Abstract
Neuroactive steroids such as (3α,5α)3-hydroxypregnan-20-one (3α,5α-THP, allopregnanolone) enhance the GABAergic effects of ethanol and modulate excessive drinking in rodents. Moreover, chronic ethanol consumption reduces 3α,5α-THP levels in human plasma, rat hippocampus, and mouse limbic regions. We explored the relationship between 3α,5α-THP levels in limbic brain areas and voluntary ethanol consumption in the cynomolgus monkey following daily self-administration of ethanol for 12 months and further examined the relationship to HPA axis function prior to ethanol exposure. Monkeys were subjected to scheduled induction of ethanol consumption followed by free access to ethanol or water for 22 hours/day over twelve months. Immunohistochemistry was performed using an anti-3α,5α-THP antibody. Prolonged voluntary drinking resulted in individual differences in ethanol consumption that ranged from 1.2 – 4.2 g/kg/day over 12 months. Prolonged ethanol consumption reduced cellular 3α,5α-THP immunoreactivity by 13±2% (p<0.05) in the lateral amygdala and 17±2% (p<0.05) in the basolateral amygdala. The effect of ethanol was most pronounced in heavy drinkers that consumed ≥3 g/kg≥20% of days. Consequently, 3α,5α-THP immunoreactivity in both the lateral and basolateral amygdala was inversely correlated with average daily ethanol intake (Spearman r = −0.87 and −0.72, respectively, p<0.05). However, no effect of ethanol and no correlation between drinking and 3α,5α-THP immunoreactivity was observed in the basomedial amygdala. 3α,5α-THP immunoreactivity following ethanol exposure was also correlated with HPA axis function prior to ethanol exposure. These data indicate that voluntary ethanol drinking reduces amygdala levels of 3α,5α-THP in nonhuman primates and that amygdala 3α,5α-THP levels may be linked to HPA axis function.
Introduction
Neuroactive steroids are endogenous steroids that rapidly alter neuronal excitability via membrane receptors. These steroids are derived from cholesterol and can be synthesized de novo in the brain, the adrenal glands, and the gonads. GABAergic neuroactive steroids function as positive allosteric modulators of GABAA receptors. Among the most potent is a derivative of progesterone, (3α,5α)-3-hydroxy-pregnan-20-one (3α,5α-THP or allopregnanolone). Some neuroactive steroids, including 3α,5α-THP act at known potentiating sites within α subunits of GABAA receptors to enhance GABAergic activity (Hosie et al., 2006), producing pharmacological effects similar to those produced by administration of ethanol. Systemic administration of GABAergic neuroactive steroids exerts a variety of pharmacological responses including anxiolytic, antidepressant, anticonvulsant, sedative, anesthetic, and analgesic effects in animal models and human studies (Kavaliers, 1988; Belelli et al., 1989; Carl et al., 1990; Bitran et al., 1991; Khisti et al., 2000) that are consistent with their GABAergic actions.
Ethanol pharmacology involves various GABAergic mechanisms that contribute to many of its behavioral effects. One such mechanism is thought to involve the synthesis and availability of endogenous neuroactive steroids. Ethanol sensitivity is influenced by elevations in neuroactive steroids that enhance the GABAergic effects of ethanol (for review, see (Morrow et al., 2006)). Systemic administration of ethanol at doses of 1.3 g/kg or greater increases both plasma and brain levels of 3α,5α-THP and its precursors in Sprague-Dawley rats (VanDoren et al., 2000; Boyd et al., 2010b; Porcu et al., 2010). In contrast, acute ethanol administration does not alter plasma GABAergic neuroactive steroids measured in cynomolgus monkeys, where the maximal dose tested was 1.5 g/kg (Porcu et al., 2010).
Chronic ethanol consumption (patients who met DSM-IV criteria for alcohol abuse) reduces plasma 3α,5α-THP levels in human alcoholics (Romeo et al., 1996), but not Sprague Dawley rats (14-day alcohol diet between 6-7.5g/kg) (Janis et al., 1998). However, ethanol-dependent male rats (2-month oral administration of 6g/kg ethanol) show tolerance to ethanol induction of circulating 3α,5α-THP levels and decreased levels of 3α,5α-THP in cerebral cortex and hippocampus (Cagetti et al., 2004). Ethanol dependent C57BL/6J mice (4-week ethanol vapor inhalation) show decreased levels of 3α,5α-THP in lateral amygdala, ventral tegmental area, and prefrontal cortex, but increases in CA3 hippocampus, and no change in several other limbic regions (Maldonado-Devincci et al., 2014). Alterations in ethanol-induced neuroactive steroid levels in specific brain regions may contribute to ethanol tolerance and the propensity to drink greater amounts of ethanol.
Long-term ethanol exposure is difficult to model in rodent studies due to their short life spans. It is also challenging to model daily drinking doses and patterns in rodents that are similar to those achieved by human alcoholics. Nonhuman primates are important for the study of complex biomedical disease processes, due to anatomical, physiological, genetic, and behavioral similarities to humans. Cynomolgus macaques (Macaca fascicularis) freely self-administer intoxicating levels of ethanol with similar drinking patterns to those seen in humans (Grant et al., 2008), making them a good model to study the effects of chronic ethanol consumption. Overall, the effects of ethanol in cynomolgus monkeys differ from rodent models in several aspects and suggest that studies in non-human primates provide unique insights that may have relevance for human alcoholism.
Chronic ethanol self-administration by the cynomolgus macaque significantly shifts GABA potency, but not the efficacy, for basolateral amygdala GABAA receptors (Floyd et al., 2004). Long-term ethanol self-administration selectively reduces expression of α2, α3, and α1 subunit mRNAs without substantially influencing α4 subunit expression in basolateral amygdala (Floyd et al., 2004) indicating that alterations in expression of the subunits may be regulating ethanol-induced changes in GABAA receptor function. Similarly, prolonged intermittent ethanol drinking in cynomolgus monkeys decreases GABAergic inhibitory synaptic transmission while concomitantly increasing glutamatergic excitatory transmission in the putamen (Cuzon Carlson et al., 2011). These studies indicate that chronic ethanol leads to changes in GABAA receptor function and expression, potentially altering neuroactive steroid modulation in specific brain regions.
The amygdala plays an important role in drug seeking and is associated with affective behaviors that contribute to the abuse of drugs, including ethanol (Janak et al., 2015). As chronic ethanol administration results in a broad range of changes in 3α,5α-THP levels across the central nervous system of mice and rats (vide supra), the present study examined the effects of long-term voluntary ethanol consumption on 3α,5α-THP levels in the amygdala and blood of male cynomolgus monkey. This is the first study of 3α,5α-THP levels in cynomolgus monkey brain.
Materials and Methods
Animals
Monkeys (Macaca fascicularis, 50 to 62 months of age, weight 3.84 to 5.74 kg, n = 9) were purchased from a commercial vendor (World Wide Primates, Miami, FL) and placed into a center for disease control quarantine facility for 2 months upon arrival at Wake Forest University School of Medicine (Winston-Salem, NC). Upon release from quarantine, the monkeys lived in the laboratory with visual, auditory, and olfactory contact with at least three other conspecifics. The monkeys were housed individually in quadrant cages (1.6×0.8×0.8m) in a room with constant temperature (68 to 72F), humidity (65%) and a 12-hour light cycle (lights on at 7:00 AM). For the duration of the experiment the monkeys were weighed weekly. During the first month, the monkeys were acclimated to the laboratory personnel, then trained to provide their leg through an opening in the cage front for blood collection via saphenous or femoral venipuncture for the assays of plasma steroids. Each step in the behavioral training was considered complete when the animals performed the behavior readily and with minimal observable distress. Briefly, twice a day each monkey was trained with positive reinforcement (given fresh fruit) to move to the front of the cage and present its leg through an opening in the cage (10×10 cm). As the animal became comfortable with this behavior, the animal’s upper leg at the femoral triangle was lightly pricked with a dental pick to simulate a needle stick before advancing to the actual blood draw. Once the animal was comfortable with this, a 3 cc blood sample was drawn through a 22-gauge needle into an EDTA-coated vacutainer tube. All primate handling procedures were performed in accordance with the NIH and were approved by Wake Forest University IACUC in accordance with the Commission on Life Sciences, National Research Council (1996) Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington).
Induction of Ethanol Self-Administration
Monkeys were trained to operate a drinking panel in daily 60-minutes sessions and then induced to drink water and later ethanol (4% w/v in water). A separate group of control monkeys did not undergo the ethanol induction protocol, but were similarly trained and housed without access to ethanol. The monkey cages contained an active panel, fluid, and food; availability was signaled through illumination of the white, green, and red stimulus lights, respectively. Initially, fluid was available through a single drinking spout, and one press on the push panel resulted in delivery of a 1 gram banana-flavored pellet (Research Diets Incorporated, New Brunswick, NJ). Monkeys were trained to press the panel for food delivery for their meals; responding was not required in the delivery of pellets. Training was complete (approximately 2 to 3 weeks) once the monkey reliably drank from the spout, and received all available food pellets by responding on the push panel. The session variables were controlled by a computer program (BioticMicro, Clemmons, NC) that created a “record” each time a pellet was delivered, drinking occurred (fluid displacement measured), or a specific condition took place, such as achieving the required induction volume. During data analysis, consumption totals and timing of events were processed to full session totals. During the induction phase, the FT schedule of pellet delivery continued until the monkey drank a predetermined volume of either water or 4% (w/v) ethanol. The monkeys were induced to drink water for 30 days (the volume corresponding to an ethanol dose of 1.5 g/kg from 4% w/v ethanol; range was 150 to 227 ml). After 30 days, ethanol induction sessions began with ethanol (4% w/v) as the only fluid available. The monkeys were induced to drink 0.5 g/kg ethanol per day (range 52 to 74 ml), 1 g/kg/day (range 110 to 147 ml), and finally 1.5 g/kg/day (range 170 to 223 ml) for 30 consecutive days at each dose.
Chronic Ethanol Self-Administration
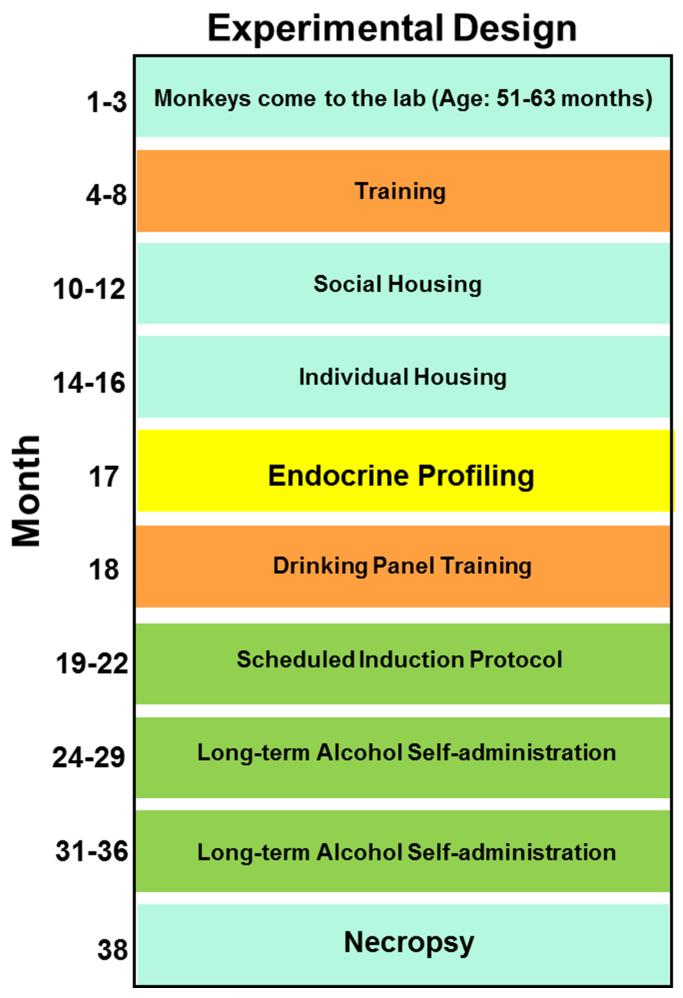
Following the 120 days of induction, the scheduled pellet delivery was discontinued. For 12 months, ethanol and water were always available and food was available in meals during a daily 22-hour session. Sessions began at 11:00 AM and ended at 9:00 AM the following day. When the sessions ended, the technical staff entered the room and downloaded the data, replenished the fluid reservoirs and pellets in the feeder. The 4% (w/v) ethanol was made fresh daily with deionized water, and the same source was used for the water reservoirs. If housing cages needed to be washed, the panels were removed from the cages at this time and cleaned by hand. All tubing was replaced as needed. Prior to the beginning of each session, the operation of the panel and the function of all stimulus lights, pellet dispensers, and response inputs were checked and replaced if necessary. Monkeys were fed fresh fruit during this time (Vivian, 2001). Of the 3570 sessions (357 sessions per monkey, 10 monkeys) that comprised the 12 month ethanol self-administration period, a total of 87 sessions (2.4%) were removed due to technical reasons (range 6 to 11 per monkey), mostly due to animals requiring anesthesia for routine veterinarian care, but there was also one case of incorrectly preparing the ethanol and one case of a panel being disconnected at the power source. The timeline for the training procedure is depicted in Figure 1.
Figure 1.
Schematic representation of the experimental design.
Pharmacological Profiling
An extensive series of assessments of the hypothalamic-pituitary-adrenal (HPA) axis were conducted (see experimental design, figure 1). The endocrine profile (EP) was designed to reflect clinical assessments commonly available in human research protocols. There were a total of eight pharmacological challenges: naloxone (2 doses), corticotrophin releasing factor (CRF), adrenocorticotropic hormone (ACTH), dexamethasone, ethanol (2 doses) and saline. Each pharmacological challenge was conducted between 8:00am and 2:00pm, except for the dexamethasone challenge. Two challenges per week were conducted, requiring four weeks to complete the endocrine profile. Here, we report data from the saline and ethanol challenges as well as the dexamethasone suppression test in relation to the post-mortem 3α,5α-THP levels.
Saline Challenge: Saline was administered and blood samples (3 ml) were drawn at 15, 30, 60, 90, and 120 minutes and assayed for steroids, including pregnenolone, to use as baseline comparisons to measure the effects of pharmacological challenges of the HPA axis.
Dexamethasone Suppression Test: The purpose of this test was to assess the sensitivity of the hypothalamus and pituitary to negative feedback from circulating levels of cortisol (Davidson, 1984; Mossman, 1989). Since dexamethasone binds with great affinity to the cortisol receptor it was used in relatively small amounts to test the sensitivity to negative feedback (Kalin, 1984). A morning (8:00 am) blood sample was taken for a baseline measure of steroids. That evening (10:00 pm) a low dose (130 μg/kg, i.m.) of dexamethasone was administered. The next morning (8:00 am) another blood sample was taken and assayed for steroids, including pregnenolone.
Blood Sampling and Neuroactive Steroid Analysis
Blood samples were obtained and stored on ice until centrifuged, frozen at − 80 °C, and stored until use. Pregnenolone and 3α,5α-THP levels were quantified in serum or plasma (300 μl) by radioimmunoassay or gas chromatography/mass spectrometry (GC/MS), respectively, using previously established methods (Porcu et al., 2006; Porcu et al., 2009).
Tissue Processing
Monkeys underwent a state-of-the-art necropsy protocol (Davenport et al., 2014) and the brain was removed, cut into 3 blocks and frozen in isopentane at −35°C. The brain blocks were stored in −80°C conditions until they were processed for histology. The temporal pole containing the amygdala was cut from the brain block, subsequently post-fixed in 4% paraformaldehyde for 24 hr at 4°C, and then stored in 30% sucrose until they were sectioned at 40 μm on a freezing microtome.
Immunohistochemistry
Amygdala were obtained and post-fixed in 4% paraformaldehyde for 24 hr at 4°C, then stored in 30% sucrose/PBS until the tissue block sank to the bottom of the container, at which point they were sectioned coronally at 40 μm on a freezing microtome and stored at 30°C until used for antibody staining. Immunohistochemistry was performed on free-floating sections (6 to 8 sections/animal/brain region) using an affinity purified 3α,5α-THP sheep antibody, that we have previously characterized by radioimmunoassay (VanDoren et al., 2000) and immunohistochemistry (Cook et al., 2014a). We have shown low cross-reactivity of this antibody with other 5α-reduced pregnane neurosteroids and their precursors (Cook et al., 2014a). No detergents or organic solvents were used to prevent the possibility of neuroactive steroid leeching. Sections were rinsed in PBS, followed by incubation in 1% hydrogen peroxide to block endogenous peroxidase activity. The tissue was blocked in 10% normal rabbit serum (Vector Laboratories, Burlingame, CA) in PBS followed by incubation in the sheep affinity purified anti-3α,5α-THP antiserum (purchased from Dr. R.H. Purdy) at a 1:2, 500 dilution for 48 hours at 4°C. Sections were rinsed in PBS and then incubated in a rabbit anti-sheep biotinylated secondary antibody (1:200; Vector Laboratories) for 60 minutes. Sections were then rinsed in PBS, avidin biotin amplification was performed using a Vectastain Elite ABC kit (Vector Laboratories), and immunoreactivity was visualized with 3,3′-diaminobenzidine (Sigma-Aldrich) using the manufacturers’ recommended protocol.
IHC Analyses
Brain-region immunoreactivity was visualized with an Olympus CX41 light microscope (Olympus America, Center Valley, PA), images were captured with a digital camera (Regita model; QImaging, Burnaby, BC), and analyzed using Bioquant (Nashville, TN) image analysis to obtain linear integrated optical density for immunoreactivity assessment. The microscope, camera, and software were background corrected to eliminate nonspecific labeling and normalized to preset light levels to ensure fidelity of data acquisition. Positive pixel count of immunoreactivity was quantified from a circumscribed field, delineated as a brain region, divided by the area of the region in square millimeters, and expressed as pixels/ mm2. Data from 6 to 8 alternate sequential sections per animal per brain region from a single hemisphere were used to average 1 value per monkey. The experimenter was blind to the condition of each animal when analyses were conducted.
Statistics
Within each experiment, raw pixel densities (pixels/mm2) were analyzed using Student’s t-test for comparisons between ethanol naïve controls and ethanol-consuming monkeys. Data for each brain region were analyzed using a one-way analysis of variance (ANOVA) (Statistica; StatSoft Inc., Tulsa, OK), with alcohol consumption levels as a factor (3: control, heavy, non-heavy). Post-hoc analyses were conducted with the Newman-Keuls test. Nonparametric correlations (Spearman) were performed between individual drinking levels (average daily drinking g/kg) and raw pixel densities (pixels/mm2). Total areas under curve (AUC) are the definite integrals of pregnenolone concentrations in blood plasma across time and were calculated using pregnenolone levels obtained at various time points and analyzed with the unpaired student-t test using Prism (Graphpad Software; La Jolla, CA). This measure allows an evaluation of the response to pharmacological challenge across time.
Results
Chronic ethanol consumption reduces 3α,5α-THP in lateral amygdala
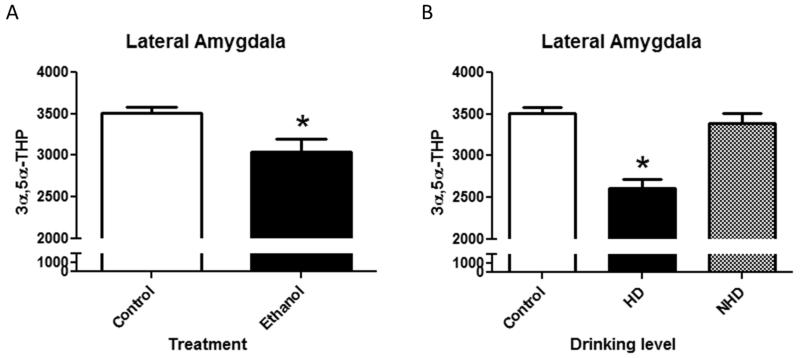
Following 12 months of daily voluntary access to ethanol, we assessed 3α,5α-THP immunoreactivity in the lateral amygdala and compared them to control animals that were similarly housed, but without access to ethanol. Overall, EtOH-consuming monkeys showed lower 3α,5α-THP immunoreactivity compared to control animals (Figure 2a). Analysis of raw pixel density indicates a reduction (−13±2%; t(18)=2.79, p<0.05) of cellular 3α,5α-THP immunoreactivity in the lateral amygdala of monkeys that chronically consumed ethanol. EtOH-consuming animals were categorized into “heavy” and “non-heavy” drinkers (~50% of each in the monkey population studied here) based on individual levels of drinking. Heavy drinkers were defined by having an ethanol consumption of ≥ 3 g/kg for ≥ 20% of days whereas animals under that threshold were classified as non-heavy drinkers. We assessed 3α,5α-THP immunoreactivity values in the lateral amygdala and found the effect of ethanol to be driven by heavy drinkers [F(2,16)=18.52, p<0.05]. Monkeys classified as heavy drinkers displayed significantly reduced (−25±3%; p<0.05) 3α,5α-THP immunoreactivity compared to controls (Figure 2b), while non-heavy drinkers showed no effect of long-term ethanol consumption. When compared to those animals classified as non-heavy drinkers, 3α,5α-THP immunoreactivity in the lateral amygdala of heavy drinkers was also reduced (−23±3%; p<0.05) (Figure 2b). These data indicate that those animals that consistently consumed the most ethanol displayed the greatest reductions in 3α,5α-THP immunoreactivity in the lateral amygdala.
Figure 2.
(A) Effects of chronic ethanol exposure on 3α,5α-THP immunoreactivity in the lateral amygdala in control (clear bars) or ethanol (EtOH)-drinking monkeys (black bars). (B) Effect of drinking levels on 3α,5α-THP immunoreactivity in the lateral amygdala in heavy drinkers (HD) and non-heavy drinkers (NH). Data depicted are mean positive pixels/mm2±SEM. *p < 0.05 compared to control.
Average daily drinking is correlated with 3α,5α-THP levels in lateral amygdala
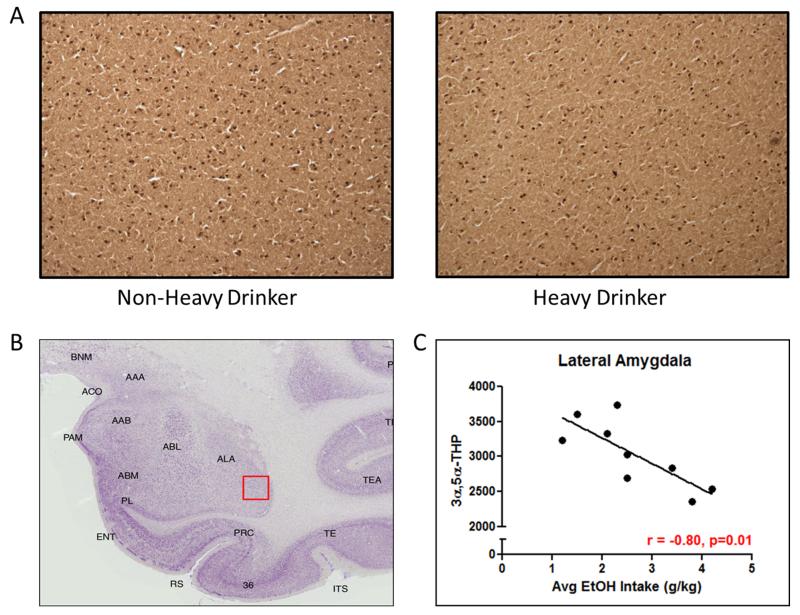
Monkeys consumed on average between 1.17 and 4.25 g/kg of ethanol daily. 3α,5α-THP immunoreactivity was assessed in the lateral amygdala and results were compared against the average daily consumption of ethanol. 3α,5α-THP immunoreactivity values in the lateral amygdala were inversely correlated (r=−0.80, p<0.05) with voluntary ethanol consumption (average daily ethanol intake [g/kg]) after 12 months of daily drinking (Figure 3c). These data indicate that the level of consumption maintained during free access plays an important role in the reduction of 3α,5α-THP immunoreactivity in the lateral amygdala.
Figure 3.
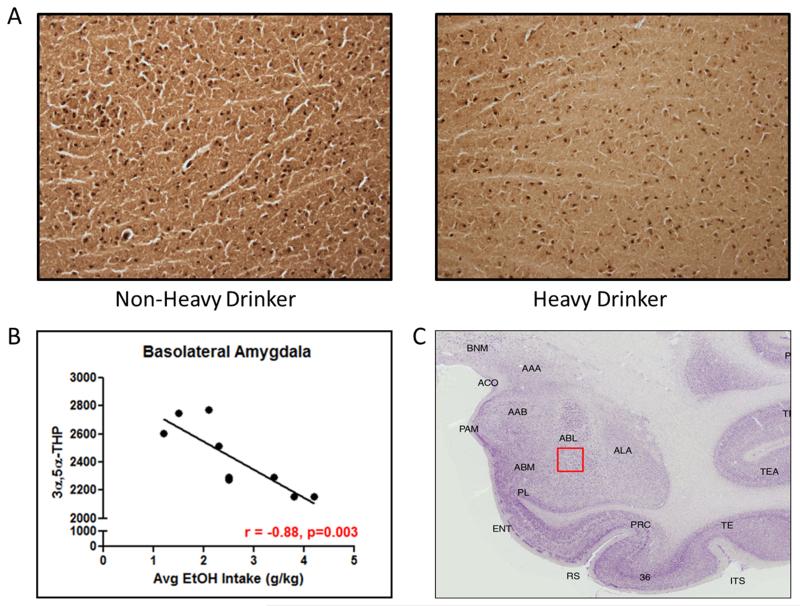
(A) Representative photomicrographs (4x) of cellular 3α,5α-THP immunoreactivity in coronal slices of lateral amygdala from non-heavy and heavy drinking cynomolgus monkeys following 12 months of voluntary access to ethanol. (B) Atlas image of cynomolgus monkey amygdala. The red box indicates the location of the representative photomicrograph within the lateral amygdala. (C) Negative correlation between average daily ethanol consumption and 3α,5α-THP immunoreactivity (r=−0.87, p<0.05).
Chronic ethanol consumption reduces 3α,5α-THP in basolateral amygdala
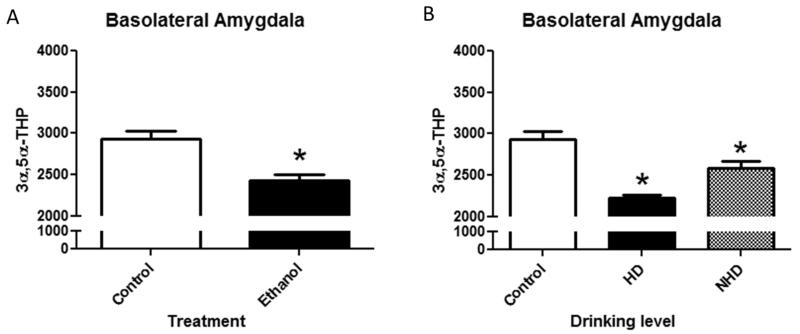
Next we assessed 3α,5α-THP immunoreactivity in the basolateral amygdala following 12 months of voluntary chronic ethanol consumption. Similar to the changes found in the lateral amygdala, analysis of raw pixel density indicates a significant reduction (−17±2%; t(18)=2.79, p<0.05) in 3α,5α-THP immunoreactivity in the basolateral amygdala of monkeys that chronically consumed ethanol (Figure 4a). We assessed the percent of control of 3α,5α-THP immunoreactivity values in the basolateral amygdala and found the greatest reductions in heavy drinkers [F(2,16)=10.1, p<0.05]. Heavy drinkers displayed significantly reduced (−22±2%; p<0.05) 3α,5α-THP immunoreactivity (Figure 4b) compared to controls. Non-heavy drinkers also showed a significant reduction (−9±1%; p<0.05) in 3α,5α-THP immunoreactivity compared to controls. These data indicate that those animals that consumed the most ethanol displayed the greatest reductions in 3α,5α-THP immunoreactivity in the basolateral amygdala.
Figure 4.
(A) Effects of chronic ethanol exposure on 3α,5α-THP immunoreactivity in the basolateral amygdala in control (clear bars) or ethanol (EtOH)-drinking monkeys (black bars). (B) Effect of drinking levels on 3α,5α-THP immunoreactivity in the basolateral amygdala in heavy drinkers (HD) and non-heavy drinkers (NH). Data depicted are mean positive pixels/mm2±SEM. *p < 0.05 compared to control.
Average daily drinking is correlated with 3α,5α-THP levels in basolateral amygdala
3α,5α-THP immunoreactivity was assessed in the basolateral amygdala and results were compared against the average daily consumption of ethanol. 3α,5α-THP immunoreactivity values in the basolateral amygdala were inversely correlated (r=−0.88, p<0.05) with voluntary ethanol consumption (average daily ethanol intake [g/kg]) after 12 months of voluntary drinking (Figure 5b). Similar to the lateral amygdala, changes in 3α,5α-THP immunoreactivity in the basolateral amygdala appear to be proportional to the average amount of ethanol consumed.
Figure 5.
(A) Representative photomicrographs (4x) of cellular 3α,5α-THP immunoreactivity in coronal slices of basolateral amygdala from non-heavy and heavy drinking cynomolgus monkeys following 12 months of voluntary access to ethanol. (B) Atlas image of cynomolgus monkey amygdala. The red box indicates the location of the representative photomicrograph within the basolateral amygdala. (C) Negative correlation between average daily ethanol consumption and 3α,5α-THP immunoreactivity (r=−0.78, p<0.05).
Chronic ethanol consumption does not alter 3α,5α-THP immunoreactivity in basomedial amygdala
A third sub-region of the amygdala, the basomedial amygdala, was also assessed for changes in 3α,5α-THP immunoreactivity following 12 months of free access to ethanol. There were no significant changes in 3α,5α-THP immunoreactivity in the basomedial amygdala of ethanol-consuming monkeys compared to control animals (Figure 6).
Figure 6.
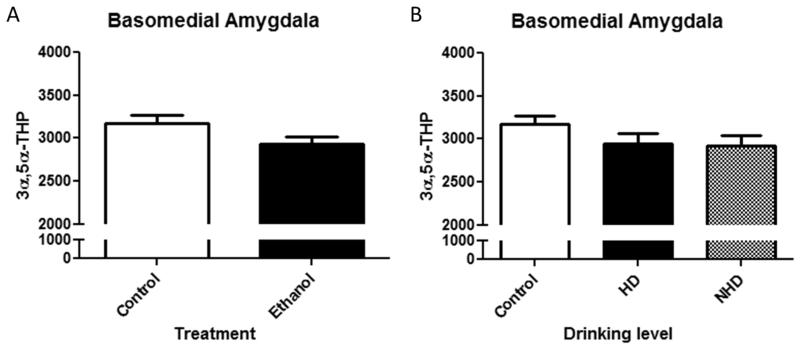
(A) Effects of chronic ethanol exposure on 3α,5α-THP immunoreactivity in the basomedial amygdala in control (clear bars) or ethanol (EtOH)-drinking monkeys (black bars). (B) Effect of drinking levels on 3α,5α-THP immunoreactivity in the basomedial amygdala in heavy drinkers (HD) and non-heavy drinkers (NH). Data depicted are mean positive pixels/mm2±SEM. *p < 0.05 compared to control.
Average daily drinking is not correlated with 3α,5α-THP levels in basomedial amygdala
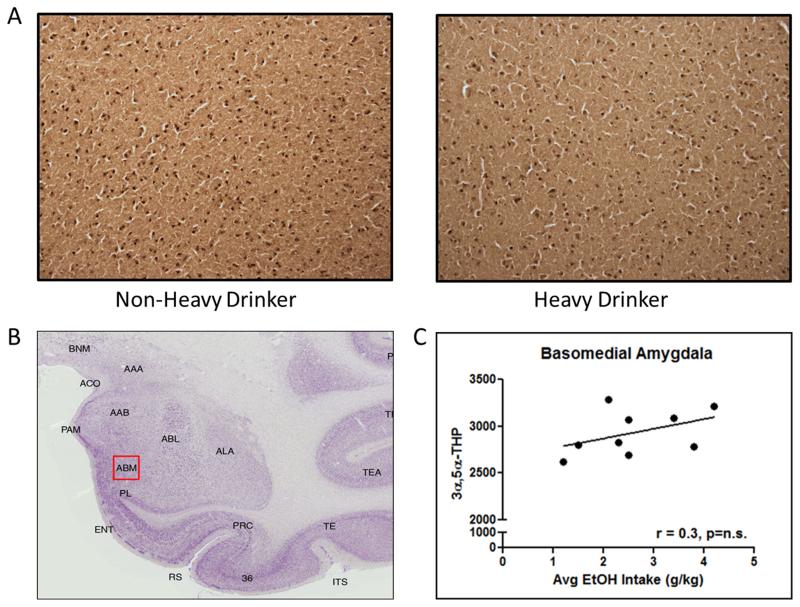
3α,5α-THP immunoreactivity was assessed in the basomedial amygdala. There was no correlation between 3α,5α-THP immunoreactivity values in the basomedial amygdala and voluntary ethanol consumption (average daily ethanol intake [g/kg]) after 12 months of voluntary drinking (Figure 7c). Unlike results found in the lateral and basolateral amygdala, levels of 3α,5α-THP immunoreactivity in the basomedial amygdala does not appear to be influenced by the amount of ethanol consumed.
Figure 7.
(A) Representative photomicrographs (4x) of cellular 3α,5α-THP immunoreactivity in coronal slices of basomedial amygdala from non-heavy and heavy drinking cynomolgus monkeys following 12 months of voluntary access to ethanol. (B) Atlas image of cynomolgus monkey amygdala. The red box indicates the location of the representative photomicrograph within the basomedial amygdala. (C) Lack of correlation between average daily ethanol consumption and 3α,5α-THP immunoreactivity.
Chronic ethanol consumption reduces circulating 3α,5α-THP levels
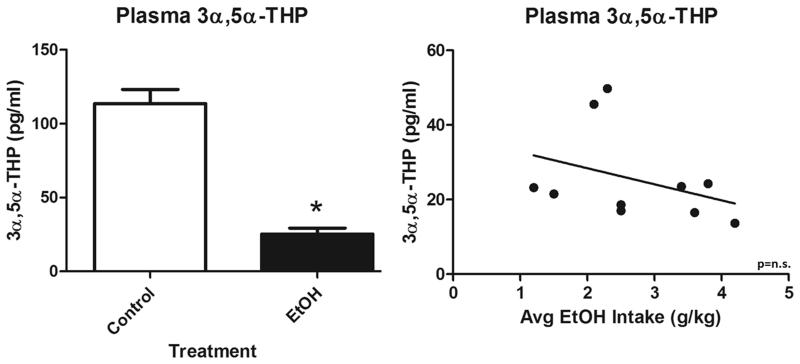
3α,5α-THP was assessed in circulating plasma following 12 months of voluntary chronic ethanol consumption and compared to control animals without access to ethanol. Ethanol-drinking monkeys showed a dramatic reduction (77.7±9.5%; t(19)=8.2, p<0.0001) of 3α,5α-THP compared to control animals (Figure 8a). There was no correlation between 3α,5α-THP levels in plasma after necropsy and voluntary ethanol consumption (average daily ethanol intake [g/kg]) after 12 months of voluntary drinking (Figure 8b). There was also no correlation between circulating 3α,5α-THP levels and amygdala 3α,5α-THP in any of the sub-regions studied (data not shown).
Figure 8.
(A) Effects of chronic ethanol exposure on 3α,5α-THP in circulating plasma in control (clear bar) or ethanol-drinking monkeys (black bar). (B) Lack of correlation between average daily ethanol consumption and plasma 3α,5α-THP. Data depicted are in pg/ml. *p < 0.0001 compared to control.
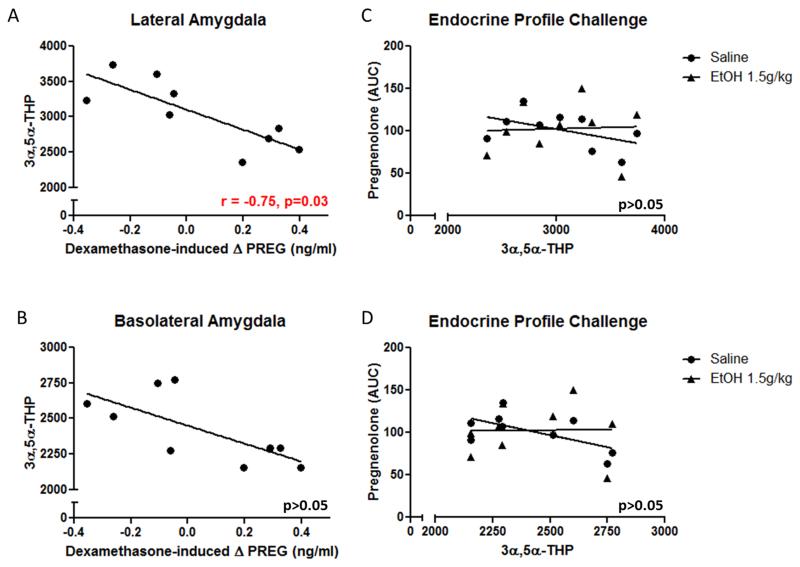
Post-mortem lateral amygdala 3α,5α-THP immunoreactivity correlates with dexamethasone-induced changes in circulating pregnenolone levels prior to ethanol exposure
Previously, it was reported that dexamethasone-induced changes in circulating pregnenolone levels in ethanol-naïve cynomolgus monkeys were correlated with subsequent alcohol intake (Porcu et al., 2006). Extending these findings, this study revealed that post-mortem levels of 3α,5α-THP in the lateral amygdala, but not basolateral amygdala, correlated with dexamethasone-induced changes in circulating pregnenolone levels (Figure 9a,b; r=−0.75, p<0.05). Dexamethasone-induced changes in plasma pregnenolone levels prior to ethanol exposure were predictive of post-mortem 3α,5α-THP levels in the lateral amygdala.
Figure 9.
(A) Dexamethasone-induced delta PREG in plasma prior to ethanol exposure is predictive of post-mortem 3α,5α-THP immunoreactivity in lateral amygdala. (B) Dexamethasone-induced delta PREG is not predictive of post-mortem 3α,5α-THP levels in BLA. Basal PREG and EtOH (1.5g/kg)-induced PREG in ethanol-naïve monkeys do not correlate with post-mortem 3α,5α-THP immunoreactivity in (C) lateral amygdala and (D) basolateral. EtOH, ethanol; AUC, Area Under Curve; PREG, pregnenolone.
We then wanted to determine if circulating levels of pregnenolone in response to saline or acute ethanol (1.5g/kg) challenges prior to ethanol exposure could be linked to post-mortem levels of 3α,5α-THP following long-term ethanol consumption. In the regions that showed reductions in 3α,5α-THP (lateral amygdala and basolateral amygdala) following chronic ethanol consumption, no correlation was observed between post-mortem amygdalar 3α,5α-THP and circulating pregnenolone after saline or acute ethanol administration to the ethanol-naïve monkey (Figure 9c and d).
Discussion
The present set of experiments demonstrate decreased 3α,5α-THP immunoreactivity in both the lateral and basolateral amygdala as well as plasma, following chronic ethanol consumption in male cynomolgus monkeys. Further, 3α,5α-THP immunoreactivity in the lateral and basolateral amygdala are inversely correlated with average daily ethanol consumption. Divided functionally into subdivisions, the amygdala receives inputs from various cortical and subcortical areas and projects to various other brain regions. The lateral amygdala receives inputs from the cortex and the hippocampus, as well as projecting to other amygdalar sub-regions and reciprocally back to input structures (Pitkanen et al., 1997; Faber et al., 2001). The basolateral amygdala sends inhibitory and excitatory projections to the lateral amygdala (Pitkanen et al., 1997). The basolateral amygdala mediates some emotions (such as anxiety) and is involved in drug craving and drug-related relapse (See et al., 2003). Both the lateral and basolateral amygdala project to the central nucleus of the amygdala, a region of the extended amygdala identified as a specific site of action for both the acute positive reinforcement of drugs of abuse and for the negative reinforcement associated with abstinence from drugs (Koob et al., 2001). GABAergic transmission in the central nucleus of the amygdala is thought to play a role in regulating alcohol intake in ethanol-dependent rats (Hyytia et al., 1995; Roberto et al., 2004). These amygdalar structures are known to be highly involved in the neurocircuitry mediating ethanol seeking and consumption, suggesting that 3α,5α-THP levels in the amygdala may regulate voluntary ethanol consumption.
Ethanol-induced reductions in neuroactive steroids would be expected to alter neurotransmission in the brain. Changes in local neuroactive steroid levels have been shown to alter neuron function following acute ethanol exposure (Sanna et al., 2004; Follesa et al., 2006; Tokuda et al., 2011). It is unclear exactly how neuroactive steroids influence neurotransmission, but available data suggests that alterations in 3α,5α-THP within a cell can alter the excitability of that particular cell (Akk et al., 2007). In the lateral amygdala, reduced GABAergic tone leads to a state of hyperexcitability (Danober et al., 1998). The primary neurons within the lateral and basolateral amygdala are pyramidal cells (Faber et al., 2001; Muller et al., 2006) whose excitation to efferent connections is regulated by GABAergic interneurons (Muller et al., 2006). A reduction in 3α,5α-THP levels in multiple sub-regions of the amygdala would be expected to decrease the inhibitory tone of these interneurons and to increase the activity of glutamatergic excitatory neurons (Iwata et al., 2013) resulting in an increase in excitation both within the amygdala and at its projection targets. Indeed, in the basolateral amygdala of Sprague-Dawley rats, chronic ethanol consumption and withdrawal increases glutamatergic synaptic and receptor function in principal neurons (Diaz et al., 2011). Recent work in the central nucleus of the amygdala has shown that chronic intermittent ethanol alters cell-type-specific tonic signaling leading to selective disinhibition that may alter the amygdala output and contribute to changes in behavior associated with ethanol dependence (Herman et al., 2014). Increases in excitatory transmission as a result of chronic ethanol-induced reductions in 3α,5α-THP may account for altered processing of sensory inputs.
Neuroactive steroids produce discriminative stimulus effects in macaque monkeys that are similar to ethanol. The GABAergic neuroactive steroids 3α,5α-THP, 3α,5β-THP, and 3α,5α-androsterone produce a discriminative stimulus effect that mimics that of both relatively low (1.0 g/kg) and higher (2.0 g/kg) doses of ethanol. In addition, increased circulating progesterone (a precursor of the 3α,5α- and 3α,5β- neuroactive steroids) during the luteal phase of the menstrual cycle in female monkeys produces increased sensitivity to the perception of ethanol, suggesting additive effects of circulating neuroactive steroids with administered ethanol (Grant et al., 1997). GABAergic neuroactive steroids can alter both ethanol reinforcement and ethanol consumption in rat models. Both pregnenolone (a precursor of all classes of neuroactive steroids) and the synthetic GABAergic neuroactive steroid 3,5-20-oxo-pregnane-3-carboxylic acid dose dependently reduce ethanol self-administration without producing sedation (O'Dell et al., 2005; Besheer et al., 2010). Recent studies in alcohol-preferring (P) rats utilizing adenovirus-mediated delivery of the steroidogenic enzyme cytochrome P450scc showed that local increases in the enzyme led to increased 3α,5α-THP immunoreactivity in the ventral tegmental area and reduced long-term operant ethanol self-administration (Cook et al., 2014b). These studies are consistent with our observation in the cynomolgus monkeys that high ethanol consumption was associated with low tissue levels of 3α,5α-THP in lateral and basolateral amygdala. Since elevations of neuroactive steroids in brain are associated with increased ethanol sensitivity in macaques and reduced drinking in rat models, the observed reductions in amygdala levels of 3α,5α-THP may have contributed to increased drinking in those monkeys that exhibited the reduced levels of 3α,5α-THP. Overall, the data suggest that low amygdala levels of 3α,5α-THP is either permissive or directly involved in heavy drinking.
The mechanisms by which 3α,5α-THP could influence ethanol consumption are unknown. One possibility, which may be secondary to the GABAergic effects on neurotransmission, is that 3α,5α-THP signaling could lead to altered amygdala circuit behavior to influence drinking (Herman et al., 2014). Alternatively, a growing body of evidence suggests that 3α,5α-THP has an inhibitory role in neuroimmune signaling that accounts for protection in studies of traumatic brain injury, Alzheimer’s disease, multiple sclerosis, and Nieman Pick Type C disease (Griffin et al., 2004; He et al., 2004; Brinton, 2013; Noorbakhsh et al., 2014). Furthermore, recent evidence suggests that inhibition of TLR-4 signaling modulates ethanol consumption in alcohol-preferring P rats (Liu et al., 2011; June et al., 2015) as well as C57BL/6J mice (Blednov et al., 2011). Hence, further work is required to elucidate the mechanistic relationship between ethanol drinking and 3α,5α-THP levels in monkey amygdala.
Previous studies have demonstrated that chronic ethanol exposure alters neuroactive steroid levels in rodent models of ethanol dependence. Indeed, ethanol-dependent male rats display reduced levels of 3α,5α-THP in hippocampus (Cagetti et al., 2004) and there is tolerance to the ability of ethanol to increase 3α,5α-THP levels in both cortex and hippocampus (Janis et al., 1998; Boyd et al., 2010a). Similar to the effects of ethanol in cynomolgus monkeys, chronic intermittent ethanol exposure in C57BL/6J mice results in decreased 3α,5α-THP immunoreactivity in sub-regions of prefrontal cortex, amygdala, ventral tegmental area, nucleus accumbens, and dorsal striatum (Maldonado-Devincci et al., 2014). The current study extends these findings to the non-human primate. The effects of ethanol on 3α,5α-THP levels in the plasma of monkeys are similar to effects that were previously reported in human alcoholics (Romeo et al., 1996). Ethanol consumption in the cynomolgus monkeys produced circulating 3α,5α-THP levels that were greatly reduced, uniform, and near the limit of detection. Thus, it was not surprising that there was no correlation between these levels and ethanol consumption. The dramatic reduction may indicate that measurement of plasma 3α,5α-THP levels may serve as a biomarker of alcohol abuse. Since we found differential effects of ethanol on 3α,5α-THP across amygdala sub-regions, it is also unsurprising that plasma levels of 3α,5α-THP are not correlated with levels in any specific brain region.
Porcu et al. 2006 reported the results of a pharmacological challenge with naloxone, (CRF), dexamethasone, (ACTH) and ethanol. To summarize, naloxone increased pregnenolone levels, while CRF appeared to increase metabolism of pregnenolone to deoxycorticosterone (DOC). ACTH, administered after dexamethasone, reduced pregnenolone levels, despite an increase in plasma cortisol. Ethanol did not alter pregnenolone levels. There was a positive correlation between dexamethasone-induced changes in plasma pregnenolone levels in ethanol-naïve cynomolgus monkeys and the average daily subsequent ethanol consumption during a twelve-month period of free access (Porcu et al., 2006). This suggested that adrenal output of pregnenolone under negative feedback of the HPA axis is predictive of subsequent alcohol consumption. Neuronal inhibition exerted by GABAergic neurons represents a major mechanism regulating HPA axis activity in the brain (for review see (Biggio et al., 2014). Here we show that dexamethasone-induced suppression of circulating pregnenolone in ethanol naïve monkeys was negatively correlated with post-mortem 3α,5α-THP levels in the lateral amygdala of ethanol-exposed monkeys. Thus, when ethanol naive, individuals that have a robust negative feedback of circulating pregnenolone to dexamethasone will likely become heavy drinkers and have low amygdala levels of 3α,5α-THP. What is not known is if low amygdala 3α,5α-THP content preceded or was a consequence of chronic heavy alcohol consumption. Nevertheless, the intriguing relationship between circulating pregnenolone response to dexamethasone and ethanol consumption suggests a link between HPA function and drinking behavior. Further, these findings may help in efforts to identify objective, quantifiable risk factors for heavy drinking that can be translated to clinical settings.
The cynomolgus monkey is a powerful model for researching chronic ethanol consumption because of their genetic homology with humans and, similar to humans, they freely self-administer intoxicating levels of ethanol and exhibit a 3-fold variation in average drinking (Grant et al., 2003). This model of oral ethanol self-administration in monkeys results in ~35% of individuals defined by average daily ethanol intake of at least 3.0g/kg/day, or “heavy drinkers.” In our studies ~50% of the animals displayed the “heavy-drinking” phenotype and had average intakes and blood ethanol concentrations similar to those reported for alcoholic men given 20 to 60 consecutive days of free access to ethanol 24 hr/day (Majchrowicz et al., 1970; Mello et al., 1970). These monkeys also exhibit similar absorption and metabolism of ethanol to humans (Green et al., 1999). This distribution allowed us to assess the effects of chronic ethanol consumption on varying levels of drinking, which better represents a human population where only some people who have free access will become alcoholics or “heavy-drinkers”. NIAAA defines “heavy drinking” in humans as 5 or more drinks on the same occasion on each of 5 or more days in the past 30 days. This definition for heavy drinking results in human consumption of <1g/kg/day. The monkeys in this study clearly met the human criteria for heavy drinking.
It has been suggested that ethanol-induced elevations of GABAergic neuroactive steroids protect against the risk for ethanol dependence (Morrow et al., 2006). Neuroactive steroids have been shown to contribute to the discriminative stimulus effects of ethanol in monkeys (Shelton et al., 2002), as well as subjective effects of ethanol in humans (Pierucci-Lagha et al., 2006; Covault et al., 2014). Diminished elevations of GABAergic neuroactive steroids following ethanol exposure may account for reduced sensitivity to the anxiolytic, sedative, anticonvulsant, cognitive-impairing, and discriminative stimulus properties of ethanol following chronic exposure (Morrow, 2006). Reduced sensitivity to ethanol is associated with greater risk for the development of alcoholism in individuals with genetic vulnerability to alcoholism (Schuckit, 2009). The results of the present experiments indicate that ethanol consumption leads to significant reductions of 3α,5α-THP in both lateral and basolateral amygdala and that the level of drinking plays an important role in these changes. Multiple brain regions likely contribute to elevated consumption following chronic ethanol and further work to explore the relationship between chronic ethanol consumption and 3α,5α-THP levels in other limbic brain structures of cynomolgus monkeys is ongoing.
The present study reports reduced levels of 3α,5α-THP in the lateral and basolateral amygdala following long-term voluntary ethanol consumption in male cynomolgus monkeys. While little is known about the relationship between chronic ethanol consumption and neuroactive steroids in non-human primates, we propose that alterations in ethanol-induced neuroactive steroid levels in specific brain regions may contribute to ethanol tolerance and the propensity to drink greater amounts of ethanol. Neuroactive steroids may influence ethanol consumptive behavior following chronic ethanol administration, but the nature of this relationship remains unknown.
Acknowledgements
This work was supported by NIH grants U01-AA020935 (ALM), AA019431 (KAG, JBD), T32-ES007126, and the UNC Bowles Center for Alcohol Studies.
Footnotes
Author Contributions:
MCB, ALM, KAG were responsible for the study concept and design. KAG was responsible for all animal procedures. MCB, AMD, PP, TKO, and JBD contributed to the acquisition of animal data. MCB, AMD, PP, TKO, KAG, and ALM conducted the data analysis and interpretation of findings. MCB drafted the manuscript. AMD, PP, TKO, JBD, KAG, and ALM provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
The authors have no conflicts of interest to declare.
References
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O'Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G, Pisu MG, Biggio F, Serra M. Allopregnanolone modulation of HPA axis function in the adult rat. Psychopharmacology (Berl) 2014;231:3437–3444. doi: 10.1007/s00213-014-3521-6. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, Morrow AL. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. J Neurochem. 2010a;115:142–152. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, Porcu P, Morrow AL. Ethanol induction of steroidogenesis in rat adrenal and brain is dependent upon pituitary ACTH release and de novo adrenal StAR synthesis. J Neurochem. 2010b;112:784–796. doi: 10.1111/j.1471-4159.2009.06509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Carl P, Hogskilde S, Nielsen JW, Sorensen MB, Lindholm M, Karlen B, et al. Pregnanolone emulsion. A preliminary pharmacokinetic and pharmacodynamic study of a new intravenous anaesthetic agent. Anaesthesia. 1990;45:189–197. doi: 10.1111/j.1365-2044.1990.tb14683.x. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O'Buckley TK, Morrow AL. Ethanol Administration Produces Divergent Changes in GABAergic Neuroactive Steroid Immunohistochemistry in the Rat Brain. Alcohol Clin Exp Res. 2014a;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Werner DF, Maldonado-Devincci AM, Leonard MN, Fisher KR, O'Buckley TK, et al. Overexpression of the steroidogenic enzyme cytochrome P450 side chain cleavage in the ventral tegmental area increases 3alpha,5alpha-THP and reduces long-term operant ethanol self-administration. J Neurosci. 2014b;34:5824–5834. doi: 10.1523/JNEUROSCI.4733-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR. Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology (Berl) 2014;231:3609–3618. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, et al. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36:2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danober L, Pape HC. Mechanisms and functional significance of a slow inhibitory potential in neurons of the lateral amygdala. Eur J Neurosci. 1998;10:853–867. doi: 10.1046/j.1460-9568.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- Davenport AT, Grant KA, Szeliga KT, Friedman DP, Daunais JB. Standardized method for the harvest of nonhuman primate tissue optimized for multiple modes of analyses. Cell and tissue banking. 2014;15:99–110. doi: 10.1007/s10561-013-9380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Lipper S, Zung WWK, Strickland R, Krishnan R, Mahorney S. Validation of four definitions of melancholia by the dexamethasone suppression test. Am J Psychiatry. 1984;141:1220–1223. doi: 10.1176/ajp.141.10.1220. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Callister RJ, Sah P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. Journal of Neurophysiology. 2001;85:714–723. doi: 10.1152/jn.2001.85.2.714. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Talani G, Murru L, Serra M, Sanna E, et al. Neurosteroids, GABAA receptors, and ethanol dependence. Psychopharmacology. 2006;186:267–280. doi: 10.1007/s00213-005-0126-0. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fascicularis) Alcohol Clin Exp Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2014 doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Iwata S, Wakita M, Shin MC, Fukuda A, Akaike N. Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal. Brain Research Bulletin. 2013;93:39–46. doi: 10.1016/j.brainresbull.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, et al. CRF-Amplified Neuronal TLR4/MCP-1 Signaling Regulates Alcohol Self-Administration. Neuropsychopharmacology. 2015;40:1549–1559. doi: 10.1038/npp.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Acute behavioral stress affects the dexamethasone suppresion test in rhesus monkeys. Biol Psychiatry. 1984;19:113–117. [PubMed] [Google Scholar]
- Kavaliers M. Inhibitory influences of the adrenal steroid, 3α,5α-tetrahydroxycorticosterone on aggression and defeat-induced analgesia in mice. Psychopharmacology. 1988;95:488–492. doi: 10.1007/BF00172960. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr., et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majchrowicz E, Mendelson JH. Blood concentrations of acetaldehyde and ethanol in chronic alcoholics. Science. 1970;168:1100–1102. doi: 10.1126/science.168.3935.1100. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Cook JB, O'Buckley TK, Morrow DH, McKinley RE, Lopez MF, et al. Chronic intermittent ethanol exposure and withdrawal alters (3α,5α)-3-hydroxy-pregnan-20-one immunostaining in cortical and limbic brain regions of C57BL/6J mice. Alcohol Clin Exp Res. 2014 doi: 10.1111/acer.12530. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173:101–116. [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman D, Somoza E. Maximizing diagnostic information from the dexamethasone suppression test. An approach to criterion selection using receiver operating characteristic analysis. Arch Gen Psychiatry. 1989;46:653–660. doi: 10.1001/archpsyc.1989.01810070079013. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Baker GB, Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Frontiers in cellular neuroscience. 2014;8:134. doi: 10.3389/fncel.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, et al. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosciences. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Porcu P, Rogers LSM, Morrow AL, Grant KA. Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol Biochem Behav. 2006;84:618–627. doi: 10.1016/j.pbb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, et al. Simultaneous quantification of GABAergic 3α,5α/3α,5β neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, et al. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E, Curatolo P, di Michele F, Spalletta G, Pompili E, Furnari C, et al. Neurosteroid alterations in mood disorders associated with alcohol withdrawal. In: Genazzani AR, Petraglia F, Purdy RH, editors. The brain: source and target for sex steroid hormones. 1 edn The Parthenon Group; New York: 1996. pp. 113–122. [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res. 2002;26:747–757. [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]