Abstract
Objective
We investigated tissue turnover in healthy and osteoarthritic cartilage. We challenge long held views that osteoarthritis (OA) is dominated by a similar turnover process in all joints and present evidence that hip and knee cartilage respond very differently to OA.
Methods
D- and L-Aspartate (Asp) were quantified for whole cartilage, collagen and non-collagenous components of cartilage obtained at the time of joint replacement. We computed the Asp racemization ratio (Asp-RR=D/D+L Asp), reflecting the proportion of old to total protein, for each component.
Results
Compared with hip OA, knee OA collagen fibrils (P<0.0001), collagen (p=0.007), and non-collagenous proteins (p=0.0003) had significantly lower age-adjusted mean Asp-RRs consistent with elevated protein synthesis in knee OA. Knee OA collagen had a mean hydroxyproline/proline (H/P) ratio of 1.2 consistent with the presence of type III collagen whereas hip OA collagen had a mean H/P ratio of 0.99 consistent with type II collagen. Based on Asp-RR, the relative age was significantly different in knee and hip OA (p<0.0005); on average OA knees were estimated to be 30yrs ‘younger’, and OA hips 10yrs ‘older’ than non-OA.
Conclusions
The metabolic response to OA was strikingly different by joint site. Knee OA cartilage evinced an anabolic response that appeared to be absent in hip OA cartilage. These results challenge the long held view that OA cartilage is capable of only minimal repair and that collagen loss is irreversible.
Keywords: Osteoarthritis, cartilage turnover, racemization, collagen
Introduction
Due to the presence of an asymmetrical carbon atom, all amino acids (AAs) except glycine exist in nature in two non-superimposable, mirror image L- and D-racemic forms. Although racemic forms share almost identical chemical properties, differing only in their optical properties, only L-AAs are incorporated into proteins. This stereochemical selection is important for the correct folding of proteins. However, racemized AA (D-AAs) spontaneously form post-translationally and build up slowly over time in long-lived extracellular matrix proteins. Racemized AAs have been detected in a wide array of proteins and tissues including tooth dentin, bone, cartilage, skin, intervertebral disc, brain and eye lens proteins[1–7]. The rate of racemization is influenced by environmental factors such as temperature, pH and ionic strength as well as the AA R-group[8]. Racemization is a spontaneous clock-like physical process used to estimate the age of a wide range of biological materials including silk[9], shells[10] and teeth[11]; it has the added advantage over carbon dating of requiring only very small amounts of sample.
Although AA racemization is slow, Asp racemization occurs on a biologically relevant timescale and is the most readily and reliably detected racemized AA. Asp racemization occurs spontaneously through a succinimide intermediate (Figure 1A). Acid hydrolysis, required to release AAs, causes spontaneous deamidation of Asn to Asp; therefore, racemized Asp represents a combination of racemized Asn and Asp. Rates of D-Asp racemization for tooth dentin (tissue devoid of turnover), are estimated to be 7.7–8.3×10−4/yr[6].
Figure 1. Correlation of Asp racemization ratio with subject age for OA whole cartilage and extracted matrix components.
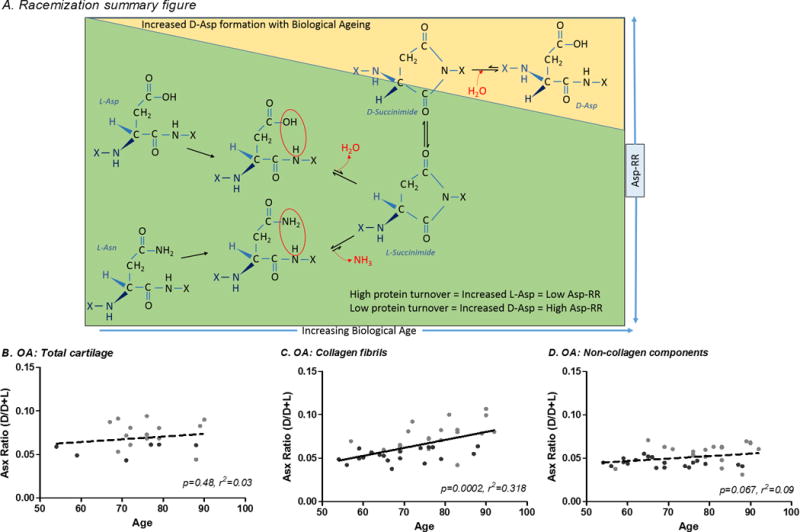
(A) Process of Asp racemization through a succinimide intermediate. Asp racemization ratio (Asp-RR=D-Asp/D+L Asp) was determined for OA cartilage including: (B) whole cartilage (full thickness macroscopically normal cartilage), (C) collagen fibrils (cartilage extensively extracted with Gu-HCl to remove soluble cartilage proteins leaving mainly collagen) and, (D) non-collagen cartilage components (proteins extractable from cartilage by Gu-HCl, mainly aggrecan). Briefly, Hydrolysates were buffered with 0.4M boric acid (pH9.0) and derivatized with o-phthaldialdehyde and N-tertiarybutyloxycarbonyl-L-cysteine (Sigma-Aldrich, USA) in methanol. Resulting fluorescent derivatives were separated by reverse-phase HPLC. A total of 100μl of derivatized sample was injected onto a Chromolith RP-18e 100×4.6mm column (VWR International LLC, USA) at a 1ml/min flow rate with fluorescent detection (excitation 344nm, emission 443nm) using the following gradient: 30min 9.5–15.5% buffer B (100% acetonitrile); 5min 60% buffer B column wash. Mobile phase consisted of 0.2M acetic acid adjusted to pH6.0 with NaOH. The Asp racemization ratio (Asp-RR) was defined as D/D+L Asp.
We investigated racemized AAs as a method of estimating protein turnover in joint tissues[7]. Given uniform turnover throughout a tissue, the ratio of racemized AAs to total AAs is unaffected by catabolism but decreased by anabolism. When catabolism and anabolism are at steady state, this ratio reflects protein/matrix turnover. For instance, under slow turnover conditions, the ratio will rise with age; under fast turnover conditions, the ratio will fall with age; when turnover matches the racemization rate, the ratio will remain unchanged with age. D-Asp increase with age at an estimated rate of 2.58×10−4/yr for type II collagen of normal cartilage indicative of a slowly turning over component[12]. AA racemization reveals differential protein turnover in osteoarthritic articular and meniscal cartilages[6, 7, 13]. In disease states, such as osteoarthritis (OA), where the matrix is no longer in a steady state, lower quantities of racemized AAs relative to normal tissue can be considered to reflect high turnover in attempted repair of the cartilage with new protein synthesis.
Cartilage matrix is enriched for two main molecular components, namely type II collagen and aggrecan, comprising 60% and 40% by dry weight, respectively[14]. Turnover and synthesis of matrix molecules in normal cartilage are slow but in equilibrium. Based on analysis of human femoral head cartilage, the predicted half-life is 25yrs for aggrecan[15] and at least 120yrs for collagen[12, 16]. Upon cytokine stimulation, proteoglycan is rapidly lost[17–19] but rapidly replaced[18]. In contrast, collagen is much less readily broken down but generally believed to be minimally replaced, so is considered to represent an irreversible step in cartilage degradation[20]. Our previous data suggested that for Cartilage Oligomeric Matrix Protein (COMP), turnover was higher in knee OA than hip OA cartilage[21]. The goal of this study was to understand the reparative responses in OA through analyses of D-Asp in soluble (primarily aggrecan) and insoluble (primarily collagen) matrix components from knee and hip OA cartilage.
Methods
Samples and demographics
Articular cartilage specimens were obtained under Duke Institutional Review Board approval as anonymized waste surgical specimens from knee and hip arthroplasties performed at Duke University Medical Center to alleviate symptoms of OA. Non-arthritic control cartilages were obtained either from the National Disease Research Interchange (Philadelphia, PA), or trauma reconstructive surgery from patients without clinical evidence of OA as determined by the surgeon and/or the laboratory researcher in charge of specimen processing. Study sample demographics are summarized in Table 1.
Table 1.
Sample demographics.
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cartilage | Collagen Fibrils | Non-collagenous proteins | ||||||||||
|
|
||||||||||||
| Sample | n | Mean Age ±SD (yrs) |
Range (yrs) |
Female (%) |
n | Mean Age ±SD (yrs) |
Range (yrs) |
Female (%) |
n | Mean Age ±SD (yrs) |
Range (yrs) |
Female (%) |
| OA knee | 5 | 68.8 ±9.8 | 54–77 | 2 (40.0%) |
18 | 68.7 ±10.1 | 54–88 | 9 (50.0%) |
18 | 68.7 ±10.1 | 54–90 | 12 (66.7%) |
| OA hip | 7 | 80.7 ±8.2 | 71–90 | 3 (42.9%) |
18 | 78.4 ±9.3 | 57–92 | 9 (50.0%) |
17 | 78.9 ±9.6 | 57–92 | 7 (41.2%) |
| Non-OA knee | 11 | 51.0 ±25.1 | 17–83 | 4 (36.4%) |
5 | 28.6 ±8.0 | 20–36 | 3 (60.0%) |
4 | 30.8 ±7.4 | 20–36 | 2 (50.0%) |
| Non-OA hip | 5 | 63.4 ±23.4 | 23–82 | 2 (40.0%) |
7 | 67.6 ±21.0 | 23–87 | 3 (42.9%) |
4 | 76.8 ±9.2 | 23–87 | 3 (75.0%) |
| Combined Non-OA joints | 16 | 54.8 ±24.5 | 17–83 | 6 (37.5%) |
12 | 51.3 ±25.8 | 20–87 | 6 (50.0%) |
8 | 53.8 ±25.8 | 20–87 | 5 (62.5%) |
All samples were independent with only one sample, either hip or knee, analyzed per person.
Sample definitions
We analysed 4 cartilage fractions: ‘whole cartilage’ defined as full thickness, macroscopically normal cartilage remote from any lesions; ‘non-collagen components’ of cartilage defined as soluble proteins extractable by guanidine hydrochloride (GuHCl); ‘collagen fibrils’ defined as the residual insoluble collagen and collagen-associated proteins remaining after depletion of soluble proteins by GuHCL extraction; and ‘collagen’ fraction defined as the residual collagen after trypsin digestion of the collagen fibril.
Sample preparation
‘Whole cartilage’ was prepared as described previously[7]. Briefly, full thickness cartilage was pulverized under liquid nitrogen and stored at −80°C. Soluble ‘non-collagen components’ were extracted as previously described[22]; pulverized cartilage was extracted twice for 24h at 4°C with 4M GuHCl, sodium acetate pH4.0, with protease inhibitor cocktail (Sigma-Aldrich, USA), and extractions combined and stored at −80°C. Two sequential extractions with Gu-HCl were previously demonstrated to be sufficient to remove all soluble detectable GAG and protein from cartilage[21]. The insoluble residue (‘collagen fibrils’) was washed overnight at 4°C with excess GuHCl prior to 5 washes in dH2O over a 24h period at 4°C, vacuum desiccated and stored at −80°C. Previous analysis of collagen fibres by LC-MS demonstrated residual cartilage proteins that were not extractable by Gu-HCl[21]. The collagen fraction was prepared as previously described[6, 23] with the following modification: ‘collagen fibrils’ were digested with 1%w:v trypsin in Eagle’s Minimal Essential Medium (0.5ml/0.02g dry weight collagen fibrils) overnight at 37°C to digest non-collagenous proteins associated with collagen fibrils. A trypsin only control was included to correct for L- and D-Asp contributed by trypsin or as a result of sample processing; L- and D-Asp in the control were negligible and subtracted prior to calculating Asp-RR. Insoluble collagen was extensively washed in ddH2O prior to desiccation and stored at −80°C.
Type II collagen purification
Using the method described previously[24], purified type II collagen was isolated from non-OA hip cartilage[24]. Briefly, 3g of hip cartilage was pulverized and extracted twice in 200ml 4M GuHCl, 0.05M Tris, pH7.5 for 24h at 4°C. The residual cartilage was washed extensively in excess ddH2O prior to pepsin digestion (1:10w:w pepsin:cartilage) in 200ml of 3%v:v acetic acid for 24h at 4°C. Supernatants were collected by centrifugation and the type II collagen was specifically precipitated with 0.7M NaCl overnight at 4°C. Precipitated collagen was resuspended in 3%v:v acetic acid, dialyzed into ddH2O, desiccated and stored at −20°C. Purity was determined by SDS-PAGE revealing a single protein band of 140 kDa consistent with α1(II) collagen; no L- or D-Asp were detectable (by HPLC as described below) in the cartilage free control demonstrating that no Asp was contributed by pepsin.
Racemized Asp quantification
Asp concentrations were determined as described previously with minor modifications[7] (see Figure 1 legend). ‘Whole cartilage’ and the ‘collagen fibril’ fraction were hydrolyzed at a ratio of 20mg/ml sample:6M HCl; ‘non-collagen components’ were hydrolyzed at a ratio of 0.1mg/ml protein:6M HCl. All samples were hydrolyzed for 6h at 105°C, rapidly neutralized on ice with 6M NaOH and stored at −80°C. Great care was taken to standardize hydrolysis conditions across samples, including hydrolysis temperature and duration. Additionally, samples were randomized and hydrolyzed blinded to sample type to prevent group or treatment bias.
Hydroxyproline (Hyp) and proline (Pro) determination
Hyp and Pro levels were determined as previously described[25].
Statistical methods
Age associations were determined by linear regression analysis (Prism5, GraphPad Software Inc). Asp-RR was compared across joint sites by multivariable regression (JMP Pro 10.0, SAS Institute Inc) with age, gender and joint location as independent variables. The biological age for an OA specimen was determined by interpolation from an age by Asp-RR linear regression analysis of non-OA cartilage. The relative age was determined by subtracting the biological age of the sample from the known chronological age of the sample with significance by joint site determined by Mann Whitney test (Prism5). The method of calculating half-lives of collagen for OA knee and hip cartilage is described in Supplementary Table 1.
Results
Racemization of cartilage components with age and osteoarthritis
Due to slow tissue turnover, Asp-RR increases significantly with age in normal healthy cartilage[6, 16]. Asp-RR of whole non-OA cartilage was significantly associated with age (r2=0.51, p=0.0019, Figure 4A) with similar trends for knee and hip. In contrast, Asp-RR of whole OA cartilage was not correlated with age (p=0.25, r2=0.132, Figure 1B), in agreement with our previous study[7]. Asp-RRs from whole cartilage of OA knees were generally distributed below the age regression line while OA hips tended to be distributed above, suggesting that OA knee cartilage accumulates D-Asp at a slower rate consistent with a higher level of protein synthesis.
Figure 4. Calculated relative age of OA knee and OA hip cartilage matrix components.
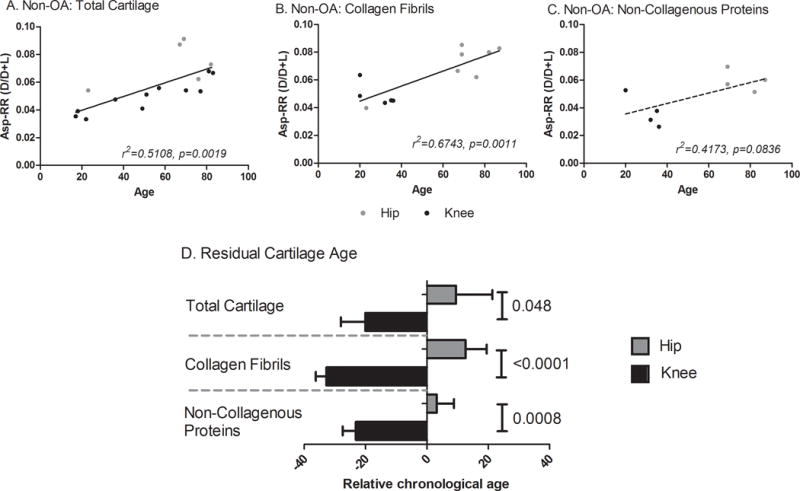
To determine the biological relevance of the difference in racemization levels between OA hip and OA knee cartilage, we interpolated the biological age of an OA tissue specimen from the corresponding correlation of Asp racemization ratio with age for non-OA joints. Panels A–C show the age-related Asp-RRs for no-OA total cartilage (A), collagen fibrils (B) and non-collagenous proteins (C). The interpolated biological age was subtracted from the known chronological age of the sample to estimate the relative age due to disease. A positive relative age means the tissue is older than would be expected (lower protein turnover) while a negative value means the tissue is younger than expected (higher protein turnover). The Mann-Whitney test was used to test for significant differences in relative ages of cartilage by joint site. (D) OA knees were on average 30yrs ‘younger’ and hips 10yrs ‘older’ than non-OA joints. Of note, both OA knee collagen and non-collagenous components were ‘younger’ than the corresponding components of non-OA cartilage.
Cartilage consists of two main pools of proteins, insoluble collagen and soluble non-collagenous proteins; we investigated both these fractions independently to determine whether their turnover in OA cartilage differed. Asp-RR of OA collagen fibrils was significantly correlated with age (p=0.0003, r2=0.321, Figure 1C). A clear joint site bias was observed for OA collagen fibrils with OA knee falling below the regression line, again consistent with higher protein turnover in knee OA relative to hip OA. Asp-RR for OA non-collagen components was also correlated with age (p=0.049, r2=0.109, Figure 1D). As the OA hip samples tended to be older than the knee, we selected an age-matched subset (n=24) of samples and repeated the analysis. The same joint bias was observed although the sample set was too small and/or too constrained in age range to demonstrate the age association for non-collagenous proteins (supplementary Figure 1). These data suggest that knee OA cartilage undergoes an accelerated level of synthesis of all major protein components relative to hip OA cartilage. Furthermore, these data unmask a hitherto unappreciated capacity for repair in knee OA, including a capacity for synthesis of collagen.
Joint site differences in protein turnover with osteoarthritis
As we observed a joint site bias in Asp-RR of OA cartilage with age, we investigated this further by multivariable regression to evaluate Asp-RR by joint site, adjusted for age and gender (Figure 2). The mean Asp-RR was significantly lower in knee OA compared with hip OA for whole cartilage (p=0.043, Figure 2A), collagen fibrils (p<0.0036, Figure 2B) and non-collagenous cartilage components (p=0.009, Figure 2C). We observed similar significant findings using an age-matched subset of samples (supplemental Figure 1). These data are consistent with a faster rate of protein turnover in knee OA compared with hip OA for whole cartilage and the major individual components of cartilage.
Figure 2. Scatter dot plot for Asp racemization ratio (Asp-RR) by joint site.
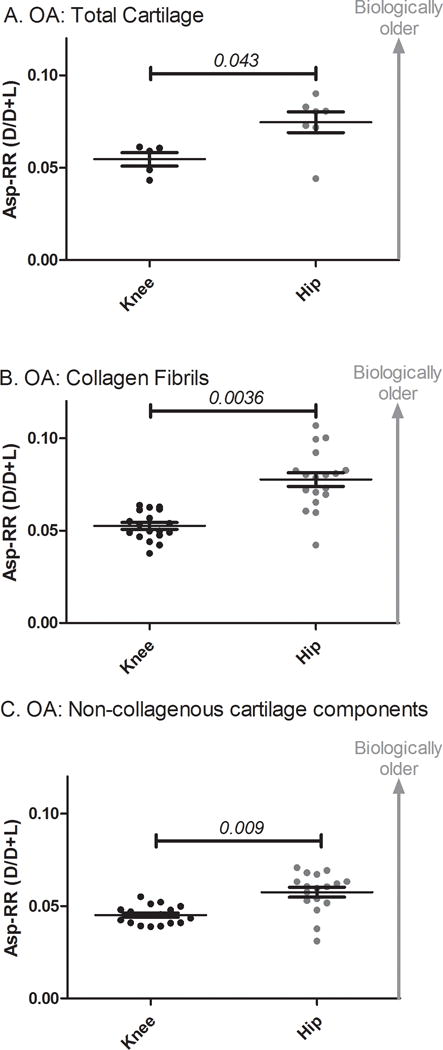
Knee OA total cartilage (A), collagen fibrils (B) and non-collagenous cartilage components (C) had significantly lower Asp-RRs than hip OA. There were significant differences between the age groups for cartilage from the different joint sites so age adjusted p-values were calculated using age, gender and joint site as covariates in a multivariable linear regression.
Collagen turnover in osteoarthritic cartilage
Based on OA collagen fibril analyses, we clearly demonstrated a difference in relative protein turnover rates of knee and hip. However, it was not evident from these data whether this difference was attributable to collagen, limited to the non-collagenous proteins associated with collagen fibrils, or both. To investigate this question, we selected an age-matched subset of samples (OA hips and knees) and treated the collagen fibrils with trypsin, in a similar manner to other studies[6, 23], to remove non-collagenous proteins associated with the collagen fibrils (Figure 3, Table 2). In this subset of OA samples, the mean Asp-RR of collagen fibrils, prior to trypsin digestion, was significantly lower in knees than hips (p=0.0002), confirming that this subsample was representative of the total sample set. After trypsin treatment, the residual insoluble collagen from OA knees also had a significantly lower mean Asp-RR than OA hips (p=0.007) consistent with higher levels of collagen synthesis in OA knees than OA hips.
Figure 3. Asp racemization ratios of the collagen fraction of OA cartilage by joint site.
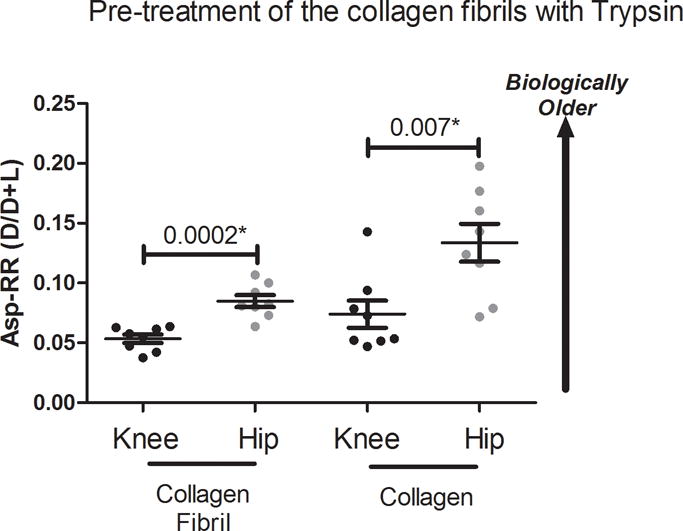
To confirm a difference in racemization levels for cartilage collagen from OA hips and OA knees, we treated a subset of the cartilage collagen fibril samples with trypsin to digest and remove proteins associated with the collagen fibril. We selected 8 OA hip and 8 OA knee samples of similar ages (p=0.12 for age difference). As with all other cartilage fractions, we observed that OA knee collagen was significantly younger than OA hip collagen. Demographics: OA hips 78.1 ±7.2yrs, 69–90yrs, 3 females; OA knees 72.9 ±10.8yrs, 56–88yrs, 4 females.
Table 2.
Cartilage collagen and fibril associated non-collagenous protein characteristics.
|
|
|||
|---|---|---|---|
| OA Hip mean ±SD |
OA Knee mean ±SD |
P-value | |
| Collagen Asp-RR | 0.13 ±0.05 | 0.073 ±0.03 | 0.006 |
| Collagen Hyp/Pro ratio | 0.99 ±0.05 | 1.19 ±0.22 | 0.029 |
| Released mM Total Asp/Hyp | 0.0050 ±0.004 | 0.043 ±0.031 | 0.003 |
| Released Asp-RR | 0.107 ±0.05 | 0.057 ±0.02 | 0.0127 |
| Released Hyp/Total Hyp | 0.008 ±0.008 | 0.012 ±0.006 | 0.27 |
Collagen fibrils were treated to remove any associated non-collagenous proteins. The negligible amounts of L- and D-Asp in the trypsin only control were subtracted from the sample data prior to calculating the sample Asp racemization ratio (Asp-RR=D-Asp/D-Asp+L-Asp). Hydroxyproline (Hyp) and Proline (Pro) concentrations were determined for both the released proteins and the insoluble collagens. Total Asp concentration represents the sum of measured L- and D- Asp. A subset of age-matched OA hip and OA knee samples were selected for to minimize confounding by demographics (see Figure 3 legend).
Trypsin digestion of collagen fibrils yielded more total Asp (p=0.003, Table 2) from knee than hip OA suggesting more protein associated with knee OA collagen fibrils. No GAG release was detected after trypsin treatment consistent with our expectation that all detectable GAG was removed by GuHCl extraction. As observed for all other cartilage protein fractions, the proteins released from knee OA collagen fibrils had a significantly lower Asp-RR (p=0.013, Table 2) than those released from hip. We observed no significant difference (p=0.27) in the small amounts of Hyp detectable in the medium suggesting that the majority of collagen in both OA hip and knee was incorporated into fibrils in a trypsin resistant, triple helical form. To confirm that AAs can racemize within the sterically hindered triple helical domain of collagen, we purified type II collagen by pepsin digestion of pooled non-OA hip cartilage; pepsin digestion removes the telopeptides allowing purification of the collagen triple helical domain. Purified collagen from a non-OA hip (87yrs) had an Asp-RR of 0.058 clearly demonstrating that racemization can occur in the collagen triple helical region. Interestingly, this ratio was lower than for OA hip suggesting that non-OA hip was biologically younger than OA hip. Acidic environments can potentially accelerate racemization. As the pepsin extraction protocol required the collagen to be in an acidic environment for up to 72h at 4°C, we incubated purified type II collagen in 3% acetic acid for a further 72h to confirm that the extraction protocol was not artificially generating the observed D-Asp. We observed no significant effect of this further incubation on the collagen Asp-RR (0.054), suggesting that the triple helical domain of type II collagen was subject to racemization in vivo rather than in vitro.
Hyp/Pro ratios differ by collagen type[26]. Interestingly, we observed a significantly higher mean Hyp/Pro ratio in the knee (ratio 1.19±0.22, p=0.029) compared with the hip (ratio 0.99±0.05) OA collagen fraction (Table 2). Based on published literature[26] and our own experimentally determined Hyp/Pro ratio for non-OA purified collagen of 0.99, these results suggested that hip OA was predominantly composed of type II collagen. In contrast, the Hyp/Pro ratio of knee OA collagen suggested the presence of other fibular collagens, possibly type III collagen, whose published Hyp/Pro ratio is 1.2–1.3[26]. Alternatively, type II collagen from OA knees may be ‘overhydroxylated’. Notably, the mean Hyp/Pro ratio of OA knees and hips did not match that of type I collagen (found in fibrocartilage) of 0.84–0.92[26].
Changes in the relative age of cartilage with osteoarthritis
To gain an appreciation of the biological relevance by estimating the relative age differences due to differences in turnover, Asp-RR of OA hip and knee was compared to Asp-RR of healthy non-OA samples. Asp-RR of cartilage components from non-OA cartilage was associated with age: total cartilage, p=0.0019, r2=0.51 (Figure 4A); collagen fibrils, p=0.001, r2=0.67 (Figure 4B); and a trend for non-collagen components of cartilage, p=0.084, r2=0.42 (Figure 4C). By interpolation from the Asp-RR versus age regression analyses of non-OA specimens, we determined a biological age for the OA specimens. The relative age (difference between the biological age and the chronological age for a specimen, Figure 4D) allowed a much clearer visualization of the mean changes in protein turnover in OA hips and knees. Of note, a negative relative age denotes that cartilage or its components appear younger in age than would be expected based upon the age of the patient; such a condition would arise as a result of protein synthesis, presumably in attempted repair, compared to normal. Conversely, a positive relative age would be indicative of more aged cartilage components, most likely arising from a repressed state of synthesis and repair relative to normal. Compared to non-OA, for all OA cartilages and cartilage components, we observed a younger mean relative age for knee OA and an older mean relative age for hip OA The mean relative age of total cartilage for knee OA was −20.1±17.8yrs and for hip OA was +9.5±31.4yrs; therefore, knee OA cartilage was significantly younger than hip OA cartilage by an estimated mean 29.6yrs (P=0.048). The mean relative age of collagen fibrils for knee OA was −32.8±14.8yrs and for hip OA was +12.6±1.9yrs; therefore, knee collagen fibrils were significantly biologically younger than hip by an estimated mean 45.4yrs (P<0.0001). Similar results were obtained for non-collagenous proteins; the mean relative age of non-collagenous proteins for knee OA was estimated to be −23.2±18.6yrs and for hip OA was +3.3±23.2yrs; therefore, knee non-collagenous proteins were also significantly biologically younger than hip by an estimated mean 26.5yrs (p=0.0008).
Based on our racemization data, the calculated mean t1/2 (half-life) of collagen turnover was 28.3±4.0yrs for OA knees at 37°C and 86.9±15.2yrs for OA hips at 37°C (p<0.0001) (see Supplementary Table 1). Since racemization is a physical process, physical factors, such as pH or temperature can impact rates of racemization. We therefore considered the possibility that temperature differences in knees and hips might account, at least in part, for different amounts of racemized amino acids in cartilages from these different joints. We therefore re-estimated the half-life of collagen turnover based on a conservative assumption of an intra-articular temperature of 33°C in knees, representing a 4°C lower intra-articular temperature than for hips. The calculated mean t1/2 of collagen turnover for OA knee at 33°C was 75.6±10.6; this was still significantly shorter than the mean t1/2 for OA hip collagen (p=0.012) (Supplementary Table 1). Taken together, these data suggest that the turnover and attempted repair process in knee OA cartilage is highly coordinated and controlled with elevated turnover for all joint components compared with OA hip.
Discussion
This is the first study to challenge the established paradigm in cartilage biology that collagen loss is irreversible. Through analyses of racemized D-Asp in cartilage proteins, we discovered a significant difference in repair responses at knees and hips. On average, the level of apparent repair of cartilage components in knee OA rendered them 3 decades ‘younger’ on a biological scale than non-OA cartilage, hip OA components were on average, an estimated decade ‘older’ than non-OA. We believe this difference represents a difference in protein turnover and specifically, the attempted cartilage repair response. We inferred from these data an increase in protein synthesis in knee OA that was absent in hip OA cartilage, suggesting that the mechanisms of disease progression are very different between hip and knee.
It is unclear why the hip does not mount a repair response similar to the knee; this could have profound effects upon the development of future treatment strategies for different joint sites. For instance, both knee and hip OA should be targeted with anti-catabolic therapies while only hip OA may require companion anabolic therapies; the apparent upregulated repair response of knee OA cartilage, unmasked through these analyses, may be sufficient to effect repair if cartilage degradation is attenuated. Due to limited numbers of non-OA samples across a broad age range, we were unable to definitively confirm whether difference between OA knee and OA hip turnover is a consequence solely of the disease process or also reflects differences in normal cartilage biology. For instance, normal knee cartilage could potentially turnover at an accelerated rate independent of disease. In this case, the estimated half-life of collagen of 120yrs (based on normal hip cartilage) would be shorter in normal knee cartilage.
Although chondrocytes in 3D cultures produce both type II collagen and GAG[27], the very long half-life of cartilage collagen, >100yrs[12, 16], has suggested that collagen loss represents an irreversible process. To understand which proteins are capable of being replaced, we fractionated cartilage into non-collagenous components and collagen fibrils, representing mainly aggrecan and collagen respectively. Treatment of the collagen fibrils with trypsin released only very low levels of Hyp with no significant difference between the OA knee and OA hip; these results suggested that the majority of collagens at both joint sites were trypsin insensitive and in a triple helical confirmation. However, the Asp-RR data clearly demonstrated that collagen, collagen associated proteins, and non-collagenous proteins were all being synthesized and incorporated into the OA knee more rapidly than OA hip. This means that in some circumstances, cartilage collagen loss does not necessarily imply an irreversible alteration, raising the hope that early identification and further treatment of OA could lead to the future regeneration of functional cartilage, thus lessening the need for joint replacement surgery.
Further evidence for a difference in the attempted repair responses of knee and hip OA came from differences in the Hyp/Pro content of OA knee and OA hip collagen. We currently do not understand this important joint site difference in the cartilage repair response although the OA hip result is consistent with the work of Hui-Chou et al who demonstrated that the Hyp/hydroxylysine ratio in collagen of degenerated articular cartilage from dysplastic canine hip joints remained unchanged[28]. The altered Hyp/Pro ratio observed in knee OA versus normal and hip OA cartilage could reflect a joint specific difference in Hyp incorporated into type II collagen. In non-OA normal cartilage, the predominant collagen is type II with small amounts of type I in the superficial zone[29]. Knee OA collagen had a significantly higher Hyp/Pro ratio (1.19) than purified type II collagen (0.99); as type I collagen has an even lower reported Hyp/Pro ratio (0.8–0.92), we concluded that type I fibrocartilage was unlikely to account for the type of repair response in knee OA. Type III collagen has a higher Hyp/Pro ratio (1.2–1.3)[26] and therefore is a worthy candidate to explain the higher Hyp/Pro ratio in knee OA collagen. This conclusion is consistent with the work of Eyre et al who demonstrated significant production of type III collagen in the OA knee, which is believed to be consistent with a wound healing response[30]. In addition, type III collagen has been identified as indicative of knee OA by biochemical biomarker analyses[31]. Although termed the ‘HELIXII’ biomarker, this knee OA associated epitope was subsequently discovered to be a collagen III epitope[32]. The regulation of collagen III may provide insights into means of boosting and monitoring a repair response in OA.
Our interpretation, that the lower D-Asp content of knee OA cartilage components signified increased protein synthesis in knee OA but not hip OA cartilage, is consistent with two previous studies from our group based on AA racemization of whole cartilage and deamidation of COMP[7, 21]. This current work expands upon these findings demonstrating that all components, both non-collagenous proteins and the collagen component, are capable of being synthesized in OA knee cartilage. These results are also consistent with Nelson et al who observed increased type II collagen synthesis in OA knee cartilage based on the CPII epitope[33].
There are several possible alternative explanations for these results. Enrichment of cartilage for D-Asp may reflect the preferential loss of the superficial zone in OA hip cartilage specifically; the superficial zone is believed to be the most metabolically active region of cartilage[34], and therefore to contain the ‘youngest’ proteins. This explanation seems unlikely to account for the joint site-specific difference in Asp-RR because data suggest that cartilage degeneration in both knee and hip OA proceeds from the top down with the preferential loss of the superficial zone of cartilage for both[34]. Furthermore, in this study we used full thickness cartilage harvested from macroscopically normal regions of OA joints to avoid any confounding issues due to loss of the superficial zones. Another alternative suggestion for these results could be an accelerated rate of racemization in the physical milieu of knee OA. Since racemization is a physical process, physical factors (e.g. pH or temperature) would have to differ between these two joint sites in OA to account for the observed differences in Asp-RR. To our knowledge, no such differences have been identified that could impact rates of amino acid racemization. Even conservatively estimating collagen half-lives, assuming a higher intra-articular hip temperature, calculations still yielded significantly slower turnover rates of hip relative to knee collagen.
In summary, OA knee cartilage is biologically ‘younger’ than hip OA cartilage of a similar chronological age. We believe this to be due to a robust repair response in knee OA cartilage. Knee OA cartilage is characterized by a decrease in Asp-RR and an increase in Hyp/Pro ratio that is compatible with an alteration of collagen type, possibly due to increase in collagen type III synthesis. In contrast, no such repair response appears to be mounted in hip OA cartilage. These data suggest, at least in the OA, that cartilage components are synthesized in response to OA damage. Thus, it appears likely that not all repair is unsuccessful, as proposed by the previously suggested “frustrated repair” hypothesis[35]. Given this potential to regenerate, if diagnosed and treated early, it may be possible to prevent significant cartilage degeneration of the knee. It is important to understand the underlying cause of this difference as it could have important implications for future joint specific treatment strategies.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health Grant 5P30 AG028716 from NIA (Claude D. Pepper Older Americans Independence Centers). We acknowledge use of tissues procured by the National Disease Research Interchange (NDRI) with support from National Institutes of Health Grant 5 U42 RR006042.
Abbreviations
- HPLC
high-performance liquid chromatography
- Hyp
hydroxyproline
- Pro
proline
- GuHCl
guanidine hydrochloride
- OA
osteoarthritis
Footnotes
Author contributions
JBC and VBK were responsible for designing the study. JBC performed all experimental procedures. RDZ and MPB were responsible for supplying all the tissue necessary for this research and associated clinical diagnosis for each sample. All authors were involved in results interpretation, drafting the manuscript and approving the final version for submission.
Competing interests
The authors have no competing interests.
References
- 1.Helfman PM, Bada JL. Aspartic acid racemisation in dentine as a measure of ageing. Nature. 1976;262:279–281. doi: 10.1038/262279b0. [DOI] [PubMed] [Google Scholar]
- 2.Man EH, Sandhouse ME, Burg J, Fisher GH. Accumulation of D-aspartic acid with age in the human brain. Science. 1983;220:1407–1408. doi: 10.1126/science.6857259. [DOI] [PubMed] [Google Scholar]
- 3.Masters PM, Bada JL, Zigler JS., Jr Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature. 1977;268:71–73. doi: 10.1038/268071a0. [DOI] [PubMed] [Google Scholar]
- 4.Ohtani S. Different racemization ratios in dentin from different locations within a tooth. Growth Dev Aging. 1997;61:93–99. [PubMed] [Google Scholar]
- 5.Ritz-Timme S, Laumeier I, Collins MJ. Aspartic acid racemization: evidence for marked longevity of elastin in human skin. Br J Dermatol. 2003;149:951–959. doi: 10.1111/j.1365-2133.2003.05618.x. [DOI] [PubMed] [Google Scholar]
- 6.Sivan SS, Wachtel E, Tsitron E, Sakkee N, van der Ham F, Degroot J, et al. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J Biol Chem. 2008;283:8796–8801. doi: 10.1074/jbc.M709885200. [DOI] [PubMed] [Google Scholar]
- 7.Stabler TV, Byers SS, Zura RD, Kraus VB. Amino acid racemization reveals differential protein turnover in osteoarthritic articular and meniscal cartilages. Arthritis Res Ther. 2009;11:R34. doi: 10.1186/ar2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCudden CR, Kraus VB. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin Biochem. 2006;39:1112–1130. doi: 10.1016/j.clinbiochem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Moini M, Klauenberg K, Ballard M. Dating silk by capillary electrophoresis mass spectrometry. Anal Chem. 2011;83:7577–7581. doi: 10.1021/ac201746u. [DOI] [PubMed] [Google Scholar]
- 10.Demarchi B, Williams MG, Milner N, Russell N, Bailey G, Penkman K. Amino acid racemization dating of marine shells: A mound of possibilities. Quat Int. 2011;239:114–124. doi: 10.1016/j.quaint.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin RC, Moody H, Penkman KE, Collins MJ. The application of amino acid racemization in the acid soluble fraction of enamel to the estimation of the age of human teeth. Forensic Sci Int. 2008;175:11–16. doi: 10.1016/j.forsciint.2007.04.226. [DOI] [PubMed] [Google Scholar]
- 12.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 13.Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, et al. Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol. 2001;20:409–417. doi: 10.1016/s0945-053x(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 14.Kuettner K, Thonar E-M, Aydelotte M. Modern aspects of articular cartilage biochemistry. In: Brandt K, editor. Cartilage Changes in Osteoarthritis. Vol. 1990. Ciba Geigy; pp. 3–11. [Google Scholar]
- 15.Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- 16.Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- 17.Saklatvala J, Pilsworth LM, Sarsfield SJ, Gavrilovic J, Heath JK. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984;224:461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page Thomas DP, King B, Stephens T, Dingle JT. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991;50:75–80. doi: 10.1136/ard.50.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dingle JT, Page Thomas DP, King B, Bard DR. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis. 1987;46:527–533. doi: 10.1136/ard.46.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jubb RW, Fell HB. The breakdown of collagen by chondrocytes. J Pathol. 1980;130:159–167. doi: 10.1002/path.1711300304. [DOI] [PubMed] [Google Scholar]
- 21.Catterall JB, Hsueh MF, Stabler TV, McCudden CR, Bolognesi M, Zura R, et al. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J Biol Chem. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catterall JB, Barr D, Bolognesi M, Zura RD, Kraus VB. Post-translational aging of proteins in osteoarthritic cartilage and synovial fluid as measured by isomerized aspartate. Arthritis Res Ther. 2009;11:R55. doi: 10.1186/ar2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 24.Wu JJ, Eyre DR. Cartilage type IX collagen is cross-linked by hydroxypyridinium residues. Biochem Biophys Res Commun. 1984;123:1033–1039. doi: 10.1016/s0006-291x(84)80237-5. [DOI] [PubMed] [Google Scholar]
- 25.Kraus VB, Huebner JL, Stabler T, Flahiff CM, Setton LA, Fink C, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50:1822–1831. doi: 10.1002/art.20291. [DOI] [PubMed] [Google Scholar]
- 26.Brenner RE, Vetter U, Nerlich A, Worsdorfer O, Teller WM, Muller PK. Biochemical analysis of callus tissue in osteogenesis imperfecta type IV. Evidence for transient overmodification in collagen types I and III. J Clin Invest. 1989;84:915–921. doi: 10.1172/JCI114253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 28.Hui-Chou CS, Lust G. The type of collagen made by the articular cartilage in joints of dogs with degenerative joint disease. Coll Relat Res. 1982;2:245–256. doi: 10.1016/s0174-173x(82)80018-6. [DOI] [PubMed] [Google Scholar]
- 29.Fujioka R, Aoyama T, Takakuwa T. The layered structure of the articular surface. Osteoarthritis Cartilage. 2013;21:1092–1098. doi: 10.1016/j.joca.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Wu JJ, Weis MA, Kim LS, Eyre DR. Type III collagen, a fibril network modifier in articular cartilage. J Biol Chem. 2010;285:18537–18544. doi: 10.1074/jbc.M110.112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charni N, Juillet F, Garnero P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2005;52:1081–1090. doi: 10.1002/art.20930. [DOI] [PubMed] [Google Scholar]
- 32.Eyre DR, Weis MA. The Helix-II epitope: a cautionary tale from a cartilage biomarker based on an invalid collagen sequence. Osteoarthritis Cartilage. 2009;17:423–426. doi: 10.1016/j.joca.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson F, Dahlberg L, Laverty S, Reiner A, Pidoux I, Ionescu M, et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J Clin Invest. 1998;102:2115–2125. doi: 10.1172/JCI4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukui N, Ikeda Y, Ohnuki T, Tanaka N, Hikita A, Mitomi H, et al. Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum. 2008;58:154–163. doi: 10.1002/art.23175. [DOI] [PubMed] [Google Scholar]
- 35.Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90:463–479. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


