Abstract
Objectives
To assess the reversibility of acute kidney injury (AKI)-induced neutrophil dysfunction and to identify involved mechanisms.
Design
Controlled laboratory experiment and prospective observational clinical study.
Setting
University laboratory and hospital.
Subjects
C57BL/6 wild-type mice.
Patients
Patients with septic shock with or without AKI.
Interventions
Murine AKI was induced by i.p. injections of folic acid (nephrotoxic AKI) or by i.m. injections of glycerol (rhabdomyolysis-induced AKI). After 24h, we incubated isolated neutrophils for 3h in normal mouse serum or minimum essential medium (MEM) buffer. We further studied the effects of plasma samples from 13 patients with septic shock (with or without severe AKI) on neutrophilic-differentiated NB4 cells (NB4PMN).
Measurements and Main Results
Experimental AKI significantly inhibited neutrophil migration and intracellular actin polymerization. Plasma levels of resistin, a pro-inflammatory cytokine and uremic toxin, were significantly elevated during both forms of AKI. Incubation in serum or MEM buffer restored normal neutrophil function. Resistin by itself was able to induce AKI-like neutrophil dysfunction in vitro.
Plasma resistin was significantly higher in patients with septic shock with AKI compared to patients with septic shock alone. Compared to plasma from patients with septic shock, plasma from patients with septic shock and AKI inhibited NB4PMN migration. Even after 4d of renal replacement therapy (RRT), plasma from patients with septic shock plus AKI still showed elevated resistin levels and inhibited NB4PMN migration. Resistin inhibited NB4PMN migration and intracellular actin polymerization at concentrations seen during AKI, but not at normal physiological concentrations.
Conclusions
AKI-induced neutrophil dysfunction is reversible in vitro. However, standard RRT does not correct this defect in patients with septic shock and AKI. Resistin is greatly elevated during AKI, even with ongoing RRT, and is sufficient to cause AKI-like neutrophil dysfunction by itself.
Introduction
More than 50% of critically ill patients develop acute kidney injury (AKI).(1, 2) Increasing epidemiological evidence supports the notion that AKI is an independent risk factor for mortality, i.e. patients are dying because of and not simply with AKI.(1, 3, 4) AKI at all stages does not only affect survival but also other relevant outcomes, including length of hospital stay, readmission rates, and development of end stage kidney disease.(5, 6)
Despite a wealth of data demonstrating worse outcomes in patients with AKI, the exact underlying mechanisms remain largely unknown.
Research efforts over the past decade have shown that AKI is a systemic disease. The interactions between AKI and the immune system have become a particular focus of research activities, largely experimental studies. These efforts have been further fueled by findings of higher infection rates in patients with AKI (7–9), e.g., bacteremia, infections after cardiac surgery, and infections in patients with hematologic malignancies. Infections are a particular problem for patients on renal replacement therapy (RRT).(10, 11) Nearly 50% of all infections become apparent shortly before initiation of RRT, 40% during RRT, and approximately 10% in the period following discontinuation of RRT. Unfortunately, RRT does not seem to alter the AKI-associated risk of infection.(10, 11)
We have recently shown in various animal models as well as in a small clinical study that AKI inhibits crucial steps within the leukocyte recruitment cascade, especially slow leukocyte rolling and transmigration.(12–14) We have further shown that AKI-induced impairment of neutrophil recruitment worsens bacterial pneumonia. (14)
The effects of AKI on neutrophils appear to be mediated by intracellular changes in actin polymerization and phosphorylation of signaling pathways.(13, 14)
We have also demonstrated that neutrophils from patients with sepsis-induced AKI lack slow rolling, when compared to neutrophils from patients with sepsis but no AKI.(13)
AKI-induced renal endocrine failure is characterized by drastically reduced 1,25-dihydroxyvitamin D3 levels.(15, 16) 1,25-dihydroxyvitamin D3 activates intracellular signaling pathways, which in turn are crucial for appropriate cytoskeleton rearrangement and cell recruitment.(17, 18)
In addition to electrolyte, fluid and acid-base disturbances, AKI-induced renal exocrine failure also leads to accumulation of various uremic toxins, some of which impair leukocyte function. RRT fails to remove some of these toxins effectively.(19–21)
We now present a translational approach to assess the reversibility of AKI-induced neutrophil dysfunction under both experimental and clinical conditions. In particular, we have examined whether restoration of renal exocrine and/or endocrine function is necessary to correct neutrophil dysfunction in vitro.
Guided by findings from the first part of our study, we have then sought to identify potential mediators involved in AKI-induced neutrophil dysfunction.
Materials and Methods
Animals
The Animal Care and Use Committee of the Penn State College of Medicine approved all animal experiments. We used 10–12-week-old male wild-type C57BL/6 mice. Mice were housed in a barrier facility under specific pathogen-free conditions.
Human subjects
The University of Pittsburgh Institutional Review Board approved the study. After informed consent, blood was drawn from 8 patients with septic shock and from 5 patients with septic shock plus AKI. In patients with AKI, we collected blood immediately before initiation of RRT (5 continuous veno-venous hemodiafiltration) and on day 4 of RRT (4 continuous veno-venous hemodiafiltration, 1 intermittent hemodialysis). RRT mode and intensity was at the discretion of the attending nephrologist. Septic shock was defined according to the American College of Chest Physicians/Society of Critical Care Medicine consensus conference criteria.(22) AKI was defined according to the RIFLE criteria; only patients with RIFLE-F were included.(23) We excluded patients who were pregnant, younger than 18 years, already receiving renal replacement therapy, receiving immunosuppressive therapy or with a history of hematological malignancy.
Reagents
If not stated otherwise, we obtained all reagents from Sigma-Aldrich (St. Louis, MO, USA).
Folic Acid (FA)-induced AKI
Following our recently published protocol (14), we used i.p. injections of FA (450mg/kg dissolved in NaHCO3) to induce nephrotoxic AKI. Sham injections of NaHCO3 served as control. Animals were exsanguinated under deep anesthesia 24h later.
Rhabdomyolysis-induced AKI
As described previously (14), we injected mice with glycerol (6mg/kg) i.m. to induce rhabdomyolysis-induced (‘rhabdomyolysis’) AKI. I.m. injections of normal saline served as sham intervention (control). Animals were exsanguinated under deep anesthesia 4 or 24h later.
In both models of AKI, we measured plasma creatinine and cystatin C to assess renal function as well plasma levels of resistin, using commercially available assays, (BioVision Inc., Milpitas, CA; R&D Systems, Minneapolis, MN).
Neutrophil isolation
Neutrophils were isolated from bone marrow cells of exsanguinated animals by Percoll gradient as described previously.(14)
We used isolated neutrophils for either immediate transwell migration assays and intracellular F-actin measurements or reconstitution experiments (see below).
In vitro reconstitution experiments
For in vitro reconstitution, we incubated neutrophils in normal mouse serum or in minimal essential medium (MEM) buffer (Life Technologies, Grand Island, NY, USA) for 3h (37°C, 5% CO2). Compared to normal serum, MEM does not contain any proteins, lipids, growth factors or vitamin D3.
Our preliminary studies had shown that prolonged incubations in simple salt-buffered solutions resulted in overt cell death, excluding these buffers from further use (data not shown). Following 3h incubations, we assessed transwell migration and intracellular actin polymerization of the reconstituted neutrophils.
Transwell migration assays - murine neutrophils
As recently described (14), transwell migration of murine neutrophils was evaluated using 3 μm transwell chambers coated with fibrinogen in a chemotaxis assay. Migration without fMLP or toward fMLP was allowed for 1h (37°C, 5% CO2). All samples were run in triplicates. Transwell migration rates were calculated as the ratio of the number of cells in the lower chamber to the number of cells seeded in the upper chamber prior to migration. Cells were counted using flow cytometry (BD Accuri C6 flow cytometer, BD Biosciences, San Jose, CA, http://static.bdbiosciences.com/documents/accuri/Accuri-TB-Guide-to-Absolute-Counting.pdf). Results were calculated as percent of sample (sham control) to normalize for assay variability.(24)
A subset of isolated neutrophils from untreated animals was incubated in phosphate-buffered saline (PBS) with or without 40 ng/ml of recombinant mouse resistin (R&D Systems, Minneapolis, MN) for 1h (37°C, 5% CO2) prior to transwell migration.
Intracellular F-actin measurements - fMLP-induced actin polymerization
Isolated murine neutrophils or neutrophilic-differentiated NB4 cells (NB4PMN, see below) were incubated in PBS with 100μM N-formyl-L-methionyl-L-leucyl-L phenylalanine (fMLP) to initiate actin polymerization. Actin polymerization was stopped after 0, 30, 120 or 600 sec. in a simultaneous fixation, permeabilization, and staining step.(25) Fixation, permeabilization, and staining (FPS) buffer contained 4% Formaldehyde in PBS (Boston BioProducts, Ashland, MA), 1% Triton X-100, and 150nM AlexaFluor 647 phalloidin.(25) After incubation with FPS, 500 μL of TBS were added and the cells washed for 5 min. Cells were then re-suspended in 500 μL of TBS and kept at 4°C until flow cytometry (BD Accuri C6 flow cytometer, BD Biosciences, San Jose, CA).
Murine neutrophils were gated by staining for a neutrophil specific surface antigen (Ly6-G) as well as by their usual appearance in FSC and SSC. NB4PMN cells were gated by surface staining for CD11b, CD35 and CD71 as well as their appearance in FSC and SSC.(26) Median fluorescence intensity (MFI) was measured on at least 10,000 cells per sample. Flow cytometry data analysis was performed with FCS Express 4 (De Novo Software, Glendale, CA).
To account for different F-actin baseline levels in murine neutrophils with or without AKI, we calculated the AUC for F-actin formation from 0–30sec (AUC0–30″) for each murine sample (linear trapezoidal rule).(27)
The product of baseline MFI x 30sec was subtracted from all raw AUC0–30″.
Intracellular F-actin immunofluorescence
Isolated murine neutrophils were incubated in PBS with or without 100μM fMLP on fibrinogen-coated glass bottom microwell petri dishes (Mat Tek corporation, Ashland MA). Actin polymerization was stopped after 150 sec. using FPS buffer (see above, except for AlexaFluor 488 Phalloidin instead of AlexaFluor 647 phalloidin). Fluorescence images of the cells were obtained with a Nikon TE2000 microscope using a 60X objective, the Photo Fluor II (89 North, Burlington, VT) for illumination, an Orca-ER 1394 digital CCD camera (Hamamatsu Photonics, Bridgewater, NJ), and iVision software (Biovision Tech, Exton, PA) for acquisition. The images were processed and pseudo-colored with iVision software.
Neutrophilic differentiation of NB4 acute promyelocytic leukemia cells
We used NB4PMN cells to study the effect of plasma from septic patients with or without AKI on neutrophil function in a standardized and reproducible way.
We induced neutrophilic differentiation by incubating NB4 cells (Part #H02129, National Cell Culture Center, Minneapolis, MN) with 1μM all-trans retinoic acid (ATRA) for 6 days in RPMI 1640 with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. ATRA has been shown to induce differentiation of NB4 cell lines into morphologically mature neutrophilic granulocytes.(28, 29)
Successful differentiation into neutrophilic granulocytes prior to each experiment was tested by flow cytometry analysis of cell surface expression of CD11b, CD35 and CD71 before each assay.(30) Our preliminary functional studies have shown that NB4PMN exhibited transwell migration towards fMLP as well as intracellular actin polymerization similar to that of freshly isolated human neutrophils (data not shown).
For some experiments, NB4PMN cells were incubated for 1h in human control serum spiked with different concentrations of recombinant human resistin (20, 50 or 100ng/ml) prior to further studies.
Transwell migration assays - NB4PMN cells
NB4PMN cells were incubated in human control serum or patients samples for 1h prior to transwell migration assay. Transwell migration of NB4PMN cells was evaluated using 5 μm transwell chambers coated with fibrinogen in a chemotaxis assay. Migration without fMLP or toward fMLP was allowed for 2h (37°C, 5% CO2). The migrated cells in the bottom well were counted via flow cytometry. All samples were run in triplicates. Transwell migration of NB4PMN cells was calculated as ratio of the cell concentration in the lower chamber and the concentrations of cells seeded in the upper chamber prior to migration. Final results are presented as transwell migration ratio of NB4PMN cells incubated in patient serum samples normalized to that of NB4PMN cells simultaneously incubated in human control serum.
Statistics
All data are presented as median (interquartile range). Statistical analysis included, where indicated, test for normality (Shapiro-Wilks), paired and unpaired t-tests, one-way ANOVA with Tukey’s multiple comparisons test, two-way ANOVA with Tukey’s multiple comparisons test, Wilcoxon matched-pairs signed rank test, and Mann Whitney test. P<0.05 was considered statistically significant.
Results
Nephrotoxic AKI and rhabdomyolysis-induced AKI
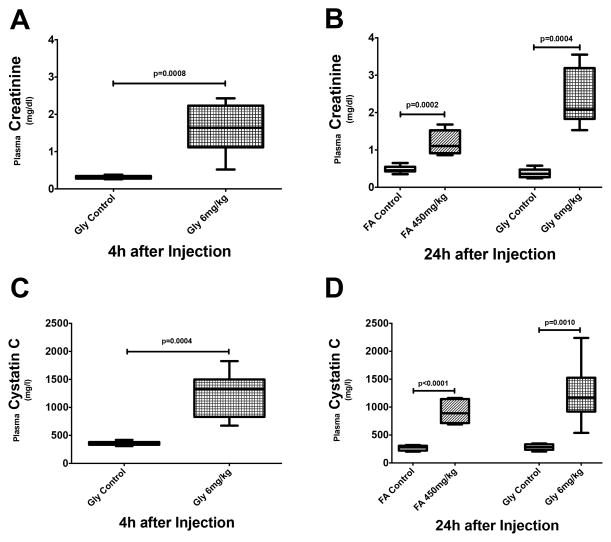
Similar to our previous study, i.p. injections of FA lead to nephrotoxic AKI within 24h.(14) AKI severity, as indicated by changes in plasma creatinine, corresponds to KDIGO stage 2 in patients with AKI (Fig. 1B).(9) I.m injections of glycerol cause rhabdomyolysis-induced AKI as early as 4h after injection (KDIGO stage 2, Fig. 1A), lasting at least as long as 24h after injection (KDIGO stage 3, Fig. 1A). Changes in plasma creatinine are similar to those in plasma cystatin C (Fig. 1C+D), supporting the use of plasma creatinine as indicator of glomerular filtration rate even in our model of rhabdomyolysis-induced AKI.
Figure 1. Renal function in murine models of nephrotoxic and rhabdomyolysis-induced AKI.
Plasma creatinine levels are elevated as early as 4 hours after injection in mice with rhabdomyolysis-induced AKI (A, n=8) and are even further increased after 24 hours (B, n=7–9). In nephrotoxic AKI (n=8), elevated plasma creatinine levels are only seen after 24 hours (B). Similar changes are seen with plasma concentrations of cystatin C (B + C).
Nephrotoxic AKI and rhabdomyolysis-induced AKI impair neutrophil transwell migration
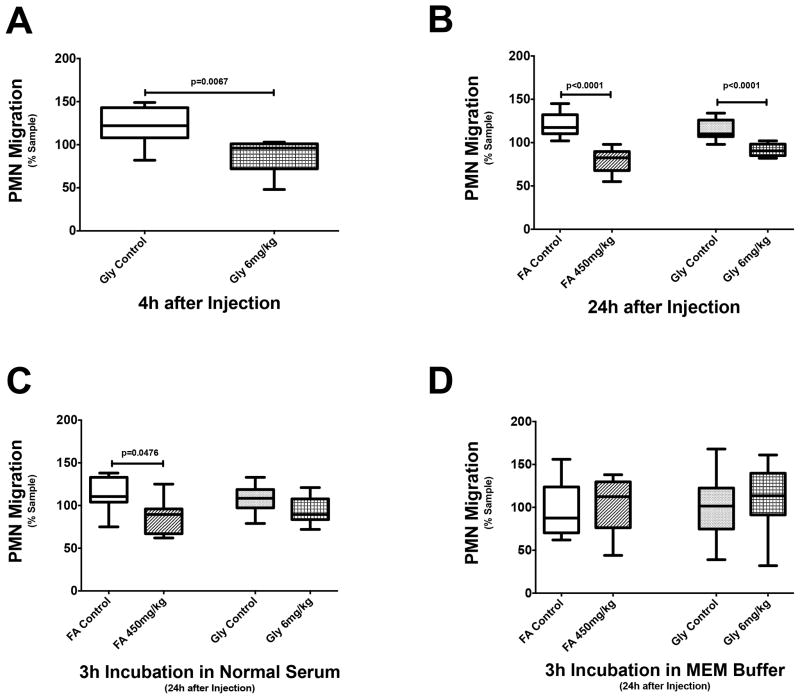
In agreement with our previous findings (14, 31), rhabdomyolysis-induced AKI significantly reduces neutrophil transwell migration as early as 4h after induction (Fig. 2A). Nephrotoxic AKI does not affect neutrophil transwell migration at 4h (data not shown) but significantly impairs neutrophil transwell migration at 24h (Fig. 2B). Both nephrotoxic and rhabdomyolysis-induced AKI attenuate neutrophil transwell migration to a similar degree.
Figure 2. Neutrophil transwell migration during AKI.
Rhabdomyolysis-induced AKI causes impaired neutrophil transwell migration as early as 4h after induction (A, n=7–8), lasting at least as long as 24h (B, n=6–9). Nephrotoxic AKI (n=6) significantly inhibits neutrophil transwell migration 24h after induction (B). Incubation in normal serum completely restores normal neutrophil transwell migration in rhabdomyolysis-induced AKI (n=8–10) but only incompletely and nephrotoxic AKI (n=8) (C). Normal neutrophil transwell migration is completely restored in both nephrotoxic AKI (n=6) and rhabdomyolysis-induced AKI (n=8–10) after incubation in MEM buffer (D).
Incubation in buffer reverses AKI-induced neutrophil transwell migration defects
3h incubation in normal mouse serum completely restores normal neutrophil transwell migration in rhabdomyolysis-induced AKI but only incompletely in nephrotoxic AKI (Fig. 2C). However, a 3h incubation in MEM buffer fully corrects neutrophil transwell migration defects in both nephrotoxic and rhabdomyolysis-induced AKI (Fig. 2D).
Incubation in normal serum mimics restoration of both renal exocrine and endocrine function, whereas incubation in MEM buffer only mimics restoration of renal exocrine function. Our findings therefore suggest that restoration of renal exocrine function is sufficient to correct AKI-induced neutrophil transwell migration defects.
Buffer is superior to normal serum for restoring neutrophil transwell migration
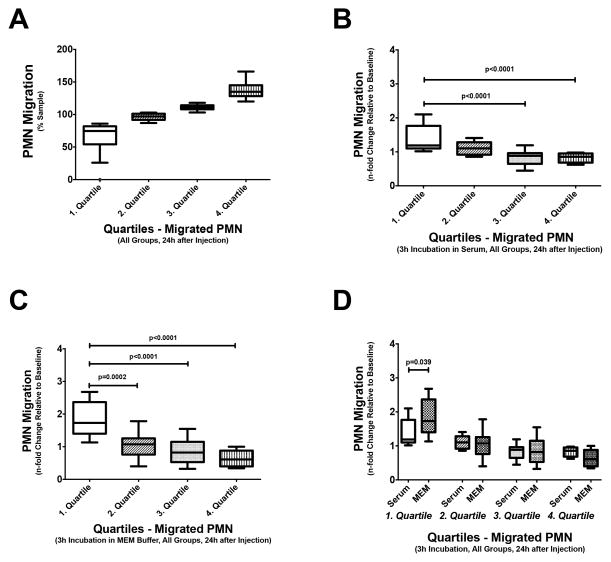
We performed additional statistical analyses to better understand the effects of MEM buffer and normal serum on restoration of normal neutrophil transwell migration, independent of the type of AKI. Combining all experimental data, we first grouped neutrophil transwell migration rates into quartiles (Fig. 3A). The first quartile reflects the lowest 25% neutrophil transwell migration rates observed, whereas the fourth quartile includes the 25% highest neutrophil transwell migration rates found. This analysis shows that incubation in both normal mouse serum and buffer has little or no effect on neutrophils with the highest migration rates, i.e. neutrophils in the fourth quartile (Fig. 3B+C). Contrary to that, incubation in normal mouse serum or buffer greatly affects neutrophils with the lowest transwell migration rates (first quartile), i.e. the neutrophils most affected by AKI. Neutrophil transwell migration rates for these cells are significantly improved. The median relative change in neutrophil transwell migration rates from before to after incubation for these cells is 1.2 and 1.7 for incubation in serum and MEM buffer, respectively. This difference in improvement of neutrophil transwell migration rates is statistically significant, indicating that MEM buffer is superior to normal serum for restoration of neutrophil function.
Figure 3. The effect of restoration of renal function on different neutrophil subpopulations in vitro.
After stratifying neutrophils from all mice (n=37) into quartiles according to their transwell migration rates (A), the effects of incubating in normal mouse serum (B) or MEM buffer (C) on each quartile of neutrophils were analyzed. Neutrophils with the highest transwell migration rates, i.e. the 4th quartile neutrophils, are not significantly affected by either incubation. The ratios of transwell migration rates after and before incubation are close to 1 (B+C). Contrary, neutrophils with the lowest transwell migration rates, i.e. the 1st quartile neutrophils, show great improvement in transwell migration. The ratios of transwell migration rates after and before incubation are significantly increased (B+C). Although a small difference, incubating poorly migrating neutrophils in MEM buffer provides significantly better transwell migration rates than incubating in normal serum (D).
Incubation in normal serum or buffer corrects actin polymerization defect
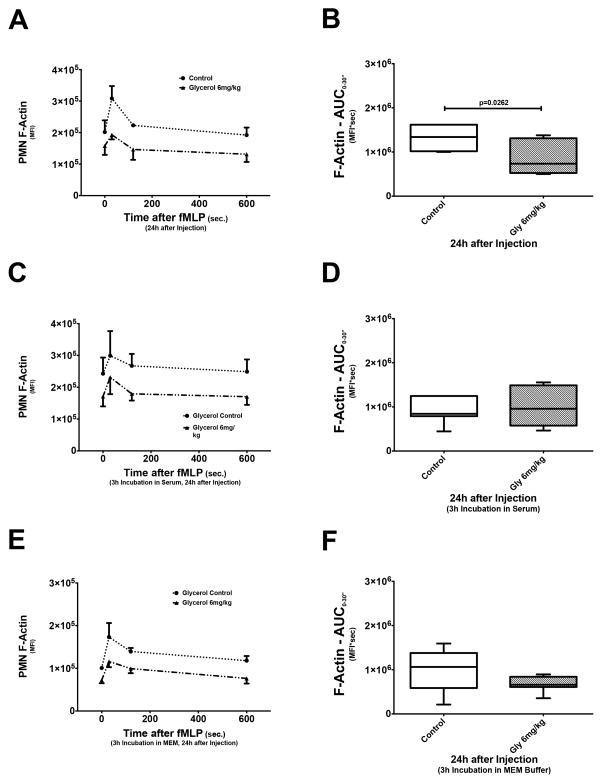
Our previous data have indicated that impaired neutrophil transwell migration during AKI is related to abnormal cytoskeleton rearrangement, in particular F-actin reorganization.(14) We here further show that both nephrotoxic and rhabdomyolysis-induced AKI result in significantly lower AUC0–30″, indicating impaired F-actin formation (Fig. 4A).(32, 33) The de-polymerization phase, i.e. decline in F-actin, is not affected by AKI (data not shown). Incubation in either normal serum or MEM buffer corrects the AKI-induced actin polymerization defect, as there are no significant AUC0–30″ differences between the groups. This is further evidence for a mechanistic link between impaired neutrophil transwell migration and defect actin polymerization during AKI.
Figure 4. Intracellular actin polymerization during AKI.
Intracellular actin polymerization in neutrophils was stimulated by in vitro incubation with fMLP and subsequently measured using flow cytometry. As seen in (A), intracellular actin, under normal conditions (control), polymerizes quickly within 30 seconds after stimulation (F-actin spike) and then depolymerizes over a longer period of time. Quantitative analysis of F-actin formation (n=7), expressed as AUC0–30″, shows that rhabdomyolysis-induced AKI significantly diminishes actin polymerization (B). Incubating neutrophils in either normal mouse serum (C) or in MEM buffer (E) restores a normal actin polymerization pattern and results in AUC0–30″ that are statistically not different from control neutrophils (D & F).
Resistin is elevated during AKI and sufficient to reproduce AKI-like effects in murine neutrophils in vitro
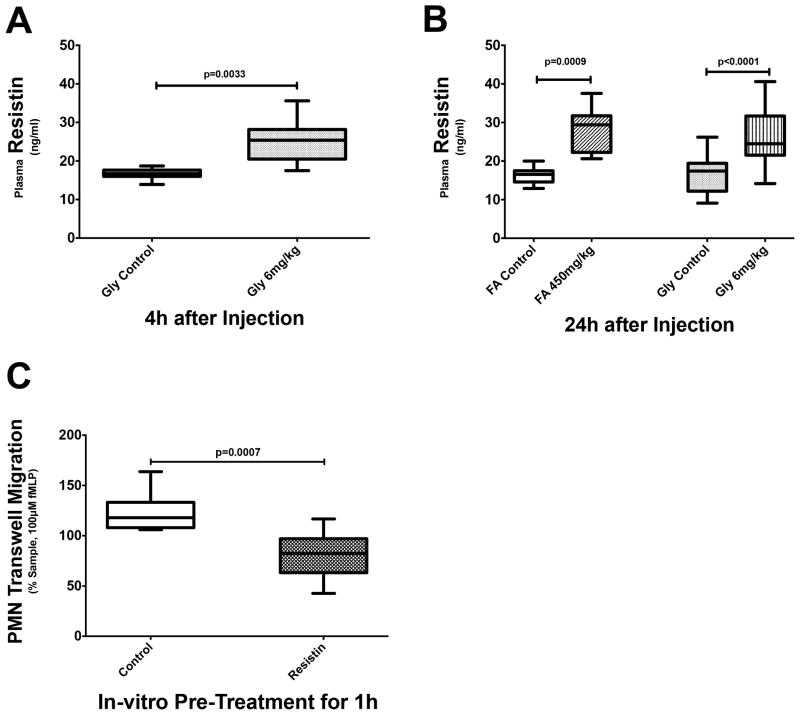
Both rhabdomyolysis-induced AKI and nephrotoxic AKI lead to elevated plasma resistin concentrations, exceeding 40 ng/ml at times (Fig. 5A+B). Plasma concentrations of resistin are elevated as early as 4h after induction of rhabdomyolysis-induced AKI. At this time point, impaired neutrophil transwell migration is also already present (Fig. 2A vs. Fig. 5A and Fig. 2B vs. Fig. 5B).
Figure 5. Resistin in murine models of nephrotoxic and rhabdomyolysis-induced AKI.
Plasma resistin levels are elevated as early as 4h after injection in mice with rhabdomyolysis-induced AKI (A) and remain elevated at 24h after induction (B). In nephrotoxic AKI, elevated plasma resistin levels are seen after 24 hours (B). (C) In vitro pretreatment with resistin significantly decreases neutrophil transwell migration rates, similar to that seen with either nephrotoxic or rhabdomyolysis-induced AKI.
Further in vitro studies show that pre-treatment of isolated neutrophils from untreated mice with recombinant resistin (40 ng/ml) is sufficient to reproduce the effects of AKI with respect to transwell migration (Fig. 5C) and F-actin formation (6A–D). Following pre-treatment, neutrophil transwell migration is impaired to a degree similar to that observed in mice with AKI (Fig. 5C). Also, resistin interferes with correct intracellular F-actin formation in fMLP-stimulated neutrophils comparable to that seen in mice with AKI (Fig. 6B+D).
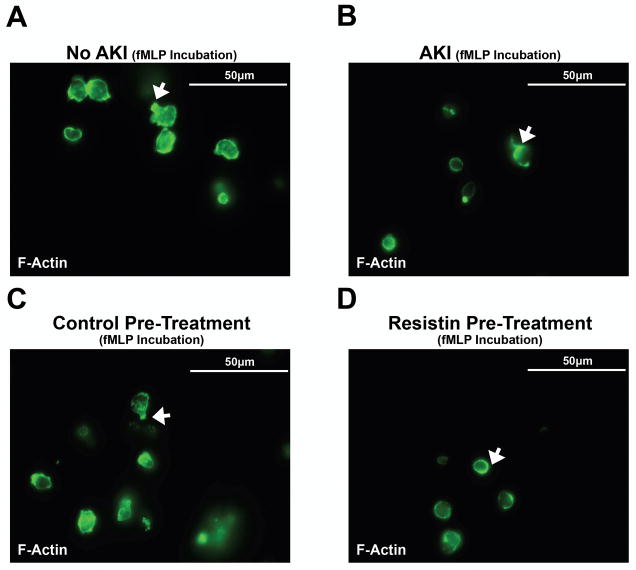
Figure 6. Immunofluorescence staining for intracellular F-actin.
Intracellular F-actin shows a normal pattern of distribution in neutrophils from control mice (A) or neutrophils undergoing control pretreatment (C). F-actin is primarily localized to the leading edge of the migrating cell, i.e. neutrophils exhibit one short and narrow lamellipodia of F-actin at one pole of the cell (arrow). Contrary to that, F-actin localization is nearly lost in neutrophils from mice with AKI (B) or neutrophils pretreated with resistin (D), i.e. neutrophils are characterized by a lack of distinctly localized F-actin formation (arrow). No formation of a leading edge can be seen in either cell type, suggesting impaired migration of neutrophils during AKI or after pretreatment with resistin.
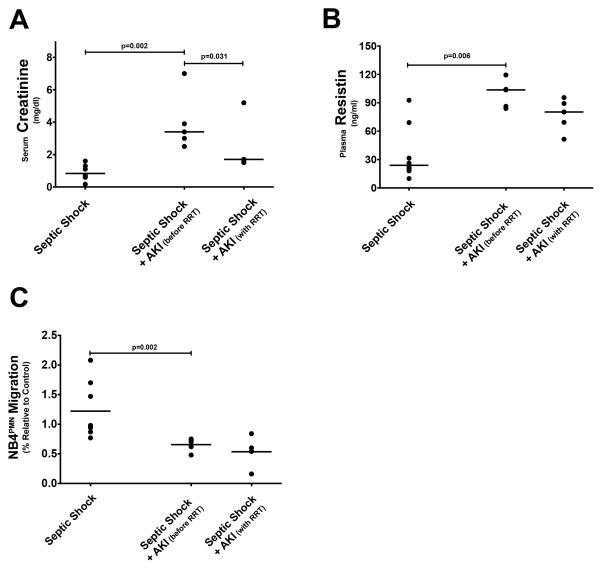
Plasma from septic patients with AKI inhibits transwell migration of NB4PMN cells
We collected blood samples from patients with septic shock as well as from patients with septic shock plus AKI (before and after 4 days of RRT). Patients with septic shock plus AKI had significantly higher serum creatinine values than patients with septic shock but no AKI (Fig. 7A). Serum creatinine levels decreased significantly with initiation of RRT (Fig. 7A) and were not different from those in patients without AKI. Patients with septic shock and AKI also had significantly higher plasma resistin levels (Fig. 7B). Interestingly, RRT does not appear to significantly affect plasma resistin levels (Fig. 7B). Plasma samples from patients with septic shock plus AKI seemingly exhibit an inhibitory effect on NB4PMN transwell migration when compared to plasma from patients with septic shock but no AKI (Fig. 7C). Similar to its effect on plasma resistin levels, RRT does not correct impaired transwell migration. Even after 4d of RRT, plasma samples from patients with septic shock plus AKI still inhibit NB4PMN transwell migration.
Figure 7. The effects of AKI in septic shock on plasma resistin and cell transwell migration.
(A) As to be expected, patients with AKI during septic shock show significantly elevated plasma creatinine concentrations when compared to those without AKI. RRT significantly reduces plasma creatinine levels by day 4. (B) Plasma resistin levels are also significantly elevated in patients with septic shock and AKI. Contrary to serum creatinine levels, our modes of RRT do not significantly lower plasma resistin levels. (C) NB4PMN transwell migration is significantly reduced in patients with septic shock and AKI. RRT fails to restore normal NB4PMN transwell migration.
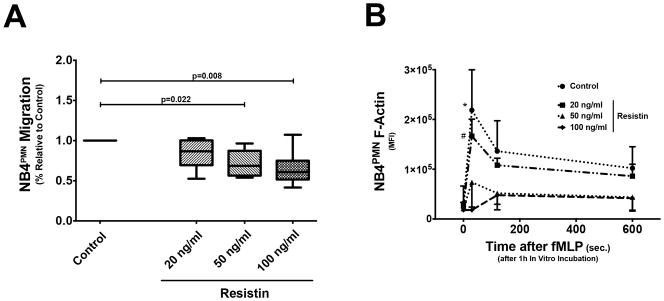
Resistin inhibits NB4PMN function only at concentrations seen during AKI
In vitro incubation of NB4PMN cells with recombinant resistin at a physiological concentration (34) does not cause abnormal transwell migration or F-actin formation (Fig. 8A + B). By contrast, recombinant resistin at concentrations seen during AKI lead to significantly reduce transwell migration and impairs F-actin formation in NB4PMN cells (Fig. 8A + B).
Figure 8. The effects of different resistin concentrations of NB4PMN transwell migration and intracellular F-actin formation.
(A) Resistin at a physiological concentration has no effect on NB4PMN transwell migration when compared to a resistin-free control. However, resistin at concentrations seen during (severe) AKI significantly inhibits transwell migration (n=8). (B) Resistin at a physiological concentration also has no effect on intracellular F-actin formation in NB4PMN cells. By contrast, resistin at concentrations seen during (severe) AKI significantly reduces intracellular F-actin formation in NB4PMN cells (n=8).
Discussion
A growing body of evidence supports the notion that patients are dying from AKI and not simply with AKI. However, the underlying factors and mechanism remain largely unknown. We have previously shown that AKI can affect innate immunity independent of changes to the cytokine homeostasis.(12–14) In particular, we have demonstrated that AKI inhibits crucial steps within the neutrophil recruitment cascade.
Here, we present a translational study to assess the reversibility of neutrophil dysfunction and ultimately identify new potential treatment pathways.
Using a nephrotoxic model and a rhabdomyolysis model of AKI, we provide evidence that AKI can impair neutrophil transwell migration as early as 4h after induction of AKI. These effects of AKI appear to last as least as long as 24h. AKI also inhibits normal intracellular F-actin formation.
Next, we sought to test whether reconstitution of either renal endocrine/exocrine function (incubation in serum) or renal exocrine function alone (incubation in MEM buffer) could reverse these defects. Our results clearly demonstrate that incubation in MEM buffer is sufficient to reverse AKI-induced neutrophil dysfunction.
Neutrophils with greatly reduced transwell migration rates benefit the most from the incubation treatment. Contrary, neutrophils with normal transmigration rates are not affected by incubation in either serum or buffer. We can therefore conclude that the incubation treatment actually corrects a deficiency rather than stimulates neutrophil transwell migration. Restoration of normal intracellular actin polymerization with incubations in either normal serum or MEM buffer provides further evidence for both the concept of reversibility and a mechanistic link between actin polymerization and neutrophil transwell migration.
Originally described as a hormone causing insulin resistance, resistin is also a well-known uremic toxin and has been shown to exhibit inhibitory effects on neutrophils at concentrations seen in patients with end-stage renal disease.(35) Resistin has further emerged as an important pro-inflammatory cytokine that can be secreted by different cell types, including monocytes, neutrophils and epithelial cells.(36, 37) Resistin correlates very well with disease severity, inflammatory cytokine and lactate levels, and serum creatinine concentrations in patients with severe sepsis and septic shock.(38–40) Moreover, resistin levels appear to be higher in ICU patients with sepsis and than in non-infected control patients.(38)
Here, both animal models of AKI lead to elevated plasma resistin levels at time points at which neutrophil transmigration is also impaired. Incubation of murine neutrophils or NB4PMN cells with recombinant resistin at concentrations similar to those found in most severe forms of AKI resulted in impaired transwell migration and F-actin formation. These findings strongly suggest that resistin is sufficient to inhibit neutrophil transwell migration. The findings also support our proposed mechanistic link between impaired actin polymerization and neutrophil transwell migration.
In a small prospective, observational study, we have been able to validate key experimental findings in patients. In patients with septic shock and AKI, plasma resistin levels are significantly elevated. Moreover, plasma resistin levels are not significantly affected by RRT. Data on the effect of RRT on plasma resistin levels are sparse. Available data suggests that intermittent hemodialysis does not significantly affect plasma resistin levels.(41)
Plasma samples from patients with septic shock plus AKI significantly inhibit NB4PMN transwell migration when compared to plasma samples from patients with septic shock alone. Also, our modes of RRT, CVVHDF and IHD, do not restore normal transwell migration. These data open a new avenue of highly interesting questions as to how resistin, a 12kDa molecule, is cleared from the circulation and whether any modes of renal replacement therapy are capable of removal.
Moreover, future efforts will also have to address the clinical relevance of elevated resistin levels in AKI and sepsis. For example, we will need studies to test the hypothesis that elevated resistin levels impair resolution of already existing infections and/or increase the risk of new infections. Ultimately, we will also need studies to assess whether removal or inhibition of resistin can improve resolution of infections and reduce the risk of new infections.
Despite its clear findings, our study has several limitations that require consideration. First and foremost, the majority of our findings are the results of in vitro experiments that require further in vivo validation regarding reproducibility and clinical relevance. Secondly, our experimental studies only include nephrotoxic and rhabdomyolysis-induced AKI. Both forms of AKI represent only a smaller portion of all cases of AKI seen in critically ill patients. We have, however, shown that other forms of experimental AKI, such as post-ischemic AKI and bilateral nephrectomy, also reduce neutrophil recruitment in vivo.(12–14) Moreover, our previous study (13) together with the present findings validate important aspects of our experimental data in patients with septic AKI, the most prevalent form of AKI in critically ill patients.(42)
In summary, our study demonstrates that removing uremic plasma and incubating neutrophils in either normal serum or MEM buffer can reverse that AKI-induced neutrophil dysfunction. This allows us to conclude that normal renal exocrine function is sufficient to restore neutrophil transwell migration in vitro. We also show that resistin, a well-known uremic toxin, is significantly elevated during AKI, and by itself can reproduce AKI-like neutrophil dysfunction. Finally, we provide evidence that neither conventional CVVHDF nor IHD can correct plasma resistin levels and restore normal transwell migration during AKI.
Acknowledgments
This work was supported by NIH grants 5K08GM081459-03 (KS), 5R01DK070910 (JAK), 5R01HL080926 (KS and JAK) and a Department of Anesthesiology - Penn State College of Medicine seed grant (KS).
Footnotes
Reprints will not be ordered
Copyright form disclosures: Dr. Singbartl received support for article research from the National Institutes of Health (NIH). Dr. Miller received support for article research from the NIH and other and disclosed off-label product use: Resistin (Investigational). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Hoste E, Schurgers M. Epidemiology of acute kidney injury: How big is the problem? Crit Care Med. 2008;36(4 Suppl):S146–151. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 2.Singbartl K, Kellum J. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 3.Metnitz P, Krenn C, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Elseviers M, Lins R, Van der Niepen P, et al. Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Crit Care. 2010;14(6):R221. doi: 10.1186/cc9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishani A, Xue J, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakar CV, Yared J-PP, Worley S, et al. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64(1):239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 8.De Waele JJ, Hoste EAJ, Blot SI. Blood Stream Infections of Abdominal Origin in the Intensive Care Unit: Characteristics and Determinants of Death. Surgical Infections. 2008;9(2):171–177. doi: 10.1089/sur.2006.063. [DOI] [PubMed] [Google Scholar]
- 9.Tumbarello M, Spanu T, Caira M, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagn Microbiol Infect Dis. 2009;64(3):320–326. doi: 10.1016/j.diagmicrobio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Hoste E, Blot S, Lameire N, et al. Effect of nosocomial bloodstream infection on the outcome of critically ill patients with acute renal failure treated with renal replacement therapy. J Am Soc Nephrol. 2004;15(2):454–462. doi: 10.1097/01.asn.0000110182.14608.0c. [DOI] [PubMed] [Google Scholar]
- 11.Reynvoet E, Vandijck D, Blot S, et al. Epidemiology of infection in critically ill patients with acute renal failure. Crit Care Med. 2009;37(7):2203–2209. doi: 10.1097/CCM.0b013e3181a03961. [DOI] [PubMed] [Google Scholar]
- 12.Zarbock A, Schmolke M, Spieker T, et al. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17(11):3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- 13.Rossaint J, Spelten O, Kassens N, et al. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80(5):493–503. doi: 10.1038/ki.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singbartl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int. 2011;80(6):633–644. doi: 10.1038/ki.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Druml W, Schwarzenhofer M, Apsner R, et al. Fat-soluble vitamins in patients with acute renal failure. Miner Electrolyte Metab. 1998;24(4):220–226. doi: 10.1159/000057374. [DOI] [PubMed] [Google Scholar]
- 16.Zehnder D, Quinkler M, Eardley KS, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74(10):1343–1353. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordonez-Moran P, Alvarez-Diaz S, Valle N, et al. The effects of 1,25-dihydroxyvitamin D3 on colon cancer cells depend on RhoA-ROCK-p38MAPK-MSK signaling. J Steroid Biochem Mol Biol. 2010;121(1–2):355–361. doi: 10.1016/j.jsbmb.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Tukaj C, Trzonkowski P, Pikula M, et al. Increased migratory properties of aortal smooth muscle cells exposed to calcitriol in culture. J Steroid Biochem Mol Biol. 2010;121(1–2):208–211. doi: 10.1016/j.jsbmb.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Clark WR, Henderson LW. Renal versus continuous versus intermittent therapies for removal of uremic toxins. Kidney Int Suppl. 2001;78:S298–303. doi: 10.1046/j.1523-1755.2001.59780298.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen G, Horl WH. Retinol binding protein isolated from acute renal failure patients inhibits polymorphonuclear leucocyte functions. Eur J Clin Invest. 2004;34(11):774–781. doi: 10.1111/j.1365-2362.2004.01418.x. [DOI] [PubMed] [Google Scholar]
- 21.Yavuz A, Tetta C, Ersoy FF, et al. Uremic toxins: a new focus on an old subject. Semin Dial. 2005;18(3):203–211. doi: 10.1111/j.1525-139X.2005.18313.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R, Ronco C, Kellum J, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goktug AN, Chai SC, Chen T. Data Analysis Approaches in High Throughput Screening. Drug Discovery. 2013 [Google Scholar]
- 25.Vicente-Manzanares M, Viton M, Sanchez-Madrid F. Measurement of the levels of polymerized actin (F-actin) in chemokine-stimulated lymphocytes and GFP-coupled cDNA transfected lymphoid cells by flow cytometry. Methods Mol Biol. 2004;239:53–68. doi: 10.1385/1-59259-435-2:53. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann A, Gerrits B, Schmidt A, et al. Proteomic cell surface phenotyping of differentiating acute myeloid leukemia cells. Blood. 2010;116(13):e26–34. doi: 10.1182/blood-2010-02-271270. [DOI] [PubMed] [Google Scholar]
- 27.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. [DOI] [PubMed] [Google Scholar]
- 28.Lanotte M, Martin-Thouvenin V, Najman S, et al. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77(5):1080–1086. [PubMed] [Google Scholar]
- 29.Hofmann A, Gerrits B, Schmidt A, et al. Proteomic cell surface phenotyping of differentiating acute myeloid leukemia cells. Blood. 2010;116(13):e26–e34. doi: 10.1182/blood-2010-02-271270. [DOI] [PubMed] [Google Scholar]
- 30.Park YS, Lim G-W, Cho K-A, et al. Improved viability and activity of neutrophils differentiated from HL-60 cells by co-culture with adipose tissue-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;423(1):19–25. doi: 10.1016/j.bbrc.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 31.Rossaint J, Spelten O, Kassens N, et al. Acute loss of renal function attenuates slow leukocyte rolling and transmigration by interfering with intracellular signaling. Kidney Int. 2011;80(5):493–503. doi: 10.1038/ki.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard TH, Oresajo CO. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985;101(3):1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bengtsson T, Orselius K, Wettero J. Role of the actin cytoskeleton during respiratory burst in chemoattractant-stimulated neutrophils. Cell Biol Int. 2006;30(2):154–163. doi: 10.1016/j.cellbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Fujinami A, Obayashi H, Ohta K, et al. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta. 2004;339(1–2):57–63. doi: 10.1016/j.cccn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Cohen G, Ilic D, Raupachova J, et al. Resistin inhibits essential functions of polymorphonuclear leukocytes. J Immunol. 2008;181(6):3761–3768. doi: 10.4049/jimmunol.181.6.3761. [DOI] [PubMed] [Google Scholar]
- 36.Bostrom EA, Tarkowski A, Bokarewa M. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochim Biophys Acta. 2009;1793(12):1894–1900. doi: 10.1016/j.bbamcr.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Johansson L, Linnér A, Sundén-Cullberg J, et al. Neutrophil-derived hyperresistinemia in severe acute streptococcal infections. J Immunol. 2009;183(6):4047–4054. doi: 10.4049/jimmunol.0901541. [DOI] [PubMed] [Google Scholar]
- 38.Koch A, Gressner OA, Sanson E, et al. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care. 2009;13(3):R95. doi: 10.1186/cc7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassiliadi D, Tzanela M, Kotanidou A, et al. Serial changes in adiponectin and resistin in critically ill patients with sepsis: associations with sepsis phase, severity, and circulating cytokine levels. J Crit Care. 2012;27(4):400–409. doi: 10.1016/j.jcrc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Macdonald SP, Stone SF, Neil CL, et al. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS One. 2014;9(10):e110678. doi: 10.1371/journal.pone.0110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippidis G, Liakopoulos V, Mertens PR, et al. Resistin serum levels are increased but not correlated with insulin resistance in chronic hemodialysis patients. Blood Purif. 2005;23:421. doi: 10.1159/000088017. [DOI] [PubMed] [Google Scholar]
- 42.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]