Abstract
Posttraumatic elbow stiffness is a disabling condition that remains challenging to treat despite improvement of our understanding of the pathogenesis of posttraumatic contractures and new treatment regimens. This review provides an update and overview of the etiology of posttraumatic elbow stiffness, its classification, evaluation, nonoperative and operative treatment, and postoperative management.
Keywords: Posttraumatic, Contracture, Elbow, Release, Stiffness
Introduction
Stiffness of the elbow after trauma is a well-recognized disabling condition that interferes with daily activities [1]. Loss of motion after elbow injury results from abnormalities of bone, soft tissue, or a combination of both, which may be present intra-articular as well as extra-articular [2, 3]. Improved understanding of the cause of stiffness has led to advances in nonoperative and operative treatment [4•, 5–11]; however, restoration of joint motion in the posttraumatic stiff elbow remains difficult and poses a challenge for surgeons [12]. In this review, we discuss the etiology of posttraumatic elbow stiffness, its classification, evaluation, and management.
Etiology
Posttraumatic stiffness of the elbow is caused by multiple factors, including soft tissue contractures, heterotopic ossification, extra- and intra-articular malunions, nonunions, and loss of articular cartilage.
Soft tissue contracture
Observations in patients with severe elbow stiffness suggested that contractures of soft tissue around the elbow, most especially the capsule, are associated with loss of motion after trauma [13]. Analyses of elbow joint capsules from patients undergoing surgery for elbow contracture have demonstrated capsular thickening [14], disorganization of collagen fiber arrangements [14], altered cytokine and enzyme levels [14, 15], and elevated myofibroblast numbers [16, 17]. Myofibroblasts have contractile and secretory properties that contribute to wound healing and tissue repair but can severely impair organ function if extra-cellular matrix protein secretion and contraction become excessive, such as in Dupuytren disease [18]. However, myofibroblasts seem absent in chronic elbow contractures (more than 5 months), suggesting that its influence is more prominent early after acute trauma [19•]. Animal models, designed to study posttraumatic stiffness and contracture, support the important role of myofibroblasts in the development of posttraumatic elbow stiffness and have identified complex interactions, such as the transforming growth factor-beta signaling pathway, which influence the differentiation and activity of myofibroblasts [20•, 21, 22].
Heterotopic ossification
Elbow stiffness may be secondary to heterotopic ossification [23]. Heterotopic ossification is defined as formation of mature lamellar bone in nonosseous tissue and can be distinguished from other pathologic bone formation, such as myositis ossificans and periarticular calcification [24]. The differentiation of progenitor cells to osteogenic precursor cells, induced by cell-mediated interactions and local microenvironment, leads to the formation of heterotopic ossification [25]. The newly formed ectopic bone restricts elbow motion and upper extremity function by a discrete block to motion. Several factors increase the risk of developing HO around the elbow, including central nervous system injury, burns, surgery (i.e., time to surgery and time to mobilization after surgery), and most commonly direct trauma [26–30].
Extra-articular malunions
Restriction of elbow motion after extra-articular malunions of the distal humerus is explained by its complex geometry. The capitellum and trochlea are translated anteriorly to the humeral diaphysis, which creates an angle between the long axis of the humerus and the distal articular segment. The lateral column follows this translation, whereas the medial column is more in line with the diaphysis. Anterior translation provides space for the coronoid process and anterior arm and forearm musculature during flexion of the elbow [31]. Compromising this relationship in treatment of distal humeral fractures can lead to loss of elbow motion [32]. A straight plate on the lateral column for fracture fixation, for example, may result in loss of anterior translation of the articular segment of the distal humerus. A plate that is precontoured to fit the lateral column helps to restore the original anatomy of the distal humerus and prevent malunion and thereby loss of elbow motion [33, 34]. The relationship between anterior translation of the distal humeral articular surface and elbow flexion after open reduction internal fixation (ORIF) has been established; however, loss of translation cannot explain the total variation in restricted elbow flexion after ORIF of a distal humerus fracture, which therefore seems to be multifactorial [35].
Intra-articular malunions
Malunion after an intra-articular fracture of the distal humerus may lead to loss of elbow motion. Malunited articular surface of the distal humerus distorts its complex articulation but may also lead to periarticular fibrosis and compromised ulnar nerve function [36]. Distortion of the geometric dimensions of the trochlea and its relationship with the greater sigmoid notch of the ulna (i.e., the ulnohumeral joint) impairs the intrinsic stability, normal kinematics, and function of the elbow [37]. Intra-articular malunion of the distal humerus can occur alone or together with nonunion [36, 38]. Malunited radial head fractures typically present with stiffness of the forearm rather than ulnohumeral stiffness or arthrosis of the radiocapitellar or proximal radioulnar joint [39].
Nonunions
Nonunion of the elbow leads commonly to elbow stiffness, which is attributed to articular distortion, intra-articular adhesions, or damage of the articular surface [36, 40]. Several factors predispose to nonunion, including patient and fracture characteristics (comminution, open fractures, high-energy injury, infection, devascularization of fracture fragments, interfragmentary defects, and metabolic or cellular abnormalities) and fracture management (inadequate fixation, interposition of soft tissue, and premature motion) [41]. Nonunions of fractures of the distal part of the humerus may be extra-articular (at the supracondylar level), intra-articular, or both intra-articular and extra-articular. Nonunions at the supracondylar level are most frequently seen [9]. Nonunions of the proximal ulna are most frequently encountered after posterior Monteggia fractures and olecranon fracture-dislocations. Olecranon nonunion may also be the result of inadequate treatment of simple fractures or osteotomy for fracture exposure [42]. Coronoid process nonunion is uncommon [43]. Nonunions of nonoperatively treated isolated radial head or neck fractures are rare [44–46] and typically do not interfere with elbow motion.
Loss of articular cartilage
Arthrosis is a common after elbow trauma and is associated with stiffness of the elbow [47–49]. Its development is attributed to a combination of biomechanical, biochemical, and, most likely, genetic factors [50]. Radiographic signs of elbow arthrosis are usually graded according to the criteria of Broberg and Morrey [51, 52], grade 0, normal joint; grade 1, slight joint-space narrowing with minimum osteophyte formation; grade 2, moderate joint-space narrowing with moderate osteophyte formation; and grade 3, severe degenerative change with gross destruction of the joint. Distal humerus fractures, including columnar, capitellum, and trochlear fractures, as well as elbow fracture-dislocations seem to be associated with moderate or severe radiographic arthrosis in the long term, whereas olecranon and radial head fractures and patient characteristics are not [53].
Classification
Posttraumatic elbow stiffness is most commonly classified based on specific structures involved (soft tissue, osseous, or combined) or anatomic location (intrinsic, extrinsic, or combined). Classification according to the structures involved is described by Kay [3], type 1, soft tissue contracture; type 2, soft tissue contracture with ossification; type 3, undisplaced articular fracture with soft tissue contracture; type 4, displaced intra-articular fracture with soft tissue contracture; and type 5, posttraumatic bony bars. Classification of posttraumatic elbow stiffness into intrinsic, extrinsic, or combined contractures has been purposed by Morrey [2]. Intrinsic contractures involve the articular surface (intra-articular adhesions, intra-articular malunions, or loss of articular cartilage), whereas extrinsic contractures do not (capsular and ligament contractures, heterotopic ossification, extra-articular malunions, and soft-tissue contractures following burns). Most posttraumatic stiff elbows have both intrinsic and extrinsic components (Fig. 1).
Fig. 1.
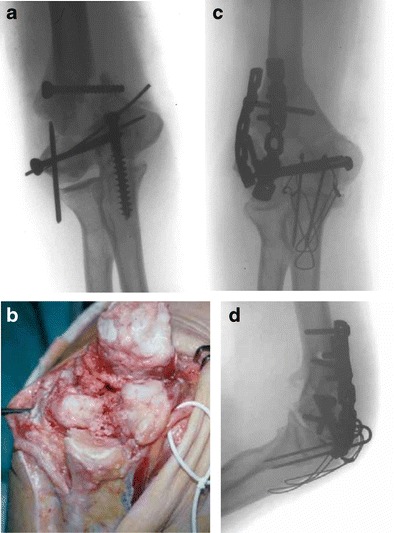
A 49-year-old woman with intra- and extra-articular nonunion of the distal humerus and contracture of soft tissue around the elbow. a Radiograph before revision surgery (anteroposterior view). b Image obtained during revision surgery. c Radiograph after revision surgery (anteroposterior view). d Radiograph after revision surgery (lateral view)
Evaluation
A thorough history of patients with posttraumatic elbow stiffness should address the original injury and initial treatment [12]. In addition, associated conditions (e.g., nervous system disorders, infections, and ipsilateral injuries) need to be recognized [12, 39]. Timing of presentation, character and progression of symptoms, and functional level before injury, which may influence decision making, must be reviewed as well. It is also recommended to discuss patients’ expectations in order to avoid disappointment due to unexpected events and outcomes. Patients might have unrealistic expectations, such as getting a perfect arm after operative treatment, while being able to depend on your arm is more important for good health.
Physical examination includes active and passive flexion-extension and pronation-supination, in which motion at the limits may be abrupt and rigid due to a bony block or compliant in case of soft tissue contracture [24]. Although most patients with a posttraumatic stiff elbow do not experience pain at rest, its presence after operative treatment might indicate a low-grade infection. Pain within the midarc of motion indicates incongruity of the joint or loss of cartilage, whereas pain at the limits of motion suggests impingement between the coronoid or olecranon process and the distal humerus [24, 48, 54]. There should be special attention for the function of the ulnar nerve during neurologic evaluation of the upper extremity, as impaired function of the ulnar nerve could be the result of elbow trauma and may lead to pain at motion [2, 12].
Anteroposterior and lateral radiographs must complement history and physical examination for full assessment [39]. The evaluation of the articular surface requires two separate anteroposterior views, one that is perpendicular to the radius and ulna and a second perpendicular to the humerus. Lateral views may be helpful for the recognition of bony impingement. The addition of computed tomography (CT), especially three-dimensional CT-based reconstructions, might be useful to identify or further characterize loose bodies, impinging osteophytes, and heterotopic ossification [23, 25, 55, 56]. In contrast, magnetic resonance imaging is not considered to be useful as heterotopic ossification and joint congruity is better defined on CT images [24]. Laboratory testing, measurement of inflammatory markers in particular, is helpful to detect infection. Elbow aspiration could be considered in case of abnormal laboratory findings or evident signs of inflammation.
Nonoperative treatment
Treatment may be indicated if loss of elbow motion interferes with activities of daily living. Most activities of daily living can be performed with 100° of elbow flexion (30° to 130°) and 100° of forearm rotation (50° of pronation and 50° of supination) [57]. However, patients may require motion beyond this average functional arc of motion. For patients presenting within 6 months after injury, nonoperative treatment with elbow splinting or manipulation under anesthesia could be considered.
Static progressive and dynamic elbow splinting can be used to regain motion in patients with posttraumatic elbow stiffness. In static progressive splinting, the joint angle stepwise increases to apply a force to contracted tissues that decreases as the tissues stretch, while in dynamic splinting, a consistent force is applied to the tissues that is maintained as the tissues stretch and improvement of motion is achieved. Both static progressive and dynamic elbow splinting help increase range of motion [58–63]. There seems no difference in improvement of flexion arc between static progressive and dynamic elbow splinting methods, and the choice of splinting protocols can be determined based on the preference of the surgeon and patient [6].
Manipulation of the elbow with patients under anesthesia can be attempted in case of radiographic evidence of osseous fracture healing. However, the outcomes of manipulation under anesthesia have only been reported for patients with elbow stiffness following surgery [64, 65]. To our knowledge, its effect has not been demonstrated in patients with elbow stiffness after trauma.
Operative treatment
In case nonoperative treatment fails or is not indicated, operative treatment can be considered. Before offering operative treatment, there must be radiographic evidence of union. In addition, the patient should demonstrate the motivation and ability to complete a challenging and prolonged rehabilitation program.
Open contracture release
Several approaches for open contracture release have been described. The preferred approach depends on the type of elbow contracture (i.e., intrinsic, extrinsic, or combined contractures), need for decompression of the ulnar nerve, location of prior elbow incisions, and location and extent of heterotopic ossification. Overall, complication rates are low [66]. The most common complications include peripheral neuropathy, postoperative infection, and recurrence of stiffness and heterotopic ossification.
The lateral approach [67] (i.e., lateral column procedure) allows arthrotomy, release of the anterior and posterior capsules, and exploration of the lateral side of the joint. However, this approach does not provide adequate exposure to address articular pathology of the medial part of the ulnohumeral joint and decompression of the ulnar nerve requires additional exposure. A curved incision is used in this procedure (i.e., a proximal one half of a Kocher incision), after which the capsule is entered at the radiohumeral joint. The lateral aspect of the capsule is excised, where the medial capsule is incised, and intra-articular adhesions and osteophytes are removed. The triceps is elevated from the distal humerus to allow release of the posterior capsule and debridement of the olecranon fossa. The olecranon tip is excised if necessary. This lateral procedure is associated with high patient satisfaction and improvement in elbow motion [67–73].
The medial approach [74] can be used to address the articular surface of the medial side of the ulnohumeral joint, decompress the ulnar nerve, remove heterotopic ossification, and release of the medial collateral ligament. This approach provides limited exposure to the lateral side of the joint. In this procedure, the incision is made medial along the midline over of the medial epicondyle. After incision, the antebrachial cutaneous nerve is protected. The ulnar nerve is mobilized and transposed anteriorly. Exposure is obtained by elevating the flexor pronator mass off the anterior aspect of the medial epicondyle. Once the anterior aspect of the capsule has been adequately exposed, it can be excised or incised if excision cannot be done safely. The medial aspect of the triceps is elevated to identify and excise the posterior capsule, release the posterior band of the medial collateral ligament, and remove osteophytes and heterotopic bone. Isolated medial approach has few indications, and it is most commonly used in addition to the lateral column procedure with satisfactory results [10, 73, 75–79].
The anterior approach [80] is limited. Neurovascular structures are at risk during this procedure, and additional release is frequently required. The anterior incision is made in a curvilinear S-shape across the antecubital skin crease, which is followed by protection of the neurovascular structures (medial and lateral antebrachial cutaneous nerves, brachial artery, median nerve, radial nerve, and musculocutaneous nerves). The interval between the common flexor origin and biceps tendon is then developed, and the brachialis muscle is dissected from the capsule. The anterior capsule is excised after adequate exposure. This procedure is predominantly indicated for isolated flexion contractures or anterior heterotopic bone [80–82].
The posterior approach [83] should be reserved for extensive releases. A posterior midline incision is used in this procedure. After the medial border of the triceps is released, the extensor mechanism is reflected and the anconeus muscles are released from the ulna. The ulnar nerve is mobilized and the posterior band of the medial collateral ligament is released [84]. As most case series combine the results of different approaches, comparison of results between different procedures is difficult. However, the results of the respective approaches demonstrate durable improvement in elbow motion [10, 75, 76, 79, 85, 86].
With regard to heterotopic ossification, open capsular release can be performed after the removal of heterotopic bone [87]. Complete ankylosis of the elbow due to heterotopic ossification requires a unique approach [8]. This approach is challenging when mature heterotopic bone encases the elbow joint, which makes it difficult to recognize the demarcation of the heterotopic bone and the original bone. For this reason, the articulation may be difficult to identify, especially on the posteromedial part of the ulnohumeral joint. An osteotome is needed to remove the heterotopic bone in layers, which is done with great care to avoid iatrogenic injury. After the heterotopic bone is resected and capsular release has been performed, the elbow is manipulated to maximize elbow motion. In case the elbow tends to subluxate or dislocate after release, hinged external fixation should be considered. Although the majority of patients with complete ankylosis secondary to heterotopic bone show good results after treatment, recurrence of severe contracture is seen in a subset of patients [8, 23].
Arthroscopic contracture release
Arthroscopic capsular release of the elbow allows debridement, synovectomy, removal of adhesions and osteophytes, and capsular release [88–92]. Arthroscopic release is challenging due to the proximity of neurovascular structures and restricted work space [93, 94]. Reported complications include nerve injury, infection, inadequate release, recurrence of stiffness, ectopic bone formation, and persistent drainage [95]. Arthroscopic capsular release is usually only considered for simple elbow contractures [1], which has been defined as elbow contractures with an arc of motion equal to or greater than 80°, no or minimal prior surgery, no prior ulnar nerve transposition, no or minimal internal fixation or hardware in place, no or minimal heterotopic ossification, and normal osseous anatomy [4]. However, with greater experience, more complex contracture releases can be performed. The demanding technique for arthroscopic elbow capsule release developed rapidly from stripping the capsule to capsulotomy and capsulectomy [96], which has shown to be safe and effective in patients with a posttraumatic elbow contracture [97–100].
Interposition arthroplasty
Interposition arthroplasty is indicated if the articular anatomy cannot be restored and reconstruction of the articular surface is necessary [49, 101–104]. It is used in younger patients as an alternative to prosthetic replacement. A posterior skin incision or prior incision is used to obtain a wide surgical exposure. The general concept is to reshape the distal humeral and ulnar articular surface through a recontouring osteotomy in order to create a new congruent joint [105]. The interposition graft is secured to the distal humerus and the collateral ligaments are reconstructed. A hinged elbow external fixator is applied to protect the interposed graft. Complications include neuropathy, discomfort at the donor site, muscle hernia, pin-site infection, and long-term failure [103, 105]. Although the majority of the patients seem content after this procedure, some consider interposition arthroplasty as a salvage procedure as it does not completely relieve pain and restore elbow motion [7, 49, 102]. Failed interposition arthroplasty may be converted to a total elbow arthroplasty.
Total elbow arthroplasty
Total elbow arthroplasty may be considered in less active and older patients if no other treatment options are available [5, 11, 48, 106–108]. The semiconstrained implant is recommended in patients with complete ankylosis and elbow stiffness [27]. Specific surgical techniques depend on the implant used, prior incisions, and the preference of the surgeon. In all cases, the ulnar nerve must be identified, released, or transposed if needed; soft tissues should be aggressively released; and bone needs to be adequately resected for optimal biomechanical conditions [11, 109]. The most recognized complications include periprosthetic fracture, loosening, mechanical failure, infection, triceps disruption, and nerve palsy [110]. Although complications are frequent, careful preoperative planning and enhanced techniques lead to improvement of function and relief of pain in a subset of patients with posttraumatic ankylosed and stiff elbows [5, 11, 48, 106, 111].
Partial elbow arthroplasty
Partial elbow replacement is rarely used for posttraumatic elbow stiffness. It might be considered in patients with loss of cartilage of the radiocapitellar joint and preserved ulnohumeral articulation [112]. Capitellar resurfacing arthroplasty may be used if radial head arthroplasty is indicated and the quality of the capitellar surface is poor. In addition, distal humeral hemiarthroplasty may be considered in case of nonunion or malunion of fractures of the distal humerus [38, 113].
Postoperative management
Postoperative management and rehabilitation programs aim to regain elbow motion, restore muscle strength, and reincorporate the arm into daily activities of living [114] and should be continued until no further improvements are made.
Most surgeons start mobilization within 48 h after open capsular release. Continuous passive motion may improve elbow motion postoperatively [81, 82]; however, its benefit in the postoperative management of elbow contracture release remains subject of discussion [115]. In addition, static progressive or dynamic splints can be used after contracture release to support the recovery of elbow motion [8, 10, 67, 71, 72, 77, 78, 82].
After interposition arthroplasty, local anesthetics are used for 24 to 48 h after surgery to allow continuous passive motion. When the external fixator is removed after 4 to 8 weeks, the elbow is examined and gently manipulated under anesthesia to determine the firmness of the endpoints, elbow joint stability, and expected elbow flexion arc. Then, progressive static splints may be used to support the rehabilitation [105].
The postoperative management after total elbow arthroplasty depends on several factors, such as the implant type, status of the ulnar nerve and triceps, and overall joint stability [116]. Splinting to regain motion is seldom indicated. When the ulnar nerve is in anatomical position, flexion could lead to compression of the nerve, which should be avoided. And in case the triceps is reflected in surgery, gravity-assisted extension is indicated for at least 4 weeks. Return to full activity with permanent restrictions can be recommended after 12 weeks [5, 108].
Conclusions
Although there have been some advances in the understanding of the pathogenesis of posttraumatic elbow contractures, the overall evidence is limited and sometimes contradictory. An improved understanding of the pathologic basis of the causes underlying posttraumatic elbow stiffness may help the development of new prevention strategies. However, current nonoperative and operative treatment regimens are considered safe and effective in patients with a posttraumatic stiff elbow, but the level of evidence of current treatment and rehabilitation programs is low. Future research will need to compare the respective treatment options (e.g., open versus arthroscopic capsular release) prospectively.
Compliance with ethical standards
Conflict of interest
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, and patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Human studies done by authors (but no animal studies)
This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Animal studies done by authors (but no human studies)
This article does not contain any studies with human subjects performed by any of the authors, with regard to the authors’ research cited in this paper and all institutional and national guidelines.
Footnotes
This article is part of the Topical Collection on Elbow Soft Tissue Surgery
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Jupiter JB, O’Driscoll SW, Cohen MS. The assessment and management of the stiff elbow. Instr Course Lect. 2003;52:93–111. [PubMed] [Google Scholar]
- 2.Morrey BF. The posttraumatic stiff elbow. Clin Orthop Relat Res. 2005;431:26–35. [PubMed] [Google Scholar]
- 3.Kay NR. Arthrolysis of the post-traumatic stiff elbow. In: Stanley D, Kay NR, editors. Surgery of the elbow. London: Arnold; 1998. pp. 228–34. [Google Scholar]
- 4.•.Blonna D, Wolf JM, Fitzsimmons JS, O’Driscoll SW. Prevention of nerve injury during arthroscopic capsulectomy of the elbow utilizing a safety-driven strategy. J Bone Joint Surg Am. 2013;95(15):1373–81. doi: 10.2106/JBJS.K.00972. [DOI] [PubMed] [Google Scholar]
- 5.Peden JP, Morrey BF. Total elbow replacement for the management of the ankylosed or fused elbow. J Bone Joint Surg Br. 2008;90(9):1198–204. doi: 10.1302/0301-620X.90B9.19967. [DOI] [PubMed] [Google Scholar]
- 6.Lindenhovius AL, Doornberg JN, Brouwer KM, Jupiter JB, Mudgal CS, Ring D. A prospective randomized controlled trial of dynamic versus static progressive elbow splinting for posttraumatic elbow stiffness. J Bone Joint Surg Am. 2012;94(8):694–700. doi: 10.2106/JBJS.J.01761. [DOI] [PubMed] [Google Scholar]
- 7.Larson AN, Morrey BF. Interposition arthroplasty with an Achilles tendon allograft as a salvage procedure for the elbow. J Bone Joint Surg Am. 2008;90(12):2714–23. doi: 10.2106/JBJS.G.00768. [DOI] [PubMed] [Google Scholar]
- 8.Ring D, Jupiter JB. Operative release of ankylosis of the elbow due to heterotopic ossification. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:2–10. doi: 10.2106/00004623-200403001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Jupiter JB. The management of nonunion and malunion of the distal humerus—a 30-year experience. J Orthop Trauma. 2008;22(10):742–50. doi: 10.1097/BOT.0b013e318188d634. [DOI] [PubMed] [Google Scholar]
- 10.Ring D, Adey L, Zurakowski D, Jupiter JB. Elbow capsulectomy for posttraumatic elbow stiffness. J Hand Surg. 2006;31(8):1264–71. doi: 10.1016/j.jhsa.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg Am. 2000;82(9):1260–8. doi: 10.2106/00004623-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.O’Driscoll SW. Clinical assessment and open and arthroscopic surgical treatment of the stiff elbow. In: Jupiter JB, editor. The stiff elbow. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. pp. 9–19. [Google Scholar]
- 13.Morrey BF. Surgical treatment of extraarticular elbow contracture. Clin Orthop Relat Res. 2000;370:57–64. doi: 10.1097/00003086-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Schimmel DR, Masuda K, Hastings H, 2nd, Muehleman C. Structural and biochemical evaluation of the elbow capsule after trauma. J Shoulder Elbow Surg. 2007;16(4):484–90. doi: 10.1016/j.jse.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Relat Res. 2005;439:228–34. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germscheid NM, Hildebrand KA. Regional variation is present in elbow capsules after injury. Clin Orthop Relat Res. 2006;450:219–24. doi: 10.1097/01.blo.0000194681.94882.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebrand KA, Zhang M, van Snellenberg W, King GJ, Hart DA. Myofibroblast numbers are elevated in human elbow capsules after trauma. Clin Orthop Relat Res. 2004;419:189–97. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•.Doornberg JN, Bosse T, Cohen MS, Jupiter JB, Ring D, Kloen P. Temporary presence of myofibroblasts in human elbow capsule after trauma. J Bone Joint Surg Am. 2014;96(5):e36. doi: 10.2106/JBJS.M.00388. [DOI] [PubMed] [Google Scholar]
- 20.•.Lake SP, Castile RM, Borinsky S, Dunham CL, Havlioglu N, Galatz LM. Development and use of an animal model to study post-traumatic stiffness and contracture of the elbow. J Orthop Res. 2016;34(2):354–64. This article describes the development and use of an animal model to study post-traumatic stiffness and contracture of the elbow. The authors claim their animal model is the first capable of examining challenges unique to the anatomically and biomechanically complex elbow joint. [DOI] [PubMed]
- 21.Hildebrand KA, Zhang M, Salo PT, Hart DA. Joint capsule mast cells and neuropeptides are increased within four weeks of injury and remain elevated in chronic stages of posttraumatic contractures. J Orthop Res. 2008;26(10):1313–9. doi: 10.1002/jor.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildebrand KA, Zhang M, Hart DA. Myofibroblast upregulators are elevated in joint capsules in posttraumatic contractures. Clin Orthop Relat Res. 2007;456:85–91. doi: 10.1097/BLO.0b013e3180312c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ring D, Jupiter JB. Operative release of complete ankylosis of the elbow due to heterotopic bone in patients without severe injury of the central nervous system. J Bone Joint Surg Am. 2003;85-A(5):849–57. doi: 10.2106/00004623-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Viola RW, Hastings H, 2nd. Treatment of ectopic ossification about the elbow. Clin Orthop Relat Res. 2000(370):65-86. [DOI] [PubMed]
- 25.Ranganathan K, Loder S, Agarwal S, Wong VC, Forsberg J, Davis TA, et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2015;97(13):1101–11. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams GD, Bellino MJ, Cheung EV. Risk factors for development of heterotopic ossification of the elbow after fracture fixation. J Shoulder Elbow Surg. 2012;21(11):1550–4. doi: 10.1016/j.jse.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 27.Bauer AS, Lawson BK, Bliss RL, Dyer GS. Risk factors for posttraumatic heterotopic ossification of the elbow: case-control study. J Hand Surg. 2012;37(7):1422–9 e1-6. doi: 10.1016/j.jhsa.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Douglas K, Cannada LK, Archer KR, Dean DB, Lee S, Obremskey W. Incidence and risk factors of heterotopic ossification following major elbow trauma. Orthopedics. 2012;35(6):e815–22. doi: 10.3928/01477447-20120525-18. [DOI] [PubMed] [Google Scholar]
- 29.Hong CC, Nashi N, Hey HW, Chee YH, Murphy D. Clinically relevant heterotopic ossification after elbow fracture surgery: a risk factors study. Orthop Traumatol Surg Res. 2015;101(2):209–13. doi: 10.1016/j.otsr.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Wiggers JK, Helmerhorst GT, Brouwer KM, Niekel MC, Nunez F, Ring D. Injury complexity factors predict heterotopic ossification restricting motion after elbow trauma. Clin Orthop Relat Res. 2014;472(7):2162–7. doi: 10.1007/s11999-013-3304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapanji IA. The physiology of the joints. Paris: Churchill Livingstone; 1982. [Google Scholar]
- 32.Ilyas A, Rehman S. Distal humerus fractures. In: Ilyas A, Rehman S, editors. Contemporary surgical management of fractures and complications. 1. New Delhi, India: Jaypee Brothers Medical Publishers; 2013. pp. 249–75. [Google Scholar]
- 33.Schemitsch EH, Tencer AF, Henley MB. Biomechanical evaluation of methods of internal fixation of the distal humerus. J Orthop Trauma. 1994;8(6):468–75. doi: 10.1097/00005131-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Yang KH, Park HW, Park SJ, Jung SH. Lateral J-plate fixation in comminuted intercondylar fracture of the humerus. Arch Orthop Trauma Surg. 2003;123(5):234–8. doi: 10.1007/s00402-003-0508-x. [DOI] [PubMed] [Google Scholar]
- 35.Brouwer KM, Lindenhovius AL, Ring D. Loss of anterior translation of the distal humeral articular surface is associated with decreased elbow flexion. J Hand Surg. 2009;34(7):1256–60. doi: 10.1016/j.jhsa.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 36.McKee M, Jupiter J, Toh CL, Wilson L, Colton C, Karras KK. Reconstruction after malunion and nonunion of intra-articular fractures of the distal humerus. Methods and results in 13 adults. J Bone Joint Surg Br. 1994;76(4):614–21. [PubMed] [Google Scholar]
- 37.An K, Zobitz ME, Morrey BF. Biomechanics of the elbow. In: Morrey BF, Sanchez-Sotelo J, editors. The elbow and its disorders. Philadelphia, PA: WB Saunders; 1993. pp. 39–66. [Google Scholar]
- 38.Lechasseur B, Laflamme M, Leclerc A, Bedard AM. Incipient malunion of an isolated humeral trochlea fracture treated with an elbow hemiarthroplasty: case report. J Hand Surg. 2015;40(2):271–5. doi: 10.1016/j.jhsa.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 39.Morrey BF. The stiff elbow with articular involvement. In: Jupiter JB, editor. The stiff elbow. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. pp. 21–30. [Google Scholar]
- 40.Schatzker J. Intraarticular malunions and nonunions. Orthop Clin North Am. 1990;21(4):743–57. [PubMed] [Google Scholar]
- 41.Gallay SH, McKee MD. Operative treatment of nonunions about the elbow. Clin Orthop Relat Res. 2000;370:87–101. doi: 10.1097/00003086-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Papagelopoulos PJ, Morrey BF. Treatment of nonunion of olecranon fractures. J Bone Joint Surg Br. 1994;76(4):627–35. [PubMed] [Google Scholar]
- 43.Liu SH, Henry M, Bowen R. Complications of type I coronoid fractures in competitive athletes: report of two cases and review of the literature. J Shoulder Elbow Surg. 1996;5(3):223–7. doi: 10.1016/S1058-2746(05)80011-4. [DOI] [PubMed] [Google Scholar]
- 44.Cobb TK, Beckenbaugh RD. Nonunion of the radial neck following fracture of the radial head and neck: case reports and a review of the literature. Orthopedics. 1998;21(3):364–8. doi: 10.3928/0147-7447-19980301-23. [DOI] [PubMed] [Google Scholar]
- 45.Faber FW, Verhaar JA. Nonunion of radial neck fracture. An unusual differential diagnosis of tennis elbow, a case report. Acta Orthop Scand. 1995;66(2):176. doi: 10.3109/17453679508995517. [DOI] [PubMed] [Google Scholar]
- 46.Horne G, Sim P. Nonunion of the radial head. J Trauma. 1985;25(5):452–3. doi: 10.1097/00005373-198505000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Lindenhovius AL, Buijze GA, Kloen P, Ring DC. Correspondence between perceived disability and objective physical impairment after elbow trauma. J Bone Joint Surg Am. 2008;90(10):2090–7. doi: 10.2106/JBJS.G.00793. [DOI] [PubMed] [Google Scholar]
- 48.Schneeberger AG, Adams R, Morrey BF. Semiconstrained total elbow replacement for the treatment of post-traumatic osteoarthrosis. J Bone Joint Surg Am. 1997;79(8):1211–22. doi: 10.2106/00004623-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Morrey BF. Post-traumatic contracture of the elbow. Operative treatment, including distraction arthroplasty. J Bone Joint Surg Am. 1990;72(4):601–18. [PubMed] [Google Scholar]
- 50.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20(10):719–25. doi: 10.1097/01.bot.0000211160.05864.14. [DOI] [PubMed] [Google Scholar]
- 51.Lindenhovius A, Karanicolas PJ, Bhandari M, Ring D. Radiographic arthrosis after elbow trauma: interobserver reliability. J Hand Surg. 2012;37(4):755–9. doi: 10.1016/j.jhsa.2011.12.043. [DOI] [PubMed] [Google Scholar]
- 52.Broberg MA, Morrey BF. Results of treatment of fracture-dislocations of the elbow. Clin Orthop Relat Res. 1987;216:109–19. [PubMed] [Google Scholar]
- 53.Guitton TG, Zurakowski D, van Dijk NC, Ring D. Incidence and risk factors for the development of radiographic arthrosis after traumatic elbow injuries. J Hand Surg. 2010;35(12):1976–80. doi: 10.1016/j.jhsa.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Chammas M. Post-traumatic osteoarthritis of the elbow. Orthop Traumatol Surg Res. 2014;100(1 Suppl):S15–24. doi: 10.1016/j.otsr.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Libicher M, Freyschmidt J. Radiological diagnosis in contracted elbow joint. Value of CT and MRI. Orthopade. 2001;30(9):593–601. doi: 10.1007/s001320170046. [DOI] [PubMed] [Google Scholar]
- 56.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. J Bone Joint Surg Br. 2004;86(3):396–403. doi: 10.1302/0301-620X.86B3.14480. [DOI] [PubMed] [Google Scholar]
- 57.Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63(6):872–7. [PubMed] [Google Scholar]
- 58.Green DP, McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J Bone Joint Surg Am. 1979;61(7):1092–5. [PubMed] [Google Scholar]
- 59.Doornberg JN, Ring D, Jupiter JB. Static progressive splinting for posttraumatic elbow stiffness. J Orthop Trauma. 2006;20(6):400–4. doi: 10.1097/00005131-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Gelinas JJ, Faber KJ, Patterson SD, King GJ. The effectiveness of turnbuckle splinting for elbow contractures. J Bone Joint Surg Br. 2000;82(1):74–8. doi: 10.1302/0301-620X.82B1.9792. [DOI] [PubMed] [Google Scholar]
- 61.Bonutti PM, Windau JE, Ables BA, Miller BG. Static progressive stretch to reestablish elbow range of motion. Clin Orthop Relat Res. 1994;303:128–34. [PubMed] [Google Scholar]
- 62.Dickson RA. Reversed dynamic slings. A new concept in the treatment of post-traumatic elbow flexion contractures. Injury. 1976;8(1):35–8. doi: 10.1016/0020-1383(76)90007-3. [DOI] [PubMed] [Google Scholar]
- 63.Shewring DJ, Beaudet M, Carvell JE. Reversed dynamic slings: results of use in the treatment of post-traumatic flexion contractures of the elbow. Injury. 1991;22(5):400–2. doi: 10.1016/0020-1383(91)90105-N. [DOI] [PubMed] [Google Scholar]
- 64.Araghi A, Celli A, Adams R, Morrey B. The outcome of examination (manipulation) under anesthesia on the stiff elbow after surgical contracture release. J Shoulder Elbow Surg. 2010;19(2):202–8. doi: 10.1016/j.jse.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 65.Duke JB, Tessler RH, Dell PC. Manipulation of the stiff elbow with patient under anesthesia. J Hand Surg. 1991;16(1):19–24. doi: 10.1016/S0363-5023(10)80005-X. [DOI] [PubMed] [Google Scholar]
- 66.Kodde IF, van Rijn J, van den Bekerom MP, Eygendaal D. Surgical treatment of post-traumatic elbow stiffness: a systematic review. J Shoulder Elbow Surg. 2013;22(4):574–80. doi: 10.1016/j.jse.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Mansat P, Morrey BF. The column procedure: a limited lateral approach for extrinsic contracture of the elbow. J Bone Joint Surg Am. 1998;80(11):1603–15. [PubMed] [Google Scholar]
- 68.Boerboom AL, de Meyier HE, Verburg AD, Verhaar JA. Arthrolysis for post-traumatic stiffness of the elbow. Int Orthop. 1993;17(6):346–9. doi: 10.1007/BF00180451. [DOI] [PubMed] [Google Scholar]
- 69.Cohen MS, Hastings H., 2nd Post-traumatic contracture of the elbow. Operative release using a lateral collateral ligament sparing approach. J Bone Joint Surg Br. 1998;80(5):805–12. doi: 10.1302/0301-620X.80B5.8528. [DOI] [PubMed] [Google Scholar]
- 70.Husband JB, Hastings H., 2nd The lateral approach for operative release of post-traumatic contracture of the elbow. J Bone Joint Surg Am. 1990;72(9):1353–8. [PubMed] [Google Scholar]
- 71.Kraushaar BS, Nirschl RP, Cox W. A modified lateral approach for release of posttraumatic elbow flexion contracture. J Shoulder Elbow Surg. 1999;8(5):476–80. doi: 10.1016/S1058-2746(99)90080-0. [DOI] [PubMed] [Google Scholar]
- 72.Gundlach U, Eygendaal D. Surgical treatment of posttraumatic stiffness of the elbow: 2-year outcome in 21 patients after a column procedure. Acta Orthop. 2008;79(1):74–7. doi: 10.1080/17453670710014798. [DOI] [PubMed] [Google Scholar]
- 73.Marti RK, Kerkhoffs GM, Maas M, Blankevoort L. Progressive surgical release of a posttraumatic stiff elbow. Technique and outcome after 2–18 years in 46 patients. Acta Orthop Scand. 2002;73(2):144–50. doi: 10.1080/000164702753671713. [DOI] [PubMed] [Google Scholar]
- 74.Mezera K, Hotchkiss RN. Fractures and dislocations of the elbow. In: Bucholz RW, Heckman JD, editors. Rockwood and Green’s fractures in adults. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 921–52. [Google Scholar]
- 75.Higgs ZC, Danks BA, Sibinski M, Rymaszewski LA. Outcomes of open arthrolysis of the elbow without post-operative passive stretching. J Bone Joint Surg Br. 2012;94(3):348–52. doi: 10.1302/0301-620X.94B3.27278. [DOI] [PubMed] [Google Scholar]
- 76.Sharma S, Rymaszewski LA. Open arthrolysis for post-traumatic stiffness of the elbow: results are durable over the medium term. J Bone Joint Surg Br. 2007;89(6):778–81. doi: 10.1302/0301-620X.89B6.18772. [DOI] [PubMed] [Google Scholar]
- 77.Tosun B, Gundes H, Buluc L, Sarlak AY. The use of combined lateral and medial releases in the treatment of post-traumatic contracture of the elbow. Int Orthop. 2007;31(5):635–8. doi: 10.1007/s00264-006-0252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan V, Daluiski A, Simic P, Hotchkiss RN. Outcome of open release for post-traumatic elbow stiffness. J Trauma. 2006;61(3):673–8. doi: 10.1097/01.ta.0000196000.96056.51. [DOI] [PubMed] [Google Scholar]
- 79.Amillo S. Arthrolysis in the relief of post-traumatic stiffness of the elbow. Int Orthop. 1992;16(2):188–90. doi: 10.1007/BF00180215. [DOI] [PubMed] [Google Scholar]
- 80.Urbaniak JR, Hansen PE, Beissinger SF, Aitken MS. Correction of post-traumatic flexion contracture of the elbow by anterior capsulotomy. J Bone Joint Surg Am. 1985;67(8):1160–4. [PubMed] [Google Scholar]
- 81.Gates HS, 3rd, Sullivan FL, Urbaniak JR. Anterior capsulotomy and continuous passive motion in the treatment of post-traumatic flexion contracture of the elbow. A prospective study. J Bone Joint Surg Am. 1992;74(8):1229–34. [PubMed] [Google Scholar]
- 82.Aldridge JM, 3rd, Atkins TA, Gunneson EE, Urbaniak JR. Anterior release of the elbow for extension loss. J Bone Joint Surg Am. 2004;86-A(9):1955–60. doi: 10.2106/00004623-200409000-00014. [DOI] [PubMed] [Google Scholar]
- 83.Bryan RS, Morrey BF. Extensive posterior exposure of the elbow. A triceps-sparing approach. Clin Orthop Relat Res. 1982(166):188-92. [PubMed]
- 84.Charalambous CP, Morrey BF. Posttraumatic elbow stiffness. J Bone Joint Surg Am. 2012;94(15):1428–37. doi: 10.2106/JBJS.K.00711. [DOI] [PubMed] [Google Scholar]
- 85.Park MJ, Chang MJ, Lee YB, Kang HJ. Surgical release for posttraumatic loss of elbow flexion. J Bone Joint Surg Am. 2010;92(16):2692–9. doi: 10.2106/JBJS.I.01367. [DOI] [PubMed] [Google Scholar]
- 86.Park MJ, Kim HG, Lee JY. Surgical treatment of post-traumatic stiffness of the elbow. J Bone Joint Surg Br. 2004;86(8):1158–62. doi: 10.1302/0301-620X.86B8.14962. [DOI] [PubMed] [Google Scholar]
- 87.Cohen MS. Heterotopic ossification of the elbow. In: Jupiter JB, editor. The stiff elbow. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. pp. 31–40. [Google Scholar]
- 88.Kamineni S, Savoie FH, 3rd, ElAttrache N. Endoscopic extracapsular capsulectomy of the elbow: a neurovascularly safe technique for high-grade contractures. Arthroscopy. 2007;23(7):789–92. doi: 10.1016/j.arthro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Kelly EW, Bryce R, Coghlan J, Bell S. Arthroscopic debridement without radial head excision of the osteoarthritic elbow. Arthroscopy. 2007;23(2):151–6. doi: 10.1016/j.arthro.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 90.Kim SJ, Kim HK, Lee JW. Arthroscopy for limitation of motion of the elbow. Arthroscopy. 1995;11(6):680–3. doi: 10.1016/0749-8063(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 91.Lapner PC, Leith JM, Regan WD. Arthroscopic debridement of the elbow for arthrofibrosis resulting from nondisplaced fracture of the radial head. Arthroscopy. 2005;21(12):1492. doi: 10.1016/j.arthro.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen D, Proper SI, MacDermid JC, King GJ, Faber KJ. Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 2006;22(8):842–9. doi: 10.1016/j.arthro.2006.04.100. [DOI] [PubMed] [Google Scholar]
- 93.Lynch GJ, Meyers JF, Whipple TL, Caspari RB. Neurovascular anatomy and elbow arthroscopy: inherent risks. Arthroscopy. 1986;2(3):190–7. doi: 10.1016/S0749-8063(86)80067-6. [DOI] [PubMed] [Google Scholar]
- 94.Gallay SH, Richards RR, O’Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthroscopy. 1993;9(1):9–13. doi: 10.1016/S0749-8063(05)80336-6. [DOI] [PubMed] [Google Scholar]
- 95.Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25–34. doi: 10.2106/00004623-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 96.Driscoll SW. Arthroscopic osteocapsular arthroplasty. In: Yamaguchi K, King G, McKee M, O’Driscoll S, editors. Advanced reconstruction elbow. 1s. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007. [Google Scholar]
- 97.Salini V, Palmieri D, Colucci C, Croce G, Castellani ML, Orso CA. Arthroscopic treatment of post-traumatic elbow stiffness. J Sports Med Phys Fitness. 2006;46(1):99–103. [PubMed] [Google Scholar]
- 98.Wu X, Wang H, Meng C, Yang S, Duan D, Xu W, et al. Outcomes of arthroscopic arthrolysis for the post-traumatic elbow stiffness. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2715–20. doi: 10.1007/s00167-014-3032-3. [DOI] [PubMed] [Google Scholar]
- 99.Nowicki KD, Shall LM. Arthroscopic release of a posttraumatic flexion contracture in the elbow: a case report and review of the literature. Arthroscopy. 1992;8(4):544–7. doi: 10.1016/0749-8063(92)90024-6. [DOI] [PubMed] [Google Scholar]
- 100.Cefo I, Eygendaal D. Arthroscopic arthrolysis for posttraumatic elbow stiffness. J Shoulder Elbow Surg. 2011;20(3):434–9. doi: 10.1016/j.jse.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 101.Regan WD, Reilly CD. Distraction arthroplasty of the elbow. Hand Clin. 1993;9(4):719–28. [PubMed] [Google Scholar]
- 102.Morrey BF. Posttraumatic stiffness: distraction arthroplasty. Orthopedics. 1992;15(7):863–9. doi: 10.3928/0147-7447-19920701-12. [DOI] [PubMed] [Google Scholar]
- 103.Wright PE, Froimson AI, Stewart MJ. Interposition arthroplasty of the elbow. In: Morrey BF, editor. The elbow and its disorders. Philadelphia, PA: W.B. Saunders; 1993. pp. 611–22. [Google Scholar]
- 104.Hausman MR, Birnbaum PS. Interposition elbow arthroplasty. Tech Hand Up Extrem Surg. 2004;8(3):181–8. doi: 10.1097/01.bth.0000137215.29223.9f. [DOI] [PubMed] [Google Scholar]
- 105.Cheng SL, Morrey BF. Treatment of the mobile, painful arthritic elbow by distraction interposition arthroplasty. J Bone Joint Surg Br. 2000;82(2):233–8. doi: 10.1302/0301-620X.82B2.9507. [DOI] [PubMed] [Google Scholar]
- 106.Figgie MP, Inglis AE, Mow CS, Figgie HE., 3rd Total elbow arthroplasty for complete ankylosis of the elbow. J Bone Joint Surg Am. 1989;71(4):513–20. [PubMed] [Google Scholar]
- 107.Aldridge JM, 3rd, Lightdale NR, Mallon WJ, Coonrad RW. Total elbow arthroplasty with the Coonrad/Coonrad-Morrey prosthesis. A 10- to 31-year survival analysis. J Bone Joint Surg Br. 2006;88(4):509–14. doi: 10.1302/0301-620X.88B4.17095. [DOI] [PubMed] [Google Scholar]
- 108.Gramstad GD, King GJ, O’Driscoll SW, Yamaguchi K. Elbow arthroplasty using a convertible implant. Tech Hand Up Extrem Surg. 2005;9(3):153–63. doi: 10.1097/01.bth.0000181293.38237.f0. [DOI] [PubMed] [Google Scholar]
- 109.Moro JK, King GJ. Total elbow arthroplasty in the treatment of posttraumatic conditions of the elbow. Clin Orthop Relat Res. 2000;370:102–14. doi: 10.1097/00003086-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 110.Kim JM, Mudgal CS, Konopka JF, Jupiter JB. Complications of total elbow arthroplasty. J Am Acad Orthop Surg. 2011;19(6):328–39. doi: 10.5435/00124635-201106000-00003. [DOI] [PubMed] [Google Scholar]
- 111.Morrey BF, Adams RA, Bryan RS. Total replacement for post-traumatic arthritis of the elbow. J Bone Joint Surg Br. 1991;73(4):607–12. doi: 10.1302/0301-620X.73B4.2071644. [DOI] [PubMed] [Google Scholar]
- 112.Heijink A, Morrey BF, Cooney WP., 3rd Radiocapitellar hemiarthroplasty for radiocapitellar arthritis: a report of three cases. J Shoulder Elbow Surg. 2008;17(2):e12–5. doi: 10.1016/j.jse.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Hohman DW, Nodzo SR, Qvick LM, Duquin TR, Paterson PP. Hemiarthroplasty of the distal humerus for acute and chronic complex intra-articular injuries. J Shoulder Elbow Surg. 2014;23(2):265–72. doi: 10.1016/j.jse.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Modabber MR, Jupiter JB. Reconstruction for post-traumatic conditions of the elbow joint. J Bone Joint Surg Am. 1995;77(9):1431–46. doi: 10.2106/00004623-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 115.Lindenhovius AL, van de Luijtgaarden K, Ring D, Jupiter J. Open elbow contracture release: postoperative management with and without continuous passive motion. J Hand Surg. 2009;34(5):858–65. doi: 10.1016/j.jhsa.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Szekeres M, King GJ. Total elbow arthroplasty. J Hand Ther. 2006;19(2):245–53. doi: 10.1197/j.jht.2006.02.010. [DOI] [PubMed] [Google Scholar]


