Abstract
Objective. To explore the possible bioactive compounds in the methanolic extracts of leaf, stem, and tuber parts of the medicinal climber, Solena amplexicaulis, using GC-MS. Methods. GC-MS analysis of the plant extracts were performed by using GC-MS-5975C [Agilent] and mass spectra of the compounds found in the extract was matched with the data in the library of National Institute of Standards and Technology (NIST). Results. Thirty-five compounds were determined to be present in the parts studied. The active principles with their retention time, molecular formula, molecular weight, peak area, structure, category of the compounds, and activities were predicted. The most prevailing compounds were phytol (38.24%) in leaf, 4-(4-ethoxyphenyl) but-3-en-2-one (56.90%) in stem, and 9,17-octadecadienal, (Z)- (21.77%) in tuber. Conclusion. This study revealed that the species S. amplexicaulis is a good source of many bioactive compounds like terpenes, triazines, esters, alkanes, alcohols, hydrocarbons, aldehydes, amides, and so forth. That justifies the traditional usage of this species.
1. Introduction
Herbal plants are valuable gift of nature for mankind and they are the source of a variety of phytochemicals which are utilized for human and animal diets also. It is capable of synthesizing an overwhelming variety of low molecular weight organic compounds called secondary metabolites, usually with unique and complex structures. The medicinal actions of plants unique to particular plant species or groups are consistent with the concept that the combination of secondary products in a particular plant is taxonomically distinct [1]. It states that around 85–90% of the world's population consumes traditional herbal medicines [2]. In recent decades, studies on phytochemical constituents of medicinal plant and its pharmacological activities have received wide attention [3–6]. WHO has emphasized the need to ensure the quality of medicinal plant products using modern techniques with the application of suitable standards. Many modern methods are adapted for identification and quantification of active principle compounds in plant materials. Of them, gas chromatography-mass spectrometry (GC-MS) has become firmly established as a key technological platform for secondary metabolite profiling in both plant and nonplant species [7, 8].
The plant species Solena amplexicaulis is commonly called creeping cucumber and belongs to the family Cucurbitaceae distributed very seldom in the dry deciduous forest and scrub jungles of Tamil Nadu [9]. The medicinal uses of this species are multifaceted. The local healers of Tamil Nadu and Andhra Pradesh are prescribing this species for many ailments owing to its effective healing property [10]. The traditional healers are prescribing the tubers, leaves, and seeds of this species for various ailments like spermatorrhoea, thermogenics, diuretics, haemorrhoids, and invigorating and it is a very good appetizer and cardiotonic [11]. The whole plant is a potential source of natural antioxidant [12, 13], antidiabetic [10], and antibacterial agent [14] also. As the leaves have good anti-inflammatory activity, it is recommended for inflammation, skin lesions, and skin diseases [15]. Crude leaf juice is used to cure jaundice [16]. Unripe fruits are eaten raw to strengthen the body [17]. The decoction of the root is taken orally to cure stomachache [18]. As the reproductive parts like seeds and tubers are exploited severely for medicinal uses, this species becomes rare sighted in its habitats of Tamil Nadu.
Despite these wide medicinal uses, no information on qualitative account of phytochemicals is available for this species. To address this lacuna, GC-MS studies were undertaken to explore the phytochemical constituents present in the leaf, stem, and tuber parts of S. amplexicaulis.
2. Materials and Methods
2.1. Collection, Identification and Preparation of Plant Materials
The leaf, stem, and tuber parts of S. amplexicaulis were collected separately from the thorny scrub jungles of Madukkarai, Coimbatore District, Tamil Nadu, India. The authenticity of the plant was confirmed in Botanical Survey of India, Southern Regional Centre, Coimbatore, by referring to the deposited specimen (Voucher specimen number: CPS 313). They were washed thoroughly in tap water, shade-dried, and then homogenized to fine powder and stored in air tight bottles.
2.2. Preparation of Extract
50 g of powdered leaf, stem, and tuber parts of S. amplexicaulis was separately extracted with 250 mL methanol at the temperature between 60 and 65°C for 24 h by using soxhlet extractor. The solvent was evaporated by rotary vacuum evaporator to obtain viscous semisolid masses. This semidry methanolic crude extract was subjected to GC-MS analysis.
2.3. GC-MS Analysis
GC-MS analysis was carried out on a 5975C Agilent equipped with a DB-5ms Agilent fused silica capillary column (30 × 0.25 mm ID; film thickness: 0.25 μm), operating in electron impact mode at 70 eV. Pure helium (99.999%) was used as carrier gas at a constant flow of 1 mL/min and an injection volume of 1 μL was employed (split ratio is 10 : 1). Mass transfer line and injector temperature were set at 230 and 250°C, respectively. The oven temperature was programmed from 70 (isothermal for 3 min) to 300°C (isothermal for 9 min) at the rate of 10°C/min. Total GC running time was 34 min and the MS detection was completed within 35 min.
By GC-MS, the compounds were separated and then they were eluted from the column and made enter into the detector which was capable of creating an electronic signal. Then they were processed by the computer for generating chromatogram. Then the compound entered into the electron ionization (mass spectroscopy) detector, where they were bombarded with a stream of electrons causing them to break apart into fragments. These fragments were actually charged ions with certain mass. The m/z (mass/charge) ratio obtained was calibrated from the graph, called the mass spectrum, and is the fingerprint of the molecule.
2.4. Identification of the Compounds
To identify the compounds, the extract was assigned for comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with the published literature. National Institute of Standards and Technology library sources were also used for matching the identified compounds from the plant materials [19, 20].
3. Results
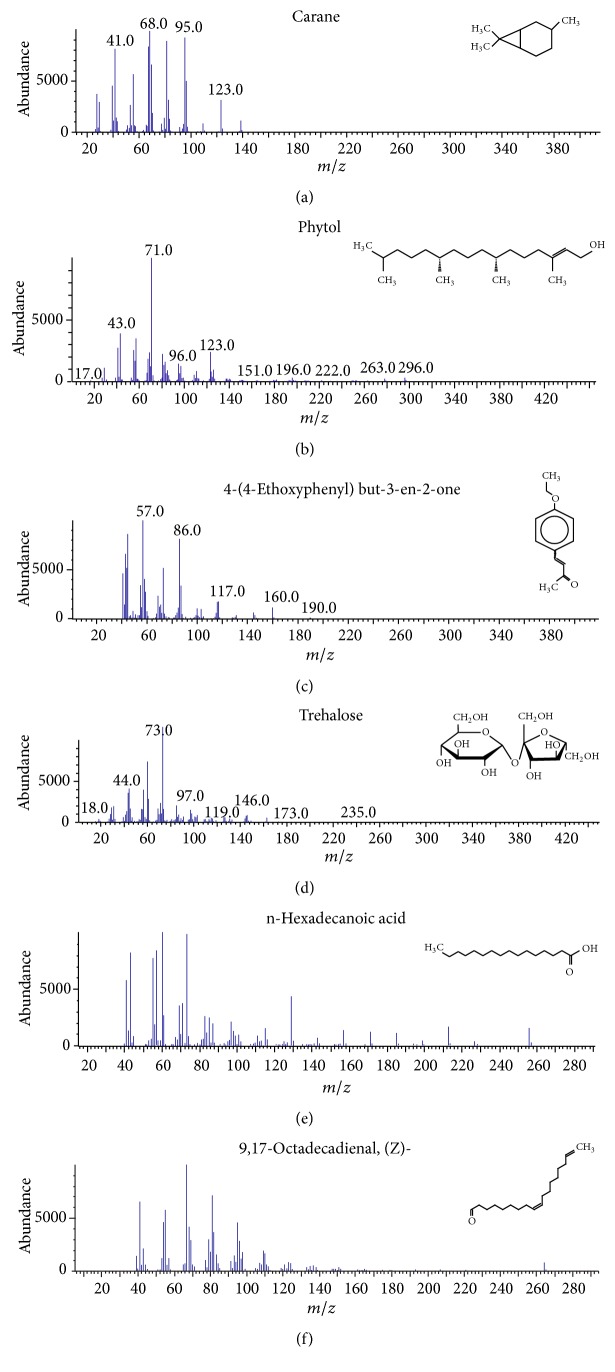
The gas chromatograms of leaf, stem, and tuber parts of S. amplexicaulis confirmed the presence of various interesting compounds with different retention times as illustrated in Figures 1, 2, and 3. These compounds were identified through mass spectrometry attached with GC. The identified compounds and their retention time, molecular formula, molecular weight, peak area (%), structure, category of the compound, and activities related with medicinal uses are given in Tables 1, 2, and 3 for leaf, stem, and tuber, respectively. The compound prediction is based on Dr. Duke's Phytochemical and Ethnobotanical Databases. Six compounds were detected in the methanolic leaf extract of S. amplexicaulis. Among them, the most prevailing major compounds were phytol, a diterpene (peak area: 38.24%) (Figure 4(a)), carane, a terpene (peak area: 18.76%) (Figure 4(b)), and 1-octanamine, an aliphatic amine (peak area: 16.16%). The methanolic stem extract of S. amplexicaulis showed the presence of fifteen different organic compounds. The major phytochemical compounds among them were 4-(4-ethoxyphenyl) but-3-en-2-one, an aliphatic acid (peak area: 56.90%) (Figure 4(c)), trehalose, sucrose (peak area: 11.49%) (Figure 4(d)), hexadecanoic acid, methyl ester, a linoleic acid ester (peak area: 6.52%), and 9-octadecenoic acid (Z)-, methyl ester, another linoleic acid ester (peak area: 6.76%). Fourteen compounds were identified in the methanolic tuber extract. In this account, 9,17-octadecadienal (Z)-, an unsaturated aldehyde (peak area: 21.77%) (Figure 4(e)), n-hexadecanoic acid, a palmitic acid (peak area: 21.75%) (Figure 4(f)), phthalic acid, di(2-propylpentyl) ester, a dicarboxylic acid ester (peak area: 9.48%), and 9,12-octadecadienoic acid (Z,Z)-, a linolenic acid (peak area: 9.35%) were the major phytochemicals on the basis of quantity.
Figure 1.
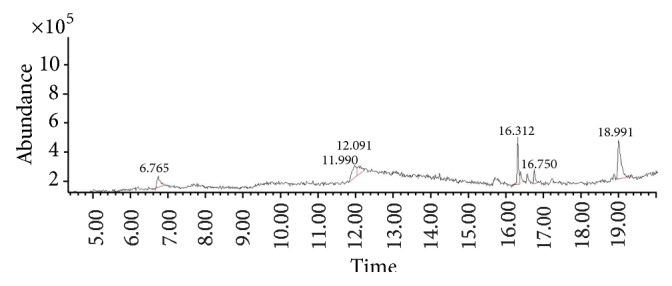
GC-MS chromatogram of methanolic leaf extract of Solena amplexicaulis.
Figure 2.
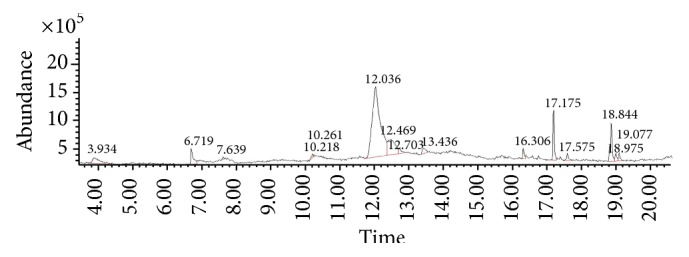
GC-MS chromatogram of methanolic stem extract of Solena amplexicaulis.
Figure 3.
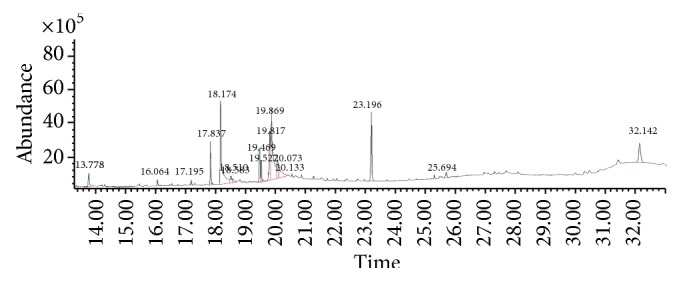
GC-MS chromatogram of methanolic tuber extract of Solena amplexicaulis.
Table 1.
Compounds identified in the methanolic leaf extract of Solena amplexicaulis by GC-MS.
| S. number | Name of the compound | RT | Molecular formula | Molecular weight | Peak area % | Structure | Category of the compound | Activity∗ |
|---|---|---|---|---|---|---|---|---|
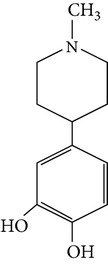
| 1 | Hexahydropyridine, 1-methyl-4-[4,5-dihydroxyphenyl]- | 6.761 | C12H17NO2 | 207.12 | 10.75 |
|
Aromatic piperidine | No activity reported |
|
| ||||||||
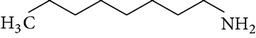
| 2 | 1-Octanamine | 11.990 | C8H19N | 129.24 | 16.16 |
|
Aliphatic amine | No activity reported |
|
| ||||||||
| 3 | 1-Tetradecanamine | 12.091 | C14H31N | 213.40 | 10.24 |

|
Aliphatic amine | No activity reported |
|
| ||||||||
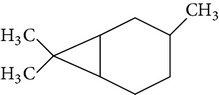
| 4 | Carane | 16.317 | C10H18 | 138.24 | 18.76 |
|
Terpene | Antifeedant, antioxidant |
|
| ||||||||
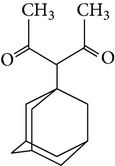
| 5 | Pentane-2,4-dione, 3-(1-adamantyl) | 16.753 | C15H22O2 | 234.33 | 5.85 |
|
Aliphatic diketone | No activity reported |
|
| ||||||||
| 6 | Phytol | 18.990 | C20H40O | 296.53 | 38.24 |
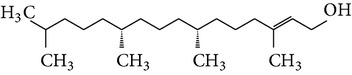
|
Diterpene | Anticancer, antioxidant, anti-inflammatory, diuretic, antitumor, chemopreventive, antimicrobial, use in vaccine formulations |
∗Source: Dr. Duke's Phytochemical and Ethnobotanical Databases (online database).
Table 2.
Compounds identified in the methanolic stem extract of Solena amplexicaulis by GC-MS.
| S. number | Name of the compound | RT | Molecular formula | Molecular weight | Peak area % | Structure | Category of the compound | Activity∗ |
|---|---|---|---|---|---|---|---|---|
| 1 | 1,3-Cyclopentanedione | 3.929 | C5H6O2 | 98.09 | 4.47 |
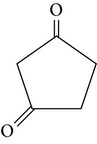
|
Cyclic diketone | No activity reported |
|
| ||||||||
| 2 | Undecane | 6.718 | C11H24 | 156.30 | 3.92 |
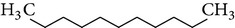
|
Alkane | Antimicrobial agents, transducer for immunosensor and its method of production. carcinogens, enzyme inhibitors, solvents |
|
| ||||||||
| 3 | 1,2,4-Triazino [5,6-E] [1,2,4]-triazine-3,6-dione, hexahydro- | 7.633 | C4H8N6O2 | 172.14 | 0.36 |
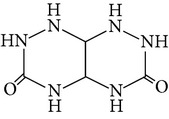
|
Triazine | No activity reported |
|
| ||||||||
| 4 | 4-Hydroxyphenyl 3-nitrobenzoate | 10.218 | C13H9NO5 | 259.21 | 0.52 |
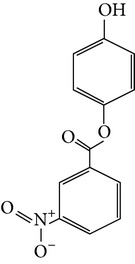
|
Aromatic nitro compound | No activity reported |
|
| ||||||||
| 5 | Taurolidine | 10.261 | C7H16N4O4S2 | 284.35 | 0.17 |
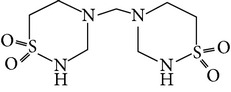
|
Taurine amino acid derivative | Antimicrobial, anti-lipopolysaccharide, anti-tumor properties, anti-infective agents, antineoplastic agents |
|
| ||||||||
| 6 | 4-(4-Ethoxyphenyl) but-3-en-2-one | 12.033 | C12H14O2 | 190.24 | 56.90 |
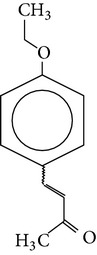
|
Aliphatic acid | No activity reported |
|
| ||||||||
| 7 | Trehalose | 12.469 | C12H22O11 | 342.29 | 11.49 |
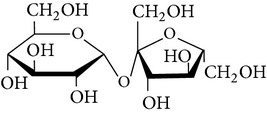
|
Sucrose | Treat amyloidosis (prevent the deposition of amyloid protein in the body) |
|
| ||||||||
| 8 | d-Glycero-d-tallo-heptose | 12.701 | C7H14O7 | 210.18 | 1.68 |
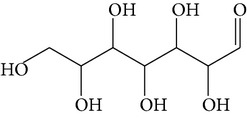
|
Aldo heptose | No activity reported |
|
| ||||||||
| 9 | Benzaldehyde, 6-hydroxy-4-methoxy-2,3-dimethyl- | 13.442 | C10H12O3 | 180.20 | 1.71 |
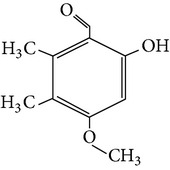
|
Aromatic benzaldehyde | No activity reported |
|
| ||||||||
| 10 | 9-Tetradecen-1-ol, acetate, (Z)- | 16.303 | C16H30O2 | 254.40 | 1.40 |
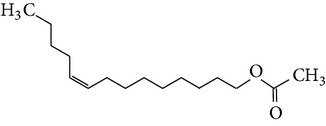
|
Aliphatic ester | No activity reported |
|
| ||||||||
| 11 | Hexadecanoic acid, methyl ester | 17.174 | C17H34O2 | 270.45 | 6.52 |
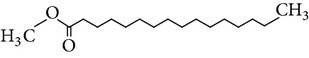
|
Linoleic acid ester | Anti-inflammatory, hypocholesterolemic, cancer preventive, hepatoprotective, nematicide, insectifuge, antihistaminic, antieczemic, antiacne, alpha reductase inhibitor, antiandrogenic, antiarthritic, anticoronary |
|
| ||||||||
| 12 | 1-Methyl-3-ethyladamantane | 17.581 | C13H22 | 178.31 | 1.37 |
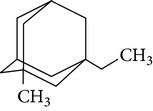
|
Bicyclic alkane | No activity reported |
|
| ||||||||
| 13 | 9-Octadecenoic acid (Z)-, methyl ester | 18.844 | C19H36O2 | 296.48 | 6.76 |
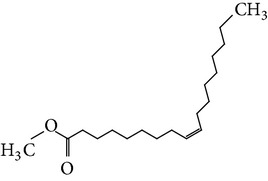
|
Linoleic acid ester | Anti-inflammatory, antiandrogenic cancer preventive, dermatitigenic hypocholesterolemic, 5-alpha reductase inhibitor, anemiagenic, insectifuge |
|
| ||||||||
| 14 | Benzaldehyde, 2-nitro-, diaminomet hylidenhydrazone | 18.975 | C8H9N5O2 | 207.18 | 1.42 |
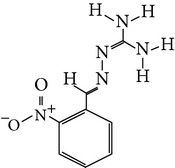
|
Nitrogen compound | Antimicrobial |
|
| ||||||||
| 15 | Heptadecanoic acid, 10-methyl-, methyl ester | 19.077 | C19H38O2 | 298.50 | 1.29 |
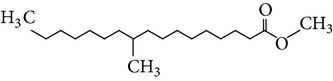
|
Fatty ester | No activity reported |
∗Source: Dr. Duke's Phytochemical and Ethnobotanical Databases (online database).
Table 3.
Compounds identified in the methanolic tuber extract of Solena amplexicaulis by GC-MS.
| S. number | Name of the compound | RT | Molecular formula | Molecular weight | Peak area % | Structure | Category of the compound | Activity∗ |
|---|---|---|---|---|---|---|---|---|
| 1 | Dodecanoic acid | 13.776 | C12H24O2 | 200.31 | 2.40 |
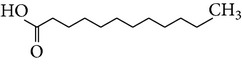
|
Fatty acids | No activity reported |
|
| ||||||||
| 2 | Tetradecanoic acid | 16.071 | C14H28O2 | 228.37 | 0.95 |
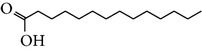
|
Myristic acid | Antioxidant, cancer preventive, nematicide, hypocholesterolemic, lubricant |
|
| ||||||||
| 3 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 17.189 | C16H22O4 | 278.34 | 0.74 |
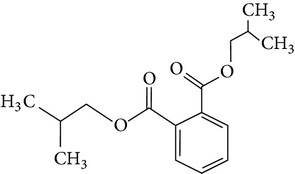
|
Phthalic ester | Used in preparation of perfumes and cosmetics, plasticized vinyl seats on furniture, cars, and clothing including jackets, raincoats, and boots and used in textiles, as dyestuffs, cosmetics, and glass making |
|
| ||||||||
| 4 | Pentadecanoic acid, 14-methyl-, methyl ester | 17.842 | C17H34O2 | 270.45 | 4.61 |
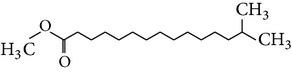
|
Fatty ester | No activity reported |
|
| ||||||||
| 5 | n-Hexadecanoic acid | 18.176 | C16H32O2 | 256.42 | 21.75 |
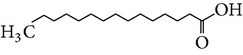
|
Palmitic acid | Antioxidant, hypocholesterolemic, nematicide, pesticide, lubricant, hemolytic inhibitor, antiandrogenic |
|
| ||||||||
| 6 | Cystodytin | 18.510 | C22H19O3N3 | 373.78 | 1.58 |
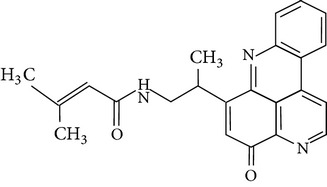
|
Aromatic alkaloid | Antiproliferative activity in human tumor cell lines |
|
| ||||||||
| 7 | 1-Decanol, 2-hexyl- | 18.583 | C16H34O | 242.44 | 1.21 |
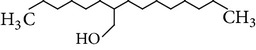
|
Aliphatic alcohols | Antimicrobial |
|
| ||||||||
| 8 | 10,13-Octadecadienoic acid, methyl ester | 19.469 | C19H34O2 | 294.47 | 4.72 |
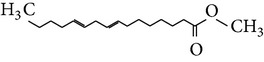
|
Linoleic acid esters | Anti-inflammatory, hypocholesterolemic, cancer preventive, hepatoprotective, nematicide, insectifuge, antieczemic, anticancer, antiarthritic, insectifuge, antihistaminic, anticoronary |
|
| ||||||||
| 9 | trans-13-Octadecenoic acid, methyl ester | 19.527 | C19H36O2 | 296.48 | 3.55 |
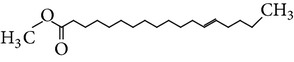
|
Linoleic acid esters | Anti-inflammatory, antiandrogenic, cancer preventive, dermatitigenic, irritant, antileukotriene—D4, hypocholesterolemic, 5-alpha reductase inhibitor, anemiagenic, insectifuge, flavor |
|
| ||||||||
| 10 | 9,12-Octadecadienoic acid (Z,Z)- | 19.817 | C18H32O2 | 280.44 | 9.35 |
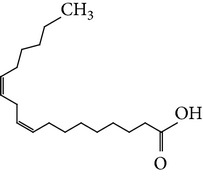
|
Linolenic acid | Anti-inflammatory, hypocholesterolemic, cancer preventive, insectifuge, antiarthritic, hepatoprotective, antiandrogenic, nematicide, antihistaminic, antieczemic |
|
| ||||||||
| 11 | 9,17-Octadecadienal, (Z)- | 19.876 | C18H32O | 264.44 | 21.77 |
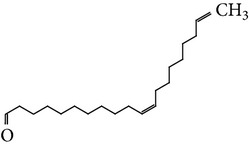
|
Unsaturated aldehyde | Antimicrobial |
|
| ||||||||
| 12 | Phthalic acid, di(2-propylpentyl) ester | 23.201 | C24H38O4 | 390.55 | 9.48 |
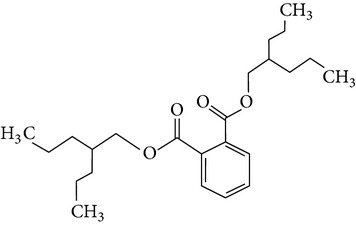
|
Dicarboxylic acid ester | Oral toxicity during pregnancy and sucking in the Long-Evans Rat |
|
| ||||||||
| 13 | Anthracene, 9-ethyl-9,10-dihydro-10-t-butyl- | 25.699 | C20H24 | 264.40 | 1.26 |
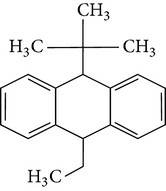
|
Hydrocarbons | No activity reported |
|
| ||||||||
| 14 | 4-Dehydroxy-N-(4,5-methylenedioxy-2-nitrobenzylidene) tyramine | 32.148 | C16H14N2O4 | 298.29 | 6.72 |
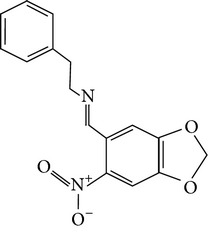
|
Tyramine derivative | No activity reported |
∗Source: Dr. Duke's Phytochemical and Ethnobotanical Databases (online database).
Figure 4.
(a) Mass spectrum of carane. (b) Mass spectrum of phytol. (c) Mass spectrum of 4-(4-ethoxyphenyl) but-3-en-2-one. (d) Mass spectrum of trehalose. (e) Mass spectrum of n-hexadecanoic acid. (f) Mass spectrum of 9,17-octadecadienal, (Z)-.
4. Discussion
The gas chromatogram shows that the relative concentrations of various compounds are getting eluted as a function of retention time. The height of the peaks indicates the relative concentrations of the compounds present in the plant. The mass spectrometer analyzes of the compounds eluted at different times to identify the nature and structure of the compounds. The large compound fragments into small compounds give rise to appearance of peaks at different m/z ratios. These mass spectra are fingerprint of that compound which can be identified from the data library.
Generally, the reliability of medicinal plant for its usage is evaluated by correlating the phytochemical compounds with their biological activities [21]. In the present study, the GC-MS analysis of the methanolic extracts of leaf, stem, and tuber parts of S. amplexicaulis altogether showed the presence of 35 compounds. In this account, the leaf extract contained six compounds among them, phytol (38.24%) is having anticancer, antioxidant, anti-inflammatory, antitumor, antimicrobial, diuretic, and chemopreventive properties and used in vaccine formulations [22, 23]. The other compound, carane (18.76%) is having antifeedant and antioxidant properties [24, 25]. The methanolic stem and tuber extracts showed the presence of greater number of 14 and 15 compounds, respectively. The six phytoconstituents, namely, undecane, taurolidine, trehalose, hexadecanoic acid methyl ester, 9-octadecenoic acid (Z)-, methyl ester, and benzaldehyde, 2-nitro-, diaminomet hylidenhydrazone in stem extracts have possessed medicinal properties [26]. Undecane, an alkane, is an antimicrobial agent, used as carcinogen [27, 28]. Similarly, the other compound, taurolidine, a taurine amino acid derivative, has antimicrobial, antilipopolysaccharidal, and antitumor properties [29, 30]. The sucrose compound, trehalose, is used for the treatment of amyloidosis [31]. The linoleic acid esters present in the stem, hexadecanoic acid methyl ester, are reported to have anti-inflammatory, cancer preventive, hepatoprotective, antiarthritic, and anticoronary properties. The other linoleic acid ester, 9-octadecenoic acid (Z)-, methyl ester, is also having anti-inflammatory, antiandrogenic, and anemiagenic properties [32]. The nitrogen compound, benzaldehyde, 2-nitro-, diaminomet hylidenhydrazone, is known to have the property of curing infectious diseases by its antimicrobial activity. In the tuber extracts, the compounds identified, namely, 10,13-octadecadienoic acid methyl ester, trans-13-octadecenoic acid, methyl ester, and 9,12-octadecadienoic acid (Z,Z)-, are possessed with anti-inflammatory and cancer preventive characters. The two compounds, namely, tetradecanoic acid and n-hexadecanoic acid, are antioxidants. The phthalic acid, 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester, is used in the preparation of perfumes and cosmetics. The unsaturated alcoholic compound, 9,17-octadecadienal, (Z)-, is reported to have antimicrobial property [33]. The study species S. amplexicaulis is endowed with various medicinal properties maybe due to the presence of all these compounds described. In a similar fashion, certain traditional medicinal plant species of Cucurbitaceae have been analyzed phytochemically by using GC-MS and suggested for drug preparation after succeeding in clinical trials [34, 35]. The therapeutic properties of the other compounds in all the three parts of S. amplexicaulis were not yet reported.
Our investigation through the present study revealed that the species S. amplexicaulis is a reliable source of bioactive compounds like fatty acid esters, alcohols, hydrocarbons, alkanes, amines, terpenes, and sugars that justify the traditional usage of this species [16–18] by the local healers in Coimbatore and Tirupur districts of Tamil Nadu, India, for various ailments. As GC-MS is the first step towards understanding the nature of active principles [36, 37], further investigation in this species is suggested for the development of novel drugs.
Acknowledgment
The authors graciously acknowledge the financial support given by University Grants Commission, New Delhi (Grant no. F. 41-415/2012(SR)), to carry out the work.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Briskin D. P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiology. 2000;124(2):507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Syed S. N., Rizvi W., Kumar A., Khan A. A., Moin S., Ahsan A. Antioxidant and hepatoprotective activity of ethanol extract of Valeriana wallichii in CCL4 treated rats. British Journal of Pharmaceutical Research. 2014;4(8):1004–1013. doi: 10.9734/BJPR/2014/7378. [DOI] [Google Scholar]
- 3.World Health Organization. WHO/EDM/TRM/2002, 21, 19. Geneva, Switzerland: World Health Organization; 2002. WHO report. [Google Scholar]
- 4.Karthika K., Thenmozhi K., Paulsamy S., Manian S. Quantification of phytochemicals and in vitro antioxidant potential of various solvent extract of certain species of Acanthaceae. International Journal of Green Pharmacy. 2014:58–64. [Google Scholar]
- 5.Kaushik P., Lal S., Rana A. C., Kaushik D. GC-MS Analysis of bioactive constituents of Pinus roxburghii Sarg. (Pinaceae) from Northern India. Research Journal of Phytochemistry. 2014;8(2):42–46. doi: 10.3923/rjphyto.2014.42.46. [DOI] [Google Scholar]
- 6.Alese M. O., Adewole O. S., Ljomone O. M., Ajayi S. A., Alese O. O. Hypoglycemic and hypolipidemic activities of methanolic extract of Sphenocentrum jollyanum on streptozotocin-induced diabetic wister rats. European Journal of Medicinal Plants. 2014;4(3):353–364. doi: 10.9734/EJMP/2014/7618. [DOI] [Google Scholar]
- 7.Robertson D. G. Metabonomics in toxicology: a review. Toxicological Sciences. 2005;85(2):809–822. doi: 10.1093/toxsci/kfi102. [DOI] [PubMed] [Google Scholar]
- 8.Kell D. B., Brown M., Davey H. M., Dunn W. B., Spasic I., Oliver S. G. Metabolic footprinting and systems biology: the medium is the message. Nature Reviews Microbiology. 2005;3(7):557–565. doi: 10.1038/nrmicro1177. [DOI] [PubMed] [Google Scholar]
- 9.Gamble J. S. Flora of the Presidency of Madras. Vol. 2. London, UK: Adlard & Sons; 1935. [Google Scholar]
- 10.Pullaiah T., Murthy K. S. R., Goud P. S. P., Kumar T. D. C., Vijayakumar R. Medicinal plants used by the tribals of Nallamalais, Eastern Ghats of India. Journal of Tropical Medicinal Plants. 2003;4(2):237–244. [Google Scholar]
- 11.Kritchevsky D. Fiber, lipids, and atherosclerosis. The American Journal of Clinical Nutrition. 1978;31(10):S65–S74. doi: 10.1093/ajcn/31.10.S65. [DOI] [PubMed] [Google Scholar]
- 12.Venkateshwaralu E., Raghuram Reddy A., Goverdhan P., Swapna Rani K., Jayapal Reddy G. In vitro and in vivo antioxidant activity of methanolic extract of Solena amplexicaulis (whole plant) International Journal of Pharmaceutical and Biological Sciences. 2011;1(4):522–533. [Google Scholar]
- 13.Karthika K., Paulsamy S., Jamuna S. Evaluation of in vitro antioxidant potential of methanolic leaf and stem extracts of Solena amplexicaulis (Lam.) Gandhi. Journal of Chemical and Pharmaceutical Research. 2012;4(6):3254–3258. [Google Scholar]
- 14.Karthika K., Paulsamy S. Antibacterial potential of traditional plant species Solena amplexicaulis (Lam.) Gandhi. against certain human pathogens. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(4):255–257. [Google Scholar]
- 15.Arun C., Satheesh Kumar R., Srinu S., Lal Babu G., Raghavendra Kumar G., Amos Babu J. Antiinflammatory activity of aqueous extract of leaves of Solena amplexicaulis . International Journal of Research in Pharmaceutical and Biomedical Sciences. 2011;2(4):1617–1619. [Google Scholar]
- 16.Rahmatullah M., Chakma P., Paul A. K., et al. A survey of preventive medicinal plants used by the Chakma residents of Hatimara (south) village of Rangamati district, Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2011;5(1):92–96. [Google Scholar]
- 17.Jeyaprakash K., Ayyanar M., Geetha K. N., Sekar T. Traditional uses of medicinal plants among the tribal people in Theni District (Western Ghats), Southern India. Asian Pacific Journal of Tropical Biomedicine. 2011;1(1):S20–S25. doi: 10.1016/S2221-1691(11)60115-9. [DOI] [Google Scholar]
- 18.Ghorbani A., Langenberger G., Feng L., Sauerborn J. Ethnobotanical study of medicinal plants utilised by Hani ethnicity in Naban River Watershed National Nature Reserve, Yunnan, China. Journal of Ethnopharmacology. 2011;134(3):651–667. doi: 10.1016/j.jep.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 19.McLafferly F. W. Registry of Mass Spectral Data. 5th. New York, NY, USA: John Wiley & Sons; 1989. [Google Scholar]
- 20.Stein S. E. National Institute of Standards and Technology (NIST) Mass Spectral Database and Software.Version 3.02. Gaithersburg, Md, USA: NIST; 1990. [Google Scholar]
- 21.Belkacem N., Djaziri R., Lahfa F., El-Haci I. A., Boucherit Z. Phytochemical screening and in vitro antioxidant activity isolated bioactive compounds from Tridax procumbens Linn. Pakistan Journal of Biological Sciences. 2013;16(24):1971–1977. doi: 10.3923/pjbs.2013.1971.1977. [DOI] [PubMed] [Google Scholar]
- 22.Sen A., Batra A. Chemical composition of methanol extract of the leaves of Melia azedarach L. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(3):42–45. [Google Scholar]
- 23.Prabhadevi V., Sahaya S. S., Johnson M., Venkatramani B., Janakiraman N. Phytochemical studies on Allamanda cathartica L. using GC-MS. Asian Pacific Journal of Tropical Biomedicine. 2012;2(2):S550–S554. doi: 10.1016/S2221-1691(12)60272-X. [DOI] [Google Scholar]
- 24.Wincza E., Lochyński S. Chemical and microbiological oxidation of (−)-cis-carane-4-one leading to chiral compounds and evaluation of their antifeedant activity. Archive for Organic Chemistry. 2012;2012(4):196–203. doi: 10.3998/ark.5550190.0013.414. [DOI] [Google Scholar]
- 25.Moniczewski A., Librowski T., Lochyński S., Strub D. Evaluation of the irritating influence of carane derivatives and their antioxidant properties in a deoxyribose degradation test. Pharmacological Reports. 2011;63(1):120–129. doi: 10.1016/S1734-1140(11)70406-6. [DOI] [PubMed] [Google Scholar]
- 26.Jegadeeswari P., Nishanthini A., Muthukumarasamy S., Mohan V. R. GC-MS analysis of bioactive components of Aristolochia krysagathra (Aristolochiaceae) Journal of Current Chemical and Pharmaceutical Sciences. 2012;2(4):226–232. [Google Scholar]
- 27.Gibka J., Kunicka-Styczyńska A., Gliński M. Antimicrobial activity of undecan-3-one, undecan-3-ol and undec-3-yl acetate. Experimental Immunology. 2009;34(3):154–157. [Google Scholar]
- 28.Styczyńska A. K., Gibka J. Antimicrobial activity of undecan-x-ones (x = 2 − 4) Polish Journal of Microbiology. 2010;59(4):301–306. [PubMed] [Google Scholar]
- 29.Blenkharn J. I. The antimicrobial activity of taurolin—a possible additive for parenteral nutrition solutions. Clinical Nutrition. 1987;6(1):35–38. [Google Scholar]
- 30.Koldehoff M., Zakrzewski. J. L. Taurolidine is effective in the treatment of central venous catheter-related bloodstream infections in cancer patients. International Journal of Antimicrobial Agents. 2004;24(5):491–495. doi: 10.1016/j.ijantimicag.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Perucho J., Casarejos M. J., Gomez A., Solano R. M., de Yébenes J. G., Mena M. A. Trehalose protects from aggravation of amyloid pathology induced by isoflurane anesthesia in APPswe mutant mice. Current Alzheimer Research. 2012;9(3):334–343. doi: 10.2174/156720512800107573. [DOI] [PubMed] [Google Scholar]
- 32.Surender S., Vinod N., Sweety J., Gupta Y. K. Evaluation of anti-inflammatory activity of plant lipids containing α-linolenic acid. Indian Journal of Experimental Biology. 2008;46(6):453–456. [PubMed] [Google Scholar]
- 33.Rajeswari G., Murugan M., Mohan V. R. GC-MS analysis of bioactive components of Hugonia mystaxL. (Linaceae) Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2013;29(29):818–824. [Google Scholar]
- 34.Lee S. Y., Eom S. H., Kim Y. K., Park N. I., Park S. U. Cucurbitane-type triterpenoids in Momordica charantia Linn. Journal of Medicinal Plants Research. 2009;3(13):1264–1269. [Google Scholar]
- 35.Gill N. S., Bali M. Isolation of anti ulcer cucurbitane type triterpenoid from the seeds of Cucurbita pepo . Research Journal of Phytochemistry. 2011;5(2):70–79. doi: 10.3923/rjphyto.2011.70.79. [DOI] [Google Scholar]
- 36.Saradha M., Paulsamy S. GC-MS analysis for bioactive compounds from methanolic leaf and stem bark extracts of Hildegardia populifolia (Roxb.) Schott & Endl. International Journal of Pharmaceutical Science Review and Research. 2013;23:328–332. [Google Scholar]
- 37.Jamuna S., Paulsamy S. GC-MS analysis for bioactive compounds in the methanolic leaf and root extracts of Hypochaeris radicata L. (Asteraceae) International Journal of Current Research. 2013;5(12):4070–4074. [Google Scholar]