Highlights
-
•
Tumor vessels form a physical barrier that hampers intratumoral T cell trafficking.
-
•
Tumor endothelial cells can directly kill T cells or suppress their activity.
-
•
Normalization of the tumor endothelial barrier enhances T cell infiltration and activity.
-
•
Tumor vascular targeting synergizes with active and adoptive immunotherapies.
Abstract
T cells play a critical role in tumor immune surveillance as evidenced by extensive mouse-tumor model studies as well as encouraging patient responses to adoptive T cell therapies and dendritic cell vaccines. It is well established that the interplay of tumor cells with their local cellular environment can trigger events that are immunoinhibitory to T cells. More recently it is emerging that the tumor vasculature itself constitutes an important barrier to T cells. Endothelial cells lining the vessels can suppress T cell activity, target them for destruction, and block them from gaining entry into the tumor in the first place through the deregulation of adhesion molecules. Here we review approaches to break this tumor endothelial barrier and enhance T cell activity.
Current Opinion in Immunology 2015, 33:55–63
This review comes from a themed issue on Tumour immunology
Edited by Hans Schreiber and Philip D Greenberg
For a complete overview see the Issue and the Editorial
Available online 6th February 2015
http://dx.doi.org/10.1016/j.coi.2015.01.011
0952-7915/© 2015 Elsevier Ltd. All rights reserved.
Introduction
T lymphocytes play a key role in tumor immune surveillance through T cell receptor (TCR)-mediated recognition of tumor associated antigens that have been processed and presented as peptides (p) at the tumor cell surface by major histocompatibility complex (MHC) molecules [1]. Activated CD8+ cytotoxic T cells are able to directly kill malignant cells upon TCR/pMHC engagement by mechanisms including perforin/granzyme secretion and FasL/Fas binding, and, along with CD4+ helper T cells, can secrete various cytokines/chemokines to direct the activities of other immune cells [2, 3]. Several clinical studies, including our own in epithelial ovarian cancer, have reported a positive correlation between patient survival and the presence of tumor infiltrating lymphocytes (TILs) [4, 5, 6, 7]. Moreover, clinically significant anti-tumor activity has been achieved for dendritic cell (DC) vaccines [8, 9] and for adoptive T cell therapies with TILs, and both TCR- and chimeric antigen receptor (CAR)-engineered T cells [10••, 11•, 12, 13••, 14••, 15••, 16, 17]. In order to improve patient outcome, important research efforts have focused on optimizing the ‘fitness’ of vaccine-induced or transferred T cells, including their state of differentiation and phenotype for enhanced persistence, proliferation, homing, etc. [18] and their receptor qualities such as specificity and binding kinetics/affinity and avidity [19, 20, 21]. In addition, the characterization of different solid tumor microenvironments and the ways in which T cell activity is inhibited, so that it may be therapeutically reversed, is a field of intense study [22•, 23, 24, 25].
Solid tumors are highly heterogeneous in nature, comprising divergent cancer cells and host stromal cells that are embedded within an extracellular matrix and nourished by an aberrant vasculature (Figure 1a). The dynamic interplay of tumor cells with their surrounding matrix and local cellular microenvironment composed of various immune cell infiltrates, fibroblasts, etc., affects gene expression and the patho-physiological characteristics of the tumor, including progression and response to therapies [26]. In general, T cells that reach the tumor bed after an initial priming in the tumor-draining lymph nodes or tumor stroma face a hostile environment, including the downregulation of MHC molecules and co-stimulatory ligands, as well as the upregulation of inhibitory receptors like programmed cell death protein ligand 1 (PD-L1) on tumor cells. They can also encounter immunosuppression by regulatory T cells (Tregs), myeloid derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), as well as a plethora of soluble inhibitory factors such as IL-6, IL-10, arginase (Arg)1, and TGFβ, various metabolites like adenosine, depleted tryptophan levels as a result of indoleamine 2,3-dioxygenase 1 (IDO-1) activity, and low pH [23, 27, 28]. However, in many instances effector T cells do not gain entry into the tumor bed in the first place because they are functionally inhibited and physically blocked by the tumor vasculature. Here we review the mechanisms by which the tumor vasculature acts as a barrier to effector T cells, the so-called tumor endothelial barrier, and different therapeutic approaches being developed to ‘break it’ or ‘normalize it’ and enhance anti-tumor T cell activity.
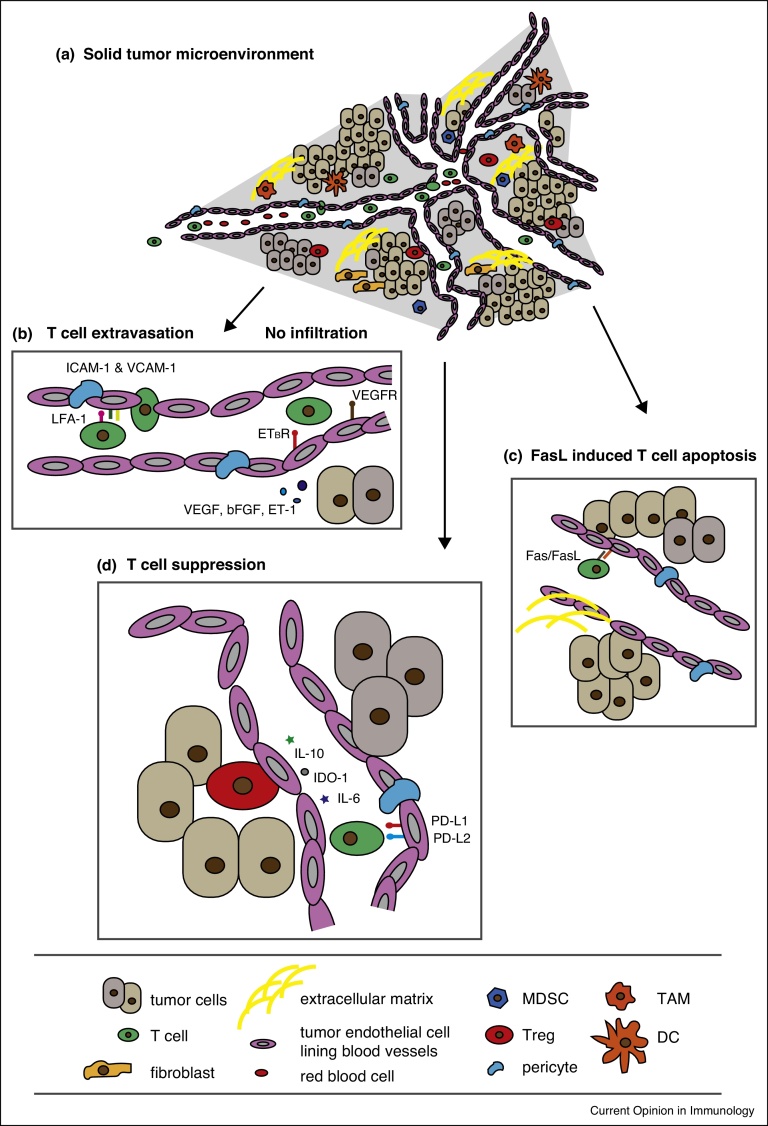
Figure 1.
The tumor microenvironment and the tumor endothelial cell barrier. (a). The tumor microenvironment is comprised of tumor cells, an aberrant vasculature lined by endothelial cells and supported by pericytes, stromal cells, an extracellular matrix, and a range of immune infiltrates including T cells, regulatory T cells (Treg), myeloid derived suppressor cells (MDSCs), tumor associated macrophages (TAMs), fibroblasts and dendritic cells (DCs). (b) T cell extravasation is dependent upon endothelial cell expression of intracellular cell adhesion molecule-1 (ICAM-1) and vasculature cell adhesion molecule-1 (VCAM-1). Tumor derived angiogenic growth factors such as VEGF and endothelin-1 (ET-1) signal through their cognate receptors, VEGFR and ETBR, respectively, to block the expression of adhesion molecules and inhibit T cell infiltration into the tumor bed. (c) The endothelium, under the influence of tumor-derived factors like VEGF, can directly inhibit T cell activation by upregulating inhibitory molecules such as PD-L1, PD-L2, IDO-1, IL-6, and IL-10, amongst many others. (d) Tumor endothelial cells can also express FasL which leads to apoptosis of Fas-expressing T cells.
The tumor vasculature is inhibitory to effector T lymphocyte responses
Tumor growth is critically dependent upon neovascularization to supply itself with nutrients. Although tumor blood vessels can be produced de novo from bone marrow-derived endothelial precursor cells, so-called vasculogenesis [29], or from tumor stem cells in a process called vascular mimicry, most are formed by the sprouting of pre-existing vessels, i.e., angiogenesis [30], promoted by an imbalance of proangiogenic factors in the microenvironment. Such factors are numerous and abundantly produced, including the most potent one, vascular endothelial growth factor-A (VEGF) [31, 32], as well as angiopoietin, basic fibroblast growth factor (bFGF), platelet-derived endothelial growth factor (PDGF), transforming growth factor (TGF)-α, fibroblast growth factor (FGF), and placental growth factor (PGF). These soluble factors act on a range of tyrosine kinase receptors like VEGFR1, VEGFR2, PDGFRA and endothelial growth factor receptor (EGFR) to initiate signaling pathways leading to angiogenesis and other biological events [33]. Compared to normal vasculatures, tumor blood vessels are characterized by having slow and irregular blood flow, an oversized diameter that varies along their length, erratic branching (the vessels are tortuous), many dysfunctional microvessels, high red blood cell flux, permeability/leakiness due to oversized pores, low or absent pericyte coverage, high compression levels due to interstitial pressure, and the vessels may lack a basement membrane or have one that is unusually thick [34]. Overall these properties result in low oxygen supply causing a state of hypoxia and an accumulation of metabolic waste that can affect immune cell function in the tumor microenvironment [35] and trigger the release and activity of proangiogenic growth factors [36, 37].
In addition, several mechanisms have been described for tumor endothelial cells (ECs) lining the vessels that specifically inhibit tumor immunity. For example, through the downregulation and/or declustering (i.e., deregulation) of intracellular adhesion molecule 1 (ICAM1) and vasculature cell adhesion molecule 1 (VCAM1), which are required for extravasation (a multistep process involving the adherence of leukocytes to ECs and their subsequent diapedesis), effector cells are unable to traverse the ECs into the tumor bed [38, 39, 40] (Figure 1b). Conversely, tumor ECs have been shown to promote Treg accumulation in the tumor through the upregulation of molecules such as common lymphatic and vasculature endothelial receptor 1 (CLEVER1) [41]. Like within the tumor itself, tumor ECs can selectively upregulate inhibitory receptors of T cell activation including PD-L1 and PD-L2 [42, 43], TIM3 [44], B7-H3 [45, 46•], B7-H4 [47], as well as IDO-1 [48, 49, 50] and other soluble inhibitory molecules such as IL-6, PGE2, IL-10 and TGFβ [51, 52, 53, 54] (Figure 1c). Moreover, tumor ECs can express the apoptosis inducing molecules TRAIL and FasL (Figure 1d), the latter of which we recently showed can selectively kill effector T cells while leaving Tregs unharmed [55, 56••, 57, 58]. Thus, overall the tumor vasculature is inhibitory to the extravasation of effector immune cells into the tumor bed and it promotes a state of immunosuppression.
Strategies to break the tumor vasculature barrier and restore anti-tumor T cell activity
Monoclonal antibody and small molecules to block proangiogenic factors and their receptors to normalize the vasculature
The VEGF/VEGFR2 axis is critical for tumor growth as it promotes proliferation, survival, migration and invasion [59]. Moreover, it confers important immunomodulatory activities including the ability to inhibit DC maturation [60, 61], and to promote the accumulation and activation of Tregs and MDSCs [62]. Consequently, VEGF expression negatively correlates with intraepithelial T cell infiltration, and it is associated with poor patient survival [4]. Not surprisingly, antiangiogenic molecules that target VEGF/VEGR2 are commonly used to treat cancer patients, and bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody (mAb) [63] was in fact the first antiangiogenic drug to be approved by the Food and Drug Administration for the treatment of various cancers including glioma and metastatic colorectal cancer [64, 65]. Some therapies like bevacizumab that target proangiogenic factors not only inhibit the sprouting of new vessels [66], but can also ‘normalize’ the vasculature. Tumor vasculature normalization, first described by Dr. Rakeesh K. Jain and colleagues, is a transient correction of structural and functional defects of the tumor blood vessels that takes place when aberrant angiogenic signaling is blocked, temporarily enabling improved oxygen and drug delivery (e.g., chemotherapy) as well as immune cell infiltration [31, 67, 68, 69]. In addition, many antiangiogenic drugs also promote overall immune responses; bevacizumab-based therapy of colorectal cancer patients, for example, has been shown to increase the peripheral B and T cell compartments [70], decrease the Treg compartment [71••], and enhance the functional maturation of DCs [72]. Small molecules targeting the tyrosine kinase domains of VEGFR and PDGFR, such as sunitinib and sorafenib, have also been approved for some advanced cancers [73] and can similarly lead to improved immune responses. A reduction in Tregs and MDSCs, for example, has been documented for metastatic renal cell carcinoma patients treated with sunitinib [74, 75, 76]. Despite initial clinical responses, however, resistance to such kinase inhibitors frequently occurs due to the redundancy of angiogenic pathways and protection of the tumor vasculature through the recruitment of proangiogenic inflammatory cells [77].
Monoclonal antibody, small molecule and cytokine treatments to restore adhesion molecule expression
T cells are dependent upon both the expression levels and clustering patterns of ICAM-1 and VCAM-1 on ECs to extravasate into the tumor. Despite the presence of TNFα in the tumor microenvironment, a pleiotropic cytokine that can activate endothelium to promote T cell adhesion [78], several mechanisms have been identified that abrogate this effect. The expression, for example, of the proangiogenic factors VEGF and bFGF downregulate adhesion molecule expression [79, 80]; this can be reversed with αVEGF and αbFGF mAb treatment [69]. Another critical signaling axis in tumors is endothelin-1 (ET-1, a 21 amino acid vasoactive peptide) and its G protein coupled receptors ETBR and ETAR. ET-1 driven signaling can activate proliferation, confer apoptosis resistance, induce VEGF expression, stimulate new blood vessel formation and promote invasion and metastasis, and it is now recognized as a common mechanism underlying the progression of many solid tumors [81, 82, 83, 84]. Through the analysis of gene expression profiles for ovarian cancers we associated the presence of ETBR with an absence of TILs, and we further demonstrated that ET-1/ETBR binding stimulates nitric oxide production, consequently decreasing ICAM-1 clustering. Moreover, in preclinical models of ovarian and lung cancer we showed that ETBR neutralization with the selective antagonist BQ-788 upregulates the expression and clustering of ICAM-1 to restore T cell adhesion, increase intratumoral T cell infiltration, and significantly improve responses following vaccination as well as adoptive T cell transfer [85••]. ETBR blockade probably also inhibits angiogenesis through suppression of tumor cell derived VEGF and the reduction of EC nitric oxide production [86, 87].
Although TNFα treatment has been shown to exert potent anti-tumor effects in animal models [88], its systemic administration in phase II clinical trials yielded minimal anti-tumor responses and was prohibitively toxic [89, 90]. New therapeutic approaches, however, have been designed to selectively deliver TNFα to tumor blood vessels. For example, TNFα coupled with the CNGRC peptide motif (NGR-TNF) that specifically interacts with CD13 (an aminopeptidase expressed by ECs of angiogenic vessels) [91], upregulates ICAM-1 and VCAM-1 expression as well as proinflammatory cytokines, enhances T-cell extravasation and intratumoral infiltration, improves the penetration of chemotherapies into the tumor and their efficacy, and enhances adoptive and active immunotherapies in various mouse tumor models [92, 93, 94•]. A similar compound, RGR-TNF, has been shown to augment active and adoptive immunotherapy in experimental pancreatic neuroendocrine tumors, in part by remodeling the vascular network with less PDGFRβ+ pericyte coverage, and in part through the polarization of tumor-resident macrophages to an M1 immunostimulatory phenotype [95•]. Another approach to upregulating tumor EC expression of adhesion molecules, including ICAM-1, VCAM-1 and E-selectin, is by αCD137 (4-1BB) agonist mAb therapy. CD137 is a costimulatory glycoprotein expressed on activated T cells, NK cells, DC cells, and, interestingly, also on human tumor blood vessels [96, 97, 98]. Thus, administration of αCD137 mAb not only directly heightens anti-tumor CD8+ T cell activity by binding to their cell surface [99•, 100, 101, 102], it also stabilizes the tumor vasculature.
Active immunization against tumor vasculature antigens
Given the genetic stability and accessibility of tumor ECs, as well as the fact that they express various angiogenic markers that are either not present or are expressed at low levels in normal vessels, the tumor vascular is an attractive target for immunization [103, 104]. The majority of immunization strategies that have been developed and tested to date in pre-clinical models are against VEGF/VEGFR2, either in the form of protein pulsed DCs [104, 105] or DNA vaccines [106, 107, 108, 109, 110, 111]. Responses, including tumor EC destruction, and the inhibition of tumor growth and metastasis, are primarily CD8+ T cell-mediated. Immunizations with both autologous and xenogeneic endothelium have also yielded encouraging preclinical responses [112, 113]. More recently, a DNA vaccine administered with tetanus toxoid and targeting tumor endothelial marker 1 (TEM1), a protein which is expressed on tumor ECs [114, 115, 116, 117, 118], as well as tumor-associated pericytes [119] and fibroblasts [120], was shown able to enhance intratumoral infiltration of endogenous CD3+ T cells as well as delay tumor progression in mice [121•].
CAR-T cell targeting of tumor vasculature antigens
Over the past few years, CAR T cells against tumor vasculature antigens, including VEGFR1, VEGFR2 and prostate specific membrane antigen (PSMA; glutamate carboxypeptidase II) [122], have also been assessed in various pre-clinical mouse models. CARs are hybrid receptors comprising an antigen-targeting moiety, typically in the form of a single chain variable antibody fragment (scFv), fused with a linker, a transmembrane domain, the intracellular signaling module of CD3ζ, and various combinations of co-stimulatory domains such as CD28, 4-1BB (CD137), and OX40 (CD134) [123]. Overall, these studies have demonstrated that vasculature-targeting CAR T cells can significantly delay tumor growth. The co-expression of IL-12 or IL-15 was also shown to enhance their efficacy through increased tumor infiltration, expansion, and in vivo survival of the transferred cells [124, 125, 126•]. Finally, the combination of CAR T cells against VEGFR2 and T cells specific for the melanoma tumor antigens gp100 and TRP-1 resulted in increased intratumoral T cells, synergistic eradication of established B16 melanoma tumors, and prolonged tumor-free survival [127••]. Thus, if the numbers of adoptively transferred anti-VEGFR2 CAR T cells are carefully escalated in the clinic, and safety mechanisms like dual/combinatorial antigen recognition and split signaling [128•, 129•], inhibitory CAR [130], or suicide gene incorporation [131, 132, 133], are implemented to minimize ‘on target, off site’ toxicity, an important consideration for this potent therapy [134], the transfer of T cell populations targeting both the tumor and its vasculature could prove highly beneficial for the treatment of advanced cancer patients.
Combinatorial treatment approaches
Administration of antiangiogenic drugs as single agents has produced only modest clinical responses with no long-term survival benefits [135, 136, 137]. Similarly, vasculature disrupting agents, drugs designed to destroy existing vessels by destabilizing microtubules etc. and create central tumor necrosis, have shown limited efficacy along with extensive toxicity, even in combination with chemotherapy (drugs that interfere with cell division by inhibiting genes involved in DNA replication or metabolism), in clinical trials [138]. When given in combination with chemotherapy, whereas, the antiangiogenic mAb bevacizumab (Avastin) led to a remarkable 5 month increase in survival time for colorectal cancer patients [139] likely because the anti-VEGF treatment normalized the tumor vasculature thus enabling enhanced oxygen and drug delivery [34]. For such outcomes, both timing and dosage of the antiangiogenic therapy is vital so that the accompanying drugs are administered when the vessels have normalized (normalization is a transient state of enhanced vessel perfusion) and so that the vasculature is not damaged to an extent that it is inhibitory to drug delivery, or leads to hypoxia and/or harms normal tissues.
Another successful combination that has been tested is vasculature targeting plus chemotherapy and active immunotherapy [140]. It is worth noting that conventional cytotoxic chemotherapeutics such as cyclophosphamide, when given at lower doses and more frequently, this is referred to as metronomic chemotherapy, can affect the endothelium and have anti-angiogenic properties themselves, through the upregulation, for example, of thrombospondin 1 (TSP1 is a component of the extracellular matrix and an endogenous inhibitor of angiogenesis [141]). Moreover, the efficacy of metronomic chemotherapy increases when administered in combination with antiangiogenic drugs [142, 143, 144]. In a recent trial we observed anti-tumor responses and clinical benefit for some patients with recurrent stage III/IV ovarian cancer vaccinated with autologous DCs pulsed with tumor cell lysate in combination with bevacizumab and oral metronomic cyclophosphamide [9, 145]. Several trials are currently underway to investigate the potential benefits of combining the anti-angiogenic drugs bevacizumab and sunitinib with immune check-point blockade antibodies (anti-PD-1 and anti-CTLA-4) and with DC vaccines [146]. It should be mentioned, however, that some combination therapies have failed. For example, in a multi-institutional clinical trial, metastatic colorectal cancer patients were randomly treated with capecitabine, oxaliplatin, and bevacizumab with or without cetuximab (a mAb against EGFR), and unfortunately, the four-drug combination led to significantly shorter progression-free survival time and inferior quality of life [147]. A similar outcome occurred for metastatic colorectal cancer patients treated with panitumumab (another anti-EGFR mAb) along with folinic acid, fluorouracil, oxaliplatin and bevacizumab. Thus, careful pre-clinical assessment of drug combinations is critical.
Conclusions
There is strong evidence that tumor infiltration by T lymphocytes is associated with good patient prognosis for many types of cancer including colorectal, ovarian, breast and melanoma. The tumor vasculature, however, constitutes an important barrier to T cells by actively blocking extravasation into the tumor through the deregulation of adhesion molecules including ICAM-1 and VCAM-1 [85••], by suppressing them with inhibitory receptors like PD-L1 and molecules such as IL-6 and IL-10, and even by targeting them for death by FasL expression [22•, 57]. Several therapeutic approaches have been developed to break the tumor endothelial barrier and have further been shown to act synergistically with active and adoptive immunotherapies. Given the heterogeneity of solid tumors (different immune cell infiltrates, stromal composition, level of vascularization, etc.), well-designed pre-clinical and clinical studies are warranted to identify optimal combinations of antiangiogenic drugs and immunotherapeutic treatments for each type. This will require extensive monitoring of changes in tumor vascularity and the patient's immunity pre- and post-treatment, determination of immune biomarkers to measure antiangiogenic responses, and continuous efforts to identify additional tumor EC targets. The optimal doses as well as the scheduling of the treatment modalities should be taken under consideration to avoid toxic side-effects and maximize clinical effectiveness. Overall there is strong evidence that targeting the tumor vasculature to enhance T cell activity improves patient outcome and tumor vasculature normalization may one day be a standard of care for the treatment of solid tumors.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest.
•• of outstanding interest.
Acknowledgments
This project was supported by NIH transformative R01CA156695, ERC Advanced grant 1400206AdG-322875, the Ovarian Cancer Research Fund, and the Leenaards Foundation.
References
- 1.Coulie P.G., et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 2.Barry M., Bleackley R.C. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy R., Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Galon J., et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q., et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 7.Gooden M.J., et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerniecki B.J., et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 9.Kandalaft L.E., et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:pe22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Rosenberg S.A., et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This clinical study demonstrated 72% objective responses following adoptive TIL transfer regardless of prior therapy.
- 11•.Chapuis A.G., et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5:174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]; This clinical study showed that IL-21-stimulated WT1-specific CD8+ T-cell clones exhibit enhanced persistence and antileukemic responses upon transfer to high-risk leukemia patients.
- 12.Robbins P.F., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Porter D.L., et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]; First clinical study illustrating that the infusion of T cells engineered with a CD19-specific CAR into a patient with chronic lymphocytic leukemia leads to complete tumor remission.
- 14••.Maude S.L., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [15] this clinical study demonstrated that CAR-modified T cells with specificity for CD19 can eradicate relapsed and refractory acute lymphoblastic leukemia and lead to durable remissions.
- 15••.Grupp S.A., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [14] this clinical study demonstrated that CAR-modified T cells with specificity for CD19 can eradicate relapsed and refractory acute lymphoblastic leukemia and lead to durable remissions.
- 16.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett D.M., et al. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinrichs C.S., et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irving M., et al. Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8+ T cell responsiveness. J Biol Chem. 2012;287:23068–23078. doi: 10.1074/jbc.M112.357673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aleksic M., et al. Dependence of T cell antigen recognition on T cell receptor–peptide MHC confinement time. Immunity. 2010;32:163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong S., et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci U S A. 2013;110:6973–6978. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Motz G.T., Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review focuses on the ways in which tumors and their vasculature exert immune suppression and highlights new therapies that seek to promote anti-tumor immunity.
- 23.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Gajewski T.F., et al. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiber R.D., Old L.J., Smyth M.J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 27.Spranger S., et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich G.A., Gabrilovich D., Sotomayor E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 30.Bergers G., Benjamin L.E. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 31.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 32.Dvorak H.F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 33.Weis S.M., Cheresh D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 34.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 35.Palazon A., et al. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res. 2012;18:1207–1213. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 36.De Bock K., Cauwenberghs S., Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Dayan F., et al. A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron. 2008;1:53–68. doi: 10.1007/s12307-008-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley K., Kansas G.S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 39.Weber C., Fraemohs L., Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 40.Ala A., Dhillon A.P., Hodgson H.J. Role of cell adhesion molecules in leukocyte recruitment in the liver and gut. Int J Exp Pathol. 2003;84:1–16. doi: 10.1046/j.1365-2613.2003.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty S., et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J Immunol. 2011;186:4147–4155. doi: 10.4049/jimmunol.1002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazanet M.M., Hughes C.C. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 43.Rodig N., et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 44.Huang D., et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zang X., et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Kraan J., et al. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br J Cancer. 2014;111:149–156. doi: 10.1038/bjc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed that CD276 can be used to discriminate malignant circulating endothelial cells from normal circulating endothelial cells.
- 47.Krambeck A.E., et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riesenberg R., et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res. 2007;13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- 49.Blaschitz A., et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS ONE. 2011;6:e21774. doi: 10.1371/journal.pone.0021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frumento G., et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulligan J.K., Young M.R. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol Immunother. 2010;59:267–277. doi: 10.1007/s00262-009-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirtskhalaishvili G., Nelson J.B. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:77–87. doi: 10.1002/1097-0045(20000615)44:1<77::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 53.Casos K., et al. Tumor cells induce COX-2 and mPGES-1 expression in microvascular endothelial cells mainly by means of IL-1 receptor activation. Microvasc Res. 2011;81:261–268. doi: 10.1016/j.mvr.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Taflin C., et al. Human endothelial cells generate Th17 and regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2011;108:2891–2896. doi: 10.1073/pnas.1011811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sata M., Walsh K. TNFalpha regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat Med. 1998;4:415–420. doi: 10.1038/nm0498-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Yu J.S., et al. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol. 2003;64:55–61. doi: 10.1007/BF02700020. [DOI] [PubMed] [Google Scholar]; This study demonstrated FasL expression on the tumor endothelial cells and its critical role in promoting the escape of tumors from T-cell-mediated immune surveillance.
- 57.Motz G.T., et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Secchiero P., Zauli G. The puzzling role of TRAIL in endothelial cell biology. Arterioscler Thromb Vasc Biol. 2008;28 doi: 10.1161/ATVBAHA.107.158451. e4, author reply e5–e6. [DOI] [PubMed] [Google Scholar]
- 59.Ellis L.M., Hicklin D.J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 60.Dikov M.M., et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 61.Gabrilovich D.I., et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 62.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrara N., et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 64.Koukourakis G.V. Has bevacizumab (Avastin) given extra therapeutic gain in metastatic colorectal cancer and malignant brain gliomas? Systematic review answering this question. Recent Pat Inflamm Allergy Drug Discov. 2012;6:70–77. doi: 10.2174/187221312798889284. [DOI] [PubMed] [Google Scholar]
- 65.Shih T., Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Miller D.W., et al. Rapid vessel regression, protease inhibition, and stromal normalization upon short-term vascular endothelial growth factor receptor 2 inhibition in skin carcinoma heterotransplants. Am J Pathol. 2005;167:1389–1403. doi: 10.1016/S0002-9440(10)61226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler F., et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Tong R.T., et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 69.Dirkx A.E., et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 2006;20:621–630. doi: 10.1096/fj.05-4493com. [DOI] [PubMed] [Google Scholar]
- 70.Manzoni M., et al. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology. 2010;79:187–196. doi: 10.1159/000320609. [DOI] [PubMed] [Google Scholar]
- 71••.Terme M., et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]; This study showed that VEGF-A/VEGFR-2 signaling blockade inhibits regulatory T-cell accumulation.
- 72.Osada T., et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gotink K.J., Verheul H.M. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adotevi O., et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother. 2010;33:991–998. doi: 10.1097/CJI.0b013e3181f4c208. [DOI] [PubMed] [Google Scholar]
- 75.Ko J.S., et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 76.Finke J.H., et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 77.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ten Hagen T.L., Seynhaeve A.L., Eggermont A.M. Tumor necrosis factor-mediated interactions between inflammatory response and tumor vascular bed. Immunol Rev. 2008;222:299–315. doi: 10.1111/j.1600-065X.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- 79.Bouzin C., Feron O. Targeting tumor stroma and exploiting mature tumor vasculature to improve anti-cancer drug delivery. Drug Resist Updat. 2007;10:109–120. doi: 10.1016/j.drup.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 80.Griffioen A.W., et al. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996;56:1111–1117. [PubMed] [Google Scholar]
- 81.Nelson J., et al. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 82.Salani D., et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spinella F., et al. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 84.Rosano L., Spinella F., Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2013;13:637–651. doi: 10.1038/nrc3546. [DOI] [PubMed] [Google Scholar]
- 85••.Buckanovich R.J., et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]; This study demonstrated that endothelin B receptor neutralization with a selective antagonist (BQ-788) restores ICAM-1 clustering, increases transendothelial T cell migration and augments tumor immunotherapy.
- 86.Rajeshkumar N.V., Rai A., Gulati A. Endothelin B receptor agonist, IRL 1620, enhances the anti-tumor efficacy of paclitaxel in breast tumor rats. Breast Cancer Res Treat. 2005;94:237–247. doi: 10.1007/s10549-005-9000-3. [DOI] [PubMed] [Google Scholar]
- 87.Cemazar M., et al. The endothelin B (ETB) receptor agonist IRL 1620 is highly vasoconstrictive in two syngeneic rat tumour lines: potential for selective tumour blood flow modification. Br J Cancer. 2005;93:98–106. doi: 10.1038/sj.bjc.6602672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palladino M.A., Jr., et al. Characterization of the antitumor activities of human tumor necrosis factor-alpha and the comparison with other cytokines: induction of tumor-specific immunity. J Immunol. 1987;138:4023–4032. [PubMed] [Google Scholar]
- 89.Lenk H., et al. Phase II clinical trial of high-dose recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1989;24:391–392. doi: 10.1007/BF00257449. [DOI] [PubMed] [Google Scholar]
- 90.Abbruzzese J.L., et al. A phase II trial of recombinant human interferon-gamma and recombinant tumor necrosis factor in patients with advanced gastrointestinal malignancies: results of a trial terminated by excessive toxicity. J Biol Response Mod. 1990;9:522–527. [PubMed] [Google Scholar]
- 91.Curnis F., et al. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 92.Curnis F., Sacchi A., Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sacchi A., et al. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. 2006;12:175–182. doi: 10.1158/1078-0432.CCR-05-1147. [DOI] [PubMed] [Google Scholar]
- 94•.Calcinotto A., et al. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687–2694. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]; This study showed that vasculature-targeted delivery of TNFa enhances the therapeutic activity of adoptive and active immunotherapy.
- 95•.Johansson A., et al. Tumor-targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci U S A. 2012;109:7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another study illustrating that the delivery of TNFα to the tumor endothelium promotes vessel remodeling and potentiates anti-tumor immune responses.
- 96.Melero I., et al. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 97.Choi B.K., et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009;182:4107–4115. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Broll K., et al. CD137 expression in tumor vessel walls, high correlation with malignant tumors. Am J Clin Pathol. 2001;115:543–549. doi: 10.1309/e343-kmyx-w3y2-10ky. [DOI] [PubMed] [Google Scholar]
- 99•.Palazon A., et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]; This work revealed that the activation of CD137 on endothelial cells induces adhesion molecule expression and augments T cell trafficking into malignant tissue.
- 100.Melero I., et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 101.Wilcox R.A., et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 102.Ito F., et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 103.Rafii S. Vaccination against tumor neovascularization: promise and reality. Cancer Cell. 2002;2:429–431. doi: 10.1016/s1535-6108(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 104.Li Y., Bohlen P., Hicklin D.J. Vaccination against angiogenesis-associated antigens: a novel cancer immunotherapy strategy. Curr Mol Med. 2003;3:773–779. doi: 10.2174/1566524033479438. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., et al. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med. 2002;195:1575–1584. doi: 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niethammer A.G., et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–1375. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 107.Luo Y., et al. FLK-1-based minigene vaccines induce T cell-mediated suppression of angiogenesis and tumor protective immunity in syngeneic BALB/c mice. Vaccine. 2007;25:1409–1415. doi: 10.1016/j.vaccine.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y.S., et al. Immunity against tumor angiogenesis induced by a fusion vaccine with murine beta-defensin 2 and mFlk-1. Clin Cancer Res. 2007;13:6779–6787. doi: 10.1158/1078-0432.CCR-07-1587. [DOI] [PubMed] [Google Scholar]
- 109.Xie K., et al. Anti-tumor effects of a human VEGFR-2-based DNA vaccine in mouse models. Genet Vaccines Ther. 2009;7:p10. doi: 10.1186/1479-0556-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuo S.G., et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol Pharm Bull. 2010;33:174–182. doi: 10.1248/bpb.33.174. [DOI] [PubMed] [Google Scholar]
- 111.Wei Y.Q., et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci U S A. 2001;98:11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okaji Y., et al. Vaccination with autologous endothelium inhibits angiogenesis and metastasis of colon cancer through autoimmunity. Cancer Sci. 2004;95:85–90. doi: 10.1111/j.1349-7006.2004.tb03175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei Y.Q., et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6:1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 114.Christian S., et al. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]
- 115.Bagley R.G., et al. Human endothelial precursor cells express tumor endothelial marker 1/endosialin/CD248. Mol Cancer Ther. 2008;7:2536–2546. doi: 10.1158/1535-7163.MCT-08-0050. [DOI] [PubMed] [Google Scholar]
- 116.Rettig W.J., et al. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A. 1992;89:10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walter-Yohrling J., et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10:2179–2189. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 118.Buckanovich R.J., et al. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol. 2007;25:852–861. doi: 10.1200/JCO.2006.08.8583. [DOI] [PubMed] [Google Scholar]
- 119.Simonavicius N., et al. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod Pathol. 2008;21:308–315. doi: 10.1038/modpathol.3801006. [DOI] [PubMed] [Google Scholar]
- 120.MacFadyen J.R., et al. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579:2569–2575. doi: 10.1016/j.febslet.2005.03.071. [DOI] [PubMed] [Google Scholar]
- 121•.Facciponte J.G., et al. Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature. J Clin Invest. 2014;124:1497–1511. doi: 10.1172/JCI67382. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report showing that therapeutic vaccination with a TEM1-specific cDNA reduces tumor vascularity, elicits endogenous anti-tumor T cell responses and limits tumor progression.
- 122.Santoro S.K., Motz S., Alatzoglou G.T., Li D., Chungsheng, Irving M., Powell D.J., Jr., Coukos G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res. 2015;3:68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chinnasamy D., et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W., et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 2013;20:970–978. doi: 10.1038/gt.2013.19. [DOI] [PubMed] [Google Scholar]
- 126•.Chinnasamy D., et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res. 2012;18:1672–1683. doi: 10.1158/1078-0432.CCR-11-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article demonstrated that targeted delivery of IL-12 into the tumor environment enhances the efficacy of VEGFR-2 redirected CAR T cells and reduces both the intratumoral and systemic CD11b(+)Gr1(+) myeloid suppressor cell subsets.
- 127••.Chinnasamy D., et al. Simultaneous targeting of tumor antigens and the tumor vasculature using T lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 2013;73:3371–3380. doi: 10.1158/0008-5472.CAN-12-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed for the first time that genetically engineered VEGFR-2 redirected CAR T cells act synergistically with tumor-specific immunotherapeutic approaches.
- 128•.Lanitis E., et al. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [129] this article shows that physical separation of T-cell activation signal 1 from costimulatory signal 2 in two CARs of differing antigen specificity retains CAR T cell anti-tumor potency and minimizes parallel reactivity against normal tissues.
- 129•.Kloss C.C., et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [128] this article demonstrated the efficacy and potential safety benefits of split CAR signaling.
- 130.Fedorov V.D., Themeli M., Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Straathof K.C., et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Di Stasi A., et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vogler I., et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol Ther. 2010;18:1330–1338. doi: 10.1038/mt.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Morgan R.A., et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang J.C., et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cobleigh M.A., et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30(Suppl. 16):117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Mayer R.J. Two steps forward in the treatment of colorectal cancer. N Engl J Med. 2004;350:2406–2408. doi: 10.1056/NEJMe048098. [DOI] [PubMed] [Google Scholar]
- 138.Hollebecque A., Massard C., Soria J.C. Vascular disrupting agents: a delicate balance between efficacy and side effects. Curr Opin Oncol. 2012;24:305–315. doi: 10.1097/CCO.0b013e32835249de. [DOI] [PubMed] [Google Scholar]
- 139.Hurwitz H., et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 140.Bellone M., Mondino A., Corti A. Vascular targeting, chemotherapy and active immunotherapy: teaming up to attack cancer. Trends Immunol. 2008;29:235–241. doi: 10.1016/j.it.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 141.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanahan D., Bergers G., Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kerbel R.S., Kamen B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 144.Cham K.K., et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103:52–60. doi: 10.1038/sj.bjc.6605727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiang C.L., et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Voron T., et al. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tol J., et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]