Abstract
Eclipta alba can be found growing wild in fallow lands of Bangladesh where it is considered as a weed by farmers. Traditional medicinal systems of the Indian subcontinent countries as well as tribal practitioners consider the plant to have diverse medicinal values and use it commonly for treatment of gastrointestinal disorders, respiratory tract disorders (including asthma), fever, hair loss and graying of hair, liver disorders (including jaundice), skin disorders, spleen enlargement, and cuts and wounds. The plant has several phytoconstituents like wedelolactone, eclalbasaponins, ursolic acid, oleanolic acid, luteolin, and apigenin. Pharmacological activities of plant extracts and individual phytoconstituents have revealed anticancer, hepatoprotective, snake venom neutralizing, anti-inflammatory, and antimicrobial properties. Phytoconstituents like wedelolactone and ursolic and oleanolic acids as well as luteolin and apigenin can form the basis of new drugs against cancer, arthritis, gastrointestinal disorders, skin diseases, and liver disorders.
1. Background
Eclipta alba (L.) Hassk. (also known as Eclipta prostrata Roxb.) belongs to the Asteraceae family and is commonly known as false daisy in English and bhringoraj or bhringraj in Bangladesh and India. It is regarded as a weed of ethnomedicinal significance. It is known in the three major forms of traditional medicinal systems in the Indian subcontinent, namely, Ayurveda, Unani, and Siddha, as bhringoraaja, bhangraa, and karissalaankanni, respectively. The Ayurvedic Pharmacopoeia of India considers the plant as hepatoprotective [1]. The full taxonomic hierarchy is shown below in Table 1.
Table 1.
Taxonomic hierarchy of E. alba.
| Kingdom | Plantae | |
| Subkingdom | Viridaeplantae | |
| Infrakingdom | Streptophyta | |
| Division | Tracheophyta | |
| Subdivision | Spermatophytina | |
| Infradivision | Angiospermae | |
| Class | Magnoliopsida | |
| Superorder | Asteranae | |
| Order | Asterales | |
| Family | Asteraceae | |
| Genus | Eclipta L. | |
| Species | Eclipta alba (L.) Hassk. |
Considering the ethnomedicinal significance of the plant, it was of interest to review the ethnopharmacological reports on the plant and selective phytoconstituents through data base searches (PubMed, Scopus, Google Scholar).
2. Reported Phytochemical Constituents
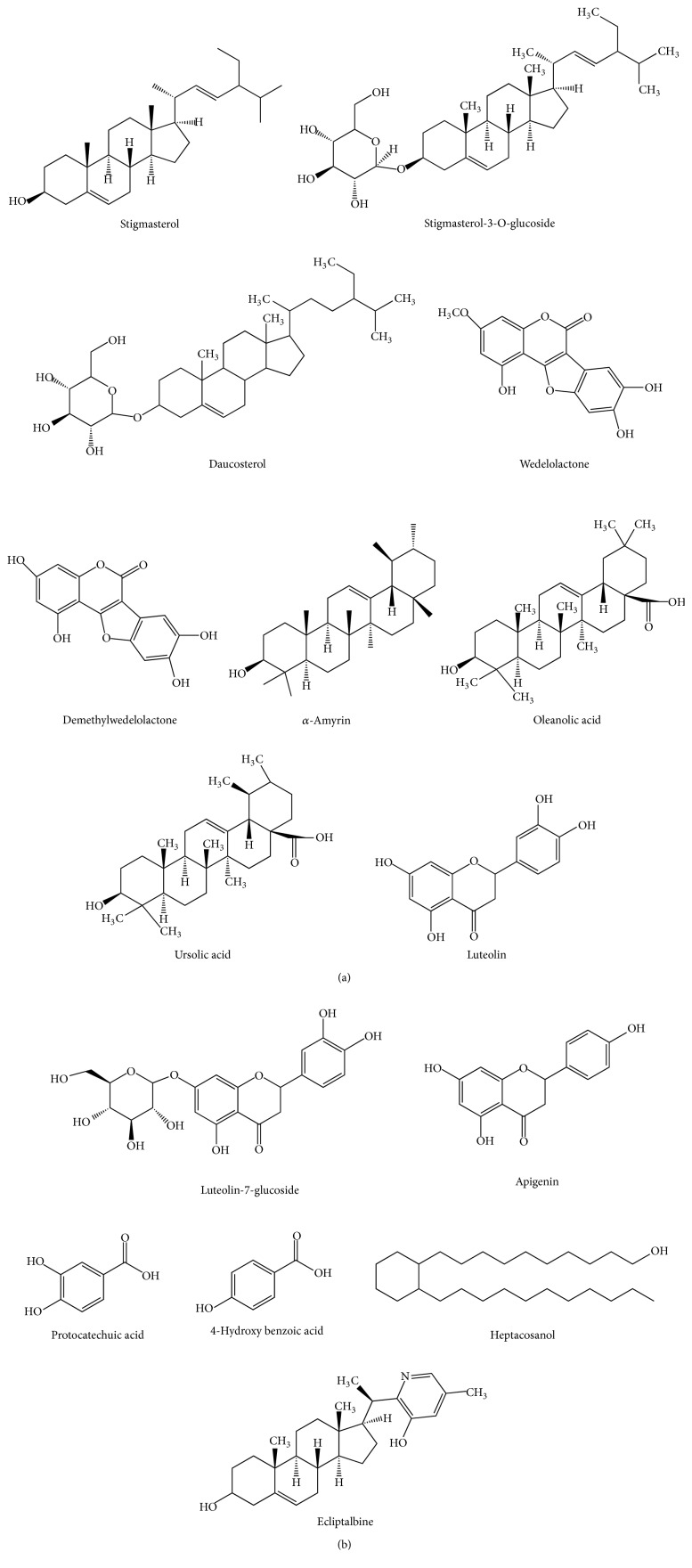
The plant reportedly contains a number of bioactive chemicals [2]. The reported constituents are shown in Table 2. The structures of several phytoconstituents are shown in Figure 1.
Table 2.
| Nature of phytoconstituent(s) | Phytoconstituent(s) |
|---|---|
| Coumestan | Wedelolactone, demethylwedelolactone, demethylwedelolactone-7-glucoside |
|
| |
| Terpenoids and their glycosides | Eclalbasaponins VII–X (taraxastane triterpene glycosides), eclalbasaponins I–VI (oleanane triterpene glycosides), eclalbosaponins I–VI (triterpene glycosides), ecliptasaponins C and D (triterpenoid glucosides), α-amyrin, oleanolic acid, ursolic acid (triterpenoids) |
|
| |
| Sterol | Stigmasterol, daucosterol, stigmasterol-3-O-glucoside |
|
| |
| Alkaloids | [(20S)(25S)-22,26-imino-cholesta-5,22(N)-dien-3β-ol] (verazine), [20-epi-3-dehydroxy-3-oxo-5,6-dihydro-4,5-dehydroverazine], [(20R)-20-pyridyl-cholesta-5-ene-3β,23-diol] (ecliptalbine), [(20R)-4β-hydroxyverazine], [4β-hydroxyverazine], [(20R)-25β-hydroxyverazine], [25β-hydroxyverazine] |
|
| |
| Flavonoids | Luteolin-7-glucoside, luteolin, apigenin, orobol (isoluteolin) |
|
| |
| Sesquiterpene lactones | 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl tiglate, 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl agelate, 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl acetate |
|
| |
| Terthienyl aldehyde | Ecliptal |
|
| |
| Fatty alcohols | Hentriacontanol, heptacosanol |
|
| |
| Volatile oils | Heptadecane, 6,10,14-trimethyl-2-pentadecanone, n-hexadecanoic acid, pentadecane, eudesma-4(14),11-diene, phytol, octadec-9-enoic ecid, 1,2-benzenediacarboxylic acid diisooctyl ester, (Z,Z)-9,12-octadecadienoic acid, (Z)-7,11-dimethyl-3-methylene-1,6,10-dodecatriene, (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene |
|
| |
| Saponins | Eclalbatin (triterpene saponin), dasyscyphin C |
|
| |
| Polyacetylinic compounds | α-Terthienylmethanol, polyacetylenes, polyacetylene substituted thiophenes |
|
| |
| Phenolic acids | Protocatechuic acid, 4-hydroxy benzoic acid |
Figure 1.
Structures of several phytoconstituents of E. alba.
3. Ethnomedicinal Reports
The plant and plant parts are used fortreatment of a variety of diseasesby folk medicinal practitioners and tribal medicinal practitioners of the Indian subcontinent. Ethnomedicinal uses of the plant have been reported from Bangladesh, India, Nepal, and Pakistan (Table 3).
Table 3.
Reported ethnomedicinal uses of E. alba.
| Location and tribe/nature of user(s) | Plant part(s) used, diseases treated, formulations |
|---|---|
| Traditional practitioners of Aligarh (1), Budaun (2), Bulandshahar (3), Farrukhabad (4), Hathras (5) districts of Western Uttar Pradesh, India [9] [Reported uses in the various districts are shown in the right column in parentheses] |
Acidity. Plant decoction is administered thrice daily with cow milk before each meal for 15 days (1–5). Alopecia. Leaf extract is given orally twice a day for 3 months (1–5). Asthma. Whole plant ash is given orally thrice daily for 3 months (1–5). Body pain. Fresh leaf extract is given orally thrice daily for 5 days or till cure (1–5). Bronchitis and pneumonia. Whole plant decoction is given orally with honey twice a day for 7 days or till cure (1–5). Burns. Whole plant extract is given orally twice a day for 7 days. Leaf paste is applied externally. This is continued till cure (1–5). Constipation. Root powder is given orally once a day for 3 days (1–5). Diarrhea and dysentery. Whole plant decoction is given orally thrice daily for 7 days or till cure (1–5). Edema. Plant extract is given twice a day for 7 days or till cure. Fever. Whole plant extract is given orally twice or thrice daily for 7 days or till cure (1–5). General weakness. Whole plant extract mixed with 3 g fruit powder of Phyllanthus emblica is given orally twice a day for 6 weeks or till the person recovers from weakness (1–5). Gingivitis. Leaf extract is given orally twice a day for 3 weeks or till cure (1–5). Hemorrhoids. Root extract is administered orally thrice daily (1, 2). Hair fall. Leaf extract is given orally twice daily with cow milk for 3 months (1–5). High blood pressure. Plant decoction is given orally twice or thrice a day for 3 months or till the patient recovers fully (1–5). Jaundice. Fresh plant extract is given orally twice or thrice daily for 3 weeks or till cure. Leaf extract along with honey is given orally twice or thrice daily for 15 days or till cure. Plant extract mixed with plant extract of Boerhavia diffusa is given orally twice a day for 15 days or till cure (1, 2). Liver enlargement. Plant extract is given orally twice or thrice daily for 1 month or till cure. This therapy is administered to adult patients only (1–5). Loss of appetite. Leaf decoction is given orally before each meal twice daily for 15 days. Leaf powder is given orally after each meal for 15 days (1, 2, 6). Palpitation of heart. Leaf extract mixed with honey is given orally twice a day for 7 days or till cure (1–5). Paronychia or whitlo. Whole plant paste is applied externally (1–5). Pimples. Fresh leaf extract is given orally twice daily with cow milk for 2 months (1–5). Premature graying of hair. Fresh leaf extract is gently applied to hair (1–5). Skin diseases. Plant paste is applied externally for 15 days in eczema. Leaf paste is applied externally to boils and extract is given orally twice daily for 15 days (1, 2, 5). Spleen enlargement. Leaf extract mixed with honey is given orally twice or thrice daily for 15 days or till cure. Urinary tract infections. Plant extract is given orally twice a day for 15 days. The extract is also used to wash genitalia externally till cure (1–5). Weakness of vision. Leaf extract is given orally twice a day with cow milk for 3 months (1–3). Wounds. Leaf extract is used to wash open wounds (1, 3, 5). Wrinkles. Leaf extract with Withania somnifera root powder is given orally with cow milk twice daily for 3 months (1–5). |
|
| |
| Local practitioners of Mount Abu in Rajasthan, India [10] | Leaves and flowers used for treatment of urinary problems, jaundice, asthma, and coughs. |
|
| |
| Local community of Jalalpur Jattan, Gujrat district, Punjab, Pakistan [11] | Leaf paste applied to treat allergy, athelete's foot and ringworm. |
|
| |
| Santal tribe residing in Thakurgaon district, Bangladesh [12] | Diabetes. Leaves of white-flowered plant are mixed with leaves of Scoparia dulcis, leaves of Cynodon dactylon and water and then boiled in an earthen vessel. The water is then strained through cloth and given to diabetic patients to be taken orally in the morning and evening on an empty stomach. |
|
| |
| Inhabitants of Mansoora, Malegaon, India [13] | Plant is used as tonic, deobstruent, emetic and considered useful in enlargement of liver and spleen. |
|
| |
| Chakma tribe of Tripura State, India [14] | Two teaspoons of leaf juice is administered daily against hepatic disorders. |
|
| |
| Local people and tribal communities of Hanumangarh District, Rajasthan, India [15] | Plant is used as hair tonic. Extracted oil is used as tonic. Leaf juice is taken orally with honey in jaundice and dysentery. The plant is considered to stimulate the digestive system, augment appetite, and improve digestion. Leaf extract is given orally with water for diarrhea. The root is considered purgative and used in conditions of liver, spleen, and dropsy. |
|
| |
| Inhabitants of Thar Desert, India [16] | Whole plant is considered deobstruent, antihepatotoxic, anticatarrhal, and febrifuge. Used in hepatitis, spleen enlargements, and skin diseases. Leaf is used to promote hair growth. Leaf extract in oil is applied to scalp before bedtime for insomnia. |
|
| |
| Local communities and ethnic groups of Bundelkhand, Uttar Pradesh, India [17] | Decoction of plant used to treat scorpion sting. |
|
| |
| Local herbalists of Samba District of Jammu and Kashmir State, India [18] | Whole plant is used in asthma, bronchitis, fever, gastric and hepatic disorders, jaundice, ulcers, wounds, sores, and leucoderma. |
|
| |
| Folk medicinal practitioners of Rampal, Bagerhat District, Bangladesh [19] | Whole plant used to treat indigestion. |
|
| |
| Tribals of Buldhana District, Maharashtra, India [20] | Whole plants and leaves used to treat wounds. |
|
| |
| Malayali tribals of Kolli Hills, Eastern Ghats, Tamil Nadu, India [21] | Whole plant juice is given orally to treat snake bite. |
|
| |
| Local people of Javadhu Hills, Tamil Nadu, India [22] | Plant is used for treatment of hepatitis. |
|
| |
| Malayaraya tribes of Vannapuram village, Idukki, Kerala, India [23] | Whole plant is used for rejuvenating hair, kidneys, and liver. |
|
| |
| Women of Kaibarta community of Assam, India [24] | Shoot juice with few drops of mustard oil or root extracts are given once daily for 3-4 days for diarrhea. |
|
| |
| Local people of Mandi Bahauddin District, Pakistan [25] | Leaf paste applied for allergy, athlete's foot and ringworm. |
|
| |
| Anyi-Ndenye pregnant women of Eastern Cote d'Ivoire, Africa [26] | Whole plant used to ensure fetal development and facilitate childbirth. |
|
| |
| Local people of Dibrugarh, Assam, India [27] | Whole plant used as tonic and for treatment of spleen enlargement. |
|
| |
| Women of Azamgarh District, Uttar Pradesh, India [28] | Whole plant juice with sugar is given to persons suffering from severe whitish dysentery. |
|
| |
| Villagers of Nizamabad District, Andhra Pradesh, India [29] | Dry plant powder is given to elderly people to provide energy. Plant paste is applied to head to blacken gray hair. |
|
| |
| Traditional herbal medical practitioners of Nagapattinam District, Tamil Nadu, India [30] | Leaf extract is applied on swellings. |
|
| |
| Local people of Birbhum District in West Bengal, India [31] | Fresh leaves are applied with sesame oil to cure baldness, elephantiasis, and headache. Juice of whole plant is applied in skin disorders on affected areas of skin. |
|
| |
| Saperas community of Khetawas, Jhajjar District, Haryana, India [32] | Treatment of snake bite. |
|
| |
| Local traditional healers of Western Uttar Pradesh, India [33] | Decoction of whole plant is given for scorpion sting. |
|
| |
| Bhil, Pawara and Pardhi tribes in Satpuda Mountain of Nandurbar, Dhule and Jalgaon district of Maharashtra, India [34] | 4-5 powdered leaves are administered with a cup of water in a single dose for 2 days for menorrhagia. |
|
| |
| Uraly tribes of Idukki District, Kerala, India [35] | Crushed leaves are applied on cuts and wounds. |
|
| |
| Local inhabitants of rural and remote areas of Kalyanpur block of Kanpur District, Uttar Pradesh, India [36] | 2–5 g leaf paste is applied on fresh cuts and wounds. |
|
| |
| Gujjar tribes in the Shivalik Hills of Haridwar, Uttarakhand, India [37] | Jaundice, premature graying and falling of hair. |
|
| |
| Tribes of Parambikulam Wildlife Sanctuary, Kerala, India [38] | Leaf paste is applied to hair to promote growth. |
|
| |
| Local people and traditional healers of Ambala District, Haryana, India [39] | Leaf decoction is put on head to cure headache. Leaf extract is given to cure asthma, cold and for hair cleaning and lice. |
|
| |
| Traditional healers and local people of Arghakhanchi District, Nepal [40] | Plant juice is applied externally in cuts and wounds. |
|
| |
| Ethnic communities of Moradabad District, Western Uttar Pradesh, India [41] | Leaf extract is applied to head to get rid of dandruff and to blacken gray hair. |
|
| |
| Tribals of Boudh District, Odisha, India [42] | Whole plant is grounded with black pepper and made into small pills. Two pills are administered twice a day to infants for treatment of jaundice and fever. |
The available ethnomedicinal reports indicate that although there are a variety of diseases treated with the plant or plant parts, the major uses are limited to treatment of gastrointestinal disorders, respiratory tract disorders (including asthma), fever, hair loss and graying of hair, liver disorders (including jaundice), skin disorders, spleen enlargement, and cuts and wounds.
4. Pharmacological Activity Reports
4.1. Hepatoprotective Activity
The protective effect of the plant against carbon tetrachloride-induced acute liver damage has been reported [3]. Coumestans (wedelolactone and demethylwedelolactone) have been mentioned as the possible components behind the protective effect on liver as well as against liver disorders; both compounds exhibited antihepatotoxic activity in assays employing CCl4—(carbon tetrachloride), GalN—(galactosamine), and phalloidin-cytotoxicity in rat hepatocytes. They also showed a significant stimulatory effect on liver cell regeneration [4]. Alcoholic extract of E. alba was found to have good antihepatotoxic activity as assessed in CCl4-induced liver damage in albino rats through liver to body weight ratio, pentobarbitone sleep time, serum levels of glutamate pyruvate transaminase (GPT) and glutamic oxaloacetic transaminase (GOT), alkaline phosphatase (ALP), and bilirubin. In CCl4-administered rats, there was an increase in liver weight, pentobarbitone sleep time, and elevated GOT, GPT, SALP, and serum bilirubin levels. The alcoholic extract at a dose of 200 mg/kg significantly reversed these effects [5].
The hepatoprotective effect of ethanol/water (1 : 1) extract (Ea) of the plant has been studied in CCl4-induced hepatotoxicity in rats. Ea significantly counteracted CCl4-induced inhibition of the hepatic microsomal drug metabolising enzyme amidopyrine N-demethylase and membrane bound glucose 6-phosphatase but failed to reverse the very high degree of inhibition of another drug metabolising enzyme aniline hydroxylase. The loss of hepatic lysosomal acid phosphatase and alkaline phosphatase by CCl4 was significantly restored by Ea. It was suggested that the hepatoprotective effect of Ea may be due to its regulating of the levels of hepatic microsomal drug metabolising enzymes [43].
The ethanolic extract of leaves of the plant has been fractionated into three parts (hot water insoluble (EaI), ethyl acetate fraction of hot water soluble (EaII), and remaining hot water soluble fraction (EaIII)) and each fraction studied for hepatoprotective activity against CCl4-induced hepatotoxicity in rats and mice. Hepatoprotective activity was determined on the basis of their effects on parameters like hexobarbitone sleep time, zoxazolamine paralysis time, bromsulfalein clearance, serum transaminases (GPT, GOT), and serum bilirubin. All the experimental parameters were increased by CCl4; fraction EaII (10–80 mg/kg, p.o.) dose-dependently and significantly reversed these increases. Fraction EaII was found to contain coumestan wedelolactone and demethylwedelolactone as major components with apigenin, luteolin, 4-hydroxybenzoic acid, and protocatechuic acid as minor constituents [44].
Hepatitis C virus (HCV) inhibitory activity has been reported for E. alba extract. Phytochemical analysis of the extract revealed the presence of three compounds, namely, wedelolactone, luteolin, and apigenin. These compounds exhibited dose-dependent inhibition of HCV replicase in vitro, and anti-HCV replication activity in the cell culture system. The results suggest that the plant or individual components have the potential to be used against HCV [45].
Ethanol extract of whole plant was tested for hepatoprotective effect against paracetamol-induced hepatotoxicity in mice. Treatment with 100 and 250 mg of the extract per 100 kg body weight showed significant reductions in paracetamol-induced serum alanine aminotransferase (ALT, also known as GOT) levels. At the same time, histopathological studies showed marked reductions in paracetamol-induced fatty degeneration and centrizonal necrosis in liver of extract-treated mice [46].
An alcoholic extract of freshly collected Eclipta alba exhibited dose-dependent (62.5–500 mg/kg p.o.) significant hepatoprotective activity against carbon tetrachloride-induced liver injury in rats and mice as determined through various tests like hexobarbitone-induced sleep, zoxazolamine-induced paralysis, bromsulfalein (BSP) clearance, serum levels of transaminases, bilirubin, and protein [47].
A combination of ethanolic extract of E. alba leaves and P. longum seeds demonstrated better hepatoprotective action against CCl4-induced hepatotoxicity in rats than either extract alone. Serum marker enzymes like alanine aminotransferase (ALT/GOT), aspartate aminotransferase (AST, also known as GOT), acid phosphatase (AP), lactate dehydrogenase (LDH), γ-glutamyl transferase (GGT), and 5′-nucleotidase were elevated with carbon tetrachloride treatment, which were restored towards normalization by the combined extract. At the same time, changes in biochemical parameters like total protein, total bilirubin, total cholesterol, triglycerides, and urea were restored to near normal levels with the combined extract [48].
Administration of fresh leaf powder (500 mg/kg) to rats was observed to lead to significant hepatoprotective action in paracetamol-induced liver toxicity in rats. Histopathological studies indicated that paracetamol-administered rat liver showed severe congestions, hydropic degeneration, and occasional necrosis, while leaf-administered rat liver showed decreased hepatocyte damage. At the same time, paracetamol-induced elevated levels of serum ALT, AST, alkaline phosphatase (ALP), LDH, and GGT as well as paracetamol-induced changes in serum proteins, bilirubin, cholesterol, and triglycerides were restored to normal levels with the leaf powder [49].
In CCl4-induced hepatotoxicity in rats, methanol extract of leaves and chloroform extract of roots of E. alba showed significant reductions of lysosomal enzymes in serum from the elevated levels induced by carbon tetrachloride. At the same time CCl4-induced elevated serum GOT, GPT, ALP, and bilirubin levels were also restored towards normalization with administration of both extracts [50].
The hepatoprotective activity of ethanol extract of E. alba whole plants was studied in rats given ethanol for 21 days. Ethanol administration led to hepatic damage as manifested by histopathological changes, increase in thiobarbituric acid-reactive substances (TBARS), decrease in reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT), and increase in glutathione peroxidase (GPx) in liver. Histopathological changes indicated that, in alcohol-treated animals, liver showed hepatocytic necrosis and inflammation in the centrilobular region with portal triaditis. These toxic effects were reversed with coadministration of extract with ethanol [51].
Aqueous extract of leaves of the plant has been found to offer hepatoprotectivity against paracetamol-induced liver damage. Paracetamol-induced increases in TBARS were reduced by the aqueous extract, and paracetamol-induced decreases in GSH were also reversed by the extract. Catalase was also decreased in paracetamol-treated groups, which was also reversed by coadministration of the extract [52].
The alcoholic and aqueous extract of E. alba leaves was tested for hepatoprotective activity against paracetamol-induced liver damage in albino rats. The alcoholic extract demonstrated significant hepatoprotective effects. The alcoholic extract-treated rats of group III revealed marked hepatoprotection as there was significant (P < 0.01) reduction in SGOT, SGPT, ALP, total bilirubin, and direct bilirubin and a significant (P < 0.01) increase in total protein and albumin as compared to paracetamol treated group [53].
The aqueous leaf extract (85%) of E. alba was examined for hepatoprotective effects in CCl4-induced hepatotoxicity in male albino rats. CCl4 induced oxidative stress in rats resulting in oxidative injury as manifested by increases in TBARS and hydroperoxides and augmented levels of serum AST, ALT, and ALP. At the same time there were depleted levels of SOD, CAT, GPx, and glutathione-S-transferase (GST). The aqueous extract given at a dose of 250 mg per kg body weight reversed these changes and brought them back to normal levels. The results suggest that increases in oxidative stress play a vital role in development of hepatic injury, which can be ameliorated through administration of aqueous extract of the leaves [54].
A polyherbal formulation (Ayush-Liv.04) containing E. alba (along with Clitoria ternatea, Asparagus racemosus, Alpinia galanga, and milk tuttam, i.e., copper containing stone) showed hepatoprotective activity against CCl4 and ethanol induced liver damage in rats. Elevated levels of serum AST, ALT, ALP, acid phosphatase, and bilirubin were significantly lowered in the polyherbal formulation-administered rats [55].
The ethanolic extract of a polyherbal formulation containing leaves of Melia azadirachta, seeds of Piper longum, and whole plants of E. alba has been evaluated for hepatoprotective effects against CCl4-induced hepatic damage in male albino rats. The substantially reduced levels of SOD, CAT, GPx, GST, and glutathione reductase (GR) due to CCl4 were restored to normal with the extract [56].
Hepatoprotective effects of ethanolic extract of E. alba leaves and leaf callus were examined in a model of CCl4-induced acute hepatotoxicity in albino rats. Liver damage was assessed by measuring serum parameters like GOT, GPT, ALP, albumin, and total protein, as well as histopathological examination. Oral administration of the extract at 250 and 300 mg/kg, respectively, showed hepatoprotective effects as demonstrated by restoring to near normal levels the serum parameters and improving hepatic lesions caused by carbon tetrachloride [57].
4.2. Hair Growth Promoting Activity
Petroleum ether and ethanol extract of E. alba has been tested in albino rats for promoting hair growth activity. The extracts were incorporated into oleaginous cream (water in oil cream base) and applied topically on shaved denuded skin of male albino rats. The extracts significantly reduced hair growth time by half, as compared to nontreated control animals. Quantitative analysis of hair growth after treatment with petroleum ether extract (5%) exhibited greater number of hair follicles in anagenic phase (69 ± 4) which were higher as compared to control (47 ± 13) [58].
The methanol extract of the plant has also been tested for its efficacy for promoting hair growth in pigmented C57/BL6 mice, preselected for their telogen phase of hair growth. In these species, the truncal epidermis lacks melanin-producing melanocytes and melanin production is strictly coupled to anagen phase of hair growth. Telogen to anagen transition was assessed following topical administration of the extract. A dose-dependent transition of telogen to anagen phase of hair growth was observed following extract treatment; with an extract dose of 3.2 mg/15 cm2, 87.5% animals showed anagen phase of growth, while with an extract dose of 1.6 mg/15 cm2, 50% of the animals showed the transition from telogen to anagen phase [59].
A polyherbal formulation containing E. alba, Hibiscus rosa-sinensis, and Nardostachys jatamansi exhibited excellent hair growth activity in Wistar albino rats. Hair growth initiation time and time required for complete hair growth were significantly reduced. Treatment with the formulation resulted in greater number of hair follicles in the anagenic phase [60].
4.3. Antidiabetic Activity
The beneficial effects of the plant in diabetes have been reported. An Ayurvedic formulation consisting of Withania somnifera, Tinospora cordifolia, Eclipta alba, Ocimum sanctum, Picrorhiza kurroa and shilajit, at doses of 100 and 200 mg/kg, p.o. administered once daily for 28 days to streptozotocin- (STZ-) induced diabetic male CF strain rats, induced a dose-related decrease in STZ hyperglycemia and attenuation of STZ induced decrease in pancreatic islet superoxide dismutase (SOD) activity. It has been suggested that the STZ-induced hyperglycemia was the consequence of decreased islet SOD in islets [61].
In alloxan-diabetic rats, oral administration of leaf suspension of E. alba (2 and 4 g/kg body weight) for 60 days resulted in significant reduction in blood glucose (from 372.0 ± 33.2 to 117.0 ± 22.8), glycosylated hemoglobin HbA(1)c, a decrease in the activities of glucose-6 phosphatase and fructose 1,6-bisphosphatase, and an increase in the activity of liver hexokinase, all of these activities being beneficial for amelioration of hyperglycemia and other diabetes-related complications [62].
The antidiabetic effect of E. alba ethanolic extract has been investigated for possible beneficial effects against hyperglycemia and diabetic nephropathy in STZ-diabetic rats. Single dose treatment of the extract was found to significantly lower blood glucose level by 17.6% after 5 h of oral administration at a dose of 250 mg/kg. Treatment of STZ-diabetic animals for 10 weeks with the above dose level significantly reduced the elevated levels of blood glucose, %HbA1C, urea, uric acid, and creatinine and significantly increased the depressed serum insulin level. The extract exerted a significant inhibitory effect on α-glucosidase in a noncompetitive manner with an IC50 value of around 54 μg per mL and was found inhibitory to eye lens aldose reductase with an IC50 value of about 4.5 μg per mL. Inhibition of α-glucosidase and aldose reductase was postulated to be the reason behind the other observed effects [63]. A bioactivity-guided isolation approach based on α-glucosidase inhibition led to the isolation of four echinocystic acid glycosides of which eclalbasaponin VI was found to be the most potent (IC50 54.2 ± 1.3 microM) [64].
4.4. Analgesic and Anti-Inflammatory Activities
Analgesic activity of alcoholic extract of E. alba has been determined through tail flick, hot plate, and writhing methods in rats and mice. In all three methods, the extract at a dose of 200 mg/kg demonstrated significant analgesic and antinociceptive effects [65].
Hydroalcoholic extract of the plant showed significant antinociceptive activity in acetic acid-induced writhing tests in rodent model at a dose of 200 mg/kg p.o. The extract further showed analgesic effects in formalin tests, with the inhibition occurring in the second phase of the response [66].
The analgesic activity of ethanol extract of E. alba whole plants as well as a total alkaloid fraction was seen in experiments with albino mice by using standard experimental models such as the tail clip method, the tail flick method, and the acetic acid induced writhing response. The results from this study showed that both the ethanol extract and the total alkaloids produced good analgesic activity in all the different models of analgesia tested. Total alkaloid fraction showed better analgesic activity than ethanolic extract [67].
The anti-inflammatory effect of the plant was evaluated using carrageenan, mediators such as histamine and serotonin induced paw oedema, and cotton pellet induced granuloma tests for their effect on acute and chronic phase inflammation models in rats. The results indicated potent anti-inflammatory activity of the plant in all the models tested [68]. Cumulatively, the reports suggest that the plant can prove valuable as both a central and peripheral analgesic agent.
4.5. Skin Diseases
Leaves of E. alba are used to get rid of ectoparasites in dogs in Trinidad and Tobago [69]. An Ayurvedic formulation containing E. alba powder has been shown to provide complete remission to 22.6% and checked the recurrence of the disease in 89.5% patients of “Vicharchika” (eczema) [70].
The antioxidant and protective effect of water extract of E. alba against ultraviolet- (UV-) irradiation-induced damage has been investigated. The extract had a potent effect in scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH), superoxide radicals, and chelating ferrous ion, exhibiting IC50 values, respectively, of 0.23 mg/mL, 0.48 mg/mL, and 1.25 mg/mL. The total phenol content of the extract was 176.45 mg gallic acid equivalents. The extract was also seen to absorb UVA and UVB irradiation and demonstrated a dose-dependent protection of HaCaT human keratinocytes and mouse fibroblasts 3T3 cells against UVB-induced cytotoxicity. The protective effect against skin cell damage was attributed to a synergistic effect between chlorogenic acid and other active components present in the extract [71].
4.6. Neuropharmacological Activities
The aqueous and hydroalcoholic extracts of E. alba have been evaluated for sedative, muscle relaxant, anxiolytic, nootropic, and antistress activities at doses of 150 and 300 mg/kg, p.o. The findings indicated nootropic activity of the aqueous extract (300 mg/kg, p.o.) and its hydrolyzed fraction (30 mg/kg, p.o.). The aqueous extract and the hydrolyzed fraction were observed to provide protection against cold restraint induced gastric ulcer formation and also normalized the white blood cell count in the milk induced leukocytosis challenge model [72].
The aqueous extract of leaves of E. alba has been examined for its memory enhancing quality. Doses of 100 and 200 mg of extract suspension in water (per kg body weight) were administered to rats to evaluate transfer latency (TL) on an elevated plus maze. This method gives a measure of acquisition and retrieval learning. Spatial habitual learning tests were conducted with mice at the aforementioned two doses. In this method, mice were placed at the center of open-field apparatus to assess spatial habitual learning, observed for 20 minutes for rearing and time spent during rearing for 30 minutes, 24 hours and 96 hours and 144 hours. The extract at both doses produced a significant decrease in TL in rats and the amount of rearing in mice. The results indicate an extract-induced improvement in cognitive functions, which was attributed to the presence of luteolins in the extract [73].
Aqueous extract of E. alba has been tested for its ability to reduce aggression through foot shock-induced aggression and water competition tests. Minimization of aggression in both tests was observed with the extract at doses of 100 and 200 mg/kg [74].
Methanolic extract of E. alba whole plant has been shown to ameliorate oxidative stress-induced mitochondrial dysfunction in an animal (rat) model of Alzheimer's disease (evaluation of short-term memory using elevated plus maze model). Mitochondrial function was determined through MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Synaptosomal fractions of scopolamine hydrobromide-treated rats exhibited a significant decrease in MTT reduction, which was prevented by the extract at a dose of 200 mg/kg. Scopolamine significantly increased transfer latency in rats indicative of amnesia or impairment of memory. Transfer latency of rats using the elevated plus maze model was also dose-dependently decreased (reversal of scopolamine-induced increase) by the extract thus demonstrating increase in memory [75]. The extract showed high phenolic and flavonoid contents, which might have contributed to amelioration of oxidative stress.
4.7. Antioxidant Activity
The methanol and hydrolyzed extract of E. alba has been assessed for its antioxidant potential in both in vitro and ex vivo models. The in vitro antioxidant activity was evaluated through 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging and nitric oxide radical inhibition activity. The ex vivo antioxidant activity was determined through lipid peroxidation inhibitory activity on mice liver homogenate by thiobarbituric acid-reactive substances (TBARS) method. The methanolic extract and hydrolyzed extract both showed potent antioxidant activity in both models in proving to be powerful scavengers of DPPH free radicals and nitric oxide radicals, as well as being inhibitors of lipid peroxidation [76]. Antioxidant activity as assessed by DPPH free radical scavenging methods has also been described for ethanol extract of the plant [77].
Methanolic and aqueous extracts of E. alba demonstrated antioxidant activity in hydrogen peroxide scavenging assays, total antioxidant capacity, and through reducing ability assay [78]. The antioxidant potential of the plant methanolic extract has been shown through DPPH free radical scavenging and 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assays [79]. The ethanolic extract of the plant also demonstrated antioxidant potential in DPPH and ABTS assays [80]. Ethanol and ethyl acetate extracts of leaves of the plant showed antioxidant activity in the ferric thiocyanate method; aqueous and hexane extracts also showed antioxidant effects but less than ethanol and ethyl acetate extracts [81].
The possible cerebroprotective and antioxidant effect of hydroalcoholic extract of E. alba has been evaluated in global cerebral ischemia in rats. The global cerebral ischemia-reperfusion injury was induced by occluding bilateral common carotid arteries (BCCA) for 30 min, followed by 4 h reperfusion. BCCA caused significant depletion in superoxide dismutase (SOD), glutathione peroxidase (GPx), reduced glutathione (GSH), catalase (CAT), glutathione-S-transferase (GST), and glutathione reductase (GR) and significant increase in malondialdehyde (MDA) in brain. Pretreatment with hydroalcoholic extract significantly reversed the levels of biochemical parameters and significantly reduced the edema and cerebral infarct size as compared to the ischemic control group [82].
4.8. Antimicrobial Activity
Various solvent (petroleum ether, benzene, chloroform, acetone, methanol, and aqueous) extracts of E. alba were found to be active against clinical isolates from oral cancer cases. These isolates included various bacteria like Staphylococcus aureus, Escherichia coli, Staphylococcus epidermis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, and Proteus vulgaris and funguses like Candida albicans and Aspergillus fumigatus [83].
Ethanol and ethyl acetate extracts of leaves of the plant have been found to be active against E. coli, K. pneumoniae, Shigella dysenteriae, Salmonella typhi, P. aeruginosa, Bacillus subtilis, and S. aureus with Minimum Inhibitory Concentrations (MIC) ranging from 4.5 to 90 μL/mL [81].
Hexane extract of aerial parts of the plant reportedly showed antibacterial activity against S. aureus, Bacillus cereus, E. coli, S. typhi, K. pneumoniae, Streptococcus pyogenes, and P. aeruginosa, whereas acetone, ethanol, methanol, and aqueous extracts showed intermediate activity against S. aureus, B. cereus, E. coli, S. typhi, K. pneumoniae, P. aeruginosa, P. mirabilis, and S. pyogenes [84]. The aqueous extract showed good activity against S. pyogenes, B. cereus, E. coli, and P. aeruginosa.
The susceptibility of various extracts of E. alba was tested against nine different test organisms using the well diffusion method. The n-butanol extract showed inhibitory activity against all nine species, namely, B. cereus, B. subtilis, C. albicans, Erwinia carotovora, E. coli, K. pneumoniae, P. aeruginosa, S. typhi, and S. aureus. Petroleum ether, dichloromethane, methanol and aqueous extracts showed varying levels of inhibition against some of these microorganisms [85].
Aqueous extract of leaves, stems, and flowers of E. alba has been screened for inhibitory activity against multiple test organisms. The leaf extract was effective against Enterobacter cloacae and K. pneumoniae; stem extract was effective against E. cloacae, Enterococcus faecalis, K. pneumoniae, and Staphylococcus saprophyticus, while flower extract was effective against P. vulgaris, S. aureus, and S. saprophyticus [86]. Aqueous and ethanolic extracts of leaves also reportedly showed moderate inhibitory activities against S. aureus, E. coli, P. vulgaris, P. aeruginosa, C. albicans, and Aspergillus niger, when tested by agar plate disc diffusion method [87].
Antimicrobial activity of petroleum ether, ethyl acetate, ethanol and aqueous extract of E. alba were screened for inhibitory activity by agar well diffusion method against B. subtilis, S. aureus, P. mirabilis, B. cereus, E. coli, Salmonella enterica serv. typhi, P. aeruginosa, S. epidermis, and C. albicans. Maximum numbers of the organisms tested were inhibited by the ethyl acetate fraction with zones of inhibition ranging from 11 ± 1 mm to 22 ± 1 mm, with maximum activity against B. cereus. The ethanol extract demonstrated maximum inhibitory activity against E. coli. Petroleum ether extract inhibited only B. cereus, while aqueous extract was found to inhibit B. subtilis, B. cereus, S. aureus, and C. albicans [88].
The antimicrobial activity of methanol, acetone, and aqueous extracts of leaves of three morphotypes of E. alba has been examined by the disc diffusion method against the Gram positive bacteria—S. aureus and B. subtilis—and Gram negative bacteria—K. pneumoniae, E. coli, and P. aeruginosa. The extracts demonstrated inhibitory activity against all bacteria against P. aeruginosa. The aqueous extract was found to be the least effective. The acetone extract showed more antimicrobial activity against S. aureus, E. coli, and K. pneumoniae than methanol extract. The methanol extract showed maximum activity against B. subtilis [89].
An aqueous extract of leaves of E. alba has been shown to inhibit the fungus Fusarium oxysporum as determined by the agar plate disc diffusion method [90]. Methanolic extract of aerial parts of the plant showed maximum inhibitory activity against S. epidermis, S. aureus, and Salmonella typhimurium. Wedelolactone, isolated from the ethyl acetate fraction of aerial parts, showed enhanced antimicrobial activity and as such can be the responsible agent behind the observed antimicrobial effects [91]. Eclalbasaponin, another phytochemical constituent of the plant, has been shown to be responsible for the inhibitory activity of the plant against B. subtilis and P. aeruginosa [92]. This inhibitory activity has been attributed to disruption of bacterial cell membrane leading to loss of bacterial cell viability.
4.9. Antimalarial Activity
The antimalarial activity of leaf extract of E. alba has been tested against Plasmodium berghei ANKA strain in mice. The methanolic leaf extract (250–750 mg/kg) produced a dose-dependent chemosuppression or schizontocidal effect during early and established infection and high mean survival time (m.s.t.) values particularly in the group administered 750 mg/kg/day of extract. The plant extract also exhibited repository activity [93].
Mosquito larvicidal and ovicidal activities of crude hexane, ethyl acetate, benzene, chloroform, and methanol extracts of the leaves of E. alba were tested against the early third-instar larvae of Anopheles stephensi (Liston) (Diptera: Culicidae). Larval mortality was observed 24 hours following extract exposure. The highest larval mortality was observed with methanol extract (LC50 = 112.56 ppm, LC90 = 220.68 ppm). Percent hatchability was also found to be inversely proportional to concentration of the extract. Mortality of 100% was exerted with methanol extract at 200 ppm. Thus this plant could prove to be useful in combating malaria through its larvicidal and ovicidal activities [94].
Larvicidal and ovicidal activities of benzene, hexane, ethyl acetate, methanol and chloroform leaf extract of E. alba against dengue vector, Aedes aegypti, has also been examined. Maximum larvicidal activity was observed with the methanol extract. The LC50 values of benzene, hexane, ethyl acetate, methanol and chloroform extract of E. alba against early third- instar larvae of A. aegypti were 151.38, 165.10, 154.88, 127.64, and 146.28 ppm, respectively. The methanol extract was found to be most effective for ovicidal activity against A. aegypti. The methanol extract exerted 100% mortality (zero hatchability) at 300 ppm [95].
The adulticidal and repellent activities of crude hexane, ethyl acetate, benzene, chloroform, and methanol extracts of leaf of E. alba were assayed for their toxicity against two important vector mosquitoes, namely, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). C. quinquefasciatus is the vector of Wuchereria bancrofti, avian malaria, and arboviruses including St. Louis encephalitis virus, Western equine encephalitis virus, and West Nile virus. All extracts showed moderate adulticide effects. The extracts also had concentration-dependent mosquito repellent activities [96]. The methanol extract of leaves of E. alba also reportedly demonstrated maximum adulticidal as well as repellent activities against A. stephensi compared to benzene, hexane, ethyl acetate, and chloroform extracts [97].
4.10. Cardiovascular Effects
The effect of administration of dried E. alba leaf powder (3 g per day) has been studied in mild hypertensive subjects. Subjects were given six capsules (500 mg powder per capsule) in three doses per day for 60 days. When compared with placebo given control groups, the results showed that Eclipta-supplemented group showed a marked reduction in mean arterial pressure by 15%, total cholesterol (17%), low-density lipoprotein fraction (24%), triglycerides (14%), very-low-density lipoprotein fraction (14%), and plasma lipid peroxides (18%). There was a marked increase in urine volume (34%), urine sodium (24%), serum vitamin C (17%), and serum tocopherols (23%) in the Eclipta-administered group. The findings indicated that leaf powder possessed diuretic, hypotensive, and hypocholesterolemic properties and helps in the alleviation of oxidative stress-induced complications in hypertensives [98].
Ethanolic extract of leaves and leaf calluses of E. alba was examined for cardiac inhibitory activity in isolated frog hearts. The extracts showed negative ionotropic and negative chronotropic effects as well as reduction in cardiac output. Callus extract demonstrated a higher cardiac inhibitory effect than leaf extract at 20 mg doses. The callus extract was also found to antagonize the effects of adrenaline [57].
4.11. Immunomodulatory Effects
The immunostimulatory effects of feeding aqueous extract of leaves of E. alba have been studied in tilapia fish (Oreochromis mossambicus). Fish were fed diet containing extract at 0, 0.01. 0.1, and 1% levels of diet. After each week, nonspecific humoral (lysozyme, antiprotease, and complement) and cellular (myeloperoxidase content, production of reactive oxygen and nitrogen species) responses and disease resistance against Aeromonas hydrophila were determined. A. hydrophila are Gram-negative straight rods with rounded ends (bacilli to coccobacilli shape) and are regarded as both fish and human pathogens. Lysozyme activity significantly increased after feeding fish with aqueous extract for 1, 2, or 3 weeks. Reactive oxygen species production and myeloperoxidase content showed significant enhancement after 1 week of feeding with aqueous extract. The percent mortality in fish following feeding the extract also diminished significantly when challenged with the pathogen [99].
The immunomodulatory responses of methanol extract of whole plant of E. alba (containing 1.6% wedelolactone) have been assessed at five dose levels (dose-response relationship) ranging from 100 to 500 mg/kg body wt. using carbon clearance, antibody titer, and cyclophosphamide immunosuppression parameters. E. alba extract significantly increased the phagocytic index and antibody titer. The F ratios of the phagocytic index and white blood cell (WBC) count were also significant [100].
4.12. Antiepilepsy Activity
Methanol extraction of leaf powder of E. alba was evaluated for its antiepileptic activity through Maximal Electroshock Test (MES) in rats. The extract was administered orally to rats for 7 days at doses of 50, 100, and 200 mg per kg body weight. One hour after the last treatment, seizures were induced in rats by delivering electroshock of 150 mA for 0.2 s with an electroconvulsiometer through a pair of ear clip electrodes. A decrease in duration of hind leg extension was taken as a parameter for anticonvulsant activity. Compared to controls, rats administered extract at different doses exhibited significant decrease in the duration of time spent in extensor phase in a dose-dependent manner. The antiepileptic activity was attributed to wedelolactone, luteolin, and β-amyrin present in the extract [101].
The anticonvulsant activity of methanol extract of E. alba leaves was studied using pentylenetetrazole- and picrotoxin-induced seizure models in mice and guinea pigs. The mechanism was further elucidated by studying the extract's GABAA receptor modulatory activity and its effect on levels of GABA (γ-amino butyric acid) in mice brain. Potent anticonvulsant activity was demonstrated by the extract when administered at doses of 10–200 mg/kg with a saturation level at 50 mg/kg. The observed anticonvulsant effect was attributed to positive modulatory effects on GABAA receptors [102]. Wedelolactone and luteolin, present in the extract, were hypothesized for giving the observed effect. Notably, GABAA receptor dysfunction contributes to epileptogenesis [103]. Wedelolactone has been reported to have selectivity and affinity towards BZD (benzodiazepine) binding site on GABAA receptors [104]. Also luteolin has neuroprotective activity and has affinity towards BZD binding site on the GABAA receptors [105].
E. alba ethanolic leaf extracts at doses of 50, 100, 200, and 400 mg/kg, p.o., were studied for anticonvulsant and muscle relaxant activity on maximal electroshock-induced seizures (MES), rotarod, and traction test, respectively, in rats. At doses of 200 and 400 mg/kg, the extract reduced seizures induced by MES, decreased the duration of tonic hind limb extension (THLE) (by 76.2 and 89.8%, resp.), and decreased motor coordination showing anticonvulsant and muscle relaxant activity [106].
E. alba ethanolic leaf extract has been shown to cause thiopental sodium-induced sleeping time in rats at an early stage and to prolong the duration of sleep at doses of 200 and 400 mg/kg. The extract at 400 mg/kg also decreased locomotor activity in rats, thus showing a sedative effect. Ursolic and oleanolic acids, present in the extract, can act as GABAA agonists and this property can be responsible for the central nervous system (CNS) depressant effect [107]. Notably, CNS depressant and antiepileptic activities have been reported for methanolic extract of leaves of Ipomoea aquatica [108].
4.13. Snake Bite
Extract of E. alba has been shown to inhibit snake venom phospholipase A2 activity of Crotalus durissus terrificus venom. The inhibitory activity has been attributed to the coumestans, wedelolactone, and demethylwedelolactone, present in the extract [109].
4.14. Anticancer Activity
The anticancer potential of hydroalcoholic extract of E. alba has been evaluated. The extract inhibited the cell proliferation in dose-dependent manner in HepG2, A498, and C6 glioma cell lines with an IC50 of 22 ± 2.9, 25 ± 3.6, and 50 ± 8.7 μg/mL, respectively. The expression of matrix metalloproteinases (MMP) 2 and 9 was downregulated significantly. Additionally, downregulation of nuclear factor κB (NFκB) was also observed. DNA damage was observed following 72 h of extract treatment, leading to apoptosis [110]. Hydroalcoholic extract of the plant also demonstrated antiproliferative activities in multidrug-resistant DR-HepG2 cells, a hepatocellular carcinoma cell line [111].
Juice obtained from E. alba was shown to inhibit the migration of HCC-S102 (hepatocellular carcinoma) cells. In various human cancer cell lines of different tissue origins (liver, lung, and breast), the juice inhibited migration of all the cell lines with IC50 values ranging from 31–70 μg/mL. Thus the plant has potential for preventing cancer metastasis [112]. Antiangiogenic activity was also demonstrated with the juice.
The ethyl acetate, methanol, and aqueous extracts of whole dried plants of E. alba were assessed for their inhibitory effects on the human lung epithelial adenocarcinoma cell line (HCC-827) using the MTT assay. Dose-dependent reductions in viable cell count were noticed with all three extracts with the ethyl acetate extract showing the most potency. All extracts induced apoptosis in the cancer cells [113].
Ethinylestradiol is widely used in various contraceptives and for treatment of metabolic and sexual disorders. It is also a genotoxic and a tumor initiating agent. The treatment of 10 μM of ethinylestradiol along with 1.02 × 10−4, 2.125 × 10−4, 3.15 × 10−4, and 4.17 × 10−4 g/mL acetone extract of E. alba leaves resulted in a significant dose-dependent decrease in the genotoxic effects induced by the treatment of 10 μM of ethinylestradiol in cultured human lymphocytes [114].
Crude methanol extract of E. alba has been shown to inhibit growth of colon cancer cells [115]. The methanolic extract of the aerial parts of the plant showed inhibitory activity on the proliferation of hepatic stellate cells or HSCs. Activity-guided fractionation led to the isolation of five oleanane-type triterpenoids, echinocystic acid, eclalbasaponin II, eclalbasaponin V, eclalbasaponin I, and eclalbasaponin III, which are all echinocystic acid derivatives. Among the five echinocystic acid derivatives isolated, echinocystic acid and eclalbasaponin II significantly inhibited the proliferation of HSCs in dose- and time-dependent manners [116].
4.15. Antiulcer Activity
The ethanolic extract of E. alba has been examined for its antiulcer effects in several ulcer models in rats, like cold resistant stress (CRS) and pylorus ligation (PL). The extract administered orally twice daily at doses of 50, 100, and 200 mg/kg was found to dose-dependently and significantly reduce ulcerative lesions. At the same time, extract administration led to significant attenuation of lipid peroxidation and elevated levels of catalase activity. Antisecretory activity of the extract was evidenced by significant reduction in gastric volume, acid output, and increase in gastric pH when compared to control (without extract) rats [117].
The methanolic extract of E. alba also showed antiulcer activity in ulcers induced in thirty- six-hour fasted Sprague Dawley rats by aspirin or ethanol or pylorus ligation plus aspirin treatment. In all the three separate experiments the group receiving oral administration of E. alba prior to ulcer induction showed highly significant reduction in the occurrence of gastric ulcers as well as gastric inflammation (after 4 h of treatment) as compared to the control groups. The extract activity was comparable to the activity of the proton pump inhibiting drug rabeprazole [118].
4.16. Anthelmintic Activity
The methanol extract of whole plant of E. alba was evaluated for its anthelmintic potential against the earthworm Pheretima posthuma at doses of 25–100 mg/mL. The extract exhibited paralysis of worms at doses of 50, 75, and 100 mg/mL and caused death of worms at 75 and 100 mg/mL [119]. The ethanol and aqueous extract also showed anthelmintic activity against P. posthuma [120].
5. Pharmacological Activity Reports on E. alba Phytoconstituents
5.1. Wedelolactone
In vitro 5-lipoxygenase inhibition by wedelolactone has been reported [121]. 5-Lipoxygenase (5-LO) catalyzes the two-step conversion of arachidonic acid to leukotriene A4 (LTA4) [122].
A study with synthetically prepared wedelolactone and derivatives showed that both the parent compound and most of the wedelolactone derivatives significantly protected primary cultured liver cells from the toxicity of CCl4, galactosamine (Galc), and phalloidin and strongly inhibited the activity of 5-lipoxygenase in porcine leukocytes. The synthetic wedelolactone was also found to have stimulatory effect on the RNA synthesis in isolated nuclei from hepatocytes [123].
Ethanolic extract of the aerial parts of E. alba has been shown to neutralize the lethal activity of the venom of South American rattlesnake (Crotalus durissus terrificus) when mixed in vitro before i.p. injection into adult Swiss mice. Three phytoconstituents isolated from the plant, namely, wedelolactone (0.54 mg/animal), sitosterol (2.3 mg/animal), and stigmasterol (2.3 mg/animal), were able to neutralize three lethal doses of the venom. Aqueous extracts of the plant inhibited the release of creatine kinase from isolated rat muscle exposed to the crude venom. This protection was also observed in vivo, when the venom was preincubated with the extract prior to injection into mice [124].
The antimyotoxic and antihemorrhagic effects of E. alba and three of its constituents, wedelolactone, sitosterol, and stigmasterol, have been investigated for their ability to protect against myotoxicity of crotalid venoms (Bothrops jararaca, Bothrops jararacussu, and Lachesis muta) and purified myotoxins (bothropstoxin, BthTX; bothropasin; and crotoxin) through quantification in vitro by the release rate of creatine kinase (CK) from rat or mouse extensor digitorum muscles and in vivo by the plasma CK activity in mice. Wedelolactone was more effective than sitosterol or stigmasterol to neutralize in vitro myotoxicity of the crotalid venoms and myotoxins. The in vivo myotoxicity of venoms and myotoxins was also neutralized by preincubation with wedelolactone. Intravenous administration of wedelolactone attenuated the increase in plasma CK activity induced by subsequent intramuscular injections of the crotalid venoms or the myotoxins. Wedelolactone inhibited the hemorrhagic effect of B. jararaca venom, as well as the phospholipase A2 activity of crotoxin and the proteolytic activity of B. jararaca venom. These effects have been attributed to antiproteolytic and antiphospholipase A2 activities of the constituents [125]. Wedelolactone also antagonized the myotoxic activity in mice of venoms from Crotalus viridis viridis and Agkistrodon contortrix laticinctus and two phospholipase A2 myotoxins, CVV myotoxin and ACL myotoxin, isolated from them [126].
Wedelolactone has been found to inhibit lipopolysaccharide- (LPS-) induced caspase-11 (an inflammatory caspase) expression in cultured cells by inhibiting NF-κB-mediated transcription. It has further been shown that wedelolactone is an inhibitor of IKK (IκB, part of the upstream NFκB signal transduction cascade), a kinase critical for activation of NF-κB by mediating phosphorylation and degradation of IκBα [127]. Wedelolactone also reportedly significantly inhibited the protein expression levels of iNOS (inducible nitric oxide synthase, produced after activation by endotoxins or cytokines and generating copious amounts of NO) and COX-2 (cyclooxygenase-2 converts arachidonic acid to prostaglandins, resulting in pain and inflammation) in LPS-stimulated RAW 264.7 cells, as well as the downstream products, including NO (nitric oxide), PGE2 (prostaglandin E2), and TNF-α (tumor necrosis factor-α). Moreover, wedelolactone also inhibited LPS-induced NF-κB p65 activation via the degradation and phosphorylation of IκB-α and subsequent translocation of the NF-κB p65 subunit to the nucleus [128]. This suggests that the compound can be used as an anti-inflammatory agent.
Bioassay-guided fractionation for anti-HIV-1 (human immunodeficiency virus 1) integrase activity led to isolation of six compounds from E. alba extract. They were identified as 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl tiglate, 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl agelate, 5-hydroxymethyl-(2,2′:5′,2′′)-terthienyl acetate, ecliptal, orobol, and wedelolactone. Wedelolactone showed maximum anti-HIV-1 integrase inhibitory activity with an IC50 value of 4.0 ± 0.2 microns. This study supports the use of E. alba in acquired immunodeficiency syndrome (AIDS) patients [129].
Wedelolactone, isolated from Wedelia chinensis, has been found to modulate the androgen receptor (AR) activation of transcription from prostate-specific antigen promoter in prostate cancer (PCa) cells [130]. The anticancer activity of wedelolactone has also been shown in androgen receptor-negative MDA-MB-231 breast cancer cells, where wedelolactone suppressed growth and induced apoptosis. Cells were arrested at the S and G2/M phase of the cell cycle with induction of DNA damage. Wedelolactone was found to interact with dsDNA and inhibited the activity of DNA topoisomerase IIα [131].
In Chlamydia trachomatis-infected HEp-2 cells (human epithelial type 2 cells, considered to originate from a human laryngeal carcinoma), wedelolactone was found to induce apoptosis in combination with LPS and polyI:C (polyinosinic:polycytidylic acid) leading to lesser viability of Chlamydia [132].
G protein-coupled receptor-35 (GPR35) has been shown to be a target of the asthma drugs cromolyn disodium and nedocromil sodium. Wedelolactone, which is antiallergic, was found to be a potent β-arrestin-biased GPR35 agonist and can be considered a potential drug against asthma [133].
Adipocyte hyperplasia is associated with obesity and arises due to adipogenic differentiation of resident multipotent stem cells in the vascular stroma of adipose tissue and remote stem cells of other organs [134]. Wedelolactone has been observed to inhibit the adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells (hAMSCs). It has been shown that this process may be mediated through the ERK (extracellular signal regulated kinase) pathway [135].
Metabolism of arachidonic acid through the 5-lipoxygenase pathway has been shown to play a critical role in the survival of prostate cancer cells. Wedelolactone has been found to kill both androgen-sensitive and androgen-independent prostate cancer cells in a dose-dependent manner by inducing apoptosis. Wedelolactone-induced apoptosis was dependent on c-Jun N-terminal kinase (c-JNK) and caspase-3. Apoptosis was triggered via downregulation of protein kinase Cε but without inhibition of Akt (protein kinase B), suggesting that a novel mechanism is at work [136]. Notably, JNK plays a critical role in death receptor-initiated extrinsic as well as mitochondrial intrinsic apoptotic pathways through modulating the activities of mitochondrial pro- and antiapoptotic proteins through distinct phosphorylation events [137]. Caspase-3 is a death protease and is frequently involved in apoptosis [138]. Downregulation of protein kinase Cε has also been shown to occur using tumor necrosis factor-related apoptosis inducing ligand (TRAIL) stimulated glioma cells; however, in this case reduced expression of Akt was also observed [139].
Wedelolactone has been reported to synergize with interferon-γ (IFN-γ) to induce apoptosis in tumor cells. The compound increased IFN-γ signaling by inhibiting STAT1 (signal transducer and activator of transcription 1 protein) dephosphorylation and prolonging STAT1 activation through specific inhibition of T-cell protein tyrosine phosphatase (TCPTP), an important tyrosine phosphatase for STAT1 dephosphorylation [140]. STAT1 has been implicated in modulating pro- and antiapoptotic genes following stress-induced responses [141].
Wedelolactone has been reported to exhibit antifibrotic effects on human hepatic stellate cell line LX-2. The compound reduced the cellular viability of LX-2 in a time- and dose-dependent manner. Wedelolactone induced apoptosis of LX-2 cells by decreasing the expression of antiapoptotic Bcl-2 and increasing the expression of proapoptotic Bax. The inhibition of activation of LX-2 cells has been demonstrated and attributed by the authors to a number of reasons including inducing Bcl-2 family involved apoptosis, upregulating phosphorylated status of ERK and JNK expressions, and inhibiting nuclear factor-κB (NF-κB) mediated activity [142].
Overall, the various reports indicate that the compound has antiinflammatory, hepatoprotective, snake venom neutralizing, anti-HIV, anticancer, and antiasthmatic effects.
5.2. Eclalbasaponins
The antiproliferative effect of eclalbasaponinsisolated from E. alba on hepatic stellate cells has previously been described [116]. Eclalbasaponin I isolated from aerial parts of the plant reportedly dose-dependently inhibited the proliferation of hepatoma cell smmc-7721 with IC50 value of 111.1703 μg/mL [143]. Eclalbasaponin VI, isolated from E. alba, has been shown to demonstrate α-glucosidase activity [64]. Eclalbasaponin, isolated from E. alba, has been shown to demonstrate antibacterial activity [92].
Echinocystic acid isolated from ethyl acetate fraction of 70% ethanol extract of the plant inhibited LPS-induced production of nitric oxide and cytokines such as tumor necrosis factor-α and interleukin-6 in RAW 264.7 macrophages. The compound also inhibited LPS-induced inducible nitric oxide synthase expression at the protein level and inducible nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) expression at the mRNA level and inhibited LPS-induced iNOS promoter binding activity. Additionally, echinocystic acid suppressed the lipopolysaccharide-induced transcriptional activity of nuclear factor-κB by blocking the nuclear translocation of p65 [144]. Thus this compound can be regarded as a potent anti-inflammatory agent.
Taken together, eclalbasaponins possess anticancer and anti-inflammatory effects.
5.3. α-Amyrin
Reports on α-amyrin are few; however, several reports are present on pharmacological effects of a combination of α- and β-amyrin (ABA) as well as α-amyrin derivatives. Antilipoxygenase activity has been reported for α-amyrin acetate, suggesting its possibly beneficial role in arthritis [145]. In adult male Wistar rats made arthritic by subplantar injection of complete Freund's adjuvant, α-amyrin palmitate caused increases in serum hyaluronate and blood granulocytes toward nonarthritic levels and corrected the moderate anemia of adjuvant arthritis [146]. The triterpenes, α-amyrin and its palmitate and linoleate esters caused growth inhibition of rat osteosarcoma cells and tadpole collagenase digestion of type I (bone) native collagen. The antiarthritic effect has been attributed to inhibition by the triterpenes of joint destruction [147].
ABA, isolated from the resin of Protium kleinii, caused dose-dependent and significant antinociception against the visceral pain in mice produced by (intraperitoneal) i.p. injection of acetic acid; i.p., p.o., intracerebroventricular (i.c.v.), or intrathecal (i.t.) administration of ABA inhibited both neurogenic and inflammatory phases of the overt nociception caused by intraplantar (i.pl.) injection of formalin; ABA given by i.p., p.o., i.t., or i.c.v. routes inhibits the neurogenic nociception induced by capsaicin. In addition, i.p. treatment with ABA was able to reduce the nociception produced by 8-bromo-cAMP (8-Br-cAMP) and by 12-O-tetradecanoylphorbol-13-acetate (TPA) or the hyperalgesia caused by glutamate [148].
Anti-inflammatory effect of α-amyrin isolated from P. kleinii has been demonstrated through its ability to inhibit both ear edema and influx of polymorphonuclear cells in response to topical application of 12-O-tetradecanoylphorbol-acetate (TPA) in mice ears [149]. In TPA-induced skin inflammation in mice, topical application of α-amyrin inhibited TPA-induced increase of prostaglandin E2 (PGE2) levels. α-Amyrin also prevented IκBα degradation, p65/RelA phosphorylation, and NF-κB activation. In addition, α-amyrin also inhibited the activation of upstream protein kinases, namely, ERK, p38 mitogen-activated protein kinase (MAPK), and protein kinase C (PKC) α. The anti-inflammatory mechanism has been proposed to be suppression of COX-2 expression through inhibition of ERK, p38MAPK, and PKCα, as well as blocking NF-κB activation [150].
ABA, isolated from Protium heptaphyllum, significantly attenuated cerulein-induced acute pancreatitis in mice. Decreases in cerulein-induced increases of tumor necrosis factor TNF-α, interleukin-6, lipase, amylase, myeloperoxidase (MPO), and TBARS were noted with administration of ABA. Moreover, ABA significantly suppressed the pancreatic edema, inflammatory cell infiltration, acinar cell necrosis, and expressions of TNFα and iNOS [151]. Similar protective effect of ABA was also seen in acute pancreatitis induced in rats with L-arginine [152].
ABA (3–100 mg/kg), isolated from Protium heptaphyllum resin, demonstrated significant antinociceptive activity against either subplantar (1.6 μg) or intracolonic application of capsaicin in mice [153]; antinociceptive activity of ABA has also been reported in mice for cyclophosphamide-induced bladder pain and intracolonic administration of mustard oil [154].
The protective effect of ABA has been demonstrated against trinitrobenzene sulphonic acid- (TNBS-) induced colitis in Swiss male mice [155]. The results indicated that ABA suppresses inflammatory cytokines and COX-2 levels possibly via inhibition of NF-κB and CREB-signaling pathways. The preventive effect of ABA against dextran sulfate-induced colitis in mice has also been demonstrated; it has been hypothesized that the cannabinoid pathway may be involved [156].
α-Amyrin acetate, isolated from Tylophora hirsuta, tested positive for antispasmodic activity on spontaneous rabbits' jejunum preparations with EC50 value of (60 ± 2) × 10(−5) M. The compound also tested positive on KCl induced contractions with EC50 value of (72 ± 3) × 10(−5) M. The compound thus can prove to be of use against gastrointestinal disorders like diarrhea and dysentery [157].
ABA, isolated from the resin of Protium heptaphyllum, manifested a hypolipidemic effect in normoglycemic mice and reduced the elevated plasma glucose levels during oral glucose tolerance tests. STZ-induced diabetic mice showed significant decreases in blood glucose (BG), total cholesterol (TC), and serum triglycerides (TGs), when treated with ABA. Histopathological studies showed the beneficial effect of ABA on pancreas in maintaining β-cell integrity. In high fat diet- (HFD-) fed mice oral administration of ABA (10, 30, and 100 mg/kg), the HFD-associated rise in serum TC and TGs was significantly less, particularly at a dose of 100 mg/kg. At this dose, there were significant decreases in very low-density lipoprotein (VLDL) and low- density lipoprotein (LDL) cholesterol and an elevation of high density lipoprotein (HDL) cholesterol. The atherogenic index was also significantly reduced by ABA [158].
α-Amyrin acetate, isolated from aerial roots of Ficus benghalensis, has been shown to reduce hyperglycemia and improve diabetic conditions in STZ-induced diabetic rats and db/db diabetic mice [159].
α-Amyrin, isolated from Rhaponticum carthamoides, has been shown to induce proliferation of human keratinocytes (HaCaT) by about 18% [160].
α-Amyrin acetate at concentrations of 1.6% caused 76.9% mortality rate in adult female A. stephensi mosquitoes. In vivo exposure of the mosquitoes to the compound was found to increase mean probing time and decrease blood engorgement time and feeding rate and a declination in fecundity, which reduced the overall survival and reproductive capacity of the malaria vector A. stephensi [161].
ABA, from the stem bark resin of Protium heptaphyllum, showed anxiolytic and antidepressant effects when tested in mice by the open-field, elevated plus maze, rotarod, forced swimming, and pentobarbital-induced sleeping time. It has been hypothesized that the anxiolytic effect may involve benzodiazepine-type receptors, while the antidepressant effect may involve noradrenergic mechanisms [162].
Hepatoprotective action of ABA has been reported against acetaminophen-induced liver injury in mice. ABA was isolated from the trunk wood resin of Protium heptaphyllum. Acetaminophen (500 mg/kg, p.o.) caused fulminant liver damage characterized by centrilobular necrosis with inflammatory cell infiltration, an increase in serum ALT and AST activities, a decrease in hepatic glutathione (GSH) and 50% mortality. Pretreatment with ABA (50 and 100 mg/kg, i.p. at 48, 24, and 2 h before acetaminophen) attenuated the acetaminophen-induced acute increase in serum ALT and AST activities, increased the depleted hepatic GSH, and considerably reduced the histopathological alterations. Furthermore, ABA potentiated the pentobarbital (50 mg/kg, i.p.) sleeping time. The results suggest that the hepatoprotective action of ABA involved possible suppression of liver cytochrome P450 and diminution in oxidative stress and toxic metabolite formation in liver [163].
ABA, isolated from Protium heptaphyllum resin, showed gastroprotective effect against ethanol-induced gastric mucosal damage in mice. Maximal gastroprotection was observed with a 100 mg/kg dose of ABA, which was almost abolished in mice with their sensory afferents chemically ablated by a neurotoxic dose of capsaicin. It has been suggested that the gastroprotective mechanism of ABA involves at least in part the activation of capsaicin-sensitive primary afferent neurons [164].
ABA, isolated from Protium heptaphyllum resin, reportedly significantly inhibited the scratching behavior induced by dextran T40 and compound 48/80 in mice. The inhibition has been attributed to a stabilizing action on mast cell membrane [165].
The available scientific literature suggests that α-amyrin and derivatives by themselves or a combination of α and β-amyrins have diverse pharmacological activities including antiarthritic, analgesic, anti-inflammatory, antispasmodic, antidiabetic, cholesterolemic, antimalarial, anxiolytic, antidepressant, hepatoprotective, and gastroprotective activities and also may be beneficial against pancreatitis and pruritus.
5.4. Miscellaneous Phytochemical Constituents of E. alba
Several phytochemical constituents of E. alba have reported multiple and diverse pharmacological activities, which will only be briefly discussed. Oleanolic acid is known for both antidiabetic and anticancer effects. It can directly modulate enzymes connected with insulin biosynthesis, secretion, and signaling [166]. Many of its effects are mediated through activation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2). Nrf2 can modulate the expression of more than 200 genes that are crucial in the metabolism of drugs and toxins, protection against oxidative stress and inflammation, and maintaining protein stability and degradation. Nrf2 can interact with tumor suppressor protein 53 (p53) and so control cell cycle and NF-κB. Through these interactions, Nrf2 plays a major role in protecting against many age-related diseases including cancer and neurodegeneration, as well as increasing longevity [167].
In tumor cells, a recent review has pointed out that the compound can modulate multiple signaling pathways, like NF-κb, AKT, signal transducer and activator of transcription 3, mammalian target of rapamycin, caspases, intercellular adhesion molecule 1, vascular endothelial growth factor, and poly (ADP-ribose) polymerase [168]. As such, oleanolic acid can be a potential preventive as well as a therapeutic agent for cancer.
Ursolic acid has been shown in various reports to have antioxidative, anticancer, and anti-inflammatory properties, although a recent review has pointed out that proinflammatory properties of the compound have also been reported in normal cells and tissues [169]. A number of reports have shown ursolic acid to have anticancer, cytotoxic, antitumor, antioxidant, anti-inflammatory, antiwrinkle, anti-HIV, acetyl cholinesterase, α-glucosidase, antimicrobial, and hepatoprotective activities (reviewed in [170]).
Oleanolic and ursolic acids have reportedly hepatoprotective, anti-inflammatory, and antihyperlipidemic properties [171]. Oleanolic and ursolic acids can also be potentially useful in neurodegenerative disorders like Alzheimer's disease [172]. A review study has described the anticancer effects of ursolic acid to include protection of cellular DNA from damage, inhibition of epidermal growth factor receptor/mitogen-activated protein kinase signal or of FoxM1 transcription factors, antiangiogenesis (which can inhibit tumor cell growth), inhibition of tumor cell migration and invasion, and inducing apoptosis in cancer cells [173]. The forkhead transcription factor or FoxM1 has been shown to bind and regulate a group of genes which are mainly involved in controlling late cell cycle events in the G2 and M phases [174]. Inhibition of FoxM1 expression has been found to diminish the proliferation and anchorage-independent growth of breast cancer cells [175].
Antioxidant, anti-inflammatory, and antiallergic activities have been described for luteolin. The compound may also have cardioprotective effect [176]. Luteolin has also been described to have the ability to block the development of cancer cells both in vitro and in vivo through offering protection from carcinogenic stimuli, inhibiting tumor cell proliferation, and induction of cell cycle arrest and/or apoptosis [177]. Furthermore, luteolin can sensitize cancer cells to therapeutic-induced cytotoxicity through a variety of mechanisms including suppression of cell cycle pathways like phosphatidylinositol 3′-kinase (PI3K)/Akt, NF-κB, and X-linked inhibitor of apoptosis protein (XIAP) and stimulating apoptosis pathways including those that induce the tumor suppressor p53 [178]. Modulation of reactive oxygen species (ROS) levels, inhibition of topoisomerases I and II, reduction of NF-κB and AP-1 activity, stabilization of p53, and inhibition of PI3K, STAT3, insulin-like growth factor 1 receptor (IGF1R), and human epidermal growth factor receptor 2 (HER2) have also been described as the causes behind the cancer chemopreventive and chemotherapeutic potential of luteolin [179]. It is to be noted that overexpression of HER2 is associated with certain aggressive forms of breast cancer.
The cardioprotective role of luteolin has been shown in cardiomyocytes following ischemia-reperfusion, suggesting that the compound can form the basis for preventing and treating cardiovascular diseases [180].
Apigenin has been described as a chemopreventive agent [181]. The compound also has antioxidant and anti-inflammatory properties. The compound may also have a beneficial effect in cardiovascular and neurological disorders [182]. A recent review has pointed out that apigenin can inhibit cancer cell growth, sensitize cancer cells to elimination by apoptosis, and hinder the development of blood vessels to serve the growing tumor. Apigenin is able to reduce cancer cell glucose uptake, inhibit remodeling of the extracellular matrix, inhibit cell adhesion molecules that participate in cancer progression (like VCAM-1 [183]), and oppose chemokine signaling pathways that direct the course of metastasis into other locations. For instance, apigenin has been shown to suppress migration and invasion of transformed cells through downregulation of C-X-C chemokine receptor 4 expression [184]. All of these effects have the net result of blocking progression and metastasis of cancer [185]. In head and neck cancers, apigenin may act through inhibiting GLUT-1 expression [186].
6. Conclusion
The plant, E. alba, is regarded by traditional medicinal practitioners as a valuable medicinal plant, particularly for the treatment of liver disorders, gastrointestinal disorders, respiratory tract disorders, hair loss, skin disorders, and fever. Scientific evidences have validated most of the claims of the ethnomedicinal uses, including that of treatment of snake bite with the plant. Various important phytochemicals have been isolated and identified from the plant. These compounds include wedelolactone, eclalbasaponins, α-amyrin, ursolic acid, oleanolic acid, luteolin, and apigenin. The available scientific reports indicate that these compounds can form the next generation of drugs to treat cancer, arthritis, liver diseases, hair loss, and snake bites.
Conflict of Interests
The authors declare that they have no competing interests.
Authors' Contribution
Rownak Jahan, Abdullah Al-Nahain, Snehali Majumder, and Mohammed Rahmatullah gathered and analyzed the available scientific reports on the plant along with ethnomedicinal reports. Mohammed Rahmatullah and Rownak Jahan analyzed the data and wrote the paper. All authors edited the paper and read and approved the final paper.
References
- 1.Khare C. P. Indian Medicinal Plants: An Illustrated Dictionary. Berlin, Germany: Springer; 2007. [Google Scholar]
- 2.Mithun N. M., Shashidhara S., Vivek Kumar R. Eclipta alba (L.) A review on its phytochemical and pharmacological profile. Pharmacologyonline. 2011;1:345–357. [Google Scholar]
- 3.Ma-Ma K., Nyunt N., Tin K. M. The protective effect of Eclipta alba on carbon tetrachloride-induced acute liver damage. Toxicology and Applied Pharmacology. 1978;45(3):723–728. doi: 10.1016/0041-008X(78)90165-5. [DOI] [PubMed] [Google Scholar]
- 4.Wagner H., Geyer B., Kiso Y., Hikino H., Rao G. S. Coumestans as the main active principles of the liver drugs Eclipta alba and Wedelia calendulacea . Planta Medica. 1986;5:370–374. [PubMed] [Google Scholar]
- 5.Murthy V. N., Reddy B. P., Venkateshwarlu V., Kokate C. K. Antihepatotoxic activity of Eclipta alba, Tephrosia purpurea and Boerhaavia diffusa . Ancient Science of Life. 1992;11:182–186. [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhyay R. K., Pandey M. B., Jha R. N., Pandey V. B. Eclalbatin, a triterpene saponin from Eclipta alba . Journal of Asian Natural Products Research. 2001;3(3):213–217. doi: 10.1080/10286020108041393. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M., Chen Y. Y., Di X. H., Liu M. Isolation and identification of ecliptasaponin D from Eclipta alba (L.) hassk. Yao Xue Xue Bao. 1997;32(8):633–634. [PubMed] [Google Scholar]
- 8.Zhang M., Chen Y. Chemical constituents of Eclipta alba (L.) Hassk. Zhongguo Zhong Yao Za Zhi. 1996;21(8):480–510. [PubMed] [Google Scholar]
- 9.Khan A. V., Khan A. A. Ethnomedicinal uses of Eclipta prostrta Linn. Indian Journal of Traditional Knowledge. 2008;7(2):316–320. [Google Scholar]
- 10.Gautam A., Batra A. Ethnomedicinal plant product used by the local traditional practitioners in Mount Abu. World Journal of Pharmaceutical Sciences. 2012;1(1):10–18. doi: 10.5497/wjp.v1.i1.10. [DOI] [Google Scholar]
- 11.Hussain K., Nisar M. F., Majeed A., Nawaz K., Bhatti K. H. Ethnomedicinal survey for important plants of Jalalpur Jattan, District Gujrat, Punjab, Pakistan. Ethnobotanical Leaflets. 2010;14:807–825. [Google Scholar]
- 12.Rahmatullah M., Mollik M. A. H., Azam A. T. M. A., et al. Ethnobotanical survey of the Santal tribe residing in Thakurgaon District, Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2009;3(4):889–898. [Google Scholar]
- 13.Ahmad W., Hasan A., Ahmad I., Zeenat F. Ethnomedicinal plants of Mansoora, Malegaon. Hamdard Medicus. 2011;54:29–40. [Google Scholar]
- 14.Das S., Choudhury M. D., Mandal S. C., Talukdar A. D. Traditional knowledge of ethnomedicinal hepatoprotective plants used by certain ethnic communities of Tripura State. Indian Journal of Fundamental and Applied Life Sciences. 2012;2:84–97. [Google Scholar]
- 15.Kapoor B. B. S., Sharma M. Ethnomedicinal aspects of some medicinal plants of Hanumangarh District of Rajasthan. Journal of Pharmaceutical and Biological Sciences. 2013;1:7–9. [Google Scholar]
- 16.Sharma A., Sharma M. S., Mishra A., Sharma S., Kumar B., Bhandari A. A review on Thar plants used in liver diseases. International Journal of Research in Pharmacy and Chemistry. 2011;1:224–236. [Google Scholar]
- 17.Kumar V., Singh P. K. Ethnomedicinal plants used as antidote for snake-bite and scorpion-sting in Bundelkhand (U.P.), India. IOSR Journal of Environmental Science, Toxicology and Food Technology. 2014;8(1):52–55. [Google Scholar]
- 18.Bhellum B. L., Singh S. Ethnomedicinal plants of district Samba of Jammu and Kashmir State (List-II) International Journal of Scientific and Research Publications. 2012;2:1–8. [Google Scholar]
- 19.Rahmatullah M., Mollik M. A. H., Paul A. K., et al. A comparative analysis of medicinal plants used to treat gastrointestinal disorders in two sub-districts of greater Khulna division, Bangladesh. Advances in Natural and Applied Sciences. 2010;4(1):22–28. [Google Scholar]
- 20.Korpenwar A. N. Ethnomedicinal plants used by the tribal's in cure of wounds in Buldhana District (MS) India. International Journal of Recent Trends in Science and Technology. 2012;3:49–53. [Google Scholar]
- 21.Vaidyanathan D., Senthilkumar M. S. S., Basha M. G. Studies on ethnomedicinal plants used by Malayali tribals in Kolli Hills of Eastern Ghats, Tamil Nadu, India. Asian Journal of Plant Science & Research. 2013;3:29–45. [Google Scholar]
- 22.David B. C., Sudarsanam G. Ethnomedicinal plant knowledge and practice of people of Javadhu hills in Tamilnadu. Asian Pacific Journal of Tropical Biomedicine. 2011;1(1):S79–S81. [Google Scholar]
- 23.Sudeesh S. Ethnomedicinal plants used by Malayaraya tribes of Vannapuram village in Idukki, Kerala, India. Indian Journal of Scientific Research and Technology. 2012;1:7–11. [Google Scholar]
- 24.Das K., Duarah P. Traditional knowledge of the women’s of Kaibarta community of Assam about the application of phyto-remedies in certain common childhood diseases. International Research Journal of Biological Sciences. 2014;3:57–63. [Google Scholar]
- 25.Nisar M. F., Ismail S., Arshad M., Majeed A., Arfan M. Ethnomedicinal flora of District Mandi Bahauddin, Pakistan. Middle-East Journal of Scientific Research. 2011;9:233–238. [Google Scholar]
- 26.Malan D. F., Neuba D. F. R. Traditional practices and medicinal plants use during pregnancy by Anyi-Ndenye women (Eastern Côte d'Ivoire) African Journal of Reproductive Health. 2011;15(1):85–93. [PubMed] [Google Scholar]
- 27.Abujam S. S., Shah R. K. Study on the ethnomedicinal system of local people of Dibrugarh, Assam. International Journal of Pharmaceutical Innovation. 2012;2:17–28. [Google Scholar]
- 28.Singh R. K., Singh A. Women's wisdom and indigenous human healthcare practices. Indian Journal of Traditional Knowledge. 2009;8(2):262–269. [Google Scholar]
- 29.Sudhakar P., Shashikanth J. Ethnomedicinal importance of some weeds grown in sugarcane crop fields of Nizamabad District, Andhra Pradesh, India. Life Sciences Leaflets. 2012;10:51–55. [Google Scholar]
- 30.Sivaranjani R., Ramakrishnan K. Traditional uses of medicinal plants in treating skin diseases in Nagapattinam District of Tamil Nadu, India. International Research Journal of Pharmacy. 2012;3:201–204. [Google Scholar]
- 31.Chakraborty N. R., Duary B. Utilization of some weeds as medicine by the local people in Birbhum District of West Bengal, India. International Journal of Bio-resource and Stress Management. 2014;5:148–152. [Google Scholar]
- 32.Panghal M., Arya V., Yadav S., Kumar S., Yadav J. P. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. Journal of Ethnobiology and Ethnomedicine. 2010;6, article 4 doi: 10.1186/1746-4269-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan A. V., Ahmed Q. U., Khan M. W., Khan A. A. Herbal cure for poisons and poisonous bites from Western Uttar Pradesh, India. Asian Pacific Journal of Tropical Disease. 2014;4(1):S116–S120. doi: 10.1016/S2222-1808(14)60425-4. [DOI] [Google Scholar]
- 34.Landge L. J., Kalse A. T. Indigenous herbal medicines used by tribal people in Satpuda Mountai. International Scientific Journal. 2014;1:65–69. [Google Scholar]
- 35.Simon S. M., Norman T. S. J., Suresh K., Ramachandran V. Ethnomedicinal plants used by the Uraly tribes of Idukki District, Kerala which are hitherto unreported in codified Ayurveda system of medicine. International Journal of Research in Ayurveda and Pharmacy. 2011;2:469–472. [Google Scholar]
- 36.Agnihotri N., Gupta A. K. Folklore medicines for cuts and wounds in Kalyanpur block of Kanpur District, Uttar Pradesh, India. PhTechMed. 2013;2:381–386. [Google Scholar]
- 37.Tewari R. C., Kotecha M., Sharma A. K., Sharma P. Ethno-medicinal heritage of Chandi Devi Hill’s of Haridwar, Uttarakhand. International Journal of Innovative Research and Development. 2013;2:233–241. [Google Scholar]
- 38.Yesodharan K., Sujana K. A. Status of ethnomedicinal plants in the Parambikulam Wildlife Sanctuary, Kerala, South India. Annals of Forestry. 2007;15(2):322–334. [Google Scholar]
- 39.Vashistha B. D., Kaur M. Floristic and ethno botanical survey of Ambala District, Haryana. International Journal of Pharma and Bio Sciences. 2013;4(2):P353–P360. [Google Scholar]
- 40.Panthi M. P., Singh A. G. Ethnobotany of Arghakhanchi District, Nepal: plants used in dermatological and cosmetic disorders. International Journal of Applied Sciences and Biotechnology. 2013;1(2):27–32. doi: 10.3126/ijasbt.v1i2.8199. [DOI] [Google Scholar]
- 41.Kumar A., Agarwal S., Singh A., Deepak D. Medico-botanical study of some weeds growing in Moradabad District of Western Uttar Pradesh in India. Indian Journal of Scientific Research. 2012;3:107–111. [Google Scholar]
- 42.Sahu C. R., Nayak R. K., Dhal N. K. Traditional herbal remedies for various diseases used by tribals of Boudh District, Odisha, India for sustainable development. International Journal of Herbal Medicine. 2013;1:12–20. [Google Scholar]
- 43.Saxena A. K., Singh B., Anand K. K. Hepatoprotective effects of Eclipta alba on subcellular levels in rats. Journal of Ethnopharmacology. 1993;40(3):155–161. doi: 10.1016/0378-8741(93)90063-B. [DOI] [PubMed] [Google Scholar]
- 44.Singh B., Saxena A. K., Chandan B. K., Agarwal S. G., Anand K. K. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian Journal of Physiology and Pharmacology. 2001;45(4):435–441. [PubMed] [Google Scholar]
- 45.Manvar D., Mishra M., Kumar S., Pandey V. N. Identification and evaluation of anti hepatitis C virus phytochemicals from Eclipta alba . Journal of Ethnopharmacology. 2012;144(3):545–554. doi: 10.1016/j.jep.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabassum N., Agrawal S. S. Hepatoprotective activity of Eclipta alba Hassk. against paracetamol induced hepatocellular damage in mice. JK Practitioner. 2004;11(4):278–280. [Google Scholar]
- 47.Singh B., Saxena A. K., Chandan B. K., Agarwal S. G., Bhatia M. S., Anand K. K. Hepatoprotective effect of ethanolic extract of Eclipta alba on experimental liver damage in rats and mice. Phytotherapy Research. 1993;7(2):154–158. doi: 10.1002/ptr.2650070212. [DOI] [Google Scholar]
- 48.Vasuki R., Hari R., Pandian S., Arumugam G. Hepatoprotective action of ethanolic extracts of Eclipta alba and Piper longum linn and their combination on CCL4 induced hepatotoxicity in rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(1):455–459. [Google Scholar]
- 49.Prabu K., Kanchana N., Sadiq A. M. Hepatoprotective effect of Eclipta alba on paracetamol induced liver toxicity in rats. Journal of Microbiology and Biotechnology Research. 2011;1:75–79. [Google Scholar]
- 50.Lal V. K., Kumar A., Kumar P., Yadav K. S. Screening of leaves and roots of Eclipta alba for hepatoprotective activity. Archives of Applied Science Research. 2010;2(1):86–94. [Google Scholar]
- 51.Arun K., Balasubramanian U. Comparative study on hepatoprotective activity of Aegle marmelos and Eclipta alba against alcohol induced in albino rats. International Journal of Environmental Science. 2011;2:389–402. [Google Scholar]
- 52.Parmar S. R., Vashrambhai P. H., Kalia K. Hepatoprotective activity of some plants extract against paracetamol induced hepatotoxicity in rats. Journal of Herbal Medicine and Toxicology. 2010;4:101–106. [Google Scholar]
- 53.Kumar K., Katiyar A. K., Swamy M., Sahni Y. P., Kumar S. Hepatoprotective effect of Eclipta alba on experimentally induced liver damage in rats. Indian Journal of Veterinary Pathology. 2013;37:159–163. [Google Scholar]
- 54.Thirumalai T., David E., Therasa V., Elumalai E. K. Restorative effect of Eclipta alba in CCl4 induced hepatotoxicity in male albino rats. Asian Pacific Journal of Tropical Disease. 2011;1(4):304–307. doi: 10.1016/S2222-1808(11)60072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayanasamy K., Selvi V. Hepatoprotective effect of a polyherbal formulation (Ayush-Liv.04) against ethanol and CCl4 induced liver damage in rats. Ancient Science of Life. 2005;25(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- 56.Kavitha M., Karimulla S. K., Kumar D., Vinoth Kumar S., Sathish Kumar R., Gurucharan M. Hepatoprotective activity ofa poly herbal extract in carbon tetra chloride intoxicated hepatotoxicity in male albino rats. International Journal of Pharma and Bio Sciences. 2011;2(3):307–312. [Google Scholar]
- 57.Zafar R., Sagar B. P. S. Hepatoprotective and cardiac inhibitory activities of ethanolic extracts from plant leaves and leaf callus of Eclipta alba . Pharmaceutical Biology. 2000;38(5):357–361. doi: 10.1076/phbi.38.5.357.5974. [DOI] [Google Scholar]
- 58.Roy R. K., Thakur M., Dixit V. K. Hair growth promoting activity of Eclipta alba in male albino rats. Archives of Dermatological Research. 2008;300(7):357–364. doi: 10.1007/s00403-008-0860-3. [DOI] [PubMed] [Google Scholar]
- 59.Datta K., Singh A. T., Mukherjee A., Bhat B., Ramesh B., Burman A. C. Eclipta alba extract with potential for hair growth promoting activity. Journal of Ethnopharmacology. 2009;124(3):450–456. doi: 10.1016/j.jep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Thorat R. M., Jadhav V. M., Kadam V. J. Development and evaluation of polyherbal formulations for hair growth-promoting activity. International Journal of PharmTech Research. 2009;1(4):1251–1254. [Google Scholar]
- 61.Bhattacharya S. K., Satyan K. S., Chakrabarti A. Effect of Trasina, an Ayurvedic herbal formulation, on pancreatic islet superoxide dismutase activity in hyperglycaemic rats. Indian Journal of Experimental Biology. 1997;35(3):297–299. [PubMed] [Google Scholar]
- 62.Ananthi J., Prakasam A., Pugalendi K. V. Antihyperglycemic activity of Eclipta alba leaf on alloxan-induced diabetic rats. Yale Journal of Biology and Medicine. 2003;76(1–6):97–102. [PMC free article] [PubMed] [Google Scholar]
- 63.Jaiswal N., Bhatia V., Srivastava S. P., Srivastava A. K., Tamrakar A. K. Antidiabetic effect of Eclipta alba associated with the inhibition of α-glucosidase and aldose reductase. Natural Product Research. 2012;26(24):2363–2367. doi: 10.1080/14786419.2012.662648. [DOI] [PubMed] [Google Scholar]
- 64.Kumar D., Gaonkar R. H., Ghosh R., Pal B. C. Bio-assay guided isolation of α-glucosidase inhibitory constituents from Eclipta alba . Natural Product Communications. 2012;7(8):989–990. [PubMed] [Google Scholar]
- 65.Pandey P. S., Upadhyay K. K., Pandey D. N. Experimental evaluation of the analgesic property of Eclipta alba (L) Hassk. Ancient Science of Life. 1997;17:36–40. [PMC free article] [PubMed] [Google Scholar]
- 66.Leal L. K. A. M., Ferreira A. A. G., Bezerra G. A., Matos F. J. A., Viana G. S. B. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. Journal of Ethnopharmacology. 2000;70(2):151–159. doi: 10.1016/S0378-8741(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 67.Sawant M., Isaac J. C., Narayanan S. Analgesic studies on total alkaloids and alcohol extracts of Eclipta alba (Linn.) Hassk. Phytotherapy Research. 2004;18(2):111–113. doi: 10.1002/ptr.1165. [DOI] [PubMed] [Google Scholar]
- 68.Kumar S. S., Sivakumar T., Chandrasekar M. J., Suresh B. Evaluation of anti-Inflammatory activity of Eclipta alba in rats. Ancient Science of Life. 2005;24:112–118. [PMC free article] [PubMed] [Google Scholar]
- 69.Lans C., Harper T., Georges K., Bridgewater E. Medicinal plants used for dogs in Trinidad and Tobago. Preventive Veterinary Medicine. 2000;45(3-4):201–220. doi: 10.1016/S0167-5877(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 70.Kaur M., Chandola H. M. Role of rasayana in cure and prevention of recurrence of vicharchika (eczema) Ayu. 2010;31(1):33–39. doi: 10.4103/0974-8520.68207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan C.-F., Huang W.-Y., Guo H.-Y., Wang B. R. Potent antioxidative and UVB protective effect of water extract of Eclipta prostrata L. The Scientific World Journal. 2014;2014:8. doi: 10.1155/2014/759039.759039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thakur V. D., Mengi S. A. Neuropharmacological profile of Eclipta alba (Linn.) Hassk. Journal of Ethnopharmacology. 2005;102(1):23–31. doi: 10.1016/j.jep.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 73.Banji O., Banji D., Annamalai A. R., Manavalan R. Investigation on the effect of Eclipta alba on animal models of learning and memory. Indian Journal of Physiology and Pharmacology. 2007;51(3):274–278. [PubMed] [Google Scholar]
- 74.Lobo O. J. F., Banji D., Annamalai A. R., Manavalan R. Evaluation of antiaggressive activity of Eclipta alba in experimental animals. Pakistan Journal of Pharmaceutical Sciences. 2008;21(2):195–199. [PubMed] [Google Scholar]
- 75.Bhaskar M., Chintamaneni M. Withania somnifera and Eclipta alba ameliorate oxidative stress induced mitochondrial dysfunction in an animal model of Alzheimer’s disease. The American Journal of Phytomedicine and Clinical Therapeutics. 2014;2:140–152. [Google Scholar]
- 76.Kaur G., Tuli R., Chintamaneni M. Antioxidant potential of methanolic and hydrolyzed extracts of Eclipta alba . Pharmacologyonline. 2009;2:947–956. [Google Scholar]
- 77.Uddin N., Rahman A., Ahmed N. U., Rana S., Akter R., Chowdhury A. M. M. A. Antioxidant, cytotoxic and antimicrobial properties of Eclipta alba ethanol extract. International Journal of Biological and Medical Research. 2010;1(4):341–346. [Google Scholar]
- 78.Swati, Bedi S., Tanuja In vitro antioxidant potential and phytochemical screening of Eclipta alba . Asian Journal of Experimental Biological Sciences. 2012;3(4):785–789. [Google Scholar]
- 79.Chandan S., Umesha S., Balamurugan V. Antileptospiral, antioxidant and DNA damaging properties of Eclipta alba and Phyllanthus amarus . Open Access Scientific Reports. 2012;1:231–238. [Google Scholar]
- 80.Baldi A., Gupta R., Panwar M. S. Evaluation of in-vitro antioxidant activity of Eclipta alba . International Journal of Pharmaceutical and Biological Archive. 2011;2:767–771. [Google Scholar]
- 81.Karthikumar S., Vigneswari K., Jegatheesan K. Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L) Scientific Research and Essay. 2007;2(4):101–104. [Google Scholar]
- 82.Mansoorali K. P., Prakash T., Kotresha D., Prabhu K., Rama Rao N. Cerebroprotective effect of Eclipta alba against global model of cerebral ischemia induced oxidative stress in rats. Phytomedicine. 2012;19(12):1108–1116. doi: 10.1016/j.phymed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Panghal M., Kaushal V., Yadav J. P. In vitro antimicrobial activity of ten medicinal plants against clinical isolates of oral cancer cases. Annals of Clinical Microbiology and Antimicrobials. 2011;10, article 21 doi: 10.1186/1476-0711-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandey M. K., Singh G. N., Sharma R. K., Lata S. Antibacterial activity of Eclipta alba (L.) hassk. Journal of Applied Pharmaceutical Science. 2011;1(7):104–107. [Google Scholar]
- 85.Bakht J., Islam A., Shafi M. Antimicrobial potentials of Eclipta alba by well diffusion method. Pakistan Journal of Botany. 2011;43:169–174. [Google Scholar]
- 86.Sandhu P. S., Kaur K., Ahmad V., et al. Screening of antimicrobial activity of aqueous extracts of leaves, flower and stem of Eclipta alba . International Journal of Drug Development and Research. 2012;4(4):142–147. [Google Scholar]
- 87.Peraman M. K., Ramalingam P., Sai B. J. N. N. Anti-inflammatory and antimicrobial activities of the extracts of Eclipta alba leaves. European Journal of Experimental Biology. 2011;1:172–177. [Google Scholar]
- 88.Borkataky M., Kakoty B. B., Saikia L. R. Proximate analysis and antimicrobial activity of Eclipta alba (L.) Hassk.—a traditionally used herb. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(1):149–154. [Google Scholar]
- 89.Saggoo M. I. S., Kaur R., Gupta R. C. Comparison of antibacterial activity of three morphotypes of medicinal herb Eclipta alba (L.) Hassk. Der Pharmacia Lettre. 2010;2:200–207. [Google Scholar]
- 90.Saraswathy N., Kumaran P. M. Evaluation of aqueous extract of Eclipta alba leaves for preservation potential against Fusarium species. The American Journal of PharmTech Research. 2012;2:645–649. [Google Scholar]
- 91.Dalal S., Kataria S. K., Sastry K. V., Rana S. V. S. Phytochemical screening of methanolic extract and antibacterial activity of active principles of hepatoprotective herb, Eclipta alba . Ethnobotanical Leaflets. 2010;14:248–258. [Google Scholar]
- 92.Ray A., Bharali P., Konwar B. K. Mode of antibacterial activity of eclalbasaponin isolated from Eclipta alba . Applied Biochemistry and Biotechnology. 2013;171(8):2003–2019. doi: 10.1007/s12010-013-0452-3. [DOI] [PubMed] [Google Scholar]
- 93.Saroj B., Shashank A., Shirshat Mahendra S., Suryaji J., Patil L. S., Deshmukh R. A. Anti-malarial activity of Eclipta alba against Plasmodium berghei infection in mice. Journal of Communicable Diseases. 2007;39(2):91–94. [PubMed] [Google Scholar]
- 94.Govindarajan M. Evaluation of indigenous plant extracts against the malarial vector, Anopheles stephensi (Liston) (Diptera: Culicidae) Parasitology Research. 2011;109(1):93–103. doi: 10.1007/s00436-010-2224-0. [DOI] [PubMed] [Google Scholar]
- 95.Govindarajan M., Karuppannan P. Mosquito larvicidal and ovicidal properties of Eclipta alba (L.) Hassk (Asteraceae) against chikungunya vector, Aedes aegypti (Linn.) (Diptera: Culicidae) Asian Pacific Journal of Tropical Medicine. 2011;4(1):24–28. doi: 10.1016/S1995-7645(11)60026-6. [DOI] [PubMed] [Google Scholar]
- 96.Govindarajan M., Sivakumar R. Adulticidal and repellent properties of indigenous plant extracts against Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) Parasitology Research. 2012;110(5):1607–1620. doi: 10.1007/s00436-011-2669-9. [DOI] [PubMed] [Google Scholar]
- 97.Govindarajan M., Sivakumar R. Mosquito adulticidal and repellent activities of botanical extracts against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae) Asian Pacific Journal of Tropical Medicine. 2011;4(12):941–947. doi: 10.1016/S1995-7645(11)60223-X. [DOI] [PubMed] [Google Scholar]
- 98.Rangineni V., Sharada D., Saxena S. Diuretic, hypotensive, and hypocholesterolemic effects of Eclipta alba in mild hypertensive subjects: a pilot study. Journal of Medicinal Food. 2007;10(1):143–148. doi: 10.1089/jmf.2006.0000. [DOI] [PubMed] [Google Scholar]
- 99.Christybapita D., Divyagnaneswari M., Michael R. D. Oral administration of Eclipta alba leaf aqueous extract enhances the non-specific immune responses and disease resistance of Oreochromis mossambicus . Fish and Shellfish Immunology. 2007;23(4):840–852. doi: 10.1016/j.fsi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Jayathirtha M. G., Mishra S. H. Preliminary immunomodulatory activities of methanol extracts of Eclipta alba and Centella asiatica . Phytomedicine. 2004;11(4):361–365. doi: 10.1078/0944711041495236. [DOI] [PubMed] [Google Scholar]
- 101.Shaikh M. F., Sancheti J., Sathaye S. Phytochemical and pharmacological investigations of Eclipta alba (Linn.) Hassak leaves for antiepileptic activity. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(4):319–323. [Google Scholar]
- 102.Shaikh M. F., Sancheti J., Sathaye S. Effect of Eclipta alba on acute seizure models: A GABAA-mediated effect. Indian Journal of Pharmaceutical Sciences. 2013;75(3):380–384. doi: 10.4103/0250-474X.117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loup F., Wieser H.-G., Yonekawa Y., Aguzzi A., Fritschy J.-M. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. The Journal of Neuroscience. 2000;20(14):5401–5419. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pôças E. S. C., Lopes D. V. S., da Silva A. J. M., et al. Structure-activity relationship of wedelolactone analogues: structural requirements for inhibition of Na+,K+-ATPase and binding to the central benzodiazepine receptor. Bioorganic and Medicinal Chemistry. 2006;14(23):7962–7966. doi: 10.1016/j.bmc.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 105.Dirscherl K., Karlstetter M., Ebert S., et al. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. Journal of Neuroinflammation. 2010;7, article 3 doi: 10.1186/1742-2094-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishra S., Jena M., Mishra S. S. Evaluation of anticonvulsant and muscle relaxant activities of Eclipta alba using animal models. Indo American Journal of Pharmaceutical Research. 2014;4:1397–1401. [Google Scholar]
- 107.Jena M., Mishra S. Sedative and antianxiety activity of ethanolic extract of Eclipta alba in albino rats. International Journal of Pharma and Bio Sciences. 2013;4(4):1–8. [Google Scholar]
- 108.Sivaraman D., Muralidaran P. CNS depressant and antiepileptic activities of the methanol extract of the leaves of Ipomoea aquatica forsk. E-Journal of Chemistry. 2010;7(4):1555–1561. doi: 10.1155/2010/503923. [DOI] [Google Scholar]
- 109.Diogo L. C., Fernandes R. S., Marcussi S., et al. Inhibition of snake venoms and phospholipases A2 by Extracts from native and genetically modified Eclipta alba: isolation of active coumestans. Basic and Clinical Pharmacology and Toxicology. 2009;104(4):293–299. doi: 10.1111/j.1742-7843.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 110.Chaudhary H., Dhuna V., Singh J., Kamboj S. S., Seshadri S. Evaluation of hydro-alcoholic extract of Eclipta alba for its anticancer potential: an in vitro study. Journal of Ethnopharmacology. 2011;136(2):363–367. doi: 10.1016/j.jep.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 111.Chaudhary H., Jena P. K., Seshadri S. Evaluation of hydro-alcoholic extract of Eclipta alba for its multidrug resistance reversal potential: an in vitro study. Nutrition and Cancer. 2013;65(5):775–780. doi: 10.1080/01635581.2013.789116. [DOI] [PubMed] [Google Scholar]
- 112.Lirdprapamongkol K., Kramb J.-P., Chokchaichamnankit D., et al. Juice of Eclipta prostrata inhibits cell migration in vitro and exhibits anti-angiogenic activity in vivo . In Vivo. 2008;22(3):363–368. [PubMed] [Google Scholar]
- 113.Chauhan N., Singh D., Painuli R. M. Screening of bioprotective properties and phytochemical analysis of various extracts of Eclipta alba whole plant. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(2):554–560. [Google Scholar]
- 114.Siddique Y. H., Ara G., Beg T., Faisal M., Afzal M. Protective role of Eclipta alba L. extract against ethinylestradiol induced genotoxic damage in cultured human lymphocytes. Alternative Medicine Studies. 2011;1:14–17. [Google Scholar]
- 115.Desireddy R. B., Sowjanya G. N., Reddy K. L. L., Sowjanya T. Screening of Eclipta alba extracts for anticancer activity. International Journal of Research and Development in Pharmacy & Life Sciences. 2012;1:203–205. [Google Scholar]
- 116.Lee M. K., Ha N. R., Yang H., Sung S. H., Kim G. H., Kim Y. C. Antiproliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine. 2008;15(9):775–780. doi: 10.1016/j.phymed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 117.Kumar S. P., Kumar B. S., Chandana V. R., Vijaykumar M., Ojha S. K., Bavani M. E. Antisecretory and antiulcer activities of Eclipta alba Linn. In rats. Proceedings of the 5th World Ayurveda Congress; December 2012; Bhopal, India. [Google Scholar]
- 118.Banerjee A., Shrivastava N., Kothari A., Padh H., Nivsarkar M. Antiulcer activity of methanol extract of Eclipta alba . Indian Journal of Pharmaceutical Sciences. 2005;67(2):165–168. [Google Scholar]
- 119.Ghule S. C., Chaudhari S. R., Chavan M. J. Anthelmintic potential of Eclipta alba (L.) Hassk against Pheretima posthuma . International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(1):143–144. [Google Scholar]
- 120.Bhinge S. D., Hogade M. G., Chavan C., Kumbhar M., Chature V. In vitro anthelmintic activity of herb extract of Eclipta prostrate L. against Pheretima posthuma . Asian Journal of Pharmaceutical and Clinical Research. 2010;3(3):229–230. [Google Scholar]
- 121.Wagner H., Fessler B. In vitro 5-lipoxygenase inhibition by Eclipta alba extracts and the coumestan derivative wedelolactone. Planta Medica. 1986;5:374–377. [PubMed] [Google Scholar]
- 122.Silverman E. S., Drazen J. M. The biology of 5-lipoxygenase: Function, structure, and regulatory mechanisms. Proceedings of the Association of American Physicians. 1999;111(6):525–536. doi: 10.1046/j.1525-1381.1999.t01-1-99231.x. [DOI] [PubMed] [Google Scholar]
- 123.Wong S. M., Antus S., Gottsegen A., et al. Wedelolactone and coumestan derivatives as new antihepatotoxic and antiphlogistic principles. Arzneimittel-Forschung. 1988;38(5):661–665. [PubMed] [Google Scholar]
- 124.Mors W. B., Do Nascimento M. C., Parente J. P., Da Silva M. H., Melo P. A., Suarez-Kurtz G. Neutralization of lethal and myotoxic activities of south american rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae) Toxicon. 1989;27(9):1003–1009. doi: 10.1016/0041-0101(89)90151-7. [DOI] [PubMed] [Google Scholar]
- 125.Melo P. A., Nascimento M. C. D., Mors W. B., Suarez-Kurtz G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon. 1994;32(5):595–603. doi: 10.1016/0041-0101(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 126.Melo P. A., Ownby C. L. Ability of wedelolactone, heparin, and para-bromophenacyl bromide to antagonize the myotoxic effects of two crotaline venoms and their PLA2 myotoxins. Toxicon. 1999;37(1):199–215. doi: 10.1016/S0041-0101(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 127.Kobori M., Yang Z., Gong D., et al. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death and Differentiation. 2004;11(1):123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- 128.Yuan F., Chen J., Sun P. P., Guan S., Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-κB Pathway in RAW 264.7 cells. Journal of Biomedical Science. 2013;20(1, article 84) doi: 10.1186/1423-0127-20-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tewtrakul S., Subhadhirasakul S., Cheenpracha S., Karalai C. HIV-1 protease and HIV-1 integrase inhibitory substances from Eclipta prostrata . Phytotherapy Research. 2007;21(11):1092–1095. doi: 10.1002/ptr.2252. [DOI] [PubMed] [Google Scholar]
- 130.Lin F.-M., Chen L.-R., Lin E.-H., et al. Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis. 2007;28(12):2521–2529. doi: 10.1093/carcin/bgm137. [DOI] [PubMed] [Google Scholar]
- 131.Benes P., Knopfova L., Trcka F., et al. Inhibition of topoisomerase IIα: novel function of wedelolactone. Cancer Letters. 2011;303(1):29–38. doi: 10.1016/j.canlet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 132.Huston W. M., Gloeckl S., de Boer L., Beagley K. W., Timms P. Apoptosis is induced in Chlamydia trachomatis-infected HEp-2 cells by the addition of a combination innate immune activation compounds and the inhibitor wedelolactone. The American Journal of Reproductive Immunology. 2011;65(5):460–465. doi: 10.1111/j.1600-0897.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 133.Deng H., Fang Y. Anti-inflammatory gallic acid and wedelolactone are G protein-coupled receptor-35 agonists. Pharmacology. 2012;89(3-4):211–219. doi: 10.1159/000337184. [DOI] [PubMed] [Google Scholar]
- 134.Qian S.-W., Li X., Zhang Y.-Y., et al. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Developmental Biology. 2010;10, article 47 doi: 10.1186/1471-213X-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lim S., Jang H.-J., Park E. H., et al. Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. Journal of Cellular Biochemistry. 2012;113(11):3436–3445. doi: 10.1002/jcb.24220. [DOI] [PubMed] [Google Scholar]
- 136.Sarveswaran S., Gautam S. C., Ghosh J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt. International Journal of Oncology. 2012;41(6):2191–2199. doi: 10.3892/ijo.2012.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dhanasekaran D. N., Reddy E. P. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Porter A. G., Jänicke R. U. Emerging roles of caspase-3 in apoptosis. Cell Death and Differentiation. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 139.Okhrimenko H., Lu W., Xiang C., Hamburger N., Kazimirsky G., Brodie C. Protein kinase C-ε regulates the apoptosis and survival of glioma cells. Cancer Research. 2005;65(16):7301–7309. doi: 10.1158/0008-5472.CAN-05-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Z., Sun X., Shen S., et al. Wedelolactone, a naturally occurring coumestan, enhances interferon-γ signaling through inhibiting STAT1 protein dephosphorylation. Journal of Biological Chemistry. 2013;288(20):14417–14427. doi: 10.1074/jbc.M112.442970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stephanou A., Latchman D. S. STAT-1: a novel regulator of apoptosis. International Journal of Experimental Pathology. 2003;84(6):239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia Y., Chen J., Cao Y., et al. Wedelolactone exhibits anti-fibrotic effects on human hepatic stellate cell line LX-2. European Journal of Pharmacology. 2013;714(1–3):105–111. doi: 10.1016/j.ejphar.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 143.Liu Q. M., Zhao H. Y., Zhong X. K., Jiang J. G. Eclipta prostrata L. phytochemicals: Isolation, structure elucidation, and their antitumor activity. Food and Chemical Toxicology. 2012;50(11):4016–4022. doi: 10.1016/j.fct.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Ryu S., Shin J.-S., Jung J. Y., et al. Echinocystic acid isolated from Eclipta prostrata suppresses Lipopolysaccharide-induced iNOS, TNF- α, and IL-6 expressions via NF-κ B inactivation in RAW 2647 macrophages. Planta Medica. 2013;79(12):1031–1037. doi: 10.1055/s-0032-1328767. [DOI] [PubMed] [Google Scholar]
- 145.Kweifio-Okai G., Macrides T. A. Antilipoxygenase activity of amyrin triterpenes. Research Communications in Chemical Pathology and Pharmacology. 1992;78(3):367–372. [PubMed] [Google Scholar]
- 146.Kweifio-Okai G., Bird D., Eu P., Carroll A. R., Ambrose R., Field B. Effect of α-amyrin palmitate on adjuvant arthritis. Drugs under Experimental and Clinical Research. 1994;20(1):1–5. [PubMed] [Google Scholar]
- 147.Kweifio-Okai G., De Munk F., Rumble B. A., Macrides T. A., Cropley M. Antiarthritic mechanisms of amyrin triterpenes. Research Communications in Molecular Pathology and Pharmacology. 1994;85(1):45–55. [PubMed] [Google Scholar]
- 148.Otuki M. F., Ferreira J., Lima F. V., et al. Antinociceptive properties of mixture of α-amyrin and β-amyrin triterpenes: evidence for participation of protein kinase C and protein kinase A pathways. Journal of Pharmacology and Experimental Therapeutics. 2005;313(1):310–318. doi: 10.1124/jpet.104.071779. [DOI] [PubMed] [Google Scholar]
- 149.Otuki M. F., Vieira-Lima F., Malheiros Â., Yunes R. A., Calixto J. B. Topical antiinflammatory effects of the ether extract from Protium kleinii and α-amyrin pentacyclic triterpene. European Journal of Pharmacology. 2005;507(1–3):253–259. doi: 10.1016/j.ejphar.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 150.Medeiros R., Otuki M. F., Avellar M. C. W., Calixto J. B. Mechanisms underlying the inhibitory actions of the pentacyclic triterpene α-amyrin in the mouse skin inflammation induced by phorbol ester 12-O-tetradecanoylphorbol-13-acetate. European Journal of Pharmacology. 2007;559(2-3):227–235. doi: 10.1016/j.ejphar.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 151.Melo C. M., Morais T. C., Tomé A. R., et al. Anti-inflammatory effect of α,β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflammation Research. 2011;60(7):673–681. doi: 10.1007/s00011-011-0321-x. [DOI] [PubMed] [Google Scholar]
- 152.Melo C. M., Carvalho K. M. M. B., de Sousa Neves J. C., et al. α,β-amyrin, a natural triterpenoid ameliorates L-arginine-induced acute pancreatitis in rats. World Journal of Gastroenterology. 2010;16(34):4272–4280. doi: 10.3748/wjg.v16.i34.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira F. A., Costa C. L. S., Chaves M. H., et al. Attenuation of capsaicin-induced acute and visceral nociceptive pain by α- and β-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. Life Sciences. 2005;77(23):2942–2952. doi: 10.1016/j.lfs.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 154.Lima R. C. P., Jr., Oliveira F. A., Gurgel L. A., et al. Attenuation of visceral nociception by α- and β- amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum, in mice. Planta Medica. 2006;72(1):34–39. doi: 10.1055/s-2005-873150. [DOI] [PubMed] [Google Scholar]
- 155.Vitor C. E., Figueiredo C. P., Hara D. B., Bento A. F., Mazzuco T. L., Calixto J. B. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, αand β-amyrin, in a mouse model of colitis. British Journal of Pharmacology. 2009;157(6):1034–1044. doi: 10.1111/j.1476-5381.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matos I., Bento A. F., Marcon R., Claudino R. F., Calixto J. B. Preventive and therapeutic oral administration of the pentacyclic triterpene α,β-amyrin ameliorates dextran sulfate sodium-induced colitis in mice: the relevance of cannabinoid system. Molecular Immunology. 2013;54(3-4):482–492. doi: 10.1016/j.molimm.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 157.Ali N. Brine shrimp cytotoxicity of crude methanol extract and antispasmodic activity of α-amyrin acetate from Tylophora hirsuta Wall. BMC Complementary and Alternative Medicine. 2013;13, article 135 doi: 10.1186/1472-6882-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Santos F. A., Frota J. T., Arruda B. R., et al. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids in Health and Disease. 2012;11, article 98 doi: 10.1186/1476-511X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh A. B., Yadav D. K., Maurya R., Srivastava A. K. Antihyperglycaemic activity of α-amyrin acetate in rats and db/db mice. Natural Product Research. 2009;23(9):876–882. doi: 10.1080/14786410802420416. [DOI] [PubMed] [Google Scholar]
- 160.Biskup E., Golebiowski M., Gniadecki R., Stepnowski P., Lojkowska E. Triterpenoid α-amyrin stimulates proliferation of human keratinocytes but does not protect them against UVB damage. Acta Biochimica Polonica. 2012;59(2):255–260. [PubMed] [Google Scholar]
- 161.Chenniappan K., Kadarkari M. Adult mortality and blood feeding behavioral effects of α-amyrin acetate, a novel bioactive compound on in vivo exposed females of Anopheles stephensi liston (Diptera: Culicidae) Parasitology Research. 2012;110(6):2117–2124. doi: 10.1007/s00436-011-2737-1. [DOI] [PubMed] [Google Scholar]
- 162.Aragão G. F., Carneiro L. M. V., Junior A. P. F., et al. A possible mechanism for anxiolytic and antidepressant effects of α- and β-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacology Biochemistry and Behavior. 2006;85(4):827–834. doi: 10.1016/j.pbb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 163.Oliveira F. A., Chaves M. H., Almeida F. R. C., et al. Protective effect of α- And β-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. Journal of Ethnopharmacology. 2005;98(1-2):103–108. doi: 10.1016/j.jep.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 164.Oliveira F. A., Vieira G. M., Jr., Chaves M. H., et al. Gastroprotective effect of the mixture of α- and β-amyrin from Protium heptaphyllum: role of capsaicin-sensitive primary afferent neurons. Planta Medica. 2004;70(8):780–782. doi: 10.1055/s-2004-827212. [DOI] [PubMed] [Google Scholar]
- 165.Oliveira F. A., Lima-Junior R. C. P., Cordeiro W. M., et al. Pentacyclic triterpenoids, α,β-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacology Biochemistry and Behavior. 2004;78(4):719–725. doi: 10.1016/j.pbb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 166.Castellano J. M., Guinda A., Delgado T., Rada M., Cayuela J. A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62(6):1791–1799. doi: 10.2337/db12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lewis K. N., Mele J., Hayes J. D., Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integrative and Comparative Biology. 2010;50(5):829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shanmugam M. K., Dai X., Kumar A. P., Tan B. K., Sethi G., Bishayee A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Letters. 2014;346:206–216. doi: 10.1016/j.canlet.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ikeda Y., Murakami A., Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Molecular Nutrition and Food Research. 2008;52(1):26–42. doi: 10.1002/mnfr.200700389. [DOI] [PubMed] [Google Scholar]
- 170.Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. Journal of Enzyme Inhibition and Medicinal Chemistry. 2011;26(5):616–642. doi: 10.3109/14756366.2010.546793. [DOI] [PubMed] [Google Scholar]
- 171.Liu J. Pharmacology of oleanolic acid and ursolic acid. Journal of Ethnopharmacology. 1995;49(2):57–68. doi: 10.1016/0378-8741(95)01310-5. [DOI] [PubMed] [Google Scholar]
- 172.Yoo K.-Y., Park S.-Y. Terpenoids as potential anti-Alzheimer's disease therapeutics. Molecules. 2012;17(3):3524–3538. doi: 10.3390/molecules17033524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zang L.-L., Wu B.-N., Lin Y., Wang J., Fu L., Tang Z.-Y. Research progress of ursolic acid’s anti-tumor actions. Chinese Journal of Integrative Medicine. 2014;20(1):72–79. doi: 10.1007/s11655-013-1541-4. [DOI] [PubMed] [Google Scholar]
- 174.Chen X., Müller G. A., Quaas M., et al. The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Molecular and Cellular Biology. 2013;33(2):227–236. doi: 10.1128/MCB.00881-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang C., Chen H., Yu L., et al. Inhibition of FOXM1 transcription factor suppresses cell proliferation and tumor growth of breast cancer. Cancer Gene Therapy. 2013;20(2):117–124. doi: 10.1038/cgt.2012.94. [DOI] [PubMed] [Google Scholar]
- 176.Seelinger G., Merfort I., Schempp C. M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Medica. 2008;74(14):1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 177.Seelinger G., Merfort I., Wölfle U., Schempp C. M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13(10):2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin Y., Shi R., Wang X., Shen H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Current Cancer Drug Targets. 2008;8(7):634–646. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Reviews in Medicinal Chemistry. 2009;9(1):31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 180.Tongda X., Li D., Jiang D. Targeting cell signaling and apoptotic pathways by luteolin: cardioprotective role in rat cardiomyocytes following ischemia/reperfusion. Nutrients. 2012;4(12):2008–2019. doi: 10.3390/nu4122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) International Journal of Oncology. 2007;30(1):233–245. [PubMed] [Google Scholar]
- 182.Shukla S., Gupta S. Apigenin: a promising molecule for cancer prevention. Pharmaceutical Research. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Piantelli M., Rossi C., Iezzi M., et al. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. Journal of Cellular Physiology. 2006;207(1):23–29. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- 184.Wang L., Kuang L., Hitron J. A., et al. Apigenin suppresses migration and invasion of transformed cells through down-regulation of C-X-C chemokine receptor 4 expression. Toxicology and Applied Pharmacology. 2013;272(1):108–116. doi: 10.1016/j.taap.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lefort É. C., Blay J. Apigenin and its impact on gastrointestinal cancers. Molecular Nutrition and Food Research. 2013;57(1):126–144. doi: 10.1002/mnfr.201200424. [DOI] [PubMed] [Google Scholar]
- 186.Bao Y.-Y., Zhou S.-H., Fan J., Wang Q.-Y. Anticancer mechanism of apigenin and the implications of GLUT-1 expression in head and neck cancers. Future Oncology. 2013;9(9):1353–1364. doi: 10.2217/fon.13.84. [DOI] [PubMed] [Google Scholar]