Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) is more frequent in males than in females. The “female protective effect” posits that females undergo greater exposure to etiological factors than males in order to develop ADHD, leading to the prediction that relatives of females with ADHD will display more ADHD behaviors. We thus tested whether cotwins of females displaying extreme ADHD traits would display more ADHD traits than cotwins of males displaying extreme ADHD traits.
Method
Parents of approximately 7,000 pairs of nonidentical twins in Sweden, and approximately 4,000 pairs of twins in England and Wales, completed dimensional assessments of ADHD traits. Probands were selected on the basis of scoring within the highest 10% of the distribution in each sample. Dimensional scores of cotwins of probands, as well as the categorical recurrence rate, were investigated by proband sex.
Results
Cotwins of female probands displayed higher mean ADHD trait scores (mean = 0.62−0.79) than cotwins of male probands (mean = 0.38−0.55) in both samples. This trend was significant in the Swedish sample (p < .01) and when the 2 samples were merged into a single, larger sample (p < .001). When the samples were merged, there was also a significant association between proband sex and cotwin’s categorical status, with more cotwins of female probands also being probands than cotwins of male probands.
Conclusion
These findings support a female protective effect against ADHD behaviors, suggesting that females require greater exposure to genetic and environmental factors associated with ADHD in order to develop the condition.
Key words: ADHD, sex differences, genetics, twin study
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition characterized by excessive hyperactivity and impulsivity, inattentiveness, or a combination of these symptoms.1 Epidemiological studies suggest that, overall, ADHD affects between 5% and 7% of the population.2 Notably, ADHD appears to be substantially more common in males than in females. A study of 10 European countries, for instance, indicated that males with ADHD outnumbered females with ADHD by ratios from 2 to 1 to 16 to 1.3 The excess of males with ADHD has been further confirmed by meta-analyses, with 4 times as many males as females thought to be affected.2, 4
A number of twin studies have established that ADHD is among the most heritable of neuropsychiatric conditions.5, 6, 7, 8, 9 The high heritability of ADHD does not vary markedly whether it is conceptualized as a categorical, diagnosed condition5, 6 or treated as a continuous trait in the general population,7, 9 thus indicating that severe forms of ADHD may be linked genetically with milder, subclinical traits of ADHD in the general population. Such studies, however, have yet to shed light on the reasons why ADHD appears to be so much more common in males than in females.
One possible explanation for the sharp sex discrepancy in ADHD prevalence is a putative “female protective effect” model. Under this model, females would be predicted to require greater exposure than males to genetic and environmental factors associated with ADHD in order to display sufficient ADHD behaviors to warrant a diagnosis, thus meaning that fewer females than males would be expected to be diagnosed with ADHD.10, 11 As such, one would expect more causal factors to be present in the families of females with ADHD, leading to the prediction that ADHD and ADHD behaviors would be more prevalent in the relatives of females with ADHD. The female protective effect is presently receiving considerable attention in relation to autism spectrum disorders (ASD), which are similarly male-biased conditions. In 1 study, for example, the fraternal cotwins of females displaying a high degree of autistic traits displayed more autistic traits than did cotwins of males with high degrees of autistic traits, and were also more likely to display high scores themselves.12
Very few studies have tested for the existence of a female protective effect against ADHD. A recent Swedish investigation suggested that merely having a female cotwin is associated with displaying a greater degree of ADHD traits than having a male cotwin, although this study did not take account of the degree to which the index twin displayed ADHD symptoms.13 In 1 study of a US-based twin sample, traits of ADHD were examined in the cotwins of individuals displaying a high degree of traits of ADHD. Cotwins of females displaying extreme ADHD traits displayed significantly greater ADHD-like behaviors than cotwins of males displaying extreme traits of ADHD.14 Of note, however, the effect was not present for the cotwins of the most severely affected twins, perhaps owing to the small effect size and lower number of twins displaying the very highest scores.
As a consequence, we aimed to test for the existence of a female protective effect against ADHD behaviors in 2 independent, large-scale European twin samples. We first tested whether the cotwins of females displaying extreme degrees of ADHD traits would exhibit more continuous ADHD traits than the cotwins of high-scoring males. Second, we sought to test whether high-scoring female twins would be more likely to have a high-scoring cotwin than high-scoring male twins. The 1 previous study documenting this effect reported that the effect size was small14; thus, we not only aimed to test for the female protective effect against ADHD in our 2 samples independently, but also pooled the 2 samples to increase power. We expected, in light of existing evidence, to find evidence of a female protective effect against ADHD behaviors.13, 14
Method
Participants
Data were collected from participants in 2 representative, community-based twin studies. The Child and Adolescent Twin Study in Sweden (CATSS) is a study of twins born in Sweden since 1992. Initially, the twins were contacted in connection with their ninth birthdays.15 For the present study, data were collected from twins participating in CATSS when they were aged 9 years. The second sample comprised participants in the Twins Early Development Study (TEDS); TEDS is a sample of twins born in England and Wales between 1994 and 1996.16 Data for the present study were collected from TEDS participants when twins were aged 8 years. TEDS and CATSS are representative of the populations of England and Wales and of Sweden, respectively.15, 16
Both CATSS and TEDS comprise both monozygotic (MZ) and dizygotic (DZ) twins, although only DZ twins were included in this study owing to the fact that the genetic resemblance of 2 DZ twins within a pair is the same as the resemblance between 2 singleton siblings (approximately 50% of their segregating DNA code on average). Both same-sex and opposite-sex DZ twins were included. In CATSS, families of 6,817 pairs of DZ twins returned data, whereas 4,309 participating families in TEDS returned data. In CATSS, exclusions were conducted for known brain injuries and chromosomal syndromes (n = 113), leaving 6,704 DZ twin pairs. Participants in TEDS were excluded for genetic and chromosomal syndromes, extreme perinatal complications, and missing first contact data (n = 254), leaving 4,055 pairs of DZ twins. Combined, there were 10,759 DZ twin pairs across the 2 samples.
CATSS has ethical approval from the Karolinska Institutet Ethical Review Board, and TEDS has ethical approval from the King’s College London, Institute of Psychiatry, Psychology and Neuroscience Ethics Committee.
Measures
In CATSS, the ADHD modules of the Autism-Tics, ADHD, and Other Comorbidities inventory (A-TAC)17 were administered to parents of the twins over the telephone. There are 2 ADHD modules, assessing hyperactivity/impulsivity and inattentiveness, comprising a total of 19 items that correspond closely to DSM-IV criteria for ADHD.1 Each item comprised a question, answered “yes” (for a score of 1), “yes, to some extent” (for a score of 0.5), or “no” (for a score of 0). Thus, the maximum possible score was 19. In the sample of DZ twins used in the present study, the A-TAC ADHD module had strong internal consistency (α = 0.92). A prior study reported strong construct validity for the scale, with 92% sensitivity and 75% specificity for detecting ADHD.18 The A-TAC also comprises 2 subscales, assessing ADHD subtypes: Hyperactivity/Impulsivity (10 items) and Inattention (9 items).
Parents of twins participating in TEDS completed the ADHD subscale of the Conners’ Parent Rating Scale–Revised (Conners ADHD).19 The measure was mailed to parents of the twins, who completed and returned it. The Conners ADHD measure comprises 18 items that are closely linked with the DSM-IV criteria for ADHD.1 Each item comprised a statement in response to which the parents rated, on a 0 to 3 scale, the extent to which each item was true of their children. The maximum possible score was 54. In the present study, the Conners ADHD showed strong internal consistency (α = 0.91). Previously, individuals with ADHD have been shown to score more highly on the measure than controls,19 supporting its construct validity. As with the A-TAC, Conners ADHD comprises Hyperactivity/Impulsivity and Inattention subscales (9 items each).
Data Analysis
Proband Selection
In both samples, 1 twin was randomly selected as the “index twin.” All other twins were cotwins. Probands were selected as the index twins scoring within the highest 10% of the A-TAC and Conners ADHD distributions, with such a cut-off designed to maximize statistical power while capturing severe-enough cases. Thus, probands in CATSS were selected on the basis of A-TAC scores of 6.5 or more, whereas TEDS probands were defined as index twins scoring at least 23 on the Conners ADHD. Subsequently, analyses were repeated using more conservative cut-offs of 9.5 on the A-TAC and 28 on the Conners ADHD. These cut-offs were designed to capture the highest scoring 5% of each sample, thus testing for a female protective effect in relation to even more extreme scores. Due to the lack of sex-specific diagnostic criteria for ADHD, the same cut-offs were used to select probands, regardless of sex. The number of probands, split by sex, is given in Table 1.
Table 1.
Numbers of Probands in Studies
| Measure | Sample | 5% |
10% |
||
|---|---|---|---|---|---|
| Male, n (%) | Female, n (%) | Male, n (%) | Female, n (%) | ||
| Total ADHD | CATSS | 227 (70) | 95 (30) | 450 (69) | 201 (31) |
| TEDS | 146 (70) | 63 (30) | 291 (68) | 138 (32) | |
| Merged | 373 (70) | 158 (30) | 741 (69) | 339 (31) | |
| Hyperactivity/Impulsivity | CATSS | 208 (68) | 100 (32) | 382 (63) | 229 (37) |
| TEDS | 142 (69) | 65 (31) | 242 (63) | 140 (37) | |
| Merged | 350 (68) | 165 (32) | 624 (63) | 369 (37) | |
| Inattention | CATSS | 238 (69) | 109 (31) | 468 (66) | 237 (34) |
| TEDS | 141 (71) | 57 (29) | 255 (69) | 116 (31) | |
| Merged | 379 (70) | 166 (30) | 723 (67) | 353 (33) | |
Note: ADHD = attention-deficit/hyperactivity disorder; CATSS = Child and Adolescent Twin Study in Sweden; TEDS = Twins Early Development Study.
Statistical Analysis
To test whether cotwins of female probands would display higher ADHD trait scores than cotwins of male probands, 3×2 between-subjects analysis of variance (ANOVA) was used. Proband status of the index twin (male proband, female proband, or control) was the grouping variable, with cotwins’ ADHD trait scores acting as the outcome variable. An omnibus test initially compared scores across cotwins of male probands, female probands, and controls, before planned comparisons compared the scores of cotwins of male and female probands. Individual p values were adjusted for multiple comparisons within each sample using the Bonferroni correction. All reported p values are adjusted in this manner. Effect sizes were summarized using Cohen’s d. Effect sizes were interpreted using Cohen’s criteria,20 with d of 0.20 to 0.49 considered a small effect, 0.50 to 0.79 medium, and greater than 0.80 large.
To test whether the sex of the probands was associated with whether or not their cotwin would also be a proband, categorical analyses were used. Using the above identified cut-offs, cotwins were classified as either “affected” (i.e., scoring above a given cut-off) or “unaffected” (i.e., scoring below a given cut-off). Then χ2 tests of association were used to test whether cotwin status was significantly associated with proband sex. Effect sizes were summarized using odds ratios (ORs).
Analyses were first conducted separately in CATSS and TEDS. To bolster statistical power, a third set of analyses was performed on the 2 samples combined. The Conners ADHD and A-TAC were both heavily, positively skewed and were therefore log transformed before analysis (Table 2). All cotwin scores used in analyses were standardized by sex of the cotwin, thus ensuring that cotwin sex was controlled for and allowing easier comparability of findings across samples. All analyses were performed in R.21
Table 2.
Descriptive Statistics
| Measure | Cronbach’s α | Possible Range of Scores | Mean Full Sample (SD) | Mean Males (SD) | Mean Females (SD) | Skew |
|---|---|---|---|---|---|---|
| A-TACa | 0.92 | 0−19 | 2.10 (3.21) | 2.54 (3.54) | 1.62 (2.73) | 2.34 |
| Conners ADHDb | 0.91 | 0−54 | 10.84 (9.00) | 12.67 (9.71) | 9.04 (7.85) | 1.37 |
| A-TAC Hyp/Impc | 0.89 | 0−10 | 0.99 (1.69) | 1.17 (1.85) | 0.81 (1.48) | 2.53 |
| A-TAC Inattend | 0.90 | 0−9 | 1.03 (1.73) | 1.28 (1.91) | 0.78 (1.49) | 2.23 |
| Conners Hyp/Impe | 0.89 | 0−27 | 5.57 (4.93) | 6.39 (5.28) | 4.78 (4.41) | 1.33 |
| Conners Inattenf | 0.91 | 0−27 | 5.27 (5.04) | 6.29 (5.45) | 4.26 (4.37) | 1.41 |
Note: ADHD = attention-deficit/hyperactivity disorder; A-TAC = Autism-Tics and Other Comorbidities Inventory; Conners ADHD = ADHD subscale of the Conners’ Parent Rating Scale; Hyp = hyperactivity; Imp = impulsivity; Inatten = inattention.
Mean A-TAC scores were significantly higher for males than females in the full sample (t6539.22 = 11.90, p < .001, d = 0.29).
Mean Conners ADHD scores were significantly higher for males than females in the full sample (t3843.64 = 13.08, p < .001, d = 0.42).
Mean A-TAC Hyperactivity/Impulsivity scores were significantly higher for males than females in the full sample (t6431.70 = 8.95, p < .001, d = 0.22).
Mean A-TAC Inattention scores were significantly higher for males than females in the full sample (t6369.05 = 12.08, p < .001, d = 0.30).
Mean Conners ADHD Hyperactivity/Impulsivity scores were significantly higher for males than females in the full sample (t3890.60 = 10.50, d = 0.34).
Mean Connors ADHD Inattention scores were significantly higher for males than females in the full sample, t(3829.86) = 13.02, p < .001.
Post hoc analyses subsequently tested for a female protective effect against specific ADHD behaviors. All of the analyses detailed above were repeated on the Hyperactivity/Impulsivity and Inattention subscales of the A-TAC and Conners ADHD. Because these analyses were post hoc, p values were not adjusted for multiple comparisons.
Results
Descriptive statistics for the A-TAC and Conners ADHD are given in Table 2.
Analysis of Continuous Scores
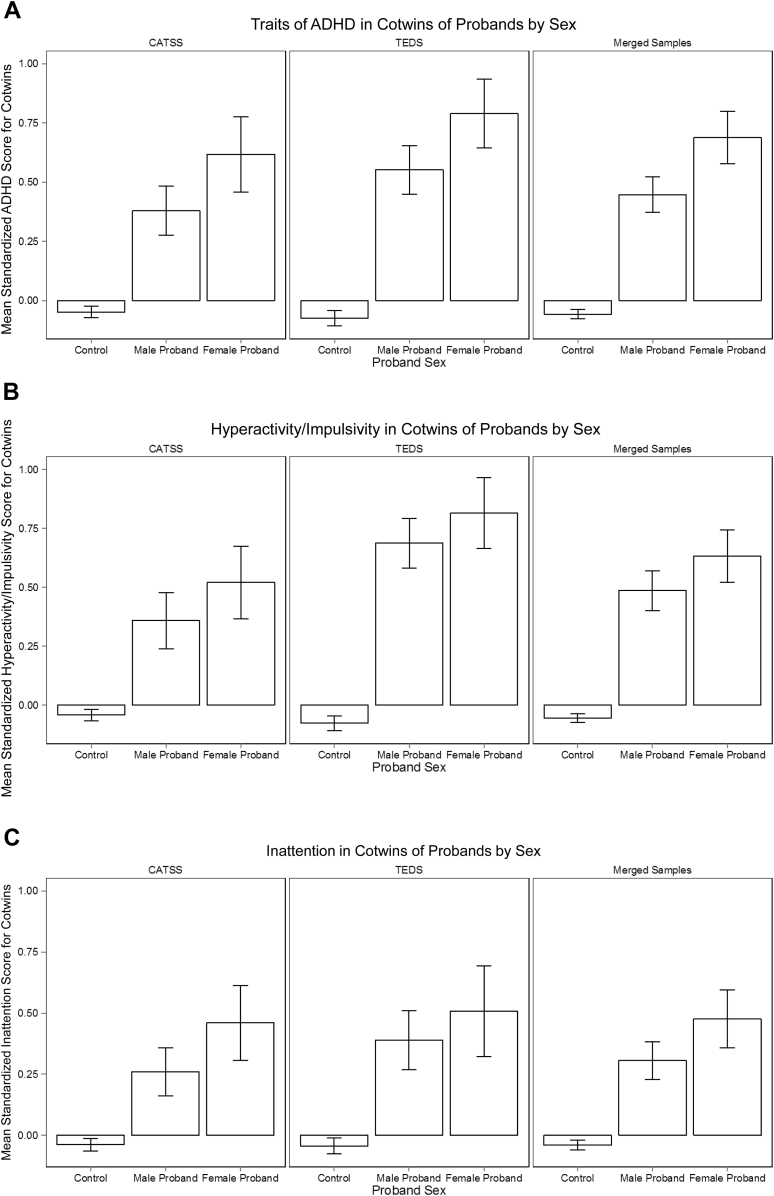
Mean standardized scores of cotwins of male probands, female probands, and controls are all shown in Figure 1 for the analyses using the 10% cut-offs. In CATSS, scores differed significantly across the 3 groups (F2,6688 = 79.35, p < .01), with cotwins of female probands scoring highest (mean = 0.62), followed by cotwins of male probands (mean = 0.38), and cotwins of controls (mean = −0.05). Specifically, cotwins of female probands displayed significantly higher A-TAC scores than cotwins of male probands (t6688 = −2.84, p < .01), with a modest effect size of d = 0.07.
Figure 1.
Mean cotwin scores for (a) full-scale attention-deficit/hyperactivity disorder (ADHD), (b) hyperactivity/impulsivity, and (c) inattention for the highest scoring 10% in all analyses. Note: Error bars represent standard deviations. CATSS = Child and Adolescent Twin Study in Sweden; TEDS = Twins Early Development Study.
Similarly in TEDS, Conners ADHD scores differed significantly across cotwins of male probands (mean = 0.55), cotwins of female probands (mean = 0.79), and cotwins of controls (mean = −0.07) (F2,4040 = 102.30, p < .001). Planned contrasts, however, indicated that mean Conners ADHD scores were not significantly elevated in cotwins of female probands relative to cotwins of male probands (t4040 = −2.36, p = .08, d = 0.07), despite a trend in this direction.
Merging the 2 samples produced the same pattern; mean ADHD trait scores differed significantly in the 3 groups (F2,10731 = 175.90, p < .001), with cotwins of female probands showing the highest ADHD trait scores (mean = 0.69), followed by cotwins of male probands (mean = 0.45), and controls (mean = −0.06). The planned contrast confirmed that mean ADHD trait scores were significantly higher for cotwins of female probands than cotwins of male probands (t10731 = −3.73, p < .001, d = 0.07).
All mean ADHD trait scores for cotwins of probands selected under the more severe, 5% cut-offs are given in Table 3. Merging the 2 samples produced the same pattern of results as the 10% cut-off. Index twin status exacted a significant main effect on the mean ADHD trait scores of cotwins (F2,10731 = 95.90, p < .001), with cotwins of female probands displaying the highest ADHD trait scores (mean = 0.73), followed by cotwins of male probands (mean 0.51) and controls (mean = −0.03). Mean ADHD trait scores were significantly elevated in cotwins of female probands compared with cotwins of male probands (t10731 = −2.38, p < .05, d = 0.05).
Table 3.
Analysis of Continuous Traits of Attention-Deficit/Hyperactivity Disorder (ADHD) in Cotwins
| 5% |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.43 (1.18) | 0.62 (0.89) | 0.51 (1.08) |
| Cotwin of female proband mean | 0.69 (1.23) | 0.79 (1.02) | 0.73 (1.15) |
| Cotwin of control mean | −0.03 (0.98) | −0.04 (0.99) | −0.03 (0.99) |
| Omnibus ANOVA | F2,6688 = 46.39, p < .01 | F2,4040 = 51.63, p < .001 | F2,10731 = 95.90, p < .001 |
| Planned contrast | t6688 = −2.10, p = .16, d = 0.05 | t4040 = −1.10, p = .27, d = 0.03 | t10731 = −2.38, p < .05, d = 0.05 |
| 10% |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.38 (1.11) | 0.55 (0.89) | 0.45 (1.04) |
| Cotwin of female proband mean | 0.62 (1.14) | 0.79 (0.87) | 0.69 (0.79) |
| Cotwin of control mean | −0.05 (0.97) | −0.07 (0.99) | −0.06 (0.98) |
| Omnibus ANOVA | F2,6688 = 79.35, p < .01 | F2,4040 = 102.30, p < .001 | F2,10731 = 175.90, p < .001 |
| Planned contrast | t6688 = −2.84, p < .01, d = 0.07 | t4040 = −2.36, p = .08, d = 0.07 | t10731 = −3.73, p < .001, d = 0.07 |
Note: Merged samples are analyses of both Child and Adolescent Twin Study in Sweden (CATSS) and Twins Early Development Study (TEDS), merged into a single dataset. Numbers in parentheses are standard deviations. Omnibus analysis of variance (ANOVA) is a comparison of all 3 conditions (cotwins of male probands, cotwins of female probands, and cotwins of controls); planned contrast is a comparison of cotwins of male probands and cotwins of female probands.
Using a 5% cut-off, the same pattern emerged in each individual sample. In CATSS, mean A-TAC scores differed significantly across cotwins of male probands (mean = 0.43), female probands (mean = 0.69), and controls (mean = −0.03) (F2,6688 = 46.39, p < .01); however, mean A-TAC scores for cotwins of female probands were not significantly higher than mean A-TAC scores for cotwins of male probands (t6688 = −2.10, p = .16, d = 0.05). The same result emerged for TEDS. Although the main effect of index twin status was significant (F2,4040 = 51.63, p < .001), with cotwins of female probands showing the highest mean Conners ADHD scores (mean = 0.79), followed by cotwins of male probands (mean = 0.62) and controls (−0.04), mean Conners ADHD scores for cotwins of female probands were not significantly higher than mean scores for cotwins of male probands (t4040 = −1.10, p = .27, d = 0.03).
Analysis of Categorical Recurrence
Table 4 shows the number of affected and unaffected cotwins by proband sex for each sample and cut-off. Using a 10% cut-off to select probands, the association between proband sex and cotwin status was significant only when CATSS and TEDS were merged (χ21 = 5.21, p < .05, OR = 0.70 [95% CI: 0.54/0.94]), with a greater proportion of cotwins of female probands (29%) than cotwins of male probands (22%) showing higher ADHD trait scores.
Table 4.
Analyses of Categorical Recurrence Rates
| CATSS | 5% |
10% |
||
|---|---|---|---|---|
| Cotwin “Affected” | Cotwin “Unaffected” | Cotwin “Affected” | Cotwin “Unaffected” | |
| Male proband | 28 (12) | 199 (88) | 40 (9) | 410 (91) |
| Female proband | 20 (21) | 75 (79) | 30 (15) | 171 (85) |
| χ21 = 3.35, p = .14, OR = 0.53 [0.28/0.99] | χ21 = 4.67, p = .06, OR = 0.57 [0.34/0.92] | |||
| TEDS | 5% |
10% |
||
|---|---|---|---|---|
| Cotwin “Affected” | Cotwin “Unaffected” | Cotwin “Affected” | Cotwin “Unaffected” | |
| Male proband | 32 (22) | 114 (78) | 83 (29) | 208 (71) |
| Female proband | 21 (33) | 42 (67) | 52 (38) | 86 (62) |
| χ21 = 2.46, p = .24, OR = 0.56 [0.29/1.08] | χ21 = 3.23, p = .14, OR = 0.66 [0.43/1.01] | |||
| Merged Samples | 5% |
10% |
||
|---|---|---|---|---|
| Cotwin “Affected” | Cotwin “Unaffected” | Cotwin “Affected” | Cotwin “Unaffected” | |
| Male proband | 60 (16) | 313 (84) | 163 (22) | 578 (78) |
| Female proband | 41 (26) | 117 (74) | 97 (29) | 242 (71) |
| χ21 = 6.38, p < .05, OR = 0.54 [0.35/0.86] | χ21 = 5.21, p < .05, OR = 0.70 [0.52/0.94] | |||
Note: Data are shown as n (%) except where noted and in brackets, which are 95% CIs. The percentages 5% and 10% indicate which cut-off was used to select probands in each analysis (highest scoring 10% of each sample or highest scoring 5% of each sample). Merged samples are analyses of both Child and Adolescent Twin Study in Sweden (CATSS) and Twins Early Development Study (TEDS) merged into a single dataset. OR = odds ratio.
In CATSS, a greater proportion of cotwins of female probands scoring above the 10% also scored above the cut-off (15% of cotwins of female probands compared with 9% of cotwins of male probands). The association was small and nonsignificant, however (χ21 = 4.67, p = .06, OR = 0.57 [95% CI: 0.34/0.92]). Similarly in TEDS, 38% of cotwins of female probands scored above the 10% cut-off compared with 29% of cotwins of male probands, although this association was again small and failed to reach significance (χ21 = 3.23, p = .14, OR = 0.66 [95% CI: 0.43/1.01]).
The findings followed the same pattern when a cut-off that selected 5% of index twins as probands was used. When CATSS and TEDS were merged, there was a significant association between proband sex and cotwin status (χ21 = 6.38, p ≤ .05, OR = 0.54 [95% CI: 0.35/0.86]), with a greater proportion of cotwins of female probands (26%) than cotwins of male probands (16%) showing a pronounced degree of ADHD traits. In CATSS alone, more cotwins of female probands (21%) than cotwins of male probands (12%) were affected, yet this association was not significant (χ21 = 3.35, p = .14, OR = 0.53 [95% CI: 0.28/0.99]). The same was true of TEDS: more cotwins of female probands (33%) than cotwins of male probands (22%) were affected, yet this seeming association was not significant (χ21 = 2.46, p = .24, OR = 0.56 [95% CI: 0.29/1.08]).
Subscale Analyses
Figures 1b and 1c show the mean scores of cotwins of probands on the A-TAC and Conners ADHD subscales (Hyperactivity/Impulsivity and Inattention), with probands scoring within the highest 10% of the subscales. In CATSS, TEDS, and the combined cohorts, cotwins of female probands displayed the highest mean Hyperactivity/Impulsivity scores, followed by cotwins of male probands and cotwins of controls. In all analyses, the mean scores of cotwins of female probands were significantly higher than those of the other 2 groups (p < .05).
Inattention followed the same pattern, as shown in Figure 1c. Cotwins of female probands displayed the highest mean Inattention score, followed by cotwins of male probands and cotwins of controls. The mean scores of cotwins of female probands were significantly higher than both other groups of cotwins in all 3 analyses (p < .05).
These results are shown in full in Table S1, Table S2, Table S3, Table S4, available online.
Discussion
This investigation sought to test whether a female protective effect can account for the substantially elevated prevalence of ADHD in males relative to females.2, 3, 4 The results of this study lend partial credence to a female protective effect hypothesis for ADHD. In line with the results of an existing US study14 and our hypotheses, there was some evidence to indicate that the cotwins of females displaying an extreme degree of characteristic ADHD behaviors displayed more such behaviors themselves than did the cotwins of males showing an extreme degree of ADHD traits. Furthermore, cotwins of females with particularly high ADHD trait scores were more likely to display an extreme degree of ADHD behaviors than were the cotwins of males. As such, these findings tentatively indicate that a female protective effect could be a potentially viable model to help understand the development of ADHD.
Our findings provide a platform for future research into the genetic basis of ADHD to build upon. Although twin studies of ADHD have consistently supported its high heritability,5, 6, 7, 8, 9 elucidating the precise genetic mechanisms underpinning ADHD has proved elusive.22 The female protective effect model provides an opportunity to raise further research questions in such research. For example, genes can be divided into high-impact and low-impact sets.23 One possibility is that females with ADHD are more likely to inherit higher impact genes associated with ADHD, which are rarer. To illustrate, ASD also more commonly affects males than females,24 and recent twin and family studies support a female protective effect against ASD.12, 25 A genetic study then indicated that females with ASD displayed a higher degree of larger copy number variants, which were more likely to be maternally inherited.11 Similar studies of ADHD may well prove useful in furthering our understanding of the etiology of ADHD.
Indeed, although our study did not investigate any specific etiological mechanisms associated with ADHD, our findings suggest that investigating the degree of exposure to etiological factors associated with ADHD in males and females with the condition may be a worthwhile future research direction. Although the above example mentioned larger, rarer copy number variants, one might also test whether females with ADHD exhibit a greater number of smaller, common genetic variants. Indeed, in using polygenic scores, which have yielded useful insights in the genetic architecture of ADHD,26 one could investigate whether females with ADHD display a greater degree of common genetic variants associated with ADHD than males with ADHD.27
One could also extend this to causal environmental factors. Although twin studies indicate that genetic factors seem to outweigh environmental factors in the etiology of ADHD,5, 6, 7, 8, 9 research has implicated certain environmental exposures with ADHD. For instance, lower birth weight is thought to be a causal environmental factor in ADHD.28, 29 It may be that females with ADHD undergo greater exposure to such factors compared with males; for instance, could females with ADHD display an even lower birth weight than males with ADHD?
The presence of a female protective effect against ADHD behaviors also has implications for clinical practice. If clinicians take account of family history when diagnosing ADHD, it may be beneficial also to take into account the sex of any previously affected relatives, under the assumption that relatives of females with ADHD are more likely to exhibit ADHD symptoms than relatives of males with ADHD. The caveat to this assertion, however, is that our findings are based only on twin data. The female protective effect against ADHD needs to be replicated in alternative, non-twin samples before such a conclusion can be decisively drawn. For instance, a recent study of ASD found that siblings of female non-twins with ASD were more likely to have ASD than siblings of male non-twins.25 Such studies of non-twin relatives are now needed in relation to ADHD.
It does need to be noted that the overall sizes of the effects reported here, where significant, were weak. The effect reported in this article is less than half the size of the female protective effect in relation to autism reported in a similar study.12 Indeed, significant findings emerged only for the more severe cut-off of 5% when the 2 samples used were merged to create a larger sample. The small effect size seen here is consistent with that reported previously,14 and so it is quite clear that subsequent studies testing the female protective effect model of ADHD are going to require large samples.
The small female protective effect seen here does, nevertheless, stress the need not to discount alternative explanations for the increased number of males with ADHD relative to females. There is very limited research considering phenotypic differences between males and females with ADHD. For instance, 1 study investigated sex differences in ADHD across 10 European countries, and reported that females with ADHD displayed more emotional difficulties.3 Furthermore, the DSM-IV criteria for ADHD, upon which our measures were based, are based exclusively on observations of males.1, 30, 31
Two further possibilities cannot be discounted from our study. Although our findings lend support to the notion of a female protective effect against ADHD, it is not mutually exclusive to the hypothesis that males have more risk factors for ADHD. It is also, in theory, possible that rater contrast effects drove the higher scores seen in cotwins of female probands. Rater contrast effects refer to the scenario whereby parent ratings of 1 twin are influenced by how they view their cotwin.32 To create the observed pattern of results, parents would need to have shown a stronger rater contrast effect on the cotwins (who were both male and female) of male probands than of female probands. In twin analyses of the A-TAC and Conners ADHD scale, rater contrast effects have been modeled and shown to be modest,9, 33 suggesting that rater contrast effects are unlikely to be an adequate explanation for our findings.
In addition to the caveat regarding the small effect size, our study did have further limitations that need taking into account. Proband status was ascertained through the use of dimensional questionnaire measures, as opposed to in-depth assessments of ADHD. The use of this approach would, however, have come at the cost of the large sample size. As mentioned above, only twins were used in this study. Although we removed MZ twins to ensure that the genetic relatedness of the relatives in our sample was similar to that of fraternal siblings, it is important to know whether these findings extend to non-twin relatives in the future. In defense of our use of a twin sample, on the other hand, there is evidence to indicate that ADHD traits are not elevated in twins’ relatives to singletons.34
Finally, our study did not take comorbidity into account. Females with ADHD are more likely to present with additional disorders, such as anxiety and depression, than males with ADHD.35 If one assumes a certain degree of common causal factors across ADHD and other neuropsychiatric disorders, as supported by recent twin studies,36, 37 then it is possible that females manifest with different symptoms at lower levels of exposure to etiological factors, with ADHD emerging only after greater exposure. This could be tested in the future using ADHD polygenic risk scores, for example.
To a certain degree, this study indicates that females are protected against behaviors characteristic of ADHD. Although our findings do not speak to any specific mechanisms through which this effect may operate, this study indicates that further research on the female protective effect model is warranted in relation to ADHD, with a view to identify the specific biological basis of this effect. If the effect holds across multiple epidemiological methods, then it represents a plausible explanation for why fewer females than males develop ADHD, as well as assisting in the diagnostic process.
Acknowledgments
The authors are indebted to the participants in CATSS and TEDS for making this study possible. The authors thank Robert Plomin, PhD, King’s College London, for the TEDS collaboration.
Footnotes
An interview with the author is available by podcast at www.jaacap.org or by scanning the QR code to the right.
The Child and Adolescent Twin Study in Sweden (CATSS) is funded by the Swedish Council for Health, Working Life and Welfare and the Swedish Research Council. Twins Early Development Study (TEDS) is funded by the Medical Research Council (grant G0901245, and previously G0500079, to Robert Plomin).
Disclosure: Dr. Larsson has served as a speaker for Eli Lilly and Co. and Shire, and has received a research grant from Shire. Drs. Taylor, Lichtenstein, Anckarsäter, Greven, and Ronald report no biomedical financial interests or potential conflicts of interest.
Supplemental Material
Table S1.
Analyses of Hyperactivity/Impulsivity Continuous Scores
| Continuous Scores (5%) |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.36 (1.19) | 0.74 (0.85) | 0.51 (1.08) |
| Cotwin of female proband mean | 0.72 (1.23) | 0.99 (0.85) | 0.83 (1.10) |
| Cotwin of control mean | −0.02 (0.98) | −0.04 (0.99) | −0.03 (0.98) |
| Omnibus ANOVA | F2,6696 = 41.65, p < .001 | F2,4038 = 77.06, p < .001 | F2,10737 = 109.60, p < .001 |
| Planned contrast | t6696 = −3.04, p < .01, d = 0.04 | t4038 = −1.76, p = .08, d = 0.05 | t10737 = −10.06, p < .001, d = 0.19 |
| Continuous Scores (10%) |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.36 (1.18) | 0.69 (0.84) | 0.49 (1.07) |
| Cotwin of female proband mean | 0.52 (1.18) | 0.81 (0.90) | 0.63 (1.09) |
| Cotwin of control mean | −0.04 (0.97) | −0.08 (0.98) | −0.06 (0.97) |
| Omnibus ANOVA | F2,6696 = 62.08, p < .001 | F2,4038 = 121.20, p < .001 | F2,10737 = 167.30, p < .001 |
| Planned contrast | t6696 = −7.66, p < .001, d = 0.09 | t4038 = −11.85, p < .001, d = 0.37 | t10737 = −13.31, p < .001, d = 0.26 |
Note: Numbers in parentheses are standard deviations. ANOVA = analysis of variance; CATSS = Child and Adolescent Twin Study in Sweden; TEDS = Twins Early Development Study.
Table S2.
Analyses of Hyperactivity/Impulsivity Categorical Recurrence Rates
| CATSS | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 23 (11) | 185 (89) | 41 (11) | 341 (89) |
| Female proband | 18 (18) | 82 (82) | 30 (13) | 199 (87) |
| χ21 = 2.25, p = .13, OR = 0.57 [0.29−1.11] | χ21 = 0.57, p = .45, OR = 0.80 [0.48−1.31] | |||
| TEDS | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 30 (21) | 112 (79) | 62 (25) | 180 (75) |
| Female proband | 19 (29) | 46 (71) | 50 (36) | 90 (64) |
| χ21 = 1.20, p = .27, OR = 0.64 [0.33−1.27] | χ21 = 3.89, p = .05, OR = 0.62 [0.40−0.97] | |||
| Merged Samples | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 53 (15) | 297 (85) | 131 (21) | 493 (79) |
| Female proband | 37 (22) | 128 (78) | 101 (27) | 268 (73) |
| χ21 = 3.63, p = .06, OR = 0.68 [0.39−0.99] | χ21 = 4.92, p < .05, OR = 0.71 [0.52−0.95] | |||
Note: Data are shown as n (%) except where noted and in brackets, which are 95% CIs. CATSS = Child and Adolescent Twin Study in Sweden; OR = odds ratio; TEDS = Twins Early Development Study.
Table S3.
Analyses of Inattention Continuous Scores
| 5% |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.30 (1.09) | 0.31 (1.01) | 0.30 (1.06) |
| Cotwin of female proband mean | 0.57 (1.28) | 0.44 (1.15) | 0.53 (1.24) |
| Cotwin of control mean | −0.02 (0.99) | −0.02 (0.99) | −0.02 (0.99) |
| Omnibus ANOVA | F2,6691 = 29.71, p < .001 | F2,4038 = 13.17, p < .001 | F2,10732 = 42.56, p < .001 |
| Planned contrast | t6691 = −2.37, p < .05, d = 0.03 | t4038 = −0.84, p = .40, d = 0.03 | t10732 = −6.18, p < .001, d = 0.12 |
| 10% |
|||
|---|---|---|---|
| CATSS | TEDS | Merged Samples | |
| Cotwin of male proband mean | 0.26 (1.09) | 0.39 (0.97) | 0.31 (1.05) |
| Cotwin of female proband mean | 0.46 (1.20) | 0.51 (1.00) | 0.48 (1.13) |
| Cotwin of control mean | −0.04 (0.98) | −0.04 (0.99) | −0.04 (0.98) |
| Omnibus ANOVA | F2,6691 = 46.07, p < .001 | F2,4038 = 38.50, p < .001 | F2,10732 = 83.02, p < .001 |
| Planned contrast | t6691 = −6.28, p < .001, d = 0.15 | t4038 = −6.75, p < .001, d = 0.21 | t10732 = −9.06, p < .001, d = 0.17 |
Note: Numbers in parentheses are standard deviations. ANOVA = analysis of variance; CATSS = Child and Adolescent Twin Study in Sweden; TEDS = Twins Early Development Study.
Table S4.
Analyses of Inattention Categorical Recurrence Rates
| CATSS | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 22 (9) | 216 (91) | 80 (17) | 388 (83) |
| Female proband | 22 (20) | 87 (80) | 56 (26) | 181 (74) |
| χ21 = 7.12, p < .05, OR = 0.40 [0.21−0.76] | χ21 = 3.91, p = .05, OR = 0.67 [0.45−0.98] | |||
| TEDS | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 14 (10) | 127 (90) | 45 (18) | 210 (82) |
| Female proband | 13 (23) | 44 (77) | 30 (26) | 86 (74) |
| χ21 = 4.67, p < .05, OR = 0.37 [0.16−0.85] | χ21 = 2.85, p = .09, OR = 0.61 [0.36−1.04] | |||
| Merged Samples | 5% |
10% |
||
|---|---|---|---|---|
| “Affected” Cotwin | “Unaffected” Cotwin | “Affected” Cotwin | “Unaffected” Cotwin | |
| Male proband | 36 (9) | 343 (91) | 125 (17) | 598 (83) |
| Female proband | 35 (21) | 131 (79) | 86 (32) | 267 (68) |
| χ21 = 12.67, p < .001, OR = 0.39 [0.24−0.65] | χ21 = 7.09, p < .05, OR = 0.65 [0.48−0.89] | |||
Note: Data are shown as n (%) except where noted and in brackets, which are 95% CIs. CATSS = Child and Adolescent Twin Study in Sweden; OR = odds ratio; TEDS = Twins Early Development Study.
References
- 1.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Willcutt E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–499. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nøvik T.S., Hervas A., Ralston S.J. Influence of gender on attention-deficit/hyperactivity disorder in Europe—ADORE. Eur Child Adolesc Psychiatry. 2006;15(Suppl 1):15–24. doi: 10.1007/s00787-006-1003-z. [DOI] [PubMed] [Google Scholar]
- 4.Catalá-López F., Peiró S., Ridao M., Sanfélix-Gimeno G., Gènova-Maleras R., Catalá M.A. Prevalence of attention deficit hyperactivity disorder among children and adolescents in Spain: a systematic review and meta-analysis of epidemiological studies. BMC Psychiatry. 2012;12:168. doi: 10.1186/1471-244X-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy F., Hay D.A., McStephen M., Wood C., Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sherman D.K., Iacono W.G., McGue M.K. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. J Am Acad Child Adolesc Psychiatry. 1997;36:745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Polderman T.J., Derks E.M., Hudziak J.J., Verhulst F.C., Posthuma D., Boomsma D.I. Across the continuum of attention skills: a twin study of the SWAN ADHD rating scale. J Child Psychol Psychiatry. 2007;48:1080–1087. doi: 10.1111/j.1469-7610.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 8.Greven C.U., Rijsdijk F.V., Plomin R. A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattention show substantial genetic overlap but also genetic specificity. J Abnorm Child Psychol. 2011;39:265–275. doi: 10.1007/s10802-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 9.Larsson H., Anckarsäter H., Råstam M., Chang Z., Lichtenstein P. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. J Child Psychol Psychiatry. 2012;53:73–80. doi: 10.1111/j.1469-7610.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- 10.Skuse D.H. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr Res. 2000;47:9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jacquemont S., Coe B.P., Hersch M. A higher mutational burden in females supports a ‘female protective model’ in neurodevelopmental disorders. Am J Hum Genet. 2014;94:415–425. doi: 10.1016/j.ajhg.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson E.B., Lichtenstein P., Anckarsäter H., Happé F., Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson J.M., Lundström S., Lichtenstein P., Bejerot S., Eriksson E. Effect of cotwin gender on neurodevelopmental symptoms: a twin register study. Mol Autism. 2016;7:8. doi: 10.1186/s13229-016-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee S.H., Waldman I.D. Etiology of sex differences in the prevalence of ADHD: an examination of inattention and hyperactivity-impulsivity. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:60–64. doi: 10.1002/ajmg.b.20131. [DOI] [PubMed] [Google Scholar]
- 15.Anckarsäter H., Lundström S., Kollberg L. The Child and Adolescent Twin Study in Sweden (CATSS) Twin Res Hum Genet. 2011;14:495–508. doi: 10.1375/twin.14.6.495. [DOI] [PubMed] [Google Scholar]
- 16.Haworth C.M., Davis O.S., Plomin R. Twins Early Development Study (TEDS): a genetically sensitive investigation of cognitive and behavioral development from childhood to young adulthood. Twin Res Hum Gent. 2013;16:117–125. doi: 10.1017/thg.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson S.L., Svanström Röjvall A., Råstam M., Gillberg C., Gillberg C., Anckarsäter H. Psychiatric telephone interview with parents for screening of childhood autism-tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. Br J Psychiatry. 2005;187:262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- 18.Larson T., Anckarsäter H., Gillberg C. The autism-tics, AD/HD and other comorbidities inventory (A-TAC): further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conners C.K., Sitarenios G., Parker J.D., Epstein J.N. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 21.R Core Team . R Foundation for Statistical Computing; Vienna: 2015. R: A language and environment for statistical computing. [Google Scholar]
- 22.Ebejer J.L., Duffy D.L., van der Werf J. Genome-wide association study of inattention and hyperactivity-impulsivity measured as quantitative traits. Twin Res Hum Genet. 2013;16:560–574. doi: 10.1017/thg.2013.12. [DOI] [PubMed] [Google Scholar]
- 23.Zuk O., Schaffner S.F., Samocha K. Searching for missing heritability: designing rare variant association studies. Proc Natl Acad Sci U S A. 2014;111 doi: 10.1073/pnas.1322563111. 455-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron-Cohen S., Scott F.J., Allison C. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194:500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- 25.Werling D.M., Geschwind D.H. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol Autism. 2015;6:27. doi: 10.1186/s13229-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin J., Hamshere M.L., Stergiakouli E., O’Donovan M.C., Thapar A. Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol Psychiatry. 2014;76:664–671. doi: 10.1016/j.biopsych.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wray N.R., Lee S.H., Mehta D., Vinkhuyzen A.A., Dudbridge F., Middeldorp C.M. Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55:1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson E., Sjölander A., Almqvist C. Birth weight as an independent predictor of ADHD symptoms: a within-twin pair analysis. J Child Psychol Psychiatry. 2015;56:453–459. doi: 10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultman C.M., Torrång A., Tuvblad C., Cnattingius S., Larsson J.O., Lichtenstein P. Birth weight and attention-deficit/hyperactivity symptoms in childhood and early adolescence: a prospective Swedish twin study. J Am Acad Child Adolesc Psychiatry. 2007;46:370–377. doi: 10.1097/01.chi.0000246059.62706.22. [DOI] [PubMed] [Google Scholar]
- 30.Rucklidge J.J. Gender differences in ADHD: implications for psychosocial treatment. Expert Rev Neurother. 2008;8:643–655. doi: 10.1586/14737175.8.4.643. [DOI] [PubMed] [Google Scholar]
- 31.Williamson D., Johnston C. Gender differences in adults with attention-deficit/hyperactivity disorder: a narrative review. Clin Psychol Rev. 2015;40:15–27. doi: 10.1016/j.cpr.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Neale M.C., Maes H.H.M. Kluwer Academic Publishers; Dordrecht, Netherlands: 2004. Methodology for Genetic Studies of Twins and Families. [Google Scholar]
- 33.Ronald A., Simonoff E., Kuntsi J., Asherson P., Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry. 2008;49:535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 34.Moilanen I., Linna S., Ebeling H. Are twins’ behavioural/emotional problems different from singletons? Eur Child Adolesc Psychiatry. 1999;8(Suppl 4):62–67. doi: 10.1007/pl00010702. [DOI] [PubMed] [Google Scholar]
- 35.Dalsgaard S., Mortensen P.B., Frydenberg M., Thomsen P.H. Conduct problems, gender and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. Br J Psychiatry. 2001;181:416–421. doi: 10.1192/bjp.181.5.416. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson E., Anckarsäter H., Gillberg C., Lichtenstein P. Different neurodevelopmental symptoms have a common genetic etiology. J Child Psychol Psychiatry. 2013;54:1356–1365. doi: 10.1111/jcpp.12113. [DOI] [PubMed] [Google Scholar]
- 37.Pettersson E., Larsson H., Lichtenstein P. Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol Psychiatry. 2015 Aug 25 doi: 10.1038/mp.2015.116. http://dx.doi.org/10.1038/mp.2015.116 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]