Abstract
Chronic psychosocial stress is a key player in the onset and aggravation of mental diseases, including psychosis. Although a strong association between this psychiatric condition and other medical co-morbidities has been recently demonstrated, few data on the link between psychosis and bone homeostasis are actually available. The aim of this study was to investigate whether chronic psychosocial stress induced by 4 or 7 weeks of social isolation in drug-naïve male Wistar rats could alter bone homeostasis in terms of bone thickness, mineral density and content, as well as markers of bone formation and resorption (sclerostin, cathepsin K, and CTX-I). We found that bone mineral density was increased in rats exposed to 7 weeks of social isolation, while no differences were detected in bone mineral content and area. Moreover, 7 weeks of social isolation lead to increase of femur thickness with respect to controls, suggesting the development of a hyperostosis condition. Isolated rats showed no changes in sclerostin levels, a marker of bone formation, compared to grouped animals. Conversely, bone resorption markers were significantly altered after 7 weeks of social isolation in terms of decrease in cathepsin K and increase of CTX-I. No alterations were found after 4 weeks of isolation rearing. Our observations suggest that chronic psychosocial stress might affect bone homeostasis, more likely independently from drug treatment. Thus, the social isolation model might help to identify possible new therapeutic targets to treat the burden of chronic psychosocial stress and to attempt alternative therapy choices.
Keywords: social rearing isolation, bone homeostasis, cathepsin K, sclerostin, CTX-I
Introduction
Chronic psychosocial stress is an important risk factor in the onset and aggravation of mental disorders, including psychosis. Thus, evidences from both clinical trials and rodent models show that humans at strong risk for psychosis or animals with behavioral and neuropathological alterations, reminiscent to psychotic symptoms, experienced high levels of psychosocial stress (Wisely et al., 2010; Pruessner et al., 2011; Mhillaj et al., 2015; Schiavone et al., 2015).
Accumulating evidence suggests that psychosis is also associated to alterations in skeletal status compared to healthy population (Montejo, 2010; Nousen et al., 2013). However, bone metabolism dysregulation in psychotic patients (Halbreich, 2007; Lin et al., 2015) has only recently received attention. In this context, a decrease in bone mass is significantly more common in patients with schizophrenia compared to control subjects (Stubbs et al., 2014). Skeletal fragility has been associated to pharmacological treatment, in particular to antipsychotic drugs (O’Keane and Meaney, 2005; Kishimoto et al., 2012). Thus, low bone mineral density (BMD) values have been observed in medicated psychotic patients (Baastrup et al., 1980; Halbreich et al., 1995), and a drug-induced decrease in BMD has been attributed mostly to hyper-prolactinemia and its consequences (Meaney and O’keane, 2002). Several studies on the relationship between antipsychotic drugs and BMD reduction in psychotic patients produced controversial results. Indeed, while several reports evidenced a statistically significant association (O’Keane and Meaney, 2005; Meaney and O’keane, 2007), others did not describe this relationship (Abraham et al., 2003; Becker et al., 2003; Howes et al., 2005; Bergemann et al., 2008). It is not clear whether psychotic patients are predisposed to certain alterations or whether these abnormalities are mainly treatment side-effects. Drug-naïve patients suffering from first psychotic episode actually represent a good opportunity to investigate the possible link between medication and the development of skeletal disorders. Unfortunately, only few studies are available from the pre-antipsychotic period, and problems with diagnosis and methodological concerns make their interpretation difficult (Crews et al., 2013). In particular, some studies have included as “drug-free” patients who in reality had received medication for a short period of time. Actually, in some cases, participants with psychosis were allowed to receive anti-anxiety medication (Fernandez-Egea et al., 2009). Since experimental methods and confounding variables can be minimized in animal models, the present study was designed to assess the potential development and prevalence of bone status abnormalities in the post-weaning social isolation rat model of psychosis, which provides a non-pharmacological tool to induce neurobiological and behavioral changes in animals, reminiscent of what observed in psychotic subjects (Bakshi and Geyer, 1999; Lapiz et al., 2003; Fone and Porkess, 2008).
Here, we investigate whether and how bone homeostasis could be impaired in an animal model of chronic psychosocial stress. To this purpose, we compared the skeletal status in animals reared either in social isolation (4 and 7 weeks) or in social groups, by using dual-energy X-ray (DEXA) absorptiometry. A microscopy investigation was also performed to assess possible bone thickness variation. Finally, to evaluate the activity of bone remodeling process, biochemical markers of bone formation (serum sclerostin) and resorption (serum cathepsin K and CTX-I) were measured.
Materials and Methods
Animals
Adult male and female Wistar rats (Harlan, S. Pietro al Natisone) weighting 250–280 g were housed at constant room temperature (22 ± 1°C) and relative humidity (55 ± 5%) under a 12 h light/dark cycle (lights on from 7:00 AM to 7:00 PM) for at least 7 days before the experiments. Food and water were available ad libitum. Procedures involving animals and their care were conducted in conformity with the institutional guidelines of the Italian Ministry of Health (D.L. 26/2014), the Guide for the Care and Use of Laboratory Animals: Eight Edition, the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2004), the Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the protection of animals used for scientific purposes. The experimental protocol was approved by the Italian Ministry of Health. Approval number was not applicable. All procedures involving animals were conducted in accordance to ARRIVE guidelines. Animal welfare was monitored daily through the entire period of experimental procedures. No signs of distress were evidenced and all efforts were made to minimize the number of animals used and their suffering.
Social Isolation Protocol
For the social isolation procedure, one male and two females were housed together for mating (Leng et al., 2004). The social isolation procedure was performed on 20 male rats. This sample size was established based on literature evidence about this animal model. At weaning (postnatal day 21), pups were separated from their mothers and reared either as isolated rats (ISO; one rat per cage) or in reared in group control rats (GRP; three to four rats per cage). To avoid a litter effect, each litter contributed only one subject to the GRP and one subject to the ISO. All animals were reared in Plexiglas cages (ISO: 40.0 cm × 27.0 cm × 20.0 cm; GRP: 59.0 cm × 38.5 cm × 20.0 cm). Animals were disturbed only for cleaning purposes, which consisted of changing the cage once a week for ISO and GRP. Both ISO and GRP rats were housed in the same room, so that ISO rats maintained a visual, auditory, and olfactory contact with the other animals. All experiments were conducted at the end of 4 and 7 weeks of isolation rearing. For post-mortem analyses, animals were deeply anesthetized with Equithesin (3.6 ml/kg; composition: 1.2 g sodium pentobarbital; 5.3 g chloral hydrate; 2.7 g MgSO4; 49.5 ml propylene glycol; 12.5 ml ethanol, 58 ml distilled water) and euthanized by decapitation, as previously described (Tucci et al., 2014).
Blindness of the Study
Researchers performing analysis were blind with respect to the rearing conditions. Indeed, it was not possible to deduce from the labeling whether an animal was isolated or not. The social isolation procedure was performed in a dedicated part of the animal facility, not accessible to the investigators during the entire period of the social isolation protocol. The blinding of the data was maintained until the analysis was terminated.
In Vivo Dual Energy X-ray Absorptiometry Analysis
A total of 20 animals (10 control and 10 isolated rats) were anesthetized by an intraperitoneal injection of Equithesin. The rationale for choice of these specific anesthetic and route of administration was established based on our experience and literature evidence. BMD (g/cm2), bone mineral content (BMC, g) and bone area (cm2) were measured in each animal by means of DEXA with a body scan densitometer (Hologic Dexa Bone Densitometer, Hologic Italia S.R.L., Rome, Italy). Before measurements, body calibration scans were performed with the Hologic phantom for small animals. Animals were positioned ventrally with the forelimbs away from the trunk to scan the whole body. The appropriate software program for small animals (DEXA; L & R Hip Software Ver. 11.1 for Windows) was used. DEXA scans were performed at post-weaning weeks 4 and 7. After the scan, three regions of interest (ROI) were marked, namely the right femur (R1), the T9–L5 vertebrae (R2) and the L1–L6 vertebrae (R3). All animal images were scanned and analyzed by the same operator.
After DEXA scanning, rats were euthanized by decapitation. Blood was collected into heparinized serum tubes. Serum samples were allowed to clot for 60 min followed by centrifugation at 4000 rpm for 10 min. Samples were stored at -80°C for later use. Then, the right femur was removed, cleaned, weighted, and stored in 4% formaldehyde at 4°C until further analysis.
Bone Thickness Measurement
Bone thickness values were calculated on transverse femur sections (0,2 cm) of control (n = 5) and isolated rats (n = 6), as previously described (Pelletier et al., 2004; Hayami et al., 2006). Briefly, femur sections were obtained by using an electric cutter and then stained with diaminobenzidine (DAB), chromogen (DAB betazoid chromogen kit, Biocare medical Concord, CA, USA; 1 ml per sample) and H2O2 revealing solution (30 μl per sample). After two washes in PBS, the femur sections were mounted on a glass support and analyzed for bone thickness values by using the Nikon Upright Microscope Eclipse software.
Measurement of Serum Biochemical Parameters
Sera from five control and five isolated rats were analyzed for sclerostin, CTX-I and cathepsin K using ELISA kits provided by Cloud-Clone Corporation (Houston, TX, USA). Assays were performed according to the manufacturer’s instructions. Each sample analysis was performed in duplicate to avoid inter-assay variations.
Statistical Analysis
All statistical analyses were performed using Graph Pad® 6.0 for Windows. Data were analyzed by Student’s t-test. After data conversion to natural logarithm, regression analyses were performed as previously reported (Prentice et al., 1994). Regression analyses were performed using Microsoft Office Excel 2013 version. Differences were considered significant only when P-values were less than 0.05.
Results
Body Weight
Rats were weighed weekly throughout the whole experiment. The initial and final weights are shown in Table 1. There was no difference in weight between grouped and isolated rats at any stage of the experimental period (4 weeks: P = 0.590 and P = 0.802 for initial and final weight, respectively; 7 weeks: P = 0.085 and P = 0.350 for initial and final weight, respectively). Thus, social isolation did not induce alterations of total body weight, when maintained at a standard chow diet.
Table 1.
Effect of social isolation on rat body weight.
| A | ||
| Initial body weight | Body weight after 4 W | |
| GRP | 45.09 ± 1.02 | 221.10 ± 8.22 |
| ISO | 45.85 ± 0.92 | 218.90 ± 4.33 |
| B | ||
| Initial body weight | Body weight after 7 W | |
| GRP | 48.26 ± 1.34 | 350.90 ± 7.94 |
| ISO | 45.56 ± 0.71 | 341.20 ± 6.41 |
Body weight of isolated (ISO) and grouped (GRP) rats at the beginning of the study (initial body weight) and after either 4 weeks of social isolation (A) or 7 weeks of social isolation (B).
All animals increased their weight during the experiment. The body weight gain of isolated rats during the 4 and 7 weeks’ experimental period was similar to that noted in the control groups. Though individual food intake in grouped rats was not measured, total food supply in isolated animals was similar to that in the grouped rats. All animals in each group survived throughout the experiment and they showed no obvious clinical signs of morbidity.
Impact of Social Isolation on Bone Mass Parameters
Bone mineral density and BMC were evaluated by DEXA in both experimental groups after a period of 4 and 7 weeks of social isolation. Four weeks of social isolation did not induce any alterations (data not shown). Conversely, 7 weeks of social isolation significantly enhanced BMD in R1 (6,5% of increase in BMD for ISO compared to GRP; Figure 1A, unpaired t-test, P = 0.023), R2 (11,3% of increase in BMD for ISO compared to GRP; Figure 1B, unpaired t-test, P = 0.040) and R3 (12,2 % of increase in BMD for ISO compared to GRP; Figure 1C, unpaired t-test, P = 0.025) body scans. However, BMC and bone area were not affected by experimental procedure (Figures 2 and 3A–C, unpaired t-test, P = 0.064, P = 0.097, P = 0.64 for R1, R2, and R3, respectively). To verify whether the results regarding BMD were a consequence of our study, we performed a regression analyses of BMC versus bone area. After natural logarithm normalization, statistical analyses revealed that those parameters were not proportional (coefficient: 0.21, standard error: 1.47, stat t: 1.45, P: 0.89).
FIGURE 1.
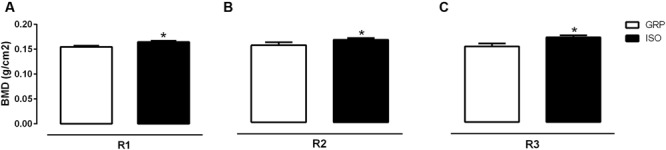
Effect of social isolation on bone mineral density (BMD): Effect of 7 weeks of social isolation rearing on BMD (g/cm2) in region of interest femur (R1) (A), T9–L5 vertebrae (R2) (B) and L1–L6 vertebrae R3 (C); ∗P < 0.05, isolated (ISO) versus grouped (GRP) rats (n = 10 per group), unpaired student t-test.
FIGURE 2.
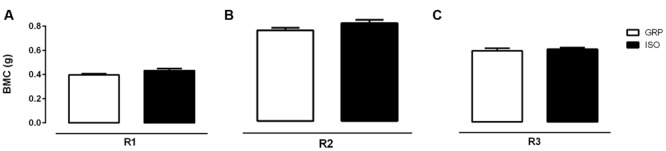
Effect of social isolation on bone mineral content (BMC): Effect of 7 weeks of social isolation rearing on BMC (g) in region of interest femur (R1) (A), T9–L5 vertebrae (R2) (B) and L1–L6 vertebrae R3 (C); P > 0.05, isolated (ISO) versus grouped (GRP) rats (n = 10 per group), unpaired student t-test.
FIGURE 3.
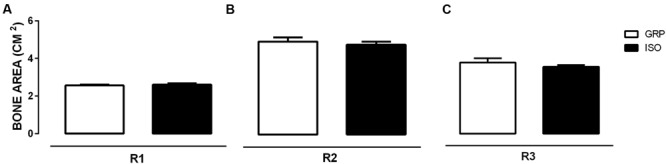
Effect of social isolation on bone area: Effect of 7 weeks of social isolation rearing on bone area (cm2) in region of interest femur (R1) (A), T9–L5 vertebrae (R2) (B) and L1–L6 vertebrae R3 (C); P > 0.05, isolated (ISO) versus grouped (GRP) rats (n = 10 per group), unpaired student t-test.
Effects of Social Isolation on Bone Thickness
To investigate the possible effects of 7 weeks of social isolation on bone thickness, transverse sections from isolated and control rat femora were obtained, colored by DAB-histochemistry, and microscopically analyzed for thickness. A period of 7 weeks of social isolation induced a significant increase of femur thickness compared to control animals (unpaired t-test, P = 0.003; Figures 4A,B), suggesting the development of a hyperostosis condition induced by chronic psychosocial stress.
FIGURE 4.
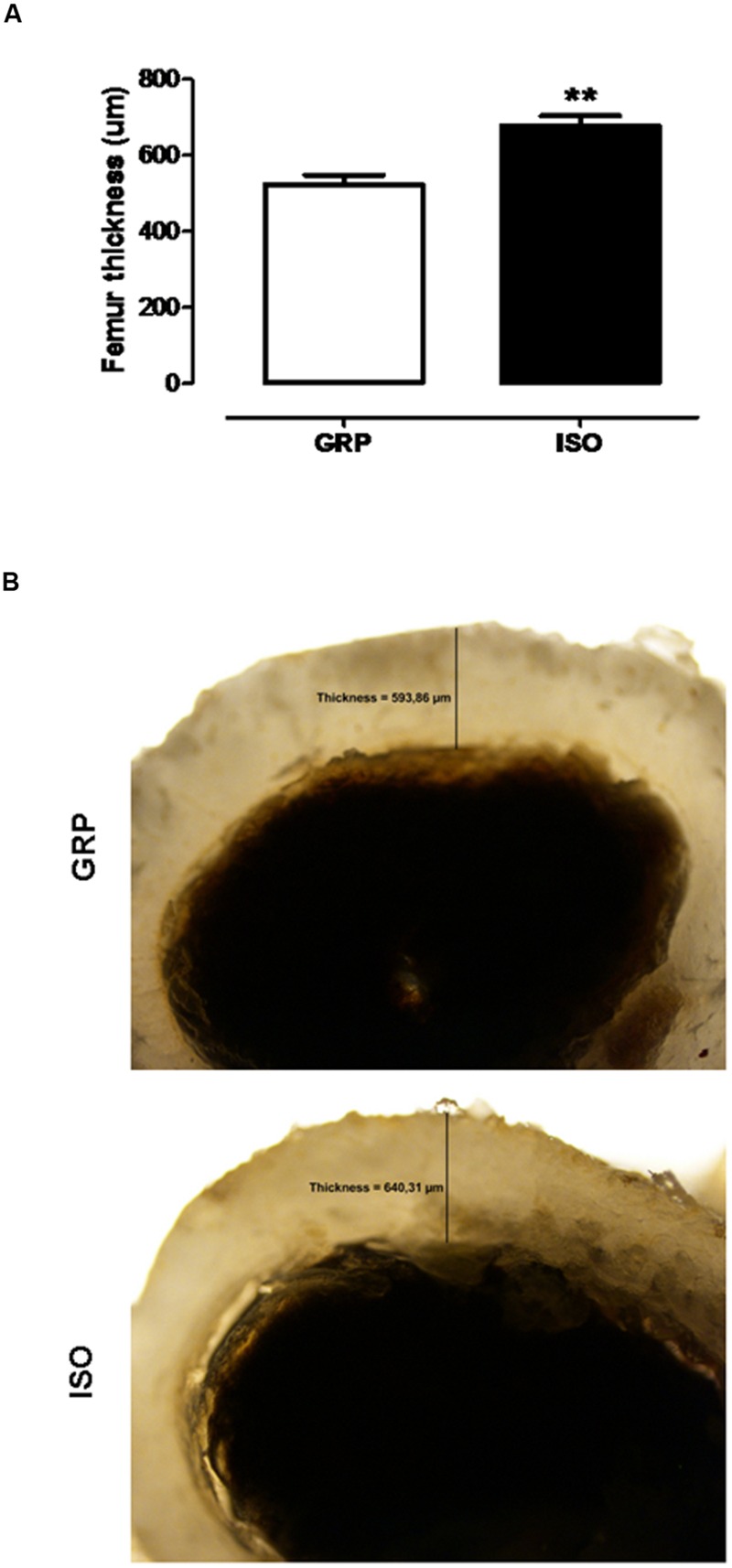
Effect of social isolation on femur thickness: Effect of 7 weeks of social isolation rearing on rat femur thickness (μm) (A), ∗∗P < 0.01, isolated (ISO) versus grouped (GRP) rats (n = 5–6 per group), unpaired student t-test. DAB-histochemistry of transverse femur section (0.2 cm) of GRP (mean thickness 593.86 μm) and ISO (mean thickness 640.31 μm) rats (B).
Alterations in Bone Formation and Resorption Markers Induced by Social Isolation
To investigate the possible pathological alterations in markers of bone formation and resorption, serum levels of cathepsin K, CTX-I, and sclerostin were determined by ELISA procedure.
A period of 4 weeks of social isolation did not alter any of these three markers with respect to control animals (data not shown, unpaired t-test, P = 0.76, P = 0.305, P = 0.241 for cathepsin K, CTX-I, and sclerostin, respectively), but a significant decrease of cathepsin K (Figure 5A, unpaired t-test, P = 0.030) as well as a significant increase of CTX-I (Figure 5B, unpaired t-test, P = 0.044) were observed after 7 weeks of social isolation. Sclerostin levels remained stable after the same isolation period compared to controls (Figure 5C, unpaired t-test, P = 0.143).
FIGURE 5.
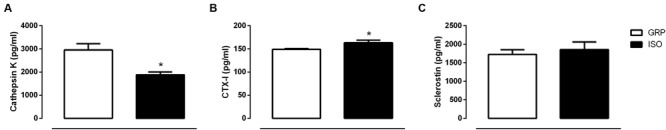
Effect of social isolation on markers of bone remodeling: Effect of 7 weeks of social isolation rearing on serum cathepsin K levels (pg/ml) (A), cross-linking telopeptide of type I collagen CTX-I (pg/ml) (B) and sclerostin (pg/ml) (C). ∗P < 0.05, isolated (ISO) versus grouped (GRP) rats (n = 5 per group), unpaired student t-test.
Discussion
To the best of our knowledge, this study provides the first in vivo evidence for bone status alterations in a rat model of psychosis, the post weaning social isolation. We found that 7 weeks of social isolation lead to increased BMD values either in femoral, trabecular 1 or trabecular 2 sections. This was accompanied by increased cortical femoral thickness, at the same time point. We also identified a significant reduction of serum cathepsin K levels and increased CTX-I serum concentrations after 7 weeks of social isolation, while sclerostin concentrations remained stable. No effects were observed after 4 weeks of isolation rearing.
Bone mineral density in isolated animals increased without corresponding changes either in BMC or bone area. It is known that BMD value can be considered an artificially derived index (Prentice et al., 1994). Therefore, the finding of an increased BMD in isolated animals, without concomitant changes in both BMC and bone area, prompted us to verify whether this observation might have resulted from an artifact. To this purpose, the use of a regression analysis of BMC on bone area, after the conversion of the variables to natural logarithms (Prentice et al., 1990; Van Loan et al., 1998), was necessary to obtain a regression coefficient, providing, finally, the appropriate power coefficient. Thus, by determining the power relation between BMC and bone area, we assessed the degree whereby BMD increase did not match a concomitant increase in BMC and bone area. After BMC and bone area conversion to natural logarithms, BMC was regressed against bone area. The obtained regression coefficient value (0,21) supported the fact that, under our experimental conditions, BMC and bone area were not directly proportional to each other. These results appeared to be in line with previous reports showing that BMC is an independent variable, not directly proportional to bone area. Consequently, a variation in BMC or bone area is not reflected in a corresponding and concomitant alteration in BMD, meaning that changes in BMC and bone area can be observed independently of each other (Svendsen et al., 1993; Prentice et al., 1994; Van Loan et al., 1998). Further on, our findings were also supported by our results on sclerostin levels. Thus, the lack of alterations in serum sclerostin concentrations were in favor of no observed alterations either in BMC and bone area. Indeed, sclerostin is known to be predominately expressed by osteocytes, displaying anti-anabolic effects on bone formation (Gooi et al., 2010; Atkins et al., 2011; Kogawa et al., 2013; Compton and Lee, 2014). In this regard, sclerostin knockout mice have been demonstrated to exhibit an increase in bone mass (Lewiecki, 2014; Ryan et al., 2015).
Rat hyperactivity in a novel environment has been used as a translational feature of psychotic agitation (Miyakawa et al., 2003; Powell and Miyakawa, 2006; Porsolt et al., 2010; Jones et al., 2011). Accordingly, we have previously demonstrated that social isolation in rodents is associated to increased spontaneous locomotor activity (Schiavone et al., 2012; Colaianna et al., 2013). Such hyperactivity could account for the observed increase of BMD. Further on, our results are in line with previous studies emphasizing the importance of physical activity for bone health (Bourrin et al., 1995; Iwamoto et al., 2005; Morgan and Weiss Jarrett, 2011; Heidemann et al., 2015; Ryan and Shaw, 2015). On the other hand, sedentary behavior and physical inactivity may change the body composition, including BMD (Heidemann et al., 2013). Thus, gain in BMD might respond to physical hyperactivity in terms of increased mechanical loading in isolated rats.
After performing a microscopy evaluation of bone thickness in control and isolated rats, we observed a significant increase of femoral cortex thickness after 7 weeks of isolation rearing, suggesting that social isolation significantly affects bone formation, inducing a hyperostosis-like pattern.
The mechanism by which social isolation induced a hyperostosis condition is not yet clear, but the present results raise the possibility that an imbalance between osteoclastic bone resorption and osteoblastic bone formation is involved. Indeed, to assess directly whether isolation rearing affects bone remodeling, as the above data suggest, we measured serum markers of bone turnover, such as cathepsin K and CTX-I. As expected, our data showed that isolation rearing induced a significant decrease of serum cathepsin K levels. Normal bone resorption critically depends on the synthesis and secretion of cathepsin K, a protease, predominantly expressed in osteoclasts, with a prominent role in bone remodeling (Troen, 2004; Yasuda et al., 2005; Brix et al., 2008). Osteoclasts isolated from cathepsin K knockout mice exhibit impaired bone resorption in vitro (Troen, 2004). On the other hand, cathepsin K hyperactivity has been linked to osteoporosis (Kiviranta et al., 2001). Thus, in our experimental conditions, we hypothesize that the influence of social isolation on BMD could, at least in part, be mediated by a reduction in cathepsin K levels, given that bone resorption depends upon the synthesis of cathepsin K by osteoclasts. Intriguingly, an important role of cathepsin K deficiency on learning and memory processes, as well as novelty seeking behavior, has been recently identified. In particular, cathepsin K deficient mice exhibited marked memory impairments in behavioral assessments, as indicated by their inability to discriminate the introduction of a novel object into a familiar environment (Dauth et al., 2011). Likewise, we have previously demonstrated that, while control rats were able to recognize the novel object from the familiar one over the 7-week period, the exploratory activity of isolated animals was compromised (Schiavone et al., 2012; Colaianna et al., 2013). Moreover, analysis of postmortem brain samples from schizophrenic patients has shown up-regulation of cathepsin K (Bernstein et al., 2007). However, the observed increased expression of cathepsin K might likely be the effect of the long-term medication, rather than a result of the underlying disease. Unfortunately, no data on the expression of cathepsin K in drug-naive psychotic patients exist. Thus, in the light of the above findings, we propose that cathepsin K activity disruption, during development and adulthood, exerts a substantial impact on the functional integrity of the rat brain, eventually resulting in learning and memory deficits, as well as in bone homeostasis impairments. In this regard, we suggest that social isolation could provide a useful tool to better understand the role that cathepsin K seems to play in the brain, in addition to its function in the turnover of bone tissue.
Degradation products derived from osteoclastic resorption of the bone matrix could be used as a sensitive index of the resorption process. High specificity as a bone resorption marker is provided by a biochemical assay for degradation fragments of the CTX-I (Okabe et al., 2001).
Indeed, in our study, this represented another important factor that underwent significant increase in isolated animals. Despite an increase in CTX-I levels, the end effect observed in our experimental conditions was an increase in BMD and bone thickness. With respect to this aspect, it should be taken into account that CTX-I is eliminated by kidney filtration. In this context, it has been recently demonstrated that early life stress, such as maternal separation and social isolation, is associated to renal dysfunctions in rats (Loria et al., 2013). Importantly, it has also been reported that an increase of oxidative stress in rodents could lead to chronic renal dysfunctions (Aragno et al., 2003; Atessahin et al., 2007; Shah and Iqbal, 2010). Accordingly, we previously demonstrated the presence of increased oxidative stress in isolated animals (Schiavone et al., 2009). Thereby, it could be hypothesized that, in isolated animals, the increase in CTX-I levels does not depend directly on bone resorption as a primary phenomenon. Rather, it may arise from a possible isolation-induced renal dysfunction and decreased urinary excretion. Further, bone resorption, associated to CTX-I increase, might be biologically balanced by bone formation induced by hyperlocomotion (Falcai et al., 2015; Sioen et al., 2015), which is a specific behavioral trait of isolated animals, as previously reported by our group and others (Fone and Porkess, 2008; Levine et al., 2008; Schiavone et al., 2009, 2012). The interaction and the counterbalance between these two aspects (possible renal failure and hyperlocomotion) might explain, at least in part, why, despite an increase in CTX-I levels, isolated rats showed increased bone thickness and BMD.
In the context of possible cathepsin K, CTX-I and sclerostin interactions, several pathophysiological pathways should be considered. Among them, we have previously reported a crucial role of the NADPH oxidase NOX2-derived central and peripheral oxidative stress in the pathogenesis of neuropathological alterations induced by social isolation (Schiavone et al., 2009, 2012, 2015). Thus, we could not exclude that increased oxidative stress in isolated rats might contribute to alterations of bone homeostasis. This hypothesis is also supported by previous investigations, showing that osteoclastogenesis is stimulated, mediated and regulated by reactive oxygen species (Kondo et al., 2013; Callaway and Jiang, 2015). Importantly, a previous study reported a crucial role of NOX2 enzyme in bone resorption, thus osteoclasts from NOX2 knock-out mice produced the same amount of superoxide and do not exhibit signs of osteopetrosis (Yang et al., 2001). Other NOX enzymes, such as NOX1 and NOX4 have also been shown to play a role in regulation of bone homeostasis (Yang et al., 2004; Sasaki et al., 2009). Mitochondrial-derived oxidative stress (Guha et al., 2016) as well as inflammatory pathways (Pislar and Kos, 2014) leading to pathological modifications of dopaminergic function should also be considered. Furthermore, it should be taken into account that the primary outcomes of the social isolation are changes in the levels of stress mediators. Importantly, many of these stress mediators have profound effects on bone homeostasis, including regulation of osteoclast differentiation and activity. These include mediators of hypothalamic pituitary-adrenal (HPA) axis response (mainly cortisol in humans and corticosterone in rodents) and sympathetic neurotransmitters (catecholamines, neuropeptide Y). In this regard, we previously showed that psychosocial stress determines alterations of the HPA-axis functioning (Colaianna et al., 2013). In particular, elevations in the hypothalamic levels of corticotropin-releasing factor and plasmatic adrenocorticotropic hormone were observed from 4 weeks of social isolation, and increased levels of plasmatic and salivary corticosterone were found at a later time point of social isolation (7 weeks). We also showed that chronic stress resulted in altered monoamine levels that vary according to brain area and rat strain (Trabace et al., 2012). Another crucial stress mediator, whose increase has been shown in the social isolation model (Thorsell et al., 2006), is the neuropeptide Y. Thus a central role of this peptide in the coordination of bone mass and weight, in the control of osteoblast function and regulation of bone mass as well as in bone remodeling under chronic stress conditions has been demonstrated (Elefteriou, 2008; Baldock et al., 2009).
To our knowledge, there are no published reports on skeletal status related to chronic social stress-induced psychosis. Among several comorbidities associated with psychosis, bone health has only recently received attention. Indeed, only few studies have focused on bone homeostasis in psychosis and suggest that the pathology seems to be associated with low BMD in humans (Meyer and Lehman, 2006). In a Danish case-control study, antipsychotic treatment was associated with an increase in overall risk of fracture (Vestergaard et al., 2006). Similarly, Renn et al. (2009) showed that schizophrenic patients had lower bone mass than young community population, in terms of bone density measured as broadband ultrasound attenuation. Moreover, an increased risk of osteoporosis and fragility fractures in medicated psychotic patients has been reported (Partti et al., 2010; Graham et al., 2011). Significantly lower calcaneal ultrasound values were also observed in subjects treated with antipsychotic or mood-stabilizing compounds (Partti et al., 2010). In the same line, clozapine, a common antipsychotic drug, exerts adverse skeletal effects in rodents (Costa et al., 2011).
All available data are referred to medicated patients and the observed effects on bone homeostasis are usually considered to be an adverse effect of antipsychotic therapy. Interestingly, our findings suggest that, at least in part, the skeletal status abnormalities could represent a pre-existing condition. Accordingly, Kaifu et al. (2003) demonstrated, in an elegant work, that DAP12-/- mice, characterized by a combination of bone alterations and psychotic symptoms, developed an increased bone mass, in which bone resorption was impaired because of decreased osteoclastic activity. On the other hand, the mutant mice also exhibited a reduced startle response, as well as an impaired pre-pulse inhibition to acoustic stimuli, indicating the deficits commonly observed in several neuropsychiatric diseases, such as schizophrenia in humans. In this context, our data could have important implications since they may contribute to recognize that, in antipsychotic-naive subjects, possible skeletal status alterations might be caused by high levels of chronic psychological stress. The animal model paradigm used in our study permitted to exclude all factors that undoubtedly contribute to alter BMD in psychotic patients, firstly therapy, but also unhealthy lifestyle behaviors, including unhealthy diet, alcoholism or smoking.
Conclusion
Our observations suggest that chronic psychosocial stress might impair bone integrity. Thus, before the beginning of a new anti-psychotic treatment, several patient-related clinical aspects should be taken into account, such as the need to control the coexistence of obesity and hypertension. Moreover, bone health status of psychotic patients should be deeply evaluated before the introduction of a new antipsychotic therapy. To this purpose, the social isolation model might represent a useful tool to better investigate molecular mechanisms leading to hyperostosis and to possibly define new therapeutic strategies.
Author Contributions
Study design: LT and VC. Study conduct: SS, MM, EM, MB, and PT. Data collection: SS, MM, EM, MB, and PT. Data analysis: SS, MM, EM, MB, and PT. Data interpretation: SS, MM, EM, PT, ADG, FC, and NM. Drafting manuscript: SS, MM, EM, and LT. Revising manuscript content: SS, MM, EM, MB, CC, PT, ADG, FC, NM, LT, and VC. Approving final version of the manuscript: SS, MM, EM, MB, CC, PT, ADG, FC, NM, LT, and VC. LT takes responsibility for the integrity of the data analysis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by PRIN 2011 from the Italian “Ministero dell’Istruzione, dell’Università e della Ricerca” (MIUR) to PT (grant number: BNMXM_006), by FIR 2015-2018 from Apulia Region to SS and by FIR 2015-2018 from Apulia Region to MM.
References
- Abraham A., Watson S., Young A. H. (2003). Glucocorticoid receptor dysfunction: consequences for the pathophysiology and treatment of mood disorders. Indian J. Psychiatry 45 5–14. [PMC free article] [PubMed] [Google Scholar]
- Aragno M., Cutrin J. C., Mastrocola R., Perrelli M. G., Restivo F., Poli G., et al. (2003). Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: attenuation by dehydroepiandrosterone. Kidney Int. 64 836–843. 10.1046/j.1523-1755.2003.00152.x [DOI] [PubMed] [Google Scholar]
- Atessahin A., Ceribasi A. O., Yuce A., Bulmus O., Cikim G. (2007). Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin. Pharmacol. Toxicol. 100 121–126. 10.1111/j.1742-7843.2006.00015.x [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Rowe P. S., Lim H. P., Welldon K. J., Ormsby R., Wijenayaka A. R., et al. (2011). Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM- dependent mechanism. J. Bone. Miner. Res. 26 1425–1436. 10.1002/jbmr.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baastrup P. C., Christiansen C., Transbol I. (1980). Calcium metabolism in schizophrenic patients on long-term neuroleptic therapy. Neuropsychobiology 6 56–59. [DOI] [PubMed] [Google Scholar]
- Bakshi V. P., Geyer M. A. (1999). Ontogeny of isolation rearing-induced deficits in sensorimotor gating in rats. Physiol. Behav. 67 385–392. [DOI] [PubMed] [Google Scholar]
- Baldock P. A., Lee N. J., Driessler F., Lin S., Allison S., Stehrer B., et al. (2009). Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS ONE 4:e8415 10.1371/journal.pone.0008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Liver O., Mester R., Rapoport M., Weizman A., Weiss M. (2003). Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J. Clin. Psychiatry 64 761–766. [DOI] [PubMed] [Google Scholar]
- Bergemann N., Parzer P., Mundt C., Auler B. (2008). High bone turnover but normal bone mineral density in women suffering from schizophrenia. Psychol. Med. 38 1195–1201. 10.1017/S003329170800319X [DOI] [PubMed] [Google Scholar]
- Bernstein H. G., Bukowska A., Dobrowolny H., Bogerts B., Lendeckel U. (2007). Cathepsin K and schizophrenia. Synapse 61 252–253. 10.1002/syn.20358 [DOI] [PubMed] [Google Scholar]
- Bourrin S., Palle S., Genty C., Alexandre C. (1995). Physical exercise during remobilization restores a normal bone trabecular network after tail suspension-induced osteopenia in young rats. J. Bone. Miner. Res. 10 820–828. 10.1002/jbmr.5650100520 [DOI] [PubMed] [Google Scholar]
- Brix K., Dunkhorst A., Mayer K., Jordans S. (2008). Cysteine cathepsins: cellular roadmap to different functions. Biochimie 90 194–207. 10.1016/j.biochi.2007.07.024 [DOI] [PubMed] [Google Scholar]
- Callaway D. A., Jiang J. X. (2015). Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 33 359–370. 10.1007/s00774-015-0656-654 [DOI] [PubMed] [Google Scholar]
- Colaianna M., Schiavone S., Zotti M., Tucci P., Morgese M. G., Backdahl L., et al. (2013). Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxid. Redox Signal. 18 1385–1399. 10.1089/ars.2012.4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton J. T., Lee F. Y. (2014). A review of osteocyte function and the emerging importance of sclerostin. J. Bone. Joint Surg. Am. 96 1659–1668. 10.2106/JBJS.M.01096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. L., Smith G., Watson M., Lin J. M., Callon K., Gamble G., et al. (2011). The atypical anti-psychotic clozapine decreases bone mass in rats in vivo. Schizophr. Res. 126 291–297. 10.1016/j.schres.2010.11.024 [DOI] [PubMed] [Google Scholar]
- Crews M., Lally J., Gardner-Sood P., Howes O., Bonaccorso S., Smith S., et al. (2013). Vitamin D deficiency in first episode psychosis: a case-control study. Schizophr. Res. 150 533–537. 10.1016/j.schres.2013.08.036 [DOI] [PubMed] [Google Scholar]
- Dauth S., Sirbulescu R. F., Jordans S., Rehders M., Avena L., Oswald J., et al. (2011). Cathepsin K deficiency in mice induces structural and metabolic changes in the central nervous system that are associated with learning and memory deficits. BMC Neurosci. 12:74 10.1186/1471-2202-12-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F. (2008). Regulation of bone remodeling by the central and peripheral nervous system. Arch. Biochem. Biophys. 473 231–236. 10.1016/j.abb.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcai M. J., Zamarioli A., Leoni G. B., De Sousa Neto M. D., Volpon J. B. (2015). Swimming activity prevents the unloading induced loss of bone mass, architecture, and strength in rats. BioMed Res. Int. 2015 507848 10.1155/2015/507848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E., Bernardo M., Donner T., Conget I., Parellada E., Justicia A., et al. (2009). Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br. J. Psychiatry 194 434–438. 10.1192/bjp.bp.108.052605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone K. C., Porkess M. V. (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 32 1087–1102. 10.1016/j.neubiorev.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Gooi J. H., Pompolo S., Karsdal M. A., Kulkarni N. H., Kalajzic I., McAhren S. H., et al. (2010). Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 46 1486–1497. 10.1016/j.bone.2010.02.018 [DOI] [PubMed] [Google Scholar]
- Graham S. M., Howgate D., Anderson W., Howes C., Heliotis M., Mantalaris A., et al. (2011). Risk of osteoporosis and fracture incidence in patients on antipsychotic medication. Expert Opin. Drug Saf. 10 575–602. 10.1517/14740338.2011.560112 [DOI] [PubMed] [Google Scholar]
- Guha M., Srinivasan S., Koenigstein A., Zaidi M., Avadhani N. G. (2016). Enhanced osteoclastogenesis by mitochondrial retrograde signaling through transcriptional activation of the cathepsin K gene. Ann. N. Y. Acad. Sci. 1364 52–62. 10.1111/nyas.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U. (2007). Osteoporosis, schizophrenia and antipsychotics: the need for a comprehensive multifactorial evaluation. CNS Drugs 21 641–657. [DOI] [PubMed] [Google Scholar]
- Halbreich U., Rojansky N., Palter S., Hreshchyshyn M., Kreeger J., Bakhai Y., et al. (1995). Decreased bone mineral density in medicated psychiatric patients. Psychosom. Med. 57 485–491. [DOI] [PubMed] [Google Scholar]
- Hayami T., Pickarski M., Zhuo Y., Wesolowski G. A., Rodan G. A., Duong Le T. (2006). Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38 234–243. 10.1016/j.bone.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Heidemann M., Holst R., Schou A. J., Klakk H., Husby S., Wedderkopp N., et al. (2015). The influence of anthropometry and body composition on children’s bone health: the childhood health, activity and motor performance school (the CHAMPS) study, Denmark. Calcif. Tissue Int. 96 97–104. 10.1007/s00223-014-9941-9949 [DOI] [PubMed] [Google Scholar]
- Heidemann M., Molgaard C., Husby S., Schou A. J., Klakk H., Moller N. C., et al. (2013). The intensity of physical activity influences bone mineral accrual in childhood: the childhood health, activity and motor performance school (the CHAMPS) study, Denmark. BMC Pediatr. 13:32 10.1186/1471-2431-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O. D., Wheeler M. J., Meaney A. M., O’keane V., Fogelman I., Blake G., et al. (2005). Bone mineral density and its relationship to prolactin levels in patients taking antipsychotic treatment. J. Clin. Psychopharmacol. 25 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J., Takeda T., Sato Y. (2005). Effect of treadmill exercise on bone mass in female rats. Exp. Anim. 54 1–6. [DOI] [PubMed] [Google Scholar]
- Jones C. A., Watson D. J., Fone K. C. (2011). Animal models of schizophrenia. Br. J. Pharmacol. 164 1162–1194. 10.1111/j.1476-5381.2011.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaifu T., Nakahara J., Inui M., Mishima K., Momiyama T., Kaji M., et al. (2003). Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 111 323–332. 10.1172/JCI16923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., De Hert M., Carlson H. E., Manu P., Correll C. U. (2012). Osteoporosis and fracture risk in people with schizophrenia. Curr. Opin. Psychiatry 25 415–429. 10.1097/YCO.0b013e328355e1ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviranta R., Morko J., Uusitalo H., Aro H. T., Vuorio E., Rantakokko J. (2001). Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J. Bone. Miner. Res. 16 1444–1452. 10.1359/jbmr.2001.16.8.1444 [DOI] [PubMed] [Google Scholar]
- Kogawa M., Wijenayaka A. R., Ormsby R. T., Thomas G. P., Anderson P. H., Bonewald L. F., et al. (2013). Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 28 2436–2448. 10.1002/jbmr.2003 [DOI] [PubMed] [Google Scholar]
- Kondo H., Takeuchi S., Togari A. (2013). beta-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species. Am. J. Physiol. Endocrinol. Metab. 304 E507–515. 10.1152/ajpendo.00191.2012 [DOI] [PubMed] [Google Scholar]
- Lapiz M. D., Fulford A., Muchimapura S., Mason R., Parker T., Marsden C. A. (2003). Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci. Behav. Physiol. 33 13–29. [DOI] [PubMed] [Google Scholar]
- Leng A., Feldon J., Ferger B. (2004). Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol. Biochem. Behav. 77 371–379. [DOI] [PubMed] [Google Scholar]
- Levine J. B., Leeder A. D., Parekkadan B., Berdichevsky Y., Rauch S. L., Smoller J. W., et al. (2008). Isolation rearing impairs wound healing and is associated with increased locomotion and decreased immediate early gene expression in the medial prefrontal cortex of juvenile rats. Neuroscience 151 589–603. 10.1016/j.neuroscience.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewiecki E. M. (2014). Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther. Adv. Musculoskelet. Dis. 6 48–57. 10.1177/1759720X13510479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Lin C. Y., Huang T. L., Wang H. S., Chang Y. C., Lane H. Y. (2015). Sex-specific factors for bone density in patients with schizophrenia. Int. Clin. Psychopharmacol. 30 96–102. 10.1097/YIC.0000000000000062 [DOI] [PubMed] [Google Scholar]
- Loria A. S., Yamamoto T., Pollock D. M., Pollock J. S. (2013). Early life stress induces renal dysfunction in adult male rats but not female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304 R121–129. 10.1152/ajpregu.00364.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney A. M., O’keane V. (2002). Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia. Life Sci. 71 979–992. [DOI] [PubMed] [Google Scholar]
- Meaney A. M., O’keane V. (2007). Bone mineral density changes over a year in young females with schizophrenia: relationship to medication and endocrine variables. Schizophr. Res. 93 136–143. 10.1016/j.schres.2007.01.013 [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Lehman D. (2006). Bone mineral density in male schizophrenia patients: a review. Ann. Clin. Psychiatry 18 43–48. 10.1080/10401230500464687 [DOI] [PubMed] [Google Scholar]
- Mhillaj E., Morgese M. G., Trabace L. (2015). Early life and oxidative stress in psychiatric disorders: what can we learn from animal models? Curr. Pharm. Des. 21 1396–1403. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Leiter L. M., Gerber D. J., Gainetdinov R. R., Sotnikova T. D., Zeng H., et al. (2003). Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 100 8987–8992. 10.1073/pnas.1432926100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montejo A. L. (2010). The need for routine physical health care in schizophrenia. Eur. Psychiatry 25(Suppl. 2) S3–S5. 10.1016/S0924-9338(10)71699-71690 [DOI] [PubMed] [Google Scholar]
- Morgan A., Weiss Jarrett J. (2011). Markers of bone turnover across a competitive season in female athletes: a preliminary investigation. J. Sports Med. Phys. Fitness 51 515–524. [PubMed] [Google Scholar]
- Nousen E. K., Franco J. G., Sullivan E. L. (2013). Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology 98 254–266. 10.1159/000355632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe R., Nakatsuka K., Inaba M., Miki T., Naka H., Masaki H., et al. (2001). Clinical evaluation of the Elecsys beta-CrossLaps serum assay, a new assay for degradation products of type I collagen C-tlopeptides. Clin. Chem. 47 1410–1414. [PubMed] [Google Scholar]
- O’Keane V., Meaney A. M. (2005). Antipsychotic drugs: a new risk factor for osteoporosis in young women with schizophrenia? J. Clin. Psychopharmacol. 25 26–31. [DOI] [PubMed] [Google Scholar]
- Partti K., Heliovaara M., Impivaara O., Perala J., Saarni S. I., Lonnqvist J., et al. (2010). Skeletal status in psychotic disorders: a population-based study. Psychosom. Med. 72 933–940. 10.1097/PSY.0b013e3181f7abd3 [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Boileau C., Brunet J., Boily M., Lajeunesse D., Reboul P., et al. (2004). The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone 34 527–538. 10.1016/j.bone.2003.11.021 [DOI] [PubMed] [Google Scholar]
- Pislar A., Kos J. (2014). Cysteine cathepsins in neurological disorders. Mol. Neurobiol. 49 1017–1030. 10.1007/s12035-013-8576-8576 [DOI] [PubMed] [Google Scholar]
- Porsolt R. D., Moser P. C., Castagne V. (2010). Behavioral indices in antipsychotic drug discovery. J. Pharmacol. Exp. Ther. 333 632–638. 10.1124/jpet.110.166710 [DOI] [PubMed] [Google Scholar]
- Powell C. M., Miyakawa T. (2006). Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry 59 1198–1207. 10.1016/j.biopsych.2006.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A., Laskey M. A., Shaw J., Cole T. J., Fraser D. R. (1990). Bone mineral content of Gambian and British children aged 0–36 months. Bone Miner. 10 211–224. 10.1016/0169-6009(90)90263-F [DOI] [PubMed] [Google Scholar]
- Prentice A., Parsons T. J., Cole T. J. (1994). Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am. J. Clin. Nutr. 60 837–842. [DOI] [PubMed] [Google Scholar]
- Pruessner M., Iyer S. N., Faridi K., Joober R., Malla A. K. (2011). Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr. Res. 129 29–35. 10.1016/j.schres.2011.03.022 [DOI] [PubMed] [Google Scholar]
- Renn J. H., Yang N. P., Chueh C. M., Lin C. Y., Lan T. H., Chou P. (2009). Bone mass in schizophrenia and normal populations across different decades of life. BMC Musculoskelet. Disord. 10:1 10.1186/1471-2474-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Shaw C. N. (2015). Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proc. Natl. Acad. Sci. U.S.A. 112 372–377. 10.1073/pnas.1418646112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan Z. C., Craig T. A., McGee-Lawrence M., Westendorf J. J., Kumar R. (2015). Alterations in vitamin D metabolite, parathyroid hormone and fibroblast growth factor-23 concentrations in sclerostin-deficient mice permit the maintenance of a high bone mass. J. Steroid Biochem. Mol. Biol. 148 225–231. 10.1016/j.jsbmb.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Yamamoto H., Tominaga K., Masuda K., Kawai T., Teshima-Kondo S., et al. (2009). NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J. Med. Invest. 56 33–41. [DOI] [PubMed] [Google Scholar]
- Schiavone S., Colaianna M., Curtis L. (2015). Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr. Pharm. Des. 21 1404–1412. [DOI] [PubMed] [Google Scholar]
- Schiavone S., Jaquet V., Sorce S., Dubois-Dauphin M., Hultqvist M., Backdahl L., et al. (2012). NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl. Psychiatry 2 e111 10.1038/tp.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone S., Sorce S., Dubois-Dauphin M., Jaquet V., Colaianna M., Zotti M., et al. (2009). Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol. Psychiatry 66 384–392. 10.1016/j.biopsych.2009.04.033 [DOI] [PubMed] [Google Scholar]
- Shah M. D., Iqbal M. (2010). Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem. Toxicol. 48 3345–3353. 10.1016/j.fct.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Sioen I., Michels N., Polfliet C., De Smet S., D’haese S., Roggen I., et al. (2015). The influence of dairy consumption, sedentary behaviour and physical activity on bone mass in Flemish children: a cross-sectional study. BMC Public Health 15:717 10.1186/s12889-015-2077-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B., De Hert M., Sepehry A. A., Correll C. U., Mitchell A. J., Soundy A., et al. (2014). A meta-analysis of prevalence estimates and moderators of low bone mass in people with schizophrenia. Acta Psychiatr. Scand. 130 470–486. 10.1111/acps.12313 [DOI] [PubMed] [Google Scholar]
- Svendsen O. L., Hassager C., Christiansen C. (1993). Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am. J. Med. 95 131–140. 10.1016/0002-9343(93)90253-L [DOI] [PubMed] [Google Scholar]
- Thorsell A., Slawecki C. J., El Khoury A., Mathe A. A., Ehlers C. L. (2006). The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol. Biochem. Behav. 83 28–34. 10.1016/j.pbb.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Trabace L., Zotti M., Colaianna M., Morgese M. G., Schiavone S., Tucci P., et al. (2012). Neurochemical differences in two rat strains exposed to social isolation rearing. Acta Neuropsychiatr. 24 286–295. 10.1111/j.1601-5215.2011.00627.x [DOI] [PubMed] [Google Scholar]
- Troen B. R. (2004). The role of cathepsin K in normal bone resorption. Drug News Perspect. 17 19–28. [DOI] [PubMed] [Google Scholar]
- Tucci P., Mhillaj E., Morgese M. G., Colaianna M., Zotti M., Schiavone S., et al. (2014). Memantine prevents memory consolidation failure induced by soluble beta amyloid in rats. Front Behav. Neurosci. 8:332 10.3389/fnbeh.2014.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loan M. D., Johnson H. L., Barbieri T. F. (1998). Effect of weight loss on bone mineral content and bone mineral density in obese women. Am. J. Clin. Nutr. 67 734–738. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. (2006). Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporos. Int. 17 807–816. 10.1007/s00198-005-0065-y [DOI] [PubMed] [Google Scholar]
- Wisely J. A., Wilson E., Duncan R. T., Tarrier N. (2010). Pre-existing psychiatric disorders, psychological reactions to stress and the recovery of burn survivors. Burns 36 183–191. 10.1016/j.burns.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Yang S., Madyastha P., Bingel S., Ries W., Key L. (2001). A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 276 5452–5458. 10.1074/jbc.M001004200 [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang Y., Ries W., Key L. (2004). Expression of Nox4 in osteoclasts. J. Cell. Biochem. 92 238–248. 10.1002/jcb.20048 [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Kaleta J., Bromme D. (2005). The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 57 973–993. 10.1016/j.addr.2004.12.013 [DOI] [PubMed] [Google Scholar]


