Abstract
Background
Various dietary components have been studied in relation to overall mortality; however, little is known about the relationship between the inflammatory potential of overall diet and mortality.
Materials and Methods
We examined the association between the dietary inflammatory index (DII) and mortality in the National Health and Nutrition Examination Survey (NHANES) III follow-up study. The DII was computed from baseline dietary intake assessed using 24-hour dietary recalls (1988–94). Mortality was determined from the National Death Index records through 2006. Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence interval (95% CI). During the follow-up, 2795 deaths were identified, including 1233 due to cardiovascular disease (CVD), and 615 due to cancer, 158 of which were due to digestive-tract cancers.
Results
Multivariate Cox proportional hazards regression analyses, adjusting for age, race, diabetes status, hypertension, physical activity, body mass index (BMI), poverty index and smoking, revealed positive associations between higher DII scores and mortality. Comparing subjects in DII Tertile 3 vs Tertile 1, significant associations were noted for all-cause mortality (HRTertile3vs1=1.34; 95%CI 1.19–1.51, Ptrend<0.0001), CVD mortality (HRTertile3vs1=1.46; 95%CI 1.18–1.81, Ptrend=0.0006), cancer mortality (HRTertile3vs1=1.46; 95%CI 1.10–1.96, Ptrend=0.01), and digestive-tract cancer mortality (HR Tertile3vs1=2.10; 95%CI 1.15–3.84, Ptrend=0.03)
Conclusion
These results indicate that a pro-inflammatory diet, as indicated by higher DII scores, was associated with higher risk of all-cause, CVD, and cancer mortality.
Introduction
Inflammation is a result of the body’s response to tissue insult or injury, or the presence of inflammatory stimulants [1,2]. The acute inflammatory response represents an important step in the process of wound healing and tissue regeneration that, under normal circumstances, will lead to recovery over a few days [3,4]. Chronic inflammation is known to be associated with common epithelial, especially colorectal, cancers, [5–7]. Worldwide, cardiovascular disease (CVD) is the leading cause of mortality, accounting for about half of the deaths among adults [8]. In the United States, more than 80 million people suffer from CVD and an average of about one million Americans die from CVD each year [9,10]. There is growing evidence that specific dietary components influence inflammation [11–13] and all-cause, cancer and CVD mortality [14–17].
Research into the role of diet in inflammation and mortality suggests that diet represents a complicated set of exposures which often interact, and whose cumulative effect modifies both inflammatory responses and health outcomes. A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet, a higher score indicates a pro-inflammatory diet [18]. It has been construct-validated with various inflammatory markers, including C-reactive protein (CRP) [19,20], interleukin-6 (IL-6) [21–23] and tumor necrosis factor alpha (TNFα) receptor 2 expression [23]. Additionally, increasing DII scores have been shown to be associated with the increased glucose intolerant and dyslipidemic components of metabolic syndrome [24,20], decreased bone mineral density in Iran [25], shift work status in a large population-based survey in the USA [26], increased risk of asthma in Australia [21], colorectal cancer in case-control studies in Spain and Italy [27] and in cohort studies in USA [28–30] and pancreatic, esophageal, endometrial hepatocellular and prostate cancers in Italy [31–35].
Two studies so far have reported on the association between the DII and mortality, and that was in a cohort of females only [36,37]. The purpose of the current study is to examine the association between the DII and all-cause, overall cancer, digestive-tract cancer, and CVD mortality in a large prospective cohort of a nationally representative population, the National Health and Nutrition Examination Survey (NHANES III), including both males and females. Our hypothesis is that a higher DII score (indicating a pro-inflammatory diet) increases risk of dying from all-cause, CVD, and cancer.
Methods
Subjects providing data for this analysis were participants in the NHANES III (1988–1994), which is a nationally representative sample of the civilian, non-institutionalized US population. Details of the survey design have been reported previously [38]. The current study was restricted to participants above 19 years of age at baseline, with complete data on mortality outcomes, diet, and relevant covariates (n = 12,366).
In the NHANES III cohort study, mortality information is derived on the basis of a probabilistic match between NHANES III and the National Death Index records through 31 December 2006 by the National Center for Health Statistics. For overall mortality, we included deaths from all causes. For cancer-specific mortality, we included deaths from malignant neoplasms which were coded from C00-C97 in the International Classification of Diseases, 10th Edition, Clinical Modification System codes (ICD-10). For digestive-tract cancers, we included malignant neoplasms from the front of the mouth to the rectum and malignant neoplasms of pancreas and hepato-biliary system (ICD-10=C00-C16, C18-C22, C25). For CVD-related mortality, we used ICD-10=I00-I178.
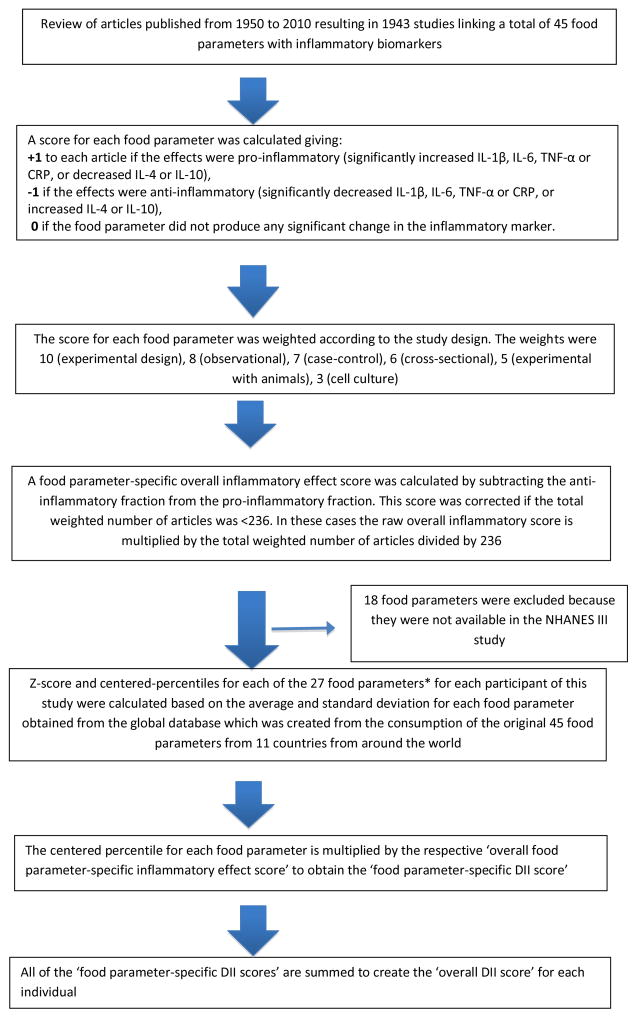
Dietary information was obtained from one in-person 24-hour diet recall (24HR) with the use of a personal computer-based, automated, interactive data collection and coding system that was developed by the University of Minnesota’s Nutrition Coordinating Center, and conducted by trained interviewers. The 24HR-derived dietary data were used to calculate DII scores for all participants. A complete description of the DII is available elsewhere [18] The DII is based on literature published through 2010 linking diet to inflammation. Developing the DII involved reviewing and scoring nearly 2000 scientific articles on cell culture and laboratory animal experiments, and cross-sectional, longitudinal and intervention trials in humans of 45 different food parameters and six inflammatory markers [i.e., CRP, IL-1β, IL-4, IL-6, IL-10, and TNF-α]. Results of each study on each of the 45 food parameters were scored by assigning a “+1” if a pro-inflammatory effect was reported, a “−1” if an anti-inflammatory effect was reported, or a “0” if there was no effect of the food parameter on inflammation (Figure 1). Scores were weighted by type of study design, with human clinical trials receiving the highest weight. Using these weighted values, the pro- and anti-inflammatory fractions for each food parameter were calculated. The “food parameter-specific overall inflammatory effect score” was then calculated by: 1) dividing the weighted pro- and anti-inflammatory articles by total weighted number of articles and 2) subtracting the anti-inflammatory fraction from the pro-inflammatory fraction. An adjustment was made for those food parameters with a less robust pool of literature.
Figure 1.
Sequence of steps in creating the dietary inflammatory index (DII) in the NHANES III study
To compare dietary intake of each of the food parameters to a standard amount, a world intake database was created using nutrition monitoring data from 11 different regions around the world, and a world mean intake and standard deviation was calculated for each of the 45 food parameters. To calculate DII in NHANES III, the dietary intake data were first linked to the world database that provided a robust estimate of a mean and standard deviation for each parameter [18]. These then became the multipliers to express an individual’s exposure relative to the “standard global mean” as a z-score. This was achieved by subtracting the “standard global mean” from the amount reported by subjects and dividing this value by the standard deviation. To minimize the effect of “right skewing,” this value was then converted to a centered percentile score. The centered percentile score for each food parameter for each individual was then multiplied by the respective food parameter-specific overall inflammatory effect score, which is derived from the literature review described above, in order to obtain a food parameter-specific DII score for an individual. All of the food parameter-specific DII scores are then summed to create the overall DII score for each participant in the study [18]. For this study, 27 of the 45 food parameters were available for DII calculation: energy, carbohydrate, protein, fat, alcohol, fiber, cholesterol, saturated fatty acid, mono-unsaturated fatty acid, poly-unsaturated fatty acid, niacin, thiamin, riboflavin, vitamin B12, vitamin B6, iron, magnesium, zinc, selenium, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, beta carotene, omega 6 and omega 3. Data were not available on 18 food parameters: anthocyanidins, eugenol, flavan3ol, flavones, flavonols, flavonones, isoflavones, caffeine, garlic, ginger, onion, saffron, turmeric, pepper, thyme/oregano, rosemary, tea and trans-fat. .DII= b1*n1+b2*n2...........b27*n27, where “b” refers to the literature-derived inflammatory effect score for each of the evaluable food parameters and “n” refers to the food parameter-specific centered percentiles, which were derived from the NHANES III dietary data. A description of validation work, including both dietary recalls and a structured questionnaire similar to an FFQ, also is available in separate publication [18]. A detailed schematic of this methodology is provided in Figure 1.
Associations with DII for demographic factors, lifestyle factors, self-reported diabetes mellitus, and anthropometric characteristics were examined using ANOVA models or χ2 tests (see Table 1 for specific variables). DII was analyzed both as a continuous variable and by tertiles of exposure in relation to all-cause, CVD, overall cancer, and digestive-tract cancer mortality. Hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated using Cox proportional hazards regression models, adjusting only for age in the first model. In the second model, additional adjustment was made for body mass index [BMI = weight (kg)/height (m)2], smoking status (smoker/ non smoker), sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American and others), diabetes status (yes/no), hypertension status (yes/no), physical activity (number of moderate to vigorous intensity activities in the past month), medical history of CVD (presence or absence of coronary heart disease or congestive cardiac failure) and poverty index (PI) (low income status=0.000≤PI<1.300, middle income status =1.301≤PI≥3.500, high income status= PI>3.500). Physical activity was assessed by questionnaire; PI was used to capture socioeconomic status, the poverty to income ratio is an index of poverty status which is calculated by dividing family income by a poverty threshold specific to family size. The covariates were chosen a priori, as they had been shown previously to be strong risk factors for the outcomes of interest in this cohort.
Table 1.
Distribution of continuous and categorical baseline characteristics of NHANES III cohort across tertilesa of DII
| Characteristicsb | Tertile 1 (N=4183) (−5.60 to −0.22) | Tertile 2 (N=4136) (−0.21 to 2.02) | Tertile 3 (N=4119) (2.03 to 4.83) | P-value |
|---|---|---|---|---|
| Age (yrs) | 47.06±18.9 | 47.05±19.1 | 48.10±19.3 | 0.009 |
| BMI (kg/m2) | 26.51±4.9 | 26.7±5.2 | 26.9±5.4 | 0.0001 |
| C-Reactive Protein (mg/l) | 2.94±1.8 | 3.11±1.9 | 3.21±2.1 | <0.0001 |
| Energy intake (kcals/day) | 2836.3±1209.4 | 2038.7±739.6 | 1442.3±594.4 | <0.001 |
| Grains (serv/day) | 8.75 ±5.0 | 6.38±3.4 | 4.80±2.7 | <0.0001 |
| Fruits (serv/day) | 2.42±2.87 | 1.41±1.86 | 0.78±1.25 | <0.0001 |
| Vegetables (serv/day) | 4.82±3.17 | 2.78±2.0 | 1.53±1.40 | <0.0001 |
| Meat (serv/day) | 2.9±2.1 | 2.3±1.6 | 1.5±1.1 | <0.0001 |
| Sex | <0.0001 | |||
| Males | 2638 (43.7) | 1958 (32.4) | 1439 (23.8) | |
| Females | 1545 (24.1) | 2178 (34.0) | 2680 (41.9) | |
| Income status based on poverty indexc,d | <0.0001 | |||
| Low | 1091 (27.9) | 1297 (33.2) | 1521 (38.9) | |
| Middle | 1871 (33.5) | 1872 (33.5) | 1841 (33.0) | |
| High | 1221 (41.5) | 967 (32.8) | 757 (25.7) | |
| Race/Ethnicity | <0.0001 | |||
| Non-Hispanic White | 1998 (37.3) | 1770 (33.0) | 1591 (29.7) | |
| Non-Hispanic Black | 839 (25.9) | 1076 (33.1) | 1330 (41.0) | |
| Mexican-American | 1181 (35.3) | 1119 (33.5) | 1041 (31.2) | |
| Others | 165 (33.5) | 171(34.5) | 157 (31.9) | |
| Hypertension | 1033 (32.3) | 1043 (32.7) | 1118 (35.0) | 0.03 |
| Diabetes | 1798 (32.5) | 1859 (33.6) | 1869 (33.8) | 0.44 |
| Mortality status | <0.0001 | |||
| Total Deaths | 852 (30.4) | 944 (33.7) | 1005 (35.9) | |
| Cancer deaths | 192 (31.1) | 190 (30.8) | 235 (38.1) | 0.02 |
| Digestive-tract cancer deaths | 46 (29.1) | 45 (28.5) | 67 (42.4) | 0.04 |
| CVD deaths | 368 (29.8) | 430 (34.8) | 437 (35.4) | 0.004 |
Tertile 1 is most anti-inflammatory group and tertile 3 is the most pro-inflammatory group.
Continuous variables examined using ANOVA presented as mean±s.d. Categorical variables examined using Chi-square test presented as n (%).
Poverty Index (PI) is a calculated variable based on family income and family size using tables published each year by the Bureau of the Census in a series “Current Population reports” on poverty in the United States.
Low income status=0.000≤PI<1.300, middle income status =1.301≤PI≥3.500, high income status= PI>3.500.
Participants with extreme energy intake values (≤500 kcals and ≥5000 kcals, n=307), with abnormal BMI values (>50 kg/m2, n=53) or PI values (>10, n=1235), or who had a history of cancer at baseline (n=461) were excluded. Some of the excluded participants met more than one exclusion criteria; hence, there was overlap. Energy was not included in the model because energy is accounted for as a parameter of the DII. A test for linear trend was conducted by including the median value for each DII tertile as a continuous term into the regression model. The assumption of proportional hazards was tested by adding to the model an interaction term between follow-up time and DII; there was no evidence that these assumptions were violated. Sensitivity analyses were conducted by excluding participants with diabetes and cardiovascular diseases at baseline and another sensitivity analyses was conducted adjusting for history of CVD status in the multivariate model. Statistical tests were performed using SAS® 9.3, (SAS Institute Inc., Cary, NC); all statistical tests were two-sided and p<0.05 was considered statistically significant.
Results
The mean DII score in this study was 0.73, SD ±2.20 and the value ranged from a maximally pro-inflammatory score of +5.83 to maximally anti-inflammatory score of −5.60 Participants in tertile 1 (−5.60 to −0.22) have the least inflammatory diet and participants in tertile 3 (+2.03 to +4.83) have the most pro-inflammatory diet. Subjects in the third tertile had significantly higher BMI and CRP values and were older than subjects in the first tertile. They also had significantly lower energy intakes, total servings of dietary fiber, total grains, total fruits, vegetables, and meat compared to those in tertile 1 of DII (Table 1). Intake of the following dietary variables decreased across DII tertiles (Table 1): Individuals in DII tertile 3 had higher percentage of females, non-Hispanic Blacks, people with low PI scores and more hypertension than those in DII tertile 1 (Table 1). There was a higher percentage of deaths observed in tertile 3 compared to tertile 1 for all-cause mortality (35.9% vs 30.4 %), overall cancer mortality (38.1% vs 31.1%), digestive-tract cancer mortality (42.4 % vs 29.1%) and CVD mortality (35.4% vs 29.8%) (Table 1). During the follow-up period (mean ± SD = 13.5 ± 4.0 years), 2801 total deaths were identified, including 617 malignant cancer deaths, 158 digestive-tract cancer deaths, and 1235 CVD deaths. When analyses were carried out using DII as a continuous variable, a 1-unit increment in DII (corresponding to 0.5 standard deviation increase) showed significant positive associations with risk of overall mortality after adjusting for age (HR=1.05; 95%CI 1.02 – 1.08). After additional adjustment for sex, race, diabetes status, hypertension, physical activity, BMI, PI and smoking, the HR was slightly attenuated (1.04; 95%CI 1.02 – 1.07). For analyses focusing on deaths due to specific causes, a significant positive association was observed with CVD mortality after adjustment for covariates (HR=1.06; 95%CI 1.02 – 1.09). For malignant cancer mortality and digestive-tract cancer mortality, HRs for DII were in the hypothesized direction as was observed for all-cause and CVD mortality; however, results did not achieve statistical significance, consistent with the smaller number of cases (Table 2).
Table 2.
Hazards ratio for mortality outcomes with DII as continuous and as tertiles
| Overall Mortality (N=2795) | Cancer Mortality (N=615) | Digestive-tract cancer mortalityc (N=158) | Cardiovascular disease mortality (N=1233) | |||||
|---|---|---|---|---|---|---|---|---|
| HRa (95% CI) | HRb(95 %CI) | HRa(95 %CI) | HRb(95 %CI) | HRa(95 %CI) | HRb(95 %CI) | HRa(95 %CI) | HRb(95 %CI) | |
| DII (continuous) | 1.05 (1.02, 1.08) | 1.04 (1.02, 1.07) | 1.05 (0.98, 1.12) | 1.04 (0.97,1.11) | 1.09 (0.95, 1.25) | 1.08 (0.95, 1.22) | 1.05(1.02, 1.09) | 1.06 (1.02, 1.09) |
| Tertile 1 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Tertile 2 | 1.24 (1.10, 1.40) | 1.23 (1.10, 1.37) | 1.32 (1.00, 1.74) | 1.30 (0.99, 1.70) | 1.29 (0.72, 2.31) | 1.31 (0.76, 2.26) | 1.26 (1.08, 1.49) | 1.28 (1.09, 1.50) |
| Tertile 3 | 1.37 (1.20, 1.56) | 1.34 (1.19, 1.51) | 1.49 (1.12, 2.00) | 1.46 (1.10, 1.96) | 2.14 (1.13, 4.05) | 2.10 (1.15, 3.84) | 1.43 (1.16, 1.77) | 1.46 (1.18, 1.81) |
| p-trend | <0.0001 | <0.0001 | 0.007 | 0.01 | 0.03 | 0.03 | 0.0008 | 0.0006 |
Age-adjusted
Additionally adjusted for sex, race, diabetes status, hypertension, physical activity, BMI, poverty index and smoking
Includes cancers from beginning of oral cavity to rectum and cancers of pancreas and hepato-biliary system.
Analysis with DII categorized as tertiles revealed significantly higher risk for subjects in the third tertile compared to those in the first tertile for overall mortality (HR=1.34; 95%CI 1.19 – 1.51, Ptrend<0.0001); for malignant cancer mortality (HR=1.46; 95%CI 1.10 – 1.96, Ptrend=0.01); digestive cancer mortality (HR=2.10; 95%CI 1.15 – 3.84, Ptrend=0.03) and CVD mortality (HR=1.46; 95%CI 1.18 – 1.81, Ptrend=0.0006) (Table 2). No significant interactions were observed between DII and either sex or BMI for all types of mortality.
We also carried out sensitivity analyses excluding participants with CVD and diabetes at baseline and the results did not alter substantially (overall mortality; HRTertile3vs1=1.33; 95%CI 1.14 – 1.55, Ptrend<0.0001). Also, additionally adjusting for baseline CVD status showed a marginal change in the HR for overall mortality (HRTertile3vs1=1.31; 95%CI 1.16 – 1.48, Ptrend<0.0001),
Discussion
In this large, nationally representative, prospective cohort study, the consumption of a more pro-inflammatory diet, as reflected by higher DII scores, was associated with increased risk of deaths from all-cause, CVD, cancer and digestive-tract cancer. Compared to subjects in tertile 1, those in tertile 3 were 34% more likely to die from any cause, 46% more like to die from all cancers, 110% more likely to die from digestive-tract cancers, and 46% more likely to die from CVD. We also observed a significantly increasing trend in CRP concentrations across tertiles of DII. These results are consistent with the epidemiology of colorectal cancer, which is known to be strongly related to inflammation [39–42], and represents the majority of digestive-tract cancers.
The DII is different from other dietary indices, virtually all of which fall into three main categories: 1). Those derived from specific dietary prescriptions based on some external standard [e.g., Healthy Eating Index (HEI) which was derived from the adherence to the US Dietary guidelines] [43]; 2). Those derived empirically from findings within particular study populations [e.g., computing a pattern using principal component analysis (PCA)][44] or 3) Those that link to particular cultural patterns of dietary intake (e.g., the Mediterranean diet score) [45]. Unlike all of these other indices, the DII 1) is grounded in peer-reviewed literature focusing specifically on inflammation; 2) can be adapted to virtually any dietary assessment method that provides estimates of nutrient intake; and 3) is standardized to dietary intake from representative populations around the world, thus facilitating easy quantitative comparisons across studies.
Studies have been conducted to examine various dietary patterns and indices in relation to mortality [46–48]. In a study conducted in the NHANES III cohort study, HEI was found to be inversely associated with all-cause and CVD mortality [47]. In a study conducted in the NIH-American Association of Retired Professionals (NIH-AARP) cohort, the Mediterranean diet score was associated with reduced all-cause and cause-specific mortality [46], while another report from the NIH-AARP study showed various indices [HEI-2010, the Alternative Healthy Eating Index-2010 (AHEI-2010), the alternate Mediterranean Diet (aMED), and Dietary Approaches to Stop Hypertension (DASH)] to be protective against all-cause, CVD and cancer mortality [49]. In the Whitehall cohort study, which was conducted in United Kingdom with a predominantly White population, AHEI was not associated with cancer mortality or non-cancer/non-CVD mortality [48].
Previous studies also have examined the effect of specific food items, such as red meat [50], and nutrients, such as magnesium [51] and vitamin E [52] on mortality. In a meta-analyses, red meat intake, especially processed meat, was found to be associated with increased all-cause mortality [50]. We also acknowledge that increasing intake of red meat in the lowest DII tertile is in the opposite direction of what would be expected; however, it should be noted that red meat is one among several other food items that affect inflammation; there are other food items such as vegetables, fruits, fish which exert a stronger anti-inflammatory effect and their distributions across the DII tertiles are similar to what is expected. The red meat observation may be explained by the fact that red meat eaters eat more of everything, including many of these anti-inflammatory foods. Because the DII takes into account the diet as a whole, red meat eaters could have an anti-inflammatory DII score if they ate sufficient quantities of other components (e.g., vegetable, spices) that contribute significantly to the anti-inflammatory effect of the entire diet. No association was observed between magnesium and calcium and cancer-related mortality in the EPIC-Heidelberg study [51]. In a prospective study conducted by Pocobelli et al., vitamin E was found to significantly reduce CVD mortality; however, no association was observed with cancer mortality [52]. A limitation of studies that focused on only one nutrient or food group is that these foods or nutrients are consumed with other food items and nutrients; thus, dietary intercorrelations may attenuate or accentuate the actual effects of the food group or nutrient under study. A very high correlation between nutrients among foods can result in instability in risk estimation and possible loss of statistical power. In formulating the DII [53,54], an entirely different approach was taken by focusing on the functional effects of foods and nutrients. As such, it relies on extensive review and careful scoring of the medical literature in specific relation to inflammation. Also, it standardizes individuals’ dietary intakes of pro- and anti-inflammatory food constituents to world referent values (for ease of comparison with findings from other, diverse studies). As a composite of up to 45 food parameters (here 27), the DII obviates problems with these intercorrelations to a large extent. While individual nutrients may be correlated with one another, the overall diet scores like DII may be only minimally correlated with other factors. We have found this when we have used a two-stage modeling technique in other populations (e.g., South India) [55–57] and for analyses of dietary and tobacco exposures within the US [58]. While highly inter-correlated factors may present problems with model fitting when considered singly, when combined the aggregate scores presented no such problems in terms of inter-correlations.
One of the possible mechanisms for the inverse associations observed in this study might be through the effect of pro-inflammatory diet on insulin resistance by increasing systemic inflammation [59,60]. Consumption of food items such as meat and butter have been shown to increase systemic inflammation by increasing levels of high-sensitivity CRP, E-selectin and soluble vascular cell adhesion molecule-1 [59], which then are responsible for increasing insulin resistance [60]. Insulin resistance caused by increasing circulating levels of insulin, triglycerides, and non-esterified fatty acids [61,62], is associated with digestive-tract cancers and CVD, all of which, if left uncontrolled, result in progressive disease leading to death. As mentioned previously, there are various dietary factors that have different effects on inflammation; for example, red meat consumption increases inflammation and green leafy vegetables reduce inflammation [62,61]. Supporting our findings, previous work in the NHANES III examining diet and mortality has shown significant inverse associations between anti-inflammatory food parameters such as selenium [63] and magnesium [17] intake and mortality. A significant positive association was observed between sodium intake and sugar intake and CVD mortality [16,64], while no association was observed between meat intake and mortality [65].
Our study has several strengths. NHANES III Study is a large, prospective, multiethnic, nationally representative study well-characterized with data on multiple risk factors and confounders. This study had a long follow-up with a large number of events for the outcomes studied. Nevertheless, there are limitations. The main limitation of this study was that the estimation of dietary intake was based on single self-reported 24HR. It is well known that differences observed in dietary intake consist of both intra- and inter-person sources of variance [66]. A single 24HR may not be an adequate reflection of usual diet due to day-to-day and other sources of intra-person variability [67]. Consequently, resulting data can lead to potential misclassification bias. In a prospective study such as NHANES, it is likely that the bias introduced will be random and this will generally lead to a higher likelihood of results consistent with the null hypothesis of no effect [68,69]. Dietary assessment was available only at one time point. Participants’ dietary habits might have changed during the follow-up period. However, previous studies have reported that dietary pattern classification is moderately stable over time [70–75].
In conclusion, these results indicate that individuals who consumed a more pro-inflammatory diet were at increased risk of dying from all causes, CVD, and cancer compared to individuals who consumed a more anti-inflammatory diet. The fact that we had access to just a single 24HR increases the likelihood of these results being biased towards the null hypothesis of no effect. Future work should include more robust measures of dietary intake, including multiple 24HR. Other future steps might include investigating how the DII behaves longitudinally in an intervention trial among individuals who have had cancer or CVD to examine if improvement in the DII scores over time is associated with subsequent improved survival. It also would be interesting to examine how DII fares in predicting mortality in studies outside the United States reflecting different populations, and how it compares with other indices in relation to all-cause, CVD, and cancer mortality.
Acknowledgments
Funding: Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is a paid employee of CHI.
References
- 1.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15(17):1949–1955. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 2.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57(11):4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:6–21. discussion 22–28, 49–52, 266–269. [PubMed] [Google Scholar]
- 4.Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–175. doi: 10.1111/j.1749-6632.2008.03985.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung Y-C, Chang Y-F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. Journal of Surgical Oncology. 2003;83(4):222–226. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 6.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 7.Toriola AT, Cheng TY, Neuhouser ML, Wener MH, Zheng Y, Brown E, Miller JW, Song X, Beresford SA, Gunter MJ, Caudill MA, Ulrich CM. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int J Cancer. 2013;132(11):2648–2658. doi: 10.1002/ijc.27942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. The global burden of disease: 2004 update. WHO; 2008. [Google Scholar]
- 9.Calabro P, Golia E, Yeh ET. CRP and the risk of atherosclerotic events. Semin Immunopathol. 2009;31(1):79–94. doi: 10.1007/s00281-009-0149-4. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 11.de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011;54(11):2755–2767. doi: 10.1007/s00125-011-2285-3. [DOI] [PubMed] [Google Scholar]
- 12.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8(10):2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 13.Luciano M, Mottus R, Starr JM, McNeill G, Jia X, Craig LC, Deary IJ. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun. 2012;26(5):717–720. doi: 10.1016/j.bbi.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. The American journal of clinical nutrition. 2014;99(2):320–327. doi: 10.3945/ajcn.113.073148. ajcn.113.073148 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2014 doi: 10.1016/j.clnu.2014.03.011. S0261-5614(14)00086-7 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III) J Gen Intern Med. 2008;23(9):1297–1302. doi: 10.1007/s11606-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. doi: 10.1186/1741-7015-11-187. 1741-7015-11-187 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public health nutrition. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood L, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical & Experimental Allergy. 2014 doi: 10.1111/cea.12323. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Huybrecht I. Association between dietary inflammatory index and inflammatory markers in Asklepios study. British Journal of Nutrition. 2014 doi: 10.1017/S000711451400395X. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of Epidemiology. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. doi: http://dx.doi.org/10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkerwi Aa, Shivappa N, Crichton G, Hébert JR. No significant independent relationships with cardiometabolic biomarkers were detected in the Observation of Cardiovascular Risk Factors in Luxembourg study population. Nutrition Research. doi: 10.1016/j.nutres.2014.07.017. http://dx.doi.org/10.1016/j.nutres.2014.07.017. [DOI] [PMC free article] [PubMed]
- 25.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. The British journal of nutrition. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hebert JR. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2014;56(2):145–148. doi: 10.1097/JOM.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, Hebert JR, Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. Genes & nutrition. 2015;10(1):447. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Prizment AE, Blair CK, Jacobs DR, Jr, Steck SE, Hebert JR. Dietary inflammatory index and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(11):2383–2392. doi: 10.1158/1055-9965.EPI-14-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, Wactawski-Wende J, Ockene JK, Hebert JR. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer causes & control : CCC. 2015;26(3):399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hébert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health–American Association of Retired Persons Diet and Health Study. British Journal of Nutrition FirstView. 2015:1–9. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. The British journal of nutrition. 2014:1–6. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. The British journal of nutrition. 2014:1–7. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La Vecchia C, Serraino D. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. The British journal of nutrition. 2015:1–8. doi: 10.1017/S0007114515004419. [DOI] [PubMed] [Google Scholar]
- 34.Shivappa N, Hébert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, Rossi M. Dietary inflammatory index and endometrial cancer risk in an Italian case–control study. British Journal of Nutrition FirstView. 2015:1–9. doi: 10.1017/S0007114515004171. [DOI] [PubMed] [Google Scholar]
- 35.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer causes & control : CCC. 2015;26(10):1439–1447. doi: 10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- 36.Shivappa N, Blair C, Prizment A, Jacobs D, Jr, Steck S, Hébert J. Association between inflammatory potential of diet and mortality in the Iowa Women’s Health study. European journal of nutrition. 2015:1–12. doi: 10.1007/s00394-015-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shivappa N, Harris H, Wolk A, Hebert JR. Association between inflammatory potential of diet and mortality among women in the Swedish Mammography Cohort. European journal of nutrition. 2015 doi: 10.1007/s00394-015-1005-z. [DOI] [PubMed] [Google Scholar]
- 38.Plan and operation of the Third National Health and Nutrition Examination Survey 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;1(32):1–407. [PubMed] [Google Scholar]
- 39.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer.[see comment] JAMA. 2004;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 40.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Research. 2006;66(4):2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 41.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S Japan Public Health Center-Based Prospective Study G. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(4):690–695. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 42.Nikiteas NI, Tzanakis N, Gazouli M, Rallis G, Daniilidis K, Theodoropoulos G, Kostakis A, Peros G. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World Journal of Gastroenterology. 2005;11(11):1639–1643. doi: 10.3748/wjg.v11.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 44.Miller PE, Lazarus P, Lesko SM, Muscat JE, Harper G, Cross AJ, Sinha R, Ryczak K, Escobar G, Mauger DT, Hartman TJ. Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr. 2010;140(7):1267–1273. doi: 10.3945/jn.110.121780. 110.121780 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16(8):559–568. doi: 10.1016/j.numecd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167(22):2461–2468. doi: 10.1001/archinte.167.22.2461. 167/22/2461 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Rathod AD, Bharadwaj AS, Badheka AO, Kizilbash M, Afonso L. Healthy Eating Index and mortality in a nationally representative elderly cohort. Arch Intern Med. 2012;172(3):275–277. doi: 10.1001/archinternmed.2011.1031. 172/3/275 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, Singh-Manoux A, Ritchie K, Shipley MJ, Kivimaki M. Alternative Healthy Eating Index and mortality over 18 y of follow-up: results from the Whitehall II cohort. The American journal of clinical nutrition. 2011;94(1):247–253. doi: 10.3945/ajcn.111.013128. ajcn.111.013128 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J Nutr. 2014 doi: 10.3945/jn.113.189407. jn.113.189407 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179(3):282–289. doi: 10.1093/aje/kwt261. kwt261 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Li K, Kaaks R, Linseisen J, Rohrmann S. Dietary calcium and magnesium intake in relation to cancer incidence and mortality in a German prospective cohort (EPIC-Heidelberg) Cancer Causes Control. 2011;22(10):1375–1382. doi: 10.1007/s10552-011-9810-z. [DOI] [PubMed] [Google Scholar]
- 52.Pocobelli G, Peters U, Kristal AR, White E. Use of supplements of multivitamins, vitamin C, and vitamin E in relation to mortality. Am J Epidemiol. 2009;170(4):472–483. doi: 10.1093/aje/kwp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in high-sensitivity c-reactive protein. J Nutr. 2009;139(12):2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutrition. 2013;10:1–9. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hebert JR. Relationship of vegetarianism to child growth in South India. The American journal of clinical nutrition. 1985;42(6):1246–1254. doi: 10.1093/ajcn/42.6.1246. [DOI] [PubMed] [Google Scholar]
- 56.Hebert JR. Effects of water quality and water quantity on nutritional status: findings from a south Indian community. Bulletin of the World Health Organization. 1985;63(1):145–155. [PMC free article] [PubMed] [Google Scholar]
- 57.Hebert JR. Water quality and water quantity and wasting in south India. Tropical and geographical medicine. 1984;36(4):375–381. [PubMed] [Google Scholar]
- 58.Hebert JR, Toporoff E. Dietary Exposures and Other Factors of Possible Prognostic-Significance in Relation to Tumor Size and Nodal Involvement in Early-Stage Breast-Cancer. Int J Epidemiol. 1989;18(3):518–526. doi: 10.1093/Ije/18.3.518. [DOI] [PubMed] [Google Scholar]
- 59.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary Patterns and Markers of Systemic Inflammation among Iranian Women. The Journal of Nutrition. 2007;137(4):992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 60.Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome: The Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 61.Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr Cancer. 2000;37(1):19–26. doi: 10.1207/S15327914NC3701_2. [DOI] [PubMed] [Google Scholar]
- 62.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1271–1279. [PubMed] [Google Scholar]
- 63.Eaton CB, Abdul Baki AR, Waring ME, Roberts MB, Lu B. The association of low selenium and renal insufficiency with coronary heart disease and all-cause mortality: NHANES III follow-up study. Atherosclerosis. 2010;212(2):689–694. doi: 10.1016/j.atherosclerosis.2010.07.008. S0021-9150(10)00511-3 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–524. doi: 10.1001/jamainternmed.2013.13563. 1819573 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kappeler R, Eichholzer M, Rohrmann S. Meat consumption and diet quality and mortality in NHANES III. European journal of clinical nutrition. 2013;67(6):598–606. doi: 10.1038/ejcn.2013.59. ejcn201359 [pii] [DOI] [PubMed] [Google Scholar]
- 66.Hebert JR, Gupta PC, Mehta H, Ebbeling CB, Bhonsle RR, Varghese F. Sources of variability in dietary intake in two distinct regions of rural India: implications for nutrition study design and interpretation. European journal of clinical nutrition. 2000;54(6):479–486. doi: 10.1038/sj.ejcn.1601042. [DOI] [PubMed] [Google Scholar]
- 67.Hebert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Advances in nutrition. 2014;5(4):447–455. doi: 10.3945/an.114.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 69.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103(14):1086–1092. doi: 10.1093/jnci/djr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jain M, Howe GR, Harrison L, Miller AB. A study of repeatability of dietary data over a seven-year period. Am J Epidemiol. 1989;129(2):422–429. doi: 10.1093/oxfordjournals.aje.a115146. [DOI] [PubMed] [Google Scholar]
- 71.Jensen OM, Wahrendorf J, Rosenqvist A, Geser A. The reliability of questionnaire-derived historical dietary information and temporal stability of food habits in individuals. Am J Epidemiol. 1984;120(2):281–290. doi: 10.1093/oxfordjournals.aje.a113891. [DOI] [PubMed] [Google Scholar]
- 72.Lindsted KD, Kuzma JW. Long-term (24-year) recall reliability in cancer cases and controls using a 21-item food frequency questionnaire. Nutr Cancer. 1989;12(2):135–149. doi: 10.1080/01635588909514012. [DOI] [PubMed] [Google Scholar]
- 73.Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR., Jr Diet quality indexes and mortality in postmenopausal women: the Iowa Women’s Health Study. The American journal of clinical nutrition. 2013;98(2):444–453. doi: 10.3945/ajcn.112.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, Kromhout D, Jacobs DR., Jr Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. The American journal of clinical nutrition. 2012;95(3):580–586. doi: 10.3945/ajcn.111.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson FE, Metzner HL, Lamphiear DE, Hawthorne VM. Characteristics of individuals and long term reproducibility of dietary reports: the Tecumseh Diet Methodology Study. J Clin Epidemiol. 1990;43(11):1169–1178. doi: 10.1016/0895-4356(90)90018-k. 0895-4356(90)90018-K [pii] [DOI] [PubMed] [Google Scholar]