Abstract
Objective. The aim of the current study was to examine the relationships among age, ethnicity, and endogenous pain facilitation using temporal summation (TS) responses to mechanical and heat stimuli.
Design. The present study assessed hyperalgesia and pain facilitation to thermal and mechanical stimuli at the knee and distal sites in 98 pain-free men and women. Participants were drawn from two ethnic groups [African-American (AA) and non-Hispanic white (NHW)] and two age groups (19–35 and 45–85).
Results. Significant main effects of ethnicity were demonstrated for both mechanical and heat modalities (all P’s ≤ 0.05), suggesting that AA participants, relative to NHW counterparts, demonstrated enhanced hyperalgesia. Age differences (older > younger) in hyperalgesia were found in mechanical pain ratings only. Results indicated that mechanical pain ratings significantly increased from first to maximal pain as a function of both age group and ethnicity (all P’s ≤ 0.05), and a significant ethnicity by age interaction for TS of mechanical pain was found at the forearm (P < 0.05) and trended toward significance at the knee (P = 0.071). Post-hoc tests suggested that results were primarily driven by the older AA participants, who demonstrated the greatest mechanical TS. Additionally, evidence of differences in heat TS due to both ethnicity alone (all P’s ≤ 0.05) and minority aging was also found.
Conclusions. This study provides evidence suggesting that older AAs demonstrate enhanced pain facilitatory processes, which is important because this group may be at increased risk for development of chronic pain. These results underscore the necessity of testing pain modulatory mechanisms when addressing questions related to pain perception and minority aging.
Keywords: Pain, Hyperalgesia, Facilitation, Ethnicity, Aging, Minority Aging
Introduction
Previous clinical studies of age-related differences in the prevalence of chronic non-cancer pain have reported that chronic pain and associated disability generally increase with age [1]. Evidence that as many as 50% of older individuals suffer from at least one type of “persistent, bothersome pain complaint” [1] is a key indication that older individuals are at heightened risk to develop chronic pain. Furthermore, the prevalence of chronic non-cancer pain has also been found to be greater in older African Americans (AAs) as compared to older non-Hispanic whites (NHWs) [2–4]. One explanation for increased pain in older AAs has been that minority aging is associated with greater sensitivity to noxious stimuli. However, previous studies that have used experimental pain protocols [i.e., quantitative sensory testing (QST)] to examine age differences in pain processing have produced mixed results. Several reviews of the literature have reported that, in contrast to individuals’ reports of their clinical pain, unidimensional measures of pain processing evaluated during QST such as pain threshold and tolerance increased, decreased, or remained unchanged over individuals’ life spans [1,5–10]. Consequently, it appears that unidimensional QST measures of pain processing may not be sufficient to optimally characterize age-related differences in clinical pain. Dynamic forms of QST, such as tests of endogenous pain facilitation, may be better suited to address the association of minority aging with pain processing [11,12].
Aging AAs consistently demonstrate hyperalgesia, or increased sensitivity, in response to suprathreshold noxious stimuli when compared to NHWs of various ages [8,13–16]. Alteration in endogenous pain modulation is one possible factor contributing to hyperalgesic changes as AAs age, particularly involving temporal summation (TS) of pain. This endogenous pain facilitatory mechanism is considered a perceptual manifestation of enhanced central excitability and is common to many chronic pain conditions, resulting in the perception of increased pain despite constant or even reduced peripheral afferent input [17]. It has been suggested that age-related decline in the function of A-delta fibers—fibers implicated in TS of pain—affects pain perception in older groups [18]. However, only a few studies have directly investigated the effects of minority aging on endogenous pain facilitatory processes such as TS. A recent study by our group showed greater TS in both mechanical and heat procedures in older AAs compared to both middle-aged and older NHWs [19], as well as greater heat TS in older AAs compared to middle-aged AA participants; however, adults younger than age 45 were not examined in this study. To our knowledge, no study has examined ethnic differences in TS across the adult lifespan. If older AAs demonstrate enhanced TS relative to older NHWs as well as younger AAs, it may be that minority aging effects on pain processing emerge relatively late in life. Further, these minority aging TS effects may be linked to differences in central nervous system processing of painful stimuli that, in turn, may be associated with hyperalgesia and chronic pain conditions later on.
The incidence of pain in minority aging populations produces an enormous burden for both the patient and the healthcare system. It is proposed that pain perception changes throughout the lifespan, and that minority groups are particularly affected by changes in endogenous pain facilitation [20]. To our knowledge, the vast majority of previous research examining experimental pain perception has considered aging effects and ethnic differences independent of one another (e.g., [1,8,14,21,22]). Thus, our aim was to examine the associations of minority aging with both unidimensional (hyperalgesia) and dynamic endogenous pain facilitatory processes (TS). Hyperalgesia and TS of pain were examined using mechanical and heat stimuli applied in a counterbalanced order to the knee and ipsilateral hand (mechanical) or forearm (heat), pursuant to previous studies demonstrating ethnic differences in mechanical and heat perception [19,23]. We hypothesized that age and ethnicity would interact in relation to hyperalgesic pain responses and TS, such that older AA participants would experience the greatest hyperalgesia—that is, exhibit highest pain ratings, and most pronounced endogenous pain facilitation of any group.
Methods
Participants and Assignment to Groups
A total of 50 young, healthy persons were recruited from the University of Alabama at Birmingham (UAB) community. These participants were limited to adults aged 19–35 who reported their ethnicity as either NHW or AA. The participants were divided equally by ethnicity, with 25 participants in each group. Participants were divided by sex in nearly equal proportions (52% female). This group of young participants was combined with 48 older, healthy individuals previously recruited as participants in the Understanding Pain and Limitations in Osteoarthritic Disease (UPLOAD) study, conducted at the UAB. These participants were chosen specifically because they had no evidence of symptomatic knee osteoarthritis, in contrast to the patients enrolled in the UPLOAD study (not included in this study). The UPLOAD participants included healthy individuals of AA (N = 22) or NHW (N = 26) ethnicities. The ages of the older UPLOAD sample ranged from 45 to 82, with more females than males (71%). Thus, the total study sample size with both age groups combined is 98 participants. A priori power analyses revealed that the combined N of 98 exceeded the necessary sample size to obtain a power of 0.95. Age groups of 19–35 and 45 + were chosen to polarize age groups, and is supported by research suggesting that age differences in endogenous pain modulation begin to emerge in middle age [24]. Identical protocols were used for evaluating pain in both groups, with the evaluation of the younger cohort based on the original UPLOAD study. Each protocol was approved by the UAB Institutional Review Board, in accordance with ethical research conduct guidelines. Written informed consent was obtained from participants and they were compensated for their involvement.
Healthy participants were chosen for this study in large part to eliminate extraneous factors that may influence pain perception, including pre-existing pain conditions, chronic systemic medical disorders, or psychiatric diagnoses. Inclusion of only healthy participants helped to ensure a more pure examination of the characteristics associated with aging and ethnicity. To this end, additional criteria for inclusion included an absence of comorbid conditions, with the most important considerations involving no evidence of: 1) uncontrolled high blood pressure or heart disease, 2) a chronic pain condition (as assessed by endorsement of pain in any body region for longer than 3 months) or acute pain (as assessed by endorsement of current pain in any body region), 3) decreased peripheral sensitization (assessed by direct questioning about neuropathy diagnoses and altered sensation, i.e. numbness and tingling in the hands and feet), and 4) a diagnosed and/or medicated psychiatric disorder. Additional exclusionary questions included evidence of knee pain “on most days” for the past 4 weeks, knee pain while climbing down stairs or walking down slopes, swelling in one or both knees, and a prior diagnosis of knee osteoarthritis. The older adult sample was confirmed free of pain and decreased peripheral sensitization by the study rheumatologist, as this group is at higher risk for arthritis or other chronic pain conditions; this was deemed unnecessary for the younger, healthy adults. These exclusion criteria were consistent with the UPLOAD adults collected previously. Other rule-outs include history of seizures, severe eczema, rheumatic disease, or any other chronic medical condition that may interfere with typical pain perception. Interested parties were screened with a Health History questionnaire to ensure that these criteria were met.
Procedures
All participants underwent a single QST assessment session that lasted approximately 3.5 hours. The QST sessions were carried out at the Center for Clinical and Translational Science-affiliated Clinical Research Unit at UAB. Upon arrival, the participant confirmed demographic information (i.e., self-reported ethnicity, age, and sex), underwent a series of physical measurements as assessed by a nurse (including height, weight, and baseline blood pressure), and completed a battery of questionnaires to evaluate mood and psychological well-being. Following these tasks, the participants completed a QST protocol to assess pain processing. All participants underwent the same set of procedures, though the order of TS tasks (heat and mechanical) was counterbalanced across participants to negate order effects.
Covariates
Some factors are likely evident even in healthy participants that relate to pain processing, such as demographic differences and sub-clinical variability in psychological factors. To account for these, other important information (as noted above) was collected from participants prior to QST. Demographic factors including sex, body mass index (BMI), and indicators of socioeconomic status have all been shown to contribute to the experience of pain [25–28]. Psychosocial factors have also been demonstrated to influence experimental pain, such as depressed mood and subjective experiences of discrimination [29,30].
Self-Report Measures
Center for Epidemiological Studies—Depression Scale
The Center for Epidemiological Studies–Depression Scale (CES-D) is a 20-item measure of symptoms of depression that has been shown to be reliable and valid in both general [31]and clinical populations [32], including when used in minority groups [33,34]. It has been reported that the CES-D is generally accepted as a useful tool for screening depressive symptomatology [34], and was therefore used to characterize depressive symptoms in the current study. Scores above 16 are indicative of increasingly high levels of depressive symptoms.
Experiences of Discrimination
The Experiences of Discrimination (EOD) measure was administered to assess participants’ self-reported experiences of racial discrimination across nine different situations. Participants were asked to indicate the frequency of each experience (from “never” to “four or more times”), how they responded to the situation, and the extent to which they worry about discrimination for themselves and their ethnic group. Examples of situations assessed in this measure include “at work,” “on the street or in a public setting,” and “from the police or in the courts,” among others. The EOD has shown good reliability and validity across multiple ethnic groups, including African Americans and non-Hispanic whites [35]. The EOD was included to specifically examine whether experiences of discrimination contributed to ethnic group differences in endogenous pain processing.
Quantitative Sensory Testing
Mechanical Testing
Participants underwent a mechanical procedure designed to assess hyperalgesia and TS pain using a nylon monofilament (Touchtest Sensory Evaluator 6.65). This filament is calibrated to bend at 300 g of pressure. Each contact was administered at identical pressure (300 g) throughout the duration of mechanical testing. First pain was assessed at the back of the hand and at the knee by a single contact of the monofilament. Participants provided a verbal rating of pain intensity on the 0–100 scale immediately after contact. Then, to assess TS, 10 contacts were administered at a rate of 1 contact per second. Participants provided a rating of pain intensity for the worst of the 10 contacts, regardless of which contact was the most intense. The entire trial was repeated twice at each site with no break between trials, and initial sites of contact were counterbalanced. Verbal ratings for the single and multiple contacts were averaged across the two trials to establish an index of mechanical pain at each site. Within these procedures, both hyperalgesia (pain sensitivity) and TS of pain (facilitation from initial to maximum contact) were assessed.
Heat Testing
Each individual underwent a series of heat stimuli at ascending strengths of 44°C, 46°C, and 48°C. The heat stimulus was delivered using a Medoc Thermal Sensory Analyzer-II system (Ramat Yishai, Israel), which includes a peltier-element-based stimulator with a 1.6 × 1.6 centimeter contact area. To induce heat pain, the contact was placed at three points along the subject’s forearm, then three sites at the knee. For all trials, the sensation began at a warm baseline temperature of 35°C, and then ramped to the target temperature five times in rapid succession. The target temperature was delivered for 1 second per heat stimulus, with a 2.5 second inter-pulse interval during which the contact area returns to baseline temperature. At the peak of each pulse, the subject provided a rating of the pain they experienced on the 0–100 scale. On this numeric rating scale, 0 = “no pain” and 100 = “the most intense pain imaginable”. Each trial continued for the full five pulses unless the participant withdrew by rating a pulse ‘100’ or saying ‘stop’. Again, both hyperalgesia (pain sensitivity) and TS of pain (facilitation from initial to maximum contact) were assessed with these procedures. The stimulus site at both the forearm and the knee was varied to account for possible local sensitization, meaning that no two trials immediately repeated on the same area.
Data Analysis
Traditional indices derived from the assessment of thermal and mechanical pain stimuli reported in the literature include the use of the first pain rating [36], mean pain ratings [37], the final pain rating [38], the maximal pain rating [17], and the maximal pain rating minus the first pain rating [21]. It is important to note that these indices of quantitative sensory testing likely reflect different aspects of pain perception including hyperalgesia and TS, and as a result, are not interchangeable. Thus, for all hyperalgesia analyses, the main effects of ethnicity and age group on pain ratings were assessed as an index of hyperalgesia. Similar to Edwards et al. (2003), TS of mechanical and heat pain in this study was assessed by evaluating the difference between the first pain rating and the maximal pain rating, as this reflects the slope or magnitude of the TS response [39]. TS-related pain facilitation effects are demonstrated if the maximal pain rating is significantly greater than the first pain rating. These TS and hyperalgesia analyses were carried out across the various trials of heat pain stimuli and mechanical pain stimuli.
Zero-order relationships among the covariates and indices of hyperalgesia and TS were examined using Pearson correlations. Due to unequal sample sizes across ethnic and age groups, the parametric assumptions of data normality and homogeneity of variance were visually inspected and tested using Shapiro-Wilk statistics and Levene’s tests, respectively. Normality was violated for all analyses (Shapiro-Wilk statistics: all P’s ≤ 0.05), as was homogeneity of variance (Levene’s test: all P’s ≤ 0.05) and the violations were not resolved with logarithmic transformation. A series of repeated measures analyses of variance (RM-ANOVAs) were performed to examine 1) the main effects of ethnic group and age group on pain ratings (i.e., hyperalgesia) and 2) the interactions among ethnic group, age group, and the repeated mechanical/heat stimuli on pain ratings (i.e., minority aging effects on temporal summation). Significant interaction effects were further analyzed by post-hoc tests using Tukey’s HSD to compare the four groups by ethnicity and age (i.e., old-AA, young-AA, old-NHW, young-NHW). All data were analyzed using SPSS (Statistical Package for the Social Sciences) at a statistical significance level of P < 0.05 unless multiple comparisons necessitated a control for type 1 error rate inflation.
Results
Characteristics of the Study Sample
Characteristics of the 98 healthy adults who participated in the study are presented in Table 1. The mean age of the younger group was 23.7 (SD = 4.2; range 19–34), with an approximately equal number of men and women (52% female). The mean age of the older group was 53.3 (SD = 5.7; range 45–82), with somewhat higher female participation (71%). While the ethnic representation in the younger group is equal, there are slightly more NHWs present in the older group (54.2%). Resting blood pressures across the entire sample fell into the normotensive to pre-hypertensive range. The overall mean BMI was 29.06. BMI measurements differed significantly by ethnicity, such that AAs were characterized by greater mean BMI compared to NHWs. Older participants also had significantly higher BMIs compared to their younger counterparts. None reported taking any prescribed or over-the-counter pain medications prior to the study session in response to direct questioning, in which the participant was asked to name any medications taken in the past two days. Education varied across the sample, with NHW participants reporting slightly more years of education than their AA counterparts.
Table 1.
Descriptive sample characteristics
| Variable | Overall | NHW younger | NHW older | AA younger | AA older |
|---|---|---|---|---|---|
| N | 98 | 25 | 26 | 25 | 22 |
| Age (years) | 38.16 (15.66) | 24.60 (4.04) | 53.50 (6.68) | 22.76 (4.14) | 52.95 (4.43) |
| Range | 19–82 | 20–34 | 45–82 | 19–31 | 45–67 |
| Gender (%F) | 61.2% | 52% | 69.2% | 52% | 72.7% |
| BMI | 29.06 (8.18) | 24.54 (3.67) | 28.28 (5.60) | 29.75 (9.52) | 34.11 (9.95) |
| Education | |||||
| < 12 years | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.5%) |
| High school | 38 (38.8%) | 5 (20%) | 7 (26.9%) | 17 (68%) | 9 (40.9%) |
| 2-year degree | 13 (13.3%) | 2 (8%) | 3 (11.5%) | 2 (8%) | 6 (27.3%) |
| 4-year degree | 30 (30.6%) | 12 (48%) | 8 (30.8%) | 6 (24%) | 4 (18.2%) |
| MA | 14 (14.3%) | 6 (24%) | 7 (26.9%) | 0 (0%) | 1 (4.5%) |
| PhD | 2 (2.0%) | 0 (0%) | 1 (3.8%) | 0 (0%) | 1 (4.5%) |
| CES-D | |||||
| Mean (SD) | 12.28 (8.21) | 15.56 (5.64) | 6.58 (7.33) | 18.44 (4.81) | 8.27 (8.33) |
| Range | 0–31 | 0–25 | 0–30 | 12–27 | 0–31 |
| EOD | |||||
| Mean (SD) | 4.59 (7.00) | 1.24 (2.28) | 1.94 (4.13) | 7.46 (7.47) | 8.25 (9.50) |
| Range | 0–30 | 0–7.5 | 0–12.5 | 0–22.5 | 0–30 |
NHW = Non-Hispanic White, AA = African-American, CES-D = Center for Epidemiologic Studies Depression Scale, EOD = Experiences of Discrimination.
Scores on the CES-D, an index of depressive symptoms, ranged from 0 to 31 overall. The older group reported significantly lower scores (M = 7.4, SD = 7.7) on average than the younger cohort (M = 17.0, SD = 5.4). On the EOD, which measures experiences of discrimination, the AA participants reported significantly higher frequency of perceived discriminatory events (M = 7.8, SD = 8.4) than the NHW participants (M = 1.6, SD = 3.3).
In each RM-ANOVA, gender, BMI, education, CES-D score, and EOD score were included as covariates. Correlations for each modality and body site are shown in Table 2. There was a small amount of missing data for two of the 98 participants. These cases were deleted listwise, which resulted in a final sample size of 96 participants for inclusion in subsequent analyses.
Table 2.
Correlations between ethnicity, age group, pain testing, and covariates
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ethnicity | ||||||||||||||
| 2 | Age Group | −0.042 | |||||||||||||
| 3 | TS-Punctate: Hand | 0.302** | 0.387** | ||||||||||||
| 4 | TS-Punctate: Knee | 0.194 | 0.483** | 0.784** | |||||||||||
| 5 | TS-Heat: 44°C, Arm | 0.170 | 0.175 | 0.213* | 0.352** | ||||||||||
| 6 | TS-Heat: 46°C, Arm | −0.089 | −0.015 | 0.172 | 0.176 | 0.279** | |||||||||
| 7 | TS-Heat: 48°C, Arm | −0.102 | 0.169 | 0.406** | 0.393** | 0.229* | 0.315** | ||||||||
| 8 | TS-Heat: 44°C, Knee | 0.006 | 0.101 | 0.235* | 0.238* | 0.279** | 0.225* | 0.367** | |||||||
| 9 | TS-Heat: 46°C, Knee | −0.143 | 0.077 | 0.257* | 0.252* | 0.314** | 0.598** | 0.503** | 0.352** | ||||||
| 10 | TS-Heat: 48°C, Knee | −0.139 | −0.003 | 0.263** | 0.212* | 0.313** | 0.560** | 0.550** | 0.186 | 0.641** | |||||
| 11 | Gender | 0.009 | 0.193 | 0.104 | 0.220* | 0.129 | 0.188 | 0.215* | 0.056 | 0.037 | 0.049 | ||||
| 12 | BMI | 0.326** | 0.230* | 0.081 | 0.098 | −0.017 | −0.132 | −0.050 | 0.042 | −0.014 | −0.031 | 0.122 | |||
| 13 | EOD | 0.447** | 0.035 | 0.182 | 0.032 | 0.114 | −0.065 | −0.014 | 0.009 | 0.003 | −0.015 | −0.076 | 0.124 | ||
| 14 | CES-D | 0.165 | −0.591** | −0.252* | −0.200* | −0.089 | −0.095 | −0.103 | −0.179 | −0.170 | −0.067 | −0.101 | −0.120 | 0.050 | |
| 15 | Education | −0.419** | 0.072 | −0.131 | −0.095 | −0.180 | −0.074 | −0.014 | −0.104 | −0.133 | −0.033 | 0.040 | −0.166 | 0.019 | −0.176 |
CES-D = Center for Epidemiologic Studies Depression Scale, EOD = Experiences of Discrimination. *P < 0.05, **P < 0.01.
Responses to Mechanical Stimuli
Hyperalgesia
Results of RM-ANOVAs are shown in Table 3. A main effect of ethnicity emerged at both the hand and the knee, such that AA participants experienced greater hyperalgesia than their NHW counterparts (knee: P < 0.01, hand: P < 0.001). Additionally, a main effect of age group was found that demonstrated hyperalgesia for older adults relative to their younger counterparts at both the hand and the knee (both P’s < 0.001).
Table 3.
Repeated measures ANOVA results for punctate temporal summation, both sites
| NHW younger | NHW older | AA younger | AA older | Ethnicity | Age group | Contact* ethnicity | Contact* age group | Contact*age group* ethnicity | |
|---|---|---|---|---|---|---|---|---|---|
| Hand | P < 0.001** | P < 0.001** | P < 0.01** | P = 0.001** | P < 0.05** | ||||
| First | 2.72 (2.74) | 4.35 (4.64) | 4.34 (4.22) | 11.05 (15.35) | |||||
| Max | 5.60 (4.76) | 14.17 (14.86) | 11.42 (12.55) | 38.33 (28.81) | |||||
| Knee | P < 0.01** | P < 0.001** | P < 0.05** | P < 0.001** | P = 0.071*** | ||||
| First | 5.33 (5.49) | 10.75 (13.47) | 6.00 (6.90) | 19.36 (22.12) | |||||
| Max | 10.04 (7.78) | 28.50 (28.90) | 13.34 (11.17) | 51.60 (29.25) |
This model is adjusted for covariates gender, BMI, education, CES-D (depressive symptoms) score, and EOD (experiences of discrimination) score. **Indicate significance levels of P <0.05; ***indicate significance level at P <0.10.
Temporal Summation
Results of these analyses are also shown in Table 3, including group means for each site. The three-way interaction between ethnicity, age group, and mechanical contact (indicating TS) was significant at the hand [F(1, 87) = 5.39, P = 0.023], though only trending toward significance at the knee [F(1, 87 = 3.37, P = 0.071]. An interaction of ethnicity and mechanical contact was significant at both sites [hand: F(1, 87) = 8.54, P = 0.004; knee: F(1, 87) = 5.41, P = 0.022], indicating that AA participants demonstrated greater mechanical pain TS relative to the NHW participants (i.e., reported higher increases in pain intensity ratings in response to the repeated mechanical contacts). Similarly, the main effect of age group was significant at both sites, demonstrating that older participants also demonstrated greater TS of mechanical pain compared to the young participants [hand: F(1, 87) = 11.02, P = 0.001; knee: F(1, 87) = 25.22, P < 0.001].
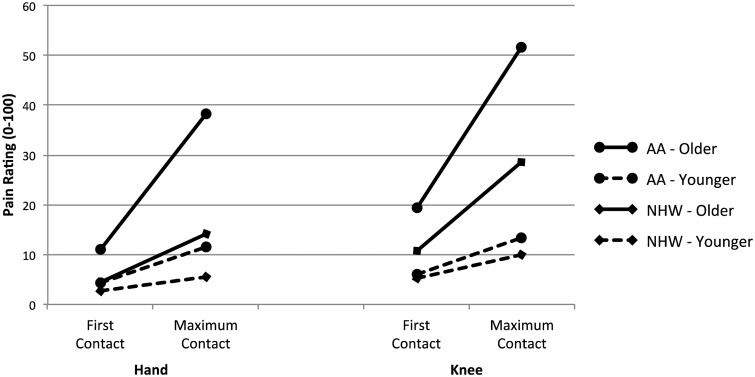
Post-hoc comparisons using the Tukey HSD test were conducted to evaluate the interaction, as depicted in Figure 1. Results indicated that the mean TS-mechanical for the older AA group was significantly higher than each of the other three groups at both the hand and the knee (all P’s < 0.001). Neither group of younger participants nor the older NHW group differed among themselves at the hand (all P’s > 0.05). However, at the knee, the older NHWs reported significantly greater TS-mechanical than the younger NHW participants (P = 0.024). Older AAs consistently demonstrate significant increases in pain ratings from the first contact to their maximal pain contact in contrast to the other three groups.
Figure 1.
Punctate first and maximum contact.
Responses to Heat Stimuli
Hyperalgesia
Results of RM-ANOVAs are shown in Tables 4 and 5. A main effect of ethnicity emerged that indicated differences at both the hand and the knee at all temperatures, such that AA participants experienced greater hyperalgesia than their NHW counterparts (all P’s < 0.05). In contrast, no differences existed between age groups (all P’s > 0.05).
Table 4.
Repeated measures ANOVA results for heat temporal summation at the forearm
| NHW younger | NHW older | AA younger | AA older | Ethnicity | Age group | Pulse* ethnicity | Pulse* age group | Pulse* age group* ethnicity | |
|---|---|---|---|---|---|---|---|---|---|
| 44°C | P < 0.05 | P = 0.671 | P = 0.373 | P = 0.167 | P = 0.667 | ||||
| First | 22.96 (19.64) | 22.15 (21.23) | 28.08 (20.06) | 33.09 (28.62) | |||||
| Max | 23.08 (20.21) | 24.35 (23.31) | 30.20 (22.50) | 38.09 (29.99) | |||||
| 46°C | P < 0.01 | P = 0.279 | P = 0.467 | P = 0.409 | P = 0.998 | ||||
| First | 24.96 (21.05) | 27.77 (24.38) | 33.68 (22.12) | 42.18 (34.19) | |||||
| Max | 28.96 (22.27) | 31.69 (26.08) | 36.48 (23.35) | 44.73 (31.24) | |||||
| 48°C | P < 0.05 | P = 0.486 | P = 0.254 | P = 0.169 | P < 0.05 | ||||
| First | 30.29 (21.27) | 33.88 (27.42) | 44.12 (28.82) | 43.27 (36.57) | |||||
| Max | 41.08 (24.63) | 43.50 (31.65) | 46.13 (27.89) | 56.77 (34.13) |
This model is adjusted for covariates gender, BMI, education, CES-D (depressive symptoms) score, and EOD (experiences of discrimination) score.
Table 5.
Repeated measures ANOVA results for heat temporal summation at the knee
| NHW younger | NHW older | AA younger | AA older | Ethnicity | Age group | Pulse* ethnicity | Pulse* age group | Pulse*age group*ethnicity | |
|---|---|---|---|---|---|---|---|---|---|
| 44°C | P < 0.01 | P = 0.472 | P = 0.646 | P = 0.960 | P < 0.01 | ||||
| First | 16.88 (18.36) | 18.85 (20.15) | 24.84 (22.36) | 29.00 (30.89) | |||||
| Max | 19.21 (20.51) | 19.00 (21.25) | 23.76 (21.04) | 32.91 (28.11) | |||||
| 46°C | P < 0.01 | P = 0.358 | P < 0.05 | P = 0.814 | P = 0.976 | ||||
| First | 2300 (21.63) | 25.27 (22.74) | 30.68 (22.64) | 35.91 (31.23) | |||||
| Max | 28.38 (23.66) | 32.62 (24.89) | 34.12 (23.52) | 40.59 (28.65) | |||||
| 48°C | P < 0.05 | P = 0.256 | P = 0.090 | P = 0.642 | P = 0.273 | ||||
| First | 29.04 (25.03) | 31.69 (25.57) | 40.44 (25.08) | 43.55 (34.93) | |||||
| Max | 42.71 (27.67) | 42.42 (29.10) | 47.52 (27.61) | 53.59 (30.59) |
This model is adjusted for covariates gender, BMI, education, CES-D (depressive symptoms) score, EOD (experiences of discrimination) score, and site (UF vs. UAB).
TS-Heat
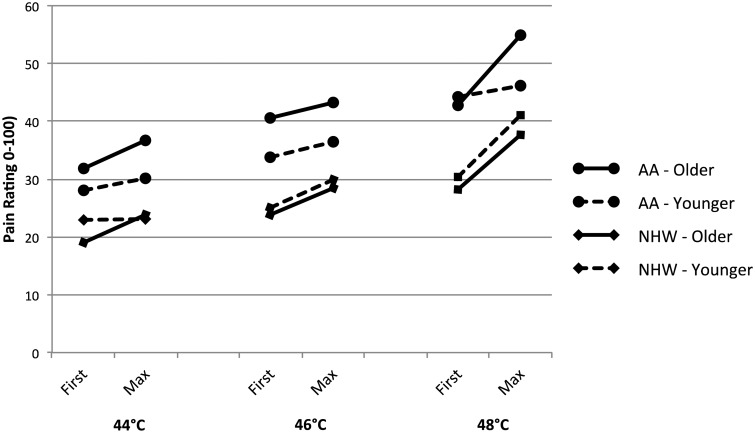
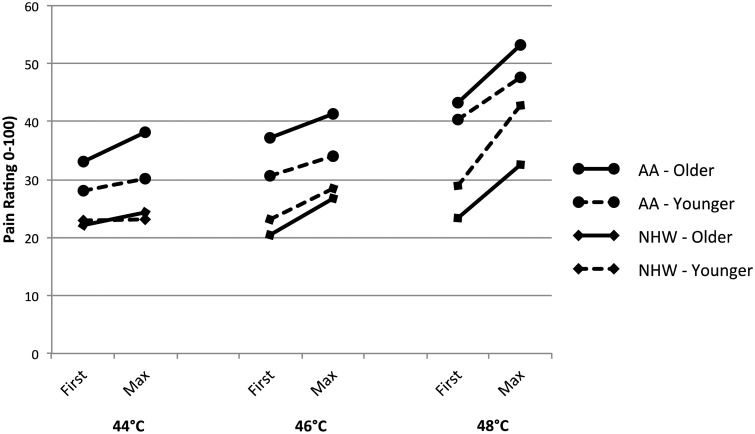
Results of the TS-Heat RM-ANOVAs, including group means for each site, are also shown in Tables 4 (hand) and 5 (knee). No significant differences in TS-Heat consistently appeared as a function of either age or ethnicity (indicated by interactions with heat pulse), though one temperature at the knee was significant when considering ethnicity [46°C: F(1, 87) = 5.09, P = 0.027]. Results were inconsistent for the three-way interaction effects of age group, ethnicity, and heat pulse. A significant interaction was evident at the knee when using 44°C [F(1, 87) = 8.15, P = 0.005] as well as at the forearm when using 48°C [F(1, 87) = 5.39, P = 0.023]. These results tentatively suggest that interaction effects may be influential in TS-heat, but that they are less strong and consistent than in mechanical stimulation (Figures 2 and 3).
Figure 2.
Heat first and maximum pulse ratings at forearm.
Figure 3.
Heat first and maximum pulse ratings at knee.
Discussion
The aim of the present study was to simultaneously assess the relationships among demographic variables (ethnicity and age) and pain responses to mechanical and thermal heat stimuli (hyperalgesia and endogenous pain facilitatory processes). Our hypotheses were partially confirmed. Hyperalgesia and increased TS for mechanical pain stimuli was demonstrated by AAs compared to NHWs as well as older compared to younger participants. In particular, older AA participants reported the highest overall pain ratings and greatest increase in pain for mechanical stimulation at both the knee and the hand. AA participants also demonstrated hyperalgesia while experiencing heat stimuli relative to their NHW counterparts. Indeed, older African Americans consistently reported the highest pain ratings for both first pain and maximum pain for both modalities compared to the other groups. Age alone was not related to hyperalgesia to heat pain at any of the stimulus intensities, nor to TS of heat. During the mechanical TS procedure, the three-way interaction of ethnicity, age group, and mechanical contact was significant at the hand and approached significance at the knee. Additionally, the interaction was present to a lesser extent when using heat TS. This interaction was primarily driven by the older AA participants, who demonstrated dramatically increased pain facilitation when compared to all other groups. Notably, mechanical TS for older AA participants appears to be comparable at the hand and knee; however, older NHW participants reported greater TS at the knee, which impacts the significance of the relationships assessed. Taken together, these data indicate that minority status, sometimes compounded by aging, is important for pain facilitation of multiple modalities. Additionally, the hyperalgesia results suggest that ethnicity is consistently important when evaluating enhanced pain sensitivity irrespective of age. To our knowledge, this is the first study to evaluate multiple modalities of pain facilitation in ethnically diverse age groups.
Though our hypotheses were partially confirmed, the pattern of facilitatory pain results differed by the modality of pain testing used. When experiencing mechanical stimuli, older AA participants demonstrated hyperalgesia and TS in response to the contacts. However, with thermal stimuli, all AA participants demonstrated hyperalgesic responses to the heat pulses but not heightened TS in comparison to their NHW counterparts, regardless of age. In fact, no age differences consistently existed in the heat TS results at all, which is in contrast with previous literature [1,21,39]. Physiological differences in processing heat and mechanical stimuli may contribute to these results. In general, TS is considered the result of repetitive peripheral stimulation involving both A-delta and C fibers, which contribute to different aspects of TS-related pain. A-delta fibers are often implicated in the sharp but brief experiences during the stimulus presentation, while C fibers contribute more heavily to the perception of increased pain over time. These fibers carry painful information from the periphery to the spinal cord and then on to supraspinal mediators [40]. Thus, a greater increase in TS is suggested to be indicative of increased central sensitivity. Particularly in mechanical TS, the secondary perception of dull pain increasing in intensity over time is induced by C-fibers, but is also further mediated by nociceptive A-fibers [41]. Mechanical TS is also thought to utilize a high contribution of A-fibers in the “pricking” sensation the monofilament produces, an experience unique to mechanical TS and not typically reported during the heat probes [41]. Indeed, previous research has suggested age-related decline in the function of A-delta fibers [18]. Given possible differences in the involvement of A-delta and C fibers between heat and mechanical TS, the modality of noxious stimuli administered may be especially important to consider when evaluating pain facilitation in minority aging groups. To date, both aging and ethnic differences in mechanical TS remain largely unexplored. Little to no previous literature had been conducted to evaluate ethnic differences in heat TS, but findings regarding suprathreshold hyperalgesia do support this study’s ethnic differentiation in heat pain [19]. Thus, mechanical procedures and their physiological underpinnings may be particularly relevant for assessment of temporal summation. In particular, this mechanical TS data suggest there may be changes in these pain facilitatory fibers specific to minority individuals as they age. Taken together, these results underscore the importance of including pain facilitatory measures in a QST session to further evaluate ethnic differences.
In addition to ethnicity and aging, it is likely that multiple other biopsychosocial factors affect pain perception. In an effort to account for some of these factors, multiple variables were included in all of the data analytic models including gender, BMI, education level, depressive symptoms, and experiences of discrimination. However, other variables not included in this study might affect the relationship between minority status, aging, and pain. For example, psychological factors such as stress [42], anxiety [43], pain hypervigilance [44], and optimism [45] have been related to pain perception in experimental pain sessions. Behavioral factors such as sleep disturbances have also recently been explored in relation to pain perception [46,47]. Though further investigation was not possible with the current study’s limited sample size, we plan to conduct additional studies with larger samples in the future. With an initial relationship between ethnicity, aging, and endogenous pain facilitation established, future research is warranted to evaluate these and many other factors as potential mediators of pain perception within the minority aging population.
Due to the relatively few studies that have directly addressed minority aging differences in pain facilitation, it is not yet possible to conclude that clinically relevant differences in endogenous pain processing exist between older AA populations and their younger and/or NHW counterparts. However, the potential clinical implications of our findings, particularly for the older minority participants, are underscored by previous research that has related QST responses to clinical pain outcomes [11]. A 2005 review suggests that QST sessions relate to clinical experience in many ways, including group differences in pain perception, cross-sectional associations between QST responses and clinical pain, and prediction of treatment outcomes. For instance, it has been demonstrated that more adaptive endogenous pain processing is associated with less clinical pain and better physical functioning [48,49]. Even among healthy individuals, QST responses and self-reported day-to-day pain symptoms have been linked [11]. This supports the careful use of QST data to inform group differences in both clinical and non-clinical pain experiences. As previously described, minority populations and older individuals often experience a higher prevalence and severity of chronic pain across a variety of clinical conditions than their counterparts, and it may be that altered endogenous pain facilitation plays a contributory role [13,50–52]. Additional research on this topic is needed to confirm or refute such a hypothesis. Establishing these differences may be particularly relevant for the clinical practice of pain management as multiple investigations have revealed ethnic- and age-related disparities in the perception, assessment, and treatment of acute and chronic pain [13,53–57], leaving those groups vulnerable to less effective pain treatment. Consequences of under- or untreated pain, particularly in older adults, include psychological symptoms (anxiety, depression, isolation), cognitive impairment, poor sleep, increased fall risk, and decreased recreational activities, as well as increased use of healthcare resources [58]. Thus, continued investigation into underlying mechanisms of ethnic- and age-related pain will be impactful for addressing disparities in clinical practice.
Several limitations are worthy of being mentioned when considering the findings of this study. The use of a 0–100 pain rating scale to assess hyperalgesia and temporal summation may have introduced a possible ceiling effect, especially for the heat stimuli. Second, as stated in the methods, all outcome variables violated the assumption of normality, even after performing transformations. Though this is likely common in literature involving pain ratings with healthy participants, it is still an important consideration given that it may limit the generalizability of our findings. Third, the study does not include a specific SES variable in the model. This is because the younger group likely reported inconsistent data regarding their financial income with their living quarters, as most lived at college while variably reporting their own or their parents’ income. Many students expressed confusion at how to indicate their socioeconomic status (SES) status, accounting for financial income and number of people in the home they share at college. Previous research has suggested that SES and education are highly related [59]; therefore, inclusion of a commonly accepted SES measure in this study may have helped to strengthen our conclusions. Thus, these results should be interpreted carefully.
Limitations notwithstanding, this study lends support for the incorporation of tests of pain modulatory mechanisms in addition to unidimensional measures when addressing questions related to minority aging and pain perception. Subsequent research should include tests of pain inhibition as well as facilitation to more fully characterize minority aging-related changes in endogenous pain modulation. Further, it may be that enhanced pain facilitatory processes place AAs at greater risk for developing chronic pain as they age. Although previous research indicates that ethnicity and age relate to unidimensional pain measures, results of comprehensive research into minority aging differences in dynamic pain responses is less clear. It will be important to more directly assess the clinical relevance of these findings in the future. If additional research substantiates that tests of dynamic pain processing are relevant for clinical pain outcomes [11], targeting treatments toward improving modulation may help reduce the burden of chronic pain currently experienced by older minority groups. Better understanding of the relationship between ethnicity- and age-related changes in endogenous pain facilitation could result in more effective, individually tailored pain management techniques. Given the U.S. Census Bureau’s recent report stating that by 2030 the number of people >65 years is predicted to reach 86.7 million (21% of all Americans), there is the potential for a large impact on a growing population.
Funding sources: Supported by the NIH/NIA Grant R01AG033906, the University of Alabama at Birmingham Center for Clinical and Translational Science grant UL1-TR000165 from the National Center for Advancing Translational Sciences and the National Center for Research Resources, and the University of Alabama at Birmingham Center for Aging Research Scholarship in Aging.
Conflict of interest: All authors declare that there are no conflicts of interest with this study.
References
- 1. Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med 2001;7(3):433–56, v–vi. [DOI] [PubMed] [Google Scholar]
- 2. Breitbart W, McDonald M V, Rosenfeld B, et al. Pain in ambulatory AIDS patients. I: Pain characteristics and medical correlates. Pain 1996;68(2-3):315–21. [DOI] [PubMed] [Google Scholar]
- 3. Carey TS, Garrett JM. The relation of race to outcomes and the use of health care services for acute low back pain. Spine 2003; 28(4):390–4. [DOI] [PubMed] [Google Scholar]
- 4. Faucett J, Gordon N, Levine J. Differences in postoperative pain severity among four ethnic groups. J Pain Symptom Manage 1994;9(6):383–9. [DOI] [PubMed] [Google Scholar]
- 5. Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain 2004;20(4):227–39. [DOI] [PubMed] [Google Scholar]
- 6. Harkins SW, Price DD, Martelli M. Effects of age on pain perception: Thermonociception. J Gerontol 1986;41(1):58–63. [DOI] [PubMed] [Google Scholar]
- 7. Collins LG, Stone LA. Pain sensitivity, age and activity level in chronic schizophrenics and in normals. Br J Psychiatry 1966;112(482):33–5. [Google Scholar]
- 8. Walsh NE, Schoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold pressor test. Am J Phys Med Rehabil 1989;68(1):6–11. [DOI] [PubMed] [Google Scholar]
- 9. Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: Differences according to age, sex and race. Psychosom Med 1972;34(6):548–56. [DOI] [PubMed] [Google Scholar]
- 10. Neri M, Agazzani E. Aging and right-left asymmetry in experimental pain measurement. Pain 1984;19(1):43–8. [DOI] [PubMed] [Google Scholar]
- 11. Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: Multiple domains of clinical relevance. Pain 2005;114(3):315–9. [DOI] [PubMed] [Google Scholar]
- 12. Van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain 2010;11(5):408–19. [DOI] [PubMed] [Google Scholar]
- 13. Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med 2003;4(3):277–94. [DOI] [PubMed] [Google Scholar]
- 14. Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med 2000;62(4):517–23. [DOI] [PubMed] [Google Scholar]
- 15. Campbell CM, France CR, Robinson ME, et al. Ethnic differences in diffuse noxious inhibitory controls. J Pain 2008;9(8):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. J Clin Invest 1944;23(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001;91(1-2):165–75. [DOI] [PubMed] [Google Scholar]
- 18. Chakour MC, Gibson SJ, Bradbeer M, Helme RD. The effect of age on A delta- and C-fibre thermal pain perception. Pain 1996;64(1):143–52. [DOI] [PubMed] [Google Scholar]
- 19. Riley JL, Cruz-Almeida Y, Glover TL, et al. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain 2014;15(3):272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morris MC, Walker L, Bruehl S, et al. Race effects on temporal summation to heat pain in youth. Pain 2015;156(5):917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults 2003;101:155–65. [DOI] [PubMed] [Google Scholar]
- 22. Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain 2005. Jan;113(1-2):20–6. [DOI] [PubMed] [Google Scholar]
- 23. Cruz-Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol 2014;66(7):1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain 2007;23(6):506–10. [DOI] [PubMed] [Google Scholar]
- 25. Fillingim RB. Sex, gender, and pain: Women and men really are different. Curr Rev Pain 2000;4(1):24–30. [DOI] [PubMed] [Google Scholar]
- 26. Riley JL, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain 1998;74(2-3):181–7. [DOI] [PubMed] [Google Scholar]
- 27. Goodin BR, Bulls HW, Herbert MS, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: Ethnic differences. Psychosom Med 2014;76(4):302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schludermann E, Zubek JP. Effect of age on pain sensitivity. Percep Motor Skills 1962;14:295–301. [Google Scholar]
- 29. Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: A systematic review of the literature with meta-analysis. Psychosom Med 2003;65(3):369–75. [DOI] [PubMed] [Google Scholar]
- 30. Goodin BR, Pham QT, Glover TL, et al. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol 2013;32(11):1117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 32. Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res 1999;46(5):437–43. [DOI] [PubMed] [Google Scholar]
- 33. Long Foley K, Reed PS, Mutran EJ, DeVellis RF. Measurement adequacy of the CES-D among a sample of older African-Americans. Psychiatry Res 2002;109(1):61–9. [DOI] [PubMed] [Google Scholar]
- 34. Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. Int J Psychiatry Med 2001;31(1):25–40. [DOI] [PubMed] [Google Scholar]
- 35. Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61(7):1576–96. [DOI] [PubMed] [Google Scholar]
- 36. Staud R, Robinson ME, Vierck CJ, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain 2003. Jan;101(1-2):167–74. [DOI] [PubMed] [Google Scholar]
- 37. Vierck CJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol 1997;78(2):992–1002. [DOI] [PubMed] [Google Scholar]
- 38. Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Med 2007;8(6):514–20. [DOI] [PubMed] [Google Scholar]
- 39. Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci 2001;56(3):M180–5. [DOI] [PubMed] [Google Scholar]
- 40. Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005;115(3):410–8. [DOI] [PubMed] [Google Scholar]
- 41. Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain 1999;122 (Pt 1):2245–57. [DOI] [PubMed] [Google Scholar]
- 42. Reinhardt T, Kleindienst N, Treede R-D, Bohus M, Schmahl C. Individual modulation of pain sensitivity under stress. Pain Med 2013;14(5):676–85. [DOI] [PubMed] [Google Scholar]
- 43. Keogh E, Cochrane M. Anxiety sensitivity, cognitive biases, and the experience of pain. J Pain 2002;3(4):320–9. [DOI] [PubMed] [Google Scholar]
- 44. Herbert MS, Goodin BR, Pero ST, et al. Pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity among adults with symptomatic knee osteoarthritis. Ann Behav Med 2014;48(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodin BR, Bulls HW. Optimism and the experience of pain: Benefits of seeing the glass as half full. Curr Pain Headache Rep 2013;17(5):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol 2012;91(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain 2013;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edwards RR, Doleys DM, Lowery D, Fillingim RB. Pain tolerance as a predictor of outcome following multidisciplinary treatment for chronic pain: Differential effects as a function of sex. Pain 2003;106(3):419–26. [DOI] [PubMed] [Google Scholar]
- 49. Edwards RR, Ness TJ, Weigent D, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain 2003;106(3):427–37. [DOI] [PubMed] [Google Scholar]
- 50. Picavet HSJ, Hazes JMW. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis 2003;62(7):644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Green CR, Baker TA, Sato Y, Washington TL, Smith EM. Race and chronic pain: A comparative study of young black and white Americans presenting for management. J Pain 2003;4(4):176–83. [DOI] [PubMed] [Google Scholar]
- 52. Green CR, Ndao-Brumblay SK, Nagrant AM, Baker TA, Rothman E. Race, age, and gender influences among clusters of African American and white patients with chronic pain. J Pain 2004;5(3):171–82. [DOI] [PubMed] [Google Scholar]
- 53. Dobscha SK, Soleck GD, Dickinson KC, et al. Associations between race and ethnicity and treatment for chronic pain in the VA. J Pain 2009;10(10):1078–87. [DOI] [PubMed] [Google Scholar]
- 54. Todd KH, Deaton C, Goe L. Ethnicity and analgesic practice. Ann Emerg Med 2000;35(1):11–6. [DOI] [PubMed] [Google Scholar]
- 55. Todd KH, Lee T, Hoffman JR. The effect of ethnicity on physician estimates of pain severity in patients with isolated extremity trauma. JAMA 2015;271(12):925–8. [PubMed] [Google Scholar]
- 56. Hwang U, Belland LK, Handel DA, et al. Is all pain is treated equally? A multicenter evaluation of acute pain care by age. Pain Int Assoc Study Pain 2014;155(12):2568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: Non-Hispanic Whites, Non-Hispanic Blacks, and Hispanics. J Pain 2007;8(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain. Annu Rev Clin Psychol 2011;7:411–34. [DOI] [PubMed] [Google Scholar]
- 59. Kington RS, Smith JP. Socioeconomic status and racial and ethnic differences in functional status associated with chronic diseases. Am J Public Health 1997;87(5):805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]