Abstract
AIM: To investigate the molecular or cellular mechanisms related to the infection of epithelial colonic mucosa by pks-positive Escherichia coli (E. coli) using optical imaging.
METHODS: We choose to evaluate the tumor metabolic activity using a fluorodeoxyglucose analogue as 2-deoxyglucosone fluorescent probes and to correlate it with tumoral volume (mm3). Inflammation measuring myeloperoxidase (MPO) activity and reactive oxygen species production was monitored by a bioluminescent (BLI) inflammation probe and related to histological examination and MPO levels by enzyme-linked immunosorbent assay (ELISA) on tumor specimens. The detection and quantitation of these two signals were validated on a xenograft model of human colon adenocarcinoma epithelial cells (HCT116) in nude mice infected with a pks-positive E. coli. The inflammatory BLI signal was validated intra-digestively in the colitis-CEABAC10 DSS models, which mimicked Crohn’s disease.
RESULTS: Using a 2-deoxyglucosone fluorescent probe, we observed a high and specific HCT116 tumor uptake in correlation with tumoral volume (P = 0.0036). Using the inflammation probe targeting MPO, we detected a rapid systemic elimination and a significant increase of the BLI signal in the pks-positive E. coli-infected HCT116 xenograft group (P < 0.005). ELISA confirmed that MPO levels were significantly higher (1556 ± 313.6 vs 234.6 ± 121.6 ng/mL P = 0.001) in xenografts infected with the pathogenic E. coli strain. Moreover, histological examination of tumor samples confirmed massive infiltration of pks-positive E. coli-infected HCT116 tumors by inflammatory cells compared to the uninfected group. These data showed that infection with the pathogenic E. coli strain enhanced inflammation and ROS production in tumors before tumor growth. Moreover, we demonstrated that the intra-digestive monitoring of inflammation is feasible in a reference colitis murine model (CEABAC10/DSS).
CONCLUSION: Using BLI and fluorescence optical imaging, we provided tools to better understand host-pathogen interactions at the early stage of disease, such as inflammatory bowel disease and colorectal cancer.
Keywords: Colorectal carcinoma, Escherichia coli, Colibactin, Myeloperoxidase, In vivo optical imaging
Core tip: Approximately 15% of cancers are related to infectious agents. Colorectal cancer (CRC) is thus a complex association of non-neoplastic and tumoral cells and a large amount of microorganisms. Recent studies reported that pks-positive Escherichia coli (E. coli) strains are more frequently detected in CRC, suggesting their possible role in tumor development. Optical imaging has emerged as a powerful tool in translational cancer research, providing new possibilities for the spatiotemporal monitoring of carcinogenesis in mouse models. It may be particularly helpful in better understanding the in vivo host-pathogen-interactions in tumor development. This is the first study to use optical imaging to explore CRC carcinogenesis and associated pathogenic E. coli.
INTRODUCTION
Colorectal cancer (CRC) is the third most frequently diagnosed cancer worldwide[1]. Despite recent advances in therapeutic care, CRC remains the second cause of cancer-related death after lung neoplasia and is responsible for over 600000 deaths annually[1,2]. It is a multifactorial disease, strongly associated with genetic and environmental factors that favor tumor development[3]. Approximately 15% of cancers can be related to infectious agents[4,5], such as Helicobacter pylori (H. pylori) and gastric cancer[6]. Colorectal cancer thus involves a complex association of non-neoplastic and tumoral cells and a large amount of microorganisms. Gut microbiota, a bacterial community of over 100 trillion microbial cells, plays a major role in colorectal carcinogenesis. Indeed, high bacterial density in the colon (1012 commensal bacteria/g of intestinal contents) compared to the small intestine (102 commensal bacteria/g of intestinal contents) is correlated with a higher risk of cancer development[7]. Gut microbiota dysbiosis has recently been linked to CRC[8-13], and several bacteria are involved in colorectal carcinogenesis, such as Streptococcus bovis[14,15], Enterococcus spp.[16], H. pylori[17-19], Bacteroides fragilis[20,21], Clostridium septicum[22], Fusobacterium spp.[23,24] and Escherichia coli (E. coli)[25,26].
E. coli is a commensal bacteria of the human gut microbiota that plays a major role in maintaining intestinal homeostasis[27]. Some strains became pathogenic, carrying virulence factors and producing toxins, such as cyclomodulins. These toxins can affect differentiation, apoptosis, and cell proliferation by interfering with the eukaryotic cell cycle and/or inducing DNA damage. Particularly, one of these toxins, the colibactin, is encoded by the pks genomic island and can lead to the creation of double-strand DNA breaks and thus induce the chromosomal instability involved in CRC[28,29]. Recent studies reported that pks-positive E. coli is more frequently detected in CRC patients, suggesting a possible role in tumor development[30-32]. Various independent studies showed that pks-positive-E. coli exhibit procarcinogenic properties in murine models, such as the multiple intestinal neoplasia (Min) mice model[33], azoxymethane (AOM)-treated Il10-/- mice[34] and AOM/DSS models[26]. Thereby, some pathogenic E. coli strains involved in colon carcinogenesis are now emerging. Nevertheless, mechanisms of action remain to be clarified, particularly in in vivo models.
The aim of the present study was to investigate in vivo the molecular or cellular mechanisms related to the infection of epithelial mucosa by pks-positive E. coli using 2D optical imaging. Indeed, optical imaging is emerging as a new powerful sensitive technology for the non-invasive spatiotemporal visualization of carcinogenesis in mice models, and it may help to better understand the host-pathogen interactions in colorectal tumor development[35-37]. Because chronic inflammation and reactive oxygen species (ROS) production are key factors in bacteria and CRC interactions, we chose to evaluate pks-positive E. coli infection on these mechanisms using commercial, available and validated probes[31,38-40]. Indeed, inflammation could play a key role in the development of dysbiosis related to CRC[40]. E. coli is also the most characterized bacteria associated with inflammatory bowel disease, which is a known risk factor for CRC[41,42]. Moreover, Raisch et al[38] demonstrated that E. coli in colon cancer induces a significant increase in COX-2 expression in macrophages, the predominant type of immune cell that infiltrates tumors. Moreover, macrophages and other immune cells infiltrate the tumors, release myeloperoxidase (MPO), and produce ROS by several chemical reactions. Arthur et al[31,39] investigated in vivo the complex interplay between inflammation, bacteria and carcinogenesis and suggested that chronic inflammation is essential for tumor development by maintaining the expression of pks island genes. ROS production has also been reported in many suspected mechanisms related to CRC development. Neutrophils and macrophages, which are present in inflamed tissues such as colon tumors, are major providers of ROS. Maddocks et al[40,43] described a possible interaction between E. coli and the DNA repair system with elevated ROS levels. Because ROS oxidizes the luminescent probe and thus produces proportional light that is detectable in vivo with an optical imager[44,45], we choose to monitor the inflammatory pathway and ROS production using on a bioluminescent (BLI) approach. The monitoring of inflammation was first performed and validated on a colitis murine model (CEABAC10/DSS mice). Then, by this approach, we showed, on a xenograft murine model, that pks-positive-E. coli significantly induces oxidative stress and inflammation before stimulating HCT116-tumor growth. While monitoring longitudinal inflammation, we choose to assess tumor growth by determining tumor metabolic activity with a fluorescent tool based on the fluorodeoxyglucose analogue 2-deoxyglucosone.
MATERIALS AND METHODS
Animal models
Studies were performed in accordance with the French Regional Ethical Animal Use Committee (No. CEEA-02). All mouse models were housed in specific pathogen-free conditions (22 ± 2 °C, 50% humidity, 12 h light/12 h dark) in the animal care facility of the Université d’Auvergne, Clermont-Ferrand, France.
HCT116 xenograft models
The HCT116 colorectal cancer cells were maintained as monolayers in culture flasks using culture medium consisting of McCoy’s 5a Medium (Modified) supplemented with 10% FCS (Biowest, Nuaillé, France), 2 mmol/L glutamine and 1% antibiotics. All the cells were grown at 37 °C in a humidified incubator containing 5% CO2.
Xenografts of human CRC were induced in male nude mice (Swiss nu/nu), weighting 26-33 g at the time of injection (7 wk old, Charles Rivers). We excluded female nude mice in order to avoid a possible hormonal influence. A total of 10 male nude mice were divided into two groups: Non-infected control xenograft (n = 5) and pks-positive E. coli-infected xenograft (n = 5). According to the infected xenograft, HCT116 cells were mixed with pks-positive E. coli as previously described by Cougnoux et al[34]. Beforehand, bacteria were grown at 37 °C in Luria-Bertani medium. Pks-positive E. coli is an ampicillin- and kanamycin-resistant E. coli strain named 11G5, isolated from a patient presenting with colon cancer and previously presented by Bonnet et al[33]. We used human colon adenocarcinoma epithelial cells (HCT116) to establish the xenograft models.
Then, animals anesthetized by isoflurane inhalation were inoculated with 2 × 106 HCT116 cells embedded in growth factor-reduced Matrigel (Becton Dickinson) by dorsal subcutaneous injection at day 0 of the experiment.
Tumor size was assessed two times per week and tumor volume was obtained according to the following formula: (width2 × length)/2 = V (mm3). The longest diameter (L) and maximum diameter (W) perpendicular to the direction of the longest diameter were determined using a caliper. Mice were sacrificed at 35 d post-injection, and the xenograft was collected from all animals and subjected to histologic examination and enzyme-linked immunosorbent assay (ELISA).
Colitis-CEABAC10 DSS models
Six CEABAC10 transgenic mice in an in vivo model mimicking colitis and Crohn’s disease[46] were used to monitor intra-digestive inflammation. They were divided into two groups. Mice from the same generation were used for experimentation. One group (n = 3) received one cycle of dextran sodium sulfate (DSS) in drinking water for 6 d at 1% (DSS-treated mice group) as described previously in Denizot et al[46]. The other group received only drinking water (n = 3; DSS-).
Optical imaging
For both BLI and fluorescence imaging acquisition, all animals were imaged using a dedicated high-sensitivity peltier-cooled (-90 °C) backlit charge-coupled device camera (IVIS Spectrum®, Perkin Elmer, United States). All acquisitions were performed under the same exposure conditions according to fluorescence or BLI imaging, with acquisition settings (binning and duration) set up depending upon the signal at the time of acquisition.
Prior to imaging, animals were anesthetized with 2%-3% isoflurane in an induction chamber; then, 2% isoflurane in air/O2 was continuously delivered via a nose cone system in the dark box of the imaging system (delivered gas to up to 5 mice). To limit auto-fluorescence related to melanin, mice were shaved before all imaging procedures (except nude mice, which are hairless).
We used the XénoLight Rediject 2-DG-750 fluorescent probe or the XénoLight Rediject Inflammation chemiluminescent probe (Perkin Elmer, United States) to monitor metabolic activity or inflammation (MPO and ROS detection), respectively.
To monitor inflammation in HCT116-grafted nude mice at 20 d and 34 d post-xenograft, we administered by intraperitoneal (i.p.) injection of 150 μL of the XenoLight Rediject Inflammation probe per mouse. Mice were then imaged 10 min post-injection (exposure time of 5 min). In the colitis CEABAC10 model, imaging was performed 6 d after the DSS cycle. To monitor inflammation at depth and limit the decrease of the BLI signal intensity in this model, we administered by an intravenous (i.v.) injection of 150 μL/mouse. With i.v. injection, the best time to image the animal was immediately post-injection (exposure time of 5 min).
For tumor metabolic activity, we administered by an intravenous injection of 100 μL/mouse and imaged them 3 h after 2-DG-750 probe injection using one filter set (excitation: 745 nm, emission: 820 nm) and a high-throughput epi-illumination acquisition mode. All nude mice were imaged individually at 17 d and 24 d post-xenograft.
Quantitative analysis of imaging was performed using Living Image® Software (Caliper Life Science, United States) with the region of interest delineated manually over organs exhibiting probe accumulation. Every image series had the same scales, set manually, to facilitate the visual comparison of signal intensity at each time point. For the BLI signal, photon flux was expressed as the average radiance in p/s/cm2/sr. Fluorescence emission was also normalized to photons per second per centimeter squared per steradian (p/s/cm2/sr).
Histological analysis
After mouse sacrifice, tumor pieces were fixed in formol solution. Paraffin-embedded sections were cut into 5-μm slices, and tissue sections were prepared for hematoxylin-eosin-safran staining and routine pathological analysis with focus on the mitotic index, infiltrating cells and tumor necrosis. Sample preparations and observations were made in the Centre Imagerie Cellulaire Santé platform (Clermont-Ferrand).
MPO activity determination
After mouse sacrifice, tumor pieces were frozen in liquid nitrogen and stored at -80 °C until used. We performed an enzyme-linked immunosorbent assay to determine the levels of MPO (ng/mL) in all the tumors according to the manufacturer’s instructions (R and D systems). Data were standardized on whole protein extracts stained with Coomassie blue.
Statistical analysis
Graph Pad Prism 5 STATA (StataCorp) was used for all statistical analysis. Unpaired Student’s t test was used for the comparisons of the 2 groups. We determined the Pearson’s correlation coefficient r to assess the degree of correlation. We considered P values of < 0.05 to be statistically significant.
RESULTS
Metabolic activity of the CRC xenograft model
In HCT116-grafted nude models, we confirmed a rapid systemic elimination of the 2-deoxyglucosone fluorescent probe with a very weak whole body uptake 180 min after probe-injection (Figure 1A). Moreover, a high and specific HCT116 tumor uptake was evidenced for each tumor 17 and 34 d post-graft. We demonstrated an increase of signal intensity over time, reflecting tumor growth. The tumor uptake of the 2-deoxyglucosone fluorescent signal was significantly (P = 0.0036) correlated with tumor volume, as determined using a caliper (Figure 1B). The in vivo monitoring of HCT116 tumor growth by the 2-deoxyglucosone fluorescent probe was efficient. No difference in tumor uptake was observed between uninfected and pks-positive E. coli-infected HCT116 cells (data not shown), as previously described with caliper determination by Cougnoux et al[34] at 34 d post-xenograft.
Figure 1.
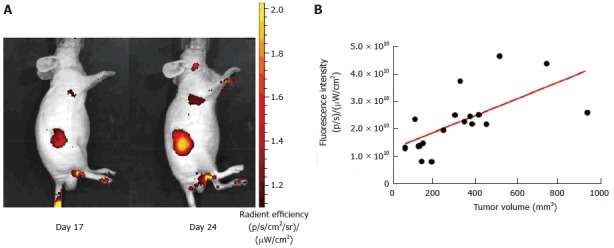
Correlation of tumor volume (HCT116 cells xenograft) and 2-DG-750 fluorescence signal uptake. A: Representative animal images showing the increase in fluorescence signal intensity with tumor development (day 17 and day 24 post-xenograft); B: Correlation between tumor development determined using a caliper and increase in fluorescence signal intensity (Pearson’s correlation factor r = 0.4197; P = 0.0036).
We tested 2-deoxyglucosone fluorescent imaging on Colitis-CEABAC10 DSS models. We did not observe any fluorescent signal in vivo in mice, reflecting that the targeting probe is specific for tumor cells (data not shown).
Pks-positive E. coli in vivo induces inflammation in the CRC xenograft model
Using the inflammation probe, all nude mice were imaged 20 and 34 d post-xenograft. We detected a rapid systemic elimination in all mice and a strong BLI signal in HCT116 tumors in the infected group 10 min after probe injection (Figure 2A and B). Figure 2A and B clearly show that the intensity of the BLI signal (average radiance in p/s/cm2) was stronger in xenografts infected with the pathogenic pks-positive E. coli strain compared to uninfected ones at each time point investigated (20 and 34 d post-xenograft). Quantitation confirmed a significant increase of the BLI signal in the infected tumors 20 d (P = 0.0132, Figure 2C) and 34 d (P = 0.0006, Figure 2D) after the xenograft.
Figure 2.
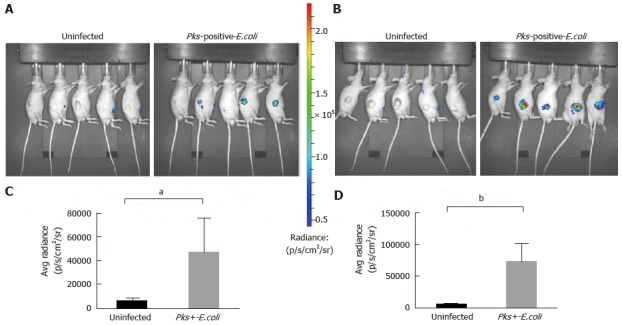
Significant increase of inflammation in HCT116 tumors infected with Escherichia coli strains measured by optical imaging using the inflammation probe. In vivo BLI imaging was performed at day 20 (A) and day 34 (B) post-HCT116 xenograft in nude mice (n = 5 animals with uninfected xenograft; n = 5 animals in pks-positive E. coli-infected xenograft). BLI images comparing uninfected to pks-positive E. coli-infected xenografts are shown on day 20 (A) and day 34 (B) post-xenograft. Intensity of emission is represented as the pseudocolor image. A sharp increase in the BLI signal was seen in the pks-positive E. coli-infected xenograft group at each time point. The BLI signal detected 10 min after i.p. probe injection was quantified from the ROI drawn manually. Photon flux was expressed as the average radiance in p/s/cm2/sr. Graphs reveal a statistically significant increase in the BLI signal in the pks-positive E. coli-infected tumors: day 20, aP = 0.0132 (C) and day 34, bP = 0.0006 (D). BLI: Bioluminescent; E. coli: Escherichia coli; i.p.: Intraperitoneal.
Monitoring intra-digestive BLI signals in Colitis-CEABAC10 DSS models
Then, we analyzed the monitoring of intra-digestive BLI signals using the inflammation probe in the CEABAC10 colitis mouse model. We induced intra-digestive inflammation using DSS in the first group, while control mice received only drinking water. To visualize BLI imaging in deep tissues in vivo in mice, the probe was injected intravenously. DSS group imaging showed a high BLI signal in DSS animals, reflecting severe intra-digestive inflammation (DSS+) relative to the untreated group (DSS-) (Figure 3). We demonstrated that monitoring intradigestive inflammation with the BLI signal is feasible and consistent.
Figure 3.
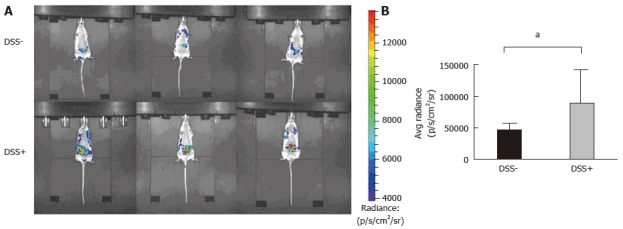
Monitoring of intra-digestive bioluminescent signals using the inflammation probe in the CEABAC10 mouse model. One mouse group was treated with 1% dextran sodium sulfate (DSS-treated mice group) in drinking water for 6 d (n = 3), while the other group received only drinking water (n = 3; DSS-). Mice were subjected to one cycle of DSS and then imaging with the inflammation probe before sacrifice. Mice were intravenously injected with the inflammation probe, and the BLI signal was detected immediately after injection. DSS-treated mouse group acquisition (A) showed severe intra-digestive inflammation was correlated with a significant increase in the BLI signal on graphs (B) compared to the DSS group (aP = 0.03). BLI: Bioluminescent; DSS: Dextran sodium sulfate.
Histological characterization
The histologic analysis of tumor samples indicated and confirmed that tumor cells in pks-positive E. coli-infected xenografts were surrounded by a remarkable infiltration of activated phagocytes (Figure 4C and D) compared to the uninfected group (Figure 4A and B). In addition, tumor necrosis was observed, especially in the pks-positive E. coli-infected group (Figure 4C). Moreover, HCT116 are characterized by megalocytosis and the progressive enlargement of the cell body and nucleus in pks-positive E. coli-infected xenografts. Histological examination confirmed the data from BLI imaging (inflammation probe).
Figure 4.
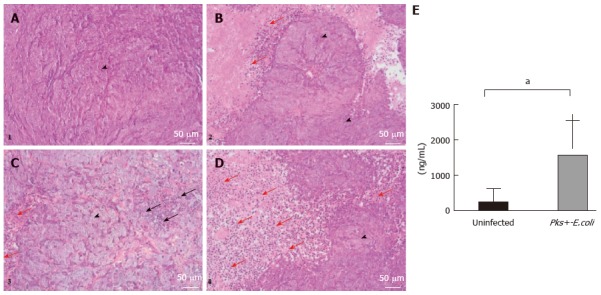
Histological and molecular analyses of HCT116 tumor samples. A-D: Histological examination of representative HCT116 tumor samples. Xenografts were harvested, paraffin embedded and processed for hematoxylin/eosin/safran. A and B are representative histological examinations from the uninfected xenograft group. C and D are representative histological examinations from the pks-positive-E. coli infected xenograft group. We noted that HCT116 tumor cells (arrowheads) infected with pathogenic E. coli strains are characterized by megalocytosis and progressive enlargement of the cell body and nucleus. Tumor cells in the pks-positive E. coli-infected xenograft group were surrounded by a remarkable infiltration of inflammatory cells (red arrow) compared to the uninfected xenograft group. Tumor necrosis was observed, especially in the infected xenograft group (black arrow). (Scale bars: 50 μm = × 20); E: MPO levels by enzyme-linked immunosorbent assay (ELISA) on HCT116 tumor specimens. An ELISA test was performed on tumor specimens after mouse sacrifice (day 34 post-xenograft). MPO standardized levels were significantly higher (1556 ± 313.6 vs 234.6 ± 121.6, aP = 0.001) in xenografts infected with pathogenic pks-positive E. coli strains. E. coli: Escherichia coli; MPO: Myeloperoxidase.
MPO levels by ELISA
To confirm the quantitation of inflammation imaging, we assessed MPO activity on HCT116-xenograft-tumor specimens by performing an ELISA. MPO levels (ng/mL) were significantly higher (1556 ± 313.6 vs 234 ± 121.6, P = 0.001) in xenografts infected with the pks-positive E. coli strain. These results showed that the pathogenic E. coli strain enhanced MPO release compared to uninfected xenografts and confirmed the data from BLI imaging.
DISCUSSION
Optical imaging appears to be a powerful, highly sensitive tool in translational cancer research, providing new possibilities for in vivo molecular imaging and allowing a better understanding of host-pathogen interactions in several tumor processes. Some studies have reported the pro-carcinogenic activities of pks-positive E. coli in murine models[26,33,34]. Most of these studies required animal sacrifice and did not allow longitudinal investigation. Ideally, it would be useful to non-invasively, longitudinally monitor these procarcinogenic processes. Here, we report the first study that utilized optical imaging in these settings. More precisely, we focused on CRC carcinogenesis and pathogenic E. coli association.
We described a specific accumulation of the 2-deoxyglucosone fluorescent probe to the tumor site (CRC xenograft model), thus establishing a correlation between tumor volume and fluorescent signal intensity. We showed that this method provides an effective tool to assess longitudinal data on CRC tumor growth in vivo. Moreover, in our experimental conditions, xenografts infected with pks-positive E. coli exhibited comparable development to uninfected ones, confirming results reported for the same experimental conditions by Cougnoux et al[34]. However, they observed a significant increase in tumor volume induced by colibactin, starting from day 44 after the xenograft. In the present study, we chose to evaluate inflammation and ROS production at an early stage, before the effect of colibactin on tumor cells proliferation. Indeed, Arthur et al[39] suggested that inflammation is necessary for E. coli’s cancer-promoting activity, probably through the enhancement of its resilience among gut microbiota in the intestine. Our results showed that the BLI signal significantly increases with bacterial infection. Pathogenic E. coli seemed to enhance inflammation and ROS production, which could participate in carcinogenesis. Using luminol-based BLI, we showed that pks-positive E. coli induced oxidative stress, which is involved in carcinogenesis process. We confirmed this observation on histological examination, which showed that inflammatory cells were mostly recruited in infected xenografts. Tumor necrosis also appeared in the pks-positive E. coli group. Moreover, a significant increase of MPO activity, which led to ROS production by infiltrating immune cells, was confirmed with an ELISA on HCT116-cells tumor specimens. Finally, these data showed that pks-positive-E. coli induced inflammation and ROS production at an early stage after infection, and could thus be an important mechanism involved in pro-carcinogenic activity.
To validate the use of luminol-based BLI imaging to monitor inflammation and oxidative stress, we used an in vivo colitis model (CEABAC10/DSS). We proved that monitoring inflammation in deep tissues is feasible and effective. This suggests that optical imaging should be tested on other murine models (APCmin/+, AOM-IL10-/-, AOM-DSS) used to determine the pro-carcinogenic proprieties of pks-positive E. coli strains, and it may facilitate a better understanding of how pathogenic bacteria impact the carcinogenesis process by various mechanisms. Using CEABAC10 models mimicking Crohn’s disease, we suggest that optical imaging is an effective method in inflammatory bowel disease research.
In conclusion, by using optical imaging, particularly the BLI approach, we provided additional tools to better understand host-pathogen interactions in digestive pathology, including CRC and inflammatory bowel disease.
COMMENTS
Background
Colorectal carcinoma (CRC) is a complex association of non-neoplastic and tumoral cells and a large amount of microorganisms. Escherichia coli (E. coli) is a consistent commensal of the human gut microbiota but some pathogenic strains have acquired the ability to produce toxins as cyclomodulins that can interfere with eukaryotic cell cycle or directly induce DNA damages. It was observed that cyclomudulin-producing-E. coli are more frequently detected on CRC patients and exhibit procarcinogenic properties on murine models.
Research frontiers
Novel imaging techniques like optical imaging could be a powerful tool in translational cancer. Particularly, in vivo optical imaging is an innovative tool for non-invasive, spatiotemporal and quantitative monitoring of carcinogenesis process in murine models. It may help to better understand the host-pathogen interactions in colorectal tumor development.
Innovations and breakthroughs
This study investigates the in vivo mechanisms of epithelial colonic mucosa infection by cyclomodulin-positive-E. coli. Because chronic inflammation and reactive oxygen species (ROS) production are key factors in bacteria and CRC interactions, the authors choose to evaluate cyclomodulin-positive-E. coli infection on these mechanisms using optical imaging and commercial, available and validated probes. By using this technique, the authors provided tools to better understand host-pathogen interactions on murine models at the early stage of disease, such as inflammatory bowel disease and CRC.
Applications
By using optical imaging, particularly the bioluminescent approach, the authors provided additional tools to better understand host-pathogen interactions in digestive pathology, including CRC and inflammatory bowel disease. The data suggest that cyclomodulin-positive-E. coli induced inflammation and ROS production at an early stage after infection, and could thus be an important mechanism involved in pro-carcinogenic activity of these bacteria.
Terminology
Oxidative stress reflects an imbalance between the systemic manifestation of ROS and the cellular biological system’s ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. In humans, oxidative stress is thought to be involved in the development of several cancers.
Peer-review
Good interesting novel study. The experiments were well designed.
Footnotes
Supported by Veziant J was supported by «année-recherche» grants from the Ministère de la Santé and the Faculté de Médecine de Clermont-Ferrand; Gagnière J was supported by a “Nuovo Soldati Foundation for Cancer Research” grant.
Institutional review board statement: Not concerned.
Institutional animal care and use committee statement: The studies were performed in accordance with the French Regional Ethical Animal Use Committee (No. CEEA-02).
Conflict-of-interest statement: No.
Data sharing statement: Not concerned.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 28, 2015
First decision: October 8, 2015
Article in press: March 25, 2016
P- Reviewer: Cao GW, Erem HH, Kodaz H S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011;12:504–512. doi: 10.1016/S1470-2045(10)70186-8. [DOI] [PubMed] [Google Scholar]
- 5.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 6.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 7.Irrazábal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell. 2014;54:309–320. doi: 10.1016/j.molcel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317–328. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 10.Jobin C. Microbial dysbiosis, a new risk factor in colorectal cancer. Med Sci (Paris) 2013;29:582–585. doi: 10.1051/medsci/2013296010. [DOI] [PubMed] [Google Scholar]
- 11.Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, Delchier JC. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease. Therap Adv Gastroenterol. 2013;6:215–229. doi: 10.1177/1756283X12473674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20. doi: 10.3389/fmicb.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdulamir AS, Hafidh RR, Abu Bakar F. The association of Streptococcus bovis/gallolyticus with colorectal tumors: the nature and the underlying mechanisms of its etiological role. J Exp Clin Cancer Res. 2011;30:11. doi: 10.1186/1756-9966-30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 16.Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23:1298–1303. doi: 10.1111/j.1440-1746.2008.05490.x. [DOI] [PubMed] [Google Scholar]
- 17.Grahn N, Hmani-Aifa M, Fransén K, Söderkvist P, Monstein HJ. Molecular identification of Helicobacter DNA present in human colorectal adenocarcinomas by 16S rDNA PCR amplification and pyrosequencing analysis. J Med Microbiol. 2005;54:1031–1035. doi: 10.1099/jmm.0.46122-0. [DOI] [PubMed] [Google Scholar]
- 18.Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship. World J Surg Oncol. 2007;5:51. doi: 10.1186/1477-7819-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zumkeller N, Brenner H, Zwahlen M, Rothenbacher D. Helicobacter pylori infection and colorectal cancer risk: a meta-analysis. Helicobacter. 2006;11:75–80. doi: 10.1111/j.1523-5378.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 20.Housseau F, Sears CL. Enterotoxigenic Bacteroides fragilis (ETBF)-mediated colitis in Min (Apc+/-) mice: a human commensal-based murine model of colon carcinogenesis. Cell Cycle. 2010;9:3–5. doi: 10.4161/cc.9.1.10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 22.Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer - a reminder. World J Surg Oncol. 2009;7:73. doi: 10.1186/1477-7819-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoy AN, Araújo-Pérez F, Azcárate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swidsinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, Lochs H. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–286. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 26.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leser TD, Mølbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ Microbiol. 2009;11:2194–2206. doi: 10.1111/j.1462-2920.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 28.Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 29.Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA. 2010;107:11537–11542. doi: 10.1073/pnas.1001261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur JC, Jobin C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes. 2013;4:253–258. doi: 10.4161/gmic.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prorok-Hamon M, Friswell MK, Alswied A, Roberts CL, Song F, Flanagan PK, Knight P, Codling C, Marchesi JR, Winstanley C, et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut. 2014;63:761–770. doi: 10.1136/gutjnl-2013-304739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 2014;20:859–867. doi: 10.1158/1078-0432.CCR-13-1343. [DOI] [PubMed] [Google Scholar]
- 34.Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Déchelotte P, Bonnet M, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. 2014;63:1932–1942. doi: 10.1136/gutjnl-2013-305257. [DOI] [PubMed] [Google Scholar]
- 35.Razkin J, Josserand V, Boturyn D, Jin ZH, Dumy P, Favrot M, Coll JL, Texier I. Activatable fluorescent probes for tumour-targeting imaging in live mice. ChemMedChem. 2006;1:1069–1072. doi: 10.1002/cmdc.200600118. [DOI] [PubMed] [Google Scholar]
- 36.Riedel SS, Mottok A, Brede C, Bäuerlein CA, Jordán Garrote AL, Ritz M, Mattenheimer K, Rosenwald A, Einsele H, Bogen B, et al. Non-invasive imaging provides spatiotemporal information on disease progression and response to therapy in a murine model of multiple myeloma. PLoS One. 2012;7:e52398. doi: 10.1371/journal.pone.0052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J, Purchio T, Contag PR. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis. 2003;20:733–744. doi: 10.1023/b:clin.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 38.Raisch J, Rolhion N, Dubois A, Darfeuille-Michaud A, Bringer MA. Intracellular colon cancer-associated Escherichia coli promote protumoral activities of human macrophages by inducing sustained COX-2 expression. Lab Invest. 2015;95:296–307. doi: 10.1038/labinvest.2014.161. [DOI] [PubMed] [Google Scholar]
- 39.Arthur JC, Gharaibeh RZ, Mühlbauer M, Perez-Chanona E, Uronis JM, McCafferty J, Fodor AA, Jobin C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. doi: 10.1038/ncomms5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One. 2009;4:e5517. doi: 10.1371/journal.pone.0005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 42.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 43.Maddocks OD, Scanlon KM, Donnenberg MS. An Escherichia coli effector protein promotes host mutation via depletion of DNA mismatch repair proteins. MBio. 2013;4:e00152–e00113. doi: 10.1128/mBio.00152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, Ratner L, Piwnica-Worms D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med. 2009;15:455–461. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alshetaiwi HS, Balivada S, Shrestha TB, Pyle M, Basel MT, Bossmann SH, Troyer DL. Luminol-based bioluminescence imaging of mouse mammary tumors. J Photochem Photobiol B. 2013;127:223–228. doi: 10.1016/j.jphotobiol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Denizot J, Sivignon A, Barreau F, Darcha C, Chan HF, Stanners CP, Hofman P, Darfeuille-Michaud A, Barnich N. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm Bowel Dis. 2012;18:294–304. doi: 10.1002/ibd.21787. [DOI] [PubMed] [Google Scholar]


