Abstract
Objective
Visceral adipose tissue is a major site for expression of pro-inflammatory and pro-coagulant genes during acute systemic inflammation. In this study, we tested whether loss of fat mass by dietary restriction (DR) would remove the major source of these factors resulting in improved tolerance to sepsis and endotoxemia.
Design
Prospective, laboratory controlled experiments.
Setting
Aging and Critical Care Research Laboratory in a university hospital.
Subjects
Middle-aged (12 month-old) male C57BL/6 mice.
Interventions
Mice were subjected to 40% DR for three weeks followed by induction of abdominal sepsis or endotoxemia by intraperitoneal injection with cecal slurry or lipopolysaccharide (LPS), respectively.
Measurements and main results
Compared to freely-fed mice, DR mice exhibited dramatically improved survival (80% vs 0% after sepsis, p<0.001;86% vs 12%after endotoxemia, p=0.013) and significantly reduced visceral fat-derived mRNA expression of interleukin-6 (IL-6), thrombospondin-1, plasminogen activator inhibitor-1, and tissue factor which positively correlated with fat mass. Plasma levels of IL-6 were significantly reduced by DR and correlated with adipose IL-6 mRNAlevels and fat mass (p<0.001, R2=0.64 and 0.89). In vitro culture of visceral fat explants from naïve DR mice showed significantly reduced IL-6 secretion compared to that from freely-fed mice in response to LPS. Analysis of major adipose immune cell populations by flow cytometry demonstrated that macrophages were the only cell population reduced by DR and that CD11c+/CD206+ (M2-type) and CD11c-/CD206- (double negative) macrophages, in addition to T cells, are the major immune cell populations that produce IL-6 in middle-age during systemic inflammation.
Conclusions
Short-term DR drastically improved the survival outcome of middle-aged mice during both polymicrobial sepsis and sterile endotoxemia. Improved survival was accompanied by a significantly attenuated inflammatory response in adipose tissue, which is likely due to alterations of both fat mass quantity and qualitative changes including a reduction in macrophage populations.
Keywords: adipose tissue, caloric/dietary restriction, endotoxemia, macrophage, mortality, sepsis
Introduction
Visceral adipose tissue is a major source of pro-inflammatory and pro-coagulant factors during acute systemic inflammation. We recently reported increased expression of pro-inflammatory cytokines TNFα, IL-1α, IL-1β, and IL-6, and pro-coagulant factors plasminogen activator inhibitors (PAI)-1 and -2, thrombospondin-1 (Thbs-1), and tissue factor (TF) in visceral adipose tissue of normal-weight mice under acute systemic inflammation (1). We further showed that degree of induction of most of these factors was significantly augmented in adipose tissues with age, and that these adipose factors are mainly produced by stromal vascular fraction (SVF) cells, rather than adipocytes (1). Within the SVF, which includes various immune cells as well as preadipocytes and vascular cells, it is largely unknown which specific cell types produce the above inflammatory and pro-coagulant genes during acute systemic inflammation.
Caloric or dietary restriction (DR) is a well-established and heavily studied area of research best known for its effects on lifespan extension of laboratory animals (2, 3). DR has also been shown to improve resistance to various acute stressors including surgical trauma, exposure to heat, and response to toxic drugs (4). DR studies are typically carried out over long periods of time (several months to years), however, the potential benefits of short-term DR have recently become of interest (5). In fact, surgical patients have shown postoperative benefits by short term preoperative reduced caloric intake (6). Despite the obvious decrease in fat mass due to DR, the effects of DR on adipose tissue physiology and the benefit of these changes in modulating response to acute illness are not well studied.
In the current study, we hypothesized that a reduction of fat mass in normal weight middle-aged mice would lead to an improved outcome with respect to survival and adipose tissue physiology during acute systemic inflammation induced by endotoxemia and experimental sepsis. The major objectives of this study were to: (1) examine the effects of short-term dietary restriction (DR) on sepsis-mediated mortality, (2) correlate an altered pro-inflammatory and pro-coagulant profile of adipose tissue with improved outcome during acute systemic inflammation, and (3) identify changes in immune cell populations in adipose tissue during DR.
Materials and Methods
Animals and Husbandry
Twelve month-old male C57BL/6 mice were obtained from the National Institute on Aging. These mice are the human equivalent of a 40-45 year old person (7). All mice were maintained in an environment under controlled temperature (21–23°C), humidity (30–70%), and lighting (14 hours light/10 hours dark) with free access to drinking water and chow (Rodent Diet No. 2500, LabDiet, St. Louis, MO) prior to experimentation. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and performed in accord with the National Institutes of Health guidelines for ethical animal treatment.
Dietary restriction
Short-term dietary restriction (DR) was achieved by restricting food intake by 40% for a period of approximately three weeks. All mice were fed ad libitum (AL) for one week, and food consumption monitored for each cage. Subsequently, mice were assigned to either the DR or AL group, and the DR mice received 40% less food each day than their average daily food intake for the previous week. This method of DR, when carried out for long periods of time, has proven to extend the lifespan of laboratory animals without causing undernutrition (8). All mice were weighed daily immediately prior to feeding. Lean and fat mass of each mouse were measured by EchoMRI Body Composition Analyzer (EchoMRI LLC, Houston, TX) before and after three weeks of DR.
Induction of abdominal sepsis and endotoxemia
Acute polymicrobial abdominal sepsis was induced by intraperitoneal (i.p.) injection with the minimum lethal dose (LD100) of cecal slurry (CS), prepared as we recently described in detail (9). Acute systemic inflammation was induced by injection with lipopolysaccharide (LPS) derived from Pseudomonas aeruginosa (Sigma-Aldrich, St. Louis, MO). LPS was dissolved in physiological saline and administered i.p. with a dose of 2.5mg/kg as used in previous studies (1, 10-12). The dose of LPS given to DR mice was calculated based on original body weight prior to weight loss. Body temperature, as a measure of disease severity (13), was monitored by rectal temperature probe (Precision Thermometer 4600, YSI Inc., Yellow Springs, OH). Surviving animals were euthanized after being monitored for at least 10 days. For plasma analysis in survival study mice, 30μL of blood was drawn from the tail vein. For tissue harvesting, separate mice were injected with LPS and sacrificed 6h later as follows. Mice were deeply anesthetized by isoflurane inhalation, laparotomy was performed and blood was collected from the inferior vena cava with a heparin-coated needle and syringe. Subsequently, the inferior vena cava was cut, and the entire vasculature was perfused with saline through the cardiac ventricles, for the purpose of eliminating circulating cells from tissues to be analyzed. Epididymal adipose tissues were harvested, weighed, flash frozen in liquid nitrogen, and stored at -80°C.
RNA isolation, sample preparation and quantitative real-time RT-PCR
Frozen epididymal adipose tissues were homogenized with TRIzol reagent (Invitrogen, Carlsbad, CA) and total cellular RNA was purified using PureLink RNA Mini Kit (Life Technologies, Grand Island, NY). The concentration of the RNA was determined by measuring the absorbance at 260 and 280 nm using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE) and integrity was confirmed through visualization of 18S and 28S RNA bands using an Agilent 2100 Bioanalyzer (Aligent Technologies, Santa Clara, CA). Equivalent amounts of RNA were reverse-transcribed into cDNA using SuperScript III First-Strand Synthesis SuperMix (Life Technologies) according to the manufacturer's protocol. Primers were designed using the Universal ProbeLibrary (Roche Applied Science, Indianapolis, IN) and purchased from Integrated DNA Technologies (Coralville, IA). The qRT-PCR reaction was performed on Roche Light Cycler 480 using the hydrolysis probe format with LightCycler 480 Probes Master mix. Target gene expression was normalized to hypoxanthineguanine phosphoribosyl transferase (HPRT) expression as an endogenous control, and fold change was calculated as 2-(ΔΔCT), using the mean ΔCT of the AL group as a calibrator.
In vitro adipose tissue explant culture
Explant culture of adipose tissue was performed as we recently described (12). Briefly, epididymal adipose tissues were cultured in vitro, treated with LPS (10 μg/mL), and the culture medium sampled at designated time points. After the final sampling, adipose tissue fragments were collected for determination of DNA content.
Plasma and culture medium cytokine analysis
Plasma samples were obtained by centrifugation of blood at 2,500 × g for 15 minutes at 4°C and stored at -80°C until use. Plasma and culture medium were analyzed in duplicate by enzyme-linked immunosorbent assay (ELISA) for quantification of IL-6 (Thermo Scientific, Rockford, IL), PAI-1 (Molecular Innovations, Novi, MI), leptin and adiponectin (R&D Systems, Minneapolis, MN) using the protocols recommended by the manufacturer. In addition, multiplex assay was performed to quantify the plasma concentrations of TNFα, IL-1β, and IL-10 (Luminex, Life Technologies, Grand Island, NY). Culture medium cytokine data was adjusted by the DNA content of adipose tissues in each sample.
Stromal vascular fraction (SVF) cell isolation
SVF cells from epididymal adipose tissue were purified as we previously described (1, 14). Red blood cells were lysed from pelleted cells and remaining cells were washed with Cell Staining Buffer (BioLegend, San Diego, CA), passed through a 70μm cell strainer, and counted using a hemocytometer.
Analyses of immune cell populations by flow cytometry
Purified SVF cells were blocked with anti-mouse CD16/32 antibody for 10 minutes on ice. For analysis of immune cell populations, cells were further incubated for 30 minutes on ice in the dark with the following anti-mouse antibodies obtained from BioLegend: APC-CD11b, APC-Cy7-CD11c, PE-CD206 or PE/Cy7-CD206, PerCP/Cy5.5-Ly6G, FITC-CD3, PerCP/Cy5.5-CD19, APC/Cy7-NK1.1. After antibody conjugation, cells were washed with Cell Staining Buffer and analyzed on a Becton-Dickinson LSRII analytical flow cytometer. For analysis of intracellular IL-6, purified SVF cells obtained by pooling the SVF of two AL mice were incubated with 1× Brefeldin A solution (BioLegend) for 4h in culture medium at 37°C. Blocking and cell surface staining were performed as described above followed by fixation and permeabilization using Fixation/Permeabilization Reagents and the protocol recommended by the manufacturer (eBioscience, San Diego, CA). Intracellular staining of IL-6 was performed using anti-mouse PE-IL-6 and negative control (PE-Rat IgG1, κ Isotype control). Cells were analyzed using iCyt Synergy Cell Sorter (Sony Biotechnology San Jose, CA). Analysis of flow cytometry data was performed using FlowJo data analysis software (FlowJo, LLC, Ashland, OR).
Statistical Analysis
Statistical analysis was performed using SigmaPlot Statistical Software, Version 11.0 (Systat Software, Chicago, IL). Survival curves were analyzed by Kaplan Meier LogRank test. For all other comparisons, data are expressed as means and standard deviations, and statistical significance was analyzed by student's t-test or two-way analysis of variance when appropriate. Post-hoc analysis was carried out using Holm-Sidak method. A p value less than 0.05 was considered statistically significant.
Results
Analysis of body weight loss and body composition changes during dietary restriction
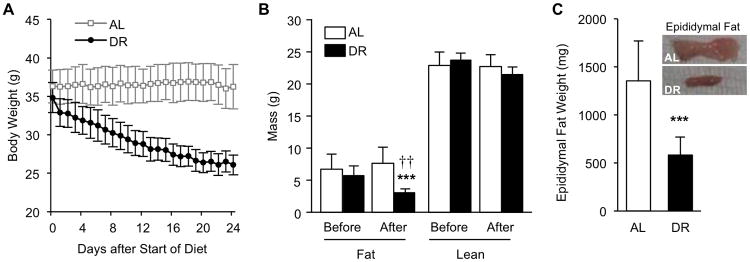
Mice were randomized into freely-fed (ad libitum, AL, n=10) and 40% dietary restricted (DR, n=10) groups, and DR was carried out for approximately 3 weeks. Initial body weight of the two groups was not significantly different (36.5 ± 2.2 and 35.1 ± 1.9, p=0.179). While AL mice maintained their body weight with no significant change, DR mice lost on average 25.1% of their original body weight (9.0 ± 1.0g grams lost, p<0.001, compared to pre-diet weight)during the 3 weeks (Fig. 1A). Body composition analysis by EchoMRI showed a significant decrease in total body fat by DR (p<0.001) and a significant difference in total body fat mass between AL and DR mice after the DR period (p=0.007, Fig. 1B). Lean mass was not significantly changed by DR (p=0.140). Epididymal fat pads were significantly smaller in DR mice compared to AL mice (p<0.001, Fig. 1C); retroperitoneal fat also showed reduced size by DR (data not shown). These data confirm that short-term DR led to a significant reduction in total body fat mass while lean mass remained largely unaffected.
Figure 1.
Analysis of body weight and body composition in ad libitum (AL) fed mice and mice under short-term 40% dietary restriction (DR). (A) Daily body weight measurements of DR (n=8) and AL (n=8) mice during the DR period (prior to sepsis/endotoxemia induction). (B) Fat and lean mass weight before and after DR assessed by Echo MRI. (C) Total epididymal fat weight at sacrifice and representative macroscopic images. * indicates statistical significance compared to pre-DR measurements, † indicates statistical significance compared to AL group. Two and three symbols indicate p< 0.01 and 0.001, respectively.
Animals are rescued from lethal polymicrobial sepsis and endotoxemia by dietary restriction
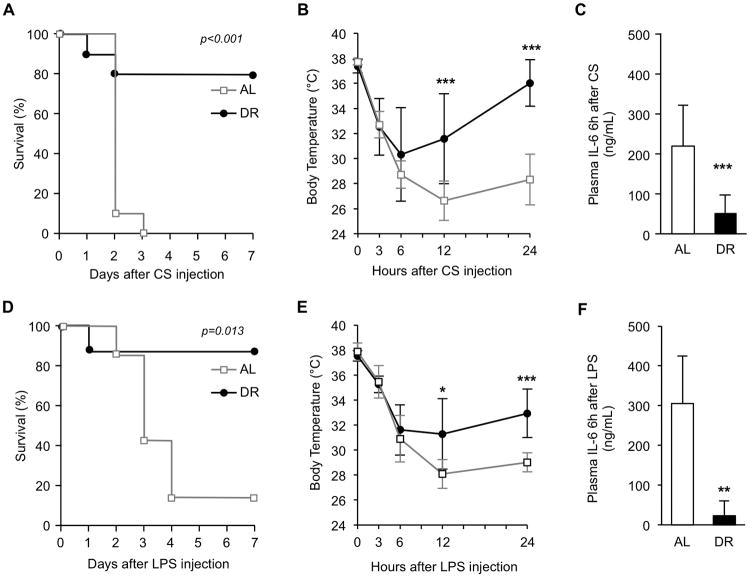
In a separate experiment, mice were again randomized into AL and 40% DR groups, and DR was carried out for 3 weeks prior to induction of experimental sepsis by injection with the minimum LD100 of cecal slurry (CS, 200 μL). While 100% of AL animals died, DR resulted in only 20% mortality (p<0.001, Fig. 2A). Degree of acute hypothermia was not significantly different between the two groups during the first 6h after CS injection; however, DR mice started to recover their body temperatures by 12h which led to a significant difference in body temperature between AL and DR mice at 12h and 24h (p <0.001, Fig. 2B). Plasma IL-6 concentration 6h after sepsis induction, a well-known predictive marker of mortality (15), was significantly reduced by DR (p<0.001, Fig. 2C), and strongly correlated with improved survival.
Figure 2.
Effects of short-term dietary restriction (DR) on survival during polymicrobial sepsis and endotoxemia. (A) After three weeks of DR (n=10) or ad libitum feeding (AL) (n=10), mice were injected with cecal slurry (CS, 200 μL, i.p) and survival monitored for one week. (B) Body temperature during the first 24h of sepsis. (C) Plasma IL-6 concentration, 6h after CS injection. (D) After three weeks of DR (n=8) or AL-feeding (n=7), mice were injected with LPS (2.5mg/kg, i.p) and survival monitored for one week. (E) Body temperature during the first 24h of endotoxemia. (F) Plasma IL-6 concentration, 6h after LPS injection. Values are the mean ± SD. * indicates statistical significance compared to AL group. One, two, and three symbols indicate p<0.05, 0.01, and 0.001, respectively.
To examine whether the DR-mediated survival benefit was due to improved immune function, we further tested whether DR would rescue mice during sterile endotoxemia. LPS (2.5 mg/kg) was injected to AL and DR mice based on original (pre-diet) body weight. DR resulted in a significant improvement in survival rate: 88% for DR vs 14% for AL (p=0.013, Fig. 2D). Similar to CS-induced sepsis, acute hypothermia was not significantly different between the two groups for the first 6h after LPS injection; however, between 12 and 24h, the body temperature of AL mice continued to decline while DR mice maintained their body temperature which led to a significant difference in body temperature between AL and DR mice at 12h and 24h (p=0.015 and <0.001, respectively, Fig. 2E). Plasma IL-6 concentration 6h after LPS injection was also lower in DR mice (p<0.01, Fig. 2F).
Altered gene expression of pro-inflammatory and pro-coagulant factors in adipose tissue from dietary restricted mice during endotoxemia
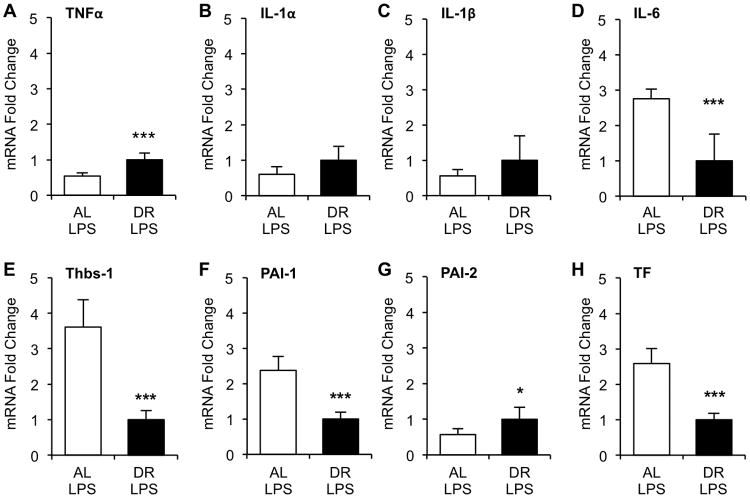
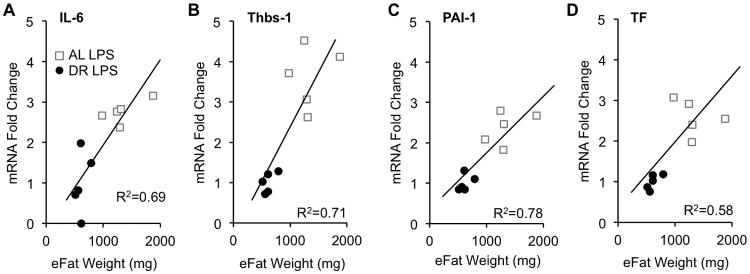
Since DR seemed to have equally beneficial effects on survival in both CS-induced sepsis and LPS-induced endotoxemia models, we characterized changes in adipose tissue gene expression using only the LPS model. We previously reported that pro-inflammatory factors TNFα, IL-1α, IL-1β, and IL-6, and pro-coagulant factors thrombospondin-1 (Thbs-1), PAI-1, PAI-2, and tissue factor (TF) are significantly induced in adipose tissues during endotoxemia (1). Here, we assessed the effects of DR on LPS-induced expression of these genes (Fig. 3). Relative mRNA levels of IL-6, Thbs-1, PAI-1, and TF were significantly reduced more than 2-fold in the DR group (p<0.001), while TNFα, IL-1α, IL-1β, and PAI-2 were either slightly increased (< 2-fold) or not changed by DR. Expression levels of the four genes (IL-6, Thbs-1, PAI-1, TF) which were reduced by DR positively correlated with weight of the epididymal fat pads (eFat) (Fig 4).
Figure 3.
Expression of inflammatory and coagulant genes in adipose tissue of dietary restricted (DR) and ad libitum (AL)-fed mice during endotoxemia. After three weeks of DR, DR and AL-fed mice (n=5 each) were injected with LPS (2.5mg/kg, i.p.) and sacrificed 6h later. Epididymal adipose tissues were harvested and extracted RNA processed and subjected to real-time qRT-PCR analysis for expression of pro-inflammatory factors (A) TNFα, (B) IL-1α, (C) IL-1β, (D)IL-6, and pro-coagulant factors (E) thrombospondin-1 (Thbs-1), (F) plasminogen activator inhibitor (PAI)-1, (G) PAI-2, (H) tissue factor (TF). Values are the mean ± SD, * indicates statistical significance compared to AL group. One and three symbols indicate p< 0.05 and 0.001, respectively.
Figure 4.
Correlation between fat mass and adipose-derived gene expression among genes which are downregulated by dietary restriction (DR). Gene expression of (A) IL-6, (B) Thbs-1, (C) PAI-1, and (D) TF from epididymal adipose tissues (eFat) were plotted against the total weight of both fat pads.
Plasma protein analysis of dietary restricted versus ad libitum-fed mice during endotoxemia
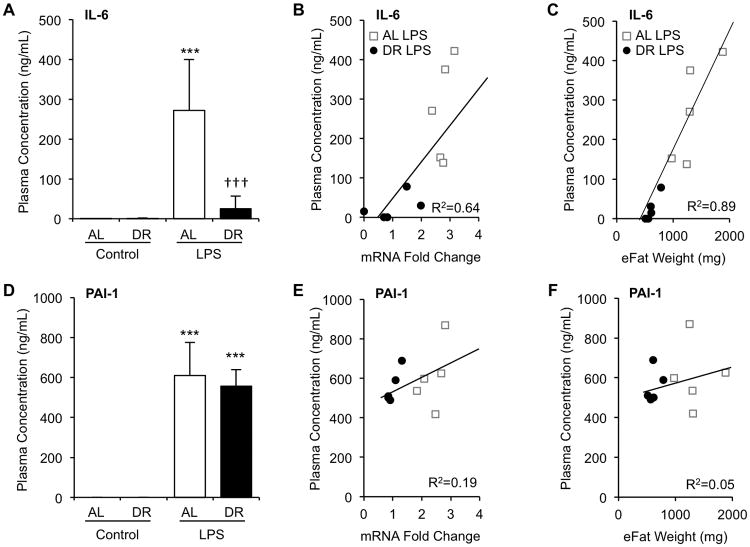
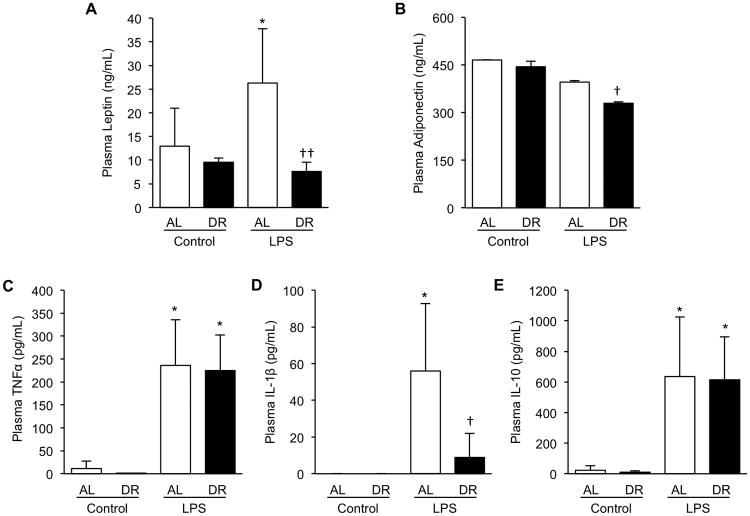
Circulating concentration of IL-6 was significantly induced by LPS in AL-fed mice (0.1 ± 0.2to 271.8 ±127.9 ng/mL, p<0.001). In contrast, only some DR mice exhibited increased IL-6 levels by LPS, thus average induced level of IL-6 in DR mice was significantly lower (0.5 ± 0.9 to 24.8 ±32.5 ng/mL, p=0.26). Induced plasma concentration of IL-6 at 6h was more than 10-fold lower in DR compared to AL mice (p=0.008, Fig. 5A). Circulating IL-6 concentration showed a strong positive correlation with adipose tissue mRNA level of IL-6 (R2=0.64, Fig. 5B) and epididymal adipose tissue weight (R2=0.89, Fig. 5C) confirming that visceral adipose tissue is a major source of this cytokine. Plasma concentration of PAI-1 was significantly increased by LPS in both AL and DR mice (Fig. 5D, 609.8 ± 165.9 and 555.6 ± 83.7 ng/mL at 6h, respectively, p<0.001 for both groups); however degree of induction was not significantly different between the two groups (p=0.53). Plasma PAI-1 concentration of non-injected control AL and DR mice was below the detection limit of the assay. Plasma PAI-1 showed a very weak positive correlation with both mRNA level (Fig 5E, R2=0.19) and epididymal fat weight (Fig 5F, R2=0.05), indicating a significant contribution to circulating PAI-1 levels by non-adipose tissues. In addition to IL-6 and PAI-1, plasma levels of adipokines, adiponectin and leptin, pro-inflammatory cytokines, TNFα and IL-1β, and anti-inflammatory cytokine IL-10 were evaluated (Fig 6). LPS-mediated induction of leptin and IL-1β were blunted by DR, while TNFα and IL-10 showed no difference between AL and DR mice. Adiponectin was significantly reduced by DR with a slightly more profound reduction in the DR group.
Figure 5.
Plasma IL-6, not PAI-1, correlates with adipose tissue gene expression and adiposity. After three weeks of DR, mice were injected with LPS (2.5mg/kg, i.p.) and sacrificed 6h later. Plasma was obtained by centrifugation of heparinized blood drawn from the inferior vena cava and subjected to analysis by ELISA. (A) Plasma IL-6 concentration. (B) Correlation of plasma IL-6 concentration with mRNA level of IL-6. (C) Correlation of plasma IL-6 concentration with weight of epididymal fat pads (eFat). (D) Plasma PAI-1 concentration. (E) Correlation of plasma PAI-1 concentration with mRNA level of PAI-1. (F) Correlation of plasma PAI-1 concentration with weight of eFat pads. Values are the mean ± SD, n=3 for non-injected controls, n=5 for LPS injected animals, * indicates statistical significance compared to non-injected controls of the same treatment group, † indicates statistical significance compared to AL LPS group. Three symbols indicate p< 0.001.
Figure 6.
Plasma protein analysis of dietary restricted (DR) and ad libitum (AL)-fed mice during endotoxemia. After three weeks of DR, mice were injected with LPS (2.5mg/kg, i.p.) and sacrificed 6h later. Plasma was obtained by centrifugation of heparinized blood drawn from the inferior vena cava and subjected to analysis by ELISA. (A) Plasma adiponectin concentration, (B) Plasma leptin concentration, (C) Plasma TNFα concentration, (D) Plasma IL-1β concentration, (E) Plasma IL-10 concentration. Values are the mean ± SD, n=3 for non-injected controls, n=5 for LPS injected animals, * indicates statistical significance compared to non-injected controls of the same treatment group, † indicates statistical significance compared to AL LPS group. One and two symbols indicate p< 0.05 and 0.01, respectively.
IL-6 release from in vitro cultured adipose tissue is substantially reduced by dietary restriction
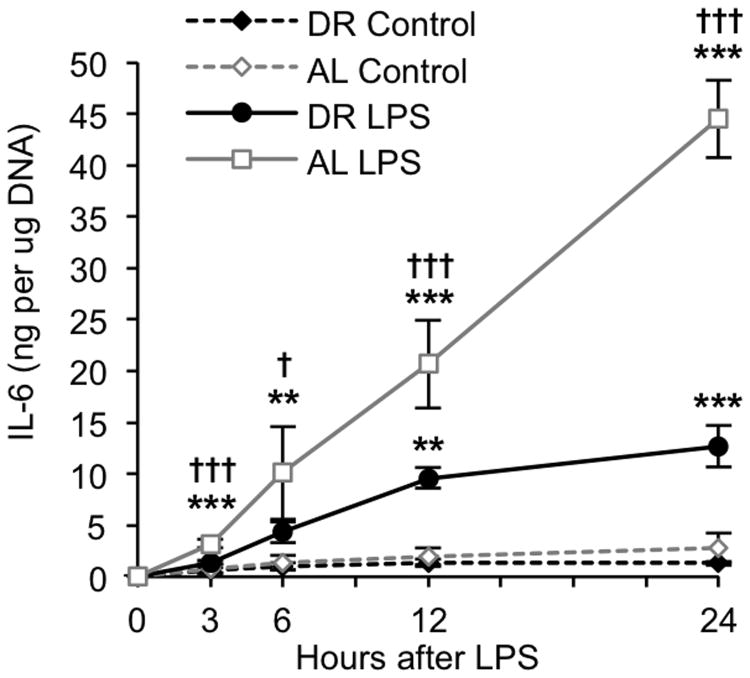
To further examine whether cytokine production by adipose tissue is suppressed by DR, equal sized pieces of epididymal adipose tissues were harvested from naïve AL and DR mice and cultured in vitro. Cultures were treated with LPS and the medium sampled at designated time points for analysis of IL-6. Control cultures received an equal volume of vehicle (PBS) which did not stimulate cytokine secretion in either group. IL-6 secretion was detectable as early as 3h after LPS treatment in adipose tissues from both groups, and a significant difference in IL-6 secretion between AL and DR mice was observed for each time-point from 3-24h. Approximately 45 ng of IL-6 per μg of adipose tissue DNA was secreted into the medium by LPS-treated adipose tissues from AL mice over a 24h period, while less than 15 ng of IL-6 per μg of DNA was secreted into the medium by LPS-treated adipose tissues from DR mice (Fig. 7). These data clearly indicate that LPS-induced IL-6 production occurs within adipose tissues (independent of systemic inflammation), and that the actions of DR to suppress IL-6 production are mediated by resident adipose cells.
Figure 7.
Effects of dietary restriction (DR) on IL-6 release from in vitro cultured adipose tissues. Explant cultures of epididymal adipose tissue from naïve DR and AL-fed mice were treated with LPS (10μg/mL) in vitro and the medium sampled at multiple time-points for analysis of IL-6 by ELISA. Cytokine levels were adjusted for adipose tissue DNA content. Data are expressed as the mean ± SD, n=3 per group and time-point. * indicates a statistically significant change as compared to the 0h time-point of the same group. † indicates a statistically significant difference between DR and AL at the same time-point. One, two, and three symbols indicate p<0.05, 0.01, and 0.001, respectively.
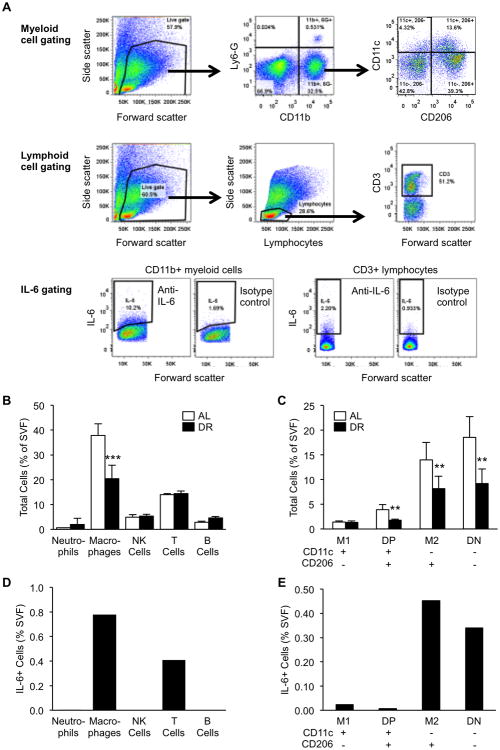
Macrophage populations are reduced in the stromal vascular fraction of adipose tissue by dietary restriction
To determine whether resident immune cell populations in adipose tissue are altered by 3 weeks of DR, we purified SVF cells from epididymal adipose tissues of naïve DR and AL mice and compared their major cell populations by flow cytometry. The gating strategy for myeloid cells and lymphoid cells is depicted in Figure 8A. Among major types of immune cells including, neutrophils (CD11b+/Ly6G+), macrophages (CD11b+/Ly6G-), NK cells (CD3-/NK1.1+), T cells (CD3+), and B cells (CD19+), only macrophages showed a significant decrease in abundance after DR (p<0.001, Fig. 8B). Other cell types showed no significant change in abundance. We further delineated macrophage subpopulations by the markers CD11c and CD206 as previously described (16, 17). Resident (M2-type, CD11c-/CD206+) and double negative (DN, CD11c-/CD206-) are the major macrophage subpopulations present in adipose tissue of middle-aged mice. Compared to AL, adipose tissues of DR mice showed reduced numbers of M2, DN and double positive (DP, CD11c+/CD206+), but not M1-type (CD11c+/CD206-) macrophages (p=0.004, 0.004, 0.005, and 0.18, respectively, Fig. 8C).
Figure 8.
Flow cytometric analysis of dietary restriction (DR)-mediated changes in adipose immune cell populations and identification of IL-6 producing cell types. (A) Gating strategy for delineation of myeloid cells and lymphoid cells. (B-C) Cell populations were quantified as a percentage of total SVF cells obtained from AL and DR mice. Data are expressed as the mean ± SD, n=5 per group and time-point. * indicates a statistically significant change as compared to the AL group. Two and three symbols indicate p<0.01 and 0.001, respectively. (D-E) IL-6 positive cells were quantified as a percentage of total SVF cells after adjusting for isotype control expression. Data are derived from SVF cells pooled from multiple AL-fed mice. IL-6 positive cells were not detected in NK or B cells.
Identification of IL-6 producing immune cells in adipose stromal vascular fraction
We previously reported that LPS-induced IL-6 is predominately expressed by SVF cells of adipose tissue rather than adipocytes (1); however, the exact cell types among the SVF which produce IL-6 in mice under acute systemic inflammation was not determined. By purifying SVF cells from epididymal adipose tissue of LPS-injected middle-aged AL mice, we show that the major IL-6 producing immune cells are macrophages (CD11b+/Ly6G-) and T cells (CD3+)(Fig. 8D). A small amount of neutrophils were positive for IL-6, while IL-6 was not detected in B cells or NK cells. Among macrophage subtypes, M2 (CD11c-/CD206+) and DN (CD11c-/CD206-) showed by far the most IL-6 positive cells (Fig. 8E).
Discussion
This study is the first to demonstrate a dramatic improvement in survival rates during polymicrobial sepsis and endotoxemia by short-term dietary restriction (DR). Additionally, this study is the first report of improved adipose tissue physiology in mice with acute systemic inflammation under DR as short as 3 weeks. Since adipose tissue is a major source of multiple inflammatory cytokines and thrombotic factors during acute inflammation, preventing unnecessary overproduction of these factors by DR appears to significantly enhance survival during sepsis and endotoxemia. While DR is unlikely to be a clinically relevant therapeutic approach for sepsis, understanding the specific aspects of DR which are beneficial for survival during acute stress may lead to future therapeutic or preventative targets for patients.
Results from previous studies attempting to evaluate effects of DR on sepsis outcome are contradicting and inconclusive. Early studies by Kang et al. reported increased mortality after CLP-induced sepsis in mice under 50-75% DR compared to AL mice (18, 19). Another study by Bermudes et al. reported significantly higher mortality to CLP in mice under a 72-hour-long fast compared to non-fasted mice (20). The dietary regimens used in these studies are extremely harsh and probably more harmful than beneficial for lean young mice. Sun et al. (21) found that 40% DR accelerated mortality of mice after CLP-induced sepsis while Hasegawa et al. (22) reported a reduced mortality in alternate-day fed mice as compared to AL; however, these two studies reported survival of animals for up to 48h only, and thus the effects of DR on final survival rates of sepsis were inconclusive. An earlier study by Peck et al. evaluated the effects of 50%DR in combination with different protein amounts on A/J mice survival after induction of bacteremia by Salmonella (23). This study found a tendency toward increased survival rates by DR although the data largely varied by different protein contents. Although none of these studies investigated the changes in adipose tissues by DR, it is possible that these studies did not find clear benefits of DR because very young lean mice were used. Our study provides important information in addition to these previous studies, in that just 3-weeks of 40% DR proved to dramatically increase survival to both polymicrobial sepsis and sterile endotoxemia. Increased survival by DR in the endotoxemia model suggests that the beneficial effects of DR are not likely due to improved immune function to eliminate bacteria since this model does not involve live bacteria. Further, this is the first study to investigate DR-mediated changes in adipose tissue gene expression in mice under acute systemic inflammation and correlate those with improved survival. Thirdly, we used non-obese mature middle-aged mice, which are an age-group much more represented in the clinical population of sepsis patients. Since middle-aged mice have more abdominal fat than lean young mice, it is possible that their benefit from DR was more pronounced.
Among the various fat depots within the body, visceral adipose tissue has become recognized as the “bad fat” for a number of reasons. Most notably, its ability to produce and secrete a variety of inflammatory cytokines and hormones, and its multiple anatomical locations within the viscera contribute to this reputation (24-27). In addition to location, there are multiple suggestions that it is the quality of the adipose tissue rather than the quantity which contributes most to adipose tissue hyper-inflammation or dysfunction (11, 12, 28-31). For example, in our recent study using explant cultures of adipose tissue from young (4-month) and aged (24-month) mice, LPS treatment induced a stronger inflammatory response in tissues taken from aged mice, indicating that aged fat, regardless of quantity or systemic contribution from the aging body, is prone to an exaggerated inflammatory response upon acute insult (12). In the current study we show that both quantity and quality play a role in the improved adipose tissue inflammatory and coagulant profile mediated by DR. LPS-induced expression levels of IL-6, Thbs-1, PAI-1, and TF in epididymal adipose tissue were significantly reduced by DR. Since these data were based on the comparison of equal amounts of mRNA from adipose tissue of AL vs DR mice, the results clearly indicate that the observed gene expression changes are due to a reduced inflammatory nature by DR and not necessarily reflective of total fat mass. In addition, we found a strong positive correlation between fat mass and gene expression levels, suggesting that the degree of fat mass reduction has an influence on alterations in inflammatory nature. Taken together, adipose tissues from DR mice produce significantly lower levels of certain cytokines and pro-coagulant factors regardless of total fat mass, indicative of qualitative changes within adipose tissue by DR; however, total amount of fat mass would also contribute.
Our conclusion may seem contradictory to recent clinical data in which lower mortality rates have been observed for overweight and obese critically ill patients compared to those with a normal BMI. Coined the “obesity paradox”, this clinical observation is only an association and several studies in this area have led to mixed results. A recent systematic review of relevant literature specific for sepsis (32) found that more studies have reported no association between sepsis mortality and obesity (33-35) or an increase in mortality in obese patients (36), than those that have reported decreased mortality with obesity (37-39). Thus the current data regarding whether or not obesity is protective during sepsis are inconclusive.
TNFα, IL-1β and IL-6 are major players in mediating a variety of cellular responses during acute stress and sepsis. Each of these cytokines is expressed in adipose tissue to some degree and show significantly augmented levels during systemic inflammation (1, 40). Here we report that among these cytokines, only IL-6 shows reduced mRNA levels in adipose tissue by DR. This pattern of expression is well reflected in the plasma levels of IL-6 during sepsis and endotoxemia, which is convincing because IL-6 expression under acute stress is predominately derived from adipose tissue (11). On the contrary, the effects of DR on induced adipose tissue mRNA levels of TNFα and IL-1β were not similar to plasma levels of these cytokines; this is likely due to the fact that these two factors are derived largely from the lung and spleen with relatively low levels in adipose tissue (12). TNFα mRNA was modestly increased in the DR group (less than 2-fold), and IL-1β and IL-1αmRNA also tended to be increased by DR though the change was not significant. LPS-induced circulating levels of TNFα, IL-1β, and IL-6 were previously shown to be blunted by 40% DR in young mice (41). Our analysis confirms these findings in middle-aged mice with a far greater fold reduction in levels of IL-6 and IL-1β. Plasma levels of TNFα were not different by DR in our study; however, we only measured plasma levels 6h after LPS. While Matsuzaki et al. propose that decreased levels of these cytokines in the DR group are due to decreased synthesis and secretion by macrophages, Vega et al. reported no difference in TNFα or IL-6 secretion from isolated peritoneal macrophages of AL vs DR middle-aged mice (42). Secretion of IL-1β from macrophages was blunted by DR in their study. Collectively, these data suggest that circulating levels of IL-1β may be largely derived from non-adipose macrophages, while IL-6 is mostly derived from adipose stromal cells. Levels of TNFα seem unrelated to either macrophages or adipose cells.
Adipose tissue macrophages (ATMs) are suspected to be the major source of multiple cytokines including IL-6 in obesity and chronic inflammation (31); however the roles ATMs play during acute inflammation is unclear. Previous studies have identified four unique subpopulations of ATMs including classical infiltrating M1-type inflammatory macrophages (CD11c+/CD206-), resident quiescent M2-type macrophages (CD11c-/CD206+), and newly identified subgroups which express both markers (double-positive (DP), CD11c+/CD206+) or are double negative (DN, CD11c-/CD206-)(16, 17, 43-45). While most of these macrophage subpopulations are greatly increased in obesity (17, 44), age-related changes are minimal, but include a decrease in M2 and an increase in DN macrophages (16, 43). We previously reported that macrophage infiltration to visceral adipose tissue is not evident during acute systemic inflammation induced by LPS; however this study could not eliminate the possibility that a subpopulation of resident ATMs contributes to LPS-mediated inflammation in adipose tissue (1). In the present study we examined the effects of DR on major immune cell populations and ATM subpopulations in non-obese middle-aged mice. ATMs were the only cell type which decreased in numbers by DR. Further, we found that DR resulted in a decrease in each of the characterized ATM subpopulations with the exception of M1 (CD11c+/CD206-classical macrophages). Zeyda et al. reported that IL-6 gene expression is highest in CD11c+ ATMs during high-fat diet-induced obesity (17). Here we found that macrophages and T cells are the major resident immune cells that produce IL-6 in fat tissues of middle-aged mice under acute systemic inflammation. Among the ATM subpopulations, the proportion of IL-6 positive cells was highest in M2 and DN ATMs. While M1 ATMs may produce higher amounts of IL-6 per cell, the total pool of IL-6 from ATMs may be mostly derived from M2 and DN populations since these two IL-6 producing cell types are much more abundant than M1. Non-immune cells, including preadipocytes and endothelial cells are also known producers of IL-6 (27, 30, 46), thus these cell types may contribute to overall IL-6 from adipose tissue; however the abundance of these cell types is unlikely to be changed during the 3-weeks of DR.
A limitation of our study is that we focused our evaluation on adipose tissue, thus we cannot exclude the possible contribution by additional mechanisms (i.e. decreased metabolism, decreased apoptosis, increased cellular protection) to the observed effects of DR to reduce acute inflammation in other organs independent of changes in adipose tissue quality and quantity. Further complex mechanistic studies are warranted to determine whether changes in adiposity and adipose tissue physiology are directly related to improved outcome during sepsis.
Conclusions
In summary, we report for the first time that, short-term dietary restriction dramatically improves survival in middle-aged mice under acute systemic inflammation induced by both polymicrobial peritonitis and sterile endotoxemia. Such a significant increase in resistance to sepsis or endotoxemia has not been previously shown by any intervention. Improved survival with dietary restriction is accompanied by reduced adiposity and alterations in adipose tissue gene expression of inflammatory and coagulant factors. These phenotypic changes are likely a result of changes in fat mass as well as cellular composition and an overall improvement in adipose tissue physiology.
Acknowledgments
The authors acknowledge additional funding support from National Institutes of General Medical Sciences NIH grant 8 P20 GM103527 for use of EchoMRI facilities. The UK Flow Cytometry & Cell Sorting core facility is supported in part by the Office of the Vice President for Research, the Markey Cancer Center and an NCI Center Core Support Grant (P30 CA177558) to the University of Kentucky Markey Cancer Center.
Financial Support: This research was supported by National Institute on Aging/National Institutes of Health RO1 R01 AG039732 grant awarded to H.S., R36 AG038547 grant awarded to M.S., and an NCI Center Core Support Grant P30 CA177558.
Footnotes
Copyright form disclosures: Drs. Saito, Starr, and Steele received support for article research from the National Institutes of Health (NIH). Dr. Cohen disclosed that he does not have any potential conflicts of interest.
References
- 1.Starr ME, Hu Y, Stromberg AJ, et al. Gene expression profile of mouse white adipose tissue during inflammatory stress: age-dependent upregulation of major procoagulant factors. Aging cell. 2013;12(2):194–206. doi: 10.1111/acel.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masoro EJ. Overview of caloric restriction and ageing. Mechanisms of ageing and development. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. J Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- 4.Masoro EJ. Hormesis and the antiaging action of dietary restriction. Experimental gerontology. 1998;33(1-2):61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 5.Robertson LT, Mitchell JR. Benefits of short-term dietary restriction in mammals. Experimental gerontology. 2013;48(10):1043–1048. doi: 10.1016/j.exger.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Ginhoven TM, Dik WA, Mitchell JR, et al. Dietary restriction modifies certain aspects of the postoperative acute phase response. The Journal of surgical research. 2011;171(2):582–589. doi: 10.1016/j.jss.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Flurkey K, Currer J, Harrison D. The Mouse in Biomedical Research. In: F JG, editor. Mouse Models in Aging Research. 2007. pp. 637–672. [Google Scholar]
- 8.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starr ME, Steele AM, Saito M, et al. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PloS one. 2014;9(12):e115705. doi: 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starr ME, Ueda J, Takahashi H, et al. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood. 2010;115(23):4886–4893. doi: 10.1182/blood-2009-10-246678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64(7):723–730. doi: 10.1093/gerona/glp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr ME, Saito M, Evers BM, et al. Age-Associated Increase in Cytokine Production During Systemic Inflammation-II: The Role of IL-1beta in Age-Dependent IL-6 Upregulation in Adipose Tissue. The journals of gerontology Series A, Biological sciences and medical sciences. 2015 doi: 10.1093/gerona/glu197. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Sherwood ER, Varma TK, et al. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mechanisms of ageing and development. 2003;124(10-12):1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Mustain WC, Starr ME, Valentino JD, et al. Inflammatory cytokine gene expression in mesenteric adipose tissue during acute experimental colitis. PloS one. 2013;8(12):e83693. doi: 10.1371/journal.pone.0083693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remick DG, Bolgos GR, Siddiqui J, et al. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lumeng CN, Liu J, Geletka L, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. Journal of immunology. 2011;187(12):6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeyda M, Gollinger K, Kriehuber E, et al. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. International journal of obesity. 2010;34(12):1684–1694. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- 18.Kang W, Saito H, Fukatsu K, et al. Effects of tyrosine kinase signaling inhibition on survival after cecal ligation and puncture in diet-restricted mice. JPEN Journal of parenteral and enteral nutrition. 2001;25(6):291–297. doi: 10.1177/0148607101025006291. discussion 298. [DOI] [PubMed] [Google Scholar]
- 19.Kang W, Saito H, Fukatsu K, et al. Diet restriction impairs extracellular signal-regulated kinase activation of peritoneal exudative cells after N-formyl-methionyl-leucyl-phenylalanine stimulation in a murine peritonitis model. JPEN Journal of parenteral and enteral nutrition. 2002;26(5):259–264. doi: 10.1177/0148607102026005259. discussion 264. [DOI] [PubMed] [Google Scholar]
- 20.Bermudes FA, Dettoni JB, Pereira FE. Effects of short term fasting on the evolution of fecal peritonitis in mice. Acta cirurgica brasileira/Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2011;26(3):181–185. doi: 10.1590/s0102-86502011000300005. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, Muthukumar AR, Lawrence RA, et al. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clinical and diagnostic laboratory immunology. 2001;8(5):1003–1011. doi: 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa A, Iwasaka H, Hagiwara S, et al. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. The Journal of surgical research. 2012;174(1):136–141. doi: 10.1016/j.jss.2010.11.883. [DOI] [PubMed] [Google Scholar]
- 23.Peck MD, Babcock GF, Alexander JW. The role of protein and calorie restriction in outcome from Salmonella infection in mice. JPEN Journal of parenteral and enteral nutrition. 1992;16(6):561–565. doi: 10.1177/0148607192016006561. [DOI] [PubMed] [Google Scholar]
- 24.Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 25.Rytka JM, Wueest S, Schoenle EJ, et al. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60(1):56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 27.Hocking SL, Wu LE, Guilhaus M, et al. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes. 2010;59(12):3008–3016. doi: 10.2337/db10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. Journal of immunology. 2007;179(7):4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 29.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 30.Tchkonia T, Morbeck DE, Von Zglinicki T, et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity. 2015;23(3):512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: A systematic review. Journal of critical care. 2015;30(3):518–524. doi: 10.1016/j.jcrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Kuperman EF, Showalter JW, Lehman EB, et al. The impact of obesity on sepsis mortality: a retrospective review. BMC infectious diseases. 2013;13:377. doi: 10.1186/1471-2334-13-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arabi YM, Dara SI, Tamim HM, et al. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Critical care. 2013;17(2):R72. doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaulton TG, Weiner MG, Morales KH, et al. The effect of obesity on clinical outcomes in presumed sepsis: a retrospective cohort study. Internal and emergency medicine. 2014;9(2):213–221. doi: 10.1007/s11739-013-1002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huttunen R, Laine J, Lumio J, et al. Obesity and smoking are factors associated with poor prognosis in patients with bacteraemia. BMC infectious diseases. 2007;7:13. doi: 10.1186/1471-2334-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prescott HC, Chang VW, O'Brien JM, Jr, et al. Obesity and 1-year outcomes in older Americans with severe sepsis. Critical care medicine. 2014;42(8):1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurzinger B, Dunser MW, Wohlmuth C, et al. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wiener klinische Wochenschrift. 2010;122(1-2):31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 39.Wacharasint P, Boyd JH, Russell JA, et al. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Critical care. 2013;17(3):R122. doi: 10.1186/cc12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leuwer M, Welters I, Marx G, et al. Endotoxaemia leads to major increases in inflammatory adipokine gene expression in white adipose tissue of mice. Pflugers Archiv: European journal of physiology. 2009;457(4):731–741. doi: 10.1007/s00424-008-0564-8. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki J, Kuwamura M, Yamaji R, et al. Inflammatory responses to lipopolysaccharide are suppressed in 40% energy-restricted mice. The Journal of nutrition. 2001;131(8):2139–2144. doi: 10.1093/jn/131.8.2139. [DOI] [PubMed] [Google Scholar]
- 42.Vega VL, De Cabo R, De Maio A. Age and caloric restriction diets are confounding factors that modify the response to lipopolysaccharide by peritoneal macrophages in C57BL/6 mice. Shock. 2004;22(3):248–253. doi: 10.1097/01.shk.0000133590.09659.a1. [DOI] [PubMed] [Google Scholar]
- 43.Garg SK, Delaney C, Shi H, et al. Changes in adipose tissue macrophages and T cells during aging. Critical reviews in immunology. 2014;34(1):1–14. doi: 10.1615/critrevimmunol.2013006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaul ME, Bennett G, Strissel KJ, et al. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59(5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Current opinion in clinical nutrition and metabolic care. 2011;14(4):341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey-Downs LC, Tucsek Z, Toth P, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(7):780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]