Abstract
We describe the case of a 79-year-old man who presented with an enlarging mass on his chest wall. He had a history of thoracoplasty performed 55 years ago for treatment of pulmonary tuberculosis. The mass was subsequently proven to be the result of empyema neccesitans caused by reactivation tuberculosis. Empyema neccesitans is a well described entity in which an empyema spontaneously decompresses by dissecting into the chest wall and extrathoracic soft tissues. This can occur following necrotizing pneumonia, including pyogenic or tuberculus, or pulmonary abscess. Complications from collapse therapy for tuberculosis can be encountered decades following the surgery, however, empyema necessitans due to reactivation tuberculosis is rare. This case affords the opportunity to review the goals, techniques, and radiologic appearance of thoracoplasty.
Abbreviations: CT, computed tomography; TB, tuberculosis
Introduction
Empyema neccesitans is a well described entity in which an empyema spontaneously decompresses by dissecting into the chest wall and extrathoracic soft tissues. This can occur following necrotizing pneumonia, including pyogenic or tuberculous, or pulmonary abscess [1]. This unique case illustrates reactivation mycobacterium tuberculosis presenting as empyema necessitans 55 years following pneumonectomy and thoracoplasty for the treatment of primary tuberculosis.
Case Report
A 79-year-old male presented to his primary care physician with the chief complaint of an enlarging lump on his chest. He first noticed it 2-3 days prior to presentation, and thought it had increased in size over that time. He had no pain, fevers, chills, or malaise.
The patient's past medical history was significant for tuberculosis which was treated with left pneumonectomy and thoracoplasty in 1950. He had oxygen dependent chronic obstructive pulmonary disease.
On physical examination, he had an erythematous lump on his left anterolateral chest wall. It was fluctuant and mildly tender. His temperature was 99.2 degrees Farinheit.
Needle aspiration of the lump yielded pus. An incision and drainage was performed, which revealed copious pus and a large abscess cavity, estimated to measure approximately 15 × 7 cm. On exploration and palpation of the wound, there was concern for communication with the pleural space.
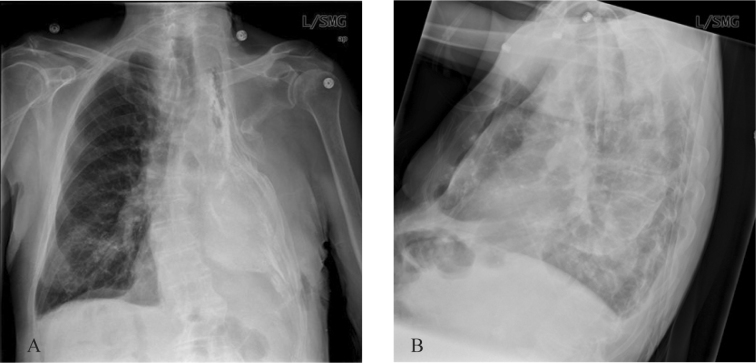
A chest radiograph was obtained (Fig. 1). This demonstrated truncation and deformation of the upper left ribs with collapse of the chest wall and volume loss of the left hemithorax. The left hemithorax was nearly completely opacified and plaque like calcifications were present along the chest wall. A small amount of air was present.
Figure 1A-B.
79-year-old man with reactivation tuberculosis and empyema necessitans 55 years following thoracoplasty. PA and lateral CXR demonstrate left upper rib resections, collapse of the left chest wall, opacification and volume loss of the left hemithorax and chest wall calcifications.
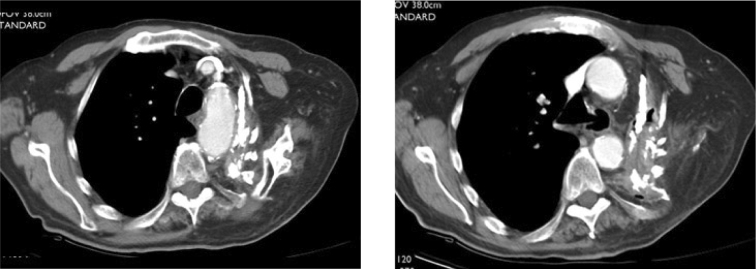
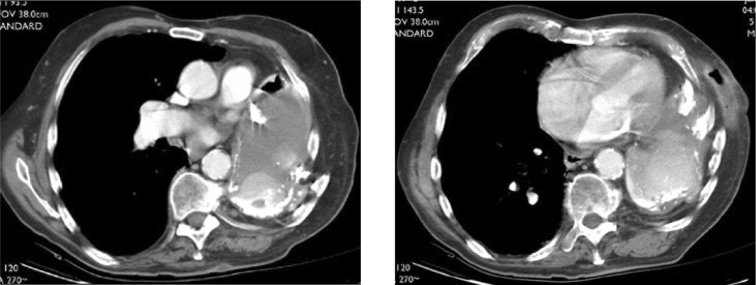
Subsequently, computed tomography of the chest was performed (Fig. 2). This demonstrated absence of most of the left first through 7th ribs and distortion and collapse of the left upper thorax. The upper chest wall was apposed to the superior mediastinum. The hemithorax was peripherally calcified and filled with fluid and several air-fluid levels. Lobular areas of high attenuation tissue were contiguous with the chest wall, mediastinum and diaphragmatic surfaces. The subcutaneous abscess cavity was filled with air and fluid.
Figures 2A-B.
79-year-old man with reactivation tuberculosis and empyema necessitans 55 years following thoracoplasty. Axial CT images of the chest show resections of left upper ribs, distortion and collapse of the left upper thorax and apposition of the chest wall to the superior mediastinum.
Intraoperative bronchoscopy revealed a normal right bronchus and intact left bronchial stump without evidence of bronchopleural fistula. A left thoracotomy incision was made and 2 left ribs were resected. The pleural space was filled with 400cc of pus, white necrotic material, and lobules of granular, clay-like yellow material. Following extraction of this material, an Eloesser flap was created by sewing the skin to the parietal pleura, creating a 4 × 10cm opening for pleural drainage. The patient tolerated the surgery well. 2+ Acid fast bacilli were present on AFB stain of the pleural fluid. Appropriate anti-tuberculous therapy was initiated.
Discussion
Thoracoplasty for the treatment of mycobacterium tuberculosis (TB) is the operative removal of the skeletal support of a portion of the chest, with the goal to cause lung cavity collapse and pleural space obliteration by apposition of the chest wall and the mediastinum. It is now rarely performed in developed countries, predominately due to the development of successful antimicrobial agents for mycobacterium tuberculosis. In contrast, the procedure is still fairly commonly used in developing countries. Between 1992 and 1997, 139 thoracoplasty procedures were performed at L.R.S. Institute of TB and Allied Diseases in New Delhi, India. 86 of these were for the treatment of TB [3]. Poor patient compliance, drug resistance, and a poor health care delivery system have been implicated as contributing to the ongoing need for this procedure [3].
Current indications for thoracoplasty include: tuberculous empyema, drug resistant TB (in whom lung resectional surgeries aren't possible or effective), refractory pyogenic empyema, post-operative empyema with bronchopleural fistula, infected post resectional space not closing with drainage alone, and concomitant tailoring thoracoplasty in conjunction with lung resection [3, 4]. With the advent of multi-drug resistant TB, thoracoplasty may again be employed with greater frequency in the primary treatment of TB.
Surgical Technique and History
Understanding the radiographic appearance of the post thoracoplasty chest requires knowledge of how the procedure is performed.
Thoracoplasty was performed as far back as the early 1900s and was the primary operation for the treatment of TB; lung resection was supplementary. The procedure was successful, with sputum conversion occurring approximately 75% of the time [5]. In the 1940's, lung resection was becoming more common, however thoracoplasty remained the treatment of choice for TB until the introduction of effective antimicrobial therapy [3, 6].
Conventional extra pleural posterolateral subperiosteal thoracoplasty was described by John Alexander in the 1930s. This involved sequential subperiosteal resection of the posterolateral aspects of the ribs and transverse processes. The initial surgery involved resection of ribs 1-3, with subsequent surgeries resecting the 4th and 5th, then 6th and 7th ribs [7]. This resulted in compression of the pulmonary parenchyma and collapse of apical lung cavities. Successful cavity closure was achieved 93% of the time, and operative mortality was reported to be 10% [4]. The thoracoplasty procedure was refined and evolved over time, with several different types described:
Conventional: (Alexander type)
Staged subperiosteal, extrapleural resection of the posterolateral aspects of a sufficient number of ribs and portions of the corresponding transverse processes to obliterate the intrathoracic space. This is often extensive, with resection of up to 11 ribs. The anterior portions of the lower ribs are left intact, or resected in a sloping format to preserve cosmesis and structural integrity [4].
Schede:
This involves the resection of ribs, intercostal muscles, endothoracic fascia and parietal pleura; the entire chest wall is removed, leaving only extrathoracic muscles and skin [4]. This results in gross deformity of the chest and spine. It is rarely used in current practice due to this deformity, but occasionally must be performed in cases of severely calcified chest walls [8].
Plombage:
This does not involve rib resection. The lung and pleura are separated from the chest well in the extrapleural space. Plombage material such as paraffin, naphthalene balls and polyethylene balls or packs are placed in the space to achieve lung collapse [5, 8].
Tailoring:
Tailoring involves the resection of enough ribs to decrease the intrathoracic volume. This is performed when it is anticipated that insufficient lung volume will remain to fill the pleural space following lung resection.
A general principle of thoracoplasty is to leave the first rib in place to minimize cosmetic deformity by maintaining structural integrity of the neck, shoulder and upper thorax [3, 8]. The apex of the lung and soft tissues are retracted downward to obliterate the underlying space and approximate the soft tissues to the mediastinum (apicolysis). Postoperatively, compression dressing is applied to the chest wall to maintain the approximation of soft tissues to the mediastinum, to maintain obliteration of the pleural space [3].
Radiographic Appearance
The radiographic appearance will vary depending on the surgical technique chosen and the extent of rib resection. The more cephalad ribs should appear truncated or be absent, and the corresponding transverse processes may be absent. The remaining ribs typically appear distorted and vertically oriented. The volume of the hemithorax should be significantly diminished. If concomitant pneumonectomy has been performed, the thorax should be opacified. Mediastinal shift will occur towards the side of the thoracoplasty. Shift of the mediastinum away from the operative side may indicate accumulation of material in the post thoracoplasty space. No air should be present within the hemithorax, and the presence of this should alert one to the potential for an empyema or bronchopleural fistula.
Conclusion
This case is unique in that it represents a complication of collapse therapy for tuberculosis which occurred 55 years from the time of operation. To my knowledge, no cases of tuberculous empyema necessitans due to reactivation TB have been reported. This case illustrates that reactivation TB can occur decades following the primary infection. The expected appearance of the post-thoracoplasty chest can be understood by understanding the surgical techniques and goals of thoracoplasty, as discussed. This knowledge will aid in the interpretation of these complex radiographs, and increase the ability to detect complications.
Figures 2C-D.
79-year-old man with reactivation tuberculosis and empyema necessitans 55 years following thoracoplasty. Axial CT images of the chest show the hemithorax opacified with fluid, peripheral lobular areas of high attenuation tissue, and calcification. A subcutaneous abscess cavity is present.
Footnotes
Published: August 12, 2008
References
- 1.Chaiyasate K, Hramiec J. Images in Clinical Medicine. Tuberculosis empyema necessitatis. NEJM. 2005 March 3;352(9):e8. doi: 10.1056/NEJMicm040373. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Dewan RK, Singh S, Kumar A, Meena BK. Thoracoplasty: An Obsolete Procedure? The Indian Journal of Chest Diseases & Allied Sciences. 1999 April-June;41(2):83–88. [PubMed] [PubMed] [Google Scholar]
- 4.Hopkins RA, Ungerleider RM, Staub EW, Yound WG. The Modern Use of Thoracoplasty. The Annals of thoracic surgery. 1985 Aug;40(2):181–187. doi: 10.1016/s0003-4975(10)60016-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Barker WL. Thoracoplasty. Chest Surgery Clinics of North America, August. 1994;4(3):593–615. [PubMed] [PubMed] [Google Scholar]
- 6.Pomerantz M, Mault JR. History of Resectional Surgery for Tuberculosis and Other Mycobacterial Infections. Chest Surgery Clinics of North America, February. 2000;10(1):131–133. [PubMed] [PubMed] [Google Scholar]
- 7.Alexander J. The collapse therapy of pulmonary tuberculosis. CC Thomas; Springfield, IL: 1937. [Google Scholar]
- 8.Peppas G, Molnar TF, Jeyasingham K, Kirk AB. Thoracoplasty in the Context of Current Surgical Practice. Annals of Thoracic Surgery. 1993;56:903–909. doi: 10.1016/0003-4975(93)90353-j. [PubMed] [DOI] [PubMed] [Google Scholar]
Uncited Reference
- 2.Weissberg D, Weissberg D. Late Complications of Collapse Therapy for Pulmonary Tuberculosis. Chest. 2001 Sep;120(3):696–697. doi: 10.1378/chest.120.3.847. [PubMed] [DOI] [PubMed] [Google Scholar]