesters, and poly (ε-caprolactone) are examples of biodegradable synthetic polymers that have been extensively explored as nanoparticle drug delivery vehicles for topical applications. These have been described in the following sections.
2.2.1. Tyrosine-derived polymeric nanospheres (TyroSpheres)
Tyrosine-derived polymeric nanospheres (abbreviated TyroSpheres) are synthesized from a family of fully-degradable, ABA-type triblock co-polymers made of poly(ethylene glycol), oligomers of desaminotyrosyltyrosine alkyl esters, and several different, naturally occurring dicarboxylic acids such as succinic acid, adipic acid, suberic acid, or sebacic acid [27]. These tyrosine-derived copolymers spontaneously self-assemble in aqueous media to form nanospheres with a hydrodynamic diameter of approximately 70 nm [27]. These nanospheres form strong complexes with hydrophobic molecules, as demonstrated using the model compound DAF, and clinically used antitumor agent paclitaxel, a powerful chemotherapeutic drug [28]. In contrast, TyroSpheres do not strongly associate with hydrophilic compounds such as fluorescein [29,30]. Batheja et al. demonstrated that a gel formulation of TyroSpheres loaded with Nile red (a hydrophobic model drug) significantly enhanced skin permeation (1.4–1.8 fold) compared to an aqueous dispersion of Nile red-loaded TyroSpheres, both in vitro and in vivo [31]. As these TyroSpheres are suitable only for hydrophobic drugs, they have been extensively studied for the binding and delivery of various therapeutic agents that are hydrophobic and water-insoluble, for example curcumin (unpublished data), cyclosporine A [32], sildenafil [33], rolipram [34] and paclitaxel [30,35,36]. Moreover, these nanospheres are stable for about 6 months, after which time they degrade, most likely through hydrolysis of their ester bonds [28]. Drug release from TyroSpheres is dependent on the properties of the drug, the binding and loading efficiencies, and the pH of the solution [37]. For example, Goyal et al. showed that the release of cyclosporine A was inversely proportional to the amount of drug loaded in the TyroSpheres [32].
In vitro cytotoxicity assays in serum-free media showed no significant decrease in cell metabolic activity at concentrations up to 2 mg/mL, demonstrating that empty TyroSpheres are non-cytotoxic [27]. Further studies have also shown that TyroSpheres can deliver agents into the skin [29,31] and have no detrimental effects on the skin's morphology [31]. Paclitaxel-loaded TyroSpheres have been shown to effectively deliver paclitaxel to tumor cells as determined using an in vitro cell killing assay [37]. In addition, in vivo studies were performed to compare the toxicity and anti-tumor efficacy of paclitaxel administered intravenously using TyroSpheres or commercially available Cremophor® EL in two breast cancer murine xenograft models [30]. It was shown that animals treated with paclitaxel-loaded Cremophor® EL lost significant weight and failed to recover following treatment, whereas those treated with paclitaxel-loaded TyroSpheres gained weight normally. Moreover, the anti-tumor efficacy of the two treatments was the same. In addition, a larger volume of distribution and longer half-life were measured for animals treated with paclitaxel-loaded TyroSpheres. These data suggest that TyroSpheres could be used as a more effective delivery vehicle for paclitaxel with fewer side effects for the treatment of breast tumors. Moreover, through modification of the triblock copolymer, ligands could be covalently attached to the TyroSpheres that could target drug-loaded TyroSpheres to specific recipient cells, thereby reducing unwanted side effects to non-targeted cells [35].
TyroSpheres also offer versatility in their storage and handling properties since they can be prepared as a liquid formulation [29], dry formulation [33,34] or incorporated into a gel to prevent runoff when used topically [31]. Tyrosine-derived nanospheres offer a promising tool for the topical skin delivery of lipophilic drugs and personal care agents — as may be desired in the treatment of dermatological conditions such as skin cancer, psoriasis, eczema, microbial infection, sunscreens and cosmetics.
2.2.2. Poly (lactic-co-glycolic acid) nanoparticles
Poly (lactic-co-glycolic acid) (PLGA) has been extensively studied in topical drug delivery applications because it is biocompatible, biodegradable, widely used in medical devices and pharmaceutical formulations approved by the FDA, commercially available with different molecular weights and copolymer ratios, able to encapsulate a wide range of drugs from hydrophobic to lipophilic, and capable of surface modification for site-specific drug delivery. Furthermore, PLGA can be formulated as nanoparticles using several different methods, such as solvent displacement, solvent diffusion, emulsification–evaporation, and phase-inversion; with sizes ranging from 10 to 1000 nm. PLGA nanoparticles have been incorporated into gels using hydroxypropyl methylcellulose (HPMC) or Carbopol® to further enhance the penetration of drugs [38] into the deeper layers of the skin by increasing contact time with the skin and reducing the loss of water from the skin.
2.2.3. Poly(ε-caprolactone) nanoparticles
Poly(ε-caprolactone) (PCL) is another synthetic polymer that is widely employed for the preparation of nanoparticle formulations due to its biocompatibility, biodegradability and favorable rheological properties, including a low glass transition temperature. The degradation of PCL is slower than other polyesters due to its semi-crystalline structure (erosion rates: poly(glycolic acid) > PLGA > poly(L-lactic acid) > PCL) [39]. As a result, PCL is often modified [40] to change its biodegradability to meet specifications of the targeted clinical application (see review by Abedalwafa et al. [41]).
2.3. Lipid-based nanoparticles
2.3.1. Solid-lipid nanoparticles (SLNs)
SLNs are made of solid lipids with an average diameter between 50 and 1,000 nm [42] and prepared using three main components: lipid(s), surfactant(s), and water. The type of lipids used to prepare SLN is very broad, including triglycerides (e.g., tristearin), partial glycerides, fatty acids (e.g., stearic acid), steroids (e.g., cholesterol) and waxes (e.g., cetyl palmitate) [43]. Surfactants are typically required to stabilize the lipid dispersion. SLNs were developed in the early 1990s and are still considered promising drug carrier systems, especially for applications that require sustained release. SLNs can be prepared without organic solvents. In addition, they are biodegradable, biocompatible, physicochemically stable, have low toxicity, and can be produced at large industrial scales at relatively low cost. Jeon et al. prepared surface-modified SLNs containing retinyl palmitate and showed that these negatively-charged SLNs enhanced the cutaneous distribution of retinyl palmitate 4.8-fold as compared to neutral SLNs [44].
2.3.2. Liposomes
Liposomes are spherical vesicles made of lipids, the major component of the cell membrane. Liposomes have at least one bilayered lipid membrane and can be loaded with drugs. The average diameter of liposomes varies from 50 to 300 nm [45]. To date, about 600 clinical trials have involved lipid-derived drug delivery systems [46]. Overall, lipids reduce the interaction of drugs with serum proteins and thereby increase the circulation time of drugs in the body, preventing rapid clearance and providing drug delivery to the targeted site [45]. Liposomes can be prepared using: (i) thin film hydration [47], (ii) reverse-phase evaporation [48], (iii) solvent injection [49], (iv) detergent depletion [50], (v) supercritical fluid [51], (vi) size reduction sonication [52], (vii) high-pressure homogenization [52], and (viii) low-pressure extrusion [53]. Liposomes are able to provide sustained [54] and triggered [55] release of their payload, however the correlation between in vitro and in vivo release kinetics has been poor [56].
2.4. Metallic and silica nanoparticles
A variety of metallic nanoparticles have captured much attention due to their effective application to various areas of science and technology. One example is silver nanoparticles (AgNPs), which have been shown to have antiseptic and antimicrobial activity against gram-positive and gram-negative bacteria [57]. AgNPs (ranging in size from ~1–100 nm) can be prepared using a variety of methods: (i) chemical synthesis, (ii) physical dispersion, (iii) photochemical synthesis, and (iv) biological synthesis [58]. Recently, Zhang et al. reported on three multistep methods used to prepare silver nanostructures with well-controlled shapes: (i) double reductant method, (ii) etching technique, and (iii) construction of core-shell nanostructures [59]. These nanoparticles had excellent optical properties. Another example is gold nanoparticles (AuNPs), which exhibit a unique combination of physical, chemical, optical, and electronic properties for drug delivery. They are easy to prepare in a range of sizes (1–150 nm), are biocompatible, and can be conjugated to other molecules. AuNPs of ~5 nm diameter have been reported to diffuse though the SC of intact mouse skin [60]. Other examples are the use of metallic nanoparticles, such as titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles, in cosmetics and sunscreens for ultraviolet radiation protection [61]. Recently, cerium oxide nanoparticles have also gained attention in wound healing applications [62]. The sizes of the metallic nanoparticles can be optimized to be large enough to reflect and refract the high energy, very short wavelengths of ultraviolet A and B (~290–400 nm), while being too small to reflect the lower energy, longer wavelengths of visible light (~400–700 nm). In this way they can remain invisible on the skin [63].
2.5. Dendrimers
Dendrimers are a class of highly branched, monodispersed, synthetic macromolecules with a well-defined composition and structure. The most commonly referenced dendrimers used in nanomedicine are polyamidoamines (PAMAM), poly(l-lysine) (PLL) scaffold dendrimers, polyesters (PGLSA-OH), polypropylimines (PPI), poly(2,2-bis (hydroxymethyl)propionic acid) scaffold dendrimers (bis-MPA), and aminobis (methylenephosphonic acid) scaffold dendrimers. Dendrimer-based NPs have been widely studied because of the scientific and technological interest relating to their possible applications in a broad range of areas, such as catalysis, optics, electronics, and biomedical applications [64]. The tunable surface chemistry of dendrimers allows their assembly onto various nanoparticles [65]. For example, magnetic iron oxide nanoparticles have been stabilized using dendrimers [65]. Dendrimer nanoparticles are usually smaller than 5 nm and can be prepared using fast reduction and nucleation chemistry [66]. Perumal et al. [67] showed that dendrimers could be used to enhance the skin permeation of hydrophilic drugs. Dendrimer size, molecular weight, surface charge and hydrophobicity are key parameters guiding topical drug delivery and skin permeation [68].
3. Topical applications of nanoparticles
3.1. Acne
Acne vulgaris is the most common skin disease. It is associated with an elevated rate of sebum secretion, which manifests as mild-severe inflammatory lesions on areas of the body containing large numbers of sebaceous follicles such as the face, back and chest [69]. Acne is classified as mild, moderate, or severe based on the number and type of lesions. Treatment options vary with the stage and intensity of the disease. In general, topical treatment is preferred for mild and moderate acne, whereas systemic therapy is used to treat severe cases [70]. Topical therapy includes the use of antibiotics, retinoids (vitamin A), and combinations thereof. The retinoids often irritate the skin, and can cause dryness, peeling/exfoliation and photosensitivity with resultant hyperpigmentation. The alternative treatment (prolonged use of antibiotics) can lead to bacterial resistance. Because of these complications, nanoparticles are being developed to provide gradual and sustained delivery of these drug(s) into the skin. Comparable or increased effectiveness with concomitantly reduced topical side effects are the key criteria used to evaluate the utility of nanoparticulate formulations of acne drugs [71]. Table 1 lists the drugs used to treat acne by topical application of nanoparticle systems. These new formulations have been shown to provide efficacy, tolerability, compliance, and cosmetic acceptability [72,73].
3.2. Infection
The rate at which infectious diseases and drug-resistant pathogens are emerging is a matter of serious concern. Despite increased knowledge of microbial pathogenesis and a wide repertoire of antimicrobial therapies, the morbidity and mortality resulting from microbial infections remains high. Therefore, there is a clinical need for novel strategies and new antimicrobial agents to treat infections.
A recent review has reported on the use of nanoparticle systems as antimicrobial and antinfective agents [74]. For example, AgNPs have been studied as antimicrobials, where it has been shown that AgNPs with diameters less than 20 nm can attach to sulfur-containing proteins of bacterial cell membranes. This increases the permeability of the bacterial membrane, causing death to the bacteria [75]. The antibacterial properties are related to the total surface area of the nanoparticles, where smaller particles with larger surface-to-volume ratios have greater antibacterial activity [76]. It has been demonstrated that composites of AgNPs and polymer gels have increased bactericidal activity compared to higher concentrations of AgNPs alone [77].
AuNPs have been used to coat a wide variety of surfaces, such as implants, wound dressings, and glass surfaces for maintaining antimicrobial surfaces [78]. AuNPs have optical absorption in the near-infrared region and can destroy bacteria via photothermal heating [79]. AuNPs generate holes in the cell wall, resulting in the leakage of cell contents and cell death. It is also possible that AuNPs bind to the DNA of bacteria and inhibit the uncoiling and transcription of DNA [80]. Stable conjugates of AuNPs coated with antibiotics have demonstrated bactericidal activity against both Gram-positive and Gram-negative bacteria [81].
Other metal oxide nanoparticles have also been studied for antibacterial activity. For example, halogen-treated magnesium oxide (MgO) nanoparticles have demonstrated antimicrobial activity against bacteria and spores [82]. Copper oxide (CuO) nanoparticles have a high antimicrobial activity against Bacillus subtilis [83]. This has been attributed to the greater abundance of amines and carboxyl groups on the cell surface of B. subtilis for which Cu has a high affinity. Cu ions may also interact with DNA molecules, intercalate with nucleic acid strands, and/or disrupt biochemical processes [83,84]. Titanium dioxide (TiO2) nanoparticles have also been explored to fight infection. It has been suggested that UV irradiated nanostructured TiO2 surfaces can be used as effective disinfectants, eliminating pathogenic microorganisms on food-contacting surfaces [85]. The major disadvantage of using TiO2 in this way is that UV light is required to activate the photocatalyst, which initiates the killing of bacteria and viruses. Zinc oxide (ZnO) nanoparticles have been shown to generate hydrogen peroxide (H2O2) from surfaces and thereby inhibit bacterial growth. It is presumed that by decreasing the particle size, the number of ZnO powder particles per unit volume of powder slurry increases, resulting in an increased surface area with an increased capacity for the generation of H2O2. Another possible mechanism is that released Zn2+ ions can directly damage the cell membrane and interact with intracellular contents [86]. Table 1 lists metal-based nanoparticles that have been explored to treat infection.
3.3. Skin cancer
Skin cancer is the most common form of malignancy, although it is not the deadliest [87]. The most common forms of skin cancer are basal cell carcinoma (~2.8 million U.S. cases annually [88]), and squamous cell carcinoma (~700,000 U.S. cases annually [89]). Malignant melanoma, on the other hand, represents only a very small proportion of skin cancers (~74,000 U.S. cases annually [90]), but accounts for the vast majority of skin cancer deaths. Excision is the current standard treatment for localized skin cancers, and in the case of melanoma this can involve extensive surgeries with deep dissections and wide margins, often requiring soft tissue reconstruction. Furthermore, skin cancers may metastasize to regional lymph nodes and distant sites (particularly in the case of melanoma, and sometimes with squamous cell carcinoma) [91]. Nanoparticles may provide targeted and effective chemotherapeutic delivery to these sites, improving treatment efficacy. Treatments for metastatic malignant melanoma have recently incorporated nanoparticular formulations of cytostatic compounds, as reviewed in 2014 by Berciano-Guerrero et al. [92]. Liposomes, SLNs, polymeric nanospheres and dendrimers are all nanoparticles that have been explored as topical delivery systems for treating skin cancer. Iwamoto et al. recently reviewed the types of drugs and delivery systems used in skin cancer chemotherapy [93]. Table 1 provides a list of nanoparticle-based formulations developed for the treatment of melanoma.
Liposomes exhibit excellent circulation, penetration, and diffusion properties [94]. Early research demonstrated that liposomes are retained in the tumor interstitial fluid in close proximity to tumor vessels [95]. Currently, several liposome-based drug delivery formulations are in clinical use for treating cancer, including melanoma [96]. Several other liposomal carriers for chemotherapeutic drugs are currently in clinical trials [97]. Moreover, major advances with cationic liposomes have led to the successful delivery of small interfering RNA (siRNA) [98]. Finally, theragnostic liposomes offer a new approach for loading a wide variety of diagnostic nanoparticles with anticancer drugs [99].
SLNs can protect drugs against degradation, and provide control over drug release kinetics. SLNs releasing docetaxel have been shown to improve efficacy against colorectal (C-26) and malignant melanoma (A-375) cell lines in vitro and in vivo. SLNs containing cholesteryl butyrate have been shown to inhibit the ability of human umbilical vein endothelial cells to adhere to cancer cell lines derived from human melanoma, colorectal, breast, and prostate cancers. This approach offers promise by interfering with the vascularity needed for tumors to grow [100].
Polymeric micelles are usually more stable in blood as compared to liposomes and other surfactant micelles. Due to their considerably large size, polymeric micelle systems can be used to co-deliver two or more drugs for combinational treatment modalities, such as radiation agents and anticancer drugs [101]. The use of polymeric micelles was recently investigated for the treatment of mice with melanoma and it was shown that the animals treated with drug-loaded polymeric micelles inhibited tumor growth and increased the survival rate as compared to animals treated with free drug [101].
Dendrimers have also been successfully used in immunotherapy and radio-immunotherapy of various types of tumors [102], including melanoma [103] and cutaneous squamous cell carcinoma [104]. However, the controlled release of drugs from dendrimers remains a significant challenge. New developments in polymer and dendrimer chemistry have provided a new class of molecules called dendronized polymers, which are linear polymers that bear dendrons at each repeat unit. Dendrons are monodisperse, wedged-shaped sections that make up the structure of dendrimers. Dendrimers and dendrons both possess three key architectural features; 1) an initiator core, 2) interior branching units, and 3) a function of generation (G) level. These molecules have an increased circulation time, and may offer drug delivery advantages such as targeted and sustained delivery.
3.4. Inflammatory diseases
Inflammation is characteristic of many dermatologic disease processes and affects cutaneous physiology, such as the skin's barrier function. This disruption can serve as a portal of entry for microbes, allergens and other pro-inflammatory stimuli, which can cause further inflammation. While many of these disease processes are not fully understood, therapies that modulate inflammation have been shown to significantly decrease morbidity [105]. Inflammatory stimuli are omnipresent in the environment, from microbes that colonize the body, to UV rays, and even commonly ingested foods [106]. These factors can result in cutaneous inflammation with varying degrees of physiological and esthetic impact, such as psoriasis, eczema, atopic dermatitis, rosacea, lichen planus, erythroderma, Stevens–Johnson syndrome and toxic epidermal necrolysis. According to statistics provided by the National Psoriasis Foundation, psoriasis is the most prevalent autoimmune disease in the U.S. with as many as 7.5 million Americans and 125 million people worldwide affected. Nanotechnology has provided an alternative approach for the delivery of drugs that can reduce inflammation and its effects on the skin's barrier function and esthetics. For example, Kilfoyle and co-workers have shown that TyroSpheres are able to provide dose-controlled delivery of paclitaxel, an anti-proliferative agent, and that the paclitaxel is preferentially deposited in the epidermis [36]. These results suggest that paclitaxel-loaded TyroSpheres are a promising approach to the treatment of psoriasis, while minimizing systemic exposure to paclitaxel. Table 1 lists the types of nanoparticle-based formulations used to topically treat inflammatory diseases or conditions of the skin.
3.5. Cosmetics
Many skin conditions, such as dry skin, oily skin, hyper/hypopigmentation, vascular deformities, and photoaging, are major concerns in the field of cosmetic dermatology. A variety of products have been formulated to address these issues [107]. However, there has been much controversy regarding the safety of the topical application of nanoparticles revolving around whether nanoparticles get absorbed systemically, evade immunologic defense mechanisms, form complexes with proteins, and/or induce the formation of free radicals, ultimately damaging healthy skin cells. This controversy is particularly evident with regard to the use of nanoparticles in sunscreens (which are regulated as drugs in the U.S.).
ZnO and TiO2 nanoparticles have traditionally been used in sunscreens because of their ability to reflect ultraviolet (UV) light [108]. In recent years, nanosized forms of ZnO and TiO2 have been optimized to be more transparent, less viscous (more spreadable) [109], and easier to blend, while maintaining their potency against UV irradiation [110]. However, their toxicity profiles need to be assessed since smaller sized particles will likely have different chemical, optical, magnetic, and structural properties [111]. Due to the safety concerns around metal nanoparticles in sunscreens, Cross et al. performed a penetration study using a Franz-cell diffusion setup [112]. ZnO nanoparticles were applied topically to cadaver skin and after 24 h, 0.03% of the applied ZnO nanoparticles were detected in the upper SC, with 0% in the lower SC and epidermis. These data suggest that it is unlikely that ZnO nanoparticles penetrated through the epidermis and into the dermis in this model.
Vitamin A, in various natural and synthetic retinoid forms has been explored for the treatment of skin aging and hyperpigmented lesions [113], however, certain forms such as retinoic acid cause irritation, burning, scaling and/or dermatitis, limiting their acceptance by patients. Retinaldehyde and retinol are considerably less irritating. The retinyl palmitate and acetate esters are the least irritating forms of Vitamin A, most probably because they are the natural storage forms of Vitamin A in the skin. In order to improve efficacy and minimize the aforementioned side effects, various novel drug delivery systems, including nanoparticles, have been developed. For example, Yamaguchi et al. [114] observed the effect of nano-scale tretinoin (ali-trans retinoic acid, atRA) on photo-damaged skin and showed reduced irritation and inflammation.
Coenzyme Q10 is a naturally occurring antioxidant used widely in cosmetics [115]. It is a lipid-soluble provitamin with low solubility in aqueous solutions (logP > 10) [116]. Therefore, SLNs, liposomes and other formulations have been explored as drug delivery vehicles for Coenzyme Q10 to facilitate penetration into the deeper layers of skin [117].
Octyl methoxycinnamate (OMC), also called ethylhexyl methoxycinnamate, is used as an active ingredient in sunscreens [118]. Puglia et al. [119] incorporated two components, OMC and diethyltoluamide, into SLNs and used differential scanning calorimetry to determine the percutaneous absorption. This study provided evidence that the topical application of SLN delivery systems could reduce their systemic absorption, and enhance their safety and efficacy for this application. However, when exposed to sunlight, OMC can change from the primary transform to the cis-form, resulting in a reduced UVB filtering efficiency [120]. Table 1 summarizes the various types of nanoformulations used in cosmetic applications.
3.6. Ophthalmology
The ocular surface epithelium, tear film, and blood-ocular barriers interfere with the penetration of topically applied drugs to the eye. Nanoparticles offer several desirable characteristics when dealing with ocular drug delivery: (i) They can improve the penetration of large, poorly water-soluble molecules, e.g., the delivery of glucocorticoid or immunosuppressive drugs for the treatment of immune-related, vision-threatening diseases, such as uveitis or scleritis [121]. (ii) They can increase the contact time of the administered drug with its target tissue, e.g., brimonidine for the treatment of glaucomaor corticosteroids for autoimmune uveitis [122]. (iii) Nanoparticles can be used to reduce side effects of highly potent drugs. (iv) They can reduce issues with patient non-compliance, e.g., glaucoma requires treatment with daily application of eye drops, which often adversely affects the anterior structure of the eye [123]. Wadhawa et al. reported a significant reduction in the intraocular pressure without irritation of rabbit eyes treated with chitosan-hyaluronic acid nanoparticles loaded with timolol compared to standard timolol eye-drops or empty nanoparticles [124]. Table 1 lists the various types of nanoparticle formulations studied for the topical treatment of eye diseases.
3.7. Chronic wound healing
In the past, the topical treatment of cutaneous wounds has focused on infection [125], but in more recent years, researchers have developed new multifunctional nanosystems with the potential to provide more comprehensive therapeutic effects [126]. Chronic wounds fail to complete the normal wound healing cascade, resulting in delayed or failed wound closure [127]. These recalcitrant wounds occur most commonly in association with diabetes, peripheral arterial disease and infection. Unlike acute wounds, there is no standard treatment for chronic wounds. Treatment options include gels and occlusive dressings that retain moisture, surgical debridement to remove dead tissue and debris, skin substitutes, vacuum-assisted wound closure, hyperbaric oxygen therapy, compression bandages, and topical drugs [128]. Nanoparticles have been explored to deliver silver and morphine to chronic wounds, to inhibit bacterial colonization, and to reduce pain [129]. In addition, nanoparticles have been used to encapsulate and protect therapeutic agents from degradation in the highly proteolytic wound environment [130]. Vasoactive agents, such as nitric oxide, have also been stabilized and released using nanoparticle technologies to prevent or treat ischemia [131]. Recently, Chigurupati et al. reported on the topical use of cerium oxide nanoparticles for healing full-thickness dermal wounds in mice via a mechanism that enhances the proliferation and migration of various skin cells. The results suggest that cerium nanoparticles penetrated into the wound tissue and reduced oxidative damage to cellular membranes and proteins [62]. Curcumin has also been explored in antimicrobial and wound healing applications [132]. Krausz et al. demonstrated that curcumin-loaded nanoparticles inhibited microbial growth and enhanced wound healing in an in vivo murine wound model [133]. Table 1 lists the various types of nanoparticle formulations explored to treat chronic wounds.
4. Electrospun nanofibers for drug delivery
4.1. Introduction to electrospinning
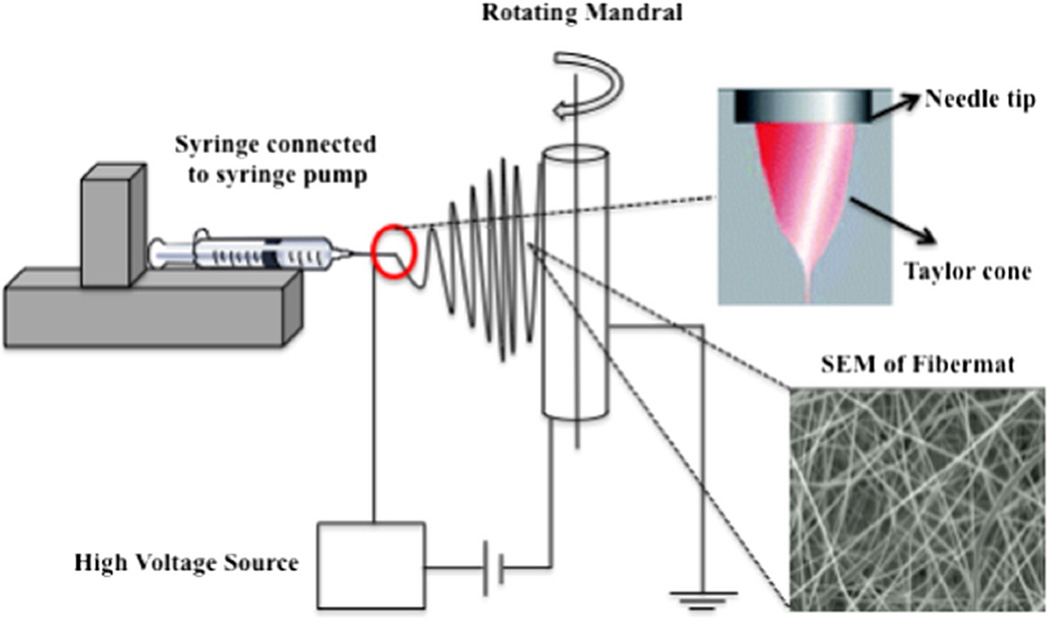
Electrospinning is a relatively simple, user-friendly and inexpensive process used to fabricate nanofibers. Electrospinning was first patented by Cooley in 1900 [134], and has gained popularity in the last 20 years for use in medical applications, including nanofiber drug delivery systems, tissue engineering scaffolds, filtration membranes, biosensors, and enzyme immobilizers. The typical setup includes at least one syringe pump, a polymer solution with or without drug, a high voltage supply, a source electrode, and a collector electrode (Fig. 2). During the electrospinning process, an electric field is applied to a viscous polymer solution, inducing charge within the polymer and needle. When the charge repulsion force exceeds the surface tension, a jet is initiated from the needle tip (referred to as a “Taylor cone”) that creates extremely thin, elongated droplets with very high surface area. As the solvent rapidly evaporates from these droplets, nanosized fibers are formed that are attracted to an oppositely charged or grounded collector, where a nonwoven, solid, fibrous mat is deposited. The collector may be stationary or rotating and/or translating. Fig. 2 shows a scanning electron micrograph of an electrospun fiber mat. In the electrospinning process, fiber formation is dependent on a variety of solution and process parameters, as reviewed by Bhardwaj et al. [135]. These include polymer concentration (Fig. 3), polymer molecular weight, solution viscosity, surface tension, conductivity of the polymer, solvent, field strength/voltage, flow rate of the feed, type of collector, and distance between the needle tip and the collector. This process can produce fibers with a diameter ranging from 2 nm to greater than 5 µm, but most commonly fiber diameters from about 10 to several hundreds of nanometers are reported in the literature [136]. Through the optimization of these parameters and atmospheric conditions (e.g., humidity and temperature), electrospinning can be used to produce nanofibers with a desired diameter and morphology for a targeted clinical application.
Fig. 2.
Schematic of a standard horizontal electrospinning setup including at least one syringe pump, a polymer solution with or without drug, a high voltage supply, a source electrode, and a collector electrode. (Note, Taylor cone adapted from ref.: [137]).
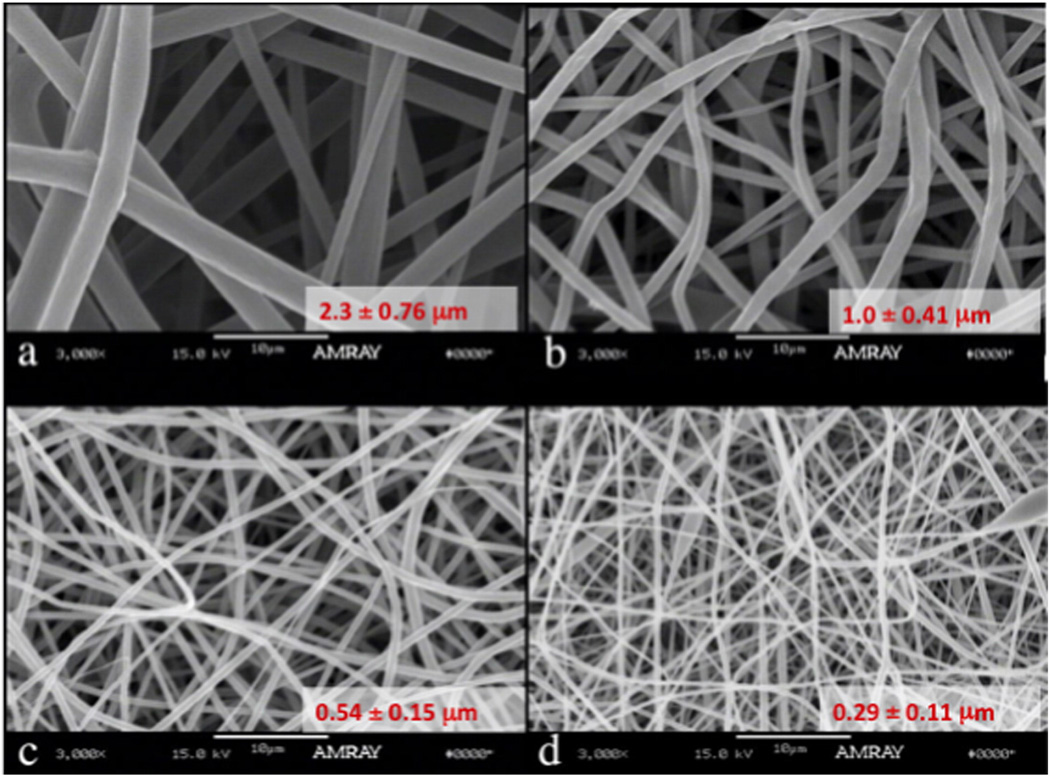
Fig. 3.
Tyrosine-derived polycarbonate fiber mats were electrospun using a standard setup and glacial acetic acid as the solvent. The fiber diameter, morphology and overall porosity were affected by changes in the solution parameters, in this case, polymer concentration in the feed. Images are courtesy of the New Jersey Center for Biomaterials and were obtained by Charles Florek.
Fig. 2 represents the standard electrospinning process used to produce drug delivery systems. The drug is either co-dissolved or finely dispersed in the polymer solution. Variations of this setup include coaxial spinning, emulsion electrospinning, and the use of dual spinnerets. Coaxial spinning is a modified electrospinning setup where two different solutions (e.g., one containing polymer and the other containing drug) are fed through a co-axial capillary channel to produce nanofibers with a core-shell structure (drug in the core and polymer in the shell) [138]. Emulsion electrospinning uses a surfactant to separate the distinct phases (e.g., organic vs. acqueous) and produce nanofibers with a core-shell structure [139]. Coaxial and emulsion spinning provide alternative approaches to electrospinning materials that are not able to be electrospun via standard setups. Co-axial electrospinning can also be used to protect therapeutic molecules from denaturation or degradation. An alternative approach, using dual spinnerets, allows the user to simultaneously electrospin two polymer solutions onto a single collector [140]. The distance between the spinnerets is an additional process parameter that could be optimized by the user.
4.2. Electrospun nanofibers as topical drug delivery systems
Electrospun fiber mats offer several desirable features as drug delivery systems. (i) The electrospinning process can be used to fabricate nanofibers from a wide variety of solutions of both natural polymers (e.g., chitosan, fibronectin, gelatin, collagen, silk, ethyl cellulose) and synthetic polymers (e.g., PLA, PGA, PLGA, tyrosine-derived polycarbonates, polycaprolactone, polyurethane, poly(vinyl pyrrolidone), poly(vinyl alcohol)), or combinations thereof as reviewed by Bhardwaj et al. [135]. (ii) Fiber mats have high surface area-to-volume ratios that provide efficient delivery of both hydrophilic and hydrophobic drugs [141]. (iii) The drug release profile can be tuned to meet the specific clinical application by modulating a variety of parameters, such as the drug to polymer ratio, fiber diameter, morphology, and/or porosity [142]. Functionalization of the nanofibers (e.g., surface graft polymerization) is another approach used to modulate drug release, as reviewed by Yoo et al. [12]. (iv) Electrospun fiber mats can provide sustained release, which reduces the frequency of topical application to increase patient compliance. (v) Electrospun fiber mats have high porosity with interconnectivity, which can play a critical role in mass transport [143,144]. (vi) Nanofiber meshes are malleable, making them suitable for topical drug delivery applications. (vii) Fiber mats can be incorporated into wound dressings, as part of a drug-releasing wound treatment technology.
Although electrospinning has been developed into an industrial scale process for the manufacture of HEPA filters and insulating materials, the exact reproducibility required for the production medical devices is still a challenge. Modifications, such as bubble electrospinning [145] and porous hollow tubes [146], have been explored to scale up the electrospinning process.
5. Areas of potential clinical use for the topical application of electrospun nanofiber drug delivery systems
5.1. Infected or highly contaminated wounds
Infection of cutaneous wounds can lead to delayed healing time, longer hospital stay, and death in some cases. The local delivery of antibiotics can be beneficial in the treatment of infection. Various antibiotic agents have been impregnated into electrospun fibers and shown to have bactericidal activity in vitro. Some examples are described below and summarized in Table 2.
Table 2.
Types of polymeric fiber mats and their clinical uses as topical drug delivery vehicles.
| Therapeutic molecule class |
Example molecules | Polymer delivery system | Solvent | Electrospinning setup |
Fiber diameter^ |
In vitro drug release |
Refs |
|---|---|---|---|---|---|---|---|
| Antimicrobial | Fusidic acid | Poly(lactide-co-glycolide) | Dichloromethane | Standard | 200 nm–2 µm | 9–15 days | [151,152] |
| Linezolid | Poly(ε-caprolactone) | Acetone | Standard | 450–800 nm | 1–14 days | [153] | |
| Poly(L-lactic acid) | Hexafluoroisopropanol | Standard, co-axial | 260 nm–2 µm | 1 — at least 5 days | [178] | ||
| Tetracycline hydrochloride | Poly(ethylene-co-vinyl acetate), poly(lactic acid), and their blends |
Chloroform/methanol | Standard | 1–6 µm | Hours to at least 5 days | [255] | |
| Iodine | Poly(vinyl pyrrolidone) (PVP) and poly(ethylene oxide)/PVP |
Water | Standard* | 230–730 nm | Data not available | [155] | |
| Cefazolin | Poly(lactide-co-glycolide) | Tetrahydrofuran/dimethylformamide | Standard | 450–500 nm | Data not available | [147] | |
| Mupirocin | Poly(L-lactic acid) | Hexafluoroisopropanol | Standard, dual | Data not available | At least 3 days | [140] | |
| Silver nanoparticles | Poly(ε-caprolactone) | Acetone | Standard | 2–4.3 µm | Data not available | [156] | |
| Zein | Citric acid in ethanol | Standard | 350–500 nm | Data not available | [157] | ||
| Poly(dopamine methacrylamide-co-methyl methacrylate) |
Dimethylformamide | Standard | 800 nm | At least 7 days | [158] | ||
| Gentamycin sulfate | Polycaprolactone | Chloroform (shell)/distilled water (core) |
Coaxial | 153 nm–1.7 µm | 7 days | [149] | |
| Cefoxitin sodium | Poly(lactic-co-glycolic acid) (PLGA) | Dimethylformamide/water | Standard | 260 nm | 7 days | [148] | |
| Antifungal | Itraconazole | Polyurethane | Dimethylformamide | Standard | 300 nm–2 µm | 24 h | [141] |
| Antioxidant | Vitamin E | Silk fibroin | Water | Standard | 380–723 nm | 3 days | [172] |
| Green tea polyphenols | Poly(lactide-co-glycolide) | Acetone/dimethylformamide | Standard | 780 nm | 10–14 days | [177] | |
| Vitamin A | Cellulose acetate | Acetone/dimethylacetamide | Standard | 250 nm | 6 days | [173] | |
| Resveratrol | Polycaprolactone | Chloroform (shell)/ethanol(core) | Coaxial | 147 nm–1.6 µm | 7 days | [149] | |
| Anti-proliferative | Dexamethasone | Poly(lactide-co-glycolide) | Acetone/dimethylformamide | Standard | 780 nm | At least 25 days | [177] |
| Local anesthetic | Bupivacaine | Poly(lactide-co-glycolide) | Hexafluoroisopropanol | Standard | 800 nm–1 µm | 12 days | [174] |
| Vancomycin Gentamicin |
Poly(lactide-co-glycolide)/collagen | Hexafluoroisopropanol | Standard, multiple |
55–314 nm | At least 21 days | [175] | |
| Lidocaine | Poly(L-lactic acid) | Hexafluoroisopropanol | Standard, dual | Data not available | 15 days 1 h–3 days |
[140] | |
| Wound healing | Epidermal growth factor | Poly(ε-caprolactone)/hyaluronic acid | Chloroform/formic acid/water | Standard | 150 nm | At least 30 days | [161] |
| Basic fibroblast growth factor | Poly(ethylene glycol)-Poly(DL-lactide) | Chloroform/PBS | Standard | 780 nm | 25 days | [165] | |
| Hyaluronic acid | Sodium hydroxide/dimethylformamide | Dual | 486 nm | 5 days | [167] | ||
| Ketanserin | Polyurethane | Dimethylacetamide | Standard | 500 nm–2 µm | 24 h | [141] | |
| Vascular endothelial growth factor |
Gelatin nanoparticles in hyaluronic acid | Sodium hydroxide/dimethylformamide | Dual | 486–534 nm | 28 days | [167] | |
| Platelet-derived growth factor | Gelatin nanoparticles in collagen | Acetic acid | 28 days | ||||
| Endothelial growth factor | Collagen | Acetic acid | 21 days |
Polymer was electrospun using a standard electrospinning setup, however, therapeutic molecule was loaded after.
Fiber diameter is reported as a range if the authors investigated the effects of electrospinning parameters on fiber morphology.
Katti et al. showed that cefazolin, an antibiotic, can be loaded into PLGA nanofibers using a standard electrospinning setup [147]. Verrek et al. used nonbiodegradable polyurethane-based nanofibers as a topical delivery system for itraconazole, a hydrophobic anti-fungal agent. This system provided ultrafast release (<24 h) of the payload, with a linear relationship to the square root of time, indicating diffusional release [141]. Kim et al. demonstrated the sustained release of cefoxitin sodium, a hydrophilic antibiotic, for up to one week, from electrospun copolymers of PLGA and polyethylene glycol (PEG), and confirmed the bioactivity of the released drug in an in vitro Staphylococcus aureus inhibition assay [148]. Huang et al. used co-axial electrospinning to incorporate gentamycin sulfate, an antibacterial agent, into polyurethane fibers, where the in vitro release followed zero-order kinetics for up to 7 days [149]. Mupirocin is an antibiotic commonly used to treat cutaneous infections, and typically formulated as a 2% cream or ointment that requires application three times a day for up to 10 days. Thakur et al. fabricated poly(L-lactic acid) (PLLA) fiber mats containing mupirocin and lidocaine hydrochloride (an anesthetic) using both a single and a dual spinneret setup, and showed that the setup significantly affected that the burst release profile of mupirocin [140]. In addition, the presence of both mupirocin and lidocaine hydrochloride in a single fiber mat changed the release kinetics of at least one of the drugs. This study demonstrated that the dual spinneret setup was preferred over the single spinneret to produce prototype dressings to treat infected wounds. Ciprofloxacin, a fluoroquinolone antibiotic, is effective against both Gram-positive Staphylococcus (S) and Gram-negative Pseudomonas (P) bacteria that cause cutaneous infections. Jannesari et al. demonstrated that the release of ciprofloxacin ranged from a few days from poly (vinyl alcohol) fibers to over 80 days from poly (vinyl acetate) fibers. The release profile could be tuned by blending poly (vinyl alcohol) and poly(vinyl acetate) [150]. Said et al. demonstrated the release of fusidic acid, an antimicrobial agent, from PLGA nanofibers over 9–15 days, which proved efficacious against Pseudomonas aureus, Pseudomonas aeruginosa, and methicillin-resistant S. aureus (MRSA) [151]. This group also incorporated a bioburden-triggered release mechanism [152]. Linezolid is a synthetic antimicrobial that was shown to inhibit S. aureus when released from PCL fiber mats [153]. Alhusein et al. reported on the controlled release of tetracycline hydrochloride from triple-layered electrospun fiber mats, where the two outer layers consisted of PCL and the inner layer consisted of poly(ethyleneco-vinyl acetate). It was shown that the nanofibers were effective at killing biofilms formed by S. aureus [154]. Poly (vinyl pyrrolidone) – iodine complexes are known to have broad-spectrum antimicrobial activity and as a result, have been explored as a treatment for various cutaneous injuries. Ignatova et al. showed that photo-crosslinked poly(vinyl pyrrolidone) or poly(vinyl pyrrolidone)/poly(ethylene oxide) fiber mats containing iodine had biocidal activity against S. aureus, Escherichia coli (E. coli), and Candida albicans (a type of fungus) [155].
The incorporation of metals into electrospun fiber mats has also been used to kill microbes, target bacterial colonization, and inhibit contamination. Thomas et al. incorporated biosynthesized AgNPs into PCL nanofibers via electrospinning and demonstrated their antimicrobial effects against Staphylococcus epidermidis and Staphylococcus haemolyticus [156]. Similarly, Dashdorj et al. incorporated AgNPs into zein (a corn protein) nanofibers and showed bactericidal activity against S. aureus and E. coli [157]. GhavamiNejad et al. used electrospinning to form AgNPs on the surface of poly(dopamine methacrylamide-co-methyl methacrylate) (MADO) fibers [158]. The fibers released ~13% of the Ag payload within 1 day, followed by a slower release for the next 6 days. In addition, the fiber mats had bactericidal activity against E. coli, S. aureus, and P. auregenosa. An in vivo rat study confirmed the effectiveness of AgNPs-MADO fiber mats, where full thickness wounds treated for 15 days showed higher wound healing rates (92%) compared to controls (51%) and MADO-alone fiber mats (65%). Similarly, Woo et al. developed a bilayered construct with an upper layer consisting of TiO2-loaded chitosan nanofibers and a lower layer consisting of human adipose-derived extracellular matrix [159]. The bilayered construct was shown to have in vitro bactericidal activity against E. coli and S. aureus and inhibit bacterial penetration and accelerate wound healing in vivo in a rat model.
5.2. Chronic wounds/wound healing
The treatment of cutaneous wounds, such as burns, traumatic cutaneous injuries, and chronic wounds is currently a significant clinical challenge. Generally, normal cutaneous wound healing is categorized into three time-dependent phases: (i) inflammation, starts immediately after injury; (ii) new tissue formation, overlaps with the first stage; and (iii) tissue reorganization, where scar tissue is formed and may remodel for a year or more [160]. Typically, in cases of deep burns, the normal wound healing is not possible, as there is no source of cells remaining for regeneration. In chronic wounds, the inflammation stage often proceeds far too long, resulting in wounds that remain open for months to years. In these types of wounds, dressings play an important role to (i) protect the wound; (ii) maintain a moist wound environment; (iii) protect the wound from contamination with exogenous microorganisms; and (iv) accelerate wound closure. The ideal wound dressing should serve as a physical barrier between the wound and the external environment, but be permeable to oxygen. Electrospun fiber mats have properties that are suitable for wound healing applications, such as (i) a highly porous membrane structure that facilitates the exchange of liquids and gases with the environment; (ii) well-interconnected pores that are required to protect the wound from bacterial penetration; (iii) high surface area; and (iv) can provide the local release of drugs into the skin. The electrospun fiber mats can be used as drug delivery vehicles that aid in wound healing.
Many groups have reported on the use of electrospun fiber mats as matrices for wound healing (summarized in Table 2). For example, Wang et al. reported on electrospun core-shell fibers made of PCL/hyaluronic acid containing epidermal growth factor (EGF). This formulation accelerated wound closure in vivo [161]. Several other groups have also reported on the impregnation of EGF [162–164], basic fibroblast growth factor (bFGF) [165,166], vascular endothelial growth factor (VEGF) [167], and platelet-derived growth factor-BB (PDGF-BB) [167] into electrospun fiber mats as a strategy for accelerating wound closure. Systemic and topical administration of ketanserin, a selective S2-serotonin antagonist, accelerated wound healing of diabetic foot ulcers [168,169]. Macri et al. incorporated a fibronectin-derived peptide (P12) that holds promise in the treatment of burns [170] into tyrosine-derived polycarbonates using a standard electrospinning setup. It was shown that both polymer composition and drug loading significantly affected in vitro drug release [142]. Recently, Peh et al. impregnated a cocktail of compounds (Vitamin C, hydrocortisone, insulin, triiodothyronine, EGF, and Vitamin D3) into electrospun PLGA/collagen fiber mats and tested their potential utility in wound healing applications. These researchers also confirmed the bioactivity of the released molecules in vitro [171].
5.3. Cosmetics/skin care
Electrospun fiber mats have been developed as cosmetic skin care masks due to their ability to be molded to the topography of the skin [135]. For skin care and renewal applications, various therapeutic molecules could be incorporated into the nanofibers (summarized in Table 2). For example, Sheng et al. impregnated silk fibroin nanofibers with Vitamin E, an antioxidant and stabilizer of biological membranes [172]. All formulations exhibited a burst release for the first 30 min followed by slow release for the next 72 h. In vitro studies also showed that Vitamin E-loaded silk fibroin fiber mats enhanced mouse skin fibroblast (L929 cells) viability under oxidative stress. In addition, Taepaiboon et al. demonstrated the potential use of electrospun Vitamin A (retinoic acid)-loaded fibers in cosmetic applications [173].
5.4. Anesthetics
Local anesthetics, such as lidocaine hydrochloride or bupivacaine, are commonly used for pain management during surgical procedures. Most commonly, these require multiple injections to infiltrate a region. Weldon et al. developed electrospun fibers that provided sustained local analgesia using bupivacaine [174]. Efficacy was demonstrated in a rat model where local analgesia was reported in 9 of 10 animals treated with bupivacaine-loaded fibers, with an onset of effect at day 1 and the maximum noted at day 3, followed by loss of the effect between 7 and 9 days. Chen et al. developed a sandwich-structure electrospun fiber mat (two outer layers were made of PLGA/collagen fibers, and the inner layer consisted of PLGA fibers containing three drugs), which released gentamycin and vancomycin (antibiotics) and lidocaine (anesthetic) for more than 3 weeks and demonstrated promising repair of infected wounds, in an in vivo rat model [175]. Table 2 summarizes examples of fiber mats used as topical drug delivery vehicles for anesthetics.
5.5. Keloids
A keloid is an excessive and abnormal type of scar tissue, which is firm, fibrous and extends beyond the normal boundaries of the healing cutaneous wound. It results from an overgrowth of granulation tissue produced by fibroblasts, but unlike a hypertrophic scar, a keloid grows beyond the margins of the original injury. Dexamethasone (DEX) has been shown to induce keloid regression by suppressing endogenous (not exogenous) VEGF expression and the proliferation of fibroblasts [176]. Li et al. impregnated DEX into PLGA fibers using a standard electrospinning setup and showed ~40% release of the payload within 10 days, followed by very slow release for another 15 days (t = 25 days) [177]. Keloids treated with DEX-loaded PLGA fiber mats, in a mouse model, were significantly reduced in size compared to silicon gel sheet-treated controls. To further enhance this reduction in size, green tea polyphenols (GTP)were co-spun with DEX and PLGA to fabricate DEX/GTP-loaded PLGA fibermats. It was shown that the addition of hydrophilic GTP into the PLGA fiber mat enhanced the release of hydrophobic DEX from the fiber mats. Further, the addition of GTP significantly enhanced the disappearance of distinguishing keloid characteristics in the mouse model, as noted by histological analyses [177]. Table 2 summarizes examples of fiber mats used as topical drug delivery vehicles for keloids.
5.6. Electrospun sutures
Sutures can provoke pain, inflammatory reactions, or infection. Therefore, researchers have explored the use of electrospinning to produce drug-loaded ultrafine fibers as sutures, aiming to reduce these side effects (Table 2). He et al. incorporated an antibiotic, tetracycline hydrochloride (tetracycline-HCl), into PLLA fibrous sutures to minimize bacterial colonization [178]. It was shown that tetracycline HCl-loaded PLLA fibers electrospun from a standard setup released 30% of its payload within the first 8 h, followed by very slow release kinetics. On the other hand, core-shell tetracycline HCl-loaded PLLA exhibited a slight burst within the first 2 h, followed by sustained release for at least 5 days. Although the breaking strength of these fibers was three times less than a commercial suture, electrospinning is a promising approach for the production of drug-loaded, fibrous sutures. Hu et al. developed braided PLLA nanofiber sutures as a drug delivery system for cefotaxime sodium, a semisynthetic third-generation antibiotic, and showed effective bactericidal activity against E. coli and S. aureus [179].
6. Formulation of nanoparticles and nanofibers for topical application in the clinic
Topical drug delivery vehicles (e.g., nanoparticles and nanofibers) can provide therapeutic action directly to the targeted site, potentially reducing unwanted systemic side effects. There is a variety of ways that nanoparticles can be formulated for topical use in the clinic, for example, as solution/liquid formulations, dry formulations, or viscous formulations (i.e., creams, lotions, gels, ointments). The medium used to suspend the nanoparticles is typically selected with a specific clinical application in mind. This medium should be biocompatible and used to facilitate percutaneous absorption. The suspending medium may also alter the release kinetics of the drug from the nanoparticles [32]. A recent review highlights the considerations employed for the development of topical dermatologic formulations [180].
Fiber mats can be applied directly onto a wound as drug-eluting dressings due to their malleable handling properties. The fiber mats may be covered with other clinically used, non-adherent bandages to maintain a moist wound environment. A recent review focuses on the design of electrospun fiber mats for tissue engineering, drug delivery and wound healing applications [181].
7. Conclusion
From the plethora of research involving nanoparticles in the past two decades, it is clear that nanoparticles have the potential to effectively deliver drugs across the skin barrier. Solid lipid nanoparticles and liposomes offer potential value as topical drug delivery systems in addition to polymeric and metal-based nanoparticles. Electrospun nanofibers have also shown great promise in the area of wound healing and as antimicrobial dressings. However, as demonstrated by the small number of advanced clinical studies, the clinical impact of nanoparticles and fibermats as topical or transdermal drug delivery systems has been limited. It seems that the translation of a very extensive global research effort into clinically used products has been slow. Although nanotechnology has proven promise in topical applications, a greater emphasis is needed on quantitative studies that can relate the dose and exposure of nanoparticle to nanoparticle penetration and therapeutic efficacy. More quantitative studies are warranted in the area of nanoparticle toxicity. Also, comparisons are lacking between healthy skin versus diseased skin in the context of nanoparticle penetration efficiency. Little is known about the mechanisms of nanoparticle transport through the three main skin layers. Another interesting observation is that very few studies use commercially relevant controls, e.g., the effect of a nanoparticular drug formulation is often compared against no treatment or drug-free formulation instead of using the current clinical “gold standard” as a point of reference. Consequently, there are many interesting unanswered questions and technical challenges that provide significant opportunity for further investigative studies.
Acknowledgments
This work was supported by the Department of Defense as part of the Joint Warfighter Medical Research Program (JWMRP) effort, under Award No. W81XWH-14-1-0100. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The authors gratefully acknowledge the suggestions made by Dr. Bozena Michniak-Kohn to improve the skin section of the review. Chem Bio Draw Ultra 14 licensed to Rutgers University was used for generating some of the figures.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Prost-Squarcioni C. Histology of skin and hair follicle. Med. Sci. 2006;22:131–137. doi: 10.1051/medsci/2006222131. [DOI] [PubMed] [Google Scholar]
- 2.Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003;42:1–36. doi: 10.1016/s0163-7827(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 3.Bhandari KH, Lee DX, Newa M, Sung Y, II, Kim JS, Kim DD, Kim J-A, Yoo BK, Woo JS, Lyoo WS, Lee JH, Choi HG, Yong CS. Evaluation of skin permeation and accumulation profiles of a highly lipophilic fatty ester. Arch. Pharm. Res. 2008;31:242–249. doi: 10.1007/s12272-001-1148-8. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Batheja P, Song Y, Wertz P, Michniak-Kohn B. Effects of growth conditions on the barrier properties of a human skin equivalent. Pharm. Res. 2009;26:1689–1700. doi: 10.1007/s11095-009-9879-1. [DOI] [PubMed] [Google Scholar]
- 6.Naik A, Pec htold LARM, Potts RO, Guy RH. Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. J. Control. Release. 1995;37:299–306. [Google Scholar]
- 7.Kumar R, Philip A. Modified transdermal technologies: breaking the barriers of drug permeation via the skin. Trop. J. Pharm. Res. 2007;6:633–644. [Google Scholar]
- 8.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escobar-Chávez JJ, Rodriguez-Cruz IM, Dominguez-Delgado CL. Chemical and physical enhancers for transdermal drug delivery. Pharmacology. 2012 [Google Scholar]
- 10.Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticlesbased topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013;5:205–218. doi: 10.1002/wnan.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Bansal R, Gupta S, Jindal N, Jindal A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013;4:267–272. doi: 10.4103/2229-5178.120635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo HS, Kim TG, Park TG. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009;61:1033–1042. doi: 10.1016/j.addr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Jin MX, Hu QH. Characterization and application in bioadhesive drug delivery system of chitosan. Cent. South Pharm. 2008;6:324–327. [Google Scholar]
- 14.Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomedicine. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodnar M, Hartmann JF, Borbely J. Preparation and characterization of chitosanbased nanoparticles. Biomacromolecules. 2005;6:2521–2527. doi: 10.1021/bm0502258. [DOI] [PubMed] [Google Scholar]
- 16.El-Shabouri MH. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002;249:101–108. doi: 10.1016/s0378-5173(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 17.de Moura MR, Aouada FA, Mattoso LH. Preparation of chitosan nanoparticles using methacrylic acid. J. Colloid Interface Sci. 2008;321:477–483. doi: 10.1016/j.jcis.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28:2233–2243. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Cheng MH, Huang YX, Zhou HJ, Liu Z, Li JF. Rapid preparation and characterization of chitosan nanoparticles for oligonucleotide. Curr. Appl. Phys. 2010;10:797–800. [Google Scholar]
- 20.Kim DG, Jeong YI, Choi C, Roh SH, Kang SK, Jang MK, Nah JW. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 2006;319:130–138. doi: 10.1016/j.ijpharm.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Prabaharan M, Mano JF. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005;12:41–57. doi: 10.1080/10717540590889781. [DOI] [PubMed] [Google Scholar]
- 22.Kashyap N, Viswanad B, Sharma G, Bhardwaj V, Ramarao P, Ravi Kumar MN. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials. 2007;28:2051–2060. doi: 10.1016/j.biomaterials.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim MM, Abd-Elgawad AH, Soliman OA, Jablonski MM. Natural bioadhesive biodegradable nanoparticle-based topical ophthalmic formulations for management of glaucoma. Transl. Vis. Sci. Technol. 2015;4:12. doi: 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman AJ, Phan J, Schairer DO, Champer J, Qin M, Pirouz A, Blecher-Paz K, Oren A, Liu PT, Modlin RL, Kim J. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: a targeted therapy for cutaneous pathogens. J. Investig. Dermatol. 2013;133:1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S, Bellare JR, Banerjee R. Protein based nanoparticles as platforms for aspirin delivery for ophthalmologic applications. Colloids Surf. B Biointerfaces. 2012;93:161–168. doi: 10.1016/j.colsurfb.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 27.Nardin C, Bolikal D, Kohn J. Nontoxic block copolymer nanospheres: design and characterization. Langmuir. 2004;20:11721–11725. doi: 10.1021/la0490285. [DOI] [PubMed] [Google Scholar]
- 28.Sheihet L, Dubin RA, Devore D, Kohn J. Hydrophobic drug delivery by self-assembling triblock copolymer-derived nanospheres. Biomacromolecules. 2005;6:2726–2731. doi: 10.1021/bm050212u. [DOI] [PubMed] [Google Scholar]
- 29.Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int. J. Pharm. 2008;350:312–319. doi: 10.1016/j.ijpharm.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Sheihet L, Garbuzenko OB, Bushman J, Gounder MK, Minko T, Kohn J. Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: in vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor. Eur. J. Pharm. Sci. 2012;45:320–329. doi: 10.1016/j.ejps.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release. 2011;149:159–167. doi: 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Goyal R, Macri L, Kohn J. Formulation strategy for the delivery of cyclosporine A: comparison of two polymeric nanospheres. Sci. Rep. 2015;5:13065. doi: 10.1038/srep13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel N, Salmasi A, Dinizo M, Goyal R, Hannan J, Lee GT, Kohn J, Bivalacqua TJ, Kim IY. Development of a nanosphere based delivery of sildenafil to sites of nerve injury for prevention of post-prostatectomy erectile dysfunction using a rat model of cavernous nerve injury. J. Urol. 2014;4:e481. [Google Scholar]
- 34.Patel N, Salmasi A, Dinizo M, Faiena I, Goyal R, Lee GT, Hannan JL, Bivalacqua TJ, Kohn J, Kim IY. Efficacy of rolipram loaded nanospheres in localized delivery to sites of nerve injury for prevention of post-prostatectomy erectile dysfunction using a rat model of cavernous nerve injury. J. Urol. 2015;4:e766–e767. [Google Scholar]
- 35.Bushman J, Vaughan A, Sheihet L, Zhang Z, Costache M, Kohn J. Functionalized nanospheres for targeted delivery of paclitaxel. J. Control. Release. 2013;171:315–321. doi: 10.1016/j.jconrel.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Kilfoyle BE, Sheihet L, Zhang Z, Laohoo M, Kohn J, Michniak-Kohn BB. Development of paclitaxel-TyroSpheres for topical skin treatment. J. Control. Release. 2012;163:18–24. doi: 10.1016/j.jconrel.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheihet L, Piotrowska K, Dubin RA, Kohn J, Devore D. Effect of tyrosine-derived triblock copolymer compositions on nanosphere self-assembly and drug delivery. Biomacromolecules. 2007;8:998–1003. doi: 10.1021/bm060860t. [DOI] [PubMed] [Google Scholar]
- 38.Kapoor DN, Bhatia A, Kaur R, Sharma R, Kaur G, Dhawan S. PLGA: a unique polymer for drug delivery. Ther. Deliv. 2015;6:41–58. doi: 10.4155/tde.14.91. [DOI] [PubMed] [Google Scholar]
- 39.Dash TK, Konkimalla VB. Poly-epsilon-caprolactone based formulations for drug delivery and tissue engineering: a review. J. Control. Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 40.Shahin M, Lavasanifar A. Novel self-associating poly(ethylene oxide)-bpoly(epsilon-caprolactone) based drug conjugates and nano-containers for paclitaxel delivery. Int. J. Pharm. 2010;389:213–222. doi: 10.1016/j.ijpharm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Abedalwafa M, Wang F, Wang L, Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev. Adv. Mater. Sci. 2013;34:123–140. [Google Scholar]
- 42.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery — a review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 43.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 44.Jeon HS, Seo JE, Kim MS, Kang MH, Oh DH, Jeon SO, Seong Hoon J, Choi YW, Lee S. A retinyl palmitate-loaded solid lipid nanoparticle system: effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013;452:311–320. doi: 10.1016/j.ijpharm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014;103:29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scalia S, Franceschinis E, Bertelli D, Iannuccelli V. Comparative evaluation of the effect of permeation enhancers, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Skin Pharmacol. Physiol. 2013;26:57–67. doi: 10.1159/000345210. [DOI] [PubMed] [Google Scholar]
- 47.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 48.Szoka F, Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. U. S. A. 1978;75:4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentine P, Bourel-Bonnet L, Frisch B. Modified and derived ethanol injection toward liposomes: development of the process. J. Liposome Res. 2013;23:11–19. doi: 10.3109/08982104.2012.717298. [DOI] [PubMed] [Google Scholar]
- 50.Lasch J, Weissig V, Brandl M. Preparation of liposomes. Oxford/New York: Oxford University Press; 2003. [Google Scholar]
- 51.Frederiksen L, Anton K, van Hoogevest P, Keller HR, Leuenberger H. Preparation of liposomes encapsulating water-soluble compounds using supercritical carbon dioxide. J. Pharm. Sci. 1997;86:921–928. doi: 10.1021/js960403q. [DOI] [PubMed] [Google Scholar]
- 52.Barnadas-Rodriguez R, Sabes M. Factors involved in the production of liposomes with a high-pressure homogenizer. Int. J. Pharm. 2001;213:175–186. doi: 10.1016/s0378-5173(00)00661-x. [DOI] [PubMed] [Google Scholar]
- 53.Rameez S, Bamba I, Palmer AF. Large scale production of vesicles by hollow fiber extrusion: a novel method for generating polymersome encapsulated hemoglobin dispersions. Langmuir. 2010;26:5279–5285. doi: 10.1021/la9036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin. Pharmacokinet. 2006;45:1153–1176. doi: 10.2165/00003088-200645120-00002. [DOI] [PubMed] [Google Scholar]
- 55.Oude Blenke E, Mastrobattista E, Schiffelers RM. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013;10:1399–1410. doi: 10.1517/17425247.2013.805742. [DOI] [PubMed] [Google Scholar]
- 56.Shrestha H, Bala R, Arora S. Lipid-based drug delivery systems. J. Pharm. 2014;2014:10. doi: 10.1155/2014/801820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marin S, Vlasceanu GM, Tiplea RE, Bucur IR, Lemnaru M, Marin MM, Grumezescu AM. Applications and toxicity of silver nanoparticles: a recent review. Curr. Top. Med. Chem. 2015;15:1596–1604. doi: 10.2174/1568026615666150414142209. [DOI] [PubMed] [Google Scholar]
- 58.Singh R, Shedbalkar UU, Wadhwani SA, Chopade BA. Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015;99:4579–4593. doi: 10.1007/s00253-015-6622-1. [DOI] [PubMed] [Google Scholar]
- 59.Zhang T, Song YJ, Zhang XY, Wu JY. Synthesis of silver nanostructures by multistep methods. Sensors. 2014;14:5860–5889. doi: 10.3390/s140405860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y, Yu F, Park YS, Wang J, Shin MC, Chung HS, Yang VC. Co-administration of protein drugs with gold nanoparticles to enable percutaneous delivery. Biomaterials. 2010;31:9086–9091. doi: 10.1016/j.biomaterials.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robichaud CO, Uyar AE, Darby MR, Zucker LG, Wiesner MR. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 2009;43:4227–4233. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- 62.Chigurupati S, Mughal MR, Okun E, Das S, Kumar A, McCaffery M, Seal S, Mattson MP. Effects of cerium oxide nanoparticles on the growth of keratinocytes, fibroblasts and vascular endothelial cells in cutaneous wound healing. Biomaterials. 2013;34:2194–2201. doi: 10.1016/j.biomaterials.2012.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anselmann R. Nanoparticles and nanolayers in commercial applications. J. Nanoparticle Res. 2001;3:329–336. [Google Scholar]
- 64.Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv. Drug Deliv. Rev. 2013;65:1316–1330. doi: 10.1016/j.addr.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Shi X, Thomas TP, Myc LA, Kotlyar A, Baker JR., Jr Synthesis, characterization, and intracellular uptake of carboxyl-terminated poly(amidoamine) dendrimer-stabilized iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2007;9:5712–5720. doi: 10.1039/b709147h. [DOI] [PubMed] [Google Scholar]
- 66.Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK. Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001;34:181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- 67.Venuganti VV, Perumal OP. Poly(amidoamine) dendrimers as skin penetration enhancers: Influence of charge, generation, and concentration. J. Pharm. Sci. 2009;98:2345–2356. doi: 10.1002/jps.21603. [DOI] [PubMed] [Google Scholar]
- 68.Parat A, Bordeianu C, Dib H, Garofalo A, Walter A, Begin-Colin S, Felder-Flesch D. Dendrimer-nanoparticle conjugates in nanomedicine. Nanomedicine. 2015;10:977–992. doi: 10.2217/nnm.14.196. [DOI] [PubMed] [Google Scholar]
- 69.Vyas A, Kumar Sonker A, Gidwani B. Carrier-based drug delivery system for treatment of acne. The Scientific World JOURNAL. 2014;2014:276260. doi: 10.1155/2014/276260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castro GA, Ferreira LA. Novel vesicular and particulate drug delivery systems for topical treatment of acne. Expert Opin. Drug Deliv. 2008;5:665–679. doi: 10.1517/17425247.5.6.665. [DOI] [PubMed] [Google Scholar]
- 71.Reis CP, Martinho N, Rosado C, Fernandes AS, Roberto A. Design of polymeric nanoparticles and its applications as drug delivery systems for acne treatment. Drug Dev. Ind. Pharm. 2014;40:409–417. doi: 10.3109/03639045.2013.767826. [DOI] [PubMed] [Google Scholar]
- 72.Webster GF, Leyden JJ, Gross JA. Results of a Phase III, double-blind, randomized, parallel-group, non-inferiority study evaluating the safety and efficacy of isotretinoin-Lidose in patients with severe recalcitrant nodular acne. J. Drugs Dermatol. 2014;13:665–670. [PubMed] [Google Scholar]
- 73.Muller S, Ebnother M, Itin P. Green nail syndrome (Pseudomonas aeruginosa nail infection): two cases successfully treated with topical nadifloxacin, an acne medication. Case Reports Dermatol. 2014;6:180–184. doi: 10.1159/000365863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Basnet P, Skalko-Basnet N. Nanodelivery systems for improved topical antimicrobial therapy. Curr. Pharm. Des. 2013;19:7237–7243. doi: 10.2174/138161281941131219124856. [DOI] [PubMed] [Google Scholar]
- 75.Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalishwaralal K, BarathManiKanth S, Pandian SR, Deepak V, Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces. 2010;79:340–344. doi: 10.1016/j.colsurfb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol. Pharm. 2009;6:1388–1401. doi: 10.1021/mp900056g. [DOI] [PubMed] [Google Scholar]
- 78.Zheng Y, Xiao M, Jiang S, Ding F, Wang J. Coating fabrics with gold nanorods for colouring, UV-protection, and antibacterial functions. Nanoscale. 2013;5:788–795. doi: 10.1039/c2nr33064d. [DOI] [PubMed] [Google Scholar]
- 79.Addae E, Dong X, McCoy E, Yang C, Chen W, Yang L. Investigation of antimicrobial activity of photothermal therapeutic gold/copper sulfide core/shell nanoparticles to bacterial spores and cells. J. Biol. Eng. 2014;8:11. doi: 10.1186/1754-1611-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 81.Cui Y, Zhao Y, Tian Y, Zhang W, Lu X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 82.Leung YH, Ng AM, Xu X, Shen Z, Gethings LA, Wong MT, Chan CM, Guo MY, Ng YH, Djurisic AB, Lee PK, Chan WK, Yu LH, Phillips DL, Ma AP, Leung FC. Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small. 2014;10:1171–1183. doi: 10.1002/smll.201302434. [DOI] [PubMed] [Google Scholar]
- 83.Ahamed M, Alhadlaq HA, Khan MM, Karuppiah P, Aldhabi NA. Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles. J. Nanomater. 2014;2014:4. [Google Scholar]
- 84.Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomedicine. 2013;8:4467–4479. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carre G, Hamon E, Ennahar S, Estner M, Lett MC, Horvatovich P, Gies JP, Keller V, Keller N, Andre P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014;80:2573–2581. doi: 10.1128/AEM.03995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009;107:1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- 87.Misak H, Zacharias N, Song Z, Hwang S, Man KP, Asmatulu R, Yang SY. Skin cancer treatment by albumin/5-Fu loaded magnetic nanocomposite spheres in a mouse model. J. Biotechnol. 2013;164:130–136. doi: 10.1016/j.jbiotec.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Mohan SV, Chang AL. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr. Dermatol. Rep. 2014;3:40–45. doi: 10.1007/s13671-014-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 90.Surveillance Epidemiology and End Results (SEER) Bethesda, MD: National Cancer Institute; 2015. [Accessed 3 Sep 2015]. SEER Cancer Statistics Factsheets: Melanoma of the Skin. http://seer.cancer.gov/statfacts/html/melan.html. [Google Scholar]
- 91.Lu YG, Wang YY, Yang YD, Zhang XC, Gao Y, Yang Y, Zhang JB, Li GL. Efficacy of topical ALA-PDT combined with excision in the treatment of skin malignant tumor. Photodiagn. Photodyn. Ther. 2014;11:122–126. doi: 10.1016/j.pdpdt.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Berciano-Guerrero MA, Montesa-Pino A, Castaneda-Penalvo G, Munoz-Fernandez L, Rodriguez-Flores J. Nanoparticles in melanoma. Curr. Med. Chem. 2014;21:3701–3716. doi: 10.2174/0929867321666140716092512. [DOI] [PubMed] [Google Scholar]
- 93.Iwamoto T. Clinical application of drug delivery systems in cancer chemotherapy: review of the efficacy and side effects of approved drugs. Biol. Pharm. Bull. 2013;36:715–718. doi: 10.1248/bpb.b12-01102. [DOI] [PubMed] [Google Scholar]
- 94.Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J. Drug Target. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 95.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 96.Zhang H, Gong W, Wang ZY, Yuan SJ, Xie XY, Yang YF, Yang Y, Wang SS, Yang DX, Xuan ZX, Mei XG. Preparation, characterization, and pharmacodynamics of thermosensitive liposomes containing docetaxel. J. Pharm. Sci. 2014;103:2177–2183. doi: 10.1002/jps.24019. [DOI] [PubMed] [Google Scholar]
- 97.Strieth S, Dunau C, Michaelis U, Jager L, Gellrich D, Wollenberg B, Dellian M. Phase I/II clinical study on safety and antivascular effects of paclitaxel encapsulated in cationic liposomes for targeted therapy in advanced head and neck cancer. Head Neck. 2014;36:976–984. doi: 10.1002/hed.23397. [DOI] [PubMed] [Google Scholar]
- 98.Yano J, Hirabayashi K, Nakagawa S, Yamaguchi T, Nogawa M, Kashimori I, Naito H, Kitagawa H, Ishiyama K, Ohgi T, Irimura T. Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin. Cancer Res. 2004;10:7721–7726. doi: 10.1158/1078-0432.CCR-04-1049. [DOI] [PubMed] [Google Scholar]
- 99.Muthu MS, Feng SS. Theranostic liposomes for cancer diagnosis and treatment: current development and pre-clinical success. Expert Opin. Drug Deliv. 2013;10:151–155. doi: 10.1517/17425247.2013.729576. [DOI] [PubMed] [Google Scholar]
- 100.Dianzani C, Zara GP, Maina G, Pettazzoni P, Pizzimenti S, Rossi F, Gigliotti CL, Ciamporcero ES, Daga M, Barrera G. Drug delivery nanoparticles in skin cancers. BioMed Res. Int. 2014;2014:895986. doi: 10.1155/2014/895986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coimbra M, Rijcken CJ, Stigter M, Hennink WE, Storm G, Schiffelers RM. Antitumor efficacy of dexamethasone-loaded core-crosslinked polymeric micelles. J. Control. Release. 2012;163:361–367. doi: 10.1016/j.jconrel.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 102.Nazir S, Hussain T, Ayub A, Rashid U, MacRobert AJ. Nanomaterials in combating cancer: therapeutic applications and developments. Nanomedicine. 2014;10:19–34. doi: 10.1016/j.nano.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Shao R, Zhang XD, Chen C. Applications of nanotechnology formelanoma treatment, diagnosis, and theranostics. Int. J. Nanomedicine. 2013;8:2677–2688. doi: 10.2147/IJN.S45429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Battah S, Balaratnam S, Casas A, O'Neill S, Edwards C, Batlle A, Dobbin P, MacRobert AJ. Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Mol. Cancer Ther. 2007;6:876–885. doi: 10.1158/1535-7163.MCT-06-0359. [DOI] [PubMed] [Google Scholar]
- 105.Cooke J. When antibiotics can be avoided in skin inflammation and bacterial colonization: a review of topical treatments. Curr. Opin. Infect. Dis. 2014;27:125–129. doi: 10.1097/QCO.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 106.Nicolaou A, Pilkington SM, Rhodes LE. Ultraviolet-radiation induced skin inflammation: dissecting the role of bioactive lipids. Chem. Phys. Lipids. 2011;164:535–543. doi: 10.1016/j.chemphyslip.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Antonio JR, Antonio CR, Cardeal IL, Ballavenuto JM, Oliveira JR. Nanotechnology in dermatology. An. Bras. Dermatol. 2014;89:126–136. doi: 10.1590/abd1806-4841.20142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol. Photoimmunol. Photomed. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 109.Singh P, Nanda A. Enhanced sun protection of nano-sized metal oxide particles over conventional metal oxide particles: an in vitro comparative study. Int. J. Cosmet. Sci. 2014;36:273–283. doi: 10.1111/ics.12124. [DOI] [PubMed] [Google Scholar]
- 110.Ryu HJ, Seo MY, Jung SK, Maeng EH, Lee SY, Jang DH, Lee TJ, Jo KY, Kim YR, Cho KB, Kim MK, Lee BJ, Son SW. Zinc oxide nanoparticles: a 90-day repeateddose dermal toxicity study in rats. Int. J. Nanomedicine. 2014;9(Suppl. 2):137–144. doi: 10.2147/IJN.S57930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. Sunscreen: development,. efficacy, and controversies. J. Am. Acad. Dermatol. 2013;69:867. doi: 10.1016/j.jaad.2013.08.022. (e861-814; quiz 881-862) [DOI] [PubMed] [Google Scholar]
- 112.Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol. Physiol. 2007;20:148–154. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- 113.Darlenski R, Surber C, Fluhr JW. Topical retinoids in the management of photodamaged skin: from theory to evidence-based practical approach. Br. J. Dermatol. 2010;163:1157–1165. doi: 10.1111/j.1365-2133.2010.09936.x. [DOI] [PubMed] [Google Scholar]
- 114.Yamaguchi Y, Nakamura N, Nagasawa T, Kitagawa A, Matsumoto K, Soma Y, Matsuda T, Mizoguchi M, Igarashi R. Enhanced skin regeneration by nanoegg formulation of all-trans retinoic acid. Pharmazie. 2006;61:117–121. [PubMed] [Google Scholar]
- 115.Hojerova J. Coenzyme Q10—its importance, properties and use in nutrition and cosmetics. Ceska Slov. Farm. 2000;49:119–123. [PubMed] [Google Scholar]
- 116.Zhou H, Yue Y, Liu G, Li Y, Zhang J, Yan Z, Duan M. Characterisation and skin distribution of lecithin-based coenzyme Q10-loaded lipid nanocapsules. Nanoscale Res. Lett. 2010;5:1561–1569. doi: 10.1007/s11671-010-9677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao GD, Sun R, Ni SL, Xia Q. Development and characterisation of a novel chitosan-coated antioxidant liposome containing both coenzyme Q10 and alphalipoic acid. J. Microencapsul. 2015;32:157–165. doi: 10.3109/02652048.2014.973072. [DOI] [PubMed] [Google Scholar]
- 118.Manova E, von Goetz N, Hungerbuehler K. Aggregate consumer exposure to UV filter ethylhexyl methoxycinnamate via personal care products. Environ. Int. 2015;74:249–257. doi: 10.1016/j.envint.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 119.Puglia C, Bonina F, Castelli F, Micieli D, Sarpietro MG. Evaluation of percutaneous absorption of the repellent diethyltoluamide and the sunscreen ethylhexyl pmethoxycinnamate-loaded solid lipid nanoparticles: an in-vitro study. J. Pharm. Pharmacol. 2009;61:1013–1019. doi: 10.1211/jpp/61.08.0004. [DOI] [PubMed] [Google Scholar]
- 120.Vettor M, Perugini P, Scalia S, Conti B, Genta I, Modena T, Pavanetto F. Poly(D, L-lactide) nanoencapsulation to reduce photoinactivation of a sunscreen agent. Int. J. Cosmet. Sci. 2008;30:219–227. doi: 10.1111/j.1468-2494.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 121.Agarwal R, Iezhitsa I, Agarwal P, Abdul Nasir NA, Razali N, Alyautdin R, Ismail NM. Liposomes in topical ophthalmic drug delivery: an update. Drug Deliv. 2014:1–17. doi: 10.3109/10717544.2014.943336. [DOI] [PubMed] [Google Scholar]
- 122.Gan L, Wang J, Jiang M, Bartlett H, Ouyang D, Eperjesi F, Liu J, Gan Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today. 2013;18:290–297. doi: 10.1016/j.drudis.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 123.Venkatraman S, Wong T. How can nanoparticles be used to overcome the challenges of glaucoma treatment? Nanomedicine. 2014;9:1281–1283. doi: 10.2217/nnm.14.85. [DOI] [PubMed] [Google Scholar]
- 124.Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Chitosan and its role in ocular therapeutics. Mini Rev. Med. Chem. 2009;9:1639–1647. doi: 10.2174/138955709791012292. [DOI] [PubMed] [Google Scholar]
- 125.Gunasekaran T, Nigusse T, Dhanaraju MD. Silver nanoparticles as real topical bullets for wound healing. J. Am. Coll. Clin. Wound Spec. 2011;3:82–96. doi: 10.1016/j.jcws.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gainza G, Villullas S, Pedraz JL, Hernandez RM, Igartua M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine. 2015;11:1551–1573. doi: 10.1016/j.nano.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 127.Han G. State-of-the-art wound healing: skin substitutes for chronic wounds. Cutis. 2014;93:E13–E16. [PubMed] [Google Scholar]
- 128.Toy LW, Macera L. Evidence-based review of silver dressing use on chronic wounds. J. Am. Acad. Nurse Pract. 2011;23:183–192. doi: 10.1111/j.1745-7599.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 129.Alexander SJ. Time to get serious about assessing — and managing — psychosocial issues associated with chronic wounds. Curr. Opin. Support. Palliat. Care. 2013;7:95–100. doi: 10.1097/SPC.0b013e32835bf2a3. [DOI] [PubMed] [Google Scholar]
- 130.Koria P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs. 2012;26:163–175. doi: 10.2165/11631850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 131.Quinn JF, Whittaker MR, Davis TP. Delivering nitric oxide with nanoparticles. J. Control. Release. 2015;205:190–205. doi: 10.1016/j.jconrel.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Cheppudira B, Fowler M, McGhee L, Greer A, Mares A, Petz L, Devore D, Loyd DR, Clifford JL. Curcumin: a novel therapeutic for burn pain and wound healing. Expert Opin. Investig. Drugs. 2013;22:1295–1303. doi: 10.1517/13543784.2013.825249. [DOI] [PubMed] [Google Scholar]
- 133.Krausz AE, Adler BL, Cabral V, Navati M, Doerner J, Charafeddine RA, Chandra D, Liang H, Gunther L, Clendaniel A, Harper S, Friedman JM, Nosanchuk JD, Friedman AJ. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomedicine. 2015;11:195–206. doi: 10.1016/j.nano.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cooley JF. Improved Methods of and Apparatus for Electrically Separating the Relatively Volatile Liquid Component from the Component of Relatively Fixed Substances of Composite Fluids. 1900 [Google Scholar]
- 135.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 136.Verreck G, Chun I, Peeters J, Rosenblatt J, Brewster ME. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003;20:810–817. doi: 10.1023/a:1023450006281. [DOI] [PubMed] [Google Scholar]
- 137.Wallace GG, Higgins MJ, Moulton SE, Wang C. Nanobionics: the impact of nanotechnology on implantable medical bionic devices. Nanoscale. 2012;4:4327–4347. doi: 10.1039/c2nr30758h. [DOI] [PubMed] [Google Scholar]
- 138.Qian W, Yu DG, Li Y, Liao YZ, Wang X, Wang L. Dual drug release electrospun core-shell nanofibers with tunable dose in the second phase. Int. J. Mol. Sci. 2014;15:774–786. doi: 10.3390/ijms15010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang Y, Li X, Cui W, Zhou S, Tan R, Wang C. Structural stability and release profiles of proteins from core-shell poly (DL-lactide) ultrafine fibers prepared by emulsion electrospinning. J. Biomed. Mater. Res. A. 2008;86:374–385. doi: 10.1002/jbm.a.31595. [DOI] [PubMed] [Google Scholar]
- 140.Thakur RA, Florek CA, Kohn J, Michniak BB. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008;364:87–93. doi: 10.1016/j.ijpharm.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 141.Verreck G, Chun I, Rosenblatt J, Peeters J, Dijck AV, Mensch J, Noppe M, Brewster ME. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release. 2003;92:349–360. doi: 10.1016/s0168-3659(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 142.Macri LK, Sheihet L, Singer AJ, Kohn J, Clark RA. Ultrafast and fast bioerodible electrospun fiber mats for topical delivery of a hydrophilic peptide. J. Control. Release. 2012;161:813–820. doi: 10.1016/j.jconrel.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 143.Jiang H, Fang D, Hsiao B, Chu B, Chen W. Preparation and characterization of ibuprofen-loaded poly(lactide-co-glycolide)/poly(ethylene glycol)-g-chitosan electrospun membranes. J. Biomater. Sci. Polym. Ed. 2004;15:279–296. doi: 10.1163/156856204322977184. [DOI] [PubMed] [Google Scholar]
- 144.Nair LS, Bhattacharyya S, Laurencin CT. Development of novel tissue engineering scaffolds via electrospinning. Expert. Opin. Biol. Ther. 2004;4:659–668. doi: 10.1517/14712598.4.5.659. [DOI] [PubMed] [Google Scholar]
- 145.Kong H, He J. A modified bubble elctrospinning for fabrication of nanofibers. J. Nano Res. 2013;23:125–128. [Google Scholar]
- 146.Varabhas JS, Chase CG, Reneker DH. Electrospun nanofibers from a porous hollow tube. Polymer. 2008;49:4226–4229. [Google Scholar]
- 147.Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J. Biomed. Mater. Res. B Appl. Biomater. 2004;70:286–296. doi: 10.1002/jbm.b.30041. [DOI] [PubMed] [Google Scholar]
- 148.Kim K, Luu YK, Chang C, Fang D, Hsiao BS, Chu B, Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Huang ZM, He CL, Yang A, Zhang Y, Han XJ, Yin J, Wu Q. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J. Biomed. Mater. Res. A. 2006;77:169–179. doi: 10.1002/jbm.a.30564. [DOI] [PubMed] [Google Scholar]
- 150.Jannesari M, Varshosaz J, Morshed M, Zamani M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrousmats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomedicine. 2011;6:993–1003. doi: 10.2147/IJN.S17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. Antimicrobial PLGA ultrafine fibers: interaction with wound bacteria. Eur. J. Pharm. Biopharm. 2011;79:108–118. doi: 10.1016/j.ejpb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 152.Said SS, El-Halfawy OM, El-Gowelli HM, Aloufy AK, Boraei NA, El-Khordagui LK. Bioburden-responsive antimicrobial PLGA ultrafine fibers for wound healing. Eur. J. Pharm. Biopharm. 2012;80:85–94. doi: 10.1016/j.ejpb.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 153.Tammaro L, Saturnino C, D'Aniello S, Vigliotta G, Vittoria V. Polymorphic solidification of Linezolid confined in electrospun PCL fibers for controlled release in topical applications. Int. J. Pharm. 2015;490:32–38. doi: 10.1016/j.ijpharm.2015.04.070. [DOI] [PubMed] [Google Scholar]
- 154.Alhusein N, De Bank PA, Blagbrough IS, Bolhuis A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly(ethylene-co-vinyl acetate) Drug Deliv. Transl. Res. 2013;3:531–541. doi: 10.1007/s13346-013-0164-9. [DOI] [PubMed] [Google Scholar]
- 155.Ignatova M, Markova N, Manolova N, Rashkov I. Antibacterial and antimycotic activity of a cross-linked electrospun poly(vinyl pyrrolidone)-iodine complex and a poly(ethylene oxide)/poly(vinyl pyrrolidone)-iodine complex. J. Biomater. Sci. Polym. Ed. 2008;19:373–386. doi: 10.1163/156856208783721056. [DOI] [PubMed] [Google Scholar]
- 156.Thomas R, Soumya KR, Mathew J, Radhakrishnan EK. Inhibitory effect of silver nanoparticle fabricated urinary catheter on colonization efficiency of Coagulase Negative Staphylococci. J. Photochem. Photobiol. B. 2015;149:68–77. doi: 10.1016/j.jphotobiol.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 157.Dashdorj U, Reyes MK, Unnithan AR, Tiwari AP, Tumurbaatar B, Park CH, Kim CS. Fabrication and characterization of electrospun zein/Ag nanocomposite mats for wound dressing applications. Int. J. Biol. Macromol. 2015;80:1–7. doi: 10.1016/j.ijbiomac.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 158.GhavamiNejad A, Rajan Unnithan A, Ramachandra Kurup Sasikala A, Samarikhalaj M, Thomas RG, Jeong YY, Nasseri S, Murugesan P, Wu D, Hee Park C, Kim CS. Mussel-inspired electrospun nanofibers functionalized with size-controlled silver nanoparticles for wound dressing application. ACS Appl. Mater. Interfaces. 2015;7:12176–12183. doi: 10.1021/acsami.5b02542. [DOI] [PubMed] [Google Scholar]
- 159.Woo CH, Choi YC, Choi JS, Lee HY, Cho YW. A bilayer composite composed of TiO2-incorporated electrospun chitosan membrane and human extracellular matrix sheet as a wound dressing. J. Biomater. Sci. Polym. Ed. 2015;26:841–854. doi: 10.1080/09205063.2015.1061349. [DOI] [PubMed] [Google Scholar]
- 160.Macri L, Clark RA. Tissue engineering for cutaneous wounds: selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol. Physiol. 2009;22:83–93. doi: 10.1159/000178867. [DOI] [PubMed] [Google Scholar]
- 161.Wang Z, Qian Y, Li L, Pan L, Njunge LW, Dong L, Yang L. Evaluation of emulsion electrospun polycaprolactone/hyaluronan/epidermal growth factor nanofibrous scaffolds for wound healing. J. Biomater. Appl. 2015 doi: 10.1177/0885328215586907. [DOI] [PubMed] [Google Scholar]
- 162.Gumusderelioglu M, Dalkiranoglu S, Aydin RS, Cakmak S. A novel dermal substitute based on biofunctionalized electrospun PCL nanofibrous matrix. J. Biomed. Mater. Res. A. 2011;98:461–472. doi: 10.1002/jbm.a.33143. [DOI] [PubMed] [Google Scholar]
- 163.Norouzi M, Shabani I, Ahvaz HH, Soleimani M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J. Biomed. Mater. Res. A. 2015;103:2225–2235. doi: 10.1002/jbm.a.35355. [DOI] [PubMed] [Google Scholar]
- 164.Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009;5:2570–2578. doi: 10.1016/j.actbio.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials. 2011;32:4243–4254. doi: 10.1016/j.biomaterials.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 166.Zou J, Yang Y, Liu Y, Chen F, Li X. Release kinetics and cellular profiles for bFGF-loaded electrospun fibers: Effect of the conjugation density and molecular weight of heparin. Polymer. 2011;52:3357–3367. [Google Scholar]
- 167.Lai HJ, Kuan CH, Wu HC, Tsai JC, Chen TM, Hsieh DJ, Wang TW. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014;10:4156–4166. doi: 10.1016/j.actbio.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Apelqvist J, Castenfors J, Larsson J, Stenstrom A, Persson G. Ketanserin in the treatment of diabetic foot ulcer with severe peripheral vascular disease. Int. Angiol. 1990;9:120–124. [PubMed] [Google Scholar]
- 169.Quatresooz P, Kharfi M, Paquet P, Vroome V, Cauwenbergh G, Pierard GE. Healing effect of ketanserin on chronic leg ulcers in patients with diabetes. J. Eur. Acad. Dermatol. Venereol. 2006;20:277–281. doi: 10.1111/j.1468-3083.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 170.Zhu J, Lin F, Brown DA, Clark RA. A fibronectin peptide redirects PDGF-BB/PDGFR complexes to macropinocytosis-like internalization and augments PDGF-BB survival signals. J. Investig. Dermatol. 2014;134:921–929. doi: 10.1038/jid.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peh P, Lim NS, Blocki A, Chee SM, Park HC, Liao S, Chan C, Raghunath M. Simultaneous delivery of highly diverse bioactive compounds from blend electrospun fibers for skin wound healing. Bioconjug. Chem. 2015;26:1348–1358. doi: 10.1021/acs.bioconjchem.5b00123. [DOI] [PubMed] [Google Scholar]
- 172.Sheng X, Fan L, He C, Zhang K, Mo X, Wang H. Vitamin E-loaded silk fibroin nanofibrous mats fabricated by green process for skin care application. Int. J. Biol. Macromol. 2013;56:49–56. doi: 10.1016/j.ijbiomac.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 173.Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur. J. Pharm. Biopharm. 2007;67:387–397. doi: 10.1016/j.ejpb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 174.Weldon CB, Tsui JH, Shankarappa SA, Nguyen VT, Ma M, Anderson DG, Kohane DS. Electrospun drug-eluting sutures for local anesthesia. J. Control. Release. 2012;161:903–909. doi: 10.1016/j.jconrel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen DW, Liao JY, Liu SJ, Chan EC. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: an in vitro and in vivo study. Int. J. Nanomedicine. 2012;7:763–771. doi: 10.2147/IJN.S29119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J. Investig. Dermatol. 2006;126:1264–1271. doi: 10.1038/sj.jid.5700274. [DOI] [PubMed] [Google Scholar]
- 177.Li J, Fu R, Li L, Yang G, Ding S, Zhong Z, Zhou S. Co-delivery of dexamethasone and green tea polyphenols using electrospun ultrafine fibers for effective treatment of keloid. Pharm. Res. 2014;31:1632–1643. doi: 10.1007/s11095-013-1266-2. [DOI] [PubMed] [Google Scholar]
- 178.He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J. Biomed. Mater. Res. A. 2009;89:80–95. doi: 10.1002/jbm.a.32004. [DOI] [PubMed] [Google Scholar]
- 179.Hu W, Huang ZM, Liu XY. Development of braided drug-loaded nanofiber sutures. Nanotechnology. 2010;21:315104. doi: 10.1088/0957-4484/21/31/315104. [DOI] [PubMed] [Google Scholar]
- 180.Chang RK, Raw A, Lionberger R, Yu L. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013;15:41–52. doi: 10.1208/s12248-012-9411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goh YF, Shakir I, Hussain R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013;48:3027–3054. [Google Scholar]
- 182.Jain AK, Jain A, Garg NK, Agarwal A, Jain A, Jain SA, Tyagi RK, Jain RK, Agrawal H, Agrawal GP. Adapalene loaded solid lipid nanoparticles gel: an effective approach for acne treatment. Colloids Surf. B Biointerfaces. 2014;121:222–229. doi: 10.1016/j.colsurfb.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 183.Guo C, Khengar RH, Sun M, Wang Z, Fan A, Zhao Y. Acid-responsive polymeric nanocarriers for topical adapalene delivery. Pharm. Res. 2014;31:3051–3059. doi: 10.1007/s11095-014-1398-z. [DOI] [PubMed] [Google Scholar]
- 184.Raza K, Singh B, Singal P, Wadhwa S, Katare OP. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf. B Biointerfaces. 2013;105:67–74. doi: 10.1016/j.colsurfb.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 185.Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int. J. Pharm. 2007;328:191–195. doi: 10.1016/j.ijpharm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 186.Raza K, Singh B, Singla N, Negi P, Singal P, Katare OP. Nano-lipoidal carriers of isotretinoin with anti-aging potential: formulation, characterization and biochemical evaluation. J. Drug Target. 2013;21:435–442. doi: 10.3109/1061186X.2012.761224. [DOI] [PubMed] [Google Scholar]
- 187.Ridolfi DM, Marcato PD, Justo GZ, Cordi L, Machado D, Duran N. Chitosan-solid lipid nanoparticles as carriers for topical delivery of tretinoin. Colloids Surf. B Biointerfaces. 2012;93:36–40. doi: 10.1016/j.colsurfb.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 188.Ourique AF, Melero A, de Bona da Silva C, Schaefer UF, Pohlmann AR, Guterres SS, Lehr CM, Kostka KH, Beck RC. Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur. J. Pharm. Biopharm. 2011;79:95–101. doi: 10.1016/j.ejpb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 189.Raza K, Singh B, Lohan S, Sharma G, Negi P, Yachha Y, Katare OP. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013;456:65–72. doi: 10.1016/j.ijpharm.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 190.Pokharkar VB, Mendiratta C, Kyadarkunte AY, Bhosale SH, Barhate GA. Skin delivery aspects of benzoyl peroxide-loaded solid lipid nanoparticles for acne treatment. Ther. Deliv. 2014;5:635–652. doi: 10.4155/tde.14.31. [DOI] [PubMed] [Google Scholar]
- 191.Fluhr JW, Barsom O, Gehring W, Gloor M. Antibacterial efficacy of benzoyl peroxide in phospholipid liposomes. A vehicle-controlled, comparative study in patients with papulopustular acne. Dermatology. 1999;198:273–277. doi: 10.1159/000018129. [DOI] [PubMed] [Google Scholar]
- 192.Castro GA, Oliveira CA, Mahecha GA, Ferreira LA. Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch. Dermatol. Res. 2011;303:513–520. doi: 10.1007/s00403-011-1130-3. [DOI] [PubMed] [Google Scholar]
- 193.Stecova J, Mehnert W, Blaschke T, Kleuser B, Sivaramakrishnan R, Zouboulis CC, Seltmann H, Korting HC, Kramer KD, Schafer-Korting M. Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: particle characterisation and skin uptake. Pharm. Res. 2007;24:991–1000. doi: 10.1007/s11095-006-9225-9. [DOI] [PubMed] [Google Scholar]
- 194.Dominguez-Delgado CL, Rodriguez-Cruz IM, Escobar-Chavez JJ, Calderon-Lojero IO, Quintanar-Guerrero D, Ganem A. Preparation and characterization of triclosan nanoparticles intended to be used for the treatment of acne. Eur. J. Pharm. Biopharm. 2011;79:102–107. doi: 10.1016/j.ejpb.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 195.Honzak L, Sentjurc M. Development of liposome encapsulated clindamycin for treatment of acne vulgaris. Pflugers Arch. 2000;440:R44–R45. [PubMed] [Google Scholar]
- 196.Bhalerao SS, RajeHarshal A. Preparation, optimization, characterization, and stability studies of salicylic acid liposomes. Drug Dev. Ind. Pharm. 2003;29:451–467. doi: 10.1081/ddc-120018380. [DOI] [PubMed] [Google Scholar]
- 197.Yang D, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, Zhang L. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009;30:6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kumar R, Singh B, Bakshi G, Katare OP. Development of liposomal systems of finasteride for topical applications: design, characterization, and in vitro evaluation. Pharm. Dev. Technol. 2007;12:591–601. doi: 10.1080/10837450701481181. [DOI] [PubMed] [Google Scholar]
- 199.Xu Y, Yang X, Xu X. Preparation and evaluation of tea tree oil liposome. West China J. Pharm. Sci. 2006 [Google Scholar]
- 200.Jigar V, Vishal G, Tejas G, Vishal C, Umesh U. Formulation and characterization of topical gel of erythromycin entrapped into niosomes. Int. J. PharmTech Res. 2011;3:5. [Google Scholar]
- 201.Czermak P, Steinle T, Ebrahimi M, Schmidts T, Runkel F. Membrane-assisted production of S1P loaded SLNs for the treatment of acne vulgaris. Desalination. 2010;250:4. [Google Scholar]
- 202.Saravanan M, Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf. B Biointerfaces. 2010;77:214–218. doi: 10.1016/j.colsurfb.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 203.Matai I, Sachdev A, Dubey P, Kumar SU, Bhushan B, Gopinath P. Antibacterial activity and mechanism of Ag-ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces. 2014;115:359–367. doi: 10.1016/j.colsurfb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 204.Perni S, Piccirillo C, Pratten J, Prokopovich P, Chrzanowski W, Parkin IP, Wilson M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials. 2009;30:89–93. doi: 10.1016/j.biomaterials.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 205.Lima E, Guerra R, Lara V, Guzman A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Cent. J. 2013;7:11. doi: 10.1186/1752-153X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Roy AS, Parveen A, Koppalkar AR, Prasad M. Effect of Nano-Titanium Dioxide with Different Antibiotics against Methicillin-Resistant Staphylococcus aureus. J. Biomater. Nanobiotechnol. 2010:5. [Google Scholar]
- 207.Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomedicine. 2012;7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnology. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug. Chem. 2009;20:1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- 210.Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res. Lett. 2008;3:5. [Google Scholar]
- 211.Sun L, Singh AK, Vig K, Pillai SR, Singh SR. Silver nanoparticles inhibit replication of respiratory syncytial virus. J. Biotechnol. 2008;4:10. [Google Scholar]
- 212.Di Gianvincenzo P, Marradi M, Martinez-Avila OM, Bedoya LM, Alcami J, Penades S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg. Med. Chem. Lett. 2010;20:2718–2721. doi: 10.1016/j.bmcl.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 213.Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 214.Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5:382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 215.Haghighi F, Mohammadi SR, Mohammadi P, Hosseinkhani S, Shipour R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans Biofilms. Infect. Epidemiol. Med. 2013;1:6. [Google Scholar]
- 216.Guo S, Wang Y, Miao L, Xu Z, Lin CH, Huang L. Turning a water and oil insoluble cisplatin derivative into a nanoparticle formulation for cancer therapy. Biomaterials. 2014;35:7647–7653. doi: 10.1016/j.biomaterials.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang Q, Aires DJ, Cai S, Fraga GR, Zhang D, Li CZ, Forrest ML. In vivo efficacy of nano hyaluronan-conjugated cisplatin for treatment of murine melanoma. J. Drugs Dermatol. 2014;13:283–287. [PMC free article] [PubMed] [Google Scholar]
- 218.Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7957–7961. doi: 10.1073/pnas.0902857106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anti-Cancer Drugs. 1999;10:767–776. [PubMed] [Google Scholar]
- 220.Falvo E, Tremante E, Fraioli R, Leonetti C, Zamparelli C, Boffi A, Morea V, Ceci P, Giacomini P. Antibody-drug conjugates: targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale. 2013;5:12278–12285. doi: 10.1039/c3nr04268e. [DOI] [PubMed] [Google Scholar]
- 221.Bedikian AY, Papadopoulos NE, Kim KB, Vardeleon A, Smith T, Lu B, Deitcher SR. A pilot study with vincristine sulfate liposome infusion in patients with metastatic melanoma. Melanoma Res. 2008;18:400–404. doi: 10.1097/CMR.0b013e328311aaa1. [DOI] [PubMed] [Google Scholar]
- 222.Yuan M, Qiu Y, Zhang L, Gao H, He Q. Targeted delivery of transferrin and TAT comodified liposomes encapsulating both paclitaxel and doxorubicin for melanoma. Drug Deliv. 2015:1–13. doi: 10.3109/10717544.2015.1040527. [DOI] [PubMed] [Google Scholar]
- 223.Taveira SF, Araujo LM, de Santana DC, Nomizo A, de Freitas LA, Lopez RF. Development of cationic solid lipid nanoparticles with factorial design-based studies for topical administration of doxorubicin. J. Biomed. Nanotechnol. 2012;8:219–228. doi: 10.1166/jbn.2012.1383. [DOI] [PubMed] [Google Scholar]
- 224.Jin YJ, Termsarasab U, Ko SH, Shim JS, Chong S, Chung SJ, Shim CK, Cho HJ, Kim DD. Hyaluronic acid derivative-based self-assembled nanoparticles for the treatment of melanoma. Pharm. Res. 2012;29:3443–3454. doi: 10.1007/s11095-012-0839-9. [DOI] [PubMed] [Google Scholar]
- 225.Al-Jamal KT, Al-Jamal WT, Wang JT, Rubio N, Buddle J, Gathercole D, Zloh M, Kostarelos K. Cationic poly-L-lysine dendrimer complexes doxorubicin and delays tumor growth in vitro and in vivo. ACS Nano. 2013;7:1905–1917. doi: 10.1021/nn305860k. [DOI] [PubMed] [Google Scholar]
- 226.Yadav D, Anwar MF, Garg V, Kardam H, Beg MN, Suri S, Gaur S, Asif M. Development of polymeric nanopaclitaxel and comparison with free paclitaxel for effects on cell proliferation of MCF-7 and B16F0 carcinoma cells. Asian Pac. J. Cancer Prev. 2014;15:2335–2340. doi: 10.7314/apjcp.2014.15.5.2335. [DOI] [PubMed] [Google Scholar]
- 227.Kalariya M, Padhi BK, Chougule M, Misra A. Clobetasol propionate solid lipid nanoparticles cream for effective treatment of eczema: formulation and clinical implications. Indian J. Exp. Biol. 2005;43:233–240. [PubMed] [Google Scholar]
- 228.Zhang J, Smith E. Percutaneous permeation of betamethasone 17-valerate incorporated in lipid nanoparticles. J. Pharm. Sci. 2011;100:896–903. doi: 10.1002/jps.22329. [DOI] [PubMed] [Google Scholar]
- 229.Ilves M, Palomaki J, Vippola M, Lehto M, Savolainen K, Savinko T, Alenius H. Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part. Fibre Toxicol. 2014;11:38. doi: 10.1186/s12989-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E, Sahudin S. Downregulation of immunological mediators in 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions by hydrocortisone-loaded chitosan nanoparticles. Int. J. Nanomedicine. 2014;9:5143–5156. doi: 10.2147/IJN.S71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rosado C, Silva C, Reis CP. Hydrocortisone-loaded poly(epsilon-caprolactone) nano-particles for atopic dermatitis treatment. Pharm. Dev. Technol. 2013;18:710–718. doi: 10.3109/10837450.2012.712537. [DOI] [PubMed] [Google Scholar]
- 232.Asz J, Asz D, Moushey R, Seigel J, Mallory SB, Foglia RP. Treatment of toxic epidermal necrolysis in a pediatric patient with a nanocrystalline silver dressing. J. Pediatr. Surg. 2006;41:e9–e12. doi: 10.1016/j.jpedsurg.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 233.Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNFalpha siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J. Control. Release. 2013;170:51–63. doi: 10.1016/j.jconrel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marianecci C, Rinaldi F, Di Marzio L, Mastriota M, Pieretti S, Celia C, Paolino D, Iannone M, Fresta M, Carafa M. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int. J. Nanomedicine. 2014;9:635–651. doi: 10.2147/IJN.S55066. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 235.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Osmond-McLeod MJ, Oytam Y, Kirby JK, Gomez-Fernandez L, Baxter B, McCall MJ. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology. 2014;8(Suppl. 1):72–84. doi: 10.3109/17435390.2013.855832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.van der Merwe D, Tawde S, Pickrell JA, Erickson LE. Nanocrystalline titanium dioxide and magnesium oxide in vitro dermal absorption in human skin. Cutan. Ocul. Toxicol. 2009;28:78–82. doi: 10.1080/15569520902914926. [DOI] [PubMed] [Google Scholar]
- 238.Xue C, Wu J, Lan F, Liu W, Yang X, Zeng F, Xu H. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J. Nanosci. Nanotechnol. 2010;10:8500–8507. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- 239.Durand L, Habran N, Henschel V, Amighi K. Encapsulation of ethylhexyl methoxycinnamate, a light-sensitive UV filter, in lipid nanoparticles. J. Microencapsul. 2010;27:714–725. doi: 10.3109/02652048.2010.513455. [DOI] [PubMed] [Google Scholar]
- 240.Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10:808–819. doi: 10.1208/s12249-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Quinteros D, Vicario-de-la-Torre M, Andres-Guerrero V, Palma S, Allemandi D, Herrero-Vanrell R, Molina-Martinez IT. Hybrid formulations of liposomes and bioadhesive polymers improve the hypotensive effect of the melatonin analogue 5-MCA-NAT in rabbit eyes. PLoS One. 2014;9:e110344. doi: 10.1371/journal.pone.0110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang F, Chen L, Zhang D, Jiang S, Shi K, Huang Y, Li R, Xu Q. Methazolamideloaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: vitro and vivo study. J. Drug Target. 2014;22:849–858. doi: 10.3109/1061186X.2014.939983. [DOI] [PubMed] [Google Scholar]
- 243.Yang H, Leffler CT. Hybrid dendrimer hydrogel/poly(lactic-co-glycolic acid) nanoparticle platform: an advanced vehicle for topical delivery of antiglaucoma drugs and a likely solution to improving compliance and adherence in glaucoma management. J. Ocul. Pharmacol. Ther. 2013;29:166–172. doi: 10.1089/jop.2012.0197. [DOI] [PubMed] [Google Scholar]
- 244.Souto EB, Doktorovova S, Gonzalez-Mira E, Egea MA, Garcia ML. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr. Eye Res. 2010;35:537–552. doi: 10.3109/02713681003760168. [DOI] [PubMed] [Google Scholar]
- 245.Sabzevari A, Adibkia K, Hashemi H, De Geest BG, Mohsenzadeh N, Atyabi F, Ghahremani MH, Khoshayand MR, Dinarvand R. Improved anti-inflammatory effects in rabbit eye model using biodegradable poly beta-amino ester nanoparticles of triamcinolone acetonide. Invest. Ophthalmol. Vis. Sci. 2013;54:5520–5526. doi: 10.1167/iovs.13-12296. [DOI] [PubMed] [Google Scholar]
- 246.Lee SY, Tong L. Lipid-containing lubricants for dry eye: a systematic review. Optom. Vis. Sci. 2012;89:1654–1661. doi: 10.1097/OPX.0b013e31826f32e0. [DOI] [PubMed] [Google Scholar]
- 247.Yavuz B, Bozdag Pehlivan S, Unlu N. An overview on dry eye treatment: approaches for cyclosporin a delivery. The Scientific World JOURNAL. 2012;2012:194848. doi: 10.1100/2012/194848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang Z, Xu L, Chen H, Li X. Rapamycin-loaded poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) nanoparticles: preparation, characterization and potential application in corneal transplantation. J. Pharm. Pharmacol. 2014;66:557–563. doi: 10.1111/jphp.12089. [DOI] [PubMed] [Google Scholar]
- 249.Wang F, Chen L, Jiang S, He J, Zhang X, Peng J, Xu Q, Li R. Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box-Behnken design. J. Pharm. Pharmacol. 2014;24:171–181. doi: 10.3109/08982104.2014.891231. [DOI] [PubMed] [Google Scholar]
- 250.Gonzalez L, Loza RJ, Han KY, Sunoqrot S, Cunningham C, Purta P, Drake J, Jain S, Hong S, Chang JH. Nanotechnology in corneal neovascularization therapy—a review. J. Ocul. Pharmacol. Ther. 2013;29:124–134. doi: 10.1089/jop.2012.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kim JE, Lee J, Jang M, Kwak MH, Go J, Kho EK, Song SH, Sung JE, Lee J, Hwang DY. Accelerated healing of cutaneous wounds using phytochemically stabilized gold nanoparticle deposited hydrocolloid membranes. Biomater. Sci. 2015;3:509–519. doi: 10.1039/c4bm00390j. [DOI] [PubMed] [Google Scholar]
- 252.Sankar R, Baskaran A, Shivashangari KS, Ravikumar V. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J. Mater. Sci. Mater. Med. 2015;26:5543. doi: 10.1007/s10856-015-5543-y. [DOI] [PubMed] [Google Scholar]
- 253.Ferreira AM, Mattu C, Ranzato E, Ciardelli G. Bioinspired porous membranes containing polymer nanoparticles for wound healing. J. Biomed. Mater. Res. A. 2014;102:4394–4405. doi: 10.1002/jbm.a.35121. [DOI] [PubMed] [Google Scholar]
- 254.Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin. Ther. 2013;35:312–320. e315. doi: 10.1016/j.clinthera.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 255.Kenawy el R, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE. Release of tetracycline hydrochloride from electrospun poly(ethylene-covinylacetate), poly(lactic acid), and a blend. J. Control. Release. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]